User login
SHORT TAKES
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Don’t let GDM history limit contraception choices
WASHINGTON – Women with a history of gestational diabetes can use any contraceptive method safely, and those with uncomplicated pregestational diabetes can also consider all methods, said Anne Burke, MD, director of the family planning division at Johns Hopkins University, Baltimore.
“The real cautions, and some red lights,” apply to those with vascular sequelae, diabetes of 20 years’ duration or more, and patients with other vascular disease in addition to diabetes, she said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“The contraception options for women with diabetes aren’t necessarily terribly limited compared to the options for other women,” Dr. Burke said. “And the big take home is that, across the board, .”
The U.S. Medical Eligibility Criteria for Contraceptive Use issued by the Centers for Disease Control and Prevention assign a 1-4 rating for each method for women with certain characteristics or medical conditions. Category 1 indicates no restrictions, and category 2 means that the advantages generally outweigh theoretical or proven risks. Category 3 indicates that such risks usually outweigh the advantages, and category 4 means there is an unacceptable risk.
The assignment to category 2 instead of category 1 can reflect either limitations in the overall amount of data available or the lack of strong randomized studies “whereas the data [otherwise] seem to support safety,” Dr. Burke said. “And sometimes, it relates to most studies saying one thing and another being a little inconsistent.”
Uncomplicated pregestational diabetes
This is important to understand because all the methods for women with uncomplicated pregestational diabetes (no evidence of vascular disease or end-organ damage) are classified as category 2, except for the copper IUD and emergency contraception, which are in category 1. The document distinguishes between insulin-dependent and non–insulin-dependent diabetes, but the recommendations do not differ between the two categories, she said.
Progestin-only contraceptives appear to have little effect on short- or long-term diabetes control, hemostatic markers, or the lipid profile in women with uncomplicated diabetes. Combined hormonal contraception appears to have no effect on long-term diabetes control or progression to retinopathy; there may be changes in the lipid profile and hemostatic markers, but “mostly within normal values, and in some cases, in a favorable direction,” said Dr. Burke of the department of gynecology and obstetrics at the university.
GDM history
In women who have had gestational diabetes, all methods are in category 1 of the Medical Eligibility Criteria (MEC). While “there have been a couple of question marks” with progestin-only contraceptives and the later development of diabetes, “it seems that there’s really not an increased risk,” she said. Nor does there seem to be an increased risk of developing later diabetes with combined hormonal contraception.
In general, the data backing the MEC come from a limited number of studies, and “few that are rigorously done,” she said. “So the recommendations reflect consensus [that is] based on the best available information.”
Severe or long-standing disease
Data are especially limited for women with more severe and/or long-standing disease, as these women have been excluded from studies. There is enough knowledge, however, to make the hypoestrogenic effects of the depot medroxyprogesterone acetate (DMPA) injectable (Depo-Provera) concerning. “It has a pretty hefty dose of a particular type of progestin that significantly suppresses the hypothalamic-pituitary-ovarian axis – more than other progestin-only methods,” she said. “And we may see some unfavorable lipid changes and changes in carbohydrate metabolism.”
The effects of the DMPA injectable, which is in category 3 for these women, may persist for several months – or longer – after discontinuation, Dr. Burke said. The levonorgestrel IUD, on the other hand, has little effect on diabetes control, hemostatic markers, or lipids; it is in category 2 for these women.
A recently published database analysis found that diabetic users of the DMPA injectable had a hazard ratio for venous thromboembolism of 4.6, compared with IUD users, Dr. Burke said. The study included patients with type 1 and type 2 diabetes (Diabetes Care 2017;40:233-8).
Combined hormonal contraception is assigned to categories 3 and 4 for women with complicated or long-standing diabetes, in part because of thrombosis risk, which “as we know, is slightly elevated even for healthy women,” Dr. Burke said. “There are still quite a few methods that are safe to use without reservation, so here is where we start to move away from combined hormonal methods.”
In addition to the Medical Eligibility Criteria, the CDC has another document, the U.S. Selected Practice Recommendations for Contraceptive Use, also last updated in 2016, which offers “helpful” advice on precontraception tests to perform, timing after pregnancy for starting contraceptive methods, and other issues, Dr. Burke said.
Dr. Burke reported receiving research funding from Bayer, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Ibis Reproductive Health.
WASHINGTON – Women with a history of gestational diabetes can use any contraceptive method safely, and those with uncomplicated pregestational diabetes can also consider all methods, said Anne Burke, MD, director of the family planning division at Johns Hopkins University, Baltimore.
“The real cautions, and some red lights,” apply to those with vascular sequelae, diabetes of 20 years’ duration or more, and patients with other vascular disease in addition to diabetes, she said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“The contraception options for women with diabetes aren’t necessarily terribly limited compared to the options for other women,” Dr. Burke said. “And the big take home is that, across the board, .”
The U.S. Medical Eligibility Criteria for Contraceptive Use issued by the Centers for Disease Control and Prevention assign a 1-4 rating for each method for women with certain characteristics or medical conditions. Category 1 indicates no restrictions, and category 2 means that the advantages generally outweigh theoretical or proven risks. Category 3 indicates that such risks usually outweigh the advantages, and category 4 means there is an unacceptable risk.
The assignment to category 2 instead of category 1 can reflect either limitations in the overall amount of data available or the lack of strong randomized studies “whereas the data [otherwise] seem to support safety,” Dr. Burke said. “And sometimes, it relates to most studies saying one thing and another being a little inconsistent.”
Uncomplicated pregestational diabetes
This is important to understand because all the methods for women with uncomplicated pregestational diabetes (no evidence of vascular disease or end-organ damage) are classified as category 2, except for the copper IUD and emergency contraception, which are in category 1. The document distinguishes between insulin-dependent and non–insulin-dependent diabetes, but the recommendations do not differ between the two categories, she said.
Progestin-only contraceptives appear to have little effect on short- or long-term diabetes control, hemostatic markers, or the lipid profile in women with uncomplicated diabetes. Combined hormonal contraception appears to have no effect on long-term diabetes control or progression to retinopathy; there may be changes in the lipid profile and hemostatic markers, but “mostly within normal values, and in some cases, in a favorable direction,” said Dr. Burke of the department of gynecology and obstetrics at the university.
GDM history
In women who have had gestational diabetes, all methods are in category 1 of the Medical Eligibility Criteria (MEC). While “there have been a couple of question marks” with progestin-only contraceptives and the later development of diabetes, “it seems that there’s really not an increased risk,” she said. Nor does there seem to be an increased risk of developing later diabetes with combined hormonal contraception.
In general, the data backing the MEC come from a limited number of studies, and “few that are rigorously done,” she said. “So the recommendations reflect consensus [that is] based on the best available information.”
Severe or long-standing disease
Data are especially limited for women with more severe and/or long-standing disease, as these women have been excluded from studies. There is enough knowledge, however, to make the hypoestrogenic effects of the depot medroxyprogesterone acetate (DMPA) injectable (Depo-Provera) concerning. “It has a pretty hefty dose of a particular type of progestin that significantly suppresses the hypothalamic-pituitary-ovarian axis – more than other progestin-only methods,” she said. “And we may see some unfavorable lipid changes and changes in carbohydrate metabolism.”
The effects of the DMPA injectable, which is in category 3 for these women, may persist for several months – or longer – after discontinuation, Dr. Burke said. The levonorgestrel IUD, on the other hand, has little effect on diabetes control, hemostatic markers, or lipids; it is in category 2 for these women.
A recently published database analysis found that diabetic users of the DMPA injectable had a hazard ratio for venous thromboembolism of 4.6, compared with IUD users, Dr. Burke said. The study included patients with type 1 and type 2 diabetes (Diabetes Care 2017;40:233-8).
Combined hormonal contraception is assigned to categories 3 and 4 for women with complicated or long-standing diabetes, in part because of thrombosis risk, which “as we know, is slightly elevated even for healthy women,” Dr. Burke said. “There are still quite a few methods that are safe to use without reservation, so here is where we start to move away from combined hormonal methods.”
