User login
Where do you stand on the Middle East conflict?
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
AI in medicine has a major Cassandra problem
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.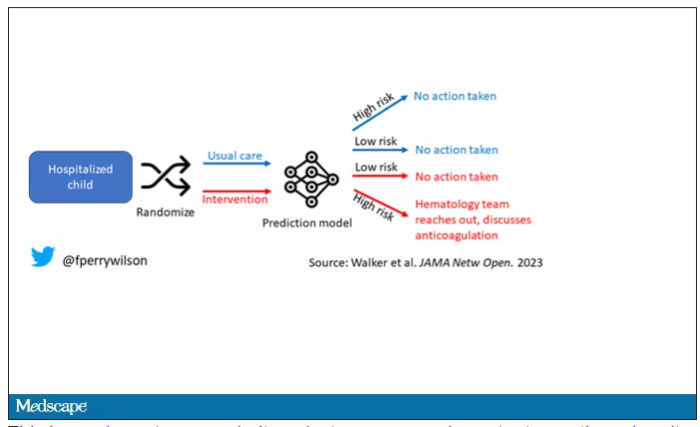
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.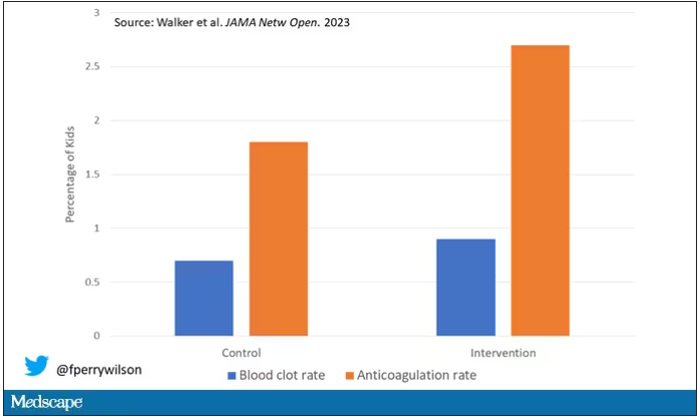
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.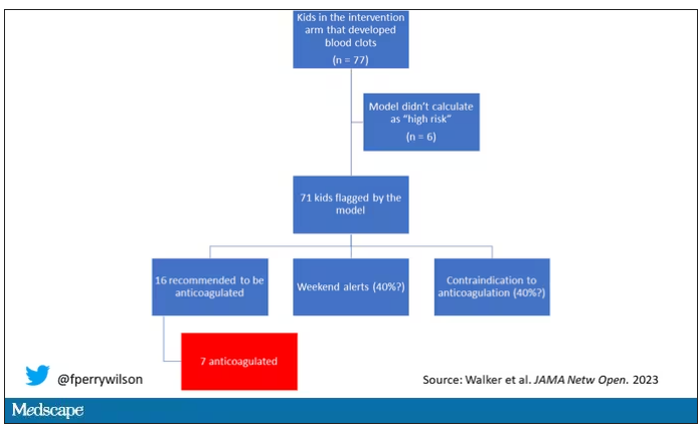
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Asthma severity higher among LGBTQ+ population
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT CHEST 2023
Scientists find the ‘on’ switch for energy-burning brown fat
A process your body uses to stay warm in cool weather could one day lead to new therapies for obesity.
Scientists have, for the first time, mapped the precise nerve pathways that activate brown fat, or brown adipose tissue (BAT), a specialized fat that generates heat. Low temperatures kick brown fat into gear, helping the body keep its temperature and burning calories in the process.
“It has long been speculated that activating this type of fat may be useful in treating obesity and related metabolic conditions,” said Preethi Srikanthan, MD, an endocrinologist and professor of medicine who oversaw the research at the UCLA School of Medicine. “The challenge has been finding a way of selectively stimulating [it].”
Brown fat is different from the fat typically linked to obesity: the kind that accumulates around the belly, hips, and thighs. That’s white fat. White fat stores energy; brown fat burns it. That’s because brown fat cells have more mitochondria, a part of the cell that generates energy.
