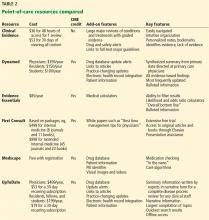
User login
Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
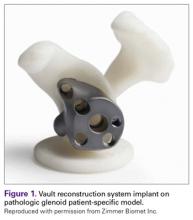
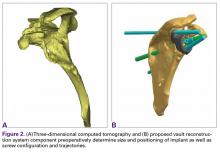
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
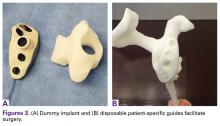
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
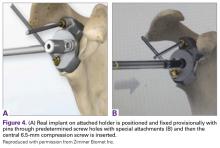
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
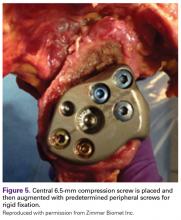
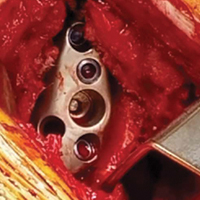
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
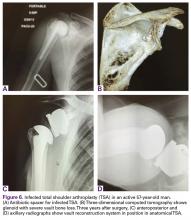
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
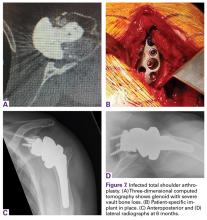
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
High-Resolution Wireless Ultrasound
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
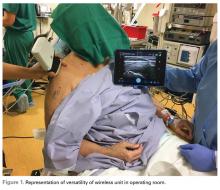
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
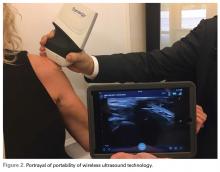
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
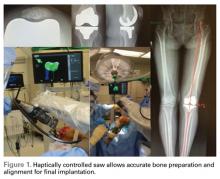
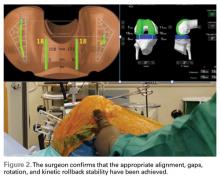
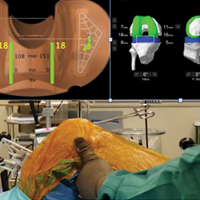
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Bedbugs: Helping your patient through an infestation
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
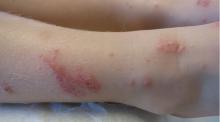
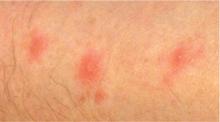
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
KEY POINTS
- The increase in pyrethroid resistance, the ban of DDT, the ease and frequency of travel, and the increased population density in large cities have led to an exponential rise in the incidence of bedbug infection.
- Once the diagnosis is suggested, patients deserve symptomatic treatment, and extermination of the pests becomes essential, though time-consuming, costly, and often problematic.
- Measures to eliminate infestation and prevent spread include early detection, identification of the pest, patient education, and professional eradication.
Bedbugs: Awareness is key
In 2004, knowing of my medical interest in arthropods, a resident came to my office to discuss an “unusual case” of pubic louse infestation seen at another hospital: a middle-aged woman had been afflicted for months with a skin eruption with excoriation and impetigo that involved the arms and legs but not the pubic area.
In a bag, the resident had a dead insect, 5 mm in length, with a brown, lens-shaped body and short hairs on the pronotum that were visible with a hand lens. An attending dermatologist at the other hospital had identified the insect—incorrectly—as a pubic louse.
With deference, I informed the resident that I did not share the opinion that this was a pubic louse, unless the insect represented a new “Cleveland variant” of the species (a reference to the 1975 Bruce Maness sci-fi film, The Tomato That Ate Cleveland). Rather, I stated, “I believe this is a bedbug, but I have not seen many specimens.”
In hindsight, these words were prophetic, for since 2004, the incidence of bedbug infestations has remarkably surged.1 The trend has not abated, making the review by Ibrahim et al in this issue of the Cleveland Clinic Journal of Medicine timely for all practitioners.2
BEDBUGS ARE BACK…
Bedbugs have plagued man for millennia. In 1939, it was estimated that 4 million Londoners (in a city of 8.5 million) were bitten by bedbugs each night.3 However, as Ibrahim et al describe, long-acting pesticides introduced during World War II dramatically reduced infestation rates. By 1997, some college entomology programs reported difficulty in locating a single teaching specimen.4
The modern resurgence of bedbugs is multifactorial, including a ban on long-acting pesticides such as dichlorodiphenyltrichloroethane (DDT), as well as population growth and increased travel. In days past, bedbug infestations may have pertained to hygiene and social status. But today, travel is a major factor in the resurgence, and bedbugs now affect a broader segment of the population, including the affluent—something that must be kept in mind in the clinical setting.5
…AND THEY’RE EVERYWHERE
Many prominent US cities are experiencing near-epidemic numbers of bedbug infestations (Table 1). Bedbug infestations occur not only in homes and hotel rooms, but also in hospitals,6 office buildings,7 movie theaters,8 schools,9 and even on subways and trains,10 expanding the number of people potentially exposed.
Understanding that bedbugs affect more than people who are in bed, or with hygiene challenges, Ibrahim et al describe the presentation of bedbug bites—useful information for all practitioners, regardless of medical specialty.
Bedbugs bite skin that is exposed during sleep (ie, the distal extremities and the head, face, and neck). Quasilinear bites, in groups of three (the notorious “breakfast, lunch, and dinner” sign) are a good clue to remember. Unusually exuberant reactions to bedbug bites may be confused with autoimmune bullous conditions or primary vasculitides.11
NOT ALL WHO ARE BITTEN HAVE REACTIONS
Intricate entomologic studies have shown that substances in bedbug saliva drive bite reactions.12,13 However, as Ibrahim et al mention, not all bites provoke a reaction in all persons.
This fact cannot be overstated, for providers in primary care and urgent and emergency care settings may have learned to ask questions about scabies such as, “Are other persons in the household similarly affected?” While it is uncommon for a person with scabies not to present with visible skin lesions, one does not want to misinterpret this historical detail in the setting of bedbug bites. If one person in a household has lesions and another does not, this does not exclude a bedbug infestation!
Ibrahim et al emphasize that treatment of bedbug bites is supportive in nature. Most often, extermination in the home or any other setting should be conducted by professionals. During travel, prevention by inspection is widely advocated.5 There has been interest in using oral ivermectin in affected patients to adversely affect the bedbug colony, but to date, early experiments have encountered daunting pharmacokinetic concerns.14
PSYCHOLOGICAL AND OTHER SEQUELAE
A final consideration in bedbug infestations is any lasting sequelae beyond the bites themselves. Bedbug infestations severe enough to cause anemia and exacerbate or trigger adverse cardiac events have been reported.15 While bedbugs carry human pathogens such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, hepatitis B virus, Bartonella quintana, and Trypanosoma cruzi, Ibrahim et al correctly inform the reader that there are no compelling reports of transmission of these diseases via bedbug bites.16
However, there may be lasting psychological sequelae. Anxiety, hypervigilance, insomnia, avoidance behaviors, and personal dysfunction can persist, even long after the infestation has been eradicated.
Bedbugs are a national and even global health problem worthy of familiarity by all healthcare providers, regardless of specialty. In this regard, Ibrahim et al succinctly and accurately provide a functional and clinically useful guide.
- Alalawi AH. Bed bugs epidemic in the United States. Entomol Ornithol Herpetol 2015; 4:143–148.
- Ibrahim O, Syed UM, Tomecki KJ. Bedbugs: a practical review. Clev Clin J Med 2017; 84:207–211.
- Velten H. Beastly London—A History of Animals in the City. London: Reaktion Books. November 15, 2013. p. 221.
- Snetsinger R. Bed bugs and other bugs. In: Moreland D, editor. Mallis Handbook of Pest Control: The Behavior, Life History, and Control of House Pests, 8th edition. Cleveland, OH: GIE Publishers, 1997:392–424.
- Kolb A, Needham GR, Neyman KM, High WA. Bedbugs. Dermatol Ther 2009; 22:347–352.
- Totten V, Charbonneau H, Hoch W, Shah C, Sheele J. The cost of decontaminating an ED after finding a bed bug: results from a single academic medical center. Am J Emerg Med 2016; 34:649.
- Baumblatt JA, Dunn JR, Schaffner W, Moncayo AC, Stull-Lane A, Jones TF. An outbreak of bed bug infestation in an office building. J Environ Health 2014; 76:16–18.
- Chalupka S. Preventing bedbug infestation. AAOHN J 2010; 58:500.
- Scisicione P. Bed bugs: they are back! The role of the school nurse in bed bug management. NASN Sch Nurse 2012; 27:268–273.
- Anders D, Brocker EB, Hamm H. Cimex lectularius—an unwelcome train attendant. Eur J Dermatol 2010; 20:239–240.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Potter MF, Haynes KF, Deutsch M, et al. The sensitivity spectrum: human reactions to bed bug bites. Pest Control Technology Magazine 2010; 70–75.
- Reinhardt K, Kempke D, Naylor RA, Siva-Jothy MT. Sensitivity to bites by the bedbug, Cimex lectularius. Med Vet Entomol 2009; 23:163–166.
- Sheele JM, Anderson JF, Tran TD, et al. Ivermectin causes Cimex lectularius (bedbug) morbidity and mortality. J Emerg Med 2013; 45:433–440.
- Paulke-Korinek M, Széll M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Ho D, Lai O, Glick S, Jagdeo J. Lack of evidence that bedbugs transmit pathogens to humans. J Am Acad Dermatol 2016; 74:1261.
In 2004, knowing of my medical interest in arthropods, a resident came to my office to discuss an “unusual case” of pubic louse infestation seen at another hospital: a middle-aged woman had been afflicted for months with a skin eruption with excoriation and impetigo that involved the arms and legs but not the pubic area.
In a bag, the resident had a dead insect, 5 mm in length, with a brown, lens-shaped body and short hairs on the pronotum that were visible with a hand lens. An attending dermatologist at the other hospital had identified the insect—incorrectly—as a pubic louse.
With deference, I informed the resident that I did not share the opinion that this was a pubic louse, unless the insect represented a new “Cleveland variant” of the species (a reference to the 1975 Bruce Maness sci-fi film, The Tomato That Ate Cleveland). Rather, I stated, “I believe this is a bedbug, but I have not seen many specimens.”
In hindsight, these words were prophetic, for since 2004, the incidence of bedbug infestations has remarkably surged.1 The trend has not abated, making the review by Ibrahim et al in this issue of the Cleveland Clinic Journal of Medicine timely for all practitioners.2
BEDBUGS ARE BACK…
Bedbugs have plagued man for millennia. In 1939, it was estimated that 4 million Londoners (in a city of 8.5 million) were bitten by bedbugs each night.3 However, as Ibrahim et al describe, long-acting pesticides introduced during World War II dramatically reduced infestation rates. By 1997, some college entomology programs reported difficulty in locating a single teaching specimen.4
The modern resurgence of bedbugs is multifactorial, including a ban on long-acting pesticides such as dichlorodiphenyltrichloroethane (DDT), as well as population growth and increased travel. In days past, bedbug infestations may have pertained to hygiene and social status. But today, travel is a major factor in the resurgence, and bedbugs now affect a broader segment of the population, including the affluent—something that must be kept in mind in the clinical setting.5
…AND THEY’RE EVERYWHERE
Many prominent US cities are experiencing near-epidemic numbers of bedbug infestations (Table 1). Bedbug infestations occur not only in homes and hotel rooms, but also in hospitals,6 office buildings,7 movie theaters,8 schools,9 and even on subways and trains,10 expanding the number of people potentially exposed.
Understanding that bedbugs affect more than people who are in bed, or with hygiene challenges, Ibrahim et al describe the presentation of bedbug bites—useful information for all practitioners, regardless of medical specialty.
Bedbugs bite skin that is exposed during sleep (ie, the distal extremities and the head, face, and neck). Quasilinear bites, in groups of three (the notorious “breakfast, lunch, and dinner” sign) are a good clue to remember. Unusually exuberant reactions to bedbug bites may be confused with autoimmune bullous conditions or primary vasculitides.11
NOT ALL WHO ARE BITTEN HAVE REACTIONS
Intricate entomologic studies have shown that substances in bedbug saliva drive bite reactions.12,13 However, as Ibrahim et al mention, not all bites provoke a reaction in all persons.
This fact cannot be overstated, for providers in primary care and urgent and emergency care settings may have learned to ask questions about scabies such as, “Are other persons in the household similarly affected?” While it is uncommon for a person with scabies not to present with visible skin lesions, one does not want to misinterpret this historical detail in the setting of bedbug bites. If one person in a household has lesions and another does not, this does not exclude a bedbug infestation!
Ibrahim et al emphasize that treatment of bedbug bites is supportive in nature. Most often, extermination in the home or any other setting should be conducted by professionals. During travel, prevention by inspection is widely advocated.5 There has been interest in using oral ivermectin in affected patients to adversely affect the bedbug colony, but to date, early experiments have encountered daunting pharmacokinetic concerns.14
PSYCHOLOGICAL AND OTHER SEQUELAE
A final consideration in bedbug infestations is any lasting sequelae beyond the bites themselves. Bedbug infestations severe enough to cause anemia and exacerbate or trigger adverse cardiac events have been reported.15 While bedbugs carry human pathogens such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, hepatitis B virus, Bartonella quintana, and Trypanosoma cruzi, Ibrahim et al correctly inform the reader that there are no compelling reports of transmission of these diseases via bedbug bites.16
However, there may be lasting psychological sequelae. Anxiety, hypervigilance, insomnia, avoidance behaviors, and personal dysfunction can persist, even long after the infestation has been eradicated.
Bedbugs are a national and even global health problem worthy of familiarity by all healthcare providers, regardless of specialty. In this regard, Ibrahim et al succinctly and accurately provide a functional and clinically useful guide.
In 2004, knowing of my medical interest in arthropods, a resident came to my office to discuss an “unusual case” of pubic louse infestation seen at another hospital: a middle-aged woman had been afflicted for months with a skin eruption with excoriation and impetigo that involved the arms and legs but not the pubic area.
In a bag, the resident had a dead insect, 5 mm in length, with a brown, lens-shaped body and short hairs on the pronotum that were visible with a hand lens. An attending dermatologist at the other hospital had identified the insect—incorrectly—as a pubic louse.
With deference, I informed the resident that I did not share the opinion that this was a pubic louse, unless the insect represented a new “Cleveland variant” of the species (a reference to the 1975 Bruce Maness sci-fi film, The Tomato That Ate Cleveland). Rather, I stated, “I believe this is a bedbug, but I have not seen many specimens.”
In hindsight, these words were prophetic, for since 2004, the incidence of bedbug infestations has remarkably surged.1 The trend has not abated, making the review by Ibrahim et al in this issue of the Cleveland Clinic Journal of Medicine timely for all practitioners.2
BEDBUGS ARE BACK…
Bedbugs have plagued man for millennia. In 1939, it was estimated that 4 million Londoners (in a city of 8.5 million) were bitten by bedbugs each night.3 However, as Ibrahim et al describe, long-acting pesticides introduced during World War II dramatically reduced infestation rates. By 1997, some college entomology programs reported difficulty in locating a single teaching specimen.4
The modern resurgence of bedbugs is multifactorial, including a ban on long-acting pesticides such as dichlorodiphenyltrichloroethane (DDT), as well as population growth and increased travel. In days past, bedbug infestations may have pertained to hygiene and social status. But today, travel is a major factor in the resurgence, and bedbugs now affect a broader segment of the population, including the affluent—something that must be kept in mind in the clinical setting.5
…AND THEY’RE EVERYWHERE
Many prominent US cities are experiencing near-epidemic numbers of bedbug infestations (Table 1). Bedbug infestations occur not only in homes and hotel rooms, but also in hospitals,6 office buildings,7 movie theaters,8 schools,9 and even on subways and trains,10 expanding the number of people potentially exposed.
Understanding that bedbugs affect more than people who are in bed, or with hygiene challenges, Ibrahim et al describe the presentation of bedbug bites—useful information for all practitioners, regardless of medical specialty.
Bedbugs bite skin that is exposed during sleep (ie, the distal extremities and the head, face, and neck). Quasilinear bites, in groups of three (the notorious “breakfast, lunch, and dinner” sign) are a good clue to remember. Unusually exuberant reactions to bedbug bites may be confused with autoimmune bullous conditions or primary vasculitides.11
NOT ALL WHO ARE BITTEN HAVE REACTIONS
Intricate entomologic studies have shown that substances in bedbug saliva drive bite reactions.12,13 However, as Ibrahim et al mention, not all bites provoke a reaction in all persons.
This fact cannot be overstated, for providers in primary care and urgent and emergency care settings may have learned to ask questions about scabies such as, “Are other persons in the household similarly affected?” While it is uncommon for a person with scabies not to present with visible skin lesions, one does not want to misinterpret this historical detail in the setting of bedbug bites. If one person in a household has lesions and another does not, this does not exclude a bedbug infestation!
Ibrahim et al emphasize that treatment of bedbug bites is supportive in nature. Most often, extermination in the home or any other setting should be conducted by professionals. During travel, prevention by inspection is widely advocated.5 There has been interest in using oral ivermectin in affected patients to adversely affect the bedbug colony, but to date, early experiments have encountered daunting pharmacokinetic concerns.14
PSYCHOLOGICAL AND OTHER SEQUELAE
A final consideration in bedbug infestations is any lasting sequelae beyond the bites themselves. Bedbug infestations severe enough to cause anemia and exacerbate or trigger adverse cardiac events have been reported.15 While bedbugs carry human pathogens such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, hepatitis B virus, Bartonella quintana, and Trypanosoma cruzi, Ibrahim et al correctly inform the reader that there are no compelling reports of transmission of these diseases via bedbug bites.16
However, there may be lasting psychological sequelae. Anxiety, hypervigilance, insomnia, avoidance behaviors, and personal dysfunction can persist, even long after the infestation has been eradicated.
Bedbugs are a national and even global health problem worthy of familiarity by all healthcare providers, regardless of specialty. In this regard, Ibrahim et al succinctly and accurately provide a functional and clinically useful guide.
- Alalawi AH. Bed bugs epidemic in the United States. Entomol Ornithol Herpetol 2015; 4:143–148.
- Ibrahim O, Syed UM, Tomecki KJ. Bedbugs: a practical review. Clev Clin J Med 2017; 84:207–211.
- Velten H. Beastly London—A History of Animals in the City. London: Reaktion Books. November 15, 2013. p. 221.
- Snetsinger R. Bed bugs and other bugs. In: Moreland D, editor. Mallis Handbook of Pest Control: The Behavior, Life History, and Control of House Pests, 8th edition. Cleveland, OH: GIE Publishers, 1997:392–424.
- Kolb A, Needham GR, Neyman KM, High WA. Bedbugs. Dermatol Ther 2009; 22:347–352.
