User login
Future Hospitalist: Top 10 tips for carrying out a successful quality improvement project
Editor’s Note: This column is a quarterly feature written by members of the Physicians in Training Committee. It aims to encourage and educate students, residents, and early career hospitalists.
One of the biggest challenges early career hospitalists, residents and medical students face in launching their first quality improvement (QI) project is knowing how and where to get started. QI can be highly rewarding, but it can also take valuable time and resources without guarantees of sustainable improvement. In this article, we outline 10 key factors to consider when starting a new project.
1. Frame your project so that it aligns with your hospital’s current goals
Choose a project with your hospital’s goals in mind. Securing resources such as health IT, financial, or staffing support will prove difficult unless you get buy-in from hospital leadership. If your project does not directly address hospital goals, frame the purpose to demonstrate that it still fits with leadership priorities. For example, though improving handoffs from daytime to nighttime providers may not be a specific goal, leadership should appreciate that this project is expected to improve patient safety.
2. Be SMART about goals
Many QI projects fail because the scope of the initial project is too large, unrealistic, or vague. Creating a clear and focused aim statement and keeping it “SMART” (Specific, Measurable, Achievable, Realistic, and Timely) will bring structure to the project.1 “We will reduce Congestive Heart Failure readmissions on 5 medicine units at our hospital by 2.5% in 6 months” is an example of a SMART aim statement.
3. Involve the right people from the start
QI project disasters often start with the wrong team. Select members based on who is needed and not who is available. It is critical to include representatives or “champions” from each area that will be affected. People will buy into a new methodology much more quickly if they were engaged in its development or know that respected members in their area were involved.
4. Use a simple, systematic approach to guide improvement work
Various QI models exist and each offers a systematic approach for assessing and improving care services. The Model for Improvement developed by the Associates in Process Improvement2 is a simple and powerful framework for quality improvement that asks three questions: (1) What are we trying to accomplish? (2) How will we know a change is an improvement? (3) What changes can we make that will result in improvement? The model incorporates Plan-Do-Study-Act (PDSA) cycles to test changes on a small scale.
5. Good projects start with good background data
6. Choose interventions that are high impact, low effort
People will more easily change if the change itself is easy. So consider the question “does this intervention add significant work?” The best interventions change a process without causing undue burden to the clinicians and staff involved.
7. If you can’t measure it, you can’t improve it
After implementation, collect enough data to know whether the changes made improved the process. Study outcome, process, and balancing measures. If possible, use data already being collected by your institution. While it is critical to have quantitative measures, qualitative data such as surveys and observations can also enrich understanding.
Example: Increasing early discharges in medical unit.
Outcome measure – This is the desired outcome that the project aims to improve. This may be the percentage of discharges before noon (DBN) or the average discharge time.
Process measure – This is a measure of a specific change made to improve the outcome metric. The discharge orders may need to be placed earlier in the electronic medical record to improve DBN. This average discharge order time is an example of a process measure.
Balance measure – This metric evaluates whether the intended outcome is leading to unintended consequences. For example, tracking the readmission rate is an important balance measure to assess whether improved DBN is associated with rushed discharges and possible unsafe transitions.
8. Communicate project goals and progress
9. Manage resistance to change
“People responsible for planning and implementing change often forget that while the first task of change management is to understand the destination and how to get there, the first task of transition management is to convince people to leave home.” – William Bridges
Inertia is powerful. We may consider our continuous performance improvement initiative as “the next big thing” but others may not share this enthusiasm. We therefore need to build a compelling reason for others to become engaged and accept major changes to work flow. Different strategies may be needed depending on your audience. Though for some, data and a rational analysis will be persuasive, for others the emotional argument will be the most motivating. Share personal anecdotes and use patient stories. In addition, let providers know “what’s in it for them.” Some may have a personal interest in your project or may need QI experience for career advancement; others might be motivated by the possibilities for scholarship arising from this work.
10. Make the work count twice
Consider QI as a scholarly initiative from the start to bring rigor to the project at all phases. Describe the project in an abstract or manuscript once improvements have been made. Publication is a great way to boost team morale and help make a business case for future improvement work. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines provide an excellent framework for designing and writing up an improvement project.4 The guidelines focus on why the project was started, what was done, what was found, and what the findings mean.
Driving change is challenging, and it is tempting to jump ahead to “fixing the problem.” But implementing a successful QI project requires intelligent direction, strategic planning, and skillful execution. It is our hope that following the above tips will help you develop the best possible ideas and approach implementation in a systematic way, ultimately leading to meaningful change.
