User login
RNA-based biopsy test bests NCCN risk stratification for PC prognosis
ORLANDO – A genetic assay for prostate cancer typically used after radical prostatectomy could be used earlier, at the time of diagnostic biopsy testing, to classify patients as low, intermediate, and high risk for metastasis and disease-specific mortality, new research reveals.
Based on an approximately 1-mm biopsy sample, the Decipher Prostate Cancer Classifier assesses the activity of 22 genes relevant to prostate cancer. In a multicenter study of 175 patients, investigators found the 5-year risk for metastatic disease was 5.0% among patients classified as low risk by Decipher, 9.3% in the intermediate-risk group, and 23.4% in the high-risk patients.
“It turns out NCCN [National Comprehensive Cancer Network] risk groups can also provide this kind of risk stratification … so why do we need the extra test?” lead author Paul L. Nguyen, MD, of Dana-Farber Cancer Institute in Boston said here at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. Because, he added, Decipher provides “significant prognostic information for distant metastases beyond clinical variables alone,” even after controlling for prostate-specific antigen level, Gleason score, and treatment type, Dr. Nguyen said.
The Decipher RNA–based test also improved the c-index for predicting likelihood of distant metastases, with a 0.75 correlation, compared with 0.66 with NCCN risk stratification and 0.66 based on Cancer of the Prostate Risk Assessment score. “So this adds to what we already know, and it helps us decide which patients are going to develop metastases.”
Decipher’s prognostic value emerged regardless of first line therapy. A total 100 patients received radiation and androgen therapy at Dana-Farber and another 75 underwent radical prostatectomy at the Cleveland Clinic or Johns Hopkins University, Baltimore. Decipher classified 13% of patients as low risk, 51% as intermediate risk, and 34% as high risk. Because prostate tumors can be heterogeneous, researchers chose the highest-grade biopsy sample for each patient.
Local Therapy for High-Risk Patients?
A meeting attendee asked if a patient is “known to be high risk on biopsy, and has a 23% chance of metastasis after treatment, why treat with local treatment in the first place?”
“For these patients, we’re meeting them up front and they have a high risk of disease, a 23% chance of metastasis, I think we’re going to throw everything we can at them,” Dr. Nguyen said. Multiple randomized controlled trials indicate intensifying therapy can improve outcomes and that local therapy contributes to overall survival in these patients, he added. “For these patients who have very high risk disease, we have enough randomized data to show local therapy is still important. The next thing we need to do is work on personalizing their systemic therapy, and figuring out how to integrate these novel systemic therapies based on their genomic scores.”
Disease-Specific Survival
Eleven participants in the study died from prostate cancer. The only variable associated with prostate-specific disease mortality was the Decipher classification, with a hazard ratio of 1.57 for every 10% increase in the score on a univariate model (P = .02).
Dr. Nguyen and his coinvestigators also assessed 5-year prostate cancer specific mortality. They found a 9.4% rate in the Decipher high-risk group, compared with 0% in both the intermediate- and low-risk groups.
“Okay, we have this data. How do we incorporate this test into our practices?” Dr. Nguyen asked. Because the low-risk patients only comprised 13% of the study population, he was unable to state that this group could be directed to active surveillance based on the findings.
What about NCCN intermediate risk? Should these people treated with dose-escalated radiation therapy also be given short-course hormone therapy? “So far we have not seen a survival improvement, and we’re awaiting a definitive trial,” Dr. Nguyen said.
Prognostic, Not Predictive
Could the high-risk classification help physicians decide among prostatectomy, radiation, and long-course hormone therapy versus enrolling patients in a clinical trial to test a novel agent? “Perhaps, and there is some rationale for thinking in that direction,” Dr. Nguyen said. “But it is important to understand the difference between a prognostic and predictive biomarker. We’ve shown Decipher has prognostic value for identifying patients at risk for distant metastases and death.” In contrast, randomized controlled trials would be required to identify a predictive marker that ultimately could guide choice of treatment in an individual, he said.
“Robust markers are needed to see who needs treatment, and which treatment is best for primary and metastatic prostate cancer,” said study discussant Angelo DeMarzo, MD, PhD, of Johns Hopkins University. He asked Dr. Nguyen about the next best step in his research.
“Our paper was mostly intermediate- and high-risk patients; I would personally love to learn more about which patients need long-course, short-course, or no hormone treatment,” Dr. Nguyen said. He would also like to conduct randomized trials to assess any role of Decipher classification for active surveillance, and for guiding treatment intensification versus de-escalation for those patients who receive therapy.
ORLANDO – A genetic assay for prostate cancer typically used after radical prostatectomy could be used earlier, at the time of diagnostic biopsy testing, to classify patients as low, intermediate, and high risk for metastasis and disease-specific mortality, new research reveals.
Based on an approximately 1-mm biopsy sample, the Decipher Prostate Cancer Classifier assesses the activity of 22 genes relevant to prostate cancer. In a multicenter study of 175 patients, investigators found the 5-year risk for metastatic disease was 5.0% among patients classified as low risk by Decipher, 9.3% in the intermediate-risk group, and 23.4% in the high-risk patients.
“It turns out NCCN [National Comprehensive Cancer Network] risk groups can also provide this kind of risk stratification … so why do we need the extra test?” lead author Paul L. Nguyen, MD, of Dana-Farber Cancer Institute in Boston said here at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. Because, he added, Decipher provides “significant prognostic information for distant metastases beyond clinical variables alone,” even after controlling for prostate-specific antigen level, Gleason score, and treatment type, Dr. Nguyen said.
The Decipher RNA–based test also improved the c-index for predicting likelihood of distant metastases, with a 0.75 correlation, compared with 0.66 with NCCN risk stratification and 0.66 based on Cancer of the Prostate Risk Assessment score. “So this adds to what we already know, and it helps us decide which patients are going to develop metastases.”
Decipher’s prognostic value emerged regardless of first line therapy. A total 100 patients received radiation and androgen therapy at Dana-Farber and another 75 underwent radical prostatectomy at the Cleveland Clinic or Johns Hopkins University, Baltimore. Decipher classified 13% of patients as low risk, 51% as intermediate risk, and 34% as high risk. Because prostate tumors can be heterogeneous, researchers chose the highest-grade biopsy sample for each patient.
