User login
Discharges Against Medical Advice
Patients leave the hospital against medical advice (AMA) for a variety of reasons. The AMA rate is approximately 1% nationally but substantially higher at safety-net hospitals and has rapidly increased over the past decade.1-5 The principle that patients have the right to make choices about their healthcare, up to and including whether to leave the hospital against the advice of medical staff, is well-established law and a foundation of medical ethics.6 In practice, however, AMA discharges are often emotionally charged for both patients and providers, and, in the high-stress setting of AMA discharge, providers may be confused about their roles.7-9
The demographics of patients who leave AMA have been well described. Compared with conventionally discharged patients, AMA patients are younger, more likely to be male, and more likely a marginalized ethnic or racial minority.10-14 Patients with mental illnesses and addiction issues are overrepresented in AMA discharges, and complicated capacity assessments and limited resources may strain providers.7,8,15,16 Studies have repeatedly shown higher rates of readmission and mortality for AMA patients than for conventionally discharged patients.17-21 Whether AMA discharge is a marker for other prognostic factors that bode poorly for patients or contributes to negative outcomes, data suggest this group of patients is vulnerable, having mortality rates up to 40% higher 1 year after discharge, relative to conventionally discharged patients.12
Several models of standardized best practice approaches for AMA have been proposed by bioethicists.6,22,23 Although details of these approaches vary, all involve assessing the patient’s decision-making capacity, clarifying the risks of AMA discharge, addressing factors that might be prompting the discharge, formulating an alternative outpatient treatment plan or “next best” option, and documenting extensively. A recent study found patients often gave advance warning of an AMA discharge, but physicians rarely prepared by arranging follow-up care.8 The investigators hypothesized that providers might not have known what they were permitted to arrange for AMA patients, or might have thought that providing “second best” options went against their principles. The investigators noted that nurses might have become aware of AMA risk sooner than physicians did but could not act on this awareness by preparing medications and arranging follow-up.
Translating models of best practice care for AMA patients into clinical practice requires buy-in from bedside providers, not just bioethicists. Given the study findings that providers have misconceptions about their roles in the AMA discharge,7 it is prudent to investigate providers’ current practices, beliefs, and concerns about AMA discharges before introducing a new approach.
The present authors conducted a mixed-methods cross-sectional study of the state of AMA discharges at Highland Hospital (Oakland, California), a 236-bed county hospital and trauma center serving a primarily underserved urban patient population. The aim of this study was to assess current provider practices for AMA discharges and provider perceptions and knowledge about AMA discharges, ultimately to help direct future educational interventions with medical providers or hospital policy changes needed to improve the quality of AMA discharges.
METHODS
Phase 1 of this study involved identifying AMA patients through a review of data from Highland Hospital’s electronic medical records for 2014. These data included discharge status (eg, AMA vs other discharge types). The hospital’s floor clerk distinguishes between absent without official leave (AWOL; the patient leaves without notifying a provider) and AMA discharge. Discharges designated AWOL were excluded from the analyses.
In phase 2, a structured chart review (Appendix A) was performed for all patients identified during phase 1 as being discharged AMA in 2014. In these reviews, further assessment was made of patient and visit characteristics in hospitalizations that ended in AMA discharge, and of providers’ documentation of AMA discharges—that is, whether several factors were documented (capacity; predischarge indication that patient might leave AMA; reason for AMA; and indications that discharge medications, transportation, and follow-up were arranged). These visit factors were reviewed because the literature has identified them as being important markers for AMA discharge safety.6,8 Two research assistants, under the guidance of Dr. Stearns, reviewed the charts. To ensure agreement across chart reviews with respect to subjective questions (eg, whether capacity was adequately documented), the group reviewed the first 10 consecutive charts together; there was full agreement on how to classify the data of interest. Throughout the study, whenever a research assistant asked how to classify particular patient data, Dr. Stearns reviewed the data, and the research team made a decision together. Additional data, for AMA patients and for all patients admitted to Highland Hospital, were obtained from the hospital’s data warehouse, which pools data from within the health system.
Phase 3 involved surveying healthcare providers who were involved in patient care on the internal medicine and trauma surgery services at the hospital. These providers were selected because chart review revealed that the vast majority of patients who left AMA in 2014 were on one of these services. Surveys (Appendix B) asked participant providers to identify their role at the hospital, to provide a self-assessment of competence in various aspects of AMA discharge, to voice opinions about provider responsibilities in arranging follow-up for AMA patients, and to make suggestions about the AMA process. The authors designed these surveys, which included questions about aspects of care that have been highlighted in the AMA discharge literature as being important for AMA discharge safety.6,8,22,23 Surveys were distributed to providers at internal medicine and trauma surgery department meetings and nursing conferences. Data (without identifying information) were analyzed, and survey responses kept anonymous.
The Alameda Health System Institutional Review Board approved this project. Providers were given the option of writing their name and contact information at the top of the survey in order to be entered into a drawing to receive a prize for completion.
We performed statistical analyses of the patient charts and physician survey data using Stata (version 14.0, Stata Corp., College Station, Texas). We analyzed both patient- and encounter-level data. In demographic analyses, this approach prevented duplicate counting of patients who left AMA multiple times. Patient-level analyses compared the demographic characteristics of AMA patients and patients discharged conventionally from the hospital in 2014. In addition, patients with either 1 or multiple AMA discharges were compared to identify characteristics that might be linked to highest risk of recurrent AMA discharge in the hope that early identification of these patients might facilitate providers’ early awareness and preparation for follow-up care or hospitalization alternatives. We used ANOVAs for continuous variables and tests of proportions for categorical variables. On the encounter level, analyses examined data about each admission (eg, AMA forms signed, follow-up arrangements made, capacity documented, etc.) for all AMA discharges. We employed chi square tests to identify variations in healthcare provider survey responses. A P value < 0.05 was used as the significance cut-off point.
Staged logistic regression analyses, adjusted for demographic characteristics, were performed to assess the association between risk of leaving AMA (yes or no) and demographic characteristics and the association between risk of leaving AMA more than once (yes or no) and health-related characteristics.
RESULTS
Demographic, Clinical, and Utilization Characteristics
Of the 12,036 Highland Hospital admissions in 2014, 319 (2.7%) ended with an AMA discharge. Of the 8207 individual patients discharged, 268 left AMA once, and 29 left AMA multiple times. Further review of the Admissions, Discharges, and Transfers Report generated from the electronic medical record revealed that 15 AWOL discharges were misclassified as AMA discharges.
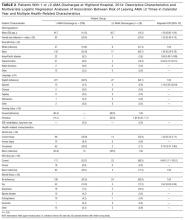
Compared with patients discharged conventionally, AMA patients were significantly younger; more likely to be male, to self-identify as Black/African American, and to be English-speaking; and less likely to self-identify as Asian/Pacific Islander or Hispanic/Latino or to be Chinese- or Spanish-speaking (Table 1). They were also more likely than all patients admitted to Highland to be homeless (15.7% vs 8.7%; P < 0.01). Multivariate regression analysis revealed persistent age and sex disparities, but racial disparities were mitigated in adjusted analyses (Appendix C). Language disparities persisted only for Spanish speakers, who had a significantly lower rate of AMA discharge, even in adjusted analyses.

The majority of AMA patients were on the internal medicine service (63.5%) or the trauma surgery service (24.8%). Regarding admission diagnosis, 17.2% of AMA patients were admitted for infections, 5.0% for drug or alcohol intoxication or withdrawal, 38.9% for acute noninfectious illnesses, 16.7% for decompensation of chronic disease, 18.4% for injuries or trauma, and 3.8% for pregnancy complications or labor. Compared with patients who left AMA once, patients who left AMA multiple times had higher rates of heavy alcohol use (53.9% vs 30.9%; P = 0.01) and illicit drug use (88.5% vs 53.7%; P < 0.001) (Table 2). In multivariate analyses, the increased odds of leaving AMA more than once persisted for current heavy illicit drug users compared with patients who had never engaged in illicit drug use.
Discharge Characteristics and Documentation
Providers documented a patient’s plan to leave AMA before actual discharge 17.3% of the time. The documented plan to leave had to indicate that the patient was actually considering leaving. For example, “Patient is eager to go home” was not enough to qualify as a plan, but “Patient is thinking of leaving” qualified. For 84.3% of AMA discharges, the hospital’s AMA form was signed and was included in the medical record. Documentation showed that medications were prescribed for AMA patients 21.4% of the time, follow-up was arranged 25.7% of the time, and follow-up was pending arrangement 14.8% of the time. The majority of AMA patients (71.4%) left during daytime hours. In 29.6% of AMA discharges, providers documented AMA patients had decision-making capacity.
Readmission After AMA Discharge
Of the 268 AMA patients, 67.7% were not readmitted within the 6 months after AMA, 24.5% had 1 or 2 readmissions, and the rest had 3 or more readmissions (1 patient had 15). In addition, 35.8% returned to the emergency department within 30 days, and 16.4% were readmitted within 30 days. In 2014, the hospital’s overall 30-day readmission rate was 10.8%. Of the patients readmitted within 6 months after AMA, 23.5% left AMA again at the next visit, 9.4% left AWOL, and 67.1% were discharged conventionally.
Drivers of Premature Discharge
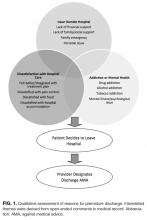
Qualitative analysis of the 35.5% of patient charts documenting a reason for leaving the hospital revealed 3 broad, interrelated themes (Figure 1). The first theme, dissatisfaction with hospital care, included chart notations such as “His wife couldn’t sleep in the hospital room” and “Not satisfied with all-liquid diet.” The second theme, urgent personal issues, included comments such as “He has a very important court date for his children” and “He needed to take care of immigration forms.” The third theme, mental health and substance abuse issues, included notations such as “He wants to go smoke” and “Severe anxiety and prison flashbacks.”
Provider Self-Assessment and Beliefs
The survey was completed by 178 healthcare providers: 49.4% registered nurses, 19.1% trainee physicians, 20.8% attending physicians, and 10.7% other providers, including chaplains, social workers, and clerks. Regarding self-assessment of competency in AMA discharges, 94% of providers agreed they were comfortable assessing capacity, and 94% agreed they were comfortable talking with patients about the risks of leaving AMA (Figure 2). Nurses were more likely than trainee physicians to agree they knew what to do for patients who lacked capacity (74% vs 49%; P = 0.02). Most providers (70%) agreed they usually knew why their patients were leaving AMA; in this self-assessment, there were no significant differences between types of providers.
Regarding follow-up, attending physicians and trainee physicians demonstrated more agreement than nurses that AMA patients should receive medications and follow-up (94% and 84% vs 64%; P < 0.05). Nurses were more likely than attending physicians to say patients should lose their rights to hospital follow-up because of leaving AMA (38% vs 6%; P < 0.01). A minority of providers (37%) agreed transportation should be arranged. Addiction was the most common driver of AMA discharge (35%), followed by familial obligations (19%), dissatisfaction with hospital care (16%), and financial concerns (15%).
