User login
Knee Pain, Heart Strain?
ANSWER
The correct interpretation includes normal sinus rhythm with left ventricular hypertrophy (LVH) and possible left atrial enlargement. Criteria for LVH include high voltages in the limb leads (R wave in lead I and S wave in lead III ≥ 25 mm) or the precordial leads (S wave in V1 and R wave in V6 ≥ 35 mm). Left atrial enlargement and ST-T wave abnormalities are often seen with LVH. The notched P wave in lead II and biphasic P wave in V1 raise suspicion for left atrial involvement. Finally, repolarization of a hypertrophic left ventricle following systole is responsible for the tall T waves seen in leads
ANSWER
The correct interpretation includes normal sinus rhythm with left ventricular hypertrophy (LVH) and possible left atrial enlargement. Criteria for LVH include high voltages in the limb leads (R wave in lead I and S wave in lead III ≥ 25 mm) or the precordial leads (S wave in V1 and R wave in V6 ≥ 35 mm). Left atrial enlargement and ST-T wave abnormalities are often seen with LVH. The notched P wave in lead II and biphasic P wave in V1 raise suspicion for left atrial involvement. Finally, repolarization of a hypertrophic left ventricle following systole is responsible for the tall T waves seen in leads
ANSWER
The correct interpretation includes normal sinus rhythm with left ventricular hypertrophy (LVH) and possible left atrial enlargement. Criteria for LVH include high voltages in the limb leads (R wave in lead I and S wave in lead III ≥ 25 mm) or the precordial leads (S wave in V1 and R wave in V6 ≥ 35 mm). Left atrial enlargement and ST-T wave abnormalities are often seen with LVH. The notched P wave in lead II and biphasic P wave in V1 raise suspicion for left atrial involvement. Finally, repolarization of a hypertrophic left ventricle following systole is responsible for the tall T waves seen in leads
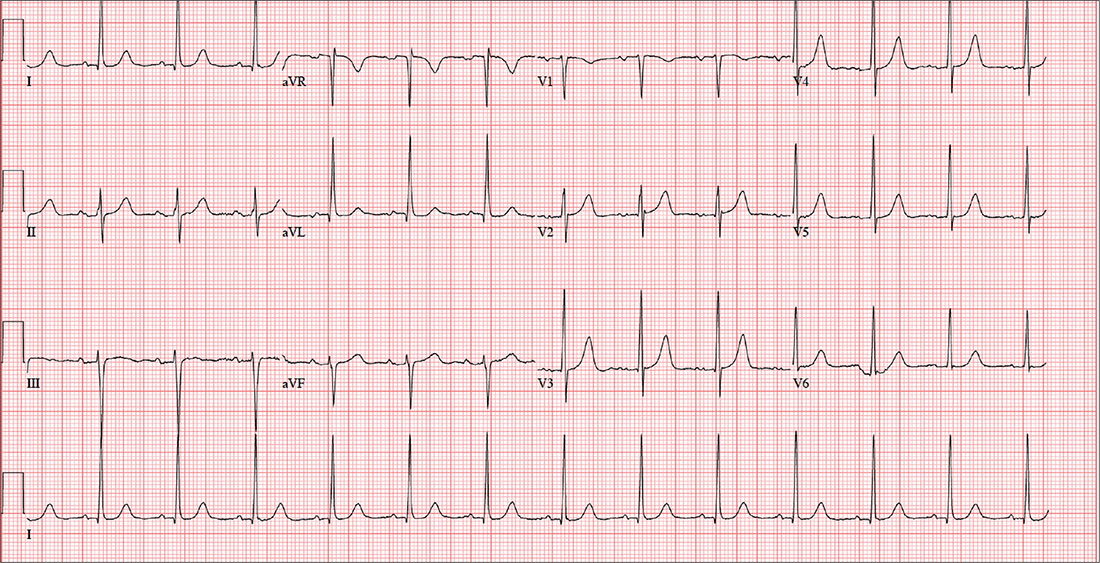
A 47-year-old man presents for preoperative exam prior to right knee arthroplasty. He twisted his knee while training for a triathlon; MRI showed a bucket handle tear of the medial meniscus.
The patient has been very active throughout his life. Medical history is remarkable for essential hypertension. He has no history of chest pain, palpitations, shortness of breath, syncope, or near-syncope.
Current medications include metoprolol XL (25 mg/d)—which he hasn’t taken in five days, since he hasn’t been able to pick up his refill—and ibuprofen (600 mg tid, prn for knee pain). He denies illicit drug use.
The patient works as an accountant and is married with two children. His parents and grandparents are all alive and well. He has never smoked tobacco but does use marijuana socially on weekends. He also has one to two glasses of wine each night.
Review of systems is noncontributory: no recent colds or flu, bowel or bladder dysfunction, or weight changes. Vital signs include a blood pressure of 138/80 mm Hg; pulse, 80 beats/min; respiratory rate, 14 breaths/min-1; and temperature, 98°F. His weight is 194 lb and his height, 75 in. Pertinent physical findings include pain on palpation of the medial aspect of the right knee and a positive McMurray sign.
Bloodwork, a chest x-ray, and an ECG are obtained. The ECG shows a ventricular rate of 79 beats/min; PR interval, 184 ms; QRS duration, 76 ms; QT/QTc intervals, 382/438 ms; P axis, 48°; R axis, –29°; and T axis, 33°. What is your interpretation of this ECG?
New federal health IT leadership, same goals
WASHINGTON – Although the leadership at the Office of the National Coordinator for Health Information Technology is new, the focus of the federal office – reducing physician burden and improving interoperability of electronic heath records – remains the same.
“One priority is on the whole question of burden of [EHR] usability,” said Don Rucker, MD, the new national coordinator, at a July 11 press briefing. “The other is interoperability. We’ve obviously spent a lot of money collectively in the country on these systems, and there’s a widespread dissatisfaction with the level of interoperability.”
“We are looking at documentation and the whole quality framework around value-based purchasing,” he said. “For a lot of practices now, this has become a challenge that we just have to think about what’s the win. At some point, the expense of complying with the quality measures is a much greater expense than the innate value of the quality measures. ”
EHRs “have become symbolic of physician administrative burden, but by no means are they the whole cause,” John Flemming, MD, ONC deputy assistant secretary for health technology reform, said at the briefing. “The physician, particularly in an independent practice, must manage the practice. So he or she is the CEO. They are also on the assembly line, seeing patients. Now with EHRs, they have to be the data input person as well. It’s time consuming.”
