User login
Osteoarthritis in hip or knee can increase diabetes risk
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
FROM THE CRA SCIENTIFIC CONFERENCE
Key clinical point:
Major finding: The risk for diabetes was significantly elevated for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees.
Data source: Population-based cohort study of 16,362 individuals without diabetes at baseline.
Disclosures: The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Hypertension in SLE pregnancy: Is it lupus flare or preeclampsia?
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
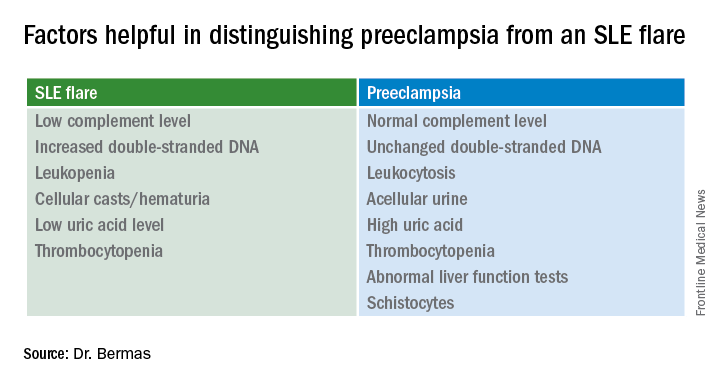
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.
It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.

It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.

It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Flu activity levels high in 23 states
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.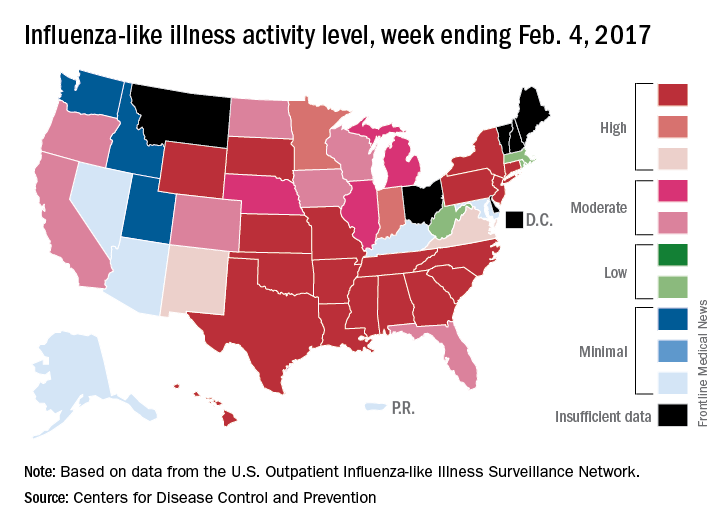
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
Why can’t my patient have that miracle drug?
Modern medicine is truly blessed. Dermatology is no exception. With the development of more precise medications, our patients with severe psoriasis and atopic dermatitis no longer have to suffer in misery and social isolation. There is new hope for patients with metastatic melanoma. I recently watched President Jimmy Carter – a man with melanoma in his brain, certainly dead except for the advent of new drugs that are truly miraculous – release a rehabilitated sea turtle.
What is the drawback to such miracles? Cost! The cost of these medications can be extraordinary (hundreds of thousands of dollars a year); and guess what, everyone wants and needs their insurance plans to foot the bill for them. Biosimilars are not going to solve the cost issue, since biologic drugs are difficult to manufacture and get approved. The biosimilars are pricing in at only 5%-10% less than costs of the original biologic.
Drug costs obviously drive part of the increase in health care premiums. Insurance companies often make drug coverage as difficult as possible, which makes sense from the insurers’ point of view. They require prior authorizations, have restricted formularies, or even insist patients switch biologics in midstream for cost savings or because of manufacturer rebates.
Sometimes a patient has an adverse event, or even dies, because of insurance plan delays. How can this be legal? Isn’t this the practice of medicine? There ought to be a law!
There is a law. Meet the Employee Retirement Income Security Act (ERISA) of 1974 (Meyer JA. ERISA Preemption: Protecting Employer Laboratories of Health Care Reform. Washington, DC: New Directions for Policy; 1995).
ERISA not only protects pensions (and established individual retirement accounts) but also health benefits. ERISA restricts compensation in lawsuits against insurers to the value of the services withheld or delayed and supersedes state laws (State regulation of managed care and the Employee Retirement Income Security Act. Mariner WK, N Engl J Med. 1996 Dec 26;335[26]:1986-90). This minimal payout makes such lawsuits unattractive to law firms. This is why insurers have become so bold in ignoring physician requests for treatment of their patients. The insurers attitude is: “Go ahead, sue me! You won’t get anything!”
Now, as physicians, we are not only patient advocates, but we also must be husbanders of scarce resources. Should we not pursue 100% clearance of that patient with psoriasis? This issue is worth debating, as the medical reimbursement pond gets sucked dry by medication costs.
Still, if you really hate prior authorizations, demented formularies, step therapy, drug denials, and outright stalling of medical care, you should ask Congress to amend ERISA. In writing about Justice Ruth Bader Ginsburg’s concurring Supreme Court opinion in a 2004 case regarding ERISA (Aetna Health Inc. v. Davila 542 U.S. 200), legal expert David S. Senoff said that amending ERISA “may be the only mechanism to provide patients with adequate compensation for damages as a result of coverage decisions by employer-sponsored health plans.” (Senoff DS. An anticipated decision with far-reaching results. Legal Intelligencer. 2004;230:5-7).
Amending ERISA is not going to happen in our current political environment. I’m not even sure I would want it to happen, since it would raise insurance costs even higher, and could make insurance unaffordable for many more people. Still, you and your patients deserve to know the cause of medication denials. Also, I suspect you have no idea how much an insurance executive will twitch (and a liberal member of Congress will smile) when you mention the possibility of amending ERISA. So if you are having a particularly acrimonious argument with an insurance executive about patient drug coverage, pull this nuke out of your arsenal and rap him or her with it.