Local Therapy for High-Risk Patients?
A meeting attendee asked if a patient is “known to be high risk on biopsy, and has a 23% chance of metastasis after treatment, why treat with local treatment in the first place?”
“For these patients, we’re meeting them up front and they have a high risk of disease, a 23% chance of metastasis, I think we’re going to throw everything we can at them,” Dr. Nguyen said. Multiple randomized controlled trials indicate intensifying therapy can improve outcomes and that local therapy contributes to overall survival in these patients, he added. “For these patients who have very high risk disease, we have enough randomized data to show local therapy is still important. The next thing we need to do is work on personalizing their systemic therapy, and figuring out how to integrate these novel systemic therapies based on their genomic scores.”
Disease-Specific Survival
Eleven participants in the study died from prostate cancer. The only variable associated with prostate-specific disease mortality was the Decipher classification, with a hazard ratio of 1.57 for every 10% increase in the score on a univariate model (P = .02).
Dr. Nguyen and his coinvestigators also assessed 5-year prostate cancer specific mortality. They found a 9.4% rate in the Decipher high-risk group, compared with 0% in both the intermediate- and low-risk groups.
“Okay, we have this data. How do we incorporate this test into our practices?” Dr. Nguyen asked. Because the low-risk patients only comprised 13% of the study population, he was unable to state that this group could be directed to active surveillance based on the findings.
What about NCCN intermediate risk? Should these people treated with dose-escalated radiation therapy also be given short-course hormone therapy? “So far we have not seen a survival improvement, and we’re awaiting a definitive trial,” Dr. Nguyen said.
Prognostic, Not Predictive
Could the high-risk classification help physicians decide among prostatectomy, radiation, and long-course hormone therapy versus enrolling patients in a clinical trial to test a novel agent? “Perhaps, and there is some rationale for thinking in that direction,” Dr. Nguyen said. “But it is important to understand the difference between a prognostic and predictive biomarker. We’ve shown Decipher has prognostic value for identifying patients at risk for distant metastases and death.” In contrast, randomized controlled trials would be required to identify a predictive marker that ultimately could guide choice of treatment in an individual, he said.
“Robust markers are needed to see who needs treatment, and which treatment is best for primary and metastatic prostate cancer,” said study discussant Angelo DeMarzo, MD, PhD, of Johns Hopkins University. He asked Dr. Nguyen about the next best step in his research.
“Our paper was mostly intermediate- and high-risk patients; I would personally love to learn more about which patients need long-course, short-course, or no hormone treatment,” Dr. Nguyen said. He would also like to conduct randomized trials to assess any role of Decipher classification for active surveillance, and for guiding treatment intensification versus de-escalation for those patients who receive therapy.
ORLANDO – A genetic assay for prostate cancer typically used after radical prostatectomy could be used earlier, at the time of diagnostic biopsy testing, to classify patients as low, intermediate, and high risk for metastasis and disease-specific mortality, new research reveals.
Based on an approximately 1-mm biopsy sample, the Decipher Prostate Cancer Classifier assesses the activity of 22 genes relevant to prostate cancer. In a multicenter study of 175 patients, investigators found the 5-year risk for metastatic disease was 5.0% among patients classified as low risk by Decipher, 9.3% in the intermediate-risk group, and 23.4% in the high-risk patients.
“It turns out NCCN [National Comprehensive Cancer Network] risk groups can also provide this kind of risk stratification … so why do we need the extra test?” lead author Paul L. Nguyen, MD, of Dana-Farber Cancer Institute in Boston said here at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. Because, he added, Decipher provides “significant prognostic information for distant metastases beyond clinical variables alone,” even after controlling for prostate-specific antigen level, Gleason score, and treatment type, Dr. Nguyen said.
The Decipher RNA–based test also improved the c-index for predicting likelihood of distant metastases, with a 0.75 correlation, compared with 0.66 with NCCN risk stratification and 0.66 based on Cancer of the Prostate Risk Assessment score. “So this adds to what we already know, and it helps us decide which patients are going to develop metastases.”
Decipher’s prognostic value emerged regardless of first line therapy. A total 100 patients received radiation and androgen therapy at Dana-Farber and another 75 underwent radical prostatectomy at the Cleveland Clinic or Johns Hopkins University, Baltimore. Decipher classified 13% of patients as low risk, 51% as intermediate risk, and 34% as high risk. Because prostate tumors can be heterogeneous, researchers chose the highest-grade biopsy sample for each patient.
Local Therapy for High-Risk Patients?
A meeting attendee asked if a patient is “known to be high risk on biopsy, and has a 23% chance of metastasis after treatment, why treat with local treatment in the first place?”
“For these patients, we’re meeting them up front and they have a high risk of disease, a 23% chance of metastasis, I think we’re going to throw everything we can at them,” Dr. Nguyen said. Multiple randomized controlled trials indicate intensifying therapy can improve outcomes and that local therapy contributes to overall survival in these patients, he added. “For these patients who have very high risk disease, we have enough randomized data to show local therapy is still important. The next thing we need to do is work on personalizing their systemic therapy, and figuring out how to integrate these novel systemic therapies based on their genomic scores.”
Disease-Specific Survival
Eleven participants in the study died from prostate cancer. The only variable associated with prostate-specific disease mortality was the Decipher classification, with a hazard ratio of 1.57 for every 10% increase in the score on a univariate model (P = .02).
Dr. Nguyen and his coinvestigators also assessed 5-year prostate cancer specific mortality. They found a 9.4% rate in the Decipher high-risk group, compared with 0% in both the intermediate- and low-risk groups.
“Okay, we have this data. How do we incorporate this test into our practices?” Dr. Nguyen asked. Because the low-risk patients only comprised 13% of the study population, he was unable to state that this group could be directed to active surveillance based on the findings.
What about NCCN intermediate risk? Should these people treated with dose-escalated radiation therapy also be given short-course hormone therapy? “So far we have not seen a survival improvement, and we’re awaiting a definitive trial,” Dr. Nguyen said.
