User login
Pregnancy not a barrier to providing cutaneous surgery
NEW YORK – For some dermatologists, surgical care of the pregnant patient represents an area of uncertainty. But with few exceptions, dermatologists can continue with business as usual for their pregnant patients, according to Keith Harrigill, MD.
Dr. Harrigill, a dermatologist who previously was a practicing obstetrician-gynecologist, delineated the safe zones of dermatologic surgery in these patients at the summer meeting of the American Academy of Dermatology.
About 2% of pregnant women will require nonobstetric surgery and about 75,000 pregnant women in the United States will have surgery annually, he said. Appendectomies and other emergent abdominal surgery account for a large proportion of these cases; dermatologic surgeries are not included in these figures, and cutaneous procedures in pregnant women are not usually tracked. The literature on dermatologic treatments during pregnancy is “scant,” said Dr. Harrigill, a dermatologist in private practice in Birmingham, Ala.
However, it’s known that one-third of women with melanoma are of childbearing age, and melanoma accounts for 8% of the malignancies diagnosed during pregnancy, with a rate estimated at 0.14 to 2.8 per 1,000 live births, he said.
Since some women will have to address potentially serious skin issues during pregnancy, what’s safe, and what isn’t? Dr. Harrigill said that the American College of Obstetrics and Gynecology has provided guidance with an April 2017 opinion, prepared in conjunction with the American Society for Anesthesia, on nonobstetric surgery during pregnancy (Obstet Gynecol. 2017;129:777-8).
The opinion primarily focuses on major surgery. “What we do – cutaneous surgeries – they consider to be minor surgery,” he said. But even with major procedures, the good news is that “there’s no increase in birth defects in fetal exposure to anesthesia at any age,” he noted.
Dr. Harrigill’s approach, which conforms to the general guidance provided by the opinion, is to think of dermatologic procedures in three categories: urgent, nonurgent, and elective. Urgent procedures might include biopsying and treating a lesion suspicious for melanoma or an aggressive nonmelanoma skin cancer, or controlling a friable, bleeding pyogenic granuloma. “Do these right away,” he said.
Nonurgent procedures, such as treatment of a nodular basal cell carcinoma, should be done during the second trimester, when possible. Elective procedures, such as a scar excision, should be deferred until after delivery.
Dermatologists can almost always achieve adequate pain control with local anesthesia alone, said Dr. Harrigill, pointing out that local anesthesia is “the safest known way to give anesthesia during pregnancy.”
However, when thinking about even a remote risk of teratogenesis, it’s important to understand that fetal organogenesis occurs from day 15 to day 56, and that before 15 days, adverse events are limited to spontaneous abortion. So it’s particularly important to avoid teratogenic medications during the first 2 months of gestation, Dr. Harrigill said.
Part of the concern, he noted, is that it’s ethically problematic to perform large randomized trials in pregnant women, so the guidelines regarding surgery and medication safety are drawn from retrospective studies, registries, meta-analyses, and expert consensus.
Still, according to the ACOG guidelines, “a pregnant woman should never be denied indicated surgery, regardless of trimester.”
There’s no reason to risk delaying a diagnosis of malignancy in a pregnant patient, Dr. Harrigill said. “My dermatologic surgery approach is to biopsy anything that is clinically suspicious for malignancy, at any gestational age.”
When performing biopsies in pregnant patients, he uses the same protocol as he uses with any other patient. Skin preparation can be done with either isopropyl alcohol or chlorhexidine. Some practitioners avoid using povidone iodine because of a theoretical risk of fetal hypothyroidism.
For anesthesia, Dr. Harrigill noted that lidocaine is generally considered safe in pregnancy. He is also comfortable using epinephrine, despite the theoretical concern of uterine artery spasm, for which “studies are lacking.” The relatively minute amount of epinephrine used in dermatologic anesthesia, he said, is not likely to have an impact on such a large vessel.
Prilocaine is generally safe, and combination creams with prilocaine are fine to use, he said. Diphenhydramine is also safe to use. However, he advised avoiding long-acting anesthetic agents, such as mepivacaine and bupivacaine.
His advice regarding sedation? “Don’t do it.” Dr. Harrigill said he doesn’t use sedation in the office for his nonpregnant patients, either.
Before about 20 weeks of pregnancy, Dr. Harrigill said not to worry about how the patient is positioned. But after that, the lateral decubitus position is best because it keeps the gravid uterus from compressing the great vessels.
“Pregnant women are prone to fainting due to progesterone-mediated vasodilation,” he said. Dermatologists can work with their office staff to keep these patients well hydrated, and make sure they get in and out of chairs and off exam tables slowly.
No changes are needed in excision or suturing techniques. Because cicatrization is delayed in pregnant women, Dr. Harrigill uses longer-lasting absorbable sutures with high tensile strength, especially when performing procedures on the trunk or abdomen. This means that his closures will use delayed-absorption epidermal sutures with running nylon pull-through subcuticular sutures as well. He will leave these in for 5-7 days longer than usual.
Pregnant women are not at a higher risk of infection than the general population, so he follows the standard procedures here as well. If an antibiotic is indicated, penicillin, a cephalosporin, azithromycin, and erythromycin base are all logical choices.
To be avoided are sulfamethoxazole/trimethoprim, which carries a risk of feta hyperbilirubinemia, especially when given in the second trimester; doxycycline and tetracycline, which can cause permanent brown discoloration of the teeth; and fluoroquinolones, which have been associated with cartilage defects.
For analgesia, acetaminophen is an option. Ibuprofen and salicylates should be avoided, especially at the end of pregnancy when their administration is associated with premature closure of the ductus arteriosus, and, possibly, placental abruption, Dr. Harrigill noted.
However, short-term use of opioids is generally considered safe for the fetus. If larger doses are given just before delivery, the neonate may experience respiratory depression. This scenario is unlikely to be faced by the dermatologist, noted Dr. Harrigill. “I use these without reservation” in terms of fetal risk, he said.
Collaboration is key when caring for pregnant patients, said Dr. Harrigill, who recommends consulting the obstetrician of record for any procedures other than a simple biopsy or shave removal.
Dr. Harrigill reported that he had no conflicts of interest.
[email protected]
On Twitter @karioakes
NEW YORK – For some dermatologists, surgical care of the pregnant patient represents an area of uncertainty. But with few exceptions, dermatologists can continue with business as usual for their pregnant patients, according to Keith Harrigill, MD.
Dr. Harrigill, a dermatologist who previously was a practicing obstetrician-gynecologist, delineated the safe zones of dermatologic surgery in these patients at the summer meeting of the American Academy of Dermatology.
About 2% of pregnant women will require nonobstetric surgery and about 75,000 pregnant women in the United States will have surgery annually, he said. Appendectomies and other emergent abdominal surgery account for a large proportion of these cases; dermatologic surgeries are not included in these figures, and cutaneous procedures in pregnant women are not usually tracked. The literature on dermatologic treatments during pregnancy is “scant,” said Dr. Harrigill, a dermatologist in private practice in Birmingham, Ala.
However, it’s known that one-third of women with melanoma are of childbearing age, and melanoma accounts for 8% of the malignancies diagnosed during pregnancy, with a rate estimated at 0.14 to 2.8 per 1,000 live births, he said.
Since some women will have to address potentially serious skin issues during pregnancy, what’s safe, and what isn’t? Dr. Harrigill said that the American College of Obstetrics and Gynecology has provided guidance with an April 2017 opinion, prepared in conjunction with the American Society for Anesthesia, on nonobstetric surgery during pregnancy (Obstet Gynecol. 2017;129:777-8).
The opinion primarily focuses on major surgery. “What we do – cutaneous surgeries – they consider to be minor surgery,” he said. But even with major procedures, the good news is that “there’s no increase in birth defects in fetal exposure to anesthesia at any age,” he noted.
Dr. Harrigill’s approach, which conforms to the general guidance provided by the opinion, is to think of dermatologic procedures in three categories: urgent, nonurgent, and elective. Urgent procedures might include biopsying and treating a lesion suspicious for melanoma or an aggressive nonmelanoma skin cancer, or controlling a friable, bleeding pyogenic granuloma. “Do these right away,” he said.
Nonurgent procedures, such as treatment of a nodular basal cell carcinoma, should be done during the second trimester, when possible. Elective procedures, such as a scar excision, should be deferred until after delivery.
Dermatologists can almost always achieve adequate pain control with local anesthesia alone, said Dr. Harrigill, pointing out that local anesthesia is “the safest known way to give anesthesia during pregnancy.”
However, when thinking about even a remote risk of teratogenesis, it’s important to understand that fetal organogenesis occurs from day 15 to day 56, and that before 15 days, adverse events are limited to spontaneous abortion. So it’s particularly important to avoid teratogenic medications during the first 2 months of gestation, Dr. Harrigill said.