In addition to the Medical Eligibility Criteria, the CDC has another document, the U.S. Selected Practice Recommendations for Contraceptive Use, also last updated in 2016, which offers “helpful” advice on precontraception tests to perform, timing after pregnancy for starting contraceptive methods, and other issues, Dr. Burke said.
Dr. Burke reported receiving research funding from Bayer, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Ibis Reproductive Health.
WASHINGTON – Women with a history of gestational diabetes can use any contraceptive method safely, and those with uncomplicated pregestational diabetes can also consider all methods, said Anne Burke, MD, director of the family planning division at Johns Hopkins University, Baltimore.
“The real cautions, and some red lights,” apply to those with vascular sequelae, diabetes of 20 years’ duration or more, and patients with other vascular disease in addition to diabetes, she said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“The contraception options for women with diabetes aren’t necessarily terribly limited compared to the options for other women,” Dr. Burke said. “And the big take home is that, across the board, .”
The U.S. Medical Eligibility Criteria for Contraceptive Use issued by the Centers for Disease Control and Prevention assign a 1-4 rating for each method for women with certain characteristics or medical conditions. Category 1 indicates no restrictions, and category 2 means that the advantages generally outweigh theoretical or proven risks. Category 3 indicates that such risks usually outweigh the advantages, and category 4 means there is an unacceptable risk.
The assignment to category 2 instead of category 1 can reflect either limitations in the overall amount of data available or the lack of strong randomized studies “whereas the data [otherwise] seem to support safety,” Dr. Burke said. “And sometimes, it relates to most studies saying one thing and another being a little inconsistent.”
Uncomplicated pregestational diabetes
This is important to understand because all the methods for women with uncomplicated pregestational diabetes (no evidence of vascular disease or end-organ damage) are classified as category 2, except for the copper IUD and emergency contraception, which are in category 1. The document distinguishes between insulin-dependent and non–insulin-dependent diabetes, but the recommendations do not differ between the two categories, she said.
Progestin-only contraceptives appear to have little effect on short- or long-term diabetes control, hemostatic markers, or the lipid profile in women with uncomplicated diabetes. Combined hormonal contraception appears to have no effect on long-term diabetes control or progression to retinopathy; there may be changes in the lipid profile and hemostatic markers, but “mostly within normal values, and in some cases, in a favorable direction,” said Dr. Burke of the department of gynecology and obstetrics at the university.
GDM history
In women who have had gestational diabetes, all methods are in category 1 of the Medical Eligibility Criteria (MEC). While “there have been a couple of question marks” with progestin-only contraceptives and the later development of diabetes, “it seems that there’s really not an increased risk,” she said. Nor does there seem to be an increased risk of developing later diabetes with combined hormonal contraception.
In general, the data backing the MEC come from a limited number of studies, and “few that are rigorously done,” she said. “So the recommendations reflect consensus [that is] based on the best available information.”
Severe or long-standing disease
Data are especially limited for women with more severe and/or long-standing disease, as these women have been excluded from studies. There is enough knowledge, however, to make the hypoestrogenic effects of the depot medroxyprogesterone acetate (DMPA) injectable (Depo-Provera) concerning. “It has a pretty hefty dose of a particular type of progestin that significantly suppresses the hypothalamic-pituitary-ovarian axis – more than other progestin-only methods,” she said. “And we may see some unfavorable lipid changes and changes in carbohydrate metabolism.”
The effects of the DMPA injectable, which is in category 3 for these women, may persist for several months – or longer – after discontinuation, Dr. Burke said. The levonorgestrel IUD, on the other hand, has little effect on diabetes control, hemostatic markers, or lipids; it is in category 2 for these women.
A recently published database analysis found that diabetic users of the DMPA injectable had a hazard ratio for venous thromboembolism of 4.6, compared with IUD users, Dr. Burke said. The study included patients with type 1 and type 2 diabetes (Diabetes Care 2017;40:233-8).
Combined hormonal contraception is assigned to categories 3 and 4 for women with complicated or long-standing diabetes, in part because of thrombosis risk, which “as we know, is slightly elevated even for healthy women,” Dr. Burke said. “There are still quite a few methods that are safe to use without reservation, so here is where we start to move away from combined hormonal methods.”
In addition to the Medical Eligibility Criteria, the CDC has another document, the U.S. Selected Practice Recommendations for Contraceptive Use, also last updated in 2016, which offers “helpful” advice on precontraception tests to perform, timing after pregnancy for starting contraceptive methods, and other issues, Dr. Burke said.
Dr. Burke reported receiving research funding from Bayer, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Ibis Reproductive Health.
EXPERT ANALYSIS FROM DPSG-NA 2017
Infections increase risk of idiopathic VTE
Infection and infection sites have been found to be associated with a significant increased risk of venous thromboembolism, according to results of a population-based, matched, case-control analysis of medical records covering the 13-year period 1988-2000.
Dr. Kevin P. Cohoon and his colleagues at the Mayo Clinic, Rochester, Minn., developed models using conditional logistic regression analysis to stratify the risk associated with specific infections and infection sites.
Five hundred thirteen (39.4%) cases and 189 (12.7%) controls had an infection within the previous 92 days (odds ratio, 4.5; P less than .0001). Known VTE risk factors and potentially confounding variables were used in the adjusted univariate and multivariate models, as reported in the American Journal of Medicine (2017. doi: 10.1016/j.amjmed.2017.09.015).
Dr. Cohoon and his colleagues reported that univariate analysis showed “most infection sites were strongly associated with venous thromboembolism” and the adjusted multivariate model resulted in 2.4-fold (P less than .0001) higher odds for VTE incidence, compared with uninfected controls.
Adjusted multivariate analysis ranked the odds of VTE according to specific infections. Dr. Cohoon and his colleagues reported that this modeling showed that the “highest magnitude of risk, compared with no infection, was imparted by intra-abdominal infection (OR, 18) followed by oral infection (OR, 12), systematic blood stream infection (OR, 11), lower respiratory infection such as pneumonia (OR, 3.6), and symptomatic urinary tract infection (OR, 2.2).”
The researchers concluded that their findings may allow for further refinement of inpatient VTE risk-prediction models such as the Padua prediction score and “future studies are required to assess the utility of venous thromboembolism prophylaxis among outpatients with high venous thromboembolism risk infections.”
The authors reported that they had no conflicts of interest.
Infection and infection sites have been found to be associated with a significant increased risk of venous thromboembolism, according to results of a population-based, matched, case-control analysis of medical records covering the 13-year period 1988-2000.
Dr. Kevin P. Cohoon and his colleagues at the Mayo Clinic, Rochester, Minn., developed models using conditional logistic regression analysis to stratify the risk associated with specific infections and infection sites.
Five hundred thirteen (39.4%) cases and 189 (12.7%) controls had an infection within the previous 92 days (odds ratio, 4.5; P less than .0001). Known VTE risk factors and potentially confounding variables were used in the adjusted univariate and multivariate models, as reported in the American Journal of Medicine (2017. doi: 10.1016/j.amjmed.2017.09.015).
Dr. Cohoon and his colleagues reported that univariate analysis showed “most infection sites were strongly associated with venous thromboembolism” and the adjusted multivariate model resulted in 2.4-fold (P less than .0001) higher odds for VTE incidence, compared with uninfected controls.
Adjusted multivariate analysis ranked the odds of VTE according to specific infections. Dr. Cohoon and his colleagues reported that this modeling showed that the “highest magnitude of risk, compared with no infection, was imparted by intra-abdominal infection (OR, 18) followed by oral infection (OR, 12), systematic blood stream infection (OR, 11), lower respiratory infection such as pneumonia (OR, 3.6), and symptomatic urinary tract infection (OR, 2.2).”
The researchers concluded that their findings may allow for further refinement of inpatient VTE risk-prediction models such as the Padua prediction score and “future studies are required to assess the utility of venous thromboembolism prophylaxis among outpatients with high venous thromboembolism risk infections.”
