User login
Breast cancer recurrence risk substantial after endocrine treatment
Women who stop adjuvant endocrine therapy after 5 years are still at substantial risk of distant recurrence over the next 15 years, even if their tumors were small, according to results of a recent meta-analysis of 88 clinical trials.
For women with T1N0 disease, the annual rate of distant recurrence was approximately 1% each year during 5-20 years, resulting in a cumulative risk of distant recurrence of 13%, authors of the meta-analysis reported (N Engl J Med. 2017 Nov. 8. doi: 10.1056/NEJMoa1701830).
Tumor diameter and nodal status was associated with the risk of distant recurrence during the later years and was approximately additive, with the risk increasing from 13% for T1N0 to 41% for T2N4–9 disease, wrote investigator Hongchao Pan, PhD, of the Nuffield Department of Population Health, University of Oxford, England, and his coauthors.
“Recognition of the magnitude of the long-term risks of ER-positive disease can help women and their health care professionals decide whether to extend therapy beyond 5 years and whether to persist if adverse events occur,” the authors wrote in the report.
The meta-analysis by Dr. Pan and his colleagues included 62,923 women with ER-positive breast cancer who were free of disease after 5 years of scheduled endocrine therapy.
They had hoped to identify a subgroup of women with a recurrence risks so small that the risk of additional side effects caused by extending endocrine therapy would outweigh any potential benefits of that additional treatment. However, the finding of measurable risk even in the women with T1N0 disease led them to recommend that extending endocrine therapy at least be considered for all patients.
“An absolute reduction of a few percentage points in the risk of distant metastases over the next 15 years might well be possible even for such low-risk women, with correspondingly greater absolute benefits for women with larger tumors or node-positive disease,” they wrote.
Whether reducing risk translates into improved survival remains to be seen.
As of now, “reliable trial evidence is not yet available” to confirm the clinical benefit of extending endocrine therapy beyond 5 years, the authors noted.
Cancer Research UK and others funded the study. Senior author Daniel F. Hayes, MD, reported grant support from Eli Lilly, Janssen Research & Development, Veridex, Puma, Pfizer, and AstraZeneca, among other disclosures. Full disclosures for all authors were provided on the NEJM website.
“This study reaffirms the potential for recurrences very late after the original diagnosis, an observation made with other datasets as well. This pattern of recurrence is most consistent with hormone-sensitive breast cancer,” William J. Gradishar, MD, said in an interview.
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology & professor of medicine at Northwestern University, Chicago.
“This study reaffirms the potential for recurrences very late after the original diagnosis, an observation made with other datasets as well. This pattern of recurrence is most consistent with hormone-sensitive breast cancer,” William J. Gradishar, MD, said in an interview.
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology & professor of medicine at Northwestern University, Chicago.
“This study reaffirms the potential for recurrences very late after the original diagnosis, an observation made with other datasets as well. This pattern of recurrence is most consistent with hormone-sensitive breast cancer,” William J. Gradishar, MD, said in an interview.
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology & professor of medicine at Northwestern University, Chicago.
Women who stop adjuvant endocrine therapy after 5 years are still at substantial risk of distant recurrence over the next 15 years, even if their tumors were small, according to results of a recent meta-analysis of 88 clinical trials.
For women with T1N0 disease, the annual rate of distant recurrence was approximately 1% each year during 5-20 years, resulting in a cumulative risk of distant recurrence of 13%, authors of the meta-analysis reported (N Engl J Med. 2017 Nov. 8. doi: 10.1056/NEJMoa1701830).
Tumor diameter and nodal status was associated with the risk of distant recurrence during the later years and was approximately additive, with the risk increasing from 13% for T1N0 to 41% for T2N4–9 disease, wrote investigator Hongchao Pan, PhD, of the Nuffield Department of Population Health, University of Oxford, England, and his coauthors.
“Recognition of the magnitude of the long-term risks of ER-positive disease can help women and their health care professionals decide whether to extend therapy beyond 5 years and whether to persist if adverse events occur,” the authors wrote in the report.
The meta-analysis by Dr. Pan and his colleagues included 62,923 women with ER-positive breast cancer who were free of disease after 5 years of scheduled endocrine therapy.
They had hoped to identify a subgroup of women with a recurrence risks so small that the risk of additional side effects caused by extending endocrine therapy would outweigh any potential benefits of that additional treatment. However, the finding of measurable risk even in the women with T1N0 disease led them to recommend that extending endocrine therapy at least be considered for all patients.
“An absolute reduction of a few percentage points in the risk of distant metastases over the next 15 years might well be possible even for such low-risk women, with correspondingly greater absolute benefits for women with larger tumors or node-positive disease,” they wrote.
Whether reducing risk translates into improved survival remains to be seen.
As of now, “reliable trial evidence is not yet available” to confirm the clinical benefit of extending endocrine therapy beyond 5 years, the authors noted.
Cancer Research UK and others funded the study. Senior author Daniel F. Hayes, MD, reported grant support from Eli Lilly, Janssen Research & Development, Veridex, Puma, Pfizer, and AstraZeneca, among other disclosures. Full disclosures for all authors were provided on the NEJM website.
Women who stop adjuvant endocrine therapy after 5 years are still at substantial risk of distant recurrence over the next 15 years, even if their tumors were small, according to results of a recent meta-analysis of 88 clinical trials.
For women with T1N0 disease, the annual rate of distant recurrence was approximately 1% each year during 5-20 years, resulting in a cumulative risk of distant recurrence of 13%, authors of the meta-analysis reported (N Engl J Med. 2017 Nov. 8. doi: 10.1056/NEJMoa1701830).
Tumor diameter and nodal status was associated with the risk of distant recurrence during the later years and was approximately additive, with the risk increasing from 13% for T1N0 to 41% for T2N4–9 disease, wrote investigator Hongchao Pan, PhD, of the Nuffield Department of Population Health, University of Oxford, England, and his coauthors.
“Recognition of the magnitude of the long-term risks of ER-positive disease can help women and their health care professionals decide whether to extend therapy beyond 5 years and whether to persist if adverse events occur,” the authors wrote in the report.