Part of the concern, he noted, is that it’s ethically problematic to perform large randomized trials in pregnant women, so the guidelines regarding surgery and medication safety are drawn from retrospective studies, registries, meta-analyses, and expert consensus.
Still, according to the ACOG guidelines, “a pregnant woman should never be denied indicated surgery, regardless of trimester.”
There’s no reason to risk delaying a diagnosis of malignancy in a pregnant patient, Dr. Harrigill said. “My dermatologic surgery approach is to biopsy anything that is clinically suspicious for malignancy, at any gestational age.”
When performing biopsies in pregnant patients, he uses the same protocol as he uses with any other patient. Skin preparation can be done with either isopropyl alcohol or chlorhexidine. Some practitioners avoid using povidone iodine because of a theoretical risk of fetal hypothyroidism.
For anesthesia, Dr. Harrigill noted that lidocaine is generally considered safe in pregnancy. He is also comfortable using epinephrine, despite the theoretical concern of uterine artery spasm, for which “studies are lacking.” The relatively minute amount of epinephrine used in dermatologic anesthesia, he said, is not likely to have an impact on such a large vessel.
Prilocaine is generally safe, and combination creams with prilocaine are fine to use, he said. Diphenhydramine is also safe to use. However, he advised avoiding long-acting anesthetic agents, such as mepivacaine and bupivacaine.
His advice regarding sedation? “Don’t do it.” Dr. Harrigill said he doesn’t use sedation in the office for his nonpregnant patients, either.
Before about 20 weeks of pregnancy, Dr. Harrigill said not to worry about how the patient is positioned. But after that, the lateral decubitus position is best because it keeps the gravid uterus from compressing the great vessels.
“Pregnant women are prone to fainting due to progesterone-mediated vasodilation,” he said. Dermatologists can work with their office staff to keep these patients well hydrated, and make sure they get in and out of chairs and off exam tables slowly.
No changes are needed in excision or suturing techniques. Because cicatrization is delayed in pregnant women, Dr. Harrigill uses longer-lasting absorbable sutures with high tensile strength, especially when performing procedures on the trunk or abdomen. This means that his closures will use delayed-absorption epidermal sutures with running nylon pull-through subcuticular sutures as well. He will leave these in for 5-7 days longer than usual.
Pregnant women are not at a higher risk of infection than the general population, so he follows the standard procedures here as well. If an antibiotic is indicated, penicillin, a cephalosporin, azithromycin, and erythromycin base are all logical choices.
To be avoided are sulfamethoxazole/trimethoprim, which carries a risk of feta hyperbilirubinemia, especially when given in the second trimester; doxycycline and tetracycline, which can cause permanent brown discoloration of the teeth; and fluoroquinolones, which have been associated with cartilage defects.
For analgesia, acetaminophen is an option. Ibuprofen and salicylates should be avoided, especially at the end of pregnancy when their administration is associated with premature closure of the ductus arteriosus, and, possibly, placental abruption, Dr. Harrigill noted.
However, short-term use of opioids is generally considered safe for the fetus. If larger doses are given just before delivery, the neonate may experience respiratory depression. This scenario is unlikely to be faced by the dermatologist, noted Dr. Harrigill. “I use these without reservation” in terms of fetal risk, he said.
Collaboration is key when caring for pregnant patients, said Dr. Harrigill, who recommends consulting the obstetrician of record for any procedures other than a simple biopsy or shave removal.
Dr. Harrigill reported that he had no conflicts of interest.
[email protected]
On Twitter @karioakes
NEW YORK – For some dermatologists, surgical care of the pregnant patient represents an area of uncertainty. But with few exceptions, dermatologists can continue with business as usual for their pregnant patients, according to Keith Harrigill, MD.
Dr. Harrigill, a dermatologist who previously was a practicing obstetrician-gynecologist, delineated the safe zones of dermatologic surgery in these patients at the summer meeting of the American Academy of Dermatology.
About 2% of pregnant women will require nonobstetric surgery and about 75,000 pregnant women in the United States will have surgery annually, he said. Appendectomies and other emergent abdominal surgery account for a large proportion of these cases; dermatologic surgeries are not included in these figures, and cutaneous procedures in pregnant women are not usually tracked. The literature on dermatologic treatments during pregnancy is “scant,” said Dr. Harrigill, a dermatologist in private practice in Birmingham, Ala.
However, it’s known that one-third of women with melanoma are of childbearing age, and melanoma accounts for 8% of the malignancies diagnosed during pregnancy, with a rate estimated at 0.14 to 2.8 per 1,000 live births, he said.
Since some women will have to address potentially serious skin issues during pregnancy, what’s safe, and what isn’t? Dr. Harrigill said that the American College of Obstetrics and Gynecology has provided guidance with an April 2017 opinion, prepared in conjunction with the American Society for Anesthesia, on nonobstetric surgery during pregnancy (Obstet Gynecol. 2017;129:777-8).
The opinion primarily focuses on major surgery. “What we do – cutaneous surgeries – they consider to be minor surgery,” he said. But even with major procedures, the good news is that “there’s no increase in birth defects in fetal exposure to anesthesia at any age,” he noted.
Dr. Harrigill’s approach, which conforms to the general guidance provided by the opinion, is to think of dermatologic procedures in three categories: urgent, nonurgent, and elective. Urgent procedures might include biopsying and treating a lesion suspicious for melanoma or an aggressive nonmelanoma skin cancer, or controlling a friable, bleeding pyogenic granuloma. “Do these right away,” he said.
Nonurgent procedures, such as treatment of a nodular basal cell carcinoma, should be done during the second trimester, when possible. Elective procedures, such as a scar excision, should be deferred until after delivery.
Dermatologists can almost always achieve adequate pain control with local anesthesia alone, said Dr. Harrigill, pointing out that local anesthesia is “the safest known way to give anesthesia during pregnancy.”
However, when thinking about even a remote risk of teratogenesis, it’s important to understand that fetal organogenesis occurs from day 15 to day 56, and that before 15 days, adverse events are limited to spontaneous abortion. So it’s particularly important to avoid teratogenic medications during the first 2 months of gestation, Dr. Harrigill said.
Part of the concern, he noted, is that it’s ethically problematic to perform large randomized trials in pregnant women, so the guidelines regarding surgery and medication safety are drawn from retrospective studies, registries, meta-analyses, and expert consensus.
Still, according to the ACOG guidelines, “a pregnant woman should never be denied indicated surgery, regardless of trimester.”
There’s no reason to risk delaying a diagnosis of malignancy in a pregnant patient, Dr. Harrigill said. “My dermatologic surgery approach is to biopsy anything that is clinically suspicious for malignancy, at any gestational age.”
When performing biopsies in pregnant patients, he uses the same protocol as he uses with any other patient. Skin preparation can be done with either isopropyl alcohol or chlorhexidine. Some practitioners avoid using povidone iodine because of a theoretical risk of fetal hypothyroidism.
For anesthesia, Dr. Harrigill noted that lidocaine is generally considered safe in pregnancy. He is also comfortable using epinephrine, despite the theoretical concern of uterine artery spasm, for which “studies are lacking.” The relatively minute amount of epinephrine used in dermatologic anesthesia, he said, is not likely to have an impact on such a large vessel.
Prilocaine is generally safe, and combination creams with prilocaine are fine to use, he said. Diphenhydramine is also safe to use. However, he advised avoiding long-acting anesthetic agents, such as mepivacaine and bupivacaine.
His advice regarding sedation? “Don’t do it.” Dr. Harrigill said he doesn’t use sedation in the office for his nonpregnant patients, either.
Before about 20 weeks of pregnancy, Dr. Harrigill said not to worry about how the patient is positioned. But after that, the lateral decubitus position is best because it keeps the gravid uterus from compressing the great vessels.
“Pregnant women are prone to fainting due to progesterone-mediated vasodilation,” he said. Dermatologists can work with their office staff to keep these patients well hydrated, and make sure they get in and out of chairs and off exam tables slowly.
No changes are needed in excision or suturing techniques. Because cicatrization is delayed in pregnant women, Dr. Harrigill uses longer-lasting absorbable sutures with high tensile strength, especially when performing procedures on the trunk or abdomen. This means that his closures will use delayed-absorption epidermal sutures with running nylon pull-through subcuticular sutures as well. He will leave these in for 5-7 days longer than usual.
Pregnant women are not at a higher risk of infection than the general population, so he follows the standard procedures here as well. If an antibiotic is indicated, penicillin, a cephalosporin, azithromycin, and erythromycin base are all logical choices.
To be avoided are sulfamethoxazole/trimethoprim, which carries a risk of feta hyperbilirubinemia, especially when given in the second trimester; doxycycline and tetracycline, which can cause permanent brown discoloration of the teeth; and fluoroquinolones, which have been associated with cartilage defects.
For analgesia, acetaminophen is an option. Ibuprofen and salicylates should be avoided, especially at the end of pregnancy when their administration is associated with premature closure of the ductus arteriosus, and, possibly, placental abruption, Dr. Harrigill noted.