Dr. Reyna is assistant professor in the division of hospital medicine and unit medical director at Mount Sinai Medical Center in New York City. She is a Certified Clinical Microsystems Coach. Dr. Burger is associate professor and associate program director, internal medicine residency, at Mount Sinai Beth Israel. He is on the faculty for the SGIM Annual Meeting Precourse on QI and is head of the high value care committee at the department of medicine at Mount Sinai Beth Israel. Dr. Cho is assistant professor and director of quality and safety in the division of hospital medicine at Mount Sinai. He is a senior fellow at the Lown Institute.
References
1. MacLeod L. Making SMART goals smarter. Physician Exec. 2012 Mar-Apr;38(2):68-70, 72.
2. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition). San Francisco: Jossey-Bass Publishers; 2009.
3. Nelson EC, Batalden PB, Godfrey MM. Quality By Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
4. Ogrinc G, Davies L, Goodman D et.al. SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2015 Sep 14.
Editor’s Note: This column is a quarterly feature written by members of the Physicians in Training Committee. It aims to encourage and educate students, residents, and early career hospitalists.
One of the biggest challenges early career hospitalists, residents and medical students face in launching their first quality improvement (QI) project is knowing how and where to get started. QI can be highly rewarding, but it can also take valuable time and resources without guarantees of sustainable improvement. In this article, we outline 10 key factors to consider when starting a new project.
1. Frame your project so that it aligns with your hospital’s current goals
Choose a project with your hospital’s goals in mind. Securing resources such as health IT, financial, or staffing support will prove difficult unless you get buy-in from hospital leadership. If your project does not directly address hospital goals, frame the purpose to demonstrate that it still fits with leadership priorities. For example, though improving handoffs from daytime to nighttime providers may not be a specific goal, leadership should appreciate that this project is expected to improve patient safety.
2. Be SMART about goals
Many QI projects fail because the scope of the initial project is too large, unrealistic, or vague. Creating a clear and focused aim statement and keeping it “SMART” (Specific, Measurable, Achievable, Realistic, and Timely) will bring structure to the project.1 “We will reduce Congestive Heart Failure readmissions on 5 medicine units at our hospital by 2.5% in 6 months” is an example of a SMART aim statement.
3. Involve the right people from the start
QI project disasters often start with the wrong team. Select members based on who is needed and not who is available. It is critical to include representatives or “champions” from each area that will be affected. People will buy into a new methodology much more quickly if they were engaged in its development or know that respected members in their area were involved.
4. Use a simple, systematic approach to guide improvement work
Various QI models exist and each offers a systematic approach for assessing and improving care services. The Model for Improvement developed by the Associates in Process Improvement2 is a simple and powerful framework for quality improvement that asks three questions: (1) What are we trying to accomplish? (2) How will we know a change is an improvement? (3) What changes can we make that will result in improvement? The model incorporates Plan-Do-Study-Act (PDSA) cycles to test changes on a small scale.
5. Good projects start with good background data
6. Choose interventions that are high impact, low effort
People will more easily change if the change itself is easy. So consider the question “does this intervention add significant work?” The best interventions change a process without causing undue burden to the clinicians and staff involved.
7. If you can’t measure it, you can’t improve it
After implementation, collect enough data to know whether the changes made improved the process. Study outcome, process, and balancing measures. If possible, use data already being collected by your institution. While it is critical to have quantitative measures, qualitative data such as surveys and observations can also enrich understanding.
Example: Increasing early discharges in medical unit.
Outcome measure – This is the desired outcome that the project aims to improve. This may be the percentage of discharges before noon (DBN) or the average discharge time.
Process measure – This is a measure of a specific change made to improve the outcome metric. The discharge orders may need to be placed earlier in the electronic medical record to improve DBN. This average discharge order time is an example of a process measure.
Balance measure – This metric evaluates whether the intended outcome is leading to unintended consequences. For example, tracking the readmission rate is an important balance measure to assess whether improved DBN is associated with rushed discharges and possible unsafe transitions.
8. Communicate project goals and progress
9. Manage resistance to change
“People responsible for planning and implementing change often forget that while the first task of change management is to understand the destination and how to get there, the first task of transition management is to convince people to leave home.” – William Bridges
Inertia is powerful. We may consider our continuous performance improvement initiative as “the next big thing” but others may not share this enthusiasm. We therefore need to build a compelling reason for others to become engaged and accept major changes to work flow. Different strategies may be needed depending on your audience. Though for some, data and a rational analysis will be persuasive, for others the emotional argument will be the most motivating. Share personal anecdotes and use patient stories. In addition, let providers know “what’s in it for them.” Some may have a personal interest in your project or may need QI experience for career advancement; others might be motivated by the possibilities for scholarship arising from this work.
