User login
3-D stereophotogrammetry helps detect progression of craniofacial morphea
TOPLINE:
over time.
METHODOLOGY:
- Existing tools that detect disease progression in patients with CM are limited.
- In a prospective cohort study, researchers evaluated the use of 3-D stereophotogrammetry, a noninvasive, radiation-free imaging modality, to detect disease progression in 27 consecutive patients with CM seen at Boston Children’s Hospital and Brigham and Women’s Hospital from April 1, 2019, to March 1, 2023.
- After clinical and 3-D stereophotogrammetry assessments were performed at 2- to 12-month intervals, the 3-D images were rated by an expert (a board-certified plastic craniofacial surgeon) and a nonexpert (a board-certified dermatologist) as demonstrating progression or no progression.
- Kappa coefficients were used to calculate inter-rater reliability.
TAKEAWAY:
- Most of the study participants (73%) were female, their median age was 14 years (range, 5-40 years), and each underwent 3-D stereophotogrammetry imaging at least two times spaced a median of 3 months apart.
- On the basis of clinical assessments during the 48-month study period, 10 patients (37%) experienced progression of their disease.
- 3-D stereophotogrammetry not only corroborated clinical impressions of disease progression with strong inter-rater reliability (kappa = 0.80; 95% confidence interval, 0.61-0.99), but it also detected occult progression of asymmetry not noted on clinical examination in three additional patients.
- In subgroup analyses, assessment of 3-D images demonstrated substantial to near-perfect inter-rater reliability in patients with Fitzpatrick skin types IV-VI.
IN PRACTICE:
“Further work is necessary to validate this measure in a larger cohort and to guide its incorporation into medical decision-making for patients with CM,” the researchers wrote.
SOURCE:
Katharina S. Shaw, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
The sample was small, and a criterion standard for assessing CM was lacking.
DISCLOSURES:
The researchers reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
over time.
METHODOLOGY:
- Existing tools that detect disease progression in patients with CM are limited.
- In a prospective cohort study, researchers evaluated the use of 3-D stereophotogrammetry, a noninvasive, radiation-free imaging modality, to detect disease progression in 27 consecutive patients with CM seen at Boston Children’s Hospital and Brigham and Women’s Hospital from April 1, 2019, to March 1, 2023.
- After clinical and 3-D stereophotogrammetry assessments were performed at 2- to 12-month intervals, the 3-D images were rated by an expert (a board-certified plastic craniofacial surgeon) and a nonexpert (a board-certified dermatologist) as demonstrating progression or no progression.
- Kappa coefficients were used to calculate inter-rater reliability.
TAKEAWAY:
- Most of the study participants (73%) were female, their median age was 14 years (range, 5-40 years), and each underwent 3-D stereophotogrammetry imaging at least two times spaced a median of 3 months apart.
- On the basis of clinical assessments during the 48-month study period, 10 patients (37%) experienced progression of their disease.
- 3-D stereophotogrammetry not only corroborated clinical impressions of disease progression with strong inter-rater reliability (kappa = 0.80; 95% confidence interval, 0.61-0.99), but it also detected occult progression of asymmetry not noted on clinical examination in three additional patients.
- In subgroup analyses, assessment of 3-D images demonstrated substantial to near-perfect inter-rater reliability in patients with Fitzpatrick skin types IV-VI.
IN PRACTICE:
“Further work is necessary to validate this measure in a larger cohort and to guide its incorporation into medical decision-making for patients with CM,” the researchers wrote.
SOURCE:
Katharina S. Shaw, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
The sample was small, and a criterion standard for assessing CM was lacking.
DISCLOSURES:
The researchers reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
over time.
METHODOLOGY:
- Existing tools that detect disease progression in patients with CM are limited.
- In a prospective cohort study, researchers evaluated the use of 3-D stereophotogrammetry, a noninvasive, radiation-free imaging modality, to detect disease progression in 27 consecutive patients with CM seen at Boston Children’s Hospital and Brigham and Women’s Hospital from April 1, 2019, to March 1, 2023.
- After clinical and 3-D stereophotogrammetry assessments were performed at 2- to 12-month intervals, the 3-D images were rated by an expert (a board-certified plastic craniofacial surgeon) and a nonexpert (a board-certified dermatologist) as demonstrating progression or no progression.
- Kappa coefficients were used to calculate inter-rater reliability.
TAKEAWAY:
- Most of the study participants (73%) were female, their median age was 14 years (range, 5-40 years), and each underwent 3-D stereophotogrammetry imaging at least two times spaced a median of 3 months apart.
- On the basis of clinical assessments during the 48-month study period, 10 patients (37%) experienced progression of their disease.
- 3-D stereophotogrammetry not only corroborated clinical impressions of disease progression with strong inter-rater reliability (kappa = 0.80; 95% confidence interval, 0.61-0.99), but it also detected occult progression of asymmetry not noted on clinical examination in three additional patients.
- In subgroup analyses, assessment of 3-D images demonstrated substantial to near-perfect inter-rater reliability in patients with Fitzpatrick skin types IV-VI.
IN PRACTICE:
“Further work is necessary to validate this measure in a larger cohort and to guide its incorporation into medical decision-making for patients with CM,” the researchers wrote.
SOURCE:
Katharina S. Shaw, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
The sample was small, and a criterion standard for assessing CM was lacking.
DISCLOSURES:
The researchers reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Pediatric sleep-disordered breathing linked to multilevel risk factors
In the first study evaluating pediatric sleep-disordered breathing (SDB) from both indoor environment and neighborhood perspectives, multilevel risk factors were revealed as being associated with SDB-related symptoms. Beyond known associations with environmental tobacco smoke (ETS), .
Although it has been well known that pediatric SDB affects low socioeconomic status (SES) children disproportionately, the roles of multilevel risk factor drivers including individual health, household SES, indoor exposures to environmental tobacco smoke, pests, and neighborhood characteristics have not been well studied, Gueye-Ndiaye et al. wrote in CHEST Pulmonary.
Pediatric SDB, a known risk factor for many health, neurobehavioral, and functional outcomes, includes habitual snoring and obstructive sleep apnea and may contribute to health disparities. Adenotonsillar hypertrophy and obesity are the most commonly recognized risk factors for SDB in generally healthy school-aged children. A role for other risk factors, however, is suggested by the fact that Black children have a fourfold increased risk for obstructive sleep apnea (OSA), compared with White children, unexplained by obesity, and have decreased response to treatment of OSA with adenotonsillectomy, compared with White children. Several studies point in the direction of neighborhood disadvantages as factors in heightened SDB prevalence or severity, Gueye-Ndiaye et al. stated.
The authors performed cross-sectional analyses on data recorded from 303 children (aged 6-12 years) enrolled in the Environmental Assessment of Sleep Youth (EASY) study from 2018 to 2022. Among them, 39% were Hispanic, Latino, Latina, or Spanish origin, 30% were Black or African American, 22% were White, and 11% were other. Maternal education attainment of a high school diploma or less was reported in 27%, and 65% of the sample lived in disadvantaged neighborhoods. Twenty-eight percent of children met criteria for objective SDB (Apnea-Hypopnea Index/Oxygen Desaturation Index ≥ 5/hr). Exposure documentation was informed by caregiver reports, assays of measured settled dust from the child’s bedroom, and neighborhood-level census data from which the Childhood Opportunity Index characterizing neighborhood disadvantage (ND) was derived. The study primary outcome was the SDB-related symptom burden assessed by the OSA-18 questionnaire total score.
Compared with children with no adverse indoor exposures to ETS and pests, children with such exposures had an approximately 4-12 point increase in total OSA-18 scores, and the increase among those with exposure to both ETS and pests was about 20 points (approximately a 1.3 standard deviation increase), Gueye-Ndiaye et al. reported.
In models adjusted for age, sex, minority race, and ethnicity, low maternal education was associated with a 7.55 (95% confidence interval, 3.44-11.66; P < .01) increased OSA-18 score. In models adjusted for sociodemographics including maternal education, history of asthma and allergic rhinitis were associated with a 13.63 (95% CI, 9.44-17.82; P < .01) and a 6.95 (95% CI, 2.62-11.29; P < .02) increased OSA-18 score, respectively. The authors noted that prior Canadian studies have shown OSA to be three times as likely in children with mothers reporting less than a high school education than in children with university educated mothers.
Speculating on the drivers of this association, they noted that the poor air quality due to tobacco smoke and allergen exposures to rodents, mold, and cockroaches are known contributors to asthma symptoms. Despite the differing pathogenesis of OSA and asthma, they suggest overlapping risk factors. Irritants and allergens may exacerbate SDB by stimulating immune responses manifested as adenotonsillar hypertrophy and by amplifying nasopharyngeal inflammation, adversely affecting upper airway patency. While ETS was not common in the sample, it was associated strongly with SDB. Gueye-Ndiaye et al. also showed associations between pest exposure, bedroom dust, and SDB symptoms. The findings, they concluded, support the importance of household- and bedroom-environmental conditions and sleep health.
OSA-18 scores were also elevated by about 7-14 points with allergic rhinitis and asthma, respectively. The findings, Gueye-Ndiaye et al. stated, underscore that asthma prevention strategies can be leveraged to address SDB disparities. No amplification of pest exposure effects, however, was found for asthma or allergic rhinitis.
“This is an incredibly important study, one that adds to our understanding of the risk factors that contribute to pediatric sleep health disparities,” said assistant professor of pediatrics Anne C. Coates, MD, Tufts University, Boston. “We have previously understood risk factors for sleep-disordered breathing like adenotonsillar hypertrophy, but this adds other elements like environmental tobacco smoke, pests, and home and neighborhood factors,” she told this news organization. “One of the most important takeaways is that beyond the importance of accurate diagnosis, there is the importance of advocating for our patients to ensure that they have the healthiest homes and neighborhoods. We need to inspire our colleagues to be advocates – for example – for pest mitigation, for antismoking policies, for every policy preventing the factors that contribute to the burden of disease.”
Dr. Coates is coauthor of “Advocacy and Health Equity: The Role of the Pediatric Pulmonologist,” currently in press (Clinics in Chest Medicine), and a member of the CHEST Physician Editorial Board.
The authors noted that a study limitation was that the sample was from one geographic area (Boston). Neither the authors nor Dr. Coates listed any conflicts.