However, short-term use of opioids is generally considered safe for the fetus. If larger doses are given just before delivery, the neonate may experience respiratory depression. This scenario is unlikely to be faced by the dermatologist, noted Dr. Harrigill. “I use these without reservation” in terms of fetal risk, he said.
Collaboration is key when caring for pregnant patients, said Dr. Harrigill, who recommends consulting the obstetrician of record for any procedures other than a simple biopsy or shave removal.
Dr. Harrigill reported that he had no conflicts of interest.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
Right Paraduodenal Hernia
Paraduodenal hernia, also called mesocolic hernia, is a type of internal hernia that is thought to be caused by a congenital defect involving abnormal retroperitoneal fixation of the mesentery due to abnormal rotation of the midgut.1 Internal hernias account for only 1% of all hernias, and paraduodenal hernias make up 50% of those.2
Paraduodenal hernias can be classified as left or right with left being far more common than right, 75% and 25%, respectively.2 Due to the fixation abnormalities in the midgut, fossae are formed that help to classify left vs right paraduodenal hernias. Herniation through Landzert fossae results in a left paraduodenal hernia with the primary constituents of the hernia sac being the inferior mesenteric artery and vein.1 This result is due to an in utero defect of the small intestine herniated between the inferior mesenteric vein and posterior parietal attachments of the descending mesocolon to the retroperitoneal.3
In a right paraduodenal hernia, herniation occurs through Waldeyer fossae with the main contents of the hernia sac being the iliocolic, right colic, and middle colic vessels within the anterior wall and the superior mesenteric artery along the medial border of the hernia.1 Since there is a failure of rotation around the superior mesenteric artery, the majority of the small intestine remains to the right of the superior mesenteric artery, resulting in the small intestine being trapped between the posteriolateral peritoneum.3 Regardless of the type of paraduodenal hernia, patients usually will present with symptoms of small bowel obstruction. In these types of hernias, a computed tomography (CT) scan with IV contrast may suggest evidence of obstruction between the duodenum and jejunum, but this may be unclear. Although rare, clinical suspicion of paraduodenal hernia is necessary to prevent ensuing complications and mortality.
Case Presentation
A 43-year-old man presented to the emergency department with symptoms that included nausea, vomiting, intermittent epigastric abdominal pain, and obstipation, which were suggestive of a small bowel obstruction. The patient reported similar intermittent episodes over the past 10 years that had resolved without surgery. The patient had no history of abdominal surgeries. A nasogastric tube was inserted and immediately drew out a significant amount of bilious contents. A CT scan indicated an obstruction at the proximal jejunum with suspicion of an internal hernia.
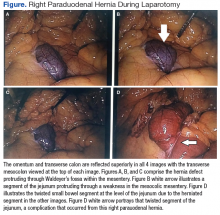
The patient underwent exploratory laparotomy soon after, which confirmed a right paraduodenal hernia (Figure). The surgery began laproscopically by retracting the omentum and transverse colon cranially to expose the ligament of Treitz. The hernia defect was identified on the mesentery where the proximal jejunum twisted on itself in a loop. The hernia was untwisted, and adhesions were removed. The posterior attachment of the hernia sac was freed with harmonic cautery and blunt dissection along with its attachment to the ligament of Treitz. In the process of freeing the herniation, a 1-cm enterotomy ensued, which did not contain succus or spillage of luminal contents at that time. Due to difficulties in visualizing the remainder of the small bowel, the procedure was converted to a laparotomy. This allowed complete freeing of the twisted loop of bowel.
Afterward, there was succus and bile draining from the enterotomy, so it was closed transversely in 2 layers, making sure there was a lumen between the layers. The first and second parts of the duodenum were examined followed by palpitation of the duodenal sweep. The remainder of the small bowel was visualized to the cecum, and the retroperitoneal space was dissected out of the hernia sac space. The abdomen was irrigated, and the omentum was draped back over the intestines. The fascia was closed followed by skin reapproximation with staples. The patient experienced an uneventful recovery and was discharged on day 6 with resolution of his symptoms.
Discussion
Paraduodenal hernias are a type of internal hernia and a rare cause of intestinal obstruction accounting for about 0.5% of all hernias. Right paraduodenal hernias are far less common than left paraduodenal hernias and occur due to a defect in the jejunum mesentery called Waldeyer fossae.4 This is located at the third part of the duodenum and behind the superior mesenteric artery.4 Symptoms of paraduodenal hernias are nonspecific and may include nausea, vomiting, and intermittent cramping. Symptoms of obstruction can be intermittent due to the small bowel herniating through the fossae and then retracting.1 Computed tomography has good specificity and aides in the diagnosis of an internal hernia, but physicians must have a high index of suspicion as well.5
Definitive diagnosis and treatment of paraduodenal hernias involves laparoscopy or exploratory laparotomy to visualize the internal hernia and its surrounding sac.4,5 All hernias should be repaired to prevent strangulation of the bowel, but internal hernias are even more important to fix because these hernias may not present until there is severe injury to the bowel.5 On identification of the paraduodenal hernia, it is important to release the bowel from the hernia sac, free up adhesions, and place small bowel segments back into the correct anatomical position.4,5
In the event of bowel injury, resection with reanastomosis is indicated. Careful dissection is important to prevent injury to the superior mesenteric artery, which supplies most of the small bowel and ascending colon.4,5 Injury to the superior mesenteric artery could lead to ischemia and gangrenous bowel.2 Immediate detection and early surgery intervention of these congenital hernias can prevent such complications.2 The literature includes reports of paraduodenal hernias with complications of gangrenous bowel that required small bowel resection.2 These complications further emphasize the need to proceed immediately with surgery if a paraduodenal hernia is suspected.
Conclusion
This rare cause of bowel obstruction was documented in order to emphasize the importance of having a high clinical suspicion for a paraduodenal hernia. This particular patient with no history of abdominal surgeries had previously dealt with bowel obstruction and would likely have this complication again without surgical intervention. Patients with paraduodenal hernias also are at risk for bowel ischemia, other high-risk complications, and even death.5 Although a CT scan provided information about an approximate location of the obstruction, laparoscopy confirmed the diagnosis. Going into the operation with paraduodenal hernia in the differential allowed the surgeon to be prepared for the appropriate anatomy involved with this procedure to minimize damage to important structures, such as the superior mesenteric artery and its branches.
1. Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Saunders; 2012.
2. Fukada T, Mukai H, Shimamura F, Furukawa T, Miyazaki M. A causal relationship between right paraduodenal hernia and superior mesenteric artery syndrome: a case report. J Med Case Rep. 2010;4:159.
3. Skandalakis JE. Peritoneum, omenta, and internal hernias. In: Skandalakis JE, Colborn GL, eds. Skandalakis Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. 1st ed. Athens, Greece: Paschalidis Medical Publications; 2004:chap 10.
4. Papaziogas B, Souparis A, Makris J, Alexandrakis A, Papaziogas T. Surgical images: soft tissue. Right paraduodenal hernia. Can J Surg. 2004;47(3):195-196.
5. Manfredelli S, Andrea Z, Stefano P, et al. Rare small bowel obstruction: right paraduodenal hernia. Case report. Int J Surg Case Rep. 2013;4(4):412-415.
Paraduodenal hernia, also called mesocolic hernia, is a type of internal hernia that is thought to be caused by a congenital defect involving abnormal retroperitoneal fixation of the mesentery due to abnormal rotation of the midgut.1 Internal hernias account for only 1% of all hernias, and paraduodenal hernias make up 50% of those.2
Paraduodenal hernias can be classified as left or right with left being far more common than right, 75% and 25%, respectively.2 Due to the fixation abnormalities in the midgut, fossae are formed that help to classify left vs right paraduodenal hernias. Herniation through Landzert fossae results in a left paraduodenal hernia with the primary constituents of the hernia sac being the inferior mesenteric artery and vein.1 This result is due to an in utero defect of the small intestine herniated between the inferior mesenteric vein and posterior parietal attachments of the descending mesocolon to the retroperitoneal.3
In a right paraduodenal hernia, herniation occurs through Waldeyer fossae with the main contents of the hernia sac being the iliocolic, right colic, and middle colic vessels within the anterior wall and the superior mesenteric artery along the medial border of the hernia.1 Since there is a failure of rotation around the superior mesenteric artery, the majority of the small intestine remains to the right of the superior mesenteric artery, resulting in the small intestine being trapped between the posteriolateral peritoneum.3 Regardless of the type of paraduodenal hernia, patients usually will present with symptoms of small bowel obstruction. In these types of hernias, a computed tomography (CT) scan with IV contrast may suggest evidence of obstruction between the duodenum and jejunum, but this may be unclear. Although rare, clinical suspicion of paraduodenal hernia is necessary to prevent ensuing complications and mortality.
