User login
Understanding the brexpiprazole therapeutic window: Why more isn’t always better
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
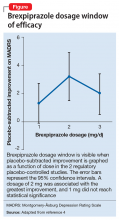
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
Hepatitis C among the mentally ill: Review and treatment update
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
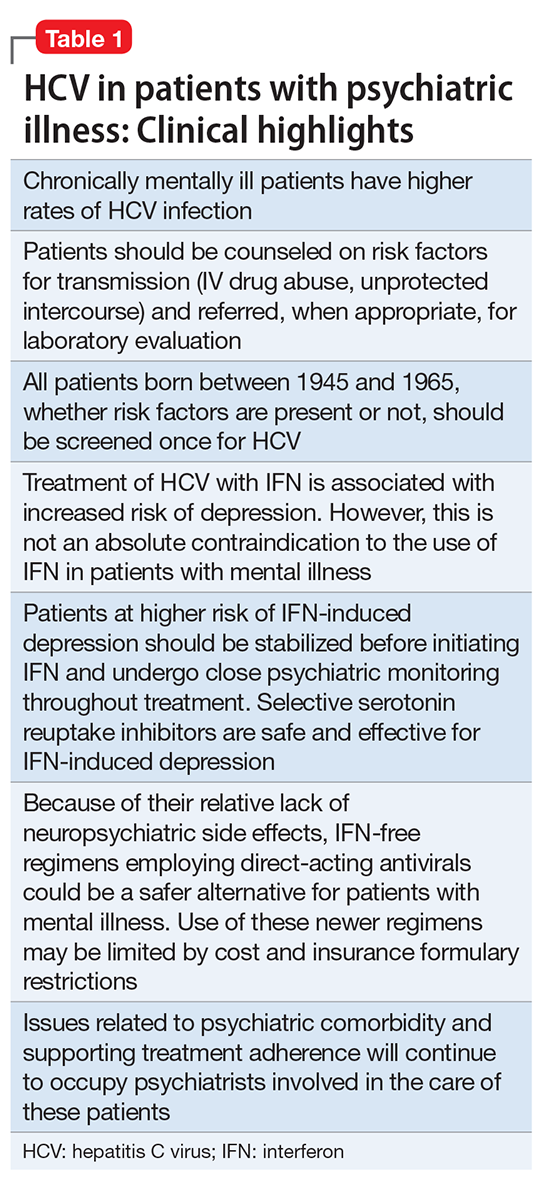
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
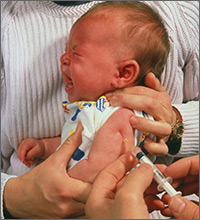
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
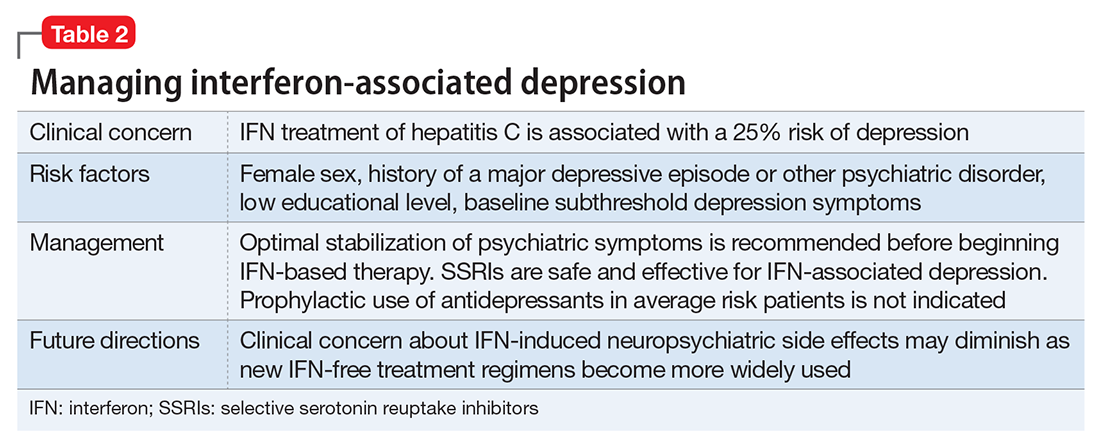
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
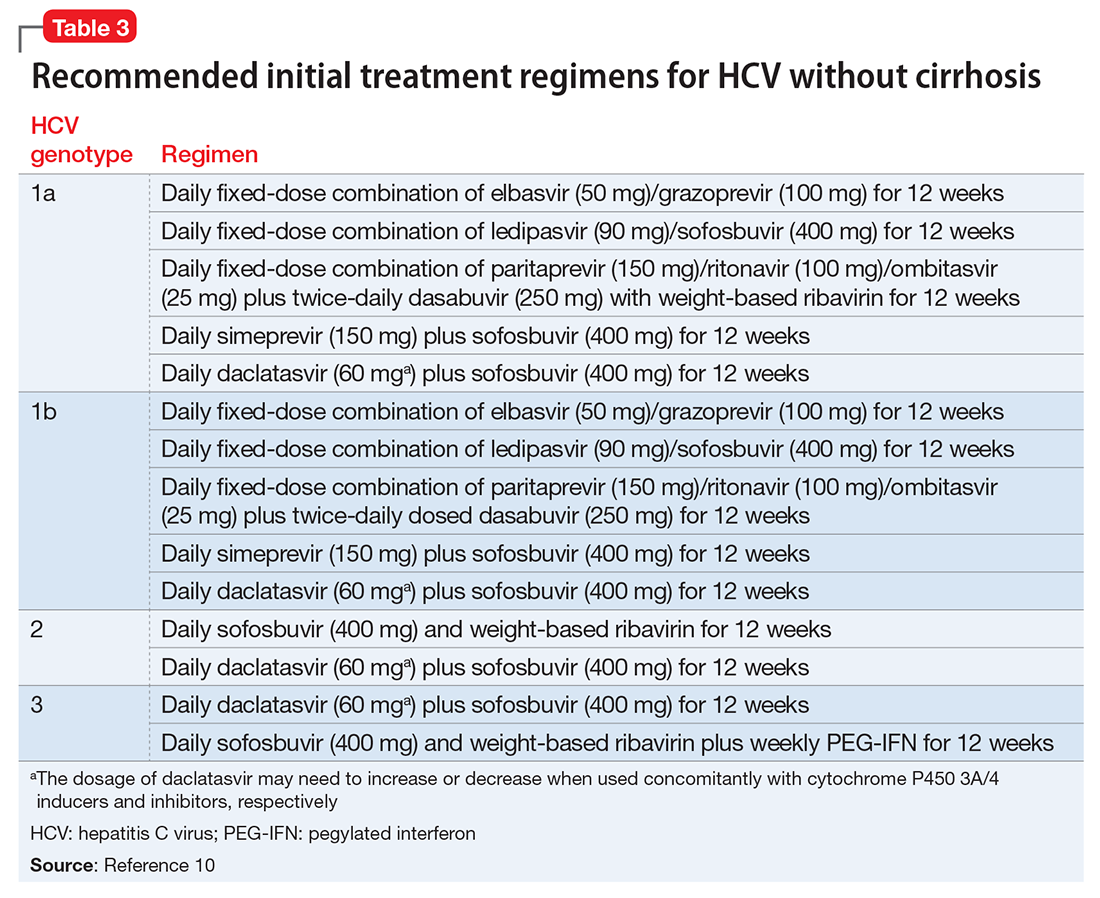
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).

CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.

CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).

Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).

CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.

CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).

Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
Social withdrawal and confusion in an inmate with schizoaffective disorder
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
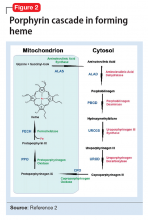
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.
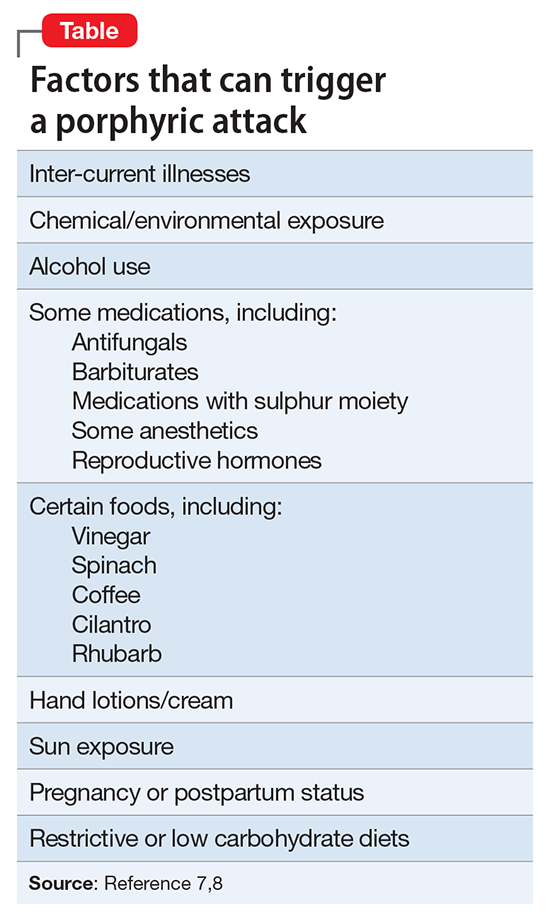
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
What is your liability for involuntary commitment based on faulty information?
Dear Dr. Mossman,
Last week, I hospitalized a patient against her will, based in part on what her family members told me she had threatened to do. The patient threatened to sue me and said I should have known that her relatives were lying. What if my patient is right? Could I face liability if I involuntarily hospitalized her based on bad collateral information?
Submitted by “Dr. R”
In all U.S. states, laws permit psychiatrists to involuntarily hospitalize persons who pose a danger to themselves or others because of mental illness.1 But taking this step can be tough. Deciding to hospitalize a patient against her will involves weighing her wants and freedom against your duty to look out for her long-term welfare and the community’s safety.2,3 Often, psychiatrists make these decisions under pressure because the family wants something done immediately, other patients also need attention, the clinical picture is incomplete, or potential dispositions (eg, crisis care and inpatient beds) are limited.3 Given such constraints, you can’t always make perfect decisions.
Dr. R’s question has 2 parts:
- What liabilities can a clinician face if a patient is wrongfully committed?
- What liabilities could arise from relying on inaccurate information or making a false petition in order to hospitalize a patient?
We hope that as you and Dr. R read our answers, you’ll have a clearer understanding of:
- the rationale for civil commitment
- how patients, doctors, and courts view civil commitment
- the role of collateral information in decision-making
- relevant legal concepts and case law.
Rationale for civil commitment
For centuries, society has used civil commitment as one of its legal methods for intervening when persons pose a danger to themselves or others because of their mental illness.4 Because incapacitation or death could result from a “false-negative” decision to release a dangerous patient, psychiatrists err on the side of caution and tolerate many “false-positive” hospitalizations of persons who wouldn’t have hurt anyone.5
We can never know if a patient would have done harm had she not been hospitalized. Measures of suicidality and hostility tend to subside during involuntary hospital treatment.6 After hospitalization, many patients cite protection from harm as a reason they are thankful for their treatment.7-9 Some involuntary inpatients want to be hospitalized but hide this for conscious or unconscious reasons,10,11 and involuntary treatment sometimes is the only way to help persons whose illness-induced anosognosia12 prevents them from understanding why they need treatment.13 Involuntary inpatient care leads to modest symptom reduction14,15 and produces treatment outcomes no worse than those of non-coerced patients.10
Patients’ views
Patients often view commitment as unjustified.16 They and their advocates object to what some view as the ultimate infringement on civil liberty.7,17 By its nature, involuntary commitment eliminates patients’ involvement in a major treatment decision,8 disempowers them,18 and influences their relationship with the treatment team.15
Some involuntary patients feel disrespected by staff members8 or experience inadvertent psychological harm, including “loss of self-esteem, identity, self-control, and self-efficacy, as well as diminished hope in the possibility of recovery.”15 Involuntary hospitalization also can have serious practical consequences. Commitment can lead to social stigma, loss of gun rights, increased risks of losing child custody, housing problems, and possible disqualification from some professions.19
Having seen many involuntary patients undergo a change of heart after treatment, psychiatrist Alan Stone proposed the “Thank You Theory” of civil commitment: involuntary hospitalization can be justified by showing that the patient is grateful after recovering.20 Studies show, however, that gratitude is far from universal.1
How coercion is experienced often depends on how it is communicated. The less coercion patients perceive, the better they feel about the treatment they received.21 Satisfaction is important because it leads to less compulsory readmission,22 and dissatisfaction makes malpractice lawsuits more likely.23
Commitment decision-making
States’ laws, judges’ attitudes, and court decisions establish each jurisdiction’s legal methods for instituting emergency holds and willingness to tolerate “false-positive” involuntary hospitalization,4,24 all of which create variation between and within states in how civil commitment laws are applied. As a result, clinicians’ decisions are influenced “by a range of social, political, and economic factors,”25 including patients’ sex, race, age, homelessness, employment status, living situation, diagnoses, previous involuntary treatment, and dissatisfaction with mental health treatment.22,26-32 Furthermore, the potential for coercion often blurs the line between an offer of voluntary admission and an involuntary hospitalization.18
Collateral information
Psychiatrists owe each patient a sound clinical assessment before deciding to initiate involuntarily hospitalization. During a psychiatric crisis, a patient might not be forthcoming or could have impaired memory or judgment. Information from friends or family can help fill in gaps in a patient’s self-report.33 As Dr. R’s question illustrates, adequate assessment often includes seeking information from persons familiar with the patient.1 A report on the Virginia Tech shootings by the Virginia Office of the Inspector General describes how collateral sources can provide otherwise missing evidence of dangerousness,34 and it often leads clinicians toward favoring admission.35
Yet clinicians should regard third-party reports with caution.36 As one attorney warns, “Psychiatrists should be cautious of the underlying motives of well-meaning family members and relatives.”37 If you make a decision to hospitalize a patient involuntarily based on collateral information that turns out to be flawed, are you at fault and potentially liable for harm to the patient?
False petitions and liability
If you’re in a situation similar to the one Dr. R describes, you can take solace in knowing that courts generally provide immunity to a psychiatrist who makes a reasonable, well-intentioned decision to commit someone. The degree of immunity offered varies by jurisdiction. Table 1 provides examples of immunity language from several states’ statutes.
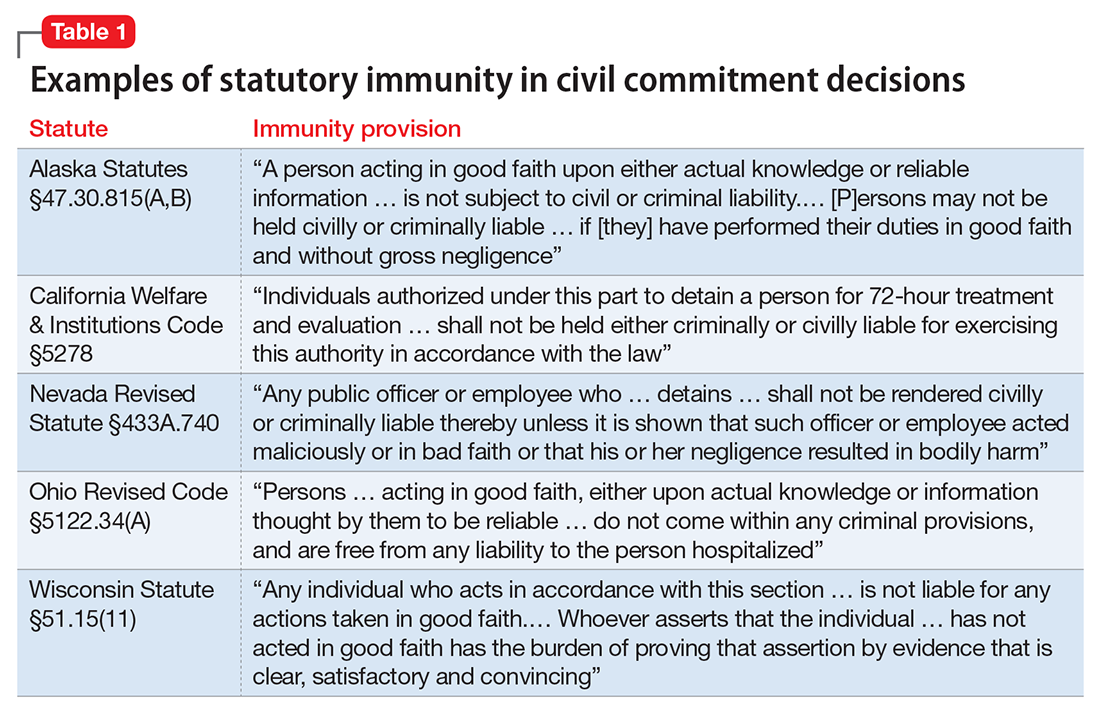
Many states’ statutes also lay out the potential consequences if a psychiatrist takes action to involuntarily hospitalize someone in bad faith or with malicious intent. In some jurisdictions, such actions can lead to criminal sanctions against the doctor or against the party who made a false petition (eg, a devious family member) (Table 2). Commenting on Texas’s statute, attorney Jeffrey Anderson explains, “The touchstone for causes of action based upon a wrongful civil commitment require that the psychiatrist[’s] conduct be found to be unreasonable and negligent. [Immunity…] still requires that a psychiatrist[’s] diagnosis of a patient[’s] threat to harm himself or others be a reasonable and prudent one.”37
The immunity extended through such statutes usually is limited to claims arising directly from the detention. For example, in the California case of Jacobs v Grossmont Hospital, a patient under a 72-hour hold fell and fractured her leg, and she sought damages. The trial court dismissed the suit under the immunity statute applicable to commitment decisions, but the appellate court held that “the immunity did not extend to other negligent acts.… The trial court erred in assuming that … the hospital was exempt from all liability for any negligence that occurred during the lawful hold.”38
Bingham v Cedars-Sinai Health Systems illustrates how physicians can lose immunity.39 A nurse contacted her supervisor to report a colleague who had stolen narcotics from work and compromised patient care. In response, the supervisor, hospital, and several physicians agreed to have her involuntarily committed. Later, it was confirmed that the colleague had taken the narcotics. She later sued the hospital system, claiming—in addition to malpractice—retaliation, invasion of privacy, assault and battery, false imprisonment, defamation, intentional infliction of emotional distress, disability-based harassment, and violation of her civil rights. Citing California’s immunity statute, the trial court granted summary judgment to the clinicians and hospital system. On appeal, however, the appellate court reversed the judgment, holding that the defendants had not shown that “the decision to detain Bingham was based on probable cause, a prerequisite to the exemption from liability,” and that Bingham had some legitimate grounds for her lawsuit.
A key point for Dr. R to consider is that, although some states provide immunity if the psychiatrist’s admitting decision was based on an evaluation “performed in good faith,”40 other states’ immunity provisions apply only if the psychiatrist had probable cause to make a decision to detain.41
Ways to reduce liability risk
Although an involuntary hospitalization could have an uncertain basis, psychiatrists can reduce the risk of legal liability for their decisions. Good documentation is important. Admitting psychiatrists usually make sound decisions, but the corresponding documentation frequently lacks clinical justification.42-44 As the rate of appropriate documentation of admission decision-making improves, the rate of commitment falls,44 and patients’ legal rights enjoy greater protection.43 Poor communication can decrease the quality of care and increase the risk of a malpractice lawsuit.45 This is just one of many reasons why you should explain your reasons for involuntary hospitalization and inform patients of the procedures for judicial review.8,9 Table 3 summarizes other steps to reduce liability risk when committing patients to the hospital.1,8,15,21,33,35-37,42,45-47
1. Pinals DA, Mossman D. Evaluation for civil commitment: best practices for forensic mental health assessments. New York, NY: Oxford University Press; 2011.
2. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgemont). 2010;7(10):30-40.
3. Hedman LC, Petrila J, Fisher WH, et al. State laws on emergency holds for mental health stabilization. Psychiatr Serv. 2016;67(5):529-535.
4. Groendyk Z. “It takes a lot to get into Bellevue”: a pro-rights critique of New York’s involuntary commitment law. Fordham Urban Law J. 2013;40(1):548-585.
5. Brooks RA. U.S. psychiatrists’ beliefs and wants about involuntary civil commitment grounds. Int J Law Psychiatry. 2006;29(1):13-21.
6. Giacco D, Priebe S. Suicidality and hostility following involuntary hospital treatment. PLoS One. 2016;11(5):e0154458. doi: 10.1371/journal.pone.0154458.
7. Wyder M, Bland R, Herriot A, et al. The experiences of the legal processes of involuntary treatment orders: tension between the legal and medical frameworks. Int J Law Psychiatry. 2015;38:44-50.
8. Valenti E, Giacco D, Katasakou C, et al. Which values are important for patients during involuntary treatment? A qualitative study with psychiatric inpatients. J Med Ethics. 2014;40(12):832-836.
9. Katsakou C, Rose D, Amos T, et al. Psychiatric patients’ views on why their involuntary hospitalisation was right or wrong: a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2012;42(7):1169-1179.
10. Kaltiala-Heino R, Laippala P, Salokangas RK. Impact of coercion on treatment outcome. Int J Law Psychiatry. 1997;20(3):311-322.
11. Hoge SK, Lidz CW, Eisenberg M, et al. Perceptions of coercion in the admission of voluntary and involuntary psychiatric patients. Int J Law Psychiatry. 1997;20(2):167-181.
12. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):10-17. 13. Gordon S. The danger zone: how the dangerousness standard in civil commitment proceedings harms people with serious mental illness. Case Western Reserve Law Review. 2016;66(3):657-700.
14. Kallert TW, Katsakou C, Adamowski T, et al. Coerced hospital admission and symptom change—a prospective observational multi-centre study. PLoS One. 2011;6(11):e28191. doi: 10.1371/journal.pone.0028191.
15. Danzer G, Wilkus-Stone A. The give and take of freedom: the role of involuntary hospitalization and treatment in recovery from mental illness. Bull Menninger Clin. 2015;79(3):255-280.
16. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
17. Amidov T. Involuntary commitment is unnecessary and discriminatory. In: Berlatsky N, ed. Mental illness. Farmington Hills, MI: Greenhaven Press; 2016;140-145.
18. Monahan J, Hoge SK, Lidz C, et al. Coercion and commitment: understanding involuntary mental hospital admission. Int J Law Psychiatry. 1995;18(3):249-263.
19. Guest Pryal KR. Heller’s scapegoats. North Carolina Law Review. 2015;93(5):1439-1473.
20. Stone AA. Mental health and law: a system in transition. Washington, DC: U.S. Government Printing Office; 1975:75-176.
21. Katsakou C, Bowers L, Amos T, et al. Coercion and treatment satisfaction among involuntary patients. Psychiatr Serv. 2010;61(3):286-292.
22. Setkowski K, van der Post LF, Peen J, et al. Changing patient perspectives after compulsory admission and the risk of re-admission during 5 years of follow-up: the Amsterdam study of acute psychiatry IX. Int J Soc Psychiatry. 2016;62(6):578-588.
23. Stelfox HT, Gandhi TK, Orav EJ, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
24. Goldman A. Continued overreliance on involuntary commitment: the need for a less restrictive alternative. J Leg Med. 2015;36(2):233-251.
25. Fisher WH, Grisso T. Commentary: civil commitment statutes—40 years of circumvention. J Am Acad Psychiatry Law. 2010;38(3):365-368.
26. Curley A, Agada E, Emechebe A, et al. Exploring and explaining involuntary care: the relationship between psychiatric admission status, gender and other demographic and clinical variables. Int J Law Psychiatry. 2016;47:53-59.
27. Muroff JR, Jackson JS, Mowbray CT, et al. The influence of gender, patient volume and time on clinical diagnostic decision making in psychiatric emergency services. Gen Hosp Psychiatry. 2007;29(6):481-488.
28. Muroff JR, Edelsohn GA, Joe S, et al. The role of race in diagnostic and disposition decision making in a pediatric psychiatric emergency service. Gen Hosp Psychiatry. 2008;30(3):269-276.
29. Unick GJ, Kessell E, Woodard EK, et al. Factors affecting psychiatric inpatient hospitalization from a psychiatric emergency service. Gen Hosp Psychiatry. 2011;33(6):618-625.
30. Ng XT, Kelly BD. Voluntary and involuntary care: three-year study of demographic and diagnostic admission statistics at an inner-city adult psychiatry unit. Int J Law Psychiatry. 2012;35(4):317-326.
31. Lo TT, Woo BK. The impact of unemployment on utilization of psychiatric emergency services. Gen Hosp Psychiatry. 2011;33(3):e7-e8. doi: 10.1016/j.genhosppsych.2010.10.010.
32. van der Post LFM, Peen J, Dekker JJ. A prediction model for the incidence of civil detention for crisis patients with psychiatric illnesses; the Amsterdam study of acute psychiatry VII. Soc Psychiatry Psychiatr Epidemiol. 2014;49(2):283-290.
33. Heilbrun K, NeMoyer A, King C, et al. Using third-party information in forensic mental health assessment: a critical review. Court Review. 2015;51(1):16-35.
34. Mass shootings at Virginia Tech, April 16, 2007 report of the Virginia Tech Review Panel presented to Timothy M. Kaine, Governor, Commonwealth of Virginia. http://cdm16064.contentdm.oclc.org/cdm/ref/collection/p266901coll4/id/904. Accessed February 2, 2017.
35. Segal SP, Laurie TA, Segal MJ. Factors in the use of coercive retention in civil commitment evaluations in psychiatric emergency services. Psychiatr Serv. 2001;52(4):514-520.
36. Lincoln A, Allen MH. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
37. Anderson JC. How I decided to sue you: misadventures in psychiatry. Reprinted in part from: Moody CE, Smith MT, Maedgen BJ. Litigation of psychiatric malpractice claims. Presented at: Medical Malpractice Conference; April 15, 1993; San Antonio, TX. http://www.texaslawfirm.com/Articles/How_I_Decided_to_Sue_You__Misadventrues_in_Psychiatry.pdf. Accessed December 27, 2016.
38. Jacobs v Grossmont Hospital, 108 Cal App 4th 69, 133 Cal Rptr 2d9 (2003).
39. Bingham v Cedars Sinai Health Systems, WL 2137442, Cal App 2 Dist (2004).
40. Ohio Revised Code §5122.34.
41. California Welfare & Institutions Code §5150(E).
42. Hashmi A, Shad M, Rhoades HM, et al. Involuntary detention: do psychiatrists clinically justify continuing involuntary hospitalization? Psychiatr Q. 2014;85(3):285-293.
43. Brayley J, Alston A, Rogers K. Legal criteria for involuntary mental health admission: clinician performance in recording grounds for decision. Med J Aust. 2015;203(8):334.
44. Perrigo TL, Williams KA. Implementation of an evidence based guideline for assessment and documentation of the civil commitment process. Community Ment Health J. 2016;52(8):1033-1036.
45. Mor S, Rabinovich-Einy O. Relational malpractice. Seton Hall Law Rev. 2012;42(2):601-642.
46. Tate v Kaiser Foundation Hospitals, WL 176625, U.S. Dist. LEXIS 5891 (CD Cal 2014).
47. Ranieri V, Madigan K, Roche E, et al. Caregivers’ perceptions of coercion in psychiatric hospital admission. Psychiatry Res. 2015;22(3)8:380-385.
Dear Dr. Mossman,
Last week, I hospitalized a patient against her will, based in part on what her family members told me she had threatened to do. The patient threatened to sue me and said I should have known that her relatives were lying. What if my patient is right? Could I face liability if I involuntarily hospitalized her based on bad collateral information?
Submitted by “Dr. R”
In all U.S. states, laws permit psychiatrists to involuntarily hospitalize persons who pose a danger to themselves or others because of mental illness.1 But taking this step can be tough. Deciding to hospitalize a patient against her will involves weighing her wants and freedom against your duty to look out for her long-term welfare and the community’s safety.2,3 Often, psychiatrists make these decisions under pressure because the family wants something done immediately, other patients also need attention, the clinical picture is incomplete, or potential dispositions (eg, crisis care and inpatient beds) are limited.3 Given such constraints, you can’t always make perfect decisions.
Dr. R’s question has 2 parts:
- What liabilities can a clinician face if a patient is wrongfully committed?
- What liabilities could arise from relying on inaccurate information or making a false petition in order to hospitalize a patient?
We hope that as you and Dr. R read our answers, you’ll have a clearer understanding of:
- the rationale for civil commitment
- how patients, doctors, and courts view civil commitment
- the role of collateral information in decision-making
- relevant legal concepts and case law.
Rationale for civil commitment
For centuries, society has used civil commitment as one of its legal methods for intervening when persons pose a danger to themselves or others because of their mental illness.4 Because incapacitation or death could result from a “false-negative” decision to release a dangerous patient, psychiatrists err on the side of caution and tolerate many “false-positive” hospitalizations of persons who wouldn’t have hurt anyone.5
We can never know if a patient would have done harm had she not been hospitalized. Measures of suicidality and hostility tend to subside during involuntary hospital treatment.6 After hospitalization, many patients cite protection from harm as a reason they are thankful for their treatment.7-9 Some involuntary inpatients want to be hospitalized but hide this for conscious or unconscious reasons,10,11 and involuntary treatment sometimes is the only way to help persons whose illness-induced anosognosia12 prevents them from understanding why they need treatment.13 Involuntary inpatient care leads to modest symptom reduction14,15 and produces treatment outcomes no worse than those of non-coerced patients.10
Patients’ views
Patients often view commitment as unjustified.16 They and their advocates object to what some view as the ultimate infringement on civil liberty.7,17 By its nature, involuntary commitment eliminates patients’ involvement in a major treatment decision,8 disempowers them,18 and influences their relationship with the treatment team.15
Some involuntary patients feel disrespected by staff members8 or experience inadvertent psychological harm, including “loss of self-esteem, identity, self-control, and self-efficacy, as well as diminished hope in the possibility of recovery.”15 Involuntary hospitalization also can have serious practical consequences. Commitment can lead to social stigma, loss of gun rights, increased risks of losing child custody, housing problems, and possible disqualification from some professions.19
Having seen many involuntary patients undergo a change of heart after treatment, psychiatrist Alan Stone proposed the “Thank You Theory” of civil commitment: involuntary hospitalization can be justified by showing that the patient is grateful after recovering.20 Studies show, however, that gratitude is far from universal.1
How coercion is experienced often depends on how it is communicated. The less coercion patients perceive, the better they feel about the treatment they received.21 Satisfaction is important because it leads to less compulsory readmission,22 and dissatisfaction makes malpractice lawsuits more likely.23
Commitment decision-making
States’ laws, judges’ attitudes, and court decisions establish each jurisdiction’s legal methods for instituting emergency holds and willingness to tolerate “false-positive” involuntary hospitalization,4,24 all of which create variation between and within states in how civil commitment laws are applied. As a result, clinicians’ decisions are influenced “by a range of social, political, and economic factors,”25 including patients’ sex, race, age, homelessness, employment status, living situation, diagnoses, previous involuntary treatment, and dissatisfaction with mental health treatment.22,26-32 Furthermore, the potential for coercion often blurs the line between an offer of voluntary admission and an involuntary hospitalization.18
Collateral information
Psychiatrists owe each patient a sound clinical assessment before deciding to initiate involuntarily hospitalization. During a psychiatric crisis, a patient might not be forthcoming or could have impaired memory or judgment. Information from friends or family can help fill in gaps in a patient’s self-report.33 As Dr. R’s question illustrates, adequate assessment often includes seeking information from persons familiar with the patient.1 A report on the Virginia Tech shootings by the Virginia Office of the Inspector General describes how collateral sources can provide otherwise missing evidence of dangerousness,34 and it often leads clinicians toward favoring admission.35
Yet clinicians should regard third-party reports with caution.36 As one attorney warns, “Psychiatrists should be cautious of the underlying motives of well-meaning family members and relatives.”37 If you make a decision to hospitalize a patient involuntarily based on collateral information that turns out to be flawed, are you at fault and potentially liable for harm to the patient?
False petitions and liability
If you’re in a situation similar to the one Dr. R describes, you can take solace in knowing that courts generally provide immunity to a psychiatrist who makes a reasonable, well-intentioned decision to commit someone. The degree of immunity offered varies by jurisdiction. Table 1 provides examples of immunity language from several states’ statutes.