10. Make the work count twice
Consider QI as a scholarly initiative from the start to bring rigor to the project at all phases. Describe the project in an abstract or manuscript once improvements have been made. Publication is a great way to boost team morale and help make a business case for future improvement work. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines provide an excellent framework for designing and writing up an improvement project.4 The guidelines focus on why the project was started, what was done, what was found, and what the findings mean.
Driving change is challenging, and it is tempting to jump ahead to “fixing the problem.” But implementing a successful QI project requires intelligent direction, strategic planning, and skillful execution. It is our hope that following the above tips will help you develop the best possible ideas and approach implementation in a systematic way, ultimately leading to meaningful change.
Dr. Reyna is assistant professor in the division of hospital medicine and unit medical director at Mount Sinai Medical Center in New York City. She is a Certified Clinical Microsystems Coach. Dr. Burger is associate professor and associate program director, internal medicine residency, at Mount Sinai Beth Israel. He is on the faculty for the SGIM Annual Meeting Precourse on QI and is head of the high value care committee at the department of medicine at Mount Sinai Beth Israel. Dr. Cho is assistant professor and director of quality and safety in the division of hospital medicine at Mount Sinai. He is a senior fellow at the Lown Institute.
References
1. MacLeod L. Making SMART goals smarter. Physician Exec. 2012 Mar-Apr;38(2):68-70, 72.
2. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition). San Francisco: Jossey-Bass Publishers; 2009.
3. Nelson EC, Batalden PB, Godfrey MM. Quality By Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
4. Ogrinc G, Davies L, Goodman D et.al. SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2015 Sep 14.
Editor’s Note: This column is a quarterly feature written by members of the Physicians in Training Committee. It aims to encourage and educate students, residents, and early career hospitalists.
One of the biggest challenges early career hospitalists, residents and medical students face in launching their first quality improvement (QI) project is knowing how and where to get started. QI can be highly rewarding, but it can also take valuable time and resources without guarantees of sustainable improvement. In this article, we outline 10 key factors to consider when starting a new project.
1. Frame your project so that it aligns with your hospital’s current goals
Choose a project with your hospital’s goals in mind. Securing resources such as health IT, financial, or staffing support will prove difficult unless you get buy-in from hospital leadership. If your project does not directly address hospital goals, frame the purpose to demonstrate that it still fits with leadership priorities. For example, though improving handoffs from daytime to nighttime providers may not be a specific goal, leadership should appreciate that this project is expected to improve patient safety.
2. Be SMART about goals
Many QI projects fail because the scope of the initial project is too large, unrealistic, or vague. Creating a clear and focused aim statement and keeping it “SMART” (Specific, Measurable, Achievable, Realistic, and Timely) will bring structure to the project.1 “We will reduce Congestive Heart Failure readmissions on 5 medicine units at our hospital by 2.5% in 6 months” is an example of a SMART aim statement.
3. Involve the right people from the start
QI project disasters often start with the wrong team. Select members based on who is needed and not who is available. It is critical to include representatives or “champions” from each area that will be affected. People will buy into a new methodology much more quickly if they were engaged in its development or know that respected members in their area were involved.
4. Use a simple, systematic approach to guide improvement work
Various QI models exist and each offers a systematic approach for assessing and improving care services. The Model for Improvement developed by the Associates in Process Improvement2 is a simple and powerful framework for quality improvement that asks three questions: (1) What are we trying to accomplish? (2) How will we know a change is an improvement? (3) What changes can we make that will result in improvement? The model incorporates Plan-Do-Study-Act (PDSA) cycles to test changes on a small scale.
5. Good projects start with good background data
6. Choose interventions that are high impact, low effort
People will more easily change if the change itself is easy. So consider the question “does this intervention add significant work?” The best interventions change a process without causing undue burden to the clinicians and staff involved.
7. If you can’t measure it, you can’t improve it
After implementation, collect enough data to know whether the changes made improved the process. Study outcome, process, and balancing measures. If possible, use data already being collected by your institution. While it is critical to have quantitative measures, qualitative data such as surveys and observations can also enrich understanding.
Example: Increasing early discharges in medical unit.
Outcome measure – This is the desired outcome that the project aims to improve. This may be the percentage of discharges before noon (DBN) or the average discharge time.
Process measure – This is a measure of a specific change made to improve the outcome metric. The discharge orders may need to be placed earlier in the electronic medical record to improve DBN. This average discharge order time is an example of a process measure.
Balance measure – This metric evaluates whether the intended outcome is leading to unintended consequences. For example, tracking the readmission rate is an important balance measure to assess whether improved DBN is associated with rushed discharges and possible unsafe transitions.