Case Presentation
A 43-year-old man presented to the emergency department with symptoms that included nausea, vomiting, intermittent epigastric abdominal pain, and obstipation, which were suggestive of a small bowel obstruction. The patient reported similar intermittent episodes over the past 10 years that had resolved without surgery. The patient had no history of abdominal surgeries. A nasogastric tube was inserted and immediately drew out a significant amount of bilious contents. A CT scan indicated an obstruction at the proximal jejunum with suspicion of an internal hernia.
The patient underwent exploratory laparotomy soon after, which confirmed a right paraduodenal hernia (Figure). The surgery began laproscopically by retracting the omentum and transverse colon cranially to expose the ligament of Treitz. The hernia defect was identified on the mesentery where the proximal jejunum twisted on itself in a loop. The hernia was untwisted, and adhesions were removed. The posterior attachment of the hernia sac was freed with harmonic cautery and blunt dissection along with its attachment to the ligament of Treitz. In the process of freeing the herniation, a 1-cm enterotomy ensued, which did not contain succus or spillage of luminal contents at that time. Due to difficulties in visualizing the remainder of the small bowel, the procedure was converted to a laparotomy. This allowed complete freeing of the twisted loop of bowel.
Afterward, there was succus and bile draining from the enterotomy, so it was closed transversely in 2 layers, making sure there was a lumen between the layers. The first and second parts of the duodenum were examined followed by palpitation of the duodenal sweep. The remainder of the small bowel was visualized to the cecum, and the retroperitoneal space was dissected out of the hernia sac space. The abdomen was irrigated, and the omentum was draped back over the intestines. The fascia was closed followed by skin reapproximation with staples. The patient experienced an uneventful recovery and was discharged on day 6 with resolution of his symptoms.
Discussion
Paraduodenal hernias are a type of internal hernia and a rare cause of intestinal obstruction accounting for about 0.5% of all hernias. Right paraduodenal hernias are far less common than left paraduodenal hernias and occur due to a defect in the jejunum mesentery called Waldeyer fossae.4 This is located at the third part of the duodenum and behind the superior mesenteric artery.4 Symptoms of paraduodenal hernias are nonspecific and may include nausea, vomiting, and intermittent cramping. Symptoms of obstruction can be intermittent due to the small bowel herniating through the fossae and then retracting.1 Computed tomography has good specificity and aides in the diagnosis of an internal hernia, but physicians must have a high index of suspicion as well.5
Definitive diagnosis and treatment of paraduodenal hernias involves laparoscopy or exploratory laparotomy to visualize the internal hernia and its surrounding sac.4,5 All hernias should be repaired to prevent strangulation of the bowel, but internal hernias are even more important to fix because these hernias may not present until there is severe injury to the bowel.5 On identification of the paraduodenal hernia, it is important to release the bowel from the hernia sac, free up adhesions, and place small bowel segments back into the correct anatomical position.4,5
In the event of bowel injury, resection with reanastomosis is indicated. Careful dissection is important to prevent injury to the superior mesenteric artery, which supplies most of the small bowel and ascending colon.4,5 Injury to the superior mesenteric artery could lead to ischemia and gangrenous bowel.2 Immediate detection and early surgery intervention of these congenital hernias can prevent such complications.2 The literature includes reports of paraduodenal hernias with complications of gangrenous bowel that required small bowel resection.2 These complications further emphasize the need to proceed immediately with surgery if a paraduodenal hernia is suspected.
Conclusion
This rare cause of bowel obstruction was documented in order to emphasize the importance of having a high clinical suspicion for a paraduodenal hernia. This particular patient with no history of abdominal surgeries had previously dealt with bowel obstruction and would likely have this complication again without surgical intervention. Patients with paraduodenal hernias also are at risk for bowel ischemia, other high-risk complications, and even death.5 Although a CT scan provided information about an approximate location of the obstruction, laparoscopy confirmed the diagnosis. Going into the operation with paraduodenal hernia in the differential allowed the surgeon to be prepared for the appropriate anatomy involved with this procedure to minimize damage to important structures, such as the superior mesenteric artery and its branches.
Paraduodenal hernia, also called mesocolic hernia, is a type of internal hernia that is thought to be caused by a congenital defect involving abnormal retroperitoneal fixation of the mesentery due to abnormal rotation of the midgut.1 Internal hernias account for only 1% of all hernias, and paraduodenal hernias make up 50% of those.2
Paraduodenal hernias can be classified as left or right with left being far more common than right, 75% and 25%, respectively.2 Due to the fixation abnormalities in the midgut, fossae are formed that help to classify left vs right paraduodenal hernias. Herniation through Landzert fossae results in a left paraduodenal hernia with the primary constituents of the hernia sac being the inferior mesenteric artery and vein.1 This result is due to an in utero defect of the small intestine herniated between the inferior mesenteric vein and posterior parietal attachments of the descending mesocolon to the retroperitoneal.3
In a right paraduodenal hernia, herniation occurs through Waldeyer fossae with the main contents of the hernia sac being the iliocolic, right colic, and middle colic vessels within the anterior wall and the superior mesenteric artery along the medial border of the hernia.1 Since there is a failure of rotation around the superior mesenteric artery, the majority of the small intestine remains to the right of the superior mesenteric artery, resulting in the small intestine being trapped between the posteriolateral peritoneum.3 Regardless of the type of paraduodenal hernia, patients usually will present with symptoms of small bowel obstruction. In these types of hernias, a computed tomography (CT) scan with IV contrast may suggest evidence of obstruction between the duodenum and jejunum, but this may be unclear. Although rare, clinical suspicion of paraduodenal hernia is necessary to prevent ensuing complications and mortality.
Case Presentation
A 43-year-old man presented to the emergency department with symptoms that included nausea, vomiting, intermittent epigastric abdominal pain, and obstipation, which were suggestive of a small bowel obstruction. The patient reported similar intermittent episodes over the past 10 years that had resolved without surgery. The patient had no history of abdominal surgeries. A nasogastric tube was inserted and immediately drew out a significant amount of bilious contents. A CT scan indicated an obstruction at the proximal jejunum with suspicion of an internal hernia.
The patient underwent exploratory laparotomy soon after, which confirmed a right paraduodenal hernia (Figure). The surgery began laproscopically by retracting the omentum and transverse colon cranially to expose the ligament of Treitz. The hernia defect was identified on the mesentery where the proximal jejunum twisted on itself in a loop. The hernia was untwisted, and adhesions were removed. The posterior attachment of the hernia sac was freed with harmonic cautery and blunt dissection along with its attachment to the ligament of Treitz. In the process of freeing the herniation, a 1-cm enterotomy ensued, which did not contain succus or spillage of luminal contents at that time. Due to difficulties in visualizing the remainder of the small bowel, the procedure was converted to a laparotomy. This allowed complete freeing of the twisted loop of bowel.
Afterward, there was succus and bile draining from the enterotomy, so it was closed transversely in 2 layers, making sure there was a lumen between the layers. The first and second parts of the duodenum were examined followed by palpitation of the duodenal sweep. The remainder of the small bowel was visualized to the cecum, and the retroperitoneal space was dissected out of the hernia sac space. The abdomen was irrigated, and the omentum was draped back over the intestines. The fascia was closed followed by skin reapproximation with staples. The patient experienced an uneventful recovery and was discharged on day 6 with resolution of his symptoms.
Discussion
Paraduodenal hernias are a type of internal hernia and a rare cause of intestinal obstruction accounting for about 0.5% of all hernias. Right paraduodenal hernias are far less common than left paraduodenal hernias and occur due to a defect in the jejunum mesentery called Waldeyer fossae.4 This is located at the third part of the duodenum and behind the superior mesenteric artery.4 Symptoms of paraduodenal hernias are nonspecific and may include nausea, vomiting, and intermittent cramping. Symptoms of obstruction can be intermittent due to the small bowel herniating through the fossae and then retracting.1 Computed tomography has good specificity and aides in the diagnosis of an internal hernia, but physicians must have a high index of suspicion as well.5
Definitive diagnosis and treatment of paraduodenal hernias involves laparoscopy or exploratory laparotomy to visualize the internal hernia and its surrounding sac.4,5 All hernias should be repaired to prevent strangulation of the bowel, but internal hernias are even more important to fix because these hernias may not present until there is severe injury to the bowel.5 On identification of the paraduodenal hernia, it is important to release the bowel from the hernia sac, free up adhesions, and place small bowel segments back into the correct anatomical position.4,5
In the event of bowel injury, resection with reanastomosis is indicated. Careful dissection is important to prevent injury to the superior mesenteric artery, which supplies most of the small bowel and ascending colon.4,5 Injury to the superior mesenteric artery could lead to ischemia and gangrenous bowel.2 Immediate detection and early surgery intervention of these congenital hernias can prevent such complications.2 The literature includes reports of paraduodenal hernias with complications of gangrenous bowel that required small bowel resection.2 These complications further emphasize the need to proceed immediately with surgery if a paraduodenal hernia is suspected.
