User login
CTCs help predict breast cancer outcomes in neoadjuvant setting
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Overall survival was associated with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs).
Data source: A meta-analysis of data for 2,156 patients.
Disclosures: This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
Adjuvant chemo prolonged survival after radical nephroureterectomy
Adjuvant chemotherapy prolonged survival after radical nephroureterectomy by nearly a year, compared with observation alone, among patients with locally advanced or positive regional lymph node upper tract urothelial carcinoma, researchers reported.
After a median follow-up period of 49 months, median overall survival was 47 months with adjuvant chemotherapy and 36 months with observation alone (P less than .001), reported Thomas Seisen, MD, of Harvard Medical School, Boston, and his associates.
This analysis included 3,253 patients with pT3/T4 and/or pN+ upper tract urothelial carcinoma from the National Cancer Database. A total of 762 (23%) patients received adjuvant chemotherapy within 90 days after surgery, while 2,491 (77%) patients underwent observation only (J Clin Oncol. 2017 Jan 3. doi: 10.1200/JCO.2016.69.414).
Kaplan Meier analyses yielded 5-year adjusted overall survival rates of 44% and 36%, respectively. Adjuvant chemotherapy conferred a significant overall survival benefit in a Cox proportional hazards regression analysis (hazard ratio, 0.77; 95% confidence interval, 0.68 to 0.88), and the effect held up in tests designed to minimize selection bias – including propensity score adjustment (HR, 0.82; 0.73 to 0.93), stratification (HR, 0.84; 0.74 to 0.95), and matching (HR, 0.84; 0.75 to 0.95).
The effect persisted across subgroups stratified by age, gender, comorbidity burden, pathologic stage, and surgical margin status, and there was no significant variability in treatment effects, the researchers said. The findings are subject to “the usual biases related to the observational study design,” but pending level 1 evidence, they inform the management of patients with advanced upper tract urothelial carcinoma who undergo radical nephroureterectomy, the researchers concluded.
The work was supported by the Vattikuti Urology Institute, the Conquer Cancer Foundation of the American Society of Clinical Oncology, and the Prostate Cancer Foundation. Dr. Seisen had no relevant financial disclosures.
Adjuvant chemotherapy prolonged survival after radical nephroureterectomy by nearly a year, compared with observation alone, among patients with locally advanced or positive regional lymph node upper tract urothelial carcinoma, researchers reported.
After a median follow-up period of 49 months, median overall survival was 47 months with adjuvant chemotherapy and 36 months with observation alone (P less than .001), reported Thomas Seisen, MD, of Harvard Medical School, Boston, and his associates.
This analysis included 3,253 patients with pT3/T4 and/or pN+ upper tract urothelial carcinoma from the National Cancer Database. A total of 762 (23%) patients received adjuvant chemotherapy within 90 days after surgery, while 2,491 (77%) patients underwent observation only (J Clin Oncol. 2017 Jan 3. doi: 10.1200/JCO.2016.69.414).
Kaplan Meier analyses yielded 5-year adjusted overall survival rates of 44% and 36%, respectively. Adjuvant chemotherapy conferred a significant overall survival benefit in a Cox proportional hazards regression analysis (hazard ratio, 0.77; 95% confidence interval, 0.68 to 0.88), and the effect held up in tests designed to minimize selection bias – including propensity score adjustment (HR, 0.82; 0.73 to 0.93), stratification (HR, 0.84; 0.74 to 0.95), and matching (HR, 0.84; 0.75 to 0.95).
The effect persisted across subgroups stratified by age, gender, comorbidity burden, pathologic stage, and surgical margin status, and there was no significant variability in treatment effects, the researchers said. The findings are subject to “the usual biases related to the observational study design,” but pending level 1 evidence, they inform the management of patients with advanced upper tract urothelial carcinoma who undergo radical nephroureterectomy, the researchers concluded.
The work was supported by the Vattikuti Urology Institute, the Conquer Cancer Foundation of the American Society of Clinical Oncology, and the Prostate Cancer Foundation. Dr. Seisen had no relevant financial disclosures.
Adjuvant chemotherapy prolonged survival after radical nephroureterectomy by nearly a year, compared with observation alone, among patients with locally advanced or positive regional lymph node upper tract urothelial carcinoma, researchers reported.
After a median follow-up period of 49 months, median overall survival was 47 months with adjuvant chemotherapy and 36 months with observation alone (P less than .001), reported Thomas Seisen, MD, of Harvard Medical School, Boston, and his associates.
This analysis included 3,253 patients with pT3/T4 and/or pN+ upper tract urothelial carcinoma from the National Cancer Database. A total of 762 (23%) patients received adjuvant chemotherapy within 90 days after surgery, while 2,491 (77%) patients underwent observation only (J Clin Oncol. 2017 Jan 3. doi: 10.1200/JCO.2016.69.414).
Kaplan Meier analyses yielded 5-year adjusted overall survival rates of 44% and 36%, respectively. Adjuvant chemotherapy conferred a significant overall survival benefit in a Cox proportional hazards regression analysis (hazard ratio, 0.77; 95% confidence interval, 0.68 to 0.88), and the effect held up in tests designed to minimize selection bias – including propensity score adjustment (HR, 0.82; 0.73 to 0.93), stratification (HR, 0.84; 0.74 to 0.95), and matching (HR, 0.84; 0.75 to 0.95).
The effect persisted across subgroups stratified by age, gender, comorbidity burden, pathologic stage, and surgical margin status, and there was no significant variability in treatment effects, the researchers said. The findings are subject to “the usual biases related to the observational study design,” but pending level 1 evidence, they inform the management of patients with advanced upper tract urothelial carcinoma who undergo radical nephroureterectomy, the researchers concluded.
The work was supported by the Vattikuti Urology Institute, the Conquer Cancer Foundation of the American Society of Clinical Oncology, and the Prostate Cancer Foundation. Dr. Seisen had no relevant financial disclosures.
Key clinical point: Adjuvant chemotherapy prolonged survival after radical nephroureterectomy by nearly a year, compared with observation alone, among patients with locally advanced or positive regional lymph node upper tract urothelial carcinoma.
Major finding: After a median follow-up period of 49 months, median overall survival was 47 months with adjuvant chemotherapy and 36 months with observation alone (P less than .001).
Data source: An analysis of 3,253 patients with pT3/T4 and/or pN+ upper tract urothelial carcinoma from the National Cancer Database.
Disclosures: The work was supported by the Vattikuti Urology Institute, the Conquer Cancer Foundation of the American Society of Clinical Oncology, and the Prostate Cancer Foundation. Dr. Seisen had no relevant financial disclosures.
Alexa
How many calories are there in a cheeseburger? (Yes, I too am looking forward to a svelter 2017). The answer, according to my new assistant, is 300 calories. She knows the dose of acetaminophen for a 10-year-old, 65-pound child is 325 mg every 4-6 hours. She also plays George Michael, reorders my Dentyne Ice gum, and turns off the lights. She is Alexa of Amazon’s Echo, the intelligent personal assistant.
Echo and Google Home are popular voice-assisted home appliances. Amazon has built a natural language processing system, so to use it, you simply say, “Alexa,” pause, then ask your question (What’s the weather in New York?) or deliver your command (Play Spotify). It’s hands free, so you can interface while typing, reading, or cooking dinner.
Some medical centers, such as the Boston Children’s Hospital, are leading the way to make voice-assisted technology useful in health care. Their KidsMD app, for example, gives Alexa the “skill” to offer simple health advice regarding their children’s fever and medication dosing. I found this Alexa skill interesting but rudimentary. Most of the advice was reasonable; however, the scope is small and the responses glitchy. For example, when I asked Alexa what to do for a feverish 2-month-old, it advised me to contact my doctor then immediately followed this with recommended antipyretic medication dosing. Although we physicians understand the child must see a doctor, some parents might be confused and choose only to administer the medication. As with any new digital health technology, the team at Boston Children’s are continually iterating and improving based upon feedback.
I found Alexa currently has a few other skills for health care. For example, a skill called Marvee functions as a “care companion” to help aging family members and their caregivers. Another skill, Health Care Genius, helps patients decipher healthcare terminology by asking questions such as, “What is a deductible?”
The potential of voice-assisted technology in clinical and home health care settings is limitless, and I expect this segment to grow dramatically. Here are a few examples:
1. Physicians can ask for real-time help such as: What are treatment options for juvenile dermatomyositis? Order doxycycline 100 mg by mouth, twice daily, quantity sufficient 10 days.
2. Physicians might also use it to dictate notes intelligently, and even extract patient instructions directly from the notes to be emailed to the patient.
3. Surgeons could command an MRI to be viewed without having to scrub out.
4. Bedridden or chronically ill patients could use it to refill medications, make doctor appointments, or contact a caregiver in an emergency.
5. Patients could receive customized instructions, such as the answer to “How often do I change my surgical bandage?”
For all its potential, voice-assisted personal assistants have a long way to go. It would be a mistake to think these won’t be integrated into the entire health care chain from care to wellness, but it will be awhile before we get there.
Interestingly, when I asked my Apple Siri how many calories are in a cheeseburger, she reported 500, which is much more than Alexa’s 300. Which is why, for now, devices like Alexa are ideal for ordering a pizza hands free from your recliner. Just don’t ask how many calories are in it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.
How many calories are there in a cheeseburger? (Yes, I too am looking forward to a svelter 2017). The answer, according to my new assistant, is 300 calories. She knows the dose of acetaminophen for a 10-year-old, 65-pound child is 325 mg every 4-6 hours. She also plays George Michael, reorders my Dentyne Ice gum, and turns off the lights. She is Alexa of Amazon’s Echo, the intelligent personal assistant.
Echo and Google Home are popular voice-assisted home appliances. Amazon has built a natural language processing system, so to use it, you simply say, “Alexa,” pause, then ask your question (What’s the weather in New York?) or deliver your command (Play Spotify). It’s hands free, so you can interface while typing, reading, or cooking dinner.
Some medical centers, such as the Boston Children’s Hospital, are leading the way to make voice-assisted technology useful in health care. Their KidsMD app, for example, gives Alexa the “skill” to offer simple health advice regarding their children’s fever and medication dosing. I found this Alexa skill interesting but rudimentary. Most of the advice was reasonable; however, the scope is small and the responses glitchy. For example, when I asked Alexa what to do for a feverish 2-month-old, it advised me to contact my doctor then immediately followed this with recommended antipyretic medication dosing. Although we physicians understand the child must see a doctor, some parents might be confused and choose only to administer the medication. As with any new digital health technology, the team at Boston Children’s are continually iterating and improving based upon feedback.
I found Alexa currently has a few other skills for health care. For example, a skill called Marvee functions as a “care companion” to help aging family members and their caregivers. Another skill, Health Care Genius, helps patients decipher healthcare terminology by asking questions such as, “What is a deductible?”
The potential of voice-assisted technology in clinical and home health care settings is limitless, and I expect this segment to grow dramatically. Here are a few examples:
1. Physicians can ask for real-time help such as: What are treatment options for juvenile dermatomyositis? Order doxycycline 100 mg by mouth, twice daily, quantity sufficient 10 days.
2. Physicians might also use it to dictate notes intelligently, and even extract patient instructions directly from the notes to be emailed to the patient.
3. Surgeons could command an MRI to be viewed without having to scrub out.
4. Bedridden or chronically ill patients could use it to refill medications, make doctor appointments, or contact a caregiver in an emergency.
5. Patients could receive customized instructions, such as the answer to “How often do I change my surgical bandage?”
For all its potential, voice-assisted personal assistants have a long way to go. It would be a mistake to think these won’t be integrated into the entire health care chain from care to wellness, but it will be awhile before we get there.
Interestingly, when I asked my Apple Siri how many calories are in a cheeseburger, she reported 500, which is much more than Alexa’s 300. Which is why, for now, devices like Alexa are ideal for ordering a pizza hands free from your recliner. Just don’t ask how many calories are in it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.
How many calories are there in a cheeseburger? (Yes, I too am looking forward to a svelter 2017). The answer, according to my new assistant, is 300 calories. She knows the dose of acetaminophen for a 10-year-old, 65-pound child is 325 mg every 4-6 hours. She also plays George Michael, reorders my Dentyne Ice gum, and turns off the lights. She is Alexa of Amazon’s Echo, the intelligent personal assistant.
Echo and Google Home are popular voice-assisted home appliances. Amazon has built a natural language processing system, so to use it, you simply say, “Alexa,” pause, then ask your question (What’s the weather in New York?) or deliver your command (Play Spotify). It’s hands free, so you can interface while typing, reading, or cooking dinner.
Some medical centers, such as the Boston Children’s Hospital, are leading the way to make voice-assisted technology useful in health care. Their KidsMD app, for example, gives Alexa the “skill” to offer simple health advice regarding their children’s fever and medication dosing. I found this Alexa skill interesting but rudimentary. Most of the advice was reasonable; however, the scope is small and the responses glitchy. For example, when I asked Alexa what to do for a feverish 2-month-old, it advised me to contact my doctor then immediately followed this with recommended antipyretic medication dosing. Although we physicians understand the child must see a doctor, some parents might be confused and choose only to administer the medication. As with any new digital health technology, the team at Boston Children’s are continually iterating and improving based upon feedback.
I found Alexa currently has a few other skills for health care. For example, a skill called Marvee functions as a “care companion” to help aging family members and their caregivers. Another skill, Health Care Genius, helps patients decipher healthcare terminology by asking questions such as, “What is a deductible?”
The potential of voice-assisted technology in clinical and home health care settings is limitless, and I expect this segment to grow dramatically. Here are a few examples:
1. Physicians can ask for real-time help such as: What are treatment options for juvenile dermatomyositis? Order doxycycline 100 mg by mouth, twice daily, quantity sufficient 10 days.
2. Physicians might also use it to dictate notes intelligently, and even extract patient instructions directly from the notes to be emailed to the patient.
3. Surgeons could command an MRI to be viewed without having to scrub out.
4. Bedridden or chronically ill patients could use it to refill medications, make doctor appointments, or contact a caregiver in an emergency.
5. Patients could receive customized instructions, such as the answer to “How often do I change my surgical bandage?”
For all its potential, voice-assisted personal assistants have a long way to go. It would be a mistake to think these won’t be integrated into the entire health care chain from care to wellness, but it will be awhile before we get there.
Interestingly, when I asked my Apple Siri how many calories are in a cheeseburger, she reported 500, which is much more than Alexa’s 300. Which is why, for now, devices like Alexa are ideal for ordering a pizza hands free from your recliner. Just don’t ask how many calories are in it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.
‘Weekend warrior’ exercise pattern sufficient to cut mortality
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).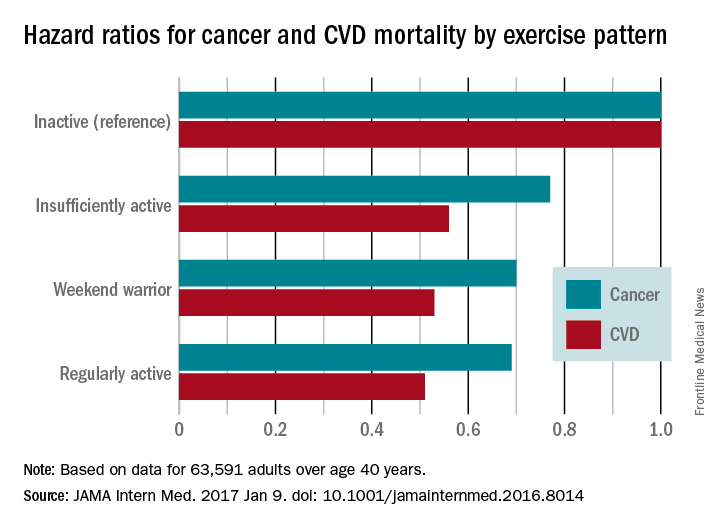
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce mortality risks.
Major finding: Compared with inactive participants, the hazard ratio for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
Data source: A pooled analysis of eight household surveillance studies in England and Scotland during 1994-2012, involving 63,591 adults older than 40 years.
Disclosures: The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
Study highlights need to address vitamin D deficiency in epilepsy patients
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: Neurologists ordered vitamin D levels in 22% of patients; another 12% were already ordered by another physician.
Data source: A retrospective review of 190 patients who had a diagnosis of epilepsy or seizures, were currently on antiepileptic medications, and whose most recent neurology visit occurred between 2009 and 2015.
Disclosures: Dr. Afgan reported having no financial disclosures.
PERSIST-2 might allay some concerns about pacritinib in myelofibrosis
SAN DIEGO – Late-breaking results from the phase III PERSIST-2 trial may ease at least some safety concerns surrounding the use of pacritinib in patients with myelofibrosis, investigators said at the annual meeting of the American Society of Hematology.
In February 2016, the Food and Drug Administration imposed a clinical hold on studies of pacritinib in the wake of concerns about excess deaths, cardiotoxicities, and hemorrhage. But in the final data analysis presented at ASH, rates of these outcomes were low and similar among patients randomized to pacritinib once daily, pacritinib twice daily, or best available treatment for myelofibrosis, including ruxolitinib, lead investigator John Mascarenhas, MD, said.
Indeed, more patients died of progressive disease after stopping pacritinib than died of treatment-associated adverse events, said Dr. Mascarenhas of Icahn School of Medicine at Mount Sinai, New York.
Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CFS1R. In the previous phase III PERSIST-1 trial, patients who received pacritinib had a fourfold greater probability of experiencing at least a 35% decrease in splenic volume than did patients who received best available treatment (P = .003).
PERSIST-2 also yielded clear efficacy signals, particularly when patients took pacritinib twice daily, said Dr. Mascarenhas. Between baseline and week 24, splenic volume dropped by at least 35% in 22% of these patients (95% confidence interval, 13%-33%), compared with 3% of patients on best available treatment (95% CI, 0.3%-10%; P = .001). Patients who took pacritinib twice daily also had a 32% (95% CI, 22%-44%) chance of experiencing at least a 50% drop in symptoms such as fatigue, bone pain, itching, and abdominal pain, compared with 14% (95% CI, 7%-24%) of patients on best available treatment (P = .01).
Demographic and disease risk characteristics did not significantly affect the chances of reaching these coprimary endpoints, Dr. Mascarenhas noted. “My humble opinion as a clinical investigator is that [pacritinib] is an effective drug, with a favorable benefit-to-risk ratio,” he said.
Several hematologists who were not involved in this trial agreed. “I don’t see why you are nervous [about presenting these results],” noted hematologist Kanti Rai, MD, of the Feinstein Institute for Medical Research in Manhasset, N.Y., told Dr. Mascarenhas during the discussion after the data were presented, prompting laughter from the audience.
It remains to be seen whether the FDA will find the data convincing enough to lift the clinical hold on pacritinib. Ruxolitinib (Jakafi) is approved to treat splenomegaly and symptom burden in myelofibrosis but is associated with dose-limiting cytopenias and cannot be used in patients with platelet counts of less than 50,000/mcL.
PERSIST-2 compared pacritinib 400 mg once daily with pacritinib 200 mg twice daily and best available treatment, including ruxolitinib, in patients with primary or secondary myelofibrosis and less than 100,000 platelets/mcL. About half of the study patients had less than 50,000 platelets/mcL, and more than 40% had previously received ruxolitinib.
When the clinical hold on pacritinib went into effect, 221 patients had reached the 24-week designated study endpoint and were included in the intention-to-treat analysis, Dr. Mascarenhas reported. Censored Kaplan-Meier curves of overall survival favored pacritinib over best available treatment, although the difference in survival rates did not reach statistical significance (hazard ratio, 0.68; 95% CI, 0.3-1.5). A total of 9% of patients in the twice-daily pacritinib group died, compared with 14% of patients receiving pacritinib once daily or best available treatment.
Twice-daily pacritinib most often led to diarrhea (48% of patients), nausea (32%), thrombocytopenia (34%), and anemia (24%). Overall rates of serious treatment-emergent adverse events were seen in 47% of the two pacritinib groups and in 31% of patients receiving best available treatment. The most common serious treatment-emergent adverse event with twice daily pacritinib was anemia (8% of patients), followed by thrombocytopenia and pneumonia (6%). Heart failure, atrial fibrillation, and cardiac arrest were rare and similar across all three treatment groups, as were epistaxis and subdural hematoma.
This is the first randomized, controlled trial of patients with myelofibrosis and thrombocytopenia, according to Dr. Mascarenhas. “This was a patient population with low platelets and at risk of poor outcomes, and they did pretty well,” he said. “There really is no therapeutic option for patients with myelofibrosis and low platelets, and [pacritinib] offers patients in this vulnerable situation an opportunity for symptom relief. I hope to see it move forward.”
CTI Biopharma sponsored the study. Dr. Mascarenhas disclosed research funding from CTI Biopharma.
SAN DIEGO – Late-breaking results from the phase III PERSIST-2 trial may ease at least some safety concerns surrounding the use of pacritinib in patients with myelofibrosis, investigators said at the annual meeting of the American Society of Hematology.
In February 2016, the Food and Drug Administration imposed a clinical hold on studies of pacritinib in the wake of concerns about excess deaths, cardiotoxicities, and hemorrhage. But in the final data analysis presented at ASH, rates of these outcomes were low and similar among patients randomized to pacritinib once daily, pacritinib twice daily, or best available treatment for myelofibrosis, including ruxolitinib, lead investigator John Mascarenhas, MD, said.
Indeed, more patients died of progressive disease after stopping pacritinib than died of treatment-associated adverse events, said Dr. Mascarenhas of Icahn School of Medicine at Mount Sinai, New York.
Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CFS1R. In the previous phase III PERSIST-1 trial, patients who received pacritinib had a fourfold greater probability of experiencing at least a 35% decrease in splenic volume than did patients who received best available treatment (P = .003).
PERSIST-2 also yielded clear efficacy signals, particularly when patients took pacritinib twice daily, said Dr. Mascarenhas. Between baseline and week 24, splenic volume dropped by at least 35% in 22% of these patients (95% confidence interval, 13%-33%), compared with 3% of patients on best available treatment (95% CI, 0.3%-10%; P = .001). Patients who took pacritinib twice daily also had a 32% (95% CI, 22%-44%) chance of experiencing at least a 50% drop in symptoms such as fatigue, bone pain, itching, and abdominal pain, compared with 14% (95% CI, 7%-24%) of patients on best available treatment (P = .01).
Demographic and disease risk characteristics did not significantly affect the chances of reaching these coprimary endpoints, Dr. Mascarenhas noted. “My humble opinion as a clinical investigator is that [pacritinib] is an effective drug, with a favorable benefit-to-risk ratio,” he said.
Several hematologists who were not involved in this trial agreed. “I don’t see why you are nervous [about presenting these results],” noted hematologist Kanti Rai, MD, of the Feinstein Institute for Medical Research in Manhasset, N.Y., told Dr. Mascarenhas during the discussion after the data were presented, prompting laughter from the audience.
It remains to be seen whether the FDA will find the data convincing enough to lift the clinical hold on pacritinib. Ruxolitinib (Jakafi) is approved to treat splenomegaly and symptom burden in myelofibrosis but is associated with dose-limiting cytopenias and cannot be used in patients with platelet counts of less than 50,000/mcL.
PERSIST-2 compared pacritinib 400 mg once daily with pacritinib 200 mg twice daily and best available treatment, including ruxolitinib, in patients with primary or secondary myelofibrosis and less than 100,000 platelets/mcL. About half of the study patients had less than 50,000 platelets/mcL, and more than 40% had previously received ruxolitinib.
When the clinical hold on pacritinib went into effect, 221 patients had reached the 24-week designated study endpoint and were included in the intention-to-treat analysis, Dr. Mascarenhas reported. Censored Kaplan-Meier curves of overall survival favored pacritinib over best available treatment, although the difference in survival rates did not reach statistical significance (hazard ratio, 0.68; 95% CI, 0.3-1.5). A total of 9% of patients in the twice-daily pacritinib group died, compared with 14% of patients receiving pacritinib once daily or best available treatment.
Twice-daily pacritinib most often led to diarrhea (48% of patients), nausea (32%), thrombocytopenia (34%), and anemia (24%). Overall rates of serious treatment-emergent adverse events were seen in 47% of the two pacritinib groups and in 31% of patients receiving best available treatment. The most common serious treatment-emergent adverse event with twice daily pacritinib was anemia (8% of patients), followed by thrombocytopenia and pneumonia (6%). Heart failure, atrial fibrillation, and cardiac arrest were rare and similar across all three treatment groups, as were epistaxis and subdural hematoma.
This is the first randomized, controlled trial of patients with myelofibrosis and thrombocytopenia, according to Dr. Mascarenhas. “This was a patient population with low platelets and at risk of poor outcomes, and they did pretty well,” he said. “There really is no therapeutic option for patients with myelofibrosis and low platelets, and [pacritinib] offers patients in this vulnerable situation an opportunity for symptom relief. I hope to see it move forward.”
CTI Biopharma sponsored the study. Dr. Mascarenhas disclosed research funding from CTI Biopharma.
SAN DIEGO – Late-breaking results from the phase III PERSIST-2 trial may ease at least some safety concerns surrounding the use of pacritinib in patients with myelofibrosis, investigators said at the annual meeting of the American Society of Hematology.
In February 2016, the Food and Drug Administration imposed a clinical hold on studies of pacritinib in the wake of concerns about excess deaths, cardiotoxicities, and hemorrhage. But in the final data analysis presented at ASH, rates of these outcomes were low and similar among patients randomized to pacritinib once daily, pacritinib twice daily, or best available treatment for myelofibrosis, including ruxolitinib, lead investigator John Mascarenhas, MD, said.
Indeed, more patients died of progressive disease after stopping pacritinib than died of treatment-associated adverse events, said Dr. Mascarenhas of Icahn School of Medicine at Mount Sinai, New York.
Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CFS1R. In the previous phase III PERSIST-1 trial, patients who received pacritinib had a fourfold greater probability of experiencing at least a 35% decrease in splenic volume than did patients who received best available treatment (P = .003).
PERSIST-2 also yielded clear efficacy signals, particularly when patients took pacritinib twice daily, said Dr. Mascarenhas. Between baseline and week 24, splenic volume dropped by at least 35% in 22% of these patients (95% confidence interval, 13%-33%), compared with 3% of patients on best available treatment (95% CI, 0.3%-10%; P = .001). Patients who took pacritinib twice daily also had a 32% (95% CI, 22%-44%) chance of experiencing at least a 50% drop in symptoms such as fatigue, bone pain, itching, and abdominal pain, compared with 14% (95% CI, 7%-24%) of patients on best available treatment (P = .01).
Demographic and disease risk characteristics did not significantly affect the chances of reaching these coprimary endpoints, Dr. Mascarenhas noted. “My humble opinion as a clinical investigator is that [pacritinib] is an effective drug, with a favorable benefit-to-risk ratio,” he said.
Several hematologists who were not involved in this trial agreed. “I don’t see why you are nervous [about presenting these results],” noted hematologist Kanti Rai, MD, of the Feinstein Institute for Medical Research in Manhasset, N.Y., told Dr. Mascarenhas during the discussion after the data were presented, prompting laughter from the audience.
It remains to be seen whether the FDA will find the data convincing enough to lift the clinical hold on pacritinib. Ruxolitinib (Jakafi) is approved to treat splenomegaly and symptom burden in myelofibrosis but is associated with dose-limiting cytopenias and cannot be used in patients with platelet counts of less than 50,000/mcL.
PERSIST-2 compared pacritinib 400 mg once daily with pacritinib 200 mg twice daily and best available treatment, including ruxolitinib, in patients with primary or secondary myelofibrosis and less than 100,000 platelets/mcL. About half of the study patients had less than 50,000 platelets/mcL, and more than 40% had previously received ruxolitinib.
When the clinical hold on pacritinib went into effect, 221 patients had reached the 24-week designated study endpoint and were included in the intention-to-treat analysis, Dr. Mascarenhas reported. Censored Kaplan-Meier curves of overall survival favored pacritinib over best available treatment, although the difference in survival rates did not reach statistical significance (hazard ratio, 0.68; 95% CI, 0.3-1.5). A total of 9% of patients in the twice-daily pacritinib group died, compared with 14% of patients receiving pacritinib once daily or best available treatment.
Twice-daily pacritinib most often led to diarrhea (48% of patients), nausea (32%), thrombocytopenia (34%), and anemia (24%). Overall rates of serious treatment-emergent adverse events were seen in 47% of the two pacritinib groups and in 31% of patients receiving best available treatment. The most common serious treatment-emergent adverse event with twice daily pacritinib was anemia (8% of patients), followed by thrombocytopenia and pneumonia (6%). Heart failure, atrial fibrillation, and cardiac arrest were rare and similar across all three treatment groups, as were epistaxis and subdural hematoma.
This is the first randomized, controlled trial of patients with myelofibrosis and thrombocytopenia, according to Dr. Mascarenhas. “This was a patient population with low platelets and at risk of poor outcomes, and they did pretty well,” he said. “There really is no therapeutic option for patients with myelofibrosis and low platelets, and [pacritinib] offers patients in this vulnerable situation an opportunity for symptom relief. I hope to see it move forward.”
CTI Biopharma sponsored the study. Dr. Mascarenhas disclosed research funding from CTI Biopharma.
AT ASH 2016
Key clinical point: Pacritinib topped best available treatments for myelofibrosis and was not associated with increased risk of death or cardiac or bleeding events.
Major finding: Rates of death, cardiac events, and bleeding events were low and similar among groups. Splenic volume dropped by at least 35% in 22% of patients receiving twice daily pacritinib, compared with 3% of patients on best available treatment, including ruxolitinib (P = .001). Total symptom scores fell by at least 50% in 32% of patients receiving twice daily pacritinib and 14% of patients on best available treatment (P = .01).
Data source: A randomized phase III trial comparing pacritinib 400 mg once daily, pacritinib 200 mg twice daily, and best available treatment, including ruxolitinib, for 24 weeks in 221 patients with primary or secondary myelofibrosis and less than 100,000 platelets/mcL.
Disclosures: CTI Biopharma sponsored the study. Dr. Mascarenhas disclosed research funding from CTI Biopharma.
IPV boost after initial OPV offers sustained protection to at least 11 months
Protection against the poliovirus is lower at 1 month but remains sustained at 6 and 11 months after an inactivated poliovirus vaccine (IPV) boost following initial oral poliovirus vaccination (OPV), according to Jacob John, MD, of Christian Medical College, Vellore, Tamil Nadu, India, and his associates.
In a randomized controlled trial from Nov. 4 and Dec. 17, 2014, 900 healthy children from ages 1 to 4 years were randomly assigned between three study groups. The groups had the children receive IPV boost at 5 months (arm A), at enrollment (arm B), or no vaccine (arm C). Poliovirus shedding in stool 7 days after challenge, determined by Fisher’s exact test, was significantly lower in arms A and B, compared with C (risk ratio, 0.68; P = .003, RR, 0.70; P = .006 for arm A vs. C and B vs. C, respectively). The reduction in shedding was more marked for serotype 3 (RR, 0.60; P = .004, RR, 0.54; P = .001 respectively) than for serotype 1 (RR, 0.72; P = .057, RR, 0.80; P = .215, respectively).
It was noted that 41 serious adverse events (11 in arm A, 17 in arm B, and 13 in arm C), including 2 deaths in arm A, were reported during the trial. However, the reported adverse events were classified as unrelated, and the deaths were from leukemia and from viral hemorrhagic fever.
“The boost to intestinal immunity against poliovirus that results from administration of IPV to OPV-vaccinated children is sustained at 6 and 11 months. It is clear that IPV is playing an increasingly important role in the polio endgame as the world transitions away from the use of OPV,” the researchers concluded. “Every effort needs to be made to ensure supply of this vaccine to meet this expanding role.”
Find the full study in the Journal of Infectious Diseases 2016. doi: 10.1093/infdis/jiw595.
Protection against the poliovirus is lower at 1 month but remains sustained at 6 and 11 months after an inactivated poliovirus vaccine (IPV) boost following initial oral poliovirus vaccination (OPV), according to Jacob John, MD, of Christian Medical College, Vellore, Tamil Nadu, India, and his associates.
In a randomized controlled trial from Nov. 4 and Dec. 17, 2014, 900 healthy children from ages 1 to 4 years were randomly assigned between three study groups. The groups had the children receive IPV boost at 5 months (arm A), at enrollment (arm B), or no vaccine (arm C). Poliovirus shedding in stool 7 days after challenge, determined by Fisher’s exact test, was significantly lower in arms A and B, compared with C (risk ratio, 0.68; P = .003, RR, 0.70; P = .006 for arm A vs. C and B vs. C, respectively). The reduction in shedding was more marked for serotype 3 (RR, 0.60; P = .004, RR, 0.54; P = .001 respectively) than for serotype 1 (RR, 0.72; P = .057, RR, 0.80; P = .215, respectively).
It was noted that 41 serious adverse events (11 in arm A, 17 in arm B, and 13 in arm C), including 2 deaths in arm A, were reported during the trial. However, the reported adverse events were classified as unrelated, and the deaths were from leukemia and from viral hemorrhagic fever.
“The boost to intestinal immunity against poliovirus that results from administration of IPV to OPV-vaccinated children is sustained at 6 and 11 months. It is clear that IPV is playing an increasingly important role in the polio endgame as the world transitions away from the use of OPV,” the researchers concluded. “Every effort needs to be made to ensure supply of this vaccine to meet this expanding role.”
Find the full study in the Journal of Infectious Diseases 2016. doi: 10.1093/infdis/jiw595.
Protection against the poliovirus is lower at 1 month but remains sustained at 6 and 11 months after an inactivated poliovirus vaccine (IPV) boost following initial oral poliovirus vaccination (OPV), according to Jacob John, MD, of Christian Medical College, Vellore, Tamil Nadu, India, and his associates.
In a randomized controlled trial from Nov. 4 and Dec. 17, 2014, 900 healthy children from ages 1 to 4 years were randomly assigned between three study groups. The groups had the children receive IPV boost at 5 months (arm A), at enrollment (arm B), or no vaccine (arm C). Poliovirus shedding in stool 7 days after challenge, determined by Fisher’s exact test, was significantly lower in arms A and B, compared with C (risk ratio, 0.68; P = .003, RR, 0.70; P = .006 for arm A vs. C and B vs. C, respectively). The reduction in shedding was more marked for serotype 3 (RR, 0.60; P = .004, RR, 0.54; P = .001 respectively) than for serotype 1 (RR, 0.72; P = .057, RR, 0.80; P = .215, respectively).