8. Communicate project goals and progress
9. Manage resistance to change
“People responsible for planning and implementing change often forget that while the first task of change management is to understand the destination and how to get there, the first task of transition management is to convince people to leave home.” – William Bridges
Inertia is powerful. We may consider our continuous performance improvement initiative as “the next big thing” but others may not share this enthusiasm. We therefore need to build a compelling reason for others to become engaged and accept major changes to work flow. Different strategies may be needed depending on your audience. Though for some, data and a rational analysis will be persuasive, for others the emotional argument will be the most motivating. Share personal anecdotes and use patient stories. In addition, let providers know “what’s in it for them.” Some may have a personal interest in your project or may need QI experience for career advancement; others might be motivated by the possibilities for scholarship arising from this work.
10. Make the work count twice
Consider QI as a scholarly initiative from the start to bring rigor to the project at all phases. Describe the project in an abstract or manuscript once improvements have been made. Publication is a great way to boost team morale and help make a business case for future improvement work. The Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines provide an excellent framework for designing and writing up an improvement project.4 The guidelines focus on why the project was started, what was done, what was found, and what the findings mean.
Driving change is challenging, and it is tempting to jump ahead to “fixing the problem.” But implementing a successful QI project requires intelligent direction, strategic planning, and skillful execution. It is our hope that following the above tips will help you develop the best possible ideas and approach implementation in a systematic way, ultimately leading to meaningful change.
Dr. Reyna is assistant professor in the division of hospital medicine and unit medical director at Mount Sinai Medical Center in New York City. She is a Certified Clinical Microsystems Coach. Dr. Burger is associate professor and associate program director, internal medicine residency, at Mount Sinai Beth Israel. He is on the faculty for the SGIM Annual Meeting Precourse on QI and is head of the high value care committee at the department of medicine at Mount Sinai Beth Israel. Dr. Cho is assistant professor and director of quality and safety in the division of hospital medicine at Mount Sinai. He is a senior fellow at the Lown Institute.
References
1. MacLeod L. Making SMART goals smarter. Physician Exec. 2012 Mar-Apr;38(2):68-70, 72.
2. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition). San Francisco: Jossey-Bass Publishers; 2009.
3. Nelson EC, Batalden PB, Godfrey MM. Quality By Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
4. Ogrinc G, Davies L, Goodman D et.al. SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2015 Sep 14.
AAD president sees the specialty as ‘a bright star on the dance floor’
NEW YORK – In a plenary session at the American Academy of Dermatology summer meeting, the AAD president offered an upbeat view of the profession, likening his role in leading the 19,000-member organization to that of a dancer and comparing the specialty itself to “a bright star on the dance floor.”
The specialty, however, is facing an uncertain future. “As the music changes, so must the dance,” Henry Lim, MD, told attendees. “And so it is with American medicine today. Successfully transitioning, adapting to those changes, is especially challenging for all of medicine, including for our specialty.”
Dr. Lim’s remarks came hours after President Donald Trump’s effort to dismantle the Affordable Care Act had failed. “We are in the middle of a health care system in deep turmoil and uncertainty – as you all saw from the vote this morning,” said Dr. Lim, whose 1-year term began in March.
Dermatology is assuming an ever-greater role as the U.S. population ages, he said. “The fastest-growing segment is people over 85 and last year Hallmark reported it sold 85,000 ‘Happy 100th Birthday’ cards.”
He cited the AAD’s Burden of Skin Disease Report, which found that nearly half of Americans over the age of 65 have at least one skin disease. That may not, however, translate into job security for dermatologists, he cautioned.
“A most concerning statistic from that report is that two in every three patients with skin disease are being treated by nondermatologists,” he said. Those practitioners include primary care physicians, pediatricians, hospitalists, nurse practitioners, and physician assistants. “We all know a major reason for it is access,” said Dr. Lim, who told a reporter prior to his speech that the academy has taken no position on whether it is for or against the Affordable Care Act.
But, he added in his speech, “we have been continuing to meet with individual members of Congress, Health and Human Services leadership, and the FDA – tackling issues eroding our ability to care for patients.”
Dr. Lim, chair emeritus of the department of dermatology and senior vice president for academic affairs at Henry Ford Health System in Detroit, cited in-office compounding, step therapy, narrow network funding for medical research, and scope of practice as examples.
“Listening is the key to understanding,” he noted, and the academy is doing just that. He and the rest of the academy’s leadership have visited with a number of state societies to listen to their concerns. “It is clear to me that, while we have handled many issues well, there are areas where we as an academy can do better,” he said.
Dr. Lim cited the need to “enhance our efforts in advocacy and to improve our communication, including our social media presence.”
The academy itself is in strong shape, with more than 90% of practicing dermatologists as members, he said. That places the AAD among the top specialty societies and means that future growth will likely come from international outreach.