The meta-analysis by Dr. Pan and his colleagues included 62,923 women with ER-positive breast cancer who were free of disease after 5 years of scheduled endocrine therapy.
They had hoped to identify a subgroup of women with a recurrence risks so small that the risk of additional side effects caused by extending endocrine therapy would outweigh any potential benefits of that additional treatment. However, the finding of measurable risk even in the women with T1N0 disease led them to recommend that extending endocrine therapy at least be considered for all patients.
“An absolute reduction of a few percentage points in the risk of distant metastases over the next 15 years might well be possible even for such low-risk women, with correspondingly greater absolute benefits for women with larger tumors or node-positive disease,” they wrote.
Whether reducing risk translates into improved survival remains to be seen.
As of now, “reliable trial evidence is not yet available” to confirm the clinical benefit of extending endocrine therapy beyond 5 years, the authors noted.
Cancer Research UK and others funded the study. Senior author Daniel F. Hayes, MD, reported grant support from Eli Lilly, Janssen Research & Development, Veridex, Puma, Pfizer, and AstraZeneca, among other disclosures. Full disclosures for all authors were provided on the NEJM website.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Among women with early-stage, estrogen-receptor (ER)–positive breast cancer who stop adjuvant endocrine therapy after 5 years, distant recurrences happened at a steady rate over the ensuing 15 years.
Major finding: Distant recurrence risk ranged from 10% to 41%, depending on tumor diameter and nodal status (TN) and tumor grade.
Data source: A meta-analysis of 88 trials including 62,923 women with ER-positive breast cancer who were disease free after 5 years of scheduled endocrine therapy.
Disclosures: The study was funded by Cancer Research UK and others. Senior author Daniel F. Hayes, MD, reported grant support from Eli Lilly, Janssen Research & Development, Veridex, Puma, Pfizer, and AstraZeneca, among other disclosures. Full disclosures for all authors were provided on the NEJM website.
Neoantigen profiling predicts response to immunotherapy
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
FROM NATURE
Key clinical point: Proof-of-concept studies show that mathematical modeling of neoantigens can be used to predict tumor responses to immune checkpoint inhibitors.
Major finding: Neoantigen quality may be a better biomarker for guiding immunotherapy than tumor genomic profiling.
Data source: Basic science reports focusing on neoantigens and their potential influence on tumor interactions with the immune system.
Disclosures: The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
State legislative update: Maternal mortality tops concerns
The American Congress of Obstetricians and Gynecologists held its State Legislative Roundtable in late October in Arlington, Va., with ob.gyns. and their lobbyists from 46 states. This is the largest number of states ever represented at the roundtable event, and it reflects the increased participation and engagement in policy making by women’s health care providers.
Attendees also discussed an increasing number of policies that focused on the exclusion of family planning providers from Medicaid. Some states have passed legislation that excludes Planned Parenthood and other qualified providers from participating in state-funded programs. These efforts raise serious concerns about access to care.
Susan Stone, DNSc, the president-elect of the American College of Nurse-Midwives (ACNM) – who was a guest at the meeting – discussed midwifery issues and shared the group’s top legislative priorities with a focus on issues and states in which there could be collaboration between ACOG and the ACNM. This discussion was continued in the breakout sessions, where a smaller group of attendees discussed a variety of issues including oversight, licensing requirements, and collaborative practices.
Another topic for the breakout sessions was the Maternal Mortality Review Committees. With an estimated 700 women dying of pregnancy-related causes in the United States every year and an additional 65,000 women experiencing serious health complications, the creation of a Maternal Mortality Review Committee in each state is a top priority. State representatives discussed this legislation and reviewed how to work with state medical societies, other medical organizations, and advocacy groups to enact this legislation. ACOG has written a proposal that will be presented to the American Medical Association in order to get their support for the passage of state legislation to create Maternal Mortality Review Committees.
Contraception and abortion access continued to be hot topics of discussion. Some states have passed laws that would protect or expand contraceptive coverage and access to abortion regardless of changes that may occur at the federal level. A few states have passed legislation that allows pharmacists to prescribe hormonal contraception. Over-the-counter access to long-term hormonal contraception has not been approved by the Food and Drug Administration and is not currently available.
Many ACOG advocates are lobbying to block state efforts to restrict abortion access, such as laws that ban abortion after 20 weeks, which have been passed in many states. A few states have passed bills that criminalize physicians who perform abortions after 20 weeks. Some states have passed or are considering legislation that defines life as beginning at conception, also referred to as “personhood” legislation. However, other states have blocked bills that would have forced physicians to tell women that a medication abortion can be “reversed.”
During a media workshop, attendees discussed interactions with the media and the use of digital media to advance legislative issues. Throughout the Roundtable, attendees tweeted using the hashtag #ACOGLegWork. The success of #ACOGLegWork resulted in the hashtag trending on Twitter. Ob.gyns. were urged to follow @ACOGAction, ACOG’s advocacy Twitter account, and to try Twitter on their own.
The next meeting of the ACOG State Legislative Roundtable will be Oct. 27-28, 2018, in Nashville, Tenn.
Dr. Bohon is an ob.gyn. in private practice in Washington. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
The American Congress of Obstetricians and Gynecologists held its State Legislative Roundtable in late October in Arlington, Va., with ob.gyns. and their lobbyists from 46 states. This is the largest number of states ever represented at the roundtable event, and it reflects the increased participation and engagement in policy making by women’s health care providers.
Attendees also discussed an increasing number of policies that focused on the exclusion of family planning providers from Medicaid. Some states have passed legislation that excludes Planned Parenthood and other qualified providers from participating in state-funded programs. These efforts raise serious concerns about access to care.
Susan Stone, DNSc, the president-elect of the American College of Nurse-Midwives (ACNM) – who was a guest at the meeting – discussed midwifery issues and shared the group’s top legislative priorities with a focus on issues and states in which there could be collaboration between ACOG and the ACNM. This discussion was continued in the breakout sessions, where a smaller group of attendees discussed a variety of issues including oversight, licensing requirements, and collaborative practices.