Dr. Coldiron is past president of the American Academy of Dermatology. He is currently in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Modern medicine is truly blessed. Dermatology is no exception. With the development of more precise medications, our patients with severe psoriasis and atopic dermatitis no longer have to suffer in misery and social isolation. There is new hope for patients with metastatic melanoma. I recently watched President Jimmy Carter – a man with melanoma in his brain, certainly dead except for the advent of new drugs that are truly miraculous – release a rehabilitated sea turtle.
What is the drawback to such miracles? Cost! The cost of these medications can be extraordinary (hundreds of thousands of dollars a year); and guess what, everyone wants and needs their insurance plans to foot the bill for them. Biosimilars are not going to solve the cost issue, since biologic drugs are difficult to manufacture and get approved. The biosimilars are pricing in at only 5%-10% less than costs of the original biologic.
Drug costs obviously drive part of the increase in health care premiums. Insurance companies often make drug coverage as difficult as possible, which makes sense from the insurers’ point of view. They require prior authorizations, have restricted formularies, or even insist patients switch biologics in midstream for cost savings or because of manufacturer rebates.
Sometimes a patient has an adverse event, or even dies, because of insurance plan delays. How can this be legal? Isn’t this the practice of medicine? There ought to be a law!
There is a law. Meet the Employee Retirement Income Security Act (ERISA) of 1974 (Meyer JA. ERISA Preemption: Protecting Employer Laboratories of Health Care Reform. Washington, DC: New Directions for Policy; 1995).
ERISA not only protects pensions (and established individual retirement accounts) but also health benefits. ERISA restricts compensation in lawsuits against insurers to the value of the services withheld or delayed and supersedes state laws (State regulation of managed care and the Employee Retirement Income Security Act. Mariner WK, N Engl J Med. 1996 Dec 26;335[26]:1986-90). This minimal payout makes such lawsuits unattractive to law firms. This is why insurers have become so bold in ignoring physician requests for treatment of their patients. The insurers attitude is: “Go ahead, sue me! You won’t get anything!”
Now, as physicians, we are not only patient advocates, but we also must be husbanders of scarce resources. Should we not pursue 100% clearance of that patient with psoriasis? This issue is worth debating, as the medical reimbursement pond gets sucked dry by medication costs.
Still, if you really hate prior authorizations, demented formularies, step therapy, drug denials, and outright stalling of medical care, you should ask Congress to amend ERISA. In writing about Justice Ruth Bader Ginsburg’s concurring Supreme Court opinion in a 2004 case regarding ERISA (Aetna Health Inc. v. Davila 542 U.S. 200), legal expert David S. Senoff said that amending ERISA “may be the only mechanism to provide patients with adequate compensation for damages as a result of coverage decisions by employer-sponsored health plans.” (Senoff DS. An anticipated decision with far-reaching results. Legal Intelligencer. 2004;230:5-7).
Amending ERISA is not going to happen in our current political environment. I’m not even sure I would want it to happen, since it would raise insurance costs even higher, and could make insurance unaffordable for many more people. Still, you and your patients deserve to know the cause of medication denials. Also, I suspect you have no idea how much an insurance executive will twitch (and a liberal member of Congress will smile) when you mention the possibility of amending ERISA. So if you are having a particularly acrimonious argument with an insurance executive about patient drug coverage, pull this nuke out of your arsenal and rap him or her with it.
Dr. Coldiron is past president of the American Academy of Dermatology. He is currently in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Modern medicine is truly blessed. Dermatology is no exception. With the development of more precise medications, our patients with severe psoriasis and atopic dermatitis no longer have to suffer in misery and social isolation. There is new hope for patients with metastatic melanoma. I recently watched President Jimmy Carter – a man with melanoma in his brain, certainly dead except for the advent of new drugs that are truly miraculous – release a rehabilitated sea turtle.
What is the drawback to such miracles? Cost! The cost of these medications can be extraordinary (hundreds of thousands of dollars a year); and guess what, everyone wants and needs their insurance plans to foot the bill for them. Biosimilars are not going to solve the cost issue, since biologic drugs are difficult to manufacture and get approved. The biosimilars are pricing in at only 5%-10% less than costs of the original biologic.
Drug costs obviously drive part of the increase in health care premiums. Insurance companies often make drug coverage as difficult as possible, which makes sense from the insurers’ point of view. They require prior authorizations, have restricted formularies, or even insist patients switch biologics in midstream for cost savings or because of manufacturer rebates.
Sometimes a patient has an adverse event, or even dies, because of insurance plan delays. How can this be legal? Isn’t this the practice of medicine? There ought to be a law!
There is a law. Meet the Employee Retirement Income Security Act (ERISA) of 1974 (Meyer JA. ERISA Preemption: Protecting Employer Laboratories of Health Care Reform. Washington, DC: New Directions for Policy; 1995).
ERISA not only protects pensions (and established individual retirement accounts) but also health benefits. ERISA restricts compensation in lawsuits against insurers to the value of the services withheld or delayed and supersedes state laws (State regulation of managed care and the Employee Retirement Income Security Act. Mariner WK, N Engl J Med. 1996 Dec 26;335[26]:1986-90). This minimal payout makes such lawsuits unattractive to law firms. This is why insurers have become so bold in ignoring physician requests for treatment of their patients. The insurers attitude is: “Go ahead, sue me! You won’t get anything!”
Now, as physicians, we are not only patient advocates, but we also must be husbanders of scarce resources. Should we not pursue 100% clearance of that patient with psoriasis? This issue is worth debating, as the medical reimbursement pond gets sucked dry by medication costs.
