User login
Listen up: Acoustic device useful for diabetic foot ulcers
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
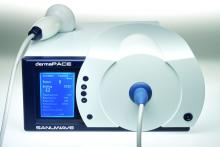
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
A Sore Subject
For several years, a 60-year-old woman has had a nonhealing, asymptomatic “sore” on her upper right cheek. The lesion causes no pain or discomfort; it bothers her simply because it will not go away. It does occasionally bleed.
Several attempts at treatment—including antibiotic ointment, peroxide, and topical alcohol— have failed. A dermatologist once treated the lesion with cryotherapy; this initially reduced its size, but the effect didn’t last.
The patient admits to “worshipping the sun” as a youngster, tanning at every opportunity. Several family members, including her sister and mother, have had skin cancer.
EXAMINATION
A 6.5-mm, round, red nodule is located on the mid-upper right cheek, below the eye. On closer inspection, it appears glassy and translucent, with several obvious telangiectasias. It is surprisingly firm on palpation, though not at all tender to touch.
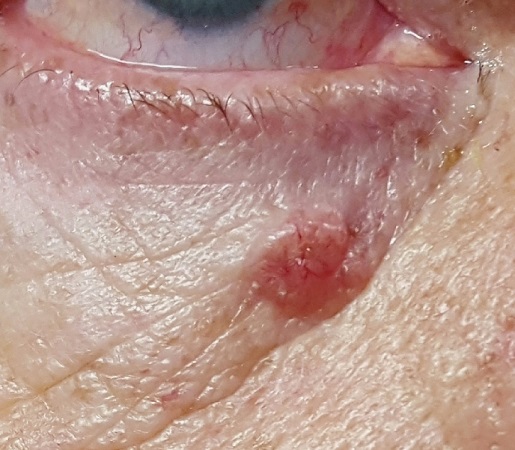
Elsewhere, the patient’s fair skin has abundant evidence of sun damage, including wrinkles, discoloration, and focal telangiectasias. No other lesions are seen. No nodes are palpable in her head or neck.
With the patient’s permission, and after discussion of the indications, procedure, alternatives, and risks, the lesion is removed by curettement. It is quite friable and shallow, allowing complete removal. The area is left to heal by secondary intention, and the specimen is submitted to pathology.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the suspected diagnosis: noduloulcerative basal cell carcinoma (BCC). By far the most common type of skin cancer in the world, noduloulcerative BCCs are caused by overexposure to the sun.
As obvious as this diagnosis seemed to be, biopsy was warranted to confirm it and to rule out several other items in the differential. The latter include squamous cell carcinoma (which has far more harmful potential than BCC), sebaceous carcinoma, Merkel cell carcinoma, and melanoma.
Not all BCCs are created equal—certain clinical and histologic characteristics predict a more aggressive course. While most BCCs require surgical excision, other treatment options do exist. In this patient’s case, the size and location of the lesion lent it to curettement and healing by secondary intention. Other methods could have included immunotherapy with imiquimod, antitumor creams (eg, 5-fluorouracil), or even radiation therapy. Her lesion was small and well-defined enough that Mohs surgery was not indicated—though some might disagree with that assessment.
TAKE-HOME LEARNING POINTS
- Like all basal cell carcinomas (BCCs), ulcerative BCC—the most common type—is caused by overexposure to the sun.
- BCCs are often mistaken for infection or other sore, which delays the diagnosis.
- Nonhealing lesions should be considered cancerous until proven otherwise.
- Though this diagnosis was fairly obvious, biopsy is necessary to rule out other items in the differential—some of which (ie, Merkel cell carcinoma, melanoma) are potentially fatal.
For several years, a 60-year-old woman has had a nonhealing, asymptomatic “sore” on her upper right cheek. The lesion causes no pain or discomfort; it bothers her simply because it will not go away. It does occasionally bleed.
Several attempts at treatment—including antibiotic ointment, peroxide, and topical alcohol— have failed. A dermatologist once treated the lesion with cryotherapy; this initially reduced its size, but the effect didn’t last.
The patient admits to “worshipping the sun” as a youngster, tanning at every opportunity. Several family members, including her sister and mother, have had skin cancer.
EXAMINATION
A 6.5-mm, round, red nodule is located on the mid-upper right cheek, below the eye. On closer inspection, it appears glassy and translucent, with several obvious telangiectasias. It is surprisingly firm on palpation, though not at all tender to touch.

Elsewhere, the patient’s fair skin has abundant evidence of sun damage, including wrinkles, discoloration, and focal telangiectasias. No other lesions are seen. No nodes are palpable in her head or neck.
With the patient’s permission, and after discussion of the indications, procedure, alternatives, and risks, the lesion is removed by curettement. It is quite friable and shallow, allowing complete removal. The area is left to heal by secondary intention, and the specimen is submitted to pathology.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the suspected diagnosis: noduloulcerative basal cell carcinoma (BCC). By far the most common type of skin cancer in the world, noduloulcerative BCCs are caused by overexposure to the sun.
As obvious as this diagnosis seemed to be, biopsy was warranted to confirm it and to rule out several other items in the differential. The latter include squamous cell carcinoma (which has far more harmful potential than BCC), sebaceous carcinoma, Merkel cell carcinoma, and melanoma.
Not all BCCs are created equal—certain clinical and histologic characteristics predict a more aggressive course. While most BCCs require surgical excision, other treatment options do exist. In this patient’s case, the size and location of the lesion lent it to curettement and healing by secondary intention. Other methods could have included immunotherapy with imiquimod, antitumor creams (eg, 5-fluorouracil), or even radiation therapy. Her lesion was small and well-defined enough that Mohs surgery was not indicated—though some might disagree with that assessment.
TAKE-HOME LEARNING POINTS
- Like all basal cell carcinomas (BCCs), ulcerative BCC—the most common type—is caused by overexposure to the sun.
- BCCs are often mistaken for infection or other sore, which delays the diagnosis.
- Nonhealing lesions should be considered cancerous until proven otherwise.
- Though this diagnosis was fairly obvious, biopsy is necessary to rule out other items in the differential—some of which (ie, Merkel cell carcinoma, melanoma) are potentially fatal.