Another topic for the breakout sessions was the Maternal Mortality Review Committees. With an estimated 700 women dying of pregnancy-related causes in the United States every year and an additional 65,000 women experiencing serious health complications, the creation of a Maternal Mortality Review Committee in each state is a top priority. State representatives discussed this legislation and reviewed how to work with state medical societies, other medical organizations, and advocacy groups to enact this legislation. ACOG has written a proposal that will be presented to the American Medical Association in order to get their support for the passage of state legislation to create Maternal Mortality Review Committees.
Contraception and abortion access continued to be hot topics of discussion. Some states have passed laws that would protect or expand contraceptive coverage and access to abortion regardless of changes that may occur at the federal level. A few states have passed legislation that allows pharmacists to prescribe hormonal contraception. Over-the-counter access to long-term hormonal contraception has not been approved by the Food and Drug Administration and is not currently available.
Many ACOG advocates are lobbying to block state efforts to restrict abortion access, such as laws that ban abortion after 20 weeks, which have been passed in many states. A few states have passed bills that criminalize physicians who perform abortions after 20 weeks. Some states have passed or are considering legislation that defines life as beginning at conception, also referred to as “personhood” legislation. However, other states have blocked bills that would have forced physicians to tell women that a medication abortion can be “reversed.”
During a media workshop, attendees discussed interactions with the media and the use of digital media to advance legislative issues. Throughout the Roundtable, attendees tweeted using the hashtag #ACOGLegWork. The success of #ACOGLegWork resulted in the hashtag trending on Twitter. Ob.gyns. were urged to follow @ACOGAction, ACOG’s advocacy Twitter account, and to try Twitter on their own.
The next meeting of the ACOG State Legislative Roundtable will be Oct. 27-28, 2018, in Nashville, Tenn.
Dr. Bohon is an ob.gyn. in private practice in Washington. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
The American Congress of Obstetricians and Gynecologists held its State Legislative Roundtable in late October in Arlington, Va., with ob.gyns. and their lobbyists from 46 states. This is the largest number of states ever represented at the roundtable event, and it reflects the increased participation and engagement in policy making by women’s health care providers.
Attendees also discussed an increasing number of policies that focused on the exclusion of family planning providers from Medicaid. Some states have passed legislation that excludes Planned Parenthood and other qualified providers from participating in state-funded programs. These efforts raise serious concerns about access to care.
Susan Stone, DNSc, the president-elect of the American College of Nurse-Midwives (ACNM) – who was a guest at the meeting – discussed midwifery issues and shared the group’s top legislative priorities with a focus on issues and states in which there could be collaboration between ACOG and the ACNM. This discussion was continued in the breakout sessions, where a smaller group of attendees discussed a variety of issues including oversight, licensing requirements, and collaborative practices.
Another topic for the breakout sessions was the Maternal Mortality Review Committees. With an estimated 700 women dying of pregnancy-related causes in the United States every year and an additional 65,000 women experiencing serious health complications, the creation of a Maternal Mortality Review Committee in each state is a top priority. State representatives discussed this legislation and reviewed how to work with state medical societies, other medical organizations, and advocacy groups to enact this legislation. ACOG has written a proposal that will be presented to the American Medical Association in order to get their support for the passage of state legislation to create Maternal Mortality Review Committees.
Contraception and abortion access continued to be hot topics of discussion. Some states have passed laws that would protect or expand contraceptive coverage and access to abortion regardless of changes that may occur at the federal level. A few states have passed legislation that allows pharmacists to prescribe hormonal contraception. Over-the-counter access to long-term hormonal contraception has not been approved by the Food and Drug Administration and is not currently available.
Many ACOG advocates are lobbying to block state efforts to restrict abortion access, such as laws that ban abortion after 20 weeks, which have been passed in many states. A few states have passed bills that criminalize physicians who perform abortions after 20 weeks. Some states have passed or are considering legislation that defines life as beginning at conception, also referred to as “personhood” legislation. However, other states have blocked bills that would have forced physicians to tell women that a medication abortion can be “reversed.”
During a media workshop, attendees discussed interactions with the media and the use of digital media to advance legislative issues. Throughout the Roundtable, attendees tweeted using the hashtag #ACOGLegWork. The success of #ACOGLegWork resulted in the hashtag trending on Twitter. Ob.gyns. were urged to follow @ACOGAction, ACOG’s advocacy Twitter account, and to try Twitter on their own.
The next meeting of the ACOG State Legislative Roundtable will be Oct. 27-28, 2018, in Nashville, Tenn.
Dr. Bohon is an ob.gyn. in private practice in Washington. She is an ACOG state legislative chair from the District of Columbia and a member of the Ob.Gyn. News Editorial Advisory Board. She reported having no relevant financial disclosures.
VIDEO: Consider depression in patients with psoriasis
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Nebulized LABA safe for long-term use in COPD
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
AT CHEST 2017
Key clinical point: The long-term safety of formoterol fumarate inhaled solution was confirmed in an FDA-mandated randomized trial in patients with moderate to severe COPD.
Major finding: Formoterol fumarate was noninferior to placebo for the primary safety endpoint of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Data source: Multicenter, randomized, double-blind, placebo-controlled trial including 1,071 patients with moderate or severe COPD, with at least one exacerbation recorded in the last year.
Disclosures: Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
Interhospital Transfer and Receipt of Specialty Procedures
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
© 2017 Society of Hospital Medicine
617-732-7072; E-mail: [email protected]
Primary Care Provider Preferences for Communication with Inpatient Teams: One Size Does Not Fit All
As the hospitalist’s role in medicine grows, the transition of care from inpatient to primary care providers (PCPs, including primary care physicians, nurse practitioners, or physician assistants), becomes increasingly important. Inadequate communication at this transition is associated with preventable adverse events leading to rehospitalization, disability, and death.1-
Providing PCPs access to the inpatient electronic health record (EHR) may reduce the need for active communication. However, a recent survey of PCPs in the general internal medicine division of an academic hospital found a strong preference for additional communication with inpatient providers, despite a shared EHR.5
We examined communication preferences of general internal medicine PCPs at a different academic institution and extended our study to include community-based PCPs who were both affiliated and unaffiliated with the institution.
