User login
Ginseng Derivatives May Protect Against Flu
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Need an Add-on to Metformin? Consider This
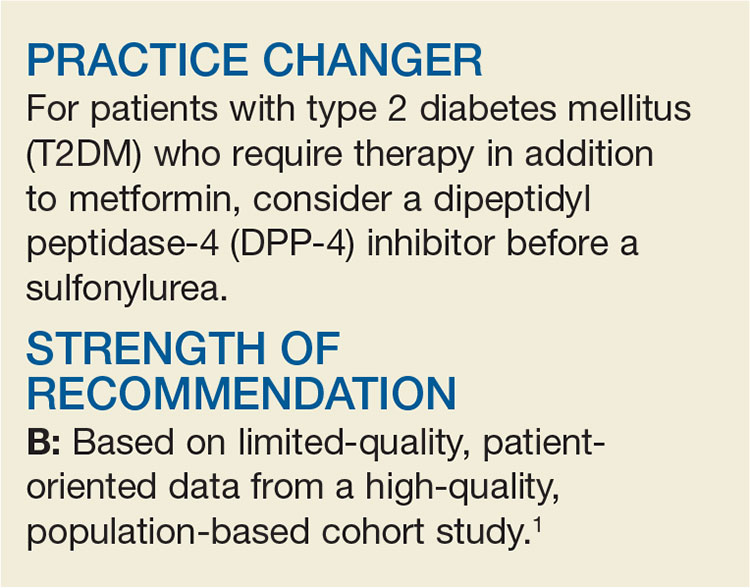
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.

A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.

A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
More readmissions with delays in discharge summaries
Clinical question: Is there an association between time to completion of discharge summary and hospital readmission?
Background: Thirty-day hospital readmission is one of the quality indicators for inpatient care and a higher rate can result in monetary penalties. Several interventions aimed at reducing this occurrence have been studied in different settings with variable success. Timely completion of discharge summary can possibly affect readmissions by providing crucial information to outpatient providers caring for patients across the care continuum.
Study design: Retrospective cohort study.
Setting: Johns Hopkins University, Baltimore.
Bottom line: Delays in completion of discharge summaries was significantly associated with higher rates of hospital readmission. It’s unclear however whether timely completion is a surrogate indicator of other important causative factors.
Citations: Hoyer EH, Odonkor CA, Bhatia SN, et al. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland. J Hosp Med. 2016; 11(6):393-400.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: Is there an association between time to completion of discharge summary and hospital readmission?
Background: Thirty-day hospital readmission is one of the quality indicators for inpatient care and a higher rate can result in monetary penalties. Several interventions aimed at reducing this occurrence have been studied in different settings with variable success. Timely completion of discharge summary can possibly affect readmissions by providing crucial information to outpatient providers caring for patients across the care continuum.
Study design: Retrospective cohort study.
Setting: Johns Hopkins University, Baltimore.
Bottom line: Delays in completion of discharge summaries was significantly associated with higher rates of hospital readmission. It’s unclear however whether timely completion is a surrogate indicator of other important causative factors.
Citations: Hoyer EH, Odonkor CA, Bhatia SN, et al. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland. J Hosp Med. 2016; 11(6):393-400.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: Is there an association between time to completion of discharge summary and hospital readmission?
Background: Thirty-day hospital readmission is one of the quality indicators for inpatient care and a higher rate can result in monetary penalties. Several interventions aimed at reducing this occurrence have been studied in different settings with variable success. Timely completion of discharge summary can possibly affect readmissions by providing crucial information to outpatient providers caring for patients across the care continuum.
Study design: Retrospective cohort study.
Setting: Johns Hopkins University, Baltimore.
Bottom line: Delays in completion of discharge summaries was significantly associated with higher rates of hospital readmission. It’s unclear however whether timely completion is a surrogate indicator of other important causative factors.
Citations: Hoyer EH, Odonkor CA, Bhatia SN, et al. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland. J Hosp Med. 2016; 11(6):393-400.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
AKI is common in young, critically ill adults
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
In CLL, specific mutation is key to ibrutinib resistance
Acquired BTKC481S and PLCG2 mutations led to ibrutinib resistance in chronic lymphocytic leukemia (CLL), investigators reported online in the Journal of Clinical Oncology.