After dissecting the necks of eight human cadavers, Dr. Srikanthan and her team traced the sympathetic nerve branches in the fat pad above the collarbone – where the largest depot of brown fat in adults is stored. They stained the nerves, took samples, and viewed them under a microscope.
They found that nerves from brown fat traveled to the third and fourth cranial nerves of the brain, bundles of nerve fibers that control blinking and some eye movements.
In a previous case study, damage to these nerves appeared to block a chemical tracer from reaching brown fat. , Dr. Srikanthan said.
A possible mechanism for Ozempic?
Brown fat has already been linked to at least one breakthrough in obesity treatment. Some evidence suggests that popular medications like semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro) may affect brown fat activity. These belong to a class of drugs known as glucagon-like peptide-1 (GLP-1) receptor agonists. They work by mimicking the hormone GLP-1, which is released in the gut and brain in response to eating glucose (sugary foods or drinks).
“GLP-1 agonists have been shown to increase [brown fat] activity in rodents and humans, but likely indirectly, via activation of specific regions in the brain,” explained Varman Samuel, MD, PhD, an associate professor of medicine at the Yale School of Medicine, New Haven, Conn., and chief of endocrinology for the VA Connecticut Healthcare System.
The scientific literature is divided on this, but there is enough evidence to support further inquiry, Dr. Srikanthan said. Her team has begun a study to examine that link.
Opening the door to future obesity treatments
But their discovery means other new treatments could be on the horizon.
Previous research had shown that the sympathetic nervous system drives brown fat activity. But now that the UCLA scientists have revealed the exact nerves connecting brown fat to the sympathetic nervous system, we could find ways to stimulate those pathways to activate brown fat – without stimulating the many organs (such as the heart and stomach) also connected to this vast network of nerves, Dr. Srikanthan said.
Methods for doing that could include medication, electrical stimulation, or heat therapy, according to the study.
Still, there is reason to temper expectations. “[Brown fat] depots, while highly metabolically active, are quite small,” Dr. Samuel said. “So, the overall contribution to whole-body energy balance in humans will likely be small.”
On the other hand, that prediction doesn’t account for what we don’t know.
“We’re learning more about how tissues communicate with each other, beyond the release of hormones or metabolites,” Dr. Samuel said. Activating brown fat could trigger “signals that help coordinate whole-body energy metabolism.”
A version of this article first appeared on WebMD.com.
A process your body uses to stay warm in cool weather could one day lead to new therapies for obesity.
Scientists have, for the first time, mapped the precise nerve pathways that activate brown fat, or brown adipose tissue (BAT), a specialized fat that generates heat. Low temperatures kick brown fat into gear, helping the body keep its temperature and burning calories in the process.
“It has long been speculated that activating this type of fat may be useful in treating obesity and related metabolic conditions,” said Preethi Srikanthan, MD, an endocrinologist and professor of medicine who oversaw the research at the UCLA School of Medicine. “The challenge has been finding a way of selectively stimulating [it].”
Brown fat is different from the fat typically linked to obesity: the kind that accumulates around the belly, hips, and thighs. That’s white fat. White fat stores energy; brown fat burns it. That’s because brown fat cells have more mitochondria, a part of the cell that generates energy.
After dissecting the necks of eight human cadavers, Dr. Srikanthan and her team traced the sympathetic nerve branches in the fat pad above the collarbone – where the largest depot of brown fat in adults is stored. They stained the nerves, took samples, and viewed them under a microscope.
They found that nerves from brown fat traveled to the third and fourth cranial nerves of the brain, bundles of nerve fibers that control blinking and some eye movements.
In a previous case study, damage to these nerves appeared to block a chemical tracer from reaching brown fat. , Dr. Srikanthan said.
A possible mechanism for Ozempic?
Brown fat has already been linked to at least one breakthrough in obesity treatment. Some evidence suggests that popular medications like semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro) may affect brown fat activity. These belong to a class of drugs known as glucagon-like peptide-1 (GLP-1) receptor agonists. They work by mimicking the hormone GLP-1, which is released in the gut and brain in response to eating glucose (sugary foods or drinks).