In the first study evaluating pediatric sleep-disordered breathing (SDB) from both indoor environment and neighborhood perspectives, multilevel risk factors were revealed as being associated with SDB-related symptoms. Beyond known associations with environmental tobacco smoke (ETS), .
Although it has been well known that pediatric SDB affects low socioeconomic status (SES) children disproportionately, the roles of multilevel risk factor drivers including individual health, household SES, indoor exposures to environmental tobacco smoke, pests, and neighborhood characteristics have not been well studied, Gueye-Ndiaye et al. wrote in CHEST Pulmonary.
Pediatric SDB, a known risk factor for many health, neurobehavioral, and functional outcomes, includes habitual snoring and obstructive sleep apnea and may contribute to health disparities. Adenotonsillar hypertrophy and obesity are the most commonly recognized risk factors for SDB in generally healthy school-aged children. A role for other risk factors, however, is suggested by the fact that Black children have a fourfold increased risk for obstructive sleep apnea (OSA), compared with White children, unexplained by obesity, and have decreased response to treatment of OSA with adenotonsillectomy, compared with White children. Several studies point in the direction of neighborhood disadvantages as factors in heightened SDB prevalence or severity, Gueye-Ndiaye et al. stated.
The authors performed cross-sectional analyses on data recorded from 303 children (aged 6-12 years) enrolled in the Environmental Assessment of Sleep Youth (EASY) study from 2018 to 2022. Among them, 39% were Hispanic, Latino, Latina, or Spanish origin, 30% were Black or African American, 22% were White, and 11% were other. Maternal education attainment of a high school diploma or less was reported in 27%, and 65% of the sample lived in disadvantaged neighborhoods. Twenty-eight percent of children met criteria for objective SDB (Apnea-Hypopnea Index/Oxygen Desaturation Index ≥ 5/hr). Exposure documentation was informed by caregiver reports, assays of measured settled dust from the child’s bedroom, and neighborhood-level census data from which the Childhood Opportunity Index characterizing neighborhood disadvantage (ND) was derived. The study primary outcome was the SDB-related symptom burden assessed by the OSA-18 questionnaire total score.
Compared with children with no adverse indoor exposures to ETS and pests, children with such exposures had an approximately 4-12 point increase in total OSA-18 scores, and the increase among those with exposure to both ETS and pests was about 20 points (approximately a 1.3 standard deviation increase), Gueye-Ndiaye et al. reported.
In models adjusted for age, sex, minority race, and ethnicity, low maternal education was associated with a 7.55 (95% confidence interval, 3.44-11.66; P < .01) increased OSA-18 score. In models adjusted for sociodemographics including maternal education, history of asthma and allergic rhinitis were associated with a 13.63 (95% CI, 9.44-17.82; P < .01) and a 6.95 (95% CI, 2.62-11.29; P < .02) increased OSA-18 score, respectively. The authors noted that prior Canadian studies have shown OSA to be three times as likely in children with mothers reporting less than a high school education than in children with university educated mothers.
Speculating on the drivers of this association, they noted that the poor air quality due to tobacco smoke and allergen exposures to rodents, mold, and cockroaches are known contributors to asthma symptoms. Despite the differing pathogenesis of OSA and asthma, they suggest overlapping risk factors. Irritants and allergens may exacerbate SDB by stimulating immune responses manifested as adenotonsillar hypertrophy and by amplifying nasopharyngeal inflammation, adversely affecting upper airway patency. While ETS was not common in the sample, it was associated strongly with SDB. Gueye-Ndiaye et al. also showed associations between pest exposure, bedroom dust, and SDB symptoms. The findings, they concluded, support the importance of household- and bedroom-environmental conditions and sleep health.
OSA-18 scores were also elevated by about 7-14 points with allergic rhinitis and asthma, respectively. The findings, Gueye-Ndiaye et al. stated, underscore that asthma prevention strategies can be leveraged to address SDB disparities. No amplification of pest exposure effects, however, was found for asthma or allergic rhinitis.
“This is an incredibly important study, one that adds to our understanding of the risk factors that contribute to pediatric sleep health disparities,” said assistant professor of pediatrics Anne C. Coates, MD, Tufts University, Boston. “We have previously understood risk factors for sleep-disordered breathing like adenotonsillar hypertrophy, but this adds other elements like environmental tobacco smoke, pests, and home and neighborhood factors,” she told this news organization. “One of the most important takeaways is that beyond the importance of accurate diagnosis, there is the importance of advocating for our patients to ensure that they have the healthiest homes and neighborhoods. We need to inspire our colleagues to be advocates – for example – for pest mitigation, for antismoking policies, for every policy preventing the factors that contribute to the burden of disease.”
Dr. Coates is coauthor of “Advocacy and Health Equity: The Role of the Pediatric Pulmonologist,” currently in press (Clinics in Chest Medicine), and a member of the CHEST Physician Editorial Board.
The authors noted that a study limitation was that the sample was from one geographic area (Boston). Neither the authors nor Dr. Coates listed any conflicts.
In the first study evaluating pediatric sleep-disordered breathing (SDB) from both indoor environment and neighborhood perspectives, multilevel risk factors were revealed as being associated with SDB-related symptoms. Beyond known associations with environmental tobacco smoke (ETS), .
Although it has been well known that pediatric SDB affects low socioeconomic status (SES) children disproportionately, the roles of multilevel risk factor drivers including individual health, household SES, indoor exposures to environmental tobacco smoke, pests, and neighborhood characteristics have not been well studied, Gueye-Ndiaye et al. wrote in CHEST Pulmonary.
Pediatric SDB, a known risk factor for many health, neurobehavioral, and functional outcomes, includes habitual snoring and obstructive sleep apnea and may contribute to health disparities. Adenotonsillar hypertrophy and obesity are the most commonly recognized risk factors for SDB in generally healthy school-aged children. A role for other risk factors, however, is suggested by the fact that Black children have a fourfold increased risk for obstructive sleep apnea (OSA), compared with White children, unexplained by obesity, and have decreased response to treatment of OSA with adenotonsillectomy, compared with White children. Several studies point in the direction of neighborhood disadvantages as factors in heightened SDB prevalence or severity, Gueye-Ndiaye et al. stated.
The authors performed cross-sectional analyses on data recorded from 303 children (aged 6-12 years) enrolled in the Environmental Assessment of Sleep Youth (EASY) study from 2018 to 2022. Among them, 39% were Hispanic, Latino, Latina, or Spanish origin, 30% were Black or African American, 22% were White, and 11% were other. Maternal education attainment of a high school diploma or less was reported in 27%, and 65% of the sample lived in disadvantaged neighborhoods. Twenty-eight percent of children met criteria for objective SDB (Apnea-Hypopnea Index/Oxygen Desaturation Index ≥ 5/hr). Exposure documentation was informed by caregiver reports, assays of measured settled dust from the child’s bedroom, and neighborhood-level census data from which the Childhood Opportunity Index characterizing neighborhood disadvantage (ND) was derived. The study primary outcome was the SDB-related symptom burden assessed by the OSA-18 questionnaire total score.
Compared with children with no adverse indoor exposures to ETS and pests, children with such exposures had an approximately 4-12 point increase in total OSA-18 scores, and the increase among those with exposure to both ETS and pests was about 20 points (approximately a 1.3 standard deviation increase), Gueye-Ndiaye et al. reported.
In models adjusted for age, sex, minority race, and ethnicity, low maternal education was associated with a 7.55 (95% confidence interval, 3.44-11.66; P < .01) increased OSA-18 score. In models adjusted for sociodemographics including maternal education, history of asthma and allergic rhinitis were associated with a 13.63 (95% CI, 9.44-17.82; P < .01) and a 6.95 (95% CI, 2.62-11.29; P < .02) increased OSA-18 score, respectively. The authors noted that prior Canadian studies have shown OSA to be three times as likely in children with mothers reporting less than a high school education than in children with university educated mothers.
Speculating on the drivers of this association, they noted that the poor air quality due to tobacco smoke and allergen exposures to rodents, mold, and cockroaches are known contributors to asthma symptoms. Despite the differing pathogenesis of OSA and asthma, they suggest overlapping risk factors. Irritants and allergens may exacerbate SDB by stimulating immune responses manifested as adenotonsillar hypertrophy and by amplifying nasopharyngeal inflammation, adversely affecting upper airway patency. While ETS was not common in the sample, it was associated strongly with SDB. Gueye-Ndiaye et al. also showed associations between pest exposure, bedroom dust, and SDB symptoms. The findings, they concluded, support the importance of household- and bedroom-environmental conditions and sleep health.
OSA-18 scores were also elevated by about 7-14 points with allergic rhinitis and asthma, respectively. The findings, Gueye-Ndiaye et al. stated, underscore that asthma prevention strategies can be leveraged to address SDB disparities. No amplification of pest exposure effects, however, was found for asthma or allergic rhinitis.
“This is an incredibly important study, one that adds to our understanding of the risk factors that contribute to pediatric sleep health disparities,” said assistant professor of pediatrics Anne C. Coates, MD, Tufts University, Boston. “We have previously understood risk factors for sleep-disordered breathing like adenotonsillar hypertrophy, but this adds other elements like environmental tobacco smoke, pests, and home and neighborhood factors,” she told this news organization. “One of the most important takeaways is that beyond the importance of accurate diagnosis, there is the importance of advocating for our patients to ensure that they have the healthiest homes and neighborhoods. We need to inspire our colleagues to be advocates – for example – for pest mitigation, for antismoking policies, for every policy preventing the factors that contribute to the burden of disease.”
Dr. Coates is coauthor of “Advocacy and Health Equity: The Role of the Pediatric Pulmonologist,” currently in press (Clinics in Chest Medicine), and a member of the CHEST Physician Editorial Board.
The authors noted that a study limitation was that the sample was from one geographic area (Boston). Neither the authors nor Dr. Coates listed any conflicts.
FROM CHEST PULMONARY
What the first authorized DNA cancer risk test can and can’t tell you
A novel DNA test system that assesses a person’s genetic predisposition for certain cancers – the first of its kind granted marketing authorization by the Food and Drug Administration – may become a valuable new public health tool.
The Common Hereditary Cancers Panel (Invitae) was approved late September following FDA review under the De Novo process, a regulatory pathway for new types of low- to moderate-risk devices.