DISCUSSION
The demographic characteristics of AMA patients in this study are similar to those identified in other studies, showing overrepresentation of young male patients.12,14 Homeless patients were also overrepresented in the AMA discharge population at Highland Hospital—a finding that has not been consistently reported in prior studies, and that warrants further examination. In adjusted analyses, Spanish speakers had a lower rate of AMA discharge, and there were no racial variations. This is consistent with another study’s finding: that racial disparities in AMA discharge rates were largely attributable to confounders.24 Language differences may result from failure of staff to fully explain the option of AMA discharge to non-English speakers, or from fear of immigration consequences after AMA discharge. Further investigation of patient experiences is needed to identify factors that contribute to demographic variations in AMA discharge rates.25,26
Of the patients who left AMA multiple times, nearly all were actively using illicit drugs. In a recent study conducted at a safety-net hospital in Vancouver, Canada, 43% of patients with illicit drug use and at least 1 hospitalization left AMA at least once during the 6-year study period.11 Many factors might explain this correlation—addiction itself, poor pain control for patients with addiction issues, fears about incarceration, and poor treatment of drug users by healthcare staff.15 Although the medical literature highlights deficits in pain control for patients addicted to opiates, proposed solutions are sparse and focus on perioperative pain control and physician prescribing practices.27,28 At safety-net hospitals in which addiction is a factor in many hospitalizations, there is opportunity for new research in inpatient pain control for patients with substance dependence. In addition, harm reduction strategies—such as methadone maintenance for hospitalized patients with opiate dependence and abscess clinics as hospitalization alternatives for injection-associated infection treatment—may be key in improving safety for patients.11,15,29
Comparing the provider survey and chart review results highlights discordance between provider beliefs and clinical practice. Healthcare providers at Highland Hospital considered themselves competent in assessing capacity and talking with patients about the risks of AMA discharge. In practice, however, capacity was documented in less than a third of AMA discharges. Although the majority of providers thought medications and follow-up should be arranged for patients, arrangements were seldom made. This may be partially attributable to limited resources for making these arrangements. Average time to “third next available” primary care appointment within the county health system that includes Highland was 44.6 days for established patients during the period of study; for new primary care patients, the average wait for an appointment was 2 to 3 months. Highland has a same-day clinic, but inpatient providers are discouraged from using it as a postdischarge clinic for patients who would be better served in primary care. Medications and transportation are easily arranged during daytime hours but are not immediately available at night. In addition, some of this discrepancy may be attributable to the limited documentation rather than to provider failure to achieve their own benchmarks of quality care for AMA patients.
Documentation in AMA discharges is key for multiple reasons. Most AMA patients in this study signed an AMA form, and it could be that the rate of documenting decision-making capacity was low because providers thought a signed AMA form was adequate documentation of capacity and informed consent. In numerous court cases, however, these forms were found to be insufficient evidence of informed consent (lacking other supportive documentation) and possibly to go against the public good.30 In addition, high rates of repeat emergency department visits and readmissions for AMA patients, demonstrated here and in other studies, highlight the importance of careful documentation in informing subsequent providers about hospital returnees’ ongoing issues.17-19
This study also demonstrated differences between nurses and physicians in their beliefs about arranging follow-up for AMA patients. Nurses were less likely than physicians to think follow-up arrangements should be made for AMA patients and more likely to say these patients should lose the right to follow-up because of the AMA discharge. For conventional discharges, nurses provide patients with significantly more discharge education than interns or hospitalists do.31 This discrepancy highlights an urgent need for the education and involvement of nurses as stakeholders in the challenging AMA discharge process. Although the percentage of physicians who thought they were not obligated to provide medications and arrange follow-up for AMA patients was lower than the percentage of nurses, these beliefs contradict best practice guidelines for AMA discharges,22,23 and this finding calls attention to the need for interventions to improve adherence to professional and ethical guidelines in this aspect of clinical practice.
Providers showed a lack of familiarity with practice guidelines regarding certain aspects of the AMA discharge process. For example, most providers thought they should not have to arrange transportation for AMA patients, even though both the California Hospital Association Guidelines and the Highland Hospital internal policy on AMA discharges recommend arranging appropriate transportation.32 This finding suggests a need for educational interventions to ensure providers are informed about state and hospital policies, and a need to include both physicians and nurses in policymaking so theory can be tied to practice.
This study was limited to a single center with healthcare provider and patient populations that might not be generalizable to other settings. In the retrospective chart review, the authors were limited to information documented in the medical record, which might not accurately reflect the AMA discharge process. As they surveyed a limited number of social workers, case managers, and others who play an important role in the AMA discharge process, their data may lack varying viewpoints.
Overall, these data suggest providers at this county hospital generally agreed in principle with the best practice guidelines proposed by bioethicists for AMA discharges. In practice, however, providers were not reliably following these guidelines. Future interventions—including provider education on best practice guidelines for AMA discharge, provider involvement in policymaking, supportive templates for guiding documentation of AMA discharges, and improving access to follow-up care—will be key in improving the safety and health outcomes of AMA patients.
Acknowledgments
The authors thank Kelly Aguilar, Kethia Chheng, Irene Yen, and the Research Advancement and Coordination Initiative at Alameda Health System for important contributions to this project.
Disclosures
Highland Hospital Department of Medicine internal grant 2015.23 helped fund this research. A portion of the data was presented as a poster at the University of California San Francisco Health Disparities Symposium; October 2015; San Francisco, CA. Two posters from the data were presented at Hospital Medicine 2016, March 2016; San Diego, CA.
1. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. PubMed
2. Stranges E, Wier L, Merrill C, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. August 2009. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed November 30, 2016. PubMed
3. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
4. O’Hara D, Hart W, McDonald I. Leaving hospital against medical advice. J Qual Clin Pract. 1996;16(3):157-164. PubMed
5. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
6. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
7. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
8. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
9. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
10. Katzenellenbogen JM, Sanfilippo FM, Hobbs MS, et al. Voting with their feet—predictors of discharge against medical advice in Aboriginal and non-Aboriginal ischaemic heart disease inpatients in Western Australia: an analytic study using data linkage. BMC Health Serv Res. 2013;13:330. PubMed
11. Ti L, Milloy MJ, Buxton J, et al. Factors associated with leaving hospital against medical advice among people who use illicit drugs in Vancouver, Canada. PLoS One. 2015;10(10):e0141594. PubMed
12. Yong TY, Fok JS, Hakendorf P, Ben-Tovim D, Thompson CH, Li JY. Characteristics and outcomes of discharges against medical advice among hospitalised patients. Intern Med J. 2013;43(7):798-802. PubMed
13. Tabatabaei SM, Sargazi Moakhar Z, Behmanesh Pour F, Shaare Mollashahi S, Zaboli M. Hospitalized pregnant women who leave against medical advice: attributes and reasons. Matern Child Health J. 2016;20(1):128-138. PubMed
14. Aliyu ZY. Discharge against medical advice: sociodemographic, clinical and financial perspectives. Int J Clin Pract. 2002;56(5):325-327. PubMed
15. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. PubMed
16. Targum SD, Capodanno AE, Hoffman HA, Foudraine C. An intervention to reduce the rate of hospital discharges against medical advice. Am J Psychiatry. 1982;139(5):657-659. PubMed
17. Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS One. 2011;6(9):e24459. PubMed
18. Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926-929. PubMed
19. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. PubMed
20. Hwang SW, Li J, Gupta R, Chien V, Martin RE. What happens to patients who leave hospital against medical advice? CMAJ. 2003;168(4):417-420. PubMed
21. Onukwugha E, Mullins CD, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. PubMed
22. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda. J Gen Intern Med. 2013;28(12):1657-1662. PubMed
23. Berger JT. Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403-408. PubMed
24. Franks P, Meldrum S, Fiscella K. Discharges against medical advice: are race/ethnicity predictors? J Gen Intern Med. 2006;21(9):955-960. PubMed
25. Hicks LS, Ayanian JZ, Orav EJ, et al. Is hospital service associated with racial and ethnic disparities in experiences with hospital care? Am J Med. 2005;118(5):529-535. PubMed
26. Hicks LS, Tovar DA, Orav EJ, Johnson PA. Experiences with hospital care: perspectives of black and Hispanic patients. J Gen Intern Med. 2008;23(8):1234-1240. PubMed
27. McCreaddie M, Lyons I, Watt D, et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19-20):2730-2740. PubMed
28. Carroll IR, Angst MS, Clark JD. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29(6):576-591. PubMed
29. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
30. Levy F, Mareiniss DP, Iacovelli C. The importance of a proper against-medical-advice (AMA) discharge: how signing out AMA may create significant liability protection for providers. J Emerg Med. 2012;43(3):516-520. PubMed
31. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
32. Joint Commission on Accreditation of Healthcare Organizations. Title 22, California Code of Regulations, §70707.3.
Patients leave the hospital against medical advice (AMA) for a variety of reasons. The AMA rate is approximately 1% nationally but substantially higher at safety-net hospitals and has rapidly increased over the past decade.1-5 The principle that patients have the right to make choices about their healthcare, up to and including whether to leave the hospital against the advice of medical staff, is well-established law and a foundation of medical ethics.6 In practice, however, AMA discharges are often emotionally charged for both patients and providers, and, in the high-stress setting of AMA discharge, providers may be confused about their roles.7-9
The demographics of patients who leave AMA have been well described. Compared with conventionally discharged patients, AMA patients are younger, more likely to be male, and more likely a marginalized ethnic or racial minority.10-14 Patients with mental illnesses and addiction issues are overrepresented in AMA discharges, and complicated capacity assessments and limited resources may strain providers.7,8,15,16 Studies have repeatedly shown higher rates of readmission and mortality for AMA patients than for conventionally discharged patients.17-21 Whether AMA discharge is a marker for other prognostic factors that bode poorly for patients or contributes to negative outcomes, data suggest this group of patients is vulnerable, having mortality rates up to 40% higher 1 year after discharge, relative to conventionally discharged patients.12
Several models of standardized best practice approaches for AMA have been proposed by bioethicists.6,22,23 Although details of these approaches vary, all involve assessing the patient’s decision-making capacity, clarifying the risks of AMA discharge, addressing factors that might be prompting the discharge, formulating an alternative outpatient treatment plan or “next best” option, and documenting extensively. A recent study found patients often gave advance warning of an AMA discharge, but physicians rarely prepared by arranging follow-up care.8 The investigators hypothesized that providers might not have known what they were permitted to arrange for AMA patients, or might have thought that providing “second best” options went against their principles. The investigators noted that nurses might have become aware of AMA risk sooner than physicians did but could not act on this awareness by preparing medications and arranging follow-up.
Translating models of best practice care for AMA patients into clinical practice requires buy-in from bedside providers, not just bioethicists. Given the study findings that providers have misconceptions about their roles in the AMA discharge,7 it is prudent to investigate providers’ current practices, beliefs, and concerns about AMA discharges before introducing a new approach.
The present authors conducted a mixed-methods cross-sectional study of the state of AMA discharges at Highland Hospital (Oakland, California), a 236-bed county hospital and trauma center serving a primarily underserved urban patient population. The aim of this study was to assess current provider practices for AMA discharges and provider perceptions and knowledge about AMA discharges, ultimately to help direct future educational interventions with medical providers or hospital policy changes needed to improve the quality of AMA discharges.
METHODS
Phase 1 of this study involved identifying AMA patients through a review of data from Highland Hospital’s electronic medical records for 2014. These data included discharge status (eg, AMA vs other discharge types). The hospital’s floor clerk distinguishes between absent without official leave (AWOL; the patient leaves without notifying a provider) and AMA discharge. Discharges designated AWOL were excluded from the analyses.