Dr. Fleming is a family physician from Louisiana and a former Republican member of congress.
Dr. Rucker acknowledged that reducing the burden of EHRs has been discussed for quite a long time now. He recalled beginning working with them in his private practice back in 1988 and figured, based on the quick rate of technological innovation demonstrated in Silicon Valley, the issues would be solved by 1992 or 1993 at the latest.
“Right now, [EHRs] are really about documentation, about billing, but that is a funny kind of beast,” he said. “Every other industry uses their enterprise computer software to do automation, to become more efficient. We are the only business that I am aware of to have used computers to become less efficient. ... I think part of what we are trying to do ... is let some of these newer technologies that will actually reduce costs, reduce variance, have those technologies have an entrée into some of these data collections that are out there.”
WASHINGTON – Although the leadership at the Office of the National Coordinator for Health Information Technology is new, the focus of the federal office – reducing physician burden and improving interoperability of electronic heath records – remains the same.
“One priority is on the whole question of burden of [EHR] usability,” said Don Rucker, MD, the new national coordinator, at a July 11 press briefing. “The other is interoperability. We’ve obviously spent a lot of money collectively in the country on these systems, and there’s a widespread dissatisfaction with the level of interoperability.”
“We are looking at documentation and the whole quality framework around value-based purchasing,” he said. “For a lot of practices now, this has become a challenge that we just have to think about what’s the win. At some point, the expense of complying with the quality measures is a much greater expense than the innate value of the quality measures. ”
EHRs “have become symbolic of physician administrative burden, but by no means are they the whole cause,” John Flemming, MD, ONC deputy assistant secretary for health technology reform, said at the briefing. “The physician, particularly in an independent practice, must manage the practice. So he or she is the CEO. They are also on the assembly line, seeing patients. Now with EHRs, they have to be the data input person as well. It’s time consuming.”
Dr. Fleming is a family physician from Louisiana and a former Republican member of congress.
Dr. Rucker acknowledged that reducing the burden of EHRs has been discussed for quite a long time now. He recalled beginning working with them in his private practice back in 1988 and figured, based on the quick rate of technological innovation demonstrated in Silicon Valley, the issues would be solved by 1992 or 1993 at the latest.
“Right now, [EHRs] are really about documentation, about billing, but that is a funny kind of beast,” he said. “Every other industry uses their enterprise computer software to do automation, to become more efficient. We are the only business that I am aware of to have used computers to become less efficient. ... I think part of what we are trying to do ... is let some of these newer technologies that will actually reduce costs, reduce variance, have those technologies have an entrée into some of these data collections that are out there.”
WASHINGTON – Although the leadership at the Office of the National Coordinator for Health Information Technology is new, the focus of the federal office – reducing physician burden and improving interoperability of electronic heath records – remains the same.
“One priority is on the whole question of burden of [EHR] usability,” said Don Rucker, MD, the new national coordinator, at a July 11 press briefing. “The other is interoperability. We’ve obviously spent a lot of money collectively in the country on these systems, and there’s a widespread dissatisfaction with the level of interoperability.”
“We are looking at documentation and the whole quality framework around value-based purchasing,” he said. “For a lot of practices now, this has become a challenge that we just have to think about what’s the win. At some point, the expense of complying with the quality measures is a much greater expense than the innate value of the quality measures. ”
EHRs “have become symbolic of physician administrative burden, but by no means are they the whole cause,” John Flemming, MD, ONC deputy assistant secretary for health technology reform, said at the briefing. “The physician, particularly in an independent practice, must manage the practice. So he or she is the CEO. They are also on the assembly line, seeing patients. Now with EHRs, they have to be the data input person as well. It’s time consuming.”
Dr. Fleming is a family physician from Louisiana and a former Republican member of congress.
Dr. Rucker acknowledged that reducing the burden of EHRs has been discussed for quite a long time now. He recalled beginning working with them in his private practice back in 1988 and figured, based on the quick rate of technological innovation demonstrated in Silicon Valley, the issues would be solved by 1992 or 1993 at the latest.
“Right now, [EHRs] are really about documentation, about billing, but that is a funny kind of beast,” he said. “Every other industry uses their enterprise computer software to do automation, to become more efficient. We are the only business that I am aware of to have used computers to become less efficient. ... I think part of what we are trying to do ... is let some of these newer technologies that will actually reduce costs, reduce variance, have those technologies have an entrée into some of these data collections that are out there.”
Hexavalent hepatitis B vaccination mostly immunogenic 10 years later
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
FROM VACCINE
Three-drug combo keeps early RA at bay long term
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: A DAS28 less than 2.6 was achieved in 88.2% who received a methotrexate-based triple combination versus 72.1% with methotrexate monotherapy at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
Data source: Two historical cohorts of early arthritis patients treated with methotrexate alone (n = 296) or a combination of methotrexate, hydroxychloroquine, and triamcinolone (n = 157) in routine care.
Disclosures: The study presenter had no disclosures to report. An independent commentator has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
FDA okays ClearLLab test for hematologic cancer detection
Beckman Coulter has been authorized to market its ClearLLab Reagents (T1, T2, B1, B2, M) tests for use with flow cytometry to detect leukemias, lymphomas, and myeloproliferative disorders in blood, bone marrow, and lymph nodes, according to the U.S. Food and Drug Administration.
A study evaluating the efficacy of the test compared the test results (n = 279) with clinical evaluations at four independent clinical sites. The results matched the diagnoses 93.4% of the time and correctly detected cancer 84.2% of the time.
“This represents a major step forward for the hematology-oncology community,” Alberto Gutierrez, PhD, of the FDA’s Center for Devices and Radiological Health said in the FDA’s release. “Laboratories and health care professionals now have access to an FDA-validated test that provides consistent results to aid in the diagnoses of these serious cancers.”
The approval coincides with criteria for ongoing evaluation of the ClearLLab tests and approval of future tests. The release notes that the ClearLLab test results must be reviewed by a trained professional.
Beckman Coulter has been authorized to market its ClearLLab Reagents (T1, T2, B1, B2, M) tests for use with flow cytometry to detect leukemias, lymphomas, and myeloproliferative disorders in blood, bone marrow, and lymph nodes, according to the U.S. Food and Drug Administration.
A study evaluating the efficacy of the test compared the test results (n = 279) with clinical evaluations at four independent clinical sites. The results matched the diagnoses 93.4% of the time and correctly detected cancer 84.2% of the time.
