User login
Clinical benefit persists for some with mRCC after stopping immune checkpoint blockade
ORLANDO – Some people with advanced kidney cancer who respond to immune checkpoint inhibitor therapy and subsequently stop because of immune-related adverse events may continue to see a clinical benefit for 6 months or longer, a retrospective, multicenter study reveals.
In fact, investigators labeled 42% of 19 patients with metastatic renal cell carcinoma (mRCC) “durable responders” to checkpoint inhibitor blockade. “What we’ve demonstrated is that, despite patients stopping their treatment, there is a subset who continue to have disease in check and controlled despite [their] not being on any therapy,” Rana R. McKay, MD, of the University of California, San Diego, said.
“Our subset was small, only 19 patients, so the next step is to validate our findings in larger study, and actually conduct a prospective trial to assess if discontinuation of therapy is worthwhile to investigate in this population,” Dr. McKay said during a press briefing prior to the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
PD-1/PDL-1 inhibitors demonstrate efficacy against an expanding list of malignancies, Dr. McKay said. The current standard is to administer these agents on a continuous basis until cancer progression or toxicity occurs. However, the study raises the possibility of intentionally discontinuing their use in some patients in the future, primarily because PD-1/PD-L1 inhibitors are associated with a wide range of side effects. Most concerning are immune-related adverse events “which are thought to be due to immune system activation,” she said.
“These drugs work to reinvigorate the immune response, and one of the unintended consequences is … they may also elicit an autoimmune response against one or more organs in the body,” said Sumanta Pal, MD, of City of Hope Medical Center in Duarte, Calif. and moderator of the press briefing.
“There was a wide spectrum of adverse events affecting different organ systems,” Dr. McKay said, “including pneumonitis, myositis, nephritis, hepatitis, pericarditis, and myocarditis, just to name a few.” A total of 84% of patients required steroids to treat immune-related adverse events, 11% needed additional immunosuppressant agents to treat their symptoms, and 53% have ongoing toxicity.
“If a patient has immune-related side effects, the impact can be serious,” Dr. Pal said. “This [study] certainly supports the premise that those individuals who experience immune related side effects could have a tangible benefit from the drug nonetheless.”
Median patient age was 68 years, 74% were male and 26% had aggressive disease. In the durable responders group, the median time on treatment was 11 months and median time off treatment was 20 months. In contrast, the patients whose cancer worsened immediately after therapy cessation were treated a median 4 months and were off treatment a median of only 2 months. A total of 63% received either PD-1 or PD-L1 monotherapy and the remainder received one of these inhibitors in combination with other systemic therapies.
Prospective studies are warranted to determine approaches to customize immunotherapy based on response, Dr. McKay said. A phase II study is planned to assess optimized management of nivolumab therapy based on response in patients with mRCC, she added
ORLANDO – Some people with advanced kidney cancer who respond to immune checkpoint inhibitor therapy and subsequently stop because of immune-related adverse events may continue to see a clinical benefit for 6 months or longer, a retrospective, multicenter study reveals.
In fact, investigators labeled 42% of 19 patients with metastatic renal cell carcinoma (mRCC) “durable responders” to checkpoint inhibitor blockade. “What we’ve demonstrated is that, despite patients stopping their treatment, there is a subset who continue to have disease in check and controlled despite [their] not being on any therapy,” Rana R. McKay, MD, of the University of California, San Diego, said.
“Our subset was small, only 19 patients, so the next step is to validate our findings in larger study, and actually conduct a prospective trial to assess if discontinuation of therapy is worthwhile to investigate in this population,” Dr. McKay said during a press briefing prior to the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
PD-1/PDL-1 inhibitors demonstrate efficacy against an expanding list of malignancies, Dr. McKay said. The current standard is to administer these agents on a continuous basis until cancer progression or toxicity occurs. However, the study raises the possibility of intentionally discontinuing their use in some patients in the future, primarily because PD-1/PD-L1 inhibitors are associated with a wide range of side effects. Most concerning are immune-related adverse events “which are thought to be due to immune system activation,” she said.
“These drugs work to reinvigorate the immune response, and one of the unintended consequences is … they may also elicit an autoimmune response against one or more organs in the body,” said Sumanta Pal, MD, of City of Hope Medical Center in Duarte, Calif. and moderator of the press briefing.
“There was a wide spectrum of adverse events affecting different organ systems,” Dr. McKay said, “including pneumonitis, myositis, nephritis, hepatitis, pericarditis, and myocarditis, just to name a few.” A total of 84% of patients required steroids to treat immune-related adverse events, 11% needed additional immunosuppressant agents to treat their symptoms, and 53% have ongoing toxicity.
“If a patient has immune-related side effects, the impact can be serious,” Dr. Pal said. “This [study] certainly supports the premise that those individuals who experience immune related side effects could have a tangible benefit from the drug nonetheless.”
Median patient age was 68 years, 74% were male and 26% had aggressive disease. In the durable responders group, the median time on treatment was 11 months and median time off treatment was 20 months. In contrast, the patients whose cancer worsened immediately after therapy cessation were treated a median 4 months and were off treatment a median of only 2 months. A total of 63% received either PD-1 or PD-L1 monotherapy and the remainder received one of these inhibitors in combination with other systemic therapies.
Prospective studies are warranted to determine approaches to customize immunotherapy based on response, Dr. McKay said. A phase II study is planned to assess optimized management of nivolumab therapy based on response in patients with mRCC, she added
ORLANDO – Some people with advanced kidney cancer who respond to immune checkpoint inhibitor therapy and subsequently stop because of immune-related adverse events may continue to see a clinical benefit for 6 months or longer, a retrospective, multicenter study reveals.
In fact, investigators labeled 42% of 19 patients with metastatic renal cell carcinoma (mRCC) “durable responders” to checkpoint inhibitor blockade. “What we’ve demonstrated is that, despite patients stopping their treatment, there is a subset who continue to have disease in check and controlled despite [their] not being on any therapy,” Rana R. McKay, MD, of the University of California, San Diego, said.
“Our subset was small, only 19 patients, so the next step is to validate our findings in larger study, and actually conduct a prospective trial to assess if discontinuation of therapy is worthwhile to investigate in this population,” Dr. McKay said during a press briefing prior to the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
PD-1/PDL-1 inhibitors demonstrate efficacy against an expanding list of malignancies, Dr. McKay said. The current standard is to administer these agents on a continuous basis until cancer progression or toxicity occurs. However, the study raises the possibility of intentionally discontinuing their use in some patients in the future, primarily because PD-1/PD-L1 inhibitors are associated with a wide range of side effects. Most concerning are immune-related adverse events “which are thought to be due to immune system activation,” she said.
“These drugs work to reinvigorate the immune response, and one of the unintended consequences is … they may also elicit an autoimmune response against one or more organs in the body,” said Sumanta Pal, MD, of City of Hope Medical Center in Duarte, Calif. and moderator of the press briefing.
“There was a wide spectrum of adverse events affecting different organ systems,” Dr. McKay said, “including pneumonitis, myositis, nephritis, hepatitis, pericarditis, and myocarditis, just to name a few.” A total of 84% of patients required steroids to treat immune-related adverse events, 11% needed additional immunosuppressant agents to treat their symptoms, and 53% have ongoing toxicity.
“If a patient has immune-related side effects, the impact can be serious,” Dr. Pal said. “This [study] certainly supports the premise that those individuals who experience immune related side effects could have a tangible benefit from the drug nonetheless.”
Median patient age was 68 years, 74% were male and 26% had aggressive disease. In the durable responders group, the median time on treatment was 11 months and median time off treatment was 20 months. In contrast, the patients whose cancer worsened immediately after therapy cessation were treated a median 4 months and were off treatment a median of only 2 months. A total of 63% received either PD-1 or PD-L1 monotherapy and the remainder received one of these inhibitors in combination with other systemic therapies.
Prospective studies are warranted to determine approaches to customize immunotherapy based on response, Dr. McKay said. A phase II study is planned to assess optimized management of nivolumab therapy based on response in patients with mRCC, she added
Key clinical point: A subset of patients with metastatic renal cell carcinoma see a durable benefit after stopping therapy with immune checkpoint inhibitors due to immune related adverse events.
Major finding: Just over 40% of patients experienced a durable response to therapy of 6 months or longer after stopping therapy with an immune checkpoint inhibitor.
Data source: Retrospective study of 19 patients conducted at five academic medical centers.
Disclosures: The Dana-Farber/Harvard Cancer Center Kidney SPORE, and the Trust Family, Michael Brigham, and Loker Pin funded this study. Rana R. McKay, MD, receives institutional research funding from Pfizer and Bayer.
CMS proposal seeks to stabilize individual insurance market
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
Opinions vary considerably on withdrawing drugs in clinically inactive JIA
A wide range of attitudes and practices for the process of withdrawing medications in pediatric patients with clinically inactive juvenile idiopathic arthritis (JIA) exist among clinician members of the Childhood Arthritis and Rheumatology Research Alliance (CARRA), according to findings from an anonymous survey.
The cross-sectional, electronic survey found that respondents varied in the amount of time they thought was necessary to spend in clinically inactive disease before beginning withdrawal of medications and in the amount of time to spend during tapering or stopping medications, for both methotrexate and biologics.
To better understand how clinicians care for patients with clinically inactive disease, the investigators emailed the survey to 388 clinician members of the CARRA in the United States and Canada over a 4-week period during November-December 2015 (J Rheumatol. 2017 Feb 1. doi: 10.3899/jrheum.161078).
The survey, which the investigators thought to be “the first comprehensive evaluation of influential factors and approaches for the clinical management of children with clinically inactive JIA,” did not include questions about systemic JIA, inflammatory bowel disease, psoriasis, and uveitis in order “to simplify responses and encourage participation, because in practice, the manifestations and outcomes of these diseases could substantially influence treatment decisions for children with JIA.”
They received complete responses from 124 of the 132 clinicians who responded to the survey email. Of the 121 respondents who reported taking clinical care of patients with JIA, 87% were physicians, and the same number reported taking care of pediatric patients only. About three-quarters spent half of their professional time in clinical care, and about half had more than 10 years of post-training clinical experience.
When deciding about withdrawing JIA medications, more than one-half of respondents said that the time that a patient spent in clinically inactive disease and a history of drug toxicity are very important factors. Most participants ranked those two factors most highly and most often among their top five factors for decision making.
Respondents also commonly ranked these factors as important:
- JIA duration before attaining clinically inactive disease.
- Patient/family preferences.
- Presence of JIA-related damage.
- JIA category.
The factors that consistently appeared in responses fit into three clusters that included JIA features and time spent in clinically inactive disease (JIA category and total disease duration), JIA severity and resistance to treatment (disease duration before clinically inactive disease, number of drugs needed to attain inactivity, joint damage, and a history of sacroiliac or temporomandibular disease), and the patient’s experience (drug toxicity and family preference).
The respondents indicated that they would be least likely to stop medications for children with rheumatoid factor (RF)–positive polyarthritis (85%), which is “consistent with prior studies showing that RF-positive polyarthritis is associated with higher rates of flares than other JIA categories,” the investigators wrote. However, respondents said they would be most likely to stop medications for children with persistent oligoarthritis (87%) “even though rates of flares in this category appear similar to other JIA types. This method may reflect a belief that flares in children with persistent oligoarticular JIA will be less severe and easier to control.”
When patients met all criteria for clinically inactive disease for a “sufficient amount of time” and families were interested in stopping medications, some factors continued to make respondents reluctant to withdraw medications. These factors were most often a history of erosions (81%), asymptomatic joint abnormalities on ultrasound or MRI (72%), and failure of multiple prior disease-modifying antirheumatic drugs or biologics to control disease (64%). The definition of clinically inactive disease is a composite of no active arthritis, uveitis, or systemic JIA symptoms; the best possible clinical global assessment; inflammatory markers normal or elevated for reasons other than JIA; and no more than 15 minutes of joint stiffness.
A little over half of respondents said they would wait until clinically inactive disease had lasted 12 months before considering stopping or tapering methotrexate or biologic monotherapy, but a substantial minority said they would wait for only 6 months for methotrexate (31%) or biologic monotherapy (23%). A smaller number would wait for 18 months for methotrexate (13%) or biologics (18%), and another 3%-5% said they could not give a time frame.
The strategies varied for how actual withdrawing of medications occurred. Most methotrexate monotherapy withdrawals involved tapering over 2-6 months, one-third over longer periods, and the fewest reported tapering for less than 2 months (7%) or immediate withdrawal (17%).
Withdrawal of biologics was generally said to occur more gradually than with methotrexate, with one-third of respondents citing over 2-6 months, a quarter more slowly, and another 29% in less than 2 months or immediately. Some wrote that they preferred spacing out the interval between doses, but none decreased the dose. When children took combination therapy with methotrexate plus a biologic, 63% said that they began tapering or stopping methotrexate first, but a quarter said that the order was strongly context dependent, and the most commonly cited reason for deciding was history of toxicity or intolerance.
Imaging played a role in less than half of the decisions to withdraw medications, with it being used often by 9% and sometimes by 36%. And while it’s assumed that patients and family consideration played an important role in decision making, only 25% of respondents reported using specific patient-reported outcomes in deciding to withdraw medications.
The study was funded by grants from Rutgers Biomedical and Health Sciences and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
A wide range of attitudes and practices for the process of withdrawing medications in pediatric patients with clinically inactive juvenile idiopathic arthritis (JIA) exist among clinician members of the Childhood Arthritis and Rheumatology Research Alliance (CARRA), according to findings from an anonymous survey.
The cross-sectional, electronic survey found that respondents varied in the amount of time they thought was necessary to spend in clinically inactive disease before beginning withdrawal of medications and in the amount of time to spend during tapering or stopping medications, for both methotrexate and biologics.
To better understand how clinicians care for patients with clinically inactive disease, the investigators emailed the survey to 388 clinician members of the CARRA in the United States and Canada over a 4-week period during November-December 2015 (J Rheumatol. 2017 Feb 1. doi: 10.3899/jrheum.161078).
The survey, which the investigators thought to be “the first comprehensive evaluation of influential factors and approaches for the clinical management of children with clinically inactive JIA,” did not include questions about systemic JIA, inflammatory bowel disease, psoriasis, and uveitis in order “to simplify responses and encourage participation, because in practice, the manifestations and outcomes of these diseases could substantially influence treatment decisions for children with JIA.”
They received complete responses from 124 of the 132 clinicians who responded to the survey email. Of the 121 respondents who reported taking clinical care of patients with JIA, 87% were physicians, and the same number reported taking care of pediatric patients only. About three-quarters spent half of their professional time in clinical care, and about half had more than 10 years of post-training clinical experience.
When deciding about withdrawing JIA medications, more than one-half of respondents said that the time that a patient spent in clinically inactive disease and a history of drug toxicity are very important factors. Most participants ranked those two factors most highly and most often among their top five factors for decision making.
Respondents also commonly ranked these factors as important:
- JIA duration before attaining clinically inactive disease.
- Patient/family preferences.
- Presence of JIA-related damage.
- JIA category.
The factors that consistently appeared in responses fit into three clusters that included JIA features and time spent in clinically inactive disease (JIA category and total disease duration), JIA severity and resistance to treatment (disease duration before clinically inactive disease, number of drugs needed to attain inactivity, joint damage, and a history of sacroiliac or temporomandibular disease), and the patient’s experience (drug toxicity and family preference).
The respondents indicated that they would be least likely to stop medications for children with rheumatoid factor (RF)–positive polyarthritis (85%), which is “consistent with prior studies showing that RF-positive polyarthritis is associated with higher rates of flares than other JIA categories,” the investigators wrote. However, respondents said they would be most likely to stop medications for children with persistent oligoarthritis (87%) “even though rates of flares in this category appear similar to other JIA types. This method may reflect a belief that flares in children with persistent oligoarticular JIA will be less severe and easier to control.”
When patients met all criteria for clinically inactive disease for a “sufficient amount of time” and families were interested in stopping medications, some factors continued to make respondents reluctant to withdraw medications. These factors were most often a history of erosions (81%), asymptomatic joint abnormalities on ultrasound or MRI (72%), and failure of multiple prior disease-modifying antirheumatic drugs or biologics to control disease (64%). The definition of clinically inactive disease is a composite of no active arthritis, uveitis, or systemic JIA symptoms; the best possible clinical global assessment; inflammatory markers normal or elevated for reasons other than JIA; and no more than 15 minutes of joint stiffness.
A little over half of respondents said they would wait until clinically inactive disease had lasted 12 months before considering stopping or tapering methotrexate or biologic monotherapy, but a substantial minority said they would wait for only 6 months for methotrexate (31%) or biologic monotherapy (23%). A smaller number would wait for 18 months for methotrexate (13%) or biologics (18%), and another 3%-5% said they could not give a time frame.
The strategies varied for how actual withdrawing of medications occurred. Most methotrexate monotherapy withdrawals involved tapering over 2-6 months, one-third over longer periods, and the fewest reported tapering for less than 2 months (7%) or immediate withdrawal (17%).
Withdrawal of biologics was generally said to occur more gradually than with methotrexate, with one-third of respondents citing over 2-6 months, a quarter more slowly, and another 29% in less than 2 months or immediately. Some wrote that they preferred spacing out the interval between doses, but none decreased the dose. When children took combination therapy with methotrexate plus a biologic, 63% said that they began tapering or stopping methotrexate first, but a quarter said that the order was strongly context dependent, and the most commonly cited reason for deciding was history of toxicity or intolerance.
Imaging played a role in less than half of the decisions to withdraw medications, with it being used often by 9% and sometimes by 36%. And while it’s assumed that patients and family consideration played an important role in decision making, only 25% of respondents reported using specific patient-reported outcomes in deciding to withdraw medications.
The study was funded by grants from Rutgers Biomedical and Health Sciences and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
A wide range of attitudes and practices for the process of withdrawing medications in pediatric patients with clinically inactive juvenile idiopathic arthritis (JIA) exist among clinician members of the Childhood Arthritis and Rheumatology Research Alliance (CARRA), according to findings from an anonymous survey.
The cross-sectional, electronic survey found that respondents varied in the amount of time they thought was necessary to spend in clinically inactive disease before beginning withdrawal of medications and in the amount of time to spend during tapering or stopping medications, for both methotrexate and biologics.
To better understand how clinicians care for patients with clinically inactive disease, the investigators emailed the survey to 388 clinician members of the CARRA in the United States and Canada over a 4-week period during November-December 2015 (J Rheumatol. 2017 Feb 1. doi: 10.3899/jrheum.161078).
The survey, which the investigators thought to be “the first comprehensive evaluation of influential factors and approaches for the clinical management of children with clinically inactive JIA,” did not include questions about systemic JIA, inflammatory bowel disease, psoriasis, and uveitis in order “to simplify responses and encourage participation, because in practice, the manifestations and outcomes of these diseases could substantially influence treatment decisions for children with JIA.”
They received complete responses from 124 of the 132 clinicians who responded to the survey email. Of the 121 respondents who reported taking clinical care of patients with JIA, 87% were physicians, and the same number reported taking care of pediatric patients only. About three-quarters spent half of their professional time in clinical care, and about half had more than 10 years of post-training clinical experience.
When deciding about withdrawing JIA medications, more than one-half of respondents said that the time that a patient spent in clinically inactive disease and a history of drug toxicity are very important factors. Most participants ranked those two factors most highly and most often among their top five factors for decision making.
Respondents also commonly ranked these factors as important:
- JIA duration before attaining clinically inactive disease.
- Patient/family preferences.
- Presence of JIA-related damage.
- JIA category.
The factors that consistently appeared in responses fit into three clusters that included JIA features and time spent in clinically inactive disease (JIA category and total disease duration), JIA severity and resistance to treatment (disease duration before clinically inactive disease, number of drugs needed to attain inactivity, joint damage, and a history of sacroiliac or temporomandibular disease), and the patient’s experience (drug toxicity and family preference).
The respondents indicated that they would be least likely to stop medications for children with rheumatoid factor (RF)–positive polyarthritis (85%), which is “consistent with prior studies showing that RF-positive polyarthritis is associated with higher rates of flares than other JIA categories,” the investigators wrote. However, respondents said they would be most likely to stop medications for children with persistent oligoarthritis (87%) “even though rates of flares in this category appear similar to other JIA types. This method may reflect a belief that flares in children with persistent oligoarticular JIA will be less severe and easier to control.”
When patients met all criteria for clinically inactive disease for a “sufficient amount of time” and families were interested in stopping medications, some factors continued to make respondents reluctant to withdraw medications. These factors were most often a history of erosions (81%), asymptomatic joint abnormalities on ultrasound or MRI (72%), and failure of multiple prior disease-modifying antirheumatic drugs or biologics to control disease (64%). The definition of clinically inactive disease is a composite of no active arthritis, uveitis, or systemic JIA symptoms; the best possible clinical global assessment; inflammatory markers normal or elevated for reasons other than JIA; and no more than 15 minutes of joint stiffness.
A little over half of respondents said they would wait until clinically inactive disease had lasted 12 months before considering stopping or tapering methotrexate or biologic monotherapy, but a substantial minority said they would wait for only 6 months for methotrexate (31%) or biologic monotherapy (23%). A smaller number would wait for 18 months for methotrexate (13%) or biologics (18%), and another 3%-5% said they could not give a time frame.