These mutations preceded 85% of clinical relapses, appearing a median of 9.3 months beforehand, Jennifer A. Woyach, MD, and her associates from the Ohio State University, Columbus, concluded from a retrospective study of 308 patients. In a separate prospective study of 112 patients, acquired BTKC481S mutation and clonal expansion preceded all eight cases of relapse, they said. “Relapse of CLL after ibrutinib is an issue of increasing clinical significance,” they concluded. “We show that mutations in Bruton tyrosine kinase (BTK) and PLCG2 appear early and have the potential to be used as a biomarker for future relapse, suggesting an opportunity for intervention.”
Ibrutinib has transformed the CLL treatment landscape, but patients face poor outcomes if they relapse with Richter transformation or develop progressive disease. Past work has linked ibrutinib resistance to acquired mutations in BTK at the binding site of ibrutinib and in PLCG2 located just downstream. But the scope of ibrutinib resistance in CLL and key mutational players were unknown (J Clin Oncol. 2017. doi: 10.1200/JCO.2016.70.2282).
To fill that gap, the researchers retrospectively analyzed data from four sequential ibrutinib CLL trials at the Ohio State University. The separate prospective analysis involved analyzing the entire BTK and PLCG2 coding regions every 3 months.
In the retrospective study, patients had received a median of 3 and up to 16 prior therapies. Given the median follow-up period of 3.4 years, about 19% of patients experienced clinical relapse within 4 years of starting ibrutinib, the researchers estimated (95% confidence interval, 14%-24%). Deep sequencing by Ion Torrent (Life Technologies) identified mutations in BTKC481S and/or PLCG2, in 40 of 47 (85%) relapses. In 31 cases, BTKC481S was the sole mutation. Mutational burdens varied among patients, but generally correlated with CLL progression in peripheral blood versus primarily nodal relapse.
At baseline, 172 (58%) of retrospective study participants had complex cytogenetics, 52% had del(13q), 40% had del(17p), and 21% had MYC abnormality. Median age was 65 years (range, 26-91 years) and 70% of patients were female. Multivariable analyses linked transformation to complex karyotype (hazard ratio, 5.0; 95% CI, 1.5-16.5) and MYC abnormality (HR, 2.5; 95% CI, 1.0-4.7), and linked progressive CLL to age younger than 65 years, complex karyotype, and del(17)(p13.1).
Richter transformation usually occurred within 2 years of starting ibrutinib and had a cumulative 4-year incidence of 10%, the investigators also reported. Patients survived a median of only 3.9 months after stopping ibrutinib because of transformation. The cumulative rate of progressive CLL was higher (19.1%), but early progression was rare, and patients who stopped ibrutinib because of progression survived longer (median, 22.7 months).
In the prospective study, all eight patients with BTKC481S who had not yet clinically relapsed nonetheless had increasing frequency of this mutation over time, the investigators reported. Together, the findings confirm BTK and PLCG2 mutations as the key players in CLL resistance to ibrutinib, they stated. Perhaps most importantly, they reveal “a prolonged period of asymptomatic clonal expression” in CLL that precedes clinical relapse and provides a window of opportunity to target these cells with novel therapies in clinical trials, they wrote.
Given that ibrutinib was approved for use in relapsed CLL only 2 years ago, “We are likely just starting to see the first emergence of relapse in the community setting,” the researchers concluded. “Enhanced knowledge of both the molecular and clinical mechanisms of relapse may allow for strategic alterations in monitoring and management that could change the natural history of ibrutinib resistance.”
Funding sources included the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, the Four Winds Foundation, the Leukemia and Lymphoma Society, Pelotonia, and the National Cancer Institute. Pharmacyclics also provided partial support. Dr. Woyach disclosed ties to Janssen, Acerta Pharma, Karyopharm Therapeutics, and MorphoSys, and a provisional patent related to C481S detection.
Acquired BTKC481S and PLCG2 mutations led to ibrutinib resistance in chronic lymphocytic leukemia (CLL), investigators reported online in the Journal of Clinical Oncology.
