User login
Allergic contact dermatitis
THE COMPARISON
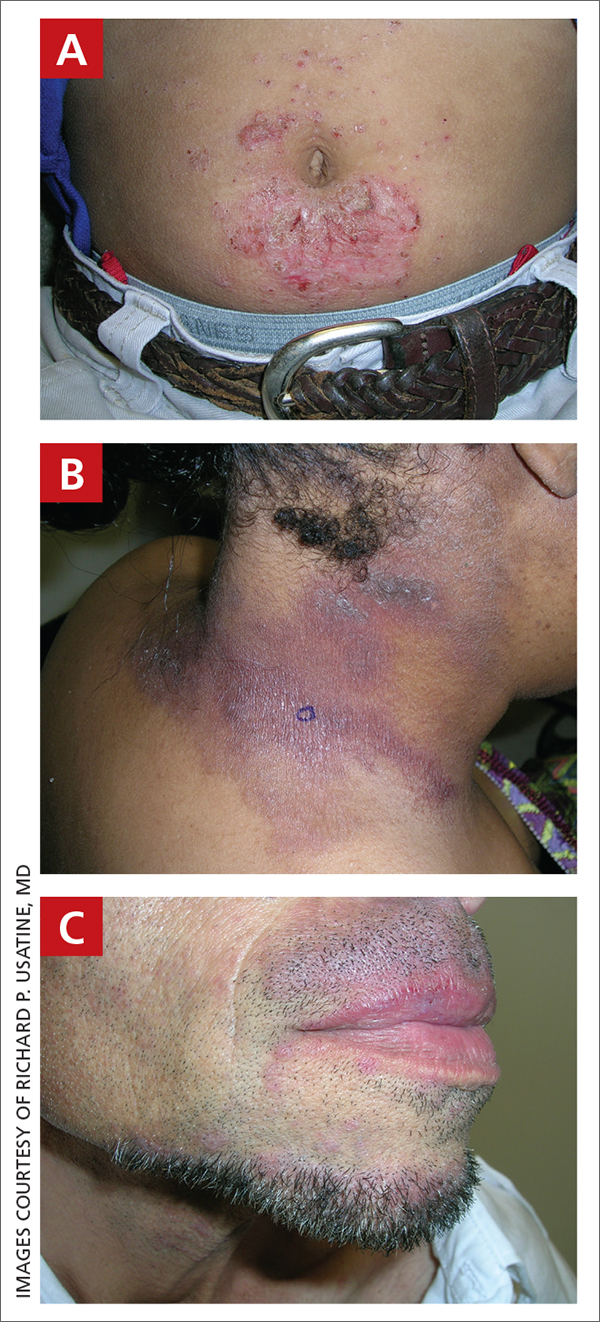
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
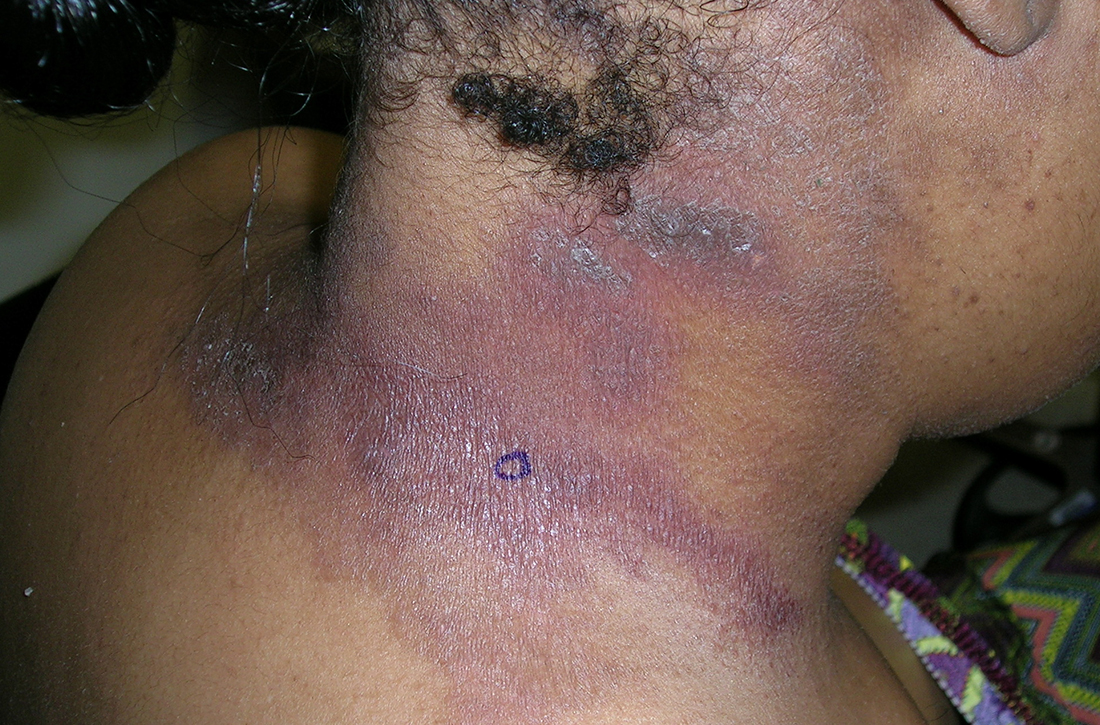
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
New AGA podcast series explores the latest in C. difficile
AGA’s new on-demand program, “C. difficile: Preparing the Field for Change,” is a six-part podcast series that outlines effective approaches to patient-centered care that will transform your practice.
Each 30-minute episode delves into a different topic – from microbiome therapy and FMT to documenting patient history – that will help you improve patient outcomes and reduce the risk of complications.
Tune in and subscribe to our channel Inside Scope wherever you listen to podcasts (Apple or Google). To claim CME credit for listening, visit AGA University (agau.gastro.org).
Episode breakdown
Risk factors
Dr. Paul Feuerstadt and Dr. Sahil Khanna cover risk factors for initial and recurrent C. difficile infection.
Microbiota changes
Dr. Paul Feuerstadt and Dr. Sahil Khanna describe the shifts in the microbiota with C. difficile infection.
Reconciling guideline differences for testing and treatment
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Colleen Kelly to discuss how to reconcile guideline differences for testing and treatment of C. difficile.
Case management and transitions of care
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Rebecca Perez and Cheri Lattimer to discuss case management and transitions of care in C. difficile infection.
Impact of rCDI on patients and their lives
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Dr. Kevin Garey and a patient to discuss the impact of recurrent CDI on patients and their lives.
FMT and new microbiome therapies
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Jessica Allegretti to discuss FMT and new microbiome therapies.
This series is supported by educational grants from Aimmune Therapeutics, Seres Therapeutics, and Ferring Pharmaceuticals.
AGA’s new on-demand program, “C. difficile: Preparing the Field for Change,” is a six-part podcast series that outlines effective approaches to patient-centered care that will transform your practice.
Each 30-minute episode delves into a different topic – from microbiome therapy and FMT to documenting patient history – that will help you improve patient outcomes and reduce the risk of complications.
Tune in and subscribe to our channel Inside Scope wherever you listen to podcasts (Apple or Google). To claim CME credit for listening, visit AGA University (agau.gastro.org).
Episode breakdown
Risk factors
Dr. Paul Feuerstadt and Dr. Sahil Khanna cover risk factors for initial and recurrent C. difficile infection.
Microbiota changes
Dr. Paul Feuerstadt and Dr. Sahil Khanna describe the shifts in the microbiota with C. difficile infection.
Reconciling guideline differences for testing and treatment
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Colleen Kelly to discuss how to reconcile guideline differences for testing and treatment of C. difficile.
Case management and transitions of care
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Rebecca Perez and Cheri Lattimer to discuss case management and transitions of care in C. difficile infection.
Impact of rCDI on patients and their lives
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Dr. Kevin Garey and a patient to discuss the impact of recurrent CDI on patients and their lives.
FMT and new microbiome therapies
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Jessica Allegretti to discuss FMT and new microbiome therapies.
This series is supported by educational grants from Aimmune Therapeutics, Seres Therapeutics, and Ferring Pharmaceuticals.
AGA’s new on-demand program, “C. difficile: Preparing the Field for Change,” is a six-part podcast series that outlines effective approaches to patient-centered care that will transform your practice.
Each 30-minute episode delves into a different topic – from microbiome therapy and FMT to documenting patient history – that will help you improve patient outcomes and reduce the risk of complications.
Tune in and subscribe to our channel Inside Scope wherever you listen to podcasts (Apple or Google). To claim CME credit for listening, visit AGA University (agau.gastro.org).
Episode breakdown
Risk factors
Dr. Paul Feuerstadt and Dr. Sahil Khanna cover risk factors for initial and recurrent C. difficile infection.
Microbiota changes
Dr. Paul Feuerstadt and Dr. Sahil Khanna describe the shifts in the microbiota with C. difficile infection.
Reconciling guideline differences for testing and treatment
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Colleen Kelly to discuss how to reconcile guideline differences for testing and treatment of C. difficile.
Case management and transitions of care
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Rebecca Perez and Cheri Lattimer to discuss case management and transitions of care in C. difficile infection.
Impact of rCDI on patients and their lives
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guests Dr. Kevin Garey and a patient to discuss the impact of recurrent CDI on patients and their lives.
FMT and new microbiome therapies
Dr. Paul Feuerstadt and Dr. Sahil Khanna are joined by guest Dr. Jessica Allegretti to discuss FMT and new microbiome therapies.
This series is supported by educational grants from Aimmune Therapeutics, Seres Therapeutics, and Ferring Pharmaceuticals.
How Medical Education Is Evolving in the Wake of the COVID-19 Pandemic
Question: What doubles every 2 months and takes more than a decade and a half to reach its ultimate destination?
Answer: Medical knowledge.
In 2011, researchers projected that by 2020, medical knowledge would double every 73 days. Also in 2011, investigators estimated that clinical research takes 17 years to translate from bench to bedside.
This “fast-slow” paradox became more relevant than ever in 2020, when the coronavirus pandemic brought the world to a near standstill. Stakeholders in undergraduate, postgraduate, and continuing medical education (CME) were suddenly faced with choices that had been discussed theoretically but not yet applied on a wide scale: How do we deliver education if in-person instruction is not an option?
Organized medicine and the clinical community made choices based on groundwork that had been laid prior to the pandemic. The medical community acted quickly out of necessity, implementing novel learning methods that are now being utilized and that need to be assessed in an ongoing manner.
The Backdrop
Medical education has long been dominated by an in-person, didactic model anchored in teacher-centered, classroom-based learning. This design has been firmly entrenched for more than 100 years, since the publication of the Flexner report in 1910, which established the standard of 4 years of medical education. Prior to 2020, many experts acknowledged that alternative practices and emerging technologies should play a role in medical education, but indecision abounded, perhaps because there was no real-world catalyst for reform. Thus, despite various attempts, the adoption of alternative forms of teaching moved slowly.