Dr. Lim called on members to join the effort by taking to the dance floor themselves and participating. “Ask not what dermatology can do for you, ask what you can do for dermatology,” he concluded. “With the leadership of our academy listening to all of you and working together with all of you, I’m confident that dermatology will continue to be a bright star on the dance floor.”
NEW YORK – In a plenary session at the American Academy of Dermatology summer meeting, the AAD president offered an upbeat view of the profession, likening his role in leading the 19,000-member organization to that of a dancer and comparing the specialty itself to “a bright star on the dance floor.”
The specialty, however, is facing an uncertain future. “As the music changes, so must the dance,” Henry Lim, MD, told attendees. “And so it is with American medicine today. Successfully transitioning, adapting to those changes, is especially challenging for all of medicine, including for our specialty.”
Dr. Lim’s remarks came hours after President Donald Trump’s effort to dismantle the Affordable Care Act had failed. “We are in the middle of a health care system in deep turmoil and uncertainty – as you all saw from the vote this morning,” said Dr. Lim, whose 1-year term began in March.
Dermatology is assuming an ever-greater role as the U.S. population ages, he said. “The fastest-growing segment is people over 85 and last year Hallmark reported it sold 85,000 ‘Happy 100th Birthday’ cards.”
He cited the AAD’s Burden of Skin Disease Report, which found that nearly half of Americans over the age of 65 have at least one skin disease. That may not, however, translate into job security for dermatologists, he cautioned.
“A most concerning statistic from that report is that two in every three patients with skin disease are being treated by nondermatologists,” he said. Those practitioners include primary care physicians, pediatricians, hospitalists, nurse practitioners, and physician assistants. “We all know a major reason for it is access,” said Dr. Lim, who told a reporter prior to his speech that the academy has taken no position on whether it is for or against the Affordable Care Act.
But, he added in his speech, “we have been continuing to meet with individual members of Congress, Health and Human Services leadership, and the FDA – tackling issues eroding our ability to care for patients.”
Dr. Lim, chair emeritus of the department of dermatology and senior vice president for academic affairs at Henry Ford Health System in Detroit, cited in-office compounding, step therapy, narrow network funding for medical research, and scope of practice as examples.
“Listening is the key to understanding,” he noted, and the academy is doing just that. He and the rest of the academy’s leadership have visited with a number of state societies to listen to their concerns. “It is clear to me that, while we have handled many issues well, there are areas where we as an academy can do better,” he said.
Dr. Lim cited the need to “enhance our efforts in advocacy and to improve our communication, including our social media presence.”
The academy itself is in strong shape, with more than 90% of practicing dermatologists as members, he said. That places the AAD among the top specialty societies and means that future growth will likely come from international outreach.
Dr. Lim called on members to join the effort by taking to the dance floor themselves and participating. “Ask not what dermatology can do for you, ask what you can do for dermatology,” he concluded. “With the leadership of our academy listening to all of you and working together with all of you, I’m confident that dermatology will continue to be a bright star on the dance floor.”
NEW YORK – In a plenary session at the American Academy of Dermatology summer meeting, the AAD president offered an upbeat view of the profession, likening his role in leading the 19,000-member organization to that of a dancer and comparing the specialty itself to “a bright star on the dance floor.”
The specialty, however, is facing an uncertain future. “As the music changes, so must the dance,” Henry Lim, MD, told attendees. “And so it is with American medicine today. Successfully transitioning, adapting to those changes, is especially challenging for all of medicine, including for our specialty.”
Dr. Lim’s remarks came hours after President Donald Trump’s effort to dismantle the Affordable Care Act had failed. “We are in the middle of a health care system in deep turmoil and uncertainty – as you all saw from the vote this morning,” said Dr. Lim, whose 1-year term began in March.
Dermatology is assuming an ever-greater role as the U.S. population ages, he said. “The fastest-growing segment is people over 85 and last year Hallmark reported it sold 85,000 ‘Happy 100th Birthday’ cards.”
He cited the AAD’s Burden of Skin Disease Report, which found that nearly half of Americans over the age of 65 have at least one skin disease. That may not, however, translate into job security for dermatologists, he cautioned.
“A most concerning statistic from that report is that two in every three patients with skin disease are being treated by nondermatologists,” he said. Those practitioners include primary care physicians, pediatricians, hospitalists, nurse practitioners, and physician assistants. “We all know a major reason for it is access,” said Dr. Lim, who told a reporter prior to his speech that the academy has taken no position on whether it is for or against the Affordable Care Act.
But, he added in his speech, “we have been continuing to meet with individual members of Congress, Health and Human Services leadership, and the FDA – tackling issues eroding our ability to care for patients.”
Dr. Lim, chair emeritus of the department of dermatology and senior vice president for academic affairs at Henry Ford Health System in Detroit, cited in-office compounding, step therapy, narrow network funding for medical research, and scope of practice as examples.