METHODS
Between October 2015 and June 2016, we surveyed PCPs from 3 practice groups with institutional affiliation or proximity to The Johns Hopkins Hospital: all general internal medicine faculty with outpatient practices (“academic,” 2 practice sites, n = 35), all community-based PCPs affiliated with the health system (“community,” 36 practice sites, n = 220), and all PCPs from an unaffiliated managed care organization (“unaffiliated,” 5 practice sites ranging from 0.3 to 4 miles from The Johns Hopkins Hospital, n = 29).
All groups have work-sponsored e-mail services. At the time of the survey, both the academic and community groups used an EHR that allowed access to inpatient laboratory and radiology data and discharge summaries. The unaffiliated group used paper health records. The hospital faxes discharge summaries to all PCPs who are identified by patients.
The investigators and representatives from each practice group collaborated to develop 15 questions with mutually exclusive answers to evaluate PCP experiences with and preferences for communication with inpatient teams. The survey was constructed and administered through Qualtrics’ online platform (Qualtrics, Provo, UT) and distributed via e-mail. The study was reviewed and acknowledged by the Johns Hopkins institutional review board as quality improvement activity.
The survey contained branching logic. Only respondents who indicated preference for communication received questions regarding preferred mode of communication. We used the preferred mode of communication for initial contact from the inpatient team in our analysis. χ2 and Fischer’s exact tests were performed with JMP 12 software (SAS Institute Inc, Cary, NC).
RESULTS
Fourteen (40%) academic, 43 (14%) community, and 16 (55%) unaffiliated PCPs completed the survey, for 73 total responses from 284 surveys distributed (26%).
Among the 73 responding PCPs, 31 (42%) reported receiving notification of admission during “every” or “almost every” hospitalization, with no significant variation across practice groups (P = 0.5).
Across all groups, 64 PCPs (88%) preferred communication at 1 or more points during hospitalizations (panel A of Figure). “Both upon admission and prior to discharge” was selected most frequently, and there were no differences between practice groups (P = 0.2).
Preferred mode of communication, however, differed significantly between groups (panel B of Figure). The academic group had a greater preference for telephone (54%) than the community (8%; P < 0.001) and unaffiliated groups (8%; P < 0.001), the community group a greater preference for EHR (77%) than the academic (23%; P = 0.002) and unaffiliated groups (0%; P < 0.001), and the unaffiliated group a greater preference for fax (58%) than the other groups (both 0%; P < 0.001).
DISCUSSION
Our findings add to previous evidence of low rates of communication between inpatient providers and PCPs6 and a preference from PCPs for communication during hospitalizations despite shared EHRs.5 We extend previous work by demonstrating that PCP preferences for mode of communication vary by practice setting. Our findings lead us to hypothesize that identifying and incorporating PCP preferences may improve communication, though at the potential expense of standardization and efficiency.
There may be several reasons for the differing communication preferences observed. Most academic PCPs are located near or have admitting privileges to the hospital and are not in clinic full time. Their preference for the telephone may thus result from interpersonal relationships born from proximity and greater availability for telephone calls, or reduced fluency with the EHR compared to full-time community clinicians.
The unaffiliated group’s preference for fax may reflect a desire for communication that integrates easily with paper charts and is least disruptive to workflow, or concerns about health information confidentiality in e-mails.
Our study’s generalizability is limited by a low response rate, though it is comparable to prior studies.7 The unaffiliated group was accessed by convenience (acquaintance with the medical director); however, we note it had the highest response rate (55%).
In summary, we found low rates of communication between inpatient providers and PCPs, despite a strong preference from most PCPs for such communication during hospitalizations. PCPs’ preferred mode of communication differed based on practice setting. Addressing PCP communication preferences may be important to future care transition interventions.
Disclosure
The authors report no conflicts of interest.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-174. PubMed
2. Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646-651. PubMed
3. van Walraven C, Mamdani M, Fang J, Austin PC. Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624-631. PubMed
4. Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency M. J Hosp Med. 2009;4(6):364-370. PubMed
5. Sheu L, Fung K, Mourad M, Ranji S, Wu E. We need to talk: Primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307-310. PubMed
6. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297(8):831-841. PubMed
7. Pantilat SZ, Lindenauer PK, Katz PP, Wachter RM. Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001(9B);111:15-20. PubMed
As the hospitalist’s role in medicine grows, the transition of care from inpatient to primary care providers (PCPs, including primary care physicians, nurse practitioners, or physician assistants), becomes increasingly important. Inadequate communication at this transition is associated with preventable adverse events leading to rehospitalization, disability, and death.1-
Providing PCPs access to the inpatient electronic health record (EHR) may reduce the need for active communication. However, a recent survey of PCPs in the general internal medicine division of an academic hospital found a strong preference for additional communication with inpatient providers, despite a shared EHR.5
We examined communication preferences of general internal medicine PCPs at a different academic institution and extended our study to include community-based PCPs who were both affiliated and unaffiliated with the institution.
METHODS
Between October 2015 and June 2016, we surveyed PCPs from 3 practice groups with institutional affiliation or proximity to The Johns Hopkins Hospital: all general internal medicine faculty with outpatient practices (“academic,” 2 practice sites, n = 35), all community-based PCPs affiliated with the health system (“community,” 36 practice sites, n = 220), and all PCPs from an unaffiliated managed care organization (“unaffiliated,” 5 practice sites ranging from 0.3 to 4 miles from The Johns Hopkins Hospital, n = 29).
All groups have work-sponsored e-mail services. At the time of the survey, both the academic and community groups used an EHR that allowed access to inpatient laboratory and radiology data and discharge summaries. The unaffiliated group used paper health records. The hospital faxes discharge summaries to all PCPs who are identified by patients.
The investigators and representatives from each practice group collaborated to develop 15 questions with mutually exclusive answers to evaluate PCP experiences with and preferences for communication with inpatient teams. The survey was constructed and administered through Qualtrics’ online platform (Qualtrics, Provo, UT) and distributed via e-mail. The study was reviewed and acknowledged by the Johns Hopkins institutional review board as quality improvement activity.