Pre-pandemic efforts
In 2017, the American Medication Association issued a report calling for “one of the most complete curricular reforms since the Flexner Report.” It urged leaders to “rethink nearly every facet of physician training,” including “greater emphasis on new technology.” The report also suggested a 14-month pre-rotation program focused on the core medical knowledge necessary to practice in a hospital setting, along with work in a primary care setting once every other week.
Before the pandemic, “blended learning” (digital and live) and “flipped classroom” approaches were assessed. A meta-analysis comparing a blended learning format to traditional classroom model programs found that blended learning resulted in better knowledge outcomes. In the flipped classroom approach, non-classroom individual or group activities replace in-class instruction after pre-class self-preparation with provided resources. A meta-analysis of 28 comparative studies showed that the flipped classroom approach resulted in improved learning compared to traditional methods. Additionally, bite-sized learning approaches have been implemented and evaluated, showing improvement in immediate knowledge recall.
Barriers to widespread implementation
Despite the need to increase medical knowledge dissemination and implement approaches proven to do so effectively, barriers to adoption are well documented. Obstacles include time limitations, inadequate technical skills, insufficient infrastructure, and a wide variety in and range of expertise of both learners and institutional strategies. There are also differences in effective techniques for teaching various topics based on the content. Some topics require knowledge-based training, whereas others fall more easily into skills-based training.
Additionally, when it comes to new evidence that needs to be translated to clinical evaluation and delivery, there is ongoing debate about the established peer-review process, which is rigorous but time-consuming vs the open-access publication process, which can disseminate information more quickly but is prone to error.
Proposed solutions
Proposed solutions to these barriers include improving educator skills, offering incentives for innovative content development, cultivating better institutional strategies, and achieving buy-in from all stakeholders. Also important is thoughtful adaptation of content to various electronic formats, such as audiovisual presentation of educational material, social media content, and gamification of content, as well as ongoing assessment of both education delivery and consumption—followed by rapid pivoting when necessary.
Despite these clearly identified challenges and thoughtful solutions, change was relatively slow until March 2020.
The Trigger
With medical knowledge expanding so rapidly, imagine if medical education moved slowly or came to a complete halt when a worldwide pandemic was declared, the effects would have been catastrophic. COVID pushed organized medicine and the healthcare community to accelerate the adoption of novel technological approaches to keep the medical knowledge pipeline flowing at a relatively reasonable— if not ideal—rate.
Challenges the pandemic produced, along with potential mitigation strategies, are outlined below.
Economic consequences: The pandemic resulted in lost income for training programs and decreased funding for graduate medical education.
Possible solution: Creating budget allowances to adopt new technologies
Impact on diversity, equity, and inclusion: COVID-19 amplified existing implicit and explicit biases in society, particularly in the field of medicine. Women trainees and individuals from disadvantaged backgrounds were disproportionately impacted.
Possible solution: Creating programs that increase awareness of the subtle nature of implicit bias and the outsized impact it can have on certain segments of the population, and offering resources to mitigate stressors such as childcare and access to technology solutions
Impact on mental health and wellness: Working through the pandemic was challenging professionally, and the pandemic also exposed individuals to stigma, loneliness, and behavioral health issues (eg, mood and sleeping disorders), which created challenges in personal lives as well. These challenges lasted well over 2 years and have a clear ongoing impact.
Possible solution: Providing accessible behavioral health resources, regularly assessing and addressing burnout, and regularly cycling trainees off of high-intensity rotations
Education delivery challenges: The sudden cancellation of in-person classes and training, from medical school lectures to rotations, created uncertainty. In-person rounds and bedside learning were significantly restricted. Moreover, as the need to perform clinical duties during the pandemic increased, time for teaching decreased. Some areas were more heavily impacted than others (eg, instruction around elective surgeries, outpatient medicine, and non-critical care training).
Possible solution: Digitizing education delivery and developing other innovative methods to compensate for a lack of face-to-face instruction
Sudden need for rapid information dissemination: The limits of traditional peer review were tested during the pandemic. Managing individuals infected with the novel coronavirus created a situation where the clinical community needed scientific information quickly, increasing the risk of misinformation.
Possible solution: Disseminating information as quickly as possible by leveraging public-private partnerships and government investment in high-quality science while maintaining peer review integrity to ensure rigorous evaluation
The Evidence
Early evidence is emerging about efforts undertaken during the pandemic to maintain adequate levels of preclinical learning, clinical training, and CME.
Preclinical learning: Virtual formats are generally accepted, and interactive discussion is preferred. But be aware of potential stressors.
A cross-sectional study involving 173 histology and pathology students at European University Cyprus found that preclinical medical education is possible via virtual learning. The pandemic forced respondents to adapt immediately to emergency remote teaching. Survey results found the concept was generally well accepted, though some stressors (eg, poor internet connection) impacted perception. Most histology and pathology students (58% and 68%, respectively) said they would prefer blended learning in the future, compared with all-live (39% and 28%, respectively), or all-virtual (4% and 5%, respectively) classrooms.
In a systematic review of 13 studies that compared digital learning with live classroom education for medical and nursing students, investigators from China found that standalone digital models are as effective as conventional modalities for improving knowledge and practice. Moreover, students preferred interactive discussion to a straight lecture format when participating online.
Clinical training: Virtual clerkships work, but a blended approach seems preferable.
In a study involving 16 third-year medical students in the general surgery clerkship at Cleveland Clinic, respondents reported their experience before and after participating in a case-based virtual surgery clerkship program. Students were significantly more confident that they could independently assess a surgical consult after taking the course. Average scores of curriculum-based surgical knowledge increased as well.
In an assessment of alternative approaches to clinical clerkships involving 42 students, investigators from China evaluated the impact of using simulated electronic health records (EHRs) for inpatient training and electronic problem-based learning and virtual interviews for outpatient training. Students using simulated EHRs felt it improved their ability to write in and summarize the record. Those who participated in electronic problem-based learning and virtual interviewing said their interviewing and counseling skills improved. However, students also noted traditional clinical clerkships are better for certain types of learning, suggesting that a blended approach is preferred.
CME: Virtual CME is accepted and improves performance, but barriers remain, including a preference for face-to-face networking.
Researchers reviewed 2,007 post-activity responses from clinicians who participated in online CME at a South Korean hospital. Of the 1332 participants who reported their satisfaction level, 85% reported being satisfied with the format and content. Among all respondents, nearly 9 in 10 said that the content would influence the way they practice. Of the 611 participants who responded to a follow-up survey 3 months later, 78% said they made changes in their clinical practice based on what they learned.
However, many clinicians prefer in-person CME. A Canadian-based memory clinic held 5 interprofessional education sessions and reported on participant experience; 3 of the sessions occurred live before March 2020 and 2 were held via videoconference once the pandemic was declared. Ratings of satisfaction, relevance, knowledge acquisition, and knowledge application were similar in both groups, but the virtual sessions were rated as less enjoyable and lacking in networking opportunities. In-person learning was preferred.
Primary care clinicians in Portugal evaluated a CME digital platform and reported several barriers, including time constraints, perceived excessive work, lack of digital competence, lack of motivation, and emotional factors.
The Future
Although challenges remain, changes due to the pandemic have been implemented in medical training and have shown preliminary success in certain domains. Medical education is rapidly evolving, and as we move further from the pandemic, diligent ongoing evaluation is needed to assess the best use of technology and various innovative teaching modalities. Keeping medical education learner-centered and instituting timely course correction if certain modalities of knowledge/skill delivery are found to be ineffective will be key to ensuring the robustness of training for future generations.
Question: What doubles every 2 months and takes more than a decade and a half to reach its ultimate destination?
Answer: Medical knowledge.
In 2011, researchers projected that by 2020, medical knowledge would double every 73 days. Also in 2011, investigators estimated that clinical research takes 17 years to translate from bench to bedside.
This “fast-slow” paradox became more relevant than ever in 2020, when the coronavirus pandemic brought the world to a near standstill. Stakeholders in undergraduate, postgraduate, and continuing medical education (CME) were suddenly faced with choices that had been discussed theoretically but not yet applied on a wide scale: How do we deliver education if in-person instruction is not an option?
Organized medicine and the clinical community made choices based on groundwork that had been laid prior to the pandemic. The medical community acted quickly out of necessity, implementing novel learning methods that are now being utilized and that need to be assessed in an ongoing manner.
The Backdrop
Medical education has long been dominated by an in-person, didactic model anchored in teacher-centered, classroom-based learning. This design has been firmly entrenched for more than 100 years, since the publication of the Flexner report in 1910, which established the standard of 4 years of medical education. Prior to 2020, many experts acknowledged that alternative practices and emerging technologies should play a role in medical education, but indecision abounded, perhaps because there was no real-world catalyst for reform. Thus, despite various attempts, the adoption of alternative forms of teaching moved slowly.
Pre-pandemic efforts
In 2017, the American Medication Association issued a report calling for “one of the most complete curricular reforms since the Flexner Report.” It urged leaders to “rethink nearly every facet of physician training,” including “greater emphasis on new technology.” The report also suggested a 14-month pre-rotation program focused on the core medical knowledge necessary to practice in a hospital setting, along with work in a primary care setting once every other week.
Before the pandemic, “blended learning” (digital and live) and “flipped classroom” approaches were assessed. A meta-analysis comparing a blended learning format to traditional classroom model programs found that blended learning resulted in better knowledge outcomes. In the flipped classroom approach, non-classroom individual or group activities replace in-class instruction after pre-class self-preparation with provided resources. A meta-analysis of 28 comparative studies showed that the flipped classroom approach resulted in improved learning compared to traditional methods. Additionally, bite-sized learning approaches have been implemented and evaluated, showing improvement in immediate knowledge recall.
Barriers to widespread implementation
Despite the need to increase medical knowledge dissemination and implement approaches proven to do so effectively, barriers to adoption are well documented. Obstacles include time limitations, inadequate technical skills, insufficient infrastructure, and a wide variety in and range of expertise of both learners and institutional strategies. There are also differences in effective techniques for teaching various topics based on the content. Some topics require knowledge-based training, whereas others fall more easily into skills-based training.
Additionally, when it comes to new evidence that needs to be translated to clinical evaluation and delivery, there is ongoing debate about the established peer-review process, which is rigorous but time-consuming vs the open-access publication process, which can disseminate information more quickly but is prone to error.
