User login
Researchers find higher opioid use among cancer survivors
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
Analysis reveals poor outcomes in refractory DLBCL
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Cancer the most common diagnosis in palliative care patients
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.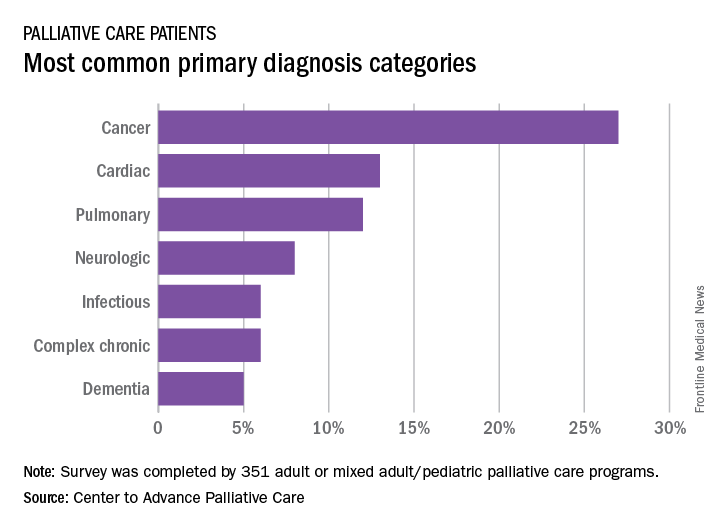
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
Safety alert for intragastric balloon systems
The Food and Drug Administration announced a safety alert on Aug. 10, 2017, for liquid-filled intragastric balloon systems, as they have caused five reports of unanticipated deaths that occurred from 2016 to present in patients.
The cause or incidence of patient death is still unknown, and the FDA has not been able to definitively attribute the deaths to the devices or the insertion procedures for these devices. All five reports show that patient deaths occurred within a month or less of balloon placement. In three of the reports, death occurred as soon as 1-3 days after balloon placement. The FDA has also received two additional reports of deaths in the same time period related to potential complications associated with balloon treatment.
The FDA continues to recommend that health care providers closely monitor patients treated with these devices for complications. Any adverse events related to intragastric balloon systems should be reported through MedWatch. The FDA will keep the public informed as new information becomes available.
Read the full safety alert on the FDA’s website.
The Food and Drug Administration announced a safety alert on Aug. 10, 2017, for liquid-filled intragastric balloon systems, as they have caused five reports of unanticipated deaths that occurred from 2016 to present in patients.
The cause or incidence of patient death is still unknown, and the FDA has not been able to definitively attribute the deaths to the devices or the insertion procedures for these devices. All five reports show that patient deaths occurred within a month or less of balloon placement. In three of the reports, death occurred as soon as 1-3 days after balloon placement. The FDA has also received two additional reports of deaths in the same time period related to potential complications associated with balloon treatment.
The FDA continues to recommend that health care providers closely monitor patients treated with these devices for complications. Any adverse events related to intragastric balloon systems should be reported through MedWatch. The FDA will keep the public informed as new information becomes available.
Read the full safety alert on the FDA’s website.
The Food and Drug Administration announced a safety alert on Aug. 10, 2017, for liquid-filled intragastric balloon systems, as they have caused five reports of unanticipated deaths that occurred from 2016 to present in patients.
The cause or incidence of patient death is still unknown, and the FDA has not been able to definitively attribute the deaths to the devices or the insertion procedures for these devices. All five reports show that patient deaths occurred within a month or less of balloon placement. In three of the reports, death occurred as soon as 1-3 days after balloon placement. The FDA has also received two additional reports of deaths in the same time period related to potential complications associated with balloon treatment.
The FDA continues to recommend that health care providers closely monitor patients treated with these devices for complications. Any adverse events related to intragastric balloon systems should be reported through MedWatch. The FDA will keep the public informed as new information becomes available.
Read the full safety alert on the FDA’s website.
Psoriasis IL-17 Inhibitors: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Psoriasis Oral Therapy Update: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dermatopathology Pearls: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Neuromodulation Patient Outcomes: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Eye disease affects 1 in 5 adults with severe atopic dermatitis
Results of a large cohort study in Denmark found that adults with atopic dermatitis (AD) were significantly more likely to be affected by certain ocular conditions, compared with those who did not have AD.
“Keratitis, conjunctivitis, and keratoconus as well as cataracts in patients younger than 50 years occurred more frequently in patients with AD and in a disease severity–dependent manner,” concluded the authors, who wrote that as far as they know, this is the largest study conducted to date of ocular disorders in adults with AD.