The survey contained branching logic. Only respondents who indicated preference for communication received questions regarding preferred mode of communication. We used the preferred mode of communication for initial contact from the inpatient team in our analysis. χ2 and Fischer’s exact tests were performed with JMP 12 software (SAS Institute Inc, Cary, NC).
RESULTS
Fourteen (40%) academic, 43 (14%) community, and 16 (55%) unaffiliated PCPs completed the survey, for 73 total responses from 284 surveys distributed (26%).
Among the 73 responding PCPs, 31 (42%) reported receiving notification of admission during “every” or “almost every” hospitalization, with no significant variation across practice groups (P = 0.5).
Across all groups, 64 PCPs (88%) preferred communication at 1 or more points during hospitalizations (panel A of Figure). “Both upon admission and prior to discharge” was selected most frequently, and there were no differences between practice groups (P = 0.2).
Preferred mode of communication, however, differed significantly between groups (panel B of Figure). The academic group had a greater preference for telephone (54%) than the community (8%; P < 0.001) and unaffiliated groups (8%; P < 0.001), the community group a greater preference for EHR (77%) than the academic (23%; P = 0.002) and unaffiliated groups (0%; P < 0.001), and the unaffiliated group a greater preference for fax (58%) than the other groups (both 0%; P < 0.001).
DISCUSSION
Our findings add to previous evidence of low rates of communication between inpatient providers and PCPs6 and a preference from PCPs for communication during hospitalizations despite shared EHRs.5 We extend previous work by demonstrating that PCP preferences for mode of communication vary by practice setting. Our findings lead us to hypothesize that identifying and incorporating PCP preferences may improve communication, though at the potential expense of standardization and efficiency.
There may be several reasons for the differing communication preferences observed. Most academic PCPs are located near or have admitting privileges to the hospital and are not in clinic full time. Their preference for the telephone may thus result from interpersonal relationships born from proximity and greater availability for telephone calls, or reduced fluency with the EHR compared to full-time community clinicians.
The unaffiliated group’s preference for fax may reflect a desire for communication that integrates easily with paper charts and is least disruptive to workflow, or concerns about health information confidentiality in e-mails.
Our study’s generalizability is limited by a low response rate, though it is comparable to prior studies.7 The unaffiliated group was accessed by convenience (acquaintance with the medical director); however, we note it had the highest response rate (55%).
In summary, we found low rates of communication between inpatient providers and PCPs, despite a strong preference from most PCPs for such communication during hospitalizations. PCPs’ preferred mode of communication differed based on practice setting. Addressing PCP communication preferences may be important to future care transition interventions.
Disclosure
The authors report no conflicts of interest.
As the hospitalist’s role in medicine grows, the transition of care from inpatient to primary care providers (PCPs, including primary care physicians, nurse practitioners, or physician assistants), becomes increasingly important. Inadequate communication at this transition is associated with preventable adverse events leading to rehospitalization, disability, and death.1-
Providing PCPs access to the inpatient electronic health record (EHR) may reduce the need for active communication. However, a recent survey of PCPs in the general internal medicine division of an academic hospital found a strong preference for additional communication with inpatient providers, despite a shared EHR.5
We examined communication preferences of general internal medicine PCPs at a different academic institution and extended our study to include community-based PCPs who were both affiliated and unaffiliated with the institution.
METHODS
Between October 2015 and June 2016, we surveyed PCPs from 3 practice groups with institutional affiliation or proximity to The Johns Hopkins Hospital: all general internal medicine faculty with outpatient practices (“academic,” 2 practice sites, n = 35), all community-based PCPs affiliated with the health system (“community,” 36 practice sites, n = 220), and all PCPs from an unaffiliated managed care organization (“unaffiliated,” 5 practice sites ranging from 0.3 to 4 miles from The Johns Hopkins Hospital, n = 29).
All groups have work-sponsored e-mail services. At the time of the survey, both the academic and community groups used an EHR that allowed access to inpatient laboratory and radiology data and discharge summaries. The unaffiliated group used paper health records. The hospital faxes discharge summaries to all PCPs who are identified by patients.
The investigators and representatives from each practice group collaborated to develop 15 questions with mutually exclusive answers to evaluate PCP experiences with and preferences for communication with inpatient teams. The survey was constructed and administered through Qualtrics’ online platform (Qualtrics, Provo, UT) and distributed via e-mail. The study was reviewed and acknowledged by the Johns Hopkins institutional review board as quality improvement activity.
The survey contained branching logic. Only respondents who indicated preference for communication received questions regarding preferred mode of communication. We used the preferred mode of communication for initial contact from the inpatient team in our analysis. χ2 and Fischer’s exact tests were performed with JMP 12 software (SAS Institute Inc, Cary, NC).
RESULTS
Fourteen (40%) academic, 43 (14%) community, and 16 (55%) unaffiliated PCPs completed the survey, for 73 total responses from 284 surveys distributed (26%).
Among the 73 responding PCPs, 31 (42%) reported receiving notification of admission during “every” or “almost every” hospitalization, with no significant variation across practice groups (P = 0.5).
Across all groups, 64 PCPs (88%) preferred communication at 1 or more points during hospitalizations (panel A of Figure). “Both upon admission and prior to discharge” was selected most frequently, and there were no differences between practice groups (P = 0.2).
Preferred mode of communication, however, differed significantly between groups (panel B of Figure). The academic group had a greater preference for telephone (54%) than the community (8%; P < 0.001) and unaffiliated groups (8%; P < 0.001), the community group a greater preference for EHR (77%) than the academic (23%; P = 0.002) and unaffiliated groups (0%; P < 0.001), and the unaffiliated group a greater preference for fax (58%) than the other groups (both 0%; P < 0.001).
DISCUSSION
Our findings add to previous evidence of low rates of communication between inpatient providers and PCPs6 and a preference from PCPs for communication during hospitalizations despite shared EHRs.5 We extend previous work by demonstrating that PCP preferences for mode of communication vary by practice setting. Our findings lead us to hypothesize that identifying and incorporating PCP preferences may improve communication, though at the potential expense of standardization and efficiency.