“Listening is the key to understanding,” he noted, and the academy is doing just that. He and the rest of the academy’s leadership have visited with a number of state societies to listen to their concerns. “It is clear to me that, while we have handled many issues well, there are areas where we as an academy can do better,” he said.
Dr. Lim cited the need to “enhance our efforts in advocacy and to improve our communication, including our social media presence.”
The academy itself is in strong shape, with more than 90% of practicing dermatologists as members, he said. That places the AAD among the top specialty societies and means that future growth will likely come from international outreach.
Dr. Lim called on members to join the effort by taking to the dance floor themselves and participating. “Ask not what dermatology can do for you, ask what you can do for dermatology,” he concluded. “With the leadership of our academy listening to all of you and working together with all of you, I’m confident that dermatology will continue to be a bright star on the dance floor.”
AT THE 2017 AAD SUMMER MEETING
Patrick Conway leaves CMS for Blue Cross and Blue Shield
Patrick Conway, MD deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services, is departing his government post to take the reigns of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
In an Aug. 8 statement, Blue Cross NC announced that Dr. Conway will start as the insurer’s new president and CEO on Oct. 1. Blue Cross NC’s role in transforming the health care system in North Carolina is both a model for other plans and a system that Dr. Conway is excited to further improve, he said in a statement.
Blue Cross NC Board of Trustees Chair Frank Holding Jr. called Dr. Conway a national and international leader in health system transformation, quality, and innovation who will further advance Blue Cross NC’s goals.
“His unique experiences as a health care provider and as a leader of the world’s largest health care payor will help Blue Cross NC fulfill its mission to improve the health and well-being of our customers and communities,” Mr. Holding said in the statement.
Dr. Conway joined CMS in 2011 as the agency’s chief medical officer and ultimately became the agency’s deputy administrator for innovation and quality and director of the Center for Medicare and Medicaid Innovation. Following President Obama’s departure from office, Dr. Conway took over as acting CMS administrator for then-CMS principal deputy administrator Andy Slavitt until new administrator Seema Verma assumed the position in March.
A longtime pediatric hospitalist, Dr. Conway was selected as a Master of Hospital Medicine by the Society of Hospital Medicine. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014. Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
[email protected]
On Twitter @legal_med
Patrick Conway, MD deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services, is departing his government post to take the reigns of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
In an Aug. 8 statement, Blue Cross NC announced that Dr. Conway will start as the insurer’s new president and CEO on Oct. 1. Blue Cross NC’s role in transforming the health care system in North Carolina is both a model for other plans and a system that Dr. Conway is excited to further improve, he said in a statement.
Blue Cross NC Board of Trustees Chair Frank Holding Jr. called Dr. Conway a national and international leader in health system transformation, quality, and innovation who will further advance Blue Cross NC’s goals.
“His unique experiences as a health care provider and as a leader of the world’s largest health care payor will help Blue Cross NC fulfill its mission to improve the health and well-being of our customers and communities,” Mr. Holding said in the statement.
Dr. Conway joined CMS in 2011 as the agency’s chief medical officer and ultimately became the agency’s deputy administrator for innovation and quality and director of the Center for Medicare and Medicaid Innovation. Following President Obama’s departure from office, Dr. Conway took over as acting CMS administrator for then-CMS principal deputy administrator Andy Slavitt until new administrator Seema Verma assumed the position in March.
A longtime pediatric hospitalist, Dr. Conway was selected as a Master of Hospital Medicine by the Society of Hospital Medicine. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014. Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
[email protected]
On Twitter @legal_med
Patrick Conway, MD deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services, is departing his government post to take the reigns of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
In an Aug. 8 statement, Blue Cross NC announced that Dr. Conway will start as the insurer’s new president and CEO on Oct. 1. Blue Cross NC’s role in transforming the health care system in North Carolina is both a model for other plans and a system that Dr. Conway is excited to further improve, he said in a statement.
Blue Cross NC Board of Trustees Chair Frank Holding Jr. called Dr. Conway a national and international leader in health system transformation, quality, and innovation who will further advance Blue Cross NC’s goals.
“His unique experiences as a health care provider and as a leader of the world’s largest health care payor will help Blue Cross NC fulfill its mission to improve the health and well-being of our customers and communities,” Mr. Holding said in the statement.
Dr. Conway joined CMS in 2011 as the agency’s chief medical officer and ultimately became the agency’s deputy administrator for innovation and quality and director of the Center for Medicare and Medicaid Innovation. Following President Obama’s departure from office, Dr. Conway took over as acting CMS administrator for then-CMS principal deputy administrator Andy Slavitt until new administrator Seema Verma assumed the position in March.