The investigators also found an elevated risk of a keratitis diagnosis among patients with mild AD (hazard ratio, 1.66; 95% confidence interval, 1.15-2.40) and those with severe AD (HR, 3.17; 95% CI, 2.31-4.35). Severe AD was associated with an elevated risk of keratoconus (HR, 10.01; 95% CI, 5.02-19.96),
Cataracts and glaucoma were not more common among those with AD overall. However, cataracts were increased among those under age 50 years with mild and severe AD, which were significant associations for both, but not among those over age 50 with AD. There were no differences for glaucoma risk associated with AD by age.
The investigators acknowledged that the study could not capture the reasons why anti-inflammatory ocular medicines were prescribed and that such medicines could have been prescribed for conditions other than the ocular conditions.
Capturing the risk of ocular diseases in AD is important, they wrote. They referred to “emerging concern” about the incidence of conjunctivitis with “near-future” biologic treatments for AD and the potential for long-term consequences. They referred to adverse event data from randomized clinical trials of dupilumab, an interleukin-4 receptor–alpha antagonist, approved by the Food and Drug Administration in March 2017 for treatment of moderate to severe AD, which included more cases of conjunctivitis among those treated with the biologic, compared with those on placebo (N Engl J Med. 2016 Dec 15;375:2335-48). A “weak trend” for more cases of conjunctivitis was also reported among treated patients with an IL-13 inhibitor, lebrikizumab, in a phase 2 study of adults with AD, they wrote.
Treatments targeting IL-4 receptor–alpha have been shown to result in increased blood eosinophil counts, and “these elevations might have clinical effects,” Dr. Thyssen and his colleagues wrote, adding: “Notably, eosinophils are pathognomonic for allergic eye disease.”
Dr. Thyssen disclosed funding from the Lundbeck Foundation and honoraria from Roche, Sanofi Genzyme, and LEO Pharma. Three other authors on the study reported research funding and/or honoraria from pharmaceutical firms.
Results of a large cohort study in Denmark found that adults with atopic dermatitis (AD) were significantly more likely to be affected by certain ocular conditions, compared with those who did not have AD.
“Keratitis, conjunctivitis, and keratoconus as well as cataracts in patients younger than 50 years occurred more frequently in patients with AD and in a disease severity–dependent manner,” concluded the authors, who wrote that as far as they know, this is the largest study conducted to date of ocular disorders in adults with AD.
The investigators also found an elevated risk of a keratitis diagnosis among patients with mild AD (hazard ratio, 1.66; 95% confidence interval, 1.15-2.40) and those with severe AD (HR, 3.17; 95% CI, 2.31-4.35). Severe AD was associated with an elevated risk of keratoconus (HR, 10.01; 95% CI, 5.02-19.96),
Cataracts and glaucoma were not more common among those with AD overall. However, cataracts were increased among those under age 50 years with mild and severe AD, which were significant associations for both, but not among those over age 50 with AD. There were no differences for glaucoma risk associated with AD by age.
The investigators acknowledged that the study could not capture the reasons why anti-inflammatory ocular medicines were prescribed and that such medicines could have been prescribed for conditions other than the ocular conditions.
Capturing the risk of ocular diseases in AD is important, they wrote. They referred to “emerging concern” about the incidence of conjunctivitis with “near-future” biologic treatments for AD and the potential for long-term consequences. They referred to adverse event data from randomized clinical trials of dupilumab, an interleukin-4 receptor–alpha antagonist, approved by the Food and Drug Administration in March 2017 for treatment of moderate to severe AD, which included more cases of conjunctivitis among those treated with the biologic, compared with those on placebo (N Engl J Med. 2016 Dec 15;375:2335-48). A “weak trend” for more cases of conjunctivitis was also reported among treated patients with an IL-13 inhibitor, lebrikizumab, in a phase 2 study of adults with AD, they wrote.
Treatments targeting IL-4 receptor–alpha have been shown to result in increased blood eosinophil counts, and “these elevations might have clinical effects,” Dr. Thyssen and his colleagues wrote, adding: “Notably, eosinophils are pathognomonic for allergic eye disease.”
Dr. Thyssen disclosed funding from the Lundbeck Foundation and honoraria from Roche, Sanofi Genzyme, and LEO Pharma. Three other authors on the study reported research funding and/or honoraria from pharmaceutical firms.
Results of a large cohort study in Denmark found that adults with atopic dermatitis (AD) were significantly more likely to be affected by certain ocular conditions, compared with those who did not have AD.
“Keratitis, conjunctivitis, and keratoconus as well as cataracts in patients younger than 50 years occurred more frequently in patients with AD and in a disease severity–dependent manner,” concluded the authors, who wrote that as far as they know, this is the largest study conducted to date of ocular disorders in adults with AD.