For several years, a 60-year-old woman has had a nonhealing, asymptomatic “sore” on her upper right cheek. The lesion causes no pain or discomfort; it bothers her simply because it will not go away. It does occasionally bleed.
Several attempts at treatment—including antibiotic ointment, peroxide, and topical alcohol— have failed. A dermatologist once treated the lesion with cryotherapy; this initially reduced its size, but the effect didn’t last.
The patient admits to “worshipping the sun” as a youngster, tanning at every opportunity. Several family members, including her sister and mother, have had skin cancer.
EXAMINATION
A 6.5-mm, round, red nodule is located on the mid-upper right cheek, below the eye. On closer inspection, it appears glassy and translucent, with several obvious telangiectasias. It is surprisingly firm on palpation, though not at all tender to touch.

Elsewhere, the patient’s fair skin has abundant evidence of sun damage, including wrinkles, discoloration, and focal telangiectasias. No other lesions are seen. No nodes are palpable in her head or neck.
With the patient’s permission, and after discussion of the indications, procedure, alternatives, and risks, the lesion is removed by curettement. It is quite friable and shallow, allowing complete removal. The area is left to heal by secondary intention, and the specimen is submitted to pathology.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the suspected diagnosis: noduloulcerative basal cell carcinoma (BCC). By far the most common type of skin cancer in the world, noduloulcerative BCCs are caused by overexposure to the sun.
As obvious as this diagnosis seemed to be, biopsy was warranted to confirm it and to rule out several other items in the differential. The latter include squamous cell carcinoma (which has far more harmful potential than BCC), sebaceous carcinoma, Merkel cell carcinoma, and melanoma.
Not all BCCs are created equal—certain clinical and histologic characteristics predict a more aggressive course. While most BCCs require surgical excision, other treatment options do exist. In this patient’s case, the size and location of the lesion lent it to curettement and healing by secondary intention. Other methods could have included immunotherapy with imiquimod, antitumor creams (eg, 5-fluorouracil), or even radiation therapy. Her lesion was small and well-defined enough that Mohs surgery was not indicated—though some might disagree with that assessment.
TAKE-HOME LEARNING POINTS
- Like all basal cell carcinomas (BCCs), ulcerative BCC—the most common type—is caused by overexposure to the sun.
- BCCs are often mistaken for infection or other sore, which delays the diagnosis.
- Nonhealing lesions should be considered cancerous until proven otherwise.
- Though this diagnosis was fairly obvious, biopsy is necessary to rule out other items in the differential—some of which (ie, Merkel cell carcinoma, melanoma) are potentially fatal.
Gastric bypass T2D benefit can fade over time
but the effect diminished over time, according to findings published Jan. 16 in JAMA.
In a randomized study of 113 obese patients with diabetes, about 50% of those who received gastric bypass in addition to lifestyle and medical management achieved the composite endpoint of a hemoglobin A1c (HbA1c) value of less than 7%, an LDL cholesterol level of less than 100 mg/dL, and a systolic blood pressure of less than 130 mm Hg after 1 year, reported Sayeed Ikramuddin, MD, FACS, of the department of surgery at the University of Minnesota, Minneapolis, and his coauthors. For comparison, just 16% in the lifestyle/medical management group achieved the endpoint (difference, 34%; 95% confidence interval, 14%-54%; P = .003) .
At 5 years’ follow-up, about 23% of patients in the gastric bypass group and 4% in the lifestyle/medical management group achieved the composite triple endpoint (difference, 19%; 95% CI, 4%-34%; P = .01), the authors reported.
The study included 120 patients at four sites in the United States and Taiwan, 7 of whom either died or were lost to follow-up before completion of the study. Participants had an HbA1c level of 8% or higher and a body mass index between 30 and 39.9 kg/m2.
Patients were randomized to receive either 2 years of lifestyle and medical management alone or in conjunction with standardized Roux-en-Y gastric bypass. During the first 2 years of intervention, patients were told to record weight, exercise, and food intake and were prescribed 325 minutes of physical activity per week. Participants also met regularly with a trained interventionist and an endocrinologist and were given pharmacologic therapy for hyperglycemia, cholesterol, and hypertension, the authors said. Aside from usual visits with a primary physician, all study interventions ceased after the initial 2-year period.
At baseline, the group that received only lifestyle/medical management had a mean BMI of 34.4 and HbA1c level of 9.6%, compared with a mean BMI of 34.9 and HbA1c level of 9.6% in the gastric bypass group.
Primary endpoint success rates decreased in both groups between years 1 and 3, going from 50% to 23% in the gastric bypass group and from 16% to 4% in the lifestyle/medical management group, but it remained stable from year 3 through year 5, Dr. Ikramuddin and his coauthors said in the report.
Overall, 26% of patients who had gastric bypass surgery during the first year achieved the triple endpoint at 5 years, compared with 8% of those who did not have surgery (difference, 18%; 95% CI, 6%-32%; P = .04).
The mean weight loss for participants in the gastric bypass group was 21.8% at 5 years, compared with 9.6% in the lifestyle/medical management group (difference, 12.2%; 95% CI, 8.9%-15.5%).
The results suggest that “gastric bypass provides significant benefit but with a smaller and less durable effect size than what is seen in the evaluation of glycemic control alone,” the authors wrote.
“Because the effect size diminished over 5 years, further follow-up is needed to understand the durability of the improvement,” Dr. Ikramuddin and his colleagues concluded.
Dr. Ikramuddin disclosed relationships with Novo Nordisk, USGI Medical, Medica, Metamodix, Medtronic, ReShape Medical, and EnteroMedics.
SOURCE: Ikramuddin S. JAMA. 2018;319(3):266-278.
but the effect diminished over time, according to findings published Jan. 16 in JAMA.
In a randomized study of 113 obese patients with diabetes, about 50% of those who received gastric bypass in addition to lifestyle and medical management achieved the composite endpoint of a hemoglobin A1c (HbA1c) value of less than 7%, an LDL cholesterol level of less than 100 mg/dL, and a systolic blood pressure of less than 130 mm Hg after 1 year, reported Sayeed Ikramuddin, MD, FACS, of the department of surgery at the University of Minnesota, Minneapolis, and his coauthors. For comparison, just 16% in the lifestyle/medical management group achieved the endpoint (difference, 34%; 95% confidence interval, 14%-54%; P = .003) .