It was noted that 41 serious adverse events (11 in arm A, 17 in arm B, and 13 in arm C), including 2 deaths in arm A, were reported during the trial. However, the reported adverse events were classified as unrelated, and the deaths were from leukemia and from viral hemorrhagic fever.
“The boost to intestinal immunity against poliovirus that results from administration of IPV to OPV-vaccinated children is sustained at 6 and 11 months. It is clear that IPV is playing an increasingly important role in the polio endgame as the world transitions away from the use of OPV,” the researchers concluded. “Every effort needs to be made to ensure supply of this vaccine to meet this expanding role.”
Find the full study in the Journal of Infectious Diseases 2016. doi: 10.1093/infdis/jiw595.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Leadership Initiatives in Patient-Centered Transgender Care
Patient-centered care is of fundamental importance when caring for the transgender population due to the well-established history of social stigma and systemic discrimination. Therefore, nursing education is mandated to equip graduates with culturally competent patient-centered care skills.1 In 2009, the Institute of Medicine (IOM) in partnership with the Robert Wood Johnson Foundation (RWJF) launched The Future of Nursing initiative, which outlined the major role nursing should play in transforming the health care system to meet the health care needs of diverse U.S. populations.
The initiative produced a blueprint of action-focused institutional recommendations at the local, state, and national levels that would facilitate the reforms necessary to transform the U.S. health care system. One of the recommendations of the IOM report was to increase opportunities for nurses to manage and lead collaborative efforts with physicians and other health care team members in the areas of systems redesign and research, to improve practice environments and health systems.2
The VHA is the largest integrated health care system in the U.S., serving more than 8.76 million veterans at more than 1,700 facilities. The VHA has an organizational structure that uses centralized control in Washington, DC, and branches out to 18 regional networks that are divided into local facilities in 50 states, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, and the Philippines. This type of structure is known for promoting efficient standardization of processes and procedures across an organization.3
The VHA Blueprint for Excellence envisions the promotion of a positive culture of service and the advancement of health care innovations necessary to create an environment that all veterans deserve.4 To that end, the VHA can be a promising health care institution through which patient-centered initiatives can be standardized, promulgated nationally, and replicated as a model for the country and international health systems. However, it is important to note that the bureaucratic organizational structure of the VHA's national integrated system of care is based on a systemwide standardization effort.5 Therefore, more time may be required to implement organizational changes.
Transgender populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, this population experiences high rates of suicide, HIV/AIDS, substance use disorder, poverty, and homelessness.6 Due to the growing evidence of health disparities and negative health outcomes affecting transgender populations, the federal government has identified transgender patient care and outcomes as a major health concern and priority in the Healthy People Initiative 2020.2,7,8
In 2012, the VHA issued a directive mandating services for transgender veterans.9 Nevertheless, health care staff significantly lack the knowledge, skills, and cultural competencies that are vital in transgender care.
This article reviews the prevalence and demographics of the transgender population, social challenges, global health concerns, and public health policies. The article also examines how the doctor of nursing practice (DNP)-prepared nurse leader can provide transformational nursing leadership to facilitate culturally competent, patient-centered initiatives to improve access and services for transgender individuals in the VHA and provide a model for change in transgender population health.
Definitions
Gender is a behavioral, cultural, or psychological trait assigned by society that is associated with male or female sex. Sex denotes the biologic differences between males and females. Transgender is an umbrella term used to describe people whose gender identity or gender expression is different from that of their sex assigned at birth. Transsexualism is a subset of transgender persons who have taken steps to self-identify or transition to look like their preferred gender.
Demographics
Estimates of the prevalence of transgenderism are roughly drawn from less rigorous methods, such as the combination of parents who report transgenderism in children, the number of adults reportedly seeking clinical care (such as cross-sex or gender-affirming hormone therapy), and the number of surgical interventions reported in different countries.10 A meta-analysis of 21 studies concluded that the ratio of transsexuals (individuals who are altering or have already altered their birth sex) was predominantly 1:14,705 adult males and 1:38,461 adult females.11 Since all transgender persons do not identify as transsexual, these figures do not provide a precise estimation of the number of transgender persons worldwide.
About 700,000, or 0.3%, of the adult population in the U.S. identify themselves as transgender, and an estimated 134,300 identify as transgender veterans.6,12 The transgender population in the U.S. is estimated to be 55% white, 16% African American, 21% Hispanic, and 8% other races.13 The U.S. census data noted that the transgender population was geographically located across the nation. Transgender persons are more likely to be single, never married, divorced, and more educated but with significantly less household income.2 Data to provide an accurate reflection of the number of transgender people in the U.S. are lacking. Some transgender individuals also may identify as lesbian, gay, or bisexual, making population-based estimation even more challenging and difficult.
Transgender persons who have transitioned may not have changed their names or changed their identified sex on official Social Security records, which the Social Security Administration allows only if there is evidence that genital sexual reassignment surgery was performed.14 The number of transgender adults requesting treatment continues to rise.10
Social and Health Challenges
Transgender people face many challenges because of their gender identity. Surveys assessing the living conditions of transgender people have found that 43% to 60% report high levels of physical violence.15 By comparison, the National Intimate Partner and Sexual Violence Survey found that interpersonal violence and sexual violence were reported by lesbian and gay individuals at equal or higher levels than that reported by heterosexuals. Forty-four percent of lesbian women, 35% of heterosexual women, 29% of heterosexual men, and 26% of gay men reported experiencing rape or physical violence.16 A study in Spain reported 59% of transgender people experienced patterns of harassment, and in Canada, 34% of transgender people lived below the poverty level.17,18
In the U.S., the National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants provided extensive data on challenges experienced by transgender people.6 Discrimination was frequently experienced in accessing health care. Due to transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.6 The same study also reported that as many as 41% live in extreme poverty with incomes of less than $10,000 per year reported. Twenty-six percent were physically assaulted, and 10% experienced sexual violence. More than 25% of the transgender population misused drugs or alcohol to cope with mistreatment.6
In the U.S., HIV infection rates for transgender individuals were more than 4 times (2.64%) the rate of the general population (0.6%).6 Internationally, there is a high prevalence of HIV in transgender women. The prevalence rate of HIV in U.S. transgender women was 21.74% of the estimated U.S. adult transgender population of about 700,000.19 One in 4 people living with HIV in the U.S. are women.20
Suicide attempt rates are extremely high among transgender people. A suicide rate of 22% to 43% has been reported across Europe, Canada, and the U.S.21 Depression and anxiety were commonly noted as a result of discrimination and social stigma. In the U.S., transgender persons reported high rates of depression, with 41% reporting attempted suicide compared with 1.6% of the general population.6 Access to health care services, such as mental health, psychosocial support, and stress management are critical for this vulnerable population.22
Health Policies
Since 1994, the UK has instituted legal employment protections for the transgender population. In the UK, transgender persons, including military and prisoners, have health care coverage that includes sexual reassignment surgery as part of the UK's National Health Service.23
In the U.S., the federal policy of "Don't Ask, Don't Tell" barring transgender persons from serving openly in the military was repealed in June 2016. This policy historically has had a silencing effect on perpetuating institutionalized biases.24 This remains problematic even after veterans have transitioned from military service to the VA for civilian care.
Between 2006 and 2013, the reported prevalence and incidence of transgender-related diagnoses in the VA have steadily increased with 40% of new diagnoses occurring since 2011.25 In fiscal year 2013, there were 32.9 per 100,000 veterans with transgender-related diagnoses.25 Health care staff, in particular health care providers (HCPs), can play a critical role in reducing health disparities and unequal treatment.26
With the passage of the U.S. Affordable Care Act (ACA), health insurance coverage for transgender persons is now guaranteed by law, and health disparities within the transgender population can begin to be properly addressed. The ACA offers the ability to purchase health insurance, possibly qualify for Medicaid, or obtain subsidies to purchase health insurance. Insurance coverage is accessible without regard to discrimination or preexisting conditions.27 As of May 2014, the Medicare program covered medically necessary hormone therapy and sex reassignment surgery.13 While VA benefits cover hormone therapy for transgender veterans, sex reassignment surgery is not currently a covered benefit.28 The ACA now increases access to primary care, preventative care, mental health services, and community health programs not previously available in the transgender community.
Healthy People 2020 Goals
One of the Healthy People 2020 stated goals is to improve the health and wellness of transgender people.29 The objective is to increase the number of population-based data collection systems used to monitor transgender people from the baseline of 2 to a total of 4 by 2020. The data systems would be assigned to collect relevant data, such as mental health; HIV status; illicit drug, alcohol, and tobacco use; cervical and breast cancer screening; health insurance coverage; and access to health care.
Health Care Staff Readiness
Transgender persons face health care challenges with major health disparities due to their gender identity. Transgender persons as a defined population are not well understood by HCPs. In a survey, 50% of transgender respondents reported that they had to teach their medical provider about transgender care.6 Negative perceptions of transgender persons are well established and have contribute to the poor health care access and services that transgender persons receive. Transgender persons are often denied access to care, denied visitation rights, and are hesitant to share information for fear of bureaucratic exclusion or isolation.
There is a lack of evidence-based studies to guide care and help HCPs gain greater understanding of this population's unique needs.30 Additionally, a significant lack of knowledge, skills, cultural competence, and awareness exist in providing transgender care. Research on nursing attitudes concerning transgender care consistently found negative attitudes, and physicians also frequently reported witnessing derogatory comments and discriminatory care from colleagues.31,32 The study by Carabez and colleagues found that practicing nurses rarely received the proper education or training in transgender health issues, and many were unaware of the needs of this population.33 In addition, many HCPs were uncomfortable working with transgender patients. Physicians also expressed knowledge deficits on gender identity disorders due to a lack of training and ethical concerns about their roles in providing gender-transitioning treatment.26
Although the VHA directive states that transgender services and treatment should be standardized, the VHA has not approved, defined, or endorsed specific standards of care or clinical guidelines within the organization for transgender care, further heightening HCP concerns.9 The clinical practice guidelines available for addressing preventive care for transgender patients are primarily based on consensus of expert opinion.34 Expert opinion has produced the Standards of Care (SOC) for the Health of Transsexual, Transgender, and Gender Nonconforming People, published by the World Professional Association for Transgender Health (WPATH) and cited by the IOM as the major clinical practice guidelines for providing care to transgender individuals.2 Transgender care at the VHA is guided by the WPATH standards of care.35
The VHA has created national educational programs and policies with targeted goals to provide uniform, culturally competent, patient-centered care. Online transgender health presentations are available, and at least 15 VHA facilities have transgender support groups.30 While the VHA supports a patient-centered philosophy for transgender patient care, many facilities do not currently have organizational initiatives that enhance clinical preparation of HCPs or have sufficiently modified the environment to better accommodate the health care needs of transgender veterans.
DNP Preparation
The DNP terminal degree provides nurses with doctoral-level training in organizational and systems leadership, leading quality improvement, and implementing systemwide initiatives by using scientific findings to drive processes that improve quality of care for a changing patient population.36 Preparation in research analysis of evidence-based interventions also is essential to evaluating practice patterns, patient outcomes, and systems of care that can identify gaps in practice. Training in health care policy and advocacy, information systems, patient care technology, and population health also is provided so that DNPs are competent to develop system strategies to transform health care through clinical prevention and health promotion.
QSEN Framework
In keeping with the IOM's Future of Nursing initiative recommendations that graduate nurses be prepared as leaders in education, practice, administration, and research, there is an increasing focus on providing graduate-level nursing education and training to ensure quality and efficiency of health outcomes.37 The Quality and Safety Education in Nursing (QSEN) project, initiated at the RWJF by Linda Cronenwett, PhD, RN, identifies a framework for knowledge, skills, and attitudes that defines the competencies that nurses need to deliver effective care to improve quality and safety within health care systems.38 These core competencies include quality improvement, safety, teamwork and collaboration, patient-centered care, evidence-based practice, and informatics. The RWJF and the American Association of Colleges of Nursing later expanded the project initiative to prepare nursing faculty to teach the QSEN competencies in graduate nursing programs.36
The DNP nurse leader is ideally suited to manage this project by applying competencies from the QSEN framework. Using open communication and mutual respect, the nurse leader is poised to effectively develop interprofessional teams to collaborate and initiate transformational changes that improve quality and patient-centered care delivered within the health care organization.
Public Health Resources
Public health resources addressing transgender patient care advocacy, public policy, community education, standards of care, cultural competency, mental health, hormone therapy, surgical interventions, reproductive health, primary care, preventative care, and research are available. For example, WPATH is an international multidisciplinary organization that has published comprehensive SOC for transgender, transsexual, and gender-nonconforming people. The seventh version of the SOC contains evidence-based guidelines for treatment.39 Additional online resources for transgender health are available from the CDC, the Center of Excellence for Transgender Health at the University of California, San Francisco; Department of Family and Community Medicine; and the National Center for Transgender Equality.13,40,41
Patient-Centered Transgender Care
The QSEN framework outlines competencies that provide applicable solutions that help prepare organizations to deliver culturally competent, patient-centered transgender care. The first step to creating patient-centered transgender care is to "analyze factors that create barriers to patient-centered care."42 The magnitude of the barriers to providing patient-centered transgender care also must be identified and understood. An assessment of individual values, beliefs, and attitudes can help to identify cultural characteristics and eliminate stereotypes that impact health practices.43
The nurse leader should solicit support from stakeholders to assess barriers to providing patient-centered transgender care at the system level. Stakeholders would include staff directly involved in patient care, such as physicians, nurse practitioners, physician assistants, registered nurses, nurse managers, nurse educators, licensed practical nurses, medical support assistants, psychologists, dieticians, and social workers. Other ancillary stakeholders with an interest in creating a patient-centered environment with positive patient outcomes include the executive leadership team of the organization, which consists of the chief of staff, director, administrative officers, and nurse executive.
The nurse leader should consult with experts in transgender care and present evidence-based research showing how deficits in staff knowledge, skills, and cultural competence negatively impact the quality of care provided to transgender persons. National data on the consequential health disparities and negative impacts on patient outcomes also should be discussed and presented to all stakeholders. The nurse leader in collaboration with the VA Office of Research and Development is ideally suited to obtain institutional review board approval of a proposal to conduct a needs assessment survey of health care staff barriers to providing patient-centered transgender care. Thereafter, the nurse leader would analyze, extract, and synthesize the data and evaluate the resources and technology available to translate this research knowledge into a clinical practice setting at the system level.44
The second solution uses the results of the survey to develop staff competency training within the organization. The nurse leader can facilitate collaboration and team building to develop practice guidelines and SOC. Competency training will prepare the staff to assist in developing strategies to improve the quality of care for transgender persons. Educationconcerning existing evidence-based clinical guidelines and SOC as well as anecdotal evidence of the needs of transgender patients should be included in competency training.45 One approach to competency training would be to trainintegrated multidisciplinary teams with expertise in transgender care to promote wellness and disease prevention.9 The nurse leader should collaborate with multiple disciplines to facilitate the development of interdisciplinary teams from nursing, medicine, social work, pharmacy, primary care, mental health, women's health, and endocrinology to participate in the Specialty Care Access Network Extension of Community Healthcare outcomes (SCAN-ECHO) training. Training can be offered by videoconferencing over several months and provides cost-effective, efficient training of providers in patient-centered transgender care.46,47 After the SCAN-ECHO program is completed, trained nursing experts could then develop a cultural sensitivity training program for nursing organizations to be offered to educate health care staff on an annual basis.
The third solution addresses the QSEN competency to "Analyze institutional features of the facilities that support or pose barriers to patient-centered care."42 Many veterans do not perceive VA environments as welcoming. In a study by Sherman and colleagues, less than one-third of veterans believed the VA environment was welcoming to sexual or gender minorities, and sexual orientation or gender identity was disclosed by only about 25% of veterans.48 Many veterans in this study felt uncomfortable disclosing their gender or sexual orientation. The majority felt that providers should not routinely ask about sexual orientation or gender identity, and 24% said they were very or somewhat uncomfortable discussing the issue. In another study, 202 VA providers were asked if they viewed the VA as welcoming, and 32% said the VA was somewhat or very unwelcoming.48
The nurse leader is trained in the essentials of health care policy advocacy, which is central to nursing practice.49 Nursing as a profession values social justice and equality, which are linked to fewer health disparities and more stable health indicators.50 Therefore, nursing can ideally provide organizational leaders by developing a culture wherein stable, patient-centered relationships can develop and thrive.
Organizational Culture
Strategies must be deployed to create an organizational culture that is welcoming, respectful, and supportive of transgender patients and family preferences. VA should develop support groups for transgender veterans in VA facilities. Support groups are helpful in diminishing stress, improving self-esteem, building confidence, and improving social relationships.51 Additionally, VA should develop community-based partnerships with other organizations that already provide institutional care and support from HCPs who support transgender persons' right to self-determination.52 These partnerships can foster environmental influences over time and lead to the development of trusting relationships between transgender veterans and the VA organization.