- Totten V, Charbonneau H, Hoch W, Shah C, Sheele J. The cost of decontaminating an ED after finding a bed bug: results from a single academic medical center. Am J Emerg Med 2016; 34:649.
- Baumblatt JA, Dunn JR, Schaffner W, Moncayo AC, Stull-Lane A, Jones TF. An outbreak of bed bug infestation in an office building. J Environ Health 2014; 76:16–18.
- Chalupka S. Preventing bedbug infestation. AAOHN J 2010; 58:500.
- Scisicione P. Bed bugs: they are back! The role of the school nurse in bed bug management. NASN Sch Nurse 2012; 27:268–273.
- Anders D, Brocker EB, Hamm H. Cimex lectularius—an unwelcome train attendant. Eur J Dermatol 2010; 20:239–240.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Potter MF, Haynes KF, Deutsch M, et al. The sensitivity spectrum: human reactions to bed bug bites. Pest Control Technology Magazine 2010; 70–75.
- Reinhardt K, Kempke D, Naylor RA, Siva-Jothy MT. Sensitivity to bites by the bedbug, Cimex lectularius. Med Vet Entomol 2009; 23:163–166.
- Sheele JM, Anderson JF, Tran TD, et al. Ivermectin causes Cimex lectularius (bedbug) morbidity and mortality. J Emerg Med 2013; 45:433–440.
- Paulke-Korinek M, Széll M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Ho D, Lai O, Glick S, Jagdeo J. Lack of evidence that bedbugs transmit pathogens to humans. J Am Acad Dermatol 2016; 74:1261.
- Alalawi AH. Bed bugs epidemic in the United States. Entomol Ornithol Herpetol 2015; 4:143–148.
- Ibrahim O, Syed UM, Tomecki KJ. Bedbugs: a practical review. Clev Clin J Med 2017; 84:207–211.
- Velten H. Beastly London—A History of Animals in the City. London: Reaktion Books. November 15, 2013. p. 221.
- Snetsinger R. Bed bugs and other bugs. In: Moreland D, editor. Mallis Handbook of Pest Control: The Behavior, Life History, and Control of House Pests, 8th edition. Cleveland, OH: GIE Publishers, 1997:392–424.
- Kolb A, Needham GR, Neyman KM, High WA. Bedbugs. Dermatol Ther 2009; 22:347–352.
- Totten V, Charbonneau H, Hoch W, Shah C, Sheele J. The cost of decontaminating an ED after finding a bed bug: results from a single academic medical center. Am J Emerg Med 2016; 34:649.
- Baumblatt JA, Dunn JR, Schaffner W, Moncayo AC, Stull-Lane A, Jones TF. An outbreak of bed bug infestation in an office building. J Environ Health 2014; 76:16–18.
- Chalupka S. Preventing bedbug infestation. AAOHN J 2010; 58:500.
- Scisicione P. Bed bugs: they are back! The role of the school nurse in bed bug management. NASN Sch Nurse 2012; 27:268–273.
- Anders D, Brocker EB, Hamm H. Cimex lectularius—an unwelcome train attendant. Eur J Dermatol 2010; 20:239–240.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Potter MF, Haynes KF, Deutsch M, et al. The sensitivity spectrum: human reactions to bed bug bites. Pest Control Technology Magazine 2010; 70–75.
- Reinhardt K, Kempke D, Naylor RA, Siva-Jothy MT. Sensitivity to bites by the bedbug, Cimex lectularius. Med Vet Entomol 2009; 23:163–166.
- Sheele JM, Anderson JF, Tran TD, et al. Ivermectin causes Cimex lectularius (bedbug) morbidity and mortality. J Emerg Med 2013; 45:433–440.
- Paulke-Korinek M, Széll M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Ho D, Lai O, Glick S, Jagdeo J. Lack of evidence that bedbugs transmit pathogens to humans. J Am Acad Dermatol 2016; 74:1261.
Staying afloat in a sea of information: Point-of-care resources
It is friday afternoon on a sunny July day. The last patient of the day, Ms. Connecticut, is an active hiker who has had Lyme disease previously. She found a tick on her ankle yesterday. She successfully removed the tick but has not brought the tick with her to the appointment. She had been hiking several times over the last week and is not certain when the tick bite occurred. Her question for you centers on the role of antibiotic prophylaxis and Lyme disease prevention.
TECHNOLOGY: PROBLEM AND SOLUTION
Physicians need to keep up with an ever-increasing stream of information—new guidelines, new medications, and updates in medical literature.1 They have to do this while seeing more patients with more chronic problems in less time and while meeting reporting requirements for meaningful use or quality measures for accountable care organizations.
Though some of these challenges are due to technology, one solution is to use technology to our advantage. While researching information in textbooks won’t drain a phone battery, carrying a textbook around is not feasible, and many textbooks (including their electronic versions) contain information that is outdated before they go to print or that is quickly outdated thereafter.2 Further, even online textbooks are currently more dense than the online resources that we review here.
Different types of resources can help task-saturated healthcare providers stay aware of new information while delivering evidence-based care. These tools—online textbooks, decision guides embedded within electronic health record systems, or even a Google search—are termed “point-of-care” resources when used at the time of patient care for decision-making in the moment.
Not all of these resources are of high quality, with reliable factual information. Researchers estimate that up to 70% of clinicians may use Wikipedia to research medical questions, and a comparison of 10 Wikipedia articles vs peer-reviewed sources on the 10 most costly diseases found that 9 of the 10 Wikipedia articles had errors.3,4
In an earlier article,5 we advocated a proactive approach to managing information, highlighting ways to scan for new information and to develop habits of extracting useful information that can then be stored and easily recovered. To complement this strategy and weed out erroneous information, physicians need reliable sources of unbiased information to efficiently answer clinical questions at the point of care.1,6
Here, to help busy clinicians choose which point-of-care resources to use, we review several of the most popular ones, examining their ease of use, key elements, strengths, and weaknesses.
WHAT MAKES A RESOURCE GOOD?
Key features that make point-of-care tools effective include:
Ease of use, with standard formats, a summary for each topic, or both
Links to original articles and concise, capsular summaries and syntheses of the data
Continuing medical education (CME) credit. Tip: when searching, add “CME” to the search string on the browser to access resources that provide this.
Institutional and individual accounts. For clinicians who work for large organizations, point-of-care products may be paid for already, or reimbursement may be available for your subscription. If unsure, ask your director of information technology or library services.
Freedom from advertisements. Many Internet sources have advertisements that either run alongside the information you want to see or, more annoyingly, pop up and require an action to move forward. There is also continuing concern about the effect of industry support on content.7 While not all of the resources that we use regularly and that we review here are ad-free, avoiding programs with high ad content helps limit the possibility of bias and the time it takes to access information. Although advertisements do bring up a risk of bias, resources with a low-level ad content can limit bias while providing free or low-cost access.
Evidence, not expert opinion. Many resources have an “about” page that explains their philosophy and the source of their information. It is vital to be sure that point-of-care databases are providing facts based on evidence.8 This page also typically addresses how authors and editors are selected and whether expert opinion is used when randomized trials are lacking.
Ease of access. Many tools can be accessed not only on computers but also through apps for smartphones and tablets. Some electronic medical records have clinical decision tools embedded in them, with varying capabilities.
Disclosure of conflict of interest. As conflicts of interest can shade recommendations, information sources should clearly disclose financial relationships that could be perceived as conflicts of interest—for example, authors writing about medications sold by companies with whom they have a financial relationship.
NO SINGLE RESOURCE DOES EVERYTHING
There are many types of tools for finding evidence-based medical information. Different tools serve different purposes. Table 1 lists “toolbox essentials” for clinicians needing to answer clinical questions during patient care.
For example, when a question about the need for a bone mineral density measurement comes up, it is useful to be able to quickly compare guidelines from different professional societies on the National Guideline Clearing House. For another example, if a patient brings in a medication in an unlabeled bottle, a pill identifier app can tell you what it is. Clinicians who can use these resources appropriately will be at an advantage in being able to use information to provide better care to their patients.
To date, no point-of-care summary source has been shown to be superior in all categories, and use may be driven by ease of navigation, clinician preference, clinical question, or past success.9,10
Reviewed below are several applications that can be used as point-of-care resources (Table 2).
CLINICAL EVIDENCE
Clinical Evidence provides systematic reviews on medical topics. Founded in 1999 by the British Medical Journal, it is available in print as the Clinical Evidence Handbook and in online desktop and smartphone formats.
More than any other source we reviewed, Clinical Evidence addresses not only the evidence that exists, but also the data that do not exist to guide decisions. Compared with 9 other point-of-care resources, Clinical Evidence was found to have the highest quality of evidence.11
Strengths of Clinical Evidence
- Uncommonly transparent in terms of source of evidence or disclosing when there is a lack of evidence.
- Clearly lists the strength and relevance of the evidence.
- Personalization. Users can add notes to articles, save personal searches, and bookmark pages for easy access later.
- Navigability. Users can easily access systematic reviews, key points, retracted papers, or guidelines.
- Intuitive organization, with information categorized as research, education, news, or campaigns.
- New content daily: podcasts, articles, videos.
Weaknesses of Clinical Evidence
- Limited topics (eg, Lyme disease was not available)
- The limited content is a challenge when needing quick information at the point of care and may cause most clinicians to use another source unless looking for comparisons of interventions.
- Cost. Subscribing to the service “on demand,” ie, to look up a single specific topic, costs $36 for 48 hours of access; monthly access or a “season ticket” allows 30 days of viewing of all content for $53. At over $600/year, this is one of the most costly of the sources we reviewed.
- Marketing of Clinical Evidence to academic institutions that support the service for faculty may limit its appeal to other clinicians.
DYNAMED
Dynamed, a clinical reference created by a group of physicians, was previously owned by the American College of Physicians and known as Smart Medicine; it is now owned by EBSCO.12 Reviewers investigate the literature for a given topic and create pithy summaries for busy clinicians. A top feature in Dynamed is its links to full articles cited for best practices or evidence-based guidelines. The company describes their content as free of expert opinion, while being unbiased and evidence-based.
Dynamed uses a 7-step algorithm for searched topics that identifies articles, assesses clinical relevance, evaluates validity of outcomes, compiles the evidence from multiple articles, and then updates the final recommendations daily.
Dynamed Plus, the new upgraded version, updates searched topics several times a day. Dynamed may be the most frequently updated point-of-care resource, with the least risk of conflict of interest, but it offers limited topics drawn from evidence-based findings.11,13–15
With the rapid doubling of the medical literature, frequent updates allow clinicians to be most current with practice guidelines. This potentially affects quality of care for antibiotic use, vaccination, health promotion, and screening as well as newly approved medications.
Strengths of Dynamed
- Large collection of topics, critically appraised, written for primary care physicians, presented in bulleted format
- The most frequently updated database11,14,15
- Can integrate with major electronic health records (eg, Epic, Allscripts, NextGen, Cerner)
- Has an area devoted to new information that changes current practice
- Chosen topic grouped with related topics in the differential diagnosis after the initial search
- Easy-to-read outline for quick access to information such as billing, diagnosis, and references
- Medical calculators
- No advertisements
- Helpful embedded tools
- Icons to print or email the article
- An icon to create a “perma-link” to topics, searches, and browse categories
- Graded evidence with a link to the grading model used
- Links to primary articles
- Patient information handouts
- Alerts for updated information
- CME credit
- Special consideration and features for medical education
- The upgraded version Dynamed Plus contains Micromedex for a medication database, expanded graphics, semantic search, concise overview for each topic, and expanded content.
Weaknesses of Dynamed
- Although the topic list is large, it is only about one-third the size of UpToDate.
- A subscription for a physician costs $395 a year. Residents can sign up for about $150, and students for just under $100.
- CME is obtainable but cumbersome; one submits the CME credits through Tufts Healthcare, which requires a second sign-on to access and track.
- Drug and nondrug treatments for diseases cannot be separated.
- Useful calculators include decision trees for clinical decision-making, but there is no way to search them—one must waste time scrolling through the topics and specialties looking for desired information.
- Major shortcoming: there is no medication reference tool unless you upgrade to Dynamed Plus.
- The expanded graphics of Dynamed Plus are difficult to view on mobile applications within the articles (they are brought up more reliably when searching just for the image).
- The use of strict evidence-based methodology without expert opinion is a strength, but limits the collection of topics without randomized controlled trials, for which turning to expert opinion may be the only option.
EVIDENCE ESSENTIALS
Evidence Essentials is a point-of-care resource from Wiley that offers a variety of content types. The website lists 13,000 medical topics; however, they are not all summary reviews as discussed in the other products above. Subject matter is reviewed 3 times a year. Comprehensive reviews number just under 800 individual topics, with the remaining content consisting of Cochrane reviews, calculators, decision support tools, POEMs (Patient-Oriented Evidence that Matters), evidence-based medical guidelines, and dermatology images (1,000).
Evidence Essentials provides some unique content including a quick evaluation and management (E/M) code-finder and calculators not only for the typical medical equations, but also for history and physical examination likelihood ratios and pretest probabilities, which are practical and an excellent teaching aid. It also offers CME along with POEMs, e-mail alerts, and a listing of upcoming topics.
Strengths of Evidence Essentials
- Relatively inexpensive at $85 a year.
- High-functioning filter system to choose to search one or multiple databases.
- Related results are listed for aid in differential diagnosis, similar to Dynamed.
- Authors, editors, and date of last review are highly visible. As in UpToDate, relevant medical calculators appear on the page.
- The likelihood and odds ratio calculators are a huge plus for clinical decision-making and putting guidelines into practice.
- “Overall bottom line” highlights key points
- Grading of evidence per topic.
- Bulleted and tabbed information for quick access.
- Tabs for information on background, prevention, diagnosis, treatment, references, guidelines, and special populations.
Weaknesses of Evidence Essentials
- Limited number of topics with comprehensive reviews.
- While you can click on any drug name and link to a choice of two drug databases, this is not included in the subscription and requires a second account.
- The resources tabs had some broken links. In our clinical example, the tab contained several videos at the top that were not related, followed by a map and tables that were relevant to Lyme disease.
- Likewise, some of the guideline references were disappointing. For example, the guideline link for Lyme disease is for the US Department of Labor Occupational Safety and Health Administration rather than a professional society.
- For the provider wanting a narrative, this is more of a bare-bones text.
FIRST CONSULT
First Consult is Elsevier’s point-of-care clinical decision product contained within ClinicalKey.
Unlike UpToDate and Dynamed, in which authors and editors read original articles and summarize or synthesize information for the learner, First Consult is a “smart” search engine that will research a question, together with associated terms and key words. Filters such as full-text availability, journal articles, and patient education can be applied.
You may need to read about your topic in a textbook first, and then, if you are looking for treatment information, find an original article through First Consult. It is available in mobile and desktop formats, and the point-of-care product, First Consult, has an app that can be downloaded and used for free for the first 60 days.
Importantly, the First Consult portion of ClinicalKey with the summary topics was rated by Shurtz and Foster13 as least current of the products we are discussing in this article. On the other hand, it was the only product that had an embedded program to assist the user in making presentations by allowing drag and drop of images and automatic citing of sources. Kim et al report that First Consult is one of the resources providers prefer.9
Strengths of First Consult
- Lengthy free trial
- Ability to access original articles from a list vs lengthy narrative
- Access to journals and books published by Elsevier
- Powerful search engine that applies associated terms automatically
- Patient education is available in different languages and font size with the ability to add instructions and even a local branding
- Can integrate with electronic health record
- Can filter results by guideline, patient education, topic overviews
- Presentation assistance.
Weaknesses of First Consult
- Time-intensive. A provider needing quick advice on treatment for a medical condition has to guess if an article or textbook will have the most up-to-date and digestible information, whereas this has already been summarized in other products. For the busy clinician, this may be prohibitive.
- Search results are limited to Elsevier products, and major journals such as the New England Journal of Medicine are not available.
- Inconsistent platform functionality. The app version was somewhat “sticky” to use, as pages did not always load efficiently, and the menu bar navigation is not ideal.
- Expensive, especially given cheaper alternatives. For example, subscribing to the specialty of internal medicine or family medicine costs $499 and provides access to 8 journals and 11 books. Extended access costs $998 and offers full-text access to 23 books and 45 journals. The complete service has a total of 400 journals, 700 books, and 2,500 procedural videos.
MEDSCAPE
Medscape, owned by parent company WebMD, has long been a popular resource. The most recent versions are available for both for Android and iOS mobile platforms. The desktop and mobile apps claim to be designed for point-of-care use, and can be downloaded at no cost after registering as a Medscape user.
Medscape has some interesting features, including a handy pill identifier tool that is new to Medscape and perfect for the “I take one blue pill for my cholesterol” moments. The drug information tools and other features work well offline.
Medscape contains a well-presented drug database and interaction checker, as well as a growing collection of evidence-based articles and videos with links to references in Medline. From the point-of-care standpoint, Medscape also offers a number of decision-making algorithms and a continuously updated medical literature and health-related newsfeed. It contains in-app medical calculators, searchable directories for providers, hospitals, and pharmacies, and CME that can be earned on the website or from the application.
The main Medscape website contains pop-up advertisements, but the mobile app has fewer. Among the occasional frustrations, updates are relatively infrequent, the content is slow to load, and the phone app can be cumbersome. Of note, in one review,11 Medscape was found to have the lowest quality of evidence.
Strengths of Medscape
- Free with registration
- Medical calculator
- Drug interaction checker
- Pill identifier
- Evidence-based information covering about 4,000 conditions with links to references in Medline
- Ability to e-mail articles for sharing or future reference
- CME
- Unique database of hospitals, providers, and pharmacies to aid in referrals or locating other healthcare professionals
- Algorithms for decision-making
- Images and videos for procedural review and learning
- Option for downloading certain databases for offline use
- Medical news helps you keep up with what patients are watching and reading.
Weaknesses of Medscape
- Advertisements (many of them pop-up)
- The content is updated less frequently than other products listed in this article
- The smartphone app can run slowly
- Quality of reviews may be a concern.
UpToDate
UpToDate (Wolters Kluwer) is used widely by medical students, residents, and fellows as well as practicing providers. It contains narrative reviews of topics written by respected experts directed at both clinicians and clinical staff. In hopes of appealing to many markets, it offers different subscription types so you can customize your choices with add-on features (UpToDate Desktop and UpToDate MobileComplete allow downloading of all content to be accessed offline), different service packages (1-, 2-, and 3-year subscriptions), and the traditional base product that provides online access.
Of the products we reviewed, UpToDate has the largest selection of medical topics, approaching 10,000.14 In some studies,10,15 it also had the fastest retrieval time for searches. It uses evidence-based graded recommendations that are updated regularly.
Some have lamented that there is too much information per topic.9 In response to early reviews, Wolters Kluwer has made significant changes in the platform and greatly improved the search engine. UpToDate has expanded to include CME and patient information, trying to become that Holy Grail of websites—a one-stop experience. For the lucky few, UpToDate integrates into some electronic health records and provides a relatively seamless experience at the point of care.