In phase 2, a structured chart review (Appendix A) was performed for all patients identified during phase 1 as being discharged AMA in 2014. In these reviews, further assessment was made of patient and visit characteristics in hospitalizations that ended in AMA discharge, and of providers’ documentation of AMA discharges—that is, whether several factors were documented (capacity; predischarge indication that patient might leave AMA; reason for AMA; and indications that discharge medications, transportation, and follow-up were arranged). These visit factors were reviewed because the literature has identified them as being important markers for AMA discharge safety.6,8 Two research assistants, under the guidance of Dr. Stearns, reviewed the charts. To ensure agreement across chart reviews with respect to subjective questions (eg, whether capacity was adequately documented), the group reviewed the first 10 consecutive charts together; there was full agreement on how to classify the data of interest. Throughout the study, whenever a research assistant asked how to classify particular patient data, Dr. Stearns reviewed the data, and the research team made a decision together. Additional data, for AMA patients and for all patients admitted to Highland Hospital, were obtained from the hospital’s data warehouse, which pools data from within the health system.
Phase 3 involved surveying healthcare providers who were involved in patient care on the internal medicine and trauma surgery services at the hospital. These providers were selected because chart review revealed that the vast majority of patients who left AMA in 2014 were on one of these services. Surveys (Appendix B) asked participant providers to identify their role at the hospital, to provide a self-assessment of competence in various aspects of AMA discharge, to voice opinions about provider responsibilities in arranging follow-up for AMA patients, and to make suggestions about the AMA process. The authors designed these surveys, which included questions about aspects of care that have been highlighted in the AMA discharge literature as being important for AMA discharge safety.6,8,22,23 Surveys were distributed to providers at internal medicine and trauma surgery department meetings and nursing conferences. Data (without identifying information) were analyzed, and survey responses kept anonymous.
The Alameda Health System Institutional Review Board approved this project. Providers were given the option of writing their name and contact information at the top of the survey in order to be entered into a drawing to receive a prize for completion.
We performed statistical analyses of the patient charts and physician survey data using Stata (version 14.0, Stata Corp., College Station, Texas). We analyzed both patient- and encounter-level data. In demographic analyses, this approach prevented duplicate counting of patients who left AMA multiple times. Patient-level analyses compared the demographic characteristics of AMA patients and patients discharged conventionally from the hospital in 2014. In addition, patients with either 1 or multiple AMA discharges were compared to identify characteristics that might be linked to highest risk of recurrent AMA discharge in the hope that early identification of these patients might facilitate providers’ early awareness and preparation for follow-up care or hospitalization alternatives. We used ANOVAs for continuous variables and tests of proportions for categorical variables. On the encounter level, analyses examined data about each admission (eg, AMA forms signed, follow-up arrangements made, capacity documented, etc.) for all AMA discharges. We employed chi square tests to identify variations in healthcare provider survey responses. A P value < 0.05 was used as the significance cut-off point.
Staged logistic regression analyses, adjusted for demographic characteristics, were performed to assess the association between risk of leaving AMA (yes or no) and demographic characteristics and the association between risk of leaving AMA more than once (yes or no) and health-related characteristics.
RESULTS
Demographic, Clinical, and Utilization Characteristics
Of the 12,036 Highland Hospital admissions in 2014, 319 (2.7%) ended with an AMA discharge. Of the 8207 individual patients discharged, 268 left AMA once, and 29 left AMA multiple times. Further review of the Admissions, Discharges, and Transfers Report generated from the electronic medical record revealed that 15 AWOL discharges were misclassified as AMA discharges.
Compared with patients discharged conventionally, AMA patients were significantly younger; more likely to be male, to self-identify as Black/African American, and to be English-speaking; and less likely to self-identify as Asian/Pacific Islander or Hispanic/Latino or to be Chinese- or Spanish-speaking (Table 1). They were also more likely than all patients admitted to Highland to be homeless (15.7% vs 8.7%; P < 0.01). Multivariate regression analysis revealed persistent age and sex disparities, but racial disparities were mitigated in adjusted analyses (Appendix C). Language disparities persisted only for Spanish speakers, who had a significantly lower rate of AMA discharge, even in adjusted analyses.

The majority of AMA patients were on the internal medicine service (63.5%) or the trauma surgery service (24.8%). Regarding admission diagnosis, 17.2% of AMA patients were admitted for infections, 5.0% for drug or alcohol intoxication or withdrawal, 38.9% for acute noninfectious illnesses, 16.7% for decompensation of chronic disease, 18.4% for injuries or trauma, and 3.8% for pregnancy complications or labor. Compared with patients who left AMA once, patients who left AMA multiple times had higher rates of heavy alcohol use (53.9% vs 30.9%; P = 0.01) and illicit drug use (88.5% vs 53.7%; P < 0.001) (Table 2). In multivariate analyses, the increased odds of leaving AMA more than once persisted for current heavy illicit drug users compared with patients who had never engaged in illicit drug use.
Discharge Characteristics and Documentation
Providers documented a patient’s plan to leave AMA before actual discharge 17.3% of the time. The documented plan to leave had to indicate that the patient was actually considering leaving. For example, “Patient is eager to go home” was not enough to qualify as a plan, but “Patient is thinking of leaving” qualified. For 84.3% of AMA discharges, the hospital’s AMA form was signed and was included in the medical record. Documentation showed that medications were prescribed for AMA patients 21.4% of the time, follow-up was arranged 25.7% of the time, and follow-up was pending arrangement 14.8% of the time. The majority of AMA patients (71.4%) left during daytime hours. In 29.6% of AMA discharges, providers documented AMA patients had decision-making capacity.
Readmission After AMA Discharge
Of the 268 AMA patients, 67.7% were not readmitted within the 6 months after AMA, 24.5% had 1 or 2 readmissions, and the rest had 3 or more readmissions (1 patient had 15). In addition, 35.8% returned to the emergency department within 30 days, and 16.4% were readmitted within 30 days. In 2014, the hospital’s overall 30-day readmission rate was 10.8%. Of the patients readmitted within 6 months after AMA, 23.5% left AMA again at the next visit, 9.4% left AWOL, and 67.1% were discharged conventionally.
Drivers of Premature Discharge
Qualitative analysis of the 35.5% of patient charts documenting a reason for leaving the hospital revealed 3 broad, interrelated themes (Figure 1). The first theme, dissatisfaction with hospital care, included chart notations such as “His wife couldn’t sleep in the hospital room” and “Not satisfied with all-liquid diet.” The second theme, urgent personal issues, included comments such as “He has a very important court date for his children” and “He needed to take care of immigration forms.” The third theme, mental health and substance abuse issues, included notations such as “He wants to go smoke” and “Severe anxiety and prison flashbacks.”
Provider Self-Assessment and Beliefs
The survey was completed by 178 healthcare providers: 49.4% registered nurses, 19.1% trainee physicians, 20.8% attending physicians, and 10.7% other providers, including chaplains, social workers, and clerks. Regarding self-assessment of competency in AMA discharges, 94% of providers agreed they were comfortable assessing capacity, and 94% agreed they were comfortable talking with patients about the risks of leaving AMA (Figure 2). Nurses were more likely than trainee physicians to agree they knew what to do for patients who lacked capacity (74% vs 49%; P = 0.02). Most providers (70%) agreed they usually knew why their patients were leaving AMA; in this self-assessment, there were no significant differences between types of providers.
Regarding follow-up, attending physicians and trainee physicians demonstrated more agreement than nurses that AMA patients should receive medications and follow-up (94% and 84% vs 64%; P < 0.05). Nurses were more likely than attending physicians to say patients should lose their rights to hospital follow-up because of leaving AMA (38% vs 6%; P < 0.01). A minority of providers (37%) agreed transportation should be arranged. Addiction was the most common driver of AMA discharge (35%), followed by familial obligations (19%), dissatisfaction with hospital care (16%), and financial concerns (15%).
DISCUSSION
The demographic characteristics of AMA patients in this study are similar to those identified in other studies, showing overrepresentation of young male patients.12,14 Homeless patients were also overrepresented in the AMA discharge population at Highland Hospital—a finding that has not been consistently reported in prior studies, and that warrants further examination. In adjusted analyses, Spanish speakers had a lower rate of AMA discharge, and there were no racial variations. This is consistent with another study’s finding: that racial disparities in AMA discharge rates were largely attributable to confounders.24 Language differences may result from failure of staff to fully explain the option of AMA discharge to non-English speakers, or from fear of immigration consequences after AMA discharge. Further investigation of patient experiences is needed to identify factors that contribute to demographic variations in AMA discharge rates.25,26
Of the patients who left AMA multiple times, nearly all were actively using illicit drugs. In a recent study conducted at a safety-net hospital in Vancouver, Canada, 43% of patients with illicit drug use and at least 1 hospitalization left AMA at least once during the 6-year study period.11 Many factors might explain this correlation—addiction itself, poor pain control for patients with addiction issues, fears about incarceration, and poor treatment of drug users by healthcare staff.15 Although the medical literature highlights deficits in pain control for patients addicted to opiates, proposed solutions are sparse and focus on perioperative pain control and physician prescribing practices.27,28 At safety-net hospitals in which addiction is a factor in many hospitalizations, there is opportunity for new research in inpatient pain control for patients with substance dependence. In addition, harm reduction strategies—such as methadone maintenance for hospitalized patients with opiate dependence and abscess clinics as hospitalization alternatives for injection-associated infection treatment—may be key in improving safety for patients.11,15,29
Comparing the provider survey and chart review results highlights discordance between provider beliefs and clinical practice. Healthcare providers at Highland Hospital considered themselves competent in assessing capacity and talking with patients about the risks of AMA discharge. In practice, however, capacity was documented in less than a third of AMA discharges. Although the majority of providers thought medications and follow-up should be arranged for patients, arrangements were seldom made. This may be partially attributable to limited resources for making these arrangements. Average time to “third next available” primary care appointment within the county health system that includes Highland was 44.6 days for established patients during the period of study; for new primary care patients, the average wait for an appointment was 2 to 3 months. Highland has a same-day clinic, but inpatient providers are discouraged from using it as a postdischarge clinic for patients who would be better served in primary care. Medications and transportation are easily arranged during daytime hours but are not immediately available at night. In addition, some of this discrepancy may be attributable to the limited documentation rather than to provider failure to achieve their own benchmarks of quality care for AMA patients.
Documentation in AMA discharges is key for multiple reasons. Most AMA patients in this study signed an AMA form, and it could be that the rate of documenting decision-making capacity was low because providers thought a signed AMA form was adequate documentation of capacity and informed consent. In numerous court cases, however, these forms were found to be insufficient evidence of informed consent (lacking other supportive documentation) and possibly to go against the public good.30 In addition, high rates of repeat emergency department visits and readmissions for AMA patients, demonstrated here and in other studies, highlight the importance of careful documentation in informing subsequent providers about hospital returnees’ ongoing issues.17-19
This study also demonstrated differences between nurses and physicians in their beliefs about arranging follow-up for AMA patients. Nurses were less likely than physicians to think follow-up arrangements should be made for AMA patients and more likely to say these patients should lose the right to follow-up because of the AMA discharge. For conventional discharges, nurses provide patients with significantly more discharge education than interns or hospitalists do.31 This discrepancy highlights an urgent need for the education and involvement of nurses as stakeholders in the challenging AMA discharge process. Although the percentage of physicians who thought they were not obligated to provide medications and arrange follow-up for AMA patients was lower than the percentage of nurses, these beliefs contradict best practice guidelines for AMA discharges,22,23 and this finding calls attention to the need for interventions to improve adherence to professional and ethical guidelines in this aspect of clinical practice.