Another community partnership of importance for the nurse leader to develop is an alliance with local universities to train nursing students in cultural competencies in transgender care at VA facilities. The U.S. population continues to diversify in race and ethnicity and cultural influences; therefore, nurses must be prepared in cultural competencies in order to provide quality care that reduces health disparities.53
Under federal law, the VHA has a data sharing agreement with the DoD. Despite the repeal of the "Don't Ask, Don't Tell" federal law, which cleared the way for transgender persons to openly serve in the military, many transgender persons may remain fearful of reprisals, such as judgment, denial of care, or loss of benefits if gender identity is disclosed.54 Given the bureaucratic structure of the VHA, the implementation of cultural changes at the system level will require a collaborative effort between multidisciplinary teams and community partnerships to transform the VA environment over time. The authors believe that on this issue, external forces must guide and lead changes within the VA system in order to develop sustainable and trusting relationships with transgender veterans.
The fourth solution is implementation of policies that "empower patients or families in all aspects of the health care process."42 Again, the nurse leader is trained and prepared to advocate for a policy that implements a Patient Bill of Rights that explicitly guarantees health care and prohibits discrimination of gender-minority veterans. This change would foster trust and confidence from transgender individuals. A study found that 83% of providers and 83% of lesbian, gay, bisexual, and transgender veterans believe that this policy change would make the VHA environment more welcoming.48 Providing transgender-affirming materials and language on standard forms also would eliminate barriers, promote patient-centered care, and empower transgender patients by creating an environment that is more inclusive of everyone.48
Conclusion
The nurse leader is well positioned to implement the QSEN framework to integrate research, practice, and policy to create a more inclusive, patient-centered health care system for transgender veterans. By using the essential principles of doctoral education for advanced nursing practice, the nurse leader is prepared to advocate for changing the organization at the systems level. The nurse leader also is equipped to direct the implementation of patient-centered transgender care initiatives by ensuring the integration of the nursing organization as a partner in strategic planning as well as the development of solutions.
The VHA Blueprint of Excellence envisions organization and collaboration to promote new relationships that serve and benefit veterans. The DNP preparation allows the nurse leader to demonstrate the ability to collaborate with VHA stakeholders and develop alliances within and outside the organization by advocating for policy changes that will be transformational in improving health care delivery and patient outcomes to vulnerable transgender veteran populations. The IOM has tasked nurse executives with creating a health care infrastructure of doctorally prepared nurses to provide patient care that is increasingly growing more complex. With an increasing number of veterans using services, VHA has prioritized an expansion in the number of doctorally prepared nurses.55
As the largest integrated health care system in the U.S., the VHA provides an ideal setting for initiating these organizational changes as a result of having developed an integrated infrastructure to collect evidence-based data at the regional (network) and state facilities and make comparisons with national benchmarks. Therefore, changes are less difficult to disseminate throughout the hierarchy of the VHA. Consequently, the VHA has been a leader in the U.S. for equity in the health care arena and provides a model for international health care systems. Finally, these changes address an urgent need to reduce health disparities, morbidity, and mortality by improving quality care and health care delivery to a vulnerable transgender population.
1. Greiner AC, Knebel E, eds. Health Professions Education: A Bridge to Quality. Washington, DC: National Academies Press; 2003.
2. Institute of Medicine. Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
3. Mintzberg H. The structuring of organizations: a synthesis of the research. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1496182 1979. Posted November 4, 2009. Accessed November 30, 2016.
4. U.S. Department of Veterans Affairs. VHA blue print for excellence. https://www.va.gov/health/docs/VHA_Blueprint_for_Excellence.pdf. Published September 21, 2014. Accessed November 30, 2016.
5. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, part 2):1573-1583.
6. Grant JM, Mottet L, Tanis JE, Harrison J, Herman J, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads /reports/reports/ntds_full.pdf. Published 2011. Accessed November 30, 2016.
7. Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objec tives/topic/lesbian-gay-bisexual-and-transgender -health. Updated November 16, 2016. Accessed November 16, 2016.
8. Institute of Medicine Committee on Lesbian Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
9. U.S. Department of Veterans Affairs. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. Washington, DC: U.S. Department of Veterans Affairs; 2013.
10. Zucker KJ, Lawrence AA. Epidemiology of gender identity disorder: recommendations for the Standards of Care of the World Professional Association for Transgender Health. Int J Transgenderism. 2009;11(1):8-18.
11. Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30(6):807-815.
12. Gates GJ, Herman JL. Transgender military service in the United States. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military -Service-May-2014.pdf. Published May 2014. Accessed November 30, 2016.
13. Flores AR, Brown TNT, and Herman JL. Race and ethnicity of adults who identify as transgender in the United States. http://williamsinstitute.law.ucla .edu/wp-content/uploads/Race-and-Ethnicity-of -Transgender-Identified-Adults-in-the-US.pdf. Published October 2016. Accessed December 13, 2016.
14. Harris BC. Likely Transgender individuals in US federal administrative records and the 2010 census. https://www.census.gov/srd/carra/15_03_Likely_Transgender_Individuals_in_ARs_and_2010Census.pdf. Published May 4, 2015. Accessed November 30, 2016.
15. Kenagy GP, Bostwick WB. Health and social service needs of transgender people in Chicago. Int J Transgenderism. 2005;8(2-3):57-66.
16. Centers for Disease Control and Prevention. National intimate partner and sexual violence survey, 2010 summary report. https://www.cdc.gov/viole nceprevention/pdf/nisvs_report2010-a.pdf. Published November 2011. Accessed December 12, 2016.
17. Bauer GR, Travers R, Scanlon K, Coleman TA. High heterogeneity of HIV-related sexual risk among transgender people in Ontario, Canada: a province-wide respondent-driven sampling survey. BMC Public Health. 2012;12(1):292-291.
18. Devis-Devis J, Pereira-Garcia S, Valencia-Peris A, Fuentes-Miguel J, López-Cañada E, Pérez-Samaniego V. Harassment patterns and risk profile in Spanish trans persons. J Homosex. 2016. [Epub ahead of print.]
19. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many -People-LGBT-Apr-2011.pdf. Published April 2011. Accessed December 1, 2016.
20. Center for Disease Control and Prevention. HIV Among Women. http://www.cdc.gov/hiv/group/gender/women/index.html. Accessed December 10, 2016.
21. Bauer GR, Scheim AI, Pyne J, Travers R, Hammond R. Intervenable factors associated with suicide risk in transgender persons: a respondent driven sampling study in Ontario, Canada. BMC Public Health. 2015;15(1):525.
22. McCann E. People who are transgender: mental health concerns. J Psychiatr Ment Health Nurs. 2015;22(1):76-81.
23. Green R. Transsexual legal rights in the United States and United Kingdom: employment, medical treatment, and civil status. Arch Sex Behav. 2010;39(1):153-160.
24. Sharpe VA, Uchendu US. Ensuring appropriate care for LGBT veterans in the Veterans Health Administration. Hastings Cent Rep. 2014;44(suppl 4):S53-S55.
25. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006-2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
26. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. "Completely out-at-sea" with "two-gender medicine": a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12(1):110.
27. U.S. Department of Health and Human Services. Key features of the affordable care act. http://www .hhs.gov/healthcare/facts-and-features/key-features -of-aca/index.html. Last reviewed November 18, 2014. Accessed December 1, 2016.
28. U.S. Department of Veterans Affairs. Federal benefits for veterans, dependents, and survivors. https://www.va.gov/opa/publications/benefits_book/Chapter_1_Health_Care_Benefits.asp. Accessed December 1, 2016.
29. HealthyPeople.gov. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bi sexual-and-transgender-health. Updated December 1, 2016. Accessed December 1, 2016.
30. Lutwak N, Byne W, Erickson-Schroth L, et al. Transgender veterans are inadequately understood by health care providers. Mil Med. 2014;179(5):483-485.
31. Dorsen C. An integrative review of nurse attitudes towards lesbian, gay, bisexual, and transgender patients. Can J Nurs Res. 2012;44(3):18-43.
32. Eliason MJ, Dibble SL, Robertson PA. Lesbian, gay, bisexual, and transgender (LGBT) physicians' experiences in the workplace. J Homosex. 2011;58(10):1355-1371.
33. Carabez R, Pellegrini M, Mankovitz A, Eliason M, Ciano M, Scott M. "Never in All My Years...": Nurses' education about LGBT health. J Prof Nurs. 2015;31(4):323-329
34. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
35. VA Boston Healthcare System. Patient Care Memorandum-11-046-LM. Management of transgender veteran patients. http://www.boston.va.gov/services/images/lgbt_patient_care_memo_transgender_care.pdf. Published May 2011. Accessed December 1, 2016.
36. Cronenwett L, Sherwood G, Pohl J, et al. Quality and safety education for advanced nursing practice. Nurs Outlook. 2009;57(6):338-348.
37. Institute of Medicine. Committee on the Robert Wood Johnson Foundation Initiative on the Future of Nursing. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: National Academies Press; 2011.
38. Smith EL, Cronenwett L, Sherwood G. Current assessments of quality and safety education in nursing. Nurs Outlook. 2007;55(3):132-137.
39. World Professional Association for Transgender Health (WPATH).The standards of care. http://www.wpath.org/site_page.cfm?pk_association _webpage_menu=1351&pk_association_web page=4655. Accessed December 1, 2016.
40. University of California San Francisco Department of Family and Community Medicine. Center of Excellence for Transgender Health. http://www.tran shealth.ucsf.edu/trans?page=home-00-00 Accessed December 1, 2016.
41. Center for Disease Control and Prevention. Lesbian, gay, bisexual and transgender health. http://www.cdc.gov/lgbthealth/transgender.htm. Accessed December 1, 2016.
42. American Association of Colleges of Nursing. QSEN education consortium: graduate-level QSEN competencies, knowledge, skills and attitudes. http://www.aacn.nche.edu/faculty/qsen/competen cies.pdf. Accessed December 1, 2016.
43. Andrews MM, Boyle JS. Transcultural Concepts in Nursing Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
44. Moran KJ, Burson R, Conrad D. The Doctor of Nursing Practice Scholarly Project: A Framework for Success. Burlington, MA: Jones & Bartlett; 2013.
45. Hanssmann C, Morrison D, Russian E, Shiu-Thornton S, Bowen D. A community-based program evaluation of community competency trainings. J Assoc Nurses AIDS Care.
46. Knapp H, Fletcher M, Taylor A, Chan K, Goetz MB. No clinic left behind: providing cost-effective in-services via distance learning. J Healthc Qual. 2011;33(5):17-24.
47. Kauth MR, Shipherd JC, Lindsay JA, Kirsh S, Knapp H, Matza L. Teleconsultation and training of VHA providers on transgender care: implementation of a multisite hub system. Telemed J E Health. 2015;21(12):1012-1018.
48. Sherman MD, Kauth MR, Ridener L, Shipherd JC, Bratkovich K, Beaulieu G. An empirical investigation of challenges and recommendations for welcoming sexual and gender minority veterans into VA care. Prof Psychol: Res Pract. 2014;45(6):433-442.
49. American Association of Colleges of Nursing. The essentials of doctoral education for advanced nursing practice. http://www.aacn.nche.edu/pub lications/position/DNPEssentials.pdf. Published October 2006. Accessed December 1, 2016.
50. Boutain DM. Social justice as a framework for professional nursing. J Nurs Educ. 2005;44(9):404-408.
51. Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84:22-29.
52. Thornhill L, Klein P. Creating environments of care with transgender communities. J Assoc Nurs AIDS Care. 2010;21(3):230-239.
53. Collins J. Nursing cultural competencies: Improving patient care quality and satisfaction. Ohio Nurses Rev. 2015;90(1):10-11.
54. Sherman MD, Kauth MR, Shipherd JC, Street RL Jr. Communication between VA providers and sexual and gender minority veterans: a pilot study. Psychol Serv. 2014;11(2):235-242.
55. Cowan L, Fasoli DR, Hagle ME, et al. Creating an infrastructure to advance nursing practice and care for veterans. Nurse Leader. 2013;11(5):33-36.
Patient-centered care is of fundamental importance when caring for the transgender population due to the well-established history of social stigma and systemic discrimination. Therefore, nursing education is mandated to equip graduates with culturally competent patient-centered care skills.1 In 2009, the Institute of Medicine (IOM) in partnership with the Robert Wood Johnson Foundation (RWJF) launched The Future of Nursing initiative, which outlined the major role nursing should play in transforming the health care system to meet the health care needs of diverse U.S. populations.
The initiative produced a blueprint of action-focused institutional recommendations at the local, state, and national levels that would facilitate the reforms necessary to transform the U.S. health care system. One of the recommendations of the IOM report was to increase opportunities for nurses to manage and lead collaborative efforts with physicians and other health care team members in the areas of systems redesign and research, to improve practice environments and health systems.2
The VHA is the largest integrated health care system in the U.S., serving more than 8.76 million veterans at more than 1,700 facilities. The VHA has an organizational structure that uses centralized control in Washington, DC, and branches out to 18 regional networks that are divided into local facilities in 50 states, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, and the Philippines. This type of structure is known for promoting efficient standardization of processes and procedures across an organization.3
The VHA Blueprint for Excellence envisions the promotion of a positive culture of service and the advancement of health care innovations necessary to create an environment that all veterans deserve.4 To that end, the VHA can be a promising health care institution through which patient-centered initiatives can be standardized, promulgated nationally, and replicated as a model for the country and international health systems. However, it is important to note that the bureaucratic organizational structure of the VHA's national integrated system of care is based on a systemwide standardization effort.5 Therefore, more time may be required to implement organizational changes.
Transgender populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, this population experiences high rates of suicide, HIV/AIDS, substance use disorder, poverty, and homelessness.6 Due to the growing evidence of health disparities and negative health outcomes affecting transgender populations, the federal government has identified transgender patient care and outcomes as a major health concern and priority in the Healthy People Initiative 2020.2,7,8
In 2012, the VHA issued a directive mandating services for transgender veterans.9 Nevertheless, health care staff significantly lack the knowledge, skills, and cultural competencies that are vital in transgender care.
This article reviews the prevalence and demographics of the transgender population, social challenges, global health concerns, and public health policies. The article also examines how the doctor of nursing practice (DNP)-prepared nurse leader can provide transformational nursing leadership to facilitate culturally competent, patient-centered initiatives to improve access and services for transgender individuals in the VHA and provide a model for change in transgender population health.
Definitions
Gender is a behavioral, cultural, or psychological trait assigned by society that is associated with male or female sex. Sex denotes the biologic differences between males and females. Transgender is an umbrella term used to describe people whose gender identity or gender expression is different from that of their sex assigned at birth. Transsexualism is a subset of transgender persons who have taken steps to self-identify or transition to look like their preferred gender.
Demographics
Estimates of the prevalence of transgenderism are roughly drawn from less rigorous methods, such as the combination of parents who report transgenderism in children, the number of adults reportedly seeking clinical care (such as cross-sex or gender-affirming hormone therapy), and the number of surgical interventions reported in different countries.10 A meta-analysis of 21 studies concluded that the ratio of transsexuals (individuals who are altering or have already altered their birth sex) was predominantly 1:14,705 adult males and 1:38,461 adult females.11 Since all transgender persons do not identify as transsexual, these figures do not provide a precise estimation of the number of transgender persons worldwide.
About 700,000, or 0.3%, of the adult population in the U.S. identify themselves as transgender, and an estimated 134,300 identify as transgender veterans.6,12 The transgender population in the U.S. is estimated to be 55% white, 16% African American, 21% Hispanic, and 8% other races.13 The U.S. census data noted that the transgender population was geographically located across the nation. Transgender persons are more likely to be single, never married, divorced, and more educated but with significantly less household income.2 Data to provide an accurate reflection of the number of transgender people in the U.S. are lacking. Some transgender individuals also may identify as lesbian, gay, or bisexual, making population-based estimation even more challenging and difficult.
Transgender persons who have transitioned may not have changed their names or changed their identified sex on official Social Security records, which the Social Security Administration allows only if there is evidence that genital sexual reassignment surgery was performed.14 The number of transgender adults requesting treatment continues to rise.10
Social and Health Challenges
Transgender people face many challenges because of their gender identity. Surveys assessing the living conditions of transgender people have found that 43% to 60% report high levels of physical violence.15 By comparison, the National Intimate Partner and Sexual Violence Survey found that interpersonal violence and sexual violence were reported by lesbian and gay individuals at equal or higher levels than that reported by heterosexuals. Forty-four percent of lesbian women, 35% of heterosexual women, 29% of heterosexual men, and 26% of gay men reported experiencing rape or physical violence.16 A study in Spain reported 59% of transgender people experienced patterns of harassment, and in Canada, 34% of transgender people lived below the poverty level.17,18
In the U.S., the National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants provided extensive data on challenges experienced by transgender people.6 Discrimination was frequently experienced in accessing health care. Due to transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.6 The same study also reported that as many as 41% live in extreme poverty with incomes of less than $10,000 per year reported. Twenty-six percent were physically assaulted, and 10% experienced sexual violence. More than 25% of the transgender population misused drugs or alcohol to cope with mistreatment.6
In the U.S., HIV infection rates for transgender individuals were more than 4 times (2.64%) the rate of the general population (0.6%).6 Internationally, there is a high prevalence of HIV in transgender women. The prevalence rate of HIV in U.S. transgender women was 21.74% of the estimated U.S. adult transgender population of about 700,000.19 One in 4 people living with HIV in the U.S. are women.20
Suicide attempt rates are extremely high among transgender people. A suicide rate of 22% to 43% has been reported across Europe, Canada, and the U.S.21 Depression and anxiety were commonly noted as a result of discrimination and social stigma. In the U.S., transgender persons reported high rates of depression, with 41% reporting attempted suicide compared with 1.6% of the general population.6 Access to health care services, such as mental health, psychosocial support, and stress management are critical for this vulnerable population.22
Health Policies
Since 1994, the UK has instituted legal employment protections for the transgender population. In the UK, transgender persons, including military and prisoners, have health care coverage that includes sexual reassignment surgery as part of the UK's National Health Service.23
In the U.S., the federal policy of "Don't Ask, Don't Tell" barring transgender persons from serving openly in the military was repealed in June 2016. This policy historically has had a silencing effect on perpetuating institutionalized biases.24 This remains problematic even after veterans have transitioned from military service to the VA for civilian care.