Proposed solutions
Proposed solutions to these barriers include improving educator skills, offering incentives for innovative content development, cultivating better institutional strategies, and achieving buy-in from all stakeholders. Also important is thoughtful adaptation of content to various electronic formats, such as audiovisual presentation of educational material, social media content, and gamification of content, as well as ongoing assessment of both education delivery and consumption—followed by rapid pivoting when necessary.
Despite these clearly identified challenges and thoughtful solutions, change was relatively slow until March 2020.
The Trigger
With medical knowledge expanding so rapidly, imagine if medical education moved slowly or came to a complete halt when a worldwide pandemic was declared, the effects would have been catastrophic. COVID pushed organized medicine and the healthcare community to accelerate the adoption of novel technological approaches to keep the medical knowledge pipeline flowing at a relatively reasonable— if not ideal—rate.
Challenges the pandemic produced, along with potential mitigation strategies, are outlined below.
Economic consequences: The pandemic resulted in lost income for training programs and decreased funding for graduate medical education.
Possible solution: Creating budget allowances to adopt new technologies
Impact on diversity, equity, and inclusion: COVID-19 amplified existing implicit and explicit biases in society, particularly in the field of medicine. Women trainees and individuals from disadvantaged backgrounds were disproportionately impacted.
Possible solution: Creating programs that increase awareness of the subtle nature of implicit bias and the outsized impact it can have on certain segments of the population, and offering resources to mitigate stressors such as childcare and access to technology solutions
Impact on mental health and wellness: Working through the pandemic was challenging professionally, and the pandemic also exposed individuals to stigma, loneliness, and behavioral health issues (eg, mood and sleeping disorders), which created challenges in personal lives as well. These challenges lasted well over 2 years and have a clear ongoing impact.
Possible solution: Providing accessible behavioral health resources, regularly assessing and addressing burnout, and regularly cycling trainees off of high-intensity rotations
Education delivery challenges: The sudden cancellation of in-person classes and training, from medical school lectures to rotations, created uncertainty. In-person rounds and bedside learning were significantly restricted. Moreover, as the need to perform clinical duties during the pandemic increased, time for teaching decreased. Some areas were more heavily impacted than others (eg, instruction around elective surgeries, outpatient medicine, and non-critical care training).
Possible solution: Digitizing education delivery and developing other innovative methods to compensate for a lack of face-to-face instruction
Sudden need for rapid information dissemination: The limits of traditional peer review were tested during the pandemic. Managing individuals infected with the novel coronavirus created a situation where the clinical community needed scientific information quickly, increasing the risk of misinformation.
Possible solution: Disseminating information as quickly as possible by leveraging public-private partnerships and government investment in high-quality science while maintaining peer review integrity to ensure rigorous evaluation
The Evidence
Early evidence is emerging about efforts undertaken during the pandemic to maintain adequate levels of preclinical learning, clinical training, and CME.
Preclinical learning: Virtual formats are generally accepted, and interactive discussion is preferred. But be aware of potential stressors.
A cross-sectional study involving 173 histology and pathology students at European University Cyprus found that preclinical medical education is possible via virtual learning. The pandemic forced respondents to adapt immediately to emergency remote teaching. Survey results found the concept was generally well accepted, though some stressors (eg, poor internet connection) impacted perception. Most histology and pathology students (58% and 68%, respectively) said they would prefer blended learning in the future, compared with all-live (39% and 28%, respectively), or all-virtual (4% and 5%, respectively) classrooms.
In a systematic review of 13 studies that compared digital learning with live classroom education for medical and nursing students, investigators from China found that standalone digital models are as effective as conventional modalities for improving knowledge and practice. Moreover, students preferred interactive discussion to a straight lecture format when participating online.
Clinical training: Virtual clerkships work, but a blended approach seems preferable.
In a study involving 16 third-year medical students in the general surgery clerkship at Cleveland Clinic, respondents reported their experience before and after participating in a case-based virtual surgery clerkship program. Students were significantly more confident that they could independently assess a surgical consult after taking the course. Average scores of curriculum-based surgical knowledge increased as well.
In an assessment of alternative approaches to clinical clerkships involving 42 students, investigators from China evaluated the impact of using simulated electronic health records (EHRs) for inpatient training and electronic problem-based learning and virtual interviews for outpatient training. Students using simulated EHRs felt it improved their ability to write in and summarize the record. Those who participated in electronic problem-based learning and virtual interviewing said their interviewing and counseling skills improved. However, students also noted traditional clinical clerkships are better for certain types of learning, suggesting that a blended approach is preferred.
CME: Virtual CME is accepted and improves performance, but barriers remain, including a preference for face-to-face networking.
Researchers reviewed 2,007 post-activity responses from clinicians who participated in online CME at a South Korean hospital. Of the 1332 participants who reported their satisfaction level, 85% reported being satisfied with the format and content. Among all respondents, nearly 9 in 10 said that the content would influence the way they practice. Of the 611 participants who responded to a follow-up survey 3 months later, 78% said they made changes in their clinical practice based on what they learned.
However, many clinicians prefer in-person CME. A Canadian-based memory clinic held 5 interprofessional education sessions and reported on participant experience; 3 of the sessions occurred live before March 2020 and 2 were held via videoconference once the pandemic was declared. Ratings of satisfaction, relevance, knowledge acquisition, and knowledge application were similar in both groups, but the virtual sessions were rated as less enjoyable and lacking in networking opportunities. In-person learning was preferred.
Primary care clinicians in Portugal evaluated a CME digital platform and reported several barriers, including time constraints, perceived excessive work, lack of digital competence, lack of motivation, and emotional factors.
The Future
Although challenges remain, changes due to the pandemic have been implemented in medical training and have shown preliminary success in certain domains. Medical education is rapidly evolving, and as we move further from the pandemic, diligent ongoing evaluation is needed to assess the best use of technology and various innovative teaching modalities. Keeping medical education learner-centered and instituting timely course correction if certain modalities of knowledge/skill delivery are found to be ineffective will be key to ensuring the robustness of training for future generations.
Question: What doubles every 2 months and takes more than a decade and a half to reach its ultimate destination?
Answer: Medical knowledge.
In 2011, researchers projected that by 2020, medical knowledge would double every 73 days. Also in 2011, investigators estimated that clinical research takes 17 years to translate from bench to bedside.
This “fast-slow” paradox became more relevant than ever in 2020, when the coronavirus pandemic brought the world to a near standstill. Stakeholders in undergraduate, postgraduate, and continuing medical education (CME) were suddenly faced with choices that had been discussed theoretically but not yet applied on a wide scale: How do we deliver education if in-person instruction is not an option?
Organized medicine and the clinical community made choices based on groundwork that had been laid prior to the pandemic. The medical community acted quickly out of necessity, implementing novel learning methods that are now being utilized and that need to be assessed in an ongoing manner.
The Backdrop
Medical education has long been dominated by an in-person, didactic model anchored in teacher-centered, classroom-based learning. This design has been firmly entrenched for more than 100 years, since the publication of the Flexner report in 1910, which established the standard of 4 years of medical education. Prior to 2020, many experts acknowledged that alternative practices and emerging technologies should play a role in medical education, but indecision abounded, perhaps because there was no real-world catalyst for reform. Thus, despite various attempts, the adoption of alternative forms of teaching moved slowly.
Pre-pandemic efforts
In 2017, the American Medication Association issued a report calling for “one of the most complete curricular reforms since the Flexner Report.” It urged leaders to “rethink nearly every facet of physician training,” including “greater emphasis on new technology.” The report also suggested a 14-month pre-rotation program focused on the core medical knowledge necessary to practice in a hospital setting, along with work in a primary care setting once every other week.
Before the pandemic, “blended learning” (digital and live) and “flipped classroom” approaches were assessed. A meta-analysis comparing a blended learning format to traditional classroom model programs found that blended learning resulted in better knowledge outcomes. In the flipped classroom approach, non-classroom individual or group activities replace in-class instruction after pre-class self-preparation with provided resources. A meta-analysis of 28 comparative studies showed that the flipped classroom approach resulted in improved learning compared to traditional methods. Additionally, bite-sized learning approaches have been implemented and evaluated, showing improvement in immediate knowledge recall.
Barriers to widespread implementation
Despite the need to increase medical knowledge dissemination and implement approaches proven to do so effectively, barriers to adoption are well documented. Obstacles include time limitations, inadequate technical skills, insufficient infrastructure, and a wide variety in and range of expertise of both learners and institutional strategies. There are also differences in effective techniques for teaching various topics based on the content. Some topics require knowledge-based training, whereas others fall more easily into skills-based training.
Additionally, when it comes to new evidence that needs to be translated to clinical evaluation and delivery, there is ongoing debate about the established peer-review process, which is rigorous but time-consuming vs the open-access publication process, which can disseminate information more quickly but is prone to error.
Proposed solutions
Proposed solutions to these barriers include improving educator skills, offering incentives for innovative content development, cultivating better institutional strategies, and achieving buy-in from all stakeholders. Also important is thoughtful adaptation of content to various electronic formats, such as audiovisual presentation of educational material, social media content, and gamification of content, as well as ongoing assessment of both education delivery and consumption—followed by rapid pivoting when necessary.
Despite these clearly identified challenges and thoughtful solutions, change was relatively slow until March 2020.
The Trigger
With medical knowledge expanding so rapidly, imagine if medical education moved slowly or came to a complete halt when a worldwide pandemic was declared, the effects would have been catastrophic. COVID pushed organized medicine and the healthcare community to accelerate the adoption of novel technological approaches to keep the medical knowledge pipeline flowing at a relatively reasonable— if not ideal—rate.
Challenges the pandemic produced, along with potential mitigation strategies, are outlined below.
Economic consequences: The pandemic resulted in lost income for training programs and decreased funding for graduate medical education.
Possible solution: Creating budget allowances to adopt new technologies
Impact on diversity, equity, and inclusion: COVID-19 amplified existing implicit and explicit biases in society, particularly in the field of medicine. Women trainees and individuals from disadvantaged backgrounds were disproportionately impacted.
Possible solution: Creating programs that increase awareness of the subtle nature of implicit bias and the outsized impact it can have on certain segments of the population, and offering resources to mitigate stressors such as childcare and access to technology solutions
Impact on mental health and wellness: Working through the pandemic was challenging professionally, and the pandemic also exposed individuals to stigma, loneliness, and behavioral health issues (eg, mood and sleeping disorders), which created challenges in personal lives as well. These challenges lasted well over 2 years and have a clear ongoing impact.
Possible solution: Providing accessible behavioral health resources, regularly assessing and addressing burnout, and regularly cycling trainees off of high-intensity rotations
Education delivery challenges: The sudden cancellation of in-person classes and training, from medical school lectures to rotations, created uncertainty. In-person rounds and bedside learning were significantly restricted. Moreover, as the need to perform clinical duties during the pandemic increased, time for teaching decreased. Some areas were more heavily impacted than others (eg, instruction around elective surgeries, outpatient medicine, and non-critical care training).