Prognostic, Not Predictive
Could the high-risk classification help physicians decide among prostatectomy, radiation, and long-course hormone therapy versus enrolling patients in a clinical trial to test a novel agent? “Perhaps, and there is some rationale for thinking in that direction,” Dr. Nguyen said. “But it is important to understand the difference between a prognostic and predictive biomarker. We’ve shown Decipher has prognostic value for identifying patients at risk for distant metastases and death.” In contrast, randomized controlled trials would be required to identify a predictive marker that ultimately could guide choice of treatment in an individual, he said.
“Robust markers are needed to see who needs treatment, and which treatment is best for primary and metastatic prostate cancer,” said study discussant Angelo DeMarzo, MD, PhD, of Johns Hopkins University. He asked Dr. Nguyen about the next best step in his research.
“Our paper was mostly intermediate- and high-risk patients; I would personally love to learn more about which patients need long-course, short-course, or no hormone treatment,” Dr. Nguyen said. He would also like to conduct randomized trials to assess any role of Decipher classification for active surveillance, and for guiding treatment intensification versus de-escalation for those patients who receive therapy.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: A genomic test accurately risk stratifies patients with prostate cancer in study.
Major finding: Five-year risk of metastasis was 5.0% in a low-risk group, 9.3% in an intermediate-risk group, and 23.4% in a high-risk group.
Data source: A multicenter trial of needle biopsy samples taken from 175 people with prostate cancer.
Disclosures: Dr, Nguyen is a consultant/advisor for Ferring, GenomeDx, and Medivation, and also receives research funding from Astellas.
Prostate cancer susceptibility loci identified
ORLANDO – , and captured an additional 6% of the familial relevant risk for prostate cancer.
In the Oncoarray stratified analysis, the researchers detected two single nucleotide polymorphisms (SNPs) that were associated with early-onset prostate cancer, defined as disease occurring in men younger than 55 years, and four that were associated with both aggressive and indolent disease.
“By the end of this year we will have a genetic test with 170 variants, and men at the top of 1% of risk group would have a 5.72% risk over the average man,” she said. “But if we incorporated in the rarer variants, which are associated with very nasty disease, could we then start to identify men who are at risk for aggressive disease, and if so, how should we intensively screen them or even treat them?”
Currently genome-wide association studies (GWAS) have identified over 100 prostate cancer susceptibility loci, which included 33% of the prostate cancer familial relative risk in Europeans.
“We already know how to stratify populations into risk groups,” said Dr. Eeles. “It is important for us to do the right tests to see if they are really going to help in terms of targeted screening.”
The heritability of prostate cancer is 57%, but currently there are no common aggressive-disease markers.
To identify further susceptibility variants, Dr. Eeles and her colleagues conducted a prostate cancer GWAS that was far larger than previous investigations. Their analysis included 46,939 prostate cancer cases and 27,910 controls of European ancestry who were genotyped using the 35,369 prostate cancer cases and 33,164 controls of European ancestry that were generated using other large-scale genotyping arrays.
Dr. Eeles explained that, because they want their data to be useful for future investigations and “you can’t type everything as just too expensive – there are millions of SNPs across the genome – we put in a GWAS backbone.”
What that means, she explained, is that, when these are typed, it can be inferred that the other genotypes are using mathematical modeling.
Their Oncoarray was a large sample consisting of various ethnic groups, but in this presentation Dr. Eeles focused on an analysis of 98.500 European samples. The number reached 145,000 because they combined it with previous results in a meta-analysis of samples derived from men of both European and Asian descent.
The identification of additional loci also will enable fine mapping efforts into the underlying biology of prostate cancer susceptibility, she added.
There was no funding source disclosed. Dr. Eeles has received honoraria from Janssen and Succinct Communications. Coauthor Peter Kraft reported a relationship with Merck, but none of the other investigators had any disclosures.
ORLANDO – , and captured an additional 6% of the familial relevant risk for prostate cancer.
In the Oncoarray stratified analysis, the researchers detected two single nucleotide polymorphisms (SNPs) that were associated with early-onset prostate cancer, defined as disease occurring in men younger than 55 years, and four that were associated with both aggressive and indolent disease.
“By the end of this year we will have a genetic test with 170 variants, and men at the top of 1% of risk group would have a 5.72% risk over the average man,” she said. “But if we incorporated in the rarer variants, which are associated with very nasty disease, could we then start to identify men who are at risk for aggressive disease, and if so, how should we intensively screen them or even treat them?”
Currently genome-wide association studies (GWAS) have identified over 100 prostate cancer susceptibility loci, which included 33% of the prostate cancer familial relative risk in Europeans.
“We already know how to stratify populations into risk groups,” said Dr. Eeles. “It is important for us to do the right tests to see if they are really going to help in terms of targeted screening.”
The heritability of prostate cancer is 57%, but currently there are no common aggressive-disease markers.
To identify further susceptibility variants, Dr. Eeles and her colleagues conducted a prostate cancer GWAS that was far larger than previous investigations. Their analysis included 46,939 prostate cancer cases and 27,910 controls of European ancestry who were genotyped using the 35,369 prostate cancer cases and 33,164 controls of European ancestry that were generated using other large-scale genotyping arrays.
Dr. Eeles explained that, because they want their data to be useful for future investigations and “you can’t type everything as just too expensive – there are millions of SNPs across the genome – we put in a GWAS backbone.”
What that means, she explained, is that, when these are typed, it can be inferred that the other genotypes are using mathematical modeling.
Their Oncoarray was a large sample consisting of various ethnic groups, but in this presentation Dr. Eeles focused on an analysis of 98.500 European samples. The number reached 145,000 because they combined it with previous results in a meta-analysis of samples derived from men of both European and Asian descent.
The identification of additional loci also will enable fine mapping efforts into the underlying biology of prostate cancer susceptibility, she added.
There was no funding source disclosed. Dr. Eeles has received honoraria from Janssen and Succinct Communications. Coauthor Peter Kraft reported a relationship with Merck, but none of the other investigators had any disclosures.
ORLANDO – , and captured an additional 6% of the familial relevant risk for prostate cancer.
In the Oncoarray stratified analysis, the researchers detected two single nucleotide polymorphisms (SNPs) that were associated with early-onset prostate cancer, defined as disease occurring in men younger than 55 years, and four that were associated with both aggressive and indolent disease.