“This represents a major step forward for the hematology-oncology community,” Alberto Gutierrez, PhD, of the FDA’s Center for Devices and Radiological Health said in the FDA’s release. “Laboratories and health care professionals now have access to an FDA-validated test that provides consistent results to aid in the diagnoses of these serious cancers.”
The approval coincides with criteria for ongoing evaluation of the ClearLLab tests and approval of future tests. The release notes that the ClearLLab test results must be reviewed by a trained professional.
Beckman Coulter has been authorized to market its ClearLLab Reagents (T1, T2, B1, B2, M) tests for use with flow cytometry to detect leukemias, lymphomas, and myeloproliferative disorders in blood, bone marrow, and lymph nodes, according to the U.S. Food and Drug Administration.
A study evaluating the efficacy of the test compared the test results (n = 279) with clinical evaluations at four independent clinical sites. The results matched the diagnoses 93.4% of the time and correctly detected cancer 84.2% of the time.
“This represents a major step forward for the hematology-oncology community,” Alberto Gutierrez, PhD, of the FDA’s Center for Devices and Radiological Health said in the FDA’s release. “Laboratories and health care professionals now have access to an FDA-validated test that provides consistent results to aid in the diagnoses of these serious cancers.”
The approval coincides with criteria for ongoing evaluation of the ClearLLab tests and approval of future tests. The release notes that the ClearLLab test results must be reviewed by a trained professional.
Sustained remission on biologics bodes well for children with JIA
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
[email protected]
On Twitter @alz_gal
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
[email protected]
On Twitter @alz_gal
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
[email protected]
On Twitter @alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Overall, 70% of those who were in remission for at least 1.5 years remained in remission after stopping their biologic.
Data source: A database review comprising 83 children.
Disclosures: Dr. Alexeeva disclosed research and grant support from numerous pharmaceutical companies.
Transcranial magnetic stimulation shows promise in autism spectrum disorder
SAN FRANCISCO – , Eric Hollander, MD, said at the annual conference of the Anxiety and Depression Association of America.
“It’s a promising tool. There’s a lot of hope. There have been a range of scattered studies. But there is still a lot more work that needs to be done in terms of defining the optimal target structures in the brain, the dose and frequency of treatment, and which symptoms respond best,” said Dr. Hollander, director of the autism and obsessive-compulsive spectrum program as well as the anxiety and depression program at Albert Einstein College of Medicine in New York.
The authors characterized transcranial magnetic stimulation (TMS) for autism spectrum disorder (ASD) as “a novel, possibly transformative approach” but added a strong cautionary note.
“The available literature on the TMS use in ASD is preliminary, composed of studies with methodological limitations. Thus, off-label clinical rTMS [repetitive TMS] use for therapeutic interventions in ASD without an investigational device exemption and outside of an IRB [institutional review board]-approved research trial is premature pending further, adequately powered and controlled trials,” according to the white paper by the TMS in ASD Consensus Group (Autism Res. 2016 Feb;9[2]:184-203).
ASD support groups are eager to see TMS developed as a treatment, Dr. Hollander said. This is largely a result of the 2016 publication of a nonfiction book entitled, “Switched On: A Memoir of Brain Change and Emotional Awakening” (New York: Spiegel & Grau, 2016). Author John Elder Robison is a high-functioning individual with ASD who describes his dramatic improvement in response to TMS therapy in an early clinical trial conducted at Boston’s Beth Israel Deaconess Medical Center.
Dr. Hollander has been extensively involved in pioneering studies of TMS for the treatment of depression – currently its only Food and Drug Administration–approved indication – as well as for obsessive-compulsive disorder. His recent work on TMS for the treatment of ASD has focused on the noninvasive therapy’s ability to favorably affect the excitatory/inhibitory imbalance that characterizes ASD. This imbalance is tied chiefly to abnormal glutamatergic and gamma-aminobutyric acid–ergic neurotransmission in the neocortex, cerebellum, hippocampus, and amygdala. The imbalance is thought to be responsible for the cognitive, sensory, learning, memory, and motor deficits, as well as increased propensity for seizures, associated with ASD.
This excitatory/inhibitory imbalance is marked by increased cortical excitability and decreased inhibition within the densely packed cortical minicolumns of neurons, which are organized into pathways and circuits.
“You can use TMS as a treatment, or you can use it as a research probe to look at these mechanisms by turning on or off pathways,” the psychiatrist explained. “These densely packed minicolumns are like wires with poor insulation, which results in impairment in the ability to distinguish a stimulus from background noise. In the pathologic condition, you’re getting a rapid firing which doesn’t really differentiate what’s a true signal from what’s background noise.”
Therapeutically, TMS can be employed to improve that signal-to-noise ratio, either by reducing excitation or increasing inhibition. Potential TMS targets in autism include the anterior cingulate cortex, the supplementary or presupplementary motor area, the dorsal medial prefrontal cortex, the dorsal lateral prefrontal cortex, and the cerebellum. More than a dozen published TMS studies – albeit open-label, uncontrolled, and featuring only handfuls of patients – have demonstrated long-lasting improvements in the two core symptom domains of ASD: reduced repetitive behaviors and improved social relatedness and interpersonal functioning, Dr. Hollander said.
A wide range of associated noncore symptoms, including disruptive behaviors such as self-injury or aggression, impulse control, social anxiety, and depression, also might be targeted.
“In our clinical practice, we tend to treat adults with ASD who have a lot of OCD [obsessive-compulsive disorder] and repetitive behavior symptoms but also mood or anxiety symptoms or PTSD [posttraumatic stress disorder] symptoms as a result of earlier bullying. You can adapt your treatment to the target symptoms, so if there’s a lot of OCD-type symptoms, you might use low-frequency TMS at 1 Hz to target the supplementary motor area. If people are coming in with depressive symptoms, you can use the dorsolateral prefrontal cortex depression target. If they have a lot of anxiety, you can target the right frontal anxiety loop with low-frequency TMS. Or with a lot of PTSD symptoms, you can use high-frequency stimulation of the dorsolateral prefrontal cortex at 20 Hz,” Dr. Hollander said.
An important caveat, however, is that ASD is associated with an increased risk of seizures and other EEG abnormalities, so low-frequency TMS generally is preferable because of its greater safety.
Another challenge is administering TMS in children.
“Kids move around a lot, so you’re probably going to be using briefer stimulation parameters like theta burst stimulation rather than longer treatment parameters,” Dr. Hollander said.