Providers showed a lack of familiarity with practice guidelines regarding certain aspects of the AMA discharge process. For example, most providers thought they should not have to arrange transportation for AMA patients, even though both the California Hospital Association Guidelines and the Highland Hospital internal policy on AMA discharges recommend arranging appropriate transportation.32 This finding suggests a need for educational interventions to ensure providers are informed about state and hospital policies, and a need to include both physicians and nurses in policymaking so theory can be tied to practice.
This study was limited to a single center with healthcare provider and patient populations that might not be generalizable to other settings. In the retrospective chart review, the authors were limited to information documented in the medical record, which might not accurately reflect the AMA discharge process. As they surveyed a limited number of social workers, case managers, and others who play an important role in the AMA discharge process, their data may lack varying viewpoints.
Overall, these data suggest providers at this county hospital generally agreed in principle with the best practice guidelines proposed by bioethicists for AMA discharges. In practice, however, providers were not reliably following these guidelines. Future interventions—including provider education on best practice guidelines for AMA discharge, provider involvement in policymaking, supportive templates for guiding documentation of AMA discharges, and improving access to follow-up care—will be key in improving the safety and health outcomes of AMA patients.
Acknowledgments
The authors thank Kelly Aguilar, Kethia Chheng, Irene Yen, and the Research Advancement and Coordination Initiative at Alameda Health System for important contributions to this project.
Disclosures
Highland Hospital Department of Medicine internal grant 2015.23 helped fund this research. A portion of the data was presented as a poster at the University of California San Francisco Health Disparities Symposium; October 2015; San Francisco, CA. Two posters from the data were presented at Hospital Medicine 2016, March 2016; San Diego, CA.
Patients leave the hospital against medical advice (AMA) for a variety of reasons. The AMA rate is approximately 1% nationally but substantially higher at safety-net hospitals and has rapidly increased over the past decade.1-5 The principle that patients have the right to make choices about their healthcare, up to and including whether to leave the hospital against the advice of medical staff, is well-established law and a foundation of medical ethics.6 In practice, however, AMA discharges are often emotionally charged for both patients and providers, and, in the high-stress setting of AMA discharge, providers may be confused about their roles.7-9
The demographics of patients who leave AMA have been well described. Compared with conventionally discharged patients, AMA patients are younger, more likely to be male, and more likely a marginalized ethnic or racial minority.10-14 Patients with mental illnesses and addiction issues are overrepresented in AMA discharges, and complicated capacity assessments and limited resources may strain providers.7,8,15,16 Studies have repeatedly shown higher rates of readmission and mortality for AMA patients than for conventionally discharged patients.17-21 Whether AMA discharge is a marker for other prognostic factors that bode poorly for patients or contributes to negative outcomes, data suggest this group of patients is vulnerable, having mortality rates up to 40% higher 1 year after discharge, relative to conventionally discharged patients.12
Several models of standardized best practice approaches for AMA have been proposed by bioethicists.6,22,23 Although details of these approaches vary, all involve assessing the patient’s decision-making capacity, clarifying the risks of AMA discharge, addressing factors that might be prompting the discharge, formulating an alternative outpatient treatment plan or “next best” option, and documenting extensively. A recent study found patients often gave advance warning of an AMA discharge, but physicians rarely prepared by arranging follow-up care.8 The investigators hypothesized that providers might not have known what they were permitted to arrange for AMA patients, or might have thought that providing “second best” options went against their principles. The investigators noted that nurses might have become aware of AMA risk sooner than physicians did but could not act on this awareness by preparing medications and arranging follow-up.
Translating models of best practice care for AMA patients into clinical practice requires buy-in from bedside providers, not just bioethicists. Given the study findings that providers have misconceptions about their roles in the AMA discharge,7 it is prudent to investigate providers’ current practices, beliefs, and concerns about AMA discharges before introducing a new approach.
The present authors conducted a mixed-methods cross-sectional study of the state of AMA discharges at Highland Hospital (Oakland, California), a 236-bed county hospital and trauma center serving a primarily underserved urban patient population. The aim of this study was to assess current provider practices for AMA discharges and provider perceptions and knowledge about AMA discharges, ultimately to help direct future educational interventions with medical providers or hospital policy changes needed to improve the quality of AMA discharges.
METHODS
Phase 1 of this study involved identifying AMA patients through a review of data from Highland Hospital’s electronic medical records for 2014. These data included discharge status (eg, AMA vs other discharge types). The hospital’s floor clerk distinguishes between absent without official leave (AWOL; the patient leaves without notifying a provider) and AMA discharge. Discharges designated AWOL were excluded from the analyses.
In phase 2, a structured chart review (Appendix A) was performed for all patients identified during phase 1 as being discharged AMA in 2014. In these reviews, further assessment was made of patient and visit characteristics in hospitalizations that ended in AMA discharge, and of providers’ documentation of AMA discharges—that is, whether several factors were documented (capacity; predischarge indication that patient might leave AMA; reason for AMA; and indications that discharge medications, transportation, and follow-up were arranged). These visit factors were reviewed because the literature has identified them as being important markers for AMA discharge safety.6,8 Two research assistants, under the guidance of Dr. Stearns, reviewed the charts. To ensure agreement across chart reviews with respect to subjective questions (eg, whether capacity was adequately documented), the group reviewed the first 10 consecutive charts together; there was full agreement on how to classify the data of interest. Throughout the study, whenever a research assistant asked how to classify particular patient data, Dr. Stearns reviewed the data, and the research team made a decision together. Additional data, for AMA patients and for all patients admitted to Highland Hospital, were obtained from the hospital’s data warehouse, which pools data from within the health system.
Phase 3 involved surveying healthcare providers who were involved in patient care on the internal medicine and trauma surgery services at the hospital. These providers were selected because chart review revealed that the vast majority of patients who left AMA in 2014 were on one of these services. Surveys (Appendix B) asked participant providers to identify their role at the hospital, to provide a self-assessment of competence in various aspects of AMA discharge, to voice opinions about provider responsibilities in arranging follow-up for AMA patients, and to make suggestions about the AMA process. The authors designed these surveys, which included questions about aspects of care that have been highlighted in the AMA discharge literature as being important for AMA discharge safety.6,8,22,23 Surveys were distributed to providers at internal medicine and trauma surgery department meetings and nursing conferences. Data (without identifying information) were analyzed, and survey responses kept anonymous.
The Alameda Health System Institutional Review Board approved this project. Providers were given the option of writing their name and contact information at the top of the survey in order to be entered into a drawing to receive a prize for completion.
We performed statistical analyses of the patient charts and physician survey data using Stata (version 14.0, Stata Corp., College Station, Texas). We analyzed both patient- and encounter-level data. In demographic analyses, this approach prevented duplicate counting of patients who left AMA multiple times. Patient-level analyses compared the demographic characteristics of AMA patients and patients discharged conventionally from the hospital in 2014. In addition, patients with either 1 or multiple AMA discharges were compared to identify characteristics that might be linked to highest risk of recurrent AMA discharge in the hope that early identification of these patients might facilitate providers’ early awareness and preparation for follow-up care or hospitalization alternatives. We used ANOVAs for continuous variables and tests of proportions for categorical variables. On the encounter level, analyses examined data about each admission (eg, AMA forms signed, follow-up arrangements made, capacity documented, etc.) for all AMA discharges. We employed chi square tests to identify variations in healthcare provider survey responses. A P value < 0.05 was used as the significance cut-off point.
Staged logistic regression analyses, adjusted for demographic characteristics, were performed to assess the association between risk of leaving AMA (yes or no) and demographic characteristics and the association between risk of leaving AMA more than once (yes or no) and health-related characteristics.
RESULTS
Demographic, Clinical, and Utilization Characteristics
Of the 12,036 Highland Hospital admissions in 2014, 319 (2.7%) ended with an AMA discharge. Of the 8207 individual patients discharged, 268 left AMA once, and 29 left AMA multiple times. Further review of the Admissions, Discharges, and Transfers Report generated from the electronic medical record revealed that 15 AWOL discharges were misclassified as AMA discharges.
Compared with patients discharged conventionally, AMA patients were significantly younger; more likely to be male, to self-identify as Black/African American, and to be English-speaking; and less likely to self-identify as Asian/Pacific Islander or Hispanic/Latino or to be Chinese- or Spanish-speaking (Table 1). They were also more likely than all patients admitted to Highland to be homeless (15.7% vs 8.7%; P < 0.01). Multivariate regression analysis revealed persistent age and sex disparities, but racial disparities were mitigated in adjusted analyses (Appendix C). Language disparities persisted only for Spanish speakers, who had a significantly lower rate of AMA discharge, even in adjusted analyses.

The majority of AMA patients were on the internal medicine service (63.5%) or the trauma surgery service (24.8%). Regarding admission diagnosis, 17.2% of AMA patients were admitted for infections, 5.0% for drug or alcohol intoxication or withdrawal, 38.9% for acute noninfectious illnesses, 16.7% for decompensation of chronic disease, 18.4% for injuries or trauma, and 3.8% for pregnancy complications or labor. Compared with patients who left AMA once, patients who left AMA multiple times had higher rates of heavy alcohol use (53.9% vs 30.9%; P = 0.01) and illicit drug use (88.5% vs 53.7%; P < 0.001) (Table 2). In multivariate analyses, the increased odds of leaving AMA more than once persisted for current heavy illicit drug users compared with patients who had never engaged in illicit drug use.
Discharge Characteristics and Documentation
Providers documented a patient’s plan to leave AMA before actual discharge 17.3% of the time. The documented plan to leave had to indicate that the patient was actually considering leaving. For example, “Patient is eager to go home” was not enough to qualify as a plan, but “Patient is thinking of leaving” qualified. For 84.3% of AMA discharges, the hospital’s AMA form was signed and was included in the medical record. Documentation showed that medications were prescribed for AMA patients 21.4% of the time, follow-up was arranged 25.7% of the time, and follow-up was pending arrangement 14.8% of the time. The majority of AMA patients (71.4%) left during daytime hours. In 29.6% of AMA discharges, providers documented AMA patients had decision-making capacity.
Readmission After AMA Discharge
Of the 268 AMA patients, 67.7% were not readmitted within the 6 months after AMA, 24.5% had 1 or 2 readmissions, and the rest had 3 or more readmissions (1 patient had 15). In addition, 35.8% returned to the emergency department within 30 days, and 16.4% were readmitted within 30 days. In 2014, the hospital’s overall 30-day readmission rate was 10.8%. Of the patients readmitted within 6 months after AMA, 23.5% left AMA again at the next visit, 9.4% left AWOL, and 67.1% were discharged conventionally.
Drivers of Premature Discharge
Qualitative analysis of the 35.5% of patient charts documenting a reason for leaving the hospital revealed 3 broad, interrelated themes (Figure 1). The first theme, dissatisfaction with hospital care, included chart notations such as “His wife couldn’t sleep in the hospital room” and “Not satisfied with all-liquid diet.” The second theme, urgent personal issues, included comments such as “He has a very important court date for his children” and “He needed to take care of immigration forms.” The third theme, mental health and substance abuse issues, included notations such as “He wants to go smoke” and “Severe anxiety and prison flashbacks.”