A longtime pediatric hospitalist, Dr. Conway was selected as a Master of Hospital Medicine by the Society of Hospital Medicine. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014. Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
[email protected]
On Twitter @legal_med
Social Interaction May Enhance Patient Survival After Chemotherapy
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Perplexingly Purple
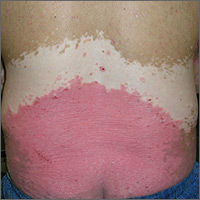
A 55-year-old woman presents for evaluation of a widespread rash that first appeared several months ago. The rash has resisted treatment with various OTC products—antifungal cream (tolnaftate), triple-antibiotic cream, and tea tree oil—and continues to itch terribly at times.
Her primary care provider prescribed oral terbinafine (250 mg/d) for a proposed fungal etiology after viewing the rash with a Wood lamp and performing a KOH prep. A one-month course yielded no relief.
The patient claims to be in good health otherwise. She does admit to going through a stressful period involving job loss, divorce, and care of her aging parents.
EXAMINATION
Multiple papulosquamous papules, nodules, and plaques are located on the patient’s arms, legs, wrists, and sacrum. The lesions range from pinpoint to several centimeters and oval to polygonal. They have a striking purple appearance. On closer inspection, many have a shiny, whitish sheen on the surface.

What is the diagnosis?
DISCUSSION
This is a classic case of lichen planus (LP), an unusual papulosquamous condition of unknown etiology. In addition to the mentioned areas, it can affect the mucosal surfaces, scalp, genitals, nails, and internal organs.
There is no evidence that the cause is infectious; rather, it appears to be triggered by a reaction to an unknown (possibly autoimmune) antigen. LP targets specific cells and produces a lichenoid reaction, in which the upper level of the dermis is broken down by an apoptotic process. This produces a characteristic sawtooth pattern at the dermoepidermal junction by a pathognomic lymphocytic infiltrate. The surface of the affected skin has a shiny, frosty appearance, also seen in other lichenoid conditions (eg, lichen sclerosus et atrophicus).
Other key diagnostic features are classified as “the Ps”: purple, plaquish, papular, planar (flat-topped), polygonal (multi-angular), penile, pruritic, and puzzling. The last word is key to triggering consideration of the other “Ps.”
LP that affects the scalp and mucosal surfaces can be problematic to treat. Topical steroids are the mainstay, but treatment can also include oral retinoids (acetretin, isotretinoin), methotrexate, antimalarials, and cyclosporine. Furthermore, LP can overlap with other diseases—notably lupus, for which TNF-[a] inhibitors are used with some success. For limited disease, intralesional steroid injections can be used (5 to 10 mg/cc triamcinolone).
This patient achieved good relief with a combination of topical and intralesional steroids. Fortunately, in most cases, the disease eventually resolves on its own.
TAKE-HOME LEARNING POINTS
- Lichen planus is an unusual condition, but the look and distribution seen in this case are fairly typical.
- The classic diagnostic “Ps” include: purple, papular, pruritic, plaquish, planar, and most of all puzzling.
- Wood lamp examination is useless for the most common dermatophytoses, which will not fluoresce; however, KOH preps are useful when correctly interpreted.
- In more obscure cases, a punch or shave biopsy will confirm the diagnosis.
A 55-year-old woman presents for evaluation of a widespread rash that first appeared several months ago. The rash has resisted treatment with various OTC products—antifungal cream (tolnaftate), triple-antibiotic cream, and tea tree oil—and continues to itch terribly at times.
Her primary care provider prescribed oral terbinafine (250 mg/d) for a proposed fungal etiology after viewing the rash with a Wood lamp and performing a KOH prep. A one-month course yielded no relief.
The patient claims to be in good health otherwise. She does admit to going through a stressful period involving job loss, divorce, and care of her aging parents.
EXAMINATION
Multiple papulosquamous papules, nodules, and plaques are located on the patient’s arms, legs, wrists, and sacrum. The lesions range from pinpoint to several centimeters and oval to polygonal. They have a striking purple appearance. On closer inspection, many have a shiny, whitish sheen on the surface.

What is the diagnosis?
DISCUSSION
This is a classic case of lichen planus (LP), an unusual papulosquamous condition of unknown etiology. In addition to the mentioned areas, it can affect the mucosal surfaces, scalp, genitals, nails, and internal organs.
There is no evidence that the cause is infectious; rather, it appears to be triggered by a reaction to an unknown (possibly autoimmune) antigen. LP targets specific cells and produces a lichenoid reaction, in which the upper level of the dermis is broken down by an apoptotic process. This produces a characteristic sawtooth pattern at the dermoepidermal junction by a pathognomic lymphocytic infiltrate. The surface of the affected skin has a shiny, frosty appearance, also seen in other lichenoid conditions (eg, lichen sclerosus et atrophicus).