That being said, there are more than two dozen published studies of TMS for treatment of children and adolescents, and surveys indicate that these patients generally find it quite tolerable. Dr. Hollander noted that in one study, children and adolescents ranked it somewhere between watching television and a long car ride. This placed TMS on the midrange of a tolerability scale: not as good as having a birthday party or playing a game, but better than going to the dentist, throwing up, or, in last place, getting a shot. Of the 39 youngsters, 34 indicated that they would recommend TMS to a friend.
Dr. Hollander reported receiving research funding from the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Institute of Neurological Disorders and Stroke. He serves as a consultant to roughly half a dozen pharmaceutical companies.
SAN FRANCISCO – , Eric Hollander, MD, said at the annual conference of the Anxiety and Depression Association of America.
“It’s a promising tool. There’s a lot of hope. There have been a range of scattered studies. But there is still a lot more work that needs to be done in terms of defining the optimal target structures in the brain, the dose and frequency of treatment, and which symptoms respond best,” said Dr. Hollander, director of the autism and obsessive-compulsive spectrum program as well as the anxiety and depression program at Albert Einstein College of Medicine in New York.
The authors characterized transcranial magnetic stimulation (TMS) for autism spectrum disorder (ASD) as “a novel, possibly transformative approach” but added a strong cautionary note.
“The available literature on the TMS use in ASD is preliminary, composed of studies with methodological limitations. Thus, off-label clinical rTMS [repetitive TMS] use for therapeutic interventions in ASD without an investigational device exemption and outside of an IRB [institutional review board]-approved research trial is premature pending further, adequately powered and controlled trials,” according to the white paper by the TMS in ASD Consensus Group (Autism Res. 2016 Feb;9[2]:184-203).
ASD support groups are eager to see TMS developed as a treatment, Dr. Hollander said. This is largely a result of the 2016 publication of a nonfiction book entitled, “Switched On: A Memoir of Brain Change and Emotional Awakening” (New York: Spiegel & Grau, 2016). Author John Elder Robison is a high-functioning individual with ASD who describes his dramatic improvement in response to TMS therapy in an early clinical trial conducted at Boston’s Beth Israel Deaconess Medical Center.
Dr. Hollander has been extensively involved in pioneering studies of TMS for the treatment of depression – currently its only Food and Drug Administration–approved indication – as well as for obsessive-compulsive disorder. His recent work on TMS for the treatment of ASD has focused on the noninvasive therapy’s ability to favorably affect the excitatory/inhibitory imbalance that characterizes ASD. This imbalance is tied chiefly to abnormal glutamatergic and gamma-aminobutyric acid–ergic neurotransmission in the neocortex, cerebellum, hippocampus, and amygdala. The imbalance is thought to be responsible for the cognitive, sensory, learning, memory, and motor deficits, as well as increased propensity for seizures, associated with ASD.
This excitatory/inhibitory imbalance is marked by increased cortical excitability and decreased inhibition within the densely packed cortical minicolumns of neurons, which are organized into pathways and circuits.
“You can use TMS as a treatment, or you can use it as a research probe to look at these mechanisms by turning on or off pathways,” the psychiatrist explained. “These densely packed minicolumns are like wires with poor insulation, which results in impairment in the ability to distinguish a stimulus from background noise. In the pathologic condition, you’re getting a rapid firing which doesn’t really differentiate what’s a true signal from what’s background noise.”
Therapeutically, TMS can be employed to improve that signal-to-noise ratio, either by reducing excitation or increasing inhibition. Potential TMS targets in autism include the anterior cingulate cortex, the supplementary or presupplementary motor area, the dorsal medial prefrontal cortex, the dorsal lateral prefrontal cortex, and the cerebellum. More than a dozen published TMS studies – albeit open-label, uncontrolled, and featuring only handfuls of patients – have demonstrated long-lasting improvements in the two core symptom domains of ASD: reduced repetitive behaviors and improved social relatedness and interpersonal functioning, Dr. Hollander said.
A wide range of associated noncore symptoms, including disruptive behaviors such as self-injury or aggression, impulse control, social anxiety, and depression, also might be targeted.
“In our clinical practice, we tend to treat adults with ASD who have a lot of OCD [obsessive-compulsive disorder] and repetitive behavior symptoms but also mood or anxiety symptoms or PTSD [posttraumatic stress disorder] symptoms as a result of earlier bullying. You can adapt your treatment to the target symptoms, so if there’s a lot of OCD-type symptoms, you might use low-frequency TMS at 1 Hz to target the supplementary motor area. If people are coming in with depressive symptoms, you can use the dorsolateral prefrontal cortex depression target. If they have a lot of anxiety, you can target the right frontal anxiety loop with low-frequency TMS. Or with a lot of PTSD symptoms, you can use high-frequency stimulation of the dorsolateral prefrontal cortex at 20 Hz,” Dr. Hollander said.
An important caveat, however, is that ASD is associated with an increased risk of seizures and other EEG abnormalities, so low-frequency TMS generally is preferable because of its greater safety.
Another challenge is administering TMS in children.
“Kids move around a lot, so you’re probably going to be using briefer stimulation parameters like theta burst stimulation rather than longer treatment parameters,” Dr. Hollander said.
That being said, there are more than two dozen published studies of TMS for treatment of children and adolescents, and surveys indicate that these patients generally find it quite tolerable. Dr. Hollander noted that in one study, children and adolescents ranked it somewhere between watching television and a long car ride. This placed TMS on the midrange of a tolerability scale: not as good as having a birthday party or playing a game, but better than going to the dentist, throwing up, or, in last place, getting a shot. Of the 39 youngsters, 34 indicated that they would recommend TMS to a friend.
Dr. Hollander reported receiving research funding from the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Institute of Neurological Disorders and Stroke. He serves as a consultant to roughly half a dozen pharmaceutical companies.
SAN FRANCISCO – , Eric Hollander, MD, said at the annual conference of the Anxiety and Depression Association of America.
“It’s a promising tool. There’s a lot of hope. There have been a range of scattered studies. But there is still a lot more work that needs to be done in terms of defining the optimal target structures in the brain, the dose and frequency of treatment, and which symptoms respond best,” said Dr. Hollander, director of the autism and obsessive-compulsive spectrum program as well as the anxiety and depression program at Albert Einstein College of Medicine in New York.
The authors characterized transcranial magnetic stimulation (TMS) for autism spectrum disorder (ASD) as “a novel, possibly transformative approach” but added a strong cautionary note.
“The available literature on the TMS use in ASD is preliminary, composed of studies with methodological limitations. Thus, off-label clinical rTMS [repetitive TMS] use for therapeutic interventions in ASD without an investigational device exemption and outside of an IRB [institutional review board]-approved research trial is premature pending further, adequately powered and controlled trials,” according to the white paper by the TMS in ASD Consensus Group (Autism Res. 2016 Feb;9[2]:184-203).
ASD support groups are eager to see TMS developed as a treatment, Dr. Hollander said. This is largely a result of the 2016 publication of a nonfiction book entitled, “Switched On: A Memoir of Brain Change and Emotional Awakening” (New York: Spiegel & Grau, 2016). Author John Elder Robison is a high-functioning individual with ASD who describes his dramatic improvement in response to TMS therapy in an early clinical trial conducted at Boston’s Beth Israel Deaconess Medical Center.
Dr. Hollander has been extensively involved in pioneering studies of TMS for the treatment of depression – currently its only Food and Drug Administration–approved indication – as well as for obsessive-compulsive disorder. His recent work on TMS for the treatment of ASD has focused on the noninvasive therapy’s ability to favorably affect the excitatory/inhibitory imbalance that characterizes ASD. This imbalance is tied chiefly to abnormal glutamatergic and gamma-aminobutyric acid–ergic neurotransmission in the neocortex, cerebellum, hippocampus, and amygdala. The imbalance is thought to be responsible for the cognitive, sensory, learning, memory, and motor deficits, as well as increased propensity for seizures, associated with ASD.
This excitatory/inhibitory imbalance is marked by increased cortical excitability and decreased inhibition within the densely packed cortical minicolumns of neurons, which are organized into pathways and circuits.
“You can use TMS as a treatment, or you can use it as a research probe to look at these mechanisms by turning on or off pathways,” the psychiatrist explained. “These densely packed minicolumns are like wires with poor insulation, which results in impairment in the ability to distinguish a stimulus from background noise. In the pathologic condition, you’re getting a rapid firing which doesn’t really differentiate what’s a true signal from what’s background noise.”
Therapeutically, TMS can be employed to improve that signal-to-noise ratio, either by reducing excitation or increasing inhibition. Potential TMS targets in autism include the anterior cingulate cortex, the supplementary or presupplementary motor area, the dorsal medial prefrontal cortex, the dorsal lateral prefrontal cortex, and the cerebellum. More than a dozen published TMS studies – albeit open-label, uncontrolled, and featuring only handfuls of patients – have demonstrated long-lasting improvements in the two core symptom domains of ASD: reduced repetitive behaviors and improved social relatedness and interpersonal functioning, Dr. Hollander said.
A wide range of associated noncore symptoms, including disruptive behaviors such as self-injury or aggression, impulse control, social anxiety, and depression, also might be targeted.
“In our clinical practice, we tend to treat adults with ASD who have a lot of OCD [obsessive-compulsive disorder] and repetitive behavior symptoms but also mood or anxiety symptoms or PTSD [posttraumatic stress disorder] symptoms as a result of earlier bullying. You can adapt your treatment to the target symptoms, so if there’s a lot of OCD-type symptoms, you might use low-frequency TMS at 1 Hz to target the supplementary motor area. If people are coming in with depressive symptoms, you can use the dorsolateral prefrontal cortex depression target. If they have a lot of anxiety, you can target the right frontal anxiety loop with low-frequency TMS. Or with a lot of PTSD symptoms, you can use high-frequency stimulation of the dorsolateral prefrontal cortex at 20 Hz,” Dr. Hollander said.
An important caveat, however, is that ASD is associated with an increased risk of seizures and other EEG abnormalities, so low-frequency TMS generally is preferable because of its greater safety.
Another challenge is administering TMS in children.
“Kids move around a lot, so you’re probably going to be using briefer stimulation parameters like theta burst stimulation rather than longer treatment parameters,” Dr. Hollander said.
That being said, there are more than two dozen published studies of TMS for treatment of children and adolescents, and surveys indicate that these patients generally find it quite tolerable. Dr. Hollander noted that in one study, children and adolescents ranked it somewhere between watching television and a long car ride. This placed TMS on the midrange of a tolerability scale: not as good as having a birthday party or playing a game, but better than going to the dentist, throwing up, or, in last place, getting a shot. Of the 39 youngsters, 34 indicated that they would recommend TMS to a friend.
Dr. Hollander reported receiving research funding from the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Institute of Neurological Disorders and Stroke. He serves as a consultant to roughly half a dozen pharmaceutical companies.
EXPERT ANALYSIS FROM THE ANXIETY AND DEPRESSION CONFERENCE 2017
Enasidenib monotherapy responses in 37% with relapsed/refractory AML and IDH2 mutations
MADRID –
Among 214 patients treated at a dose level of 100 mg daily, the overall response rate was 37%, including 20.1% complete responses (CRs) and 7.9% complete responses with incomplete recovery of platelets (CRp) or incomplete hematologic recovery (CRi), reported Eytan M. Stein, MD, an internist and hematologic oncologist at the Memorial Sloan Kettering Cancer Center in New York.
“In patients with relapsed/refractory AML (with IDH2 mutations), most of whom had received multiple prior AML treatments, enasidenib induced durable CRs that were associated with overall survival of greater than 8 months,” he said at the annual congress of the European Hematology Association.
The IDH2 gene encodes for isocitrate dehydrogenase 2, which is an enzyme of the citric acid cycle. An estimated 8%-15% of patients with AML have mutations in IDH2 that cause intracellular accumulation of beta-hydroxyglutarate, which leads to blockage of myeloblast differentiation through a variety of mechanisms. The primary mechanism of action of enasidenib appears to be through its action on differentiation, rather than through cytotoxicity, Dr. Stein said.
He reported updated results from the fully enrolled cohorts of the phase 1/2 study. Earlier data from the study were reported at the 2017 annual meeting of the American Society of Clinical Oncology and in a paper published concurrently in Blood.
In the study, the investigators first enrolled 113 patients with advanced hematologic malignancies with IDH2 mutations and treated them with cumulative daily doses of enasidenib ranging from 50 to 650 mg.
In a phase 1 expansion study at the established dose of 100 mg daily, 126 patients with IDH2 mutations were enrolled in one of four cohorts: patients aged 60 or older with relapsed or refractory AML or AML patients of any age who experienced a relapse after undergoing a bone marrow transplant (BMT); patients under age 60 except those with post-BMT relapses; patients with previously untreated AML who were 60 years or older who declined the standard of care; and patients with other hematologic malignancies who were ineligible for other study arms.
The study also included a phase 2 expansion cohort of 106 patients with relapsed/refractory AML with IDH2 mutations treated with enasidenib 100 mg daily. The data cutoff was Oct. 14, 2016.
Among 214 patients treated at the 100-mg/day dose, the ORR was 37%, including 20.1% with a CR, 7.9% with a CRp or CRi, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state.
The median time to first response was 1.9 months, and the median time to CR was 3.7 months.
Clinicians should wait until patients have received at least four cycles of the drug before determining whether they should be continued on the drug or switched to another therapy, Dr. Stein said.
After 30 months of follow-up, overall survival (OS) among the 281 patients with relapsed/refractory AML with IDH2 mutations who were treated with enasidenib at any dose level was 8.4 months. Among the 214 treated at the 100-mg daily dose level, the median OS was 8.3 months.
When the investigators looked at OS by best response, they saw that patients who had a CR had a median OS of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months.
Patients generally tolerated the drug well. Most adverse events were grade 1 or 2 in severity.
An increase in blood bilirubin was the most frequent grade 3 or 4 adverse event, occurring in 8% of all patients. The effect was caused by an off-target reaction and was not associated with elevations in liver enzymes or evidence of liver damage, Dr. Stein said.
Grade 3 or 4 dyspnea occurred in 6% of patients, and 7% of all patients had serious treatment-related IDH-inhibitor–associated differentiation syndrome (IDH-DS). This syndrome presents with symptoms similar to those of retinoic acid syndrome, which occurs during treatment for acute promyelocytic leukemia.
Enasidenib is being explored in a phase 3 study comparing enasidenib monotherapy with conventional care in patients with late-stage AML and in combination with other agents and regimens in phase 1/2 studies in patients with newly diagnosed AML with IDH2 mutations.
Enasidenib has been granted priority review by the U.S. Food and Drug Administration for relapsed/refractory AML with an IDH2 mutation and has been given a Prescription Drug User Fee Act action date of Aug. 30, 2017, according to Celgene.
The study was funded by Celgene. Dr. Stein disclosed a consulting/advisory role with the company, research funding, and travel expenses.
MADRID –
Among 214 patients treated at a dose level of 100 mg daily, the overall response rate was 37%, including 20.1% complete responses (CRs) and 7.9% complete responses with incomplete recovery of platelets (CRp) or incomplete hematologic recovery (CRi), reported Eytan M. Stein, MD, an internist and hematologic oncologist at the Memorial Sloan Kettering Cancer Center in New York.
“In patients with relapsed/refractory AML (with IDH2 mutations), most of whom had received multiple prior AML treatments, enasidenib induced durable CRs that were associated with overall survival of greater than 8 months,” he said at the annual congress of the European Hematology Association.
The IDH2 gene encodes for isocitrate dehydrogenase 2, which is an enzyme of the citric acid cycle. An estimated 8%-15% of patients with AML have mutations in IDH2 that cause intracellular accumulation of beta-hydroxyglutarate, which leads to blockage of myeloblast differentiation through a variety of mechanisms. The primary mechanism of action of enasidenib appears to be through its action on differentiation, rather than through cytotoxicity, Dr. Stein said.
He reported updated results from the fully enrolled cohorts of the phase 1/2 study. Earlier data from the study were reported at the 2017 annual meeting of the American Society of Clinical Oncology and in a paper published concurrently in Blood.
In the study, the investigators first enrolled 113 patients with advanced hematologic malignancies with IDH2 mutations and treated them with cumulative daily doses of enasidenib ranging from 50 to 650 mg.
In a phase 1 expansion study at the established dose of 100 mg daily, 126 patients with IDH2 mutations were enrolled in one of four cohorts: patients aged 60 or older with relapsed or refractory AML or AML patients of any age who experienced a relapse after undergoing a bone marrow transplant (BMT); patients under age 60 except those with post-BMT relapses; patients with previously untreated AML who were 60 years or older who declined the standard of care; and patients with other hematologic malignancies who were ineligible for other study arms.
The study also included a phase 2 expansion cohort of 106 patients with relapsed/refractory AML with IDH2 mutations treated with enasidenib 100 mg daily. The data cutoff was Oct. 14, 2016.
Among 214 patients treated at the 100-mg/day dose, the ORR was 37%, including 20.1% with a CR, 7.9% with a CRp or CRi, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state.
The median time to first response was 1.9 months, and the median time to CR was 3.7 months.
Clinicians should wait until patients have received at least four cycles of the drug before determining whether they should be continued on the drug or switched to another therapy, Dr. Stein said.
After 30 months of follow-up, overall survival (OS) among the 281 patients with relapsed/refractory AML with IDH2 mutations who were treated with enasidenib at any dose level was 8.4 months. Among the 214 treated at the 100-mg daily dose level, the median OS was 8.3 months.
When the investigators looked at OS by best response, they saw that patients who had a CR had a median OS of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months.
Patients generally tolerated the drug well. Most adverse events were grade 1 or 2 in severity.
An increase in blood bilirubin was the most frequent grade 3 or 4 adverse event, occurring in 8% of all patients. The effect was caused by an off-target reaction and was not associated with elevations in liver enzymes or evidence of liver damage, Dr. Stein said.
Grade 3 or 4 dyspnea occurred in 6% of patients, and 7% of all patients had serious treatment-related IDH-inhibitor–associated differentiation syndrome (IDH-DS). This syndrome presents with symptoms similar to those of retinoic acid syndrome, which occurs during treatment for acute promyelocytic leukemia.
Enasidenib is being explored in a phase 3 study comparing enasidenib monotherapy with conventional care in patients with late-stage AML and in combination with other agents and regimens in phase 1/2 studies in patients with newly diagnosed AML with IDH2 mutations.
Enasidenib has been granted priority review by the U.S. Food and Drug Administration for relapsed/refractory AML with an IDH2 mutation and has been given a Prescription Drug User Fee Act action date of Aug. 30, 2017, according to Celgene.
The study was funded by Celgene. Dr. Stein disclosed a consulting/advisory role with the company, research funding, and travel expenses.
MADRID –
Among 214 patients treated at a dose level of 100 mg daily, the overall response rate was 37%, including 20.1% complete responses (CRs) and 7.9% complete responses with incomplete recovery of platelets (CRp) or incomplete hematologic recovery (CRi), reported Eytan M. Stein, MD, an internist and hematologic oncologist at the Memorial Sloan Kettering Cancer Center in New York.