Between 2006 and 2013, the reported prevalence and incidence of transgender-related diagnoses in the VA have steadily increased with 40% of new diagnoses occurring since 2011.25 In fiscal year 2013, there were 32.9 per 100,000 veterans with transgender-related diagnoses.25 Health care staff, in particular health care providers (HCPs), can play a critical role in reducing health disparities and unequal treatment.26
With the passage of the U.S. Affordable Care Act (ACA), health insurance coverage for transgender persons is now guaranteed by law, and health disparities within the transgender population can begin to be properly addressed. The ACA offers the ability to purchase health insurance, possibly qualify for Medicaid, or obtain subsidies to purchase health insurance. Insurance coverage is accessible without regard to discrimination or preexisting conditions.27 As of May 2014, the Medicare program covered medically necessary hormone therapy and sex reassignment surgery.13 While VA benefits cover hormone therapy for transgender veterans, sex reassignment surgery is not currently a covered benefit.28 The ACA now increases access to primary care, preventative care, mental health services, and community health programs not previously available in the transgender community.
Healthy People 2020 Goals
One of the Healthy People 2020 stated goals is to improve the health and wellness of transgender people.29 The objective is to increase the number of population-based data collection systems used to monitor transgender people from the baseline of 2 to a total of 4 by 2020. The data systems would be assigned to collect relevant data, such as mental health; HIV status; illicit drug, alcohol, and tobacco use; cervical and breast cancer screening; health insurance coverage; and access to health care.
Health Care Staff Readiness
Transgender persons face health care challenges with major health disparities due to their gender identity. Transgender persons as a defined population are not well understood by HCPs. In a survey, 50% of transgender respondents reported that they had to teach their medical provider about transgender care.6 Negative perceptions of transgender persons are well established and have contribute to the poor health care access and services that transgender persons receive. Transgender persons are often denied access to care, denied visitation rights, and are hesitant to share information for fear of bureaucratic exclusion or isolation.
There is a lack of evidence-based studies to guide care and help HCPs gain greater understanding of this population's unique needs.30 Additionally, a significant lack of knowledge, skills, cultural competence, and awareness exist in providing transgender care. Research on nursing attitudes concerning transgender care consistently found negative attitudes, and physicians also frequently reported witnessing derogatory comments and discriminatory care from colleagues.31,32 The study by Carabez and colleagues found that practicing nurses rarely received the proper education or training in transgender health issues, and many were unaware of the needs of this population.33 In addition, many HCPs were uncomfortable working with transgender patients. Physicians also expressed knowledge deficits on gender identity disorders due to a lack of training and ethical concerns about their roles in providing gender-transitioning treatment.26
Although the VHA directive states that transgender services and treatment should be standardized, the VHA has not approved, defined, or endorsed specific standards of care or clinical guidelines within the organization for transgender care, further heightening HCP concerns.9 The clinical practice guidelines available for addressing preventive care for transgender patients are primarily based on consensus of expert opinion.34 Expert opinion has produced the Standards of Care (SOC) for the Health of Transsexual, Transgender, and Gender Nonconforming People, published by the World Professional Association for Transgender Health (WPATH) and cited by the IOM as the major clinical practice guidelines for providing care to transgender individuals.2 Transgender care at the VHA is guided by the WPATH standards of care.35
The VHA has created national educational programs and policies with targeted goals to provide uniform, culturally competent, patient-centered care. Online transgender health presentations are available, and at least 15 VHA facilities have transgender support groups.30 While the VHA supports a patient-centered philosophy for transgender patient care, many facilities do not currently have organizational initiatives that enhance clinical preparation of HCPs or have sufficiently modified the environment to better accommodate the health care needs of transgender veterans.
DNP Preparation
The DNP terminal degree provides nurses with doctoral-level training in organizational and systems leadership, leading quality improvement, and implementing systemwide initiatives by using scientific findings to drive processes that improve quality of care for a changing patient population.36 Preparation in research analysis of evidence-based interventions also is essential to evaluating practice patterns, patient outcomes, and systems of care that can identify gaps in practice. Training in health care policy and advocacy, information systems, patient care technology, and population health also is provided so that DNPs are competent to develop system strategies to transform health care through clinical prevention and health promotion.
QSEN Framework
In keeping with the IOM's Future of Nursing initiative recommendations that graduate nurses be prepared as leaders in education, practice, administration, and research, there is an increasing focus on providing graduate-level nursing education and training to ensure quality and efficiency of health outcomes.37 The Quality and Safety Education in Nursing (QSEN) project, initiated at the RWJF by Linda Cronenwett, PhD, RN, identifies a framework for knowledge, skills, and attitudes that defines the competencies that nurses need to deliver effective care to improve quality and safety within health care systems.38 These core competencies include quality improvement, safety, teamwork and collaboration, patient-centered care, evidence-based practice, and informatics. The RWJF and the American Association of Colleges of Nursing later expanded the project initiative to prepare nursing faculty to teach the QSEN competencies in graduate nursing programs.36
The DNP nurse leader is ideally suited to manage this project by applying competencies from the QSEN framework. Using open communication and mutual respect, the nurse leader is poised to effectively develop interprofessional teams to collaborate and initiate transformational changes that improve quality and patient-centered care delivered within the health care organization.
Public Health Resources
Public health resources addressing transgender patient care advocacy, public policy, community education, standards of care, cultural competency, mental health, hormone therapy, surgical interventions, reproductive health, primary care, preventative care, and research are available. For example, WPATH is an international multidisciplinary organization that has published comprehensive SOC for transgender, transsexual, and gender-nonconforming people. The seventh version of the SOC contains evidence-based guidelines for treatment.39 Additional online resources for transgender health are available from the CDC, the Center of Excellence for Transgender Health at the University of California, San Francisco; Department of Family and Community Medicine; and the National Center for Transgender Equality.13,40,41
Patient-Centered Transgender Care
The QSEN framework outlines competencies that provide applicable solutions that help prepare organizations to deliver culturally competent, patient-centered transgender care. The first step to creating patient-centered transgender care is to "analyze factors that create barriers to patient-centered care."42 The magnitude of the barriers to providing patient-centered transgender care also must be identified and understood. An assessment of individual values, beliefs, and attitudes can help to identify cultural characteristics and eliminate stereotypes that impact health practices.43
The nurse leader should solicit support from stakeholders to assess barriers to providing patient-centered transgender care at the system level. Stakeholders would include staff directly involved in patient care, such as physicians, nurse practitioners, physician assistants, registered nurses, nurse managers, nurse educators, licensed practical nurses, medical support assistants, psychologists, dieticians, and social workers. Other ancillary stakeholders with an interest in creating a patient-centered environment with positive patient outcomes include the executive leadership team of the organization, which consists of the chief of staff, director, administrative officers, and nurse executive.
The nurse leader should consult with experts in transgender care and present evidence-based research showing how deficits in staff knowledge, skills, and cultural competence negatively impact the quality of care provided to transgender persons. National data on the consequential health disparities and negative impacts on patient outcomes also should be discussed and presented to all stakeholders. The nurse leader in collaboration with the VA Office of Research and Development is ideally suited to obtain institutional review board approval of a proposal to conduct a needs assessment survey of health care staff barriers to providing patient-centered transgender care. Thereafter, the nurse leader would analyze, extract, and synthesize the data and evaluate the resources and technology available to translate this research knowledge into a clinical practice setting at the system level.44
The second solution uses the results of the survey to develop staff competency training within the organization. The nurse leader can facilitate collaboration and team building to develop practice guidelines and SOC. Competency training will prepare the staff to assist in developing strategies to improve the quality of care for transgender persons. Educationconcerning existing evidence-based clinical guidelines and SOC as well as anecdotal evidence of the needs of transgender patients should be included in competency training.45 One approach to competency training would be to trainintegrated multidisciplinary teams with expertise in transgender care to promote wellness and disease prevention.9 The nurse leader should collaborate with multiple disciplines to facilitate the development of interdisciplinary teams from nursing, medicine, social work, pharmacy, primary care, mental health, women's health, and endocrinology to participate in the Specialty Care Access Network Extension of Community Healthcare outcomes (SCAN-ECHO) training. Training can be offered by videoconferencing over several months and provides cost-effective, efficient training of providers in patient-centered transgender care.46,47 After the SCAN-ECHO program is completed, trained nursing experts could then develop a cultural sensitivity training program for nursing organizations to be offered to educate health care staff on an annual basis.
The third solution addresses the QSEN competency to "Analyze institutional features of the facilities that support or pose barriers to patient-centered care."42 Many veterans do not perceive VA environments as welcoming. In a study by Sherman and colleagues, less than one-third of veterans believed the VA environment was welcoming to sexual or gender minorities, and sexual orientation or gender identity was disclosed by only about 25% of veterans.48 Many veterans in this study felt uncomfortable disclosing their gender or sexual orientation. The majority felt that providers should not routinely ask about sexual orientation or gender identity, and 24% said they were very or somewhat uncomfortable discussing the issue. In another study, 202 VA providers were asked if they viewed the VA as welcoming, and 32% said the VA was somewhat or very unwelcoming.48
The nurse leader is trained in the essentials of health care policy advocacy, which is central to nursing practice.49 Nursing as a profession values social justice and equality, which are linked to fewer health disparities and more stable health indicators.50 Therefore, nursing can ideally provide organizational leaders by developing a culture wherein stable, patient-centered relationships can develop and thrive.
Organizational Culture
Strategies must be deployed to create an organizational culture that is welcoming, respectful, and supportive of transgender patients and family preferences. VA should develop support groups for transgender veterans in VA facilities. Support groups are helpful in diminishing stress, improving self-esteem, building confidence, and improving social relationships.51 Additionally, VA should develop community-based partnerships with other organizations that already provide institutional care and support from HCPs who support transgender persons' right to self-determination.52 These partnerships can foster environmental influences over time and lead to the development of trusting relationships between transgender veterans and the VA organization.
Another community partnership of importance for the nurse leader to develop is an alliance with local universities to train nursing students in cultural competencies in transgender care at VA facilities. The U.S. population continues to diversify in race and ethnicity and cultural influences; therefore, nurses must be prepared in cultural competencies in order to provide quality care that reduces health disparities.53
Under federal law, the VHA has a data sharing agreement with the DoD. Despite the repeal of the "Don't Ask, Don't Tell" federal law, which cleared the way for transgender persons to openly serve in the military, many transgender persons may remain fearful of reprisals, such as judgment, denial of care, or loss of benefits if gender identity is disclosed.54 Given the bureaucratic structure of the VHA, the implementation of cultural changes at the system level will require a collaborative effort between multidisciplinary teams and community partnerships to transform the VA environment over time. The authors believe that on this issue, external forces must guide and lead changes within the VA system in order to develop sustainable and trusting relationships with transgender veterans.
The fourth solution is implementation of policies that "empower patients or families in all aspects of the health care process."42 Again, the nurse leader is trained and prepared to advocate for a policy that implements a Patient Bill of Rights that explicitly guarantees health care and prohibits discrimination of gender-minority veterans. This change would foster trust and confidence from transgender individuals. A study found that 83% of providers and 83% of lesbian, gay, bisexual, and transgender veterans believe that this policy change would make the VHA environment more welcoming.48 Providing transgender-affirming materials and language on standard forms also would eliminate barriers, promote patient-centered care, and empower transgender patients by creating an environment that is more inclusive of everyone.48
Conclusion
The nurse leader is well positioned to implement the QSEN framework to integrate research, practice, and policy to create a more inclusive, patient-centered health care system for transgender veterans. By using the essential principles of doctoral education for advanced nursing practice, the nurse leader is prepared to advocate for changing the organization at the systems level. The nurse leader also is equipped to direct the implementation of patient-centered transgender care initiatives by ensuring the integration of the nursing organization as a partner in strategic planning as well as the development of solutions.
The VHA Blueprint of Excellence envisions organization and collaboration to promote new relationships that serve and benefit veterans. The DNP preparation allows the nurse leader to demonstrate the ability to collaborate with VHA stakeholders and develop alliances within and outside the organization by advocating for policy changes that will be transformational in improving health care delivery and patient outcomes to vulnerable transgender veteran populations. The IOM has tasked nurse executives with creating a health care infrastructure of doctorally prepared nurses to provide patient care that is increasingly growing more complex. With an increasing number of veterans using services, VHA has prioritized an expansion in the number of doctorally prepared nurses.55
As the largest integrated health care system in the U.S., the VHA provides an ideal setting for initiating these organizational changes as a result of having developed an integrated infrastructure to collect evidence-based data at the regional (network) and state facilities and make comparisons with national benchmarks. Therefore, changes are less difficult to disseminate throughout the hierarchy of the VHA. Consequently, the VHA has been a leader in the U.S. for equity in the health care arena and provides a model for international health care systems. Finally, these changes address an urgent need to reduce health disparities, morbidity, and mortality by improving quality care and health care delivery to a vulnerable transgender population.
Patient-centered care is of fundamental importance when caring for the transgender population due to the well-established history of social stigma and systemic discrimination. Therefore, nursing education is mandated to equip graduates with culturally competent patient-centered care skills.1 In 2009, the Institute of Medicine (IOM) in partnership with the Robert Wood Johnson Foundation (RWJF) launched The Future of Nursing initiative, which outlined the major role nursing should play in transforming the health care system to meet the health care needs of diverse U.S. populations.
The initiative produced a blueprint of action-focused institutional recommendations at the local, state, and national levels that would facilitate the reforms necessary to transform the U.S. health care system. One of the recommendations of the IOM report was to increase opportunities for nurses to manage and lead collaborative efforts with physicians and other health care team members in the areas of systems redesign and research, to improve practice environments and health systems.2
The VHA is the largest integrated health care system in the U.S., serving more than 8.76 million veterans at more than 1,700 facilities. The VHA has an organizational structure that uses centralized control in Washington, DC, and branches out to 18 regional networks that are divided into local facilities in 50 states, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, and the Philippines. This type of structure is known for promoting efficient standardization of processes and procedures across an organization.3
The VHA Blueprint for Excellence envisions the promotion of a positive culture of service and the advancement of health care innovations necessary to create an environment that all veterans deserve.4 To that end, the VHA can be a promising health care institution through which patient-centered initiatives can be standardized, promulgated nationally, and replicated as a model for the country and international health systems. However, it is important to note that the bureaucratic organizational structure of the VHA's national integrated system of care is based on a systemwide standardization effort.5 Therefore, more time may be required to implement organizational changes.
Transgender populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, this population experiences high rates of suicide, HIV/AIDS, substance use disorder, poverty, and homelessness.6 Due to the growing evidence of health disparities and negative health outcomes affecting transgender populations, the federal government has identified transgender patient care and outcomes as a major health concern and priority in the Healthy People Initiative 2020.2,7,8
In 2012, the VHA issued a directive mandating services for transgender veterans.9 Nevertheless, health care staff significantly lack the knowledge, skills, and cultural competencies that are vital in transgender care.
This article reviews the prevalence and demographics of the transgender population, social challenges, global health concerns, and public health policies. The article also examines how the doctor of nursing practice (DNP)-prepared nurse leader can provide transformational nursing leadership to facilitate culturally competent, patient-centered initiatives to improve access and services for transgender individuals in the VHA and provide a model for change in transgender population health.
Definitions
Gender is a behavioral, cultural, or psychological trait assigned by society that is associated with male or female sex. Sex denotes the biologic differences between males and females. Transgender is an umbrella term used to describe people whose gender identity or gender expression is different from that of their sex assigned at birth. Transsexualism is a subset of transgender persons who have taken steps to self-identify or transition to look like their preferred gender.
Demographics
Estimates of the prevalence of transgenderism are roughly drawn from less rigorous methods, such as the combination of parents who report transgenderism in children, the number of adults reportedly seeking clinical care (such as cross-sex or gender-affirming hormone therapy), and the number of surgical interventions reported in different countries.10 A meta-analysis of 21 studies concluded that the ratio of transsexuals (individuals who are altering or have already altered their birth sex) was predominantly 1:14,705 adult males and 1:38,461 adult females.11 Since all transgender persons do not identify as transsexual, these figures do not provide a precise estimation of the number of transgender persons worldwide.
About 700,000, or 0.3%, of the adult population in the U.S. identify themselves as transgender, and an estimated 134,300 identify as transgender veterans.6,12 The transgender population in the U.S. is estimated to be 55% white, 16% African American, 21% Hispanic, and 8% other races.13 The U.S. census data noted that the transgender population was geographically located across the nation. Transgender persons are more likely to be single, never married, divorced, and more educated but with significantly less household income.2 Data to provide an accurate reflection of the number of transgender people in the U.S. are lacking. Some transgender individuals also may identify as lesbian, gay, or bisexual, making population-based estimation even more challenging and difficult.