The strategies varied for how actual withdrawing of medications occurred. Most methotrexate monotherapy withdrawals involved tapering over 2-6 months, one-third over longer periods, and the fewest reported tapering for less than 2 months (7%) or immediate withdrawal (17%).
Withdrawal of biologics was generally said to occur more gradually than with methotrexate, with one-third of respondents citing over 2-6 months, a quarter more slowly, and another 29% in less than 2 months or immediately. Some wrote that they preferred spacing out the interval between doses, but none decreased the dose. When children took combination therapy with methotrexate plus a biologic, 63% said that they began tapering or stopping methotrexate first, but a quarter said that the order was strongly context dependent, and the most commonly cited reason for deciding was history of toxicity or intolerance.
Imaging played a role in less than half of the decisions to withdraw medications, with it being used often by 9% and sometimes by 36%. And while it’s assumed that patients and family consideration played an important role in decision making, only 25% of respondents reported using specific patient-reported outcomes in deciding to withdraw medications.
The study was funded by grants from Rutgers Biomedical and Health Sciences and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Key clinical point:
Major finding: A little over half of respondents said they would wait until clinically inactive disease had lasted 12 months before considering stopping or tapering methotrexate or biologic monotherapy.
Data source: A survey of 121 of 388 CARRA members involved in clinical care of JIA patients.
Disclosures: The study was funded by grants from Rutgers Biomedical and Health Sciences and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Bundled payment for gastrointestinal hemorrhage
The Medicare Access and Chips Reauthorization Act (MACRA) is now law; it passed with bipartisan, virtually unanimous support in both chambers of Congress. MACRA replaced the Sustainable Growth Rate formula for physician reimbursement and replaced it with a pathway to value-based payment. This law will alter our practices more than the Affordable Care Act and to an extent not seen since the passage of the original Medicare Act. Practices that continue to hang on to our traditional colonoscopy-based fee-for-service reimbursement model will increasingly be marginalized (or discounted) by Medicare, commercial payers, and regional health systems. To thrive in the coming decade, innovative practices will move toward alternative payment models. Many practices have risk-linked bundled payments for colonoscopy, but this step is only for the interim. Long-term success will come to practices that understand the implications of episode payments, specialty medical homes, and total cost of care. Do not wait for the finances to magically appear – start now to build infrastructure. In this month’s article, Dr. Mehta provides a detailed description of how a practice might construct a bundled payment for a common inpatient disorder. No one is paying for this yet, but it will come. Now is not the time to be a “WIMP” (Gastroenterology. 2016;150:295-9).
John I. Allen, MD, MBA, AGAF
Editor in Chief
In January 2016, the Centers for Medicare & Medicaid Services (CMS) launched the Comprehensive Care for Joint Replacement (CJR) model. This payment model aims to improve the value of care provided to Medicare beneficiaries for hip and knee replacement surgery during the inpatient stay and 90-day period after discharge by holding hospitals accountable for cost and quality.1 It includes hospitals in 67 geographic areas across the United States and marks the first time that a postacute bundled payment model is mandatory for traditional Medicare patients. Although this may not seem to be relevant for gastroenterology, it marks an important signal by CMS that there will likely be more episode-payment models in the future.
Gastroenterologists have not been primary drivers or participants in these models, but gastrointestinal hemorrhage is included as 1 of the 48 clinical conditions for the postacute bundled payment program. In addition, CMS recently announced that clinical episode-based payment for GI hemorrhage will be included in hospital inpatient quality reporting (IQR) for fiscal year 2019.4 This is an opportunity for the field of gastroenterology to take a leadership role in an alternate payment model as it has for colonoscopy bundled payment,5 but it requires an understanding of the history of postacute bundled payments and the opportunities for and challenges to applying this model to GI hemorrhage. In this article, I will describe insights from our health system’s experience in evaluating different postacute bundled payment programs and participating in a GI bundled payment program.
Inpatient and postacute bundled payments
A bundled payment refers to a situation in which hospitals and physicians are incentivized to coordinate care for an episode of care across the continuum and eliminate unnecessary spending. In 1983, Medicare initiated a type of bundled payment for Part A spending on inpatient hospital care by creating prospective payment that is based on diagnosis-related groups (DRGs). This was a response to the rising cost of inpatient care resulting from retrospective payment that is based on hospital charges. Because hospitals would get paid the same amount for similar conditions, it resulted in shortened length of stay and reduction in the rise of inpatient costs, along with no measurable impact on quality of care.6 This was followed by prospective payment for outpatient hospital fees and skilled nursing facility (SNF) care as a result of the Balanced Budget Act of 1997. Medicare built on this by bundling physician and hospital fees through demonstration projects in coronary artery bypass graft surgery from 1991 to 1996 and orthopedic and cardiovascular surgery from 2009 to 2012, both resulting in reduced costs and no measurable impact on quality.
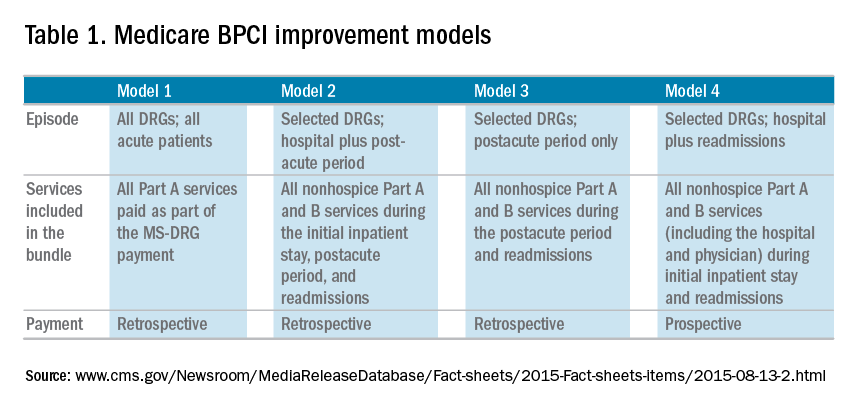
The Bundled Payment for Care Improvement (BPCI) program built on these results in 2013 by expanding to include Part A and B services rendered up to 90 days after discharge, and as of January 2016, it includes 1,574 participants across the country. On a voluntary basis, hospitals, physician groups, and postacute providers and conveners were able to participate in 1 of 4 bundled payment models that were anchored on an inpatient for any of 48 clinical conditions that were based on MS-DRG (Table 1).
• Model 1 defined the episode as the inpatient hospital stay and bundled the facility and physician fees, similar to prior demonstration projects.
• Model 2 is a retrospective bundled payment for Part A and B services in the inpatient hospital stay and up to 90 days after discharge.
• Model 3 is a retrospective model that starts after hospital discharge and includes up to 90 days. (Models 1-3 maintain the current payment structure and retrospectively compare the actual reimbursement with target values that are based on historical data for that hospital with a 2%-3% payment reduction.)
• Model 4 makes a single, prospectively determined global payment to a hospital that encompasses all services during the hospital stay.
Opportunities in inpatient and postacute bundled payments
Participation in bundled payments requires a new set of analytic and organizational capabilities.
• The first step is to identify the patient population on the basis of inclusion and exclusion criteria and to measure the current cost of care through external claims data and internal hospital data. This includes payments for hospital inpatient services, physician fees, postacute care, readmissions, other Part B services, and home health services. The biggest opportunity for postacute bundles is shifting site of service from postacute care to lower-cost settings and reducing readmission rates.
• Subsequently, they need to identify areas of opportunity to reduce expenditure, while also demonstrating consistent or improved quality and outcomes.
• On the basis of this, the team can identify variation in care within the cohort and in comparison with benchmarks across the country.
• After identifying areas of opportunity, the team needs to develop strategies to improve value such as care pathways, information technology tools, care coordination, and remote services.
Of the 48 clinical conditions in BPCI, 4 could be described as related to GI: esophagitis, gastroenteritis, and other digestive disorders (Medicare Severity–Diagnosis Related Group [MS-DRG] 391, 392); gastrointestinal hemorrhage (MS-DRG 377, 378, 379); gastrointestinal obstruction (MS-DRG 388, 389, 390); and major bowel procedure (MS-DRG 329, 330, 331). After evaluating the GI bundles, it was apparent that these were created for billing purposes and were not clinically intuitive, which is why our institution immediately excluded the broad category of esophagitis, gastroenteritis, and other digestive disorders. GI obstruction and major bowel surgery relate to the care of gastroenterologists, but surgeons are typically primary drivers of care for these patients. Thus, we believed that GI hemorrhage was most appropriate because gastroenterologists drive care for this condition, and there is substantial evidence about established guidelines and pathways during this episode.
Bundled payment for gastrointestinal hemorrhage
We built a multidisciplinary team of physicians, data analysts, clinical documentation specialists, and care managers to start developing a plan for improving the value of care in this population. This included data about readmissions and site of postacute care for this population, which were supplemented by chart review of financial outliers and readmissions. We quickly learned about some of the challenges to medical bundles and the GI hemorrhage bundle in particular. It is difficult to identify these patients early in the hospital stay because inclusion is based on a billing code. Many of these patients also have cardiovascular disease, cancer, or cirrhosis, which makes it hard to identify which patients will end up with primary GI hemorrhage coding until after the patient is discharged. They are also on many different inpatient services; in our hospital, there were at least 12 different admitting services. In addition, almost one-third of the patients actually had an admission before this hospitalization, often for different clinical conditions.
Most importantly, it was very challenging to develop protocols to improve the value of care in this population. Most of the patients had many comorbid conditions, so a GI hemorrhage pathway alone would not be sufficient to alter care. The two main areas of opportunity for cost savings in postacute bundled payments are postacute site of service and readmissions, both of which are hard to change for medical GI patients. For medical patients, they have many comorbidities before admission, so postacute site of service is typically driven by which site they were admitted from. This is different from surgical patients who are in SNF or rehabilitation facilities for limited time frames, and there may be more discretion to shift to lower cost settings. In addition, readmissions have not been studied much in GI hemorrhage, so it is not clear how to improve them. On the basis of these factors and the limited sample size for this condition, our health system opted to stop taking financial risk for this population.
Future opportunities for gastroenterology
However, the latest CMS Inpatient Prospective Payment System rule describes the implementation of a new quality metric for hospital IQR called the Gastrointestinal Hemorrhage Clinical Episode-Based Payment. This would hold hospitals accountable for the cost of care for GI hemorrhage admissions plus the 90 days after discharge, similar to model 2 of BPCI. This announcement, as well as the launch of mandatory orthopedic bundles, demonstrates that hospital reimbursement is shifting toward an expansion of bundled payments to include the postacute time frame. This is manifested in postacute bundles, episode-based payment, and readmission penalties. This reignited our GI hemorrhage episode team’s efforts, but with a broader purpose.
