User login
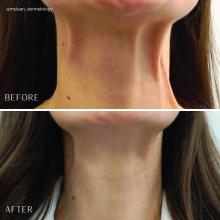
Treatment of the neck and lower face with botulinum toxin
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
Many young adults with type 2 diabetes skip medications
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
Patch testing finds higher prevalence of ACD among children with AD
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Zuranolone: FAQs for clinicians and patients
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
Low-certainty evidence supports probiotics for IBS
or very low certainty, with many studies suffering from bias, according to a recent review and meta-analysis.
These shortcomings in the probiotic research landscape should be kept in mind when making treatment recommendations, reported researchers who were led by Alexander C. Ford, MBChB, of the Leeds Gastroenterology Institute, University of Leeds (England). They suggested these issues need to be addressed in the methodology of future clinical trials.
“Although multiple probiotics have been tested in IBS in randomized clinical trials, understanding of which probiotics may be beneficial is limited,” the investigators wrote in Gastroenterology.
They noted that previous efforts – including their own – to meta-analyze these findings have been hindered by a scarcity of trial data coupled with heterogeneity across probiotic strains, combinations, and doses, resulting in clinical uncertainty.
“Making recommendations concerning which probiotics, or combinations of probiotics, are beneficial according to IBS subtype or individual symptom has been difficult to date,” they wrote.
To narrow this knowledge gap, the researchers conducted an updated systematic review and meta-analysis with newly identified trials.
“There is continued interest in the role of probiotics in the management of IBS, as evidenced by the publication of more than 20 new randomized clinical trials since the prior version of this meta-analysis in 2018,” they wrote.
The new dataset included 82 RCTs comprising 10,332 patients with IBS. Along with safety, three separate efficacy endpoints were evaluated: global symptoms, abdominal pain, and abdominal bloating or distension.
For global symptoms, moderate-certainty evidence supported the efficacy of Escherichia coli strains; low-certainty data supported Lactobacillus plantarum 299V and other Lactobacillus strains; and very-low-certainty evidence supported Bacillus, LacClean Gold S, and Duolac 7s strains, and combination probiotics.
For abdominal pain, low-certainty evidence supported Bifidobacterium strains and Saccharomyces cerevisiae I-3856. Very-low-certainty data supported Lactobacillus, Saccharomyces, and Bacillus strains, and combination probiotics.
Very-low-certainty evidence supported the benefits of Bacillus strains and combination probiotics for alleviating abdominal bloating or distension.
In a safety analysis of 55 trials involving more than 7,000 patients, risk of adverse events was no higher for probiotics than placebo.
“Our analyses provide some support for the use of certain probiotics in IBS, and also for particular strains for specific symptoms,” the investigators wrote. “However, there is a paucity of data for their use in patients with IBS-C [IBS with constipation], with only seven RCTs reporting efficacy in this subtype, and no evidence of efficacy in any of these analyses. Their use in patients with IBS-C is, therefore, not supported by current evidence.”
A broader discussion in the publication called out the general lack of high certainty evidence in this area of clinical research.
“Only 24 of 82 eligible RCTs were low risk of bias across all domains, and there was significant heterogeneity between trials in many of our analyses, as well as evidence of publication bias, or other small study effects, in some of our analyses,” the researchers wrote. “The fact that few of the included studies were low risk of bias across all domains should be borne in mind when making treatment recommendations.”
The investigators disclosed relationships with Salix, Biocodex, 4D Pharma, and others.
IBS patients frequently inquire about probiotics. As a clinician, this can be difficult to address. A search of the literature yields numerous small trials. Turning to the guidelines does not help, as the AGA Clinical Practice Guidelines on Probiotics offer no recommendations for IBS because of the low quality of evidence. Nevertheless, we have patients who want to try probiotics. Some of these patients have had inadequate responses to first-line therapies and/or prefer a nonpharmacologic approach.
For example, the strain with the most trials was Lactobacillus plantarum 299V. The dose used (10 billion CFU once daily) is commercially available (Jarrow Formulas Ideal Bowel Support® LP299V®). Bacillus strains were also promising for global symptoms, abdominal pain and bloating. Two trials used the same strain and dose, Bacillus coagulans MTCC 5856, 2 billion CFU once daily, also commercially available (LactoSpore). Both can be purchased via major online retailers for $10-$13 for a 30-day supply. I am glad to have something to recommend however conditionally.