“GLP-1 agonists have been shown to increase [brown fat] activity in rodents and humans, but likely indirectly, via activation of specific regions in the brain,” explained Varman Samuel, MD, PhD, an associate professor of medicine at the Yale School of Medicine, New Haven, Conn., and chief of endocrinology for the VA Connecticut Healthcare System.
The scientific literature is divided on this, but there is enough evidence to support further inquiry, Dr. Srikanthan said. Her team has begun a study to examine that link.
Opening the door to future obesity treatments
But their discovery means other new treatments could be on the horizon.
Previous research had shown that the sympathetic nervous system drives brown fat activity. But now that the UCLA scientists have revealed the exact nerves connecting brown fat to the sympathetic nervous system, we could find ways to stimulate those pathways to activate brown fat – without stimulating the many organs (such as the heart and stomach) also connected to this vast network of nerves, Dr. Srikanthan said.
Methods for doing that could include medication, electrical stimulation, or heat therapy, according to the study.
Still, there is reason to temper expectations. “[Brown fat] depots, while highly metabolically active, are quite small,” Dr. Samuel said. “So, the overall contribution to whole-body energy balance in humans will likely be small.”
On the other hand, that prediction doesn’t account for what we don’t know.
“We’re learning more about how tissues communicate with each other, beyond the release of hormones or metabolites,” Dr. Samuel said. Activating brown fat could trigger “signals that help coordinate whole-body energy metabolism.”
A version of this article first appeared on WebMD.com.
A process your body uses to stay warm in cool weather could one day lead to new therapies for obesity.
Scientists have, for the first time, mapped the precise nerve pathways that activate brown fat, or brown adipose tissue (BAT), a specialized fat that generates heat. Low temperatures kick brown fat into gear, helping the body keep its temperature and burning calories in the process.
“It has long been speculated that activating this type of fat may be useful in treating obesity and related metabolic conditions,” said Preethi Srikanthan, MD, an endocrinologist and professor of medicine who oversaw the research at the UCLA School of Medicine. “The challenge has been finding a way of selectively stimulating [it].”
Brown fat is different from the fat typically linked to obesity: the kind that accumulates around the belly, hips, and thighs. That’s white fat. White fat stores energy; brown fat burns it. That’s because brown fat cells have more mitochondria, a part of the cell that generates energy.
After dissecting the necks of eight human cadavers, Dr. Srikanthan and her team traced the sympathetic nerve branches in the fat pad above the collarbone – where the largest depot of brown fat in adults is stored. They stained the nerves, took samples, and viewed them under a microscope.
They found that nerves from brown fat traveled to the third and fourth cranial nerves of the brain, bundles of nerve fibers that control blinking and some eye movements.
In a previous case study, damage to these nerves appeared to block a chemical tracer from reaching brown fat. , Dr. Srikanthan said.
A possible mechanism for Ozempic?
Brown fat has already been linked to at least one breakthrough in obesity treatment. Some evidence suggests that popular medications like semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro) may affect brown fat activity. These belong to a class of drugs known as glucagon-like peptide-1 (GLP-1) receptor agonists. They work by mimicking the hormone GLP-1, which is released in the gut and brain in response to eating glucose (sugary foods or drinks).
“GLP-1 agonists have been shown to increase [brown fat] activity in rodents and humans, but likely indirectly, via activation of specific regions in the brain,” explained Varman Samuel, MD, PhD, an associate professor of medicine at the Yale School of Medicine, New Haven, Conn., and chief of endocrinology for the VA Connecticut Healthcare System.
The scientific literature is divided on this, but there is enough evidence to support further inquiry, Dr. Srikanthan said. Her team has begun a study to examine that link.
Opening the door to future obesity treatments
But their discovery means other new treatments could be on the horizon.
Previous research had shown that the sympathetic nervous system drives brown fat activity. But now that the UCLA scientists have revealed the exact nerves connecting brown fat to the sympathetic nervous system, we could find ways to stimulate those pathways to activate brown fat – without stimulating the many organs (such as the heart and stomach) also connected to this vast network of nerves, Dr. Srikanthan said.