Gastroenterologists can take a leadership role in responding to episode-based payments as a way for us to demonstrate value in our collaboration with hospitals, health systems, and payers. The focus on cardiovascular disease as part of readmission penalties and core measures has allowed our cardiology colleagues to partner closely with service lines, learn about episode-based care, and garner resources to build and lead disease and episode teams. Because patients do not fit into the different clinical areas in mutually exclusive categories, we will need to collaborate with other specialties to care for the overlap with other conditions. Many heart failure and myocardial infarction patients will get readmitted for GI hemorrhage, and many GI hemorrhage patients will have concomitant cardiovascular disease or cancer. This suggests that future strategies need to integrate efforts of service lines and that there is greater opportunity for gastroenterologists than just the GI bundles.
Gastroenterologists should also participate in a proactive way. Any new payment mechanism will have some flaws in implementation, so it is more important to do what is right from a clinical standpoint rather than focusing too much on the specific billing code or payment model. These models are evolving, and we have an opportunity to have impact on future implementation. This starts with identifying and including patients from a clinical perspective rather than focusing on specific insurance types that participate in bundled payments. Some examples to improve the value of care in GI hemorrhage include creating evidence-based care pathways that span the episode of care, structured documentation after endoscopy for risk stratification, integrating pathways into the workflow of providers through the electronic health record, and increased coordination between specialties across the continuum of care. Other diagnoses that might be included in future bundles include cirrhosis, bowel obstruction, and inflammatory bowel disease. We can also learn from successful efforts in other clinical specialties that have identified variations in care and implemented a multi-modal strategy to improving care and measuring impact.
References
1. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time? N Engl J Med. 2015;373[14]:1291-3.
2. U.S. Department of Health and Human Services. Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. January 26, 2015. Available from: http://www.hhs.gov/about/news/2015/01/26/better-smarter-healthier-in-historic-announcement-hhs-sets-clear-goals-and-timeline-for-shifting-medicare-reimbursements-from-volume-to-value.html. Accessed June 28, 2016.
3. Patel, K., Presser, E., George, M., et al. Shifting away from fee-for-service: Alternative approaches to payment in gastroenterology. Clin Gastroenterol Hepatol. 2016;14[4]:497-506.
4. Medicare FY 2016 IPPS final rule. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2016-IPPS-Final-Rule-Home-Page.html. Accessed June 28, 2016.
5. Ketover, S.R. Bundled payment for colonoscopy. Clin Gastroenterol Hepatol. 2013;11[5]:454-7.
6. Coulam, R.F., Gaumer, G.L. Medicare’s prospective payment system: a critical appraisal. Health Care Financ Rev Annu Suppl. 1991:45-77.
Dr. Mehta is in the division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, and Penn Medicine Center for Health Care Innovation, University of Pennsylvania, Philadelphia. The author discloses no conflicts of interest.
The Medicare Access and Chips Reauthorization Act (MACRA) is now law; it passed with bipartisan, virtually unanimous support in both chambers of Congress. MACRA replaced the Sustainable Growth Rate formula for physician reimbursement and replaced it with a pathway to value-based payment. This law will alter our practices more than the Affordable Care Act and to an extent not seen since the passage of the original Medicare Act. Practices that continue to hang on to our traditional colonoscopy-based fee-for-service reimbursement model will increasingly be marginalized (or discounted) by Medicare, commercial payers, and regional health systems. To thrive in the coming decade, innovative practices will move toward alternative payment models. Many practices have risk-linked bundled payments for colonoscopy, but this step is only for the interim. Long-term success will come to practices that understand the implications of episode payments, specialty medical homes, and total cost of care. Do not wait for the finances to magically appear – start now to build infrastructure. In this month’s article, Dr. Mehta provides a detailed description of how a practice might construct a bundled payment for a common inpatient disorder. No one is paying for this yet, but it will come. Now is not the time to be a “WIMP” (Gastroenterology. 2016;150:295-9).
John I. Allen, MD, MBA, AGAF
Editor in Chief
In January 2016, the Centers for Medicare & Medicaid Services (CMS) launched the Comprehensive Care for Joint Replacement (CJR) model. This payment model aims to improve the value of care provided to Medicare beneficiaries for hip and knee replacement surgery during the inpatient stay and 90-day period after discharge by holding hospitals accountable for cost and quality.1 It includes hospitals in 67 geographic areas across the United States and marks the first time that a postacute bundled payment model is mandatory for traditional Medicare patients. Although this may not seem to be relevant for gastroenterology, it marks an important signal by CMS that there will likely be more episode-payment models in the future.
Gastroenterologists have not been primary drivers or participants in these models, but gastrointestinal hemorrhage is included as 1 of the 48 clinical conditions for the postacute bundled payment program. In addition, CMS recently announced that clinical episode-based payment for GI hemorrhage will be included in hospital inpatient quality reporting (IQR) for fiscal year 2019.4 This is an opportunity for the field of gastroenterology to take a leadership role in an alternate payment model as it has for colonoscopy bundled payment,5 but it requires an understanding of the history of postacute bundled payments and the opportunities for and challenges to applying this model to GI hemorrhage. In this article, I will describe insights from our health system’s experience in evaluating different postacute bundled payment programs and participating in a GI bundled payment program.
Inpatient and postacute bundled payments
A bundled payment refers to a situation in which hospitals and physicians are incentivized to coordinate care for an episode of care across the continuum and eliminate unnecessary spending. In 1983, Medicare initiated a type of bundled payment for Part A spending on inpatient hospital care by creating prospective payment that is based on diagnosis-related groups (DRGs). This was a response to the rising cost of inpatient care resulting from retrospective payment that is based on hospital charges. Because hospitals would get paid the same amount for similar conditions, it resulted in shortened length of stay and reduction in the rise of inpatient costs, along with no measurable impact on quality of care.6 This was followed by prospective payment for outpatient hospital fees and skilled nursing facility (SNF) care as a result of the Balanced Budget Act of 1997. Medicare built on this by bundling physician and hospital fees through demonstration projects in coronary artery bypass graft surgery from 1991 to 1996 and orthopedic and cardiovascular surgery from 2009 to 2012, both resulting in reduced costs and no measurable impact on quality.
The Bundled Payment for Care Improvement (BPCI) program built on these results in 2013 by expanding to include Part A and B services rendered up to 90 days after discharge, and as of January 2016, it includes 1,574 participants across the country. On a voluntary basis, hospitals, physician groups, and postacute providers and conveners were able to participate in 1 of 4 bundled payment models that were anchored on an inpatient for any of 48 clinical conditions that were based on MS-DRG (Table 1).
• Model 1 defined the episode as the inpatient hospital stay and bundled the facility and physician fees, similar to prior demonstration projects.
• Model 2 is a retrospective bundled payment for Part A and B services in the inpatient hospital stay and up to 90 days after discharge.
• Model 3 is a retrospective model that starts after hospital discharge and includes up to 90 days. (Models 1-3 maintain the current payment structure and retrospectively compare the actual reimbursement with target values that are based on historical data for that hospital with a 2%-3% payment reduction.)
• Model 4 makes a single, prospectively determined global payment to a hospital that encompasses all services during the hospital stay.

Opportunities in inpatient and postacute bundled payments
Participation in bundled payments requires a new set of analytic and organizational capabilities.
• The first step is to identify the patient population on the basis of inclusion and exclusion criteria and to measure the current cost of care through external claims data and internal hospital data. This includes payments for hospital inpatient services, physician fees, postacute care, readmissions, other Part B services, and home health services. The biggest opportunity for postacute bundles is shifting site of service from postacute care to lower-cost settings and reducing readmission rates.
• Subsequently, they need to identify areas of opportunity to reduce expenditure, while also demonstrating consistent or improved quality and outcomes.
• On the basis of this, the team can identify variation in care within the cohort and in comparison with benchmarks across the country.
• After identifying areas of opportunity, the team needs to develop strategies to improve value such as care pathways, information technology tools, care coordination, and remote services.
Of the 48 clinical conditions in BPCI, 4 could be described as related to GI: esophagitis, gastroenteritis, and other digestive disorders (Medicare Severity–Diagnosis Related Group [MS-DRG] 391, 392); gastrointestinal hemorrhage (MS-DRG 377, 378, 379); gastrointestinal obstruction (MS-DRG 388, 389, 390); and major bowel procedure (MS-DRG 329, 330, 331). After evaluating the GI bundles, it was apparent that these were created for billing purposes and were not clinically intuitive, which is why our institution immediately excluded the broad category of esophagitis, gastroenteritis, and other digestive disorders. GI obstruction and major bowel surgery relate to the care of gastroenterologists, but surgeons are typically primary drivers of care for these patients. Thus, we believed that GI hemorrhage was most appropriate because gastroenterologists drive care for this condition, and there is substantial evidence about established guidelines and pathways during this episode.
Bundled payment for gastrointestinal hemorrhage
We built a multidisciplinary team of physicians, data analysts, clinical documentation specialists, and care managers to start developing a plan for improving the value of care in this population. This included data about readmissions and site of postacute care for this population, which were supplemented by chart review of financial outliers and readmissions. We quickly learned about some of the challenges to medical bundles and the GI hemorrhage bundle in particular. It is difficult to identify these patients early in the hospital stay because inclusion is based on a billing code. Many of these patients also have cardiovascular disease, cancer, or cirrhosis, which makes it hard to identify which patients will end up with primary GI hemorrhage coding until after the patient is discharged. They are also on many different inpatient services; in our hospital, there were at least 12 different admitting services. In addition, almost one-third of the patients actually had an admission before this hospitalization, often for different clinical conditions.
Most importantly, it was very challenging to develop protocols to improve the value of care in this population. Most of the patients had many comorbid conditions, so a GI hemorrhage pathway alone would not be sufficient to alter care. The two main areas of opportunity for cost savings in postacute bundled payments are postacute site of service and readmissions, both of which are hard to change for medical GI patients. For medical patients, they have many comorbidities before admission, so postacute site of service is typically driven by which site they were admitted from. This is different from surgical patients who are in SNF or rehabilitation facilities for limited time frames, and there may be more discretion to shift to lower cost settings. In addition, readmissions have not been studied much in GI hemorrhage, so it is not clear how to improve them. On the basis of these factors and the limited sample size for this condition, our health system opted to stop taking financial risk for this population.
Future opportunities for gastroenterology
However, the latest CMS Inpatient Prospective Payment System rule describes the implementation of a new quality metric for hospital IQR called the Gastrointestinal Hemorrhage Clinical Episode-Based Payment. This would hold hospitals accountable for the cost of care for GI hemorrhage admissions plus the 90 days after discharge, similar to model 2 of BPCI. This announcement, as well as the launch of mandatory orthopedic bundles, demonstrates that hospital reimbursement is shifting toward an expansion of bundled payments to include the postacute time frame. This is manifested in postacute bundles, episode-based payment, and readmission penalties. This reignited our GI hemorrhage episode team’s efforts, but with a broader purpose.
