User login
Anemia, iron deficit common in rheumatic disease pregnancy
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to findings from a longitudinal cohort study.
METHODOLOGY:
- Researchers analyzed data from 368 pregnancies in women with rheumatic diseases during the period 2014-2022; nearly two-thirds (62%) had a connective tissue disease, 16% had rheumatoid arthritis or juvenile idiopathic arthritis, 14% had spondyloarthritis, 3% had vasculitis, and 7% had other diseases.
- Patients were aged 17-44 years, with a median age of 32 years at the time of birth.
- Researchers examined the frequency of anemia and iron deficiency and the impact of anemia on adverse maternal and child outcomes.
TAKEAWAY:
- The prevalence of iron deficiency was 28%, 51%, and 62% in the first, second, and third trimesters, respectively.
- The prevalence of anemia was 18%, 27%, and 33% in the first, second, and third trimesters, respectively.
- There was an increased risk for fetal complications such as malformation, infections, small for gestational age, neonatal lupus, preterm birth, and abortion or stillbirth in association with maternal connective tissue disease (odds ratio, 2.14) and also with low maternal hemoglobin levels and maternal iron deficiency (ORs, 0.52 and 0.86, respectively).
- Lower maternal hemoglobin levels were associated with an increased risk for maternal complications (OR, 1.47) such as flare with adaption of rheumatic medication and pregnancy-related adverse events (preeclampsia, gestational diabetes, bleeding complications, and thromboembolism), but patients with connective tissue disease had a lower risk for maternal complications (OR, 0.51); mean serum ferritin had no significant impact on maternal complications (OR, 1.02).
IN PRACTICE:
“Patients with rheumatic diseases suffer more often and already in early pregnancy from iron deficiency,” the researchers write. Therefore, early identification of anemia and iron deficiency in this population could inform prepregnancy counseling.
SOURCE:
The lead author on the study was Ann-Christin Pecher, MD, of University Hospital Tübingen, Germany. The study was published online in Joint Bone Spine.
LIMITATIONS:
The findings were limited by the use of a single dataset that might not be representative of all pregnant patients with rheumatic diseases. Other limitations included the lack of a standardized approach to iron supplementation.
DISCLOSURES:
The study was supported by a grant from the Medical Faculty of Tübingen Clinician-Scientist to the lead author. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA denies approval for patisiran in ATTR cardiomyopathy, despite panel nod
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
Illicit steroids: If MDs don’t ask, patients won’t tell
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Redefining CVD risk: Cardiovascular-kidney-metabolic (CKM) syndrome
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Breast, GYN Cancers Diagnosed at Lower Ages in VA Than Community
CHICAGO—A new report offers a picture of patients seeking care for breast and gynecological cancers within the US Department of Veterans Affairs (VA) health care system: They are somewhat younger than their counterparts in the general population, and those with breast cancer are much more likely to be men.
It is not clear whether these numbers are purely a reflection of the unique population within the VA or whether there is a more complicated explanation. Researchers also found that more than half of those with newly diagnosed breast, cervical, and ovarian cancers lived in the South compared with few (8%-13%) who lived in the Northeast.
The study findings were released in a poster at the 2023 annual meeting of the Association of VA Hematology/Oncology. Sarah Colonna, MD, study coauthor and medical director of breast oncology for the national VA, said in an interview that it is important to understand statistics regarding breast and gynecological cancer within the VA, especially as the system focuses more on patients with the conditions. And, she said, the wave of women who joined the military in recent decades are getting older and more likely to need oncology care. “We know women veterans are coming: They’re aging, and they’re going to get cancer.”
Colonna and colleagues examined statistics from the VA Corporate Data Warehouse to determine how many veterans were newly diagnosed with breast, uterine, ovarian, cervical, and vulvovaginal cancer in 2021 and 2022. The researchers compared their findings with 2020 statistics about the general population from the SEER database.
Within the VA, there were 3304 cases of breast cancer (mean age, 59 years; range, 23-99; mean body mass index [BMI], 31), 344 cases of cervical cancer (mean age, 46 years; range, 22-90; mean BMI, 29), 177 cases of ovarian cancer (mean age, 57 years; range, 24-80; mean BMI, 29), 365 cases of uterine cancer (mean age, 60 years; range, 24-85; mean BMI, 35), and 32 cases of vaginal/vulvar cancer (mean age, 56 years; range, 24-75; mean BMI, 31).