Many states’ statutes also lay out the potential consequences if a psychiatrist takes action to involuntarily hospitalize someone in bad faith or with malicious intent. In some jurisdictions, such actions can lead to criminal sanctions against the doctor or against the party who made a false petition (eg, a devious family member) (Table 2). Commenting on Texas’s statute, attorney Jeffrey Anderson explains, “The touchstone for causes of action based upon a wrongful civil commitment require that the psychiatrist[’s] conduct be found to be unreasonable and negligent. [Immunity…] still requires that a psychiatrist[’s] diagnosis of a patient[’s] threat to harm himself or others be a reasonable and prudent one.”37
The immunity extended through such statutes usually is limited to claims arising directly from the detention. For example, in the California case of Jacobs v Grossmont Hospital, a patient under a 72-hour hold fell and fractured her leg, and she sought damages. The trial court dismissed the suit under the immunity statute applicable to commitment decisions, but the appellate court held that “the immunity did not extend to other negligent acts.… The trial court erred in assuming that … the hospital was exempt from all liability for any negligence that occurred during the lawful hold.”38
Bingham v Cedars-Sinai Health Systems illustrates how physicians can lose immunity.39 A nurse contacted her supervisor to report a colleague who had stolen narcotics from work and compromised patient care. In response, the supervisor, hospital, and several physicians agreed to have her involuntarily committed. Later, it was confirmed that the colleague had taken the narcotics. She later sued the hospital system, claiming—in addition to malpractice—retaliation, invasion of privacy, assault and battery, false imprisonment, defamation, intentional infliction of emotional distress, disability-based harassment, and violation of her civil rights. Citing California’s immunity statute, the trial court granted summary judgment to the clinicians and hospital system. On appeal, however, the appellate court reversed the judgment, holding that the defendants had not shown that “the decision to detain Bingham was based on probable cause, a prerequisite to the exemption from liability,” and that Bingham had some legitimate grounds for her lawsuit.
A key point for Dr. R to consider is that, although some states provide immunity if the psychiatrist’s admitting decision was based on an evaluation “performed in good faith,”40 other states’ immunity provisions apply only if the psychiatrist had probable cause to make a decision to detain.41
Ways to reduce liability risk
Although an involuntary hospitalization could have an uncertain basis, psychiatrists can reduce the risk of legal liability for their decisions. Good documentation is important. Admitting psychiatrists usually make sound decisions, but the corresponding documentation frequently lacks clinical justification.42-44 As the rate of appropriate documentation of admission decision-making improves, the rate of commitment falls,44 and patients’ legal rights enjoy greater protection.43 Poor communication can decrease the quality of care and increase the risk of a malpractice lawsuit.45 This is just one of many reasons why you should explain your reasons for involuntary hospitalization and inform patients of the procedures for judicial review.8,9 Table 3 summarizes other steps to reduce liability risk when committing patients to the hospital.1,8,15,21,33,35-37,42,45-47

Dear Dr. Mossman,
Last week, I hospitalized a patient against her will, based in part on what her family members told me she had threatened to do. The patient threatened to sue me and said I should have known that her relatives were lying. What if my patient is right? Could I face liability if I involuntarily hospitalized her based on bad collateral information?
Submitted by “Dr. R”
In all U.S. states, laws permit psychiatrists to involuntarily hospitalize persons who pose a danger to themselves or others because of mental illness.1 But taking this step can be tough. Deciding to hospitalize a patient against her will involves weighing her wants and freedom against your duty to look out for her long-term welfare and the community’s safety.2,3 Often, psychiatrists make these decisions under pressure because the family wants something done immediately, other patients also need attention, the clinical picture is incomplete, or potential dispositions (eg, crisis care and inpatient beds) are limited.3 Given such constraints, you can’t always make perfect decisions.
Dr. R’s question has 2 parts:
- What liabilities can a clinician face if a patient is wrongfully committed?
- What liabilities could arise from relying on inaccurate information or making a false petition in order to hospitalize a patient?
We hope that as you and Dr. R read our answers, you’ll have a clearer understanding of:
- the rationale for civil commitment
- how patients, doctors, and courts view civil commitment
- the role of collateral information in decision-making
- relevant legal concepts and case law.
Rationale for civil commitment
For centuries, society has used civil commitment as one of its legal methods for intervening when persons pose a danger to themselves or others because of their mental illness.4 Because incapacitation or death could result from a “false-negative” decision to release a dangerous patient, psychiatrists err on the side of caution and tolerate many “false-positive” hospitalizations of persons who wouldn’t have hurt anyone.5
We can never know if a patient would have done harm had she not been hospitalized. Measures of suicidality and hostility tend to subside during involuntary hospital treatment.6 After hospitalization, many patients cite protection from harm as a reason they are thankful for their treatment.7-9 Some involuntary inpatients want to be hospitalized but hide this for conscious or unconscious reasons,10,11 and involuntary treatment sometimes is the only way to help persons whose illness-induced anosognosia12 prevents them from understanding why they need treatment.13 Involuntary inpatient care leads to modest symptom reduction14,15 and produces treatment outcomes no worse than those of non-coerced patients.10
Patients’ views
Patients often view commitment as unjustified.16 They and their advocates object to what some view as the ultimate infringement on civil liberty.7,17 By its nature, involuntary commitment eliminates patients’ involvement in a major treatment decision,8 disempowers them,18 and influences their relationship with the treatment team.15
Some involuntary patients feel disrespected by staff members8 or experience inadvertent psychological harm, including “loss of self-esteem, identity, self-control, and self-efficacy, as well as diminished hope in the possibility of recovery.”15 Involuntary hospitalization also can have serious practical consequences. Commitment can lead to social stigma, loss of gun rights, increased risks of losing child custody, housing problems, and possible disqualification from some professions.19
Having seen many involuntary patients undergo a change of heart after treatment, psychiatrist Alan Stone proposed the “Thank You Theory” of civil commitment: involuntary hospitalization can be justified by showing that the patient is grateful after recovering.20 Studies show, however, that gratitude is far from universal.1
How coercion is experienced often depends on how it is communicated. The less coercion patients perceive, the better they feel about the treatment they received.21 Satisfaction is important because it leads to less compulsory readmission,22 and dissatisfaction makes malpractice lawsuits more likely.23
Commitment decision-making
States’ laws, judges’ attitudes, and court decisions establish each jurisdiction’s legal methods for instituting emergency holds and willingness to tolerate “false-positive” involuntary hospitalization,4,24 all of which create variation between and within states in how civil commitment laws are applied. As a result, clinicians’ decisions are influenced “by a range of social, political, and economic factors,”25 including patients’ sex, race, age, homelessness, employment status, living situation, diagnoses, previous involuntary treatment, and dissatisfaction with mental health treatment.22,26-32 Furthermore, the potential for coercion often blurs the line between an offer of voluntary admission and an involuntary hospitalization.18
Collateral information
Psychiatrists owe each patient a sound clinical assessment before deciding to initiate involuntarily hospitalization. During a psychiatric crisis, a patient might not be forthcoming or could have impaired memory or judgment. Information from friends or family can help fill in gaps in a patient’s self-report.33 As Dr. R’s question illustrates, adequate assessment often includes seeking information from persons familiar with the patient.1 A report on the Virginia Tech shootings by the Virginia Office of the Inspector General describes how collateral sources can provide otherwise missing evidence of dangerousness,34 and it often leads clinicians toward favoring admission.35
Yet clinicians should regard third-party reports with caution.36 As one attorney warns, “Psychiatrists should be cautious of the underlying motives of well-meaning family members and relatives.”37 If you make a decision to hospitalize a patient involuntarily based on collateral information that turns out to be flawed, are you at fault and potentially liable for harm to the patient?
False petitions and liability
If you’re in a situation similar to the one Dr. R describes, you can take solace in knowing that courts generally provide immunity to a psychiatrist who makes a reasonable, well-intentioned decision to commit someone. The degree of immunity offered varies by jurisdiction. Table 1 provides examples of immunity language from several states’ statutes.

Many states’ statutes also lay out the potential consequences if a psychiatrist takes action to involuntarily hospitalize someone in bad faith or with malicious intent. In some jurisdictions, such actions can lead to criminal sanctions against the doctor or against the party who made a false petition (eg, a devious family member) (Table 2). Commenting on Texas’s statute, attorney Jeffrey Anderson explains, “The touchstone for causes of action based upon a wrongful civil commitment require that the psychiatrist[’s] conduct be found to be unreasonable and negligent. [Immunity…] still requires that a psychiatrist[’s] diagnosis of a patient[’s] threat to harm himself or others be a reasonable and prudent one.”37
The immunity extended through such statutes usually is limited to claims arising directly from the detention. For example, in the California case of Jacobs v Grossmont Hospital, a patient under a 72-hour hold fell and fractured her leg, and she sought damages. The trial court dismissed the suit under the immunity statute applicable to commitment decisions, but the appellate court held that “the immunity did not extend to other negligent acts.… The trial court erred in assuming that … the hospital was exempt from all liability for any negligence that occurred during the lawful hold.”38
Bingham v Cedars-Sinai Health Systems illustrates how physicians can lose immunity.39 A nurse contacted her supervisor to report a colleague who had stolen narcotics from work and compromised patient care. In response, the supervisor, hospital, and several physicians agreed to have her involuntarily committed. Later, it was confirmed that the colleague had taken the narcotics. She later sued the hospital system, claiming—in addition to malpractice—retaliation, invasion of privacy, assault and battery, false imprisonment, defamation, intentional infliction of emotional distress, disability-based harassment, and violation of her civil rights. Citing California’s immunity statute, the trial court granted summary judgment to the clinicians and hospital system. On appeal, however, the appellate court reversed the judgment, holding that the defendants had not shown that “the decision to detain Bingham was based on probable cause, a prerequisite to the exemption from liability,” and that Bingham had some legitimate grounds for her lawsuit.
A key point for Dr. R to consider is that, although some states provide immunity if the psychiatrist’s admitting decision was based on an evaluation “performed in good faith,”40 other states’ immunity provisions apply only if the psychiatrist had probable cause to make a decision to detain.41
Ways to reduce liability risk
Although an involuntary hospitalization could have an uncertain basis, psychiatrists can reduce the risk of legal liability for their decisions. Good documentation is important. Admitting psychiatrists usually make sound decisions, but the corresponding documentation frequently lacks clinical justification.42-44 As the rate of appropriate documentation of admission decision-making improves, the rate of commitment falls,44 and patients’ legal rights enjoy greater protection.43 Poor communication can decrease the quality of care and increase the risk of a malpractice lawsuit.45 This is just one of many reasons why you should explain your reasons for involuntary hospitalization and inform patients of the procedures for judicial review.8,9 Table 3 summarizes other steps to reduce liability risk when committing patients to the hospital.1,8,15,21,33,35-37,42,45-47