Validation of the prescription-only in vitro test was based on assessments of more than 9,000 clinical samples, which demonstrated accuracy of at least 99% for all tested variants in 47 genes known to be associated with an increased risk of developing certain cancers, including breast, ovarian, uterine, prostate, colorectal, gastric, pancreatic as well as melanoma.
How the test system works
Next-generation sequencing assesses germline human genomic DNA extracted from a single blood sample collected at the point of care, such as a doctor’s office, and is sent to a laboratory for analysis.
Specifically, the system aims to detect substitutions, small insertion and deletion alterations, and copy number variants in the panel of 47 targeted genes.
Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological health, explained in an FDA press release announcing the marketing authorization.
Clinical interpretation is based on evidence from the published literature, prediction programs, public databases, and Invitae’s own variants database, the FDA statement explained.
What the test can do
Not only can the Common Hereditary Cancer Panel identify genetic variants that increase an individual’s risk of certain cancers, the panel can also help identify potential cancer-related hereditary variants in patients already diagnosed with cancer.
The most clinically significant genes the test system can detect include BRCA1 and BRCA2, which have known associations with hereditary breast and ovarian cancer syndrome; Lynch syndrome–associated genes including MLH1, MSH2, MSH6, PMS2, and EPCAM; CDH1, which is largely associated with hereditary diffuse gastric cancer and lobular breast cancer; and STK11, which is associated with Peutz-Jeghers syndrome.
“Patients should speak with a health care professional, such as a genetic counselor, to discuss any personal/family history of cancer, as such information can be helpful in interpreting test results,” the FDA advised.
What the test can’t do
The test is not intended to identify or evaluate all known genes tied to a person’s potential predisposition for cancer. The test is also not intended for cancer screening or prenatal testing.
For these reasons, and because genetics are not the only factor associated with developing cancer, negative test results could lead to misunderstanding among some patients about their cancer risk.
“Results are intended to be interpreted within the context of additional laboratory results, family history, and clinical findings,” the company wrote in a statement.
Test safety
Risks associated with the test include the possibility of false positive and false negative results and the potential for people to misunderstand what the results mean about their risk for cancer.
A false sense of assurance after a false negative result might, for instance, lead patients to forgo recommended surveillance or clinical management, whereas false positive test results could lead to inappropriate decision-making and undesirable consequences.
“These risks are mitigated by the analytical performance validation, clinical validation, and appropriate labeling of this test,” the agency explained.
Along with the De Novo authorization, the FDA is establishing special controls to define requirements for these tests. For instance, accuracy must be 99% or higher for positive agreement and at least 99.9% for negative agreement with a validated, independent method.
Public health implications
The information gleaned from this tool can “help guide physicians to provide appropriate monitoring and potential therapy, based on discovered variants,” Dr. Shuren said.
The marketing authorization of Invitae’s test established a new regulatory category, which “means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process,” the FDA explained.
A version of this article first appeared on Medscape.com.
A novel DNA test system that assesses a person’s genetic predisposition for certain cancers – the first of its kind granted marketing authorization by the Food and Drug Administration – may become a valuable new public health tool.
The Common Hereditary Cancers Panel (Invitae) was approved late September following FDA review under the De Novo process, a regulatory pathway for new types of low- to moderate-risk devices.
Validation of the prescription-only in vitro test was based on assessments of more than 9,000 clinical samples, which demonstrated accuracy of at least 99% for all tested variants in 47 genes known to be associated with an increased risk of developing certain cancers, including breast, ovarian, uterine, prostate, colorectal, gastric, pancreatic as well as melanoma.
How the test system works
Next-generation sequencing assesses germline human genomic DNA extracted from a single blood sample collected at the point of care, such as a doctor’s office, and is sent to a laboratory for analysis.
Specifically, the system aims to detect substitutions, small insertion and deletion alterations, and copy number variants in the panel of 47 targeted genes.
Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological health, explained in an FDA press release announcing the marketing authorization.
Clinical interpretation is based on evidence from the published literature, prediction programs, public databases, and Invitae’s own variants database, the FDA statement explained.
What the test can do
Not only can the Common Hereditary Cancer Panel identify genetic variants that increase an individual’s risk of certain cancers, the panel can also help identify potential cancer-related hereditary variants in patients already diagnosed with cancer.
The most clinically significant genes the test system can detect include BRCA1 and BRCA2, which have known associations with hereditary breast and ovarian cancer syndrome; Lynch syndrome–associated genes including MLH1, MSH2, MSH6, PMS2, and EPCAM; CDH1, which is largely associated with hereditary diffuse gastric cancer and lobular breast cancer; and STK11, which is associated with Peutz-Jeghers syndrome.
“Patients should speak with a health care professional, such as a genetic counselor, to discuss any personal/family history of cancer, as such information can be helpful in interpreting test results,” the FDA advised.
What the test can’t do
The test is not intended to identify or evaluate all known genes tied to a person’s potential predisposition for cancer. The test is also not intended for cancer screening or prenatal testing.
For these reasons, and because genetics are not the only factor associated with developing cancer, negative test results could lead to misunderstanding among some patients about their cancer risk.
“Results are intended to be interpreted within the context of additional laboratory results, family history, and clinical findings,” the company wrote in a statement.
Test safety
Risks associated with the test include the possibility of false positive and false negative results and the potential for people to misunderstand what the results mean about their risk for cancer.
A false sense of assurance after a false negative result might, for instance, lead patients to forgo recommended surveillance or clinical management, whereas false positive test results could lead to inappropriate decision-making and undesirable consequences.
“These risks are mitigated by the analytical performance validation, clinical validation, and appropriate labeling of this test,” the agency explained.
Along with the De Novo authorization, the FDA is establishing special controls to define requirements for these tests. For instance, accuracy must be 99% or higher for positive agreement and at least 99.9% for negative agreement with a validated, independent method.
Public health implications
The information gleaned from this tool can “help guide physicians to provide appropriate monitoring and potential therapy, based on discovered variants,” Dr. Shuren said.
The marketing authorization of Invitae’s test established a new regulatory category, which “means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process,” the FDA explained.
A version of this article first appeared on Medscape.com.
A novel DNA test system that assesses a person’s genetic predisposition for certain cancers – the first of its kind granted marketing authorization by the Food and Drug Administration – may become a valuable new public health tool.
The Common Hereditary Cancers Panel (Invitae) was approved late September following FDA review under the De Novo process, a regulatory pathway for new types of low- to moderate-risk devices.
Validation of the prescription-only in vitro test was based on assessments of more than 9,000 clinical samples, which demonstrated accuracy of at least 99% for all tested variants in 47 genes known to be associated with an increased risk of developing certain cancers, including breast, ovarian, uterine, prostate, colorectal, gastric, pancreatic as well as melanoma.
How the test system works
Next-generation sequencing assesses germline human genomic DNA extracted from a single blood sample collected at the point of care, such as a doctor’s office, and is sent to a laboratory for analysis.
Specifically, the system aims to detect substitutions, small insertion and deletion alterations, and copy number variants in the panel of 47 targeted genes.
Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological health, explained in an FDA press release announcing the marketing authorization.
Clinical interpretation is based on evidence from the published literature, prediction programs, public databases, and Invitae’s own variants database, the FDA statement explained.
What the test can do
Not only can the Common Hereditary Cancer Panel identify genetic variants that increase an individual’s risk of certain cancers, the panel can also help identify potential cancer-related hereditary variants in patients already diagnosed with cancer.
The most clinically significant genes the test system can detect include BRCA1 and BRCA2, which have known associations with hereditary breast and ovarian cancer syndrome; Lynch syndrome–associated genes including MLH1, MSH2, MSH6, PMS2, and EPCAM; CDH1, which is largely associated with hereditary diffuse gastric cancer and lobular breast cancer; and STK11, which is associated with Peutz-Jeghers syndrome.
“Patients should speak with a health care professional, such as a genetic counselor, to discuss any personal/family history of cancer, as such information can be helpful in interpreting test results,” the FDA advised.
What the test can’t do
The test is not intended to identify or evaluate all known genes tied to a person’s potential predisposition for cancer. The test is also not intended for cancer screening or prenatal testing.
For these reasons, and because genetics are not the only factor associated with developing cancer, negative test results could lead to misunderstanding among some patients about their cancer risk.
“Results are intended to be interpreted within the context of additional laboratory results, family history, and clinical findings,” the company wrote in a statement.
Test safety
Risks associated with the test include the possibility of false positive and false negative results and the potential for people to misunderstand what the results mean about their risk for cancer.
A false sense of assurance after a false negative result might, for instance, lead patients to forgo recommended surveillance or clinical management, whereas false positive test results could lead to inappropriate decision-making and undesirable consequences.
“These risks are mitigated by the analytical performance validation, clinical validation, and appropriate labeling of this test,” the agency explained.
Along with the De Novo authorization, the FDA is establishing special controls to define requirements for these tests. For instance, accuracy must be 99% or higher for positive agreement and at least 99.9% for negative agreement with a validated, independent method.
Public health implications
The information gleaned from this tool can “help guide physicians to provide appropriate monitoring and potential therapy, based on discovered variants,” Dr. Shuren said.
The marketing authorization of Invitae’s test established a new regulatory category, which “means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process,” the FDA explained.
A version of this article first appeared on Medscape.com.