Gastroenterologists can take a leadership role in responding to episode-based payments as a way for us to demonstrate value in our collaboration with hospitals, health systems, and payers. The focus on cardiovascular disease as part of readmission penalties and core measures has allowed our cardiology colleagues to partner closely with service lines, learn about episode-based care, and garner resources to build and lead disease and episode teams. Because patients do not fit into the different clinical areas in mutually exclusive categories, we will need to collaborate with other specialties to care for the overlap with other conditions. Many heart failure and myocardial infarction patients will get readmitted for GI hemorrhage, and many GI hemorrhage patients will have concomitant cardiovascular disease or cancer. This suggests that future strategies need to integrate efforts of service lines and that there is greater opportunity for gastroenterologists than just the GI bundles.
Gastroenterologists should also participate in a proactive way. Any new payment mechanism will have some flaws in implementation, so it is more important to do what is right from a clinical standpoint rather than focusing too much on the specific billing code or payment model. These models are evolving, and we have an opportunity to have impact on future implementation. This starts with identifying and including patients from a clinical perspective rather than focusing on specific insurance types that participate in bundled payments. Some examples to improve the value of care in GI hemorrhage include creating evidence-based care pathways that span the episode of care, structured documentation after endoscopy for risk stratification, integrating pathways into the workflow of providers through the electronic health record, and increased coordination between specialties across the continuum of care. Other diagnoses that might be included in future bundles include cirrhosis, bowel obstruction, and inflammatory bowel disease. We can also learn from successful efforts in other clinical specialties that have identified variations in care and implemented a multi-modal strategy to improving care and measuring impact.
References
1. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time? N Engl J Med. 2015;373[14]:1291-3.
2. U.S. Department of Health and Human Services. Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. January 26, 2015. Available from: http://www.hhs.gov/about/news/2015/01/26/better-smarter-healthier-in-historic-announcement-hhs-sets-clear-goals-and-timeline-for-shifting-medicare-reimbursements-from-volume-to-value.html. Accessed June 28, 2016.
3. Patel, K., Presser, E., George, M., et al. Shifting away from fee-for-service: Alternative approaches to payment in gastroenterology. Clin Gastroenterol Hepatol. 2016;14[4]:497-506.
4. Medicare FY 2016 IPPS final rule. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2016-IPPS-Final-Rule-Home-Page.html. Accessed June 28, 2016.
5. Ketover, S.R. Bundled payment for colonoscopy. Clin Gastroenterol Hepatol. 2013;11[5]:454-7.
6. Coulam, R.F., Gaumer, G.L. Medicare’s prospective payment system: a critical appraisal. Health Care Financ Rev Annu Suppl. 1991:45-77.
Dr. Mehta is in the division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, and Penn Medicine Center for Health Care Innovation, University of Pennsylvania, Philadelphia. The author discloses no conflicts of interest.
The Medicare Access and Chips Reauthorization Act (MACRA) is now law; it passed with bipartisan, virtually unanimous support in both chambers of Congress. MACRA replaced the Sustainable Growth Rate formula for physician reimbursement and replaced it with a pathway to value-based payment. This law will alter our practices more than the Affordable Care Act and to an extent not seen since the passage of the original Medicare Act. Practices that continue to hang on to our traditional colonoscopy-based fee-for-service reimbursement model will increasingly be marginalized (or discounted) by Medicare, commercial payers, and regional health systems. To thrive in the coming decade, innovative practices will move toward alternative payment models. Many practices have risk-linked bundled payments for colonoscopy, but this step is only for the interim. Long-term success will come to practices that understand the implications of episode payments, specialty medical homes, and total cost of care. Do not wait for the finances to magically appear – start now to build infrastructure. In this month’s article, Dr. Mehta provides a detailed description of how a practice might construct a bundled payment for a common inpatient disorder. No one is paying for this yet, but it will come. Now is not the time to be a “WIMP” (Gastroenterology. 2016;150:295-9).
John I. Allen, MD, MBA, AGAF
Editor in Chief
In January 2016, the Centers for Medicare & Medicaid Services (CMS) launched the Comprehensive Care for Joint Replacement (CJR) model. This payment model aims to improve the value of care provided to Medicare beneficiaries for hip and knee replacement surgery during the inpatient stay and 90-day period after discharge by holding hospitals accountable for cost and quality.1 It includes hospitals in 67 geographic areas across the United States and marks the first time that a postacute bundled payment model is mandatory for traditional Medicare patients. Although this may not seem to be relevant for gastroenterology, it marks an important signal by CMS that there will likely be more episode-payment models in the future.
Gastroenterologists have not been primary drivers or participants in these models, but gastrointestinal hemorrhage is included as 1 of the 48 clinical conditions for the postacute bundled payment program. In addition, CMS recently announced that clinical episode-based payment for GI hemorrhage will be included in hospital inpatient quality reporting (IQR) for fiscal year 2019.4 This is an opportunity for the field of gastroenterology to take a leadership role in an alternate payment model as it has for colonoscopy bundled payment,5 but it requires an understanding of the history of postacute bundled payments and the opportunities for and challenges to applying this model to GI hemorrhage. In this article, I will describe insights from our health system’s experience in evaluating different postacute bundled payment programs and participating in a GI bundled payment program.
Inpatient and postacute bundled payments
A bundled payment refers to a situation in which hospitals and physicians are incentivized to coordinate care for an episode of care across the continuum and eliminate unnecessary spending. In 1983, Medicare initiated a type of bundled payment for Part A spending on inpatient hospital care by creating prospective payment that is based on diagnosis-related groups (DRGs). This was a response to the rising cost of inpatient care resulting from retrospective payment that is based on hospital charges. Because hospitals would get paid the same amount for similar conditions, it resulted in shortened length of stay and reduction in the rise of inpatient costs, along with no measurable impact on quality of care.6 This was followed by prospective payment for outpatient hospital fees and skilled nursing facility (SNF) care as a result of the Balanced Budget Act of 1997. Medicare built on this by bundling physician and hospital fees through demonstration projects in coronary artery bypass graft surgery from 1991 to 1996 and orthopedic and cardiovascular surgery from 2009 to 2012, both resulting in reduced costs and no measurable impact on quality.
The Bundled Payment for Care Improvement (BPCI) program built on these results in 2013 by expanding to include Part A and B services rendered up to 90 days after discharge, and as of January 2016, it includes 1,574 participants across the country. On a voluntary basis, hospitals, physician groups, and postacute providers and conveners were able to participate in 1 of 4 bundled payment models that were anchored on an inpatient for any of 48 clinical conditions that were based on MS-DRG (Table 1).
• Model 1 defined the episode as the inpatient hospital stay and bundled the facility and physician fees, similar to prior demonstration projects.
• Model 2 is a retrospective bundled payment for Part A and B services in the inpatient hospital stay and up to 90 days after discharge.
• Model 3 is a retrospective model that starts after hospital discharge and includes up to 90 days. (Models 1-3 maintain the current payment structure and retrospectively compare the actual reimbursement with target values that are based on historical data for that hospital with a 2%-3% payment reduction.)
• Model 4 makes a single, prospectively determined global payment to a hospital that encompasses all services during the hospital stay.

Opportunities in inpatient and postacute bundled payments
Participation in bundled payments requires a new set of analytic and organizational capabilities.
• The first step is to identify the patient population on the basis of inclusion and exclusion criteria and to measure the current cost of care through external claims data and internal hospital data. This includes payments for hospital inpatient services, physician fees, postacute care, readmissions, other Part B services, and home health services. The biggest opportunity for postacute bundles is shifting site of service from postacute care to lower-cost settings and reducing readmission rates.
• Subsequently, they need to identify areas of opportunity to reduce expenditure, while also demonstrating consistent or improved quality and outcomes.
• On the basis of this, the team can identify variation in care within the cohort and in comparison with benchmarks across the country.
• After identifying areas of opportunity, the team needs to develop strategies to improve value such as care pathways, information technology tools, care coordination, and remote services.
Of the 48 clinical conditions in BPCI, 4 could be described as related to GI: esophagitis, gastroenteritis, and other digestive disorders (Medicare Severity–Diagnosis Related Group [MS-DRG] 391, 392); gastrointestinal hemorrhage (MS-DRG 377, 378, 379); gastrointestinal obstruction (MS-DRG 388, 389, 390); and major bowel procedure (MS-DRG 329, 330, 331). After evaluating the GI bundles, it was apparent that these were created for billing purposes and were not clinically intuitive, which is why our institution immediately excluded the broad category of esophagitis, gastroenteritis, and other digestive disorders. GI obstruction and major bowel surgery relate to the care of gastroenterologists, but surgeons are typically primary drivers of care for these patients. Thus, we believed that GI hemorrhage was most appropriate because gastroenterologists drive care for this condition, and there is substantial evidence about established guidelines and pathways during this episode.
Bundled payment for gastrointestinal hemorrhage
We built a multidisciplinary team of physicians, data analysts, clinical documentation specialists, and care managers to start developing a plan for improving the value of care in this population. This included data about readmissions and site of postacute care for this population, which were supplemented by chart review of financial outliers and readmissions. We quickly learned about some of the challenges to medical bundles and the GI hemorrhage bundle in particular. It is difficult to identify these patients early in the hospital stay because inclusion is based on a billing code. Many of these patients also have cardiovascular disease, cancer, or cirrhosis, which makes it hard to identify which patients will end up with primary GI hemorrhage coding until after the patient is discharged. They are also on many different inpatient services; in our hospital, there were at least 12 different admitting services. In addition, almost one-third of the patients actually had an admission before this hospitalization, often for different clinical conditions.
Most importantly, it was very challenging to develop protocols to improve the value of care in this population. Most of the patients had many comorbid conditions, so a GI hemorrhage pathway alone would not be sufficient to alter care. The two main areas of opportunity for cost savings in postacute bundled payments are postacute site of service and readmissions, both of which are hard to change for medical GI patients. For medical patients, they have many comorbidities before admission, so postacute site of service is typically driven by which site they were admitted from. This is different from surgical patients who are in SNF or rehabilitation facilities for limited time frames, and there may be more discretion to shift to lower cost settings. In addition, readmissions have not been studied much in GI hemorrhage, so it is not clear how to improve them. On the basis of these factors and the limited sample size for this condition, our health system opted to stop taking financial risk for this population.
Future opportunities for gastroenterology
However, the latest CMS Inpatient Prospective Payment System rule describes the implementation of a new quality metric for hospital IQR called the Gastrointestinal Hemorrhage Clinical Episode-Based Payment. This would hold hospitals accountable for the cost of care for GI hemorrhage admissions plus the 90 days after discharge, similar to model 2 of BPCI. This announcement, as well as the launch of mandatory orthopedic bundles, demonstrates that hospital reimbursement is shifting toward an expansion of bundled payments to include the postacute time frame. This is manifested in postacute bundles, episode-based payment, and readmission penalties. This reignited our GI hemorrhage episode team’s efforts, but with a broader purpose.
Gastroenterologists can take a leadership role in responding to episode-based payments as a way for us to demonstrate value in our collaboration with hospitals, health systems, and payers. The focus on cardiovascular disease as part of readmission penalties and core measures has allowed our cardiology colleagues to partner closely with service lines, learn about episode-based care, and garner resources to build and lead disease and episode teams. Because patients do not fit into the different clinical areas in mutually exclusive categories, we will need to collaborate with other specialties to care for the overlap with other conditions. Many heart failure and myocardial infarction patients will get readmitted for GI hemorrhage, and many GI hemorrhage patients will have concomitant cardiovascular disease or cancer. This suggests that future strategies need to integrate efforts of service lines and that there is greater opportunity for gastroenterologists than just the GI bundles.
Gastroenterologists should also participate in a proactive way. Any new payment mechanism will have some flaws in implementation, so it is more important to do what is right from a clinical standpoint rather than focusing too much on the specific billing code or payment model. These models are evolving, and we have an opportunity to have impact on future implementation. This starts with identifying and including patients from a clinical perspective rather than focusing on specific insurance types that participate in bundled payments. Some examples to improve the value of care in GI hemorrhage include creating evidence-based care pathways that span the episode of care, structured documentation after endoscopy for risk stratification, integrating pathways into the workflow of providers through the electronic health record, and increased coordination between specialties across the continuum of care. Other diagnoses that might be included in future bundles include cirrhosis, bowel obstruction, and inflammatory bowel disease. We can also learn from successful efforts in other clinical specialties that have identified variations in care and implemented a multi-modal strategy to improving care and measuring impact.
References
1. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time? N Engl J Med. 2015;373[14]:1291-3.
2. U.S. Department of Health and Human Services. Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. January 26, 2015. Available from: http://www.hhs.gov/about/news/2015/01/26/better-smarter-healthier-in-historic-announcement-hhs-sets-clear-goals-and-timeline-for-shifting-medicare-reimbursements-from-volume-to-value.html. Accessed June 28, 2016.
3. Patel, K., Presser, E., George, M., et al. Shifting away from fee-for-service: Alternative approaches to payment in gastroenterology. Clin Gastroenterol Hepatol. 2016;14[4]:497-506.
4. Medicare FY 2016 IPPS final rule. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2016-IPPS-Final-Rule-Home-Page.html. Accessed June 28, 2016.
5. Ketover, S.R. Bundled payment for colonoscopy. Clin Gastroenterol Hepatol. 2013;11[5]:454-7.
6. Coulam, R.F., Gaumer, G.L. Medicare’s prospective payment system: a critical appraisal. Health Care Financ Rev Annu Suppl. 1991:45-77.
Dr. Mehta is in the division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, and Penn Medicine Center for Health Care Innovation, University of Pennsylvania, Philadelphia. The author discloses no conflicts of interest.
Malpractice 2017: Do we need reform?
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”
But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”

But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Malpractice reforms long espoused by Thomas E. Price, MD, Health & Human Services secretary, are fostering debate on whether national fixes are necessary in an improving liability landscape. Claims against health providers have decreased twofold and doctors nationwide have seen their premiums decrease steadily for a decade. But the numbers tell just part of the story, liability experts say.
“Most health policy and medical malpractice scholars would not describe the environment we’re in right now as a state of crisis, at least not compared to what we observed a decade ago,” said Anupam B. Jena, MD, PhD, health care policy professor at Harvard University, Boston, and a medical liability researcher.“But I would argue that malpractice is still a salient fact for most physicians. What’s much more important than focusing on changes over time is taking a static perspective and understanding the risk that a physician faces when it comes to malpractice.”
During just over 12 years in Congress, Dr. Price (R-Ga.) has consistently advocated for tort reform, proposing legislation that would restructure the lawsuit process. He supports damage caps and has recommended the creation of health care tribunals that would hear cases only after medical review. He has also proposed the development of national practice guidelines for doctors, which if followed could be used defensively.
“From exorbitant malpractice insurance premiums to the remarkably expensive practice of defensive medicine, it is my experience that the current culture of litigation costs patients hundreds of billions of dollars,” Dr. Price wrote in a 2009 commentary about his Empowering Patients First Act. “These costs do nothing to provide better care, but rather serve only as a defense against unyielding personal injury lawyers ...When malpractice suits are brought through specialized courts and viewed through the perspective of medically appropriate care, rather than a lottery mentality, we will see a decline in frivolous lawsuits.”
Data show lawsuits down
Claims data however, show that lawsuits against doctors are on the decline – and have been for more than 10 years. The rate of paid claims against physicians dropped from 18.6 to 9.9 paid claims per 1,000 physicians between 2002 and 2013, according to 2014 study by Michelle Mello of Stanford (Calif.) University and colleagues (JAMA. 2014;312(20):2146-2155. doi:10.1001/jama.2014.10705). Another analysis found that claims decreased from 12 claims per 100 doctors in 2003 to 6 per 100 doctors in 2016, according to a study of claims from The Doctors Company database.
The sharp decline in claims is directly affecting premiums, which have remained nearly unchanged for years, said Michael Matray, editor of the Medical Liability Monitor (MLM), which tracks premium rates. In 2003, rate increases of 49% were commonplace, he noted, with some states reporting rises of 100%. But premiums started to fall in 2006, he said.
“We are in the midst of a 10-year soft market, the longest we’ve ever had,” Mr. Matray said in an interview. “In 2006, we started to soften. We have been soft or flat with no change since. It’s definitely unprecedented.”
In 2016, U.S. internists paid an average premium of $15,853, compared to an average premium payment of $19,900 in 2006 without inflation adjustment, according to this news organization’s analysis of MLM data. General surgeons meanwhile, paid an average of $52,905 in premiums in 2016, compared to a 2006 average of $68,186. Ob.gyns. paid an average premium of $72,999 in 2016, a drop from $93,230 in 2006.
“Generally speaking, it’s great for doctors,” Mr. Matray said. “Even if you remove the inflationary factor, there are some states where they are paying less now than they were 10 years ago.”

But stable premiums do not mean inexpensive insurance, noted Mike Stinson, vice president of government relations and public policy for the PIAA, a national trade association for medical liability insurers.
“They’ve certainly come down over the last few years,” Mr. Stinson said in an interview. “But they certainly are not low.”
South Florida doctors for example, are paying among the highest premiums in the nation despite an overall drop in the last 10 years. Internists in Dade County, Fla., paid $47,707 for insurance in 2016, $27,000 less than in 2006, according to MLM data. Coverage is still is not affordable, said Jason M. Goldman, MD, an internist in private practice in Coral Springs, Fla., and governor for the American College of Physicians Florida chapter.
The premiums are ridiculous,” he said in an interview. “That’s one of the reasons I went bare. Having medical insurance makes you a target, because lawyers think, ‘We can sue the insurance company and the insurance company is going to pay.’ ”
State reforms driving lawsuit decline
A combination of state reforms and patient safety initiatives are fueling the lawsuit down slide, said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2016 MLM Survey report.
More than 25 states now have laws that enforce caps on noneconomic damages in medical liability cases, and in the last 15 years, a large majority have upheld such limits, Mr. Greve said. In addition, a number of states require a certificate of merit before suits can move forward, which mandate that a qualified physician verify that a defendant’s actions were likely negligent.
Patient safety programs such as internal patient safety committees, enhanced provider education, safety protocols, and communication and resolution programs, are also making an impact.
“There have been many efforts toward thinking innovatively about how we should be compensating patients more fairly and more quickly,” said Harvard’s Dr. Jena, who practices at Massachusetts General Hospital. “There are early disclosure programs that many hospitals have adopted as a movement toward that goal. The system is evolving.”
The high expense of filing a medical malpractice lawsuit is another factor detouring lawsuits, Mr. Greve added.
“It’s very expensive for [plaintiffs’ attorneys] to pursue these cases,” he said. “They have to invest a large amount of money into them and they can’t afford to take cases that have questionable liability, or they’re going to end up shelling out a lot of money and not getting anything in return.”
Malpractice system remains broken
While lawsuit and premium data paint a positive picture, the medical malpractice system remains dysfunctional for doctors and patients, said PIAA’s Mr. Stinson.
A majority of medical malpractice claims against physicians are determined to be nonmeritorious yet an average lawsuit takes roughly 4 years to resolve, he noted. Suits against doctors are dismissed by the court 54% of the time across all specialties, and among cases that go to trial, 80% end in the physician’s favor, according to a 2012 study by Dr. Jena and colleagues (JAMA Intern Med. 2012 Jun. 11 doi: 10.1001/jamainternmed.2012.1416)
“We think that’s a huge amount of resources both in time and money and human capital to get poured into cases where no payment is ever deemed appropriate,” Mr. Stinson said. “We would like to see [malpractice] reforms in part because we think you can weed out some of these claims in advance, so more of the resources can be used to determine whether or not there was negligence and if so, making that patient whole as fast as possible.”
Regardless of declining claims, most physicians will still be sued in their lifetime, Dr. Jena said. By age 65, 75% of physicians in low-risk specialties have faced a malpractice claim, and 99% of doctors in high-risk specialties have been sued, according to a 2011 study by Dr. Jena and colleagues (N Engl J Med. 2011 Aug. 18. doi: 10.1056/NEJMsa1012370)
“What really matters for physicians is not only whether they win or lose a malpractice suit, but whether they are sued in the first place,” Dr. Jena said. “Lawsuits happen quite often and over the course of a physician’s career they are quite common. So whether or not we’re in a state of malpractice crisis or not, the lifetime risk for physicians is quite real.”
The cost of defending claims continues to rise, noted Richard E. Anderson, MD, chair and CEO of The Doctors Company. At the same time, multi-million dollar malpractice verdicts have become more common.
“There’s still way too much malpractice litigation,” Dr. Anderson said. “The majority of it is fruitless, and the total cost burden borne by physicians has not decreased as much as the frequency because of the ongoing increase in severity.”
“The bottom line is defensive medicine is a huge and growing cost to all Americans,” he said. “We’re all on the hook for America’s rising health care bills. And as my increasingly infirm Baby Boomer generation gets older and weaker and sicker, those bills are going to rise that much faster. So the notion advanced by some so-called experts that we can nonetheless afford to spend a half trillion dollars a year or more on largely unnecessary testing driven in significant part by fear of litigation and giant outlier verdicts seems rather inane, and it suggests perhaps that those experts aren’t so expert.”
What would Dr. Price do?
Opinions are mixed about whether Dr. Price’s proposed reforms are the right changes for the medical liability system.
Expert panels to review claims for validity are a promising suggestion, Dr. Jena said. “Administrative courts to help identify malpractice cases that are truly malpractice early on is a good idea,” he said. “We want to have a system that prevents less meritorious cases from soaking up resources.”
But the idea may be easier said than done, said Dr. Anderson of The Doctors Company. “The devil is always in the details There is a lot of merit in [health courts] and they make a lot of sense. However, the notion of going from where we are today to an untested system, which would have to be a compromise between adversaries, would be a very challenging one.”
National clinical standards for physicians to follow and use as a safeguard could backfire, Mr. Stinson said. Bureaucracy could slow the guidelines from being promptly updated, and the standards could fail to keep up with the latest medical developments. As doctors know, medicine is not a one-size-fits-all approach, he added.
“[Guidelines] could encourage doctors to practice cookbook medicine, where they’re just going to follow the standard along without being given the opportunity to use their training and clinical experience to see whether that’s actually in the patient’s best interest,” Mr. Stinson said. “We certainly wouldn’t want to see a situation where a doctor feels diverting from a guidelines is in a patient’s best interest, but they don’t dare do it because it could subject them to a lawsuit.”
When enacting malpractice reforms, it will be critical to evaluate the intervention first to ensure the right objections are met, Dr. Jena added.
“At the end of the day, if the [legal] environment is such that it’s uncomfortable to practice medicine, that’s going to have implications for who goes into medicine and implications for ordering tests and procedures,” he said. “Any reform that attempts to make the process more efficient is a good idea because it lowers cost to the system and makes the experience better for patients and physicians.”
This article was updated 2/15/17.
[email protected]
On Twitter @legal_med
Hepatitis Outlook: January 2017
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering the major hepatitis viruses.
Hepatitis A virus vaccine provides protective antibody levels 20 years after childhood vaccination, according to a study of Alaska Native persons.
Outcome measures that reflect the entire cycle of hepatitis care are needed to assist both clinicians and administrators in improving quality and value of care, according to an analysis in Hepatology.
Prophylactic antiviral therapy management is necessary for hepatitis B surface antigen–positive breast cancer patients undergoing chemotherapy, a recent study revealed, in spite of high correlation with lamivudine-resistant hepatitis B virus variants with tyrosine-methionine-aspartate-aspartate motif-mutations.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody–positive donor organs from 2005 to 2014, and stable reporting of HCV-positive donor organs and HIV-positive recipients.
The proportion of anti-HCV–positive patients in Poland decreased from 2004 to 2014, according to a study reported in Eurosurveillance.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance, even after demographic, lifestyle, and metabolic factors are controlled for.
Hepatitis A virus vaccination had increased effectiveness for postexposure prophylaxis in HA outbreaks, similar to that of immunoglobulin, and offered long-term protection, according to a study in Human Vaccines & Immunotherapeutics. The authors said the result supports the preferential use of vaccination to avoid secondary cases.
Hepatitis C virus subtype 3b and 6a subepidemics in China are currently not under control, according to a report in the Journal of Viral Hepatitis, and new epidemic waves may emerge given the rapid increase in international travel.
Strong, consistent evidence exists that Western health professionals miss opportunities for hepatitis B virus testing and vaccination of Chinese migrant populations, a recent study revealed.
New research shows that laboratory of genetics and physiology 2, a retinoic acid–inducible gene I–like receptor, plays an essential role in hepatitis C virus infection–induced interferon responses.
A study in Hepatology revealed that the hepatitis C virus uses the protein NS5B to specifically suppress the tumor suppressor NORE1A, facilitating viral replication and elevated Ras signaling.
Combined hepatitis A and B vaccine could stimulate both high level of anti-hepatitis A and anti-hepatitis Bs antibodies and not increase adverse events, a recent study revealed, providing a new choice for hepatitis B booster.
A study in Hepatology reports that proanthocyanidin, an oligomeric flavonoid, and its analogs represent a new class of anti-hepatitis B virus agents that directly target the preS1 region of the HBV large surface protein. These agents could contribute to the development of a potent, well-tolerated, and broadly active inhibitor of HBV infection.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
A Lancet study found that one administration of RG-101, a hepatocyte targeted N-acetylgalactosamine conjugated anti–miR-122 oligonucleotide, was well tolerated and resulted in substantial viral load reduction in all treated patients within 4 weeks, and sustained virologic response in three patients for 76 weeks.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A liver biopsy and antiviral therapy should be strongly considered when treating hepatitis B e antigen–positive patients with a normal or minimally elevated ALT level, low HBV DNA level, and age greater than 35 years, according to a study in the Journal of Viral Hepatitis.
Serum long intergenic noncoding RNA-p21 could serve as a potential biomarker of liver fibrosis in chronic hepatitis B virus infection patients, according to a Chinese study.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering the major hepatitis viruses.
Hepatitis A virus vaccine provides protective antibody levels 20 years after childhood vaccination, according to a study of Alaska Native persons.
Outcome measures that reflect the entire cycle of hepatitis care are needed to assist both clinicians and administrators in improving quality and value of care, according to an analysis in Hepatology.
Prophylactic antiviral therapy management is necessary for hepatitis B surface antigen–positive breast cancer patients undergoing chemotherapy, a recent study revealed, in spite of high correlation with lamivudine-resistant hepatitis B virus variants with tyrosine-methionine-aspartate-aspartate motif-mutations.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody–positive donor organs from 2005 to 2014, and stable reporting of HCV-positive donor organs and HIV-positive recipients.
The proportion of anti-HCV–positive patients in Poland decreased from 2004 to 2014, according to a study reported in Eurosurveillance.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance, even after demographic, lifestyle, and metabolic factors are controlled for.
Hepatitis A virus vaccination had increased effectiveness for postexposure prophylaxis in HA outbreaks, similar to that of immunoglobulin, and offered long-term protection, according to a study in Human Vaccines & Immunotherapeutics. The authors said the result supports the preferential use of vaccination to avoid secondary cases.
Hepatitis C virus subtype 3b and 6a subepidemics in China are currently not under control, according to a report in the Journal of Viral Hepatitis, and new epidemic waves may emerge given the rapid increase in international travel.
Strong, consistent evidence exists that Western health professionals miss opportunities for hepatitis B virus testing and vaccination of Chinese migrant populations, a recent study revealed.
New research shows that laboratory of genetics and physiology 2, a retinoic acid–inducible gene I–like receptor, plays an essential role in hepatitis C virus infection–induced interferon responses.
A study in Hepatology revealed that the hepatitis C virus uses the protein NS5B to specifically suppress the tumor suppressor NORE1A, facilitating viral replication and elevated Ras signaling.
Combined hepatitis A and B vaccine could stimulate both high level of anti-hepatitis A and anti-hepatitis Bs antibodies and not increase adverse events, a recent study revealed, providing a new choice for hepatitis B booster.
A study in Hepatology reports that proanthocyanidin, an oligomeric flavonoid, and its analogs represent a new class of anti-hepatitis B virus agents that directly target the preS1 region of the HBV large surface protein. These agents could contribute to the development of a potent, well-tolerated, and broadly active inhibitor of HBV infection.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
A Lancet study found that one administration of RG-101, a hepatocyte targeted N-acetylgalactosamine conjugated anti–miR-122 oligonucleotide, was well tolerated and resulted in substantial viral load reduction in all treated patients within 4 weeks, and sustained virologic response in three patients for 76 weeks.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A liver biopsy and antiviral therapy should be strongly considered when treating hepatitis B e antigen–positive patients with a normal or minimally elevated ALT level, low HBV DNA level, and age greater than 35 years, according to a study in the Journal of Viral Hepatitis.
Serum long intergenic noncoding RNA-p21 could serve as a potential biomarker of liver fibrosis in chronic hepatitis B virus infection patients, according to a Chinese study.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering the major hepatitis viruses.
Hepatitis A virus vaccine provides protective antibody levels 20 years after childhood vaccination, according to a study of Alaska Native persons.
Outcome measures that reflect the entire cycle of hepatitis care are needed to assist both clinicians and administrators in improving quality and value of care, according to an analysis in Hepatology.
Prophylactic antiviral therapy management is necessary for hepatitis B surface antigen–positive breast cancer patients undergoing chemotherapy, a recent study revealed, in spite of high correlation with lamivudine-resistant hepatitis B virus variants with tyrosine-methionine-aspartate-aspartate motif-mutations.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody–positive donor organs from 2005 to 2014, and stable reporting of HCV-positive donor organs and HIV-positive recipients.
The proportion of anti-HCV–positive patients in Poland decreased from 2004 to 2014, according to a study reported in Eurosurveillance.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance, even after demographic, lifestyle, and metabolic factors are controlled for.
Hepatitis A virus vaccination had increased effectiveness for postexposure prophylaxis in HA outbreaks, similar to that of immunoglobulin, and offered long-term protection, according to a study in Human Vaccines & Immunotherapeutics. The authors said the result supports the preferential use of vaccination to avoid secondary cases.
Hepatitis C virus subtype 3b and 6a subepidemics in China are currently not under control, according to a report in the Journal of Viral Hepatitis, and new epidemic waves may emerge given the rapid increase in international travel.
Strong, consistent evidence exists that Western health professionals miss opportunities for hepatitis B virus testing and vaccination of Chinese migrant populations, a recent study revealed.
New research shows that laboratory of genetics and physiology 2, a retinoic acid–inducible gene I–like receptor, plays an essential role in hepatitis C virus infection–induced interferon responses.
A study in Hepatology revealed that the hepatitis C virus uses the protein NS5B to specifically suppress the tumor suppressor NORE1A, facilitating viral replication and elevated Ras signaling.
Combined hepatitis A and B vaccine could stimulate both high level of anti-hepatitis A and anti-hepatitis Bs antibodies and not increase adverse events, a recent study revealed, providing a new choice for hepatitis B booster.
A study in Hepatology reports that proanthocyanidin, an oligomeric flavonoid, and its analogs represent a new class of anti-hepatitis B virus agents that directly target the preS1 region of the HBV large surface protein. These agents could contribute to the development of a potent, well-tolerated, and broadly active inhibitor of HBV infection.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
A Lancet study found that one administration of RG-101, a hepatocyte targeted N-acetylgalactosamine conjugated anti–miR-122 oligonucleotide, was well tolerated and resulted in substantial viral load reduction in all treated patients within 4 weeks, and sustained virologic response in three patients for 76 weeks.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A liver biopsy and antiviral therapy should be strongly considered when treating hepatitis B e antigen–positive patients with a normal or minimally elevated ALT level, low HBV DNA level, and age greater than 35 years, according to a study in the Journal of Viral Hepatitis.