These mutations preceded 85% of clinical relapses, appearing a median of 9.3 months beforehand, Jennifer A. Woyach, MD, and her associates from the Ohio State University, Columbus, concluded from a retrospective study of 308 patients. In a separate prospective study of 112 patients, acquired BTKC481S mutation and clonal expansion preceded all eight cases of relapse, they said. “Relapse of CLL after ibrutinib is an issue of increasing clinical significance,” they concluded. “We show that mutations in Bruton tyrosine kinase (BTK) and PLCG2 appear early and have the potential to be used as a biomarker for future relapse, suggesting an opportunity for intervention.”
Ibrutinib has transformed the CLL treatment landscape, but patients face poor outcomes if they relapse with Richter transformation or develop progressive disease. Past work has linked ibrutinib resistance to acquired mutations in BTK at the binding site of ibrutinib and in PLCG2 located just downstream. But the scope of ibrutinib resistance in CLL and key mutational players were unknown (J Clin Oncol. 2017. doi: 10.1200/JCO.2016.70.2282).
To fill that gap, the researchers retrospectively analyzed data from four sequential ibrutinib CLL trials at the Ohio State University. The separate prospective analysis involved analyzing the entire BTK and PLCG2 coding regions every 3 months.
In the retrospective study, patients had received a median of 3 and up to 16 prior therapies. Given the median follow-up period of 3.4 years, about 19% of patients experienced clinical relapse within 4 years of starting ibrutinib, the researchers estimated (95% confidence interval, 14%-24%). Deep sequencing by Ion Torrent (Life Technologies) identified mutations in BTKC481S and/or PLCG2, in 40 of 47 (85%) relapses. In 31 cases, BTKC481S was the sole mutation. Mutational burdens varied among patients, but generally correlated with CLL progression in peripheral blood versus primarily nodal relapse.
At baseline, 172 (58%) of retrospective study participants had complex cytogenetics, 52% had del(13q), 40% had del(17p), and 21% had MYC abnormality. Median age was 65 years (range, 26-91 years) and 70% of patients were female. Multivariable analyses linked transformation to complex karyotype (hazard ratio, 5.0; 95% CI, 1.5-16.5) and MYC abnormality (HR, 2.5; 95% CI, 1.0-4.7), and linked progressive CLL to age younger than 65 years, complex karyotype, and del(17)(p13.1).
Richter transformation usually occurred within 2 years of starting ibrutinib and had a cumulative 4-year incidence of 10%, the investigators also reported. Patients survived a median of only 3.9 months after stopping ibrutinib because of transformation. The cumulative rate of progressive CLL was higher (19.1%), but early progression was rare, and patients who stopped ibrutinib because of progression survived longer (median, 22.7 months).
In the prospective study, all eight patients with BTKC481S who had not yet clinically relapsed nonetheless had increasing frequency of this mutation over time, the investigators reported. Together, the findings confirm BTK and PLCG2 mutations as the key players in CLL resistance to ibrutinib, they stated. Perhaps most importantly, they reveal “a prolonged period of asymptomatic clonal expression” in CLL that precedes clinical relapse and provides a window of opportunity to target these cells with novel therapies in clinical trials, they wrote.
Given that ibrutinib was approved for use in relapsed CLL only 2 years ago, “We are likely just starting to see the first emergence of relapse in the community setting,” the researchers concluded. “Enhanced knowledge of both the molecular and clinical mechanisms of relapse may allow for strategic alterations in monitoring and management that could change the natural history of ibrutinib resistance.”
Funding sources included the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, the Four Winds Foundation, the Leukemia and Lymphoma Society, Pelotonia, and the National Cancer Institute. Pharmacyclics also provided partial support. Dr. Woyach disclosed ties to Janssen, Acerta Pharma, Karyopharm Therapeutics, and MorphoSys, and a provisional patent related to C481S detection.
Acquired BTKC481S and PLCG2 mutations led to ibrutinib resistance in chronic lymphocytic leukemia (CLL), investigators reported online in the Journal of Clinical Oncology.
These mutations preceded 85% of clinical relapses, appearing a median of 9.3 months beforehand, Jennifer A. Woyach, MD, and her associates from the Ohio State University, Columbus, concluded from a retrospective study of 308 patients. In a separate prospective study of 112 patients, acquired BTKC481S mutation and clonal expansion preceded all eight cases of relapse, they said. “Relapse of CLL after ibrutinib is an issue of increasing clinical significance,” they concluded. “We show that mutations in Bruton tyrosine kinase (BTK) and PLCG2 appear early and have the potential to be used as a biomarker for future relapse, suggesting an opportunity for intervention.”
Ibrutinib has transformed the CLL treatment landscape, but patients face poor outcomes if they relapse with Richter transformation or develop progressive disease. Past work has linked ibrutinib resistance to acquired mutations in BTK at the binding site of ibrutinib and in PLCG2 located just downstream. But the scope of ibrutinib resistance in CLL and key mutational players were unknown (J Clin Oncol. 2017. doi: 10.1200/JCO.2016.70.2282).
To fill that gap, the researchers retrospectively analyzed data from four sequential ibrutinib CLL trials at the Ohio State University. The separate prospective analysis involved analyzing the entire BTK and PLCG2 coding regions every 3 months.
In the retrospective study, patients had received a median of 3 and up to 16 prior therapies. Given the median follow-up period of 3.4 years, about 19% of patients experienced clinical relapse within 4 years of starting ibrutinib, the researchers estimated (95% confidence interval, 14%-24%). Deep sequencing by Ion Torrent (Life Technologies) identified mutations in BTKC481S and/or PLCG2, in 40 of 47 (85%) relapses. In 31 cases, BTKC481S was the sole mutation. Mutational burdens varied among patients, but generally correlated with CLL progression in peripheral blood versus primarily nodal relapse.
At baseline, 172 (58%) of retrospective study participants had complex cytogenetics, 52% had del(13q), 40% had del(17p), and 21% had MYC abnormality. Median age was 65 years (range, 26-91 years) and 70% of patients were female. Multivariable analyses linked transformation to complex karyotype (hazard ratio, 5.0; 95% CI, 1.5-16.5) and MYC abnormality (HR, 2.5; 95% CI, 1.0-4.7), and linked progressive CLL to age younger than 65 years, complex karyotype, and del(17)(p13.1).
Richter transformation usually occurred within 2 years of starting ibrutinib and had a cumulative 4-year incidence of 10%, the investigators also reported. Patients survived a median of only 3.9 months after stopping ibrutinib because of transformation. The cumulative rate of progressive CLL was higher (19.1%), but early progression was rare, and patients who stopped ibrutinib because of progression survived longer (median, 22.7 months).
In the prospective study, all eight patients with BTKC481S who had not yet clinically relapsed nonetheless had increasing frequency of this mutation over time, the investigators reported. Together, the findings confirm BTK and PLCG2 mutations as the key players in CLL resistance to ibrutinib, they stated. Perhaps most importantly, they reveal “a prolonged period of asymptomatic clonal expression” in CLL that precedes clinical relapse and provides a window of opportunity to target these cells with novel therapies in clinical trials, they wrote.
Given that ibrutinib was approved for use in relapsed CLL only 2 years ago, “We are likely just starting to see the first emergence of relapse in the community setting,” the researchers concluded. “Enhanced knowledge of both the molecular and clinical mechanisms of relapse may allow for strategic alterations in monitoring and management that could change the natural history of ibrutinib resistance.”
Funding sources included the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, the Four Winds Foundation, the Leukemia and Lymphoma Society, Pelotonia, and the National Cancer Institute. Pharmacyclics also provided partial support. Dr. Woyach disclosed ties to Janssen, Acerta Pharma, Karyopharm Therapeutics, and MorphoSys, and a provisional patent related to C481S detection.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Acquired mutations in BTKC481S and PLCG2 predict ibrutinib resistance in chronic lymphocytic leukemia.
Major finding: These mutations appeared a median of 9.3 months before clinical relapse in 85% of cases. In a separate study, all eight CLL patients who relapsed on ibrutinib had previously developed the BTKC481S mutation with clonal expansion.
Data source: A retrospective analysis of 308 CLL patients from four ibrutinib trials, and a separate prospective study of 118 CLL patients.
Disclosures: Funding sources included the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, the Four Winds Foundation, the Leukemia and Lymphoma Society, Pelotonia, and the National Cancer Institute. Pharmacyclics also provided partial support. Dr. Woyach disclosed ties to Janssen, Acerta Pharma, Karyopharm Therapeutics, and MorphoSys, and a provisional patent related to C481S detection.
Outpatient visits for CNS polypharmacy rising among elderly
The number of outpatient visits for CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, results from a large analysis of national data showed.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” researchers led by Donovan T. Maust, MD, wrote in a research letter published online Feb. 13, 2017, in JAMA Internal Medicine. “Opioids have recently been included in a Beers measure of central nervous system polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the U.S. Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants.”
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults 65 years or older increased from 1.50 million in 2004 to 3.68 million in 2013, or 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio of 3.12; P less than .001). The largest increases were observed among rural visits and those for visits with no mental health or pain diagnoses (aOR of 4.99 and 2.65, respectively.)
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by those who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
Dr. Maust was supported by the Beeson Career Development Award Program. The other researchers reported having no financial disclosures.
The number of outpatient visits for CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, results from a large analysis of national data showed.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” researchers led by Donovan T. Maust, MD, wrote in a research letter published online Feb. 13, 2017, in JAMA Internal Medicine. “Opioids have recently been included in a Beers measure of central nervous system polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the U.S. Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants.”
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults 65 years or older increased from 1.50 million in 2004 to 3.68 million in 2013, or 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio of 3.12; P less than .001). The largest increases were observed among rural visits and those for visits with no mental health or pain diagnoses (aOR of 4.99 and 2.65, respectively.)
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by those who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
Dr. Maust was supported by the Beeson Career Development Award Program. The other researchers reported having no financial disclosures.
The number of outpatient visits for CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, results from a large analysis of national data showed.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” researchers led by Donovan T. Maust, MD, wrote in a research letter published online Feb. 13, 2017, in JAMA Internal Medicine. “Opioids have recently been included in a Beers measure of central nervous system polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the U.S. Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants.”
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults 65 years or older increased from 1.50 million in 2004 to 3.68 million in 2013, or 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio of 3.12; P less than .001). The largest increases were observed among rural visits and those for visits with no mental health or pain diagnoses (aOR of 4.99 and 2.65, respectively.)
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by those who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
Dr. Maust was supported by the Beeson Career Development Award Program. The other researchers reported having no financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Annual CNS polypharmacy visits by adults 65 years or older increased from 1.50 million in 2004 to 3.68 million in 2013.
Data source: An analysis of 97,910 outpatients aged 65 and older from the National Ambulatory Medical Care Survey (NAMCS) from 2004 through 2013.
Disclosures: Dr. Maust was supported by the Beeson Career Development Award Program. The other researchers reported having no financial disclosures.
Guideline: Prioritize nondrug therapies for low back pain
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
FROM ANNALS OF INTERNAL MEDICINE
Reversion mutations detected in cfDNA could guide HGSC treatment
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Five of 30 patients – all in the recurrent disease group – had reversion mutations. Reversion mutations were detected in the cell-free DNA only from those with tumor-detected reversion.
Data source: Plasma and tumor samples from 30 women in the Australian Ovarian Cancer Study.
Disclosures: Dr. Christie reported having no conflicts of interest.
Lipid-lowering meds benefit some breast cancer patients
Patients with early-stage, hormone-receptor positive breast cancer who used cholesterol-lowering medication at baseline of a long-term randomized study had more beneficial tumor characteristics and improved outcomes, compared with nonusers.
The findings come from an observation study of a randomized, phase III, double-blind trial conducted by the Breast International Group (BIG) known as BIG 1-98, which enrolled 8,010 postmenopausal women with early-stage, hormone receptor–positive invasive breast cancer from 1998 to 2003. As reported online in the Feb. 13, 2017 issue of the Journal of Clinical Oncology, researchers measured systemic levels of total cholesterol and use of cholesterol-lowering medication at study entry and every 6 months up to 5.5 years. Endpoints of interest were disease-free survival, breast cancer–free interval, and distant recurrence–free interval.
Of the 789 patients who initiated cholesterol-lowering medication during endocrine therapy, most were assigned to letrozole monotherapy (318), followed by sequential tamoxifen-letrozole (189), letrozole-tamoxifen (176), and tamoxifen monotherapy (106).
The results showed that initiation of cholesterol-lowering medication during endocrine therapy was related to improved disease-free survival (hazard ratio 0.79; P = .01), breast cancer–free interval (HR, 0.76; P = .02), and distant recurrence–free interval (HR, 0.74; P = .03).
“The evidence from our observational study warrants consideration of a large, prospective, randomized clinical trial to confirm the value of CLM concomitant with endocrine treatment of breast cancer,” corresponding author Signe Borgquist, MD, PhD, of the division of oncology and pathology at Lund University, Sweden, and her associates concluded. “Further elucidation of the effect upon outcome of the clinical interaction between CLM and endocrine agents – both widely used by patients with breast cancer – will provide exclusive insight to future trial designs.”
The BIG 1-98 trial was supported by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). Of the 17 study authors, 7 reported having relevant financial disclosures.
[email protected]
Patients with early-stage, hormone-receptor positive breast cancer who used cholesterol-lowering medication at baseline of a long-term randomized study had more beneficial tumor characteristics and improved outcomes, compared with nonusers.
The findings come from an observation study of a randomized, phase III, double-blind trial conducted by the Breast International Group (BIG) known as BIG 1-98, which enrolled 8,010 postmenopausal women with early-stage, hormone receptor–positive invasive breast cancer from 1998 to 2003. As reported online in the Feb. 13, 2017 issue of the Journal of Clinical Oncology, researchers measured systemic levels of total cholesterol and use of cholesterol-lowering medication at study entry and every 6 months up to 5.5 years. Endpoints of interest were disease-free survival, breast cancer–free interval, and distant recurrence–free interval.
Of the 789 patients who initiated cholesterol-lowering medication during endocrine therapy, most were assigned to letrozole monotherapy (318), followed by sequential tamoxifen-letrozole (189), letrozole-tamoxifen (176), and tamoxifen monotherapy (106).
The results showed that initiation of cholesterol-lowering medication during endocrine therapy was related to improved disease-free survival (hazard ratio 0.79; P = .01), breast cancer–free interval (HR, 0.76; P = .02), and distant recurrence–free interval (HR, 0.74; P = .03).
“The evidence from our observational study warrants consideration of a large, prospective, randomized clinical trial to confirm the value of CLM concomitant with endocrine treatment of breast cancer,” corresponding author Signe Borgquist, MD, PhD, of the division of oncology and pathology at Lund University, Sweden, and her associates concluded. “Further elucidation of the effect upon outcome of the clinical interaction between CLM and endocrine agents – both widely used by patients with breast cancer – will provide exclusive insight to future trial designs.”
The BIG 1-98 trial was supported by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). Of the 17 study authors, 7 reported having relevant financial disclosures.
[email protected]
Patients with early-stage, hormone-receptor positive breast cancer who used cholesterol-lowering medication at baseline of a long-term randomized study had more beneficial tumor characteristics and improved outcomes, compared with nonusers.
The findings come from an observation study of a randomized, phase III, double-blind trial conducted by the Breast International Group (BIG) known as BIG 1-98, which enrolled 8,010 postmenopausal women with early-stage, hormone receptor–positive invasive breast cancer from 1998 to 2003. As reported online in the Feb. 13, 2017 issue of the Journal of Clinical Oncology, researchers measured systemic levels of total cholesterol and use of cholesterol-lowering medication at study entry and every 6 months up to 5.5 years. Endpoints of interest were disease-free survival, breast cancer–free interval, and distant recurrence–free interval.
Of the 789 patients who initiated cholesterol-lowering medication during endocrine therapy, most were assigned to letrozole monotherapy (318), followed by sequential tamoxifen-letrozole (189), letrozole-tamoxifen (176), and tamoxifen monotherapy (106).
The results showed that initiation of cholesterol-lowering medication during endocrine therapy was related to improved disease-free survival (hazard ratio 0.79; P = .01), breast cancer–free interval (HR, 0.76; P = .02), and distant recurrence–free interval (HR, 0.74; P = .03).
“The evidence from our observational study warrants consideration of a large, prospective, randomized clinical trial to confirm the value of CLM concomitant with endocrine treatment of breast cancer,” corresponding author Signe Borgquist, MD, PhD, of the division of oncology and pathology at Lund University, Sweden, and her associates concluded. “Further elucidation of the effect upon outcome of the clinical interaction between CLM and endocrine agents – both widely used by patients with breast cancer – will provide exclusive insight to future trial designs.”
The BIG 1-98 trial was supported by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). Of the 17 study authors, 7 reported having relevant financial disclosures.
[email protected]
FROM THE JOURNAL OF CLINICAL ONCOLOGY
New AJCC guidance brings melanoma staging changes
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR