User login
Charting a new course in sepsis management
A drug overdose victim is admitted to a hospital. Providers focus on treating the overdose and blame it for some of the patient’s troubling vital signs, including low blood pressure and increased heart rate. Prior to admission, however, the patient had vomited and aspirated, leading to an infection. In fact, the patient is developing sepsis.
This real-world incident is but one of many ways that sepsis can fool hospitalists and other providers, often with rapidly deteriorating and deadly consequences. A range of quality improvement (QI) projects, however, are demonstrating how earlier identification and treatment may help to set a new course for addressing a condition that has remained stubbornly difficult to manage.
Devin J. Horton, MD, an academic hospitalist at University Hospital in Salt Lake City, sometimes compares sepsis to acute MI to illustrate the difficulty of early detection. A patient complaining of chest pain immediately sets in motion a well-rehearsed chain of events. “But the patient doesn’t look at you and say, ‘You know, I think I’m having SIRS [systemic inflammatory response syndrome] criteria in the setting of infection,’ ” he said. “And yet, the mortality of severe septic shock is at least as bad as acute myocardial infarction.” The trick is generating the same sense of urgency without a clear warning.
The location in a hospital also can present a major obstacle for early identification. Hospitalist Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis, calls hospital wards the “third space” of sepsis care, after the ICU and ED. “A lot of the historical improvement efforts and research has really focused on streamlining care in the ICU and streamlining care in the emergency department,” he said. Often, however, sepsis or septic shock isn’t recognized until a patient is admitted to a medical or surgical ward.
Observational studies by the Surviving Sepsis Campaign suggested that patients diagnosed on the floor had mortality rates comparable to and substantially higher than theoretically sicker patients diagnosed in the ICU and ED, respectively.1 “That was kind of a sea change for a lot of people and really articulated what a lot of us on the wards had been feeling,” Dr. Odden said. “We can’t simply apply the lessons that we’ve learned from the emergency department and the ICU to the wards if we’re going to provide the right care for these patients,” he said.
Dueling definitions
Better sepsis care in hospital wards will require a better understanding of shifting management guidelines. Confusing and contradictory definitions haven’t helped. In October 2015, the Centers for Medicare & Medicaid Services instituted its Sepsis Core Measure (SEP-1) for Medicare, requiring every hospital to audit a percentage of patients treated with best-practice 3- and 6-hour bundles for severe sepsis and septic shock. The SEP-1 measure uses the traditional definition of severe sepsis as two or more SIRS criteria, a suspected or proven infection, and organ dysfunction.
A separate set of guidelines issued by the international Sepsis-3 task force in February 2016, by contrast, concluded that the term “severe sepsis” is redundant.2 The update defines sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection” and asserts that the condition can be represented by an increase in the SOFA (Sequential Organ Failure Assessment) score of 2 or more points.
For hospital wards, the task force recommended a bedside scoring system called qSOFA (quickSOFA) for adult patients with a suspected infection. The risk stratification tool may help rapidly identify those who are likely to have poorer outcomes typical of sepsis if they meet two of the following three clinical criteria: a “respiratory rate of 22 [breaths]/min or greater, altered mentation, or systolic blood pressure of 100 mm Hg or less.”
CMS doesn’t recognize the Sepsis-3 definition at all and multiple providers have described widespread skepticism and uncertainty over how to reconcile it with the prior definition. Dr. Odden says the dueling definitions have “caused a tremendous amount of confusion” over diagnoses, the necessary sense of urgency, and whether severe sepsis is still a recognized entity. “When people aren’t speaking the same language with the same terminology, there is enormous opportunity for miscommunication to occur,” he said.
Obtaining reliable scores is another matter. The qSOFA blood pressure score generally is measured accurately, he said. On noncritical care units, though, nurses aren’t always trained to consistently and accurately document a patient’s mental status. Likewise, he said, documentation of respiratory rate often is subjective, and an abnormal rate can be easily missed. Changing that dynamic, he stressed, will require coordination with nursing leadership to ensure more consistent and accurate measurements.
Reshaping sepsis pathways
So how can hospitals identify sepsis sooner? Some hospitals have relied more on EMR-based screening methods; others have relied more on nurses to lead the charge. Either way, Dr. Shieh said, the field is trying to encourage the use of set pathways. Almost every medical center that performs well on sepsis measures, she says, has a good screening program, a pathway implemented through an order set or nursing staff, and a highly trained sepsis team that ensures patients get the treatment they need.
At Middlesex Hospital in Middletown, Conn., a major QI project led to significant improvements in sepsis mortality, total mortality, sepsis-related serious safety events and sepsis length of stay. Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence, said the project sprang from concerns by the hospital’s Rapid Response Review Committee about some serious safety events involving a delay in sepsis diagnosis and treatment.
In 2013, the hospital documented three serious safety events related to a delay in diagnosis and treatment of sepsis. In 2014, it recorded only one event and has had none since then. From 2014 to 2015, sepsis-related mortality fell by more than 20%, saving an estimated 25 lives. Sepsis length of stay also declined. “We’re identifying them sooner and treating them sooner so they’re not getting as sick or requiring critical care and longer length of stays,” Ms. Savino said.
Dr. Odden has participated in two multicenter QI initiatives on sepsis. One, a partnership led by the Institute for Healthcare Improvement in Cambridge, Mass., and New York’s North Shore-LIJ Health System, focused on how to diagnose sepsis in hospital ward patients as quickly as possible and how to successfully deliver the 3-hour sepsis bundle.3 Beyond getting everyone on the same page regarding definitions, he said, the collaborators discussed and shared strategies for identifying patients. “One hospital would often have a solution for a problem that other hospitals could either take directly or modify based on their own understanding of their own processes,” he said.
Dr. Odden also participated in a national project sponsored by the Surviving Sepsis Campaign that focused on developing protocols for nurse-led screening processes in hospital wards. Within a pilot unit of each participating hospital, bedside nurses screened every patient for sepsis during every shift. For positive screens, the hospitals then developed protocols for order sets, like blood work and fluids.
The initiative suggested that a nurse-based, every-shift screening method might be one feasible way to identify sick patients as early as possible. “Going through the screening process really seemed to empower the nurses to take a much more active role in partnering with the physicians and in recognizing some of the early warning signs,” Dr. Odden said. The project led to other benefits as well, including improved identification of strokes, delirium, and even a gastrointestinal bleed because the “barriers in communication had been broken down,” he said.
To help medical providers recognize sepsis earlier, Dr. Shieh and her colleagues created a free game called Septris as an adjunctive teaching tool. Based on a player’s diagnosis and treatment decisions, patient outcomes either rise or fall – often rapidly. “I’m an educator and what I know is that the best way you learn is by doing,” she said. The interactive and repetitive nature of Septris, she said, helps its take-home messages stick in a player’s mind without the expense of patient simulations. Dr. Shieh said the game has been adapted for German and British medical institutions as well, and that she collects data from players around the world about their experiences and scores.
Winning interdisciplinary buy-in
To maximize the chances for success, several doctors emphasize the importance of forming an interdisciplinary task force that includes every department affected by a QI project. Ms. Savino said executive sponsorship of her hospital’s QI project was key as well. So was meeting frequently with the carefully chosen team members representing key stakeholders throughout the hospital. “It was a lot of work,” she said. “But I really think that was one reason why it was so successful. We had everybody’s buy-in, and we kept our short-term goals on track.”
Based on their success, the QI initiative has spread to two other hospitals in the University of Utah’s network. “Once the culture changes have been made and the project’s up and going, it’s kind of self-sufficient,” Dr. Horton said. “But it was so much work.” He and Dr. Graves are careful to emphasize that there are other options for sepsis-related QI efforts. “I think it is better to start something small than to believe you can’t do anything at all,” Dr. Graves said.
No matter what the size, assembling a motivated and multidisciplinary team is critical, she said. So is empowering nurses to talk to physicians about decompensating patients and other aspects of sepsis care. Being available and willing to listen to other providers also can pay big dividends. “Knowing that we cared about the project’s success was important to people working on it,” Dr. Graves said.
Despite the remaining challenges, Dr. Shieh points out that sepsis mortality rates have improved significantly, thanks in large part to more awareness and ambitious QI projects. “I do want to say that we have come a long way,” she said.
References
1. Levy MM et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014 Nov;40(11):1623-33.
2. Singer M et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-10.
3. Schorr C et al. Implementation of a multicenter performance improvement program for early detection and treatment of severe sepsis in general medical-surgical wards. J Hosp Med. 2016 Nov;11:S32-9.
4. Lee VS et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016 Sep 13;316(10):1061-72.
A drug overdose victim is admitted to a hospital. Providers focus on treating the overdose and blame it for some of the patient’s troubling vital signs, including low blood pressure and increased heart rate. Prior to admission, however, the patient had vomited and aspirated, leading to an infection. In fact, the patient is developing sepsis.
This real-world incident is but one of many ways that sepsis can fool hospitalists and other providers, often with rapidly deteriorating and deadly consequences. A range of quality improvement (QI) projects, however, are demonstrating how earlier identification and treatment may help to set a new course for addressing a condition that has remained stubbornly difficult to manage.