Conclusion
This rare cause of bowel obstruction was documented in order to emphasize the importance of having a high clinical suspicion for a paraduodenal hernia. This particular patient with no history of abdominal surgeries had previously dealt with bowel obstruction and would likely have this complication again without surgical intervention. Patients with paraduodenal hernias also are at risk for bowel ischemia, other high-risk complications, and even death.5 Although a CT scan provided information about an approximate location of the obstruction, laparoscopy confirmed the diagnosis. Going into the operation with paraduodenal hernia in the differential allowed the surgeon to be prepared for the appropriate anatomy involved with this procedure to minimize damage to important structures, such as the superior mesenteric artery and its branches.
1. Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Saunders; 2012.
2. Fukada T, Mukai H, Shimamura F, Furukawa T, Miyazaki M. A causal relationship between right paraduodenal hernia and superior mesenteric artery syndrome: a case report. J Med Case Rep. 2010;4:159.
3. Skandalakis JE. Peritoneum, omenta, and internal hernias. In: Skandalakis JE, Colborn GL, eds. Skandalakis Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. 1st ed. Athens, Greece: Paschalidis Medical Publications; 2004:chap 10.
4. Papaziogas B, Souparis A, Makris J, Alexandrakis A, Papaziogas T. Surgical images: soft tissue. Right paraduodenal hernia. Can J Surg. 2004;47(3):195-196.
5. Manfredelli S, Andrea Z, Stefano P, et al. Rare small bowel obstruction: right paraduodenal hernia. Case report. Int J Surg Case Rep. 2013;4(4):412-415.
1. Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Saunders; 2012.
2. Fukada T, Mukai H, Shimamura F, Furukawa T, Miyazaki M. A causal relationship between right paraduodenal hernia and superior mesenteric artery syndrome: a case report. J Med Case Rep. 2010;4:159.
3. Skandalakis JE. Peritoneum, omenta, and internal hernias. In: Skandalakis JE, Colborn GL, eds. Skandalakis Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. 1st ed. Athens, Greece: Paschalidis Medical Publications; 2004:chap 10.
4. Papaziogas B, Souparis A, Makris J, Alexandrakis A, Papaziogas T. Surgical images: soft tissue. Right paraduodenal hernia. Can J Surg. 2004;47(3):195-196.
5. Manfredelli S, Andrea Z, Stefano P, et al. Rare small bowel obstruction: right paraduodenal hernia. Case report. Int J Surg Case Rep. 2013;4(4):412-415.
New telehealth legislation would provide for testing, expansion
A bipartisan bill introduced in the U.S. Senate in late March 2017 would authorize the Center for Medicare & Medicaid Innovation (CMMI) to test expanded telehealth services provided to Medicare beneficiaries.
The Telehealth Innovation and Improvement Act (S.787), currently in the Senate Finance Committee, was introduced by Sen. Gary Peters (D-Mich.) and Sen. Cory Gardner (R-Colo.). A similar bill they introduced in 2015 was never enacted.
However, there are physicians hoping to see this bill or others like it granted consideration. Currently, the Centers for Medicare & Medicaid Services reimburses only for certain telemedicine services provided in rural or underserved geographic areas, but the new bill would apply in suburban and urban areas as well, based on pilot testing of models and evaluating them for cost, quality, and effectiveness. Successful models would be covered by Medicare.
“Medicare has made some provisions for specific rural sites and niche areas, but writ large, there’s no prescribed way for people to just open a telemedicine shop and begin to bill,” said Bradley Flansbaum, DO, MPH, MHM, a member of the SHM Public Policy Committee.
With the exception of telestroke and critical care, “evidence is needed for the type of setting and type of clinical problems addressed by telemedicine. It’s not been tested enough,” added Dr. Flansbaum, who holds a dual appointment in hospital medicine and population health at Geisinger Medical Center in Danville, Penn. “How does it work for routine inpatient problems and how do hospitalists use it? We haven’t seen data there and that’s where a pilot comes in.”
Dr. Mac believes it inconsistent that, in many circumstances, physicians providing services via telemedicine technology are not reimbursed by Medicare and other payers.
“The expansion and ability to provide care in more unique ways – more specialties and in more environments – has expanded more quickly than the systems of reimbursement for professional fees have and it really is a bit of a hodgepodge now,” he said. “We certainly are pleased that this is getting attention and that we have leaders pushing for this in Congress. We don’t know for sure how the final legislation (on this bill) may look but hopefully there will be some form of this that will come to fruition.”
Whether telemedicine can reduce costs while improving outcomes, or improve outcomes without increasing costs, remains unsettled. A study published in Health Affairs in March 2017 indicates that while telehealth can improve access to care, it results in greater utilization, thereby increasing costs.1
The study relied on claims data for more than 300,000 patients in the California Public Employees’ Retirement System during 2011-2013. It looked at utilization of direct-to-consumer telehealth and spending for acute respiratory illness, one of the most common reasons patients seek telehealth services. While, per episode, telehealth visits cost 50% less than did an outpatient visit and less than 5% of an emergency department visit, annual spending per individual for acute respiratory illness went up $45 because, as the authors estimated, 88% of direct-to-consumer telehealth visits represented new utilization.
Whether this would be the case for hospitalist patients remains to be tested.
“It gets back to whether or not you’re adding a necessary service or substituting a less expensive one for a more expensive one,” said Dr. Flansbaum. “Are physicians providing a needed service or adding unnecessary visits to the system?”
Jayne Lee, MD, has been a hospitalist with Eagle for nearly a decade. Before making the transition from an in-hospital physician to one treating patients from behind a robot – with assistance at the point of service from a nurse – she was working 10 shifts in a row at her home in the United States before traveling to her home in Paris. Dr. Mac offered her the opportunity to practice full time as a telehospitalist from overseas. Today, she is also the company’s chief medical officer and estimates she’s had more than 7,000 patient encounters using telemedicine technology.
Dr. Lee is licensed in multiple states – a barrier that plagues many would-be telehealth providers, but which Eagle has solved with its licensing and credentialing staff – and because she is often providing services at night to urban and rural areas, she sees a broad range of patients.
“We see things from coronary artery disease, COPD [chronic obstructive pulmonary disease] exacerbations, and diabetes-related conditions to drug overdoses and alcohol abuse,” she said. “I enjoy seeing the variety of patients I encounter every night.”
Dr. Lee has to navigate each health system’s electronic medical records and triage systems but, she says, patient care has remained the same. And she’s providing services for hospitals that may not have another hospitalist to assign.
“Our practices keep growing, a sign that hospitals are needing our services now more than ever, given that there is a physician shortage and given the financial constraints we’re seeing in the healthcare system.” she said.
References
1. Ashwood JS, Mehrota A, Cowling D, et al. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Affairs. 2017; 36(3):485-491. doi: 10.1377/hlthaff.2016.1130.
A bipartisan bill introduced in the U.S. Senate in late March 2017 would authorize the Center for Medicare & Medicaid Innovation (CMMI) to test expanded telehealth services provided to Medicare beneficiaries.
The Telehealth Innovation and Improvement Act (S.787), currently in the Senate Finance Committee, was introduced by Sen. Gary Peters (D-Mich.) and Sen. Cory Gardner (R-Colo.). A similar bill they introduced in 2015 was never enacted.
However, there are physicians hoping to see this bill or others like it granted consideration. Currently, the Centers for Medicare & Medicaid Services reimburses only for certain telemedicine services provided in rural or underserved geographic areas, but the new bill would apply in suburban and urban areas as well, based on pilot testing of models and evaluating them for cost, quality, and effectiveness. Successful models would be covered by Medicare.
“Medicare has made some provisions for specific rural sites and niche areas, but writ large, there’s no prescribed way for people to just open a telemedicine shop and begin to bill,” said Bradley Flansbaum, DO, MPH, MHM, a member of the SHM Public Policy Committee.
With the exception of telestroke and critical care, “evidence is needed for the type of setting and type of clinical problems addressed by telemedicine. It’s not been tested enough,” added Dr. Flansbaum, who holds a dual appointment in hospital medicine and population health at Geisinger Medical Center in Danville, Penn. “How does it work for routine inpatient problems and how do hospitalists use it? We haven’t seen data there and that’s where a pilot comes in.”
Dr. Mac believes it inconsistent that, in many circumstances, physicians providing services via telemedicine technology are not reimbursed by Medicare and other payers.
“The expansion and ability to provide care in more unique ways – more specialties and in more environments – has expanded more quickly than the systems of reimbursement for professional fees have and it really is a bit of a hodgepodge now,” he said. “We certainly are pleased that this is getting attention and that we have leaders pushing for this in Congress. We don’t know for sure how the final legislation (on this bill) may look but hopefully there will be some form of this that will come to fruition.”