SCLC Pathophysiology & Epidemiology
Pruritic rash and nocturnal itching
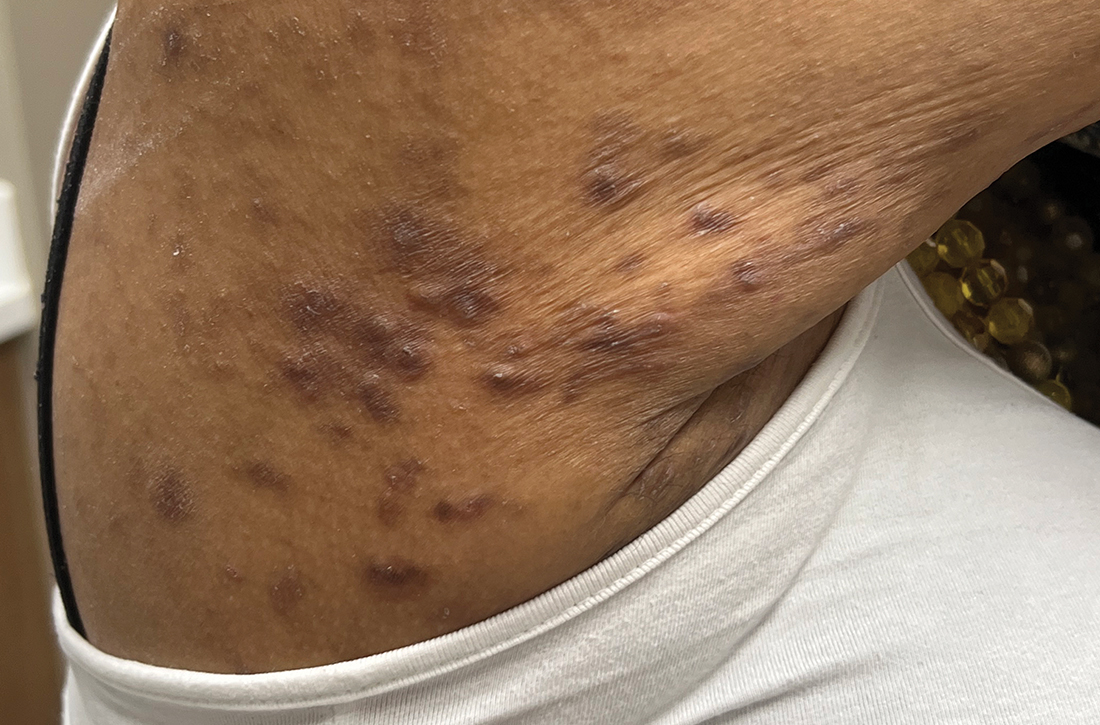
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
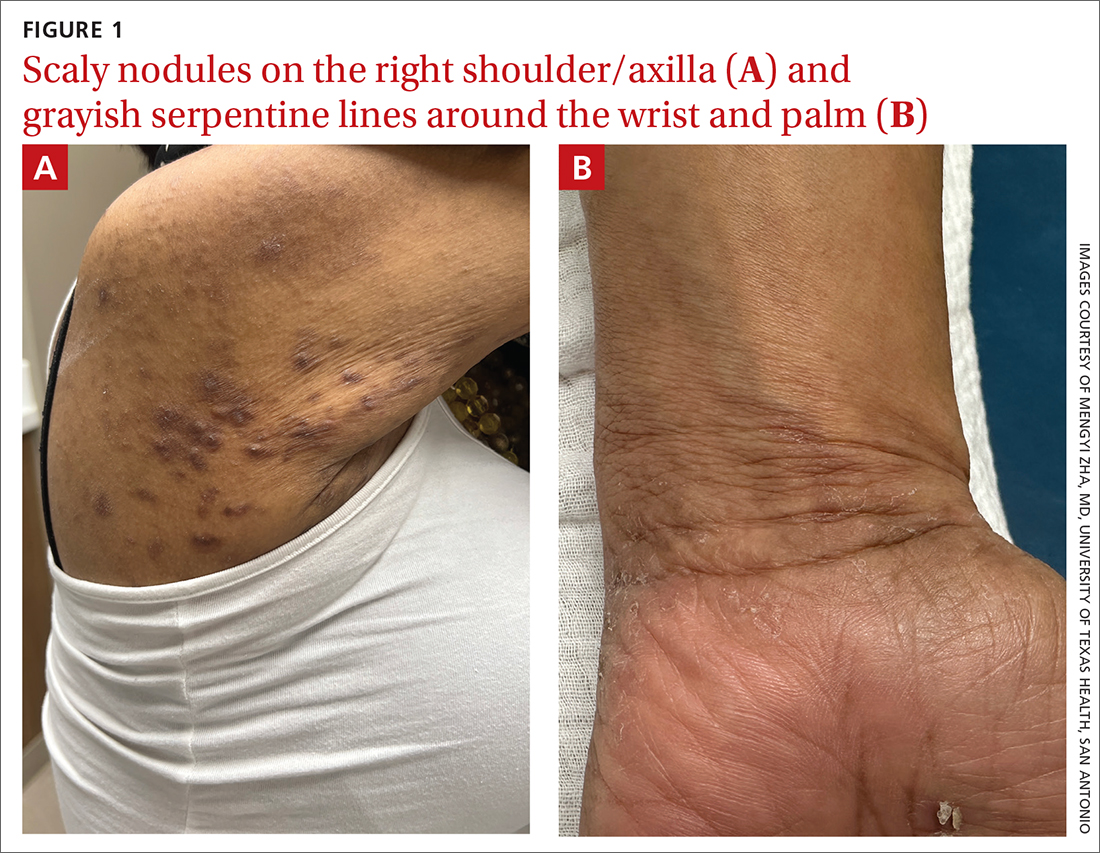
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
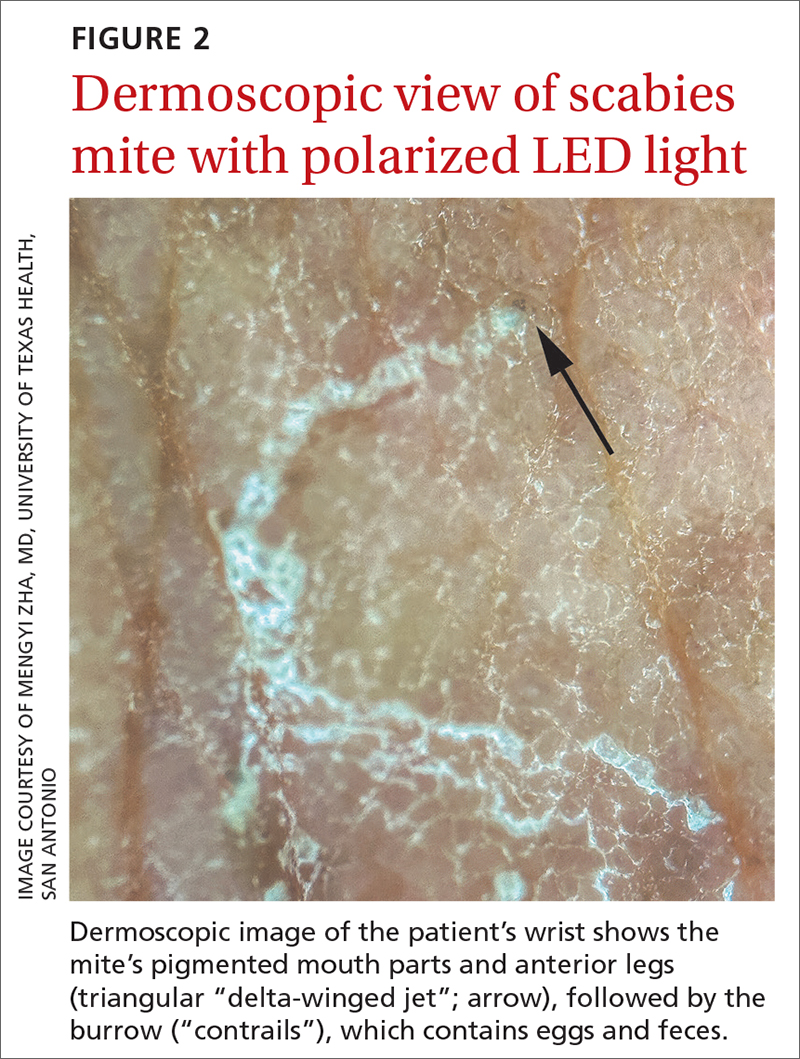
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).
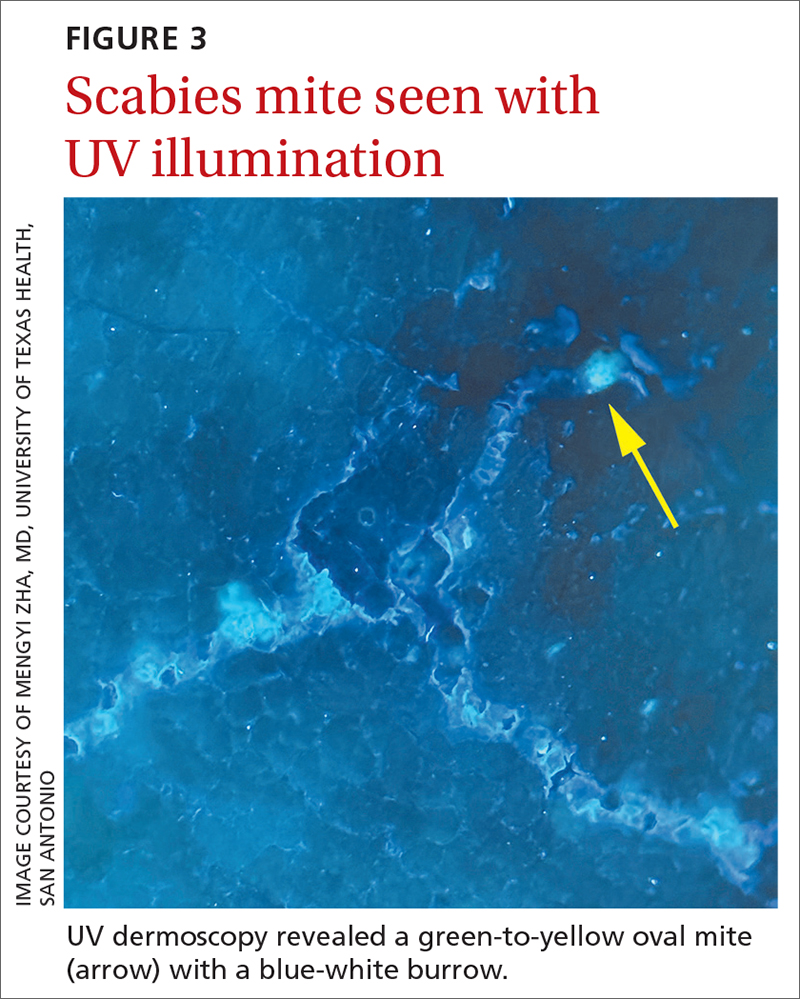
The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).
Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
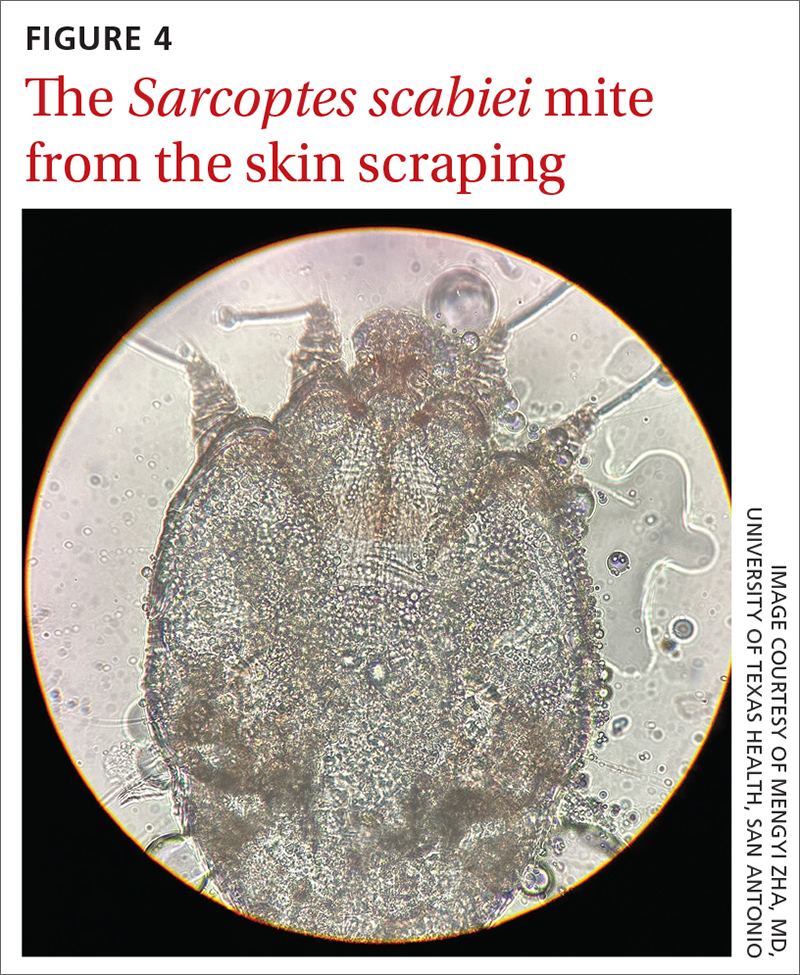
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).
UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).

The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).

Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).

UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).

The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).

Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).

UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
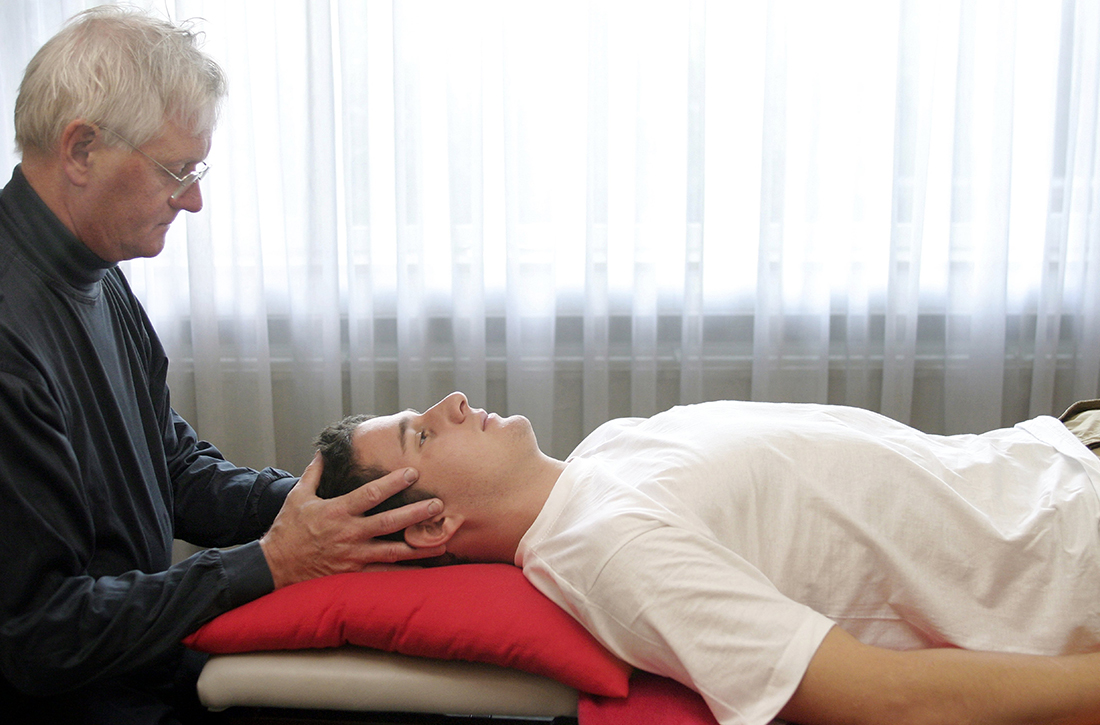
Are manual therapies effective at reducing chronic tension headache frequency in adults?
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.
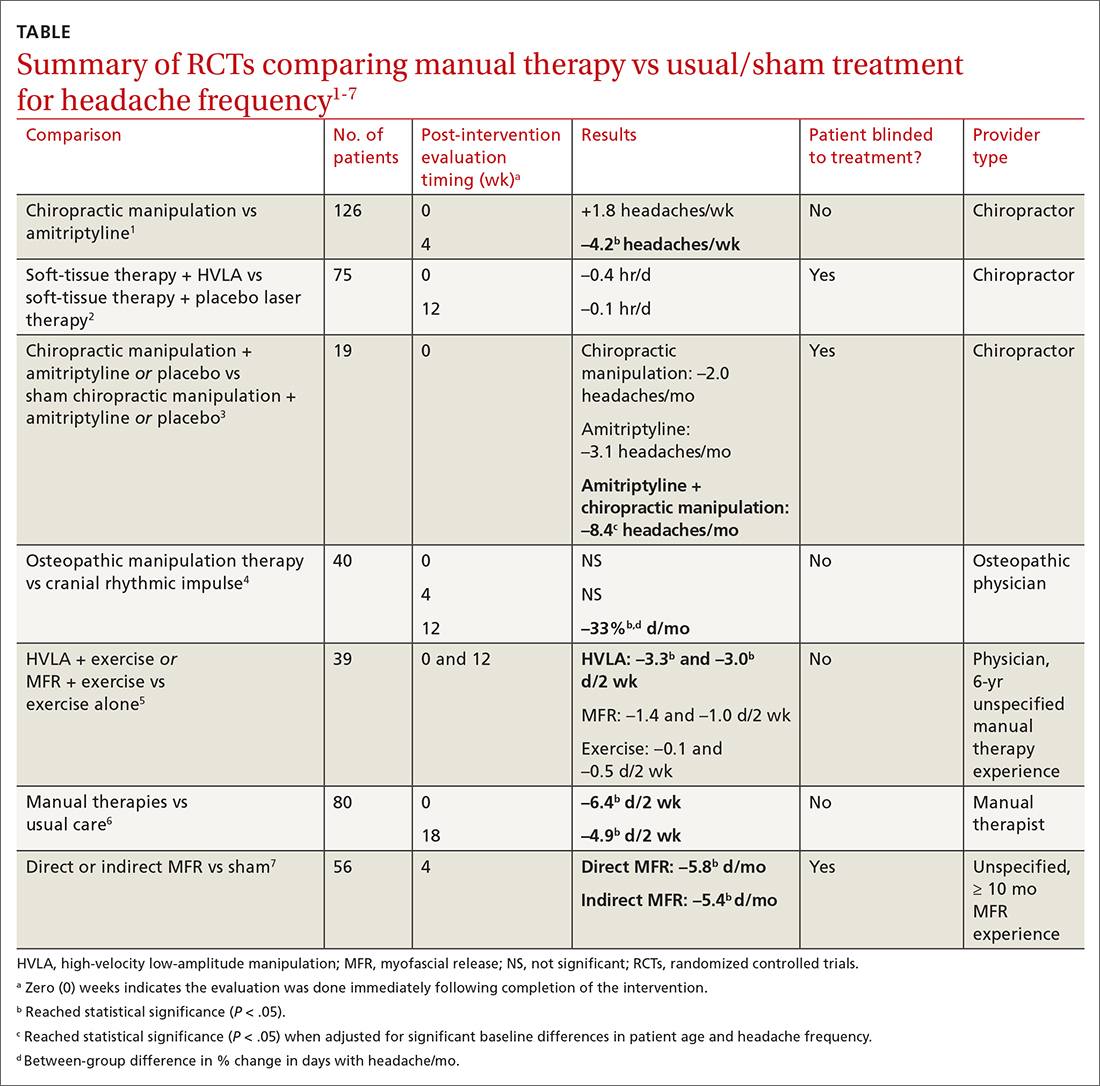
Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
EVIDENCE-BASED ANSWER:
MAYBE. Among patients with chronic tension headaches, manual therapies may reduce headache frequency more than sham manual therapy, usual care, or exercise treatments—by 1.5 to 4.2 headaches or days with headache per week (strength of recommendation, B; preponderance of evidence from primarily small, heterogeneous randomized controlled trials [RCTs]).
Not acne, but what?