Possible solution: Digitizing education delivery and developing other innovative methods to compensate for a lack of face-to-face instruction
Sudden need for rapid information dissemination: The limits of traditional peer review were tested during the pandemic. Managing individuals infected with the novel coronavirus created a situation where the clinical community needed scientific information quickly, increasing the risk of misinformation.
Possible solution: Disseminating information as quickly as possible by leveraging public-private partnerships and government investment in high-quality science while maintaining peer review integrity to ensure rigorous evaluation
The Evidence
Early evidence is emerging about efforts undertaken during the pandemic to maintain adequate levels of preclinical learning, clinical training, and CME.
Preclinical learning: Virtual formats are generally accepted, and interactive discussion is preferred. But be aware of potential stressors.
A cross-sectional study involving 173 histology and pathology students at European University Cyprus found that preclinical medical education is possible via virtual learning. The pandemic forced respondents to adapt immediately to emergency remote teaching. Survey results found the concept was generally well accepted, though some stressors (eg, poor internet connection) impacted perception. Most histology and pathology students (58% and 68%, respectively) said they would prefer blended learning in the future, compared with all-live (39% and 28%, respectively), or all-virtual (4% and 5%, respectively) classrooms.
In a systematic review of 13 studies that compared digital learning with live classroom education for medical and nursing students, investigators from China found that standalone digital models are as effective as conventional modalities for improving knowledge and practice. Moreover, students preferred interactive discussion to a straight lecture format when participating online.
Clinical training: Virtual clerkships work, but a blended approach seems preferable.
In a study involving 16 third-year medical students in the general surgery clerkship at Cleveland Clinic, respondents reported their experience before and after participating in a case-based virtual surgery clerkship program. Students were significantly more confident that they could independently assess a surgical consult after taking the course. Average scores of curriculum-based surgical knowledge increased as well.
In an assessment of alternative approaches to clinical clerkships involving 42 students, investigators from China evaluated the impact of using simulated electronic health records (EHRs) for inpatient training and electronic problem-based learning and virtual interviews for outpatient training. Students using simulated EHRs felt it improved their ability to write in and summarize the record. Those who participated in electronic problem-based learning and virtual interviewing said their interviewing and counseling skills improved. However, students also noted traditional clinical clerkships are better for certain types of learning, suggesting that a blended approach is preferred.
CME: Virtual CME is accepted and improves performance, but barriers remain, including a preference for face-to-face networking.
Researchers reviewed 2,007 post-activity responses from clinicians who participated in online CME at a South Korean hospital. Of the 1332 participants who reported their satisfaction level, 85% reported being satisfied with the format and content. Among all respondents, nearly 9 in 10 said that the content would influence the way they practice. Of the 611 participants who responded to a follow-up survey 3 months later, 78% said they made changes in their clinical practice based on what they learned.
However, many clinicians prefer in-person CME. A Canadian-based memory clinic held 5 interprofessional education sessions and reported on participant experience; 3 of the sessions occurred live before March 2020 and 2 were held via videoconference once the pandemic was declared. Ratings of satisfaction, relevance, knowledge acquisition, and knowledge application were similar in both groups, but the virtual sessions were rated as less enjoyable and lacking in networking opportunities. In-person learning was preferred.
Primary care clinicians in Portugal evaluated a CME digital platform and reported several barriers, including time constraints, perceived excessive work, lack of digital competence, lack of motivation, and emotional factors.
The Future
Although challenges remain, changes due to the pandemic have been implemented in medical training and have shown preliminary success in certain domains. Medical education is rapidly evolving, and as we move further from the pandemic, diligent ongoing evaluation is needed to assess the best use of technology and various innovative teaching modalities. Keeping medical education learner-centered and instituting timely course correction if certain modalities of knowledge/skill delivery are found to be ineffective will be key to ensuring the robustness of training for future generations.
Nonhormonal medication treatment of VMS
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.
Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
Can a novel, rapid-acting oral treatment effectively manage PPD?
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
Nano drug delivery could overcome toxicity in HCC to enable safer, more effective therapy
leading to safer treatment and better outcomes, according to a recent review.
Nanomedicines homing in on the Wnt/beta-catenin signaling pathway could be particularly impactful, Mamatha Bhat, MD, PhD, a hepatologist and clinician-scientist at Toronto General Hospital Research Institute, and colleagues reported, as this is one of the most up-regulated pathways in HCC.
To date, however, agents addressing this pathway have been hindered by off-target toxicity, suggesting that more work is needed to develop the right payload for nanoparticle delivery, the investigators wrote in Gastro Hep Advances.
“Although nanotherapeutics offers an unmatched improvement in drug delivery, due to the limited impact and treatment-resistance demonstrated by the current systemic therapies, there is currently no approved nanomedicine for the treatment of HCC,” the investigators wrote. “Therefore, it is of utmost importance to dig deeper into understanding the signaling pathways that govern hepatocarcinogenesis and identify novel targets that can be used to develop more specific and targeted nanotherapies.”
Their review focused on the Wnt/beta-catenin signaling pathway, but first, Dr. Bhat and colleagues discussed the characteristics of inorganic versus lipid nanoparticles, as these differences can determine liver uptake.
Inorganic nanoparticles have a high surface-to-volume ratio, leading to increased surface charges that enhance cellular uptake. However, they are prone to oxidation, requiring surface modifications or short circulation times to prevent degradation. These nanoparticles are limited in delivering chemotherapeutic drugs and peptides, and are not suitable for encapsulating nucleic acids.
In contrast, lipid nanoparticles are preferred for targeted delivery in HCC, according to the investigators. They have a natural affinity for apolipoprotein E (apo E), resembling lipoproteins, which aids in specific liver cell targeting. When lipid nanoparticles enter the bloodstream, they interact with apo E–rich lipoproteins like HDL cholesterol and LDL cholesterol, leading to formation of complexes recognized by LDL cholesterol receptors on liver cells. This triggers receptor-mediated endocytosis, internalizing apo E–lipid nanoparticle complexes into HCC cells.
The other major variable is the selected treatment target. Dr. Bhat and colleagues made the case for the Wnt/beta-catenin signaling pathway based on alterations found in approximately two-thirds of patients with HCC.
“Aberrant activation of this pathway and mutations in genes encoding key components are characteristic to hepatocarcinogenesis and promote tumor growth and dedifferentiation,” they wrote.
Although beta-catenin itself makes for an obvious molecular target, especially considering known associations with drug resistance, its flat structure lacks deep binding pockets that would be suitable for small-molecule inhibitors, and any available pockets may be altered by numerous posttranscriptional modifications. Instead, beta-catenin could be indirectly modulated by nanoparticle-mediated siRNA therapy, as this would allow for precise delivery of siRNA to cancer cells, minimizing off-target toxicity.
Alternative approaches could involve targeting proteasomal degradation of beta-catenin, transcriptional coactivators of beta-catenin, or different oncogenes in HCC, all of which are described in further detail in the review, along with promising preclinical findings.
“With ongoing advancements in nanotechnology, there is optimism that it will continue to play a vital role in overcoming the challenges associated with HCC management and contribute to further advancements in therapeutic outcomes for patients,” the authors concluded.
One coauthor disclosed external funding by a Mitacs Elevate postdoctoral fellowship in collaboration with Highland Therapeutics. The remaining authors disclosed no conflicts of interest.
Hepatocellular Carcinoma (HCC) remains a major health problem associate with increasing prevalence and mortality rates worldwide. Around 50-60% of HCC patients are exposed to systemic therapies during their natural history. Atezolizumab plus bevacizumab (median OS: 19.2mo, ORR 30%), and durvalumab plus tremelimumab (median OS: 16.4mo, ORR: 20%) are considered first line treatment options for advanced HCC, and sorafenib or lenvatinib are recommended for patients with any contraindication for immune checkpoint inhibitors. These therapies are indicated for ‘all comers’ an no molecular markers /personalize medicine is currently available for this cancer. The lack of precision oncology relates to the fact that the most common mutations ( i.e TERT, TP53,CTNNB1) are unactionable targets. In this scenario, advances in precision oncology are an unmet medical need.
The Wnt/B-catenin signaling pathway is a master regulator of oncogenesis in HCC and defines one of the molecular subclasses characterized by CTNNB1 mutations (~25-30%) or AXIN1 mutations (~5-10%). Most of these tumors have an immune excluded/desert phenotype. Thus, targeting this pathway is expected to provide a primary antitumoral effect along with an immune-modulatory effect rescuing cases with an immune excluded phenotype.
In this review, the authors discuss the applicability of precision oncology in HCC targeting the WNT/B-catenin pathway by inhibiting the interaction with transcriptional coactivators of B-catenin such as CBP and TCF or by enhancing the proteasomal degradation of B-catenin, reducing pathway activation, with drugs like Tankyrase inhibitors and casein kinase 1a activators. These approaches are challenging due to its associated off-target toxicity and its complexity. To overcome these caveats, the author propose to utilization of nanotechnology to deliver Wnt inhibitors, an approach that currently requires further research to refine the most promising strategies and drugs suitable for clinical implementation.
Josep M. Llovet, MD, PhD, FAASLD, director, Mount Sinai Liver Cancer Program in New York, and head of translational research in the Liver Cancer Group, Liver Unit, IDIBAPS, Hospital Clínic Barcelona. Dr. Llovet receives research support from Bayer Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen.
Hepatocellular Carcinoma (HCC) remains a major health problem associate with increasing prevalence and mortality rates worldwide. Around 50-60% of HCC patients are exposed to systemic therapies during their natural history. Atezolizumab plus bevacizumab (median OS: 19.2mo, ORR 30%), and durvalumab plus tremelimumab (median OS: 16.4mo, ORR: 20%) are considered first line treatment options for advanced HCC, and sorafenib or lenvatinib are recommended for patients with any contraindication for immune checkpoint inhibitors. These therapies are indicated for ‘all comers’ an no molecular markers /personalize medicine is currently available for this cancer. The lack of precision oncology relates to the fact that the most common mutations ( i.e TERT, TP53,CTNNB1) are unactionable targets. In this scenario, advances in precision oncology are an unmet medical need.
The Wnt/B-catenin signaling pathway is a master regulator of oncogenesis in HCC and defines one of the molecular subclasses characterized by CTNNB1 mutations (~25-30%) or AXIN1 mutations (~5-10%). Most of these tumors have an immune excluded/desert phenotype. Thus, targeting this pathway is expected to provide a primary antitumoral effect along with an immune-modulatory effect rescuing cases with an immune excluded phenotype.