Strengths of UpToDate
- One-stop shopping for information, resources, and CME
- Patient information is easy to read and accessible from the same screen
- The largest repository of medical subject matter
- Ability to cull out only pediatric or adult topics
- Searching available within a medical topic
- Tabs for quick access
- The What’s New feature allows access to practice-changing medical updates
- Medical calculators
- Drug interactions
- CME is is tracked in the system, allowing for CME credit information for hospital privileges and board certification
- Flexibility of access: can use online or download content to mobile/desktop device (the online version is easy to use, although robust wireless reception is needed; offices with slow Internet benefit from the offline feature)
- Electronic health record integration is possible with the most popular systems, such as Epic, eClinicalWorks, NextGen, and Allscripts
- Patient education and medication interaction features embedded in the electronic health record; produced in collaboration with Lexicomp
- Integrated drug database
- Alerts for updates
- References have links to full-text articles
- The date of last update is easily found for verifying information accuracy
- May be provided free for clinicians who are a part of a university or large health system.
Weaknesses of UpToDate
- Articles can be lengthy, which is both a strength and a weakness. Searches can retrieve too much information.9 High volume of text can frustrate the user trying to find bulleted, easy-to-read facts. However, for the person looking for a narrative summary, the content is organized as narrative paragraphs with appropriate headers in the left margin, and the search function is robust and powerful.
- Each topic has a “Summary of Recommendations,” but answers here often require linking back to the main text.
- Patient information is sometimes at a high literacy level.
- Costs more than Dynamed. A 1-year subscription is $499 for a physician, but you have the option of paying $53 for a 30-day recurring subscription. Residents, fellows, and students can pay $199 for 1 year or $19 for a 30-day recurring subscription.
- The requirement to download means that users need to keep their version updated on all of their computers—in each of their examination rooms, for example.
- Concerns about conflict of interest arise because authors and editors may maintain financial relationships with companies that produce medications discussed in the articles they have written.
BUILDING YOUR OWN PERSONAL ONLINE REPOSITORY
Our previous article5 reviewed how to store information using tools such as Evernote and Diigo that allow information viewed on a web page to be exported to any online repository. This can be done using extensions for a web browser or by sending the information to a custom e-mail account for these services.
For information that a provider knows he or she will need repeatedly, storage in one system is actually the easiest method. Such a system can then incorporate key information from the summary tools we have reviewed here. The ideal “electronic filing cabinet” should have several features such as a the capability to label articles by topic, to separate or sort as you see fit, and a search function to find information quickly—making it a personalized and effective point-of-care resource.
STAYING AFLOAT
Clinicians make many decisions every day. In fact, the release of How Doctors Think (both publications) has led to increased research into how clinical decisions and diagnoses are formed.16,17
With the medical literature expected to double every 73 days by 2020,18 there is an ever-widening ocean of information to sift through. With this onslaught, clinicians can no longer remain fully current. Instead, refining skills in accessing, sorting, and interpreting accurate scientific evidence efficiently is crucial to time spent actually caring for patients and coordinating their care.
Guidelines, algorithms, and comprehensive databases can aid clinicians in all aspects of care, from generating more complete differential diagnoses to managing disease-specific treatment. Individuals can first think about and list the qualities of a tool that are most important to them (eg, breadth of topics, frequency of updates, integration within their electronic health record, and cost) before focusing on a few applications or websites that meet those goals. With practice, point-of-care knowledge can become part of the everyday visit.
Effective integration into electronic health records will require design input from front-line clinicians. Otherwise, systems are prone to add too much “support” and overly rely on orthodox metrics and guidelines, resulting in alarm fatigue and frustration rather than facilitation.19–23
OUR CONCLUSIONS
Comprehensive point-of-care resources can play a significant role in helping busy clinicians provide best evidence-based care to their patients. Embedded clinical decision guides within an electronic health record are ideal, but low topic coverage has limited the usefulness of these systems.24 Here are our conclusions:
Medscape, ePocrates, and Wikipedia are probably the most popular free resources. Dynamed has offered free subscriptions to Wikipedia’s top health editors with the hopes of correcting factual errors. Medscape has excellent features but is supported by sponsored content, which raises a concern about bias and potential time-consuming distractions.
Dynamed and UpToDate have both been reported to answer more questions than other sources.12
UpToDate has the largest repository, with each topic curated by an expert or experts in that subject. This content can be dense and difficult to scan quickly at the point of care, but this is balanced by the ability to search within a medical topic, which has given it the fastest retrieval time.15 It does, however, allow authors and editors to maintain financial relationships with companies that produce medications discussed in the article.22
Dynamed has the advantage in frequency of updates, clearest conflict-of-interest policy, and the least amount of conflict of interest. Its topic list is not as extensive as UpToDate’s due to the limitation of using only evidence-based medicine without expert opinion.
First Consult has high user satisfaction, but as a point-of-care resource it can be time-consuming to find the best source for the clinical question at hand, and its expanded access is costly.9
ART AND SCIENCE
Point-of-care resources do not solve all the complicated problems of patient care, and no single resource is ideal for all situations. A busy clinician has limited time to process the evolving literature to practice the best evidence-based medicine. Effective information access, quality of care provided, and the marginal time cost required create a complex calculus. Clinical decision-making remains an art and a science,25 but these technologies help define a new era in its pursuit.
Ultimately, a clinician’s choice needs to correlate with a provider’s resources and style. This article has detailed several options available on the market today. This is a quickly evolving area of products and services. Longer term, users might consider a tool’s preferred key features when evaluating any current or future resource in order to choose the right ones for their practice.
CASE REVISITED
Before we leave for the weekend, we need a plan for Ms. Connecticut. To find appropriate recommendations for our patient, we search several of our point-of-care resources: UpToDate and Dynamed. Both resources have correct information according to the Infectious Disease Society of America (IDSA) guidelines.
UpToDate has a monograph of approximately 2,000 words on Lyme disease, which is lengthy but adds to clinical-decision making skills for a learner thinking through the decision. This service also has a patient handout highlighting the recommendations. The topic was last updated in 2016, but states that it is current with literature through January 2017.
Dynamed has bulleted information that is quicker to digest, but essentially highlights the IDSA recommendations without the thought process behind them. It too, has patient resources with links to a variety of handouts from professional organizations such as the US Centers for Disease Control and Prevention. They last updated the topic January 31, 2017.
When searching for the topic on both sites, a clinician can see the breadth of information in each program. However, this is also a detractor. Searching for Lyme disease prophylaxis on Dynamed brought up related data (that doxycycline is not FDA-approved for prophylaxis), but not the primary information. Likewise, the search under UpToDate first brought us to the patient information. Both articles have helpful tables and links to associated topics.
My partner chose the UpToDate article, in part to review the topic with a medical student. However, I used Dynamed for its quick bulleted information, as I was on call that evening and needed to return to the hospital. We both came to the same conclusion, and Ms. Connecticut chose no prophylaxis even though her home is in an endemic area. She has done well.
- Worster A, Haynes RB. How do I find a point-of-care answer to my clinical question? CJEM 2012; 14:31–35.
- Jeffery R, Navarro T, Lokker C, Haynes RB, Wilczynski NL, Farjou G. How current are leading evidence-based medical textbooks? An analytic survey of four online textbooks. J Med Internet Res 2012; 14:e175.
- ClinicalKey. Errors found in nine out of ten Wikipedia health entries. www.clinicalkey.com/info/blog/errors-in-wikipedia-health/. Accessed February 9, 2017.
- Hasty RT, Garbalosa RC, Barbato VA, et al. Wikipedia vs peer-reviewed medical literature for information about the 10 most costly medical conditions. J Am Osteopath Assoc 2014; 114:368–373.
- Mehta NB, Martin SA, Maypole J, Andrews R. Information management for clinicians. Cleve Clin J Med 2016; 83:589–595.
- Cook DA, Sorensen KJ, Hersh W, Berger RA, Wilkinson JM. Features of effective medical knowledge resources to support point of care learning: a focus group study. PLoS One 2013; 8:e80318.
- Steinbrook R. Future directions in industry funding of continuing medical education. Arch Intern Med 2011; 171:257–258.
- Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ 1999; 319:1618.
- Kim S, Noveck H, Galt J, Hogshire L, Willett L, O’Rourke K. Searching for answers to clinical questions using Google versus evidence-based summary resources: a randomized controlled crossover study. Acad Med 2014; 89:940–943.
- Ahmadi SF, Faghankhani M, Javanbakht A, et al. A comparison of answer retrieval through four evidence-based textbooks (ACP PIER, Essential Evidence Plus, First Consult, and UpToDate): a randomized controlled trial. Med Teach 2011; 33:724–730.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Shurtz S, Foster MJ. Developing and using a rubric for evaluating evidence-based medicine point-of-care tools. J Med Libr Assoc 2011; 99:247–254.
- Ketterman E, Besaw M. An evaluation of citation counts, search results, and frequency of updates in Dynamed and UpToDate. J Electron Res in Med Libr 2010; 7:273–280.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics 2014; 40:578–580.
- Montgomery K. How Doctors Think: Clinical Judgment and the Practice of Medicine. New York, NY: Oxford University Press; 2005.
- Groopman J. How Doctors Think. Boston, MA: Houghton Mifflin; 2008.
- Densen P. Challenges and opportunities facing medical education. Trans Am Clin Climatol Assoc 2010; 122:48–58.
- Kesselheim AS, Cresswell K, Phansalkar S, Bates DW, Sheikh A. Clinical decision support systems could be modified to reduce ‘alert fatigue’ while still minimizing the risk of litigation. Health Aff (Millwood) 2011; 30:2310–2317.
- Russ AL, Zillich AJ, McManus MS, Doebbeling BN, Saleem JJ. Prescribers’ interactions with medication alerts at the point of prescribing: a multi-method, in situ investigation of the human-computer interaction. Int J Med Inform 2012; 81:232–243.
- Fraccaro P, Arguello Castelerio M, Ainsworth J, Buchan I. Adoption of clinical decision support in multimorbidity: a systematic review. JMIR Med Informatics 2015; 3:e4.
- McLeod W, Eidus R, Stewart EE. Clinical decision support: using technology to identify patients’ unmet needs. Fam Pract Manag 2012; 19:22–28.
- Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med 2014; 371:1280–1283.
- Cook DA, Sorensen KJ, Nishimura RA, Ommen SR, Lloyd FJ. A comprehensive information technology system to support physician learning at the point of care. Acad Med 2015; 90:33–39.
- Woolever DR. The art and science of clinical decision making. Fam Pract Manag 2008; 15:31–36.
It is friday afternoon on a sunny July day. The last patient of the day, Ms. Connecticut, is an active hiker who has had Lyme disease previously. She found a tick on her ankle yesterday. She successfully removed the tick but has not brought the tick with her to the appointment. She had been hiking several times over the last week and is not certain when the tick bite occurred. Her question for you centers on the role of antibiotic prophylaxis and Lyme disease prevention.
TECHNOLOGY: PROBLEM AND SOLUTION
Physicians need to keep up with an ever-increasing stream of information—new guidelines, new medications, and updates in medical literature.1 They have to do this while seeing more patients with more chronic problems in less time and while meeting reporting requirements for meaningful use or quality measures for accountable care organizations.
Though some of these challenges are due to technology, one solution is to use technology to our advantage. While researching information in textbooks won’t drain a phone battery, carrying a textbook around is not feasible, and many textbooks (including their electronic versions) contain information that is outdated before they go to print or that is quickly outdated thereafter.2 Further, even online textbooks are currently more dense than the online resources that we review here.
Different types of resources can help task-saturated healthcare providers stay aware of new information while delivering evidence-based care. These tools—online textbooks, decision guides embedded within electronic health record systems, or even a Google search—are termed “point-of-care” resources when used at the time of patient care for decision-making in the moment.
Not all of these resources are of high quality, with reliable factual information. Researchers estimate that up to 70% of clinicians may use Wikipedia to research medical questions, and a comparison of 10 Wikipedia articles vs peer-reviewed sources on the 10 most costly diseases found that 9 of the 10 Wikipedia articles had errors.3,4
In an earlier article,5 we advocated a proactive approach to managing information, highlighting ways to scan for new information and to develop habits of extracting useful information that can then be stored and easily recovered. To complement this strategy and weed out erroneous information, physicians need reliable sources of unbiased information to efficiently answer clinical questions at the point of care.1,6
Here, to help busy clinicians choose which point-of-care resources to use, we review several of the most popular ones, examining their ease of use, key elements, strengths, and weaknesses.
WHAT MAKES A RESOURCE GOOD?
Key features that make point-of-care tools effective include:
Ease of use, with standard formats, a summary for each topic, or both
Links to original articles and concise, capsular summaries and syntheses of the data
Continuing medical education (CME) credit. Tip: when searching, add “CME” to the search string on the browser to access resources that provide this.
Institutional and individual accounts. For clinicians who work for large organizations, point-of-care products may be paid for already, or reimbursement may be available for your subscription. If unsure, ask your director of information technology or library services.
Freedom from advertisements. Many Internet sources have advertisements that either run alongside the information you want to see or, more annoyingly, pop up and require an action to move forward. There is also continuing concern about the effect of industry support on content.7 While not all of the resources that we use regularly and that we review here are ad-free, avoiding programs with high ad content helps limit the possibility of bias and the time it takes to access information. Although advertisements do bring up a risk of bias, resources with a low-level ad content can limit bias while providing free or low-cost access.
Evidence, not expert opinion. Many resources have an “about” page that explains their philosophy and the source of their information. It is vital to be sure that point-of-care databases are providing facts based on evidence.8 This page also typically addresses how authors and editors are selected and whether expert opinion is used when randomized trials are lacking.
Ease of access. Many tools can be accessed not only on computers but also through apps for smartphones and tablets. Some electronic medical records have clinical decision tools embedded in them, with varying capabilities.
Disclosure of conflict of interest. As conflicts of interest can shade recommendations, information sources should clearly disclose financial relationships that could be perceived as conflicts of interest—for example, authors writing about medications sold by companies with whom they have a financial relationship.
NO SINGLE RESOURCE DOES EVERYTHING
There are many types of tools for finding evidence-based medical information. Different tools serve different purposes. Table 1 lists “toolbox essentials” for clinicians needing to answer clinical questions during patient care.
For example, when a question about the need for a bone mineral density measurement comes up, it is useful to be able to quickly compare guidelines from different professional societies on the National Guideline Clearing House. For another example, if a patient brings in a medication in an unlabeled bottle, a pill identifier app can tell you what it is. Clinicians who can use these resources appropriately will be at an advantage in being able to use information to provide better care to their patients.
To date, no point-of-care summary source has been shown to be superior in all categories, and use may be driven by ease of navigation, clinician preference, clinical question, or past success.9,10
Reviewed below are several applications that can be used as point-of-care resources (Table 2).
CLINICAL EVIDENCE
Clinical Evidence provides systematic reviews on medical topics. Founded in 1999 by the British Medical Journal, it is available in print as the Clinical Evidence Handbook and in online desktop and smartphone formats.
More than any other source we reviewed, Clinical Evidence addresses not only the evidence that exists, but also the data that do not exist to guide decisions. Compared with 9 other point-of-care resources, Clinical Evidence was found to have the highest quality of evidence.11
Strengths of Clinical Evidence
- Uncommonly transparent in terms of source of evidence or disclosing when there is a lack of evidence.
- Clearly lists the strength and relevance of the evidence.
- Personalization. Users can add notes to articles, save personal searches, and bookmark pages for easy access later.
- Navigability. Users can easily access systematic reviews, key points, retracted papers, or guidelines.
- Intuitive organization, with information categorized as research, education, news, or campaigns.
- New content daily: podcasts, articles, videos.
Weaknesses of Clinical Evidence
- Limited topics (eg, Lyme disease was not available)
- The limited content is a challenge when needing quick information at the point of care and may cause most clinicians to use another source unless looking for comparisons of interventions.
- Cost. Subscribing to the service “on demand,” ie, to look up a single specific topic, costs $36 for 48 hours of access; monthly access or a “season ticket” allows 30 days of viewing of all content for $53. At over $600/year, this is one of the most costly of the sources we reviewed.
- Marketing of Clinical Evidence to academic institutions that support the service for faculty may limit its appeal to other clinicians.
DYNAMED
Dynamed, a clinical reference created by a group of physicians, was previously owned by the American College of Physicians and known as Smart Medicine; it is now owned by EBSCO.12 Reviewers investigate the literature for a given topic and create pithy summaries for busy clinicians. A top feature in Dynamed is its links to full articles cited for best practices or evidence-based guidelines. The company describes their content as free of expert opinion, while being unbiased and evidence-based.
Dynamed uses a 7-step algorithm for searched topics that identifies articles, assesses clinical relevance, evaluates validity of outcomes, compiles the evidence from multiple articles, and then updates the final recommendations daily.
Dynamed Plus, the new upgraded version, updates searched topics several times a day. Dynamed may be the most frequently updated point-of-care resource, with the least risk of conflict of interest, but it offers limited topics drawn from evidence-based findings.11,13–15
With the rapid doubling of the medical literature, frequent updates allow clinicians to be most current with practice guidelines. This potentially affects quality of care for antibiotic use, vaccination, health promotion, and screening as well as newly approved medications.
Strengths of Dynamed
- Large collection of topics, critically appraised, written for primary care physicians, presented in bulleted format
- The most frequently updated database11,14,15
- Can integrate with major electronic health records (eg, Epic, Allscripts, NextGen, Cerner)
- Has an area devoted to new information that changes current practice
- Chosen topic grouped with related topics in the differential diagnosis after the initial search
- Easy-to-read outline for quick access to information such as billing, diagnosis, and references
- Medical calculators
- No advertisements
- Helpful embedded tools
- Icons to print or email the article
- An icon to create a “perma-link” to topics, searches, and browse categories
- Graded evidence with a link to the grading model used
- Links to primary articles
- Patient information handouts
- Alerts for updated information
- CME credit
- Special consideration and features for medical education
- The upgraded version Dynamed Plus contains Micromedex for a medication database, expanded graphics, semantic search, concise overview for each topic, and expanded content.
Weaknesses of Dynamed
- Although the topic list is large, it is only about one-third the size of UpToDate.
- A subscription for a physician costs $395 a year. Residents can sign up for about $150, and students for just under $100.
- CME is obtainable but cumbersome; one submits the CME credits through Tufts Healthcare, which requires a second sign-on to access and track.
- Drug and nondrug treatments for diseases cannot be separated.
- Useful calculators include decision trees for clinical decision-making, but there is no way to search them—one must waste time scrolling through the topics and specialties looking for desired information.
- Major shortcoming: there is no medication reference tool unless you upgrade to Dynamed Plus.
- The expanded graphics of Dynamed Plus are difficult to view on mobile applications within the articles (they are brought up more reliably when searching just for the image).
- The use of strict evidence-based methodology without expert opinion is a strength, but limits the collection of topics without randomized controlled trials, for which turning to expert opinion may be the only option.