Still, if you really hate prior authorizations, demented formularies, step therapy, drug denials, and outright stalling of medical care, you should ask Congress to amend ERISA. In writing about Justice Ruth Bader Ginsburg’s concurring Supreme Court opinion in a 2004 case regarding ERISA (Aetna Health Inc. v. Davila 542 U.S. 200), legal expert David S. Senoff said that amending ERISA “may be the only mechanism to provide patients with adequate compensation for damages as a result of coverage decisions by employer-sponsored health plans.” (Senoff DS. An anticipated decision with far-reaching results. Legal Intelligencer. 2004;230:5-7).
Amending ERISA is not going to happen in our current political environment. I’m not even sure I would want it to happen, since it would raise insurance costs even higher, and could make insurance unaffordable for many more people. Still, you and your patients deserve to know the cause of medication denials. Also, I suspect you have no idea how much an insurance executive will twitch (and a liberal member of Congress will smile) when you mention the possibility of amending ERISA. So if you are having a particularly acrimonious argument with an insurance executive about patient drug coverage, pull this nuke out of your arsenal and rap him or her with it.
Dr. Coldiron is past president of the American Academy of Dermatology. He is currently in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Low-back pain: CBT, mindfulness benefits diminish over time
For patients with chronic low-back pain, the benefits of cognitive-behavioral therapy and mindfulness-based stress reduction that were reported at 6 months and 1 year largely disappeared by 2-year follow-up, according to a Research Letter to the Editor published Feb 14 in JAMA.
In a clinical trial involving 342 affected patients aged 20-70 years, participants were randomly assigned to receive CBT (113 patients), mindfulness-based stress reduction (MBSR, 116 patients), or usual care (113 patients) and followed up at 26 weeks. The two psychological interventions were delivered in 2-hour group sessions every week for 8 weeks. As previously reported, both CBT and MBSR reduced functional limitations and decreased the “bothersomeness” of the pain, as measured by scores on the modified Roland Disability Questionnaire, compared with usual care, said Daniel C. Cherkin, PhD of the Group Health Research Institute, Seattle, and his associates.
This study was limited in that few participants in the two intervention groups attended all eight of their weekly CBT or MBSR sessions, and only 60% attended six sessions.
Further study is needed to determine whether strategies to increase adherence to these therapies, or “booster sessions” added months after the eight scheduled sessions, would help maintain the short-term benefits, Dr. Cherkin and his associates said.
For patients with chronic low-back pain, the benefits of cognitive-behavioral therapy and mindfulness-based stress reduction that were reported at 6 months and 1 year largely disappeared by 2-year follow-up, according to a Research Letter to the Editor published Feb 14 in JAMA.
In a clinical trial involving 342 affected patients aged 20-70 years, participants were randomly assigned to receive CBT (113 patients), mindfulness-based stress reduction (MBSR, 116 patients), or usual care (113 patients) and followed up at 26 weeks. The two psychological interventions were delivered in 2-hour group sessions every week for 8 weeks. As previously reported, both CBT and MBSR reduced functional limitations and decreased the “bothersomeness” of the pain, as measured by scores on the modified Roland Disability Questionnaire, compared with usual care, said Daniel C. Cherkin, PhD of the Group Health Research Institute, Seattle, and his associates.
This study was limited in that few participants in the two intervention groups attended all eight of their weekly CBT or MBSR sessions, and only 60% attended six sessions.
Further study is needed to determine whether strategies to increase adherence to these therapies, or “booster sessions” added months after the eight scheduled sessions, would help maintain the short-term benefits, Dr. Cherkin and his associates said.
For patients with chronic low-back pain, the benefits of cognitive-behavioral therapy and mindfulness-based stress reduction that were reported at 6 months and 1 year largely disappeared by 2-year follow-up, according to a Research Letter to the Editor published Feb 14 in JAMA.
In a clinical trial involving 342 affected patients aged 20-70 years, participants were randomly assigned to receive CBT (113 patients), mindfulness-based stress reduction (MBSR, 116 patients), or usual care (113 patients) and followed up at 26 weeks. The two psychological interventions were delivered in 2-hour group sessions every week for 8 weeks. As previously reported, both CBT and MBSR reduced functional limitations and decreased the “bothersomeness” of the pain, as measured by scores on the modified Roland Disability Questionnaire, compared with usual care, said Daniel C. Cherkin, PhD of the Group Health Research Institute, Seattle, and his associates.
This study was limited in that few participants in the two intervention groups attended all eight of their weekly CBT or MBSR sessions, and only 60% attended six sessions.
Further study is needed to determine whether strategies to increase adherence to these therapies, or “booster sessions” added months after the eight scheduled sessions, would help maintain the short-term benefits, Dr. Cherkin and his associates said.
FROM JAMA
Key clinical point: For patients with chronic low-back pain, the benefits of cognitive-behavioral therapy and mindfulness-based stress reduction reported at 6 months and 1 year largely disappeared by 2 years.
Major finding: The differences among the three study groups in functional limitations and bothersomeness of back pain were no longer significant at 2 years, even though the proportion of participants who showed 30% or more improvement from baseline on the RDQ remained numerically higher for CBT (62%) and MBSR (55%) than for usual care (42%).
Data source: Extended follow-up of a randomized trial involving 342 adults with chronic low-back pain.
Disclosures: This work was supported by the National Center for Complementary and Integrative Health. Dr. Cherkin and his associates reported having no relevant financial disclosures.
Making single-cell RNA sequencing widely available
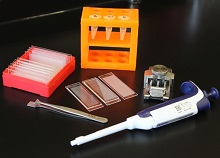
portable technology, Seq-Well, that
can prepare the RNA of many cells
for simultaneous sequencing.
Photo courtesy of Alex K. Shalek
and his colleagues
Researchers say they have developed a portable, low-cost platform for high-throughput, single-cell RNA sequencing.
The
team believes the technology, known as Seq-Well, could allow
scientists to more easily identify different cell types in blood and tissue
samples, helping them study how cancer cells respond to treatment, among other applications.