Provider Self-Assessment and Beliefs
The survey was completed by 178 healthcare providers: 49.4% registered nurses, 19.1% trainee physicians, 20.8% attending physicians, and 10.7% other providers, including chaplains, social workers, and clerks. Regarding self-assessment of competency in AMA discharges, 94% of providers agreed they were comfortable assessing capacity, and 94% agreed they were comfortable talking with patients about the risks of leaving AMA (Figure 2). Nurses were more likely than trainee physicians to agree they knew what to do for patients who lacked capacity (74% vs 49%; P = 0.02). Most providers (70%) agreed they usually knew why their patients were leaving AMA; in this self-assessment, there were no significant differences between types of providers.
Regarding follow-up, attending physicians and trainee physicians demonstrated more agreement than nurses that AMA patients should receive medications and follow-up (94% and 84% vs 64%; P < 0.05). Nurses were more likely than attending physicians to say patients should lose their rights to hospital follow-up because of leaving AMA (38% vs 6%; P < 0.01). A minority of providers (37%) agreed transportation should be arranged. Addiction was the most common driver of AMA discharge (35%), followed by familial obligations (19%), dissatisfaction with hospital care (16%), and financial concerns (15%).
DISCUSSION
The demographic characteristics of AMA patients in this study are similar to those identified in other studies, showing overrepresentation of young male patients.12,14 Homeless patients were also overrepresented in the AMA discharge population at Highland Hospital—a finding that has not been consistently reported in prior studies, and that warrants further examination. In adjusted analyses, Spanish speakers had a lower rate of AMA discharge, and there were no racial variations. This is consistent with another study’s finding: that racial disparities in AMA discharge rates were largely attributable to confounders.24 Language differences may result from failure of staff to fully explain the option of AMA discharge to non-English speakers, or from fear of immigration consequences after AMA discharge. Further investigation of patient experiences is needed to identify factors that contribute to demographic variations in AMA discharge rates.25,26
Of the patients who left AMA multiple times, nearly all were actively using illicit drugs. In a recent study conducted at a safety-net hospital in Vancouver, Canada, 43% of patients with illicit drug use and at least 1 hospitalization left AMA at least once during the 6-year study period.11 Many factors might explain this correlation—addiction itself, poor pain control for patients with addiction issues, fears about incarceration, and poor treatment of drug users by healthcare staff.15 Although the medical literature highlights deficits in pain control for patients addicted to opiates, proposed solutions are sparse and focus on perioperative pain control and physician prescribing practices.27,28 At safety-net hospitals in which addiction is a factor in many hospitalizations, there is opportunity for new research in inpatient pain control for patients with substance dependence. In addition, harm reduction strategies—such as methadone maintenance for hospitalized patients with opiate dependence and abscess clinics as hospitalization alternatives for injection-associated infection treatment—may be key in improving safety for patients.11,15,29
Comparing the provider survey and chart review results highlights discordance between provider beliefs and clinical practice. Healthcare providers at Highland Hospital considered themselves competent in assessing capacity and talking with patients about the risks of AMA discharge. In practice, however, capacity was documented in less than a third of AMA discharges. Although the majority of providers thought medications and follow-up should be arranged for patients, arrangements were seldom made. This may be partially attributable to limited resources for making these arrangements. Average time to “third next available” primary care appointment within the county health system that includes Highland was 44.6 days for established patients during the period of study; for new primary care patients, the average wait for an appointment was 2 to 3 months. Highland has a same-day clinic, but inpatient providers are discouraged from using it as a postdischarge clinic for patients who would be better served in primary care. Medications and transportation are easily arranged during daytime hours but are not immediately available at night. In addition, some of this discrepancy may be attributable to the limited documentation rather than to provider failure to achieve their own benchmarks of quality care for AMA patients.
Documentation in AMA discharges is key for multiple reasons. Most AMA patients in this study signed an AMA form, and it could be that the rate of documenting decision-making capacity was low because providers thought a signed AMA form was adequate documentation of capacity and informed consent. In numerous court cases, however, these forms were found to be insufficient evidence of informed consent (lacking other supportive documentation) and possibly to go against the public good.30 In addition, high rates of repeat emergency department visits and readmissions for AMA patients, demonstrated here and in other studies, highlight the importance of careful documentation in informing subsequent providers about hospital returnees’ ongoing issues.17-19
This study also demonstrated differences between nurses and physicians in their beliefs about arranging follow-up for AMA patients. Nurses were less likely than physicians to think follow-up arrangements should be made for AMA patients and more likely to say these patients should lose the right to follow-up because of the AMA discharge. For conventional discharges, nurses provide patients with significantly more discharge education than interns or hospitalists do.31 This discrepancy highlights an urgent need for the education and involvement of nurses as stakeholders in the challenging AMA discharge process. Although the percentage of physicians who thought they were not obligated to provide medications and arrange follow-up for AMA patients was lower than the percentage of nurses, these beliefs contradict best practice guidelines for AMA discharges,22,23 and this finding calls attention to the need for interventions to improve adherence to professional and ethical guidelines in this aspect of clinical practice.
Providers showed a lack of familiarity with practice guidelines regarding certain aspects of the AMA discharge process. For example, most providers thought they should not have to arrange transportation for AMA patients, even though both the California Hospital Association Guidelines and the Highland Hospital internal policy on AMA discharges recommend arranging appropriate transportation.32 This finding suggests a need for educational interventions to ensure providers are informed about state and hospital policies, and a need to include both physicians and nurses in policymaking so theory can be tied to practice.
This study was limited to a single center with healthcare provider and patient populations that might not be generalizable to other settings. In the retrospective chart review, the authors were limited to information documented in the medical record, which might not accurately reflect the AMA discharge process. As they surveyed a limited number of social workers, case managers, and others who play an important role in the AMA discharge process, their data may lack varying viewpoints.
Overall, these data suggest providers at this county hospital generally agreed in principle with the best practice guidelines proposed by bioethicists for AMA discharges. In practice, however, providers were not reliably following these guidelines. Future interventions—including provider education on best practice guidelines for AMA discharge, provider involvement in policymaking, supportive templates for guiding documentation of AMA discharges, and improving access to follow-up care—will be key in improving the safety and health outcomes of AMA patients.
Acknowledgments
The authors thank Kelly Aguilar, Kethia Chheng, Irene Yen, and the Research Advancement and Coordination Initiative at Alameda Health System for important contributions to this project.
Disclosures
Highland Hospital Department of Medicine internal grant 2015.23 helped fund this research. A portion of the data was presented as a poster at the University of California San Francisco Health Disparities Symposium; October 2015; San Francisco, CA. Two posters from the data were presented at Hospital Medicine 2016, March 2016; San Diego, CA.
1. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. PubMed
2. Stranges E, Wier L, Merrill C, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. August 2009. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed November 30, 2016. PubMed
3. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
4. O’Hara D, Hart W, McDonald I. Leaving hospital against medical advice. J Qual Clin Pract. 1996;16(3):157-164. PubMed
5. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
6. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
7. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
8. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
9. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
10. Katzenellenbogen JM, Sanfilippo FM, Hobbs MS, et al. Voting with their feet—predictors of discharge against medical advice in Aboriginal and non-Aboriginal ischaemic heart disease inpatients in Western Australia: an analytic study using data linkage. BMC Health Serv Res. 2013;13:330. PubMed
11. Ti L, Milloy MJ, Buxton J, et al. Factors associated with leaving hospital against medical advice among people who use illicit drugs in Vancouver, Canada. PLoS One. 2015;10(10):e0141594. PubMed
12. Yong TY, Fok JS, Hakendorf P, Ben-Tovim D, Thompson CH, Li JY. Characteristics and outcomes of discharges against medical advice among hospitalised patients. Intern Med J. 2013;43(7):798-802. PubMed
13. Tabatabaei SM, Sargazi Moakhar Z, Behmanesh Pour F, Shaare Mollashahi S, Zaboli M. Hospitalized pregnant women who leave against medical advice: attributes and reasons. Matern Child Health J. 2016;20(1):128-138. PubMed
14. Aliyu ZY. Discharge against medical advice: sociodemographic, clinical and financial perspectives. Int J Clin Pract. 2002;56(5):325-327. PubMed
15. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. PubMed
16. Targum SD, Capodanno AE, Hoffman HA, Foudraine C. An intervention to reduce the rate of hospital discharges against medical advice. Am J Psychiatry. 1982;139(5):657-659. PubMed
17. Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS One. 2011;6(9):e24459. PubMed
18. Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926-929. PubMed
19. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. PubMed
20. Hwang SW, Li J, Gupta R, Chien V, Martin RE. What happens to patients who leave hospital against medical advice? CMAJ. 2003;168(4):417-420. PubMed
21. Onukwugha E, Mullins CD, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. PubMed
22. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda. J Gen Intern Med. 2013;28(12):1657-1662. PubMed
23. Berger JT. Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403-408. PubMed
24. Franks P, Meldrum S, Fiscella K. Discharges against medical advice: are race/ethnicity predictors? J Gen Intern Med. 2006;21(9):955-960. PubMed
25. Hicks LS, Ayanian JZ, Orav EJ, et al. Is hospital service associated with racial and ethnic disparities in experiences with hospital care? Am J Med. 2005;118(5):529-535. PubMed
26. Hicks LS, Tovar DA, Orav EJ, Johnson PA. Experiences with hospital care: perspectives of black and Hispanic patients. J Gen Intern Med. 2008;23(8):1234-1240. PubMed
27. McCreaddie M, Lyons I, Watt D, et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19-20):2730-2740. PubMed
28. Carroll IR, Angst MS, Clark JD. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29(6):576-591. PubMed
29. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
30. Levy F, Mareiniss DP, Iacovelli C. The importance of a proper against-medical-advice (AMA) discharge: how signing out AMA may create significant liability protection for providers. J Emerg Med. 2012;43(3):516-520. PubMed
31. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
32. Joint Commission on Accreditation of Healthcare Organizations. Title 22, California Code of Regulations, §70707.3.
1. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. PubMed
2. Stranges E, Wier L, Merrill C, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. August 2009. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed November 30, 2016. PubMed
3. Devitt PJ, Devitt AC, Dewan M. Does identifying a discharge as “against medical advice” confer legal protection? J Fam Pract. 2000;49(3):224-227. PubMed
4. O’Hara D, Hart W, McDonald I. Leaving hospital against medical advice. J Qual Clin Pract. 1996;16(3):157-164. PubMed
5. Ibrahim SA, Kwoh CK, Krishnan E. Factors associated with patients who leave acute-care hospitals against medical advice. Am J Public Health. 2007;97(12):2204-2208. PubMed
6. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
7. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
8. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
9. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
10. Katzenellenbogen JM, Sanfilippo FM, Hobbs MS, et al. Voting with their feet—predictors of discharge against medical advice in Aboriginal and non-Aboriginal ischaemic heart disease inpatients in Western Australia: an analytic study using data linkage. BMC Health Serv Res. 2013;13:330. PubMed
11. Ti L, Milloy MJ, Buxton J, et al. Factors associated with leaving hospital against medical advice among people who use illicit drugs in Vancouver, Canada. PLoS One. 2015;10(10):e0141594. PubMed
12. Yong TY, Fok JS, Hakendorf P, Ben-Tovim D, Thompson CH, Li JY. Characteristics and outcomes of discharges against medical advice among hospitalised patients. Intern Med J. 2013;43(7):798-802. PubMed
13. Tabatabaei SM, Sargazi Moakhar Z, Behmanesh Pour F, Shaare Mollashahi S, Zaboli M. Hospitalized pregnant women who leave against medical advice: attributes and reasons. Matern Child Health J. 2016;20(1):128-138. PubMed
14. Aliyu ZY. Discharge against medical advice: sociodemographic, clinical and financial perspectives. Int J Clin Pract. 2002;56(5):325-327. PubMed
15. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. PubMed
16. Targum SD, Capodanno AE, Hoffman HA, Foudraine C. An intervention to reduce the rate of hospital discharges against medical advice. Am J Psychiatry. 1982;139(5):657-659. PubMed
17. Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS One. 2011;6(9):e24459. PubMed
18. Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926-929. PubMed
19. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. PubMed
20. Hwang SW, Li J, Gupta R, Chien V, Martin RE. What happens to patients who leave hospital against medical advice? CMAJ. 2003;168(4):417-420. PubMed
21. Onukwugha E, Mullins CD, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. PubMed
22. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda. J Gen Intern Med. 2013;28(12):1657-1662. PubMed
23. Berger JT. Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403-408. PubMed
24. Franks P, Meldrum S, Fiscella K. Discharges against medical advice: are race/ethnicity predictors? J Gen Intern Med. 2006;21(9):955-960. PubMed
25. Hicks LS, Ayanian JZ, Orav EJ, et al. Is hospital service associated with racial and ethnic disparities in experiences with hospital care? Am J Med. 2005;118(5):529-535. PubMed
26. Hicks LS, Tovar DA, Orav EJ, Johnson PA. Experiences with hospital care: perspectives of black and Hispanic patients. J Gen Intern Med. 2008;23(8):1234-1240. PubMed
27. McCreaddie M, Lyons I, Watt D, et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19-20):2730-2740. PubMed
28. Carroll IR, Angst MS, Clark JD. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29(6):576-591. PubMed
29. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
30. Levy F, Mareiniss DP, Iacovelli C. The importance of a proper against-medical-advice (AMA) discharge: how signing out AMA may create significant liability protection for providers. J Emerg Med. 2012;43(3):516-520. PubMed
31. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
32. Joint Commission on Accreditation of Healthcare Organizations. Title 22, California Code of Regulations, §70707.3.
© 2017 Society of Hospital Medicine
Routine Replacement of Peripheral Intravenous Catheters
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
© 2017 Society of Hospital Medicine
Personality disorders on the acute care unit
We all know these patients:
The young man who, when his name shows up on the ED board, everyone lets out a little groan, knowing his hospital stay will be long and tumultuous.
The middle-aged woman who seems to do want your care and attention and yet rebuffs your attempts to help her, meanwhile, making constant demands on nursing staff.
The older man who trusts no one and will not cooperate with any of his needed care, frustrating staff and physicians alike.
Caring for the patient is integral to the art of doctoring, and yet, there are some people for whom this is incredibly hard to do. They frustrate even the most seasoned professional and work their way under our skin. While their disruptive acts may feel volitional to those of us attempting to provide care, these individuals may suffer from a personality disorder.
In the hospital, a patient must to relate to, and cooperate with, a revolving team of care providers all while under some degree of physical and emotional distress. While this can be destabilizing for even the most resilient patient, for those with personality disorders, it is nearly inevitable that conflict will arise. In a recent article in the Journal of Hospital Medicine, my colleagues and I discussed the management of such patients, with a focus on evidence-based interventions (doi: 10.1002/jhm.2643).2
While the behaviors associated with personality disorders can feel deliberate and even manipulative, research shows that these disorders arise from a complex set of genetic and environmental factors. Alterations found in the serotonin system and regions of the brain involved in emotional reactivity and social processing suggest an underlying neurophysiology contributing to difficulties with interpersonal relationships seen in these disorders.3-9
Many do not realize that having a personality disorder has real implications for an individual’s healthcare outcomes; those with a personality disorder have a life expectancy nearly two decades shorter than the general population.10 While there are a number of factors that likely contribute to the effect on mortality, it has been suggested that dysfunctional personality structures may interfere with the individual’s ability to access and utilize care, resulting in higher morbidity and mortality.11
Although it can be difficult to make a formal diagnosis of a personality disorder on the acute care unit, we provide guideline for recognizing individuals based on the way in which they interact with others. Specifically, we propose a team should consider a personality disorder when the following features are present:
The patient elicits a strong emotional reaction from providers; these may vary markedly between providers
The patient’s emotional responses may appear disproportionate to the inciting event
The patient is on a number of different psychiatric medications with little relief of symptoms
The patient takes up an disproportionate amount of providers’ time
The patient externalizes blame, seeing others as the source of discomfort or distress and therefore sees others as the solution.2
When the team suspects a patient’s behavior may be driven by an underlying dysfunctional personality structure, there are a number of steps that can be taken to help facilitate care and shape behaviors. Key among these is recognizing our own complicated responses to these individuals. These patients evoke strong responses and no team member – from nurses and aides to residents and senior attendings – is immune.12-15
Reactions can range from a need to care for and protect the patient to feelings of futility or contempt.15 Other important behavioral interventions include providing consistency, reinforcing desired behaviors, offering empathy, and providing boundaries while also recognizing the importance of picking your battles.2 Of note, while medications may offer some help, there is limited evidence for use of pharmacological interventions. Although they may be somewhat helpful in addressing particular features of these disorders, such as impulsivity, affective dysregulation or cognitive-perceptual symptoms16, many of these patients end up on a cocktail of psychotropic medications with minimal evidence for their use or efficacy. Thus behavioral management remains the cornerstone of treatment.
While care of the patient with personality disorders can present unique challenges, it offers the opportunity for therapeutic intervention. By appreciating the underlying genetic and environmental factors, we are in a better position to offer empathy and support. For these patients, managing their personality disorder can be just as important as managing any of their other medical comorbidities. By taking an approach that acknowledges the emotional responses of the team while also reinforcing and facilitating positive behaviors of the patient, the hospital stay can prove therapeutic, helping these individuals to develop new skills while also getting their physical needs addressed.
Megan Riddle, MD, PhD, is based in the department of psychiatry and behavioral sciences at the University of Washington, Seattle.
NOTES
1. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA: American Psychiatric Association; 2013.
2. Riddle M, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016 Dec;11(12):873-878.
3. Bukh JD, Bock C, Kessing LV. Association between genetic polymorphisms in the serotonergic system and comorbid personality disorders among patients with first-episode depression. J Pers Disord. 2014 Jun;28(3):365-378.
4. Perez-Rodriguez MM, Weinstein S, New AS, et al. Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatr Res. 2010 Nov; 44(15):1075-1081.
5. Checknita D, Maussion G, Labonte B, et al. Monoamine oxidase: A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry. 2015 Mar;206(3):216-222.
6. Boen E, Westlye LT, Elvsashagen T, et al. Regional cortical thinning may be a biological marker for borderline personality disorder. Acta Psychiatr Scand. 2014 Sep;130(3):193-204.
7. Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci Biobehav Rev. 2013 Mar;37(3):448-470.
8. Liu H, Liao J, Jiang W, Wang W. Changes in low-frequency fluctuations in patients with antisocial personality disorder revealed by resting-state functional MRI. PLoS One. 2014 Mar 5;9(3):e89790.
9. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. 2009 Nov 30;174(2):81-88.
10. Fok ML, Hayes RD, Chang CK, Stewart R, Callard FJ, Moran P. Life expectancy at birth and all-cause mortality among people with personality disorder. J Psychosom Res. 2012 Aug;73(2):104-107.
11. Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015 Feb 21;385:717-726.
12. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978 Apr 20; 298:883-887.
13. Groves JE. Management of the borderline patient on a medical or surgical ward: The psychiatric consultant’s role. Int J Psychiatry Med. 1975;6(3):337-348.
14. Bodner E, Cohen-Fridel S, Mashiah M, et al. The attitudes of psychiatric hospital staff toward hospitalization and treatment of patients with borderline personality disorder. BMC psychiatry. 2015 Jan 22;15:2.
15. Colli A, Tanzilli A, Dimaggio G, Lingiardi V. Patient personality and therapist response: An empirical investigation. Am J Psychiatry. 2014 Jan;171(1):102-108.
16. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: Meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010 Jan;71(1):14-25.
We all know these patients:
The young man who, when his name shows up on the ED board, everyone lets out a little groan, knowing his hospital stay will be long and tumultuous.
The middle-aged woman who seems to do want your care and attention and yet rebuffs your attempts to help her, meanwhile, making constant demands on nursing staff.
The older man who trusts no one and will not cooperate with any of his needed care, frustrating staff and physicians alike.
Caring for the patient is integral to the art of doctoring, and yet, there are some people for whom this is incredibly hard to do. They frustrate even the most seasoned professional and work their way under our skin. While their disruptive acts may feel volitional to those of us attempting to provide care, these individuals may suffer from a personality disorder.
In the hospital, a patient must to relate to, and cooperate with, a revolving team of care providers all while under some degree of physical and emotional distress. While this can be destabilizing for even the most resilient patient, for those with personality disorders, it is nearly inevitable that conflict will arise. In a recent article in the Journal of Hospital Medicine, my colleagues and I discussed the management of such patients, with a focus on evidence-based interventions (doi: 10.1002/jhm.2643).2
While the behaviors associated with personality disorders can feel deliberate and even manipulative, research shows that these disorders arise from a complex set of genetic and environmental factors. Alterations found in the serotonin system and regions of the brain involved in emotional reactivity and social processing suggest an underlying neurophysiology contributing to difficulties with interpersonal relationships seen in these disorders.3-9
Many do not realize that having a personality disorder has real implications for an individual’s healthcare outcomes; those with a personality disorder have a life expectancy nearly two decades shorter than the general population.10 While there are a number of factors that likely contribute to the effect on mortality, it has been suggested that dysfunctional personality structures may interfere with the individual’s ability to access and utilize care, resulting in higher morbidity and mortality.11
Although it can be difficult to make a formal diagnosis of a personality disorder on the acute care unit, we provide guideline for recognizing individuals based on the way in which they interact with others. Specifically, we propose a team should consider a personality disorder when the following features are present:
The patient elicits a strong emotional reaction from providers; these may vary markedly between providers
The patient’s emotional responses may appear disproportionate to the inciting event
The patient is on a number of different psychiatric medications with little relief of symptoms
The patient takes up an disproportionate amount of providers’ time
The patient externalizes blame, seeing others as the source of discomfort or distress and therefore sees others as the solution.2
When the team suspects a patient’s behavior may be driven by an underlying dysfunctional personality structure, there are a number of steps that can be taken to help facilitate care and shape behaviors. Key among these is recognizing our own complicated responses to these individuals. These patients evoke strong responses and no team member – from nurses and aides to residents and senior attendings – is immune.12-15
Reactions can range from a need to care for and protect the patient to feelings of futility or contempt.15 Other important behavioral interventions include providing consistency, reinforcing desired behaviors, offering empathy, and providing boundaries while also recognizing the importance of picking your battles.2 Of note, while medications may offer some help, there is limited evidence for use of pharmacological interventions. Although they may be somewhat helpful in addressing particular features of these disorders, such as impulsivity, affective dysregulation or cognitive-perceptual symptoms16, many of these patients end up on a cocktail of psychotropic medications with minimal evidence for their use or efficacy. Thus behavioral management remains the cornerstone of treatment.