The investigators also found an elevated risk of a keratitis diagnosis among patients with mild AD (hazard ratio, 1.66; 95% confidence interval, 1.15-2.40) and those with severe AD (HR, 3.17; 95% CI, 2.31-4.35). Severe AD was associated with an elevated risk of keratoconus (HR, 10.01; 95% CI, 5.02-19.96),
Cataracts and glaucoma were not more common among those with AD overall. However, cataracts were increased among those under age 50 years with mild and severe AD, which were significant associations for both, but not among those over age 50 with AD. There were no differences for glaucoma risk associated with AD by age.
The investigators acknowledged that the study could not capture the reasons why anti-inflammatory ocular medicines were prescribed and that such medicines could have been prescribed for conditions other than the ocular conditions.
Capturing the risk of ocular diseases in AD is important, they wrote. They referred to “emerging concern” about the incidence of conjunctivitis with “near-future” biologic treatments for AD and the potential for long-term consequences. They referred to adverse event data from randomized clinical trials of dupilumab, an interleukin-4 receptor–alpha antagonist, approved by the Food and Drug Administration in March 2017 for treatment of moderate to severe AD, which included more cases of conjunctivitis among those treated with the biologic, compared with those on placebo (N Engl J Med. 2016 Dec 15;375:2335-48). A “weak trend” for more cases of conjunctivitis was also reported among treated patients with an IL-13 inhibitor, lebrikizumab, in a phase 2 study of adults with AD, they wrote.
Treatments targeting IL-4 receptor–alpha have been shown to result in increased blood eosinophil counts, and “these elevations might have clinical effects,” Dr. Thyssen and his colleagues wrote, adding: “Notably, eosinophils are pathognomonic for allergic eye disease.”
Dr. Thyssen disclosed funding from the Lundbeck Foundation and honoraria from Roche, Sanofi Genzyme, and LEO Pharma. Three other authors on the study reported research funding and/or honoraria from pharmaceutical firms.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Conjunctivitis, keratitis, and keratoconus are common in patients with atopic dermatitis, compared with the general population
Major finding: 19% of adults with severe AD received a prescription for an anti-inflammatory eye medication, compared with 4.5% of the general population.
Data source: Epidemiologic data from more than 4 million patients in Danish health care and prescription registries.
Disclosures: Four investigators disclosed outside grant funding and/or financial relationships with pharmaceutical manufacturers.
Why three strategies to correct vaccination hesitancy failed
, supporting findings in the literature that misinformation tends to linger in the memory and efforts to dislodge it may in fact reinforce it.
In a study of 120 students recruited from diverse departments of the University of Edinburgh, the Suor Orsola Benincasa University of Naples, and the Second University of Naples, 61% were women, and the mean age was 25 years. Most students had a bachelor’s (38%) or master’s degree (53%), while 11 were PhD students, reported Sara Pluviano, PhD, of the University of Edinburgh, and her associates.
The participants completed a baseline questionnaire assessing their beliefs and attitudes about immunization, and twice completed a questionnaire that had focused on general misperceptions about vaccines causing autism, vaccine side effects, and how likely they would be to give MMR vaccine to their children – immediately after an intervention and after a 7-day delay.
Students were exposed to one of four correction interventions.
In the first, participants were given a booklet confronting 10 “myths” with a number of “facts” about vaccines (myths vs. facts group). In the second, students were presented with a series of tables comparing the potential problems caused by measles, mumps, and rubella with the potential side effects caused by the MMR vaccine (graphics group). In the third intervention, participants were shown photos of unvaccinated children with measles, mumps, and rubella, in addition to the symptoms of each disease and warnings about vaccinating one’s own child (fear group). The fourth was the control condition, where participants were given two unrelated fact sheets with tips to help prevent medical errors and get safer health care (control group).
“The myths vs. facts format, at odds with its aims, induced stronger beliefs in the vaccine/autism link and in vaccines side effects over time, lending credit to the literature showing that countering false information in ways that repeat it may further contribute to its dissemination,” the researchers said. Also, “the exposure to fear appeals through images of sick children led to more increased misperceptions about vaccines causing autism. Moreover, this corrective strategy induced the strongest beliefs in vaccines side effects.
“The usage of fact/icon boxes resulted in less damage but did not bring any effective result,” they said.
“Our pattern of results thus confirms that there should be more testing of public health campaign messages,” Dr. Pluviano and her associates cautioned. “This is especially true because corrective strategies may appear effective immediately, yet backfire even after a short delay when the message they tried to convey gradually fades from memory, allowing common misconceptions to be more easily remembered and identified as true.”
Read more in PLOS One (2017 Jul 27. doi: 10.1371/journal.pone.0181640).
, supporting findings in the literature that misinformation tends to linger in the memory and efforts to dislodge it may in fact reinforce it.
In a study of 120 students recruited from diverse departments of the University of Edinburgh, the Suor Orsola Benincasa University of Naples, and the Second University of Naples, 61% were women, and the mean age was 25 years. Most students had a bachelor’s (38%) or master’s degree (53%), while 11 were PhD students, reported Sara Pluviano, PhD, of the University of Edinburgh, and her associates.
The participants completed a baseline questionnaire assessing their beliefs and attitudes about immunization, and twice completed a questionnaire that had focused on general misperceptions about vaccines causing autism, vaccine side effects, and how likely they would be to give MMR vaccine to their children – immediately after an intervention and after a 7-day delay.
Students were exposed to one of four correction interventions.
In the first, participants were given a booklet confronting 10 “myths” with a number of “facts” about vaccines (myths vs. facts group). In the second, students were presented with a series of tables comparing the potential problems caused by measles, mumps, and rubella with the potential side effects caused by the MMR vaccine (graphics group). In the third intervention, participants were shown photos of unvaccinated children with measles, mumps, and rubella, in addition to the symptoms of each disease and warnings about vaccinating one’s own child (fear group). The fourth was the control condition, where participants were given two unrelated fact sheets with tips to help prevent medical errors and get safer health care (control group).
“The myths vs. facts format, at odds with its aims, induced stronger beliefs in the vaccine/autism link and in vaccines side effects over time, lending credit to the literature showing that countering false information in ways that repeat it may further contribute to its dissemination,” the researchers said. Also, “the exposure to fear appeals through images of sick children led to more increased misperceptions about vaccines causing autism. Moreover, this corrective strategy induced the strongest beliefs in vaccines side effects.
“The usage of fact/icon boxes resulted in less damage but did not bring any effective result,” they said.
“Our pattern of results thus confirms that there should be more testing of public health campaign messages,” Dr. Pluviano and her associates cautioned. “This is especially true because corrective strategies may appear effective immediately, yet backfire even after a short delay when the message they tried to convey gradually fades from memory, allowing common misconceptions to be more easily remembered and identified as true.”
Read more in PLOS One (2017 Jul 27. doi: 10.1371/journal.pone.0181640).
, supporting findings in the literature that misinformation tends to linger in the memory and efforts to dislodge it may in fact reinforce it.
In a study of 120 students recruited from diverse departments of the University of Edinburgh, the Suor Orsola Benincasa University of Naples, and the Second University of Naples, 61% were women, and the mean age was 25 years. Most students had a bachelor’s (38%) or master’s degree (53%), while 11 were PhD students, reported Sara Pluviano, PhD, of the University of Edinburgh, and her associates.
The participants completed a baseline questionnaire assessing their beliefs and attitudes about immunization, and twice completed a questionnaire that had focused on general misperceptions about vaccines causing autism, vaccine side effects, and how likely they would be to give MMR vaccine to their children – immediately after an intervention and after a 7-day delay.
Students were exposed to one of four correction interventions.
In the first, participants were given a booklet confronting 10 “myths” with a number of “facts” about vaccines (myths vs. facts group). In the second, students were presented with a series of tables comparing the potential problems caused by measles, mumps, and rubella with the potential side effects caused by the MMR vaccine (graphics group). In the third intervention, participants were shown photos of unvaccinated children with measles, mumps, and rubella, in addition to the symptoms of each disease and warnings about vaccinating one’s own child (fear group). The fourth was the control condition, where participants were given two unrelated fact sheets with tips to help prevent medical errors and get safer health care (control group).
“The myths vs. facts format, at odds with its aims, induced stronger beliefs in the vaccine/autism link and in vaccines side effects over time, lending credit to the literature showing that countering false information in ways that repeat it may further contribute to its dissemination,” the researchers said. Also, “the exposure to fear appeals through images of sick children led to more increased misperceptions about vaccines causing autism. Moreover, this corrective strategy induced the strongest beliefs in vaccines side effects.
“The usage of fact/icon boxes resulted in less damage but did not bring any effective result,” they said.
“Our pattern of results thus confirms that there should be more testing of public health campaign messages,” Dr. Pluviano and her associates cautioned. “This is especially true because corrective strategies may appear effective immediately, yet backfire even after a short delay when the message they tried to convey gradually fades from memory, allowing common misconceptions to be more easily remembered and identified as true.”
Read more in PLOS One (2017 Jul 27. doi: 10.1371/journal.pone.0181640).
FROM PLOS ONE