The authors reported that they had no conflicts of interest.
Infection and infection sites have been found to be associated with a significant increased risk of venous thromboembolism, according to results of a population-based, matched, case-control analysis of medical records covering the 13-year period 1988-2000.
Dr. Kevin P. Cohoon and his colleagues at the Mayo Clinic, Rochester, Minn., developed models using conditional logistic regression analysis to stratify the risk associated with specific infections and infection sites.
Five hundred thirteen (39.4%) cases and 189 (12.7%) controls had an infection within the previous 92 days (odds ratio, 4.5; P less than .0001). Known VTE risk factors and potentially confounding variables were used in the adjusted univariate and multivariate models, as reported in the American Journal of Medicine (2017. doi: 10.1016/j.amjmed.2017.09.015).
Dr. Cohoon and his colleagues reported that univariate analysis showed “most infection sites were strongly associated with venous thromboembolism” and the adjusted multivariate model resulted in 2.4-fold (P less than .0001) higher odds for VTE incidence, compared with uninfected controls.
Adjusted multivariate analysis ranked the odds of VTE according to specific infections. Dr. Cohoon and his colleagues reported that this modeling showed that the “highest magnitude of risk, compared with no infection, was imparted by intra-abdominal infection (OR, 18) followed by oral infection (OR, 12), systematic blood stream infection (OR, 11), lower respiratory infection such as pneumonia (OR, 3.6), and symptomatic urinary tract infection (OR, 2.2).”
The researchers concluded that their findings may allow for further refinement of inpatient VTE risk-prediction models such as the Padua prediction score and “future studies are required to assess the utility of venous thromboembolism prophylaxis among outpatients with high venous thromboembolism risk infections.”
The authors reported that they had no conflicts of interest.
FROM THE AMERICAN JOURNAL OF MEDICINE
Key clinical point:
Major finding: A significantly greater number of patients with infections developed VTE as compared with uninfected controls (OR, 4.5; P less than .0001).
Data source: Study was a retrospective database analysis of 1,303 VTE patients and 1,494 paired controls.
Disclosures: The authors reported that they had no conflicts of interest.
Online Help for People With Alcohol Use Disorder
In any given year, < 10% of people diagnosed with alcohol use disorder receive treatment, and many do not receive the type of care that best fits their needs. Two reasons may be that they don’t know where to turn for help, or they may not know that they have more treatment options beyond a mutual help group or long-term residential rehabilitation facility.
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has developed a new online tool that may help. The “comprehensive, yet easy-to-use” Alcohol Treatment Navigator was developed “to help address the alcohol ‘treatment gap,’” said NIAAA Director George Koob, PhD. It’s based on decades of scientific research into clinical interventions and health services with input from patients, providers, and researchers.
The Navigator includes an overview of alcohol use disorder and a description of professionally led treatment options. It also gives step-by-step instructions for searching online directories of treatment providers, 10 questions to ask a provider, and signs of quality to listen for. A downloadable tool kit helps organize and simplify the search process.
In any given year, < 10% of people diagnosed with alcohol use disorder receive treatment, and many do not receive the type of care that best fits their needs. Two reasons may be that they don’t know where to turn for help, or they may not know that they have more treatment options beyond a mutual help group or long-term residential rehabilitation facility.
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has developed a new online tool that may help. The “comprehensive, yet easy-to-use” Alcohol Treatment Navigator was developed “to help address the alcohol ‘treatment gap,’” said NIAAA Director George Koob, PhD. It’s based on decades of scientific research into clinical interventions and health services with input from patients, providers, and researchers.
The Navigator includes an overview of alcohol use disorder and a description of professionally led treatment options. It also gives step-by-step instructions for searching online directories of treatment providers, 10 questions to ask a provider, and signs of quality to listen for. A downloadable tool kit helps organize and simplify the search process.
In any given year, < 10% of people diagnosed with alcohol use disorder receive treatment, and many do not receive the type of care that best fits their needs. Two reasons may be that they don’t know where to turn for help, or they may not know that they have more treatment options beyond a mutual help group or long-term residential rehabilitation facility.
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has developed a new online tool that may help. The “comprehensive, yet easy-to-use” Alcohol Treatment Navigator was developed “to help address the alcohol ‘treatment gap,’” said NIAAA Director George Koob, PhD. It’s based on decades of scientific research into clinical interventions and health services with input from patients, providers, and researchers.
The Navigator includes an overview of alcohol use disorder and a description of professionally led treatment options. It also gives step-by-step instructions for searching online directories of treatment providers, 10 questions to ask a provider, and signs of quality to listen for. A downloadable tool kit helps organize and simplify the search process.
Is MRD ready for prime time in multiple myeloma?
NEW YORK, NY—Speakers faced off over the issue of minimal residual disease (MRD) testing in multiple myeloma (MM) at Lymphoma & Myeloma 2017.
Ola Landgren, MD, PhD, of Weill Cornell Medicine in New York, New York, said, “it’s really a necessary and logical step forward to look at MRD.”
On the other hand, Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, took the clinicians’ perspective and suggested that, at this point, “we’re not yet ready to apply it to everyday practice.”
“[P]atients who have a complete response (CR) and are MRD negative have longer progression-free survival (PFS),” Dr Landgren pointed out, “and there are indications that their overall survival (OS) is better than in those patients who are just CR and MRD positive.”
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena,” Dr Richardson contended. “The question for me, as a clinician, in my clinic, is ‘Do I apply it to everyday practice?’ And I would simply suggest to you, at this point, we’re not ready for that.”
Yes—MRD is ready for prime time
Dr Landgren based his argument on 2 meta-analyses published in 2016 and 2017 that outline the importance of MRD status in newly diagnosed MM patients.
The first analysis (Landgren et al 2016) showed that MRD negativity was associated with better PFS (hazard ratio [HR]=0.35] and OS (HR=0.48) than MRD positivity.
“So using more simple language,” Dr Landgren said, “this means that MRD negativity reduces the risk of progression by 65%, and it also reduces the risk of dying by 52%.”
The second analysis (Munshi et al 2017) also associated MRD-negative status with superior survival outcomes for both PFS (HR=0.41) and OS (HR=0.57).
As further confirmation of the importance of MRD status, the International Myeloma Working Group last year published response definitions that include MRD negativity at a sensitivity of 1 in 105 cells or higher as the deepest level of treatment response in MM.
Dr Landgren drew on additional studies to support routine MRD testing in patient care.
The IFM Study Group found that, in newly diagnosed patients treated with lenalidomide, bortezomib, and dexamethasone followed by 1 year of lenalidomide maintenance, patients who received a subsequent transplant achieved superior outcomes compared to non-transplanted patients, in terms of CR (58% vs 46%) and 3-year PFS (61% vs 48%).
However, in patients who were MRD negative in both arms, the PFS rates were very similar, Dr Landgren said. And in terms of 3-year OS, there was no difference, at 88% in both arms.
The experience with daratumumab in relapsed/refractory patients exhibited a similar pattern.
The phase 3 POLLUX trial first showed that adding daratumumab to lenalidomide and dexamethasone was superior to lenalidomide and dexamethasone only, with a PFS at 18 months of 78% and 52%, respectively. This amounted to a 63% reduction in the risk of disease progression.
Investigators then took one more step forward, Dr Landgren said, and looked at MRD.
At a sensitivity of 10-5, almost 25% of patients on the 3-drug regimen were MRD negative, “which is kind of amazing,” Dr Landgren said. “This is a very big step forward.”
“If you break down the results by MRD status, which is not the primary endpoint of the study, you see very similar patterns for PFS for MRD negative patients in each of the 2 arms,” he continued.
This raises the question of whether attaining MRD negativity is more important than the treatment modality.
MRD negativity has implications for speeding drug approvals, developing more sensitive assays, and future treatment management, Dr Landgren said.