1. Pinals DA, Mossman D. Evaluation for civil commitment: best practices for forensic mental health assessments. New York, NY: Oxford University Press; 2011.
2. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgemont). 2010;7(10):30-40.
3. Hedman LC, Petrila J, Fisher WH, et al. State laws on emergency holds for mental health stabilization. Psychiatr Serv. 2016;67(5):529-535.
4. Groendyk Z. “It takes a lot to get into Bellevue”: a pro-rights critique of New York’s involuntary commitment law. Fordham Urban Law J. 2013;40(1):548-585.
5. Brooks RA. U.S. psychiatrists’ beliefs and wants about involuntary civil commitment grounds. Int J Law Psychiatry. 2006;29(1):13-21.
6. Giacco D, Priebe S. Suicidality and hostility following involuntary hospital treatment. PLoS One. 2016;11(5):e0154458. doi: 10.1371/journal.pone.0154458.
7. Wyder M, Bland R, Herriot A, et al. The experiences of the legal processes of involuntary treatment orders: tension between the legal and medical frameworks. Int J Law Psychiatry. 2015;38:44-50.
8. Valenti E, Giacco D, Katasakou C, et al. Which values are important for patients during involuntary treatment? A qualitative study with psychiatric inpatients. J Med Ethics. 2014;40(12):832-836.
9. Katsakou C, Rose D, Amos T, et al. Psychiatric patients’ views on why their involuntary hospitalisation was right or wrong: a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2012;42(7):1169-1179.
10. Kaltiala-Heino R, Laippala P, Salokangas RK. Impact of coercion on treatment outcome. Int J Law Psychiatry. 1997;20(3):311-322.
11. Hoge SK, Lidz CW, Eisenberg M, et al. Perceptions of coercion in the admission of voluntary and involuntary psychiatric patients. Int J Law Psychiatry. 1997;20(2):167-181.
12. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):10-17. 13. Gordon S. The danger zone: how the dangerousness standard in civil commitment proceedings harms people with serious mental illness. Case Western Reserve Law Review. 2016;66(3):657-700.
14. Kallert TW, Katsakou C, Adamowski T, et al. Coerced hospital admission and symptom change—a prospective observational multi-centre study. PLoS One. 2011;6(11):e28191. doi: 10.1371/journal.pone.0028191.
15. Danzer G, Wilkus-Stone A. The give and take of freedom: the role of involuntary hospitalization and treatment in recovery from mental illness. Bull Menninger Clin. 2015;79(3):255-280.
16. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
17. Amidov T. Involuntary commitment is unnecessary and discriminatory. In: Berlatsky N, ed. Mental illness. Farmington Hills, MI: Greenhaven Press; 2016;140-145.
18. Monahan J, Hoge SK, Lidz C, et al. Coercion and commitment: understanding involuntary mental hospital admission. Int J Law Psychiatry. 1995;18(3):249-263.
19. Guest Pryal KR. Heller’s scapegoats. North Carolina Law Review. 2015;93(5):1439-1473.
20. Stone AA. Mental health and law: a system in transition. Washington, DC: U.S. Government Printing Office; 1975:75-176.
21. Katsakou C, Bowers L, Amos T, et al. Coercion and treatment satisfaction among involuntary patients. Psychiatr Serv. 2010;61(3):286-292.
22. Setkowski K, van der Post LF, Peen J, et al. Changing patient perspectives after compulsory admission and the risk of re-admission during 5 years of follow-up: the Amsterdam study of acute psychiatry IX. Int J Soc Psychiatry. 2016;62(6):578-588.
23. Stelfox HT, Gandhi TK, Orav EJ, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
24. Goldman A. Continued overreliance on involuntary commitment: the need for a less restrictive alternative. J Leg Med. 2015;36(2):233-251.
25. Fisher WH, Grisso T. Commentary: civil commitment statutes—40 years of circumvention. J Am Acad Psychiatry Law. 2010;38(3):365-368.
26. Curley A, Agada E, Emechebe A, et al. Exploring and explaining involuntary care: the relationship between psychiatric admission status, gender and other demographic and clinical variables. Int J Law Psychiatry. 2016;47:53-59.
27. Muroff JR, Jackson JS, Mowbray CT, et al. The influence of gender, patient volume and time on clinical diagnostic decision making in psychiatric emergency services. Gen Hosp Psychiatry. 2007;29(6):481-488.
28. Muroff JR, Edelsohn GA, Joe S, et al. The role of race in diagnostic and disposition decision making in a pediatric psychiatric emergency service. Gen Hosp Psychiatry. 2008;30(3):269-276.
29. Unick GJ, Kessell E, Woodard EK, et al. Factors affecting psychiatric inpatient hospitalization from a psychiatric emergency service. Gen Hosp Psychiatry. 2011;33(6):618-625.
30. Ng XT, Kelly BD. Voluntary and involuntary care: three-year study of demographic and diagnostic admission statistics at an inner-city adult psychiatry unit. Int J Law Psychiatry. 2012;35(4):317-326.
31. Lo TT, Woo BK. The impact of unemployment on utilization of psychiatric emergency services. Gen Hosp Psychiatry. 2011;33(3):e7-e8. doi: 10.1016/j.genhosppsych.2010.10.010.
32. van der Post LFM, Peen J, Dekker JJ. A prediction model for the incidence of civil detention for crisis patients with psychiatric illnesses; the Amsterdam study of acute psychiatry VII. Soc Psychiatry Psychiatr Epidemiol. 2014;49(2):283-290.
33. Heilbrun K, NeMoyer A, King C, et al. Using third-party information in forensic mental health assessment: a critical review. Court Review. 2015;51(1):16-35.
34. Mass shootings at Virginia Tech, April 16, 2007 report of the Virginia Tech Review Panel presented to Timothy M. Kaine, Governor, Commonwealth of Virginia. http://cdm16064.contentdm.oclc.org/cdm/ref/collection/p266901coll4/id/904. Accessed February 2, 2017.
35. Segal SP, Laurie TA, Segal MJ. Factors in the use of coercive retention in civil commitment evaluations in psychiatric emergency services. Psychiatr Serv. 2001;52(4):514-520.
36. Lincoln A, Allen MH. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
37. Anderson JC. How I decided to sue you: misadventures in psychiatry. Reprinted in part from: Moody CE, Smith MT, Maedgen BJ. Litigation of psychiatric malpractice claims. Presented at: Medical Malpractice Conference; April 15, 1993; San Antonio, TX. http://www.texaslawfirm.com/Articles/How_I_Decided_to_Sue_You__Misadventrues_in_Psychiatry.pdf. Accessed December 27, 2016.
38. Jacobs v Grossmont Hospital, 108 Cal App 4th 69, 133 Cal Rptr 2d9 (2003).
39. Bingham v Cedars Sinai Health Systems, WL 2137442, Cal App 2 Dist (2004).
40. Ohio Revised Code §5122.34.
41. California Welfare & Institutions Code §5150(E).
42. Hashmi A, Shad M, Rhoades HM, et al. Involuntary detention: do psychiatrists clinically justify continuing involuntary hospitalization? Psychiatr Q. 2014;85(3):285-293.
43. Brayley J, Alston A, Rogers K. Legal criteria for involuntary mental health admission: clinician performance in recording grounds for decision. Med J Aust. 2015;203(8):334.
44. Perrigo TL, Williams KA. Implementation of an evidence based guideline for assessment and documentation of the civil commitment process. Community Ment Health J. 2016;52(8):1033-1036.
45. Mor S, Rabinovich-Einy O. Relational malpractice. Seton Hall Law Rev. 2012;42(2):601-642.
46. Tate v Kaiser Foundation Hospitals, WL 176625, U.S. Dist. LEXIS 5891 (CD Cal 2014).
47. Ranieri V, Madigan K, Roche E, et al. Caregivers’ perceptions of coercion in psychiatric hospital admission. Psychiatry Res. 2015;22(3)8:380-385.
1. Pinals DA, Mossman D. Evaluation for civil commitment: best practices for forensic mental health assessments. New York, NY: Oxford University Press; 2011.
2. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgemont). 2010;7(10):30-40.
3. Hedman LC, Petrila J, Fisher WH, et al. State laws on emergency holds for mental health stabilization. Psychiatr Serv. 2016;67(5):529-535.
4. Groendyk Z. “It takes a lot to get into Bellevue”: a pro-rights critique of New York’s involuntary commitment law. Fordham Urban Law J. 2013;40(1):548-585.
5. Brooks RA. U.S. psychiatrists’ beliefs and wants about involuntary civil commitment grounds. Int J Law Psychiatry. 2006;29(1):13-21.
6. Giacco D, Priebe S. Suicidality and hostility following involuntary hospital treatment. PLoS One. 2016;11(5):e0154458. doi: 10.1371/journal.pone.0154458.
7. Wyder M, Bland R, Herriot A, et al. The experiences of the legal processes of involuntary treatment orders: tension between the legal and medical frameworks. Int J Law Psychiatry. 2015;38:44-50.
8. Valenti E, Giacco D, Katasakou C, et al. Which values are important for patients during involuntary treatment? A qualitative study with psychiatric inpatients. J Med Ethics. 2014;40(12):832-836.
9. Katsakou C, Rose D, Amos T, et al. Psychiatric patients’ views on why their involuntary hospitalisation was right or wrong: a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2012;42(7):1169-1179.
10. Kaltiala-Heino R, Laippala P, Salokangas RK. Impact of coercion on treatment outcome. Int J Law Psychiatry. 1997;20(3):311-322.
11. Hoge SK, Lidz CW, Eisenberg M, et al. Perceptions of coercion in the admission of voluntary and involuntary psychiatric patients. Int J Law Psychiatry. 1997;20(2):167-181.
12. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):10-17. 13. Gordon S. The danger zone: how the dangerousness standard in civil commitment proceedings harms people with serious mental illness. Case Western Reserve Law Review. 2016;66(3):657-700.
14. Kallert TW, Katsakou C, Adamowski T, et al. Coerced hospital admission and symptom change—a prospective observational multi-centre study. PLoS One. 2011;6(11):e28191. doi: 10.1371/journal.pone.0028191.
15. Danzer G, Wilkus-Stone A. The give and take of freedom: the role of involuntary hospitalization and treatment in recovery from mental illness. Bull Menninger Clin. 2015;79(3):255-280.
16. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
17. Amidov T. Involuntary commitment is unnecessary and discriminatory. In: Berlatsky N, ed. Mental illness. Farmington Hills, MI: Greenhaven Press; 2016;140-145.
18. Monahan J, Hoge SK, Lidz C, et al. Coercion and commitment: understanding involuntary mental hospital admission. Int J Law Psychiatry. 1995;18(3):249-263.
19. Guest Pryal KR. Heller’s scapegoats. North Carolina Law Review. 2015;93(5):1439-1473.
20. Stone AA. Mental health and law: a system in transition. Washington, DC: U.S. Government Printing Office; 1975:75-176.
21. Katsakou C, Bowers L, Amos T, et al. Coercion and treatment satisfaction among involuntary patients. Psychiatr Serv. 2010;61(3):286-292.
22. Setkowski K, van der Post LF, Peen J, et al. Changing patient perspectives after compulsory admission and the risk of re-admission during 5 years of follow-up: the Amsterdam study of acute psychiatry IX. Int J Soc Psychiatry. 2016;62(6):578-588.
23. Stelfox HT, Gandhi TK, Orav EJ, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
24. Goldman A. Continued overreliance on involuntary commitment: the need for a less restrictive alternative. J Leg Med. 2015;36(2):233-251.
25. Fisher WH, Grisso T. Commentary: civil commitment statutes—40 years of circumvention. J Am Acad Psychiatry Law. 2010;38(3):365-368.
26. Curley A, Agada E, Emechebe A, et al. Exploring and explaining involuntary care: the relationship between psychiatric admission status, gender and other demographic and clinical variables. Int J Law Psychiatry. 2016;47:53-59.
27. Muroff JR, Jackson JS, Mowbray CT, et al. The influence of gender, patient volume and time on clinical diagnostic decision making in psychiatric emergency services. Gen Hosp Psychiatry. 2007;29(6):481-488.
28. Muroff JR, Edelsohn GA, Joe S, et al. The role of race in diagnostic and disposition decision making in a pediatric psychiatric emergency service. Gen Hosp Psychiatry. 2008;30(3):269-276.
29. Unick GJ, Kessell E, Woodard EK, et al. Factors affecting psychiatric inpatient hospitalization from a psychiatric emergency service. Gen Hosp Psychiatry. 2011;33(6):618-625.
30. Ng XT, Kelly BD. Voluntary and involuntary care: three-year study of demographic and diagnostic admission statistics at an inner-city adult psychiatry unit. Int J Law Psychiatry. 2012;35(4):317-326.
31. Lo TT, Woo BK. The impact of unemployment on utilization of psychiatric emergency services. Gen Hosp Psychiatry. 2011;33(3):e7-e8. doi: 10.1016/j.genhosppsych.2010.10.010.
32. van der Post LFM, Peen J, Dekker JJ. A prediction model for the incidence of civil detention for crisis patients with psychiatric illnesses; the Amsterdam study of acute psychiatry VII. Soc Psychiatry Psychiatr Epidemiol. 2014;49(2):283-290.
33. Heilbrun K, NeMoyer A, King C, et al. Using third-party information in forensic mental health assessment: a critical review. Court Review. 2015;51(1):16-35.
34. Mass shootings at Virginia Tech, April 16, 2007 report of the Virginia Tech Review Panel presented to Timothy M. Kaine, Governor, Commonwealth of Virginia. http://cdm16064.contentdm.oclc.org/cdm/ref/collection/p266901coll4/id/904. Accessed February 2, 2017.
35. Segal SP, Laurie TA, Segal MJ. Factors in the use of coercive retention in civil commitment evaluations in psychiatric emergency services. Psychiatr Serv. 2001;52(4):514-520.
36. Lincoln A, Allen MH. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
37. Anderson JC. How I decided to sue you: misadventures in psychiatry. Reprinted in part from: Moody CE, Smith MT, Maedgen BJ. Litigation of psychiatric malpractice claims. Presented at: Medical Malpractice Conference; April 15, 1993; San Antonio, TX. http://www.texaslawfirm.com/Articles/How_I_Decided_to_Sue_You__Misadventrues_in_Psychiatry.pdf. Accessed December 27, 2016.
38. Jacobs v Grossmont Hospital, 108 Cal App 4th 69, 133 Cal Rptr 2d9 (2003).
39. Bingham v Cedars Sinai Health Systems, WL 2137442, Cal App 2 Dist (2004).
40. Ohio Revised Code §5122.34.
41. California Welfare & Institutions Code §5150(E).
42. Hashmi A, Shad M, Rhoades HM, et al. Involuntary detention: do psychiatrists clinically justify continuing involuntary hospitalization? Psychiatr Q. 2014;85(3):285-293.
43. Brayley J, Alston A, Rogers K. Legal criteria for involuntary mental health admission: clinician performance in recording grounds for decision. Med J Aust. 2015;203(8):334.
44. Perrigo TL, Williams KA. Implementation of an evidence based guideline for assessment and documentation of the civil commitment process. Community Ment Health J. 2016;52(8):1033-1036.
45. Mor S, Rabinovich-Einy O. Relational malpractice. Seton Hall Law Rev. 2012;42(2):601-642.
46. Tate v Kaiser Foundation Hospitals, WL 176625, U.S. Dist. LEXIS 5891 (CD Cal 2014).
47. Ranieri V, Madigan K, Roche E, et al. Caregivers’ perceptions of coercion in psychiatric hospital admission. Psychiatry Res. 2015;22(3)8:380-385.
Prazosin and doxazosin for PTSD are underutilized and underdosed
The primary symptoms of PTSD are recurrent and include intrusive memories and dreams of the traumatic events, flashbacks, hypervigilance, irritability, sleep disturbances, and persistent avoidance of stimuli associated with the traumatic event. According to the National Comorbidity Survey, the estimated lifetime prevalence of PTSD among adults is 6.8% and is more common in women (9.7%) than men (3.6%).2 Among veterans, the prevalence of PTSD has been reported as:
- 31% among male Vietnam veterans (lifetime)
- 10% among Gulf War veterans
- 14% among Iraq and Afghanistan veterans.3
Why is PTSD overlooked in substance use?
Among individuals with SUD, 10% to 63% have comorbid PTSD.4 A recent report underscores the complexity and challenges of SUD–PTSD comorbidity.5 Most PTSD patients with comorbid SUD receive treatment only for SUD and the PTSD symptoms often are unaddressed.5 Those suffering from PTSD often abuse alcohol because they might consider it to be a coping strategy. Alcohol reduces hyperactivation of the dorsal anterior cingulate cortex caused by re-experiencing PTSD symptoms. Other substances of abuse, such as Cannabis, could suppress PTSD symptoms through alternate mechanisms (eg, endocannabinoid receptors). All of these could mask PTSD symptoms, which can delay diagnosis and treatment.
SUD is the tip of the “SUD-PTSD iceberg.” Some clinicians tend to focus on detoxification while completely ignoring the underlying psychopathology of SUD, which may be PTSD. Even during detoxification, PTSD should be aggressively treated.6 Lastly, practice guidelines for managing SUD–PTSD comorbidity are lacking.
Targeting mechanisms of action
Noradrenergic mechanisms have been strongly implicated in the pathophysiology of PTSD. However, selective serotonin reuptake inhibitors, such as sertraline and paroxetine, are the only FDA-approved pharmacotherapy options for PTSD, although their efficacy is limited, perhaps because they are serotonergic.
Prazosin, an alpha-1 (α-1) adrenergic antagonist that is FDA-approved for hypertension and benign prostatic hypertrophy, has been studied for treating nightmares in PTSD.7 Prazosin has shown efficacy for nightmares in PTSD and other daytime symptoms, such as flashbacks, hypervigilance, and irritability.8 Several studies support the efficacy of prazosin in persons suffering from PTSD.9-11 Use of lower dosages in clinical trials might explain why prazosin did not separate from placebo in some studies. (See Table summarizing studies of prazosin dosing for PTSD.)
In a study of 12,844 veterans, the mean maximum prazosin dosage reached in the first year of treatment was 3.6 mg/d, and only 14% of patients reached the minimum Veterans Affairs recommended dosage of 6 mg/d.17 The most recent (March 2009) American Psychiatric Association practice guidelines recommend prazosin, 3 to 15 mg at bedtime.18
Prazosin has a short half-life of 2 to 3 hours and duration of action of 6 to 10 hours. Therefore, its use is limited to 2 or 3 times daily dosing. Higher (30 to 50 mg) and more frequent (2 to 3 times per day) dosages8,12,13 might be needed because of the drug’s short half-life.
Doxazosin. Another α-1 adrenergic drug, doxazosin, 8 to 16 mg/d, has shown benefit for PTSD as well.14,15 Doxazosin, which has a longer half-life (16 to 30 hours), requires only once-daily dosing.16 The most common side effects of prazosin and doxazosin are dizziness, headache, and drowsiness; syncope has been reported but is rare.
Prazosin and doxazosin also are used to treat substance abuse, such as alcohol use disorder19-21 and cocaine use disorder.22,23 This “two birds with one stone” approach could become more common in clinical practice.
Until a major breakthrough in PTSD treatment emerges, prazosin and doxazosin, although off-label, are reasonable treatment approaches.
1. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428.
2. National Comorbidity Survey. 12-month prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort (n=9282). http://www.hcp.med.harvard.edu/ncs/ftpdir/NCS-R_12-month_Prevalence_Estimates.pdf. Published 2005. Accessed February 10, 2017.
3. Gradus JL. Epidemiology of PTSD. http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated February 23, 2016. Accessed February 13, 2017.
4. Debell F, Fear NT, Head M, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49(9):1401-1425.
5. Vujanovic AA, Bonn-Miller MO, Petry NM. Co-occurring posttraumatic stress and substance use: emerging research on correlates, mechanisms, and treatments-introduction to the special issue. Psychol Addict Behav. 2016;30(7):713-719.
6. Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158(8):1184-1190.
7. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
8. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
11. Raskind MA, Millard SP, Petrie EC, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80(10):736-742.
12. Koola MM, Varghese SP, Fawcett JA. High-dose prazosin for the treatment of post-traumatic stress disorder. Ther Adv Psychopharmacol. 2014;4(1):43-47.
13. Vaishnav M, Patel V, Varghese SP, et al. Fludrocortisone in posttraumatic stress disorder: effective for symptoms and prazosin-induced hypotension. Prim Care Companion CNS Disord. 2014;16(6). doi: 10.4088/PCC.14l01676.
14. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
15. Roepke S, Danker-Hopfe H, Repantis D, et al. Doxazosin, an α-1-adrenergic-receptor antagonist, for nightmares in patients with posttraumatic stress disorder and/or borderline personality disorder: a chart review. Pharmacopsychiatry. 2017;50(1):26-31.
16. Smith C, Koola MM. Evidence for using doxazosin in the treatment of posttraumatic stress disorder. Psychiatr Ann. 2016;46(9):553-555.
17. Alexander B, Lund BC, Bernardy NC, et al. Early discontinuation and suboptimal dosing of prazosin: a potential missed opportunity for veterans with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(5):e639-e644.
18. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf. Accessed February 10, 2017.
19. Qazi H, Wijegunaratne H, Savajiyani R, et al. Naltrexone and prazosin combination for posttraumatic stress disorder and alcohol use disorder. Prim Care Companion CNS Disord. 2014;16(4). doi: 10.4088/PCC.14l01638.
20. Simpson TL, Malte CA, Dietel B, et al. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39(5):808-817.
21. Kenna GA, Haass-Koffler CL, Zywiak WH, et al. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21(4):904-914.
22. Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013;131(1-2):66-70.
23. Newton TF, De La Garza R II, Brown G, et al. Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7(2):e30854.
The primary symptoms of PTSD are recurrent and include intrusive memories and dreams of the traumatic events, flashbacks, hypervigilance, irritability, sleep disturbances, and persistent avoidance of stimuli associated with the traumatic event. According to the National Comorbidity Survey, the estimated lifetime prevalence of PTSD among adults is 6.8% and is more common in women (9.7%) than men (3.6%).2 Among veterans, the prevalence of PTSD has been reported as:
- 31% among male Vietnam veterans (lifetime)
- 10% among Gulf War veterans
- 14% among Iraq and Afghanistan veterans.3
Why is PTSD overlooked in substance use?
Among individuals with SUD, 10% to 63% have comorbid PTSD.4 A recent report underscores the complexity and challenges of SUD–PTSD comorbidity.5 Most PTSD patients with comorbid SUD receive treatment only for SUD and the PTSD symptoms often are unaddressed.5 Those suffering from PTSD often abuse alcohol because they might consider it to be a coping strategy. Alcohol reduces hyperactivation of the dorsal anterior cingulate cortex caused by re-experiencing PTSD symptoms. Other substances of abuse, such as Cannabis, could suppress PTSD symptoms through alternate mechanisms (eg, endocannabinoid receptors). All of these could mask PTSD symptoms, which can delay diagnosis and treatment.
SUD is the tip of the “SUD-PTSD iceberg.” Some clinicians tend to focus on detoxification while completely ignoring the underlying psychopathology of SUD, which may be PTSD. Even during detoxification, PTSD should be aggressively treated.6 Lastly, practice guidelines for managing SUD–PTSD comorbidity are lacking.
Targeting mechanisms of action
Noradrenergic mechanisms have been strongly implicated in the pathophysiology of PTSD. However, selective serotonin reuptake inhibitors, such as sertraline and paroxetine, are the only FDA-approved pharmacotherapy options for PTSD, although their efficacy is limited, perhaps because they are serotonergic.
Prazosin, an alpha-1 (α-1) adrenergic antagonist that is FDA-approved for hypertension and benign prostatic hypertrophy, has been studied for treating nightmares in PTSD.7 Prazosin has shown efficacy for nightmares in PTSD and other daytime symptoms, such as flashbacks, hypervigilance, and irritability.8 Several studies support the efficacy of prazosin in persons suffering from PTSD.9-11 Use of lower dosages in clinical trials might explain why prazosin did not separate from placebo in some studies. (See Table summarizing studies of prazosin dosing for PTSD.)