Whether telemedicine can reduce costs while improving outcomes, or improve outcomes without increasing costs, remains unsettled. A study published in Health Affairs in March 2017 indicates that while telehealth can improve access to care, it results in greater utilization, thereby increasing costs.1
The study relied on claims data for more than 300,000 patients in the California Public Employees’ Retirement System during 2011-2013. It looked at utilization of direct-to-consumer telehealth and spending for acute respiratory illness, one of the most common reasons patients seek telehealth services. While, per episode, telehealth visits cost 50% less than did an outpatient visit and less than 5% of an emergency department visit, annual spending per individual for acute respiratory illness went up $45 because, as the authors estimated, 88% of direct-to-consumer telehealth visits represented new utilization.
Whether this would be the case for hospitalist patients remains to be tested.
“It gets back to whether or not you’re adding a necessary service or substituting a less expensive one for a more expensive one,” said Dr. Flansbaum. “Are physicians providing a needed service or adding unnecessary visits to the system?”
Jayne Lee, MD, has been a hospitalist with Eagle for nearly a decade. Before making the transition from an in-hospital physician to one treating patients from behind a robot – with assistance at the point of service from a nurse – she was working 10 shifts in a row at her home in the United States before traveling to her home in Paris. Dr. Mac offered her the opportunity to practice full time as a telehospitalist from overseas. Today, she is also the company’s chief medical officer and estimates she’s had more than 7,000 patient encounters using telemedicine technology.
Dr. Lee is licensed in multiple states – a barrier that plagues many would-be telehealth providers, but which Eagle has solved with its licensing and credentialing staff – and because she is often providing services at night to urban and rural areas, she sees a broad range of patients.
“We see things from coronary artery disease, COPD [chronic obstructive pulmonary disease] exacerbations, and diabetes-related conditions to drug overdoses and alcohol abuse,” she said. “I enjoy seeing the variety of patients I encounter every night.”
Dr. Lee has to navigate each health system’s electronic medical records and triage systems but, she says, patient care has remained the same. And she’s providing services for hospitals that may not have another hospitalist to assign.
“Our practices keep growing, a sign that hospitals are needing our services now more than ever, given that there is a physician shortage and given the financial constraints we’re seeing in the healthcare system.” she said.
References
1. Ashwood JS, Mehrota A, Cowling D, et al. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Affairs. 2017; 36(3):485-491. doi: 10.1377/hlthaff.2016.1130.
A bipartisan bill introduced in the U.S. Senate in late March 2017 would authorize the Center for Medicare & Medicaid Innovation (CMMI) to test expanded telehealth services provided to Medicare beneficiaries.
The Telehealth Innovation and Improvement Act (S.787), currently in the Senate Finance Committee, was introduced by Sen. Gary Peters (D-Mich.) and Sen. Cory Gardner (R-Colo.). A similar bill they introduced in 2015 was never enacted.
However, there are physicians hoping to see this bill or others like it granted consideration. Currently, the Centers for Medicare & Medicaid Services reimburses only for certain telemedicine services provided in rural or underserved geographic areas, but the new bill would apply in suburban and urban areas as well, based on pilot testing of models and evaluating them for cost, quality, and effectiveness. Successful models would be covered by Medicare.
“Medicare has made some provisions for specific rural sites and niche areas, but writ large, there’s no prescribed way for people to just open a telemedicine shop and begin to bill,” said Bradley Flansbaum, DO, MPH, MHM, a member of the SHM Public Policy Committee.
With the exception of telestroke and critical care, “evidence is needed for the type of setting and type of clinical problems addressed by telemedicine. It’s not been tested enough,” added Dr. Flansbaum, who holds a dual appointment in hospital medicine and population health at Geisinger Medical Center in Danville, Penn. “How does it work for routine inpatient problems and how do hospitalists use it? We haven’t seen data there and that’s where a pilot comes in.”
Dr. Mac believes it inconsistent that, in many circumstances, physicians providing services via telemedicine technology are not reimbursed by Medicare and other payers.
“The expansion and ability to provide care in more unique ways – more specialties and in more environments – has expanded more quickly than the systems of reimbursement for professional fees have and it really is a bit of a hodgepodge now,” he said. “We certainly are pleased that this is getting attention and that we have leaders pushing for this in Congress. We don’t know for sure how the final legislation (on this bill) may look but hopefully there will be some form of this that will come to fruition.”
Whether telemedicine can reduce costs while improving outcomes, or improve outcomes without increasing costs, remains unsettled. A study published in Health Affairs in March 2017 indicates that while telehealth can improve access to care, it results in greater utilization, thereby increasing costs.1
The study relied on claims data for more than 300,000 patients in the California Public Employees’ Retirement System during 2011-2013. It looked at utilization of direct-to-consumer telehealth and spending for acute respiratory illness, one of the most common reasons patients seek telehealth services. While, per episode, telehealth visits cost 50% less than did an outpatient visit and less than 5% of an emergency department visit, annual spending per individual for acute respiratory illness went up $45 because, as the authors estimated, 88% of direct-to-consumer telehealth visits represented new utilization.
Whether this would be the case for hospitalist patients remains to be tested.
“It gets back to whether or not you’re adding a necessary service or substituting a less expensive one for a more expensive one,” said Dr. Flansbaum. “Are physicians providing a needed service or adding unnecessary visits to the system?”
Jayne Lee, MD, has been a hospitalist with Eagle for nearly a decade. Before making the transition from an in-hospital physician to one treating patients from behind a robot – with assistance at the point of service from a nurse – she was working 10 shifts in a row at her home in the United States before traveling to her home in Paris. Dr. Mac offered her the opportunity to practice full time as a telehospitalist from overseas. Today, she is also the company’s chief medical officer and estimates she’s had more than 7,000 patient encounters using telemedicine technology.
Dr. Lee is licensed in multiple states – a barrier that plagues many would-be telehealth providers, but which Eagle has solved with its licensing and credentialing staff – and because she is often providing services at night to urban and rural areas, she sees a broad range of patients.
“We see things from coronary artery disease, COPD [chronic obstructive pulmonary disease] exacerbations, and diabetes-related conditions to drug overdoses and alcohol abuse,” she said. “I enjoy seeing the variety of patients I encounter every night.”
Dr. Lee has to navigate each health system’s electronic medical records and triage systems but, she says, patient care has remained the same. And she’s providing services for hospitals that may not have another hospitalist to assign.
“Our practices keep growing, a sign that hospitals are needing our services now more than ever, given that there is a physician shortage and given the financial constraints we’re seeing in the healthcare system.” she said.
References
1. Ashwood JS, Mehrota A, Cowling D, et al. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Affairs. 2017; 36(3):485-491. doi: 10.1377/hlthaff.2016.1130.
Some Transgender Women Are Reluctant to Combine Antiretroviral and Hormone Therapy
Transgender women are at high risk for HIV, but they are understandably concerned about taking both antiretroviral therapy (ART) drugs and feminizing hormone therapy (HT). In a survey of 87 transgender women at a community-based AIDS service organization in Los Angeles, more than half of those living with HIV were worried about that, and many cited their concerns as a reason for not taking anti-HIV medications, HT, or both, say researchers from National Institutes of Health (NIH) and Gilead Sciences.
Of the study participants, 69% were on some type of HT (including 25% who reported using HT without supervision from a qualified health professional). Transgender women living with HIV were more likely to use HT without supervision: 34% compared with 13% of those without HIV.
However, while 57% of the women living with HIV were concerned about drug interactions between ART and HT, only 49% of the participants had discussed the possibility with their health care team.
There is no scientific consensus on the safety and effectiveness of any combination of ART and HT in transgender women living with HIV, NIH says. Certain forms of ART and components of hormonal contraceptives interact, but because the drugs used in HT are at different dosages than in contraception, dose modifications or drug substitutions can reduce the risk of interactions.
The concerns also pose a problem for research, because transgender women may be reluctant to join clinical trials combining HT and ART. “Despite all indications that transgender women are a critical population in HIV care, very little is known about how to optimize coadministration of ART and hormonal therapies in this population,” said Jordan Lake, MD, study leader, who is continuing the research at the University of Texas Health Sciences Center at Houston.
“Making sure we are meeting the needs of transgender women living with HIV is key to addressing this pandemic,” said Judith Currier, MD, co-investigator and vice chair of the NIAID-supported AIDS Clinical Trials Network. “We need to provide an evidence-based response to these understandable concerns so that this key population and their sexual partners may reap the full benefits of effective HIV care.”
Source:
Drug interaction concerns may negatively affect HIV treatment adherence among transgender women. https://www.nih.gov/news-events/news-releases/drug-interaction-concerns-may-negatively-affect-hiv-treatment-adherence-among-transgender-women. Published July 24, 2017. Accessed August 10, 2017.
Transgender women are at high risk for HIV, but they are understandably concerned about taking both antiretroviral therapy (ART) drugs and feminizing hormone therapy (HT). In a survey of 87 transgender women at a community-based AIDS service organization in Los Angeles, more than half of those living with HIV were worried about that, and many cited their concerns as a reason for not taking anti-HIV medications, HT, or both, say researchers from National Institutes of Health (NIH) and Gilead Sciences.