There may be several reasons for the differing communication preferences observed. Most academic PCPs are located near or have admitting privileges to the hospital and are not in clinic full time. Their preference for the telephone may thus result from interpersonal relationships born from proximity and greater availability for telephone calls, or reduced fluency with the EHR compared to full-time community clinicians.
The unaffiliated group’s preference for fax may reflect a desire for communication that integrates easily with paper charts and is least disruptive to workflow, or concerns about health information confidentiality in e-mails.
Our study’s generalizability is limited by a low response rate, though it is comparable to prior studies.7 The unaffiliated group was accessed by convenience (acquaintance with the medical director); however, we note it had the highest response rate (55%).
In summary, we found low rates of communication between inpatient providers and PCPs, despite a strong preference from most PCPs for such communication during hospitalizations. PCPs’ preferred mode of communication differed based on practice setting. Addressing PCP communication preferences may be important to future care transition interventions.
Disclosure
The authors report no conflicts of interest.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-174. PubMed
2. Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646-651. PubMed
3. van Walraven C, Mamdani M, Fang J, Austin PC. Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624-631. PubMed
4. Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency M. J Hosp Med. 2009;4(6):364-370. PubMed
5. Sheu L, Fung K, Mourad M, Ranji S, Wu E. We need to talk: Primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307-310. PubMed
6. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297(8):831-841. PubMed
7. Pantilat SZ, Lindenauer PK, Katz PP, Wachter RM. Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001(9B);111:15-20. PubMed
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-174. PubMed
2. Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646-651. PubMed
3. van Walraven C, Mamdani M, Fang J, Austin PC. Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624-631. PubMed
4. Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency M. J Hosp Med. 2009;4(6):364-370. PubMed
5. Sheu L, Fung K, Mourad M, Ranji S, Wu E. We need to talk: Primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307-310. PubMed
6. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297(8):831-841. PubMed
7. Pantilat SZ, Lindenauer PK, Katz PP, Wachter RM. Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001(9B);111:15-20. PubMed
© 2017 Society of Hospital Medicine
[email protected]
Proposed In-Training Electrocardiogram Interpretation Competencies for Undergraduate and Postgraduate Trainees
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
© 2018 Society of Hospital Medicine
Public health hazard: Bring your flu to work day
Slightly more than 41% of health care personnel who had the flu during the 2014-2015 influenza season went to work while they were ill, according to an annual survey.
Physicians, however, were well above this average, with 63% reporting they had worked with an influenza-like illness (ILI); they were not quite as far above average as pharmacists, though, who had a 67% rate of “presenteeism” – the highest among all of the health care occupations included in the survey, said Sophia Chiu, MD, MPH, of the Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health, and her associates.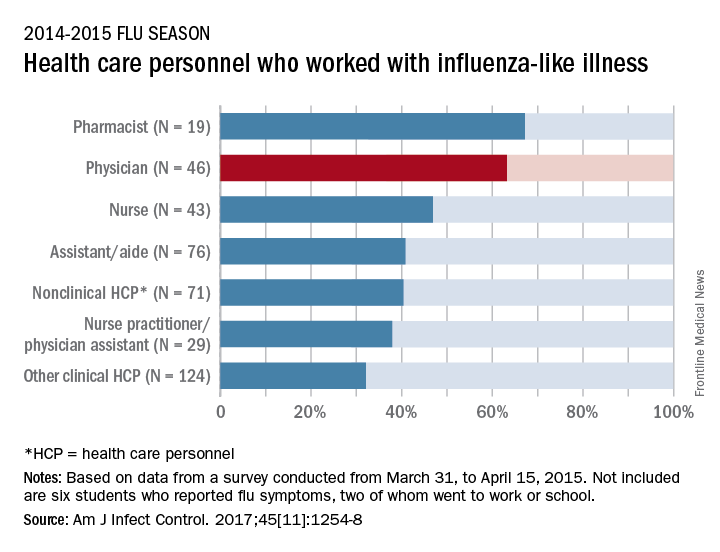
“The statistics are alarming. At least one earlier study has shown that patients who are exposed to a health care worker who is sick are five times more likely to get a health care–associated infection,” Dr. Chiu said in a separate written statement.
For the study, ILI was defined as “fever (without a specified temperature cutoff) and sore throat or cough.” The “nonclinical personnel” category included managers, food service workers, and janitors, while the “other clinical personnel” category included technicians and technologists. The annual Internet panel survey was conducted from March 31, 2015, to April 15, 2015, and 414 of its 1,914 respondents self-reported having an ILI, of whom 183 said that they worked during their illness, Dr. Chiu and her associates said.
The investigators are all CDC employees. The respondents were recruited from Internet panels operated by Survey Sampling International through a contract with Abt Associates.
Slightly more than 41% of health care personnel who had the flu during the 2014-2015 influenza season went to work while they were ill, according to an annual survey.
Physicians, however, were well above this average, with 63% reporting they had worked with an influenza-like illness (ILI); they were not quite as far above average as pharmacists, though, who had a 67% rate of “presenteeism” – the highest among all of the health care occupations included in the survey, said Sophia Chiu, MD, MPH, of the Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health, and her associates.
“The statistics are alarming. At least one earlier study has shown that patients who are exposed to a health care worker who is sick are five times more likely to get a health care–associated infection,” Dr. Chiu said in a separate written statement.
For the study, ILI was defined as “fever (without a specified temperature cutoff) and sore throat or cough.” The “nonclinical personnel” category included managers, food service workers, and janitors, while the “other clinical personnel” category included technicians and technologists. The annual Internet panel survey was conducted from March 31, 2015, to April 15, 2015, and 414 of its 1,914 respondents self-reported having an ILI, of whom 183 said that they worked during their illness, Dr. Chiu and her associates said.
The investigators are all CDC employees. The respondents were recruited from Internet panels operated by Survey Sampling International through a contract with Abt Associates.
Slightly more than 41% of health care personnel who had the flu during the 2014-2015 influenza season went to work while they were ill, according to an annual survey.
Physicians, however, were well above this average, with 63% reporting they had worked with an influenza-like illness (ILI); they were not quite as far above average as pharmacists, though, who had a 67% rate of “presenteeism” – the highest among all of the health care occupations included in the survey, said Sophia Chiu, MD, MPH, of the Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health, and her associates.