No—MRD is not ready for prime time
Dr Richardson acknowledged that MRD assessment is important. However, he pointed out a number of caveats regarding how MRD assessment would be applied in clinical practice to support his position.
“I’d simply suggest to you that, in day-to-day practice, the definition [of MRD] is somewhat fluid,” he said. “And it varies, obviously, between diseases and technology used.”
For most malignancies, Dr Richardson said, 109 to 1010 malignant cells are undetectable with conventional methods. These may or may not lead to a full clinical relapse within months or even years.
Using a sensitive technique to determine the presence of MRD could permit analysis of treatments that induce a greater depth of response or identify patients at risk of early relapse who need further treatment.
Dr Richardson enumerated hematologic malignancies that utilize MRD as secondary endpoints—acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, chronic lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma.
In chronic myeloid leukemia, MRD is used as a primary endpoint that dictates practice.
“And I would applaud the field in that area because, obviously, molecular response accepted as an endpoint by FDA for second-generation TKIs has been a bedrock of that approval process, and it now applies in clinical practice,” Dr Richardson said.
“Obviously, that’s where we’d like to be, but I’d suggest to you, just again, with a certain amount of moderation and a certain amount of caution, that we may not be quite there yet.”
Dr Richardson suggested that MRD assessment in MM is less advanced than in leukemia and lymphoma.
“[W]e are currently at the point where MRD assessments are clearly secondary endpoints, an important research tool,” he said.
Some “remarkable combination therapies,” he added, have abrogated some of the “extraordinary genetic complexity” in MM.
“The critical point here, though, is that, while we’re more successful in terms of these triplets and quadruplets and now with the introduction of monoclonal antibodies and similar approaches, we’re able to throw a bigger net around the disease,” Dr Richardson said.
“We’re not able to eradicate it completely, and cure remains, in myeloma, frankly, evasive. And I think that’s a critical point.”
Dr Richardson reviewed various strategies for molecular response monitoring, from flow cytometry to polymerase chain reaction and next-generation sequencing, noting that there is variance in applicability and sensitivity.
For example, the limits of detection among 91 labs ranged from 0.10% to 0.001%.
Dr Richardson returned to the “very robust” meta-analysis by Munshi and colleagues discussed by Dr Landgren.
While the authors’ analysis demonstrated that MRD is predictive of both longer PFS and OS, they concluded that the evidence supported MRD as an endpoint and research tool in clinical trials.
“So I would humbly suggest perhaps it’s not ready for clinical prime time yet,” Dr Richardson said.
He also referred to the IFM Study Group trial described by Dr Landgren, calling it a “critical forward effort.”
“[W]hat’s so interesting is that there was no difference in overall survival,” Dr Richardson said. “Now, that’s a very important point as we soberly look at these data and judge what they mean for each patient.”
And so Dr Richardson stood by his assessment that MRD is not yet a standard of care but may be one day. ![]()
NEW YORK, NY—Speakers faced off over the issue of minimal residual disease (MRD) testing in multiple myeloma (MM) at Lymphoma & Myeloma 2017.
Ola Landgren, MD, PhD, of Weill Cornell Medicine in New York, New York, said, “it’s really a necessary and logical step forward to look at MRD.”
On the other hand, Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, took the clinicians’ perspective and suggested that, at this point, “we’re not yet ready to apply it to everyday practice.”
“[P]atients who have a complete response (CR) and are MRD negative have longer progression-free survival (PFS),” Dr Landgren pointed out, “and there are indications that their overall survival (OS) is better than in those patients who are just CR and MRD positive.”
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena,” Dr Richardson contended. “The question for me, as a clinician, in my clinic, is ‘Do I apply it to everyday practice?’ And I would simply suggest to you, at this point, we’re not ready for that.”
Yes—MRD is ready for prime time
Dr Landgren based his argument on 2 meta-analyses published in 2016 and 2017 that outline the importance of MRD status in newly diagnosed MM patients.
The first analysis (Landgren et al 2016) showed that MRD negativity was associated with better PFS (hazard ratio [HR]=0.35] and OS (HR=0.48) than MRD positivity.
“So using more simple language,” Dr Landgren said, “this means that MRD negativity reduces the risk of progression by 65%, and it also reduces the risk of dying by 52%.”
The second analysis (Munshi et al 2017) also associated MRD-negative status with superior survival outcomes for both PFS (HR=0.41) and OS (HR=0.57).
As further confirmation of the importance of MRD status, the International Myeloma Working Group last year published response definitions that include MRD negativity at a sensitivity of 1 in 105 cells or higher as the deepest level of treatment response in MM.
Dr Landgren drew on additional studies to support routine MRD testing in patient care.
The IFM Study Group found that, in newly diagnosed patients treated with lenalidomide, bortezomib, and dexamethasone followed by 1 year of lenalidomide maintenance, patients who received a subsequent transplant achieved superior outcomes compared to non-transplanted patients, in terms of CR (58% vs 46%) and 3-year PFS (61% vs 48%).
However, in patients who were MRD negative in both arms, the PFS rates were very similar, Dr Landgren said. And in terms of 3-year OS, there was no difference, at 88% in both arms.
The experience with daratumumab in relapsed/refractory patients exhibited a similar pattern.
The phase 3 POLLUX trial first showed that adding daratumumab to lenalidomide and dexamethasone was superior to lenalidomide and dexamethasone only, with a PFS at 18 months of 78% and 52%, respectively. This amounted to a 63% reduction in the risk of disease progression.
Investigators then took one more step forward, Dr Landgren said, and looked at MRD.
At a sensitivity of 10-5, almost 25% of patients on the 3-drug regimen were MRD negative, “which is kind of amazing,” Dr Landgren said. “This is a very big step forward.”
“If you break down the results by MRD status, which is not the primary endpoint of the study, you see very similar patterns for PFS for MRD negative patients in each of the 2 arms,” he continued.
This raises the question of whether attaining MRD negativity is more important than the treatment modality.
MRD negativity has implications for speeding drug approvals, developing more sensitive assays, and future treatment management, Dr Landgren said.
No—MRD is not ready for prime time
Dr Richardson acknowledged that MRD assessment is important. However, he pointed out a number of caveats regarding how MRD assessment would be applied in clinical practice to support his position.
“I’d simply suggest to you that, in day-to-day practice, the definition [of MRD] is somewhat fluid,” he said. “And it varies, obviously, between diseases and technology used.”
For most malignancies, Dr Richardson said, 109 to 1010 malignant cells are undetectable with conventional methods. These may or may not lead to a full clinical relapse within months or even years.
Using a sensitive technique to determine the presence of MRD could permit analysis of treatments that induce a greater depth of response or identify patients at risk of early relapse who need further treatment.
Dr Richardson enumerated hematologic malignancies that utilize MRD as secondary endpoints—acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, chronic lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma.
In chronic myeloid leukemia, MRD is used as a primary endpoint that dictates practice.
“And I would applaud the field in that area because, obviously, molecular response accepted as an endpoint by FDA for second-generation TKIs has been a bedrock of that approval process, and it now applies in clinical practice,” Dr Richardson said.
“Obviously, that’s where we’d like to be, but I’d suggest to you, just again, with a certain amount of moderation and a certain amount of caution, that we may not be quite there yet.”
Dr Richardson suggested that MRD assessment in MM is less advanced than in leukemia and lymphoma.
“[W]e are currently at the point where MRD assessments are clearly secondary endpoints, an important research tool,” he said.
Some “remarkable combination therapies,” he added, have abrogated some of the “extraordinary genetic complexity” in MM.
“The critical point here, though, is that, while we’re more successful in terms of these triplets and quadruplets and now with the introduction of monoclonal antibodies and similar approaches, we’re able to throw a bigger net around the disease,” Dr Richardson said.
“We’re not able to eradicate it completely, and cure remains, in myeloma, frankly, evasive. And I think that’s a critical point.”
Dr Richardson reviewed various strategies for molecular response monitoring, from flow cytometry to polymerase chain reaction and next-generation sequencing, noting that there is variance in applicability and sensitivity.