Of the study participants, 69% were on some type of HT (including 25% who reported using HT without supervision from a qualified health professional). Transgender women living with HIV were more likely to use HT without supervision: 34% compared with 13% of those without HIV.
However, while 57% of the women living with HIV were concerned about drug interactions between ART and HT, only 49% of the participants had discussed the possibility with their health care team.
There is no scientific consensus on the safety and effectiveness of any combination of ART and HT in transgender women living with HIV, NIH says. Certain forms of ART and components of hormonal contraceptives interact, but because the drugs used in HT are at different dosages than in contraception, dose modifications or drug substitutions can reduce the risk of interactions.
The concerns also pose a problem for research, because transgender women may be reluctant to join clinical trials combining HT and ART. “Despite all indications that transgender women are a critical population in HIV care, very little is known about how to optimize coadministration of ART and hormonal therapies in this population,” said Jordan Lake, MD, study leader, who is continuing the research at the University of Texas Health Sciences Center at Houston.
“Making sure we are meeting the needs of transgender women living with HIV is key to addressing this pandemic,” said Judith Currier, MD, co-investigator and vice chair of the NIAID-supported AIDS Clinical Trials Network. “We need to provide an evidence-based response to these understandable concerns so that this key population and their sexual partners may reap the full benefits of effective HIV care.”
Source:
Drug interaction concerns may negatively affect HIV treatment adherence among transgender women. https://www.nih.gov/news-events/news-releases/drug-interaction-concerns-may-negatively-affect-hiv-treatment-adherence-among-transgender-women. Published July 24, 2017. Accessed August 10, 2017.
Transgender women are at high risk for HIV, but they are understandably concerned about taking both antiretroviral therapy (ART) drugs and feminizing hormone therapy (HT). In a survey of 87 transgender women at a community-based AIDS service organization in Los Angeles, more than half of those living with HIV were worried about that, and many cited their concerns as a reason for not taking anti-HIV medications, HT, or both, say researchers from National Institutes of Health (NIH) and Gilead Sciences.
Of the study participants, 69% were on some type of HT (including 25% who reported using HT without supervision from a qualified health professional). Transgender women living with HIV were more likely to use HT without supervision: 34% compared with 13% of those without HIV.
However, while 57% of the women living with HIV were concerned about drug interactions between ART and HT, only 49% of the participants had discussed the possibility with their health care team.
There is no scientific consensus on the safety and effectiveness of any combination of ART and HT in transgender women living with HIV, NIH says. Certain forms of ART and components of hormonal contraceptives interact, but because the drugs used in HT are at different dosages than in contraception, dose modifications or drug substitutions can reduce the risk of interactions.
The concerns also pose a problem for research, because transgender women may be reluctant to join clinical trials combining HT and ART. “Despite all indications that transgender women are a critical population in HIV care, very little is known about how to optimize coadministration of ART and hormonal therapies in this population,” said Jordan Lake, MD, study leader, who is continuing the research at the University of Texas Health Sciences Center at Houston.
“Making sure we are meeting the needs of transgender women living with HIV is key to addressing this pandemic,” said Judith Currier, MD, co-investigator and vice chair of the NIAID-supported AIDS Clinical Trials Network. “We need to provide an evidence-based response to these understandable concerns so that this key population and their sexual partners may reap the full benefits of effective HIV care.”
Source:
Drug interaction concerns may negatively affect HIV treatment adherence among transgender women. https://www.nih.gov/news-events/news-releases/drug-interaction-concerns-may-negatively-affect-hiv-treatment-adherence-among-transgender-women. Published July 24, 2017. Accessed August 10, 2017.
Tests can produce confusing results after HSCT in MM
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Insured cancer patients report ‘overwhelming’ financial distress
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.” ![]()
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.” ![]()
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.” ![]()
Methotrexate could treat myeloproliferative neoplasms
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said. ![]()
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said. ![]()
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said. ![]()
Unexplained leukocytosis in a hospitalized patient
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
Tips for avoiding potentially dangerous patients
WASHINGTON – Clinicians who treat patients with emotional and psychiatric problems must put risk management interventions in place for their safety, Jeffrey N. Younggren, PhD, said at the annual convention of the American Psychological Association.
“Many times, people lose sight of the nature of their therapeutic relationship,” said Dr. Younggren, professor of psychology at the University of Missouri in Columbia. To stay safe, clinicians must overreact, he said, just as they do with suicide risk assessments.
Dr. Younggren critiqued an American Psychological Association article on safety and offered his own recommendations. Among them:
- Think about evacuation strategies. “Don’t get between that individual and the door,” said Dr. Younggren, a clinical and forensic psychologist.
- Refuse to see patients who are inebriated or intoxicated. If such a patient shows up for an appointment in one of these conditions and refuses to leave, call the police.
- Remove yourself from physical danger. “I’m a very good ‘fall on the ground’ person,” said Dr. Younggren, who said he has been attacked by patients three times in his career. “That’s a risk management strategy.”
- Terminate patients appropriately in the absence of threats. However, “if someone threatens you, write them a letter, and you’re done,” he said.
Dr. Younggren suggested that other recommendations in the article were unrealistic, such as, don’t work alone at night, install security cameras, and learn self-defense techniques. “What does [learn self-defense techniques] mean,” he asked. “My best one is to fall down.”
Mismanagement of the therapeutic alliance can careen out of control, as it did in the case of Ensworth vs. Mullvain.
In that case, decided in 1990, Heather Ensworth, PhD, a psychologist who practiced in California, treated a patient named Cynthia Mullvain for just short of 2 years and then terminated the treatment. But Ms. Mullvain did not accept the termination and persuaded Dr. Ensworth to see her again “to resolve the termination issues to help [Mullvain] disengage from [Ensworth].”
After several harassing incidents, Dr. Ensworth terminated contact with Ms. Mullvain a second time. At this point, Dr. Ensworth sought and was granted a restraining order against the patient. Despite the restraining order, Ms. Mullvain’s harassing behavior continued. Among other things, she stalked Dr. Ensworth, sent her threatening letters, and started doing community service work at a library located about 150 feet away from Dr. Ensworth’s home, according to Dr. Ensworth’s petition seeking a second restraining order. Ultimately, the court ruled that Ms. Mullvain had “willfully engaged in a course of conduct that seriously alarmed, annoyed, or harassed Ensworth, and that Ensworth actually suffered substantial emotional distress.”
Ernest J. Bordini, PhD said that, beyond private offices, nurses and aides are at greatest risk when it comes to workplace violence. According to a report by the Occupational Health and Safety Administration (OSHA), in 2013, psychiatric aides had the highest rate among health care workers of violent injuries that led to days away from work: 590 per 10,000 full-time employees, compared with 55 such injuries per 10,000 for nursing assistants. The report said the highest risk areas were emergency departments, geriatrics, and behavioral health.
Psychiatric patients are more likely to be the victims of violence than perpetrators, but Dr. Bordini, a neuropsychologist with expertise in forensic assessment, said in an interview that he wanted to add a point.
“It is important to dismiss the notion that all psychiatric patients do not have elevated risks of assault,” he said. “Those who present with psychoses or bipolar disorder can have elevated risk, especially if they develop delusional thoughts or obsessions about the therapist or another individual. Paranoid individuals already feel threatened, and hence can strike out in anticipation.”
He said he and his colleagues are not advocating that all clinicians train in self defense or arm themselves. However, it is essential to be proactive. Falling down can work for some, Dr. Bordini said, but “experience teaches us that playing possum does not always cease an attack. I recommend de-escalation, escape, and/or self-defense plans that one has practiced, feels comfortable with, and feels confident that they can execute under stress.”
At the meeting, he said some patients are able to sense fear from the clinician. “If you’re skittish, [this will] put you at higher risk,” said Dr. Bordini, executive director of Clinical Psychology Associates of North Central Florida in Gainesville. “That sense of intuition is something you should tend to,” he said, citing The Gift of Fear (New York: Dell, 1999) by Gavin de Becker as an example of a book that explores recognizing and reacting to subtle signs of danger. “If you’re not comfortable seeing a patient, listen to that.”
Neither Dr. Younggren nor Dr. Bordini had financial disclosures.
To access OSHA’s guidelines for workplace violence in health care settings, visit https://www.osha.gov/Publications/OSHA3826.pdf. The American Medical Association’s latest policy on workplace violence can be found at https://www.ama-assn.org/ama-adopts-new-public-health-policies-improve-health-nation.
[email protected]
On Twitter @ginalhenderson
WASHINGTON – Clinicians who treat patients with emotional and psychiatric problems must put risk management interventions in place for their safety, Jeffrey N. Younggren, PhD, said at the annual convention of the American Psychological Association.
“Many times, people lose sight of the nature of their therapeutic relationship,” said Dr. Younggren, professor of psychology at the University of Missouri in Columbia. To stay safe, clinicians must overreact, he said, just as they do with suicide risk assessments.