Devin J. Horton, MD, an academic hospitalist at University Hospital in Salt Lake City, sometimes compares sepsis to acute MI to illustrate the difficulty of early detection. A patient complaining of chest pain immediately sets in motion a well-rehearsed chain of events. “But the patient doesn’t look at you and say, ‘You know, I think I’m having SIRS [systemic inflammatory response syndrome] criteria in the setting of infection,’ ” he said. “And yet, the mortality of severe septic shock is at least as bad as acute myocardial infarction.” The trick is generating the same sense of urgency without a clear warning.
The location in a hospital also can present a major obstacle for early identification. Hospitalist Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis, calls hospital wards the “third space” of sepsis care, after the ICU and ED. “A lot of the historical improvement efforts and research has really focused on streamlining care in the ICU and streamlining care in the emergency department,” he said. Often, however, sepsis or septic shock isn’t recognized until a patient is admitted to a medical or surgical ward.
Observational studies by the Surviving Sepsis Campaign suggested that patients diagnosed on the floor had mortality rates comparable to and substantially higher than theoretically sicker patients diagnosed in the ICU and ED, respectively.1 “That was kind of a sea change for a lot of people and really articulated what a lot of us on the wards had been feeling,” Dr. Odden said. “We can’t simply apply the lessons that we’ve learned from the emergency department and the ICU to the wards if we’re going to provide the right care for these patients,” he said.
Dueling definitions
Better sepsis care in hospital wards will require a better understanding of shifting management guidelines. Confusing and contradictory definitions haven’t helped. In October 2015, the Centers for Medicare & Medicaid Services instituted its Sepsis Core Measure (SEP-1) for Medicare, requiring every hospital to audit a percentage of patients treated with best-practice 3- and 6-hour bundles for severe sepsis and septic shock. The SEP-1 measure uses the traditional definition of severe sepsis as two or more SIRS criteria, a suspected or proven infection, and organ dysfunction.
A separate set of guidelines issued by the international Sepsis-3 task force in February 2016, by contrast, concluded that the term “severe sepsis” is redundant.2 The update defines sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection” and asserts that the condition can be represented by an increase in the SOFA (Sequential Organ Failure Assessment) score of 2 or more points.
For hospital wards, the task force recommended a bedside scoring system called qSOFA (quickSOFA) for adult patients with a suspected infection. The risk stratification tool may help rapidly identify those who are likely to have poorer outcomes typical of sepsis if they meet two of the following three clinical criteria: a “respiratory rate of 22 [breaths]/min or greater, altered mentation, or systolic blood pressure of 100 mm Hg or less.”
CMS doesn’t recognize the Sepsis-3 definition at all and multiple providers have described widespread skepticism and uncertainty over how to reconcile it with the prior definition. Dr. Odden says the dueling definitions have “caused a tremendous amount of confusion” over diagnoses, the necessary sense of urgency, and whether severe sepsis is still a recognized entity. “When people aren’t speaking the same language with the same terminology, there is enormous opportunity for miscommunication to occur,” he said.
Obtaining reliable scores is another matter. The qSOFA blood pressure score generally is measured accurately, he said. On noncritical care units, though, nurses aren’t always trained to consistently and accurately document a patient’s mental status. Likewise, he said, documentation of respiratory rate often is subjective, and an abnormal rate can be easily missed. Changing that dynamic, he stressed, will require coordination with nursing leadership to ensure more consistent and accurate measurements.
Reshaping sepsis pathways
So how can hospitals identify sepsis sooner? Some hospitals have relied more on EMR-based screening methods; others have relied more on nurses to lead the charge. Either way, Dr. Shieh said, the field is trying to encourage the use of set pathways. Almost every medical center that performs well on sepsis measures, she says, has a good screening program, a pathway implemented through an order set or nursing staff, and a highly trained sepsis team that ensures patients get the treatment they need.
At Middlesex Hospital in Middletown, Conn., a major QI project led to significant improvements in sepsis mortality, total mortality, sepsis-related serious safety events and sepsis length of stay. Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence, said the project sprang from concerns by the hospital’s Rapid Response Review Committee about some serious safety events involving a delay in sepsis diagnosis and treatment.
In 2013, the hospital documented three serious safety events related to a delay in diagnosis and treatment of sepsis. In 2014, it recorded only one event and has had none since then. From 2014 to 2015, sepsis-related mortality fell by more than 20%, saving an estimated 25 lives. Sepsis length of stay also declined. “We’re identifying them sooner and treating them sooner so they’re not getting as sick or requiring critical care and longer length of stays,” Ms. Savino said.
Dr. Odden has participated in two multicenter QI initiatives on sepsis. One, a partnership led by the Institute for Healthcare Improvement in Cambridge, Mass., and New York’s North Shore-LIJ Health System, focused on how to diagnose sepsis in hospital ward patients as quickly as possible and how to successfully deliver the 3-hour sepsis bundle.3 Beyond getting everyone on the same page regarding definitions, he said, the collaborators discussed and shared strategies for identifying patients. “One hospital would often have a solution for a problem that other hospitals could either take directly or modify based on their own understanding of their own processes,” he said.
Dr. Odden also participated in a national project sponsored by the Surviving Sepsis Campaign that focused on developing protocols for nurse-led screening processes in hospital wards. Within a pilot unit of each participating hospital, bedside nurses screened every patient for sepsis during every shift. For positive screens, the hospitals then developed protocols for order sets, like blood work and fluids.
The initiative suggested that a nurse-based, every-shift screening method might be one feasible way to identify sick patients as early as possible. “Going through the screening process really seemed to empower the nurses to take a much more active role in partnering with the physicians and in recognizing some of the early warning signs,” Dr. Odden said. The project led to other benefits as well, including improved identification of strokes, delirium, and even a gastrointestinal bleed because the “barriers in communication had been broken down,” he said.
To help medical providers recognize sepsis earlier, Dr. Shieh and her colleagues created a free game called Septris as an adjunctive teaching tool. Based on a player’s diagnosis and treatment decisions, patient outcomes either rise or fall – often rapidly. “I’m an educator and what I know is that the best way you learn is by doing,” she said. The interactive and repetitive nature of Septris, she said, helps its take-home messages stick in a player’s mind without the expense of patient simulations. Dr. Shieh said the game has been adapted for German and British medical institutions as well, and that she collects data from players around the world about their experiences and scores.
Winning interdisciplinary buy-in
To maximize the chances for success, several doctors emphasize the importance of forming an interdisciplinary task force that includes every department affected by a QI project. Ms. Savino said executive sponsorship of her hospital’s QI project was key as well. So was meeting frequently with the carefully chosen team members representing key stakeholders throughout the hospital. “It was a lot of work,” she said. “But I really think that was one reason why it was so successful. We had everybody’s buy-in, and we kept our short-term goals on track.”
Based on their success, the QI initiative has spread to two other hospitals in the University of Utah’s network. “Once the culture changes have been made and the project’s up and going, it’s kind of self-sufficient,” Dr. Horton said. “But it was so much work.” He and Dr. Graves are careful to emphasize that there are other options for sepsis-related QI efforts. “I think it is better to start something small than to believe you can’t do anything at all,” Dr. Graves said.
No matter what the size, assembling a motivated and multidisciplinary team is critical, she said. So is empowering nurses to talk to physicians about decompensating patients and other aspects of sepsis care. Being available and willing to listen to other providers also can pay big dividends. “Knowing that we cared about the project’s success was important to people working on it,” Dr. Graves said.
Despite the remaining challenges, Dr. Shieh points out that sepsis mortality rates have improved significantly, thanks in large part to more awareness and ambitious QI projects. “I do want to say that we have come a long way,” she said.
References
1. Levy MM et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014 Nov;40(11):1623-33.
2. Singer M et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-10.
3. Schorr C et al. Implementation of a multicenter performance improvement program for early detection and treatment of severe sepsis in general medical-surgical wards. J Hosp Med. 2016 Nov;11:S32-9.
4. Lee VS et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016 Sep 13;316(10):1061-72.
A drug overdose victim is admitted to a hospital. Providers focus on treating the overdose and blame it for some of the patient’s troubling vital signs, including low blood pressure and increased heart rate. Prior to admission, however, the patient had vomited and aspirated, leading to an infection. In fact, the patient is developing sepsis.
This real-world incident is but one of many ways that sepsis can fool hospitalists and other providers, often with rapidly deteriorating and deadly consequences. A range of quality improvement (QI) projects, however, are demonstrating how earlier identification and treatment may help to set a new course for addressing a condition that has remained stubbornly difficult to manage.
Devin J. Horton, MD, an academic hospitalist at University Hospital in Salt Lake City, sometimes compares sepsis to acute MI to illustrate the difficulty of early detection. A patient complaining of chest pain immediately sets in motion a well-rehearsed chain of events. “But the patient doesn’t look at you and say, ‘You know, I think I’m having SIRS [systemic inflammatory response syndrome] criteria in the setting of infection,’ ” he said. “And yet, the mortality of severe septic shock is at least as bad as acute myocardial infarction.” The trick is generating the same sense of urgency without a clear warning.
The location in a hospital also can present a major obstacle for early identification. Hospitalist Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis, calls hospital wards the “third space” of sepsis care, after the ICU and ED. “A lot of the historical improvement efforts and research has really focused on streamlining care in the ICU and streamlining care in the emergency department,” he said. Often, however, sepsis or septic shock isn’t recognized until a patient is admitted to a medical or surgical ward.