Other key diagnostic features are classified as “the Ps”: purple, plaquish, papular, planar (flat-topped), polygonal (multi-angular), penile, pruritic, and puzzling. The last word is key to triggering consideration of the other “Ps.”
LP that affects the scalp and mucosal surfaces can be problematic to treat. Topical steroids are the mainstay, but treatment can also include oral retinoids (acetretin, isotretinoin), methotrexate, antimalarials, and cyclosporine. Furthermore, LP can overlap with other diseases—notably lupus, for which TNF-[a] inhibitors are used with some success. For limited disease, intralesional steroid injections can be used (5 to 10 mg/cc triamcinolone).
This patient achieved good relief with a combination of topical and intralesional steroids. Fortunately, in most cases, the disease eventually resolves on its own.
TAKE-HOME LEARNING POINTS
- Lichen planus is an unusual condition, but the look and distribution seen in this case are fairly typical.
- The classic diagnostic “Ps” include: purple, papular, pruritic, plaquish, planar, and most of all puzzling.
- Wood lamp examination is useless for the most common dermatophytoses, which will not fluoresce; however, KOH preps are useful when correctly interpreted.
- In more obscure cases, a punch or shave biopsy will confirm the diagnosis.
A 55-year-old woman presents for evaluation of a widespread rash that first appeared several months ago. The rash has resisted treatment with various OTC products—antifungal cream (tolnaftate), triple-antibiotic cream, and tea tree oil—and continues to itch terribly at times.
Her primary care provider prescribed oral terbinafine (250 mg/d) for a proposed fungal etiology after viewing the rash with a Wood lamp and performing a KOH prep. A one-month course yielded no relief.
The patient claims to be in good health otherwise. She does admit to going through a stressful period involving job loss, divorce, and care of her aging parents.
EXAMINATION
Multiple papulosquamous papules, nodules, and plaques are located on the patient’s arms, legs, wrists, and sacrum. The lesions range from pinpoint to several centimeters and oval to polygonal. They have a striking purple appearance. On closer inspection, many have a shiny, whitish sheen on the surface.

What is the diagnosis?
DISCUSSION
This is a classic case of lichen planus (LP), an unusual papulosquamous condition of unknown etiology. In addition to the mentioned areas, it can affect the mucosal surfaces, scalp, genitals, nails, and internal organs.
There is no evidence that the cause is infectious; rather, it appears to be triggered by a reaction to an unknown (possibly autoimmune) antigen. LP targets specific cells and produces a lichenoid reaction, in which the upper level of the dermis is broken down by an apoptotic process. This produces a characteristic sawtooth pattern at the dermoepidermal junction by a pathognomic lymphocytic infiltrate. The surface of the affected skin has a shiny, frosty appearance, also seen in other lichenoid conditions (eg, lichen sclerosus et atrophicus).
Other key diagnostic features are classified as “the Ps”: purple, plaquish, papular, planar (flat-topped), polygonal (multi-angular), penile, pruritic, and puzzling. The last word is key to triggering consideration of the other “Ps.”
LP that affects the scalp and mucosal surfaces can be problematic to treat. Topical steroids are the mainstay, but treatment can also include oral retinoids (acetretin, isotretinoin), methotrexate, antimalarials, and cyclosporine. Furthermore, LP can overlap with other diseases—notably lupus, for which TNF-[a] inhibitors are used with some success. For limited disease, intralesional steroid injections can be used (5 to 10 mg/cc triamcinolone).
This patient achieved good relief with a combination of topical and intralesional steroids. Fortunately, in most cases, the disease eventually resolves on its own.
TAKE-HOME LEARNING POINTS
- Lichen planus is an unusual condition, but the look and distribution seen in this case are fairly typical.
- The classic diagnostic “Ps” include: purple, papular, pruritic, plaquish, planar, and most of all puzzling.
- Wood lamp examination is useless for the most common dermatophytoses, which will not fluoresce; however, KOH preps are useful when correctly interpreted.
- In more obscure cases, a punch or shave biopsy will confirm the diagnosis.
Strategy could reduce myelosuppression in AML
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Study suggests malaria is undertreated
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
Drug receives orphan designation for ocular GVHD
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Rash and skin discoloration

The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
U.S. opioid, heroin overdose deaths may be one-fifth higher than reported
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Death certificates for drug overdose that fail to specify the drug ivolved may be responsible for a significant underestimation of opioid and heroin fatality rates in the United States.
Major finding: In 2014, national opioid mortality rates were actually 24% greater than reported, and heroin overdose fatality rates were 22% higher than reported.
Data source: Analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014.
Disclosures: No conflicts of interest were declared.