Dr. Younggren critiqued an American Psychological Association article on safety and offered his own recommendations. Among them:
- Think about evacuation strategies. “Don’t get between that individual and the door,” said Dr. Younggren, a clinical and forensic psychologist.
- Refuse to see patients who are inebriated or intoxicated. If such a patient shows up for an appointment in one of these conditions and refuses to leave, call the police.
- Remove yourself from physical danger. “I’m a very good ‘fall on the ground’ person,” said Dr. Younggren, who said he has been attacked by patients three times in his career. “That’s a risk management strategy.”
- Terminate patients appropriately in the absence of threats. However, “if someone threatens you, write them a letter, and you’re done,” he said.
Dr. Younggren suggested that other recommendations in the article were unrealistic, such as, don’t work alone at night, install security cameras, and learn self-defense techniques. “What does [learn self-defense techniques] mean,” he asked. “My best one is to fall down.”
Mismanagement of the therapeutic alliance can careen out of control, as it did in the case of Ensworth vs. Mullvain.
In that case, decided in 1990, Heather Ensworth, PhD, a psychologist who practiced in California, treated a patient named Cynthia Mullvain for just short of 2 years and then terminated the treatment. But Ms. Mullvain did not accept the termination and persuaded Dr. Ensworth to see her again “to resolve the termination issues to help [Mullvain] disengage from [Ensworth].”
After several harassing incidents, Dr. Ensworth terminated contact with Ms. Mullvain a second time. At this point, Dr. Ensworth sought and was granted a restraining order against the patient. Despite the restraining order, Ms. Mullvain’s harassing behavior continued. Among other things, she stalked Dr. Ensworth, sent her threatening letters, and started doing community service work at a library located about 150 feet away from Dr. Ensworth’s home, according to Dr. Ensworth’s petition seeking a second restraining order. Ultimately, the court ruled that Ms. Mullvain had “willfully engaged in a course of conduct that seriously alarmed, annoyed, or harassed Ensworth, and that Ensworth actually suffered substantial emotional distress.”
Ernest J. Bordini, PhD said that, beyond private offices, nurses and aides are at greatest risk when it comes to workplace violence. According to a report by the Occupational Health and Safety Administration (OSHA), in 2013, psychiatric aides had the highest rate among health care workers of violent injuries that led to days away from work: 590 per 10,000 full-time employees, compared with 55 such injuries per 10,000 for nursing assistants. The report said the highest risk areas were emergency departments, geriatrics, and behavioral health.
Psychiatric patients are more likely to be the victims of violence than perpetrators, but Dr. Bordini, a neuropsychologist with expertise in forensic assessment, said in an interview that he wanted to add a point.
“It is important to dismiss the notion that all psychiatric patients do not have elevated risks of assault,” he said. “Those who present with psychoses or bipolar disorder can have elevated risk, especially if they develop delusional thoughts or obsessions about the therapist or another individual. Paranoid individuals already feel threatened, and hence can strike out in anticipation.”
He said he and his colleagues are not advocating that all clinicians train in self defense or arm themselves. However, it is essential to be proactive. Falling down can work for some, Dr. Bordini said, but “experience teaches us that playing possum does not always cease an attack. I recommend de-escalation, escape, and/or self-defense plans that one has practiced, feels comfortable with, and feels confident that they can execute under stress.”
At the meeting, he said some patients are able to sense fear from the clinician. “If you’re skittish, [this will] put you at higher risk,” said Dr. Bordini, executive director of Clinical Psychology Associates of North Central Florida in Gainesville. “That sense of intuition is something you should tend to,” he said, citing The Gift of Fear (New York: Dell, 1999) by Gavin de Becker as an example of a book that explores recognizing and reacting to subtle signs of danger. “If you’re not comfortable seeing a patient, listen to that.”
Neither Dr. Younggren nor Dr. Bordini had financial disclosures.
To access OSHA’s guidelines for workplace violence in health care settings, visit https://www.osha.gov/Publications/OSHA3826.pdf. The American Medical Association’s latest policy on workplace violence can be found at https://www.ama-assn.org/ama-adopts-new-public-health-policies-improve-health-nation.
[email protected]
On Twitter @ginalhenderson
WASHINGTON – Clinicians who treat patients with emotional and psychiatric problems must put risk management interventions in place for their safety, Jeffrey N. Younggren, PhD, said at the annual convention of the American Psychological Association.
“Many times, people lose sight of the nature of their therapeutic relationship,” said Dr. Younggren, professor of psychology at the University of Missouri in Columbia. To stay safe, clinicians must overreact, he said, just as they do with suicide risk assessments.
Dr. Younggren critiqued an American Psychological Association article on safety and offered his own recommendations. Among them:
- Think about evacuation strategies. “Don’t get between that individual and the door,” said Dr. Younggren, a clinical and forensic psychologist.
- Refuse to see patients who are inebriated or intoxicated. If such a patient shows up for an appointment in one of these conditions and refuses to leave, call the police.
- Remove yourself from physical danger. “I’m a very good ‘fall on the ground’ person,” said Dr. Younggren, who said he has been attacked by patients three times in his career. “That’s a risk management strategy.”
- Terminate patients appropriately in the absence of threats. However, “if someone threatens you, write them a letter, and you’re done,” he said.
Dr. Younggren suggested that other recommendations in the article were unrealistic, such as, don’t work alone at night, install security cameras, and learn self-defense techniques. “What does [learn self-defense techniques] mean,” he asked. “My best one is to fall down.”
Mismanagement of the therapeutic alliance can careen out of control, as it did in the case of Ensworth vs. Mullvain.
In that case, decided in 1990, Heather Ensworth, PhD, a psychologist who practiced in California, treated a patient named Cynthia Mullvain for just short of 2 years and then terminated the treatment. But Ms. Mullvain did not accept the termination and persuaded Dr. Ensworth to see her again “to resolve the termination issues to help [Mullvain] disengage from [Ensworth].”
After several harassing incidents, Dr. Ensworth terminated contact with Ms. Mullvain a second time. At this point, Dr. Ensworth sought and was granted a restraining order against the patient. Despite the restraining order, Ms. Mullvain’s harassing behavior continued. Among other things, she stalked Dr. Ensworth, sent her threatening letters, and started doing community service work at a library located about 150 feet away from Dr. Ensworth’s home, according to Dr. Ensworth’s petition seeking a second restraining order. Ultimately, the court ruled that Ms. Mullvain had “willfully engaged in a course of conduct that seriously alarmed, annoyed, or harassed Ensworth, and that Ensworth actually suffered substantial emotional distress.”
Ernest J. Bordini, PhD said that, beyond private offices, nurses and aides are at greatest risk when it comes to workplace violence. According to a report by the Occupational Health and Safety Administration (OSHA), in 2013, psychiatric aides had the highest rate among health care workers of violent injuries that led to days away from work: 590 per 10,000 full-time employees, compared with 55 such injuries per 10,000 for nursing assistants. The report said the highest risk areas were emergency departments, geriatrics, and behavioral health.
Psychiatric patients are more likely to be the victims of violence than perpetrators, but Dr. Bordini, a neuropsychologist with expertise in forensic assessment, said in an interview that he wanted to add a point.
“It is important to dismiss the notion that all psychiatric patients do not have elevated risks of assault,” he said. “Those who present with psychoses or bipolar disorder can have elevated risk, especially if they develop delusional thoughts or obsessions about the therapist or another individual. Paranoid individuals already feel threatened, and hence can strike out in anticipation.”
He said he and his colleagues are not advocating that all clinicians train in self defense or arm themselves. However, it is essential to be proactive. Falling down can work for some, Dr. Bordini said, but “experience teaches us that playing possum does not always cease an attack. I recommend de-escalation, escape, and/or self-defense plans that one has practiced, feels comfortable with, and feels confident that they can execute under stress.”
At the meeting, he said some patients are able to sense fear from the clinician. “If you’re skittish, [this will] put you at higher risk,” said Dr. Bordini, executive director of Clinical Psychology Associates of North Central Florida in Gainesville. “That sense of intuition is something you should tend to,” he said, citing The Gift of Fear (New York: Dell, 1999) by Gavin de Becker as an example of a book that explores recognizing and reacting to subtle signs of danger. “If you’re not comfortable seeing a patient, listen to that.”
Neither Dr. Younggren nor Dr. Bordini had financial disclosures.
To access OSHA’s guidelines for workplace violence in health care settings, visit https://www.osha.gov/Publications/OSHA3826.pdf. The American Medical Association’s latest policy on workplace violence can be found at https://www.ama-assn.org/ama-adopts-new-public-health-policies-improve-health-nation.
[email protected]
On Twitter @ginalhenderson
EXPERT ANALYSIS FROM THE 2017 APA CONVENTION
How to counsel women about marijuana in pregnancy
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017