In contrast, the mean ages at diagnosis for the general population were slightly higher at 63 years for breast cancer, 50 years for cervical cancer, 63 years for ovarian cancer, and 63 years for uterine cancer. Vaginal/vulvar cancer was a bit of an outlier at mean age 69 years for the general population vs 56 years for the VA population; however, the number of cases in the latter group was quite low at 32 patients.
Overall, gynecological cancers were diagnosed at an average age of 55 years among the VA population vs 61 years among the general population. Men made up 11% of breast cancer cases in the VA vs 1% in the general population. “Of course, we have 10 times the proportion of men than in the outside,” said Colonna, an oncologist with the Huntsman Cancer Institute/Wahlen VA Medical Center in Utah. That may explain the difference, “but nobody knows for sure,” she said.
Patients Within the VA with the following cancers were more likely to be Black veterans than in the general population: breast, 30% vs 12%; cervical, 20% vs 14%; ovarian, 28% vs 10%; uterine, 25% vs 12%; and vaginal/vulvar, 44% vs 10%. This could reflect the fact that 30% of women treated within the VA are Black women vs 12% in the general population, Colonna said. Unfortunately, she said, “black women with breast cancer, tend to do really poorly. They tend to get it young, and they tend to die.”
As for the geographic distribution of cases, Colonna said it represents the high numbers of veterans who live in the South, suggesting that more VA oncology resources may be needed there.
In an interview, Aditi Hazra, PhD, MPH, an assistant professor of medicine at Harvard Medical School, said the new analysis is “very valuable”: “Women are a growing proportion of the veterans who serve, and we need more data to understand the risk factors and incidents of disease in this population.” Hazra said the next step will be to control the data for risk factors and “tease out what is driving the rates in the VA.”
There is no study funding, and the authors have no disclosures. Dr. Hazra discloses that she works for the VA and has collaborated with one of the study authors.
CHICAGO—A new report offers a picture of patients seeking care for breast and gynecological cancers within the US Department of Veterans Affairs (VA) health care system: They are somewhat younger than their counterparts in the general population, and those with breast cancer are much more likely to be men.
It is not clear whether these numbers are purely a reflection of the unique population within the VA or whether there is a more complicated explanation. Researchers also found that more than half of those with newly diagnosed breast, cervical, and ovarian cancers lived in the South compared with few (8%-13%) who lived in the Northeast.
The study findings were released in a poster at the 2023 annual meeting of the Association of VA Hematology/Oncology. Sarah Colonna, MD, study coauthor and medical director of breast oncology for the national VA, said in an interview that it is important to understand statistics regarding breast and gynecological cancer within the VA, especially as the system focuses more on patients with the conditions. And, she said, the wave of women who joined the military in recent decades are getting older and more likely to need oncology care. “We know women veterans are coming: They’re aging, and they’re going to get cancer.”
Colonna and colleagues examined statistics from the VA Corporate Data Warehouse to determine how many veterans were newly diagnosed with breast, uterine, ovarian, cervical, and vulvovaginal cancer in 2021 and 2022. The researchers compared their findings with 2020 statistics about the general population from the SEER database.
Within the VA, there were 3304 cases of breast cancer (mean age, 59 years; range, 23-99; mean body mass index [BMI], 31), 344 cases of cervical cancer (mean age, 46 years; range, 22-90; mean BMI, 29), 177 cases of ovarian cancer (mean age, 57 years; range, 24-80; mean BMI, 29), 365 cases of uterine cancer (mean age, 60 years; range, 24-85; mean BMI, 35), and 32 cases of vaginal/vulvar cancer (mean age, 56 years; range, 24-75; mean BMI, 31).
In contrast, the mean ages at diagnosis for the general population were slightly higher at 63 years for breast cancer, 50 years for cervical cancer, 63 years for ovarian cancer, and 63 years for uterine cancer. Vaginal/vulvar cancer was a bit of an outlier at mean age 69 years for the general population vs 56 years for the VA population; however, the number of cases in the latter group was quite low at 32 patients.
Overall, gynecological cancers were diagnosed at an average age of 55 years among the VA population vs 61 years among the general population. Men made up 11% of breast cancer cases in the VA vs 1% in the general population. “Of course, we have 10 times the proportion of men than in the outside,” said Colonna, an oncologist with the Huntsman Cancer Institute/Wahlen VA Medical Center in Utah. That may explain the difference, “but nobody knows for sure,” she said.
Patients Within the VA with the following cancers were more likely to be Black veterans than in the general population: breast, 30% vs 12%; cervical, 20% vs 14%; ovarian, 28% vs 10%; uterine, 25% vs 12%; and vaginal/vulvar, 44% vs 10%. This could reflect the fact that 30% of women treated within the VA are Black women vs 12% in the general population, Colonna said. Unfortunately, she said, “black women with breast cancer, tend to do really poorly. They tend to get it young, and they tend to die.”
As for the geographic distribution of cases, Colonna said it represents the high numbers of veterans who live in the South, suggesting that more VA oncology resources may be needed there.
In an interview, Aditi Hazra, PhD, MPH, an assistant professor of medicine at Harvard Medical School, said the new analysis is “very valuable”: “Women are a growing proportion of the veterans who serve, and we need more data to understand the risk factors and incidents of disease in this population.” Hazra said the next step will be to control the data for risk factors and “tease out what is driving the rates in the VA.”
There is no study funding, and the authors have no disclosures. Dr. Hazra discloses that she works for the VA and has collaborated with one of the study authors.
CHICAGO—A new report offers a picture of patients seeking care for breast and gynecological cancers within the US Department of Veterans Affairs (VA) health care system: They are somewhat younger than their counterparts in the general population, and those with breast cancer are much more likely to be men.
It is not clear whether these numbers are purely a reflection of the unique population within the VA or whether there is a more complicated explanation. Researchers also found that more than half of those with newly diagnosed breast, cervical, and ovarian cancers lived in the South compared with few (8%-13%) who lived in the Northeast.
The study findings were released in a poster at the 2023 annual meeting of the Association of VA Hematology/Oncology. Sarah Colonna, MD, study coauthor and medical director of breast oncology for the national VA, said in an interview that it is important to understand statistics regarding breast and gynecological cancer within the VA, especially as the system focuses more on patients with the conditions. And, she said, the wave of women who joined the military in recent decades are getting older and more likely to need oncology care. “We know women veterans are coming: They’re aging, and they’re going to get cancer.”
Colonna and colleagues examined statistics from the VA Corporate Data Warehouse to determine how many veterans were newly diagnosed with breast, uterine, ovarian, cervical, and vulvovaginal cancer in 2021 and 2022. The researchers compared their findings with 2020 statistics about the general population from the SEER database.
Within the VA, there were 3304 cases of breast cancer (mean age, 59 years; range, 23-99; mean body mass index [BMI], 31), 344 cases of cervical cancer (mean age, 46 years; range, 22-90; mean BMI, 29), 177 cases of ovarian cancer (mean age, 57 years; range, 24-80; mean BMI, 29), 365 cases of uterine cancer (mean age, 60 years; range, 24-85; mean BMI, 35), and 32 cases of vaginal/vulvar cancer (mean age, 56 years; range, 24-75; mean BMI, 31).
In contrast, the mean ages at diagnosis for the general population were slightly higher at 63 years for breast cancer, 50 years for cervical cancer, 63 years for ovarian cancer, and 63 years for uterine cancer. Vaginal/vulvar cancer was a bit of an outlier at mean age 69 years for the general population vs 56 years for the VA population; however, the number of cases in the latter group was quite low at 32 patients.
Overall, gynecological cancers were diagnosed at an average age of 55 years among the VA population vs 61 years among the general population. Men made up 11% of breast cancer cases in the VA vs 1% in the general population. “Of course, we have 10 times the proportion of men than in the outside,” said Colonna, an oncologist with the Huntsman Cancer Institute/Wahlen VA Medical Center in Utah. That may explain the difference, “but nobody knows for sure,” she said.
Patients Within the VA with the following cancers were more likely to be Black veterans than in the general population: breast, 30% vs 12%; cervical, 20% vs 14%; ovarian, 28% vs 10%; uterine, 25% vs 12%; and vaginal/vulvar, 44% vs 10%. This could reflect the fact that 30% of women treated within the VA are Black women vs 12% in the general population, Colonna said. Unfortunately, she said, “black women with breast cancer, tend to do really poorly. They tend to get it young, and they tend to die.”
As for the geographic distribution of cases, Colonna said it represents the high numbers of veterans who live in the South, suggesting that more VA oncology resources may be needed there.
In an interview, Aditi Hazra, PhD, MPH, an assistant professor of medicine at Harvard Medical School, said the new analysis is “very valuable”: “Women are a growing proportion of the veterans who serve, and we need more data to understand the risk factors and incidents of disease in this population.” Hazra said the next step will be to control the data for risk factors and “tease out what is driving the rates in the VA.”
There is no study funding, and the authors have no disclosures. Dr. Hazra discloses that she works for the VA and has collaborated with one of the study authors.
Anti-acid meds lower strength of systemic sclerosis drug
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Every click you make, the EHR is watching you
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.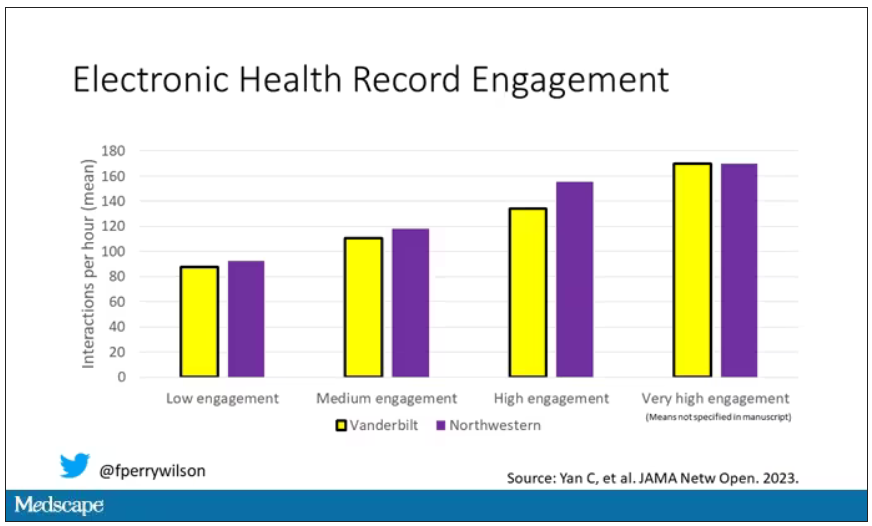
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.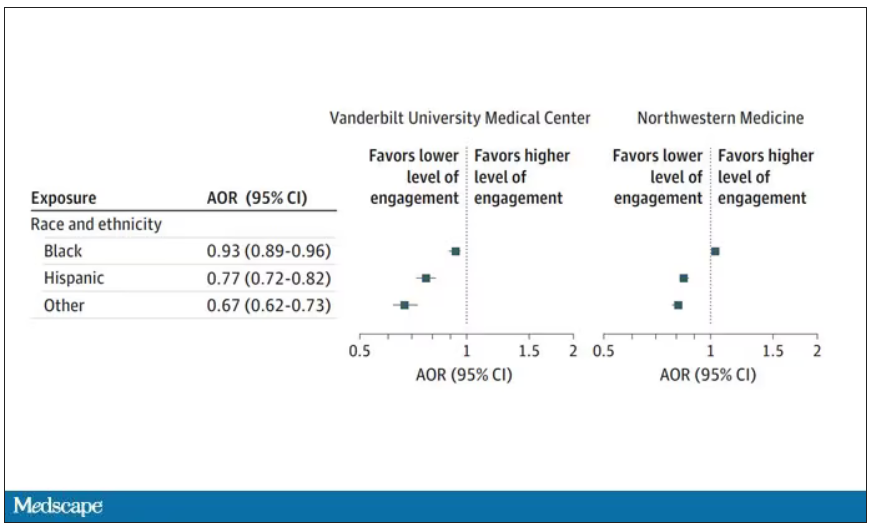
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.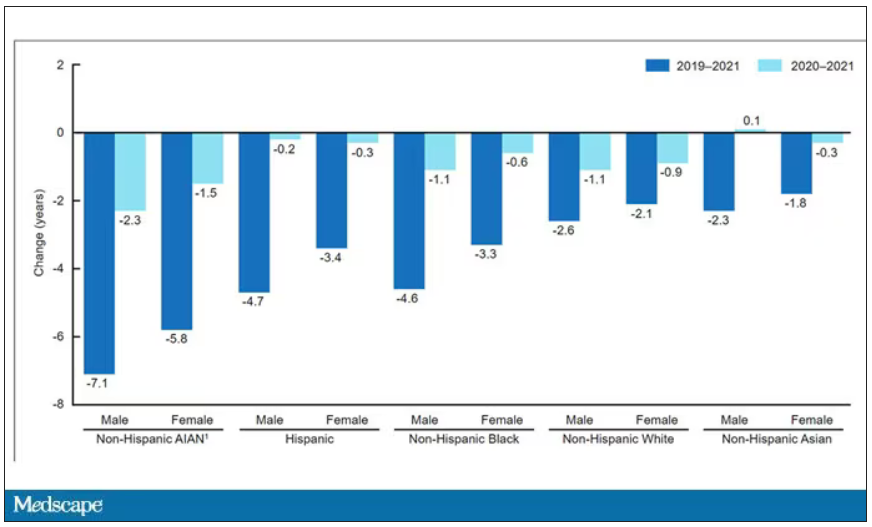
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Are women and men with rheumatism treated equally?
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
LEIPZIG, GERMANY – Women eat more healthily, visit their physician more often, and accept offers of prophylactic treatment more frequently than their male counterparts. Nevertheless, they are generally diagnosed with a rheumatic disease much later. “With systemic sclerosis for example, diagnosis occurs a whole year later than for male patients,” said Uta Kiltz, MD, senior physician at the Ruhrgebiet Rheumatism Center in Bochum, Germany, at a press conference for the annual congress of the German Society for Rheumatology.
In addition, certain markers and antibodies can be detected earlier in men’s blood – for example in systemic sclerosis. “What’s more, women exhibit a more diverse array of symptoms, which can make an unequivocal diagnosis difficult,” Dr. Kiltz explained.
Differences between the sexes in terms of disease progression and clinical presentation have been described for most rheumatic diseases. Roughly speaking, women often exhibit a much wider range of symptoms and report a higher disease burden, whereas men tend to experience a more severe progression of the disease.
Comorbidities also occur at different rates between the sexes. Whereas women with rheumatoid arthritis suffer more frequently from osteoporosis and depression, men are more likely to develop cardiovascular diseases and diabetes.
Gender-sensitive approach
Like Dr. Kiltz, Susanna Späthling-Mestekemper, MD, PhD, of the Munich-Pasing (Germany) Rheumatology Practice, also advocates a gender-sensitive approach to diagnosis and therapy. Dr. Späthling-Mestekemper referred to this during the conference, stating that women are still treated more poorly than men. The difference in treatment quality results from gaps in knowledge in the following areas:
- Sex-specific differences in the diagnosis and therapy of rheumatic diseases and in basic and clinical research
- Sex-specific differences in communication between male and female patients and between male and female physicians.
Dr. Späthling-Mestekemper used axial spondyloarthritis (axSpA) as a “prominent example” of false diagnoses. “Men more commonly fulfill the modified New York criteria – involvement of the axial skeleton, the lumbar spine, and increasing radiological progression.”
In contrast, women with axSpA exhibit the following differences:
- It is more likely for the cervical spine to be affected.
- Women are more likely to suffer from peripheral joint involvement.
- They suffer more from whole body pain.
- They have fatigue and exhaustion.
- They exhibit fewer humoral signs of inflammation (lower C-reactive protein).
- They are rarely HLA-B27 positive.
“We also have to completely rethink how we make the diagnosis in women,” said Dr. Späthling-Mestekemper. The current approach leads to women with axSpA being diagnosed much later than men. “Depending on the study, the difference can range from 7 months to 2 years,” according to Dr. Späthling-Mestekemper.
A 2018 Spanish study reported that the most common incorrect diagnoses in women with axSpA were sciatica, osteoarthritis, and fibromyalgia.
However, it is not just in axSpA that there are significant differences between men and women. There is evidence that women with systemic lupus erythematosus suffer more from musculoskeletal symptoms, while men with lupus exhibit more severe organ involvement (especially more serositis and nephritis).
For systemic sclerosis, women have the higher survival rate. They also exhibit skin involvement more frequently. Men, however, are more likely to have organ involvement, especially with the lungs.
TNF blockers
Using the example of axSpA, Dr. Späthling-Mestekemper also showed that men and women respond differently to tumor necrosis factor (TNF) blocker therapy. “The duration of therapy with TNF blockers is shorter for women: 33.4 months versus 44.9 months. They respond less to this therapy; they stop and change more frequently.”
Data from March 2023 show that, in contrast, there is no evidence of a difference in response to Janus kinase inhibitor treatment.
The presence of enthesitis has been discussed as one reason for the worse response to TNF blockers in women, since they have it more often than men do. “In fact, a better response to TNF blockers is associated with HLA-B27 positivity, with the absence of enthesitis and with TNF blocker naivety. In women, higher fat-mass index could also play a part, or even abdominal weight gain, which also increases in women after menopause,” said Dr. Späthling-Mestekemper.
She mentioned the following other potential reasons for a delayed therapy response to biological drugs in women:
- Genetic, physical, or hormonal causes
- Widespread pain or fibromyalgia
- Late diagnosis or late application of therapy, which lowers the chances of remission.
Even the science itself has shown the following sex-specific shortcomings:
- Disregarding sex-specific differences in animal-experimental studies (which, until recently, were only conducted in male mice to avoid hormone fluctuations)
- Women in clinical studies are still underrepresented: only 37% of the populations in phase 3 studies are women; 64% of studies do not describe any sex-specific differences
- Most of the data come from epidemiological analyses (not from basic research)
- Gaps in medical textbooks
Communication differences
Female patients are looking for explanations, whereas male patients describe specific symptoms. Female physicians talk, while male physicians treat. They sound like stereotypes, but they have been substantiated in multiple studies, said Dr. Späthling-Mestekemper. In general, the study results show that male patients behave in the following ways:
- Describe their symptoms in terms of specifics
- Do not like to admit having mental health issues
- Are three to five times more likely to commit suicide because of depression than women
On the other hand, female patients behave in the following ways:
- Look for an explanation for their symptoms
- Often do not have their physical symptoms taken seriously
- Are often pushed in a psychosomatic direction.
Female physicians focus on the following questions:
- Prevention, communication, shared decision-making, open-ended questions, “positive” discussions, patient self-management (chronic diseases such as diabetes: female physicians are better at reaching the therapy goals set by the ADA guidelines than male physicians)
- Psychosocial situations: consultations last 1 minute longer (10%).
Male physicians focus on the following questions:
- Medical history
- Physical examination (cardiac catheterizations after a heart attack are arranged much more commonly by male rather than female physicians)
- Diagnostics
Recognition and training
A large-scale surgical study in 2021 made a few waves. The study analyzed whether it makes a difference if women are operated on by men or by women. The results showed that women who had been operated on by men exhibited a higher level of risk after the surgery, compared with men who had been operated on by men or by women. The risk took the following forms:
- 15% higher risk for a worse surgery result
- 16% higher risk for complications
- 11% higher risk for repeat hospitalization
- 20% higher risk for a longer period of hospitalization
- 32% higher risk for mortality
The study authors provided the following potential reasons for these differences:
- Male physicians underestimate the severity of symptoms in their female patients
- Women are less comfortable indicating their postoperative pain to a male physician
- Different working style and treatment decisions between female and male physicians
- Unconsciously incorporated role patterns and preconceptions
“Our potential solutions are recognition and training. We need a personalized style of medicine; we need to have a closer look. We owe our male and female patients as much,” said Dr. Späthling-Mestekemper.
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
AT THE GERMAN RHEUMATOLOGY CONGRESS 2023