“By the end of this year we will have a genetic test with 170 variants, and men at the top of 1% of risk group would have a 5.72% risk over the average man,” she said. “But if we incorporated in the rarer variants, which are associated with very nasty disease, could we then start to identify men who are at risk for aggressive disease, and if so, how should we intensively screen them or even treat them?”
Currently genome-wide association studies (GWAS) have identified over 100 prostate cancer susceptibility loci, which included 33% of the prostate cancer familial relative risk in Europeans.
“We already know how to stratify populations into risk groups,” said Dr. Eeles. “It is important for us to do the right tests to see if they are really going to help in terms of targeted screening.”
The heritability of prostate cancer is 57%, but currently there are no common aggressive-disease markers.
To identify further susceptibility variants, Dr. Eeles and her colleagues conducted a prostate cancer GWAS that was far larger than previous investigations. Their analysis included 46,939 prostate cancer cases and 27,910 controls of European ancestry who were genotyped using the 35,369 prostate cancer cases and 33,164 controls of European ancestry that were generated using other large-scale genotyping arrays.
Dr. Eeles explained that, because they want their data to be useful for future investigations and “you can’t type everything as just too expensive – there are millions of SNPs across the genome – we put in a GWAS backbone.”
What that means, she explained, is that, when these are typed, it can be inferred that the other genotypes are using mathematical modeling.
Their Oncoarray was a large sample consisting of various ethnic groups, but in this presentation Dr. Eeles focused on an analysis of 98.500 European samples. The number reached 145,000 because they combined it with previous results in a meta-analysis of samples derived from men of both European and Asian descent.
The identification of additional loci also will enable fine mapping efforts into the underlying biology of prostate cancer susceptibility, she added.
There was no funding source disclosed. Dr. Eeles has received honoraria from Janssen and Succinct Communications. Coauthor Peter Kraft reported a relationship with Merck, but none of the other investigators had any disclosures.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Findings demonstrate the utility of high-density arrays and large sample sizes for novel genetic discovery.
Major finding: A meta-analysis from more than 145,000 men identified 65 novel prostate cancer susceptibility loci.
Data source: A large genomic study conducted to identify prostate cancer susceptibility loci.
Disclosures: There was no funding source disclosed. Dr. Eeles has received honoraria from Janssen and Succinct Communications. Coauthor Peter Kraft reported a relationship with Merck, but none of the other investigators had any disclosures to report.
PADI2: A potential therapeutic target in multiple myeloma?
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
FROM LEUKEMIA
Key clinical point:
Major finding: Increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS or multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib by malignant plasma cells.
Data source: Transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls.
Disclosures: The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Study finds Roux-en-Y safe, effective for older patients
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Older patients can safely lose weight after Roux-en-Y gastric bypass without excess mortality risk.
Major finding: At the end of follow-up, patients had lost a mean of 84% of their excess body weight, compared with 4.6% loss in controls. Survival was similar (90% of surgical patients and 93% of controls).
Data source: The retrospective study comprised 107 patients and 425 controls.
Disclosures: The investigator had no disclosures.
Malnutrition as a Fall Risk Factor
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
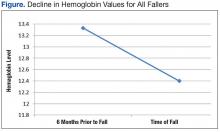
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
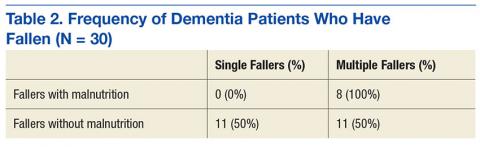
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
Group ranks TKIs according to cardiotoxicity
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
The Other Types of Diabetes: Shades of Grey
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Strength in Community
No question about it, we are entering another uncertain time in health care.
Those of us of a “certain age” may yearn for a return to the days when we surgeons faced few restrictions on our practice or our decisions. Each of us has developed his/her own perspective on the state of U.S. health care and the best path forward, most frequently shaped by our background, geographical location, and practice environment. The tendency among a great many of us has been to erect walls rather than bridges and to view our own situation as unique and more threatened than that of others. Specialists and generalists, urbanites and rural dwellers, all tend to view their own worlds as uniquely challenging.
If there was ever a need to reach out and communicate with one another and understand that the overwhelming desire of every surgeon is to provide his/her patients with the best possible care, it is now. After all, we’re all surgeons who face the same sets of challenges in the OR. There is far more in our professional lives that unites us than separates us. It is critically important that surgeons maintain open lines of communication and solidarity, no matter what other characteristics of politics or place separate us. How do we accomplish this seemingly Herculean task in the midst of the dissension and disintegration occurring all around us?
One powerful tool that can help break down barriers to honest dialogue and reinforce solidarity among surgeons is the ACS Communities. This hugely successful communication platform, which grew out of the old ACS Rural List, is now an electronic meeting place and venue for dialogue for all members of the College. The Communities is a safe place where participants, despite their many differences in specialty, location, practice type, and political views, share the common goal of improving the care available to our patients. Postings range across a wide spectrum of topics – from clinical to fiscal, social, personal and philosophical, sometimes all in the same thread. All ACS Fellows are free to sign up and post on any subject that is of concern to them, as long as their postings adhere to a baseline respect for other members. The contributions are curated by Tyler G Hughes, MD, FACS, coeditor of ACS Surgery News, but they rarely stray from the boundaries of civil discourse.
The diversity of voices on the Communities is gratifying. Midcareer surgeons, recent grads, and retired specialists all converse with each other in this space. Surgeons from different practice types and locales, including from those who work in rural critical access hospitals and those in academic medical centers, all come to discuss, ask for opinions, exchange information, and debate. These colleagues offer opinions based on long years of practice and from article of the highest quality from the literature. The whole is somehow greater than the sum of its parts. Divergent opinions add depth and breadth to any conversation and lead to a greater understanding of the entirety of a subject. I always learn something from these dialogues.
Most threads involve questions about clinical issues that have arisen in the author’s practice. One recent topic was interval cholecystectomy in which the question was raised about whether the gallbladder needs to be removed after placement of a tube cholecystostomy (usually by Interventional Radiology) for acute cholecystitis, a practice that seems to be proliferating in many areas. Over a two-week period, responses poured in from a wide variety of practice sites and types and ranged from expert opinion to references from the literature. Other threads involve less commonly encountered conditions such as coccydynia or splenic cysts or offer opinions about such “hot-button” items” as attire in the OR or music in the OR. Almost every subject is interesting and provides the reader with food for thought.