“The statistics are alarming. At least one earlier study has shown that patients who are exposed to a health care worker who is sick are five times more likely to get a health care–associated infection,” Dr. Chiu said in a separate written statement.
For the study, ILI was defined as “fever (without a specified temperature cutoff) and sore throat or cough.” The “nonclinical personnel” category included managers, food service workers, and janitors, while the “other clinical personnel” category included technicians and technologists. The annual Internet panel survey was conducted from March 31, 2015, to April 15, 2015, and 414 of its 1,914 respondents self-reported having an ILI, of whom 183 said that they worked during their illness, Dr. Chiu and her associates said.
The investigators are all CDC employees. The respondents were recruited from Internet panels operated by Survey Sampling International through a contract with Abt Associates.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
MedPAC offers more details of MIPS replacement
WASHINGTON – The Medicare Payment Advisory Commission continues to mull the specifics of its proposed recommendation to scrap the Quality Payment Program’s MIPS component.
The basics of the MIPS (Merit-based Incentive Payment System) replacement have not changed. The proposal calls for creation of a voluntary value program (VVP) that would withhold a percentage – currently 2% – of Medicare payments for physicians who are not part of an advanced alternative payment model (APM) under the Quality Payment Program of the Medicare Access and CHIP Reauthorization Act of 2015.
There would be two ways to recapture the withheld pay. The first would be to join an APM. The second would be to participate in a VVP by entering a voluntary reporting group. Under the proposal, VVPs would be at least 10 providers who would report together on population-based measures, patient experience, and cost measures, according to staff presentations given Nov. 2 at a meeting of the Medicare Payment Advisory Commission (MedPAC).
Proposed measures would be patient oriented, would encourage coordination across all providers, would promote positive change in the delivery system, and would be less burdensome to providers. Measures would be more in line with those employed by APMs – important because the overall goal would be encouraging participation in an APM rather than permanently lingering in the VVP.
To that end, MedPAC staff member Kate Bloniarz noted during the presentation of the VVP proposal that the total payments in the program “should be capped to be less attractive than joining an [advanced] APM. This comes from a general sense among commissioners that clinicians should not be able to receive large bonuses” for remaining in Medicare fee-for-service.
MedPAC staff recommended that the Centers for Medicare & Medicaid Services offer a fallback group that would provide an option to providers that would otherwise not have access to other groups to join.
Commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, suggested withholding be increased to perhaps 3%, with providers able to recoup 2% in the VVP and 3% in an APM, to further incentivize APM participation.
Staff noted that certain quality measures and process measures would be lost if MIPS were to go away, but they could be accounted for in other channels, such as through electronic health records and registries.
Most commissioners expressed support for both the repeal of MIPS and the conceptual framework for the new VVP, although many sought more details, particularly in the handling of specialists.
“I don’t know that we want to try to make the VVP do too much, especially when you get into the specialties that are very, very episodic,” said commission member Brian DeBusk, PhD, CEO of DeRoyal Industries. “The classic example would be a joint replacement. … I hope to see some specialist APMs developed in parallel and I think that is going to take some pressure off to try to make the VVP be all things to all people.”
Commission member Pat Wang, CEO of Healthfirst, offered a possible solution.
“I would suggest that we try to think about doing that in the context of something that is a little bit, perhaps, not full bore APM, but a VVP for specialists with their own metrics that are not big, gigantic readmissions,” she said. “Those are very broad population health metrics [that may not work for specialists].”
But at least two commission members voiced their dissent to the proposal as presented, with one going so far as to saying that MIPS should not be repealed.
David Nerenz, PhD, of the Henry Ford Health System, said that he had “very serious concerns about the VVP part of this proposal [and] they are such that if it comes to us as a recommendation in more or less its current form I will not support it.”
He called it “pretty significant social engineering in the structure of medical practice and I think we are doing it in the absence of what to me would be compelling evidence that this large group structure we are talking about is good.
“I also don’t see any evidence that beneficiaries find value in the set of measures we are talking about,” Dr. Nerenz added. He is on the record as supporting the repeal of MIPS.
Commission member Alice Coombs, MD, of Weymouth, Mass., was the lone voice speaking in support of MIPS: “I think MIPS has a lot of problems … but there are some things that are coming out of MIPS that are actually good.”
She called the VVP proposal “inadequate” and took issue with the measures. As a practicing physician, she said that she favors more population health measures that will affect patient outcomes.
MedPAC staff expect to have a draft recommendation prepared for discussion at the December meeting, with a final vote on what will be presented to Congress coming as soon as January.
WASHINGTON – The Medicare Payment Advisory Commission continues to mull the specifics of its proposed recommendation to scrap the Quality Payment Program’s MIPS component.
The basics of the MIPS (Merit-based Incentive Payment System) replacement have not changed. The proposal calls for creation of a voluntary value program (VVP) that would withhold a percentage – currently 2% – of Medicare payments for physicians who are not part of an advanced alternative payment model (APM) under the Quality Payment Program of the Medicare Access and CHIP Reauthorization Act of 2015.
There would be two ways to recapture the withheld pay. The first would be to join an APM. The second would be to participate in a VVP by entering a voluntary reporting group. Under the proposal, VVPs would be at least 10 providers who would report together on population-based measures, patient experience, and cost measures, according to staff presentations given Nov. 2 at a meeting of the Medicare Payment Advisory Commission (MedPAC).
Proposed measures would be patient oriented, would encourage coordination across all providers, would promote positive change in the delivery system, and would be less burdensome to providers. Measures would be more in line with those employed by APMs – important because the overall goal would be encouraging participation in an APM rather than permanently lingering in the VVP.
To that end, MedPAC staff member Kate Bloniarz noted during the presentation of the VVP proposal that the total payments in the program “should be capped to be less attractive than joining an [advanced] APM. This comes from a general sense among commissioners that clinicians should not be able to receive large bonuses” for remaining in Medicare fee-for-service.
MedPAC staff recommended that the Centers for Medicare & Medicaid Services offer a fallback group that would provide an option to providers that would otherwise not have access to other groups to join.
Commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, suggested withholding be increased to perhaps 3%, with providers able to recoup 2% in the VVP and 3% in an APM, to further incentivize APM participation.
Staff noted that certain quality measures and process measures would be lost if MIPS were to go away, but they could be accounted for in other channels, such as through electronic health records and registries.
Most commissioners expressed support for both the repeal of MIPS and the conceptual framework for the new VVP, although many sought more details, particularly in the handling of specialists.
“I don’t know that we want to try to make the VVP do too much, especially when you get into the specialties that are very, very episodic,” said commission member Brian DeBusk, PhD, CEO of DeRoyal Industries. “The classic example would be a joint replacement. … I hope to see some specialist APMs developed in parallel and I think that is going to take some pressure off to try to make the VVP be all things to all people.”
Commission member Pat Wang, CEO of Healthfirst, offered a possible solution.
“I would suggest that we try to think about doing that in the context of something that is a little bit, perhaps, not full bore APM, but a VVP for specialists with their own metrics that are not big, gigantic readmissions,” she said. “Those are very broad population health metrics [that may not work for specialists].”
But at least two commission members voiced their dissent to the proposal as presented, with one going so far as to saying that MIPS should not be repealed.
David Nerenz, PhD, of the Henry Ford Health System, said that he had “very serious concerns about the VVP part of this proposal [and] they are such that if it comes to us as a recommendation in more or less its current form I will not support it.”
He called it “pretty significant social engineering in the structure of medical practice and I think we are doing it in the absence of what to me would be compelling evidence that this large group structure we are talking about is good.
“I also don’t see any evidence that beneficiaries find value in the set of measures we are talking about,” Dr. Nerenz added. He is on the record as supporting the repeal of MIPS.
Commission member Alice Coombs, MD, of Weymouth, Mass., was the lone voice speaking in support of MIPS: “I think MIPS has a lot of problems … but there are some things that are coming out of MIPS that are actually good.”
She called the VVP proposal “inadequate” and took issue with the measures. As a practicing physician, she said that she favors more population health measures that will affect patient outcomes.
MedPAC staff expect to have a draft recommendation prepared for discussion at the December meeting, with a final vote on what will be presented to Congress coming as soon as January.
WASHINGTON – The Medicare Payment Advisory Commission continues to mull the specifics of its proposed recommendation to scrap the Quality Payment Program’s MIPS component.
The basics of the MIPS (Merit-based Incentive Payment System) replacement have not changed. The proposal calls for creation of a voluntary value program (VVP) that would withhold a percentage – currently 2% – of Medicare payments for physicians who are not part of an advanced alternative payment model (APM) under the Quality Payment Program of the Medicare Access and CHIP Reauthorization Act of 2015.
There would be two ways to recapture the withheld pay. The first would be to join an APM. The second would be to participate in a VVP by entering a voluntary reporting group. Under the proposal, VVPs would be at least 10 providers who would report together on population-based measures, patient experience, and cost measures, according to staff presentations given Nov. 2 at a meeting of the Medicare Payment Advisory Commission (MedPAC).
Proposed measures would be patient oriented, would encourage coordination across all providers, would promote positive change in the delivery system, and would be less burdensome to providers. Measures would be more in line with those employed by APMs – important because the overall goal would be encouraging participation in an APM rather than permanently lingering in the VVP.
To that end, MedPAC staff member Kate Bloniarz noted during the presentation of the VVP proposal that the total payments in the program “should be capped to be less attractive than joining an [advanced] APM. This comes from a general sense among commissioners that clinicians should not be able to receive large bonuses” for remaining in Medicare fee-for-service.
MedPAC staff recommended that the Centers for Medicare & Medicaid Services offer a fallback group that would provide an option to providers that would otherwise not have access to other groups to join.
Commission member Kathy Buto, former vice president of global health policy at Johnson & Johnson, suggested withholding be increased to perhaps 3%, with providers able to recoup 2% in the VVP and 3% in an APM, to further incentivize APM participation.
Staff noted that certain quality measures and process measures would be lost if MIPS were to go away, but they could be accounted for in other channels, such as through electronic health records and registries.
Most commissioners expressed support for both the repeal of MIPS and the conceptual framework for the new VVP, although many sought more details, particularly in the handling of specialists.
“I don’t know that we want to try to make the VVP do too much, especially when you get into the specialties that are very, very episodic,” said commission member Brian DeBusk, PhD, CEO of DeRoyal Industries. “The classic example would be a joint replacement. … I hope to see some specialist APMs developed in parallel and I think that is going to take some pressure off to try to make the VVP be all things to all people.”
Commission member Pat Wang, CEO of Healthfirst, offered a possible solution.
“I would suggest that we try to think about doing that in the context of something that is a little bit, perhaps, not full bore APM, but a VVP for specialists with their own metrics that are not big, gigantic readmissions,” she said. “Those are very broad population health metrics [that may not work for specialists].”
But at least two commission members voiced their dissent to the proposal as presented, with one going so far as to saying that MIPS should not be repealed.
David Nerenz, PhD, of the Henry Ford Health System, said that he had “very serious concerns about the VVP part of this proposal [and] they are such that if it comes to us as a recommendation in more or less its current form I will not support it.”
He called it “pretty significant social engineering in the structure of medical practice and I think we are doing it in the absence of what to me would be compelling evidence that this large group structure we are talking about is good.
“I also don’t see any evidence that beneficiaries find value in the set of measures we are talking about,” Dr. Nerenz added. He is on the record as supporting the repeal of MIPS.
Commission member Alice Coombs, MD, of Weymouth, Mass., was the lone voice speaking in support of MIPS: “I think MIPS has a lot of problems … but there are some things that are coming out of MIPS that are actually good.”
She called the VVP proposal “inadequate” and took issue with the measures. As a practicing physician, she said that she favors more population health measures that will affect patient outcomes.
MedPAC staff expect to have a draft recommendation prepared for discussion at the December meeting, with a final vote on what will be presented to Congress coming as soon as January.
AT A PUBLIC MEETING OF MEDPAC