Elizabeth (Beth) Videlock, MD, PhD is assistant professor of medicine in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California, Los Angeles, and a staff physician in gastroenterology in the Greater Los Angeles Veterans Affairs Healthcare System. She is co-lead of the neurodevelopmental and neurodegenerative diseases research program of the Goodman-Luskin Microbiome Center at UCLA. She has no relevant disclosures.
IBS patients frequently inquire about probiotics. As a clinician, this can be difficult to address. A search of the literature yields numerous small trials. Turning to the guidelines does not help, as the AGA Clinical Practice Guidelines on Probiotics offer no recommendations for IBS because of the low quality of evidence. Nevertheless, we have patients who want to try probiotics. Some of these patients have had inadequate responses to first-line therapies and/or prefer a nonpharmacologic approach.
For example, the strain with the most trials was Lactobacillus plantarum 299V. The dose used (10 billion CFU once daily) is commercially available (Jarrow Formulas Ideal Bowel Support® LP299V®). Bacillus strains were also promising for global symptoms, abdominal pain and bloating. Two trials used the same strain and dose, Bacillus coagulans MTCC 5856, 2 billion CFU once daily, also commercially available (LactoSpore). Both can be purchased via major online retailers for $10-$13 for a 30-day supply. I am glad to have something to recommend however conditionally.
Elizabeth (Beth) Videlock, MD, PhD is assistant professor of medicine in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California, Los Angeles, and a staff physician in gastroenterology in the Greater Los Angeles Veterans Affairs Healthcare System. She is co-lead of the neurodevelopmental and neurodegenerative diseases research program of the Goodman-Luskin Microbiome Center at UCLA. She has no relevant disclosures.
IBS patients frequently inquire about probiotics. As a clinician, this can be difficult to address. A search of the literature yields numerous small trials. Turning to the guidelines does not help, as the AGA Clinical Practice Guidelines on Probiotics offer no recommendations for IBS because of the low quality of evidence. Nevertheless, we have patients who want to try probiotics. Some of these patients have had inadequate responses to first-line therapies and/or prefer a nonpharmacologic approach.
For example, the strain with the most trials was Lactobacillus plantarum 299V. The dose used (10 billion CFU once daily) is commercially available (Jarrow Formulas Ideal Bowel Support® LP299V®). Bacillus strains were also promising for global symptoms, abdominal pain and bloating. Two trials used the same strain and dose, Bacillus coagulans MTCC 5856, 2 billion CFU once daily, also commercially available (LactoSpore). Both can be purchased via major online retailers for $10-$13 for a 30-day supply. I am glad to have something to recommend however conditionally.
Elizabeth (Beth) Videlock, MD, PhD is assistant professor of medicine in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California, Los Angeles, and a staff physician in gastroenterology in the Greater Los Angeles Veterans Affairs Healthcare System. She is co-lead of the neurodevelopmental and neurodegenerative diseases research program of the Goodman-Luskin Microbiome Center at UCLA. She has no relevant disclosures.
or very low certainty, with many studies suffering from bias, according to a recent review and meta-analysis.
These shortcomings in the probiotic research landscape should be kept in mind when making treatment recommendations, reported researchers who were led by Alexander C. Ford, MBChB, of the Leeds Gastroenterology Institute, University of Leeds (England). They suggested these issues need to be addressed in the methodology of future clinical trials.
“Although multiple probiotics have been tested in IBS in randomized clinical trials, understanding of which probiotics may be beneficial is limited,” the investigators wrote in Gastroenterology.
They noted that previous efforts – including their own – to meta-analyze these findings have been hindered by a scarcity of trial data coupled with heterogeneity across probiotic strains, combinations, and doses, resulting in clinical uncertainty.
“Making recommendations concerning which probiotics, or combinations of probiotics, are beneficial according to IBS subtype or individual symptom has been difficult to date,” they wrote.
To narrow this knowledge gap, the researchers conducted an updated systematic review and meta-analysis with newly identified trials.
“There is continued interest in the role of probiotics in the management of IBS, as evidenced by the publication of more than 20 new randomized clinical trials since the prior version of this meta-analysis in 2018,” they wrote.
The new dataset included 82 RCTs comprising 10,332 patients with IBS. Along with safety, three separate efficacy endpoints were evaluated: global symptoms, abdominal pain, and abdominal bloating or distension.
For global symptoms, moderate-certainty evidence supported the efficacy of Escherichia coli strains; low-certainty data supported Lactobacillus plantarum 299V and other Lactobacillus strains; and very-low-certainty evidence supported Bacillus, LacClean Gold S, and Duolac 7s strains, and combination probiotics.