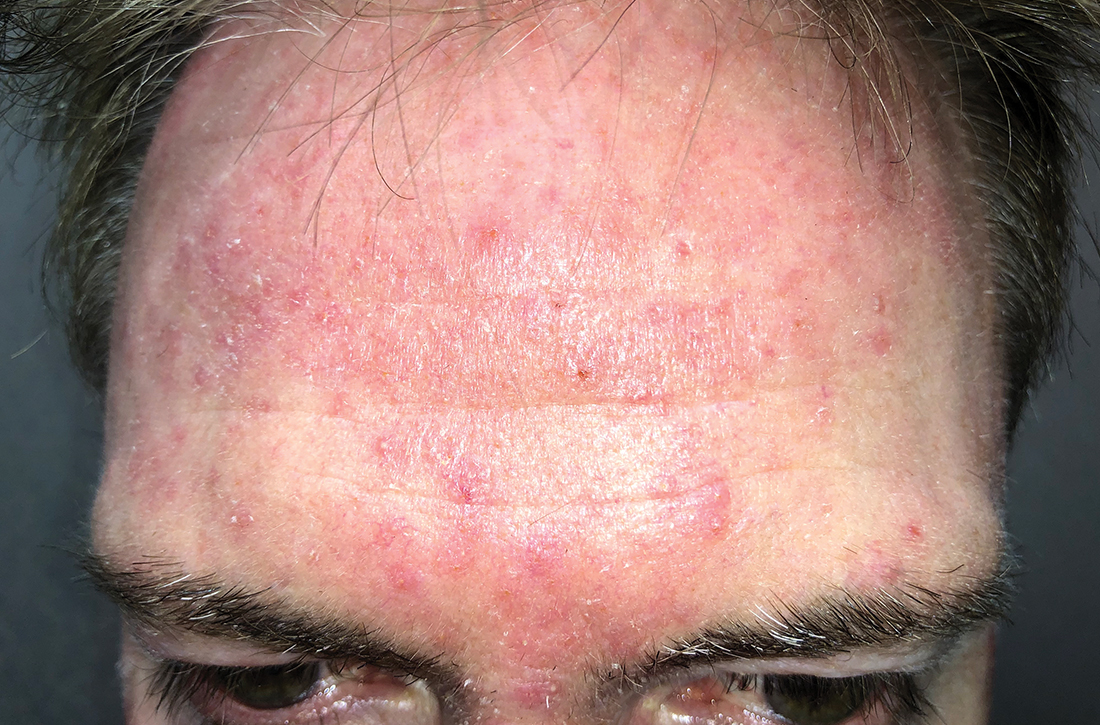
AN OTHERWISE HEALTHY
Scattered papules and pustules were present on the forehead, nose, and cheeks, with background erythema and telangiectasias (FIGURE 1). A few pinpoint crusted excoriations were noted. A sample was taken from the papules and pustules using a #15 blade and submitted for examination.
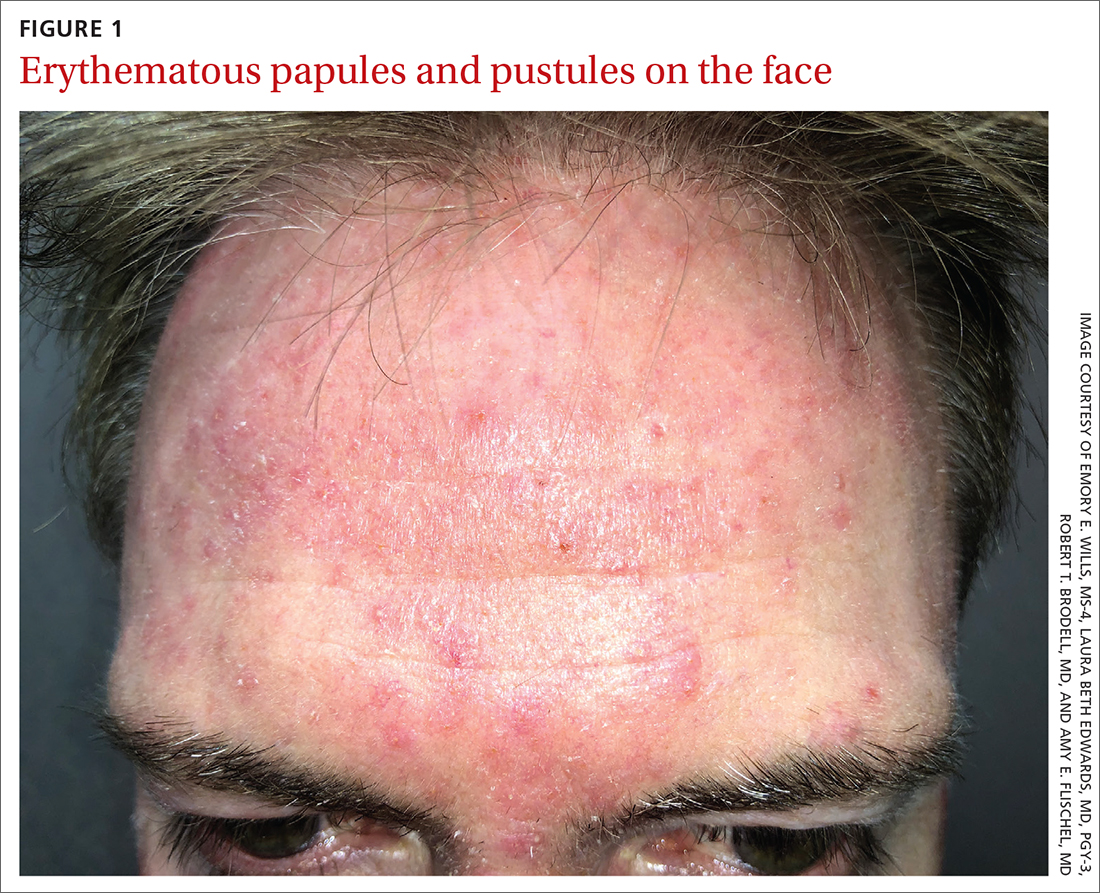
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
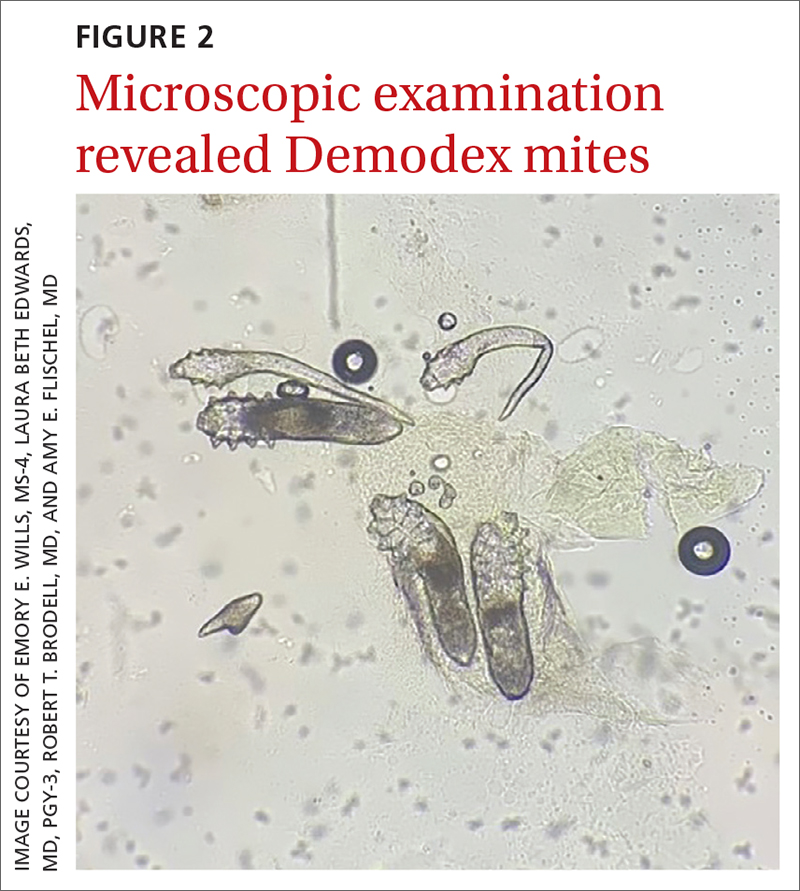
Diagnosis: Rosacea with Demodex mites
Under light microscopy, the scraping revealed Demodex mites (FIGURE 2). It has been proposed that these mites play a role in the inflammatory process seen in rosacea, although studies have yet to determine whether the inflammatory symptoms of rosacea cause the mites to proliferate or if the mites contribute to the initial inflammatory process.1,2
Demodex folliculorum and D brevis are part of normal skin flora; they are found in about 12% of all follicles and most commonly involve the face.3 They often become abundant in the presence of numerous sebaceous glands. Men have more sebaceous glands than women do, and thus run a greater risk for infestation with mites. An abnormal proliferation of Demodex mites can lead to demodicosis.
Demodex mites can be examined microscopically via the skin surface sampling technique known as scraping, which was done in this case. Samples taken from the papules and pustules utilizing a #15 blade are placed in immersion oil on a glass slide, cover-slipped, and examined by light microscopy.
Rosacea is thought to be an inflammatory disease in which the immune system is triggered by a variety of factors, including UV light, heat, stress, alcohol, hormonal influences, and microorganisms.1,4 The disease is found in up to 10% of the population worldwide.1
The diagnosis of rosacea requires at least 1 of the 2 “core features”—persistent central facial erythema or phymatous changes—or 2 of 4 “major features”: papules/pustules, ocular manifestation, flushing, and telangiectasias. There are 3 phenotypes: ocular, papulopustular, and erythematotelangiectatic.5,6
Continue to: The connection
The connection. Papulopustular and erythematotelangiectatic rosacea may be caused by a proliferation of Demodex mites and increased vascular endothelial growth factor production.2 In fact, a proliferation of Demodex is seen in almost all cases of papulopustular rosacea and more than 60% of cases of erythematotelangiectatic rosacea.2
Patient age and distribution of lesions narrowed the differential
Acne vulgaris is an inflammatory disease of the pilosebaceous units caused by increased sebum production, inflammation, and bacterial colonization (Propionibacterium acnes) of hair follicles on the face, neck, chest, and other areas. Both inflammatory and noninflammatory lesions can be present, and in serious cases, scarring can result.7 The case patient’s age and accompanying broad erythema were more consistent with rosacea than acne vulgaris.
Seborrheic dermatitis is a common skin condition usually stemming from an inflammatory reaction to a common yeast. Classic symptoms include scaling and erythema of the scalp and central face, as well as pruritus. Topical antifungals such as ketoconazole 2% cream and 2% shampoo are the mainstay of treatment.8 The broad distribution and papulopustules in this patient argue against the diagnosis of seborrheic dermatitis.
Systemic lupus erythematosus is a systemic inflammatory disease that often has cutaneous manifestations. Acute lupus manifests as an erythematous “butterfly rash” across the face and cheeks. Chronic discoid lupus involves depigmented plaques, erythematous macules, telangiectasias, and scarring with loss of normal hair follicles. These findings classically are photodistributed.9 The classic broad erythema extending from the cheeks over the bridge of the nose was not present in this patient.
Treatment is primarily topical
Mild cases of rosacea often can be managed with topical antibiotic creams. More severe cases may require systemic antibiotics such as tetracycline or doxycycline, although these are used with caution due to the potential for antibiotic resistance.
Ivermectin 1% cream is a US Food and Drug Administration–approved medication that is applied once daily for up to a year to treat the inflammatory pustules associated with Demodex mites. Although it is costly, studies have shown better results with topical ivermectin than with other topical medications (eg, metronidazole 0.75% gel or cream). However, metronidazole 0.75% gel applied twice daily and oral tetracycline 250 mg or doxycycline 100 mg daily or twice daily for at least 2 months often are utilized when the cost of topical ivermectin is prohibitive.10
Our patient was treated with a combination of doxycycline 100 mg daily for 30 days and
1. Forton FMN. Rosacea, an infectious disease: why rosacea with papulopustules should be considered a demodicosis. A narrative review. J Eur Acad Dermatol Venereol. 2022;36:987-1002. doi: 10.1111/jdv.18049
2. Forton FMN. The pathogenic role of demodex mites in rosacea: a potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol Ther (Heidelb). 2020;10:1229-1253. doi: 10.1007/s13555-020-00458-9
3. Elston DM. Demodex mites: facts and controversies. Clin Dermatol. 2010;28:502-504. doi: 10.1016/j.clindermatol.2010.03.006
4. Erbağci Z, OzgöztaŞi O. The significance of demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425. doi: 10.1046/j.1365-4362.1998.00218.x
5. Tan J, Almeida LMC, Criber B, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438. doi: 10.1111/bjd.15122
6. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155. doi: 10.1016/j.jaad.2017.08.037
7. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379:361-372. doi: 10.1016/S0140-6736(11)60321-8.
8. Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
9. Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
10. Raedler LA. Soolantra (ivermectin) 1% cream: a novel, antibiotic-free agent approved for the treatment of patients with rosacea. Am Health Drug Benefits. 2015;8(Spec Feature):122-125.
AN OTHERWISE HEALTHY
Scattered papules and pustules were present on the forehead, nose, and cheeks, with background erythema and telangiectasias (FIGURE 1). A few pinpoint crusted excoriations were noted. A sample was taken from the papules and pustules using a #15 blade and submitted for examination.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rosacea with Demodex mites
Under light microscopy, the scraping revealed Demodex mites (FIGURE 2). It has been proposed that these mites play a role in the inflammatory process seen in rosacea, although studies have yet to determine whether the inflammatory symptoms of rosacea cause the mites to proliferate or if the mites contribute to the initial inflammatory process.1,2

Demodex folliculorum and D brevis are part of normal skin flora; they are found in about 12% of all follicles and most commonly involve the face.3 They often become abundant in the presence of numerous sebaceous glands. Men have more sebaceous glands than women do, and thus run a greater risk for infestation with mites. An abnormal proliferation of Demodex mites can lead to demodicosis.
Demodex mites can be examined microscopically via the skin surface sampling technique known as scraping, which was done in this case. Samples taken from the papules and pustules utilizing a #15 blade are placed in immersion oil on a glass slide, cover-slipped, and examined by light microscopy.
Rosacea is thought to be an inflammatory disease in which the immune system is triggered by a variety of factors, including UV light, heat, stress, alcohol, hormonal influences, and microorganisms.1,4 The disease is found in up to 10% of the population worldwide.1
The diagnosis of rosacea requires at least 1 of the 2 “core features”—persistent central facial erythema or phymatous changes—or 2 of 4 “major features”: papules/pustules, ocular manifestation, flushing, and telangiectasias. There are 3 phenotypes: ocular, papulopustular, and erythematotelangiectatic.5,6
Continue to: The connection
The connection. Papulopustular and erythematotelangiectatic rosacea may be caused by a proliferation of Demodex mites and increased vascular endothelial growth factor production.2 In fact, a proliferation of Demodex is seen in almost all cases of papulopustular rosacea and more than 60% of cases of erythematotelangiectatic rosacea.2
Patient age and distribution of lesions narrowed the differential
Acne vulgaris is an inflammatory disease of the pilosebaceous units caused by increased sebum production, inflammation, and bacterial colonization (Propionibacterium acnes) of hair follicles on the face, neck, chest, and other areas. Both inflammatory and noninflammatory lesions can be present, and in serious cases, scarring can result.7 The case patient’s age and accompanying broad erythema were more consistent with rosacea than acne vulgaris.
Seborrheic dermatitis is a common skin condition usually stemming from an inflammatory reaction to a common yeast. Classic symptoms include scaling and erythema of the scalp and central face, as well as pruritus. Topical antifungals such as ketoconazole 2% cream and 2% shampoo are the mainstay of treatment.8 The broad distribution and papulopustules in this patient argue against the diagnosis of seborrheic dermatitis.
Systemic lupus erythematosus is a systemic inflammatory disease that often has cutaneous manifestations. Acute lupus manifests as an erythematous “butterfly rash” across the face and cheeks. Chronic discoid lupus involves depigmented plaques, erythematous macules, telangiectasias, and scarring with loss of normal hair follicles. These findings classically are photodistributed.9 The classic broad erythema extending from the cheeks over the bridge of the nose was not present in this patient.
Treatment is primarily topical
Mild cases of rosacea often can be managed with topical antibiotic creams. More severe cases may require systemic antibiotics such as tetracycline or doxycycline, although these are used with caution due to the potential for antibiotic resistance.
Ivermectin 1% cream is a US Food and Drug Administration–approved medication that is applied once daily for up to a year to treat the inflammatory pustules associated with Demodex mites. Although it is costly, studies have shown better results with topical ivermectin than with other topical medications (eg, metronidazole 0.75% gel or cream). However, metronidazole 0.75% gel applied twice daily and oral tetracycline 250 mg or doxycycline 100 mg daily or twice daily for at least 2 months often are utilized when the cost of topical ivermectin is prohibitive.10
Our patient was treated with a combination of doxycycline 100 mg daily for 30 days and
AN OTHERWISE HEALTHY
Scattered papules and pustules were present on the forehead, nose, and cheeks, with background erythema and telangiectasias (FIGURE 1). A few pinpoint crusted excoriations were noted. A sample was taken from the papules and pustules using a #15 blade and submitted for examination.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rosacea with Demodex mites
Under light microscopy, the scraping revealed Demodex mites (FIGURE 2). It has been proposed that these mites play a role in the inflammatory process seen in rosacea, although studies have yet to determine whether the inflammatory symptoms of rosacea cause the mites to proliferate or if the mites contribute to the initial inflammatory process.1,2

Demodex folliculorum and D brevis are part of normal skin flora; they are found in about 12% of all follicles and most commonly involve the face.3 They often become abundant in the presence of numerous sebaceous glands. Men have more sebaceous glands than women do, and thus run a greater risk for infestation with mites. An abnormal proliferation of Demodex mites can lead to demodicosis.
Demodex mites can be examined microscopically via the skin surface sampling technique known as scraping, which was done in this case. Samples taken from the papules and pustules utilizing a #15 blade are placed in immersion oil on a glass slide, cover-slipped, and examined by light microscopy.
Rosacea is thought to be an inflammatory disease in which the immune system is triggered by a variety of factors, including UV light, heat, stress, alcohol, hormonal influences, and microorganisms.1,4 The disease is found in up to 10% of the population worldwide.1
The diagnosis of rosacea requires at least 1 of the 2 “core features”—persistent central facial erythema or phymatous changes—or 2 of 4 “major features”: papules/pustules, ocular manifestation, flushing, and telangiectasias. There are 3 phenotypes: ocular, papulopustular, and erythematotelangiectatic.5,6
Continue to: The connection
The connection. Papulopustular and erythematotelangiectatic rosacea may be caused by a proliferation of Demodex mites and increased vascular endothelial growth factor production.2 In fact, a proliferation of Demodex is seen in almost all cases of papulopustular rosacea and more than 60% of cases of erythematotelangiectatic rosacea.2
Patient age and distribution of lesions narrowed the differential
Acne vulgaris is an inflammatory disease of the pilosebaceous units caused by increased sebum production, inflammation, and bacterial colonization (Propionibacterium acnes) of hair follicles on the face, neck, chest, and other areas. Both inflammatory and noninflammatory lesions can be present, and in serious cases, scarring can result.7 The case patient’s age and accompanying broad erythema were more consistent with rosacea than acne vulgaris.
Seborrheic dermatitis is a common skin condition usually stemming from an inflammatory reaction to a common yeast. Classic symptoms include scaling and erythema of the scalp and central face, as well as pruritus. Topical antifungals such as ketoconazole 2% cream and 2% shampoo are the mainstay of treatment.8 The broad distribution and papulopustules in this patient argue against the diagnosis of seborrheic dermatitis.
Systemic lupus erythematosus is a systemic inflammatory disease that often has cutaneous manifestations. Acute lupus manifests as an erythematous “butterfly rash” across the face and cheeks. Chronic discoid lupus involves depigmented plaques, erythematous macules, telangiectasias, and scarring with loss of normal hair follicles. These findings classically are photodistributed.9 The classic broad erythema extending from the cheeks over the bridge of the nose was not present in this patient.
Treatment is primarily topical
Mild cases of rosacea often can be managed with topical antibiotic creams. More severe cases may require systemic antibiotics such as tetracycline or doxycycline, although these are used with caution due to the potential for antibiotic resistance.
Ivermectin 1% cream is a US Food and Drug Administration–approved medication that is applied once daily for up to a year to treat the inflammatory pustules associated with Demodex mites. Although it is costly, studies have shown better results with topical ivermectin than with other topical medications (eg, metronidazole 0.75% gel or cream). However, metronidazole 0.75% gel applied twice daily and oral tetracycline 250 mg or doxycycline 100 mg daily or twice daily for at least 2 months often are utilized when the cost of topical ivermectin is prohibitive.10
Our patient was treated with a combination of doxycycline 100 mg daily for 30 days and
1. Forton FMN. Rosacea, an infectious disease: why rosacea with papulopustules should be considered a demodicosis. A narrative review. J Eur Acad Dermatol Venereol. 2022;36:987-1002. doi: 10.1111/jdv.18049
2. Forton FMN. The pathogenic role of demodex mites in rosacea: a potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol Ther (Heidelb). 2020;10:1229-1253. doi: 10.1007/s13555-020-00458-9
3. Elston DM. Demodex mites: facts and controversies. Clin Dermatol. 2010;28:502-504. doi: 10.1016/j.clindermatol.2010.03.006
4. Erbağci Z, OzgöztaŞi O. The significance of demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425. doi: 10.1046/j.1365-4362.1998.00218.x
5. Tan J, Almeida LMC, Criber B, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438. doi: 10.1111/bjd.15122
6. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155. doi: 10.1016/j.jaad.2017.08.037
7. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379:361-372. doi: 10.1016/S0140-6736(11)60321-8.
8. Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
9. Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
10. Raedler LA. Soolantra (ivermectin) 1% cream: a novel, antibiotic-free agent approved for the treatment of patients with rosacea. Am Health Drug Benefits. 2015;8(Spec Feature):122-125.
1. Forton FMN. Rosacea, an infectious disease: why rosacea with papulopustules should be considered a demodicosis. A narrative review. J Eur Acad Dermatol Venereol. 2022;36:987-1002. doi: 10.1111/jdv.18049
2. Forton FMN. The pathogenic role of demodex mites in rosacea: a potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol Ther (Heidelb). 2020;10:1229-1253. doi: 10.1007/s13555-020-00458-9
3. Elston DM. Demodex mites: facts and controversies. Clin Dermatol. 2010;28:502-504. doi: 10.1016/j.clindermatol.2010.03.006
4. Erbağci Z, OzgöztaŞi O. The significance of demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425. doi: 10.1046/j.1365-4362.1998.00218.x
5. Tan J, Almeida LMC, Criber B, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438. doi: 10.1111/bjd.15122
6. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155. doi: 10.1016/j.jaad.2017.08.037
7. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379:361-372. doi: 10.1016/S0140-6736(11)60321-8.
8. Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
9. Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
10. Raedler LA. Soolantra (ivermectin) 1% cream: a novel, antibiotic-free agent approved for the treatment of patients with rosacea. Am Health Drug Benefits. 2015;8(Spec Feature):122-125.
How best to diagnose and manage abdominal aortic aneurysms
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).
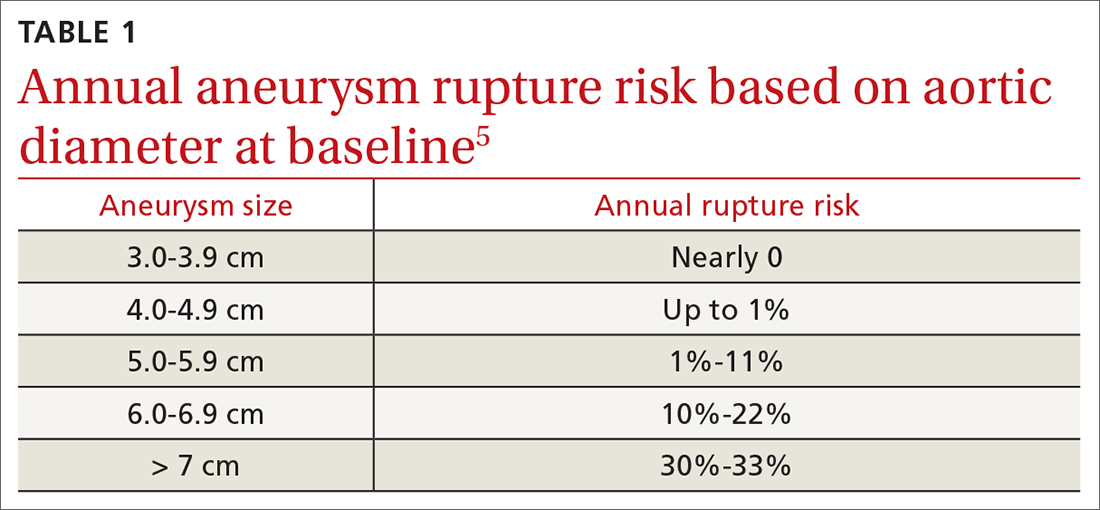
Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4
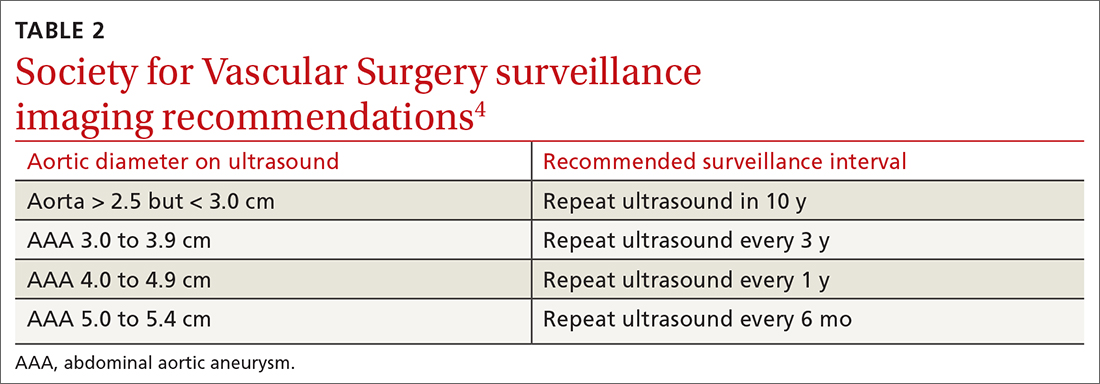
Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42
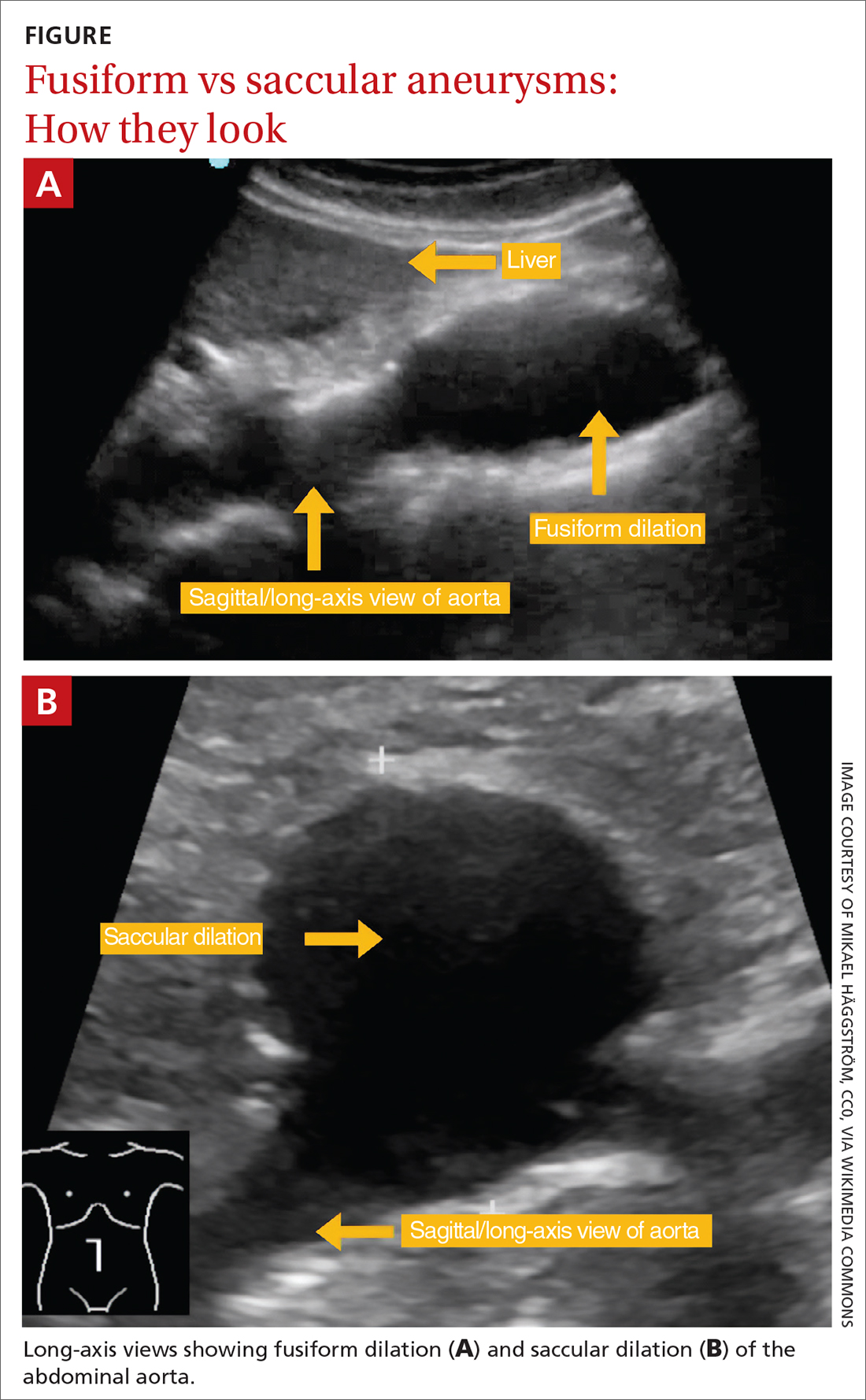
Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
PRACTICE RECOMMENDATIONS
› Perform a one-time abdominal aortic aneurysm (AAA) screening ultrasound in men ages 65 to 75 years who have ever smoked. B
› Consider performing a one-time AAA screening ultrasound in women ages 65 to 75 years who have ever smoked. C
› Prescribe high-intensity statin therapy for men and women with atherosclerotic AAA. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Updates in the Management of Erosive Esophagitis
Gastroesophageal reflux disease (GERD) encompasses various syndromes and complications associated with abnormal movement of gastric refluxate from the stomach into the esophagus, and even into the oral pharynx, lungs, and throat.
Read More
Gastroesophageal reflux disease (GERD) encompasses various syndromes and complications associated with abnormal movement of gastric refluxate from the stomach into the esophagus, and even into the oral pharynx, lungs, and throat.
Read More
Gastroesophageal reflux disease (GERD) encompasses various syndromes and complications associated with abnormal movement of gastric refluxate from the stomach into the esophagus, and even into the oral pharynx, lungs, and throat.
Read More
The HPV vaccine: Time for ObGyn physicians to up our game
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.
Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.