Transgender persons who have transitioned may not have changed their names or changed their identified sex on official Social Security records, which the Social Security Administration allows only if there is evidence that genital sexual reassignment surgery was performed.14 The number of transgender adults requesting treatment continues to rise.10
Social and Health Challenges
Transgender people face many challenges because of their gender identity. Surveys assessing the living conditions of transgender people have found that 43% to 60% report high levels of physical violence.15 By comparison, the National Intimate Partner and Sexual Violence Survey found that interpersonal violence and sexual violence were reported by lesbian and gay individuals at equal or higher levels than that reported by heterosexuals. Forty-four percent of lesbian women, 35% of heterosexual women, 29% of heterosexual men, and 26% of gay men reported experiencing rape or physical violence.16 A study in Spain reported 59% of transgender people experienced patterns of harassment, and in Canada, 34% of transgender people lived below the poverty level.17,18
In the U.S., the National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants provided extensive data on challenges experienced by transgender people.6 Discrimination was frequently experienced in accessing health care. Due to transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.6 The same study also reported that as many as 41% live in extreme poverty with incomes of less than $10,000 per year reported. Twenty-six percent were physically assaulted, and 10% experienced sexual violence. More than 25% of the transgender population misused drugs or alcohol to cope with mistreatment.6
In the U.S., HIV infection rates for transgender individuals were more than 4 times (2.64%) the rate of the general population (0.6%).6 Internationally, there is a high prevalence of HIV in transgender women. The prevalence rate of HIV in U.S. transgender women was 21.74% of the estimated U.S. adult transgender population of about 700,000.19 One in 4 people living with HIV in the U.S. are women.20
Suicide attempt rates are extremely high among transgender people. A suicide rate of 22% to 43% has been reported across Europe, Canada, and the U.S.21 Depression and anxiety were commonly noted as a result of discrimination and social stigma. In the U.S., transgender persons reported high rates of depression, with 41% reporting attempted suicide compared with 1.6% of the general population.6 Access to health care services, such as mental health, psychosocial support, and stress management are critical for this vulnerable population.22
Health Policies
Since 1994, the UK has instituted legal employment protections for the transgender population. In the UK, transgender persons, including military and prisoners, have health care coverage that includes sexual reassignment surgery as part of the UK's National Health Service.23
In the U.S., the federal policy of "Don't Ask, Don't Tell" barring transgender persons from serving openly in the military was repealed in June 2016. This policy historically has had a silencing effect on perpetuating institutionalized biases.24 This remains problematic even after veterans have transitioned from military service to the VA for civilian care.
Between 2006 and 2013, the reported prevalence and incidence of transgender-related diagnoses in the VA have steadily increased with 40% of new diagnoses occurring since 2011.25 In fiscal year 2013, there were 32.9 per 100,000 veterans with transgender-related diagnoses.25 Health care staff, in particular health care providers (HCPs), can play a critical role in reducing health disparities and unequal treatment.26
With the passage of the U.S. Affordable Care Act (ACA), health insurance coverage for transgender persons is now guaranteed by law, and health disparities within the transgender population can begin to be properly addressed. The ACA offers the ability to purchase health insurance, possibly qualify for Medicaid, or obtain subsidies to purchase health insurance. Insurance coverage is accessible without regard to discrimination or preexisting conditions.27 As of May 2014, the Medicare program covered medically necessary hormone therapy and sex reassignment surgery.13 While VA benefits cover hormone therapy for transgender veterans, sex reassignment surgery is not currently a covered benefit.28 The ACA now increases access to primary care, preventative care, mental health services, and community health programs not previously available in the transgender community.
Healthy People 2020 Goals
One of the Healthy People 2020 stated goals is to improve the health and wellness of transgender people.29 The objective is to increase the number of population-based data collection systems used to monitor transgender people from the baseline of 2 to a total of 4 by 2020. The data systems would be assigned to collect relevant data, such as mental health; HIV status; illicit drug, alcohol, and tobacco use; cervical and breast cancer screening; health insurance coverage; and access to health care.
Health Care Staff Readiness
Transgender persons face health care challenges with major health disparities due to their gender identity. Transgender persons as a defined population are not well understood by HCPs. In a survey, 50% of transgender respondents reported that they had to teach their medical provider about transgender care.6 Negative perceptions of transgender persons are well established and have contribute to the poor health care access and services that transgender persons receive. Transgender persons are often denied access to care, denied visitation rights, and are hesitant to share information for fear of bureaucratic exclusion or isolation.
There is a lack of evidence-based studies to guide care and help HCPs gain greater understanding of this population's unique needs.30 Additionally, a significant lack of knowledge, skills, cultural competence, and awareness exist in providing transgender care. Research on nursing attitudes concerning transgender care consistently found negative attitudes, and physicians also frequently reported witnessing derogatory comments and discriminatory care from colleagues.31,32 The study by Carabez and colleagues found that practicing nurses rarely received the proper education or training in transgender health issues, and many were unaware of the needs of this population.33 In addition, many HCPs were uncomfortable working with transgender patients. Physicians also expressed knowledge deficits on gender identity disorders due to a lack of training and ethical concerns about their roles in providing gender-transitioning treatment.26
Although the VHA directive states that transgender services and treatment should be standardized, the VHA has not approved, defined, or endorsed specific standards of care or clinical guidelines within the organization for transgender care, further heightening HCP concerns.9 The clinical practice guidelines available for addressing preventive care for transgender patients are primarily based on consensus of expert opinion.34 Expert opinion has produced the Standards of Care (SOC) for the Health of Transsexual, Transgender, and Gender Nonconforming People, published by the World Professional Association for Transgender Health (WPATH) and cited by the IOM as the major clinical practice guidelines for providing care to transgender individuals.2 Transgender care at the VHA is guided by the WPATH standards of care.35
The VHA has created national educational programs and policies with targeted goals to provide uniform, culturally competent, patient-centered care. Online transgender health presentations are available, and at least 15 VHA facilities have transgender support groups.30 While the VHA supports a patient-centered philosophy for transgender patient care, many facilities do not currently have organizational initiatives that enhance clinical preparation of HCPs or have sufficiently modified the environment to better accommodate the health care needs of transgender veterans.
DNP Preparation
The DNP terminal degree provides nurses with doctoral-level training in organizational and systems leadership, leading quality improvement, and implementing systemwide initiatives by using scientific findings to drive processes that improve quality of care for a changing patient population.36 Preparation in research analysis of evidence-based interventions also is essential to evaluating practice patterns, patient outcomes, and systems of care that can identify gaps in practice. Training in health care policy and advocacy, information systems, patient care technology, and population health also is provided so that DNPs are competent to develop system strategies to transform health care through clinical prevention and health promotion.
QSEN Framework
In keeping with the IOM's Future of Nursing initiative recommendations that graduate nurses be prepared as leaders in education, practice, administration, and research, there is an increasing focus on providing graduate-level nursing education and training to ensure quality and efficiency of health outcomes.37 The Quality and Safety Education in Nursing (QSEN) project, initiated at the RWJF by Linda Cronenwett, PhD, RN, identifies a framework for knowledge, skills, and attitudes that defines the competencies that nurses need to deliver effective care to improve quality and safety within health care systems.38 These core competencies include quality improvement, safety, teamwork and collaboration, patient-centered care, evidence-based practice, and informatics. The RWJF and the American Association of Colleges of Nursing later expanded the project initiative to prepare nursing faculty to teach the QSEN competencies in graduate nursing programs.36
The DNP nurse leader is ideally suited to manage this project by applying competencies from the QSEN framework. Using open communication and mutual respect, the nurse leader is poised to effectively develop interprofessional teams to collaborate and initiate transformational changes that improve quality and patient-centered care delivered within the health care organization.
Public Health Resources
Public health resources addressing transgender patient care advocacy, public policy, community education, standards of care, cultural competency, mental health, hormone therapy, surgical interventions, reproductive health, primary care, preventative care, and research are available. For example, WPATH is an international multidisciplinary organization that has published comprehensive SOC for transgender, transsexual, and gender-nonconforming people. The seventh version of the SOC contains evidence-based guidelines for treatment.39 Additional online resources for transgender health are available from the CDC, the Center of Excellence for Transgender Health at the University of California, San Francisco; Department of Family and Community Medicine; and the National Center for Transgender Equality.13,40,41
Patient-Centered Transgender Care
The QSEN framework outlines competencies that provide applicable solutions that help prepare organizations to deliver culturally competent, patient-centered transgender care. The first step to creating patient-centered transgender care is to "analyze factors that create barriers to patient-centered care."42 The magnitude of the barriers to providing patient-centered transgender care also must be identified and understood. An assessment of individual values, beliefs, and attitudes can help to identify cultural characteristics and eliminate stereotypes that impact health practices.43
The nurse leader should solicit support from stakeholders to assess barriers to providing patient-centered transgender care at the system level. Stakeholders would include staff directly involved in patient care, such as physicians, nurse practitioners, physician assistants, registered nurses, nurse managers, nurse educators, licensed practical nurses, medical support assistants, psychologists, dieticians, and social workers. Other ancillary stakeholders with an interest in creating a patient-centered environment with positive patient outcomes include the executive leadership team of the organization, which consists of the chief of staff, director, administrative officers, and nurse executive.
The nurse leader should consult with experts in transgender care and present evidence-based research showing how deficits in staff knowledge, skills, and cultural competence negatively impact the quality of care provided to transgender persons. National data on the consequential health disparities and negative impacts on patient outcomes also should be discussed and presented to all stakeholders. The nurse leader in collaboration with the VA Office of Research and Development is ideally suited to obtain institutional review board approval of a proposal to conduct a needs assessment survey of health care staff barriers to providing patient-centered transgender care. Thereafter, the nurse leader would analyze, extract, and synthesize the data and evaluate the resources and technology available to translate this research knowledge into a clinical practice setting at the system level.44
The second solution uses the results of the survey to develop staff competency training within the organization. The nurse leader can facilitate collaboration and team building to develop practice guidelines and SOC. Competency training will prepare the staff to assist in developing strategies to improve the quality of care for transgender persons. Educationconcerning existing evidence-based clinical guidelines and SOC as well as anecdotal evidence of the needs of transgender patients should be included in competency training.45 One approach to competency training would be to trainintegrated multidisciplinary teams with expertise in transgender care to promote wellness and disease prevention.9 The nurse leader should collaborate with multiple disciplines to facilitate the development of interdisciplinary teams from nursing, medicine, social work, pharmacy, primary care, mental health, women's health, and endocrinology to participate in the Specialty Care Access Network Extension of Community Healthcare outcomes (SCAN-ECHO) training. Training can be offered by videoconferencing over several months and provides cost-effective, efficient training of providers in patient-centered transgender care.46,47 After the SCAN-ECHO program is completed, trained nursing experts could then develop a cultural sensitivity training program for nursing organizations to be offered to educate health care staff on an annual basis.
The third solution addresses the QSEN competency to "Analyze institutional features of the facilities that support or pose barriers to patient-centered care."42 Many veterans do not perceive VA environments as welcoming. In a study by Sherman and colleagues, less than one-third of veterans believed the VA environment was welcoming to sexual or gender minorities, and sexual orientation or gender identity was disclosed by only about 25% of veterans.48 Many veterans in this study felt uncomfortable disclosing their gender or sexual orientation. The majority felt that providers should not routinely ask about sexual orientation or gender identity, and 24% said they were very or somewhat uncomfortable discussing the issue. In another study, 202 VA providers were asked if they viewed the VA as welcoming, and 32% said the VA was somewhat or very unwelcoming.48
The nurse leader is trained in the essentials of health care policy advocacy, which is central to nursing practice.49 Nursing as a profession values social justice and equality, which are linked to fewer health disparities and more stable health indicators.50 Therefore, nursing can ideally provide organizational leaders by developing a culture wherein stable, patient-centered relationships can develop and thrive.
Organizational Culture
Strategies must be deployed to create an organizational culture that is welcoming, respectful, and supportive of transgender patients and family preferences. VA should develop support groups for transgender veterans in VA facilities. Support groups are helpful in diminishing stress, improving self-esteem, building confidence, and improving social relationships.51 Additionally, VA should develop community-based partnerships with other organizations that already provide institutional care and support from HCPs who support transgender persons' right to self-determination.52 These partnerships can foster environmental influences over time and lead to the development of trusting relationships between transgender veterans and the VA organization.
Another community partnership of importance for the nurse leader to develop is an alliance with local universities to train nursing students in cultural competencies in transgender care at VA facilities. The U.S. population continues to diversify in race and ethnicity and cultural influences; therefore, nurses must be prepared in cultural competencies in order to provide quality care that reduces health disparities.53
Under federal law, the VHA has a data sharing agreement with the DoD. Despite the repeal of the "Don't Ask, Don't Tell" federal law, which cleared the way for transgender persons to openly serve in the military, many transgender persons may remain fearful of reprisals, such as judgment, denial of care, or loss of benefits if gender identity is disclosed.54 Given the bureaucratic structure of the VHA, the implementation of cultural changes at the system level will require a collaborative effort between multidisciplinary teams and community partnerships to transform the VA environment over time. The authors believe that on this issue, external forces must guide and lead changes within the VA system in order to develop sustainable and trusting relationships with transgender veterans.
The fourth solution is implementation of policies that "empower patients or families in all aspects of the health care process."42 Again, the nurse leader is trained and prepared to advocate for a policy that implements a Patient Bill of Rights that explicitly guarantees health care and prohibits discrimination of gender-minority veterans. This change would foster trust and confidence from transgender individuals. A study found that 83% of providers and 83% of lesbian, gay, bisexual, and transgender veterans believe that this policy change would make the VHA environment more welcoming.48 Providing transgender-affirming materials and language on standard forms also would eliminate barriers, promote patient-centered care, and empower transgender patients by creating an environment that is more inclusive of everyone.48
Conclusion
The nurse leader is well positioned to implement the QSEN framework to integrate research, practice, and policy to create a more inclusive, patient-centered health care system for transgender veterans. By using the essential principles of doctoral education for advanced nursing practice, the nurse leader is prepared to advocate for changing the organization at the systems level. The nurse leader also is equipped to direct the implementation of patient-centered transgender care initiatives by ensuring the integration of the nursing organization as a partner in strategic planning as well as the development of solutions.
The VHA Blueprint of Excellence envisions organization and collaboration to promote new relationships that serve and benefit veterans. The DNP preparation allows the nurse leader to demonstrate the ability to collaborate with VHA stakeholders and develop alliances within and outside the organization by advocating for policy changes that will be transformational in improving health care delivery and patient outcomes to vulnerable transgender veteran populations. The IOM has tasked nurse executives with creating a health care infrastructure of doctorally prepared nurses to provide patient care that is increasingly growing more complex. With an increasing number of veterans using services, VHA has prioritized an expansion in the number of doctorally prepared nurses.55
As the largest integrated health care system in the U.S., the VHA provides an ideal setting for initiating these organizational changes as a result of having developed an integrated infrastructure to collect evidence-based data at the regional (network) and state facilities and make comparisons with national benchmarks. Therefore, changes are less difficult to disseminate throughout the hierarchy of the VHA. Consequently, the VHA has been a leader in the U.S. for equity in the health care arena and provides a model for international health care systems. Finally, these changes address an urgent need to reduce health disparities, morbidity, and mortality by improving quality care and health care delivery to a vulnerable transgender population.
1. Greiner AC, Knebel E, eds. Health Professions Education: A Bridge to Quality. Washington, DC: National Academies Press; 2003.
2. Institute of Medicine. Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
3. Mintzberg H. The structuring of organizations: a synthesis of the research. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1496182 1979. Posted November 4, 2009. Accessed November 30, 2016.
4. U.S. Department of Veterans Affairs. VHA blue print for excellence. https://www.va.gov/health/docs/VHA_Blueprint_for_Excellence.pdf. Published September 21, 2014. Accessed November 30, 2016.
5. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, part 2):1573-1583.
6. Grant JM, Mottet L, Tanis JE, Harrison J, Herman J, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads /reports/reports/ntds_full.pdf. Published 2011. Accessed November 30, 2016.
7. Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objec tives/topic/lesbian-gay-bisexual-and-transgender -health. Updated November 16, 2016. Accessed November 16, 2016.
8. Institute of Medicine Committee on Lesbian Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
9. U.S. Department of Veterans Affairs. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. Washington, DC: U.S. Department of Veterans Affairs; 2013.
10. Zucker KJ, Lawrence AA. Epidemiology of gender identity disorder: recommendations for the Standards of Care of the World Professional Association for Transgender Health. Int J Transgenderism. 2009;11(1):8-18.
11. Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30(6):807-815.
12. Gates GJ, Herman JL. Transgender military service in the United States. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military -Service-May-2014.pdf. Published May 2014. Accessed November 30, 2016.
13. Flores AR, Brown TNT, and Herman JL. Race and ethnicity of adults who identify as transgender in the United States. http://williamsinstitute.law.ucla .edu/wp-content/uploads/Race-and-Ethnicity-of -Transgender-Identified-Adults-in-the-US.pdf. Published October 2016. Accessed December 13, 2016.