Other topics stray from the purely clinical or surgical to the health care system itself. One current ongoing and timely subject of a thread on the ACS General Surgery community is entitled “Care for the Vulnerable vs. Cash for the Powerful.” Opinions have been expressed from many perspectives and have truly been educational and enlightening. While the general public discourse has been fraught and divisive, the Communities discussions have been respectful and collegial. This basic unity underlying our diversity is the foundation of the Communities and it is a source of strength for the surgical profession.
The ACS Communities is the work of many hands and hearts over many years, and I am truly grateful to those who made it happen and to the ACS for sponsoring this great platform for communication.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Coeditor of ACS Surgery News.
No question about it, we are entering another uncertain time in health care.
Those of us of a “certain age” may yearn for a return to the days when we surgeons faced few restrictions on our practice or our decisions. Each of us has developed his/her own perspective on the state of U.S. health care and the best path forward, most frequently shaped by our background, geographical location, and practice environment. The tendency among a great many of us has been to erect walls rather than bridges and to view our own situation as unique and more threatened than that of others. Specialists and generalists, urbanites and rural dwellers, all tend to view their own worlds as uniquely challenging.
If there was ever a need to reach out and communicate with one another and understand that the overwhelming desire of every surgeon is to provide his/her patients with the best possible care, it is now. After all, we’re all surgeons who face the same sets of challenges in the OR. There is far more in our professional lives that unites us than separates us. It is critically important that surgeons maintain open lines of communication and solidarity, no matter what other characteristics of politics or place separate us. How do we accomplish this seemingly Herculean task in the midst of the dissension and disintegration occurring all around us?
One powerful tool that can help break down barriers to honest dialogue and reinforce solidarity among surgeons is the ACS Communities. This hugely successful communication platform, which grew out of the old ACS Rural List, is now an electronic meeting place and venue for dialogue for all members of the College. The Communities is a safe place where participants, despite their many differences in specialty, location, practice type, and political views, share the common goal of improving the care available to our patients. Postings range across a wide spectrum of topics – from clinical to fiscal, social, personal and philosophical, sometimes all in the same thread. All ACS Fellows are free to sign up and post on any subject that is of concern to them, as long as their postings adhere to a baseline respect for other members. The contributions are curated by Tyler G Hughes, MD, FACS, coeditor of ACS Surgery News, but they rarely stray from the boundaries of civil discourse.
The diversity of voices on the Communities is gratifying. Midcareer surgeons, recent grads, and retired specialists all converse with each other in this space. Surgeons from different practice types and locales, including from those who work in rural critical access hospitals and those in academic medical centers, all come to discuss, ask for opinions, exchange information, and debate. These colleagues offer opinions based on long years of practice and from article of the highest quality from the literature. The whole is somehow greater than the sum of its parts. Divergent opinions add depth and breadth to any conversation and lead to a greater understanding of the entirety of a subject. I always learn something from these dialogues.
Most threads involve questions about clinical issues that have arisen in the author’s practice. One recent topic was interval cholecystectomy in which the question was raised about whether the gallbladder needs to be removed after placement of a tube cholecystostomy (usually by Interventional Radiology) for acute cholecystitis, a practice that seems to be proliferating in many areas. Over a two-week period, responses poured in from a wide variety of practice sites and types and ranged from expert opinion to references from the literature. Other threads involve less commonly encountered conditions such as coccydynia or splenic cysts or offer opinions about such “hot-button” items” as attire in the OR or music in the OR. Almost every subject is interesting and provides the reader with food for thought.
Other topics stray from the purely clinical or surgical to the health care system itself. One current ongoing and timely subject of a thread on the ACS General Surgery community is entitled “Care for the Vulnerable vs. Cash for the Powerful.” Opinions have been expressed from many perspectives and have truly been educational and enlightening. While the general public discourse has been fraught and divisive, the Communities discussions have been respectful and collegial. This basic unity underlying our diversity is the foundation of the Communities and it is a source of strength for the surgical profession.
The ACS Communities is the work of many hands and hearts over many years, and I am truly grateful to those who made it happen and to the ACS for sponsoring this great platform for communication.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Coeditor of ACS Surgery News.
No question about it, we are entering another uncertain time in health care.
Those of us of a “certain age” may yearn for a return to the days when we surgeons faced few restrictions on our practice or our decisions. Each of us has developed his/her own perspective on the state of U.S. health care and the best path forward, most frequently shaped by our background, geographical location, and practice environment. The tendency among a great many of us has been to erect walls rather than bridges and to view our own situation as unique and more threatened than that of others. Specialists and generalists, urbanites and rural dwellers, all tend to view their own worlds as uniquely challenging.
If there was ever a need to reach out and communicate with one another and understand that the overwhelming desire of every surgeon is to provide his/her patients with the best possible care, it is now. After all, we’re all surgeons who face the same sets of challenges in the OR. There is far more in our professional lives that unites us than separates us. It is critically important that surgeons maintain open lines of communication and solidarity, no matter what other characteristics of politics or place separate us. How do we accomplish this seemingly Herculean task in the midst of the dissension and disintegration occurring all around us?
One powerful tool that can help break down barriers to honest dialogue and reinforce solidarity among surgeons is the ACS Communities. This hugely successful communication platform, which grew out of the old ACS Rural List, is now an electronic meeting place and venue for dialogue for all members of the College. The Communities is a safe place where participants, despite their many differences in specialty, location, practice type, and political views, share the common goal of improving the care available to our patients. Postings range across a wide spectrum of topics – from clinical to fiscal, social, personal and philosophical, sometimes all in the same thread. All ACS Fellows are free to sign up and post on any subject that is of concern to them, as long as their postings adhere to a baseline respect for other members. The contributions are curated by Tyler G Hughes, MD, FACS, coeditor of ACS Surgery News, but they rarely stray from the boundaries of civil discourse.
