User login
Trump Promises Funding Boost for VA and DoD
In his first address to a joint session of Congress, President Donald Trump promised “heroic veterans will get the care they so desperately need,” that he would eliminate the defense sequester, and called for “one of the largest increases in national defense spending in American history.”
“This looks like an increase in resources for us,” VA Secretary David Shulkin, MD, is reported to have told reporters early in the day at an American Legion meeting. “I'm confident this budget is going to reflect the President’s commitment to his ability to deliver on his promises to make veterans care better and stronger.” According to reports, the White House already has approved 37,000 exemptions from the federal hiring ban to help fill the VA’s 45,000 current job vacancies.
Reports also suggest that the military will receive an additional $54 billion in funding, a 10% increase. Details of where that extra money will go have not been released. The President’s military budget is less than the $640 billion budget proposed by Senator John McCain (R- AZ) and represents a 3% increase over the budget that had been projected by President Obama.
The overall impact on federal health care remains unclear. While President Trump held to his promise to repeal and replace the Affordable Care Act, to “invest in women’s health,” and to “give our state governors the resources and flexibility they need with Medicaid to make sure no one is left out,” there were few details of how those promises would be implemented and where the funding will come from.
In the address, the President outlined 4 necessary elements of an Affordable Care Act replacement:
- Americans with pre-existing conditions would have access to coverage, and a stable transition for health care exchanges enrollees;
- The use of tax credits and savings accounts for purchasing private insurance;
- Flexibility for states to expand Medicaid coverage; and
- Legal reforms that “protect patients and doctors from unnecessary costs that drive up the price of insurance and work to bring down the artificially high price of drugs and bring them down immediately.”
The FDA also drew the President’s attention. Calling the approval process “slow and burdensome,” the President charged that the FDA “keeps too many advances… from reaching those in need.” The agency is still waiting on a nomination for its commissioner position, and it is unclear how it can speed up approval while under the federal hiring freeze.
Recognizing the devastation of opioid addiction, the President also promised to “stop the drugs from pouring into our country and poisoning our youth, and we will expand treatment for those who have become so badly addicted.” The President did not specify whether treatment resources would be exempt from the hiring freeze, incorporated into the Affordable Care Act, or handled in a different manner.
In his first address to a joint session of Congress, President Donald Trump promised “heroic veterans will get the care they so desperately need,” that he would eliminate the defense sequester, and called for “one of the largest increases in national defense spending in American history.”
“This looks like an increase in resources for us,” VA Secretary David Shulkin, MD, is reported to have told reporters early in the day at an American Legion meeting. “I'm confident this budget is going to reflect the President’s commitment to his ability to deliver on his promises to make veterans care better and stronger.” According to reports, the White House already has approved 37,000 exemptions from the federal hiring ban to help fill the VA’s 45,000 current job vacancies.
Reports also suggest that the military will receive an additional $54 billion in funding, a 10% increase. Details of where that extra money will go have not been released. The President’s military budget is less than the $640 billion budget proposed by Senator John McCain (R- AZ) and represents a 3% increase over the budget that had been projected by President Obama.
The overall impact on federal health care remains unclear. While President Trump held to his promise to repeal and replace the Affordable Care Act, to “invest in women’s health,” and to “give our state governors the resources and flexibility they need with Medicaid to make sure no one is left out,” there were few details of how those promises would be implemented and where the funding will come from.
In the address, the President outlined 4 necessary elements of an Affordable Care Act replacement:
- Americans with pre-existing conditions would have access to coverage, and a stable transition for health care exchanges enrollees;
- The use of tax credits and savings accounts for purchasing private insurance;
- Flexibility for states to expand Medicaid coverage; and
- Legal reforms that “protect patients and doctors from unnecessary costs that drive up the price of insurance and work to bring down the artificially high price of drugs and bring them down immediately.”
The FDA also drew the President’s attention. Calling the approval process “slow and burdensome,” the President charged that the FDA “keeps too many advances… from reaching those in need.” The agency is still waiting on a nomination for its commissioner position, and it is unclear how it can speed up approval while under the federal hiring freeze.
Recognizing the devastation of opioid addiction, the President also promised to “stop the drugs from pouring into our country and poisoning our youth, and we will expand treatment for those who have become so badly addicted.” The President did not specify whether treatment resources would be exempt from the hiring freeze, incorporated into the Affordable Care Act, or handled in a different manner.