Methods for doing that could include medication, electrical stimulation, or heat therapy, according to the study.
Still, there is reason to temper expectations. “[Brown fat] depots, while highly metabolically active, are quite small,” Dr. Samuel said. “So, the overall contribution to whole-body energy balance in humans will likely be small.”
On the other hand, that prediction doesn’t account for what we don’t know.
“We’re learning more about how tissues communicate with each other, beyond the release of hormones or metabolites,” Dr. Samuel said. Activating brown fat could trigger “signals that help coordinate whole-body energy metabolism.”
A version of this article first appeared on WebMD.com.
FROM PLOS ONE
Fractures beget fractures at any age
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2023
Similar prognoses for contralateral axillary lymph node metastasis and oligometastatic disease in BC
Key clinical point: The prognosis for patients with breast cancer (BC) who develop contralateral axillary lymph node metastasis (CAM) is similar to that for patients developing oligometastatic disease (OM) but is considerably worse than that for patients developing locoregional recurrence (LRR).
Major finding: The overall survival (OS) and progression-free survival (PFS) outcomes in patients with CAM were similar to those in patients with OM (P = .07 and P = .97, respectively) but were significantly worse than those in patients with LRR (hazard ratio [HR] 0.47, P = .0097; and HR 0.39, P < .0001).
Study details: Findings are from a single-center retrospective study including 299 patients with BC, of whom 29, 180, and 90 patients developed CAM, OM, and LRR respectively.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhao Q et al. Contralateral axillary lymph node metastasis in breast cancer: An oligometastatic-like disease. Breast. 2023 (Oct 7). doi: 10.1016/j.breast.2023.103589
Key clinical point: The prognosis for patients with breast cancer (BC) who develop contralateral axillary lymph node metastasis (CAM) is similar to that for patients developing oligometastatic disease (OM) but is considerably worse than that for patients developing locoregional recurrence (LRR).
Major finding: The overall survival (OS) and progression-free survival (PFS) outcomes in patients with CAM were similar to those in patients with OM (P = .07 and P = .97, respectively) but were significantly worse than those in patients with LRR (hazard ratio [HR] 0.47, P = .0097; and HR 0.39, P < .0001).
Study details: Findings are from a single-center retrospective study including 299 patients with BC, of whom 29, 180, and 90 patients developed CAM, OM, and LRR respectively.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhao Q et al. Contralateral axillary lymph node metastasis in breast cancer: An oligometastatic-like disease. Breast. 2023 (Oct 7). doi: 10.1016/j.breast.2023.103589
Key clinical point: The prognosis for patients with breast cancer (BC) who develop contralateral axillary lymph node metastasis (CAM) is similar to that for patients developing oligometastatic disease (OM) but is considerably worse than that for patients developing locoregional recurrence (LRR).
Major finding: The overall survival (OS) and progression-free survival (PFS) outcomes in patients with CAM were similar to those in patients with OM (P = .07 and P = .97, respectively) but were significantly worse than those in patients with LRR (hazard ratio [HR] 0.47, P = .0097; and HR 0.39, P < .0001).
Study details: Findings are from a single-center retrospective study including 299 patients with BC, of whom 29, 180, and 90 patients developed CAM, OM, and LRR respectively.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Zhao Q et al. Contralateral axillary lymph node metastasis in breast cancer: An oligometastatic-like disease. Breast. 2023 (Oct 7). doi: 10.1016/j.breast.2023.103589
Metronomic capecitabine+pyrotinib shows clinical benefits in HER2+ metastatic BC in phase 2
Key clinical point: The combination of oral metronomic capecitabine and pyrotinib showed acceptable efficacy and tolerable safety in patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: Patients receiving metronomic capecitabine + pyrotinib had an objective response rate of 34.7% and a clinical benefit rate of 81.6%, with 4.1% and 30.6% of patients achieving complete and partial responses, respectively, which lasted for ≥ 24 weeks. The most common grade 3 adverse events were hand-foot syndrome (12.2%), diarrhea (12.2%), vomiting (4.1%), and nausea (2.0%).