Observational studies by the Surviving Sepsis Campaign suggested that patients diagnosed on the floor had mortality rates comparable to and substantially higher than theoretically sicker patients diagnosed in the ICU and ED, respectively.1 “That was kind of a sea change for a lot of people and really articulated what a lot of us on the wards had been feeling,” Dr. Odden said. “We can’t simply apply the lessons that we’ve learned from the emergency department and the ICU to the wards if we’re going to provide the right care for these patients,” he said.
Dueling definitions
Better sepsis care in hospital wards will require a better understanding of shifting management guidelines. Confusing and contradictory definitions haven’t helped. In October 2015, the Centers for Medicare & Medicaid Services instituted its Sepsis Core Measure (SEP-1) for Medicare, requiring every hospital to audit a percentage of patients treated with best-practice 3- and 6-hour bundles for severe sepsis and septic shock. The SEP-1 measure uses the traditional definition of severe sepsis as two or more SIRS criteria, a suspected or proven infection, and organ dysfunction.
A separate set of guidelines issued by the international Sepsis-3 task force in February 2016, by contrast, concluded that the term “severe sepsis” is redundant.2 The update defines sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection” and asserts that the condition can be represented by an increase in the SOFA (Sequential Organ Failure Assessment) score of 2 or more points.
For hospital wards, the task force recommended a bedside scoring system called qSOFA (quickSOFA) for adult patients with a suspected infection. The risk stratification tool may help rapidly identify those who are likely to have poorer outcomes typical of sepsis if they meet two of the following three clinical criteria: a “respiratory rate of 22 [breaths]/min or greater, altered mentation, or systolic blood pressure of 100 mm Hg or less.”
CMS doesn’t recognize the Sepsis-3 definition at all and multiple providers have described widespread skepticism and uncertainty over how to reconcile it with the prior definition. Dr. Odden says the dueling definitions have “caused a tremendous amount of confusion” over diagnoses, the necessary sense of urgency, and whether severe sepsis is still a recognized entity. “When people aren’t speaking the same language with the same terminology, there is enormous opportunity for miscommunication to occur,” he said.
Obtaining reliable scores is another matter. The qSOFA blood pressure score generally is measured accurately, he said. On noncritical care units, though, nurses aren’t always trained to consistently and accurately document a patient’s mental status. Likewise, he said, documentation of respiratory rate often is subjective, and an abnormal rate can be easily missed. Changing that dynamic, he stressed, will require coordination with nursing leadership to ensure more consistent and accurate measurements.
Reshaping sepsis pathways
So how can hospitals identify sepsis sooner? Some hospitals have relied more on EMR-based screening methods; others have relied more on nurses to lead the charge. Either way, Dr. Shieh said, the field is trying to encourage the use of set pathways. Almost every medical center that performs well on sepsis measures, she says, has a good screening program, a pathway implemented through an order set or nursing staff, and a highly trained sepsis team that ensures patients get the treatment they need.
At Middlesex Hospital in Middletown, Conn., a major QI project led to significant improvements in sepsis mortality, total mortality, sepsis-related serious safety events and sepsis length of stay. Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence, said the project sprang from concerns by the hospital’s Rapid Response Review Committee about some serious safety events involving a delay in sepsis diagnosis and treatment.
In 2013, the hospital documented three serious safety events related to a delay in diagnosis and treatment of sepsis. In 2014, it recorded only one event and has had none since then. From 2014 to 2015, sepsis-related mortality fell by more than 20%, saving an estimated 25 lives. Sepsis length of stay also declined. “We’re identifying them sooner and treating them sooner so they’re not getting as sick or requiring critical care and longer length of stays,” Ms. Savino said.
Dr. Odden has participated in two multicenter QI initiatives on sepsis. One, a partnership led by the Institute for Healthcare Improvement in Cambridge, Mass., and New York’s North Shore-LIJ Health System, focused on how to diagnose sepsis in hospital ward patients as quickly as possible and how to successfully deliver the 3-hour sepsis bundle.3 Beyond getting everyone on the same page regarding definitions, he said, the collaborators discussed and shared strategies for identifying patients. “One hospital would often have a solution for a problem that other hospitals could either take directly or modify based on their own understanding of their own processes,” he said.
Dr. Odden also participated in a national project sponsored by the Surviving Sepsis Campaign that focused on developing protocols for nurse-led screening processes in hospital wards. Within a pilot unit of each participating hospital, bedside nurses screened every patient for sepsis during every shift. For positive screens, the hospitals then developed protocols for order sets, like blood work and fluids.
The initiative suggested that a nurse-based, every-shift screening method might be one feasible way to identify sick patients as early as possible. “Going through the screening process really seemed to empower the nurses to take a much more active role in partnering with the physicians and in recognizing some of the early warning signs,” Dr. Odden said. The project led to other benefits as well, including improved identification of strokes, delirium, and even a gastrointestinal bleed because the “barriers in communication had been broken down,” he said.
To help medical providers recognize sepsis earlier, Dr. Shieh and her colleagues created a free game called Septris as an adjunctive teaching tool. Based on a player’s diagnosis and treatment decisions, patient outcomes either rise or fall – often rapidly. “I’m an educator and what I know is that the best way you learn is by doing,” she said. The interactive and repetitive nature of Septris, she said, helps its take-home messages stick in a player’s mind without the expense of patient simulations. Dr. Shieh said the game has been adapted for German and British medical institutions as well, and that she collects data from players around the world about their experiences and scores.
Winning interdisciplinary buy-in
To maximize the chances for success, several doctors emphasize the importance of forming an interdisciplinary task force that includes every department affected by a QI project. Ms. Savino said executive sponsorship of her hospital’s QI project was key as well. So was meeting frequently with the carefully chosen team members representing key stakeholders throughout the hospital. “It was a lot of work,” she said. “But I really think that was one reason why it was so successful. We had everybody’s buy-in, and we kept our short-term goals on track.”
Based on their success, the QI initiative has spread to two other hospitals in the University of Utah’s network. “Once the culture changes have been made and the project’s up and going, it’s kind of self-sufficient,” Dr. Horton said. “But it was so much work.” He and Dr. Graves are careful to emphasize that there are other options for sepsis-related QI efforts. “I think it is better to start something small than to believe you can’t do anything at all,” Dr. Graves said.
No matter what the size, assembling a motivated and multidisciplinary team is critical, she said. So is empowering nurses to talk to physicians about decompensating patients and other aspects of sepsis care. Being available and willing to listen to other providers also can pay big dividends. “Knowing that we cared about the project’s success was important to people working on it,” Dr. Graves said.
Despite the remaining challenges, Dr. Shieh points out that sepsis mortality rates have improved significantly, thanks in large part to more awareness and ambitious QI projects. “I do want to say that we have come a long way,” she said.
References
1. Levy MM et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014 Nov;40(11):1623-33.
2. Singer M et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-10.
3. Schorr C et al. Implementation of a multicenter performance improvement program for early detection and treatment of severe sepsis in general medical-surgical wards. J Hosp Med. 2016 Nov;11:S32-9.
4. Lee VS et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016 Sep 13;316(10):1061-72.
Novel JAK1 inhibitor shows promise for myeloid malignancies
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: Overall response rates with INCB052793 plus azacitidine were 67% in AML and 56% in MDS or MDS/MPN.
Study details: A phase 1/2 study involving 58 initial patients.
Disclosures: This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
Source: Zeidan A et al. ASH 2017 Abstract 640.
Fetal fibronectin testing is infrequent
Fetal fibronectin testing was linked to longer gestation periods and a lower risk of delivery occurring within 3 days of the first hospital contact, but only about half of women with symptoms of preterm labor (PTL, weeks 24-37) received any testing at all for PTL, and just 14.0% received fFN, according to an analysis of administrative claims data from Medicaid enrollees in Texas.
Fetal fibronectin (fFN) testing can help determine the probability of spontaneous preterm birth in the following 14 days.
The results reinforce the value of fFN in predicting spontaneous preterm birth, but also underline the need for additional testing.
Women who received the fFN test incurred an additional cost of $2,252, compared with those who did not receive the test, but their gestation periods lasted an average of 9 additional days. The researchers point out that a conservative cost of time spent in the neonatal intensive care unit is $3,000-$3,500 per day, so that the increased cost of testing would be likely be offset by cost reductions in preterm births.
The study comprised 29,553 women aged 21 years and older who went to the emergency department or were admitted to the hospital with PTL between Jan. 1, 2012, and May 31, 2015. During the 5 months prior to delivery, 74.0% of the subjects received a PTL diagnosis, and 26.0% were diagnosed with threatened PTL, defined as early, threatened, or false labor. Nearly half of the patients (49.8%) underwent at least one PTL test, most often transvaginal ultrasound (44.1%). Just 14.0% of patients received fFN testing.
The researchers used propensity score matching to account for baseline differences in risk factors for the two groups, creating two groups of 4,098 patients (fFN and non-fFN) for the final cohort study.
Patients who received fFN testing were less likely to deliver during the initial health care visit (45.9% vs. 59.3%; P less than .0001) and had a longer mean time to delivery (24.6 days vs. 15.2 days; P less than .0001). They were less likely to deliver within 3 days of the initial visit (49.1% vs. 63.9%; P less than .0001).