While care of the patient with personality disorders can present unique challenges, it offers the opportunity for therapeutic intervention. By appreciating the underlying genetic and environmental factors, we are in a better position to offer empathy and support. For these patients, managing their personality disorder can be just as important as managing any of their other medical comorbidities. By taking an approach that acknowledges the emotional responses of the team while also reinforcing and facilitating positive behaviors of the patient, the hospital stay can prove therapeutic, helping these individuals to develop new skills while also getting their physical needs addressed.
Megan Riddle, MD, PhD, is based in the department of psychiatry and behavioral sciences at the University of Washington, Seattle.
NOTES
1. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA: American Psychiatric Association; 2013.
2. Riddle M, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016 Dec;11(12):873-878.
3. Bukh JD, Bock C, Kessing LV. Association between genetic polymorphisms in the serotonergic system and comorbid personality disorders among patients with first-episode depression. J Pers Disord. 2014 Jun;28(3):365-378.
4. Perez-Rodriguez MM, Weinstein S, New AS, et al. Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatr Res. 2010 Nov; 44(15):1075-1081.
5. Checknita D, Maussion G, Labonte B, et al. Monoamine oxidase: A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry. 2015 Mar;206(3):216-222.
6. Boen E, Westlye LT, Elvsashagen T, et al. Regional cortical thinning may be a biological marker for borderline personality disorder. Acta Psychiatr Scand. 2014 Sep;130(3):193-204.
7. Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci Biobehav Rev. 2013 Mar;37(3):448-470.
8. Liu H, Liao J, Jiang W, Wang W. Changes in low-frequency fluctuations in patients with antisocial personality disorder revealed by resting-state functional MRI. PLoS One. 2014 Mar 5;9(3):e89790.
9. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. 2009 Nov 30;174(2):81-88.
10. Fok ML, Hayes RD, Chang CK, Stewart R, Callard FJ, Moran P. Life expectancy at birth and all-cause mortality among people with personality disorder. J Psychosom Res. 2012 Aug;73(2):104-107.
11. Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015 Feb 21;385:717-726.
12. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978 Apr 20; 298:883-887.
13. Groves JE. Management of the borderline patient on a medical or surgical ward: The psychiatric consultant’s role. Int J Psychiatry Med. 1975;6(3):337-348.
14. Bodner E, Cohen-Fridel S, Mashiah M, et al. The attitudes of psychiatric hospital staff toward hospitalization and treatment of patients with borderline personality disorder. BMC psychiatry. 2015 Jan 22;15:2.
15. Colli A, Tanzilli A, Dimaggio G, Lingiardi V. Patient personality and therapist response: An empirical investigation. Am J Psychiatry. 2014 Jan;171(1):102-108.
16. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: Meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010 Jan;71(1):14-25.
We all know these patients:
The young man who, when his name shows up on the ED board, everyone lets out a little groan, knowing his hospital stay will be long and tumultuous.
The middle-aged woman who seems to do want your care and attention and yet rebuffs your attempts to help her, meanwhile, making constant demands on nursing staff.
The older man who trusts no one and will not cooperate with any of his needed care, frustrating staff and physicians alike.
Caring for the patient is integral to the art of doctoring, and yet, there are some people for whom this is incredibly hard to do. They frustrate even the most seasoned professional and work their way under our skin. While their disruptive acts may feel volitional to those of us attempting to provide care, these individuals may suffer from a personality disorder.
In the hospital, a patient must to relate to, and cooperate with, a revolving team of care providers all while under some degree of physical and emotional distress. While this can be destabilizing for even the most resilient patient, for those with personality disorders, it is nearly inevitable that conflict will arise. In a recent article in the Journal of Hospital Medicine, my colleagues and I discussed the management of such patients, with a focus on evidence-based interventions (doi: 10.1002/jhm.2643).2
While the behaviors associated with personality disorders can feel deliberate and even manipulative, research shows that these disorders arise from a complex set of genetic and environmental factors. Alterations found in the serotonin system and regions of the brain involved in emotional reactivity and social processing suggest an underlying neurophysiology contributing to difficulties with interpersonal relationships seen in these disorders.3-9
Many do not realize that having a personality disorder has real implications for an individual’s healthcare outcomes; those with a personality disorder have a life expectancy nearly two decades shorter than the general population.10 While there are a number of factors that likely contribute to the effect on mortality, it has been suggested that dysfunctional personality structures may interfere with the individual’s ability to access and utilize care, resulting in higher morbidity and mortality.11
Although it can be difficult to make a formal diagnosis of a personality disorder on the acute care unit, we provide guideline for recognizing individuals based on the way in which they interact with others. Specifically, we propose a team should consider a personality disorder when the following features are present:
The patient elicits a strong emotional reaction from providers; these may vary markedly between providers
The patient’s emotional responses may appear disproportionate to the inciting event
The patient is on a number of different psychiatric medications with little relief of symptoms
The patient takes up an disproportionate amount of providers’ time
The patient externalizes blame, seeing others as the source of discomfort or distress and therefore sees others as the solution.2
When the team suspects a patient’s behavior may be driven by an underlying dysfunctional personality structure, there are a number of steps that can be taken to help facilitate care and shape behaviors. Key among these is recognizing our own complicated responses to these individuals. These patients evoke strong responses and no team member – from nurses and aides to residents and senior attendings – is immune.12-15
Reactions can range from a need to care for and protect the patient to feelings of futility or contempt.15 Other important behavioral interventions include providing consistency, reinforcing desired behaviors, offering empathy, and providing boundaries while also recognizing the importance of picking your battles.2 Of note, while medications may offer some help, there is limited evidence for use of pharmacological interventions. Although they may be somewhat helpful in addressing particular features of these disorders, such as impulsivity, affective dysregulation or cognitive-perceptual symptoms16, many of these patients end up on a cocktail of psychotropic medications with minimal evidence for their use or efficacy. Thus behavioral management remains the cornerstone of treatment.
While care of the patient with personality disorders can present unique challenges, it offers the opportunity for therapeutic intervention. By appreciating the underlying genetic and environmental factors, we are in a better position to offer empathy and support. For these patients, managing their personality disorder can be just as important as managing any of their other medical comorbidities. By taking an approach that acknowledges the emotional responses of the team while also reinforcing and facilitating positive behaviors of the patient, the hospital stay can prove therapeutic, helping these individuals to develop new skills while also getting their physical needs addressed.
Megan Riddle, MD, PhD, is based in the department of psychiatry and behavioral sciences at the University of Washington, Seattle.
NOTES
1. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA: American Psychiatric Association; 2013.
2. Riddle M, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016 Dec;11(12):873-878.
3. Bukh JD, Bock C, Kessing LV. Association between genetic polymorphisms in the serotonergic system and comorbid personality disorders among patients with first-episode depression. J Pers Disord. 2014 Jun;28(3):365-378.
4. Perez-Rodriguez MM, Weinstein S, New AS, et al. Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatr Res. 2010 Nov; 44(15):1075-1081.
5. Checknita D, Maussion G, Labonte B, et al. Monoamine oxidase: A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry. 2015 Mar;206(3):216-222.
6. Boen E, Westlye LT, Elvsashagen T, et al. Regional cortical thinning may be a biological marker for borderline personality disorder. Acta Psychiatr Scand. 2014 Sep;130(3):193-204.
7. Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci Biobehav Rev. 2013 Mar;37(3):448-470.
8. Liu H, Liao J, Jiang W, Wang W. Changes in low-frequency fluctuations in patients with antisocial personality disorder revealed by resting-state functional MRI. PLoS One. 2014 Mar 5;9(3):e89790.
9. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. 2009 Nov 30;174(2):81-88.
10. Fok ML, Hayes RD, Chang CK, Stewart R, Callard FJ, Moran P. Life expectancy at birth and all-cause mortality among people with personality disorder. J Psychosom Res. 2012 Aug;73(2):104-107.
11. Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015 Feb 21;385:717-726.
12. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978 Apr 20; 298:883-887.
13. Groves JE. Management of the borderline patient on a medical or surgical ward: The psychiatric consultant’s role. Int J Psychiatry Med. 1975;6(3):337-348.
14. Bodner E, Cohen-Fridel S, Mashiah M, et al. The attitudes of psychiatric hospital staff toward hospitalization and treatment of patients with borderline personality disorder. BMC psychiatry. 2015 Jan 22;15:2.
15. Colli A, Tanzilli A, Dimaggio G, Lingiardi V. Patient personality and therapist response: An empirical investigation. Am J Psychiatry. 2014 Jan;171(1):102-108.
16. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: Meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010 Jan;71(1):14-25.
Why ustekinumab dosing differs in Crohn’s disease
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
Guidelines for diagnosing TB in adults, children
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
Major finding: The clinical practice guideline includes 23 evidence-based recommendations concerning diagnostic testing for latent, pulmonary, or extrapulmonary tuberculosis in adults and children.
Data source: A compilation of 23 evidence-based recommendations about diagnostic testing for tuberculosis.
Disclosures: This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Non-MD CT Surgical Team Scientific Poster Opportunity
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
Non-MD CT Surgical Team Scientific Poster Opportunity
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition. Winning posters will be displayed at the AATS Centennial, April 29 – May 3 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
Deadline: January 20, 2017
Share:
First drug for spinal muscular atrophy approved
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
Ischemia-repairing cells fall short for treating intermittent claudication
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: At 6 months, peak walking time had increased by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, a nonsignificant difference (P = .238).
Data source: PACE, a randomized phase II trial of 82 patients with PAD and symptom-limiting intermittent claudication of the legs.
Disclosures: Dr. Perin received a research grant from the National Heart, Lung, and Blood Institute.
A Rare Association in Down Syndrome: Milialike Idiopathic Calcinosis Cutis and Palpebral Syringoma
To the Editor:
Down syndrome (DS) is associated with rare dermatological disorders, and the prevalence of some common dermatoses is greater in patients with DS. We report a case of milialike idiopathic calcinosis cutis (MICC) associated with syringomas in a patient with DS. We emphasize that MICC is one of the rare dermatoses associated with DS.
A 4-year-old girl with DS presented with a 4-mm, flesh-colored, regular-bordered, exophytic papular lesion on the left upper eyelid of 8 months' duration (Figure 1). It was clinically recognized as syringoma. On dermatologic examination of the patient, there also were 1- to 3-mm, round, whitish, painless, milialike papules on the dorsal surface of the hands and wrists (Figure 2). Some of these papules were surrounded by erythema. There was no sign of perforation. Her personal and family history were unremarkable.
Histopathologic examination of a biopsy from a milialike lesion on the hand showed a hyperkeratotic epidermis. In the dermis there was a roundish calcific nodule surrounded by a fibrovascular rim. The patient's guardians refused a biopsy from the lesion on the eyelid.
Laboratory tests including serum vitamin D, thyroid and parathyroid hormone, calcium, phosphorus, and urinary calcium levels, as well as renal function tests, were within reference range. On the basis of these clinical and histopathological findings, the patient was diagnosed with MICC and palpebral syringoma.
Many dermatoses associated with DS have been reported including elastosis perforans serpiginosa, alopecia areata, and syringomas.1-3 Sano et al4 first described MICC and syringomas in a patient with DS in 1978. Milialike idiopathic calcinosis cutis is characterized by asymptomatic, millimetric, firm, round, whitish papules that are sometimes surrounded by erythema. These papules may show perforation leading to transepidermal elimination of calcium, similar to the transdermal elimination of elastic fibrils in elastosis perforans serpiginosa. Although MICC usually is described in acral sites of children with DS, it also is reported in adults without DS and on other parts of the body.5-7
The cause of MICC is unknown. One hypothesis of the development of MICC is an increase of the calcium content in the sweat leading to calcification of the acrosyringium.8 Milia are small keratin cysts that usually develop by occlusion of the hair follicle, sweat duct, or sebaceous duct. However, milia also can occur from occlusion of the eccrine ducts where syringomas originate.9 Therefore, syringomas can be seen in association with milia and calcium deposits.5,9-11
We believe that MICC in DS may be more common than usually recognized, as the lesions often are asymptomatic. It is important to differentiate MICC from other dermatological diseases such as molluscum contagiosum, verruca plana, milia, and inclusion cysts. Histopathology and dermoscopy could aid in the accurate diagnosis of MICC.
- Dourmishev A, Miteva L, Mitev V, et al. Cutaneous aspects of Down syndrome. Cutis. 2000;66:420-424.
- Madan V, Williams J, Lear JT. Dermatological manifestations of Down's syndrome. Clin Exp Dermatol. 2006;31:623-629.
- Schepis C, Barone C, Siragusa M, et al. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205:234-238.
- Sano T, Tate S, Ishikawa C. A case of Down's syndrome associated with syringoma, milia, and subepidermal calcified nodule. Jpn J Dermatol. 1978;88:740.
- Schepis C, Siragusa M, Palazzo R, et al. Perforating milia-like idiopathic calcinosis cutis and periorbital syringomas in a girl with Down syndrome. Pediatr Dermatol. 1994;11:258-260.
- Schepis C, Siragusa M, Palazzo R, et al. Milia like idiopathic calcinosis cutis: an unusual dermatosis associated with Down syndrome. Br J Dermatol. 1996;134:143-146.
- Houtappel M, Leguit R, Sigurdsson V. Milia-like idiopathic calcinosis cutis in an adult without Down's syndrome. J Dermatol Case Rep. 2007;1:16-19.
- Eng AM, Mandrea E. Perforating calcinosis cutis presenting as milia. J Cutan Pathol. 1981;8:247-250.
- Wang KH, Chu JS, Lin YH, et al. Milium-like syringoma: a case study on histogenesis. J Cutan Pathol. 2004;31:336-340.
- Weiss E, Paez E, Greenberg AS, et al. Eruptive syringomas associated with milia. Int J Dermatol. 1995;34:193-195.
- Kim SJ, Won YH, Chun IK. Subepidermal calcified nodules and syringoma. J Eur Acad Dermatol Venereol. 1997;8:51-52.
To the Editor:
Down syndrome (DS) is associated with rare dermatological disorders, and the prevalence of some common dermatoses is greater in patients with DS. We report a case of milialike idiopathic calcinosis cutis (MICC) associated with syringomas in a patient with DS. We emphasize that MICC is one of the rare dermatoses associated with DS.
A 4-year-old girl with DS presented with a 4-mm, flesh-colored, regular-bordered, exophytic papular lesion on the left upper eyelid of 8 months' duration (Figure 1). It was clinically recognized as syringoma. On dermatologic examination of the patient, there also were 1- to 3-mm, round, whitish, painless, milialike papules on the dorsal surface of the hands and wrists (Figure 2). Some of these papules were surrounded by erythema. There was no sign of perforation. Her personal and family history were unremarkable.


Histopathologic examination of a biopsy from a milialike lesion on the hand showed a hyperkeratotic epidermis. In the dermis there was a roundish calcific nodule surrounded by a fibrovascular rim. The patient's guardians refused a biopsy from the lesion on the eyelid.
Laboratory tests including serum vitamin D, thyroid and parathyroid hormone, calcium, phosphorus, and urinary calcium levels, as well as renal function tests, were within reference range. On the basis of these clinical and histopathological findings, the patient was diagnosed with MICC and palpebral syringoma.
Many dermatoses associated with DS have been reported including elastosis perforans serpiginosa, alopecia areata, and syringomas.1-3 Sano et al4 first described MICC and syringomas in a patient with DS in 1978. Milialike idiopathic calcinosis cutis is characterized by asymptomatic, millimetric, firm, round, whitish papules that are sometimes surrounded by erythema. These papules may show perforation leading to transepidermal elimination of calcium, similar to the transdermal elimination of elastic fibrils in elastosis perforans serpiginosa. Although MICC usually is described in acral sites of children with DS, it also is reported in adults without DS and on other parts of the body.5-7
The cause of MICC is unknown. One hypothesis of the development of MICC is an increase of the calcium content in the sweat leading to calcification of the acrosyringium.8 Milia are small keratin cysts that usually develop by occlusion of the hair follicle, sweat duct, or sebaceous duct. However, milia also can occur from occlusion of the eccrine ducts where syringomas originate.9 Therefore, syringomas can be seen in association with milia and calcium deposits.5,9-11
We believe that MICC in DS may be more common than usually recognized, as the lesions often are asymptomatic. It is important to differentiate MICC from other dermatological diseases such as molluscum contagiosum, verruca plana, milia, and inclusion cysts. Histopathology and dermoscopy could aid in the accurate diagnosis of MICC.
To the Editor:
Down syndrome (DS) is associated with rare dermatological disorders, and the prevalence of some common dermatoses is greater in patients with DS. We report a case of milialike idiopathic calcinosis cutis (MICC) associated with syringomas in a patient with DS. We emphasize that MICC is one of the rare dermatoses associated with DS.
A 4-year-old girl with DS presented with a 4-mm, flesh-colored, regular-bordered, exophytic papular lesion on the left upper eyelid of 8 months' duration (Figure 1). It was clinically recognized as syringoma. On dermatologic examination of the patient, there also were 1- to 3-mm, round, whitish, painless, milialike papules on the dorsal surface of the hands and wrists (Figure 2). Some of these papules were surrounded by erythema. There was no sign of perforation. Her personal and family history were unremarkable.


Histopathologic examination of a biopsy from a milialike lesion on the hand showed a hyperkeratotic epidermis. In the dermis there was a roundish calcific nodule surrounded by a fibrovascular rim. The patient's guardians refused a biopsy from the lesion on the eyelid.
Laboratory tests including serum vitamin D, thyroid and parathyroid hormone, calcium, phosphorus, and urinary calcium levels, as well as renal function tests, were within reference range. On the basis of these clinical and histopathological findings, the patient was diagnosed with MICC and palpebral syringoma.
Many dermatoses associated with DS have been reported including elastosis perforans serpiginosa, alopecia areata, and syringomas.1-3 Sano et al4 first described MICC and syringomas in a patient with DS in 1978. Milialike idiopathic calcinosis cutis is characterized by asymptomatic, millimetric, firm, round, whitish papules that are sometimes surrounded by erythema. These papules may show perforation leading to transepidermal elimination of calcium, similar to the transdermal elimination of elastic fibrils in elastosis perforans serpiginosa. Although MICC usually is described in acral sites of children with DS, it also is reported in adults without DS and on other parts of the body.5-7
The cause of MICC is unknown. One hypothesis of the development of MICC is an increase of the calcium content in the sweat leading to calcification of the acrosyringium.8 Milia are small keratin cysts that usually develop by occlusion of the hair follicle, sweat duct, or sebaceous duct. However, milia also can occur from occlusion of the eccrine ducts where syringomas originate.9 Therefore, syringomas can be seen in association with milia and calcium deposits.5,9-11
We believe that MICC in DS may be more common than usually recognized, as the lesions often are asymptomatic. It is important to differentiate MICC from other dermatological diseases such as molluscum contagiosum, verruca plana, milia, and inclusion cysts. Histopathology and dermoscopy could aid in the accurate diagnosis of MICC.
- Dourmishev A, Miteva L, Mitev V, et al. Cutaneous aspects of Down syndrome. Cutis. 2000;66:420-424.
- Madan V, Williams J, Lear JT. Dermatological manifestations of Down's syndrome. Clin Exp Dermatol. 2006;31:623-629.
- Schepis C, Barone C, Siragusa M, et al. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205:234-238.
- Sano T, Tate S, Ishikawa C. A case of Down's syndrome associated with syringoma, milia, and subepidermal calcified nodule. Jpn J Dermatol. 1978;88:740.
- Schepis C, Siragusa M, Palazzo R, et al. Perforating milia-like idiopathic calcinosis cutis and periorbital syringomas in a girl with Down syndrome. Pediatr Dermatol. 1994;11:258-260.
- Schepis C, Siragusa M, Palazzo R, et al. Milia like idiopathic calcinosis cutis: an unusual dermatosis associated with Down syndrome. Br J Dermatol. 1996;134:143-146.
- Houtappel M, Leguit R, Sigurdsson V. Milia-like idiopathic calcinosis cutis in an adult without Down's syndrome. J Dermatol Case Rep. 2007;1:16-19.
- Eng AM, Mandrea E. Perforating calcinosis cutis presenting as milia. J Cutan Pathol. 1981;8:247-250.
- Wang KH, Chu JS, Lin YH, et al. Milium-like syringoma: a case study on histogenesis. J Cutan Pathol. 2004;31:336-340.
- Weiss E, Paez E, Greenberg AS, et al. Eruptive syringomas associated with milia. Int J Dermatol. 1995;34:193-195.
- Kim SJ, Won YH, Chun IK. Subepidermal calcified nodules and syringoma. J Eur Acad Dermatol Venereol. 1997;8:51-52.
- Dourmishev A, Miteva L, Mitev V, et al. Cutaneous aspects of Down syndrome. Cutis. 2000;66:420-424.
- Madan V, Williams J, Lear JT. Dermatological manifestations of Down's syndrome. Clin Exp Dermatol. 2006;31:623-629.
- Schepis C, Barone C, Siragusa M, et al. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205:234-238.
- Sano T, Tate S, Ishikawa C. A case of Down's syndrome associated with syringoma, milia, and subepidermal calcified nodule. Jpn J Dermatol. 1978;88:740.
- Schepis C, Siragusa M, Palazzo R, et al. Perforating milia-like idiopathic calcinosis cutis and periorbital syringomas in a girl with Down syndrome. Pediatr Dermatol. 1994;11:258-260.
- Schepis C, Siragusa M, Palazzo R, et al. Milia like idiopathic calcinosis cutis: an unusual dermatosis associated with Down syndrome. Br J Dermatol. 1996;134:143-146.
- Houtappel M, Leguit R, Sigurdsson V. Milia-like idiopathic calcinosis cutis in an adult without Down's syndrome. J Dermatol Case Rep. 2007;1:16-19.
- Eng AM, Mandrea E. Perforating calcinosis cutis presenting as milia. J Cutan Pathol. 1981;8:247-250.
- Wang KH, Chu JS, Lin YH, et al. Milium-like syringoma: a case study on histogenesis. J Cutan Pathol. 2004;31:336-340.
- Weiss E, Paez E, Greenberg AS, et al. Eruptive syringomas associated with milia. Int J Dermatol. 1995;34:193-195.
- Kim SJ, Won YH, Chun IK. Subepidermal calcified nodules and syringoma. J Eur Acad Dermatol Venereol. 1997;8:51-52.
Practice Points
- Down syndrome is associated with rare dermatological disorders and an increased prevalence of common dermatoses.
- It is important to differentiate milialike idiopathic calcinosis cutis from other dermatological diseases using histopathology and dermoscopy.