14. Harris BC. Likely Transgender individuals in US federal administrative records and the 2010 census. https://www.census.gov/srd/carra/15_03_Likely_Transgender_Individuals_in_ARs_and_2010Census.pdf. Published May 4, 2015. Accessed November 30, 2016.
15. Kenagy GP, Bostwick WB. Health and social service needs of transgender people in Chicago. Int J Transgenderism. 2005;8(2-3):57-66.
16. Centers for Disease Control and Prevention. National intimate partner and sexual violence survey, 2010 summary report. https://www.cdc.gov/viole nceprevention/pdf/nisvs_report2010-a.pdf. Published November 2011. Accessed December 12, 2016.
17. Bauer GR, Travers R, Scanlon K, Coleman TA. High heterogeneity of HIV-related sexual risk among transgender people in Ontario, Canada: a province-wide respondent-driven sampling survey. BMC Public Health. 2012;12(1):292-291.
18. Devis-Devis J, Pereira-Garcia S, Valencia-Peris A, Fuentes-Miguel J, López-Cañada E, Pérez-Samaniego V. Harassment patterns and risk profile in Spanish trans persons. J Homosex. 2016. [Epub ahead of print.]
19. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many -People-LGBT-Apr-2011.pdf. Published April 2011. Accessed December 1, 2016.
20. Center for Disease Control and Prevention. HIV Among Women. http://www.cdc.gov/hiv/group/gender/women/index.html. Accessed December 10, 2016.
21. Bauer GR, Scheim AI, Pyne J, Travers R, Hammond R. Intervenable factors associated with suicide risk in transgender persons: a respondent driven sampling study in Ontario, Canada. BMC Public Health. 2015;15(1):525.
22. McCann E. People who are transgender: mental health concerns. J Psychiatr Ment Health Nurs. 2015;22(1):76-81.
23. Green R. Transsexual legal rights in the United States and United Kingdom: employment, medical treatment, and civil status. Arch Sex Behav. 2010;39(1):153-160.
24. Sharpe VA, Uchendu US. Ensuring appropriate care for LGBT veterans in the Veterans Health Administration. Hastings Cent Rep. 2014;44(suppl 4):S53-S55.
25. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006-2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
26. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. "Completely out-at-sea" with "two-gender medicine": a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12(1):110.
27. U.S. Department of Health and Human Services. Key features of the affordable care act. http://www .hhs.gov/healthcare/facts-and-features/key-features -of-aca/index.html. Last reviewed November 18, 2014. Accessed December 1, 2016.
28. U.S. Department of Veterans Affairs. Federal benefits for veterans, dependents, and survivors. https://www.va.gov/opa/publications/benefits_book/Chapter_1_Health_Care_Benefits.asp. Accessed December 1, 2016.
29. HealthyPeople.gov. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bi sexual-and-transgender-health. Updated December 1, 2016. Accessed December 1, 2016.
30. Lutwak N, Byne W, Erickson-Schroth L, et al. Transgender veterans are inadequately understood by health care providers. Mil Med. 2014;179(5):483-485.
31. Dorsen C. An integrative review of nurse attitudes towards lesbian, gay, bisexual, and transgender patients. Can J Nurs Res. 2012;44(3):18-43.
32. Eliason MJ, Dibble SL, Robertson PA. Lesbian, gay, bisexual, and transgender (LGBT) physicians' experiences in the workplace. J Homosex. 2011;58(10):1355-1371.
33. Carabez R, Pellegrini M, Mankovitz A, Eliason M, Ciano M, Scott M. "Never in All My Years...": Nurses' education about LGBT health. J Prof Nurs. 2015;31(4):323-329
34. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
35. VA Boston Healthcare System. Patient Care Memorandum-11-046-LM. Management of transgender veteran patients. http://www.boston.va.gov/services/images/lgbt_patient_care_memo_transgender_care.pdf. Published May 2011. Accessed December 1, 2016.
36. Cronenwett L, Sherwood G, Pohl J, et al. Quality and safety education for advanced nursing practice. Nurs Outlook. 2009;57(6):338-348.
37. Institute of Medicine. Committee on the Robert Wood Johnson Foundation Initiative on the Future of Nursing. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: National Academies Press; 2011.
38. Smith EL, Cronenwett L, Sherwood G. Current assessments of quality and safety education in nursing. Nurs Outlook. 2007;55(3):132-137.
39. World Professional Association for Transgender Health (WPATH).The standards of care. http://www.wpath.org/site_page.cfm?pk_association _webpage_menu=1351&pk_association_web page=4655. Accessed December 1, 2016.
40. University of California San Francisco Department of Family and Community Medicine. Center of Excellence for Transgender Health. http://www.tran shealth.ucsf.edu/trans?page=home-00-00 Accessed December 1, 2016.
41. Center for Disease Control and Prevention. Lesbian, gay, bisexual and transgender health. http://www.cdc.gov/lgbthealth/transgender.htm. Accessed December 1, 2016.
42. American Association of Colleges of Nursing. QSEN education consortium: graduate-level QSEN competencies, knowledge, skills and attitudes. http://www.aacn.nche.edu/faculty/qsen/competen cies.pdf. Accessed December 1, 2016.
43. Andrews MM, Boyle JS. Transcultural Concepts in Nursing Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
44. Moran KJ, Burson R, Conrad D. The Doctor of Nursing Practice Scholarly Project: A Framework for Success. Burlington, MA: Jones & Bartlett; 2013.
45. Hanssmann C, Morrison D, Russian E, Shiu-Thornton S, Bowen D. A community-based program evaluation of community competency trainings. J Assoc Nurses AIDS Care.
46. Knapp H, Fletcher M, Taylor A, Chan K, Goetz MB. No clinic left behind: providing cost-effective in-services via distance learning. J Healthc Qual. 2011;33(5):17-24.
47. Kauth MR, Shipherd JC, Lindsay JA, Kirsh S, Knapp H, Matza L. Teleconsultation and training of VHA providers on transgender care: implementation of a multisite hub system. Telemed J E Health. 2015;21(12):1012-1018.
48. Sherman MD, Kauth MR, Ridener L, Shipherd JC, Bratkovich K, Beaulieu G. An empirical investigation of challenges and recommendations for welcoming sexual and gender minority veterans into VA care. Prof Psychol: Res Pract. 2014;45(6):433-442.
49. American Association of Colleges of Nursing. The essentials of doctoral education for advanced nursing practice. http://www.aacn.nche.edu/pub lications/position/DNPEssentials.pdf. Published October 2006. Accessed December 1, 2016.
50. Boutain DM. Social justice as a framework for professional nursing. J Nurs Educ. 2005;44(9):404-408.
51. Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84:22-29.
52. Thornhill L, Klein P. Creating environments of care with transgender communities. J Assoc Nurs AIDS Care. 2010;21(3):230-239.
53. Collins J. Nursing cultural competencies: Improving patient care quality and satisfaction. Ohio Nurses Rev. 2015;90(1):10-11.
54. Sherman MD, Kauth MR, Shipherd JC, Street RL Jr. Communication between VA providers and sexual and gender minority veterans: a pilot study. Psychol Serv. 2014;11(2):235-242.
55. Cowan L, Fasoli DR, Hagle ME, et al. Creating an infrastructure to advance nursing practice and care for veterans. Nurse Leader. 2013;11(5):33-36.
1. Greiner AC, Knebel E, eds. Health Professions Education: A Bridge to Quality. Washington, DC: National Academies Press; 2003.
2. Institute of Medicine. Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
3. Mintzberg H. The structuring of organizations: a synthesis of the research. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1496182 1979. Posted November 4, 2009. Accessed November 30, 2016.
4. U.S. Department of Veterans Affairs. VHA blue print for excellence. https://www.va.gov/health/docs/VHA_Blueprint_for_Excellence.pdf. Published September 21, 2014. Accessed November 30, 2016.
5. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, part 2):1573-1583.
6. Grant JM, Mottet L, Tanis JE, Harrison J, Herman J, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads /reports/reports/ntds_full.pdf. Published 2011. Accessed November 30, 2016.
7. Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objec tives/topic/lesbian-gay-bisexual-and-transgender -health. Updated November 16, 2016. Accessed November 16, 2016.
8. Institute of Medicine Committee on Lesbian Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academy of Sciences; 2011.
9. U.S. Department of Veterans Affairs. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. Washington, DC: U.S. Department of Veterans Affairs; 2013.
10. Zucker KJ, Lawrence AA. Epidemiology of gender identity disorder: recommendations for the Standards of Care of the World Professional Association for Transgender Health. Int J Transgenderism. 2009;11(1):8-18.
11. Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30(6):807-815.
12. Gates GJ, Herman JL. Transgender military service in the United States. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military -Service-May-2014.pdf. Published May 2014. Accessed November 30, 2016.
13. Flores AR, Brown TNT, and Herman JL. Race and ethnicity of adults who identify as transgender in the United States. http://williamsinstitute.law.ucla .edu/wp-content/uploads/Race-and-Ethnicity-of -Transgender-Identified-Adults-in-the-US.pdf. Published October 2016. Accessed December 13, 2016.
14. Harris BC. Likely Transgender individuals in US federal administrative records and the 2010 census. https://www.census.gov/srd/carra/15_03_Likely_Transgender_Individuals_in_ARs_and_2010Census.pdf. Published May 4, 2015. Accessed November 30, 2016.
15. Kenagy GP, Bostwick WB. Health and social service needs of transgender people in Chicago. Int J Transgenderism. 2005;8(2-3):57-66.
16. Centers for Disease Control and Prevention. National intimate partner and sexual violence survey, 2010 summary report. https://www.cdc.gov/viole nceprevention/pdf/nisvs_report2010-a.pdf. Published November 2011. Accessed December 12, 2016.
17. Bauer GR, Travers R, Scanlon K, Coleman TA. High heterogeneity of HIV-related sexual risk among transgender people in Ontario, Canada: a province-wide respondent-driven sampling survey. BMC Public Health. 2012;12(1):292-291.
18. Devis-Devis J, Pereira-Garcia S, Valencia-Peris A, Fuentes-Miguel J, López-Cañada E, Pérez-Samaniego V. Harassment patterns and risk profile in Spanish trans persons. J Homosex. 2016. [Epub ahead of print.]
19. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many -People-LGBT-Apr-2011.pdf. Published April 2011. Accessed December 1, 2016.
20. Center for Disease Control and Prevention. HIV Among Women. http://www.cdc.gov/hiv/group/gender/women/index.html. Accessed December 10, 2016.
21. Bauer GR, Scheim AI, Pyne J, Travers R, Hammond R. Intervenable factors associated with suicide risk in transgender persons: a respondent driven sampling study in Ontario, Canada. BMC Public Health. 2015;15(1):525.
22. McCann E. People who are transgender: mental health concerns. J Psychiatr Ment Health Nurs. 2015;22(1):76-81.
23. Green R. Transsexual legal rights in the United States and United Kingdom: employment, medical treatment, and civil status. Arch Sex Behav. 2010;39(1):153-160.
24. Sharpe VA, Uchendu US. Ensuring appropriate care for LGBT veterans in the Veterans Health Administration. Hastings Cent Rep. 2014;44(suppl 4):S53-S55.
25. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006-2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
26. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. "Completely out-at-sea" with "two-gender medicine": a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12(1):110.
27. U.S. Department of Health and Human Services. Key features of the affordable care act. http://www .hhs.gov/healthcare/facts-and-features/key-features -of-aca/index.html. Last reviewed November 18, 2014. Accessed December 1, 2016.
28. U.S. Department of Veterans Affairs. Federal benefits for veterans, dependents, and survivors. https://www.va.gov/opa/publications/benefits_book/Chapter_1_Health_Care_Benefits.asp. Accessed December 1, 2016.
29. HealthyPeople.gov. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bi sexual-and-transgender-health. Updated December 1, 2016. Accessed December 1, 2016.
30. Lutwak N, Byne W, Erickson-Schroth L, et al. Transgender veterans are inadequately understood by health care providers. Mil Med. 2014;179(5):483-485.
31. Dorsen C. An integrative review of nurse attitudes towards lesbian, gay, bisexual, and transgender patients. Can J Nurs Res. 2012;44(3):18-43.
32. Eliason MJ, Dibble SL, Robertson PA. Lesbian, gay, bisexual, and transgender (LGBT) physicians' experiences in the workplace. J Homosex. 2011;58(10):1355-1371.
33. Carabez R, Pellegrini M, Mankovitz A, Eliason M, Ciano M, Scott M. "Never in All My Years...": Nurses' education about LGBT health. J Prof Nurs. 2015;31(4):323-329
34. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
35. VA Boston Healthcare System. Patient Care Memorandum-11-046-LM. Management of transgender veteran patients. http://www.boston.va.gov/services/images/lgbt_patient_care_memo_transgender_care.pdf. Published May 2011. Accessed December 1, 2016.
36. Cronenwett L, Sherwood G, Pohl J, et al. Quality and safety education for advanced nursing practice. Nurs Outlook. 2009;57(6):338-348.
37. Institute of Medicine. Committee on the Robert Wood Johnson Foundation Initiative on the Future of Nursing. The Future of Nursing: Leading Change, Advancing Health. Washington, DC: National Academies Press; 2011.
38. Smith EL, Cronenwett L, Sherwood G. Current assessments of quality and safety education in nursing. Nurs Outlook. 2007;55(3):132-137.
39. World Professional Association for Transgender Health (WPATH).The standards of care. http://www.wpath.org/site_page.cfm?pk_association _webpage_menu=1351&pk_association_web page=4655. Accessed December 1, 2016.
40. University of California San Francisco Department of Family and Community Medicine. Center of Excellence for Transgender Health. http://www.tran shealth.ucsf.edu/trans?page=home-00-00 Accessed December 1, 2016.
41. Center for Disease Control and Prevention. Lesbian, gay, bisexual and transgender health. http://www.cdc.gov/lgbthealth/transgender.htm. Accessed December 1, 2016.
42. American Association of Colleges of Nursing. QSEN education consortium: graduate-level QSEN competencies, knowledge, skills and attitudes. http://www.aacn.nche.edu/faculty/qsen/competen cies.pdf. Accessed December 1, 2016.
43. Andrews MM, Boyle JS. Transcultural Concepts in Nursing Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
44. Moran KJ, Burson R, Conrad D. The Doctor of Nursing Practice Scholarly Project: A Framework for Success. Burlington, MA: Jones & Bartlett; 2013.
45. Hanssmann C, Morrison D, Russian E, Shiu-Thornton S, Bowen D. A community-based program evaluation of community competency trainings. J Assoc Nurses AIDS Care.
46. Knapp H, Fletcher M, Taylor A, Chan K, Goetz MB. No clinic left behind: providing cost-effective in-services via distance learning. J Healthc Qual. 2011;33(5):17-24.
47. Kauth MR, Shipherd JC, Lindsay JA, Kirsh S, Knapp H, Matza L. Teleconsultation and training of VHA providers on transgender care: implementation of a multisite hub system. Telemed J E Health. 2015;21(12):1012-1018.
48. Sherman MD, Kauth MR, Ridener L, Shipherd JC, Bratkovich K, Beaulieu G. An empirical investigation of challenges and recommendations for welcoming sexual and gender minority veterans into VA care. Prof Psychol: Res Pract. 2014;45(6):433-442.
49. American Association of Colleges of Nursing. The essentials of doctoral education for advanced nursing practice. http://www.aacn.nche.edu/pub lications/position/DNPEssentials.pdf. Published October 2006. Accessed December 1, 2016.
50. Boutain DM. Social justice as a framework for professional nursing. J Nurs Educ. 2005;44(9):404-408.
51. Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84:22-29.
52. Thornhill L, Klein P. Creating environments of care with transgender communities. J Assoc Nurs AIDS Care. 2010;21(3):230-239.
53. Collins J. Nursing cultural competencies: Improving patient care quality and satisfaction. Ohio Nurses Rev. 2015;90(1):10-11.
54. Sherman MD, Kauth MR, Shipherd JC, Street RL Jr. Communication between VA providers and sexual and gender minority veterans: a pilot study. Psychol Serv. 2014;11(2):235-242.
55. Cowan L, Fasoli DR, Hagle ME, et al. Creating an infrastructure to advance nursing practice and care for veterans. Nurse Leader. 2013;11(5):33-36.
Oral Rehydration Therapy for KidsA More Palatable Alternative

A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.

Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.

A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.

Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.

A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.

Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
Silver
Silver is a naturally occurring chemical element (number 47 on the periodic table) with the chemical symbol Ag, which is derived from the Latin word argentum (árgyros in Greek), from the Indo-European root “arg-,” meaning “shining,” “white,” or “gray.” It has been used for medical purposes since ancient times, with Hippocrates (circa 460-370 BCE) noting the beneficial healing and disease-altering activity of the element.1
In modern times, silver compounds – in metallic, nanocrystalline, and ionic formulations – have exhibited broad antibacterial activity and attracted interest for topical antiseptic use in wound dressings.2 Nanocrystalline silver dressings were introduced commercially as antimicrobial dressings in 1998.3 Silver is now used in dressings, catheters, cleansers, ophthalmic ointments, and myriad other medical products. In 2010 alone, an estimated 15 metric tons of silver were incorporated into medical products worldwide.4 It also is included in personal care products, textiles, and water purification devices. In topical skin preparations, the noble metal is included as colloidal silver (suspension of silver particles in an aqueous base) or nanosilver (as nanoparticles ranging from 1 nm to 100 nm in at least one dimension).5 Silver has been used to treat burns and wounds, but this discussion will be limited to acne, atopic dermatitis, and the anti-inflammatory response.