In this review, the authors discuss the applicability of precision oncology in HCC targeting the WNT/B-catenin pathway by inhibiting the interaction with transcriptional coactivators of B-catenin such as CBP and TCF or by enhancing the proteasomal degradation of B-catenin, reducing pathway activation, with drugs like Tankyrase inhibitors and casein kinase 1a activators. These approaches are challenging due to its associated off-target toxicity and its complexity. To overcome these caveats, the author propose to utilization of nanotechnology to deliver Wnt inhibitors, an approach that currently requires further research to refine the most promising strategies and drugs suitable for clinical implementation.
Josep M. Llovet, MD, PhD, FAASLD, director, Mount Sinai Liver Cancer Program in New York, and head of translational research in the Liver Cancer Group, Liver Unit, IDIBAPS, Hospital Clínic Barcelona. Dr. Llovet receives research support from Bayer Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen.
Hepatocellular Carcinoma (HCC) remains a major health problem associate with increasing prevalence and mortality rates worldwide. Around 50-60% of HCC patients are exposed to systemic therapies during their natural history. Atezolizumab plus bevacizumab (median OS: 19.2mo, ORR 30%), and durvalumab plus tremelimumab (median OS: 16.4mo, ORR: 20%) are considered first line treatment options for advanced HCC, and sorafenib or lenvatinib are recommended for patients with any contraindication for immune checkpoint inhibitors. These therapies are indicated for ‘all comers’ an no molecular markers /personalize medicine is currently available for this cancer. The lack of precision oncology relates to the fact that the most common mutations ( i.e TERT, TP53,CTNNB1) are unactionable targets. In this scenario, advances in precision oncology are an unmet medical need.
The Wnt/B-catenin signaling pathway is a master regulator of oncogenesis in HCC and defines one of the molecular subclasses characterized by CTNNB1 mutations (~25-30%) or AXIN1 mutations (~5-10%). Most of these tumors have an immune excluded/desert phenotype. Thus, targeting this pathway is expected to provide a primary antitumoral effect along with an immune-modulatory effect rescuing cases with an immune excluded phenotype.
In this review, the authors discuss the applicability of precision oncology in HCC targeting the WNT/B-catenin pathway by inhibiting the interaction with transcriptional coactivators of B-catenin such as CBP and TCF or by enhancing the proteasomal degradation of B-catenin, reducing pathway activation, with drugs like Tankyrase inhibitors and casein kinase 1a activators. These approaches are challenging due to its associated off-target toxicity and its complexity. To overcome these caveats, the author propose to utilization of nanotechnology to deliver Wnt inhibitors, an approach that currently requires further research to refine the most promising strategies and drugs suitable for clinical implementation.
Josep M. Llovet, MD, PhD, FAASLD, director, Mount Sinai Liver Cancer Program in New York, and head of translational research in the Liver Cancer Group, Liver Unit, IDIBAPS, Hospital Clínic Barcelona. Dr. Llovet receives research support from Bayer Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen.
leading to safer treatment and better outcomes, according to a recent review.
Nanomedicines homing in on the Wnt/beta-catenin signaling pathway could be particularly impactful, Mamatha Bhat, MD, PhD, a hepatologist and clinician-scientist at Toronto General Hospital Research Institute, and colleagues reported, as this is one of the most up-regulated pathways in HCC.
To date, however, agents addressing this pathway have been hindered by off-target toxicity, suggesting that more work is needed to develop the right payload for nanoparticle delivery, the investigators wrote in Gastro Hep Advances.
“Although nanotherapeutics offers an unmatched improvement in drug delivery, due to the limited impact and treatment-resistance demonstrated by the current systemic therapies, there is currently no approved nanomedicine for the treatment of HCC,” the investigators wrote. “Therefore, it is of utmost importance to dig deeper into understanding the signaling pathways that govern hepatocarcinogenesis and identify novel targets that can be used to develop more specific and targeted nanotherapies.”
Their review focused on the Wnt/beta-catenin signaling pathway, but first, Dr. Bhat and colleagues discussed the characteristics of inorganic versus lipid nanoparticles, as these differences can determine liver uptake.
Inorganic nanoparticles have a high surface-to-volume ratio, leading to increased surface charges that enhance cellular uptake. However, they are prone to oxidation, requiring surface modifications or short circulation times to prevent degradation. These nanoparticles are limited in delivering chemotherapeutic drugs and peptides, and are not suitable for encapsulating nucleic acids.
In contrast, lipid nanoparticles are preferred for targeted delivery in HCC, according to the investigators. They have a natural affinity for apolipoprotein E (apo E), resembling lipoproteins, which aids in specific liver cell targeting. When lipid nanoparticles enter the bloodstream, they interact with apo E–rich lipoproteins like HDL cholesterol and LDL cholesterol, leading to formation of complexes recognized by LDL cholesterol receptors on liver cells. This triggers receptor-mediated endocytosis, internalizing apo E–lipid nanoparticle complexes into HCC cells.
The other major variable is the selected treatment target. Dr. Bhat and colleagues made the case for the Wnt/beta-catenin signaling pathway based on alterations found in approximately two-thirds of patients with HCC.
“Aberrant activation of this pathway and mutations in genes encoding key components are characteristic to hepatocarcinogenesis and promote tumor growth and dedifferentiation,” they wrote.
Although beta-catenin itself makes for an obvious molecular target, especially considering known associations with drug resistance, its flat structure lacks deep binding pockets that would be suitable for small-molecule inhibitors, and any available pockets may be altered by numerous posttranscriptional modifications. Instead, beta-catenin could be indirectly modulated by nanoparticle-mediated siRNA therapy, as this would allow for precise delivery of siRNA to cancer cells, minimizing off-target toxicity.
Alternative approaches could involve targeting proteasomal degradation of beta-catenin, transcriptional coactivators of beta-catenin, or different oncogenes in HCC, all of which are described in further detail in the review, along with promising preclinical findings.
“With ongoing advancements in nanotechnology, there is optimism that it will continue to play a vital role in overcoming the challenges associated with HCC management and contribute to further advancements in therapeutic outcomes for patients,” the authors concluded.
One coauthor disclosed external funding by a Mitacs Elevate postdoctoral fellowship in collaboration with Highland Therapeutics. The remaining authors disclosed no conflicts of interest.
leading to safer treatment and better outcomes, according to a recent review.
Nanomedicines homing in on the Wnt/beta-catenin signaling pathway could be particularly impactful, Mamatha Bhat, MD, PhD, a hepatologist and clinician-scientist at Toronto General Hospital Research Institute, and colleagues reported, as this is one of the most up-regulated pathways in HCC.
To date, however, agents addressing this pathway have been hindered by off-target toxicity, suggesting that more work is needed to develop the right payload for nanoparticle delivery, the investigators wrote in Gastro Hep Advances.
“Although nanotherapeutics offers an unmatched improvement in drug delivery, due to the limited impact and treatment-resistance demonstrated by the current systemic therapies, there is currently no approved nanomedicine for the treatment of HCC,” the investigators wrote. “Therefore, it is of utmost importance to dig deeper into understanding the signaling pathways that govern hepatocarcinogenesis and identify novel targets that can be used to develop more specific and targeted nanotherapies.”
Their review focused on the Wnt/beta-catenin signaling pathway, but first, Dr. Bhat and colleagues discussed the characteristics of inorganic versus lipid nanoparticles, as these differences can determine liver uptake.
Inorganic nanoparticles have a high surface-to-volume ratio, leading to increased surface charges that enhance cellular uptake. However, they are prone to oxidation, requiring surface modifications or short circulation times to prevent degradation. These nanoparticles are limited in delivering chemotherapeutic drugs and peptides, and are not suitable for encapsulating nucleic acids.
In contrast, lipid nanoparticles are preferred for targeted delivery in HCC, according to the investigators. They have a natural affinity for apolipoprotein E (apo E), resembling lipoproteins, which aids in specific liver cell targeting. When lipid nanoparticles enter the bloodstream, they interact with apo E–rich lipoproteins like HDL cholesterol and LDL cholesterol, leading to formation of complexes recognized by LDL cholesterol receptors on liver cells. This triggers receptor-mediated endocytosis, internalizing apo E–lipid nanoparticle complexes into HCC cells.
The other major variable is the selected treatment target. Dr. Bhat and colleagues made the case for the Wnt/beta-catenin signaling pathway based on alterations found in approximately two-thirds of patients with HCC.
“Aberrant activation of this pathway and mutations in genes encoding key components are characteristic to hepatocarcinogenesis and promote tumor growth and dedifferentiation,” they wrote.
Although beta-catenin itself makes for an obvious molecular target, especially considering known associations with drug resistance, its flat structure lacks deep binding pockets that would be suitable for small-molecule inhibitors, and any available pockets may be altered by numerous posttranscriptional modifications. Instead, beta-catenin could be indirectly modulated by nanoparticle-mediated siRNA therapy, as this would allow for precise delivery of siRNA to cancer cells, minimizing off-target toxicity.
Alternative approaches could involve targeting proteasomal degradation of beta-catenin, transcriptional coactivators of beta-catenin, or different oncogenes in HCC, all of which are described in further detail in the review, along with promising preclinical findings.
“With ongoing advancements in nanotechnology, there is optimism that it will continue to play a vital role in overcoming the challenges associated with HCC management and contribute to further advancements in therapeutic outcomes for patients,” the authors concluded.
One coauthor disclosed external funding by a Mitacs Elevate postdoctoral fellowship in collaboration with Highland Therapeutics. The remaining authors disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
Neutrophils may offer therapeutic target for Wilson’s disease
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Two-thirds with microscopic colitis respond to bile acid sequestrants
, based on a recent retrospective study from the Mayo Clinic.
The findings support use of BAS in patients with microscopic colitis who fail first-line therapy, or have intolerance to those agents, wrote researchers who were led by Darrell S. Pardi, MD, AGAF, a gastroenterologist with Mayo Clinic, Rochester, Minn. To date, the American Gastroenterological Association (AGA) has refrained from issuing any recommendations for BAS monotherapy in microscopic colitis (MC), the study authors wrote, citing a lack of relevant data.
The AGA recommends budesonide as first-line therapy for patients with moderate to severe symptoms of microscopic colitis. However, the treatment is associated with a high rate of relapse (40%-81%) once the patient stops taking the drug. Its long-term use is associated with a risk of side effects.
“At present, there are no randomized controlled trials that have evaluated the efficacy of BAS monotherapy for MC,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Studies investigating the role of BAM [bile acid malabsorption] in MC are comprised of small cohorts and have shown inconsistent results. Patients without BAM may also respond to BAS therapy. Therefore, the role of BAM and treatment with BAS in MC merits further investigation.”