Women who received fFN testing had higher all-cause maternal health care costs ($15,238.20 vs. $12,985.80; P less than .0001).
The study did not include newborn outcomes and health care costs. “It is unknown whether use of fFN testing is associated with improved newborn outcomes; if it is, fFN testing could potentially reduce newborn-incurred costs, offsetting the costs associated with testing,” the authors wrote.
SOURCE: Barner J et al. Am J Manag Care. 2017 Dec;23(19 Suppl):S363-70.
Fetal fibronectin testing was linked to longer gestation periods and a lower risk of delivery occurring within 3 days of the first hospital contact, but only about half of women with symptoms of preterm labor (PTL, weeks 24-37) received any testing at all for PTL, and just 14.0% received fFN, according to an analysis of administrative claims data from Medicaid enrollees in Texas.
Fetal fibronectin (fFN) testing can help determine the probability of spontaneous preterm birth in the following 14 days.
The results reinforce the value of fFN in predicting spontaneous preterm birth, but also underline the need for additional testing.
Women who received the fFN test incurred an additional cost of $2,252, compared with those who did not receive the test, but their gestation periods lasted an average of 9 additional days. The researchers point out that a conservative cost of time spent in the neonatal intensive care unit is $3,000-$3,500 per day, so that the increased cost of testing would be likely be offset by cost reductions in preterm births.
The study comprised 29,553 women aged 21 years and older who went to the emergency department or were admitted to the hospital with PTL between Jan. 1, 2012, and May 31, 2015. During the 5 months prior to delivery, 74.0% of the subjects received a PTL diagnosis, and 26.0% were diagnosed with threatened PTL, defined as early, threatened, or false labor. Nearly half of the patients (49.8%) underwent at least one PTL test, most often transvaginal ultrasound (44.1%). Just 14.0% of patients received fFN testing.
The researchers used propensity score matching to account for baseline differences in risk factors for the two groups, creating two groups of 4,098 patients (fFN and non-fFN) for the final cohort study.
Patients who received fFN testing were less likely to deliver during the initial health care visit (45.9% vs. 59.3%; P less than .0001) and had a longer mean time to delivery (24.6 days vs. 15.2 days; P less than .0001). They were less likely to deliver within 3 days of the initial visit (49.1% vs. 63.9%; P less than .0001).
Women who received fFN testing had higher all-cause maternal health care costs ($15,238.20 vs. $12,985.80; P less than .0001).
The study did not include newborn outcomes and health care costs. “It is unknown whether use of fFN testing is associated with improved newborn outcomes; if it is, fFN testing could potentially reduce newborn-incurred costs, offsetting the costs associated with testing,” the authors wrote.
SOURCE: Barner J et al. Am J Manag Care. 2017 Dec;23(19 Suppl):S363-70.
Fetal fibronectin testing was linked to longer gestation periods and a lower risk of delivery occurring within 3 days of the first hospital contact, but only about half of women with symptoms of preterm labor (PTL, weeks 24-37) received any testing at all for PTL, and just 14.0% received fFN, according to an analysis of administrative claims data from Medicaid enrollees in Texas.
Fetal fibronectin (fFN) testing can help determine the probability of spontaneous preterm birth in the following 14 days.
The results reinforce the value of fFN in predicting spontaneous preterm birth, but also underline the need for additional testing.
Women who received the fFN test incurred an additional cost of $2,252, compared with those who did not receive the test, but their gestation periods lasted an average of 9 additional days. The researchers point out that a conservative cost of time spent in the neonatal intensive care unit is $3,000-$3,500 per day, so that the increased cost of testing would be likely be offset by cost reductions in preterm births.
The study comprised 29,553 women aged 21 years and older who went to the emergency department or were admitted to the hospital with PTL between Jan. 1, 2012, and May 31, 2015. During the 5 months prior to delivery, 74.0% of the subjects received a PTL diagnosis, and 26.0% were diagnosed with threatened PTL, defined as early, threatened, or false labor. Nearly half of the patients (49.8%) underwent at least one PTL test, most often transvaginal ultrasound (44.1%). Just 14.0% of patients received fFN testing.
The researchers used propensity score matching to account for baseline differences in risk factors for the two groups, creating two groups of 4,098 patients (fFN and non-fFN) for the final cohort study.
Patients who received fFN testing were less likely to deliver during the initial health care visit (45.9% vs. 59.3%; P less than .0001) and had a longer mean time to delivery (24.6 days vs. 15.2 days; P less than .0001). They were less likely to deliver within 3 days of the initial visit (49.1% vs. 63.9%; P less than .0001).
Women who received fFN testing had higher all-cause maternal health care costs ($15,238.20 vs. $12,985.80; P less than .0001).
The study did not include newborn outcomes and health care costs. “It is unknown whether use of fFN testing is associated with improved newborn outcomes; if it is, fFN testing could potentially reduce newborn-incurred costs, offsetting the costs associated with testing,” the authors wrote.
SOURCE: Barner J et al. Am J Manag Care. 2017 Dec;23(19 Suppl):S363-70.
FROM THE AMERICAN JOURNAL OF MANAGED CARE
Key clinical point: Fetal fibronectin testing in women presenting with preterm labor improves delivery outcomes, but is rarely applied in at-risk women.
Major finding: Fourteen percent of women who presented with symptoms of preterm labor received the fetal fibronectin test.
Data source: Retrospective analysis of claims data among Medicaid recipients in Texas (n = 29,553).
Disclosures: The study was funded by Hologic, which markets the Fetal Fibronectin Enzyme immunoassay and Rapid fFN Test. Some of the authors have financial ties to Hologic.
Source: Barner J et al. Am J Manag Care. 2017 Dec;23(19 Suppl):S363-70.
Iodine deficiency linked to delay in pregnancy
Iodine deficiency could lead to significant delays in becoming pregnant, according to data from a prospective cohort study published online in Human Reproduction.
Researchers followed 501 couples that were discontinuing contraception to become pregnant, for 12 months, with the woman’s iodine levels measured at the time of enrollment.
This negative impact on fecundity remained even after researchers controlled for hypo/hyperthyroidism and adjusted for body mass index and cotinine as an indicator of smoking status.
“The significant delay in time to pregnancy in that group raises serious concerns given the high prevalence of iodine deficiency in women of childbearing age,” wrote James L. Mills, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and his coauthors. Previous research has found that around one-third of American women of childbearing age have urinary iodine concentrations below 100 mcg/L, and iodine deficiency may be present in more than two-thirds of British schoolgirls.
“Although it seems incongruous that deficiency would be common in a population with high sodium intake, the likely explanation is that most sodium in the diet comes from processed food, and it appears that most salt in processed food is not iodized,” they wrote.
Women with iodine-creatinine ratios in the 50-99 mcg/g range – categorized as mildly deficient – had a smaller but nonsignificant increase in time to pregnancy, compared with women with ratios above 100 mcg/g.
Iodine deficiency is known to have effects on thyroid function, and hypothyroidism in particular is associated with infertility, the authors wrote.
“Low thyroid hormone concentrations are associated with thyrotropin-releasing hormone elevations that stimulate prolactin, which in turn interferes with GnRH pulsatility,” they wrote. “They also cause decreased granulosa cell steroid production and alterations in androgen and estrogen concentrations.”
The researchers selected couples that had recently stopped using contraception to rule out individuals with long-term fertility problems. They also used sensitive HCG pregnancy tests, and the women kept daily journals so that the time to pregnancy could be calculated accurately.
However, they did note that iodine levels were measured only at enrollment and may have varied over the course of the study. They also did not measure thyroid levels during the study.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. No conflicts of interest were declared.
SOURCE: Mills JL et al. Hum Reprod. 2018 Jan 11. doi: 10.1093/humrep/dex379.
Iodine deficiency could lead to significant delays in becoming pregnant, according to data from a prospective cohort study published online in Human Reproduction.
Researchers followed 501 couples that were discontinuing contraception to become pregnant, for 12 months, with the woman’s iodine levels measured at the time of enrollment.
This negative impact on fecundity remained even after researchers controlled for hypo/hyperthyroidism and adjusted for body mass index and cotinine as an indicator of smoking status.
“The significant delay in time to pregnancy in that group raises serious concerns given the high prevalence of iodine deficiency in women of childbearing age,” wrote James L. Mills, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and his coauthors. Previous research has found that around one-third of American women of childbearing age have urinary iodine concentrations below 100 mcg/L, and iodine deficiency may be present in more than two-thirds of British schoolgirls.
“Although it seems incongruous that deficiency would be common in a population with high sodium intake, the likely explanation is that most sodium in the diet comes from processed food, and it appears that most salt in processed food is not iodized,” they wrote.
Women with iodine-creatinine ratios in the 50-99 mcg/g range – categorized as mildly deficient – had a smaller but nonsignificant increase in time to pregnancy, compared with women with ratios above 100 mcg/g.
Iodine deficiency is known to have effects on thyroid function, and hypothyroidism in particular is associated with infertility, the authors wrote.
“Low thyroid hormone concentrations are associated with thyrotropin-releasing hormone elevations that stimulate prolactin, which in turn interferes with GnRH pulsatility,” they wrote. “They also cause decreased granulosa cell steroid production and alterations in androgen and estrogen concentrations.”
The researchers selected couples that had recently stopped using contraception to rule out individuals with long-term fertility problems. They also used sensitive HCG pregnancy tests, and the women kept daily journals so that the time to pregnancy could be calculated accurately.
However, they did note that iodine levels were measured only at enrollment and may have varied over the course of the study. They also did not measure thyroid levels during the study.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. No conflicts of interest were declared.
SOURCE: Mills JL et al. Hum Reprod. 2018 Jan 11. doi: 10.1093/humrep/dex379.
Iodine deficiency could lead to significant delays in becoming pregnant, according to data from a prospective cohort study published online in Human Reproduction.
Researchers followed 501 couples that were discontinuing contraception to become pregnant, for 12 months, with the woman’s iodine levels measured at the time of enrollment.
This negative impact on fecundity remained even after researchers controlled for hypo/hyperthyroidism and adjusted for body mass index and cotinine as an indicator of smoking status.
“The significant delay in time to pregnancy in that group raises serious concerns given the high prevalence of iodine deficiency in women of childbearing age,” wrote James L. Mills, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and his coauthors. Previous research has found that around one-third of American women of childbearing age have urinary iodine concentrations below 100 mcg/L, and iodine deficiency may be present in more than two-thirds of British schoolgirls.
“Although it seems incongruous that deficiency would be common in a population with high sodium intake, the likely explanation is that most sodium in the diet comes from processed food, and it appears that most salt in processed food is not iodized,” they wrote.
Women with iodine-creatinine ratios in the 50-99 mcg/g range – categorized as mildly deficient – had a smaller but nonsignificant increase in time to pregnancy, compared with women with ratios above 100 mcg/g.
Iodine deficiency is known to have effects on thyroid function, and hypothyroidism in particular is associated with infertility, the authors wrote.
“Low thyroid hormone concentrations are associated with thyrotropin-releasing hormone elevations that stimulate prolactin, which in turn interferes with GnRH pulsatility,” they wrote. “They also cause decreased granulosa cell steroid production and alterations in androgen and estrogen concentrations.”
The researchers selected couples that had recently stopped using contraception to rule out individuals with long-term fertility problems. They also used sensitive HCG pregnancy tests, and the women kept daily journals so that the time to pregnancy could be calculated accurately.
However, they did note that iodine levels were measured only at enrollment and may have varied over the course of the study. They also did not measure thyroid levels during the study.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. No conflicts of interest were declared.
SOURCE: Mills JL et al. Hum Reprod. 2018 Jan 11. doi: 10.1093/humrep/dex379.
FROM HUMAN REPRODUCTION
Key clinical point: Women with moderate to severe iodine deficiency could experience significant delays in time to achieving pregnancy.
Major finding: Women with iodine levels in the moderate to severe range showed a 44% lower odds ratio of becoming pregnant in any one cycle, compared with women with levels in the normal range.
Data source: Population-based prospective cohort study in 501 couples.
Disclosures: The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. No conflicts of interest were declared.
Source: Hum Reprod. 2018 Jan. doi: 10.1093/humrep/dex379.
DMARDs may hamper pneumococcal vaccine response in systemic sclerosis patients
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
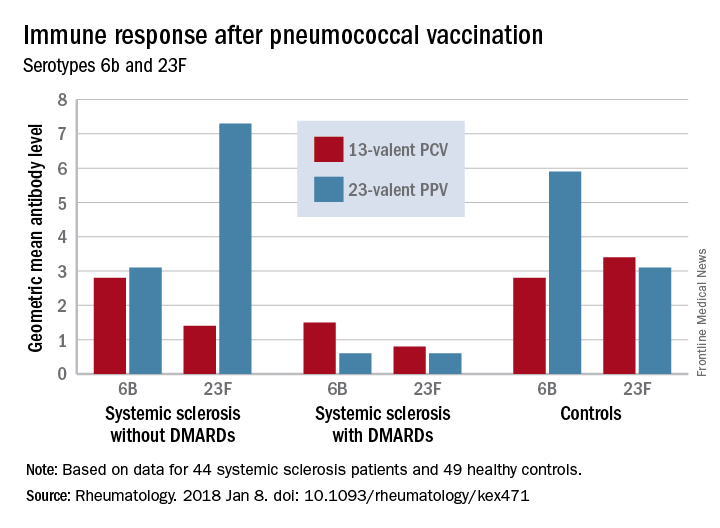
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.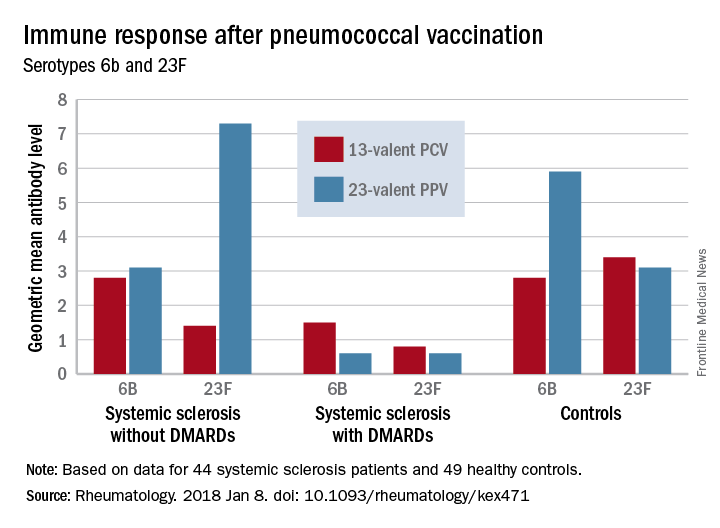
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: An increase in prevaccination antibody levels of at least twofold occurred in significantly fewer patients taking DMARDs than in patients not taking DMARDs and controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
Study details: The prospective study comprised 44 systemic sclerosis patients and 49 healthy controls.
Disclosures: None of the authors had conflicts of interest to disclose.
Source: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471
Immune-modified RECIST can help identify survival benefit from cancer immunotherapy
Cancer immunotherapy-specific response criteria not only provide improved estimates of treatment response versus standard criteria, but may also better identify patients who achieve an overall survival benefit from therapy.
Compared to standard Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, the immune-modified RECIST provided a 1%-2% greater overall response and an 8%-13% greater rate of disease control, and added 0.5-1.5 months to median progression-free survival among patients treated with the PD-L1 inhibitor atezolizumab, according to analyses of different phase 1 and 2 trials.
In addition, overall survival (OS) benefit in some of the trials could be better delineated using the immune-modified criteria, which account for unique patterns of progression sometimes experienced by patients on cancer immunotherapy, noted the study authors. The report was published in the Journal of Clinical Oncology.
Using immune-specific criteria to evaluate response to cancer immunotherapy is not a new concept. However, there are only limited data on how those criteria might apply to predictions of OS, according to lead author F. Stephen Hodi, MD, of Dana-Farber Cancer Institute, Boston, and his coauthors.
“These analyses reveal aspects of immune-modified RECIST that seem to predict OS better than RECIST v1.1, and aspects needing refinement to improve the ability to predict clinical benefit,” wrote Dr. Hodi and his colleagues.
Typical response criteria may not adequately predict the potential OS benefit of cancer immunotherapy, since patients receiving cancer immunotherapy may exhibit response patterns outside of the “classic response patterns” seen with other anticancer treatments, they noted.
In particular, some patients may experience an initial transient increase in tumor burden before responding, while in other cases, patients with responding baseline lesions might develop new lesions.
Immune-modified criteria have been developed to account for those “other patterns” that can manifest with cancer immunotherapy, the authors said.
Dr. Hodi and his colleagues sought to evaluate outcomes by RECIST vs. immune-related RECIST criteria among patients treated with atezolizumab in studies of non–small-cell lung cancer (NSCLC) and metastatic urothelial carcinoma.
In the phase 2 BIRCH study of first-line atezolizumab for NSCLC, they found that immune-related RECIST criteria appeared to predict OS better than RECIST. Median overall survival was 4.0 months longer among patients who had progressive disease (PD) by RECIST criteria within 90 days of study enrollment, versus patients who had PD by both RECIST and immune-modified RECIST at that time point, Dr. Hodi and his colleagues reported.
In the POPLAR trial of atezolizumab in NSCLC, median overall survival was 1.4 months longer for patients with PD by RECIST vs. patients with PD by both RECIST and immune-modified RECIST at 90 days, they reported.
For patients with metastatic urothelial bladder cancer treated with atezolizumab in the IMvigor210 study, median overall survival was 4.4 months longer for patients with PD by RECIST only vs. PD by both RECIST and immune-related RECIST within 180 days of enrollment, the researchers noted.
An international effort is underway to compare data sets from larger trial sets and multiple cancer immunotherapy agents, they wrote.
SOURCE: Hodi FS et al., J Clin Oncol. 2018 Jan 17. doi: 10.1200/JCO.2017.75.1644
Cancer immunotherapy-specific response criteria not only provide improved estimates of treatment response versus standard criteria, but may also better identify patients who achieve an overall survival benefit from therapy.
Compared to standard Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, the immune-modified RECIST provided a 1%-2% greater overall response and an 8%-13% greater rate of disease control, and added 0.5-1.5 months to median progression-free survival among patients treated with the PD-L1 inhibitor atezolizumab, according to analyses of different phase 1 and 2 trials.
In addition, overall survival (OS) benefit in some of the trials could be better delineated using the immune-modified criteria, which account for unique patterns of progression sometimes experienced by patients on cancer immunotherapy, noted the study authors. The report was published in the Journal of Clinical Oncology.
Using immune-specific criteria to evaluate response to cancer immunotherapy is not a new concept. However, there are only limited data on how those criteria might apply to predictions of OS, according to lead author F. Stephen Hodi, MD, of Dana-Farber Cancer Institute, Boston, and his coauthors.
“These analyses reveal aspects of immune-modified RECIST that seem to predict OS better than RECIST v1.1, and aspects needing refinement to improve the ability to predict clinical benefit,” wrote Dr. Hodi and his colleagues.
Typical response criteria may not adequately predict the potential OS benefit of cancer immunotherapy, since patients receiving cancer immunotherapy may exhibit response patterns outside of the “classic response patterns” seen with other anticancer treatments, they noted.
In particular, some patients may experience an initial transient increase in tumor burden before responding, while in other cases, patients with responding baseline lesions might develop new lesions.
Immune-modified criteria have been developed to account for those “other patterns” that can manifest with cancer immunotherapy, the authors said.
Dr. Hodi and his colleagues sought to evaluate outcomes by RECIST vs. immune-related RECIST criteria among patients treated with atezolizumab in studies of non–small-cell lung cancer (NSCLC) and metastatic urothelial carcinoma.
In the phase 2 BIRCH study of first-line atezolizumab for NSCLC, they found that immune-related RECIST criteria appeared to predict OS better than RECIST. Median overall survival was 4.0 months longer among patients who had progressive disease (PD) by RECIST criteria within 90 days of study enrollment, versus patients who had PD by both RECIST and immune-modified RECIST at that time point, Dr. Hodi and his colleagues reported.
In the POPLAR trial of atezolizumab in NSCLC, median overall survival was 1.4 months longer for patients with PD by RECIST vs. patients with PD by both RECIST and immune-modified RECIST at 90 days, they reported.
For patients with metastatic urothelial bladder cancer treated with atezolizumab in the IMvigor210 study, median overall survival was 4.4 months longer for patients with PD by RECIST only vs. PD by both RECIST and immune-related RECIST within 180 days of enrollment, the researchers noted.
An international effort is underway to compare data sets from larger trial sets and multiple cancer immunotherapy agents, they wrote.
SOURCE: Hodi FS et al., J Clin Oncol. 2018 Jan 17. doi: 10.1200/JCO.2017.75.1644
Cancer immunotherapy-specific response criteria not only provide improved estimates of treatment response versus standard criteria, but may also better identify patients who achieve an overall survival benefit from therapy.
Compared to standard Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, the immune-modified RECIST provided a 1%-2% greater overall response and an 8%-13% greater rate of disease control, and added 0.5-1.5 months to median progression-free survival among patients treated with the PD-L1 inhibitor atezolizumab, according to analyses of different phase 1 and 2 trials.
In addition, overall survival (OS) benefit in some of the trials could be better delineated using the immune-modified criteria, which account for unique patterns of progression sometimes experienced by patients on cancer immunotherapy, noted the study authors. The report was published in the Journal of Clinical Oncology.
Using immune-specific criteria to evaluate response to cancer immunotherapy is not a new concept. However, there are only limited data on how those criteria might apply to predictions of OS, according to lead author F. Stephen Hodi, MD, of Dana-Farber Cancer Institute, Boston, and his coauthors.
“These analyses reveal aspects of immune-modified RECIST that seem to predict OS better than RECIST v1.1, and aspects needing refinement to improve the ability to predict clinical benefit,” wrote Dr. Hodi and his colleagues.
Typical response criteria may not adequately predict the potential OS benefit of cancer immunotherapy, since patients receiving cancer immunotherapy may exhibit response patterns outside of the “classic response patterns” seen with other anticancer treatments, they noted.
In particular, some patients may experience an initial transient increase in tumor burden before responding, while in other cases, patients with responding baseline lesions might develop new lesions.
Immune-modified criteria have been developed to account for those “other patterns” that can manifest with cancer immunotherapy, the authors said.
Dr. Hodi and his colleagues sought to evaluate outcomes by RECIST vs. immune-related RECIST criteria among patients treated with atezolizumab in studies of non–small-cell lung cancer (NSCLC) and metastatic urothelial carcinoma.
In the phase 2 BIRCH study of first-line atezolizumab for NSCLC, they found that immune-related RECIST criteria appeared to predict OS better than RECIST. Median overall survival was 4.0 months longer among patients who had progressive disease (PD) by RECIST criteria within 90 days of study enrollment, versus patients who had PD by both RECIST and immune-modified RECIST at that time point, Dr. Hodi and his colleagues reported.
In the POPLAR trial of atezolizumab in NSCLC, median overall survival was 1.4 months longer for patients with PD by RECIST vs. patients with PD by both RECIST and immune-modified RECIST at 90 days, they reported.
For patients with metastatic urothelial bladder cancer treated with atezolizumab in the IMvigor210 study, median overall survival was 4.4 months longer for patients with PD by RECIST only vs. PD by both RECIST and immune-related RECIST within 180 days of enrollment, the researchers noted.
An international effort is underway to compare data sets from larger trial sets and multiple cancer immunotherapy agents, they wrote.
SOURCE: Hodi FS et al., J Clin Oncol. 2018 Jan 17. doi: 10.1200/JCO.2017.75.1644
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Compared to standard criteria for response evaluation, criteria developed specifically to evaluate response to cancer immunotherapy better identified patients with an overall survival (OS) benefit.
Major finding: Median OS was 4.0 and 1.4 months longer, respectively, in the BIRCH and POPLAR non–small-cell lung cancer trial among patients who had progressive disease (PD) by standard criteria only, as opposed to patients who also had PD according to the immunotherapy-specific response criteria.
Data source: Analysis of patients treated with single-agent atezolizumab in phase 1 and 2 clinical trials.
Disclosures: The study was supported by F. Hoffmann-La Roche. Authors reported disclosures related to Merck Sharp & Dohme, Novartis, Genentech/Roche, Bristol-Myers Squibb, and others.
Source: Hodi FS et al., J Clin Oncol. 2018 Jan 17. doi: 10.1200/JCO.2017.75.1644.
Papules below eyes
The FP looked at the small papules closely and recognized them as white milia cysts and flesh-colored syringomas. He explained to the patient that both conditions were benign and discussed treatment options.
Milia cysts, which appear as shiny white papules, can be extracted. This procedure is performed without local anesthesia, and the most uncomfortable part is when pressure is applied with the comedone extractor against the infraorbital bone. (The billing code for this procedure is the same as the one used for acne surgery.)
Syringomas, however, are not easily treated. New lesions can form even if some resolve. Treatment options for syringomas include topical trichloroacetic acid, cryosurgery, and electrosurgery. Because this patient’s lesions were on the eyelids, as they often are, there are risks involved.
The patient agreed to extraction of the milia cysts, so the FP removed a number of them using the tip of a Number 11 scalpel blade and a comedone extractor. The patient was happy to have them removed and said that he would think about the options for syringoma treatment at a future date.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP looked at the small papules closely and recognized them as white milia cysts and flesh-colored syringomas. He explained to the patient that both conditions were benign and discussed treatment options.
Milia cysts, which appear as shiny white papules, can be extracted. This procedure is performed without local anesthesia, and the most uncomfortable part is when pressure is applied with the comedone extractor against the infraorbital bone. (The billing code for this procedure is the same as the one used for acne surgery.)
Syringomas, however, are not easily treated. New lesions can form even if some resolve. Treatment options for syringomas include topical trichloroacetic acid, cryosurgery, and electrosurgery. Because this patient’s lesions were on the eyelids, as they often are, there are risks involved.
The patient agreed to extraction of the milia cysts, so the FP removed a number of them using the tip of a Number 11 scalpel blade and a comedone extractor. The patient was happy to have them removed and said that he would think about the options for syringoma treatment at a future date.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP looked at the small papules closely and recognized them as white milia cysts and flesh-colored syringomas. He explained to the patient that both conditions were benign and discussed treatment options.
Milia cysts, which appear as shiny white papules, can be extracted. This procedure is performed without local anesthesia, and the most uncomfortable part is when pressure is applied with the comedone extractor against the infraorbital bone. (The billing code for this procedure is the same as the one used for acne surgery.)
Syringomas, however, are not easily treated. New lesions can form even if some resolve. Treatment options for syringomas include topical trichloroacetic acid, cryosurgery, and electrosurgery. Because this patient’s lesions were on the eyelids, as they often are, there are risks involved.
The patient agreed to extraction of the milia cysts, so the FP removed a number of them using the tip of a Number 11 scalpel blade and a comedone extractor. The patient was happy to have them removed and said that he would think about the options for syringoma treatment at a future date.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Ixekizumab beats ustekinumab for fingernail psoriasis, hands down
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
REPORTING FROM THE EADV CONGRESS
Key clinical point:
Major finding: At week 24, complete clearance of fingernail psoriasis was documented in 49% of patients on ixekizumab and 23% on ustekinumab.
Study details: This secondary analysis of the randomized, multicenter, prospective, phase 3b IXORA-S trial included 189 patients with moderate to severe plaque psoriasis with fingernail involvement.
Disclosures: The study was sponsored by Eli Lilly and presented by a company employee.
Source: Dutronc Y. https://eadvgeneva2017.org
Hospitalist leader: Are you burned out? Are you resilient?
I had the privilege of teaching two seminars at the recent Society of Hospital Medicine Leadership Academy in Scottsdale, Ariz. The theme of my second seminar was “Swarm Leadership,” the topic of my September column. There seemed to be enthusiasm and interest in the topic. Participants were intrigued at the notion of leveraging instinctual responses to encourage team spirit and collective outcomes.
The key principles of these swarm-like behaviors are: 1) unity of mission, 2) generosity of spirit, 3) staying in lanes and helping others succeed in theirs, 4) no ego/no blame, and 5) a foundation of trust among those working together. Leaders create the conditions in which these behaviors are more likely to emerge. The resulting team spirit and productivity raise morale and increase the sense of work-related purpose and mission.
People expressed their sense of being burned out and overworked, even to the extent of being exploited. I was stunned at the prevalence of this sensation in the room. Not everyone spoke though many people identified with the theme.
What I heard was enough to raise the question here: For hospitalist leaders, to what extent is burnout significant enough to give it serious attention? (I want to be abundantly clear: I report observations as anecdotal and impressionistic. There is no implied critique of hospitalists on the whole nor any individual or groups.)
Burnout includes sensations of being exhausted, overburdened, underappreciated, undercompensated, cynical, and depressed. These phenomena together can affect your productivity, the quality of your work, and your endurance when the workload gets tough.
By contrast, the opposite of burnout is balance, including sensations of being engaged, enthusiastic, energetic, absorbed, challenged, and dedicated. Work is part of the equilibrium you establish in your life, which includes a variety of fulfilling and motivating experiences and accomplishments.
Ideal balance would have all the different parts of your life – from family to hobbies to work – in perfect synergy with one another. Complete burnout would have all parts of your life imploding on one another, with little room for joy, personal contentment, and professional satisfaction.
How do you assess the differences between burnout and balance? First, this is a very individual metric. What one person might consider challenging and engaging another would experience as overwhelming and alienating. When you assess a group of people, these differences are important and could inform how work assignments and heavy lifting are assigned.
During the SHM session and in private comments, people described this rise in burnout not as a personal phenomena. Rather, it results from the health system expecting more of hospitalists than they can reasonably and reliably produce. People described hospitalists getting to the breaking point with no relief in sight. What can be done about this phenomenon?
First, hold a mirror up to yourself. You cannot help others as a leader if you are not clear with your own state of burnout and balance. The questions for you – a leader of other hospitalists – include: To what extent are you burned out? If so, why? If not, why not? If you were to draw a continuum between burned out and balanced, where on that range would you place yourself? Where would others in your group or department pinpoint themselves, relative to one another, on this continuum?
How might burnout develop for hospitalist leaders? Like a car, even a high performance vehicle, you can only go so fast and so far. Push too hard on the accelerator and the vehicle begins to shake as performance declines. If your system is expecting the pace and productivity to outstrip what you consider reasonable, your performance, job satisfaction and morale drops. Impose those demands upon a group of people and the unhappiness can become infectious.
With a decline in performance comes a decline in confidence. You and your colleagues strive for top-rate outcomes. Fatigue, pressure, and unreasonable expectations challenge your ability to feel good about what you are doing. That satisfaction is part of why you chose hospital medicine and without it, you wonder about what you are doing and why you are doing it.
When you and your colleagues sense that you are unappreciated, it can spark a profound sense of disappointment. That realization could express itself in many forms, including unhappiness about pay and workload to dissatisfaction with professional support or acknowledgment. When the system on the whole is driving so fast that it cannot stop to ensure and reward good work, the rattling can have a stunting effect on performance.
When I first began teaching at SHM conferences and had hospitalists in my classes at the Harvard School of Public Health – way back when – the field was novel, revolutionary, and striving to establish a newly effective and efficient way to provide patient services. It is useful to keep these roots in perspective – hospital medicine over the arc of time – from what WAS, to what IS and eventually what WILL BE. The cleverness of hospitalist leaders has been their capacity to understand this evolution and work with it. Hospitalist medicine built opportunities in response to high costs, the lack of continuity of care, and problems of communication. It was a solution.
How might you diagnose your burnout – and that of others with whom you work – in order to build solutions? Is it a phenomenon that involves just several individuals or is it characteristic of your group as a whole? What are the causes? What are the symptoms and what are the core issues? Some are system problems in which expectations for performance – and the resources to meet those objectives – are not reasonably aligned. There is a cost for trying to reduce costs on the backs of overworked clinicians.
If this is more than an individual problem, systematically ask the question and seek systematic answers. The better you document root causes and implications, the better are you able to make a data-driven case for change. Interview, survey, and with all this, you demonstrate your concern for staff, their work, and their work experience.
Showing that you care about the professional and personal well-being and balance of your workforce, in and of itself, is the beginning of an intervention. Be honest with yourself about your own experience. And then be open to the experiences of others. As a leader, your colleagues may suggest changes you make in your own leadership that could ameliorate some of that burnout. Better communication? Improved organization? Enhanced flexibility as appropriate? These are problems you can fix.
Other solutions must be negotiated with others on the systems level. With documentation in hand, build your case for the necessary changes, whatever that might entail. Hospitalist leaders negotiated their way into respected and productive positions in the health care system. Similarly, they must negotiate the right balance now to ensure the quality, morale, and reasonable productivity of their departments and workforce.
As a hospitalist leader, you know that each day will bring its complexities, challenges, and at times, its burdens. Your objective is to encourage – for yourself, for your colleagues, and for your system – resilience that is both personal and organizational. That resilience – the ability to take a hit and bounce back – is an encouraging signal of hope and recovery, for your workforce as well as the people for whom you care. The principles of swarm leadership – reinvigorated for your group – could very well provide signposts on that everyday quest for personal and group resilience.
Leonard J. Marcus, PhD, is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration,” Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the Program for Health Care Negotiation and Conflict Resolution at the Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected].
I had the privilege of teaching two seminars at the recent Society of Hospital Medicine Leadership Academy in Scottsdale, Ariz. The theme of my second seminar was “Swarm Leadership,” the topic of my September column. There seemed to be enthusiasm and interest in the topic. Participants were intrigued at the notion of leveraging instinctual responses to encourage team spirit and collective outcomes.
The key principles of these swarm-like behaviors are: 1) unity of mission, 2) generosity of spirit, 3) staying in lanes and helping others succeed in theirs, 4) no ego/no blame, and 5) a foundation of trust among those working together. Leaders create the conditions in which these behaviors are more likely to emerge. The resulting team spirit and productivity raise morale and increase the sense of work-related purpose and mission.
People expressed their sense of being burned out and overworked, even to the extent of being exploited. I was stunned at the prevalence of this sensation in the room. Not everyone spoke though many people identified with the theme.
What I heard was enough to raise the question here: For hospitalist leaders, to what extent is burnout significant enough to give it serious attention? (I want to be abundantly clear: I report observations as anecdotal and impressionistic. There is no implied critique of hospitalists on the whole nor any individual or groups.)
Burnout includes sensations of being exhausted, overburdened, underappreciated, undercompensated, cynical, and depressed. These phenomena together can affect your productivity, the quality of your work, and your endurance when the workload gets tough.
By contrast, the opposite of burnout is balance, including sensations of being engaged, enthusiastic, energetic, absorbed, challenged, and dedicated. Work is part of the equilibrium you establish in your life, which includes a variety of fulfilling and motivating experiences and accomplishments.
Ideal balance would have all the different parts of your life – from family to hobbies to work – in perfect synergy with one another. Complete burnout would have all parts of your life imploding on one another, with little room for joy, personal contentment, and professional satisfaction.
How do you assess the differences between burnout and balance? First, this is a very individual metric. What one person might consider challenging and engaging another would experience as overwhelming and alienating. When you assess a group of people, these differences are important and could inform how work assignments and heavy lifting are assigned.
During the SHM session and in private comments, people described this rise in burnout not as a personal phenomena. Rather, it results from the health system expecting more of hospitalists than they can reasonably and reliably produce. People described hospitalists getting to the breaking point with no relief in sight. What can be done about this phenomenon?
First, hold a mirror up to yourself. You cannot help others as a leader if you are not clear with your own state of burnout and balance. The questions for you – a leader of other hospitalists – include: To what extent are you burned out? If so, why? If not, why not? If you were to draw a continuum between burned out and balanced, where on that range would you place yourself? Where would others in your group or department pinpoint themselves, relative to one another, on this continuum?
How might burnout develop for hospitalist leaders? Like a car, even a high performance vehicle, you can only go so fast and so far. Push too hard on the accelerator and the vehicle begins to shake as performance declines. If your system is expecting the pace and productivity to outstrip what you consider reasonable, your performance, job satisfaction and morale drops. Impose those demands upon a group of people and the unhappiness can become infectious.
With a decline in performance comes a decline in confidence. You and your colleagues strive for top-rate outcomes. Fatigue, pressure, and unreasonable expectations challenge your ability to feel good about what you are doing. That satisfaction is part of why you chose hospital medicine and without it, you wonder about what you are doing and why you are doing it.
When you and your colleagues sense that you are unappreciated, it can spark a profound sense of disappointment. That realization could express itself in many forms, including unhappiness about pay and workload to dissatisfaction with professional support or acknowledgment. When the system on the whole is driving so fast that it cannot stop to ensure and reward good work, the rattling can have a stunting effect on performance.
When I first began teaching at SHM conferences and had hospitalists in my classes at the Harvard School of Public Health – way back when – the field was novel, revolutionary, and striving to establish a newly effective and efficient way to provide patient services. It is useful to keep these roots in perspective – hospital medicine over the arc of time – from what WAS, to what IS and eventually what WILL BE. The cleverness of hospitalist leaders has been their capacity to understand this evolution and work with it. Hospitalist medicine built opportunities in response to high costs, the lack of continuity of care, and problems of communication. It was a solution.
How might you diagnose your burnout – and that of others with whom you work – in order to build solutions? Is it a phenomenon that involves just several individuals or is it characteristic of your group as a whole? What are the causes? What are the symptoms and what are the core issues? Some are system problems in which expectations for performance – and the resources to meet those objectives – are not reasonably aligned. There is a cost for trying to reduce costs on the backs of overworked clinicians.
If this is more than an individual problem, systematically ask the question and seek systematic answers. The better you document root causes and implications, the better are you able to make a data-driven case for change. Interview, survey, and with all this, you demonstrate your concern for staff, their work, and their work experience.
Showing that you care about the professional and personal well-being and balance of your workforce, in and of itself, is the beginning of an intervention. Be honest with yourself about your own experience. And then be open to the experiences of others. As a leader, your colleagues may suggest changes you make in your own leadership that could ameliorate some of that burnout. Better communication? Improved organization? Enhanced flexibility as appropriate? These are problems you can fix.
Other solutions must be negotiated with others on the systems level. With documentation in hand, build your case for the necessary changes, whatever that might entail. Hospitalist leaders negotiated their way into respected and productive positions in the health care system. Similarly, they must negotiate the right balance now to ensure the quality, morale, and reasonable productivity of their departments and workforce.
As a hospitalist leader, you know that each day will bring its complexities, challenges, and at times, its burdens. Your objective is to encourage – for yourself, for your colleagues, and for your system – resilience that is both personal and organizational. That resilience – the ability to take a hit and bounce back – is an encouraging signal of hope and recovery, for your workforce as well as the people for whom you care. The principles of swarm leadership – reinvigorated for your group – could very well provide signposts on that everyday quest for personal and group resilience.
Leonard J. Marcus, PhD, is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration,” Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the Program for Health Care Negotiation and Conflict Resolution at the Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected].
I had the privilege of teaching two seminars at the recent Society of Hospital Medicine Leadership Academy in Scottsdale, Ariz. The theme of my second seminar was “Swarm Leadership,” the topic of my September column. There seemed to be enthusiasm and interest in the topic. Participants were intrigued at the notion of leveraging instinctual responses to encourage team spirit and collective outcomes.
The key principles of these swarm-like behaviors are: 1) unity of mission, 2) generosity of spirit, 3) staying in lanes and helping others succeed in theirs, 4) no ego/no blame, and 5) a foundation of trust among those working together. Leaders create the conditions in which these behaviors are more likely to emerge. The resulting team spirit and productivity raise morale and increase the sense of work-related purpose and mission.
People expressed their sense of being burned out and overworked, even to the extent of being exploited. I was stunned at the prevalence of this sensation in the room. Not everyone spoke though many people identified with the theme.
What I heard was enough to raise the question here: For hospitalist leaders, to what extent is burnout significant enough to give it serious attention? (I want to be abundantly clear: I report observations as anecdotal and impressionistic. There is no implied critique of hospitalists on the whole nor any individual or groups.)
Burnout includes sensations of being exhausted, overburdened, underappreciated, undercompensated, cynical, and depressed. These phenomena together can affect your productivity, the quality of your work, and your endurance when the workload gets tough.
By contrast, the opposite of burnout is balance, including sensations of being engaged, enthusiastic, energetic, absorbed, challenged, and dedicated. Work is part of the equilibrium you establish in your life, which includes a variety of fulfilling and motivating experiences and accomplishments.
Ideal balance would have all the different parts of your life – from family to hobbies to work – in perfect synergy with one another. Complete burnout would have all parts of your life imploding on one another, with little room for joy, personal contentment, and professional satisfaction.
How do you assess the differences between burnout and balance? First, this is a very individual metric. What one person might consider challenging and engaging another would experience as overwhelming and alienating. When you assess a group of people, these differences are important and could inform how work assignments and heavy lifting are assigned.
During the SHM session and in private comments, people described this rise in burnout not as a personal phenomena. Rather, it results from the health system expecting more of hospitalists than they can reasonably and reliably produce. People described hospitalists getting to the breaking point with no relief in sight. What can be done about this phenomenon?
First, hold a mirror up to yourself. You cannot help others as a leader if you are not clear with your own state of burnout and balance. The questions for you – a leader of other hospitalists – include: To what extent are you burned out? If so, why? If not, why not? If you were to draw a continuum between burned out and balanced, where on that range would you place yourself? Where would others in your group or department pinpoint themselves, relative to one another, on this continuum?
How might burnout develop for hospitalist leaders? Like a car, even a high performance vehicle, you can only go so fast and so far. Push too hard on the accelerator and the vehicle begins to shake as performance declines. If your system is expecting the pace and productivity to outstrip what you consider reasonable, your performance, job satisfaction and morale drops. Impose those demands upon a group of people and the unhappiness can become infectious.
With a decline in performance comes a decline in confidence. You and your colleagues strive for top-rate outcomes. Fatigue, pressure, and unreasonable expectations challenge your ability to feel good about what you are doing. That satisfaction is part of why you chose hospital medicine and without it, you wonder about what you are doing and why you are doing it.
When you and your colleagues sense that you are unappreciated, it can spark a profound sense of disappointment. That realization could express itself in many forms, including unhappiness about pay and workload to dissatisfaction with professional support or acknowledgment. When the system on the whole is driving so fast that it cannot stop to ensure and reward good work, the rattling can have a stunting effect on performance.
When I first began teaching at SHM conferences and had hospitalists in my classes at the Harvard School of Public Health – way back when – the field was novel, revolutionary, and striving to establish a newly effective and efficient way to provide patient services. It is useful to keep these roots in perspective – hospital medicine over the arc of time – from what WAS, to what IS and eventually what WILL BE. The cleverness of hospitalist leaders has been their capacity to understand this evolution and work with it. Hospitalist medicine built opportunities in response to high costs, the lack of continuity of care, and problems of communication. It was a solution.
How might you diagnose your burnout – and that of others with whom you work – in order to build solutions? Is it a phenomenon that involves just several individuals or is it characteristic of your group as a whole? What are the causes? What are the symptoms and what are the core issues? Some are system problems in which expectations for performance – and the resources to meet those objectives – are not reasonably aligned. There is a cost for trying to reduce costs on the backs of overworked clinicians.
If this is more than an individual problem, systematically ask the question and seek systematic answers. The better you document root causes and implications, the better are you able to make a data-driven case for change. Interview, survey, and with all this, you demonstrate your concern for staff, their work, and their work experience.
Showing that you care about the professional and personal well-being and balance of your workforce, in and of itself, is the beginning of an intervention. Be honest with yourself about your own experience. And then be open to the experiences of others. As a leader, your colleagues may suggest changes you make in your own leadership that could ameliorate some of that burnout. Better communication? Improved organization? Enhanced flexibility as appropriate? These are problems you can fix.
Other solutions must be negotiated with others on the systems level. With documentation in hand, build your case for the necessary changes, whatever that might entail. Hospitalist leaders negotiated their way into respected and productive positions in the health care system. Similarly, they must negotiate the right balance now to ensure the quality, morale, and reasonable productivity of their departments and workforce.
As a hospitalist leader, you know that each day will bring its complexities, challenges, and at times, its burdens. Your objective is to encourage – for yourself, for your colleagues, and for your system – resilience that is both personal and organizational. That resilience – the ability to take a hit and bounce back – is an encouraging signal of hope and recovery, for your workforce as well as the people for whom you care. The principles of swarm leadership – reinvigorated for your group – could very well provide signposts on that everyday quest for personal and group resilience.
Leonard J. Marcus, PhD, is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration,” Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the Program for Health Care Negotiation and Conflict Resolution at the Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected].
Proinflammatory diet may increase colorectal cancer risk
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
FROM JAMA ONCOLOGY
Key clinical point: A diet high in foods such as processed meats, refined grains, and soda may increase the risk of colorectal cancer by promoting inflammation.
Major finding: People in the highest quintile of dietary inflammatory score had a 32% higher risk of developing colorectal cancer, compared with those in the lowest quintile.
Data source: Analysis of data from 46,804 men in the Health Professionals Follow-up Study, and 74,246 women in the Nurses’ Health Study.
Disclosures: The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute, and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
Source: JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844