EVIDENCE ESSENTIALS
Evidence Essentials is a point-of-care resource from Wiley that offers a variety of content types. The website lists 13,000 medical topics; however, they are not all summary reviews as discussed in the other products above. Subject matter is reviewed 3 times a year. Comprehensive reviews number just under 800 individual topics, with the remaining content consisting of Cochrane reviews, calculators, decision support tools, POEMs (Patient-Oriented Evidence that Matters), evidence-based medical guidelines, and dermatology images (1,000).
Evidence Essentials provides some unique content including a quick evaluation and management (E/M) code-finder and calculators not only for the typical medical equations, but also for history and physical examination likelihood ratios and pretest probabilities, which are practical and an excellent teaching aid. It also offers CME along with POEMs, e-mail alerts, and a listing of upcoming topics.
Strengths of Evidence Essentials
- Relatively inexpensive at $85 a year.
- High-functioning filter system to choose to search one or multiple databases.
- Related results are listed for aid in differential diagnosis, similar to Dynamed.
- Authors, editors, and date of last review are highly visible. As in UpToDate, relevant medical calculators appear on the page.
- The likelihood and odds ratio calculators are a huge plus for clinical decision-making and putting guidelines into practice.
- “Overall bottom line” highlights key points
- Grading of evidence per topic.
- Bulleted and tabbed information for quick access.
- Tabs for information on background, prevention, diagnosis, treatment, references, guidelines, and special populations.
Weaknesses of Evidence Essentials
- Limited number of topics with comprehensive reviews.
- While you can click on any drug name and link to a choice of two drug databases, this is not included in the subscription and requires a second account.
- The resources tabs had some broken links. In our clinical example, the tab contained several videos at the top that were not related, followed by a map and tables that were relevant to Lyme disease.
- Likewise, some of the guideline references were disappointing. For example, the guideline link for Lyme disease is for the US Department of Labor Occupational Safety and Health Administration rather than a professional society.
- For the provider wanting a narrative, this is more of a bare-bones text.
FIRST CONSULT
First Consult is Elsevier’s point-of-care clinical decision product contained within ClinicalKey.
Unlike UpToDate and Dynamed, in which authors and editors read original articles and summarize or synthesize information for the learner, First Consult is a “smart” search engine that will research a question, together with associated terms and key words. Filters such as full-text availability, journal articles, and patient education can be applied.
You may need to read about your topic in a textbook first, and then, if you are looking for treatment information, find an original article through First Consult. It is available in mobile and desktop formats, and the point-of-care product, First Consult, has an app that can be downloaded and used for free for the first 60 days.
Importantly, the First Consult portion of ClinicalKey with the summary topics was rated by Shurtz and Foster13 as least current of the products we are discussing in this article. On the other hand, it was the only product that had an embedded program to assist the user in making presentations by allowing drag and drop of images and automatic citing of sources. Kim et al report that First Consult is one of the resources providers prefer.9
Strengths of First Consult
- Lengthy free trial
- Ability to access original articles from a list vs lengthy narrative
- Access to journals and books published by Elsevier
- Powerful search engine that applies associated terms automatically
- Patient education is available in different languages and font size with the ability to add instructions and even a local branding
- Can integrate with electronic health record
- Can filter results by guideline, patient education, topic overviews
- Presentation assistance.
Weaknesses of First Consult
- Time-intensive. A provider needing quick advice on treatment for a medical condition has to guess if an article or textbook will have the most up-to-date and digestible information, whereas this has already been summarized in other products. For the busy clinician, this may be prohibitive.
- Search results are limited to Elsevier products, and major journals such as the New England Journal of Medicine are not available.
- Inconsistent platform functionality. The app version was somewhat “sticky” to use, as pages did not always load efficiently, and the menu bar navigation is not ideal.
- Expensive, especially given cheaper alternatives. For example, subscribing to the specialty of internal medicine or family medicine costs $499 and provides access to 8 journals and 11 books. Extended access costs $998 and offers full-text access to 23 books and 45 journals. The complete service has a total of 400 journals, 700 books, and 2,500 procedural videos.
MEDSCAPE
Medscape, owned by parent company WebMD, has long been a popular resource. The most recent versions are available for both for Android and iOS mobile platforms. The desktop and mobile apps claim to be designed for point-of-care use, and can be downloaded at no cost after registering as a Medscape user.
Medscape has some interesting features, including a handy pill identifier tool that is new to Medscape and perfect for the “I take one blue pill for my cholesterol” moments. The drug information tools and other features work well offline.
Medscape contains a well-presented drug database and interaction checker, as well as a growing collection of evidence-based articles and videos with links to references in Medline. From the point-of-care standpoint, Medscape also offers a number of decision-making algorithms and a continuously updated medical literature and health-related newsfeed. It contains in-app medical calculators, searchable directories for providers, hospitals, and pharmacies, and CME that can be earned on the website or from the application.
The main Medscape website contains pop-up advertisements, but the mobile app has fewer. Among the occasional frustrations, updates are relatively infrequent, the content is slow to load, and the phone app can be cumbersome. Of note, in one review,11 Medscape was found to have the lowest quality of evidence.
Strengths of Medscape
- Free with registration
- Medical calculator
- Drug interaction checker
- Pill identifier
- Evidence-based information covering about 4,000 conditions with links to references in Medline
- Ability to e-mail articles for sharing or future reference
- CME
- Unique database of hospitals, providers, and pharmacies to aid in referrals or locating other healthcare professionals
- Algorithms for decision-making
- Images and videos for procedural review and learning
- Option for downloading certain databases for offline use
- Medical news helps you keep up with what patients are watching and reading.
Weaknesses of Medscape
- Advertisements (many of them pop-up)
- The content is updated less frequently than other products listed in this article
- The smartphone app can run slowly
- Quality of reviews may be a concern.
UpToDate
UpToDate (Wolters Kluwer) is used widely by medical students, residents, and fellows as well as practicing providers. It contains narrative reviews of topics written by respected experts directed at both clinicians and clinical staff. In hopes of appealing to many markets, it offers different subscription types so you can customize your choices with add-on features (UpToDate Desktop and UpToDate MobileComplete allow downloading of all content to be accessed offline), different service packages (1-, 2-, and 3-year subscriptions), and the traditional base product that provides online access.
Of the products we reviewed, UpToDate has the largest selection of medical topics, approaching 10,000.14 In some studies,10,15 it also had the fastest retrieval time for searches. It uses evidence-based graded recommendations that are updated regularly.
Some have lamented that there is too much information per topic.9 In response to early reviews, Wolters Kluwer has made significant changes in the platform and greatly improved the search engine. UpToDate has expanded to include CME and patient information, trying to become that Holy Grail of websites—a one-stop experience. For the lucky few, UpToDate integrates into some electronic health records and provides a relatively seamless experience at the point of care.
Strengths of UpToDate
- One-stop shopping for information, resources, and CME
- Patient information is easy to read and accessible from the same screen
- The largest repository of medical subject matter
- Ability to cull out only pediatric or adult topics
- Searching available within a medical topic
- Tabs for quick access
- The What’s New feature allows access to practice-changing medical updates
- Medical calculators
- Drug interactions
- CME is is tracked in the system, allowing for CME credit information for hospital privileges and board certification
- Flexibility of access: can use online or download content to mobile/desktop device (the online version is easy to use, although robust wireless reception is needed; offices with slow Internet benefit from the offline feature)
- Electronic health record integration is possible with the most popular systems, such as Epic, eClinicalWorks, NextGen, and Allscripts
- Patient education and medication interaction features embedded in the electronic health record; produced in collaboration with Lexicomp
- Integrated drug database
- Alerts for updates
- References have links to full-text articles
- The date of last update is easily found for verifying information accuracy
- May be provided free for clinicians who are a part of a university or large health system.
Weaknesses of UpToDate
- Articles can be lengthy, which is both a strength and a weakness. Searches can retrieve too much information.9 High volume of text can frustrate the user trying to find bulleted, easy-to-read facts. However, for the person looking for a narrative summary, the content is organized as narrative paragraphs with appropriate headers in the left margin, and the search function is robust and powerful.
- Each topic has a “Summary of Recommendations,” but answers here often require linking back to the main text.
- Patient information is sometimes at a high literacy level.
- Costs more than Dynamed. A 1-year subscription is $499 for a physician, but you have the option of paying $53 for a 30-day recurring subscription. Residents, fellows, and students can pay $199 for 1 year or $19 for a 30-day recurring subscription.
- The requirement to download means that users need to keep their version updated on all of their computers—in each of their examination rooms, for example.
- Concerns about conflict of interest arise because authors and editors may maintain financial relationships with companies that produce medications discussed in the articles they have written.
BUILDING YOUR OWN PERSONAL ONLINE REPOSITORY
Our previous article5 reviewed how to store information using tools such as Evernote and Diigo that allow information viewed on a web page to be exported to any online repository. This can be done using extensions for a web browser or by sending the information to a custom e-mail account for these services.
For information that a provider knows he or she will need repeatedly, storage in one system is actually the easiest method. Such a system can then incorporate key information from the summary tools we have reviewed here. The ideal “electronic filing cabinet” should have several features such as a the capability to label articles by topic, to separate or sort as you see fit, and a search function to find information quickly—making it a personalized and effective point-of-care resource.
STAYING AFLOAT
Clinicians make many decisions every day. In fact, the release of How Doctors Think (both publications) has led to increased research into how clinical decisions and diagnoses are formed.16,17
With the medical literature expected to double every 73 days by 2020,18 there is an ever-widening ocean of information to sift through. With this onslaught, clinicians can no longer remain fully current. Instead, refining skills in accessing, sorting, and interpreting accurate scientific evidence efficiently is crucial to time spent actually caring for patients and coordinating their care.
Guidelines, algorithms, and comprehensive databases can aid clinicians in all aspects of care, from generating more complete differential diagnoses to managing disease-specific treatment. Individuals can first think about and list the qualities of a tool that are most important to them (eg, breadth of topics, frequency of updates, integration within their electronic health record, and cost) before focusing on a few applications or websites that meet those goals. With practice, point-of-care knowledge can become part of the everyday visit.
Effective integration into electronic health records will require design input from front-line clinicians. Otherwise, systems are prone to add too much “support” and overly rely on orthodox metrics and guidelines, resulting in alarm fatigue and frustration rather than facilitation.19–23
OUR CONCLUSIONS
Comprehensive point-of-care resources can play a significant role in helping busy clinicians provide best evidence-based care to their patients. Embedded clinical decision guides within an electronic health record are ideal, but low topic coverage has limited the usefulness of these systems.24 Here are our conclusions:
Medscape, ePocrates, and Wikipedia are probably the most popular free resources. Dynamed has offered free subscriptions to Wikipedia’s top health editors with the hopes of correcting factual errors. Medscape has excellent features but is supported by sponsored content, which raises a concern about bias and potential time-consuming distractions.
Dynamed and UpToDate have both been reported to answer more questions than other sources.12
UpToDate has the largest repository, with each topic curated by an expert or experts in that subject. This content can be dense and difficult to scan quickly at the point of care, but this is balanced by the ability to search within a medical topic, which has given it the fastest retrieval time.15 It does, however, allow authors and editors to maintain financial relationships with companies that produce medications discussed in the article.22
Dynamed has the advantage in frequency of updates, clearest conflict-of-interest policy, and the least amount of conflict of interest. Its topic list is not as extensive as UpToDate’s due to the limitation of using only evidence-based medicine without expert opinion.
First Consult has high user satisfaction, but as a point-of-care resource it can be time-consuming to find the best source for the clinical question at hand, and its expanded access is costly.9
ART AND SCIENCE
Point-of-care resources do not solve all the complicated problems of patient care, and no single resource is ideal for all situations. A busy clinician has limited time to process the evolving literature to practice the best evidence-based medicine. Effective information access, quality of care provided, and the marginal time cost required create a complex calculus. Clinical decision-making remains an art and a science,25 but these technologies help define a new era in its pursuit.
Ultimately, a clinician’s choice needs to correlate with a provider’s resources and style. This article has detailed several options available on the market today. This is a quickly evolving area of products and services. Longer term, users might consider a tool’s preferred key features when evaluating any current or future resource in order to choose the right ones for their practice.
CASE REVISITED
Before we leave for the weekend, we need a plan for Ms. Connecticut. To find appropriate recommendations for our patient, we search several of our point-of-care resources: UpToDate and Dynamed. Both resources have correct information according to the Infectious Disease Society of America (IDSA) guidelines.
UpToDate has a monograph of approximately 2,000 words on Lyme disease, which is lengthy but adds to clinical-decision making skills for a learner thinking through the decision. This service also has a patient handout highlighting the recommendations. The topic was last updated in 2016, but states that it is current with literature through January 2017.
Dynamed has bulleted information that is quicker to digest, but essentially highlights the IDSA recommendations without the thought process behind them. It too, has patient resources with links to a variety of handouts from professional organizations such as the US Centers for Disease Control and Prevention. They last updated the topic January 31, 2017.
When searching for the topic on both sites, a clinician can see the breadth of information in each program. However, this is also a detractor. Searching for Lyme disease prophylaxis on Dynamed brought up related data (that doxycycline is not FDA-approved for prophylaxis), but not the primary information. Likewise, the search under UpToDate first brought us to the patient information. Both articles have helpful tables and links to associated topics.
My partner chose the UpToDate article, in part to review the topic with a medical student. However, I used Dynamed for its quick bulleted information, as I was on call that evening and needed to return to the hospital. We both came to the same conclusion, and Ms. Connecticut chose no prophylaxis even though her home is in an endemic area. She has done well.
It is friday afternoon on a sunny July day. The last patient of the day, Ms. Connecticut, is an active hiker who has had Lyme disease previously. She found a tick on her ankle yesterday. She successfully removed the tick but has not brought the tick with her to the appointment. She had been hiking several times over the last week and is not certain when the tick bite occurred. Her question for you centers on the role of antibiotic prophylaxis and Lyme disease prevention.
TECHNOLOGY: PROBLEM AND SOLUTION
Physicians need to keep up with an ever-increasing stream of information—new guidelines, new medications, and updates in medical literature.1 They have to do this while seeing more patients with more chronic problems in less time and while meeting reporting requirements for meaningful use or quality measures for accountable care organizations.
Though some of these challenges are due to technology, one solution is to use technology to our advantage. While researching information in textbooks won’t drain a phone battery, carrying a textbook around is not feasible, and many textbooks (including their electronic versions) contain information that is outdated before they go to print or that is quickly outdated thereafter.2 Further, even online textbooks are currently more dense than the online resources that we review here.
Different types of resources can help task-saturated healthcare providers stay aware of new information while delivering evidence-based care. These tools—online textbooks, decision guides embedded within electronic health record systems, or even a Google search—are termed “point-of-care” resources when used at the time of patient care for decision-making in the moment.
Not all of these resources are of high quality, with reliable factual information. Researchers estimate that up to 70% of clinicians may use Wikipedia to research medical questions, and a comparison of 10 Wikipedia articles vs peer-reviewed sources on the 10 most costly diseases found that 9 of the 10 Wikipedia articles had errors.3,4
In an earlier article,5 we advocated a proactive approach to managing information, highlighting ways to scan for new information and to develop habits of extracting useful information that can then be stored and easily recovered. To complement this strategy and weed out erroneous information, physicians need reliable sources of unbiased information to efficiently answer clinical questions at the point of care.1,6
Here, to help busy clinicians choose which point-of-care resources to use, we review several of the most popular ones, examining their ease of use, key elements, strengths, and weaknesses.
WHAT MAKES A RESOURCE GOOD?
Key features that make point-of-care tools effective include:
Ease of use, with standard formats, a summary for each topic, or both
Links to original articles and concise, capsular summaries and syntheses of the data
Continuing medical education (CME) credit. Tip: when searching, add “CME” to the search string on the browser to access resources that provide this.
Institutional and individual accounts. For clinicians who work for large organizations, point-of-care products may be paid for already, or reimbursement may be available for your subscription. If unsure, ask your director of information technology or library services.
Freedom from advertisements. Many Internet sources have advertisements that either run alongside the information you want to see or, more annoyingly, pop up and require an action to move forward. There is also continuing concern about the effect of industry support on content.7 While not all of the resources that we use regularly and that we review here are ad-free, avoiding programs with high ad content helps limit the possibility of bias and the time it takes to access information. Although advertisements do bring up a risk of bias, resources with a low-level ad content can limit bias while providing free or low-cost access.
Evidence, not expert opinion. Many resources have an “about” page that explains their philosophy and the source of their information. It is vital to be sure that point-of-care databases are providing facts based on evidence.8 This page also typically addresses how authors and editors are selected and whether expert opinion is used when randomized trials are lacking.
Ease of access. Many tools can be accessed not only on computers but also through apps for smartphones and tablets. Some electronic medical records have clinical decision tools embedded in them, with varying capabilities.
Disclosure of conflict of interest. As conflicts of interest can shade recommendations, information sources should clearly disclose financial relationships that could be perceived as conflicts of interest—for example, authors writing about medications sold by companies with whom they have a financial relationship.
NO SINGLE RESOURCE DOES EVERYTHING
There are many types of tools for finding evidence-based medical information. Different tools serve different purposes. Table 1 lists “toolbox essentials” for clinicians needing to answer clinical questions during patient care.
For example, when a question about the need for a bone mineral density measurement comes up, it is useful to be able to quickly compare guidelines from different professional societies on the National Guideline Clearing House. For another example, if a patient brings in a medication in an unlabeled bottle, a pill identifier app can tell you what it is. Clinicians who can use these resources appropriately will be at an advantage in being able to use information to provide better care to their patients.
To date, no point-of-care summary source has been shown to be superior in all categories, and use may be driven by ease of navigation, clinician preference, clinical question, or past success.9,10
Reviewed below are several applications that can be used as point-of-care resources (Table 2).
CLINICAL EVIDENCE
Clinical Evidence provides systematic reviews on medical topics. Founded in 1999 by the British Medical Journal, it is available in print as the Clinical Evidence Handbook and in online desktop and smartphone formats.
More than any other source we reviewed, Clinical Evidence addresses not only the evidence that exists, but also the data that do not exist to guide decisions. Compared with 9 other point-of-care resources, Clinical Evidence was found to have the highest quality of evidence.11
Strengths of Clinical Evidence
- Uncommonly transparent in terms of source of evidence or disclosing when there is a lack of evidence.
- Clearly lists the strength and relevance of the evidence.
- Personalization. Users can add notes to articles, save personal searches, and bookmark pages for easy access later.
- Navigability. Users can easily access systematic reviews, key points, retracted papers, or guidelines.
- Intuitive organization, with information categorized as research, education, news, or campaigns.
- New content daily: podcasts, articles, videos.
Weaknesses of Clinical Evidence
- Limited topics (eg, Lyme disease was not available)
- The limited content is a challenge when needing quick information at the point of care and may cause most clinicians to use another source unless looking for comparisons of interventions.