“In patients with relapsed/refractory AML (with IDH2 mutations), most of whom had received multiple prior AML treatments, enasidenib induced durable CRs that were associated with overall survival of greater than 8 months,” he said at the annual congress of the European Hematology Association.
The IDH2 gene encodes for isocitrate dehydrogenase 2, which is an enzyme of the citric acid cycle. An estimated 8%-15% of patients with AML have mutations in IDH2 that cause intracellular accumulation of beta-hydroxyglutarate, which leads to blockage of myeloblast differentiation through a variety of mechanisms. The primary mechanism of action of enasidenib appears to be through its action on differentiation, rather than through cytotoxicity, Dr. Stein said.
He reported updated results from the fully enrolled cohorts of the phase 1/2 study. Earlier data from the study were reported at the 2017 annual meeting of the American Society of Clinical Oncology and in a paper published concurrently in Blood.
In the study, the investigators first enrolled 113 patients with advanced hematologic malignancies with IDH2 mutations and treated them with cumulative daily doses of enasidenib ranging from 50 to 650 mg.
In a phase 1 expansion study at the established dose of 100 mg daily, 126 patients with IDH2 mutations were enrolled in one of four cohorts: patients aged 60 or older with relapsed or refractory AML or AML patients of any age who experienced a relapse after undergoing a bone marrow transplant (BMT); patients under age 60 except those with post-BMT relapses; patients with previously untreated AML who were 60 years or older who declined the standard of care; and patients with other hematologic malignancies who were ineligible for other study arms.
The study also included a phase 2 expansion cohort of 106 patients with relapsed/refractory AML with IDH2 mutations treated with enasidenib 100 mg daily. The data cutoff was Oct. 14, 2016.
Among 214 patients treated at the 100-mg/day dose, the ORR was 37%, including 20.1% with a CR, 7.9% with a CRp or CRi, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state.
The median time to first response was 1.9 months, and the median time to CR was 3.7 months.
Clinicians should wait until patients have received at least four cycles of the drug before determining whether they should be continued on the drug or switched to another therapy, Dr. Stein said.
After 30 months of follow-up, overall survival (OS) among the 281 patients with relapsed/refractory AML with IDH2 mutations who were treated with enasidenib at any dose level was 8.4 months. Among the 214 treated at the 100-mg daily dose level, the median OS was 8.3 months.
When the investigators looked at OS by best response, they saw that patients who had a CR had a median OS of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months.
Patients generally tolerated the drug well. Most adverse events were grade 1 or 2 in severity.
An increase in blood bilirubin was the most frequent grade 3 or 4 adverse event, occurring in 8% of all patients. The effect was caused by an off-target reaction and was not associated with elevations in liver enzymes or evidence of liver damage, Dr. Stein said.
Grade 3 or 4 dyspnea occurred in 6% of patients, and 7% of all patients had serious treatment-related IDH-inhibitor–associated differentiation syndrome (IDH-DS). This syndrome presents with symptoms similar to those of retinoic acid syndrome, which occurs during treatment for acute promyelocytic leukemia.
Enasidenib is being explored in a phase 3 study comparing enasidenib monotherapy with conventional care in patients with late-stage AML and in combination with other agents and regimens in phase 1/2 studies in patients with newly diagnosed AML with IDH2 mutations.
Enasidenib has been granted priority review by the U.S. Food and Drug Administration for relapsed/refractory AML with an IDH2 mutation and has been given a Prescription Drug User Fee Act action date of Aug. 30, 2017, according to Celgene.
The study was funded by Celgene. Dr. Stein disclosed a consulting/advisory role with the company, research funding, and travel expenses.
AT EHA 2017
Key clinical point: Approximately 12% of patients with acute myeloid leukemia have mutations in IDH2, the target of the investigational agent enasidenib.
Major finding: Among 214 patients treated at a dose level of 100 mg daily, the overall response rate was 37%.
Data source: A phase 1/2 study in patients with relapsed/refractory AML and other hematologic malignancies with mutations in IDH2.
Disclosures: The study was funded by Celgene. Dr. Stein disclosed a consulting/advisory role with the company, research funding, and travel expenses.
Don’t forget about Zika
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
Listening for golf balls
“What did the patient say about the golf ball?” I asked my student.
The student looked blank. “Golf ball?” he asked.
“The patient said a golf ball hit him.”
“He did?”
“I showed him a precancerous red spot on his forehead, and said I could freeze it off.”
“Yes,” said the student. “Now I remember.”
“Good. Now tell me why he said it.”
The student looked lost. “Because he really was hit by a golf ball?”
“Maybe he was,” I said. “But in his 60 years, he’s been hit by a lot of things. How can he be sure the golf ball hit just that spot? And anyhow, why tell me about it? He must have thought it was important for me to know. We discussed this the other day,” I reminded him.
“Because there was trauma?”
“That’s it,” I said. “One way patients understand why things happen to them is by saying that what got sick was hit by something. They assume trauma weakens and damages the body, and disposes it to being unhealthy.
“After all,” I went on, “I had told him his spot was caused by sun exposure. But he’s had sun exposure all over his face, so why would he get a sun spot only right there? His answer: Sun damages all skin, but the part the golf ball whacked is especially susceptible.
“Is he right? I have no idea, but it’s important – to him – to think so. Not so much for this spot – we’re going to treat it anyway – but because of what he said 2 minutes later about his left shin. Remember?”
The student did not.
“He had a raised brown spot on his leg,” I reminded him. “It was just a seborrheic keratosis, not even precancerous. But he said he was always picking it.”
“Yes, he did say that,” said the student.
“So again: Why did he think I needed to know?”
“Because picking is a form of trauma, which might cause the spot to turn into something?”
“Yes, indeed,” I said. “You should train yourself to listen to these offhand remarks that seem irrelevant to you. They are relevant to the patient, or he wouldn’t say them.
Sure enough, a little later the student and I met another patient coming for a skin check. A computer scientist from a local university, he displayed a big collection of cherry angiomas on his torso, front, and back.
Looking at his belly, he said, “I know where I got those.”
“Which ones?” I asked.
He pointed to a dense collection of red spots near his navel. “A soccer ball hit me there when I was a teenager in Colombia,” he said.
Later, the student and I discussed this man’s recollection. “What makes his observation striking,” I suggested, “is not just as another example of a patient blaming body changes on trauma. It’s that he did it in a way that even a smidgen of critical thinking – the kind he applies to his professional work all the time – would show that his hypothesis makes no sense. After all, he has dozens of red spots nowhere near where the soccer ball supposedly hit him.