The study analyzed data from 282 patients (88.3% women) with microscopic colitis treated between 2010 to 2020. Bile acid malabsorption was defined by elevated serum 7⍺-hydroxy-4-cholesten-3-one or by fecal testing. After a median follow-up of 4.5 years, cholestyramine was the most prescribed BAS (64.9%), followed by colesevelam (21.6%) and colestipol (13.5%). Approximately half of the patients achieved a complete response (49.3%), while 16.3% had a partial response. Nonresponders accounted for 24.8% of the population, and 9.6% of patients did not tolerate BAS therapy. These outcomes were not significantly impacted by BAS dose or combination with other agents. Additional logistic regression analysis revealed no predictors of response.
After discontinuing BAS, 41.6% of patients had recurrence in a median of 21 weeks, ranging from 1 to 172 weeks.
“Consistent with prior studies evaluating the clinical course of MC and similar to the high rate of recurrence after discontinuation of budesonide, relapse was common after cessation of BAS therapy,” the investigators noted.
Still, the findings suggest that BAS is a valid second-line option with a favorable risk-benefit profile, and an elevated dose appears unnecessary to achieve clinical response.
“These results suggest that BAS may be considered as a treatment option in those that do not respond to first-line options or have intolerance to these agents,” the researchers wrote.
They suggested that BAS may be particularly useful as long-term maintenance therapy for patients wishing to avoid prolonged corticosteroid exposure.
“However, BAS have the potential to interfere with the administration and absorption of other medications, particularly in older populations with polypharmacy,” the researchers wrote. “Patients should thus be closely monitored for drug interactions, especially those taking concurrent medications. BAS should be administered separately from other medications to avoid the potential for drug interactions.”
Ideally, prospective studies will be conducted to confirm these findings, and to identify the subset of patients who are most likely to respond to BAS, the investigators concluded.
Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda; and has consulted for Vedanta, Seres, Phantom Pharmaceuticals, Abbvie, Immunic, and Otsuka.
Despite being increasingly recognized and diagnosed, there remains a scantiness of studies addressing therapeutic options in microscopic colitis (MC). Oral budesonide is recommended as first-line option; however, there is a high relapse rate after budesonide discontinuation, some patients are intolerant, and there is concern for steroid toxicity associated with long-term exposure.
While the cause of MC remains elusive, there is a rationale to suggest that bile acids may play a role in disease pathogenesis. Not only are BA important signaling molecules, acting in inflammation and metabolism, but also prior small studies reported on BA malabsorption co-existing in MC, with variable response rates to BA sequestrants.
This retrospective large study of 282 patients with MC showed that almost two-thirds of patients will present a complete or partial response to BA sequestrant therapy (cholestyramine, colesevelam, and colestipol). For those that relapsed following BA therapy discontinuation, re-treatment was successful in the majority of cases.
Therapy was well tolerated, however caution is needed, as it can interfere with absorption of other medications, and in the long-term also with fat-soluble vitamins. It remains to be determined which patients could benefit the most from BA therapy, since no predictors of response were identified, nor was response associated with BA malabsorption. Nonetheless, this study shows that BA therapy could be an attractive option for steroid-dependent, steroid refractory or intolerant MC patients potentially worth trying before embarking on immunosuppressive or biological therapy. It also highlights the need for carefully conducted clinical trials exploring other options beyond budesonide for this chronic and debilitating condition.
Joana Torres, MD, PhD, is a consultant gastroenterologist at Hospital Beatriz Angelo and Hospital da Luz in Portugal and assistant professor in Uniersidade de Lisboa, Portugal. She has no conflicts.
Despite being increasingly recognized and diagnosed, there remains a scantiness of studies addressing therapeutic options in microscopic colitis (MC). Oral budesonide is recommended as first-line option; however, there is a high relapse rate after budesonide discontinuation, some patients are intolerant, and there is concern for steroid toxicity associated with long-term exposure.
While the cause of MC remains elusive, there is a rationale to suggest that bile acids may play a role in disease pathogenesis. Not only are BA important signaling molecules, acting in inflammation and metabolism, but also prior small studies reported on BA malabsorption co-existing in MC, with variable response rates to BA sequestrants.
This retrospective large study of 282 patients with MC showed that almost two-thirds of patients will present a complete or partial response to BA sequestrant therapy (cholestyramine, colesevelam, and colestipol). For those that relapsed following BA therapy discontinuation, re-treatment was successful in the majority of cases.
Therapy was well tolerated, however caution is needed, as it can interfere with absorption of other medications, and in the long-term also with fat-soluble vitamins. It remains to be determined which patients could benefit the most from BA therapy, since no predictors of response were identified, nor was response associated with BA malabsorption. Nonetheless, this study shows that BA therapy could be an attractive option for steroid-dependent, steroid refractory or intolerant MC patients potentially worth trying before embarking on immunosuppressive or biological therapy. It also highlights the need for carefully conducted clinical trials exploring other options beyond budesonide for this chronic and debilitating condition.
Joana Torres, MD, PhD, is a consultant gastroenterologist at Hospital Beatriz Angelo and Hospital da Luz in Portugal and assistant professor in Uniersidade de Lisboa, Portugal. She has no conflicts.
Despite being increasingly recognized and diagnosed, there remains a scantiness of studies addressing therapeutic options in microscopic colitis (MC). Oral budesonide is recommended as first-line option; however, there is a high relapse rate after budesonide discontinuation, some patients are intolerant, and there is concern for steroid toxicity associated with long-term exposure.
While the cause of MC remains elusive, there is a rationale to suggest that bile acids may play a role in disease pathogenesis. Not only are BA important signaling molecules, acting in inflammation and metabolism, but also prior small studies reported on BA malabsorption co-existing in MC, with variable response rates to BA sequestrants.
This retrospective large study of 282 patients with MC showed that almost two-thirds of patients will present a complete or partial response to BA sequestrant therapy (cholestyramine, colesevelam, and colestipol). For those that relapsed following BA therapy discontinuation, re-treatment was successful in the majority of cases.
Therapy was well tolerated, however caution is needed, as it can interfere with absorption of other medications, and in the long-term also with fat-soluble vitamins. It remains to be determined which patients could benefit the most from BA therapy, since no predictors of response were identified, nor was response associated with BA malabsorption. Nonetheless, this study shows that BA therapy could be an attractive option for steroid-dependent, steroid refractory or intolerant MC patients potentially worth trying before embarking on immunosuppressive or biological therapy. It also highlights the need for carefully conducted clinical trials exploring other options beyond budesonide for this chronic and debilitating condition.
Joana Torres, MD, PhD, is a consultant gastroenterologist at Hospital Beatriz Angelo and Hospital da Luz in Portugal and assistant professor in Uniersidade de Lisboa, Portugal. She has no conflicts.
, based on a recent retrospective study from the Mayo Clinic.
The findings support use of BAS in patients with microscopic colitis who fail first-line therapy, or have intolerance to those agents, wrote researchers who were led by Darrell S. Pardi, MD, AGAF, a gastroenterologist with Mayo Clinic, Rochester, Minn. To date, the American Gastroenterological Association (AGA) has refrained from issuing any recommendations for BAS monotherapy in microscopic colitis (MC), the study authors wrote, citing a lack of relevant data.
The AGA recommends budesonide as first-line therapy for patients with moderate to severe symptoms of microscopic colitis. However, the treatment is associated with a high rate of relapse (40%-81%) once the patient stops taking the drug. Its long-term use is associated with a risk of side effects.
“At present, there are no randomized controlled trials that have evaluated the efficacy of BAS monotherapy for MC,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Studies investigating the role of BAM [bile acid malabsorption] in MC are comprised of small cohorts and have shown inconsistent results. Patients without BAM may also respond to BAS therapy. Therefore, the role of BAM and treatment with BAS in MC merits further investigation.”
The study analyzed data from 282 patients (88.3% women) with microscopic colitis treated between 2010 to 2020. Bile acid malabsorption was defined by elevated serum 7⍺-hydroxy-4-cholesten-3-one or by fecal testing. After a median follow-up of 4.5 years, cholestyramine was the most prescribed BAS (64.9%), followed by colesevelam (21.6%) and colestipol (13.5%). Approximately half of the patients achieved a complete response (49.3%), while 16.3% had a partial response. Nonresponders accounted for 24.8% of the population, and 9.6% of patients did not tolerate BAS therapy. These outcomes were not significantly impacted by BAS dose or combination with other agents. Additional logistic regression analysis revealed no predictors of response.
After discontinuing BAS, 41.6% of patients had recurrence in a median of 21 weeks, ranging from 1 to 172 weeks.
“Consistent with prior studies evaluating the clinical course of MC and similar to the high rate of recurrence after discontinuation of budesonide, relapse was common after cessation of BAS therapy,” the investigators noted.
Still, the findings suggest that BAS is a valid second-line option with a favorable risk-benefit profile, and an elevated dose appears unnecessary to achieve clinical response.
“These results suggest that BAS may be considered as a treatment option in those that do not respond to first-line options or have intolerance to these agents,” the researchers wrote.
They suggested that BAS may be particularly useful as long-term maintenance therapy for patients wishing to avoid prolonged corticosteroid exposure.
“However, BAS have the potential to interfere with the administration and absorption of other medications, particularly in older populations with polypharmacy,” the researchers wrote. “Patients should thus be closely monitored for drug interactions, especially those taking concurrent medications. BAS should be administered separately from other medications to avoid the potential for drug interactions.”
Ideally, prospective studies will be conducted to confirm these findings, and to identify the subset of patients who are most likely to respond to BAS, the investigators concluded.
Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda; and has consulted for Vedanta, Seres, Phantom Pharmaceuticals, Abbvie, Immunic, and Otsuka.
, based on a recent retrospective study from the Mayo Clinic.
The findings support use of BAS in patients with microscopic colitis who fail first-line therapy, or have intolerance to those agents, wrote researchers who were led by Darrell S. Pardi, MD, AGAF, a gastroenterologist with Mayo Clinic, Rochester, Minn. To date, the American Gastroenterological Association (AGA) has refrained from issuing any recommendations for BAS monotherapy in microscopic colitis (MC), the study authors wrote, citing a lack of relevant data.
The AGA recommends budesonide as first-line therapy for patients with moderate to severe symptoms of microscopic colitis. However, the treatment is associated with a high rate of relapse (40%-81%) once the patient stops taking the drug. Its long-term use is associated with a risk of side effects.
“At present, there are no randomized controlled trials that have evaluated the efficacy of BAS monotherapy for MC,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Studies investigating the role of BAM [bile acid malabsorption] in MC are comprised of small cohorts and have shown inconsistent results. Patients without BAM may also respond to BAS therapy. Therefore, the role of BAM and treatment with BAS in MC merits further investigation.”