Study details: Findings are from a prospective, single-arm phase 2 trial including 49 patients with HER2+ metastatic BC who received 500 mg oral metronomic capecitabine 3 times per day and 400 mg pyrotinib per day.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: He M, Liu J, et al. Safety and efficacy study of oral metronomic capecitabine combined with pyrotinib in HER2-positive metastatic breast cancer: A phase II trial. Breast. 2023;72:105381 (Sep 19). doi: 10.1016/j.breast.2023.103581
Key clinical point: The combination of oral metronomic capecitabine and pyrotinib showed acceptable efficacy and tolerable safety in patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: Patients receiving metronomic capecitabine + pyrotinib had an objective response rate of 34.7% and a clinical benefit rate of 81.6%, with 4.1% and 30.6% of patients achieving complete and partial responses, respectively, which lasted for ≥ 24 weeks. The most common grade 3 adverse events were hand-foot syndrome (12.2%), diarrhea (12.2%), vomiting (4.1%), and nausea (2.0%).
Study details: Findings are from a prospective, single-arm phase 2 trial including 49 patients with HER2+ metastatic BC who received 500 mg oral metronomic capecitabine 3 times per day and 400 mg pyrotinib per day.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: He M, Liu J, et al. Safety and efficacy study of oral metronomic capecitabine combined with pyrotinib in HER2-positive metastatic breast cancer: A phase II trial. Breast. 2023;72:105381 (Sep 19). doi: 10.1016/j.breast.2023.103581
Key clinical point: The combination of oral metronomic capecitabine and pyrotinib showed acceptable efficacy and tolerable safety in patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: Patients receiving metronomic capecitabine + pyrotinib had an objective response rate of 34.7% and a clinical benefit rate of 81.6%, with 4.1% and 30.6% of patients achieving complete and partial responses, respectively, which lasted for ≥ 24 weeks. The most common grade 3 adverse events were hand-foot syndrome (12.2%), diarrhea (12.2%), vomiting (4.1%), and nausea (2.0%).
Study details: Findings are from a prospective, single-arm phase 2 trial including 49 patients with HER2+ metastatic BC who received 500 mg oral metronomic capecitabine 3 times per day and 400 mg pyrotinib per day.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: He M, Liu J, et al. Safety and efficacy study of oral metronomic capecitabine combined with pyrotinib in HER2-positive metastatic breast cancer: A phase II trial. Breast. 2023;72:105381 (Sep 19). doi: 10.1016/j.breast.2023.103581
First-line palbociclib+AI improves prognosis in elderly patients with metastatic BC in real-world settings
Key clinical point: First-line therapy with palbociclib plus an aromatase inhibitor (AI) vs only AI improved survival outcomes in elderly patients (age ≥75 years) with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Patients receiving palbociclib + AI combination therapy vs only AI had a significantly improved overall survival (hazard ratio 0.66; P = .0007), real-world progression-free survival (hazard ratio 0.72; P = .0021) and prolonged time to receiving chemotherapy (hazard ratio 0.69; P = .0014).
Study details: This sub-analysis of the retrospective observational P-REALITY X cohort study included 961 patients with HR+/HER2− metastatic BC who were age ≥ 75 years and received either palbociclib + AI (32.6%) or only AI (67.4%) as first-line therapy.
Disclosures: This study was funded by Pfizer. Four authors declared being employees and stockholders of Pfizer. The other authors declared receiving research grants, consulting or advisory fees, honoraria, or sponsorship for research from Pfizer and other sources.
Source: Brufsky A et al. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front Oncol. 2023;13:1237751 (Sep 28). doi: 10.3389/fonc.2023.1237751
Key clinical point: First-line therapy with palbociclib plus an aromatase inhibitor (AI) vs only AI improved survival outcomes in elderly patients (age ≥75 years) with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Patients receiving palbociclib + AI combination therapy vs only AI had a significantly improved overall survival (hazard ratio 0.66; P = .0007), real-world progression-free survival (hazard ratio 0.72; P = .0021) and prolonged time to receiving chemotherapy (hazard ratio 0.69; P = .0014).