In his first address to a joint session of Congress, President Donald Trump promised “heroic veterans will get the care they so desperately need,” that he would eliminate the defense sequester, and called for “one of the largest increases in national defense spending in American history.”
“This looks like an increase in resources for us,” VA Secretary David Shulkin, MD, is reported to have told reporters early in the day at an American Legion meeting. “I'm confident this budget is going to reflect the President’s commitment to his ability to deliver on his promises to make veterans care better and stronger.” According to reports, the White House already has approved 37,000 exemptions from the federal hiring ban to help fill the VA’s 45,000 current job vacancies.
Reports also suggest that the military will receive an additional $54 billion in funding, a 10% increase. Details of where that extra money will go have not been released. The President’s military budget is less than the $640 billion budget proposed by Senator John McCain (R- AZ) and represents a 3% increase over the budget that had been projected by President Obama.
The overall impact on federal health care remains unclear. While President Trump held to his promise to repeal and replace the Affordable Care Act, to “invest in women’s health,” and to “give our state governors the resources and flexibility they need with Medicaid to make sure no one is left out,” there were few details of how those promises would be implemented and where the funding will come from.
In the address, the President outlined 4 necessary elements of an Affordable Care Act replacement:
- Americans with pre-existing conditions would have access to coverage, and a stable transition for health care exchanges enrollees;
- The use of tax credits and savings accounts for purchasing private insurance;
- Flexibility for states to expand Medicaid coverage; and
- Legal reforms that “protect patients and doctors from unnecessary costs that drive up the price of insurance and work to bring down the artificially high price of drugs and bring them down immediately.”
The FDA also drew the President’s attention. Calling the approval process “slow and burdensome,” the President charged that the FDA “keeps too many advances… from reaching those in need.” The agency is still waiting on a nomination for its commissioner position, and it is unclear how it can speed up approval while under the federal hiring freeze.
Recognizing the devastation of opioid addiction, the President also promised to “stop the drugs from pouring into our country and poisoning our youth, and we will expand treatment for those who have become so badly addicted.” The President did not specify whether treatment resources would be exempt from the hiring freeze, incorporated into the Affordable Care Act, or handled in a different manner.
Treating tardive dyskinesia
The Patellofemoral Compartment: Making Sense of It
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The Diagnosis and Initial Treatment of Patellofemoral Disorders
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
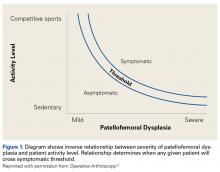
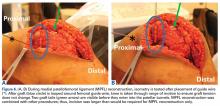
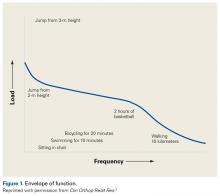
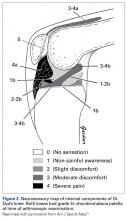
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
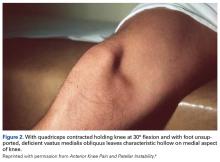
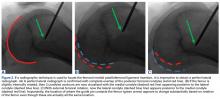
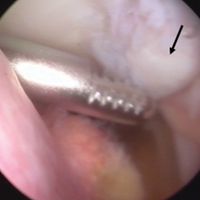
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
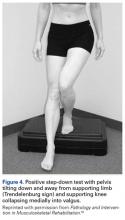
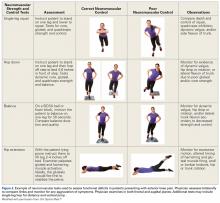
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
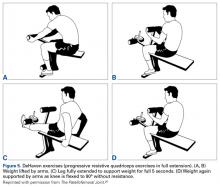
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
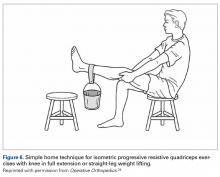
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
Correct Positioning of the Medial Patellofemoral Ligament: Troubleshooting in the Operating Room
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
Clinical Rehabilitation of Anterior Knee Pain: Current Concepts
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
Nutrition expert to heart patients: ‘Eat some cheese’
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
The Role of Medial Patellofemoral Ligament Repair and Imbrication
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
Patellofemoral Pain: An Enigma Explained by Homeostasis and Common Sense
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
A Practical Guide to Understanding and Treating Patellofemoral Pain
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.