- Cost. Subscribing to the service “on demand,” ie, to look up a single specific topic, costs $36 for 48 hours of access; monthly access or a “season ticket” allows 30 days of viewing of all content for $53. At over $600/year, this is one of the most costly of the sources we reviewed.
- Marketing of Clinical Evidence to academic institutions that support the service for faculty may limit its appeal to other clinicians.
DYNAMED
Dynamed, a clinical reference created by a group of physicians, was previously owned by the American College of Physicians and known as Smart Medicine; it is now owned by EBSCO.12 Reviewers investigate the literature for a given topic and create pithy summaries for busy clinicians. A top feature in Dynamed is its links to full articles cited for best practices or evidence-based guidelines. The company describes their content as free of expert opinion, while being unbiased and evidence-based.
Dynamed uses a 7-step algorithm for searched topics that identifies articles, assesses clinical relevance, evaluates validity of outcomes, compiles the evidence from multiple articles, and then updates the final recommendations daily.
Dynamed Plus, the new upgraded version, updates searched topics several times a day. Dynamed may be the most frequently updated point-of-care resource, with the least risk of conflict of interest, but it offers limited topics drawn from evidence-based findings.11,13–15
With the rapid doubling of the medical literature, frequent updates allow clinicians to be most current with practice guidelines. This potentially affects quality of care for antibiotic use, vaccination, health promotion, and screening as well as newly approved medications.
Strengths of Dynamed
- Large collection of topics, critically appraised, written for primary care physicians, presented in bulleted format
- The most frequently updated database11,14,15
- Can integrate with major electronic health records (eg, Epic, Allscripts, NextGen, Cerner)
- Has an area devoted to new information that changes current practice
- Chosen topic grouped with related topics in the differential diagnosis after the initial search
- Easy-to-read outline for quick access to information such as billing, diagnosis, and references
- Medical calculators
- No advertisements
- Helpful embedded tools
- Icons to print or email the article
- An icon to create a “perma-link” to topics, searches, and browse categories
- Graded evidence with a link to the grading model used
- Links to primary articles
- Patient information handouts
- Alerts for updated information
- CME credit
- Special consideration and features for medical education
- The upgraded version Dynamed Plus contains Micromedex for a medication database, expanded graphics, semantic search, concise overview for each topic, and expanded content.
Weaknesses of Dynamed
- Although the topic list is large, it is only about one-third the size of UpToDate.
- A subscription for a physician costs $395 a year. Residents can sign up for about $150, and students for just under $100.
- CME is obtainable but cumbersome; one submits the CME credits through Tufts Healthcare, which requires a second sign-on to access and track.
- Drug and nondrug treatments for diseases cannot be separated.
- Useful calculators include decision trees for clinical decision-making, but there is no way to search them—one must waste time scrolling through the topics and specialties looking for desired information.
- Major shortcoming: there is no medication reference tool unless you upgrade to Dynamed Plus.
- The expanded graphics of Dynamed Plus are difficult to view on mobile applications within the articles (they are brought up more reliably when searching just for the image).
- The use of strict evidence-based methodology without expert opinion is a strength, but limits the collection of topics without randomized controlled trials, for which turning to expert opinion may be the only option.
EVIDENCE ESSENTIALS
Evidence Essentials is a point-of-care resource from Wiley that offers a variety of content types. The website lists 13,000 medical topics; however, they are not all summary reviews as discussed in the other products above. Subject matter is reviewed 3 times a year. Comprehensive reviews number just under 800 individual topics, with the remaining content consisting of Cochrane reviews, calculators, decision support tools, POEMs (Patient-Oriented Evidence that Matters), evidence-based medical guidelines, and dermatology images (1,000).
Evidence Essentials provides some unique content including a quick evaluation and management (E/M) code-finder and calculators not only for the typical medical equations, but also for history and physical examination likelihood ratios and pretest probabilities, which are practical and an excellent teaching aid. It also offers CME along with POEMs, e-mail alerts, and a listing of upcoming topics.
Strengths of Evidence Essentials
- Relatively inexpensive at $85 a year.
- High-functioning filter system to choose to search one or multiple databases.
- Related results are listed for aid in differential diagnosis, similar to Dynamed.
- Authors, editors, and date of last review are highly visible. As in UpToDate, relevant medical calculators appear on the page.
- The likelihood and odds ratio calculators are a huge plus for clinical decision-making and putting guidelines into practice.
- “Overall bottom line” highlights key points
- Grading of evidence per topic.
- Bulleted and tabbed information for quick access.
- Tabs for information on background, prevention, diagnosis, treatment, references, guidelines, and special populations.
Weaknesses of Evidence Essentials
- Limited number of topics with comprehensive reviews.
- While you can click on any drug name and link to a choice of two drug databases, this is not included in the subscription and requires a second account.
- The resources tabs had some broken links. In our clinical example, the tab contained several videos at the top that were not related, followed by a map and tables that were relevant to Lyme disease.
- Likewise, some of the guideline references were disappointing. For example, the guideline link for Lyme disease is for the US Department of Labor Occupational Safety and Health Administration rather than a professional society.
- For the provider wanting a narrative, this is more of a bare-bones text.
FIRST CONSULT
First Consult is Elsevier’s point-of-care clinical decision product contained within ClinicalKey.
Unlike UpToDate and Dynamed, in which authors and editors read original articles and summarize or synthesize information for the learner, First Consult is a “smart” search engine that will research a question, together with associated terms and key words. Filters such as full-text availability, journal articles, and patient education can be applied.
You may need to read about your topic in a textbook first, and then, if you are looking for treatment information, find an original article through First Consult. It is available in mobile and desktop formats, and the point-of-care product, First Consult, has an app that can be downloaded and used for free for the first 60 days.
Importantly, the First Consult portion of ClinicalKey with the summary topics was rated by Shurtz and Foster13 as least current of the products we are discussing in this article. On the other hand, it was the only product that had an embedded program to assist the user in making presentations by allowing drag and drop of images and automatic citing of sources. Kim et al report that First Consult is one of the resources providers prefer.9
Strengths of First Consult
- Lengthy free trial
- Ability to access original articles from a list vs lengthy narrative
- Access to journals and books published by Elsevier
- Powerful search engine that applies associated terms automatically
- Patient education is available in different languages and font size with the ability to add instructions and even a local branding
- Can integrate with electronic health record
- Can filter results by guideline, patient education, topic overviews
- Presentation assistance.
Weaknesses of First Consult
- Time-intensive. A provider needing quick advice on treatment for a medical condition has to guess if an article or textbook will have the most up-to-date and digestible information, whereas this has already been summarized in other products. For the busy clinician, this may be prohibitive.
- Search results are limited to Elsevier products, and major journals such as the New England Journal of Medicine are not available.
- Inconsistent platform functionality. The app version was somewhat “sticky” to use, as pages did not always load efficiently, and the menu bar navigation is not ideal.
- Expensive, especially given cheaper alternatives. For example, subscribing to the specialty of internal medicine or family medicine costs $499 and provides access to 8 journals and 11 books. Extended access costs $998 and offers full-text access to 23 books and 45 journals. The complete service has a total of 400 journals, 700 books, and 2,500 procedural videos.
MEDSCAPE
Medscape, owned by parent company WebMD, has long been a popular resource. The most recent versions are available for both for Android and iOS mobile platforms. The desktop and mobile apps claim to be designed for point-of-care use, and can be downloaded at no cost after registering as a Medscape user.
Medscape has some interesting features, including a handy pill identifier tool that is new to Medscape and perfect for the “I take one blue pill for my cholesterol” moments. The drug information tools and other features work well offline.
Medscape contains a well-presented drug database and interaction checker, as well as a growing collection of evidence-based articles and videos with links to references in Medline. From the point-of-care standpoint, Medscape also offers a number of decision-making algorithms and a continuously updated medical literature and health-related newsfeed. It contains in-app medical calculators, searchable directories for providers, hospitals, and pharmacies, and CME that can be earned on the website or from the application.
The main Medscape website contains pop-up advertisements, but the mobile app has fewer. Among the occasional frustrations, updates are relatively infrequent, the content is slow to load, and the phone app can be cumbersome. Of note, in one review,11 Medscape was found to have the lowest quality of evidence.
Strengths of Medscape
- Free with registration
- Medical calculator
- Drug interaction checker
- Pill identifier
- Evidence-based information covering about 4,000 conditions with links to references in Medline
- Ability to e-mail articles for sharing or future reference
- CME
- Unique database of hospitals, providers, and pharmacies to aid in referrals or locating other healthcare professionals
- Algorithms for decision-making
- Images and videos for procedural review and learning
- Option for downloading certain databases for offline use
- Medical news helps you keep up with what patients are watching and reading.
Weaknesses of Medscape
- Advertisements (many of them pop-up)
- The content is updated less frequently than other products listed in this article
- The smartphone app can run slowly
- Quality of reviews may be a concern.
UpToDate
UpToDate (Wolters Kluwer) is used widely by medical students, residents, and fellows as well as practicing providers. It contains narrative reviews of topics written by respected experts directed at both clinicians and clinical staff. In hopes of appealing to many markets, it offers different subscription types so you can customize your choices with add-on features (UpToDate Desktop and UpToDate MobileComplete allow downloading of all content to be accessed offline), different service packages (1-, 2-, and 3-year subscriptions), and the traditional base product that provides online access.
Of the products we reviewed, UpToDate has the largest selection of medical topics, approaching 10,000.14 In some studies,10,15 it also had the fastest retrieval time for searches. It uses evidence-based graded recommendations that are updated regularly.
Some have lamented that there is too much information per topic.9 In response to early reviews, Wolters Kluwer has made significant changes in the platform and greatly improved the search engine. UpToDate has expanded to include CME and patient information, trying to become that Holy Grail of websites—a one-stop experience. For the lucky few, UpToDate integrates into some electronic health records and provides a relatively seamless experience at the point of care.
Strengths of UpToDate
- One-stop shopping for information, resources, and CME
- Patient information is easy to read and accessible from the same screen
- The largest repository of medical subject matter
- Ability to cull out only pediatric or adult topics
- Searching available within a medical topic
- Tabs for quick access
- The What’s New feature allows access to practice-changing medical updates
- Medical calculators
- Drug interactions
- CME is is tracked in the system, allowing for CME credit information for hospital privileges and board certification
- Flexibility of access: can use online or download content to mobile/desktop device (the online version is easy to use, although robust wireless reception is needed; offices with slow Internet benefit from the offline feature)
- Electronic health record integration is possible with the most popular systems, such as Epic, eClinicalWorks, NextGen, and Allscripts
- Patient education and medication interaction features embedded in the electronic health record; produced in collaboration with Lexicomp
- Integrated drug database
- Alerts for updates
- References have links to full-text articles
- The date of last update is easily found for verifying information accuracy
- May be provided free for clinicians who are a part of a university or large health system.
Weaknesses of UpToDate
- Articles can be lengthy, which is both a strength and a weakness. Searches can retrieve too much information.9 High volume of text can frustrate the user trying to find bulleted, easy-to-read facts. However, for the person looking for a narrative summary, the content is organized as narrative paragraphs with appropriate headers in the left margin, and the search function is robust and powerful.
- Each topic has a “Summary of Recommendations,” but answers here often require linking back to the main text.
- Patient information is sometimes at a high literacy level.
- Costs more than Dynamed. A 1-year subscription is $499 for a physician, but you have the option of paying $53 for a 30-day recurring subscription. Residents, fellows, and students can pay $199 for 1 year or $19 for a 30-day recurring subscription.
- The requirement to download means that users need to keep their version updated on all of their computers—in each of their examination rooms, for example.
- Concerns about conflict of interest arise because authors and editors may maintain financial relationships with companies that produce medications discussed in the articles they have written.
BUILDING YOUR OWN PERSONAL ONLINE REPOSITORY
Our previous article5 reviewed how to store information using tools such as Evernote and Diigo that allow information viewed on a web page to be exported to any online repository. This can be done using extensions for a web browser or by sending the information to a custom e-mail account for these services.
For information that a provider knows he or she will need repeatedly, storage in one system is actually the easiest method. Such a system can then incorporate key information from the summary tools we have reviewed here. The ideal “electronic filing cabinet” should have several features such as a the capability to label articles by topic, to separate or sort as you see fit, and a search function to find information quickly—making it a personalized and effective point-of-care resource.
STAYING AFLOAT
Clinicians make many decisions every day. In fact, the release of How Doctors Think (both publications) has led to increased research into how clinical decisions and diagnoses are formed.16,17
With the medical literature expected to double every 73 days by 2020,18 there is an ever-widening ocean of information to sift through. With this onslaught, clinicians can no longer remain fully current. Instead, refining skills in accessing, sorting, and interpreting accurate scientific evidence efficiently is crucial to time spent actually caring for patients and coordinating their care.
Guidelines, algorithms, and comprehensive databases can aid clinicians in all aspects of care, from generating more complete differential diagnoses to managing disease-specific treatment. Individuals can first think about and list the qualities of a tool that are most important to them (eg, breadth of topics, frequency of updates, integration within their electronic health record, and cost) before focusing on a few applications or websites that meet those goals. With practice, point-of-care knowledge can become part of the everyday visit.
Effective integration into electronic health records will require design input from front-line clinicians. Otherwise, systems are prone to add too much “support” and overly rely on orthodox metrics and guidelines, resulting in alarm fatigue and frustration rather than facilitation.19–23
OUR CONCLUSIONS
Comprehensive point-of-care resources can play a significant role in helping busy clinicians provide best evidence-based care to their patients. Embedded clinical decision guides within an electronic health record are ideal, but low topic coverage has limited the usefulness of these systems.24 Here are our conclusions:
Medscape, ePocrates, and Wikipedia are probably the most popular free resources. Dynamed has offered free subscriptions to Wikipedia’s top health editors with the hopes of correcting factual errors. Medscape has excellent features but is supported by sponsored content, which raises a concern about bias and potential time-consuming distractions.
Dynamed and UpToDate have both been reported to answer more questions than other sources.12
UpToDate has the largest repository, with each topic curated by an expert or experts in that subject. This content can be dense and difficult to scan quickly at the point of care, but this is balanced by the ability to search within a medical topic, which has given it the fastest retrieval time.15 It does, however, allow authors and editors to maintain financial relationships with companies that produce medications discussed in the article.22
Dynamed has the advantage in frequency of updates, clearest conflict-of-interest policy, and the least amount of conflict of interest. Its topic list is not as extensive as UpToDate’s due to the limitation of using only evidence-based medicine without expert opinion.
First Consult has high user satisfaction, but as a point-of-care resource it can be time-consuming to find the best source for the clinical question at hand, and its expanded access is costly.9
ART AND SCIENCE
Point-of-care resources do not solve all the complicated problems of patient care, and no single resource is ideal for all situations. A busy clinician has limited time to process the evolving literature to practice the best evidence-based medicine. Effective information access, quality of care provided, and the marginal time cost required create a complex calculus. Clinical decision-making remains an art and a science,25 but these technologies help define a new era in its pursuit.
Ultimately, a clinician’s choice needs to correlate with a provider’s resources and style. This article has detailed several options available on the market today. This is a quickly evolving area of products and services. Longer term, users might consider a tool’s preferred key features when evaluating any current or future resource in order to choose the right ones for their practice.
CASE REVISITED
Before we leave for the weekend, we need a plan for Ms. Connecticut. To find appropriate recommendations for our patient, we search several of our point-of-care resources: UpToDate and Dynamed. Both resources have correct information according to the Infectious Disease Society of America (IDSA) guidelines.
UpToDate has a monograph of approximately 2,000 words on Lyme disease, which is lengthy but adds to clinical-decision making skills for a learner thinking through the decision. This service also has a patient handout highlighting the recommendations. The topic was last updated in 2016, but states that it is current with literature through January 2017.
Dynamed has bulleted information that is quicker to digest, but essentially highlights the IDSA recommendations without the thought process behind them. It too, has patient resources with links to a variety of handouts from professional organizations such as the US Centers for Disease Control and Prevention. They last updated the topic January 31, 2017.
When searching for the topic on both sites, a clinician can see the breadth of information in each program. However, this is also a detractor. Searching for Lyme disease prophylaxis on Dynamed brought up related data (that doxycycline is not FDA-approved for prophylaxis), but not the primary information. Likewise, the search under UpToDate first brought us to the patient information. Both articles have helpful tables and links to associated topics.
My partner chose the UpToDate article, in part to review the topic with a medical student. However, I used Dynamed for its quick bulleted information, as I was on call that evening and needed to return to the hospital. We both came to the same conclusion, and Ms. Connecticut chose no prophylaxis even though her home is in an endemic area. She has done well.
- Worster A, Haynes RB. How do I find a point-of-care answer to my clinical question? CJEM 2012; 14:31–35.
- Jeffery R, Navarro T, Lokker C, Haynes RB, Wilczynski NL, Farjou G. How current are leading evidence-based medical textbooks? An analytic survey of four online textbooks. J Med Internet Res 2012; 14:e175.
- ClinicalKey. Errors found in nine out of ten Wikipedia health entries. www.clinicalkey.com/info/blog/errors-in-wikipedia-health/. Accessed February 9, 2017.
- Hasty RT, Garbalosa RC, Barbato VA, et al. Wikipedia vs peer-reviewed medical literature for information about the 10 most costly medical conditions. J Am Osteopath Assoc 2014; 114:368–373.
- Mehta NB, Martin SA, Maypole J, Andrews R. Information management for clinicians. Cleve Clin J Med 2016; 83:589–595.
- Cook DA, Sorensen KJ, Hersh W, Berger RA, Wilkinson JM. Features of effective medical knowledge resources to support point of care learning: a focus group study. PLoS One 2013; 8:e80318.
- Steinbrook R. Future directions in industry funding of continuing medical education. Arch Intern Med 2011; 171:257–258.
- Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ 1999; 319:1618.
- Kim S, Noveck H, Galt J, Hogshire L, Willett L, O’Rourke K. Searching for answers to clinical questions using Google versus evidence-based summary resources: a randomized controlled crossover study. Acad Med 2014; 89:940–943.
- Ahmadi SF, Faghankhani M, Javanbakht A, et al. A comparison of answer retrieval through four evidence-based textbooks (ACP PIER, Essential Evidence Plus, First Consult, and UpToDate): a randomized controlled trial. Med Teach 2011; 33:724–730.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Shurtz S, Foster MJ. Developing and using a rubric for evaluating evidence-based medicine point-of-care tools. J Med Libr Assoc 2011; 99:247–254.
- Ketterman E, Besaw M. An evaluation of citation counts, search results, and frequency of updates in Dynamed and UpToDate. J Electron Res in Med Libr 2010; 7:273–280.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics 2014; 40:578–580.
- Montgomery K. How Doctors Think: Clinical Judgment and the Practice of Medicine. New York, NY: Oxford University Press; 2005.
- Groopman J. How Doctors Think. Boston, MA: Houghton Mifflin; 2008.
- Densen P. Challenges and opportunities facing medical education. Trans Am Clin Climatol Assoc 2010; 122:48–58.