Study details: This sub-analysis of the retrospective observational P-REALITY X cohort study included 961 patients with HR+/HER2− metastatic BC who were age ≥ 75 years and received either palbociclib + AI (32.6%) or only AI (67.4%) as first-line therapy.
Disclosures: This study was funded by Pfizer. Four authors declared being employees and stockholders of Pfizer. The other authors declared receiving research grants, consulting or advisory fees, honoraria, or sponsorship for research from Pfizer and other sources.
Source: Brufsky A et al. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front Oncol. 2023;13:1237751 (Sep 28). doi: 10.3389/fonc.2023.1237751
Key clinical point: First-line therapy with palbociclib plus an aromatase inhibitor (AI) vs only AI improved survival outcomes in elderly patients (age ≥75 years) with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Patients receiving palbociclib + AI combination therapy vs only AI had a significantly improved overall survival (hazard ratio 0.66; P = .0007), real-world progression-free survival (hazard ratio 0.72; P = .0021) and prolonged time to receiving chemotherapy (hazard ratio 0.69; P = .0014).
Study details: This sub-analysis of the retrospective observational P-REALITY X cohort study included 961 patients with HR+/HER2− metastatic BC who were age ≥ 75 years and received either palbociclib + AI (32.6%) or only AI (67.4%) as first-line therapy.
Disclosures: This study was funded by Pfizer. Four authors declared being employees and stockholders of Pfizer. The other authors declared receiving research grants, consulting or advisory fees, honoraria, or sponsorship for research from Pfizer and other sources.
Source: Brufsky A et al. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front Oncol. 2023;13:1237751 (Sep 28). doi: 10.3389/fonc.2023.1237751
Prognostic predictors in breast cancer brain metastases after stereotactic surgery
Key clinical point: Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis in patients with metastatic breast cancer (BC) who had brain metastases and underwent stereotactic radiosurgery (SRS).
Major finding: The median overall survival (OS) was 14.8 months for the entire cohort. OS outcomes worsened in patients with estrogen receptor-negative (ER−)/human epidermal growth factor receptor 2-negative (HER2−) BC (hazard ratio [HR] 2.00; 95% CI 1.09-3.67) but were better in those with ER+/HER2+ BC (HR 0.43; 95% CI 0.19-0.96). The presence of extracranial visceral metastases (HR 2.90; 95% CI 1.53-5.50) was also associated with poor survival outcomes.
Study details: Findings are from a retrospective analysis of a cohort including 149 patients with metastatic breast cancer and brain metastases underwent received SRS.
Disclosures: This study was supported by Lundbeck Foundation, Copenhagen. The authors declared no conflicts of interest.
Source: Depner JF et al. Treating brain metastases in metastatic breast cancer: Outcomes after stereotactic radiosurgery examined in a retrospective, single-center cohort analysis. Acta Oncol. 2023 (Sep 26). doi: 10.1080/0284186X.2023.2260942
Key clinical point: Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis in patients with metastatic breast cancer (BC) who had brain metastases and underwent stereotactic radiosurgery (SRS).
Major finding: The median overall survival (OS) was 14.8 months for the entire cohort. OS outcomes worsened in patients with estrogen receptor-negative (ER−)/human epidermal growth factor receptor 2-negative (HER2−) BC (hazard ratio [HR] 2.00; 95% CI 1.09-3.67) but were better in those with ER+/HER2+ BC (HR 0.43; 95% CI 0.19-0.96). The presence of extracranial visceral metastases (HR 2.90; 95% CI 1.53-5.50) was also associated with poor survival outcomes.
Study details: Findings are from a retrospective analysis of a cohort including 149 patients with metastatic breast cancer and brain metastases underwent received SRS.
Disclosures: This study was supported by Lundbeck Foundation, Copenhagen. The authors declared no conflicts of interest.
Source: Depner JF et al. Treating brain metastases in metastatic breast cancer: Outcomes after stereotactic radiosurgery examined in a retrospective, single-center cohort analysis. Acta Oncol. 2023 (Sep 26). doi: 10.1080/0284186X.2023.2260942
Key clinical point: Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis in patients with metastatic breast cancer (BC) who had brain metastases and underwent stereotactic radiosurgery (SRS).