For abdominal pain, low-certainty evidence supported Bifidobacterium strains and Saccharomyces cerevisiae I-3856. Very-low-certainty data supported Lactobacillus, Saccharomyces, and Bacillus strains, and combination probiotics.
Very-low-certainty evidence supported the benefits of Bacillus strains and combination probiotics for alleviating abdominal bloating or distension.
In a safety analysis of 55 trials involving more than 7,000 patients, risk of adverse events was no higher for probiotics than placebo.
“Our analyses provide some support for the use of certain probiotics in IBS, and also for particular strains for specific symptoms,” the investigators wrote. “However, there is a paucity of data for their use in patients with IBS-C [IBS with constipation], with only seven RCTs reporting efficacy in this subtype, and no evidence of efficacy in any of these analyses. Their use in patients with IBS-C is, therefore, not supported by current evidence.”
A broader discussion in the publication called out the general lack of high certainty evidence in this area of clinical research.
“Only 24 of 82 eligible RCTs were low risk of bias across all domains, and there was significant heterogeneity between trials in many of our analyses, as well as evidence of publication bias, or other small study effects, in some of our analyses,” the researchers wrote. “The fact that few of the included studies were low risk of bias across all domains should be borne in mind when making treatment recommendations.”
The investigators disclosed relationships with Salix, Biocodex, 4D Pharma, and others.
or very low certainty, with many studies suffering from bias, according to a recent review and meta-analysis.
These shortcomings in the probiotic research landscape should be kept in mind when making treatment recommendations, reported researchers who were led by Alexander C. Ford, MBChB, of the Leeds Gastroenterology Institute, University of Leeds (England). They suggested these issues need to be addressed in the methodology of future clinical trials.
“Although multiple probiotics have been tested in IBS in randomized clinical trials, understanding of which probiotics may be beneficial is limited,” the investigators wrote in Gastroenterology.
They noted that previous efforts – including their own – to meta-analyze these findings have been hindered by a scarcity of trial data coupled with heterogeneity across probiotic strains, combinations, and doses, resulting in clinical uncertainty.
“Making recommendations concerning which probiotics, or combinations of probiotics, are beneficial according to IBS subtype or individual symptom has been difficult to date,” they wrote.
To narrow this knowledge gap, the researchers conducted an updated systematic review and meta-analysis with newly identified trials.
“There is continued interest in the role of probiotics in the management of IBS, as evidenced by the publication of more than 20 new randomized clinical trials since the prior version of this meta-analysis in 2018,” they wrote.
The new dataset included 82 RCTs comprising 10,332 patients with IBS. Along with safety, three separate efficacy endpoints were evaluated: global symptoms, abdominal pain, and abdominal bloating or distension.
For global symptoms, moderate-certainty evidence supported the efficacy of Escherichia coli strains; low-certainty data supported Lactobacillus plantarum 299V and other Lactobacillus strains; and very-low-certainty evidence supported Bacillus, LacClean Gold S, and Duolac 7s strains, and combination probiotics.
For abdominal pain, low-certainty evidence supported Bifidobacterium strains and Saccharomyces cerevisiae I-3856. Very-low-certainty data supported Lactobacillus, Saccharomyces, and Bacillus strains, and combination probiotics.
Very-low-certainty evidence supported the benefits of Bacillus strains and combination probiotics for alleviating abdominal bloating or distension.
In a safety analysis of 55 trials involving more than 7,000 patients, risk of adverse events was no higher for probiotics than placebo.
“Our analyses provide some support for the use of certain probiotics in IBS, and also for particular strains for specific symptoms,” the investigators wrote. “However, there is a paucity of data for their use in patients with IBS-C [IBS with constipation], with only seven RCTs reporting efficacy in this subtype, and no evidence of efficacy in any of these analyses. Their use in patients with IBS-C is, therefore, not supported by current evidence.”
A broader discussion in the publication called out the general lack of high certainty evidence in this area of clinical research.
“Only 24 of 82 eligible RCTs were low risk of bias across all domains, and there was significant heterogeneity between trials in many of our analyses, as well as evidence of publication bias, or other small study effects, in some of our analyses,” the researchers wrote. “The fact that few of the included studies were low risk of bias across all domains should be borne in mind when making treatment recommendations.”
The investigators disclosed relationships with Salix, Biocodex, 4D Pharma, and others.
FROM GASTROENTEROLOGY
Can these salt substitutes prevent complications of hypertension?
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2 The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2 The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2 The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
PRACTICE CHANGER
Consider recommending potassium-enriched salt substitutes for appropriate patients with hypertension to reduce blood pressure (BP) and risk for related cardiovascular (CV) events or mortality.
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of controlled trials. 1
Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart . 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
Feeling salty about our sodium intake
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities
AGA aims to increase awareness of exocrine pancreatic insufficiency
The update, which was led by Anna M. Buchner, MD, PhD, University of Pennsylvania, Philadelphia, includes 15 best practice advice statements based on available literature and expert opinion.
“EPI is frequently underdiagnosed and, as a result, patients are often not treated appropriately,” the authors wrote in Gastroenterology. “There is an urgent need to increase awareness of and treatment for this condition.”
To this end, the authors offered guidance spanning the patient journey, with recommendations broadly grouped into four categories: clinical features and risk factors, diagnostic strategies, treatment approaches, and disease monitoring.
Clinical features and risk factors
The CPU begins by listing the key clinical features of EPI, including bloating, excessive flatulence, fat-soluble vitamin deficiencies, protein-calorie malnutrition, steatorrhea with or without diarrhea, and weight loss.
The authors went on to suggest that EPI should also be considered in patients with high-risk clinical conditions, including previous pancreatic surgery, chronic pancreatitis, cystic fibrosis, pancreatic ductal adenocarcinoma, and relapsing acute pancreatitis.
Similarly, suspicion should be increased for individuals with moderate-risk clinical conditions, such as prior intestinal surgery, Zollinger-Ellison syndrome, longstanding diabetes mellitus, and duodenal diseases such as celiac and Crohn’s disease.
Diagnostic strategies
The primary diagnostic tool for EPI is the fecal elastase test, according to the update. Levels below 100 mcg/g indicate EPI, whereas levels between 100-200 mcg/g are considered indeterminate. The investigators noted that this test can be conducted even during pancreatic enzyme replacement therapy (PERT).
Other tests for EPI are rarely used, such as fecal fat testing, which must be performed on a high-fat diet, and quantitative testing, which is generally impractical for routine clinical use.
The authors also noted that a therapeutic trial of PERT is an unreliable method for diagnosing EPI.
“Patients with nonspecific symptoms, such as bloating, excess gas, and foul-smelling or floating stools may note some improvement in these symptoms while taking PERT, but these symptoms are nonspecific and symptomatic changes may be a placebo effect or masking other disorders, such as celiac disease, causing delays in a correct diagnosis,” they wrote.
While cross-sectional imaging methods such as CT scans, MRI, and endoscopic ultrasound play a significant role in detecting other pancreatic diseases, they cannot identify EPI. Breath tests and direct pancreatic function tests do hold promise, but they are not widely available in the United States.
Treatment strategies
Once EPI is diagnosed, treatment with PERT is indicated to prevent complications related to fat malabsorption and malnutrition.
PERT formulations are all equally effective at equivalent doses, according to the update, but non–enteric-coated preparations require concurrent H2 or proton pump inhibitor therapy. PERT should be taken during meals, with an initial adult dose of at least 40,000 USP units of lipase during each meal. Half that dose may be considered for snacks, with further dosage refinements based on meal size and fat content.
Dietary modifications may include supplementation with fat-soluble vitamins alongside smaller, more frequent, low- to moderate-fat meals. Very-low-fat diets should be avoided, the authors cautioned.
Surveillance
EPI treatment success can be identified by reduction in steatorrhea and associated gastrointestinal symptoms, as well as weight gain, improved muscle mass and function, and enhanced fat-soluble vitamin levels, Dr. Whitcomb and colleagues wrote, noting that a dual-energy x-ray absorptiometry scan also should be performed at baseline, then repeated every 1-2 years.
The update was commissioned and approved by the AGA. The investigators disclosed relationships with AbbVie, Nestlé, Regeneron, and others.
The update, which was led by Anna M. Buchner, MD, PhD, University of Pennsylvania, Philadelphia, includes 15 best practice advice statements based on available literature and expert opinion.
“EPI is frequently underdiagnosed and, as a result, patients are often not treated appropriately,” the authors wrote in Gastroenterology. “There is an urgent need to increase awareness of and treatment for this condition.”
To this end, the authors offered guidance spanning the patient journey, with recommendations broadly grouped into four categories: clinical features and risk factors, diagnostic strategies, treatment approaches, and disease monitoring.
Clinical features and risk factors
The CPU begins by listing the key clinical features of EPI, including bloating, excessive flatulence, fat-soluble vitamin deficiencies, protein-calorie malnutrition, steatorrhea with or without diarrhea, and weight loss.
The authors went on to suggest that EPI should also be considered in patients with high-risk clinical conditions, including previous pancreatic surgery, chronic pancreatitis, cystic fibrosis, pancreatic ductal adenocarcinoma, and relapsing acute pancreatitis.
Similarly, suspicion should be increased for individuals with moderate-risk clinical conditions, such as prior intestinal surgery, Zollinger-Ellison syndrome, longstanding diabetes mellitus, and duodenal diseases such as celiac and Crohn’s disease.
Diagnostic strategies
The primary diagnostic tool for EPI is the fecal elastase test, according to the update. Levels below 100 mcg/g indicate EPI, whereas levels between 100-200 mcg/g are considered indeterminate. The investigators noted that this test can be conducted even during pancreatic enzyme replacement therapy (PERT).
Other tests for EPI are rarely used, such as fecal fat testing, which must be performed on a high-fat diet, and quantitative testing, which is generally impractical for routine clinical use.
The authors also noted that a therapeutic trial of PERT is an unreliable method for diagnosing EPI.
“Patients with nonspecific symptoms, such as bloating, excess gas, and foul-smelling or floating stools may note some improvement in these symptoms while taking PERT, but these symptoms are nonspecific and symptomatic changes may be a placebo effect or masking other disorders, such as celiac disease, causing delays in a correct diagnosis,” they wrote.
While cross-sectional imaging methods such as CT scans, MRI, and endoscopic ultrasound play a significant role in detecting other pancreatic diseases, they cannot identify EPI. Breath tests and direct pancreatic function tests do hold promise, but they are not widely available in the United States.
Treatment strategies
Once EPI is diagnosed, treatment with PERT is indicated to prevent complications related to fat malabsorption and malnutrition.
PERT formulations are all equally effective at equivalent doses, according to the update, but non–enteric-coated preparations require concurrent H2 or proton pump inhibitor therapy. PERT should be taken during meals, with an initial adult dose of at least 40,000 USP units of lipase during each meal. Half that dose may be considered for snacks, with further dosage refinements based on meal size and fat content.
Dietary modifications may include supplementation with fat-soluble vitamins alongside smaller, more frequent, low- to moderate-fat meals. Very-low-fat diets should be avoided, the authors cautioned.
Surveillance
EPI treatment success can be identified by reduction in steatorrhea and associated gastrointestinal symptoms, as well as weight gain, improved muscle mass and function, and enhanced fat-soluble vitamin levels, Dr. Whitcomb and colleagues wrote, noting that a dual-energy x-ray absorptiometry scan also should be performed at baseline, then repeated every 1-2 years.
The update was commissioned and approved by the AGA. The investigators disclosed relationships with AbbVie, Nestlé, Regeneron, and others.
The update, which was led by Anna M. Buchner, MD, PhD, University of Pennsylvania, Philadelphia, includes 15 best practice advice statements based on available literature and expert opinion.
“EPI is frequently underdiagnosed and, as a result, patients are often not treated appropriately,” the authors wrote in Gastroenterology. “There is an urgent need to increase awareness of and treatment for this condition.”
To this end, the authors offered guidance spanning the patient journey, with recommendations broadly grouped into four categories: clinical features and risk factors, diagnostic strategies, treatment approaches, and disease monitoring.
Clinical features and risk factors
The CPU begins by listing the key clinical features of EPI, including bloating, excessive flatulence, fat-soluble vitamin deficiencies, protein-calorie malnutrition, steatorrhea with or without diarrhea, and weight loss.
The authors went on to suggest that EPI should also be considered in patients with high-risk clinical conditions, including previous pancreatic surgery, chronic pancreatitis, cystic fibrosis, pancreatic ductal adenocarcinoma, and relapsing acute pancreatitis.
Similarly, suspicion should be increased for individuals with moderate-risk clinical conditions, such as prior intestinal surgery, Zollinger-Ellison syndrome, longstanding diabetes mellitus, and duodenal diseases such as celiac and Crohn’s disease.
Diagnostic strategies
The primary diagnostic tool for EPI is the fecal elastase test, according to the update. Levels below 100 mcg/g indicate EPI, whereas levels between 100-200 mcg/g are considered indeterminate. The investigators noted that this test can be conducted even during pancreatic enzyme replacement therapy (PERT).
Other tests for EPI are rarely used, such as fecal fat testing, which must be performed on a high-fat diet, and quantitative testing, which is generally impractical for routine clinical use.
The authors also noted that a therapeutic trial of PERT is an unreliable method for diagnosing EPI.
“Patients with nonspecific symptoms, such as bloating, excess gas, and foul-smelling or floating stools may note some improvement in these symptoms while taking PERT, but these symptoms are nonspecific and symptomatic changes may be a placebo effect or masking other disorders, such as celiac disease, causing delays in a correct diagnosis,” they wrote.
While cross-sectional imaging methods such as CT scans, MRI, and endoscopic ultrasound play a significant role in detecting other pancreatic diseases, they cannot identify EPI. Breath tests and direct pancreatic function tests do hold promise, but they are not widely available in the United States.
Treatment strategies
Once EPI is diagnosed, treatment with PERT is indicated to prevent complications related to fat malabsorption and malnutrition.
PERT formulations are all equally effective at equivalent doses, according to the update, but non–enteric-coated preparations require concurrent H2 or proton pump inhibitor therapy. PERT should be taken during meals, with an initial adult dose of at least 40,000 USP units of lipase during each meal. Half that dose may be considered for snacks, with further dosage refinements based on meal size and fat content.
Dietary modifications may include supplementation with fat-soluble vitamins alongside smaller, more frequent, low- to moderate-fat meals. Very-low-fat diets should be avoided, the authors cautioned.
Surveillance
EPI treatment success can be identified by reduction in steatorrhea and associated gastrointestinal symptoms, as well as weight gain, improved muscle mass and function, and enhanced fat-soluble vitamin levels, Dr. Whitcomb and colleagues wrote, noting that a dual-energy x-ray absorptiometry scan also should be performed at baseline, then repeated every 1-2 years.
The update was commissioned and approved by the AGA. The investigators disclosed relationships with AbbVie, Nestlé, Regeneron, and others.
FROM GASTROENTEROLOGY
ACIP updates recommendations for influenza vaccination
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.
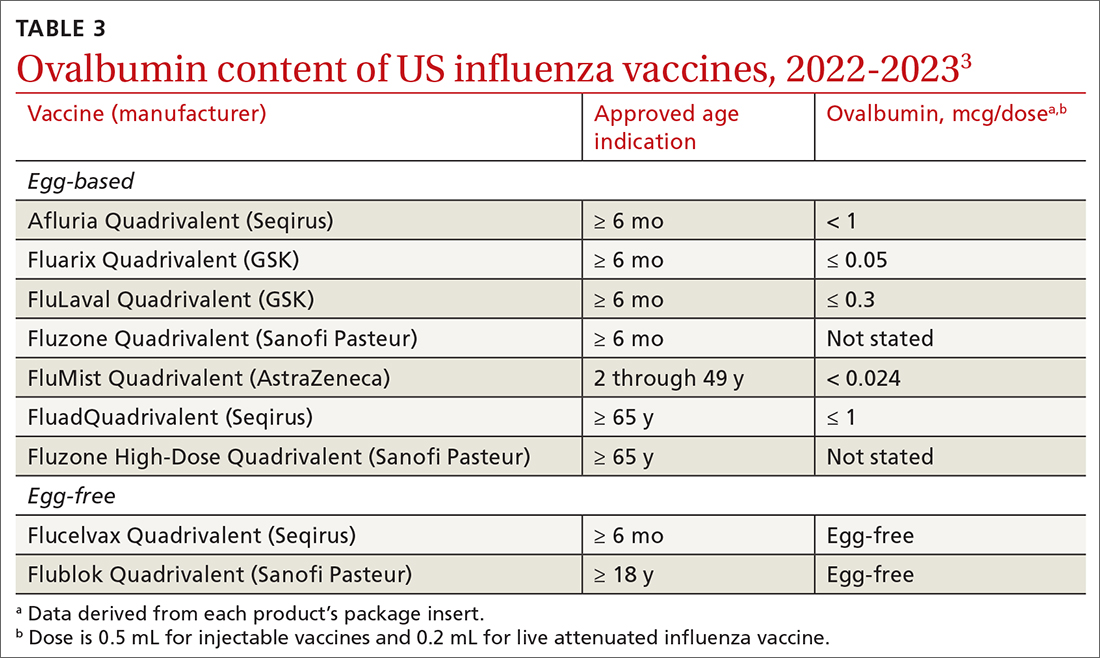
Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html