“Rather than trying to pick one marker that defines a cell type, using single-cell RNA sequencing, we can go in and look at everything a cell is expressing at a given moment,” said Alex K. Shalek, PhD, of the Massachusetts Institute of Technology in Cambridge.
“By finding common patterns across cells, we can figure out who those cells are.”
Dr Shalek and his colleagues have spent the past several years developing single-cell RNA sequencing strategies.
Now, they’ve created a new version of the technology that, they say, can rapidly analyze large numbers of cells using simple equipment.
“We’ve combined [Dr Shalek’s] technologies with some of ours in a way that makes it really accessible for researchers who want to do this type of sequencing on a range of different clinical samples and settings,” said J. Christopher Love, PhD, also of the Massachusetts Institute of Technology.
“It overcomes some of the barriers that are facing the adoption of these techniques more broadly.”
Drs Love and Shalek are the senior authors of a paper describing Seq-Well in Nature Methods.
Improving analysis
Key to sequencing RNA from large populations of cells is keeping track of which RNA transcripts came from which cell. The earliest techniques for this required sorting the cells into individual tubes or compartments of multiwell plates and then separately transforming each into a sequencing library.
That process works well but can’t handle large samples containing thousands of cells, such as blood samples or tissue biopsies, and costs between $25 and $35 per cell.
Dr Shalek and others have recently developed microfluidic techniques to help automate and parallelize the process considerably, but the amount of equipment required makes it impossible to be easily transported.
Drs Shalek and Love realized that technology Dr Love had previously developed to analyze protein secretions from single cells could be adapted to do single-cell RNA sequencing rapidly and inexpensively using a portable device.
Over the past several years, Dr Love’s lab has developed a microscale system that can isolate individual cells and measure the antibodies and other proteins that each cell secretes. The device resembles a tiny ice cube tray, with individual compartments for each cell.
Dr Love also developed a process known as microengraving that uses these trays, which can hold tens of thousands of cells, to measure each cell’s protein secretions.
To use this approach for sequencing RNA, the researchers created arrays of nanowells that each capture a single cell plus a barcoded bead to capture the RNA fragments.
The nanowells are sealed with a semipermeable membrane that allows the passage of chemicals needed to break the cells apart, while the RNA stays contained.
After the RNA binds to the beads, it is removed and sequenced. Using this process, the cost per cell is less than $1.
Uncovering unknowns
Similar to previous single-cell RNA sequencing techniques, the Seq-Well process captures and analyzes about 10% to 15% of the total number of RNA transcripts per cell.
“That is still a very rich set of information that maps to several thousand genes,” Dr Love said. “If you look at sets of these genes, you can start to understand the identity of those cells based on the sets of genes that are expressed in common.”
The researchers used Seq-Well to analyze macrophages infected with tuberculosis, allowing them to identify different pre-existing populations and responses to infection.
Dr Shalek and members of his lab also brought the technology to South Africa and analyzed tissue samples from tuberculosis- and HIV-infected patients there.
“Having a simple system that can go everywhere, I think, is going to be incredibly empowering,” Dr Shalek said.
Dr Love’s lab is now using this approach to analyze immune cells from people with food allergies, which could help researchers determine why some people are more likely to respond well to therapies designed to treat their allergies.
“There are still a lot of unknowns in chronic diseases, and these types of tools help you uncover new insights,” Dr Love said.
The research team has also joined forces with clinical investigators at Dana-Farber/Harvard Cancer Center to apply this technology toward the discovery of new combination immunotherapies for cancers. ![]()

portable technology, Seq-Well, that
can prepare the RNA of many cells
for simultaneous sequencing.
Photo courtesy of Alex K. Shalek
and his colleagues
Researchers say they have developed a portable, low-cost platform for high-throughput, single-cell RNA sequencing.
The
team believes the technology, known as Seq-Well, could allow
scientists to more easily identify different cell types in blood and tissue
samples, helping them study how cancer cells respond to treatment, among other applications.
“Rather than trying to pick one marker that defines a cell type, using single-cell RNA sequencing, we can go in and look at everything a cell is expressing at a given moment,” said Alex K. Shalek, PhD, of the Massachusetts Institute of Technology in Cambridge.
“By finding common patterns across cells, we can figure out who those cells are.”
Dr Shalek and his colleagues have spent the past several years developing single-cell RNA sequencing strategies.
Now, they’ve created a new version of the technology that, they say, can rapidly analyze large numbers of cells using simple equipment.
“We’ve combined [Dr Shalek’s] technologies with some of ours in a way that makes it really accessible for researchers who want to do this type of sequencing on a range of different clinical samples and settings,” said J. Christopher Love, PhD, also of the Massachusetts Institute of Technology.
“It overcomes some of the barriers that are facing the adoption of these techniques more broadly.”
Drs Love and Shalek are the senior authors of a paper describing Seq-Well in Nature Methods.
Improving analysis
Key to sequencing RNA from large populations of cells is keeping track of which RNA transcripts came from which cell. The earliest techniques for this required sorting the cells into individual tubes or compartments of multiwell plates and then separately transforming each into a sequencing library.
That process works well but can’t handle large samples containing thousands of cells, such as blood samples or tissue biopsies, and costs between $25 and $35 per cell.
Dr Shalek and others have recently developed microfluidic techniques to help automate and parallelize the process considerably, but the amount of equipment required makes it impossible to be easily transported.
Drs Shalek and Love realized that technology Dr Love had previously developed to analyze protein secretions from single cells could be adapted to do single-cell RNA sequencing rapidly and inexpensively using a portable device.
Over the past several years, Dr Love’s lab has developed a microscale system that can isolate individual cells and measure the antibodies and other proteins that each cell secretes. The device resembles a tiny ice cube tray, with individual compartments for each cell.
Dr Love also developed a process known as microengraving that uses these trays, which can hold tens of thousands of cells, to measure each cell’s protein secretions.
To use this approach for sequencing RNA, the researchers created arrays of nanowells that each capture a single cell plus a barcoded bead to capture the RNA fragments.
The nanowells are sealed with a semipermeable membrane that allows the passage of chemicals needed to break the cells apart, while the RNA stays contained.
After the RNA binds to the beads, it is removed and sequenced. Using this process, the cost per cell is less than $1.
Uncovering unknowns
Similar to previous single-cell RNA sequencing techniques, the Seq-Well process captures and analyzes about 10% to 15% of the total number of RNA transcripts per cell.
“That is still a very rich set of information that maps to several thousand genes,” Dr Love said. “If you look at sets of these genes, you can start to understand the identity of those cells based on the sets of genes that are expressed in common.”
The researchers used Seq-Well to analyze macrophages infected with tuberculosis, allowing them to identify different pre-existing populations and responses to infection.
Dr Shalek and members of his lab also brought the technology to South Africa and analyzed tissue samples from tuberculosis- and HIV-infected patients there.
“Having a simple system that can go everywhere, I think, is going to be incredibly empowering,” Dr Shalek said.
Dr Love’s lab is now using this approach to analyze immune cells from people with food allergies, which could help researchers determine why some people are more likely to respond well to therapies designed to treat their allergies.
“There are still a lot of unknowns in chronic diseases, and these types of tools help you uncover new insights,” Dr Love said.
The research team has also joined forces with clinical investigators at Dana-Farber/Harvard Cancer Center to apply this technology toward the discovery of new combination immunotherapies for cancers. ![]()

portable technology, Seq-Well, that
can prepare the RNA of many cells
for simultaneous sequencing.
Photo courtesy of Alex K. Shalek
and his colleagues
Researchers say they have developed a portable, low-cost platform for high-throughput, single-cell RNA sequencing.
The
team believes the technology, known as Seq-Well, could allow
scientists to more easily identify different cell types in blood and tissue
samples, helping them study how cancer cells respond to treatment, among other applications.
“Rather than trying to pick one marker that defines a cell type, using single-cell RNA sequencing, we can go in and look at everything a cell is expressing at a given moment,” said Alex K. Shalek, PhD, of the Massachusetts Institute of Technology in Cambridge.
“By finding common patterns across cells, we can figure out who those cells are.”
Dr Shalek and his colleagues have spent the past several years developing single-cell RNA sequencing strategies.
Now, they’ve created a new version of the technology that, they say, can rapidly analyze large numbers of cells using simple equipment.
“We’ve combined [Dr Shalek’s] technologies with some of ours in a way that makes it really accessible for researchers who want to do this type of sequencing on a range of different clinical samples and settings,” said J. Christopher Love, PhD, also of the Massachusetts Institute of Technology.
“It overcomes some of the barriers that are facing the adoption of these techniques more broadly.”
Drs Love and Shalek are the senior authors of a paper describing Seq-Well in Nature Methods.
Improving analysis
Key to sequencing RNA from large populations of cells is keeping track of which RNA transcripts came from which cell. The earliest techniques for this required sorting the cells into individual tubes or compartments of multiwell plates and then separately transforming each into a sequencing library.
That process works well but can’t handle large samples containing thousands of cells, such as blood samples or tissue biopsies, and costs between $25 and $35 per cell.
Dr Shalek and others have recently developed microfluidic techniques to help automate and parallelize the process considerably, but the amount of equipment required makes it impossible to be easily transported.
Drs Shalek and Love realized that technology Dr Love had previously developed to analyze protein secretions from single cells could be adapted to do single-cell RNA sequencing rapidly and inexpensively using a portable device.
Over the past several years, Dr Love’s lab has developed a microscale system that can isolate individual cells and measure the antibodies and other proteins that each cell secretes. The device resembles a tiny ice cube tray, with individual compartments for each cell.
Dr Love also developed a process known as microengraving that uses these trays, which can hold tens of thousands of cells, to measure each cell’s protein secretions.
To use this approach for sequencing RNA, the researchers created arrays of nanowells that each capture a single cell plus a barcoded bead to capture the RNA fragments.
The nanowells are sealed with a semipermeable membrane that allows the passage of chemicals needed to break the cells apart, while the RNA stays contained.
After the RNA binds to the beads, it is removed and sequenced. Using this process, the cost per cell is less than $1.
Uncovering unknowns
Similar to previous single-cell RNA sequencing techniques, the Seq-Well process captures and analyzes about 10% to 15% of the total number of RNA transcripts per cell.
“That is still a very rich set of information that maps to several thousand genes,” Dr Love said. “If you look at sets of these genes, you can start to understand the identity of those cells based on the sets of genes that are expressed in common.”
The researchers used Seq-Well to analyze macrophages infected with tuberculosis, allowing them to identify different pre-existing populations and responses to infection.
Dr Shalek and members of his lab also brought the technology to South Africa and analyzed tissue samples from tuberculosis- and HIV-infected patients there.
“Having a simple system that can go everywhere, I think, is going to be incredibly empowering,” Dr Shalek said.
Dr Love’s lab is now using this approach to analyze immune cells from people with food allergies, which could help researchers determine why some people are more likely to respond well to therapies designed to treat their allergies.
“There are still a lot of unknowns in chronic diseases, and these types of tools help you uncover new insights,” Dr Love said.
The research team has also joined forces with clinical investigators at Dana-Farber/Harvard Cancer Center to apply this technology toward the discovery of new combination immunotherapies for cancers. ![]()
How EBV causes lymphoma, other cancers

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
New research published in Nature Communications appears to explain how Epstein-Barr virus (EBV) reprograms cells into cancer cells.
Investigators said they discovered a mechanism by which EBV particles induce chromosomal instability without establishing a chronic infection, thereby conferring a risk for the development of tumors that do not necessarily carry the viral genome.
“The contribution of the viral infection to cancer development in patients with a weakened immune system is well understood,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
“But in the majority of cases, it remains unclear how an EBV infection leads to cancer development.”
With their research, Dr Delecluse and his colleagues found that BNRF1, a protein component of EBV, promotes the development of cancer. They said BNRF1 induces centrosome amplification, which is associated with chromosomal instability.
When a dividing cell comes in contact with EBV, BNRF1 frequently prompts the formation of an excessive number of centrosomes. As a result, chromosomes are no longer divided equally and accurately between daughter cells—a known cancer risk factor.
In contrast, when the investigators studied EBV deficient of BNRF1, they found the virus did not interfere with chromosome distribution to daughter cells.
The team noted that EBV normally remains silent in a few infected cells, but, occasionally, it reactivates to produce viral offspring that infects nearby cells. As a consequence, these cells come in close contact with BNRF1, thus increasing their risk of transforming into cancer cells.
“The novelty of our work is that we have uncovered a component of the viral particle as a cancer driver,” Dr Delecluse said. “All human-tumors viruses that have been studied so far cause cancer in a completely different manner.”
“Usually, the genetic material of the viruses needs to be permanently present in the infected cell, thus causing the activation of one or several viral genes that cause cancer development. However, these gene products are not present in the infectious particle itself.”
Dr Delecluse and his colleagues therefore suspect that EBV could cause cancers other than those that have already been linked to EBV. Certain cancers might not have been linked to the virus because they do not carry the viral genetic material.
“We must push forward with the development of a vaccine against EBV infection,” Dr Delecluse said. “This would be the most direct strategy to prevent an infection with the virus.”
“Our latest results show that the first infection could already be a cancer risk, and this fits with earlier work that showed an increase in the incidence of Hodgkin’s lymphoma in people who underwent an episode of infectious mononucleosis.” ![]()
G-CSF could prevent infertility in cancer patients

Granulocyte colony-stimulating factor (G-CSF) could prevent infertility in male cancer patients, according to preclinical research published in Reproductive Biology and Endocrinology.
Researchers said they found that G-CSF protects spermatogenesis after alkylating chemotherapy by stimulating the proliferation of surviving spermatogonia.
The team also found evidence to suggest that G-CSF may be useful as a fertility-restoring treatment.
The researchers have been pursuing initiatives to restore fertility in men who have lost their ability to have children as a result of cancer treatments they received as children.
While working on methods to restart sperm production, the team discovered a link between G-CSF and the absence of normal damage to reproductive ability.
“We were using G-CSF to prevent infections in our research experiments,” said study author Brian Hermann, PhD, of The University of Texas at San Antonio.
“It turned out that the drug also had the unexpected impact of guarding against male infertility.”
To test the fertility-related impact of G-CSF, the researchers treated male mice with G-CSF before and/or after treatment with busulfan.
The team then evaluated effects on spermatogenesis in these mice and control mice that only received busulfan.
G-CSF had a protective effect on spermatogenesis that was stable for at least 19 weeks after chemotherapy.
And mice treated with G-CSF for 4 days after busulfan showed modestly enhanced spermatogenic recovery compared to controls.
The researchers said these results suggest G-CSF promotes spermatogonial proliferation, leading to enhanced spermatogenic regeneration from surviving spermatogonial stem cells. ![]()

Granulocyte colony-stimulating factor (G-CSF) could prevent infertility in male cancer patients, according to preclinical research published in Reproductive Biology and Endocrinology.
Researchers said they found that G-CSF protects spermatogenesis after alkylating chemotherapy by stimulating the proliferation of surviving spermatogonia.
The team also found evidence to suggest that G-CSF may be useful as a fertility-restoring treatment.
The researchers have been pursuing initiatives to restore fertility in men who have lost their ability to have children as a result of cancer treatments they received as children.
While working on methods to restart sperm production, the team discovered a link between G-CSF and the absence of normal damage to reproductive ability.
“We were using G-CSF to prevent infections in our research experiments,” said study author Brian Hermann, PhD, of The University of Texas at San Antonio.
“It turned out that the drug also had the unexpected impact of guarding against male infertility.”
To test the fertility-related impact of G-CSF, the researchers treated male mice with G-CSF before and/or after treatment with busulfan.
The team then evaluated effects on spermatogenesis in these mice and control mice that only received busulfan.
G-CSF had a protective effect on spermatogenesis that was stable for at least 19 weeks after chemotherapy.
And mice treated with G-CSF for 4 days after busulfan showed modestly enhanced spermatogenic recovery compared to controls.
The researchers said these results suggest G-CSF promotes spermatogonial proliferation, leading to enhanced spermatogenic regeneration from surviving spermatogonial stem cells. ![]()

Granulocyte colony-stimulating factor (G-CSF) could prevent infertility in male cancer patients, according to preclinical research published in Reproductive Biology and Endocrinology.
Researchers said they found that G-CSF protects spermatogenesis after alkylating chemotherapy by stimulating the proliferation of surviving spermatogonia.
The team also found evidence to suggest that G-CSF may be useful as a fertility-restoring treatment.
The researchers have been pursuing initiatives to restore fertility in men who have lost their ability to have children as a result of cancer treatments they received as children.
While working on methods to restart sperm production, the team discovered a link between G-CSF and the absence of normal damage to reproductive ability.
“We were using G-CSF to prevent infections in our research experiments,” said study author Brian Hermann, PhD, of The University of Texas at San Antonio.
“It turned out that the drug also had the unexpected impact of guarding against male infertility.”
To test the fertility-related impact of G-CSF, the researchers treated male mice with G-CSF before and/or after treatment with busulfan.
The team then evaluated effects on spermatogenesis in these mice and control mice that only received busulfan.
G-CSF had a protective effect on spermatogenesis that was stable for at least 19 weeks after chemotherapy.
And mice treated with G-CSF for 4 days after busulfan showed modestly enhanced spermatogenic recovery compared to controls.
The researchers said these results suggest G-CSF promotes spermatogonial proliferation, leading to enhanced spermatogenic regeneration from surviving spermatogonial stem cells. ![]()
Therapy shows promise for treating hemophilia B

that can develop hemophilia B
A plant-made therapy has demonstrated safety and efficacy in dogs with hemophilia B, according to research published in Molecular Therapy.
Previously, researchers found they could produce freeze-dried lettuce cells expressing human coagulation factor IX (FIX) fused with cholera toxin B subunit (CTB).
These cells were able to prevent inhibitor formation and allergic reactions to intravenous FIX therapy in mice with hemophilia B.
With the current study, the researchers showed that lettuce cells expressing CTB-FIX were safe and could prevent anaphylaxis and inhibitor formation in dogs receiving intravenous FIX to treat hemophilia B.
“The results were quite dramatic,” said study author Henry Daniell, PhD, of the University of Pennsylvania in Philadelphia.
“We corrected blood clotting time in each of the dogs and were able to suppress antibody formation as well. All signs point to this material being ready for the clinic.”
This study made use of Dr Daniell’s patented plant-based drug-production platform, in which genetic modifications enable the growth of plants that have specified human proteins in their leaves.
The researchers grew lettuce that had been modified to produce a fusion protein of human FIX and CTB. CTB helps the fused protein cross the intestinal lining as the lettuce cells are digested by gut microbes, while the plant cell walls protect FIX from digestion.
The researchers said they were able to achieve commercial-scale production of CTB-FIX fusions expressed in lettuce chloroplasts by growing the plants in a hydroponic facility.
The team first tested their product in 2 dogs with hemophilia B. Twice a week for 10 months, the dogs consumed the freeze-dried lettuce material, which was spiked with bacon flavor and sprinkled on their food.
Observing no negative effects of the treatment, the researchers went on to a more robust study, including 4 dogs that were fed the lettuce material and 4 that served as controls.
The 4 dogs in the experimental group were fed the lettuce material for 4 weeks. At that point, they also began receiving weekly injections of FIX, which continued for 8 weeks. The control dogs only received the FIX injections.
All 4 dogs in the control group developed significant levels of antibodies against FIX, and 2 had visible anaphylactic reactions that required the administration of antihistamine.
In contrast, 3 of the 4 dogs in the experimental group had only minimal levels of one type of antibody, IgG2, and no detectable levels of IgG1 or IgE.
The fourth dog in the experimental group had only a partial response to the lettuce cells expressing CTB-FIX. The researchers believe this was due to a pre-existing antibody to human FIX.
Overall, levels of IgG2 were 32 times lower in the experimental group than in the controls.
In addition, the dogs showed no negative side effects from ingesting the lettuce material, and blood samples taken throughout the experiment revealed no signs of toxicity.
“Looking at the dogs that were fed the lettuce material, you can see it’s quite effective,” Dr Daniell said. “They either developed no antibodies to factor IX, or their antibodies went up just a little bit and then came down.”
The next steps for the researchers include additional toxicology and pharmacokinetics studies before applying for an investigational new drug application with the US Food and Drug Administration, a step they hope to take before the end of the year. ![]()

that can develop hemophilia B
A plant-made therapy has demonstrated safety and efficacy in dogs with hemophilia B, according to research published in Molecular Therapy.
Previously, researchers found they could produce freeze-dried lettuce cells expressing human coagulation factor IX (FIX) fused with cholera toxin B subunit (CTB).
These cells were able to prevent inhibitor formation and allergic reactions to intravenous FIX therapy in mice with hemophilia B.
With the current study, the researchers showed that lettuce cells expressing CTB-FIX were safe and could prevent anaphylaxis and inhibitor formation in dogs receiving intravenous FIX to treat hemophilia B.
“The results were quite dramatic,” said study author Henry Daniell, PhD, of the University of Pennsylvania in Philadelphia.
“We corrected blood clotting time in each of the dogs and were able to suppress antibody formation as well. All signs point to this material being ready for the clinic.”
This study made use of Dr Daniell’s patented plant-based drug-production platform, in which genetic modifications enable the growth of plants that have specified human proteins in their leaves.
The researchers grew lettuce that had been modified to produce a fusion protein of human FIX and CTB. CTB helps the fused protein cross the intestinal lining as the lettuce cells are digested by gut microbes, while the plant cell walls protect FIX from digestion.
The researchers said they were able to achieve commercial-scale production of CTB-FIX fusions expressed in lettuce chloroplasts by growing the plants in a hydroponic facility.
The team first tested their product in 2 dogs with hemophilia B. Twice a week for 10 months, the dogs consumed the freeze-dried lettuce material, which was spiked with bacon flavor and sprinkled on their food.
Observing no negative effects of the treatment, the researchers went on to a more robust study, including 4 dogs that were fed the lettuce material and 4 that served as controls.
The 4 dogs in the experimental group were fed the lettuce material for 4 weeks. At that point, they also began receiving weekly injections of FIX, which continued for 8 weeks. The control dogs only received the FIX injections.
All 4 dogs in the control group developed significant levels of antibodies against FIX, and 2 had visible anaphylactic reactions that required the administration of antihistamine.
In contrast, 3 of the 4 dogs in the experimental group had only minimal levels of one type of antibody, IgG2, and no detectable levels of IgG1 or IgE.
The fourth dog in the experimental group had only a partial response to the lettuce cells expressing CTB-FIX. The researchers believe this was due to a pre-existing antibody to human FIX.
Overall, levels of IgG2 were 32 times lower in the experimental group than in the controls.
In addition, the dogs showed no negative side effects from ingesting the lettuce material, and blood samples taken throughout the experiment revealed no signs of toxicity.
“Looking at the dogs that were fed the lettuce material, you can see it’s quite effective,” Dr Daniell said. “They either developed no antibodies to factor IX, or their antibodies went up just a little bit and then came down.”
The next steps for the researchers include additional toxicology and pharmacokinetics studies before applying for an investigational new drug application with the US Food and Drug Administration, a step they hope to take before the end of the year. ![]()

that can develop hemophilia B
A plant-made therapy has demonstrated safety and efficacy in dogs with hemophilia B, according to research published in Molecular Therapy.
Previously, researchers found they could produce freeze-dried lettuce cells expressing human coagulation factor IX (FIX) fused with cholera toxin B subunit (CTB).
These cells were able to prevent inhibitor formation and allergic reactions to intravenous FIX therapy in mice with hemophilia B.
With the current study, the researchers showed that lettuce cells expressing CTB-FIX were safe and could prevent anaphylaxis and inhibitor formation in dogs receiving intravenous FIX to treat hemophilia B.
“The results were quite dramatic,” said study author Henry Daniell, PhD, of the University of Pennsylvania in Philadelphia.
“We corrected blood clotting time in each of the dogs and were able to suppress antibody formation as well. All signs point to this material being ready for the clinic.”
This study made use of Dr Daniell’s patented plant-based drug-production platform, in which genetic modifications enable the growth of plants that have specified human proteins in their leaves.
The researchers grew lettuce that had been modified to produce a fusion protein of human FIX and CTB. CTB helps the fused protein cross the intestinal lining as the lettuce cells are digested by gut microbes, while the plant cell walls protect FIX from digestion.
The researchers said they were able to achieve commercial-scale production of CTB-FIX fusions expressed in lettuce chloroplasts by growing the plants in a hydroponic facility.
The team first tested their product in 2 dogs with hemophilia B. Twice a week for 10 months, the dogs consumed the freeze-dried lettuce material, which was spiked with bacon flavor and sprinkled on their food.
Observing no negative effects of the treatment, the researchers went on to a more robust study, including 4 dogs that were fed the lettuce material and 4 that served as controls.
The 4 dogs in the experimental group were fed the lettuce material for 4 weeks. At that point, they also began receiving weekly injections of FIX, which continued for 8 weeks. The control dogs only received the FIX injections.
All 4 dogs in the control group developed significant levels of antibodies against FIX, and 2 had visible anaphylactic reactions that required the administration of antihistamine.
In contrast, 3 of the 4 dogs in the experimental group had only minimal levels of one type of antibody, IgG2, and no detectable levels of IgG1 or IgE.
The fourth dog in the experimental group had only a partial response to the lettuce cells expressing CTB-FIX. The researchers believe this was due to a pre-existing antibody to human FIX.
Overall, levels of IgG2 were 32 times lower in the experimental group than in the controls.
In addition, the dogs showed no negative side effects from ingesting the lettuce material, and blood samples taken throughout the experiment revealed no signs of toxicity.
“Looking at the dogs that were fed the lettuce material, you can see it’s quite effective,” Dr Daniell said. “They either developed no antibodies to factor IX, or their antibodies went up just a little bit and then came down.”
The next steps for the researchers include additional toxicology and pharmacokinetics studies before applying for an investigational new drug application with the US Food and Drug Administration, a step they hope to take before the end of the year. ![]()
New NCI Formulary May Help Streamline Cancer Clinical Trials
Normally, negotiations to use drugs in preclinical studies and clinical trials can take as long as 18 months. But the National Cancer Institute’s (NCI) new drug formulary will allow investigators at NCI-designated cancer centers quicker access to approved and investigational agents, helping make more effective treatments available sooner.
The NCI Formulary, a public-private partnership between NCI and pharmaceutical and biotechnology companies, is one of NCI’s efforts in support of the Cancer Moonshot (ex-Vice President Biden’s call for greater collaboration and faster development of new therapies). The formulary enables NCI to act as an intermediary between investigators at cancer centers and participating pharmaceutical companies, streamlining arrangements for access to and use of drugs.
The formulary launched with 15 targeted agents from 6 pharmaceutical companies: Bristol-Myers Squibb, Eli Lilly and Company, Genentech, Kyowa Hakko Kirin, Loxo Oncology, and Xcovery Holding Company LLC.
Normally, negotiations to use drugs in preclinical studies and clinical trials can take as long as 18 months. But the National Cancer Institute’s (NCI) new drug formulary will allow investigators at NCI-designated cancer centers quicker access to approved and investigational agents, helping make more effective treatments available sooner.
The NCI Formulary, a public-private partnership between NCI and pharmaceutical and biotechnology companies, is one of NCI’s efforts in support of the Cancer Moonshot (ex-Vice President Biden’s call for greater collaboration and faster development of new therapies). The formulary enables NCI to act as an intermediary between investigators at cancer centers and participating pharmaceutical companies, streamlining arrangements for access to and use of drugs.
The formulary launched with 15 targeted agents from 6 pharmaceutical companies: Bristol-Myers Squibb, Eli Lilly and Company, Genentech, Kyowa Hakko Kirin, Loxo Oncology, and Xcovery Holding Company LLC.
Normally, negotiations to use drugs in preclinical studies and clinical trials can take as long as 18 months. But the National Cancer Institute’s (NCI) new drug formulary will allow investigators at NCI-designated cancer centers quicker access to approved and investigational agents, helping make more effective treatments available sooner.
The NCI Formulary, a public-private partnership between NCI and pharmaceutical and biotechnology companies, is one of NCI’s efforts in support of the Cancer Moonshot (ex-Vice President Biden’s call for greater collaboration and faster development of new therapies). The formulary enables NCI to act as an intermediary between investigators at cancer centers and participating pharmaceutical companies, streamlining arrangements for access to and use of drugs.
The formulary launched with 15 targeted agents from 6 pharmaceutical companies: Bristol-Myers Squibb, Eli Lilly and Company, Genentech, Kyowa Hakko Kirin, Loxo Oncology, and Xcovery Holding Company LLC.