The diversity of voices on the Communities is gratifying. Midcareer surgeons, recent grads, and retired specialists all converse with each other in this space. Surgeons from different practice types and locales, including from those who work in rural critical access hospitals and those in academic medical centers, all come to discuss, ask for opinions, exchange information, and debate. These colleagues offer opinions based on long years of practice and from article of the highest quality from the literature. The whole is somehow greater than the sum of its parts. Divergent opinions add depth and breadth to any conversation and lead to a greater understanding of the entirety of a subject. I always learn something from these dialogues.
Most threads involve questions about clinical issues that have arisen in the author’s practice. One recent topic was interval cholecystectomy in which the question was raised about whether the gallbladder needs to be removed after placement of a tube cholecystostomy (usually by Interventional Radiology) for acute cholecystitis, a practice that seems to be proliferating in many areas. Over a two-week period, responses poured in from a wide variety of practice sites and types and ranged from expert opinion to references from the literature. Other threads involve less commonly encountered conditions such as coccydynia or splenic cysts or offer opinions about such “hot-button” items” as attire in the OR or music in the OR. Almost every subject is interesting and provides the reader with food for thought.
Other topics stray from the purely clinical or surgical to the health care system itself. One current ongoing and timely subject of a thread on the ACS General Surgery community is entitled “Care for the Vulnerable vs. Cash for the Powerful.” Opinions have been expressed from many perspectives and have truly been educational and enlightening. While the general public discourse has been fraught and divisive, the Communities discussions have been respectful and collegial. This basic unity underlying our diversity is the foundation of the Communities and it is a source of strength for the surgical profession.
The ACS Communities is the work of many hands and hearts over many years, and I am truly grateful to those who made it happen and to the ACS for sponsoring this great platform for communication.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Coeditor of ACS Surgery News.
Initiating a surgical society within the ACS: The renewed Excelsior Surgical Society
At the end of World War II, surgeons who had served during the conflict gathered at the Excelsior Hotel in Rome, Italy, to discuss their experiences. This meeting was the first of what would be called the Excelsior Surgical Society. These meetings continued annually until the death of the last World War II member, Michael E. DeBakey, MD, FACS.
Visit the society’s web page for more information and to apply for membership.
Day-long meeting
In conjunction with Clinical Congress 2016, the Excelsior Surgical Society held a day-long meeting, with nearly 200 active and retired military surgeons, residents, and students in attendance. The meeting included discussion of the following topics:
• State of the Service addresses by the three General Surgery Consultants to the Army, Navy, and Air Force Surgeon Generals:
o COL Mary Edwards, MD, FACS (Army).
o CAPT Craig Shepps, MD, FACS (Navy).
o COL Gregory York, MD, FACS (Air Force).
• The John Pryor Annual Lectureship, delivered by retired Army COL Norman M. Rich, MD, FACS, department of surgery, Uniformed Services University of the Health Sciences (USUHS) and the Walter Reed National Military Medical Center (WRNMMC), Bethesda, Md.
• The Committee on Trauma Region 13 (Military Region) annual resident paper competition.
• Abstracts on various surgical topics submitted by surgeons from multiple military health care facilities across the country.
• Panel discussions on training and sustainment for surgeons in the military.
Election of Officers
At the business meeting, the following Excelsior Surgical Society Officers were elected:
• President: CAPT Eric Elster, MD, FACS, U.S. Navy, professor and chairman, department of surgery at USUHS and WRNMMC.
• Vice-President: COL Stacy Shackelford, MD, FACS, U.S. Air Force, deputy commander for clinical services/chief of the medical staff 455th Expeditionary Medical Dental Group, Craig Joint Theater Hospital Bagram Airfield, Afghanistan.
• Secretary: COL Robert B. Lim, MD, FACS, U.S. Army, chief, metabolic and advanced laparoscopic surgery, Tripler Army Medical Center, Honolulu, Hawaii.
• Treasurer: COL Kirby R. Gross, MD, FACS, U.S. Army, director, Army Trauma Training Center, University of Miami, Fla.
• Councilperson at Large, U.S. Army: COL Matthew Martin, MD, FACS, FASMBS, trauma medical director, Madigan Army Medical Center, Tacoma, Wash.
• Councilperson at Large, U.S. Navy: CPT Gordon Wisbach, MD, FACS, staff surgeon, department of general surgery, Naval Medical Center San Diego, Calif.
• Councilperson at Large, U.S. Air Force: Col Joe DuBose, MD, FACS, vascular and trauma surgeon, Travis Air Force Base, Calif.
• Councilperson at Large, Reserve/National Guard: COL Jay A. Johannigman, MD, FACS, director of the division of trauma and surgical critical care and professor of surgery at the University of Cincinnati, Ohio.
• Honorary Member: Dr. Rich, Leonard Heaton & David Packard Professor, founding chairman, department of surgery, USUHS and WRNMMC.
At the end of World War II, surgeons who had served during the conflict gathered at the Excelsior Hotel in Rome, Italy, to discuss their experiences. This meeting was the first of what would be called the Excelsior Surgical Society. These meetings continued annually until the death of the last World War II member, Michael E. DeBakey, MD, FACS.
Visit the society’s web page for more information and to apply for membership.
Day-long meeting
In conjunction with Clinical Congress 2016, the Excelsior Surgical Society held a day-long meeting, with nearly 200 active and retired military surgeons, residents, and students in attendance. The meeting included discussion of the following topics:
• State of the Service addresses by the three General Surgery Consultants to the Army, Navy, and Air Force Surgeon Generals:
o COL Mary Edwards, MD, FACS (Army).
o CAPT Craig Shepps, MD, FACS (Navy).
o COL Gregory York, MD, FACS (Air Force).
• The John Pryor Annual Lectureship, delivered by retired Army COL Norman M. Rich, MD, FACS, department of surgery, Uniformed Services University of the Health Sciences (USUHS) and the Walter Reed National Military Medical Center (WRNMMC), Bethesda, Md.
• The Committee on Trauma Region 13 (Military Region) annual resident paper competition.
• Abstracts on various surgical topics submitted by surgeons from multiple military health care facilities across the country.
• Panel discussions on training and sustainment for surgeons in the military.
Election of Officers
At the business meeting, the following Excelsior Surgical Society Officers were elected:
• President: CAPT Eric Elster, MD, FACS, U.S. Navy, professor and chairman, department of surgery at USUHS and WRNMMC.
• Vice-President: COL Stacy Shackelford, MD, FACS, U.S. Air Force, deputy commander for clinical services/chief of the medical staff 455th Expeditionary Medical Dental Group, Craig Joint Theater Hospital Bagram Airfield, Afghanistan.
• Secretary: COL Robert B. Lim, MD, FACS, U.S. Army, chief, metabolic and advanced laparoscopic surgery, Tripler Army Medical Center, Honolulu, Hawaii.
• Treasurer: COL Kirby R. Gross, MD, FACS, U.S. Army, director, Army Trauma Training Center, University of Miami, Fla.
• Councilperson at Large, U.S. Army: COL Matthew Martin, MD, FACS, FASMBS, trauma medical director, Madigan Army Medical Center, Tacoma, Wash.
• Councilperson at Large, U.S. Navy: CPT Gordon Wisbach, MD, FACS, staff surgeon, department of general surgery, Naval Medical Center San Diego, Calif.
• Councilperson at Large, U.S. Air Force: Col Joe DuBose, MD, FACS, vascular and trauma surgeon, Travis Air Force Base, Calif.
• Councilperson at Large, Reserve/National Guard: COL Jay A. Johannigman, MD, FACS, director of the division of trauma and surgical critical care and professor of surgery at the University of Cincinnati, Ohio.
• Honorary Member: Dr. Rich, Leonard Heaton & David Packard Professor, founding chairman, department of surgery, USUHS and WRNMMC.
At the end of World War II, surgeons who had served during the conflict gathered at the Excelsior Hotel in Rome, Italy, to discuss their experiences. This meeting was the first of what would be called the Excelsior Surgical Society. These meetings continued annually until the death of the last World War II member, Michael E. DeBakey, MD, FACS.
Visit the society’s web page for more information and to apply for membership.
Day-long meeting
In conjunction with Clinical Congress 2016, the Excelsior Surgical Society held a day-long meeting, with nearly 200 active and retired military surgeons, residents, and students in attendance. The meeting included discussion of the following topics:
• State of the Service addresses by the three General Surgery Consultants to the Army, Navy, and Air Force Surgeon Generals:
o COL Mary Edwards, MD, FACS (Army).
o CAPT Craig Shepps, MD, FACS (Navy).
o COL Gregory York, MD, FACS (Air Force).
• The John Pryor Annual Lectureship, delivered by retired Army COL Norman M. Rich, MD, FACS, department of surgery, Uniformed Services University of the Health Sciences (USUHS) and the Walter Reed National Military Medical Center (WRNMMC), Bethesda, Md.
• The Committee on Trauma Region 13 (Military Region) annual resident paper competition.
• Abstracts on various surgical topics submitted by surgeons from multiple military health care facilities across the country.
• Panel discussions on training and sustainment for surgeons in the military.
Election of Officers
At the business meeting, the following Excelsior Surgical Society Officers were elected:
• President: CAPT Eric Elster, MD, FACS, U.S. Navy, professor and chairman, department of surgery at USUHS and WRNMMC.
• Vice-President: COL Stacy Shackelford, MD, FACS, U.S. Air Force, deputy commander for clinical services/chief of the medical staff 455th Expeditionary Medical Dental Group, Craig Joint Theater Hospital Bagram Airfield, Afghanistan.
• Secretary: COL Robert B. Lim, MD, FACS, U.S. Army, chief, metabolic and advanced laparoscopic surgery, Tripler Army Medical Center, Honolulu, Hawaii.
• Treasurer: COL Kirby R. Gross, MD, FACS, U.S. Army, director, Army Trauma Training Center, University of Miami, Fla.
• Councilperson at Large, U.S. Army: COL Matthew Martin, MD, FACS, FASMBS, trauma medical director, Madigan Army Medical Center, Tacoma, Wash.
• Councilperson at Large, U.S. Navy: CPT Gordon Wisbach, MD, FACS, staff surgeon, department of general surgery, Naval Medical Center San Diego, Calif.
• Councilperson at Large, U.S. Air Force: Col Joe DuBose, MD, FACS, vascular and trauma surgeon, Travis Air Force Base, Calif.
• Councilperson at Large, Reserve/National Guard: COL Jay A. Johannigman, MD, FACS, director of the division of trauma and surgical critical care and professor of surgery at the University of Cincinnati, Ohio.
• Honorary Member: Dr. Rich, Leonard Heaton & David Packard Professor, founding chairman, department of surgery, USUHS and WRNMMC.
The right choice? Surgeons, confidence, and humility
It started as an offhand comment. The patient had been on the medicine service for over a week before developing acute appendicitis with an abscess and requiring an emergency open appendectomy. He was a 68-year-old man who had longstanding medical issues that had given him many opportunities to interact with physicians in the prior few years.
On the second morning after surgery, a new team of surgical residents was rounding on him. The chief resident led the group of residents and students into the patient’s room and introduced himself as being part of the surgical team. The patient smiled and stated that he knew this was a group of surgeons. When asked why, the patient reported that he could always tell when surgeons enter the room. “You enter with an air of bravado and arrogance that the medical doctors do not exude.” The surgical residents commented on this fact to me later when I rounded on the patient, and it prompted discussion of the potential positives and negatives of confidence in surgical practice.
There is no doubt in my mind that in order to be willing to put a patient through an operation, surgeons must be confident in their skills. Surgery never achieves its benefit for patients without first causing the patient some harm. Any operation requires that the surgeon impose a violent act on the patient that, in any other context, would be illegal. In order to do such things to patients, surgeons must have a high degree of confidence.
Patients also appreciate a confident surgeon. Over the years, I have known many technically excellent surgeons who have never been as busy as they might have been because they were unable to express confidence to their patients. The opposite, however, is also true. There are surgeons who become so overconfident in their abilities that they become reckless in recommending high-risk operations to patients.