For example, the limits of detection among 91 labs ranged from 0.10% to 0.001%.
Dr Richardson returned to the “very robust” meta-analysis by Munshi and colleagues discussed by Dr Landgren.
While the authors’ analysis demonstrated that MRD is predictive of both longer PFS and OS, they concluded that the evidence supported MRD as an endpoint and research tool in clinical trials.
“So I would humbly suggest perhaps it’s not ready for clinical prime time yet,” Dr Richardson said.
He also referred to the IFM Study Group trial described by Dr Landgren, calling it a “critical forward effort.”
“[W]hat’s so interesting is that there was no difference in overall survival,” Dr Richardson said. “Now, that’s a very important point as we soberly look at these data and judge what they mean for each patient.”
And so Dr Richardson stood by his assessment that MRD is not yet a standard of care but may be one day. ![]()
NEW YORK, NY—Speakers faced off over the issue of minimal residual disease (MRD) testing in multiple myeloma (MM) at Lymphoma & Myeloma 2017.
Ola Landgren, MD, PhD, of Weill Cornell Medicine in New York, New York, said, “it’s really a necessary and logical step forward to look at MRD.”
On the other hand, Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, took the clinicians’ perspective and suggested that, at this point, “we’re not yet ready to apply it to everyday practice.”
“[P]atients who have a complete response (CR) and are MRD negative have longer progression-free survival (PFS),” Dr Landgren pointed out, “and there are indications that their overall survival (OS) is better than in those patients who are just CR and MRD positive.”
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena,” Dr Richardson contended. “The question for me, as a clinician, in my clinic, is ‘Do I apply it to everyday practice?’ And I would simply suggest to you, at this point, we’re not ready for that.”
Yes—MRD is ready for prime time
Dr Landgren based his argument on 2 meta-analyses published in 2016 and 2017 that outline the importance of MRD status in newly diagnosed MM patients.
The first analysis (Landgren et al 2016) showed that MRD negativity was associated with better PFS (hazard ratio [HR]=0.35] and OS (HR=0.48) than MRD positivity.
“So using more simple language,” Dr Landgren said, “this means that MRD negativity reduces the risk of progression by 65%, and it also reduces the risk of dying by 52%.”
The second analysis (Munshi et al 2017) also associated MRD-negative status with superior survival outcomes for both PFS (HR=0.41) and OS (HR=0.57).
As further confirmation of the importance of MRD status, the International Myeloma Working Group last year published response definitions that include MRD negativity at a sensitivity of 1 in 105 cells or higher as the deepest level of treatment response in MM.
Dr Landgren drew on additional studies to support routine MRD testing in patient care.
The IFM Study Group found that, in newly diagnosed patients treated with lenalidomide, bortezomib, and dexamethasone followed by 1 year of lenalidomide maintenance, patients who received a subsequent transplant achieved superior outcomes compared to non-transplanted patients, in terms of CR (58% vs 46%) and 3-year PFS (61% vs 48%).
However, in patients who were MRD negative in both arms, the PFS rates were very similar, Dr Landgren said. And in terms of 3-year OS, there was no difference, at 88% in both arms.
The experience with daratumumab in relapsed/refractory patients exhibited a similar pattern.
The phase 3 POLLUX trial first showed that adding daratumumab to lenalidomide and dexamethasone was superior to lenalidomide and dexamethasone only, with a PFS at 18 months of 78% and 52%, respectively. This amounted to a 63% reduction in the risk of disease progression.
Investigators then took one more step forward, Dr Landgren said, and looked at MRD.
At a sensitivity of 10-5, almost 25% of patients on the 3-drug regimen were MRD negative, “which is kind of amazing,” Dr Landgren said. “This is a very big step forward.”
“If you break down the results by MRD status, which is not the primary endpoint of the study, you see very similar patterns for PFS for MRD negative patients in each of the 2 arms,” he continued.
This raises the question of whether attaining MRD negativity is more important than the treatment modality.
MRD negativity has implications for speeding drug approvals, developing more sensitive assays, and future treatment management, Dr Landgren said.
No—MRD is not ready for prime time
Dr Richardson acknowledged that MRD assessment is important. However, he pointed out a number of caveats regarding how MRD assessment would be applied in clinical practice to support his position.
“I’d simply suggest to you that, in day-to-day practice, the definition [of MRD] is somewhat fluid,” he said. “And it varies, obviously, between diseases and technology used.”
For most malignancies, Dr Richardson said, 109 to 1010 malignant cells are undetectable with conventional methods. These may or may not lead to a full clinical relapse within months or even years.
Using a sensitive technique to determine the presence of MRD could permit analysis of treatments that induce a greater depth of response or identify patients at risk of early relapse who need further treatment.
Dr Richardson enumerated hematologic malignancies that utilize MRD as secondary endpoints—acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, chronic lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma.
In chronic myeloid leukemia, MRD is used as a primary endpoint that dictates practice.
“And I would applaud the field in that area because, obviously, molecular response accepted as an endpoint by FDA for second-generation TKIs has been a bedrock of that approval process, and it now applies in clinical practice,” Dr Richardson said.
“Obviously, that’s where we’d like to be, but I’d suggest to you, just again, with a certain amount of moderation and a certain amount of caution, that we may not be quite there yet.”
Dr Richardson suggested that MRD assessment in MM is less advanced than in leukemia and lymphoma.
“[W]e are currently at the point where MRD assessments are clearly secondary endpoints, an important research tool,” he said.
Some “remarkable combination therapies,” he added, have abrogated some of the “extraordinary genetic complexity” in MM.
“The critical point here, though, is that, while we’re more successful in terms of these triplets and quadruplets and now with the introduction of monoclonal antibodies and similar approaches, we’re able to throw a bigger net around the disease,” Dr Richardson said.
“We’re not able to eradicate it completely, and cure remains, in myeloma, frankly, evasive. And I think that’s a critical point.”
Dr Richardson reviewed various strategies for molecular response monitoring, from flow cytometry to polymerase chain reaction and next-generation sequencing, noting that there is variance in applicability and sensitivity.
For example, the limits of detection among 91 labs ranged from 0.10% to 0.001%.
Dr Richardson returned to the “very robust” meta-analysis by Munshi and colleagues discussed by Dr Landgren.
While the authors’ analysis demonstrated that MRD is predictive of both longer PFS and OS, they concluded that the evidence supported MRD as an endpoint and research tool in clinical trials.
“So I would humbly suggest perhaps it’s not ready for clinical prime time yet,” Dr Richardson said.
He also referred to the IFM Study Group trial described by Dr Landgren, calling it a “critical forward effort.”
“[W]hat’s so interesting is that there was no difference in overall survival,” Dr Richardson said. “Now, that’s a very important point as we soberly look at these data and judge what they mean for each patient.”
And so Dr Richardson stood by his assessment that MRD is not yet a standard of care but may be one day. ![]()
Ferric citrate approved to treat iron-deficiency anemia
The US Food and Drug Administration (FDA) has approved ferric citrate (Auryxia) to treat iron-deficiency anemia in adults with chronic kidney disease (CKD) who are not on dialysis.
Ferric citrate was originally approved by the FDA in September 2014 for the control of serum phosphorus levels in patients with CKD who require dialysis.
The full prescribing information for the drug is available at www.Auryxia.com.
“We are pleased with the broad indication permitted by the FDA, as a first-line treatment option for adults with iron-deficiency anemia and chronic kidney disease not on dialysis,” said John Neylan, MD, senior vice president and chief medical officer of Keryx Biopharmaceuticals, Inc., the company marketing ferric citrate.
“Physicians and their patients now have a new treatment option to help manage a serious complication of this complex disease.”
The new approval of ferric citrate was based on results from a 24-week, placebo-controlled, phase 3 trial. Results from this trial were published in the Journal of the American Society of Nephrology in January.