Anti-inflammatory uses
For several decades, noble metals including silver have been known to exert anti-inflammatory activity.7-11 In the case of silver, its anti-inflammatory properties appear to be mediated by its influence on the cytokine system. Silver nanoparticles inhibit the activity of interleukin-6 (IL-6), IL-12, IL-1beta, and tumor necrosis factor–alpha (TNF-alpha). This impact on the cytokine system is responsible for the impact of silver in demonstrably alleviating symptoms of rheumatoid arthritis.3
In 2004, Bhol et al. used dinitrochlorobenzene (DNCB) to induce allergic contact dermatitis in a guinea pig model, finding that topical nanocrystalline silver cream dose-dependently decreased erythema and as effectively as topical steroids and immunosuppressants.12 The next year, Bhol and Schechter showed that nanocrystalline silver suppressed allergic contact dermatitis in mice, inhibited TNF-alpha and IL-12 expression, and induced inflammatory cell apoptosis.13
In 2008, Nadworny et al. used a porcine contact dermatitis model to investigate the anti-inflammatory activity of nanocrystalline silver. They found that nanocrystalline silver treatments reduced DNCB-induced erythema and edema, promoted apoptosis in dermal cells, and diminished matrix metalloproteinase (MMP) and proinflammatory cytokine expression.3 The investigators speculated that the lower TNF-alpha observed in the silver-treated animals occurred due to apoptosis of the inflammatory cells.
Acne
Silver acts as a bactericidal and anti-inflammatory agent, without generating free radicals, as seen with benzoyl peroxide. Therefore, it is a compelling option for responding to the presence of Propionibacterium acnes. However, silver has not been approved by the Food and Drug Administration for this use. Even though formal acne studies have not been performed with silver sulfadiazine, it has long been used “off-label” for this purpose. As suggested above, the use of silver sulfadiazine for acne is limited by the risk of sulfa allergy. Cosmetic appearance and ease of use also are limiting factors, as silver sulfadiazine preparations are characterized by a thick, white pasty consistency. Other options for use of silver to treat acne include silver-containing cleansers and textiles.
Atopic dermatitis
A 2006 study in patients with atopic dermatitis demonstrated that silver-coated textiles could significantly diminish Staphylococcus aureus density after 2 days of wearing, with the effect enduring through the end of 7 days of treatment and then 1 week after removal of the textiles.14 Within 2 weeks, objective and subjective symptoms of atopic dermatitis were significantly enhanced in association with the silver-coated textiles, compared with cotton, without measurable adverse effects. A technology called Padycare incorporates silver into micromesh material (82% polyamide, 18% Lycra) used in clothing and bedding.15 As compared with topical formulations applied directly to the skin, textiles confer certain advantages such as preventing scratching and protecting against irritating substances and allergens. Washing of silver-infused textiles is a possible disadvantage, though, as the amount of silver lost from textiles can range from a 100% loss after four washings to less than a 1% loss.16 It also is important to note that there are concerns regarding the potential of silver to leak from textiles into the water supply, and eradicating the beneficial bacteria used to treat the water.
Conclusion
Despite centuries of medical use, silver has not been approved by the FDA for any medical applications. Further study, particularly in terms of safety and efficacy, is necessary. Nevertheless, it is used off-label before and after minimally invasive dermatologic procedures (for example, dermal filling, botulinum toxin injections, chemical peeling) because of its antimicrobial and anti-inflammatory activities as well as soothing qualities for facial skin and the skin barrier. Silver appears to be particularly suitable for use as an acne therapy option due to the low risk of bacterial resistance, lack of irritation, and its preservation of the skin barrier unlike harsher options such as retinoids, antibiotics, and benzoyl peroxide.
References
1. Adv Skin Wound Care. 2006 Nov-Dec;19(9):472-4.
2. Clin Infect Dis. 2009 Nov 15;49(10):1541-9.
3. Nanomedicine. 2008 Sep;4(3):241-51.
4. J Antimicrob Chemother. 2013 Jan;68(1):131-8.
5. Nanocrystalline Silver: Use in wound care, in Current Advances in the Medical Application of Nanotechnology (Manchester, England: Bentham Books, 2012, pp. 25-31).
6. Nanomedicine. 2013 Jan;9(1):39-54.
7. Jpn J Pharmacol. 1965 Jun;15(2):131-4.
8. J Allergy Clin Immunol. 1995 Aug;96(2):251-6.
9. Inflamm Res. 2003 Dec;52(12):487-501.
10. J Nutr Environ Med. 1997;7(4):295-305.
11. Clin Exp Pharmacol Physiol. 2000 Mar;27(3):139-44.
12. Clin Exp Dermatol. 2004 May;29(3):282-7.
13. Br J Dermatol. 2005 Jun;152(6):1235-42.
14. Curr Probl Dermatol. 2006;33:152-64.
15. J Eur Acad Dermatol Venereol. 2006 May;20(5):534-41.
16. Environ Sci Technol. 2008 Jun 1;42(11):4133-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis, Neutrogena, Philosophy, Topix, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Silver is a naturally occurring chemical element (number 47 on the periodic table) with the chemical symbol Ag, which is derived from the Latin word argentum (árgyros in Greek), from the Indo-European root “arg-,” meaning “shining,” “white,” or “gray.” It has been used for medical purposes since ancient times, with Hippocrates (circa 460-370 BCE) noting the beneficial healing and disease-altering activity of the element.1
In modern times, silver compounds – in metallic, nanocrystalline, and ionic formulations – have exhibited broad antibacterial activity and attracted interest for topical antiseptic use in wound dressings.2 Nanocrystalline silver dressings were introduced commercially as antimicrobial dressings in 1998.3 Silver is now used in dressings, catheters, cleansers, ophthalmic ointments, and myriad other medical products. In 2010 alone, an estimated 15 metric tons of silver were incorporated into medical products worldwide.4 It also is included in personal care products, textiles, and water purification devices. In topical skin preparations, the noble metal is included as colloidal silver (suspension of silver particles in an aqueous base) or nanosilver (as nanoparticles ranging from 1 nm to 100 nm in at least one dimension).5 Silver has been used to treat burns and wounds, but this discussion will be limited to acne, atopic dermatitis, and the anti-inflammatory response.
Anti-inflammatory uses
For several decades, noble metals including silver have been known to exert anti-inflammatory activity.7-11 In the case of silver, its anti-inflammatory properties appear to be mediated by its influence on the cytokine system. Silver nanoparticles inhibit the activity of interleukin-6 (IL-6), IL-12, IL-1beta, and tumor necrosis factor–alpha (TNF-alpha). This impact on the cytokine system is responsible for the impact of silver in demonstrably alleviating symptoms of rheumatoid arthritis.3
In 2004, Bhol et al. used dinitrochlorobenzene (DNCB) to induce allergic contact dermatitis in a guinea pig model, finding that topical nanocrystalline silver cream dose-dependently decreased erythema and as effectively as topical steroids and immunosuppressants.12 The next year, Bhol and Schechter showed that nanocrystalline silver suppressed allergic contact dermatitis in mice, inhibited TNF-alpha and IL-12 expression, and induced inflammatory cell apoptosis.13
In 2008, Nadworny et al. used a porcine contact dermatitis model to investigate the anti-inflammatory activity of nanocrystalline silver. They found that nanocrystalline silver treatments reduced DNCB-induced erythema and edema, promoted apoptosis in dermal cells, and diminished matrix metalloproteinase (MMP) and proinflammatory cytokine expression.3 The investigators speculated that the lower TNF-alpha observed in the silver-treated animals occurred due to apoptosis of the inflammatory cells.
Acne
Silver acts as a bactericidal and anti-inflammatory agent, without generating free radicals, as seen with benzoyl peroxide. Therefore, it is a compelling option for responding to the presence of Propionibacterium acnes. However, silver has not been approved by the Food and Drug Administration for this use. Even though formal acne studies have not been performed with silver sulfadiazine, it has long been used “off-label” for this purpose. As suggested above, the use of silver sulfadiazine for acne is limited by the risk of sulfa allergy. Cosmetic appearance and ease of use also are limiting factors, as silver sulfadiazine preparations are characterized by a thick, white pasty consistency. Other options for use of silver to treat acne include silver-containing cleansers and textiles.
Atopic dermatitis
A 2006 study in patients with atopic dermatitis demonstrated that silver-coated textiles could significantly diminish Staphylococcus aureus density after 2 days of wearing, with the effect enduring through the end of 7 days of treatment and then 1 week after removal of the textiles.14 Within 2 weeks, objective and subjective symptoms of atopic dermatitis were significantly enhanced in association with the silver-coated textiles, compared with cotton, without measurable adverse effects. A technology called Padycare incorporates silver into micromesh material (82% polyamide, 18% Lycra) used in clothing and bedding.15 As compared with topical formulations applied directly to the skin, textiles confer certain advantages such as preventing scratching and protecting against irritating substances and allergens. Washing of silver-infused textiles is a possible disadvantage, though, as the amount of silver lost from textiles can range from a 100% loss after four washings to less than a 1% loss.16 It also is important to note that there are concerns regarding the potential of silver to leak from textiles into the water supply, and eradicating the beneficial bacteria used to treat the water.
Conclusion
Despite centuries of medical use, silver has not been approved by the FDA for any medical applications. Further study, particularly in terms of safety and efficacy, is necessary. Nevertheless, it is used off-label before and after minimally invasive dermatologic procedures (for example, dermal filling, botulinum toxin injections, chemical peeling) because of its antimicrobial and anti-inflammatory activities as well as soothing qualities for facial skin and the skin barrier. Silver appears to be particularly suitable for use as an acne therapy option due to the low risk of bacterial resistance, lack of irritation, and its preservation of the skin barrier unlike harsher options such as retinoids, antibiotics, and benzoyl peroxide.
References
1. Adv Skin Wound Care. 2006 Nov-Dec;19(9):472-4.
2. Clin Infect Dis. 2009 Nov 15;49(10):1541-9.
3. Nanomedicine. 2008 Sep;4(3):241-51.
4. J Antimicrob Chemother. 2013 Jan;68(1):131-8.
5. Nanocrystalline Silver: Use in wound care, in Current Advances in the Medical Application of Nanotechnology (Manchester, England: Bentham Books, 2012, pp. 25-31).
6. Nanomedicine. 2013 Jan;9(1):39-54.
7. Jpn J Pharmacol. 1965 Jun;15(2):131-4.
8. J Allergy Clin Immunol. 1995 Aug;96(2):251-6.
9. Inflamm Res. 2003 Dec;52(12):487-501.
10. J Nutr Environ Med. 1997;7(4):295-305.
11. Clin Exp Pharmacol Physiol. 2000 Mar;27(3):139-44.
12. Clin Exp Dermatol. 2004 May;29(3):282-7.
13. Br J Dermatol. 2005 Jun;152(6):1235-42.
14. Curr Probl Dermatol. 2006;33:152-64.
15. J Eur Acad Dermatol Venereol. 2006 May;20(5):534-41.
16. Environ Sci Technol. 2008 Jun 1;42(11):4133-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis, Neutrogena, Philosophy, Topix, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Silver is a naturally occurring chemical element (number 47 on the periodic table) with the chemical symbol Ag, which is derived from the Latin word argentum (árgyros in Greek), from the Indo-European root “arg-,” meaning “shining,” “white,” or “gray.” It has been used for medical purposes since ancient times, with Hippocrates (circa 460-370 BCE) noting the beneficial healing and disease-altering activity of the element.1
In modern times, silver compounds – in metallic, nanocrystalline, and ionic formulations – have exhibited broad antibacterial activity and attracted interest for topical antiseptic use in wound dressings.2 Nanocrystalline silver dressings were introduced commercially as antimicrobial dressings in 1998.3 Silver is now used in dressings, catheters, cleansers, ophthalmic ointments, and myriad other medical products. In 2010 alone, an estimated 15 metric tons of silver were incorporated into medical products worldwide.4 It also is included in personal care products, textiles, and water purification devices. In topical skin preparations, the noble metal is included as colloidal silver (suspension of silver particles in an aqueous base) or nanosilver (as nanoparticles ranging from 1 nm to 100 nm in at least one dimension).5 Silver has been used to treat burns and wounds, but this discussion will be limited to acne, atopic dermatitis, and the anti-inflammatory response.
Anti-inflammatory uses
For several decades, noble metals including silver have been known to exert anti-inflammatory activity.7-11 In the case of silver, its anti-inflammatory properties appear to be mediated by its influence on the cytokine system. Silver nanoparticles inhibit the activity of interleukin-6 (IL-6), IL-12, IL-1beta, and tumor necrosis factor–alpha (TNF-alpha). This impact on the cytokine system is responsible for the impact of silver in demonstrably alleviating symptoms of rheumatoid arthritis.3
In 2004, Bhol et al. used dinitrochlorobenzene (DNCB) to induce allergic contact dermatitis in a guinea pig model, finding that topical nanocrystalline silver cream dose-dependently decreased erythema and as effectively as topical steroids and immunosuppressants.12 The next year, Bhol and Schechter showed that nanocrystalline silver suppressed allergic contact dermatitis in mice, inhibited TNF-alpha and IL-12 expression, and induced inflammatory cell apoptosis.13
In 2008, Nadworny et al. used a porcine contact dermatitis model to investigate the anti-inflammatory activity of nanocrystalline silver. They found that nanocrystalline silver treatments reduced DNCB-induced erythema and edema, promoted apoptosis in dermal cells, and diminished matrix metalloproteinase (MMP) and proinflammatory cytokine expression.3 The investigators speculated that the lower TNF-alpha observed in the silver-treated animals occurred due to apoptosis of the inflammatory cells.
Acne
Silver acts as a bactericidal and anti-inflammatory agent, without generating free radicals, as seen with benzoyl peroxide. Therefore, it is a compelling option for responding to the presence of Propionibacterium acnes. However, silver has not been approved by the Food and Drug Administration for this use. Even though formal acne studies have not been performed with silver sulfadiazine, it has long been used “off-label” for this purpose. As suggested above, the use of silver sulfadiazine for acne is limited by the risk of sulfa allergy. Cosmetic appearance and ease of use also are limiting factors, as silver sulfadiazine preparations are characterized by a thick, white pasty consistency. Other options for use of silver to treat acne include silver-containing cleansers and textiles.
Atopic dermatitis
A 2006 study in patients with atopic dermatitis demonstrated that silver-coated textiles could significantly diminish Staphylococcus aureus density after 2 days of wearing, with the effect enduring through the end of 7 days of treatment and then 1 week after removal of the textiles.14 Within 2 weeks, objective and subjective symptoms of atopic dermatitis were significantly enhanced in association with the silver-coated textiles, compared with cotton, without measurable adverse effects. A technology called Padycare incorporates silver into micromesh material (82% polyamide, 18% Lycra) used in clothing and bedding.15 As compared with topical formulations applied directly to the skin, textiles confer certain advantages such as preventing scratching and protecting against irritating substances and allergens. Washing of silver-infused textiles is a possible disadvantage, though, as the amount of silver lost from textiles can range from a 100% loss after four washings to less than a 1% loss.16 It also is important to note that there are concerns regarding the potential of silver to leak from textiles into the water supply, and eradicating the beneficial bacteria used to treat the water.
Conclusion
Despite centuries of medical use, silver has not been approved by the FDA for any medical applications. Further study, particularly in terms of safety and efficacy, is necessary. Nevertheless, it is used off-label before and after minimally invasive dermatologic procedures (for example, dermal filling, botulinum toxin injections, chemical peeling) because of its antimicrobial and anti-inflammatory activities as well as soothing qualities for facial skin and the skin barrier. Silver appears to be particularly suitable for use as an acne therapy option due to the low risk of bacterial resistance, lack of irritation, and its preservation of the skin barrier unlike harsher options such as retinoids, antibiotics, and benzoyl peroxide.
References
1. Adv Skin Wound Care. 2006 Nov-Dec;19(9):472-4.
2. Clin Infect Dis. 2009 Nov 15;49(10):1541-9.
3. Nanomedicine. 2008 Sep;4(3):241-51.
4. J Antimicrob Chemother. 2013 Jan;68(1):131-8.
5. Nanocrystalline Silver: Use in wound care, in Current Advances in the Medical Application of Nanotechnology (Manchester, England: Bentham Books, 2012, pp. 25-31).
6. Nanomedicine. 2013 Jan;9(1):39-54.
7. Jpn J Pharmacol. 1965 Jun;15(2):131-4.
8. J Allergy Clin Immunol. 1995 Aug;96(2):251-6.
9. Inflamm Res. 2003 Dec;52(12):487-501.
10. J Nutr Environ Med. 1997;7(4):295-305.
11. Clin Exp Pharmacol Physiol. 2000 Mar;27(3):139-44.
12. Clin Exp Dermatol. 2004 May;29(3):282-7.
13. Br J Dermatol. 2005 Jun;152(6):1235-42.
14. Curr Probl Dermatol. 2006;33:152-64.
15. J Eur Acad Dermatol Venereol. 2006 May;20(5):534-41.
16. Environ Sci Technol. 2008 Jun 1;42(11):4133-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis, Neutrogena, Philosophy, Topix, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.