The study analyzed data from 282 patients (88.3% women) with microscopic colitis treated between 2010 to 2020. Bile acid malabsorption was defined by elevated serum 7⍺-hydroxy-4-cholesten-3-one or by fecal testing. After a median follow-up of 4.5 years, cholestyramine was the most prescribed BAS (64.9%), followed by colesevelam (21.6%) and colestipol (13.5%). Approximately half of the patients achieved a complete response (49.3%), while 16.3% had a partial response. Nonresponders accounted for 24.8% of the population, and 9.6% of patients did not tolerate BAS therapy. These outcomes were not significantly impacted by BAS dose or combination with other agents. Additional logistic regression analysis revealed no predictors of response.
After discontinuing BAS, 41.6% of patients had recurrence in a median of 21 weeks, ranging from 1 to 172 weeks.
“Consistent with prior studies evaluating the clinical course of MC and similar to the high rate of recurrence after discontinuation of budesonide, relapse was common after cessation of BAS therapy,” the investigators noted.
Still, the findings suggest that BAS is a valid second-line option with a favorable risk-benefit profile, and an elevated dose appears unnecessary to achieve clinical response.
“These results suggest that BAS may be considered as a treatment option in those that do not respond to first-line options or have intolerance to these agents,” the researchers wrote.
They suggested that BAS may be particularly useful as long-term maintenance therapy for patients wishing to avoid prolonged corticosteroid exposure.
“However, BAS have the potential to interfere with the administration and absorption of other medications, particularly in older populations with polypharmacy,” the researchers wrote. “Patients should thus be closely monitored for drug interactions, especially those taking concurrent medications. BAS should be administered separately from other medications to avoid the potential for drug interactions.”
Ideally, prospective studies will be conducted to confirm these findings, and to identify the subset of patients who are most likely to respond to BAS, the investigators concluded.
Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda; and has consulted for Vedanta, Seres, Phantom Pharmaceuticals, Abbvie, Immunic, and Otsuka.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Survival on the upswing in myeloma
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Back then, the treatments for MM were chemotherapy and steroids. Stem-cell transplants were on the horizon, as was a most unexpected therapy: the infamous drug thalidomide.
But in the wake of the rib facture, the health of Stanley Katz, MD, worsened and he died after 25 weeks, Dr. Berenson recalled in an interview. At that time, Dr. Katz’s horrifically shortened lifespan following diagnosis was not unusual.
About 4 decades later, hematologists like Dr. Berenson are heralding a new era in MM, a sharp reversal of the previous eras of grim prognoses.
In a new study, Dr. Berenson tracked 161 patients with MM treated at his West Hollywood, Calif., private clinic from 2006 to 2023 and found that their median survival was 136.2 months – more than 11 years. “The OS reported in this study ... is the longest reported to date in an unselected, newly diagnosed MM population,” the study authors write.
Dr. Berenson’s patients are unique: They’re largely White, and they didn’t undergo stem-cell transplants. But other recent studies also suggest that lifespans of more than 10 years are increasingly possible after MM diagnosis. Former TV news anchor Tom Brokaw, for one, has reached that point.
In fact, a pair of other hematologists say the overall survival in Dr. Berenson’s report is hardly out of the question. And, they say, patients diagnosed today could potentially live even longer, because treatments continue to improve.
“With data that’s 10 years old, we expect the median overall survival to be 10 years,” hematologist Sagar Lonial, MD, who’s been tracking survival data in MM, said in an interview. “When patients ask about my outlook, I say it’s a constantly evolving field. Things are changing fast enough that I use 10 years as a floor.”
Dr. Lonial is chair of the department of hematology and medical oncology and chief medical officer at Emory University, Atlanta, Winship Cancer Institute.
Hematologist Rafael Fonseca, MD, chief innovation officer at Mayo Clinic–Arizona, put it this way in an interview: Dr. Berenson’s results “are probably in sync with what we would anticipate with similar cohorts of patients. The reality is that we’ve seen a huge improvement in the life expectancy of patients who were diagnosed with multiple myeloma. It’s not unusual to see patients in the clinic now that are 15 or 20 years out from their diagnosis.”
According to Yale Medicine, MM accounts for 10% of blood cancers and 1%-2% of all cancers and is more common in men vs. women and Blacks vs. Whites. It’s most frequently diagnosed between the ages of 65 and 74, according to the National Cancer Institute, and the median age at diagnosis is 69.
Among the most famous American people currently battling MM are newsman Mr. Brokaw, the former NBC News anchor, and Republican Congressman Steve Scalise, majority leader of the U.S. House of Representatives and a candidate for House speaker.
Mr. Brokaw was diagnosed in 2013 while in his early 70s and has talked about his intense struggle with the disease: the infections, operations, infusions, and daily regimens of 24 pills.
“I didn’t want to be Tom Brokaw, cancer victim,” he said in 2018 at the annual meeting of the American Society of Hematology. But he opened up about his illness, and became “the multiple myeloma poster boy.”
Rep. Scalise, who’s in his late 50s, is undergoing chemotherapy. He survived being gravely wounded in an assassination attempt in 2017.
Dr. Berenson’s new study, published in Targeted Oncology, tracked 161 patients (89 women, 72 men; median age, 65.4; 125 White, 3 Black, 10 Hispanic, 15 Asian, and 8 multi-ethnic).
All started frontline treatment at Dr. Berenson’s clinic and were included if they could read consent forms and gave permission for blood draws. None underwent stem-cell transplants as part of initial therapy. Another 1,036 patients had been treated elsewhere and were not included in the study.
Over a median of 42.7 months (range, 1.9-195.1), the 1-, 3-, and 5-year survival rates were 97.5%, 85.3%, and 76.2%, respectively.
The study claims “these results are considerably better than those reported from patients enrolled in clinical trials and those from countries with national registries.”
In the interview, Dr. Berenson said the study is unique because it’s not limited like many studies to younger, healthier patients. Nor does it include those treated at other facilities, he said.
The study is unusual in other ways. Dr. Berenson said his drug regimens aren’t necessarily standard, and he doesn’t treat patients with stem-cell transplants. “I stopped transplanting in about 2000 because clearly it was not improving the length of life,” he said.
Dr. Berenson said colleagues can learn from his insistence on sensitively treating the quality of life of patients, his embrace of clinical trials with novel combinations, and his close monitoring of myeloma proteins to gauge whether patients need to rapidly switch therapies.
He noted that his drug regimens are typically off-label and vary by patient. “We’re not using as high doses of drugs like Velcade [bortezomib] or Revlimid [lenalidomide] as my colleagues. We’re not necessarily giving as many doses. Also, we’re not adding as many drugs in many cases as they are. We’re taking it slower.”
The National Comprehensive Cancer Network recommends bortezomib and lenalidomide as standard induction treatments in patients with MM who are candidates for stem-cell transplantation, a procedure it considers the “preferred approach in transplant-eligible patients.”
There are limitations to Dr. Berenson’s new study. The patients aren’t representative of people with MM as a whole: His cohort is overwhelmingly White (78%) and just 2% Black, while an estimated one-fifth of patients with MM in the United States are Black and have poorer outcomes.
Dr. Berenson also acknowledged that his patients are most likely a wealthier group, although he said it’s not feasible to ask them about income. The study provides no information about socioeconomics.
Dr. Lonial said survival of 10-11 years is fairly typical in MM, with standard-risk patients reaching 14 years.
He highlighted a 2021 Canadian study that tracked 3,030 patients with newly diagnosed MM from 2007 to 2018 (average age, 64; 58.6% men). Those who received an upfront autologous stem-cell transplant had a median overall survival of 122.0 months (95% confidence interval, 115.0-135.0 months) vs. 54.3 months (95% CI, 50.8-58.8 months) for those who didn’t get the transplants. Not surprisingly, survival dipped with each subsequent treatment regimen.
Dr. Lonial is coauthor of a 2020 study that tracked 1,000 consecutive patients (mean age, 61; 35.2% Black) with newly diagnosed myeloma who were treated with RVD (lenalidomide, bortezomib, and dexamethasone) induction therapy from 2007 to 2016. The median overall survival was 126.6 months (95% CI, 113.3-139.8 months).
Dr. Fonseca noted that the news about MM survival rates is not entirely positive. Patients with high-risk disease often die early on in their disease course, he said.
Research suggests even the youngest patients with MM may die within years of diagnosis. A 2021 French study tracked 214 patients in the 18-40 age group for 15 years (2000-2015). At 5 years, “relative survival compared with same age- and sex-matched individuals was 83.5%,” and estimated overall survival was 14.5 years.
Still, a “very, very fertile environment for the development of drugs” has made a huge difference, Dr. Fonseca said. “We’ve had about 19 FDA approvals in the last 15 or 20 years.”
He urged colleagues to keep in mind that survival drops as patients decline in a line of therapy and need to switch to another one. “It might make intuitive sense to say ‘I’m gonna save something for later. I want to keep my powder dry.’ But put your best foot forward. Always go with your best treatments first.”
This approach can play out in a decision, say, to start with a four-drug initial regimen instead of a weaker two-drug regimen, he said. “Be mindful of managing toxicities, but hit harder.”
As he noted, side effects were worse with older generations of drugs. In regard to cost, multidrug treatments can cost hundreds of thousands of dollars. Dr. Fonseca said insurance tends to cover drugs that are approved by guidelines.
Dr. Fonseca discloses relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millennium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, Regeneron, Sanofi, Adaptive Biotechnologies, Caris Life Sciences, Oncotracker, Antegene, and AZBio, and a patent in MM. Dr. Berenson discloses ties with Janssen, Amgen, Sanofi, BMS, Karyopharm, and Incyte. Dr. Lonial reports ties with TG Therapeutics, Celgene, BMS, Janssen, Novartis, GlaxoSmithKline, AbbVie, Takeda, Merck, Sanofi, Pfizer, Regeneron, and Novartis.
Nonsurgical option for more large thyroid nodule patients?
WASHINGTON – , compared with only those that were.
While more research is needed, “the risk of false negative FNA results for large nodules may not be as high as reported in previous studies if you include patients who do not have indication for surgery, such as compressive symptoms, suspicious ultrasound features, etc.,” senior author Tracy Tylee, MD, an associate professor of endocrinology at the University of Washington, Seattle, said in an interview.
The implication is that nonsurgical options such as radiofrequency ablation may be appropriate for more patients than realized, she added.
“Clinicians should consider following these patients more conservatively, either with a second FNA to confirm [the] nodule is benign or with ultrasound follow-up for 5 years with intervention only if [there are] significant changes on imaging,” she said.
The findings were presented at the annual meeting of the American Thyroid Association.
Concerns about nodules over 4 cm having high false negative rates
Management of large thyroid nodules over 4 cm that are classified as Bethesda II, indicative of being benign, is complicated by concerns of false negatives in such cases. While the false negative rate for thyroid nodules in general is approximately 3%, the rate for large nodules over 4 cm has been reported as high as 35%.