The trial enrolled 234 adults with stage 3-5, non-dialysis-dependent CKD and iron-deficiency anemia. Patients had hemoglobin levels between 9.0 g/dL and 11.5 g/dL and were intolerant to or had an inadequate response to prior treatment with oral iron supplements.
The starting dose of ferric citrate was 3 tablets per day, taken with meals. The mean dose was 5 tablets per day. Patients were not allowed to receive any intravenous or oral iron or erythropoiesis-stimulating agents.
Significantly more patients in the ferric citrate arm than the placebo arm had increases in hemoglobin levels of at least 1 g/dL at any point during the trial’s 16-week efficacy period—52.1% (61/117) and 19.1% (22/115), respectively (P<0.001).
Likewise, significantly more patients in the ferric citrate arm than the placebo arm had a sustained increase in hemoglobin of at least 0.75 g/dL over any 4-week period during the trial—48.7% (n=57) and 14.8% (n=17), respectively (P<0.001).
Serious adverse events occurred in 12.0% of patients in the ferric citrate arm and 11.2% of patients in the placebo arm. There were 2 treatment-emergent deaths in the ferric citrate arm (and none in the placebo arm), but they were not considered drug-related.
The most common (≥5%) treatment-emergent adverse events in patients who received ferric citrate were diarrhea (20.5%), constipation (18.8%), discolored feces (14.5%), nausea (11.1%), abdominal pain (6.0%), and hyperkalemia (6.8%). ![]()
The US Food and Drug Administration (FDA) has approved ferric citrate (Auryxia) to treat iron-deficiency anemia in adults with chronic kidney disease (CKD) who are not on dialysis.
Ferric citrate was originally approved by the FDA in September 2014 for the control of serum phosphorus levels in patients with CKD who require dialysis.
The full prescribing information for the drug is available at www.Auryxia.com.
“We are pleased with the broad indication permitted by the FDA, as a first-line treatment option for adults with iron-deficiency anemia and chronic kidney disease not on dialysis,” said John Neylan, MD, senior vice president and chief medical officer of Keryx Biopharmaceuticals, Inc., the company marketing ferric citrate.
“Physicians and their patients now have a new treatment option to help manage a serious complication of this complex disease.”
The new approval of ferric citrate was based on results from a 24-week, placebo-controlled, phase 3 trial. Results from this trial were published in the Journal of the American Society of Nephrology in January.
The trial enrolled 234 adults with stage 3-5, non-dialysis-dependent CKD and iron-deficiency anemia. Patients had hemoglobin levels between 9.0 g/dL and 11.5 g/dL and were intolerant to or had an inadequate response to prior treatment with oral iron supplements.
The starting dose of ferric citrate was 3 tablets per day, taken with meals. The mean dose was 5 tablets per day. Patients were not allowed to receive any intravenous or oral iron or erythropoiesis-stimulating agents.
Significantly more patients in the ferric citrate arm than the placebo arm had increases in hemoglobin levels of at least 1 g/dL at any point during the trial’s 16-week efficacy period—52.1% (61/117) and 19.1% (22/115), respectively (P<0.001).
Likewise, significantly more patients in the ferric citrate arm than the placebo arm had a sustained increase in hemoglobin of at least 0.75 g/dL over any 4-week period during the trial—48.7% (n=57) and 14.8% (n=17), respectively (P<0.001).
Serious adverse events occurred in 12.0% of patients in the ferric citrate arm and 11.2% of patients in the placebo arm. There were 2 treatment-emergent deaths in the ferric citrate arm (and none in the placebo arm), but they were not considered drug-related.
The most common (≥5%) treatment-emergent adverse events in patients who received ferric citrate were diarrhea (20.5%), constipation (18.8%), discolored feces (14.5%), nausea (11.1%), abdominal pain (6.0%), and hyperkalemia (6.8%). ![]()
The US Food and Drug Administration (FDA) has approved ferric citrate (Auryxia) to treat iron-deficiency anemia in adults with chronic kidney disease (CKD) who are not on dialysis.
Ferric citrate was originally approved by the FDA in September 2014 for the control of serum phosphorus levels in patients with CKD who require dialysis.
The full prescribing information for the drug is available at www.Auryxia.com.
“We are pleased with the broad indication permitted by the FDA, as a first-line treatment option for adults with iron-deficiency anemia and chronic kidney disease not on dialysis,” said John Neylan, MD, senior vice president and chief medical officer of Keryx Biopharmaceuticals, Inc., the company marketing ferric citrate.
“Physicians and their patients now have a new treatment option to help manage a serious complication of this complex disease.”
The new approval of ferric citrate was based on results from a 24-week, placebo-controlled, phase 3 trial. Results from this trial were published in the Journal of the American Society of Nephrology in January.
The trial enrolled 234 adults with stage 3-5, non-dialysis-dependent CKD and iron-deficiency anemia. Patients had hemoglobin levels between 9.0 g/dL and 11.5 g/dL and were intolerant to or had an inadequate response to prior treatment with oral iron supplements.
The starting dose of ferric citrate was 3 tablets per day, taken with meals. The mean dose was 5 tablets per day. Patients were not allowed to receive any intravenous or oral iron or erythropoiesis-stimulating agents.
Significantly more patients in the ferric citrate arm than the placebo arm had increases in hemoglobin levels of at least 1 g/dL at any point during the trial’s 16-week efficacy period—52.1% (61/117) and 19.1% (22/115), respectively (P<0.001).
Likewise, significantly more patients in the ferric citrate arm than the placebo arm had a sustained increase in hemoglobin of at least 0.75 g/dL over any 4-week period during the trial—48.7% (n=57) and 14.8% (n=17), respectively (P<0.001).
Serious adverse events occurred in 12.0% of patients in the ferric citrate arm and 11.2% of patients in the placebo arm. There were 2 treatment-emergent deaths in the ferric citrate arm (and none in the placebo arm), but they were not considered drug-related.
The most common (≥5%) treatment-emergent adverse events in patients who received ferric citrate were diarrhea (20.5%), constipation (18.8%), discolored feces (14.5%), nausea (11.1%), abdominal pain (6.0%), and hyperkalemia (6.8%). ![]()
FDA lifts hold on trials of universal CAR T-cell therapy
The US Food and Drug Administration (FDA) has lifted the full clinical hold on 2 phase 1 studies of UCART123, an allogeneic chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One of these studies was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The hold meant no new subjects could be enrolled in either trial, and there could be no further dosing of subjects who were already enrolled.
The hold was placed in September because the first patient treated in the BPDCN trial died. The patient developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection. This was followed by grade 4 capillary leak syndrome and grade 5 CRS.
The first patient treated in the AML trial also developed grade 4 capillary leak syndrome and grade 3 CRS, but both resolved.
Now, the FDA has lifted the hold on the trials because Cellectis, the company developing UCART123, agreed to implement the following main revisions to phase 1 UCART123 protocols:
- Decrease the cohort dose level to 6.25 x 104 UCART123 cells/kg
- Decrease the cyclophosphamide dose of the lymphodepleting regimen to 750 mg/m²/day over 3 days, with a maximum daily dose of 1.33 grams
- Include specific criteria at Day 0, the day of UCART123 infusion, such as no new uncontrolled infection after receipt of lymphodepletion, afebrile, off all but replacement dose of corticosteroids, and no organ dysfunction since eligibility screening
- Ensure the next 3 patients to be treated in each protocol will be under the age of 65
- Ensure that enrollment will be staggered across the UCART123 protocols; at least 28 days should elapse between the enrollments of 2 patients across the 2 studies.
Cellectis is currently working with investigators and clinical sites to obtain internal review board approval on the revised protocols and resume patient enrollment. ![]()
The US Food and Drug Administration (FDA) has lifted the full clinical hold on 2 phase 1 studies of UCART123, an allogeneic chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One of these studies was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The hold meant no new subjects could be enrolled in either trial, and there could be no further dosing of subjects who were already enrolled.
The hold was placed in September because the first patient treated in the BPDCN trial died. The patient developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection. This was followed by grade 4 capillary leak syndrome and grade 5 CRS.
The first patient treated in the AML trial also developed grade 4 capillary leak syndrome and grade 3 CRS, but both resolved.
Now, the FDA has lifted the hold on the trials because Cellectis, the company developing UCART123, agreed to implement the following main revisions to phase 1 UCART123 protocols:
- Decrease the cohort dose level to 6.25 x 104 UCART123 cells/kg
- Decrease the cyclophosphamide dose of the lymphodepleting regimen to 750 mg/m²/day over 3 days, with a maximum daily dose of 1.33 grams
- Include specific criteria at Day 0, the day of UCART123 infusion, such as no new uncontrolled infection after receipt of lymphodepletion, afebrile, off all but replacement dose of corticosteroids, and no organ dysfunction since eligibility screening
- Ensure the next 3 patients to be treated in each protocol will be under the age of 65
- Ensure that enrollment will be staggered across the UCART123 protocols; at least 28 days should elapse between the enrollments of 2 patients across the 2 studies.
Cellectis is currently working with investigators and clinical sites to obtain internal review board approval on the revised protocols and resume patient enrollment. ![]()
The US Food and Drug Administration (FDA) has lifted the full clinical hold on 2 phase 1 studies of UCART123, an allogeneic chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One of these studies was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The hold meant no new subjects could be enrolled in either trial, and there could be no further dosing of subjects who were already enrolled.
The hold was placed in September because the first patient treated in the BPDCN trial died. The patient developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection. This was followed by grade 4 capillary leak syndrome and grade 5 CRS.
The first patient treated in the AML trial also developed grade 4 capillary leak syndrome and grade 3 CRS, but both resolved.
Now, the FDA has lifted the hold on the trials because Cellectis, the company developing UCART123, agreed to implement the following main revisions to phase 1 UCART123 protocols:
- Decrease the cohort dose level to 6.25 x 104 UCART123 cells/kg
- Decrease the cyclophosphamide dose of the lymphodepleting regimen to 750 mg/m²/day over 3 days, with a maximum daily dose of 1.33 grams
- Include specific criteria at Day 0, the day of UCART123 infusion, such as no new uncontrolled infection after receipt of lymphodepletion, afebrile, off all but replacement dose of corticosteroids, and no organ dysfunction since eligibility screening
- Ensure the next 3 patients to be treated in each protocol will be under the age of 65
- Ensure that enrollment will be staggered across the UCART123 protocols; at least 28 days should elapse between the enrollments of 2 patients across the 2 studies.
Cellectis is currently working with investigators and clinical sites to obtain internal review board approval on the revised protocols and resume patient enrollment. ![]()
From 5K to … the End of the Driveway
ANSWER
The correct interpretation includes sinus tachycardia with complete heart block and an idioventricular rhythm. Careful review of this ECG confirms complete atrioventricular dissociation, which is indicative of complete heart block.
Sinus tachycardia is indicated by a consistent P-P interval at a rate of 110 beats/min, idioventricular rhythm with a regular (but not normal) rate, and prolonged QRS interval of 148 ms. In this patient’s case, the tachycardia was presumed to be due to his upper respiratory infection. He underwent permanent pacemaker placement and resumed his normal activities without restriction.
ANSWER
The correct interpretation includes sinus tachycardia with complete heart block and an idioventricular rhythm. Careful review of this ECG confirms complete atrioventricular dissociation, which is indicative of complete heart block.
Sinus tachycardia is indicated by a consistent P-P interval at a rate of 110 beats/min, idioventricular rhythm with a regular (but not normal) rate, and prolonged QRS interval of 148 ms. In this patient’s case, the tachycardia was presumed to be due to his upper respiratory infection. He underwent permanent pacemaker placement and resumed his normal activities without restriction.
ANSWER
The correct interpretation includes sinus tachycardia with complete heart block and an idioventricular rhythm. Careful review of this ECG confirms complete atrioventricular dissociation, which is indicative of complete heart block.
Sinus tachycardia is indicated by a consistent P-P interval at a rate of 110 beats/min, idioventricular rhythm with a regular (but not normal) rate, and prolonged QRS interval of 148 ms. In this patient’s case, the tachycardia was presumed to be due to his upper respiratory infection. He underwent permanent pacemaker placement and resumed his normal activities without restriction.
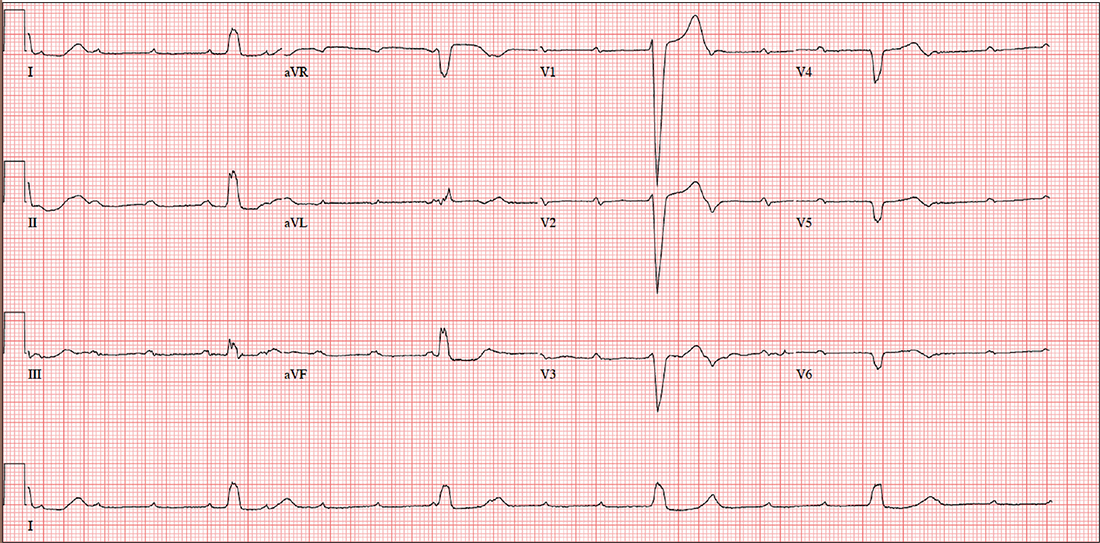
Until three weeks ago, this 74-year-old man walked three miles every day without difficulty. But now, shortness of breath forces him to stop walking before he even reaches the end of his driveway. He denies chest pain (at rest or on exertion), palpitations, dyspnea at rest, and paroxysmal nocturnal dyspnea. There have been no recent weight changes.
Four years ago, he was diagnosed with coronary artery disease after experiencing chest pain at rest. A coronary angiography revealed stenosis in the proximal right coronary artery and the second obtuse marginal branch of his circumflex coronary artery; drug-eluting stents were placed, and he has had no further symptoms. An echocardiogram performed at a routine clinic visit six months ago showed mild aortic valve sclerosis, a left ventricular ejection fraction of 64%, and no regional wall motion abnormalities.
Surgical history is also remarkable for an open reduction and stabilization of a right high ankle fracture 10 years ago. Medical history includes type 2 diabetes and hyperlipidemia; treatment has normalized his A1C and his lipid panel.
His current medication list includes metoprolol, isosorbide dinitrate, metformin, and atorvastatin. He has no known drug allergies. He is unaware of any medical issues with his parents or grandparents.
The patient has two adult sons who live abroad and visit once a year. He was married for 53 years but lost his wife to lung cancer three years ago; her diagnosis prompted him to quit his long-term smoking habit. He consumes alcohol socially, having “one or two beers with friends on the weekends,” but denies current or previous use of marijuana or nonprescribed medications.
Review of systems is remarkable for a three-day history of upper respiratory infection with cough and rhinitis. He denies any change in bowel or bladder function.
Vital signs include a blood pressure of 104/54 mm Hg; pulse, 30 beats/min; respiratory rate, 16 breaths/min-1; and temperature, 99.4°F. His weight is 169 lb and his height, 70 in.
On physical exam, you note a thin, healthy-looking male in no acute distress. Pertinent findings include internally inflamed nares and oropharynx, a few scattered rales in both lower lung fields that clear with coughing, and no wheezing. There are no palpable lymph nodes in the head or neck.
Cardiac exam reveals a regular rhythm of 30 beats/min with no murmurs, rubs, or extra heart sounds. The abdomen is soft and nontender. Peripheral pulses are strong and palpable in both upper and lower extremities, and there is no peripheral edema. The neurologic exam is intact, without evidence of diabetic neuropathy.
Given the slow heart rate observed on physical exam, an ECG is ordered. It shows a ventricular rate of 29 beats/min; no discernable PR interval; QRS duration, 148 ms; QT/QTc interval, 584/405 ms; P axis, 64°; R axis, 55°; and T axis, 83°. What is your interpretation?
Apple pie and ...
How do you feel about apple pie? Is it a concept that evokes a positive feeling for you? Even if you prefer pumpkin or blueberry? Although your attitude toward apple pie may be relevant as we approach the holidays, is it a topic worthy of discussion in a publication devoted to pediatrics?
Certainly not, but what about motherhood? How do you feel about motherhood? As someone who is devoting his or her professional energies to the health of children, you must have formed some opinions about motherhood. Although your patients are children, it is their parents – and more often their mothers – with whom you communicate, particularly in the first several years of life.
You may never have been asked that question in exactly that way before, but I suspect you have thought about it both professionally and personally. You may have considered the answer as you were deciding if, when, and how you were going to return to work after maternity leave. Or you may have been forced to consider the question in formulating an opinion in a case of contested child custody.
An opinion piece in the Wall Street Journal (“The Politicization of Motherhood,” by James Taranto, Oct. 27, 2017) suggests that how you answer my question about the biological necessity of motherhood will determine your position on one of our nation’s political divides. The article focuses on Erica Komisar, who has written a book in which she lays out evidence from the fields of neuroscience, psychology, and epigenetics supporting her view that a mother is biologically equipped to provide for the emotional development of her child (“Being There: Why Prioritizing Motherhood in the First Three Years Matters,” New York: TarcherPerigee, 2017).
I haven’t read Ms. Komisar’s book, nor am I aware of the studies she cites, but reading the article prompted me to think a bit more deeply regarding how I feel about motherhood. I guess I always have felt that there is something special that a mother can provide her children, particularly during the first 3 years of life. I don’t know whether there is a neurobiological basis for this special something, but if it is missing, the child’s emotional development can suffer. Are there situations where another person(s) can provide a substitute for this special maternal sauce? Of course, but it doesn’t always work as well as the real thing. And not every mother has an adequate amount of that certain maternal something.
As pediatricians, we are faced with two challenges. The first is to help families cope with situations in which that special maternal ingredient is absent or in short supply. Our second challenge is to help mothers who believe there is something special they can offer their children but feel guilty because, for whatever reason, they can’t be there to provide it.
I am interested to hear how you feel about motherhood ... and apple pie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Email him at [email protected].
How do you feel about apple pie? Is it a concept that evokes a positive feeling for you? Even if you prefer pumpkin or blueberry? Although your attitude toward apple pie may be relevant as we approach the holidays, is it a topic worthy of discussion in a publication devoted to pediatrics?
Certainly not, but what about motherhood? How do you feel about motherhood? As someone who is devoting his or her professional energies to the health of children, you must have formed some opinions about motherhood. Although your patients are children, it is their parents – and more often their mothers – with whom you communicate, particularly in the first several years of life.
You may never have been asked that question in exactly that way before, but I suspect you have thought about it both professionally and personally. You may have considered the answer as you were deciding if, when, and how you were going to return to work after maternity leave. Or you may have been forced to consider the question in formulating an opinion in a case of contested child custody.
An opinion piece in the Wall Street Journal (“The Politicization of Motherhood,” by James Taranto, Oct. 27, 2017) suggests that how you answer my question about the biological necessity of motherhood will determine your position on one of our nation’s political divides. The article focuses on Erica Komisar, who has written a book in which she lays out evidence from the fields of neuroscience, psychology, and epigenetics supporting her view that a mother is biologically equipped to provide for the emotional development of her child (“Being There: Why Prioritizing Motherhood in the First Three Years Matters,” New York: TarcherPerigee, 2017).
I haven’t read Ms. Komisar’s book, nor am I aware of the studies she cites, but reading the article prompted me to think a bit more deeply regarding how I feel about motherhood. I guess I always have felt that there is something special that a mother can provide her children, particularly during the first 3 years of life. I don’t know whether there is a neurobiological basis for this special something, but if it is missing, the child’s emotional development can suffer. Are there situations where another person(s) can provide a substitute for this special maternal sauce? Of course, but it doesn’t always work as well as the real thing. And not every mother has an adequate amount of that certain maternal something.
As pediatricians, we are faced with two challenges. The first is to help families cope with situations in which that special maternal ingredient is absent or in short supply. Our second challenge is to help mothers who believe there is something special they can offer their children but feel guilty because, for whatever reason, they can’t be there to provide it.
I am interested to hear how you feel about motherhood ... and apple pie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Email him at [email protected].
How do you feel about apple pie? Is it a concept that evokes a positive feeling for you? Even if you prefer pumpkin or blueberry? Although your attitude toward apple pie may be relevant as we approach the holidays, is it a topic worthy of discussion in a publication devoted to pediatrics?
Certainly not, but what about motherhood? How do you feel about motherhood? As someone who is devoting his or her professional energies to the health of children, you must have formed some opinions about motherhood. Although your patients are children, it is their parents – and more often their mothers – with whom you communicate, particularly in the first several years of life.
You may never have been asked that question in exactly that way before, but I suspect you have thought about it both professionally and personally. You may have considered the answer as you were deciding if, when, and how you were going to return to work after maternity leave. Or you may have been forced to consider the question in formulating an opinion in a case of contested child custody.
An opinion piece in the Wall Street Journal (“The Politicization of Motherhood,” by James Taranto, Oct. 27, 2017) suggests that how you answer my question about the biological necessity of motherhood will determine your position on one of our nation’s political divides. The article focuses on Erica Komisar, who has written a book in which she lays out evidence from the fields of neuroscience, psychology, and epigenetics supporting her view that a mother is biologically equipped to provide for the emotional development of her child (“Being There: Why Prioritizing Motherhood in the First Three Years Matters,” New York: TarcherPerigee, 2017).
I haven’t read Ms. Komisar’s book, nor am I aware of the studies she cites, but reading the article prompted me to think a bit more deeply regarding how I feel about motherhood. I guess I always have felt that there is something special that a mother can provide her children, particularly during the first 3 years of life. I don’t know whether there is a neurobiological basis for this special something, but if it is missing, the child’s emotional development can suffer. Are there situations where another person(s) can provide a substitute for this special maternal sauce? Of course, but it doesn’t always work as well as the real thing. And not every mother has an adequate amount of that certain maternal something.
As pediatricians, we are faced with two challenges. The first is to help families cope with situations in which that special maternal ingredient is absent or in short supply. Our second challenge is to help mothers who believe there is something special they can offer their children but feel guilty because, for whatever reason, they can’t be there to provide it.
I am interested to hear how you feel about motherhood ... and apple pie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Email him at [email protected].
Use of opioids, SSRIs linked to increased fracture risk in RA
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
AT ACR 2017
Key clinical point: When managing with opioids, even in the short-term, clinicians should be aware of the fracture risk.
Major finding: In patients with RA, concomitant use of selective serotonin reuptake inhibitors was associated with an increased risk of osteoporotic fracture (HR, 1.35; P = .003), as was opioid use (HR, 1.45 and HR, 1.79) for weak and strong agents, respectively; P less than .001 for both).
Study details: An observational study of 11,049 patients from the National Data Bank for Rheumatic Diseases.
Disclosures: Dr. Ozen reported having no disclosures.