Given that patients expect their surgeons to have confidence and surgeons actually need to be confident in order to be successful, it might be surprising that the important attribute of self-confidence does not more frequently spill over into overbearing arrogance. Perhaps the most important temporizing of surgeon overconfidence is the unfortunate inevitable consequence of surgery that complications happen to even the best surgeons. We all know that the central question of the M & M conference is, “What could you have done differently?” Whether this question is answered publicly or only in the mind of the surgeon, the contemplation of the decisions made, and their consequences, is essential for each surgeon to consider in the face of every complication.
Much as the public should want surgeons to be confident, but not too confident, they should also want their surgeons to take complications seriously, but not too seriously. It is helpful for a surgeon to think about making a different choice in the future. But it would not be helpful if, in the face of a bad outcome, a surgeon decides that he or she can no longer perform surgery.
This balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications, is challenging to teach to surgical residents and fellows. Part of the challenge is that often surgical faculty do not verbalize the challenges that we face in this realm. The perfect combination of confidence and humility is something that few of us have identified in our own lives, let alone are prepared to teach it authoritatively to others. Nevertheless, teaching the next generation of surgeons to recognize the tension between confidence and humility is worthwhile. And like their elders, they may well discover that achieving the right balance is a lifelong pursuit.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
It started as an offhand comment. The patient had been on the medicine service for over a week before developing acute appendicitis with an abscess and requiring an emergency open appendectomy. He was a 68-year-old man who had longstanding medical issues that had given him many opportunities to interact with physicians in the prior few years.
On the second morning after surgery, a new team of surgical residents was rounding on him. The chief resident led the group of residents and students into the patient’s room and introduced himself as being part of the surgical team. The patient smiled and stated that he knew this was a group of surgeons. When asked why, the patient reported that he could always tell when surgeons enter the room. “You enter with an air of bravado and arrogance that the medical doctors do not exude.” The surgical residents commented on this fact to me later when I rounded on the patient, and it prompted discussion of the potential positives and negatives of confidence in surgical practice.
There is no doubt in my mind that in order to be willing to put a patient through an operation, surgeons must be confident in their skills. Surgery never achieves its benefit for patients without first causing the patient some harm. Any operation requires that the surgeon impose a violent act on the patient that, in any other context, would be illegal. In order to do such things to patients, surgeons must have a high degree of confidence.
Patients also appreciate a confident surgeon. Over the years, I have known many technically excellent surgeons who have never been as busy as they might have been because they were unable to express confidence to their patients. The opposite, however, is also true. There are surgeons who become so overconfident in their abilities that they become reckless in recommending high-risk operations to patients.
Given that patients expect their surgeons to have confidence and surgeons actually need to be confident in order to be successful, it might be surprising that the important attribute of self-confidence does not more frequently spill over into overbearing arrogance. Perhaps the most important temporizing of surgeon overconfidence is the unfortunate inevitable consequence of surgery that complications happen to even the best surgeons. We all know that the central question of the M & M conference is, “What could you have done differently?” Whether this question is answered publicly or only in the mind of the surgeon, the contemplation of the decisions made, and their consequences, is essential for each surgeon to consider in the face of every complication.
Much as the public should want surgeons to be confident, but not too confident, they should also want their surgeons to take complications seriously, but not too seriously. It is helpful for a surgeon to think about making a different choice in the future. But it would not be helpful if, in the face of a bad outcome, a surgeon decides that he or she can no longer perform surgery.
This balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications, is challenging to teach to surgical residents and fellows. Part of the challenge is that often surgical faculty do not verbalize the challenges that we face in this realm. The perfect combination of confidence and humility is something that few of us have identified in our own lives, let alone are prepared to teach it authoritatively to others. Nevertheless, teaching the next generation of surgeons to recognize the tension between confidence and humility is worthwhile. And like their elders, they may well discover that achieving the right balance is a lifelong pursuit.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
It started as an offhand comment. The patient had been on the medicine service for over a week before developing acute appendicitis with an abscess and requiring an emergency open appendectomy. He was a 68-year-old man who had longstanding medical issues that had given him many opportunities to interact with physicians in the prior few years.
On the second morning after surgery, a new team of surgical residents was rounding on him. The chief resident led the group of residents and students into the patient’s room and introduced himself as being part of the surgical team. The patient smiled and stated that he knew this was a group of surgeons. When asked why, the patient reported that he could always tell when surgeons enter the room. “You enter with an air of bravado and arrogance that the medical doctors do not exude.” The surgical residents commented on this fact to me later when I rounded on the patient, and it prompted discussion of the potential positives and negatives of confidence in surgical practice.
There is no doubt in my mind that in order to be willing to put a patient through an operation, surgeons must be confident in their skills. Surgery never achieves its benefit for patients without first causing the patient some harm. Any operation requires that the surgeon impose a violent act on the patient that, in any other context, would be illegal. In order to do such things to patients, surgeons must have a high degree of confidence.
Patients also appreciate a confident surgeon. Over the years, I have known many technically excellent surgeons who have never been as busy as they might have been because they were unable to express confidence to their patients. The opposite, however, is also true. There are surgeons who become so overconfident in their abilities that they become reckless in recommending high-risk operations to patients.
Given that patients expect their surgeons to have confidence and surgeons actually need to be confident in order to be successful, it might be surprising that the important attribute of self-confidence does not more frequently spill over into overbearing arrogance. Perhaps the most important temporizing of surgeon overconfidence is the unfortunate inevitable consequence of surgery that complications happen to even the best surgeons. We all know that the central question of the M & M conference is, “What could you have done differently?” Whether this question is answered publicly or only in the mind of the surgeon, the contemplation of the decisions made, and their consequences, is essential for each surgeon to consider in the face of every complication.
Much as the public should want surgeons to be confident, but not too confident, they should also want their surgeons to take complications seriously, but not too seriously. It is helpful for a surgeon to think about making a different choice in the future. But it would not be helpful if, in the face of a bad outcome, a surgeon decides that he or she can no longer perform surgery.
This balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications, is challenging to teach to surgical residents and fellows. Part of the challenge is that often surgical faculty do not verbalize the challenges that we face in this realm. The perfect combination of confidence and humility is something that few of us have identified in our own lives, let alone are prepared to teach it authoritatively to others. Nevertheless, teaching the next generation of surgeons to recognize the tension between confidence and humility is worthwhile. And like their elders, they may well discover that achieving the right balance is a lifelong pursuit.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.