In a study of 12,844 veterans, the mean maximum prazosin dosage reached in the first year of treatment was 3.6 mg/d, and only 14% of patients reached the minimum Veterans Affairs recommended dosage of 6 mg/d.17 The most recent (March 2009) American Psychiatric Association practice guidelines recommend prazosin, 3 to 15 mg at bedtime.18
Prazosin has a short half-life of 2 to 3 hours and duration of action of 6 to 10 hours. Therefore, its use is limited to 2 or 3 times daily dosing. Higher (30 to 50 mg) and more frequent (2 to 3 times per day) dosages8,12,13 might be needed because of the drug’s short half-life.
Doxazosin. Another α-1 adrenergic drug, doxazosin, 8 to 16 mg/d, has shown benefit for PTSD as well.14,15 Doxazosin, which has a longer half-life (16 to 30 hours), requires only once-daily dosing.16 The most common side effects of prazosin and doxazosin are dizziness, headache, and drowsiness; syncope has been reported but is rare.
Prazosin and doxazosin also are used to treat substance abuse, such as alcohol use disorder19-21 and cocaine use disorder.22,23 This “two birds with one stone” approach could become more common in clinical practice.
Until a major breakthrough in PTSD treatment emerges, prazosin and doxazosin, although off-label, are reasonable treatment approaches.
The primary symptoms of PTSD are recurrent and include intrusive memories and dreams of the traumatic events, flashbacks, hypervigilance, irritability, sleep disturbances, and persistent avoidance of stimuli associated with the traumatic event. According to the National Comorbidity Survey, the estimated lifetime prevalence of PTSD among adults is 6.8% and is more common in women (9.7%) than men (3.6%).2 Among veterans, the prevalence of PTSD has been reported as:
- 31% among male Vietnam veterans (lifetime)
- 10% among Gulf War veterans
- 14% among Iraq and Afghanistan veterans.3
Why is PTSD overlooked in substance use?
Among individuals with SUD, 10% to 63% have comorbid PTSD.4 A recent report underscores the complexity and challenges of SUD–PTSD comorbidity.5 Most PTSD patients with comorbid SUD receive treatment only for SUD and the PTSD symptoms often are unaddressed.5 Those suffering from PTSD often abuse alcohol because they might consider it to be a coping strategy. Alcohol reduces hyperactivation of the dorsal anterior cingulate cortex caused by re-experiencing PTSD symptoms. Other substances of abuse, such as Cannabis, could suppress PTSD symptoms through alternate mechanisms (eg, endocannabinoid receptors). All of these could mask PTSD symptoms, which can delay diagnosis and treatment.
SUD is the tip of the “SUD-PTSD iceberg.” Some clinicians tend to focus on detoxification while completely ignoring the underlying psychopathology of SUD, which may be PTSD. Even during detoxification, PTSD should be aggressively treated.6 Lastly, practice guidelines for managing SUD–PTSD comorbidity are lacking.
Targeting mechanisms of action
Noradrenergic mechanisms have been strongly implicated in the pathophysiology of PTSD. However, selective serotonin reuptake inhibitors, such as sertraline and paroxetine, are the only FDA-approved pharmacotherapy options for PTSD, although their efficacy is limited, perhaps because they are serotonergic.
Prazosin, an alpha-1 (α-1) adrenergic antagonist that is FDA-approved for hypertension and benign prostatic hypertrophy, has been studied for treating nightmares in PTSD.7 Prazosin has shown efficacy for nightmares in PTSD and other daytime symptoms, such as flashbacks, hypervigilance, and irritability.8 Several studies support the efficacy of prazosin in persons suffering from PTSD.9-11 Use of lower dosages in clinical trials might explain why prazosin did not separate from placebo in some studies. (See Table summarizing studies of prazosin dosing for PTSD.)

In a study of 12,844 veterans, the mean maximum prazosin dosage reached in the first year of treatment was 3.6 mg/d, and only 14% of patients reached the minimum Veterans Affairs recommended dosage of 6 mg/d.17 The most recent (March 2009) American Psychiatric Association practice guidelines recommend prazosin, 3 to 15 mg at bedtime.18
Prazosin has a short half-life of 2 to 3 hours and duration of action of 6 to 10 hours. Therefore, its use is limited to 2 or 3 times daily dosing. Higher (30 to 50 mg) and more frequent (2 to 3 times per day) dosages8,12,13 might be needed because of the drug’s short half-life.
Doxazosin. Another α-1 adrenergic drug, doxazosin, 8 to 16 mg/d, has shown benefit for PTSD as well.14,15 Doxazosin, which has a longer half-life (16 to 30 hours), requires only once-daily dosing.16 The most common side effects of prazosin and doxazosin are dizziness, headache, and drowsiness; syncope has been reported but is rare.
Prazosin and doxazosin also are used to treat substance abuse, such as alcohol use disorder19-21 and cocaine use disorder.22,23 This “two birds with one stone” approach could become more common in clinical practice.
Until a major breakthrough in PTSD treatment emerges, prazosin and doxazosin, although off-label, are reasonable treatment approaches.
1. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428.
2. National Comorbidity Survey. 12-month prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort (n=9282). http://www.hcp.med.harvard.edu/ncs/ftpdir/NCS-R_12-month_Prevalence_Estimates.pdf. Published 2005. Accessed February 10, 2017.
3. Gradus JL. Epidemiology of PTSD. http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated February 23, 2016. Accessed February 13, 2017.
4. Debell F, Fear NT, Head M, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49(9):1401-1425.
5. Vujanovic AA, Bonn-Miller MO, Petry NM. Co-occurring posttraumatic stress and substance use: emerging research on correlates, mechanisms, and treatments-introduction to the special issue. Psychol Addict Behav. 2016;30(7):713-719.
6. Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158(8):1184-1190.
7. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
8. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
11. Raskind MA, Millard SP, Petrie EC, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80(10):736-742.
12. Koola MM, Varghese SP, Fawcett JA. High-dose prazosin for the treatment of post-traumatic stress disorder. Ther Adv Psychopharmacol. 2014;4(1):43-47.
13. Vaishnav M, Patel V, Varghese SP, et al. Fludrocortisone in posttraumatic stress disorder: effective for symptoms and prazosin-induced hypotension. Prim Care Companion CNS Disord. 2014;16(6). doi: 10.4088/PCC.14l01676.
14. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
15. Roepke S, Danker-Hopfe H, Repantis D, et al. Doxazosin, an α-1-adrenergic-receptor antagonist, for nightmares in patients with posttraumatic stress disorder and/or borderline personality disorder: a chart review. Pharmacopsychiatry. 2017;50(1):26-31.
16. Smith C, Koola MM. Evidence for using doxazosin in the treatment of posttraumatic stress disorder. Psychiatr Ann. 2016;46(9):553-555.
17. Alexander B, Lund BC, Bernardy NC, et al. Early discontinuation and suboptimal dosing of prazosin: a potential missed opportunity for veterans with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(5):e639-e644.
18. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf. Accessed February 10, 2017.
19. Qazi H, Wijegunaratne H, Savajiyani R, et al. Naltrexone and prazosin combination for posttraumatic stress disorder and alcohol use disorder. Prim Care Companion CNS Disord. 2014;16(4). doi: 10.4088/PCC.14l01638.
20. Simpson TL, Malte CA, Dietel B, et al. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39(5):808-817.
21. Kenna GA, Haass-Koffler CL, Zywiak WH, et al. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21(4):904-914.
22. Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013;131(1-2):66-70.
23. Newton TF, De La Garza R II, Brown G, et al. Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7(2):e30854.
1. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428.
2. National Comorbidity Survey. 12-month prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort (n=9282). http://www.hcp.med.harvard.edu/ncs/ftpdir/NCS-R_12-month_Prevalence_Estimates.pdf. Published 2005. Accessed February 10, 2017.
3. Gradus JL. Epidemiology of PTSD. http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated February 23, 2016. Accessed February 13, 2017.
4. Debell F, Fear NT, Head M, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49(9):1401-1425.
5. Vujanovic AA, Bonn-Miller MO, Petry NM. Co-occurring posttraumatic stress and substance use: emerging research on correlates, mechanisms, and treatments-introduction to the special issue. Psychol Addict Behav. 2016;30(7):713-719.
6. Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158(8):1184-1190.
7. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
8. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
11. Raskind MA, Millard SP, Petrie EC, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80(10):736-742.
12. Koola MM, Varghese SP, Fawcett JA. High-dose prazosin for the treatment of post-traumatic stress disorder. Ther Adv Psychopharmacol. 2014;4(1):43-47.
13. Vaishnav M, Patel V, Varghese SP, et al. Fludrocortisone in posttraumatic stress disorder: effective for symptoms and prazosin-induced hypotension. Prim Care Companion CNS Disord. 2014;16(6). doi: 10.4088/PCC.14l01676.
14. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
15. Roepke S, Danker-Hopfe H, Repantis D, et al. Doxazosin, an α-1-adrenergic-receptor antagonist, for nightmares in patients with posttraumatic stress disorder and/or borderline personality disorder: a chart review. Pharmacopsychiatry. 2017;50(1):26-31.
16. Smith C, Koola MM. Evidence for using doxazosin in the treatment of posttraumatic stress disorder. Psychiatr Ann. 2016;46(9):553-555.
17. Alexander B, Lund BC, Bernardy NC, et al. Early discontinuation and suboptimal dosing of prazosin: a potential missed opportunity for veterans with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(5):e639-e644.
18. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf. Accessed February 10, 2017.
19. Qazi H, Wijegunaratne H, Savajiyani R, et al. Naltrexone and prazosin combination for posttraumatic stress disorder and alcohol use disorder. Prim Care Companion CNS Disord. 2014;16(4). doi: 10.4088/PCC.14l01638.
20. Simpson TL, Malte CA, Dietel B, et al. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39(5):808-817.
21. Kenna GA, Haass-Koffler CL, Zywiak WH, et al. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21(4):904-914.
22. Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013;131(1-2):66-70.
23. Newton TF, De La Garza R II, Brown G, et al. Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7(2):e30854.
Hepatitis C: Screening changes, treatment advances
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4
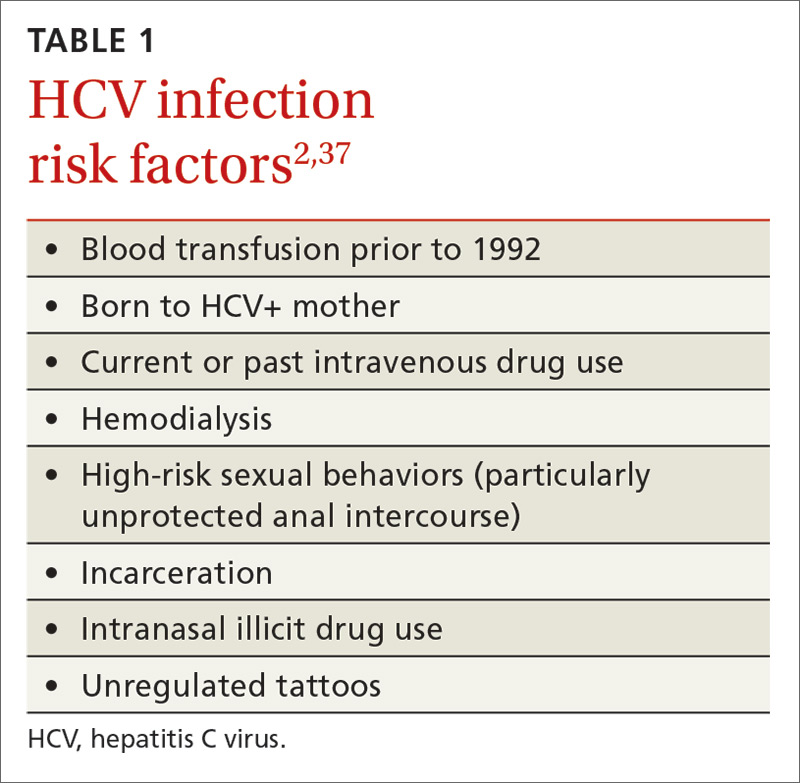
In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).
The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).
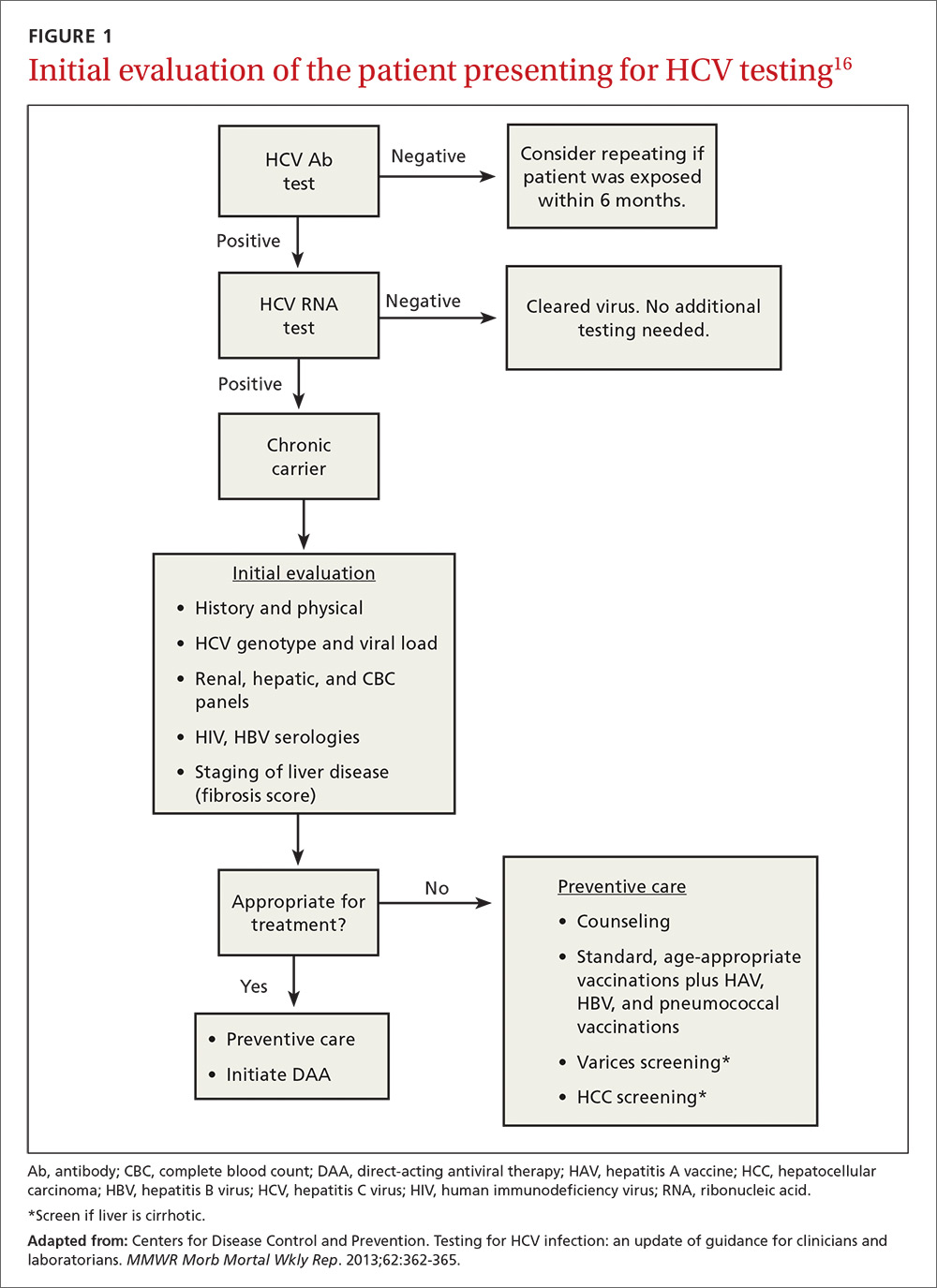
Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4

In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).

The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).

Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4

In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).

The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).

Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
PRACTICE RECOMMENDATIONS
› Offer hepatitis C virus (HCV) screening to all patients with identified risk factors, as well as anyone born between 1945 and 1965, regardless of risk factors. B
› Offer human immunodeficiency virus and hepatitis B testing, as well as hepatitis A, hepatitis B, and pneumococcal vaccinations, to all patients with chronic HCV infection. C
› Consider treatment with interferon-free direct-acting antiviral therapies for all patients with chronic HCV infection to reduce liver-related and all-cause morbidity and mortality. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The elements of pain care that the guidelines don’t address
You are probably one of the million+ physicians who received a letter from the Surgeon General urging us to use opioids judiciously,1 and you are likely familiar with the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain.2 (See JFP’s “Opioids for chronic pain: The CDC’s 12 recommendations,” 2016;65:906-909.) Most of these recommendations are common-sense practices, such as reducing doses, using alternative medications and treatments, monitoring prescribing through state databases, conducting random drug tests, consulting pain and addiction specialists, and establishing clear treatment goals.
But the guidelines only go so far. They don’t address the empathy, perseverance, and insight needed to stick with these patients and oversee their care. And they don’t directly address the patients who are already taking opioids for chronic pain when they arrive at our offices. Despite nearly 40 years of practicing family medicine, I can count on one hand the number of patients for whom I initiated opioid medication. Yet I have managed many patients with chronic pain who were already on hefty doses of narcotics when they became my patients. Rather than refuse to care for them, we should seek to understand their story, continuously try other medications and therapies, repeatedly attempt to reduce dosages, and frequently check substance databases.
Following the guidelines is no guarantee that our prescribing practices won’t be called into question. I have seen excellent family physicians censured by state licensing boards unjustifiably. One colleague was accused by a patient of “getting him addicted,” only after the physician refused to continue prescribing narcotics. Based on this single complaint, the physician had his license temporarily revoked with no due process whatsoever. He got his license back after an appeals process that took several months, cost many dollars, and inflicted significant emotional trauma. No wonder some of us just say “No” to caring for patients with chronic pain.
Perseverance and motivation. I remind myself that good, well-intentioned, and careful primary care physicians are NOT the cause of this epidemic. I encourage you to stick with these patients (lest they turn to the streets to obtain heroin laced with fentanyl), and look for sources of motivation. You may be motivated, as I was, by a physician’s story in JAMA about his 49-year-old younger sister, a vibrant, accomplished, caring woman whose chronic pain led to her death in a jail cell after she became combative in the ED.3 Had she been treated as a patient with a chronic illness, rather than a criminal with a character flaw, I suspect she would be alive today.
1. Turn the Tide: the Surgeon General’s call to end the opioid crisis. Available at: http://turnthetiderx.org/#. Accessed February 15, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed February 15, 2017.
3. Weeks WB. Hailey. JAMA. 2016;316:1975-1976.
You are probably one of the million+ physicians who received a letter from the Surgeon General urging us to use opioids judiciously,1 and you are likely familiar with the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain.2 (See JFP’s “Opioids for chronic pain: The CDC’s 12 recommendations,” 2016;65:906-909.) Most of these recommendations are common-sense practices, such as reducing doses, using alternative medications and treatments, monitoring prescribing through state databases, conducting random drug tests, consulting pain and addiction specialists, and establishing clear treatment goals.
But the guidelines only go so far. They don’t address the empathy, perseverance, and insight needed to stick with these patients and oversee their care. And they don’t directly address the patients who are already taking opioids for chronic pain when they arrive at our offices. Despite nearly 40 years of practicing family medicine, I can count on one hand the number of patients for whom I initiated opioid medication. Yet I have managed many patients with chronic pain who were already on hefty doses of narcotics when they became my patients. Rather than refuse to care for them, we should seek to understand their story, continuously try other medications and therapies, repeatedly attempt to reduce dosages, and frequently check substance databases.
Following the guidelines is no guarantee that our prescribing practices won’t be called into question. I have seen excellent family physicians censured by state licensing boards unjustifiably. One colleague was accused by a patient of “getting him addicted,” only after the physician refused to continue prescribing narcotics. Based on this single complaint, the physician had his license temporarily revoked with no due process whatsoever. He got his license back after an appeals process that took several months, cost many dollars, and inflicted significant emotional trauma. No wonder some of us just say “No” to caring for patients with chronic pain.
Perseverance and motivation. I remind myself that good, well-intentioned, and careful primary care physicians are NOT the cause of this epidemic. I encourage you to stick with these patients (lest they turn to the streets to obtain heroin laced with fentanyl), and look for sources of motivation. You may be motivated, as I was, by a physician’s story in JAMA about his 49-year-old younger sister, a vibrant, accomplished, caring woman whose chronic pain led to her death in a jail cell after she became combative in the ED.3 Had she been treated as a patient with a chronic illness, rather than a criminal with a character flaw, I suspect she would be alive today.
You are probably one of the million+ physicians who received a letter from the Surgeon General urging us to use opioids judiciously,1 and you are likely familiar with the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain.2 (See JFP’s “Opioids for chronic pain: The CDC’s 12 recommendations,” 2016;65:906-909.) Most of these recommendations are common-sense practices, such as reducing doses, using alternative medications and treatments, monitoring prescribing through state databases, conducting random drug tests, consulting pain and addiction specialists, and establishing clear treatment goals.
But the guidelines only go so far. They don’t address the empathy, perseverance, and insight needed to stick with these patients and oversee their care. And they don’t directly address the patients who are already taking opioids for chronic pain when they arrive at our offices. Despite nearly 40 years of practicing family medicine, I can count on one hand the number of patients for whom I initiated opioid medication. Yet I have managed many patients with chronic pain who were already on hefty doses of narcotics when they became my patients. Rather than refuse to care for them, we should seek to understand their story, continuously try other medications and therapies, repeatedly attempt to reduce dosages, and frequently check substance databases.
Following the guidelines is no guarantee that our prescribing practices won’t be called into question. I have seen excellent family physicians censured by state licensing boards unjustifiably. One colleague was accused by a patient of “getting him addicted,” only after the physician refused to continue prescribing narcotics. Based on this single complaint, the physician had his license temporarily revoked with no due process whatsoever. He got his license back after an appeals process that took several months, cost many dollars, and inflicted significant emotional trauma. No wonder some of us just say “No” to caring for patients with chronic pain.
Perseverance and motivation. I remind myself that good, well-intentioned, and careful primary care physicians are NOT the cause of this epidemic. I encourage you to stick with these patients (lest they turn to the streets to obtain heroin laced with fentanyl), and look for sources of motivation. You may be motivated, as I was, by a physician’s story in JAMA about his 49-year-old younger sister, a vibrant, accomplished, caring woman whose chronic pain led to her death in a jail cell after she became combative in the ED.3 Had she been treated as a patient with a chronic illness, rather than a criminal with a character flaw, I suspect she would be alive today.
1. Turn the Tide: the Surgeon General’s call to end the opioid crisis. Available at: http://turnthetiderx.org/#. Accessed February 15, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed February 15, 2017.
3. Weeks WB. Hailey. JAMA. 2016;316:1975-1976.
1. Turn the Tide: the Surgeon General’s call to end the opioid crisis. Available at: http://turnthetiderx.org/#. Accessed February 15, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed February 15, 2017.
3. Weeks WB. Hailey. JAMA. 2016;316:1975-1976.
Does giving a sweet-tasting solution before vaccine injection reduce infant crying?
EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).
1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).

EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).

1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. Oral administration of a sucrose or glucose solution before intramuscular vaccine injection reduces expected crying duration by 12 to 77 seconds following the shot (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs] and 2 RCTs).
“Cold turkey” works best for smoking cessation
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Counsel patients who want to quit smoking that abrupt smoking cessation is more effective for long-term abstinence than taking a gradual approach.
STRENGTH OF RECOMMENDATION
B: Based on one well-designed, randomized controlled trial.
Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.1
Readers weigh in on opioid epidemic
I read Dr. Unger’s guest editorial, “Staring down the opioid epidemic” (J Fam Pract. 2017;66:8) and thought that he made some good points, but as an internist for 38 years and a detox addiction specialist for the past 7 years, I have seen too much “pendulum swinging” with regard to opioids.
The state of Pennsylvania is enforcing opioid prescription laws so intensely that I now see underprescribing of needed medications by physicians and dentists. For example, I recently had dental surgery and wasn’t prescribed a narcotic. I suffered for 24 hours with ineffective nonsteroidal anti-inflammatory drugs. And a relative of mine experienced excessive pain following gynecologic cancer surgery because the surgeon wouldn’t prescribe opioids for fear of reprisal.
I would like to see someone conduct a nationwide survey of primary care physicians regarding their views on narcotics for pain so that I can better understand my colleagues’ perspectives on this issue.
Don Sesso, DO, FCCP
Gwynedd Valley, PA
In his guest editorial, Dr. Unger urged family physicians to treat patients who are addicted to opioids with buprenorphine. It’s a shame that so few of us do so.
Patients who are addicted to opioids are no more difficult to treat than patients with diabetes, yet we, as family physicians, often fail to fulfill our basic duty to respond to their illness. Using buprenorphine to help a patient who is addicted to opioids achieve sobriety is highly effective. And treating these patients is amazingly satisfying, as you’ll never have more grateful patients than these.
I began integrating buprenorphine treatment into my family practice 10 years ago. It has made me much more effective in treating my patients who are addicted to alcohol, and it has provided me with a great deal of personal satisfaction in the latter part of my career.
I challenge all family physicians to step up and do their duty to help combat the opioid epidemic.
David A. Moore, MD
Salt Lake City, Utah
I read Dr. Unger’s guest editorial, “Staring down the opioid epidemic” (J Fam Pract. 2017;66:8) and thought that he made some good points, but as an internist for 38 years and a detox addiction specialist for the past 7 years, I have seen too much “pendulum swinging” with regard to opioids.
The state of Pennsylvania is enforcing opioid prescription laws so intensely that I now see underprescribing of needed medications by physicians and dentists. For example, I recently had dental surgery and wasn’t prescribed a narcotic. I suffered for 24 hours with ineffective nonsteroidal anti-inflammatory drugs. And a relative of mine experienced excessive pain following gynecologic cancer surgery because the surgeon wouldn’t prescribe opioids for fear of reprisal.
I would like to see someone conduct a nationwide survey of primary care physicians regarding their views on narcotics for pain so that I can better understand my colleagues’ perspectives on this issue.
Don Sesso, DO, FCCP
Gwynedd Valley, PA
In his guest editorial, Dr. Unger urged family physicians to treat patients who are addicted to opioids with buprenorphine. It’s a shame that so few of us do so.
Patients who are addicted to opioids are no more difficult to treat than patients with diabetes, yet we, as family physicians, often fail to fulfill our basic duty to respond to their illness. Using buprenorphine to help a patient who is addicted to opioids achieve sobriety is highly effective. And treating these patients is amazingly satisfying, as you’ll never have more grateful patients than these.
I began integrating buprenorphine treatment into my family practice 10 years ago. It has made me much more effective in treating my patients who are addicted to alcohol, and it has provided me with a great deal of personal satisfaction in the latter part of my career.
I challenge all family physicians to step up and do their duty to help combat the opioid epidemic.
David A. Moore, MD
Salt Lake City, Utah
I read Dr. Unger’s guest editorial, “Staring down the opioid epidemic” (J Fam Pract. 2017;66:8) and thought that he made some good points, but as an internist for 38 years and a detox addiction specialist for the past 7 years, I have seen too much “pendulum swinging” with regard to opioids.
The state of Pennsylvania is enforcing opioid prescription laws so intensely that I now see underprescribing of needed medications by physicians and dentists. For example, I recently had dental surgery and wasn’t prescribed a narcotic. I suffered for 24 hours with ineffective nonsteroidal anti-inflammatory drugs. And a relative of mine experienced excessive pain following gynecologic cancer surgery because the surgeon wouldn’t prescribe opioids for fear of reprisal.
I would like to see someone conduct a nationwide survey of primary care physicians regarding their views on narcotics for pain so that I can better understand my colleagues’ perspectives on this issue.
Don Sesso, DO, FCCP
Gwynedd Valley, PA
In his guest editorial, Dr. Unger urged family physicians to treat patients who are addicted to opioids with buprenorphine. It’s a shame that so few of us do so.
Patients who are addicted to opioids are no more difficult to treat than patients with diabetes, yet we, as family physicians, often fail to fulfill our basic duty to respond to their illness. Using buprenorphine to help a patient who is addicted to opioids achieve sobriety is highly effective. And treating these patients is amazingly satisfying, as you’ll never have more grateful patients than these.
I began integrating buprenorphine treatment into my family practice 10 years ago. It has made me much more effective in treating my patients who are addicted to alcohol, and it has provided me with a great deal of personal satisfaction in the latter part of my career.
I challenge all family physicians to step up and do their duty to help combat the opioid epidemic.
David A. Moore, MD
Salt Lake City, Utah