- Kesselheim AS, Cresswell K, Phansalkar S, Bates DW, Sheikh A. Clinical decision support systems could be modified to reduce ‘alert fatigue’ while still minimizing the risk of litigation. Health Aff (Millwood) 2011; 30:2310–2317.
- Russ AL, Zillich AJ, McManus MS, Doebbeling BN, Saleem JJ. Prescribers’ interactions with medication alerts at the point of prescribing: a multi-method, in situ investigation of the human-computer interaction. Int J Med Inform 2012; 81:232–243.
- Fraccaro P, Arguello Castelerio M, Ainsworth J, Buchan I. Adoption of clinical decision support in multimorbidity: a systematic review. JMIR Med Informatics 2015; 3:e4.
- McLeod W, Eidus R, Stewart EE. Clinical decision support: using technology to identify patients’ unmet needs. Fam Pract Manag 2012; 19:22–28.
- Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med 2014; 371:1280–1283.
- Cook DA, Sorensen KJ, Nishimura RA, Ommen SR, Lloyd FJ. A comprehensive information technology system to support physician learning at the point of care. Acad Med 2015; 90:33–39.
- Woolever DR. The art and science of clinical decision making. Fam Pract Manag 2008; 15:31–36.
- Worster A, Haynes RB. How do I find a point-of-care answer to my clinical question? CJEM 2012; 14:31–35.
- Jeffery R, Navarro T, Lokker C, Haynes RB, Wilczynski NL, Farjou G. How current are leading evidence-based medical textbooks? An analytic survey of four online textbooks. J Med Internet Res 2012; 14:e175.
- ClinicalKey. Errors found in nine out of ten Wikipedia health entries. www.clinicalkey.com/info/blog/errors-in-wikipedia-health/. Accessed February 9, 2017.
- Hasty RT, Garbalosa RC, Barbato VA, et al. Wikipedia vs peer-reviewed medical literature for information about the 10 most costly medical conditions. J Am Osteopath Assoc 2014; 114:368–373.
- Mehta NB, Martin SA, Maypole J, Andrews R. Information management for clinicians. Cleve Clin J Med 2016; 83:589–595.
- Cook DA, Sorensen KJ, Hersh W, Berger RA, Wilkinson JM. Features of effective medical knowledge resources to support point of care learning: a focus group study. PLoS One 2013; 8:e80318.
- Steinbrook R. Future directions in industry funding of continuing medical education. Arch Intern Med 2011; 171:257–258.
- Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ 1999; 319:1618.
- Kim S, Noveck H, Galt J, Hogshire L, Willett L, O’Rourke K. Searching for answers to clinical questions using Google versus evidence-based summary resources: a randomized controlled crossover study. Acad Med 2014; 89:940–943.
- Ahmadi SF, Faghankhani M, Javanbakht A, et al. A comparison of answer retrieval through four evidence-based textbooks (ACP PIER, Essential Evidence Plus, First Consult, and UpToDate): a randomized controlled trial. Med Teach 2011; 33:724–730.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Prorok JC, Iserman EC, Wilczynski NL, Haynes RB. The quality, breadth, and timeliness of content updating vary substantially for 10 online medical texts: an analytic survey. J Clin Epidemiol 2012; 65:1289–1295.
- Shurtz S, Foster MJ. Developing and using a rubric for evaluating evidence-based medicine point-of-care tools. J Med Libr Assoc 2011; 99:247–254.
- Ketterman E, Besaw M. An evaluation of citation counts, search results, and frequency of updates in Dynamed and UpToDate. J Electron Res in Med Libr 2010; 7:273–280.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics 2014; 40:578–580.
- Montgomery K. How Doctors Think: Clinical Judgment and the Practice of Medicine. New York, NY: Oxford University Press; 2005.
- Groopman J. How Doctors Think. Boston, MA: Houghton Mifflin; 2008.
- Densen P. Challenges and opportunities facing medical education. Trans Am Clin Climatol Assoc 2010; 122:48–58.
- Kesselheim AS, Cresswell K, Phansalkar S, Bates DW, Sheikh A. Clinical decision support systems could be modified to reduce ‘alert fatigue’ while still minimizing the risk of litigation. Health Aff (Millwood) 2011; 30:2310–2317.
- Russ AL, Zillich AJ, McManus MS, Doebbeling BN, Saleem JJ. Prescribers’ interactions with medication alerts at the point of prescribing: a multi-method, in situ investigation of the human-computer interaction. Int J Med Inform 2012; 81:232–243.
- Fraccaro P, Arguello Castelerio M, Ainsworth J, Buchan I. Adoption of clinical decision support in multimorbidity: a systematic review. JMIR Med Informatics 2015; 3:e4.
- McLeod W, Eidus R, Stewart EE. Clinical decision support: using technology to identify patients’ unmet needs. Fam Pract Manag 2012; 19:22–28.
- Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med 2014; 371:1280–1283.
- Cook DA, Sorensen KJ, Nishimura RA, Ommen SR, Lloyd FJ. A comprehensive information technology system to support physician learning at the point of care. Acad Med 2015; 90:33–39.
- Woolever DR. The art and science of clinical decision making. Fam Pract Manag 2008; 15:31–36.
KEY POINTS
- Today, it seems impossible to keep up with all the information we need, but we can refine our skills in accessing, sorting, and interpreting accurate scientific evidence.
- The resources reviewed in this article require paid subscriptions except for Medscape, which is supported by advertising.
- Each of the resources has strengths and weaknesses. For example, UpToDate offers the most topics, but its articles tend to be too long to be practical to read at the point of care.
- Physicians should familiarize themselves with these resources and use the ones that best suit their needs.
Update on viral hepatitis in pregnancy
Viral hepatitis affects mother and child, and pregnancy can exacerbate the disease. Vertical transmission contributes significantly to the high prevalence of viral hepatitis and compromises the well-being and the prognosis in the newborn. The indications for therapy in pregnant women may differ from those in the general population, and new therapies are available.
HEPATITIS A
Hepatitis A virus (HAV) infection is associated with significant morbidity and death around the world, as 1.4 million cases are reported every year worldwide.1 However, in the United States, the prevalence has declined by 95% since HAV vaccination was introduced in 1995, and in 2013, the prevalence was 0.6 per 100,000 population.2 Acute HAV infection during pregnancy is rare. As a result, the incidence during pregnancy is difficult to ascertain.3
HAV is transmitted by the fecal-oral route from person to person contact and from contamination of food and water. Vertical transmission from pregnant mother to fetus has not been reported.4
Clinical outcomes of HAV in pregnancy
Acute HAV infection during pregnancy is rare, and teratogenicity associated with HAV has not been reported.3 The course of the disease during pregnancy is generally similar to that in nonpregnant patients, with excellent maternal and fetal outcomes in developed nations. There have been reports in developing nations of premature contractions and labor, placental separation, premature rupture of the membranes, and vaginal bleeding.5,6
Diagnosis
Routine screening for HAV is not recommended, but serologic testing by detection of anti-HAV immunoglobulin M (IgM) antibodies is done in high-risk patients suspected of having acute HAV infection.
Prevention
Prevention includes adherence to sanitary practices and active and passive immunoprophylaxis.3 Universal vaccination for pregnant mothers is not recommended,1,2,6 but vaccination is recommended for high-risk patients and mothers—those with chronic liver disease, those receiving clotting factors, those who use illegal drugs, and travelers to areas where HAV is endemic. Immune globulin is also available for postexposure prophylaxis. HAV vaccines and immune globulin are safe in pregnancy.3,6,7
Treatment
Treatment of acute HAV in pregnancy is supportive because of its benign nature; few patients require hospitalization.3
Pregnant patients with HAV can deliver vaginally, and breastfeeding is not contraindicated.8
HEPATITIS B
Hepatitis B virus (HBV) infection is a major global health problem. About 240 million people worldwide have chronic HBV infection, and more than 780,000 die every year from acute and chronic consequences.9
Vertical transmission is responsible for about half of chronic HBV infections worldwide. Thus, interruption of mother-to-child transmission is important. Universal maternal screening and passive-active immunoprophylaxis of newborns have lowered the transmission rates to between 5% and 10%. The 10% failure rate is unacceptably high and has been attributed to seropositivity for hepatitis B e antigen and a high viral load in the mother (ie, HBV DNA > 106 copies/mL). High viral load is an independent risk factor for failure of immunoprophylaxis.10 Therefore, antiviral therapy is suggested in pregnant women who have a high HBV viral load to further decrease the chance of mother-to-child transmission and to prevent failure of immunoprophylaxis.11
Clinical outcomes of HBV in pregnancy
Acute HBV infection during pregnancy is usually benign and is not associated with increased risk of death or teratogenicity.12 Symptomatic disease in the mother with acute hepatitis B includes nausea, vomiting, abdominal pain, fatigue, and jaundice.3 For the newborn, there is increased risk of low birth weight and prematurity.13
When acute HBV infection occurs early in pregnancy, the rate of perinatal transmission is about 10%, increasing to 60% if it occurs near delivery.12,13
Chronic HBV infection does not usually affect the outcome of pregnancy, but it may if the woman has cirrhosis or advanced liver disease14; however, pregnancy is very rare in women with HBV cirrhosis due to anovulation, and acute HBV flares have been described during pregnancy and postpartum.15
Pregnant patients with cirrhosis and portal hypertension are at risk of hepatic decompensation, variceal bleeding, and death.16 Risk is high with a score of 10 or more on the Model for End-stage Liver Disease scale, and is low with a score of 6 or less.17 Like nonpregnant patients, pregnant patients with cirrhosis should be monitored, and upper endoscopy should be performed in the 2nd trimester to assess for varices. A beta-blocker should be given or banding of varices should be done to avoid rupture. Rates of fetal demise, premature labor, spontaneous abortion, and stillbirth are high with portal hypertension.16
Risk of mother-to-child HBV transmission
Vertical HBV transmission can occur during the antepartum, intrapartum, and postpartum periods,18,19 but it most often occurs during the intrapartum period at the time of delivery. Without immunoprophylaxis of the newborn, the risk of mother-to-child transmission can be as high as 90% if the mother is hepatitis B e antigen-positive and has a viral load greater than 106 copies/mL. With active and passive immunoprophylaxis, the risk decreases substantially.
Screening and diagnosis
All pregnant women should be tested for hepatitis B surface antigen during the 1st trimester,20 or any time thereafter if early testing was not done, even if they were vaccinated before becoming pregnant.21
Prevention
HBV infection is best prevented before pregnancy by vaccinating the mother or, after delivery, by vaccinating the newborn. Universal vaccination of newborns has been the standard of care since the 1990s. Pregnant women should be tested early in the pregnancy; unvaccinated, uninfected women at high risk of acquiring HBV (eg, because of sexual contacts or intravenous drug use) should be vaccinated.2,3
HBV vaccine and immune globulin are both approved by the US Food and Drug Administration (FDA) for prevention of HBV infection and can be given during pregnancy and breastfeeding.3 All infants should be vaccinated for HBV at birth, and all infants born to mothers who test positive for hepatitis B s antigen should receive the HBV vaccine and the immune globulin within 12 to 24 hours after delivery. The vaccine series should be completed within 6 months.20,21 This will decrease the rate of neonatal infection.
Treatment of HBV infection in pregnancy
The main objectives of treating chronic HBV infection in pregnancy are to maintain stable liver function in the mother and to prevent neonatal infection, which may cause cirrhosis and hepatocellular carcinoma and contribute to the global burden of the disease.22 Therefore, maternal HBV DNA and liver aminotransferase levels should be tested regularly during gestation.
The current guidelines of the American Association for the Study of Liver Diseases suggest antiviral therapy to reduce the risk of perinatal transmission of HBV in pregnant women with an HBV DNA level greater than 200,000 IU/mL or greater than 106 copies/mL.23,24
In a meta-analysis,25 antiviral therapy with lamivudine, telbivudine, or tenofovir showed no apparent teratogenicity or safety concerns for maternal and fetal outcomes26 and significantly reduced the rate of mother-to-child transmission. Of these 3 drugs, telbivudine was associated with a higher rate of normalization of liver enzymes, HBV suppression, and e-antigen seroconversion.25 Lamivudine has proven the test of time in mothers co-infected with HBV and human immunodeficiency virus (HIV). However, tenofovir is considered the preferred treatment in pregnancy, owing to concerns about drug resistance to telbivudine and lamivudine and a high genetic barrier to resistance with tenofovir.26 In mothers with HBV and HIV treated with tenofovir, treatment was associated with lower bone mineral density in the newborns, with a propensity for renal injury in the mothers. No safety concerns for maternal or fetal outcomes were identified in pregnant women infected only with HBV.25
Many pregnant mothers choose to stop therapy around the time of conception because of safety concerns for the baby. In such situations, close monitoring is necessary to detect flares of HBV infection.
When the decision to treat is made, treatment should begin at 28 to 30 weeks of gestation, when organogenesis is complete and to allow enough time for HBV DNA levels to decline.
Breastfeeding is not contraindicated because antiviral drugs are minimally excreted in breast milk and are unlikely to cause toxicity. However, data are insufficient as to the long-term safety for the newborn when the mother took these drugs during pregancy and while breastfeeding.23,27
Alanine aminotransferase and HBV DNA levels should be monitored postpartum because of the possibility of a hepatitis flare. In this setting, any of the three drugs can be used.28 For mothers on therapy because of cirrhosis or an advanced histologic feature, antiviral therapy should be continued throughout pregnancy to prevent disease progression and decompensation.19,22,27
No drug therapy is necessary for pregnant carriers of HBV.
Delivery and breastfeeding
The mode of delivery does not appear to have a significant effect on the interruption of vertical transmission of HBV.29 Cesarean delivery is not recommended by the US Centers for Disease Control and Prevention (CDC)2 or the American College of Obstetricians and Gynecologists.6 Breastfeeding is encouraged if the infant has received appropriate immunoprophylaxis.6
Coinfection with hepatitis D
Coinfection with hepatitis D virus (HDV) and HBV is associated with severe acute hepatitis30,31 and increases the risk of death by a factor of 10. The World Health Organization recommends testing for HDV in pregnant women who are HBV-positive.8
Prevention of HDV infection requires prevention of HBV. The treatment of HDV in pregnancy is supportive. Pegylated interferon is successful outside pregnancy but is contraindicated during pregnancy.32 In patients with fulminant hepatic failure and end-stage liver disease, liver transplant can be lifesaving.
Take-home points
- HBV infection during pregnancy is usually benign and not severe but can be associated with an increased risk of mother-to-child transmission and progression of liver disease in the pregnant mother.
- Prevention of vertical transmission of HBV is important to reduce the burden of chronic HBV infection. Universal maternal screening early in pregnancy and passive-active immunoprophylaxis of newborns are usually sufficient to prevent vertical transmission of HBV, but antiviral therapy is needed for highly viremic mothers to further reduce the risk.
- Antiviral therapy is also indicated for pregnant women with moderate to severe hepatitis or cirrhosis to prevent disease progression and liver failure.
- Telbivudine, tenofovir, or lamivudine can be used during pregnancy, but more data are needed on the long-term safety of fetal exposure to these agents.
HEPATITIS C
The global prevalence of hepatitis C virus (HCV) infection is 2% to 3%, with 130 to 170 million HCV-positive people, most of whom are chronically infected.33 The incidence of HCV during pregnancy is 1% to 2.4%, but 3% to 5% of infected mothers transmit HCV to their child at the time of birth.6,34 Women coinfected with HIV and HCV have twice the risk of perinatal HCV transmission compared with women who have HCV infection alone.6,34
HCV infection is usually asymptomatic and is discovered either by screening high-risk patients or during evaluation of persistently elevated aminotransferase levels. Acute HCV infection during pregnancy has been reported only rarely, and most pregnant women who are infected have chronic disease with no effect on the pregnancy or the infant.6,34
Treatment
The CDC recommends that all adults (including pregnant women) born between 1945 and 1965 undergo 1-time testing for HCV without prior ascertainment of HCV risk (strong recommendation, with moderate quality of evidence).35 The most important risk factor for HCV infection is past or current injection drug use.33 Additional risk factors are similar to those for nonpregnant patients.
Because of the benign effect of HCV on the pregnancy, treatment is not recommended. To decrease the risk of maternal-child transmission, it is prudent to avoid amniocentesis, scalp instrumentation, and prolonged rupture of membranes.6
There is no vaccine or immune globulin for prevention. HCV infection should not influence the mode of delivery, and it is not a contraindication to breastfeeding.34,36,37
HEPATITIS E
Every year, 20 million cases of hepatitis E virus (HEV) infection are recorded worldwide. These numbers include 3.3 million symptomatic cases and 56,600 deaths.38 HEV infection is most common in developing countries, and pregnant women traveling to these areas are at high risk of acquiring this infection, of developing fulminant hepatitis, and of death.39 Sporadic cases not associated with travel are increasingly reported in developed countries and are attributed to immunocompromised status (due to HIV or solid-organ transplant).38,40
Modes of transmission of HEV are mainly via fecal-oral contamination and by vertical transmission.41
Diagnosis
HEV infection can be diagnosed either by detecting IgM antibody with an enzyme-linked immunosorbent assay or by detecting HEV RNA in the blood using reverse transcription polymerase chain reaction testing.42
Treatment and prevention
Hospitalization should be considered for pregnant women. Ribavirin or pegylated interferon alpha or both are effective but are contraindicated in pregnancy because of the risk of teratogenicity.41,42 Urgent liver transplant can be a successful option in acute liver failure.
Prevention relies primarily on good sanitation, clean drinking water, and avoiding raw pork and venison. Boiling and chlorination of water inactivate HEV.39,40 Pregnant women should be advised to avoid travel to highly endemic areas.
- World Health Organization (WHO).Hepatitis A fact sheet. www.who.int/mediacentre/factsheets/fs328/en/. Accessed December 7, 2016.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—statistics & surveillance. www.cdc.gov/hepatitis/statistics/2013surveillance/commentary.htm#hepatitis A. Accessed December 7, 2016.
- Rac MW, Sheffield JS. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin North Am 2014; 41:573–592.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Elinav E, Ben-Dov IZ, Shapira Y, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology 2006; 130:1129–1134.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86: Viral hepatitis in pregnancy. Obstet Gynecol 2007; 110:941–956.
- Advisory Committee on Immunization Practices. Guidelines for Vaccinating Pregnant Women. www.cdc.gov/vaccines/pregnancy/hcp/guidelines.html. Accessed December 7, 2016.
- Daudi N, Shouval D, Stein-Zamir C, Ackerman Z. Breastmilk hepatitis A virus RNA in nursing mothers with acute hepatitis A virus infection. Breastfeed Med 2012; 7:313–315.
- World Health Organization (WHO). Hepatitis B fact sheet. www.who.int/mediacentre/factsheets/fs204/en/. Accessed December 7, 2016.
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012; 19:e18–e25.
- Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis 2013; 33:138–146.
- Sookoian S. Liver disease during pregnancy: acute viral hepatitis. Ann Hepatol 2006; 5:231–236.
- Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int 2009; 29(suppl 1):133–139.
- Wong S, Chan LY, Yu V, Ho L. Hepatitis B carrier and perinatal outcome in singleton pregnancy. Am J Perinatol 1999; 16:485–488.
- Rawal BK, Parida S, Watkins RP, Ghosh P, Smith H. Symptomatic reactivation of hepatitis B in pregnancy. Lancet 1991; 337:364.
- Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman RK. Pregnancy with portal hypertension. J Clin Exp Hepatol 2014; 4:163–171.
- Westbrook RH, Yeoman AD, O'Grady JG, Harrison PM, Devlin J, Heneghan MA. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol 2011; 9:694–699.
- Cheung KW, Seto MT, Wong SF. Towards complete eradication of hepatitis B infection from perinatal transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during pregnancy. Eur J Obstet Gynecol Reproduct Biol 2013; 169:17–23.
- Pan CQ, Duan AP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012; 10:452–459.
- Mast EE, Margolis HS, Fiore AE, et al; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- US Centers for Disease Control (CDC). Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep 1988; 37:341–346, 351.
- Han GR, Xu CL, Zhao W, Yang YF. Management of chronic hepatitis B in pregnancy. World J Gastroenterol 2012; 18:4517–4521.
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–283.
- Tran TT, Ahn J, Reau N. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol 2016: 111:176–194.
- Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatol 2016; 63:319–333.
- Brown RS Jr, Verna EC, Pereira MR, et al. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the antiretroviral pregnancy registry. J Hepatol 2012; 57:953–959.
- Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol 2015; 7:1233–1237.
- Potthoff A, Rifai K, Wedemeyer H, Deterding K, Manns M, Strassburg C. Successful treatment of fulminant hepatitis B during pregnancy. Z Gastroenterol 2009; 47:667–670.
- Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus—a systematic review. Virol J 2008; 5:100.
- Price J. An update on hepatitis B, D, and E viruses. Top Antivir Med 2014; 21:157–163.
- World Health Organization (WHO). Global alert and response. Hepatitis Delta. www.who.int/csr/resources/publications/hepatitis/who_cds_csr_ncs_2001_1/en/. Accessed December 7, 2016.
- Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther 2014; 19:463–468.
- Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Curr Pharm Des 2008; 14:1646–1654.
- Floreani A. Hepatitis C and pregnancy. World J Gastroenterol 2013; 19:6714–6720.
- US Centers for Disease Control and Prevention. Viral hepatitis—CDC recommendations for specific populations and settings. www.cdc.gov/hepatitis/populations/1945-1965.htm. Accessed December 7, 2016.
- World Health Organization (WHO). Hepatitis C fact sheet. www.who.int/mediacentre/factsheets/fs164/en/. Accessed December 7, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:349–357.
- World Health Organization (WHO). Hepatitis E fact sheet. www.who.int/mediacentre/factsheets/fs280/en/. Accessed December 7, 2016.
- Velosa M, Figueiredo A, Gloria H, et al. Fulminant hepatitis E in a pregnant woman. GE Port J Gastroenterol 2013; 20:210–214.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—hepatitis E information. Hepatitis E FAQs for health professionals. www.cdc.gov/hepatitis/hev/hevfaq.htm. Accessed December 7, 2016.
- Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat 2015; 22:965–973.
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012: 379:2477–2488.
Viral hepatitis affects mother and child, and pregnancy can exacerbate the disease. Vertical transmission contributes significantly to the high prevalence of viral hepatitis and compromises the well-being and the prognosis in the newborn. The indications for therapy in pregnant women may differ from those in the general population, and new therapies are available.
HEPATITIS A
Hepatitis A virus (HAV) infection is associated with significant morbidity and death around the world, as 1.4 million cases are reported every year worldwide.1 However, in the United States, the prevalence has declined by 95% since HAV vaccination was introduced in 1995, and in 2013, the prevalence was 0.6 per 100,000 population.2 Acute HAV infection during pregnancy is rare. As a result, the incidence during pregnancy is difficult to ascertain.3
HAV is transmitted by the fecal-oral route from person to person contact and from contamination of food and water. Vertical transmission from pregnant mother to fetus has not been reported.4
Clinical outcomes of HAV in pregnancy
Acute HAV infection during pregnancy is rare, and teratogenicity associated with HAV has not been reported.3 The course of the disease during pregnancy is generally similar to that in nonpregnant patients, with excellent maternal and fetal outcomes in developed nations. There have been reports in developing nations of premature contractions and labor, placental separation, premature rupture of the membranes, and vaginal bleeding.5,6
Diagnosis
Routine screening for HAV is not recommended, but serologic testing by detection of anti-HAV immunoglobulin M (IgM) antibodies is done in high-risk patients suspected of having acute HAV infection.
Prevention
Prevention includes adherence to sanitary practices and active and passive immunoprophylaxis.3 Universal vaccination for pregnant mothers is not recommended,1,2,6 but vaccination is recommended for high-risk patients and mothers—those with chronic liver disease, those receiving clotting factors, those who use illegal drugs, and travelers to areas where HAV is endemic. Immune globulin is also available for postexposure prophylaxis. HAV vaccines and immune globulin are safe in pregnancy.3,6,7
Treatment
Treatment of acute HAV in pregnancy is supportive because of its benign nature; few patients require hospitalization.3
Pregnant patients with HAV can deliver vaginally, and breastfeeding is not contraindicated.8
HEPATITIS B
Hepatitis B virus (HBV) infection is a major global health problem. About 240 million people worldwide have chronic HBV infection, and more than 780,000 die every year from acute and chronic consequences.9
Vertical transmission is responsible for about half of chronic HBV infections worldwide. Thus, interruption of mother-to-child transmission is important. Universal maternal screening and passive-active immunoprophylaxis of newborns have lowered the transmission rates to between 5% and 10%. The 10% failure rate is unacceptably high and has been attributed to seropositivity for hepatitis B e antigen and a high viral load in the mother (ie, HBV DNA > 106 copies/mL). High viral load is an independent risk factor for failure of immunoprophylaxis.10 Therefore, antiviral therapy is suggested in pregnant women who have a high HBV viral load to further decrease the chance of mother-to-child transmission and to prevent failure of immunoprophylaxis.11
Clinical outcomes of HBV in pregnancy
Acute HBV infection during pregnancy is usually benign and is not associated with increased risk of death or teratogenicity.12 Symptomatic disease in the mother with acute hepatitis B includes nausea, vomiting, abdominal pain, fatigue, and jaundice.3 For the newborn, there is increased risk of low birth weight and prematurity.13
When acute HBV infection occurs early in pregnancy, the rate of perinatal transmission is about 10%, increasing to 60% if it occurs near delivery.12,13
Chronic HBV infection does not usually affect the outcome of pregnancy, but it may if the woman has cirrhosis or advanced liver disease14; however, pregnancy is very rare in women with HBV cirrhosis due to anovulation, and acute HBV flares have been described during pregnancy and postpartum.15
Pregnant patients with cirrhosis and portal hypertension are at risk of hepatic decompensation, variceal bleeding, and death.16 Risk is high with a score of 10 or more on the Model for End-stage Liver Disease scale, and is low with a score of 6 or less.17 Like nonpregnant patients, pregnant patients with cirrhosis should be monitored, and upper endoscopy should be performed in the 2nd trimester to assess for varices. A beta-blocker should be given or banding of varices should be done to avoid rupture. Rates of fetal demise, premature labor, spontaneous abortion, and stillbirth are high with portal hypertension.16
Risk of mother-to-child HBV transmission
Vertical HBV transmission can occur during the antepartum, intrapartum, and postpartum periods,18,19 but it most often occurs during the intrapartum period at the time of delivery. Without immunoprophylaxis of the newborn, the risk of mother-to-child transmission can be as high as 90% if the mother is hepatitis B e antigen-positive and has a viral load greater than 106 copies/mL. With active and passive immunoprophylaxis, the risk decreases substantially.
Screening and diagnosis
All pregnant women should be tested for hepatitis B surface antigen during the 1st trimester,20 or any time thereafter if early testing was not done, even if they were vaccinated before becoming pregnant.21
Prevention
HBV infection is best prevented before pregnancy by vaccinating the mother or, after delivery, by vaccinating the newborn. Universal vaccination of newborns has been the standard of care since the 1990s. Pregnant women should be tested early in the pregnancy; unvaccinated, uninfected women at high risk of acquiring HBV (eg, because of sexual contacts or intravenous drug use) should be vaccinated.2,3
HBV vaccine and immune globulin are both approved by the US Food and Drug Administration (FDA) for prevention of HBV infection and can be given during pregnancy and breastfeeding.3 All infants should be vaccinated for HBV at birth, and all infants born to mothers who test positive for hepatitis B s antigen should receive the HBV vaccine and the immune globulin within 12 to 24 hours after delivery. The vaccine series should be completed within 6 months.20,21 This will decrease the rate of neonatal infection.
Treatment of HBV infection in pregnancy
The main objectives of treating chronic HBV infection in pregnancy are to maintain stable liver function in the mother and to prevent neonatal infection, which may cause cirrhosis and hepatocellular carcinoma and contribute to the global burden of the disease.22 Therefore, maternal HBV DNA and liver aminotransferase levels should be tested regularly during gestation.
The current guidelines of the American Association for the Study of Liver Diseases suggest antiviral therapy to reduce the risk of perinatal transmission of HBV in pregnant women with an HBV DNA level greater than 200,000 IU/mL or greater than 106 copies/mL.23,24
In a meta-analysis,25 antiviral therapy with lamivudine, telbivudine, or tenofovir showed no apparent teratogenicity or safety concerns for maternal and fetal outcomes26 and significantly reduced the rate of mother-to-child transmission. Of these 3 drugs, telbivudine was associated with a higher rate of normalization of liver enzymes, HBV suppression, and e-antigen seroconversion.25 Lamivudine has proven the test of time in mothers co-infected with HBV and human immunodeficiency virus (HIV). However, tenofovir is considered the preferred treatment in pregnancy, owing to concerns about drug resistance to telbivudine and lamivudine and a high genetic barrier to resistance with tenofovir.26 In mothers with HBV and HIV treated with tenofovir, treatment was associated with lower bone mineral density in the newborns, with a propensity for renal injury in the mothers. No safety concerns for maternal or fetal outcomes were identified in pregnant women infected only with HBV.25
Many pregnant mothers choose to stop therapy around the time of conception because of safety concerns for the baby. In such situations, close monitoring is necessary to detect flares of HBV infection.
When the decision to treat is made, treatment should begin at 28 to 30 weeks of gestation, when organogenesis is complete and to allow enough time for HBV DNA levels to decline.
Breastfeeding is not contraindicated because antiviral drugs are minimally excreted in breast milk and are unlikely to cause toxicity. However, data are insufficient as to the long-term safety for the newborn when the mother took these drugs during pregancy and while breastfeeding.23,27
Alanine aminotransferase and HBV DNA levels should be monitored postpartum because of the possibility of a hepatitis flare. In this setting, any of the three drugs can be used.28 For mothers on therapy because of cirrhosis or an advanced histologic feature, antiviral therapy should be continued throughout pregnancy to prevent disease progression and decompensation.19,22,27
No drug therapy is necessary for pregnant carriers of HBV.
Delivery and breastfeeding
The mode of delivery does not appear to have a significant effect on the interruption of vertical transmission of HBV.29 Cesarean delivery is not recommended by the US Centers for Disease Control and Prevention (CDC)2 or the American College of Obstetricians and Gynecologists.6 Breastfeeding is encouraged if the infant has received appropriate immunoprophylaxis.6
Coinfection with hepatitis D
Coinfection with hepatitis D virus (HDV) and HBV is associated with severe acute hepatitis30,31 and increases the risk of death by a factor of 10. The World Health Organization recommends testing for HDV in pregnant women who are HBV-positive.8
Prevention of HDV infection requires prevention of HBV. The treatment of HDV in pregnancy is supportive. Pegylated interferon is successful outside pregnancy but is contraindicated during pregnancy.32 In patients with fulminant hepatic failure and end-stage liver disease, liver transplant can be lifesaving.
Take-home points
- HBV infection during pregnancy is usually benign and not severe but can be associated with an increased risk of mother-to-child transmission and progression of liver disease in the pregnant mother.
- Prevention of vertical transmission of HBV is important to reduce the burden of chronic HBV infection. Universal maternal screening early in pregnancy and passive-active immunoprophylaxis of newborns are usually sufficient to prevent vertical transmission of HBV, but antiviral therapy is needed for highly viremic mothers to further reduce the risk.
- Antiviral therapy is also indicated for pregnant women with moderate to severe hepatitis or cirrhosis to prevent disease progression and liver failure.
- Telbivudine, tenofovir, or lamivudine can be used during pregnancy, but more data are needed on the long-term safety of fetal exposure to these agents.
HEPATITIS C
The global prevalence of hepatitis C virus (HCV) infection is 2% to 3%, with 130 to 170 million HCV-positive people, most of whom are chronically infected.33 The incidence of HCV during pregnancy is 1% to 2.4%, but 3% to 5% of infected mothers transmit HCV to their child at the time of birth.6,34 Women coinfected with HIV and HCV have twice the risk of perinatal HCV transmission compared with women who have HCV infection alone.6,34
HCV infection is usually asymptomatic and is discovered either by screening high-risk patients or during evaluation of persistently elevated aminotransferase levels. Acute HCV infection during pregnancy has been reported only rarely, and most pregnant women who are infected have chronic disease with no effect on the pregnancy or the infant.6,34
Treatment
The CDC recommends that all adults (including pregnant women) born between 1945 and 1965 undergo 1-time testing for HCV without prior ascertainment of HCV risk (strong recommendation, with moderate quality of evidence).35 The most important risk factor for HCV infection is past or current injection drug use.33 Additional risk factors are similar to those for nonpregnant patients.
Because of the benign effect of HCV on the pregnancy, treatment is not recommended. To decrease the risk of maternal-child transmission, it is prudent to avoid amniocentesis, scalp instrumentation, and prolonged rupture of membranes.6
There is no vaccine or immune globulin for prevention. HCV infection should not influence the mode of delivery, and it is not a contraindication to breastfeeding.34,36,37
HEPATITIS E
Every year, 20 million cases of hepatitis E virus (HEV) infection are recorded worldwide. These numbers include 3.3 million symptomatic cases and 56,600 deaths.38 HEV infection is most common in developing countries, and pregnant women traveling to these areas are at high risk of acquiring this infection, of developing fulminant hepatitis, and of death.39 Sporadic cases not associated with travel are increasingly reported in developed countries and are attributed to immunocompromised status (due to HIV or solid-organ transplant).38,40
Modes of transmission of HEV are mainly via fecal-oral contamination and by vertical transmission.41
Diagnosis
HEV infection can be diagnosed either by detecting IgM antibody with an enzyme-linked immunosorbent assay or by detecting HEV RNA in the blood using reverse transcription polymerase chain reaction testing.42
Treatment and prevention
Hospitalization should be considered for pregnant women. Ribavirin or pegylated interferon alpha or both are effective but are contraindicated in pregnancy because of the risk of teratogenicity.41,42 Urgent liver transplant can be a successful option in acute liver failure.
Prevention relies primarily on good sanitation, clean drinking water, and avoiding raw pork and venison. Boiling and chlorination of water inactivate HEV.39,40 Pregnant women should be advised to avoid travel to highly endemic areas.
Viral hepatitis affects mother and child, and pregnancy can exacerbate the disease. Vertical transmission contributes significantly to the high prevalence of viral hepatitis and compromises the well-being and the prognosis in the newborn. The indications for therapy in pregnant women may differ from those in the general population, and new therapies are available.
HEPATITIS A
Hepatitis A virus (HAV) infection is associated with significant morbidity and death around the world, as 1.4 million cases are reported every year worldwide.1 However, in the United States, the prevalence has declined by 95% since HAV vaccination was introduced in 1995, and in 2013, the prevalence was 0.6 per 100,000 population.2 Acute HAV infection during pregnancy is rare. As a result, the incidence during pregnancy is difficult to ascertain.3
HAV is transmitted by the fecal-oral route from person to person contact and from contamination of food and water. Vertical transmission from pregnant mother to fetus has not been reported.4
Clinical outcomes of HAV in pregnancy
Acute HAV infection during pregnancy is rare, and teratogenicity associated with HAV has not been reported.3 The course of the disease during pregnancy is generally similar to that in nonpregnant patients, with excellent maternal and fetal outcomes in developed nations. There have been reports in developing nations of premature contractions and labor, placental separation, premature rupture of the membranes, and vaginal bleeding.5,6
Diagnosis
Routine screening for HAV is not recommended, but serologic testing by detection of anti-HAV immunoglobulin M (IgM) antibodies is done in high-risk patients suspected of having acute HAV infection.
Prevention
Prevention includes adherence to sanitary practices and active and passive immunoprophylaxis.3 Universal vaccination for pregnant mothers is not recommended,1,2,6 but vaccination is recommended for high-risk patients and mothers—those with chronic liver disease, those receiving clotting factors, those who use illegal drugs, and travelers to areas where HAV is endemic. Immune globulin is also available for postexposure prophylaxis. HAV vaccines and immune globulin are safe in pregnancy.3,6,7
Treatment
Treatment of acute HAV in pregnancy is supportive because of its benign nature; few patients require hospitalization.3
Pregnant patients with HAV can deliver vaginally, and breastfeeding is not contraindicated.8
HEPATITIS B
Hepatitis B virus (HBV) infection is a major global health problem. About 240 million people worldwide have chronic HBV infection, and more than 780,000 die every year from acute and chronic consequences.9
Vertical transmission is responsible for about half of chronic HBV infections worldwide. Thus, interruption of mother-to-child transmission is important. Universal maternal screening and passive-active immunoprophylaxis of newborns have lowered the transmission rates to between 5% and 10%. The 10% failure rate is unacceptably high and has been attributed to seropositivity for hepatitis B e antigen and a high viral load in the mother (ie, HBV DNA > 106 copies/mL). High viral load is an independent risk factor for failure of immunoprophylaxis.10 Therefore, antiviral therapy is suggested in pregnant women who have a high HBV viral load to further decrease the chance of mother-to-child transmission and to prevent failure of immunoprophylaxis.11
Clinical outcomes of HBV in pregnancy
Acute HBV infection during pregnancy is usually benign and is not associated with increased risk of death or teratogenicity.12 Symptomatic disease in the mother with acute hepatitis B includes nausea, vomiting, abdominal pain, fatigue, and jaundice.3 For the newborn, there is increased risk of low birth weight and prematurity.13
When acute HBV infection occurs early in pregnancy, the rate of perinatal transmission is about 10%, increasing to 60% if it occurs near delivery.12,13
Chronic HBV infection does not usually affect the outcome of pregnancy, but it may if the woman has cirrhosis or advanced liver disease14; however, pregnancy is very rare in women with HBV cirrhosis due to anovulation, and acute HBV flares have been described during pregnancy and postpartum.15
Pregnant patients with cirrhosis and portal hypertension are at risk of hepatic decompensation, variceal bleeding, and death.16 Risk is high with a score of 10 or more on the Model for End-stage Liver Disease scale, and is low with a score of 6 or less.17 Like nonpregnant patients, pregnant patients with cirrhosis should be monitored, and upper endoscopy should be performed in the 2nd trimester to assess for varices. A beta-blocker should be given or banding of varices should be done to avoid rupture. Rates of fetal demise, premature labor, spontaneous abortion, and stillbirth are high with portal hypertension.16
Risk of mother-to-child HBV transmission
Vertical HBV transmission can occur during the antepartum, intrapartum, and postpartum periods,18,19 but it most often occurs during the intrapartum period at the time of delivery. Without immunoprophylaxis of the newborn, the risk of mother-to-child transmission can be as high as 90% if the mother is hepatitis B e antigen-positive and has a viral load greater than 106 copies/mL. With active and passive immunoprophylaxis, the risk decreases substantially.
Screening and diagnosis
All pregnant women should be tested for hepatitis B surface antigen during the 1st trimester,20 or any time thereafter if early testing was not done, even if they were vaccinated before becoming pregnant.21
Prevention
HBV infection is best prevented before pregnancy by vaccinating the mother or, after delivery, by vaccinating the newborn. Universal vaccination of newborns has been the standard of care since the 1990s. Pregnant women should be tested early in the pregnancy; unvaccinated, uninfected women at high risk of acquiring HBV (eg, because of sexual contacts or intravenous drug use) should be vaccinated.2,3
HBV vaccine and immune globulin are both approved by the US Food and Drug Administration (FDA) for prevention of HBV infection and can be given during pregnancy and breastfeeding.3 All infants should be vaccinated for HBV at birth, and all infants born to mothers who test positive for hepatitis B s antigen should receive the HBV vaccine and the immune globulin within 12 to 24 hours after delivery. The vaccine series should be completed within 6 months.20,21 This will decrease the rate of neonatal infection.
Treatment of HBV infection in pregnancy
The main objectives of treating chronic HBV infection in pregnancy are to maintain stable liver function in the mother and to prevent neonatal infection, which may cause cirrhosis and hepatocellular carcinoma and contribute to the global burden of the disease.22 Therefore, maternal HBV DNA and liver aminotransferase levels should be tested regularly during gestation.
The current guidelines of the American Association for the Study of Liver Diseases suggest antiviral therapy to reduce the risk of perinatal transmission of HBV in pregnant women with an HBV DNA level greater than 200,000 IU/mL or greater than 106 copies/mL.23,24
In a meta-analysis,25 antiviral therapy with lamivudine, telbivudine, or tenofovir showed no apparent teratogenicity or safety concerns for maternal and fetal outcomes26 and significantly reduced the rate of mother-to-child transmission. Of these 3 drugs, telbivudine was associated with a higher rate of normalization of liver enzymes, HBV suppression, and e-antigen seroconversion.25 Lamivudine has proven the test of time in mothers co-infected with HBV and human immunodeficiency virus (HIV). However, tenofovir is considered the preferred treatment in pregnancy, owing to concerns about drug resistance to telbivudine and lamivudine and a high genetic barrier to resistance with tenofovir.26 In mothers with HBV and HIV treated with tenofovir, treatment was associated with lower bone mineral density in the newborns, with a propensity for renal injury in the mothers. No safety concerns for maternal or fetal outcomes were identified in pregnant women infected only with HBV.25
Many pregnant mothers choose to stop therapy around the time of conception because of safety concerns for the baby. In such situations, close monitoring is necessary to detect flares of HBV infection.
When the decision to treat is made, treatment should begin at 28 to 30 weeks of gestation, when organogenesis is complete and to allow enough time for HBV DNA levels to decline.
Breastfeeding is not contraindicated because antiviral drugs are minimally excreted in breast milk and are unlikely to cause toxicity. However, data are insufficient as to the long-term safety for the newborn when the mother took these drugs during pregancy and while breastfeeding.23,27
Alanine aminotransferase and HBV DNA levels should be monitored postpartum because of the possibility of a hepatitis flare. In this setting, any of the three drugs can be used.28 For mothers on therapy because of cirrhosis or an advanced histologic feature, antiviral therapy should be continued throughout pregnancy to prevent disease progression and decompensation.19,22,27
No drug therapy is necessary for pregnant carriers of HBV.
Delivery and breastfeeding
The mode of delivery does not appear to have a significant effect on the interruption of vertical transmission of HBV.29 Cesarean delivery is not recommended by the US Centers for Disease Control and Prevention (CDC)2 or the American College of Obstetricians and Gynecologists.6 Breastfeeding is encouraged if the infant has received appropriate immunoprophylaxis.6
Coinfection with hepatitis D
Coinfection with hepatitis D virus (HDV) and HBV is associated with severe acute hepatitis30,31 and increases the risk of death by a factor of 10. The World Health Organization recommends testing for HDV in pregnant women who are HBV-positive.8
Prevention of HDV infection requires prevention of HBV. The treatment of HDV in pregnancy is supportive. Pegylated interferon is successful outside pregnancy but is contraindicated during pregnancy.32 In patients with fulminant hepatic failure and end-stage liver disease, liver transplant can be lifesaving.
Take-home points
- HBV infection during pregnancy is usually benign and not severe but can be associated with an increased risk of mother-to-child transmission and progression of liver disease in the pregnant mother.
- Prevention of vertical transmission of HBV is important to reduce the burden of chronic HBV infection. Universal maternal screening early in pregnancy and passive-active immunoprophylaxis of newborns are usually sufficient to prevent vertical transmission of HBV, but antiviral therapy is needed for highly viremic mothers to further reduce the risk.
- Antiviral therapy is also indicated for pregnant women with moderate to severe hepatitis or cirrhosis to prevent disease progression and liver failure.
- Telbivudine, tenofovir, or lamivudine can be used during pregnancy, but more data are needed on the long-term safety of fetal exposure to these agents.
HEPATITIS C
The global prevalence of hepatitis C virus (HCV) infection is 2% to 3%, with 130 to 170 million HCV-positive people, most of whom are chronically infected.33 The incidence of HCV during pregnancy is 1% to 2.4%, but 3% to 5% of infected mothers transmit HCV to their child at the time of birth.6,34 Women coinfected with HIV and HCV have twice the risk of perinatal HCV transmission compared with women who have HCV infection alone.6,34
HCV infection is usually asymptomatic and is discovered either by screening high-risk patients or during evaluation of persistently elevated aminotransferase levels. Acute HCV infection during pregnancy has been reported only rarely, and most pregnant women who are infected have chronic disease with no effect on the pregnancy or the infant.6,34
Treatment
The CDC recommends that all adults (including pregnant women) born between 1945 and 1965 undergo 1-time testing for HCV without prior ascertainment of HCV risk (strong recommendation, with moderate quality of evidence).35 The most important risk factor for HCV infection is past or current injection drug use.33 Additional risk factors are similar to those for nonpregnant patients.
Because of the benign effect of HCV on the pregnancy, treatment is not recommended. To decrease the risk of maternal-child transmission, it is prudent to avoid amniocentesis, scalp instrumentation, and prolonged rupture of membranes.6
There is no vaccine or immune globulin for prevention. HCV infection should not influence the mode of delivery, and it is not a contraindication to breastfeeding.34,36,37
HEPATITIS E
Every year, 20 million cases of hepatitis E virus (HEV) infection are recorded worldwide. These numbers include 3.3 million symptomatic cases and 56,600 deaths.38 HEV infection is most common in developing countries, and pregnant women traveling to these areas are at high risk of acquiring this infection, of developing fulminant hepatitis, and of death.39 Sporadic cases not associated with travel are increasingly reported in developed countries and are attributed to immunocompromised status (due to HIV or solid-organ transplant).38,40
Modes of transmission of HEV are mainly via fecal-oral contamination and by vertical transmission.41
Diagnosis
HEV infection can be diagnosed either by detecting IgM antibody with an enzyme-linked immunosorbent assay or by detecting HEV RNA in the blood using reverse transcription polymerase chain reaction testing.42
Treatment and prevention
Hospitalization should be considered for pregnant women. Ribavirin or pegylated interferon alpha or both are effective but are contraindicated in pregnancy because of the risk of teratogenicity.41,42 Urgent liver transplant can be a successful option in acute liver failure.
Prevention relies primarily on good sanitation, clean drinking water, and avoiding raw pork and venison. Boiling and chlorination of water inactivate HEV.39,40 Pregnant women should be advised to avoid travel to highly endemic areas.
- World Health Organization (WHO).Hepatitis A fact sheet. www.who.int/mediacentre/factsheets/fs328/en/. Accessed December 7, 2016.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—statistics & surveillance. www.cdc.gov/hepatitis/statistics/2013surveillance/commentary.htm#hepatitis A. Accessed December 7, 2016.
- Rac MW, Sheffield JS. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin North Am 2014; 41:573–592.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Elinav E, Ben-Dov IZ, Shapira Y, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology 2006; 130:1129–1134.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86: Viral hepatitis in pregnancy. Obstet Gynecol 2007; 110:941–956.
- Advisory Committee on Immunization Practices. Guidelines for Vaccinating Pregnant Women. www.cdc.gov/vaccines/pregnancy/hcp/guidelines.html. Accessed December 7, 2016.
- Daudi N, Shouval D, Stein-Zamir C, Ackerman Z. Breastmilk hepatitis A virus RNA in nursing mothers with acute hepatitis A virus infection. Breastfeed Med 2012; 7:313–315.
- World Health Organization (WHO). Hepatitis B fact sheet. www.who.int/mediacentre/factsheets/fs204/en/. Accessed December 7, 2016.
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012; 19:e18–e25.
- Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis 2013; 33:138–146.
- Sookoian S. Liver disease during pregnancy: acute viral hepatitis. Ann Hepatol 2006; 5:231–236.
- Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int 2009; 29(suppl 1):133–139.
- Wong S, Chan LY, Yu V, Ho L. Hepatitis B carrier and perinatal outcome in singleton pregnancy. Am J Perinatol 1999; 16:485–488.
- Rawal BK, Parida S, Watkins RP, Ghosh P, Smith H. Symptomatic reactivation of hepatitis B in pregnancy. Lancet 1991; 337:364.
- Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman RK. Pregnancy with portal hypertension. J Clin Exp Hepatol 2014; 4:163–171.
- Westbrook RH, Yeoman AD, O'Grady JG, Harrison PM, Devlin J, Heneghan MA. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol 2011; 9:694–699.
- Cheung KW, Seto MT, Wong SF. Towards complete eradication of hepatitis B infection from perinatal transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during pregnancy. Eur J Obstet Gynecol Reproduct Biol 2013; 169:17–23.
- Pan CQ, Duan AP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012; 10:452–459.
- Mast EE, Margolis HS, Fiore AE, et al; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- US Centers for Disease Control (CDC). Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep 1988; 37:341–346, 351.
- Han GR, Xu CL, Zhao W, Yang YF. Management of chronic hepatitis B in pregnancy. World J Gastroenterol 2012; 18:4517–4521.
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–283.
- Tran TT, Ahn J, Reau N. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol 2016: 111:176–194.
- Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatol 2016; 63:319–333.
- Brown RS Jr, Verna EC, Pereira MR, et al. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the antiretroviral pregnancy registry. J Hepatol 2012; 57:953–959.
- Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol 2015; 7:1233–1237.
- Potthoff A, Rifai K, Wedemeyer H, Deterding K, Manns M, Strassburg C. Successful treatment of fulminant hepatitis B during pregnancy. Z Gastroenterol 2009; 47:667–670.
- Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus—a systematic review. Virol J 2008; 5:100.
- Price J. An update on hepatitis B, D, and E viruses. Top Antivir Med 2014; 21:157–163.
- World Health Organization (WHO). Global alert and response. Hepatitis Delta. www.who.int/csr/resources/publications/hepatitis/who_cds_csr_ncs_2001_1/en/. Accessed December 7, 2016.
- Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther 2014; 19:463–468.
- Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Curr Pharm Des 2008; 14:1646–1654.
- Floreani A. Hepatitis C and pregnancy. World J Gastroenterol 2013; 19:6714–6720.
- US Centers for Disease Control and Prevention. Viral hepatitis—CDC recommendations for specific populations and settings. www.cdc.gov/hepatitis/populations/1945-1965.htm. Accessed December 7, 2016.
- World Health Organization (WHO). Hepatitis C fact sheet. www.who.int/mediacentre/factsheets/fs164/en/. Accessed December 7, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:349–357.
- World Health Organization (WHO). Hepatitis E fact sheet. www.who.int/mediacentre/factsheets/fs280/en/. Accessed December 7, 2016.
- Velosa M, Figueiredo A, Gloria H, et al. Fulminant hepatitis E in a pregnant woman. GE Port J Gastroenterol 2013; 20:210–214.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—hepatitis E information. Hepatitis E FAQs for health professionals. www.cdc.gov/hepatitis/hev/hevfaq.htm. Accessed December 7, 2016.
- Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat 2015; 22:965–973.
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012: 379:2477–2488.
- World Health Organization (WHO).Hepatitis A fact sheet. www.who.int/mediacentre/factsheets/fs328/en/. Accessed December 7, 2016.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—statistics & surveillance. www.cdc.gov/hepatitis/statistics/2013surveillance/commentary.htm#hepatitis A. Accessed December 7, 2016.
- Rac MW, Sheffield JS. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin North Am 2014; 41:573–592.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Elinav E, Ben-Dov IZ, Shapira Y, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology 2006; 130:1129–1134.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86: Viral hepatitis in pregnancy. Obstet Gynecol 2007; 110:941–956.
- Advisory Committee on Immunization Practices. Guidelines for Vaccinating Pregnant Women. www.cdc.gov/vaccines/pregnancy/hcp/guidelines.html. Accessed December 7, 2016.
- Daudi N, Shouval D, Stein-Zamir C, Ackerman Z. Breastmilk hepatitis A virus RNA in nursing mothers with acute hepatitis A virus infection. Breastfeed Med 2012; 7:313–315.
- World Health Organization (WHO). Hepatitis B fact sheet. www.who.int/mediacentre/factsheets/fs204/en/. Accessed December 7, 2016.
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012; 19:e18–e25.
- Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis 2013; 33:138–146.
- Sookoian S. Liver disease during pregnancy: acute viral hepatitis. Ann Hepatol 2006; 5:231–236.
- Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int 2009; 29(suppl 1):133–139.
- Wong S, Chan LY, Yu V, Ho L. Hepatitis B carrier and perinatal outcome in singleton pregnancy. Am J Perinatol 1999; 16:485–488.
- Rawal BK, Parida S, Watkins RP, Ghosh P, Smith H. Symptomatic reactivation of hepatitis B in pregnancy. Lancet 1991; 337:364.
- Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman RK. Pregnancy with portal hypertension. J Clin Exp Hepatol 2014; 4:163–171.
- Westbrook RH, Yeoman AD, O'Grady JG, Harrison PM, Devlin J, Heneghan MA. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol 2011; 9:694–699.
- Cheung KW, Seto MT, Wong SF. Towards complete eradication of hepatitis B infection from perinatal transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during pregnancy. Eur J Obstet Gynecol Reproduct Biol 2013; 169:17–23.
- Pan CQ, Duan AP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012; 10:452–459.
- Mast EE, Margolis HS, Fiore AE, et al; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- US Centers for Disease Control (CDC). Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep 1988; 37:341–346, 351.
- Han GR, Xu CL, Zhao W, Yang YF. Management of chronic hepatitis B in pregnancy. World J Gastroenterol 2012; 18:4517–4521.
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–283.
- Tran TT, Ahn J, Reau N. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol 2016: 111:176–194.
- Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatol 2016; 63:319–333.
- Brown RS Jr, Verna EC, Pereira MR, et al. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the antiretroviral pregnancy registry. J Hepatol 2012; 57:953–959.
- Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol 2015; 7:1233–1237.
- Potthoff A, Rifai K, Wedemeyer H, Deterding K, Manns M, Strassburg C. Successful treatment of fulminant hepatitis B during pregnancy. Z Gastroenterol 2009; 47:667–670.
- Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus—a systematic review. Virol J 2008; 5:100.
- Price J. An update on hepatitis B, D, and E viruses. Top Antivir Med 2014; 21:157–163.
- World Health Organization (WHO). Global alert and response. Hepatitis Delta. www.who.int/csr/resources/publications/hepatitis/who_cds_csr_ncs_2001_1/en/. Accessed December 7, 2016.
- Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther 2014; 19:463–468.
- Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Curr Pharm Des 2008; 14:1646–1654.
- Floreani A. Hepatitis C and pregnancy. World J Gastroenterol 2013; 19:6714–6720.
- US Centers for Disease Control and Prevention. Viral hepatitis—CDC recommendations for specific populations and settings. www.cdc.gov/hepatitis/populations/1945-1965.htm. Accessed December 7, 2016.
- World Health Organization (WHO). Hepatitis C fact sheet. www.who.int/mediacentre/factsheets/fs164/en/. Accessed December 7, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:349–357.
- World Health Organization (WHO). Hepatitis E fact sheet. www.who.int/mediacentre/factsheets/fs280/en/. Accessed December 7, 2016.
- Velosa M, Figueiredo A, Gloria H, et al. Fulminant hepatitis E in a pregnant woman. GE Port J Gastroenterol 2013; 20:210–214.
- US Centers for Disease Control and Prevention (CDC). Viral hepatitis—hepatitis E information. Hepatitis E FAQs for health professionals. www.cdc.gov/hepatitis/hev/hevfaq.htm. Accessed December 7, 2016.
- Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat 2015; 22:965–973.
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012: 379:2477–2488.
KEY POINTS
- Preventing vertical transmission of HBV infection in pregnancy is key to decreasing the global burden of this infection. Universal maternal screening and passive-active immunoprophylaxis of newborns have reduced transmission of HBV, but the addition of antiviral therapy is necessary to further decrease immunoprophylaxis failure.
- Tenofovir, telbivudine, and lamivudine can be used safely in pregnancy without apparent teratogenicity or other harmful effects on mother or baby. But optimal outcome requires discussion of safety and the plan of care with the patient, obstetrician, and hepatologist.
- Most pregnant women with hepatitis C virus (HCV) infection have chronic disease, with no effects on the pregnancy or baby, but 3% to 5% transmit HCV to their child at the time of birth. All pregnant women at risk should be screened at the first prenatal visit. The safety and efficacy of treating pregnant women to prevent transmission to the fetus are not established; thus, treatment is not recommended for pregnant women.
Porcelain heart in a uremic patient
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
Disseminated molluscum contagiosum lesions in an HIV patient
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
Worsening migraine due to neurocysticercosis
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).
Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.
TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).

Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.


TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).

Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.


TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.