Importantly, however, most studies evaluating the issue only involve patients who have received thyroid surgery, whereas most benign nodules are not referred for surgery.
“This may overestimate the false negative FNA biopsy risk for this group,” first author Melbin Thomas, MD, also of the University of Washington, said in her talk.
To better assess the false negative rate in the broader context of large nodules that did and did not undergo surgery, Dr. Thomas and her colleagues conducted a retrospective chart review of all patients undergoing FNA biopsy at her center between 2008 and 2014 for thyroid nodules larger than 4 cm and initially classified as Bethesda II, or benign.
With a follow-up of up to 10 years, nodules were considered accurately benign if they showed benign pathology on surgical resection, if they remained benign based on repeat FNA biopsy with Bethesda II results, or if there were no changes on imaging characteristics on ultrasound after at least 2 years.
Overall, 47 nodules over 4 cm and Bethesda II cytology were included, with an average follow-up of 5 years (range 2.2-9.7 years).
Of the nodules, 23 were treated with surgery, two of which were determined to have been malignant (8.7%) and, hence, false negatives. Nine of the nodules had repeat FNA, with none found to be malignant, and 15 received repeat ultrasound, also with no malignancies.
Overall, the false negative rate including all patients was 4.3%.
“False negative FNA biopsy results were not markedly elevated if nodules greater than 4 cm are evaluated, but rates were considerably higher if limited to surgical patients,” Dr. Thomas said.
Clinicians may be compelled to perform more aggressive surgery on large but benign thyroid nodules for a number of reasons, Dr. Tylee noted.
“A concern is that we may discontinue follow-up on these larger nodules and fail to diagnose a cancer early on, before there has been extrathyroidal extension or lymph node metastases,” she said.
In such cases, patients could wind up presenting at a higher stage of disease and require more intensive therapy.
However, with a low false negative rate overall, “all of this can increase the long-term health care costs and anxiety for patients, so having a better understanding of the true benign rate for large nodules is important,” she concluded.
Commenting on the research, Rodis D. Paparodis, MD, chief of Endocrinology, Diabetes, and Metabolism Clinics, in Patras, Greece, said the findings underscore that, as a surgical procedure, “thyroidectomy should be used cautiously, only when the benefit outweighs the risk.”
In his own previous multicenter study, Dr. Paparodis conducted a review of nearly 2,500 thyroidectomies that were performed based on size or longterm slow growth despite preoperative benign FNA findings. The results showed that only 1.9% of patients had any form of thyroid cancer in the nodule that had led to surgery; however, multiple other significant cancers were often present in other locations in the gland.
“Therefore, we suggest that careful sonographic evaluation of all thyroid nodules is warranted prior to deciding and planning the extent of surgical management for multinodular goiter,” he told this news organization.
“In addition, FNA of all suspicious nodules is required as well, to avoid unnecessary surprises in surgical pathology.”
Dr. Tylee, Dr. Thomas, and Dr. Paparodis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , compared with only those that were.
While more research is needed, “the risk of false negative FNA results for large nodules may not be as high as reported in previous studies if you include patients who do not have indication for surgery, such as compressive symptoms, suspicious ultrasound features, etc.,” senior author Tracy Tylee, MD, an associate professor of endocrinology at the University of Washington, Seattle, said in an interview.
The implication is that nonsurgical options such as radiofrequency ablation may be appropriate for more patients than realized, she added.
“Clinicians should consider following these patients more conservatively, either with a second FNA to confirm [the] nodule is benign or with ultrasound follow-up for 5 years with intervention only if [there are] significant changes on imaging,” she said.
The findings were presented at the annual meeting of the American Thyroid Association.
Concerns about nodules over 4 cm having high false negative rates
Management of large thyroid nodules over 4 cm that are classified as Bethesda II, indicative of being benign, is complicated by concerns of false negatives in such cases. While the false negative rate for thyroid nodules in general is approximately 3%, the rate for large nodules over 4 cm has been reported as high as 35%.
Importantly, however, most studies evaluating the issue only involve patients who have received thyroid surgery, whereas most benign nodules are not referred for surgery.
“This may overestimate the false negative FNA biopsy risk for this group,” first author Melbin Thomas, MD, also of the University of Washington, said in her talk.
To better assess the false negative rate in the broader context of large nodules that did and did not undergo surgery, Dr. Thomas and her colleagues conducted a retrospective chart review of all patients undergoing FNA biopsy at her center between 2008 and 2014 for thyroid nodules larger than 4 cm and initially classified as Bethesda II, or benign.
With a follow-up of up to 10 years, nodules were considered accurately benign if they showed benign pathology on surgical resection, if they remained benign based on repeat FNA biopsy with Bethesda II results, or if there were no changes on imaging characteristics on ultrasound after at least 2 years.
Overall, 47 nodules over 4 cm and Bethesda II cytology were included, with an average follow-up of 5 years (range 2.2-9.7 years).
Of the nodules, 23 were treated with surgery, two of which were determined to have been malignant (8.7%) and, hence, false negatives. Nine of the nodules had repeat FNA, with none found to be malignant, and 15 received repeat ultrasound, also with no malignancies.
Overall, the false negative rate including all patients was 4.3%.
“False negative FNA biopsy results were not markedly elevated if nodules greater than 4 cm are evaluated, but rates were considerably higher if limited to surgical patients,” Dr. Thomas said.
Clinicians may be compelled to perform more aggressive surgery on large but benign thyroid nodules for a number of reasons, Dr. Tylee noted.
“A concern is that we may discontinue follow-up on these larger nodules and fail to diagnose a cancer early on, before there has been extrathyroidal extension or lymph node metastases,” she said.
In such cases, patients could wind up presenting at a higher stage of disease and require more intensive therapy.
However, with a low false negative rate overall, “all of this can increase the long-term health care costs and anxiety for patients, so having a better understanding of the true benign rate for large nodules is important,” she concluded.
Commenting on the research, Rodis D. Paparodis, MD, chief of Endocrinology, Diabetes, and Metabolism Clinics, in Patras, Greece, said the findings underscore that, as a surgical procedure, “thyroidectomy should be used cautiously, only when the benefit outweighs the risk.”
In his own previous multicenter study, Dr. Paparodis conducted a review of nearly 2,500 thyroidectomies that were performed based on size or longterm slow growth despite preoperative benign FNA findings. The results showed that only 1.9% of patients had any form of thyroid cancer in the nodule that had led to surgery; however, multiple other significant cancers were often present in other locations in the gland.
“Therefore, we suggest that careful sonographic evaluation of all thyroid nodules is warranted prior to deciding and planning the extent of surgical management for multinodular goiter,” he told this news organization.
“In addition, FNA of all suspicious nodules is required as well, to avoid unnecessary surprises in surgical pathology.”
Dr. Tylee, Dr. Thomas, and Dr. Paparodis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , compared with only those that were.
While more research is needed, “the risk of false negative FNA results for large nodules may not be as high as reported in previous studies if you include patients who do not have indication for surgery, such as compressive symptoms, suspicious ultrasound features, etc.,” senior author Tracy Tylee, MD, an associate professor of endocrinology at the University of Washington, Seattle, said in an interview.
The implication is that nonsurgical options such as radiofrequency ablation may be appropriate for more patients than realized, she added.
“Clinicians should consider following these patients more conservatively, either with a second FNA to confirm [the] nodule is benign or with ultrasound follow-up for 5 years with intervention only if [there are] significant changes on imaging,” she said.
The findings were presented at the annual meeting of the American Thyroid Association.
Concerns about nodules over 4 cm having high false negative rates
Management of large thyroid nodules over 4 cm that are classified as Bethesda II, indicative of being benign, is complicated by concerns of false negatives in such cases. While the false negative rate for thyroid nodules in general is approximately 3%, the rate for large nodules over 4 cm has been reported as high as 35%.
Importantly, however, most studies evaluating the issue only involve patients who have received thyroid surgery, whereas most benign nodules are not referred for surgery.
“This may overestimate the false negative FNA biopsy risk for this group,” first author Melbin Thomas, MD, also of the University of Washington, said in her talk.
To better assess the false negative rate in the broader context of large nodules that did and did not undergo surgery, Dr. Thomas and her colleagues conducted a retrospective chart review of all patients undergoing FNA biopsy at her center between 2008 and 2014 for thyroid nodules larger than 4 cm and initially classified as Bethesda II, or benign.
With a follow-up of up to 10 years, nodules were considered accurately benign if they showed benign pathology on surgical resection, if they remained benign based on repeat FNA biopsy with Bethesda II results, or if there were no changes on imaging characteristics on ultrasound after at least 2 years.
Overall, 47 nodules over 4 cm and Bethesda II cytology were included, with an average follow-up of 5 years (range 2.2-9.7 years).
Of the nodules, 23 were treated with surgery, two of which were determined to have been malignant (8.7%) and, hence, false negatives. Nine of the nodules had repeat FNA, with none found to be malignant, and 15 received repeat ultrasound, also with no malignancies.
Overall, the false negative rate including all patients was 4.3%.
“False negative FNA biopsy results were not markedly elevated if nodules greater than 4 cm are evaluated, but rates were considerably higher if limited to surgical patients,” Dr. Thomas said.
Clinicians may be compelled to perform more aggressive surgery on large but benign thyroid nodules for a number of reasons, Dr. Tylee noted.
“A concern is that we may discontinue follow-up on these larger nodules and fail to diagnose a cancer early on, before there has been extrathyroidal extension or lymph node metastases,” she said.
In such cases, patients could wind up presenting at a higher stage of disease and require more intensive therapy.
However, with a low false negative rate overall, “all of this can increase the long-term health care costs and anxiety for patients, so having a better understanding of the true benign rate for large nodules is important,” she concluded.
Commenting on the research, Rodis D. Paparodis, MD, chief of Endocrinology, Diabetes, and Metabolism Clinics, in Patras, Greece, said the findings underscore that, as a surgical procedure, “thyroidectomy should be used cautiously, only when the benefit outweighs the risk.”
In his own previous multicenter study, Dr. Paparodis conducted a review of nearly 2,500 thyroidectomies that were performed based on size or longterm slow growth despite preoperative benign FNA findings. The results showed that only 1.9% of patients had any form of thyroid cancer in the nodule that had led to surgery; however, multiple other significant cancers were often present in other locations in the gland.
“Therefore, we suggest that careful sonographic evaluation of all thyroid nodules is warranted prior to deciding and planning the extent of surgical management for multinodular goiter,” he told this news organization.
“In addition, FNA of all suspicious nodules is required as well, to avoid unnecessary surprises in surgical pathology.”
Dr. Tylee, Dr. Thomas, and Dr. Paparodis report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ATA 2023