Major finding: The median overall survival (OS) was 14.8 months for the entire cohort. OS outcomes worsened in patients with estrogen receptor-negative (ER−)/human epidermal growth factor receptor 2-negative (HER2−) BC (hazard ratio [HR] 2.00; 95% CI 1.09-3.67) but were better in those with ER+/HER2+ BC (HR 0.43; 95% CI 0.19-0.96). The presence of extracranial visceral metastases (HR 2.90; 95% CI 1.53-5.50) was also associated with poor survival outcomes.
Study details: Findings are from a retrospective analysis of a cohort including 149 patients with metastatic breast cancer and brain metastases underwent received SRS.
Disclosures: This study was supported by Lundbeck Foundation, Copenhagen. The authors declared no conflicts of interest.
Source: Depner JF et al. Treating brain metastases in metastatic breast cancer: Outcomes after stereotactic radiosurgery examined in a retrospective, single-center cohort analysis. Acta Oncol. 2023 (Sep 26). doi: 10.1080/0284186X.2023.2260942
Primary breast tumor surgery does not prolong survival in de novo metastatic BC shows meta-analysis
Key clinical point: Surgical removal of the primary tumor failed to prolong survival and may not be necessary in patients with de novo metastatic breast cancer (BC).
Major finding: In women with de novo metastatic BC, primary breast tumor surgery vs no surgery improved the local progression-free survival outcomes (hazard ratio [HR] 0.37; 95% CI 0.19-0.74) but not the overall survival (HR 0.93; 95% CI 0.76-1.14).
Study details: Findings are from a meta-analysis of five randomized controlled trials including 1381 patients with de novo metastatic BC, of whom 49.6% underwent primary breast tumor surgery.
Disclosures: This study did not receive any specific funding. Three authors declared having advisory roles, serving as consultants, or receiving speaker fees, consulting fees, or unrelated research grants from various sources.
Source: Villacampa G et al. Impact of primary breast surgery on overall survival of patients with de novo metastatic breast cancer: A systematic review and meta-analysis. Oncologist. 2023 (Sep 12). doi: 10.1093/oncolo/oyad266
Key clinical point: Surgical removal of the primary tumor failed to prolong survival and may not be necessary in patients with de novo metastatic breast cancer (BC).
Major finding: In women with de novo metastatic BC, primary breast tumor surgery vs no surgery improved the local progression-free survival outcomes (hazard ratio [HR] 0.37; 95% CI 0.19-0.74) but not the overall survival (HR 0.93; 95% CI 0.76-1.14).
Study details: Findings are from a meta-analysis of five randomized controlled trials including 1381 patients with de novo metastatic BC, of whom 49.6% underwent primary breast tumor surgery.
Disclosures: This study did not receive any specific funding. Three authors declared having advisory roles, serving as consultants, or receiving speaker fees, consulting fees, or unrelated research grants from various sources.
Source: Villacampa G et al. Impact of primary breast surgery on overall survival of patients with de novo metastatic breast cancer: A systematic review and meta-analysis. Oncologist. 2023 (Sep 12). doi: 10.1093/oncolo/oyad266
Key clinical point: Surgical removal of the primary tumor failed to prolong survival and may not be necessary in patients with de novo metastatic breast cancer (BC).
Major finding: In women with de novo metastatic BC, primary breast tumor surgery vs no surgery improved the local progression-free survival outcomes (hazard ratio [HR] 0.37; 95% CI 0.19-0.74) but not the overall survival (HR 0.93; 95% CI 0.76-1.14).
Study details: Findings are from a meta-analysis of five randomized controlled trials including 1381 patients with de novo metastatic BC, of whom 49.6% underwent primary breast tumor surgery.
Disclosures: This study did not receive any specific funding. Three authors declared having advisory roles, serving as consultants, or receiving speaker fees, consulting fees, or unrelated research grants from various sources.
Source: Villacampa G et al. Impact of primary breast surgery on overall survival of patients with de novo metastatic breast cancer: A systematic review and meta-analysis. Oncologist. 2023 (Sep 12). doi: 10.1093/oncolo/oyad266