At 5 years’ follow-up, about 23% of patients in the gastric bypass group and 4% in the lifestyle/medical management group achieved the composite triple endpoint (difference, 19%; 95% CI, 4%-34%; P = .01), the authors reported.
The study included 120 patients at four sites in the United States and Taiwan, 7 of whom either died or were lost to follow-up before completion of the study. Participants had an HbA1c level of 8% or higher and a body mass index between 30 and 39.9 kg/m2.
Patients were randomized to receive either 2 years of lifestyle and medical management alone or in conjunction with standardized Roux-en-Y gastric bypass. During the first 2 years of intervention, patients were told to record weight, exercise, and food intake and were prescribed 325 minutes of physical activity per week. Participants also met regularly with a trained interventionist and an endocrinologist and were given pharmacologic therapy for hyperglycemia, cholesterol, and hypertension, the authors said. Aside from usual visits with a primary physician, all study interventions ceased after the initial 2-year period.
At baseline, the group that received only lifestyle/medical management had a mean BMI of 34.4 and HbA1c level of 9.6%, compared with a mean BMI of 34.9 and HbA1c level of 9.6% in the gastric bypass group.
Primary endpoint success rates decreased in both groups between years 1 and 3, going from 50% to 23% in the gastric bypass group and from 16% to 4% in the lifestyle/medical management group, but it remained stable from year 3 through year 5, Dr. Ikramuddin and his coauthors said in the report.
Overall, 26% of patients who had gastric bypass surgery during the first year achieved the triple endpoint at 5 years, compared with 8% of those who did not have surgery (difference, 18%; 95% CI, 6%-32%; P = .04).
The mean weight loss for participants in the gastric bypass group was 21.8% at 5 years, compared with 9.6% in the lifestyle/medical management group (difference, 12.2%; 95% CI, 8.9%-15.5%).
The results suggest that “gastric bypass provides significant benefit but with a smaller and less durable effect size than what is seen in the evaluation of glycemic control alone,” the authors wrote.
“Because the effect size diminished over 5 years, further follow-up is needed to understand the durability of the improvement,” Dr. Ikramuddin and his colleagues concluded.
Dr. Ikramuddin disclosed relationships with Novo Nordisk, USGI Medical, Medica, Metamodix, Medtronic, ReShape Medical, and EnteroMedics.
SOURCE: Ikramuddin S. JAMA. 2018;319(3):266-278.
but the effect diminished over time, according to findings published Jan. 16 in JAMA.
In a randomized study of 113 obese patients with diabetes, about 50% of those who received gastric bypass in addition to lifestyle and medical management achieved the composite endpoint of a hemoglobin A1c (HbA1c) value of less than 7%, an LDL cholesterol level of less than 100 mg/dL, and a systolic blood pressure of less than 130 mm Hg after 1 year, reported Sayeed Ikramuddin, MD, FACS, of the department of surgery at the University of Minnesota, Minneapolis, and his coauthors. For comparison, just 16% in the lifestyle/medical management group achieved the endpoint (difference, 34%; 95% confidence interval, 14%-54%; P = .003) .
At 5 years’ follow-up, about 23% of patients in the gastric bypass group and 4% in the lifestyle/medical management group achieved the composite triple endpoint (difference, 19%; 95% CI, 4%-34%; P = .01), the authors reported.
The study included 120 patients at four sites in the United States and Taiwan, 7 of whom either died or were lost to follow-up before completion of the study. Participants had an HbA1c level of 8% or higher and a body mass index between 30 and 39.9 kg/m2.
Patients were randomized to receive either 2 years of lifestyle and medical management alone or in conjunction with standardized Roux-en-Y gastric bypass. During the first 2 years of intervention, patients were told to record weight, exercise, and food intake and were prescribed 325 minutes of physical activity per week. Participants also met regularly with a trained interventionist and an endocrinologist and were given pharmacologic therapy for hyperglycemia, cholesterol, and hypertension, the authors said. Aside from usual visits with a primary physician, all study interventions ceased after the initial 2-year period.
At baseline, the group that received only lifestyle/medical management had a mean BMI of 34.4 and HbA1c level of 9.6%, compared with a mean BMI of 34.9 and HbA1c level of 9.6% in the gastric bypass group.
Primary endpoint success rates decreased in both groups between years 1 and 3, going from 50% to 23% in the gastric bypass group and from 16% to 4% in the lifestyle/medical management group, but it remained stable from year 3 through year 5, Dr. Ikramuddin and his coauthors said in the report.
Overall, 26% of patients who had gastric bypass surgery during the first year achieved the triple endpoint at 5 years, compared with 8% of those who did not have surgery (difference, 18%; 95% CI, 6%-32%; P = .04).
The mean weight loss for participants in the gastric bypass group was 21.8% at 5 years, compared with 9.6% in the lifestyle/medical management group (difference, 12.2%; 95% CI, 8.9%-15.5%).
The results suggest that “gastric bypass provides significant benefit but with a smaller and less durable effect size than what is seen in the evaluation of glycemic control alone,” the authors wrote.
“Because the effect size diminished over 5 years, further follow-up is needed to understand the durability of the improvement,” Dr. Ikramuddin and his colleagues concluded.
Dr. Ikramuddin disclosed relationships with Novo Nordisk, USGI Medical, Medica, Metamodix, Medtronic, ReShape Medical, and EnteroMedics.
SOURCE: Ikramuddin S. JAMA. 2018;319(3):266-278.
FROM JAMA
Key clinical point: Adding gastric bypass surgery to lifestyle and medical management improves diabetes outcomes – but with diminished effect over time.
Major finding: Primary endpoint success rates decreased in both groups between years 1 and 3, going from 50% to 23% in the gastric bypass group and from 16% to 4% in the lifestyle/medical management group.
Data source: A randomized study of 113 patients at four sites in the United States and Taiwan with a HbA1c level of 8% or higher and a BMI of 30-39.9 kg/m2.
Disclosures: Dr. Ikramuddin disclosed relationships with Novo Nordisk, USGI Medical, Medica, Metamodix, Medtronic, ReShape Medical, and EnteroMedics
SOURCE: Ikramuddin S et al. JAMA. 2018;319(3):266-278.
Risk-stratification tool predicts severe hypoglycemia
Background: Severe hypoglycemia caused by glucose-lowering medications is a known public health and patient safety issue. Identifying patients with T2D at risk of severe hypoglycemia might facilitate interventions to offset that risk.
Study design: Prospective cohort.
Setting: Kaiser Permanente Northern California (derivation and internal validation cohort); Veterans Affairs Diabetes Epidemiology Cohort and Group Health Cooperative (external validation cohorts).
Synopsis: Through EHR data, 206,435 eligible patients with T2D were randomly split into derivation (80%) and internal validation (20%) samples. EHR data were reviewed for preselected clinical risk factors for hypoglycemia with a primary outcome of ED visit or hospital admission with a primary diagnosis of hypoglycemia over the ensuing year. A predictive tool was built based on six variables: prior hypoglycemia episodes, number of ED encounters for any reason in the prior year, insulin use, sulfonylurea use, presence of severe or end-stage kidney disease, and age. Predicted 12-month risk was categorized as high (greater than 5%), intermediate (1%-5%) or low (less than 1%). In the internal validation sample, 2.0%, 10.7%, and 87.3% of patients were categorized as high, intermediate, and low risk, respectively. Observed 12-month hypoglycemia-related health care utilization rates were 6.7%, 1.4%, and 0.2%, respectively. The external validation cohorts performed similarly.
Bottom line: A simple tool using readily available data can be used to estimate the 12-month risk of severe hypoglycemia in patients with T2D.
Citation: Karter AJ et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med. 2017 Oct 1;177(10):1461-70.
Dr. Hoegh is a hospitalist at the University of Colorado School of Medicine.
Background: Severe hypoglycemia caused by glucose-lowering medications is a known public health and patient safety issue. Identifying patients with T2D at risk of severe hypoglycemia might facilitate interventions to offset that risk.
Study design: Prospective cohort.
Setting: Kaiser Permanente Northern California (derivation and internal validation cohort); Veterans Affairs Diabetes Epidemiology Cohort and Group Health Cooperative (external validation cohorts).
Synopsis: Through EHR data, 206,435 eligible patients with T2D were randomly split into derivation (80%) and internal validation (20%) samples. EHR data were reviewed for preselected clinical risk factors for hypoglycemia with a primary outcome of ED visit or hospital admission with a primary diagnosis of hypoglycemia over the ensuing year. A predictive tool was built based on six variables: prior hypoglycemia episodes, number of ED encounters for any reason in the prior year, insulin use, sulfonylurea use, presence of severe or end-stage kidney disease, and age. Predicted 12-month risk was categorized as high (greater than 5%), intermediate (1%-5%) or low (less than 1%). In the internal validation sample, 2.0%, 10.7%, and 87.3% of patients were categorized as high, intermediate, and low risk, respectively. Observed 12-month hypoglycemia-related health care utilization rates were 6.7%, 1.4%, and 0.2%, respectively. The external validation cohorts performed similarly.
Bottom line: A simple tool using readily available data can be used to estimate the 12-month risk of severe hypoglycemia in patients with T2D.
Citation: Karter AJ et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med. 2017 Oct 1;177(10):1461-70.
Dr. Hoegh is a hospitalist at the University of Colorado School of Medicine.
Background: Severe hypoglycemia caused by glucose-lowering medications is a known public health and patient safety issue. Identifying patients with T2D at risk of severe hypoglycemia might facilitate interventions to offset that risk.
Study design: Prospective cohort.
Setting: Kaiser Permanente Northern California (derivation and internal validation cohort); Veterans Affairs Diabetes Epidemiology Cohort and Group Health Cooperative (external validation cohorts).
Synopsis: Through EHR data, 206,435 eligible patients with T2D were randomly split into derivation (80%) and internal validation (20%) samples. EHR data were reviewed for preselected clinical risk factors for hypoglycemia with a primary outcome of ED visit or hospital admission with a primary diagnosis of hypoglycemia over the ensuing year. A predictive tool was built based on six variables: prior hypoglycemia episodes, number of ED encounters for any reason in the prior year, insulin use, sulfonylurea use, presence of severe or end-stage kidney disease, and age. Predicted 12-month risk was categorized as high (greater than 5%), intermediate (1%-5%) or low (less than 1%). In the internal validation sample, 2.0%, 10.7%, and 87.3% of patients were categorized as high, intermediate, and low risk, respectively. Observed 12-month hypoglycemia-related health care utilization rates were 6.7%, 1.4%, and 0.2%, respectively. The external validation cohorts performed similarly.
Bottom line: A simple tool using readily available data can be used to estimate the 12-month risk of severe hypoglycemia in patients with T2D.
Citation: Karter AJ et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med. 2017 Oct 1;177(10):1461-70.
Dr. Hoegh is a hospitalist at the University of Colorado School of Medicine.
Five pearls target wound healing
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
EXPERT ANALYSIS FROM ODAC 2018
Hospitalist movers and shakers – Jan. 2018
Michael Rader, MD, has been named the chief medical officer of the Department of Veterans Affairs New York/New Jersey Health Care Network. The network serves upward of a half-million veterans in 76 counties in New York, New Jersey, and Pennsylvania. Dr. Rader’s appointment began on Oct. 15, 2017.
Previously, Dr. Rader was chief medical officer at Prospect East Orange (N.J.) General. In his new position, the 35-year veteran will be charged with overseeing care at VA facilities in Albany Stratton, Bath, Canandaigua, and Syracuse in New York, as well as the VA Western New York Health System, the New York Harbor Health System, the New Jersey Health Care System, Hudson Valley Healthcare System, the James J. Peters and Northport VA Medical Centers, as well as 66 community-based outpatient clinics.
The Rhode Island Medical Society has elected Bradley Collins, MD, as its new president. An internist and hospitalist, Dr. Collins practices at Miriam Hospital in Providence, R.I., where he started in 2006 as a staff hospitalist. He’s now the medical director of appeals for Lifespan at Miriam.
Dr. Collins is an assistant professor of clinical medicine at Brown University’s Alpert Medical School, while also serving as a fellow for the Society of Hospital Medicine.
Tracy Cardin, ACNP, SFHM, has been named associate director of clinical integration at Adfinitas Health, a private hospitalist company based in Maryland that serves more than 50 hospitals and post-acute care centers across the Mid-Atlantic region. Cardin is responsible for advancing the company’s training and onboarding infrastructure to support the full integration of physicians, nurse practitioners, and physician assistants into the Adfinitas care delivery model.
Jeffrey Millard, MD, has been named Patient Experience Provider of the Year by the employees and staff at Hardin Memorial Health (Elizabethtown, Ky.). Dr. Millard has been a hospitalist at Hardin Memorial Hospital since 2012.
The Patient Experience Provider of the Year award recognizes a provider who exceeds the company’s mission and vision with patients and their families, as well as with the hospital’s staff. Dr. Millard was chosen from a list of more than 800 nominations.
Benjamin Keidan, MD, has been appointed as chief medical officer for Boulder (Colo.) Community Health. Dr. Keidan advances from his previous role as medical director of quality and population health for outpatient primary care and specialty clinics.
Dr. Keidan is a former internist and hospitalist for BCH and has worked in Boulder County for the past 12 years. He is only the second CMO in BCH’s history.
Dinesh Bande, MD, has been selected as the new chair of the department of internal medicine at the University of North Dakota, Grand Forks. Dr. Bande is a clinical associate professor at the school and a hospitalist with Sanford Health.
Dr. Bande has been the clerkship director for third-year medical students at UND for the past 2 years. As chair of internal medicine, he will oversee education, research, clinical care, training, and service programs within the department.
Vicki Iannotti, MD, has been named chief medical officer at the Elizabeth Ann Seton Pediatric Center in Yonkers, N.Y. Dr. Iannotti joins Seton after spending 12 years as associate chief of pediatrics and pediatric hospital medicine at Maria Fareri Children’s Hospital in Valhalla, N.Y.
At Seton, Dr. Iannotti will oversee 100 medical professionals at the 201,000-square-foot facility, which is the largest provider of children’s acute care in the United States.
Business moves
Management Service Organization Continuum Health (Marlton, N.J.) has signed an agreement with the Mid-Atlantic region’s largest private hospitalist group, Adfinitas Health (Hanover, Md.), to be its revenue management cycle partner. Founded in 1999, Continuum Health now serves more than 1,500 providers in more than 400 locations.
Colquitt Regional Medical Center in Moultrie, Ga., has expanded its hospitalist program, adding 5 physicians to increase its total to 10 on-staff hospitalists. Colquitt Regional’s program began in 2012 with four hospitalists.
In the past, Colquitt Regional has shared hospitalists with surrounding hospitals to meet the demand for care. With the addition of the five new physicians, the hospital can provide full-time hospital medicine care with physicians employed by Colquitt Regional.
Michael Rader, MD, has been named the chief medical officer of the Department of Veterans Affairs New York/New Jersey Health Care Network. The network serves upward of a half-million veterans in 76 counties in New York, New Jersey, and Pennsylvania. Dr. Rader’s appointment began on Oct. 15, 2017.
Previously, Dr. Rader was chief medical officer at Prospect East Orange (N.J.) General. In his new position, the 35-year veteran will be charged with overseeing care at VA facilities in Albany Stratton, Bath, Canandaigua, and Syracuse in New York, as well as the VA Western New York Health System, the New York Harbor Health System, the New Jersey Health Care System, Hudson Valley Healthcare System, the James J. Peters and Northport VA Medical Centers, as well as 66 community-based outpatient clinics.
The Rhode Island Medical Society has elected Bradley Collins, MD, as its new president. An internist and hospitalist, Dr. Collins practices at Miriam Hospital in Providence, R.I., where he started in 2006 as a staff hospitalist. He’s now the medical director of appeals for Lifespan at Miriam.
Dr. Collins is an assistant professor of clinical medicine at Brown University’s Alpert Medical School, while also serving as a fellow for the Society of Hospital Medicine.
Tracy Cardin, ACNP, SFHM, has been named associate director of clinical integration at Adfinitas Health, a private hospitalist company based in Maryland that serves more than 50 hospitals and post-acute care centers across the Mid-Atlantic region. Cardin is responsible for advancing the company’s training and onboarding infrastructure to support the full integration of physicians, nurse practitioners, and physician assistants into the Adfinitas care delivery model.
Jeffrey Millard, MD, has been named Patient Experience Provider of the Year by the employees and staff at Hardin Memorial Health (Elizabethtown, Ky.). Dr. Millard has been a hospitalist at Hardin Memorial Hospital since 2012.
The Patient Experience Provider of the Year award recognizes a provider who exceeds the company’s mission and vision with patients and their families, as well as with the hospital’s staff. Dr. Millard was chosen from a list of more than 800 nominations.
Benjamin Keidan, MD, has been appointed as chief medical officer for Boulder (Colo.) Community Health. Dr. Keidan advances from his previous role as medical director of quality and population health for outpatient primary care and specialty clinics.
Dr. Keidan is a former internist and hospitalist for BCH and has worked in Boulder County for the past 12 years. He is only the second CMO in BCH’s history.
Dinesh Bande, MD, has been selected as the new chair of the department of internal medicine at the University of North Dakota, Grand Forks. Dr. Bande is a clinical associate professor at the school and a hospitalist with Sanford Health.
Dr. Bande has been the clerkship director for third-year medical students at UND for the past 2 years. As chair of internal medicine, he will oversee education, research, clinical care, training, and service programs within the department.
Vicki Iannotti, MD, has been named chief medical officer at the Elizabeth Ann Seton Pediatric Center in Yonkers, N.Y. Dr. Iannotti joins Seton after spending 12 years as associate chief of pediatrics and pediatric hospital medicine at Maria Fareri Children’s Hospital in Valhalla, N.Y.
At Seton, Dr. Iannotti will oversee 100 medical professionals at the 201,000-square-foot facility, which is the largest provider of children’s acute care in the United States.
Business moves
Management Service Organization Continuum Health (Marlton, N.J.) has signed an agreement with the Mid-Atlantic region’s largest private hospitalist group, Adfinitas Health (Hanover, Md.), to be its revenue management cycle partner. Founded in 1999, Continuum Health now serves more than 1,500 providers in more than 400 locations.
Colquitt Regional Medical Center in Moultrie, Ga., has expanded its hospitalist program, adding 5 physicians to increase its total to 10 on-staff hospitalists. Colquitt Regional’s program began in 2012 with four hospitalists.
In the past, Colquitt Regional has shared hospitalists with surrounding hospitals to meet the demand for care. With the addition of the five new physicians, the hospital can provide full-time hospital medicine care with physicians employed by Colquitt Regional.
Michael Rader, MD, has been named the chief medical officer of the Department of Veterans Affairs New York/New Jersey Health Care Network. The network serves upward of a half-million veterans in 76 counties in New York, New Jersey, and Pennsylvania. Dr. Rader’s appointment began on Oct. 15, 2017.
Previously, Dr. Rader was chief medical officer at Prospect East Orange (N.J.) General. In his new position, the 35-year veteran will be charged with overseeing care at VA facilities in Albany Stratton, Bath, Canandaigua, and Syracuse in New York, as well as the VA Western New York Health System, the New York Harbor Health System, the New Jersey Health Care System, Hudson Valley Healthcare System, the James J. Peters and Northport VA Medical Centers, as well as 66 community-based outpatient clinics.
The Rhode Island Medical Society has elected Bradley Collins, MD, as its new president. An internist and hospitalist, Dr. Collins practices at Miriam Hospital in Providence, R.I., where he started in 2006 as a staff hospitalist. He’s now the medical director of appeals for Lifespan at Miriam.
Dr. Collins is an assistant professor of clinical medicine at Brown University’s Alpert Medical School, while also serving as a fellow for the Society of Hospital Medicine.
Tracy Cardin, ACNP, SFHM, has been named associate director of clinical integration at Adfinitas Health, a private hospitalist company based in Maryland that serves more than 50 hospitals and post-acute care centers across the Mid-Atlantic region. Cardin is responsible for advancing the company’s training and onboarding infrastructure to support the full integration of physicians, nurse practitioners, and physician assistants into the Adfinitas care delivery model.
Jeffrey Millard, MD, has been named Patient Experience Provider of the Year by the employees and staff at Hardin Memorial Health (Elizabethtown, Ky.). Dr. Millard has been a hospitalist at Hardin Memorial Hospital since 2012.
The Patient Experience Provider of the Year award recognizes a provider who exceeds the company’s mission and vision with patients and their families, as well as with the hospital’s staff. Dr. Millard was chosen from a list of more than 800 nominations.
Benjamin Keidan, MD, has been appointed as chief medical officer for Boulder (Colo.) Community Health. Dr. Keidan advances from his previous role as medical director of quality and population health for outpatient primary care and specialty clinics.
Dr. Keidan is a former internist and hospitalist for BCH and has worked in Boulder County for the past 12 years. He is only the second CMO in BCH’s history.
Dinesh Bande, MD, has been selected as the new chair of the department of internal medicine at the University of North Dakota, Grand Forks. Dr. Bande is a clinical associate professor at the school and a hospitalist with Sanford Health.
Dr. Bande has been the clerkship director for third-year medical students at UND for the past 2 years. As chair of internal medicine, he will oversee education, research, clinical care, training, and service programs within the department.
Vicki Iannotti, MD, has been named chief medical officer at the Elizabeth Ann Seton Pediatric Center in Yonkers, N.Y. Dr. Iannotti joins Seton after spending 12 years as associate chief of pediatrics and pediatric hospital medicine at Maria Fareri Children’s Hospital in Valhalla, N.Y.
At Seton, Dr. Iannotti will oversee 100 medical professionals at the 201,000-square-foot facility, which is the largest provider of children’s acute care in the United States.
Business moves
Management Service Organization Continuum Health (Marlton, N.J.) has signed an agreement with the Mid-Atlantic region’s largest private hospitalist group, Adfinitas Health (Hanover, Md.), to be its revenue management cycle partner. Founded in 1999, Continuum Health now serves more than 1,500 providers in more than 400 locations.
Colquitt Regional Medical Center in Moultrie, Ga., has expanded its hospitalist program, adding 5 physicians to increase its total to 10 on-staff hospitalists. Colquitt Regional’s program began in 2012 with four hospitalists.
In the past, Colquitt Regional has shared hospitalists with surrounding hospitals to meet the demand for care. With the addition of the five new physicians, the hospital can provide full-time hospital medicine care with physicians employed by Colquitt Regional.
Transcatheter aortic valve-in-ring for mitral disease a winner
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
REPORTING FROM TCT 2017
Key clinical point: .
Major finding: Thirty-day all-cause mortality following a transcatheter valve-in-ring procedure in unacceptably high surgical-risk patients with severe mitral valve disease due to a failing annuloplasty ring was 6.8%.
Study details: This prospective observational study included 60 patients who underwent transcatheter mitral valve replacement for severe mitral valve disease, half due to a failed annuloplasty ring and half secondary to mitral annular calcification.
Disclosures: The MITRAL trial was partially supported by Edwards Lifesciences. The study presenter reported receiving a research grant from the company.
Source: Guerrero M. No abstract.
Atopic dermatitis linked to psychiatric disorders but not hospitalizations
Atopic dermatitis (AD) is associated with anxiety, depression, and suicidal thoughts but does not lead to increased psychiatric hospitalization or suicide, according to a Danish study.
While previous literature has indicated a connection between AD and depressive symptoms, these findings indicated the psychiatric burden is not severe.
A total of 9,656 patients selected from the Danish study of Functional Disorders (DanFunD) during 2011-2015 were asked to fill out a questionnaire concerning clinical diagnoses and psychiatric symptoms.
Investigators also conducted an analysis of 4,259,457 patient records gathered the Danish National Patient Register.
The 1,044 AD patients from the DanFunD were slightly younger on average, at 50 years, compared with an average of 53 years in the 8,612-person control group; there were more women (65%) in the AD group, compared with a relatively even split (53%) in the control group.
Patients with AD were more likely to have clinically diagnosed depression (odds ratio, 1.76) or anxiety (OR, 1.61) than were those in the control group.
The report was published in the journal Allergy.
Among the 568 individuals with symptoms consistent of major depressive disorder, those with AD (112) were almost twice as likely to report these symptoms on the questionnaire (OR, 1.92) and more than twice as likely to report anxiety attacks (OR, 2.32), despite differences in psychiatric hospitalizations remaining insignificant.
The investigators hypothesized the treatment methods for AD patients may be part of the reason that suicide and hospitalization rates were not higher.
“These more severe outcomes of suicide and psychiatric hospitalization could be mitigated in AD patients due to their increased general health care and psychiatric health care utilization leading to, for example, earlier and more aggressive antidepressant therapy,” according to Dr. Thyssen and his fellow investigators.
In an analysis of the general Danish population, medication was most aggressive in patients with moderate-severe AD, who were significantly more likely to use antidepressants (hazard ratio,1.24) and anxiolytic drugs (HR, 1.66), while those with milder symptoms had only a slightly increased likelihood.
Because of the observational nature of the study, investigators could not determine what the specific cause of these psychiatric symptoms were.
The DanFunD Study was supported by TrygFonden and Lundbeck Foundation. Dr. Thyssen is supported by a grant from the Lundbeck Foundation. Some of the investigators received financial support from various pharmaceutical companies or their foundations. The remaining investigators reported no relevant financial disclosures.
SOURCE: Thyssen JP et al. Allergy. 2018 Jan;73(1):214-20.
Atopic dermatitis (AD) is associated with anxiety, depression, and suicidal thoughts but does not lead to increased psychiatric hospitalization or suicide, according to a Danish study.
While previous literature has indicated a connection between AD and depressive symptoms, these findings indicated the psychiatric burden is not severe.
A total of 9,656 patients selected from the Danish study of Functional Disorders (DanFunD) during 2011-2015 were asked to fill out a questionnaire concerning clinical diagnoses and psychiatric symptoms.
Investigators also conducted an analysis of 4,259,457 patient records gathered the Danish National Patient Register.
The 1,044 AD patients from the DanFunD were slightly younger on average, at 50 years, compared with an average of 53 years in the 8,612-person control group; there were more women (65%) in the AD group, compared with a relatively even split (53%) in the control group.
Patients with AD were more likely to have clinically diagnosed depression (odds ratio, 1.76) or anxiety (OR, 1.61) than were those in the control group.
The report was published in the journal Allergy.
Among the 568 individuals with symptoms consistent of major depressive disorder, those with AD (112) were almost twice as likely to report these symptoms on the questionnaire (OR, 1.92) and more than twice as likely to report anxiety attacks (OR, 2.32), despite differences in psychiatric hospitalizations remaining insignificant.
The investigators hypothesized the treatment methods for AD patients may be part of the reason that suicide and hospitalization rates were not higher.
“These more severe outcomes of suicide and psychiatric hospitalization could be mitigated in AD patients due to their increased general health care and psychiatric health care utilization leading to, for example, earlier and more aggressive antidepressant therapy,” according to Dr. Thyssen and his fellow investigators.
In an analysis of the general Danish population, medication was most aggressive in patients with moderate-severe AD, who were significantly more likely to use antidepressants (hazard ratio,1.24) and anxiolytic drugs (HR, 1.66), while those with milder symptoms had only a slightly increased likelihood.
Because of the observational nature of the study, investigators could not determine what the specific cause of these psychiatric symptoms were.
The DanFunD Study was supported by TrygFonden and Lundbeck Foundation. Dr. Thyssen is supported by a grant from the Lundbeck Foundation. Some of the investigators received financial support from various pharmaceutical companies or their foundations. The remaining investigators reported no relevant financial disclosures.
SOURCE: Thyssen JP et al. Allergy. 2018 Jan;73(1):214-20.
Atopic dermatitis (AD) is associated with anxiety, depression, and suicidal thoughts but does not lead to increased psychiatric hospitalization or suicide, according to a Danish study.
While previous literature has indicated a connection between AD and depressive symptoms, these findings indicated the psychiatric burden is not severe.
A total of 9,656 patients selected from the Danish study of Functional Disorders (DanFunD) during 2011-2015 were asked to fill out a questionnaire concerning clinical diagnoses and psychiatric symptoms.
Investigators also conducted an analysis of 4,259,457 patient records gathered the Danish National Patient Register.
The 1,044 AD patients from the DanFunD were slightly younger on average, at 50 years, compared with an average of 53 years in the 8,612-person control group; there were more women (65%) in the AD group, compared with a relatively even split (53%) in the control group.
Patients with AD were more likely to have clinically diagnosed depression (odds ratio, 1.76) or anxiety (OR, 1.61) than were those in the control group.
The report was published in the journal Allergy.
Among the 568 individuals with symptoms consistent of major depressive disorder, those with AD (112) were almost twice as likely to report these symptoms on the questionnaire (OR, 1.92) and more than twice as likely to report anxiety attacks (OR, 2.32), despite differences in psychiatric hospitalizations remaining insignificant.
The investigators hypothesized the treatment methods for AD patients may be part of the reason that suicide and hospitalization rates were not higher.
“These more severe outcomes of suicide and psychiatric hospitalization could be mitigated in AD patients due to their increased general health care and psychiatric health care utilization leading to, for example, earlier and more aggressive antidepressant therapy,” according to Dr. Thyssen and his fellow investigators.
In an analysis of the general Danish population, medication was most aggressive in patients with moderate-severe AD, who were significantly more likely to use antidepressants (hazard ratio,1.24) and anxiolytic drugs (HR, 1.66), while those with milder symptoms had only a slightly increased likelihood.
Because of the observational nature of the study, investigators could not determine what the specific cause of these psychiatric symptoms were.
The DanFunD Study was supported by TrygFonden and Lundbeck Foundation. Dr. Thyssen is supported by a grant from the Lundbeck Foundation. Some of the investigators received financial support from various pharmaceutical companies or their foundations. The remaining investigators reported no relevant financial disclosures.
SOURCE: Thyssen JP et al. Allergy. 2018 Jan;73(1):214-20.
FROM ALLERGY
Key clinical point:
Major finding: Atopic dermatitis patients were at greater risk for depression (OR, 1.76) and anxiety (OR, 1.61).
Study details: Observational, controlled study of 9,656 Danish residents collected from the Danish study of Functional Disorders between 2011 and 2015, and an analysis of 4,259,457 patient records gathered the Danish National Patient Register.
Disclosures: The DanFunD Study was supported by TrygFonden and Lundbeck Foundation. Dr. Thyssen is supported by a grant from the Lundbeck Foundation. Some of the investigators received financial support from various pharmaceutical companies or their foundations. The remaining investigators reported no relevant financial disclosures.
Source: Thyssen JP et al. Allergy. 2018 Jan;73(1):214-20.
Pendulum swings on mesenteric venous thrombosis treatment
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
NIOSH Survey: Adherence to Best Practices is Falling Short
The health care industry sees more nonfatal occupational injury and illness than other industry sectors. One reason may be that employers and employees are not adhering to health and safety best practices, according to a recent National Institute for Occupational Safety and Health (NIOSH) survey of nearly 11,000 workers in a wide range of professional, technical, and support occupations.
The survey—the largest federally sponsored survey addressing chemical hazards in health care—found lapses everywhere. For instance, when administering aerosolized pentamidine, 69% of health care workers did not always wear protective gowns, 49% did not always wear respiratory protection, and 22% did not always wear protective gloves.
When NIOSH compared responses from respondents who administered pentamidine versus those who administered antibiotics, it found that those who administered pentamidine were more likely to be trained, familiar with employer standard procedures, and use eye/face protection and respirators. The major barrier to using personal protective equipment for those who administered either pentamidine or antibiotics include “the perception that aerosolized medications are not as dangerous as other chemicals.” Moreover, NIOSH concluded that there was “a belief” that employers do not fully appreciate the potential adverse health effects associated with exposure to the drugs, and thus do not prioritize adherence.
The survey also revealed that best practices to minimize exposure to high-level disinfectants are not universally implemented: 17% of respondents said they never received training. Of those who received training, 42% said it was > 12 months before, and 19% said employer safe handling procedures were unavailable. Nearly half of respondents did not always wear a protective gown when handling the products; 9% did not always wear protective gloves.
Among other findings: use of anesthesia machines with scavenging systems was “nearly universal.” However, adherence to other best practices was lacking. For instance, one third of health care workers who administered anesthetic gases to children and 14% of those with adult patients started the anesthetic gas flow before the delivery mask or airway mask was applied to the patient. And, 18% of respondents said they never received training. Of those who did receive training, 81% said it had been > 12 months before. Not surprisingly, NIOSH concludes that findings from the survey show that best practices have not been implemented and adherence is “not universal.”
The health care industry sees more nonfatal occupational injury and illness than other industry sectors. One reason may be that employers and employees are not adhering to health and safety best practices, according to a recent National Institute for Occupational Safety and Health (NIOSH) survey of nearly 11,000 workers in a wide range of professional, technical, and support occupations.
The survey—the largest federally sponsored survey addressing chemical hazards in health care—found lapses everywhere. For instance, when administering aerosolized pentamidine, 69% of health care workers did not always wear protective gowns, 49% did not always wear respiratory protection, and 22% did not always wear protective gloves.
When NIOSH compared responses from respondents who administered pentamidine versus those who administered antibiotics, it found that those who administered pentamidine were more likely to be trained, familiar with employer standard procedures, and use eye/face protection and respirators. The major barrier to using personal protective equipment for those who administered either pentamidine or antibiotics include “the perception that aerosolized medications are not as dangerous as other chemicals.” Moreover, NIOSH concluded that there was “a belief” that employers do not fully appreciate the potential adverse health effects associated with exposure to the drugs, and thus do not prioritize adherence.
The survey also revealed that best practices to minimize exposure to high-level disinfectants are not universally implemented: 17% of respondents said they never received training. Of those who received training, 42% said it was > 12 months before, and 19% said employer safe handling procedures were unavailable. Nearly half of respondents did not always wear a protective gown when handling the products; 9% did not always wear protective gloves.
Among other findings: use of anesthesia machines with scavenging systems was “nearly universal.” However, adherence to other best practices was lacking. For instance, one third of health care workers who administered anesthetic gases to children and 14% of those with adult patients started the anesthetic gas flow before the delivery mask or airway mask was applied to the patient. And, 18% of respondents said they never received training. Of those who did receive training, 81% said it had been > 12 months before. Not surprisingly, NIOSH concludes that findings from the survey show that best practices have not been implemented and adherence is “not universal.”
The health care industry sees more nonfatal occupational injury and illness than other industry sectors. One reason may be that employers and employees are not adhering to health and safety best practices, according to a recent National Institute for Occupational Safety and Health (NIOSH) survey of nearly 11,000 workers in a wide range of professional, technical, and support occupations.
The survey—the largest federally sponsored survey addressing chemical hazards in health care—found lapses everywhere. For instance, when administering aerosolized pentamidine, 69% of health care workers did not always wear protective gowns, 49% did not always wear respiratory protection, and 22% did not always wear protective gloves.
When NIOSH compared responses from respondents who administered pentamidine versus those who administered antibiotics, it found that those who administered pentamidine were more likely to be trained, familiar with employer standard procedures, and use eye/face protection and respirators. The major barrier to using personal protective equipment for those who administered either pentamidine or antibiotics include “the perception that aerosolized medications are not as dangerous as other chemicals.” Moreover, NIOSH concluded that there was “a belief” that employers do not fully appreciate the potential adverse health effects associated with exposure to the drugs, and thus do not prioritize adherence.
The survey also revealed that best practices to minimize exposure to high-level disinfectants are not universally implemented: 17% of respondents said they never received training. Of those who received training, 42% said it was > 12 months before, and 19% said employer safe handling procedures were unavailable. Nearly half of respondents did not always wear a protective gown when handling the products; 9% did not always wear protective gloves.
Among other findings: use of anesthesia machines with scavenging systems was “nearly universal.” However, adherence to other best practices was lacking. For instance, one third of health care workers who administered anesthetic gases to children and 14% of those with adult patients started the anesthetic gas flow before the delivery mask or airway mask was applied to the patient. And, 18% of respondents said they never received training. Of those who did receive training, 81% said it had been > 12 months before. Not surprisingly, NIOSH concludes that findings from the survey show that best practices have not been implemented and adherence is “not universal.”