Serum long intergenic noncoding RNA-p21 could serve as a potential biomarker of liver fibrosis in chronic hepatitis B virus infection patients, according to a Chinese study.
[email protected]
On Twitter @richpizzi
Legacy Society members sustain research
Research has brought so much to our specialty and advanced the science and practice of gastroenterology. Research is made possible through funding. AGA Legacy Society members are showing their gratitude for what funding and research has brought to our specialty by giving back.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
“I was at a crossroads in my career when I received funding from the AGA,” said Michael Camilleri, MD, AGAF, AGA Past President. “Having been personally a recipient of awards from the AGA Research Foundation, I believe it is now important to give back. This is one of the ways I will impact not only the careers of young colleagues but ultimately patient care, as well.”
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. More than 870 researchers have benefited from our support since 1984 – with more than 90% of AGA Research Scholar Award recipients in the past 10 years continuing on to exceptional research careers. These research grants are funded through the generosity of donors.
“To understand the fundamental mechanism of disease process, particularly chronic diseases is always a challenge, but it is critical to be able to interfere with the disease process, halt progression and hopefully achieve a cure,” remarked Kiron M. Das, MD, PhD, AGAF. “Research has to be continued, and we have to train young investigators. On behalf of my wife and myself, we want to thank the AGA Research Foundation for its commitment to promote discovery. It is critical that we support and give to the AGA Research Foundation.”
A celebration of research support
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The University Club of Chicago will be the location of the 2017 AGA Research Foundation Benefactors Dinner during DDW in Chicago. Guests will enjoy a wonderful evening in the historic setting established in 1887 to foster an appreciation for literature and the arts. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
Research has brought so much to our specialty and advanced the science and practice of gastroenterology. Research is made possible through funding. AGA Legacy Society members are showing their gratitude for what funding and research has brought to our specialty by giving back.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
“I was at a crossroads in my career when I received funding from the AGA,” said Michael Camilleri, MD, AGAF, AGA Past President. “Having been personally a recipient of awards from the AGA Research Foundation, I believe it is now important to give back. This is one of the ways I will impact not only the careers of young colleagues but ultimately patient care, as well.”
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. More than 870 researchers have benefited from our support since 1984 – with more than 90% of AGA Research Scholar Award recipients in the past 10 years continuing on to exceptional research careers. These research grants are funded through the generosity of donors.
“To understand the fundamental mechanism of disease process, particularly chronic diseases is always a challenge, but it is critical to be able to interfere with the disease process, halt progression and hopefully achieve a cure,” remarked Kiron M. Das, MD, PhD, AGAF. “Research has to be continued, and we have to train young investigators. On behalf of my wife and myself, we want to thank the AGA Research Foundation for its commitment to promote discovery. It is critical that we support and give to the AGA Research Foundation.”
A celebration of research support
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The University Club of Chicago will be the location of the 2017 AGA Research Foundation Benefactors Dinner during DDW in Chicago. Guests will enjoy a wonderful evening in the historic setting established in 1887 to foster an appreciation for literature and the arts. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
Research has brought so much to our specialty and advanced the science and practice of gastroenterology. Research is made possible through funding. AGA Legacy Society members are showing their gratitude for what funding and research has brought to our specialty by giving back.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
“I was at a crossroads in my career when I received funding from the AGA,” said Michael Camilleri, MD, AGAF, AGA Past President. “Having been personally a recipient of awards from the AGA Research Foundation, I believe it is now important to give back. This is one of the ways I will impact not only the careers of young colleagues but ultimately patient care, as well.”
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. More than 870 researchers have benefited from our support since 1984 – with more than 90% of AGA Research Scholar Award recipients in the past 10 years continuing on to exceptional research careers. These research grants are funded through the generosity of donors.
“To understand the fundamental mechanism of disease process, particularly chronic diseases is always a challenge, but it is critical to be able to interfere with the disease process, halt progression and hopefully achieve a cure,” remarked Kiron M. Das, MD, PhD, AGAF. “Research has to be continued, and we have to train young investigators. On behalf of my wife and myself, we want to thank the AGA Research Foundation for its commitment to promote discovery. It is critical that we support and give to the AGA Research Foundation.”
A celebration of research support
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The University Club of Chicago will be the location of the 2017 AGA Research Foundation Benefactors Dinner during DDW in Chicago. Guests will enjoy a wonderful evening in the historic setting established in 1887 to foster an appreciation for literature and the arts. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
When to screen asymptomatic diabetics for CAD
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
MACRA is not going away: Will you be ready?
Despite potential repeal of the Affordable Care Act under the new administration, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) and commitment to cost-effective, value-based care is here to stay.
Congress overwhelmingly passed MACRA legislation with bipartisan support in both chambers of Congress to overhaul the way physicians are reimbursed under Medicare. MACRA will eventually transition physicians toward more value-based payments. Ignore MACRA in 2017, and you will face an automatic reduction of 4% to your payments under Medicare in 2019.
You should take advantage of 2017 being a transition year during which time you can pick your pace for participation to help you increase your earning potential. If you are already reporting to the 2016 Physician Quality Reporting System (PQRS), you will be familiar with some of the 2017 participation options that could qualify you for a reimbursement incentive in 2019 under MACRA.
If you have not participated in PQRS in 2016 or previous years, you need to start gathering information for your practice to begin reporting through one of the new MACRA 2017 reporting options by Oct. 2, 2017. Quality accounts for the highest percentage of your score and will help you maximize your potential for a positive adjustment.
AGA can help – check out our MACRA resources at gastro.org/MACRA and on the AGA Community.
Despite potential repeal of the Affordable Care Act under the new administration, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) and commitment to cost-effective, value-based care is here to stay.
Congress overwhelmingly passed MACRA legislation with bipartisan support in both chambers of Congress to overhaul the way physicians are reimbursed under Medicare. MACRA will eventually transition physicians toward more value-based payments. Ignore MACRA in 2017, and you will face an automatic reduction of 4% to your payments under Medicare in 2019.
You should take advantage of 2017 being a transition year during which time you can pick your pace for participation to help you increase your earning potential. If you are already reporting to the 2016 Physician Quality Reporting System (PQRS), you will be familiar with some of the 2017 participation options that could qualify you for a reimbursement incentive in 2019 under MACRA.
If you have not participated in PQRS in 2016 or previous years, you need to start gathering information for your practice to begin reporting through one of the new MACRA 2017 reporting options by Oct. 2, 2017. Quality accounts for the highest percentage of your score and will help you maximize your potential for a positive adjustment.
AGA can help – check out our MACRA resources at gastro.org/MACRA and on the AGA Community.
Despite potential repeal of the Affordable Care Act under the new administration, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) and commitment to cost-effective, value-based care is here to stay.
Congress overwhelmingly passed MACRA legislation with bipartisan support in both chambers of Congress to overhaul the way physicians are reimbursed under Medicare. MACRA will eventually transition physicians toward more value-based payments. Ignore MACRA in 2017, and you will face an automatic reduction of 4% to your payments under Medicare in 2019.
You should take advantage of 2017 being a transition year during which time you can pick your pace for participation to help you increase your earning potential. If you are already reporting to the 2016 Physician Quality Reporting System (PQRS), you will be familiar with some of the 2017 participation options that could qualify you for a reimbursement incentive in 2019 under MACRA.
If you have not participated in PQRS in 2016 or previous years, you need to start gathering information for your practice to begin reporting through one of the new MACRA 2017 reporting options by Oct. 2, 2017. Quality accounts for the highest percentage of your score and will help you maximize your potential for a positive adjustment.
AGA can help – check out our MACRA resources at gastro.org/MACRA and on the AGA Community.
Earn credit while reading AGA journal articles
Now you can read some of your favorite AGA journal articles and receive maintenance of certification (MOC) credit at the same time.
Each issue of Clinical Gastroenterology and Hepatology (CGH)and Gastroenterology includes continuing medical education (CME) exams designated for potential CME, and now MOC as well. The exams, which are available to subscribers, are based on an article from that issue and consist of a single test with short questions, followed by a brief post-test evaluation.
AGA designates certain journal-based CME activities for AMA PRA Category 1 Credit. Successful completion of these CME activities, which includes participation in the evaluation component, enables the participant to earn up to one MOC point in the American Board of Internal Medicine’s (ABIM) MOC program. As AGA works to reform the MOC system, we recognize that many members need to earn points in the current system. Eligible participants will earn MOC points equivalent to the amount of CME credits claimed for the activity.
For more information about logging in and participating, visit the journal sites.
Ready to get started? The March exams for both CGH and Gastroenterology are now available online. You can also access past exams from each publication, but keep in mind that credit can only be earned for up to 1 year after the exam has been published.
Now you can read some of your favorite AGA journal articles and receive maintenance of certification (MOC) credit at the same time.
Each issue of Clinical Gastroenterology and Hepatology (CGH)and Gastroenterology includes continuing medical education (CME) exams designated for potential CME, and now MOC as well. The exams, which are available to subscribers, are based on an article from that issue and consist of a single test with short questions, followed by a brief post-test evaluation.
AGA designates certain journal-based CME activities for AMA PRA Category 1 Credit. Successful completion of these CME activities, which includes participation in the evaluation component, enables the participant to earn up to one MOC point in the American Board of Internal Medicine’s (ABIM) MOC program. As AGA works to reform the MOC system, we recognize that many members need to earn points in the current system. Eligible participants will earn MOC points equivalent to the amount of CME credits claimed for the activity.
For more information about logging in and participating, visit the journal sites.
Ready to get started? The March exams for both CGH and Gastroenterology are now available online. You can also access past exams from each publication, but keep in mind that credit can only be earned for up to 1 year after the exam has been published.
Now you can read some of your favorite AGA journal articles and receive maintenance of certification (MOC) credit at the same time.
Each issue of Clinical Gastroenterology and Hepatology (CGH)and Gastroenterology includes continuing medical education (CME) exams designated for potential CME, and now MOC as well. The exams, which are available to subscribers, are based on an article from that issue and consist of a single test with short questions, followed by a brief post-test evaluation.
AGA designates certain journal-based CME activities for AMA PRA Category 1 Credit. Successful completion of these CME activities, which includes participation in the evaluation component, enables the participant to earn up to one MOC point in the American Board of Internal Medicine’s (ABIM) MOC program. As AGA works to reform the MOC system, we recognize that many members need to earn points in the current system. Eligible participants will earn MOC points equivalent to the amount of CME credits claimed for the activity.
For more information about logging in and participating, visit the journal sites.
Ready to get started? The March exams for both CGH and Gastroenterology are now available online. You can also access past exams from each publication, but keep in mind that credit can only be earned for up to 1 year after the exam has been published.



