“You would think a computer scientist would notice this, but when it comes to looking at our own health, even sophisticated scientific training may not help. Instead, the thinking is: “I’ve got these red spots. Something caused them. A soccer ball hit me down there. That must be it.”
Sometimes hearing what patients say doesn’t matter; we’re not going to remove the cherry angiomas. But sometimes it does, by telling us the real reason they want something removed, which may include some guilt about their own picking, guilt they can do without.
But you would have to listen for that nuance, and listening is hard. Mostly, in medicine and in life, we hear only what we expect to hear.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected]
“What did the patient say about the golf ball?” I asked my student.
The student looked blank. “Golf ball?” he asked.
“The patient said a golf ball hit him.”
“He did?”
“I showed him a precancerous red spot on his forehead, and said I could freeze it off.”
“Yes,” said the student. “Now I remember.”
“Good. Now tell me why he said it.”
The student looked lost. “Because he really was hit by a golf ball?”
“Maybe he was,” I said. “But in his 60 years, he’s been hit by a lot of things. How can he be sure the golf ball hit just that spot? And anyhow, why tell me about it? He must have thought it was important for me to know. We discussed this the other day,” I reminded him.
“Because there was trauma?”
“That’s it,” I said. “One way patients understand why things happen to them is by saying that what got sick was hit by something. They assume trauma weakens and damages the body, and disposes it to being unhealthy.
“After all,” I went on, “I had told him his spot was caused by sun exposure. But he’s had sun exposure all over his face, so why would he get a sun spot only right there? His answer: Sun damages all skin, but the part the golf ball whacked is especially susceptible.
“Is he right? I have no idea, but it’s important – to him – to think so. Not so much for this spot – we’re going to treat it anyway – but because of what he said 2 minutes later about his left shin. Remember?”
The student did not.
“He had a raised brown spot on his leg,” I reminded him. “It was just a seborrheic keratosis, not even precancerous. But he said he was always picking it.”
“Yes, he did say that,” said the student.
“So again: Why did he think I needed to know?”
“Because picking is a form of trauma, which might cause the spot to turn into something?”
“Yes, indeed,” I said. “You should train yourself to listen to these offhand remarks that seem irrelevant to you. They are relevant to the patient, or he wouldn’t say them.
Sure enough, a little later the student and I met another patient coming for a skin check. A computer scientist from a local university, he displayed a big collection of cherry angiomas on his torso, front, and back.
Looking at his belly, he said, “I know where I got those.”
“Which ones?” I asked.
He pointed to a dense collection of red spots near his navel. “A soccer ball hit me there when I was a teenager in Colombia,” he said.
Later, the student and I discussed this man’s recollection. “What makes his observation striking,” I suggested, “is not just as another example of a patient blaming body changes on trauma. It’s that he did it in a way that even a smidgen of critical thinking – the kind he applies to his professional work all the time – would show that his hypothesis makes no sense. After all, he has dozens of red spots nowhere near where the soccer ball supposedly hit him.
“You would think a computer scientist would notice this, but when it comes to looking at our own health, even sophisticated scientific training may not help. Instead, the thinking is: “I’ve got these red spots. Something caused them. A soccer ball hit me down there. That must be it.”
Sometimes hearing what patients say doesn’t matter; we’re not going to remove the cherry angiomas. But sometimes it does, by telling us the real reason they want something removed, which may include some guilt about their own picking, guilt they can do without.
But you would have to listen for that nuance, and listening is hard. Mostly, in medicine and in life, we hear only what we expect to hear.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected]
“What did the patient say about the golf ball?” I asked my student.
The student looked blank. “Golf ball?” he asked.
“The patient said a golf ball hit him.”
“He did?”
“I showed him a precancerous red spot on his forehead, and said I could freeze it off.”
“Yes,” said the student. “Now I remember.”
“Good. Now tell me why he said it.”
The student looked lost. “Because he really was hit by a golf ball?”
“Maybe he was,” I said. “But in his 60 years, he’s been hit by a lot of things. How can he be sure the golf ball hit just that spot? And anyhow, why tell me about it? He must have thought it was important for me to know. We discussed this the other day,” I reminded him.
“Because there was trauma?”
“That’s it,” I said. “One way patients understand why things happen to them is by saying that what got sick was hit by something. They assume trauma weakens and damages the body, and disposes it to being unhealthy.
“After all,” I went on, “I had told him his spot was caused by sun exposure. But he’s had sun exposure all over his face, so why would he get a sun spot only right there? His answer: Sun damages all skin, but the part the golf ball whacked is especially susceptible.
“Is he right? I have no idea, but it’s important – to him – to think so. Not so much for this spot – we’re going to treat it anyway – but because of what he said 2 minutes later about his left shin. Remember?”
The student did not.
“He had a raised brown spot on his leg,” I reminded him. “It was just a seborrheic keratosis, not even precancerous. But he said he was always picking it.”
“Yes, he did say that,” said the student.
“So again: Why did he think I needed to know?”
“Because picking is a form of trauma, which might cause the spot to turn into something?”
“Yes, indeed,” I said. “You should train yourself to listen to these offhand remarks that seem irrelevant to you. They are relevant to the patient, or he wouldn’t say them.
Sure enough, a little later the student and I met another patient coming for a skin check. A computer scientist from a local university, he displayed a big collection of cherry angiomas on his torso, front, and back.
Looking at his belly, he said, “I know where I got those.”
“Which ones?” I asked.
He pointed to a dense collection of red spots near his navel. “A soccer ball hit me there when I was a teenager in Colombia,” he said.
Later, the student and I discussed this man’s recollection. “What makes his observation striking,” I suggested, “is not just as another example of a patient blaming body changes on trauma. It’s that he did it in a way that even a smidgen of critical thinking – the kind he applies to his professional work all the time – would show that his hypothesis makes no sense. After all, he has dozens of red spots nowhere near where the soccer ball supposedly hit him.
“You would think a computer scientist would notice this, but when it comes to looking at our own health, even sophisticated scientific training may not help. Instead, the thinking is: “I’ve got these red spots. Something caused them. A soccer ball hit me down there. That must be it.”
Sometimes hearing what patients say doesn’t matter; we’re not going to remove the cherry angiomas. But sometimes it does, by telling us the real reason they want something removed, which may include some guilt about their own picking, guilt they can do without.
But you would have to listen for that nuance, and listening is hard. Mostly, in medicine and in life, we hear only what we expect to hear.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected]