User login
Diabetes-related kidney failure down sharply in Native Americans
Kidney failure in Native Americans and Alaska Natives with diabetes has declined drastically over the last 20 years, according to new data released as part of this month’s Vital Signs report by the CDC.
“The 54 percent decline in kidney failure from diabetes followed implementation of public health and population approaches to diabetes as well as improvements in clinical care by the IHS [Indian Health Service],” said Mary L. Smith, principal deputy director of the Indian Health Service.
Of all U.S.-based populations, Native Americans are the most susceptible to diabetes and are about twice as likely as white Americans to develop diabetes. Furthermore, 69% of kidney failure deaths in Native Americans are the result of diabetes (MMWR. 2017 Jan 10. doi: 10.15585/mmwr.mm6601e1).
Since 1996, however, kidney failure has dropped more among Native Americans than any other ethnic group in the country. The 54% drop represents a decrease from 57.3 diabetes-related end-stage renal disease cases per 100,000 population in 1996 to 26.5 per 100,000 population in 2013 among U.S. adults.
“This decline is especially remarkable given the well-documented health and socioeconomic disparities in the [Native American and Alaska Natives] population, including poverty, limited health care resources, and disproportionate burden of many health problems,” wrote the authors of the Vital Signs report.
According to the report, blood sugar control among Native American populations has improved by 10%, kidney testing in diabetic Native Americans aged 65 years or older is 50% greater than Medicare diabetes patients of the same age, and the average blood pressure of Native Americans with both diabetes and hypertension was 133/76 in 2015.
“We believe these strategies can be effective in any population,” Ms. Smith stated, a sentiment that was also shared by Tom Frieden, MD, director of the CDC.
“Strong coordinated clinical care and education, community outreach and environmental changes can make a dramatic difference in reducing complications from diabetes for all Americans,” Dr. Frieden said in a statement.
Not only does diabetes persist as a significant burden on the U.S. health care system, but kidney failure in particular can be costly. Figures released by the CDC indicate that average medical costs associated with kidney failure in 2013 were as high as $82,000 per patient, with Medicare spending nearly $14 billion for kidney failure treatments in the same year.
“The findings in this report are consistent with other studies among [Native Americans and Alaska Natives] nationwide and among Pima Indians in the Southwest, which concluded that improvements in blood pressure, blood glucose, and the use of ACE inhibitors and [angiotensin II receptor blockers] played a significant role in the decline of [diabetes-related end-stage renal disease] in these populations,” the report concludes.
To ensure that kidney failure decreases continue in Native Americans, the U.S. government will continue funding diabetes screening and prevention efforts in applicable communities, assist community health care facilities to provide care for diabetes, and will establish a nationwide system for tracking chronic kidney disease. The CDC also advocates using population approaches and coordinated care to treat diabetes, advising health care professionals to “integrate kidney disease prevention and education into routine diabetes care.”
“The Indian Health Service has made tremendous progress by applying population health and team-based approaches to diabetes and kidney care,” Dr. Frieden stated.
Kidney failure in Native Americans and Alaska Natives with diabetes has declined drastically over the last 20 years, according to new data released as part of this month’s Vital Signs report by the CDC.
“The 54 percent decline in kidney failure from diabetes followed implementation of public health and population approaches to diabetes as well as improvements in clinical care by the IHS [Indian Health Service],” said Mary L. Smith, principal deputy director of the Indian Health Service.
Of all U.S.-based populations, Native Americans are the most susceptible to diabetes and are about twice as likely as white Americans to develop diabetes. Furthermore, 69% of kidney failure deaths in Native Americans are the result of diabetes (MMWR. 2017 Jan 10. doi: 10.15585/mmwr.mm6601e1).
Since 1996, however, kidney failure has dropped more among Native Americans than any other ethnic group in the country. The 54% drop represents a decrease from 57.3 diabetes-related end-stage renal disease cases per 100,000 population in 1996 to 26.5 per 100,000 population in 2013 among U.S. adults.
“This decline is especially remarkable given the well-documented health and socioeconomic disparities in the [Native American and Alaska Natives] population, including poverty, limited health care resources, and disproportionate burden of many health problems,” wrote the authors of the Vital Signs report.
According to the report, blood sugar control among Native American populations has improved by 10%, kidney testing in diabetic Native Americans aged 65 years or older is 50% greater than Medicare diabetes patients of the same age, and the average blood pressure of Native Americans with both diabetes and hypertension was 133/76 in 2015.
“We believe these strategies can be effective in any population,” Ms. Smith stated, a sentiment that was also shared by Tom Frieden, MD, director of the CDC.
“Strong coordinated clinical care and education, community outreach and environmental changes can make a dramatic difference in reducing complications from diabetes for all Americans,” Dr. Frieden said in a statement.
Not only does diabetes persist as a significant burden on the U.S. health care system, but kidney failure in particular can be costly. Figures released by the CDC indicate that average medical costs associated with kidney failure in 2013 were as high as $82,000 per patient, with Medicare spending nearly $14 billion for kidney failure treatments in the same year.
“The findings in this report are consistent with other studies among [Native Americans and Alaska Natives] nationwide and among Pima Indians in the Southwest, which concluded that improvements in blood pressure, blood glucose, and the use of ACE inhibitors and [angiotensin II receptor blockers] played a significant role in the decline of [diabetes-related end-stage renal disease] in these populations,” the report concludes.
To ensure that kidney failure decreases continue in Native Americans, the U.S. government will continue funding diabetes screening and prevention efforts in applicable communities, assist community health care facilities to provide care for diabetes, and will establish a nationwide system for tracking chronic kidney disease. The CDC also advocates using population approaches and coordinated care to treat diabetes, advising health care professionals to “integrate kidney disease prevention and education into routine diabetes care.”
“The Indian Health Service has made tremendous progress by applying population health and team-based approaches to diabetes and kidney care,” Dr. Frieden stated.
Kidney failure in Native Americans and Alaska Natives with diabetes has declined drastically over the last 20 years, according to new data released as part of this month’s Vital Signs report by the CDC.
“The 54 percent decline in kidney failure from diabetes followed implementation of public health and population approaches to diabetes as well as improvements in clinical care by the IHS [Indian Health Service],” said Mary L. Smith, principal deputy director of the Indian Health Service.
Of all U.S.-based populations, Native Americans are the most susceptible to diabetes and are about twice as likely as white Americans to develop diabetes. Furthermore, 69% of kidney failure deaths in Native Americans are the result of diabetes (MMWR. 2017 Jan 10. doi: 10.15585/mmwr.mm6601e1).
Since 1996, however, kidney failure has dropped more among Native Americans than any other ethnic group in the country. The 54% drop represents a decrease from 57.3 diabetes-related end-stage renal disease cases per 100,000 population in 1996 to 26.5 per 100,000 population in 2013 among U.S. adults.
“This decline is especially remarkable given the well-documented health and socioeconomic disparities in the [Native American and Alaska Natives] population, including poverty, limited health care resources, and disproportionate burden of many health problems,” wrote the authors of the Vital Signs report.
According to the report, blood sugar control among Native American populations has improved by 10%, kidney testing in diabetic Native Americans aged 65 years or older is 50% greater than Medicare diabetes patients of the same age, and the average blood pressure of Native Americans with both diabetes and hypertension was 133/76 in 2015.
“We believe these strategies can be effective in any population,” Ms. Smith stated, a sentiment that was also shared by Tom Frieden, MD, director of the CDC.
“Strong coordinated clinical care and education, community outreach and environmental changes can make a dramatic difference in reducing complications from diabetes for all Americans,” Dr. Frieden said in a statement.
Not only does diabetes persist as a significant burden on the U.S. health care system, but kidney failure in particular can be costly. Figures released by the CDC indicate that average medical costs associated with kidney failure in 2013 were as high as $82,000 per patient, with Medicare spending nearly $14 billion for kidney failure treatments in the same year.
“The findings in this report are consistent with other studies among [Native Americans and Alaska Natives] nationwide and among Pima Indians in the Southwest, which concluded that improvements in blood pressure, blood glucose, and the use of ACE inhibitors and [angiotensin II receptor blockers] played a significant role in the decline of [diabetes-related end-stage renal disease] in these populations,” the report concludes.
To ensure that kidney failure decreases continue in Native Americans, the U.S. government will continue funding diabetes screening and prevention efforts in applicable communities, assist community health care facilities to provide care for diabetes, and will establish a nationwide system for tracking chronic kidney disease. The CDC also advocates using population approaches and coordinated care to treat diabetes, advising health care professionals to “integrate kidney disease prevention and education into routine diabetes care.”
“The Indian Health Service has made tremendous progress by applying population health and team-based approaches to diabetes and kidney care,” Dr. Frieden stated.
Cardiologist care for newly diagnosed A-fib improves outcomes
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The incidence of ischemic stroke in patients who saw a cardiologist for their newly diagnosed atrial fibrillation was 7.6 per 1,000 person-years, significantly lower than the 8.8 per 1,000 in those cared for exclusively by primary care physicians.
Data source: The TREAT-AF study was a national retrospective cohort study of 181,161 Veterans Affairs patients with newly diagnosed AF during 2003-2012.
Disclosures: The study presenter reported having no financial conflicts of interest.
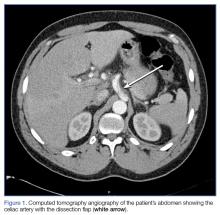
Dissection of the Celiac Artery
Case
A 41-year-old man presented to our ED with a 4-day history of epigastric pain radiating to the bilateral flanks and back. His medical history was significant for hypertension, for which he was prescribed isosorbide dinitrite 30 mg four times per day; however, he reported that he did not regularly take this medication.
The patient had visited our ED 3 days earlier with the same complaint. Since his blood pressure (BP) reading at the first ED presentation was 213/141 mm Hg, he had been admitted for hypertensive urgency. The patient’s BP was controlled with antihypertensive agents during his stay, but he continued to experience epigastric pain. A basic work-up for abdominal pain was ordered, the results of which were normal. Based on these findings, the patient’s pain was attributed to gastritis, and he was discharged home with instructions to return to the ED if his pain became worse or persisted.
At both ED presentations, the patient denied experiencing any nausea, vomiting, diarrhea, or chest pain. At the second presentation, his triage BP was 158/106 mm Hg. A chest X-ray, complete blood count (CBC), basic metabolic profile (BMP), hepatic panel, and lipase evaluation were all unremarkable, with the exception of a mild increase in creatinine to 1.38 mg/dL. A point-of-care (POC) ultrasound study of the aorta was normal.
Based on the CTA findings, a nicardipine infusion was immediately started, and the patient was admitted to the medical intensive care unit (MICU). Because his heart rate was in the range of 60 beats/min, an esmolol infusion was not required. Prior to transferring the patient to MICU, a second ultrasound study of the aorta was performed by our fellowship-trained director of emergency medicine ultrasound.
In the MICU, the patient’s BP was stabilized on hospital day 2, and he was transitioned to oral antihypertensive medications. He was also started on a heparin infusion at the recommendation of vascular surgery services.
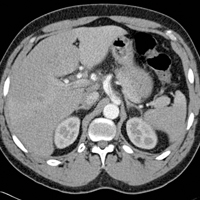
A repeat CTA of the abdomen taken on hospital day 3 showed an unchanged dissection in the celiac axis extending into the hepatic artery. The vascular surgeon recommended strict BP control, anticoagulation therapy, and a vascular surgery follow-up with a repeat CTA of the abdomen in 6 months.
On hospital day 6, repeat serial CBC, BMP, and hepatic panels revealed only slight increases in aspartate transaminase to 88 U/L and alanine aminotransferase to 117 U/L. The patient was transitioned to enoxaparin and discharged home on hospital day 6, and instructed to follow-up with his primary care physician for transition to warfarin. Unfortunately, this patient was lost to follow-up.
Discussion
Isolated DCA is a rare cause of abdominal pain. The first documented case of isolated DCA is often incorrectly attributed to Bauersfeld’s1 1947 case series on dissections,but that report described superior mesenteric artery dissection rather than a celiac artery dissection. Watson’s2 1956 dissection series is also incorrectly cited as the first DCA, but that series described a dissection of the splenic artery, which is a branch of the celiac artery. In a 1959 series, Foord and Lewis3 described what is most likely the first report of DCA as an incidental finding at autopsy. More frequent descriptions in recent years are thought to be due to the routine use of abdominal CTA.4
Dissection of the celiac artery is a rare occurrence, with less than 100 cases reported, and little evidence exists to guide its management.5 These dissections represent 36.8% of all visceral artery dissections,6 which themselves are less common than renal, carotid, and vertebral artery dissections.7 Dissection of visceral arteries occurs predominantly in men and more often in middle-aged patients.8 Risk factors for DCA are thought to mirror risk factors for dissection of other arteries, including atherosclerotic disease, hypertension, connective tissue disorders, trauma, vasculitis, and pregnancy.9-11
Signs and Symptoms
Patients with DCA typically present with sudden onset of epigastric, flank, and/or chest pain, though 50% of patients may be asymptomatic.12 This pain is easily overlooked because the physical examination and laboratory studies are typically unremarkable.13 Fortunately, DCA is rarely accompanied by fatal organ dysfunction due to collateral flow from other vessels.14
Diagnosis and Management
While CTA with contrast is considered the mainstay of diagnosis of DCA,15 optimal treatment for DCA has not been well established. Management options include medical management, operative repair, and endovascular embolization. Medical management is reserved for stable patients without signs of end organ dysfunction. Typical management involves anticoagulation with warfarin for 3 to 6 months and strict BP control accompanied by close surveillance for progression.10,13 Some clinicians have argued that anticoagulation therapy may be unnecessary and that risk factor modification and BP control alone may be sufficient.5,6 Others have advocated that surgical management should be favored in cases of persistent pain, development of aneurysm, or threatened or compromised flow to end organs.7
Point-of-Care Ultrasound
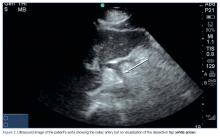
The American College of Emergency Physicians considers ultrasound of the abdominal aorta a core application of emergency ultrasound.16 While sensitivity and specificity of emergency ultrasound for abdominal aortic aneurysm are well established, data supporting its use for screening for dissections are less definitive. With a sensitivity of 67% to 80% and a specificity of 99% to 100% with visualization of an intimal flap, aortic dissection screening using ultrasound is less reliable than most emergency physicians (EPs) would prefer.17,18 There are no published data reporting the sensitivity or specificity of emergency ultrasound for DCA. However, the vascular surgery literature encourages color Doppler ultrasound as part of the initial diagnostic work-up for this rare entity.19 While this may seem like an area ripe for emergency ultrasound, it is important to note—as seen in our case—that the site of the dissection is not often seen. Instead, the use of Doppler allows a screening for an abnormal flow pattern suggestive of dissection.20
Conclusion
In our case, both resident EPs and an expert fellowship-trained emergency ultrasound attending physician were unable to visualize a dissection—even after knowledge of the lesion was established by CTA. This points out a limitation of emergency ultrasound. While a POC ultrasound may be able to effectively rule in dissections of the aorta and its branches, we cannot reliably rule out these lesions. As EPs continue to expand the use of ultrasound, it is important to balance the desire for efficiency and cost-effectiveness with a high index of suspicion, experience, and clinical acumen.
1. Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947;26(6):873-889.
2. Watson AJ. Dissecting aneurysm of arteries other than the aorta. J Pathol. 1956;72(2):439-449. doi:10.1002/path.1700720209.
3. Foord AG, Lewis RD. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch Pathol. 1959;68:553-577.
4. Neychev V, Krol E, Dietzek A. Unusual presentation and treatment of spontaneous celiac artery dissection. J Vasc Surg. 2013;58(2):491-495. doi:10.1016/j.jvs.2012.10.136.
5. DiMusto PD, Oberdoerster MM, Criado E. Isolated celiac artery dissection. J Vasc Surg. 2015;61(4):972-976. doi: 10.1016/j.jvs.2014.10.108.
6. Takayama T, Miyata T, Shirakawa M, Nagawa H. J Vasc Surg. 2008;48(2):329-333. doi:10.1016/j.jvs.2008.03.002.
7. Glehen O, Feugier P, Aleksic Y, Delannoy P, Chevalier JM. Spontaneous dissection of the celiac artery. Ann Vasc Surg. 2001;15(6):687-692.
8. Patel KS, Benshar O, Vrabie R, Patel A, Adler M, Hines G. A major pain in the … back and epigastrium: an unusual case of spontaneous celiac artery dissection. J Community Hosp Intern Med Perspect. 2014;4(5):23840. doi:10.3402/jchimp.v4.23840.
9. Kang TL, Teich DL, McGillicuddy DC. Isolated, spontaneous superior mesenteric and celiac artery dissection: case report and review of literature. J Emerg Med. 2011;40(2):e21-e25. doi:10.1016/j.jemermed.2007.12.038.
10. Galastri FL, Cavalcante RN, Motta-Leal-Filho JM, et al. Evaluation and management of symptomatic isolated spontaneous celiac trunk dissection. Vasc Med. 2015;20(4):358-363. doi:10.1177/1358863X15581447.
11. Wang HC, Chen JH, Hsiao CC, Jeng CM, Chen WL. Spontaneous dissection of the celiac artery: a case report and literature review. Am J Emerg Med. 2013;31(6):1000.e3-e5. doi:10.1016/j.ajem.2013.02.007.
12. Oh S, Cho YP, Kim JH, Shin S, Kwon TW, Ko GY. Symptomatic spontaneous celiac artery dissection treated by conservative management: serial imaging findings. Abdom Imaging. 2011;36(1):79-82. doi:10.1007/s00261-010-9657-x.
13. Wang JL, Hsieh MJ, Lee CH, Chen CC, Hsieh IC. Celiac artery dissection presenting with abdominal and chest pain. Am J Emerg Med. 2010;28(1):111.e3-e5. doi:10.1016/j.ajem.2009.02.023.
14. Takayama Y, Takao M, Inoue T, Yoshimi F, Koyama K, Nagai H. Isolated spontaneous dissection of the celiac artery: report of two cases. Ann Vasc Dis. 2014;7(1):64-67. doi:10.3400/avd.cr.13-00102.
15. Rehman AU, Almanfi A, Nadella S, Sohail U. Isolated spontaneous celiac artery dissection in a 47-year-old man with von Willebrand disease. Tex Heart Inst J. 2014;41(3):344-345. doi:10.14503/THIJ-13-3404.
16. American College of Emergency Physicians. Policy statement. Ultrasound Guidelines: Emergency, Point-of-Care, and Clinical Ultrasound Guidelines in Medicine, June 2016. https://www.acep.org/Clinical---Practice-Management/Ultrasound/. Accessed November 15, 2016.
17. Williams J, Heiner JD, Perreault MD, McArthur TJ. Aortic dissection diagnosed by ultrasound. West J Emerg Med. 2010;11(1):98-99.
18. Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
19. Woolard JD, Ammar AD. Spontaneous dissection of the celiac artery: a case report. J Vasc Surg. 2007;45(6):1256-1258.
20. Fenoglio L, Allione A, Scalabrino E, et al. Spontaneous dissection of the celiac artery: a pitfall in the diagnosis of acute abdominal pain. Presentation of two cases. Dig Dis Sci. 2004;49(7-8):1223-1227.
Case
A 41-year-old man presented to our ED with a 4-day history of epigastric pain radiating to the bilateral flanks and back. His medical history was significant for hypertension, for which he was prescribed isosorbide dinitrite 30 mg four times per day; however, he reported that he did not regularly take this medication.
The patient had visited our ED 3 days earlier with the same complaint. Since his blood pressure (BP) reading at the first ED presentation was 213/141 mm Hg, he had been admitted for hypertensive urgency. The patient’s BP was controlled with antihypertensive agents during his stay, but he continued to experience epigastric pain. A basic work-up for abdominal pain was ordered, the results of which were normal. Based on these findings, the patient’s pain was attributed to gastritis, and he was discharged home with instructions to return to the ED if his pain became worse or persisted.
At both ED presentations, the patient denied experiencing any nausea, vomiting, diarrhea, or chest pain. At the second presentation, his triage BP was 158/106 mm Hg. A chest X-ray, complete blood count (CBC), basic metabolic profile (BMP), hepatic panel, and lipase evaluation were all unremarkable, with the exception of a mild increase in creatinine to 1.38 mg/dL. A point-of-care (POC) ultrasound study of the aorta was normal.
Based on the CTA findings, a nicardipine infusion was immediately started, and the patient was admitted to the medical intensive care unit (MICU). Because his heart rate was in the range of 60 beats/min, an esmolol infusion was not required. Prior to transferring the patient to MICU, a second ultrasound study of the aorta was performed by our fellowship-trained director of emergency medicine ultrasound.
In the MICU, the patient’s BP was stabilized on hospital day 2, and he was transitioned to oral antihypertensive medications. He was also started on a heparin infusion at the recommendation of vascular surgery services.
A repeat CTA of the abdomen taken on hospital day 3 showed an unchanged dissection in the celiac axis extending into the hepatic artery. The vascular surgeon recommended strict BP control, anticoagulation therapy, and a vascular surgery follow-up with a repeat CTA of the abdomen in 6 months.
On hospital day 6, repeat serial CBC, BMP, and hepatic panels revealed only slight increases in aspartate transaminase to 88 U/L and alanine aminotransferase to 117 U/L. The patient was transitioned to enoxaparin and discharged home on hospital day 6, and instructed to follow-up with his primary care physician for transition to warfarin. Unfortunately, this patient was lost to follow-up.
Discussion
Isolated DCA is a rare cause of abdominal pain. The first documented case of isolated DCA is often incorrectly attributed to Bauersfeld’s1 1947 case series on dissections,but that report described superior mesenteric artery dissection rather than a celiac artery dissection. Watson’s2 1956 dissection series is also incorrectly cited as the first DCA, but that series described a dissection of the splenic artery, which is a branch of the celiac artery. In a 1959 series, Foord and Lewis3 described what is most likely the first report of DCA as an incidental finding at autopsy. More frequent descriptions in recent years are thought to be due to the routine use of abdominal CTA.4
Dissection of the celiac artery is a rare occurrence, with less than 100 cases reported, and little evidence exists to guide its management.5 These dissections represent 36.8% of all visceral artery dissections,6 which themselves are less common than renal, carotid, and vertebral artery dissections.7 Dissection of visceral arteries occurs predominantly in men and more often in middle-aged patients.8 Risk factors for DCA are thought to mirror risk factors for dissection of other arteries, including atherosclerotic disease, hypertension, connective tissue disorders, trauma, vasculitis, and pregnancy.9-11
Signs and Symptoms
Patients with DCA typically present with sudden onset of epigastric, flank, and/or chest pain, though 50% of patients may be asymptomatic.12 This pain is easily overlooked because the physical examination and laboratory studies are typically unremarkable.13 Fortunately, DCA is rarely accompanied by fatal organ dysfunction due to collateral flow from other vessels.14
Diagnosis and Management
While CTA with contrast is considered the mainstay of diagnosis of DCA,15 optimal treatment for DCA has not been well established. Management options include medical management, operative repair, and endovascular embolization. Medical management is reserved for stable patients without signs of end organ dysfunction. Typical management involves anticoagulation with warfarin for 3 to 6 months and strict BP control accompanied by close surveillance for progression.10,13 Some clinicians have argued that anticoagulation therapy may be unnecessary and that risk factor modification and BP control alone may be sufficient.5,6 Others have advocated that surgical management should be favored in cases of persistent pain, development of aneurysm, or threatened or compromised flow to end organs.7
Point-of-Care Ultrasound
The American College of Emergency Physicians considers ultrasound of the abdominal aorta a core application of emergency ultrasound.16 While sensitivity and specificity of emergency ultrasound for abdominal aortic aneurysm are well established, data supporting its use for screening for dissections are less definitive. With a sensitivity of 67% to 80% and a specificity of 99% to 100% with visualization of an intimal flap, aortic dissection screening using ultrasound is less reliable than most emergency physicians (EPs) would prefer.17,18 There are no published data reporting the sensitivity or specificity of emergency ultrasound for DCA. However, the vascular surgery literature encourages color Doppler ultrasound as part of the initial diagnostic work-up for this rare entity.19 While this may seem like an area ripe for emergency ultrasound, it is important to note—as seen in our case—that the site of the dissection is not often seen. Instead, the use of Doppler allows a screening for an abnormal flow pattern suggestive of dissection.20
Conclusion
In our case, both resident EPs and an expert fellowship-trained emergency ultrasound attending physician were unable to visualize a dissection—even after knowledge of the lesion was established by CTA. This points out a limitation of emergency ultrasound. While a POC ultrasound may be able to effectively rule in dissections of the aorta and its branches, we cannot reliably rule out these lesions. As EPs continue to expand the use of ultrasound, it is important to balance the desire for efficiency and cost-effectiveness with a high index of suspicion, experience, and clinical acumen.
Case
A 41-year-old man presented to our ED with a 4-day history of epigastric pain radiating to the bilateral flanks and back. His medical history was significant for hypertension, for which he was prescribed isosorbide dinitrite 30 mg four times per day; however, he reported that he did not regularly take this medication.
The patient had visited our ED 3 days earlier with the same complaint. Since his blood pressure (BP) reading at the first ED presentation was 213/141 mm Hg, he had been admitted for hypertensive urgency. The patient’s BP was controlled with antihypertensive agents during his stay, but he continued to experience epigastric pain. A basic work-up for abdominal pain was ordered, the results of which were normal. Based on these findings, the patient’s pain was attributed to gastritis, and he was discharged home with instructions to return to the ED if his pain became worse or persisted.
At both ED presentations, the patient denied experiencing any nausea, vomiting, diarrhea, or chest pain. At the second presentation, his triage BP was 158/106 mm Hg. A chest X-ray, complete blood count (CBC), basic metabolic profile (BMP), hepatic panel, and lipase evaluation were all unremarkable, with the exception of a mild increase in creatinine to 1.38 mg/dL. A point-of-care (POC) ultrasound study of the aorta was normal.
Based on the CTA findings, a nicardipine infusion was immediately started, and the patient was admitted to the medical intensive care unit (MICU). Because his heart rate was in the range of 60 beats/min, an esmolol infusion was not required. Prior to transferring the patient to MICU, a second ultrasound study of the aorta was performed by our fellowship-trained director of emergency medicine ultrasound.
In the MICU, the patient’s BP was stabilized on hospital day 2, and he was transitioned to oral antihypertensive medications. He was also started on a heparin infusion at the recommendation of vascular surgery services.
A repeat CTA of the abdomen taken on hospital day 3 showed an unchanged dissection in the celiac axis extending into the hepatic artery. The vascular surgeon recommended strict BP control, anticoagulation therapy, and a vascular surgery follow-up with a repeat CTA of the abdomen in 6 months.
On hospital day 6, repeat serial CBC, BMP, and hepatic panels revealed only slight increases in aspartate transaminase to 88 U/L and alanine aminotransferase to 117 U/L. The patient was transitioned to enoxaparin and discharged home on hospital day 6, and instructed to follow-up with his primary care physician for transition to warfarin. Unfortunately, this patient was lost to follow-up.
Discussion
Isolated DCA is a rare cause of abdominal pain. The first documented case of isolated DCA is often incorrectly attributed to Bauersfeld’s1 1947 case series on dissections,but that report described superior mesenteric artery dissection rather than a celiac artery dissection. Watson’s2 1956 dissection series is also incorrectly cited as the first DCA, but that series described a dissection of the splenic artery, which is a branch of the celiac artery. In a 1959 series, Foord and Lewis3 described what is most likely the first report of DCA as an incidental finding at autopsy. More frequent descriptions in recent years are thought to be due to the routine use of abdominal CTA.4
Dissection of the celiac artery is a rare occurrence, with less than 100 cases reported, and little evidence exists to guide its management.5 These dissections represent 36.8% of all visceral artery dissections,6 which themselves are less common than renal, carotid, and vertebral artery dissections.7 Dissection of visceral arteries occurs predominantly in men and more often in middle-aged patients.8 Risk factors for DCA are thought to mirror risk factors for dissection of other arteries, including atherosclerotic disease, hypertension, connective tissue disorders, trauma, vasculitis, and pregnancy.9-11
Signs and Symptoms
Patients with DCA typically present with sudden onset of epigastric, flank, and/or chest pain, though 50% of patients may be asymptomatic.12 This pain is easily overlooked because the physical examination and laboratory studies are typically unremarkable.13 Fortunately, DCA is rarely accompanied by fatal organ dysfunction due to collateral flow from other vessels.14
Diagnosis and Management
While CTA with contrast is considered the mainstay of diagnosis of DCA,15 optimal treatment for DCA has not been well established. Management options include medical management, operative repair, and endovascular embolization. Medical management is reserved for stable patients without signs of end organ dysfunction. Typical management involves anticoagulation with warfarin for 3 to 6 months and strict BP control accompanied by close surveillance for progression.10,13 Some clinicians have argued that anticoagulation therapy may be unnecessary and that risk factor modification and BP control alone may be sufficient.5,6 Others have advocated that surgical management should be favored in cases of persistent pain, development of aneurysm, or threatened or compromised flow to end organs.7
Point-of-Care Ultrasound
The American College of Emergency Physicians considers ultrasound of the abdominal aorta a core application of emergency ultrasound.16 While sensitivity and specificity of emergency ultrasound for abdominal aortic aneurysm are well established, data supporting its use for screening for dissections are less definitive. With a sensitivity of 67% to 80% and a specificity of 99% to 100% with visualization of an intimal flap, aortic dissection screening using ultrasound is less reliable than most emergency physicians (EPs) would prefer.17,18 There are no published data reporting the sensitivity or specificity of emergency ultrasound for DCA. However, the vascular surgery literature encourages color Doppler ultrasound as part of the initial diagnostic work-up for this rare entity.19 While this may seem like an area ripe for emergency ultrasound, it is important to note—as seen in our case—that the site of the dissection is not often seen. Instead, the use of Doppler allows a screening for an abnormal flow pattern suggestive of dissection.20
Conclusion
In our case, both resident EPs and an expert fellowship-trained emergency ultrasound attending physician were unable to visualize a dissection—even after knowledge of the lesion was established by CTA. This points out a limitation of emergency ultrasound. While a POC ultrasound may be able to effectively rule in dissections of the aorta and its branches, we cannot reliably rule out these lesions. As EPs continue to expand the use of ultrasound, it is important to balance the desire for efficiency and cost-effectiveness with a high index of suspicion, experience, and clinical acumen.
1. Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947;26(6):873-889.
2. Watson AJ. Dissecting aneurysm of arteries other than the aorta. J Pathol. 1956;72(2):439-449. doi:10.1002/path.1700720209.
3. Foord AG, Lewis RD. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch Pathol. 1959;68:553-577.
4. Neychev V, Krol E, Dietzek A. Unusual presentation and treatment of spontaneous celiac artery dissection. J Vasc Surg. 2013;58(2):491-495. doi:10.1016/j.jvs.2012.10.136.
5. DiMusto PD, Oberdoerster MM, Criado E. Isolated celiac artery dissection. J Vasc Surg. 2015;61(4):972-976. doi: 10.1016/j.jvs.2014.10.108.
6. Takayama T, Miyata T, Shirakawa M, Nagawa H. J Vasc Surg. 2008;48(2):329-333. doi:10.1016/j.jvs.2008.03.002.
7. Glehen O, Feugier P, Aleksic Y, Delannoy P, Chevalier JM. Spontaneous dissection of the celiac artery. Ann Vasc Surg. 2001;15(6):687-692.
8. Patel KS, Benshar O, Vrabie R, Patel A, Adler M, Hines G. A major pain in the … back and epigastrium: an unusual case of spontaneous celiac artery dissection. J Community Hosp Intern Med Perspect. 2014;4(5):23840. doi:10.3402/jchimp.v4.23840.
9. Kang TL, Teich DL, McGillicuddy DC. Isolated, spontaneous superior mesenteric and celiac artery dissection: case report and review of literature. J Emerg Med. 2011;40(2):e21-e25. doi:10.1016/j.jemermed.2007.12.038.
10. Galastri FL, Cavalcante RN, Motta-Leal-Filho JM, et al. Evaluation and management of symptomatic isolated spontaneous celiac trunk dissection. Vasc Med. 2015;20(4):358-363. doi:10.1177/1358863X15581447.
11. Wang HC, Chen JH, Hsiao CC, Jeng CM, Chen WL. Spontaneous dissection of the celiac artery: a case report and literature review. Am J Emerg Med. 2013;31(6):1000.e3-e5. doi:10.1016/j.ajem.2013.02.007.
12. Oh S, Cho YP, Kim JH, Shin S, Kwon TW, Ko GY. Symptomatic spontaneous celiac artery dissection treated by conservative management: serial imaging findings. Abdom Imaging. 2011;36(1):79-82. doi:10.1007/s00261-010-9657-x.
13. Wang JL, Hsieh MJ, Lee CH, Chen CC, Hsieh IC. Celiac artery dissection presenting with abdominal and chest pain. Am J Emerg Med. 2010;28(1):111.e3-e5. doi:10.1016/j.ajem.2009.02.023.
14. Takayama Y, Takao M, Inoue T, Yoshimi F, Koyama K, Nagai H. Isolated spontaneous dissection of the celiac artery: report of two cases. Ann Vasc Dis. 2014;7(1):64-67. doi:10.3400/avd.cr.13-00102.
15. Rehman AU, Almanfi A, Nadella S, Sohail U. Isolated spontaneous celiac artery dissection in a 47-year-old man with von Willebrand disease. Tex Heart Inst J. 2014;41(3):344-345. doi:10.14503/THIJ-13-3404.
16. American College of Emergency Physicians. Policy statement. Ultrasound Guidelines: Emergency, Point-of-Care, and Clinical Ultrasound Guidelines in Medicine, June 2016. https://www.acep.org/Clinical---Practice-Management/Ultrasound/. Accessed November 15, 2016.
17. Williams J, Heiner JD, Perreault MD, McArthur TJ. Aortic dissection diagnosed by ultrasound. West J Emerg Med. 2010;11(1):98-99.
18. Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
19. Woolard JD, Ammar AD. Spontaneous dissection of the celiac artery: a case report. J Vasc Surg. 2007;45(6):1256-1258.
20. Fenoglio L, Allione A, Scalabrino E, et al. Spontaneous dissection of the celiac artery: a pitfall in the diagnosis of acute abdominal pain. Presentation of two cases. Dig Dis Sci. 2004;49(7-8):1223-1227.
1. Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947;26(6):873-889.
2. Watson AJ. Dissecting aneurysm of arteries other than the aorta. J Pathol. 1956;72(2):439-449. doi:10.1002/path.1700720209.
3. Foord AG, Lewis RD. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch Pathol. 1959;68:553-577.
4. Neychev V, Krol E, Dietzek A. Unusual presentation and treatment of spontaneous celiac artery dissection. J Vasc Surg. 2013;58(2):491-495. doi:10.1016/j.jvs.2012.10.136.
5. DiMusto PD, Oberdoerster MM, Criado E. Isolated celiac artery dissection. J Vasc Surg. 2015;61(4):972-976. doi: 10.1016/j.jvs.2014.10.108.
6. Takayama T, Miyata T, Shirakawa M, Nagawa H. J Vasc Surg. 2008;48(2):329-333. doi:10.1016/j.jvs.2008.03.002.
7. Glehen O, Feugier P, Aleksic Y, Delannoy P, Chevalier JM. Spontaneous dissection of the celiac artery. Ann Vasc Surg. 2001;15(6):687-692.
8. Patel KS, Benshar O, Vrabie R, Patel A, Adler M, Hines G. A major pain in the … back and epigastrium: an unusual case of spontaneous celiac artery dissection. J Community Hosp Intern Med Perspect. 2014;4(5):23840. doi:10.3402/jchimp.v4.23840.
9. Kang TL, Teich DL, McGillicuddy DC. Isolated, spontaneous superior mesenteric and celiac artery dissection: case report and review of literature. J Emerg Med. 2011;40(2):e21-e25. doi:10.1016/j.jemermed.2007.12.038.
10. Galastri FL, Cavalcante RN, Motta-Leal-Filho JM, et al. Evaluation and management of symptomatic isolated spontaneous celiac trunk dissection. Vasc Med. 2015;20(4):358-363. doi:10.1177/1358863X15581447.
11. Wang HC, Chen JH, Hsiao CC, Jeng CM, Chen WL. Spontaneous dissection of the celiac artery: a case report and literature review. Am J Emerg Med. 2013;31(6):1000.e3-e5. doi:10.1016/j.ajem.2013.02.007.
12. Oh S, Cho YP, Kim JH, Shin S, Kwon TW, Ko GY. Symptomatic spontaneous celiac artery dissection treated by conservative management: serial imaging findings. Abdom Imaging. 2011;36(1):79-82. doi:10.1007/s00261-010-9657-x.
13. Wang JL, Hsieh MJ, Lee CH, Chen CC, Hsieh IC. Celiac artery dissection presenting with abdominal and chest pain. Am J Emerg Med. 2010;28(1):111.e3-e5. doi:10.1016/j.ajem.2009.02.023.
14. Takayama Y, Takao M, Inoue T, Yoshimi F, Koyama K, Nagai H. Isolated spontaneous dissection of the celiac artery: report of two cases. Ann Vasc Dis. 2014;7(1):64-67. doi:10.3400/avd.cr.13-00102.
15. Rehman AU, Almanfi A, Nadella S, Sohail U. Isolated spontaneous celiac artery dissection in a 47-year-old man with von Willebrand disease. Tex Heart Inst J. 2014;41(3):344-345. doi:10.14503/THIJ-13-3404.
16. American College of Emergency Physicians. Policy statement. Ultrasound Guidelines: Emergency, Point-of-Care, and Clinical Ultrasound Guidelines in Medicine, June 2016. https://www.acep.org/Clinical---Practice-Management/Ultrasound/. Accessed November 15, 2016.
17. Williams J, Heiner JD, Perreault MD, McArthur TJ. Aortic dissection diagnosed by ultrasound. West J Emerg Med. 2010;11(1):98-99.
18. Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
19. Woolard JD, Ammar AD. Spontaneous dissection of the celiac artery: a case report. J Vasc Surg. 2007;45(6):1256-1258.
20. Fenoglio L, Allione A, Scalabrino E, et al. Spontaneous dissection of the celiac artery: a pitfall in the diagnosis of acute abdominal pain. Presentation of two cases. Dig Dis Sci. 2004;49(7-8):1223-1227.
Physician Communications: Avoiding the Blame Game
A recent opinion piece in MedPage Today by an internist about poor communications between emergency physicians (EPs) and primary care physicians (PCPs) was subtitled “We’ve gotten better going from office to ER, but not the other way,” and complained about the lack of a “live, warm handoff” from EPs to PCPs of patients being discharged from EDs. Similar complaints were examined in two recent Emergency Medicine (EM) editorials (Anger Management, 2015;47[4]:149 and Broadside Journalism, 2015;47[6]:244). In the first, we noted that PCPs sometimes are angered when they are not consulted about one of their patients in the ED or about a treatment or disposition plan with which they disagree, while EPs are frustrated by the number of phone calls required to reach some PCPs or a knowledgeable covering physician.
Only 2 months later, we expressed concerns about a New York Times opinion editorial describing a young patient whose vertebral artery dissection had been “diagnosed correctly and acted on in the ED,” but then angrily criticizing an initial recommendation that the patient curtail her physical activities based on what a famous neurologist considered an erroneously interpreted vascular imaging study. (Presumably, the recommendation was by another neurologist and the interpretation by a radiologist, but all of the neurologist’s caustic criticism was directed at the EP and ED.) Although the neurologist subsequently apologized in a letter to his emergency medicine colleagues for “being quoted out of context,” few if any
We concluded the second EM editorial with the suggestion that “all physicians must be very, very careful in framing statements to the media, and should assume that their remarks will not be placed ‘in context’ or nuanced as they may have been intended....Most important, is to not disparage entire specialties or use belittling terms such as ‘ER docs’....[that] heighten...patients’ fears” of being treated in EDs.
Why another editorial about physician-to-physician miscommunications and name-calling? Because patient care is significantly affected.
The Centers for Medicare and Medicaid Services originally classified four medical specialties as “primary care” for reimbursement purposes: family medicine, internal medicine, pediatrics, and obstetrics-gynecology, and the 2010 Affordable Care Act added geriatrics. Although emergency medicine had been considered initially, it has never been categorized as a primary care specialty. That being the case, isn’t it incumbent upon us to learn as much as we can from PCPs about their ill patients en route to the ED for treatment or admission, and afterward ensure that an ED visit is part of a continuum of patient care and not an isolated episode?
In 1996, when I accepted an offer to become New York Presbyterian-Weill Cornell’s first Emergency Physician-in-Chief, I created a new position of full-time “ED follow-up nurse practitioner” to track and report test results to discharged patients and their designated PCPs. When we added a fourth unit to the ED a few years later, I designated an experienced, senior attending EP among the four on duty as the “administrative attending” (AA) who, among other tasks, took all phone calls from PCPs about patients they were sending to the ED and entered the information in the “en route” section of our electronic tracking board. In this way, important patient information, including PCP contact information, was no longer misplaced during shift changes. The AA carried a direct-dial cell phone-like device and eventually all attending EPs and the charge nurse were equipped with such phones. In a short time, most of the communications problems and complaints about incoming patients were eliminated.
But despite numerous attempts, for the reasons mentioned above, systematically ensuring effective communications with PCPs for discharged patients has proven to be a more difficult task. At present, handing off discharged patients to PCPs still depends largely on a combination of judgment, understanding, compassion, and respect.
A recent opinion piece in MedPage Today by an internist about poor communications between emergency physicians (EPs) and primary care physicians (PCPs) was subtitled “We’ve gotten better going from office to ER, but not the other way,” and complained about the lack of a “live, warm handoff” from EPs to PCPs of patients being discharged from EDs. Similar complaints were examined in two recent Emergency Medicine (EM) editorials (Anger Management, 2015;47[4]:149 and Broadside Journalism, 2015;47[6]:244). In the first, we noted that PCPs sometimes are angered when they are not consulted about one of their patients in the ED or about a treatment or disposition plan with which they disagree, while EPs are frustrated by the number of phone calls required to reach some PCPs or a knowledgeable covering physician.
Only 2 months later, we expressed concerns about a New York Times opinion editorial describing a young patient whose vertebral artery dissection had been “diagnosed correctly and acted on in the ED,” but then angrily criticizing an initial recommendation that the patient curtail her physical activities based on what a famous neurologist considered an erroneously interpreted vascular imaging study. (Presumably, the recommendation was by another neurologist and the interpretation by a radiologist, but all of the neurologist’s caustic criticism was directed at the EP and ED.) Although the neurologist subsequently apologized in a letter to his emergency medicine colleagues for “being quoted out of context,” few if any
We concluded the second EM editorial with the suggestion that “all physicians must be very, very careful in framing statements to the media, and should assume that their remarks will not be placed ‘in context’ or nuanced as they may have been intended....Most important, is to not disparage entire specialties or use belittling terms such as ‘ER docs’....[that] heighten...patients’ fears” of being treated in EDs.
Why another editorial about physician-to-physician miscommunications and name-calling? Because patient care is significantly affected.
The Centers for Medicare and Medicaid Services originally classified four medical specialties as “primary care” for reimbursement purposes: family medicine, internal medicine, pediatrics, and obstetrics-gynecology, and the 2010 Affordable Care Act added geriatrics. Although emergency medicine had been considered initially, it has never been categorized as a primary care specialty. That being the case, isn’t it incumbent upon us to learn as much as we can from PCPs about their ill patients en route to the ED for treatment or admission, and afterward ensure that an ED visit is part of a continuum of patient care and not an isolated episode?
In 1996, when I accepted an offer to become New York Presbyterian-Weill Cornell’s first Emergency Physician-in-Chief, I created a new position of full-time “ED follow-up nurse practitioner” to track and report test results to discharged patients and their designated PCPs. When we added a fourth unit to the ED a few years later, I designated an experienced, senior attending EP among the four on duty as the “administrative attending” (AA) who, among other tasks, took all phone calls from PCPs about patients they were sending to the ED and entered the information in the “en route” section of our electronic tracking board. In this way, important patient information, including PCP contact information, was no longer misplaced during shift changes. The AA carried a direct-dial cell phone-like device and eventually all attending EPs and the charge nurse were equipped with such phones. In a short time, most of the communications problems and complaints about incoming patients were eliminated.
But despite numerous attempts, for the reasons mentioned above, systematically ensuring effective communications with PCPs for discharged patients has proven to be a more difficult task. At present, handing off discharged patients to PCPs still depends largely on a combination of judgment, understanding, compassion, and respect.
A recent opinion piece in MedPage Today by an internist about poor communications between emergency physicians (EPs) and primary care physicians (PCPs) was subtitled “We’ve gotten better going from office to ER, but not the other way,” and complained about the lack of a “live, warm handoff” from EPs to PCPs of patients being discharged from EDs. Similar complaints were examined in two recent Emergency Medicine (EM) editorials (Anger Management, 2015;47[4]:149 and Broadside Journalism, 2015;47[6]:244). In the first, we noted that PCPs sometimes are angered when they are not consulted about one of their patients in the ED or about a treatment or disposition plan with which they disagree, while EPs are frustrated by the number of phone calls required to reach some PCPs or a knowledgeable covering physician.
Only 2 months later, we expressed concerns about a New York Times opinion editorial describing a young patient whose vertebral artery dissection had been “diagnosed correctly and acted on in the ED,” but then angrily criticizing an initial recommendation that the patient curtail her physical activities based on what a famous neurologist considered an erroneously interpreted vascular imaging study. (Presumably, the recommendation was by another neurologist and the interpretation by a radiologist, but all of the neurologist’s caustic criticism was directed at the EP and ED.) Although the neurologist subsequently apologized in a letter to his emergency medicine colleagues for “being quoted out of context,” few if any
We concluded the second EM editorial with the suggestion that “all physicians must be very, very careful in framing statements to the media, and should assume that their remarks will not be placed ‘in context’ or nuanced as they may have been intended....Most important, is to not disparage entire specialties or use belittling terms such as ‘ER docs’....[that] heighten...patients’ fears” of being treated in EDs.
Why another editorial about physician-to-physician miscommunications and name-calling? Because patient care is significantly affected.
The Centers for Medicare and Medicaid Services originally classified four medical specialties as “primary care” for reimbursement purposes: family medicine, internal medicine, pediatrics, and obstetrics-gynecology, and the 2010 Affordable Care Act added geriatrics. Although emergency medicine had been considered initially, it has never been categorized as a primary care specialty. That being the case, isn’t it incumbent upon us to learn as much as we can from PCPs about their ill patients en route to the ED for treatment or admission, and afterward ensure that an ED visit is part of a continuum of patient care and not an isolated episode?
In 1996, when I accepted an offer to become New York Presbyterian-Weill Cornell’s first Emergency Physician-in-Chief, I created a new position of full-time “ED follow-up nurse practitioner” to track and report test results to discharged patients and their designated PCPs. When we added a fourth unit to the ED a few years later, I designated an experienced, senior attending EP among the four on duty as the “administrative attending” (AA) who, among other tasks, took all phone calls from PCPs about patients they were sending to the ED and entered the information in the “en route” section of our electronic tracking board. In this way, important patient information, including PCP contact information, was no longer misplaced during shift changes. The AA carried a direct-dial cell phone-like device and eventually all attending EPs and the charge nurse were equipped with such phones. In a short time, most of the communications problems and complaints about incoming patients were eliminated.
But despite numerous attempts, for the reasons mentioned above, systematically ensuring effective communications with PCPs for discharged patients has proven to be a more difficult task. At present, handing off discharged patients to PCPs still depends largely on a combination of judgment, understanding, compassion, and respect.
SHM welcomes its newest members - January 2017
Justin Kimsey, Alabama
Mohammed N.Y. Shah, MD, Alaska
Katharina Beeler, MD, Arizona
Khoi Nguyen, MD, Arizona
Vinay Saini, MD, Arizona
Maria Aceves, PA-C, California
Sarvenaz Alibeigi, California
Peter Cadman, MD, California
Katrina Chapman, DO, MPH, California
Cheryll Gallardo-Villena, MD, California
Sripriya Ganesan, California
Alice Gong, MD, California
Henry Kwang, MD, California
Kevin Li, California
Anthony Murphy, MD, California
Dan Nguyen, California
Daniel Oh, California
Joon Parle, California
Katie Raffel, California
Darshana Sarathchandra, MD, California
Lifang Zhang, California
Jaime Baker, MD, Colorado
Eric Johnson, PA-C, Colorado
Juan Lessing, MD, Colorado
Benjamin Ruckman, DO, Colorado
Rehaan Shaffie, MD, Colorado
Deborah Casey, MD, Connecticut
Daniel Heacock, PA-C, Connecticut
Shabana Ansari, DO, Delaware
Madhu Prattipati, MD, Delaware
Pallavi Aneja, MD, Florida
Satcha Borgella, MD, Florida
Thendrex H. Estrella, MD, Florida
Abid Hussain, MD, Florida
Daphnee Hutchinson, DO, Florida
Muhammad Jaffer, Florida
Sue Lee, ANP, Florida
Melissa Odermann, DO, Florida
Jose Guillermo Revelo Paiz, MD, Florida
Rafael J. Rolon Rivera, MD, Florida
Eleonor Rongo, Florida
Esther Roth, Florida
Shitaye Argaw, MD, Georgia
Taryn DeGrazia, Georgia
Becca Feistritzer, Georgia
Jamal Fitts, Georgia
Kristen Flint, Georgia
Zachary Hermes, Georgia
Mukesh Kumar, Georgia
Kajal Patel, Georgia
Madeline Smith, Georgia
Wade Flowers, PharmD, Idaho
Ajay Bhandare, Illinois
Kimberly Brighton, Illinois
Hristo D. Hristov, MD, Illinois
Sidney Iriana, Illinois
Aurelian Ivan, Illinois
Ming Lee, MD, Illinois
Michelle Lundholm, Illinois
Idrees Mohiuddin, MD, Illinois
Murr Murray, Illinois
Tad Nair, MD, Illinois
Shalini Reddy, MD, Illinois
Richard Rethorst, MD, Illinois
Kelly Robertshaw, Illinois
Gracelene Wegrzyn, Illinois
Evan Yates, Illinois
Lora J. Jones McClure, MD, Indiana
Carleigh Wilson, DO, Indiana
Erin Brown, ARNP, Iowa
Adam Gray, Iowa
Paul Greco, MD, Iowa
Shelly McGurk, ACNP, ARNP, Iowa
Julie Stanik-Hutt, ACNP, CNS, PhD, Iowa
Elizabeth Cozad, DO, Kansas
Roshan Pais, Kentucky
Mark Youssef, MD, Kentucky
Heather Kahn, MD, Louisiana
Danielle Parrott, PA-C, Maine
Erica Lafferty, ACNP, Maryland
Andrea Limpuangthip, Maryland
Steven Schwartz, CCM, MD, Maryland
Eisha Azhar, MBBS, Massachusetts
Badal Kalamkar, MD, MPH, Massachusetts
Bhavya Rajanna, MD, Massachusetts
Sahib Baljinder Singh, MD, Massachusetts
Kathryn Adams, Michigan
Haseeb Aslam, MD, MBBS, Michigan
Hilda Crispin, MD, Michigan
Sharmistha Dev, MD, Michigan
Tristan Feierabend, MD, Michigan
Sonal Kamalia, MD, MBBS, Michigan
Matthew Luzum, MD, Michigan
Daniel Mitzel, MD, Michigan
Richard Raad, Michigan
Mythri Ramegowda, MD, Michigan
Katie Scally, MD, Michigan
Linden Spital, MSN, NP, Michigan
Porama Koy Thanaporn, MD, Michigan
Chanteil Ulatowski, Michigan
Tingting Xiong, MD, Michigan
Adam Zahr, Michigan
Mike Beste, MD, Minnesota
Elise Haupt, PA-C, Minnesota
Lobsang Trasar, MD, Minnesota
Kari Goan, DO, Mississippi
David C. Pierre, Mississippi
Sudheer Tangella, MD, Mississippi
Tahani Atieh, Missouri
Nicholas Arnold, Missouri
Amanda Calhoun, Missouri
Jyotirmoy Das, Missouri
Umber Dube, Missouri
Daniel Gaughan, Missouri
Woojin Joo, Missouri
Khaled Jumean, MBBS, Missouri
Salma Kazmi, MBBS, MD, Missouri
Yoon Kook (Danny) Kim, Missouri
Ryan Kronen, Missouri
Alyssa Kroner, Missouri
Randy Laine, Missouri
Edward Lee, Missouri
Cerena Leung, Missouri
Patricia Lithrow, Missouri
Brandt Lydon, Missouri
Mary Morgan Scott, Missouri
Jay Patel, Missouri
Justin Porter, Missouri
Danelle Reagin, FNP-C, Missouri
Amanda Reis, Missouri
Awik Som, Missouri
Abby Sung, Missouri
Mary Sutherland, Missouri
Maggie Wang, Missouri
Noah Wasserman, Missouri
Alexis Webber, Missouri
Ryan White, Missouri
Amy Xu, Missouri
Ran Xu, Missouri
Michael Yang, Missouri
Christopher Dietrich, MD, Montana
Jason Kunz, DO, Montana
Jodi Cantrell, MD, Nebraska
Steven Hart, MD, Nebraska
Kurt Kapels, MD, Nebraska
Brian Keegan, MD, Nebraska
Shaun Jang, MD, Nevada
Gurpinder Singh, MD, New Hampshire
Pragati Banda, MD, New Jersey
Sahai Donaldson, MBBS, New Jersey
Ashesha Mechineni, MD, New Jersey
Alisa Clark, New Mexico
Prajit Arora, MBBS, New Mexico
Crystal Cardwell, New Mexico
Landon Casaus, New Mexico
Tapuwa Mupfumira, MD, New Mexico
Eric Rightley, New Mexico
David S. Anderson, New York
Joan Bosco, MD, New York
Jessica Caro, New York
Anna Dewan, New York
Amrita Dhillon, MBBS, New York
Julia Frydman, New York
Radhika Gali, MBBS, MDS, New York
Allison Guttmann, MD, New York
Aryles Hedjar, MD, New York
Peter Janes, New York
Nadine Kalavazoff, New York
Jeffrey Lach, DO, New York
Keron Lezama, MD, New York
Yingheng Liu, New York
Taimur Mirza, New York
Cyrus Nensey, MD, New York
Nekee Pandya, MD, New York
Thushara Paul, MD, New York
Yu Sung, New York
Joel Boggan, MD, North Carolina
Angela Fletcher, North Carolina
Rebecca Gimpert, PA-C, North Carolina
Samantha Levering, PA-C, North Carolina
Nancy Martin, North Carolina
Richard Sherwood, North Carolina
Kranthi K. Sitammagari, MD, North Carolina
Aaron Swedberg, MPAS, PA-C, North Carolina
Yih-Cherng Tsai, North Carolina
Richard Bakker, MD, PhD, Ohio
Matthew Broderick, MD, Ohio
Subbaraju Budharaju, MD, MS, Ohio
Steven Bumb, MD, Ohio
Ahmed Eltelbany, MD, Ohio
Tracey Hardin, MS, Ohio
Patricia Hardman, APRN, Ohio
Michael Lewis, MD, Ohio
Volodymyr Manko, Ohio
Rebecca Stone, Ohio
Chaitanya Valluri, Ohio
Holly Wierzbicki, CNP, Ohio
Jamie Yockey, APRN, CNP, Ohio
Mahdi Mussa, MD, Oklahoma
Monica Saemz, DO, Oklahoma
Peter Ganter, MD, Oregon
Bethany Roy, MD, Oregon
Mary Clare Bohnett, Oregon
Molly Rabinowitz, Oregon
Abdullateef Abdulkareem, MD, MPH, Pennsylvania
David Ahamba, MD, MPH, Pennsylvania
David Chin, MD, Pennsylvania
Thomas Conlon, Pennsylvania
Dan Giesler, MD, Pennsylvania
Umair Randhawa, MD, Pennsylvania
Syed Yusuf, MBBS, Pennsylvania
Michael Rigatti, Pennsylvania
Thaylon Barreto, Rhode Island
Jessica Cook, MD, South Carolina
Robin Malik, MD, South Carolina
John Busigin, Tennessee
Shefali Paranjape, MD, Tennessee
Thai Dang, MD, Texas
Matthew Glover, MD, Texas
Snigdha Jain, MD, Texas
David Kellenberger, Texas
Sumeet Kumar, Texas
Kyle McClendon, PA-C, Texas
Sowjanya Mohan, Texas
Akhil D. Vats, MD, Texas
Samatha Vellanki, Texas
Lee-Anna Burgess, MD, Vermont
Rick Hildebrant, MD, Vermont
Matthew Backens, MD, Virginia
Megan Coe, Virginia
Kevin Dehaan, Virginia
Stephen Fox, Virginia
Amber Inofuentes, MD, Virginia
Jessica Keiser, MD, Virginia
Joseph Perez, MD, FAAFP, MBA, Virginia
Kanwapreet S. Saini, MD, Virginia
Erin Vipler, MD, Virginia
Naveen Voore, MBBS, Virginia
Abhishek Agarwal, MD, MBBS, Washington
Robert Cooney, MD, Washington
Cynthia Horton, MD, Washington
Rich A. Kukreja, MD, Washington
Ji Young Nam, MD, Washington
Kai Wilhelm, MD, Washington
In Kyu Yoo, Washington
Temu Brown, Wisconsin
Pablo Colon Nieves, Wisconsin
Christina Evans, PAC, Wisconsin
Swetha Karturi, MBBS, Wisconsin
Mark Babcock, DO, Wyoming
Ahmad Von Schlegell, Canada
Anand Kartha, Japan
Mohamed Sadek, Qatar
Amine Rakab, MD, Qatar
Abazar Saeed, Qatar
Joao Guerra, MD
Justin Kimsey, Alabama
Mohammed N.Y. Shah, MD, Alaska
Katharina Beeler, MD, Arizona
Khoi Nguyen, MD, Arizona
Vinay Saini, MD, Arizona
Maria Aceves, PA-C, California
Sarvenaz Alibeigi, California
Peter Cadman, MD, California
Katrina Chapman, DO, MPH, California
Cheryll Gallardo-Villena, MD, California
Sripriya Ganesan, California
Alice Gong, MD, California
Henry Kwang, MD, California
Kevin Li, California
Anthony Murphy, MD, California
Dan Nguyen, California
Daniel Oh, California
Joon Parle, California
Katie Raffel, California
Darshana Sarathchandra, MD, California
Lifang Zhang, California
Jaime Baker, MD, Colorado
Eric Johnson, PA-C, Colorado
Juan Lessing, MD, Colorado
Benjamin Ruckman, DO, Colorado
Rehaan Shaffie, MD, Colorado
Deborah Casey, MD, Connecticut
Daniel Heacock, PA-C, Connecticut
Shabana Ansari, DO, Delaware
Madhu Prattipati, MD, Delaware
Pallavi Aneja, MD, Florida
Satcha Borgella, MD, Florida
Thendrex H. Estrella, MD, Florida
Abid Hussain, MD, Florida
Daphnee Hutchinson, DO, Florida
Muhammad Jaffer, Florida
Sue Lee, ANP, Florida
Melissa Odermann, DO, Florida
Jose Guillermo Revelo Paiz, MD, Florida
Rafael J. Rolon Rivera, MD, Florida
Eleonor Rongo, Florida
Esther Roth, Florida
Shitaye Argaw, MD, Georgia
Taryn DeGrazia, Georgia
Becca Feistritzer, Georgia
Jamal Fitts, Georgia
Kristen Flint, Georgia
Zachary Hermes, Georgia
Mukesh Kumar, Georgia
Kajal Patel, Georgia
Madeline Smith, Georgia
Wade Flowers, PharmD, Idaho
Ajay Bhandare, Illinois
Kimberly Brighton, Illinois
Hristo D. Hristov, MD, Illinois
Sidney Iriana, Illinois
Aurelian Ivan, Illinois
Ming Lee, MD, Illinois
Michelle Lundholm, Illinois
Idrees Mohiuddin, MD, Illinois
Murr Murray, Illinois
Tad Nair, MD, Illinois
Shalini Reddy, MD, Illinois
Richard Rethorst, MD, Illinois
Kelly Robertshaw, Illinois
Gracelene Wegrzyn, Illinois
Evan Yates, Illinois
Lora J. Jones McClure, MD, Indiana
Carleigh Wilson, DO, Indiana
Erin Brown, ARNP, Iowa
Adam Gray, Iowa
Paul Greco, MD, Iowa
Shelly McGurk, ACNP, ARNP, Iowa
Julie Stanik-Hutt, ACNP, CNS, PhD, Iowa
Elizabeth Cozad, DO, Kansas
Roshan Pais, Kentucky
Mark Youssef, MD, Kentucky
Heather Kahn, MD, Louisiana
Danielle Parrott, PA-C, Maine
Erica Lafferty, ACNP, Maryland
Andrea Limpuangthip, Maryland
Steven Schwartz, CCM, MD, Maryland
Eisha Azhar, MBBS, Massachusetts
Badal Kalamkar, MD, MPH, Massachusetts
Bhavya Rajanna, MD, Massachusetts
Sahib Baljinder Singh, MD, Massachusetts
Kathryn Adams, Michigan
Haseeb Aslam, MD, MBBS, Michigan
Hilda Crispin, MD, Michigan
Sharmistha Dev, MD, Michigan
Tristan Feierabend, MD, Michigan
Sonal Kamalia, MD, MBBS, Michigan
Matthew Luzum, MD, Michigan
Daniel Mitzel, MD, Michigan
Richard Raad, Michigan
Mythri Ramegowda, MD, Michigan
Katie Scally, MD, Michigan
Linden Spital, MSN, NP, Michigan
Porama Koy Thanaporn, MD, Michigan
Chanteil Ulatowski, Michigan
Tingting Xiong, MD, Michigan
Adam Zahr, Michigan
Mike Beste, MD, Minnesota
Elise Haupt, PA-C, Minnesota
Lobsang Trasar, MD, Minnesota
Kari Goan, DO, Mississippi
David C. Pierre, Mississippi
Sudheer Tangella, MD, Mississippi
Tahani Atieh, Missouri
Nicholas Arnold, Missouri
Amanda Calhoun, Missouri
Jyotirmoy Das, Missouri
Umber Dube, Missouri
Daniel Gaughan, Missouri
Woojin Joo, Missouri
Khaled Jumean, MBBS, Missouri
Salma Kazmi, MBBS, MD, Missouri
Yoon Kook (Danny) Kim, Missouri
Ryan Kronen, Missouri
Alyssa Kroner, Missouri
Randy Laine, Missouri
Edward Lee, Missouri
Cerena Leung, Missouri
Patricia Lithrow, Missouri
Brandt Lydon, Missouri
Mary Morgan Scott, Missouri
Jay Patel, Missouri
Justin Porter, Missouri
Danelle Reagin, FNP-C, Missouri
Amanda Reis, Missouri
Awik Som, Missouri
Abby Sung, Missouri
Mary Sutherland, Missouri
Maggie Wang, Missouri
Noah Wasserman, Missouri
Alexis Webber, Missouri
Ryan White, Missouri
Amy Xu, Missouri
Ran Xu, Missouri
Michael Yang, Missouri
Christopher Dietrich, MD, Montana
Jason Kunz, DO, Montana
Jodi Cantrell, MD, Nebraska
Steven Hart, MD, Nebraska
Kurt Kapels, MD, Nebraska
Brian Keegan, MD, Nebraska
Shaun Jang, MD, Nevada
Gurpinder Singh, MD, New Hampshire
Pragati Banda, MD, New Jersey
Sahai Donaldson, MBBS, New Jersey
Ashesha Mechineni, MD, New Jersey
Alisa Clark, New Mexico
Prajit Arora, MBBS, New Mexico
Crystal Cardwell, New Mexico
Landon Casaus, New Mexico
Tapuwa Mupfumira, MD, New Mexico
Eric Rightley, New Mexico
David S. Anderson, New York
Joan Bosco, MD, New York
Jessica Caro, New York
Anna Dewan, New York
Amrita Dhillon, MBBS, New York
Julia Frydman, New York
Radhika Gali, MBBS, MDS, New York
Allison Guttmann, MD, New York
Aryles Hedjar, MD, New York
Peter Janes, New York
Nadine Kalavazoff, New York
Jeffrey Lach, DO, New York
Keron Lezama, MD, New York
Yingheng Liu, New York
Taimur Mirza, New York
Cyrus Nensey, MD, New York
Nekee Pandya, MD, New York
Thushara Paul, MD, New York
Yu Sung, New York
Joel Boggan, MD, North Carolina
Angela Fletcher, North Carolina
Rebecca Gimpert, PA-C, North Carolina
Samantha Levering, PA-C, North Carolina
Nancy Martin, North Carolina
Richard Sherwood, North Carolina
Kranthi K. Sitammagari, MD, North Carolina
Aaron Swedberg, MPAS, PA-C, North Carolina
Yih-Cherng Tsai, North Carolina
Richard Bakker, MD, PhD, Ohio
Matthew Broderick, MD, Ohio
Subbaraju Budharaju, MD, MS, Ohio
Steven Bumb, MD, Ohio
Ahmed Eltelbany, MD, Ohio
Tracey Hardin, MS, Ohio
Patricia Hardman, APRN, Ohio
Michael Lewis, MD, Ohio
Volodymyr Manko, Ohio
Rebecca Stone, Ohio
Chaitanya Valluri, Ohio
Holly Wierzbicki, CNP, Ohio
Jamie Yockey, APRN, CNP, Ohio
Mahdi Mussa, MD, Oklahoma
Monica Saemz, DO, Oklahoma
Peter Ganter, MD, Oregon
Bethany Roy, MD, Oregon
Mary Clare Bohnett, Oregon
Molly Rabinowitz, Oregon
Abdullateef Abdulkareem, MD, MPH, Pennsylvania
David Ahamba, MD, MPH, Pennsylvania
David Chin, MD, Pennsylvania
Thomas Conlon, Pennsylvania
Dan Giesler, MD, Pennsylvania
Umair Randhawa, MD, Pennsylvania
Syed Yusuf, MBBS, Pennsylvania
Michael Rigatti, Pennsylvania
Thaylon Barreto, Rhode Island
Jessica Cook, MD, South Carolina
Robin Malik, MD, South Carolina
John Busigin, Tennessee
Shefali Paranjape, MD, Tennessee
Thai Dang, MD, Texas
Matthew Glover, MD, Texas
Snigdha Jain, MD, Texas
David Kellenberger, Texas
Sumeet Kumar, Texas
Kyle McClendon, PA-C, Texas
Sowjanya Mohan, Texas
Akhil D. Vats, MD, Texas
Samatha Vellanki, Texas
Lee-Anna Burgess, MD, Vermont
Rick Hildebrant, MD, Vermont
Matthew Backens, MD, Virginia
Megan Coe, Virginia
Kevin Dehaan, Virginia
Stephen Fox, Virginia
Amber Inofuentes, MD, Virginia
Jessica Keiser, MD, Virginia
Joseph Perez, MD, FAAFP, MBA, Virginia
Kanwapreet S. Saini, MD, Virginia
Erin Vipler, MD, Virginia
Naveen Voore, MBBS, Virginia
Abhishek Agarwal, MD, MBBS, Washington
Robert Cooney, MD, Washington
Cynthia Horton, MD, Washington
Rich A. Kukreja, MD, Washington
Ji Young Nam, MD, Washington
Kai Wilhelm, MD, Washington
In Kyu Yoo, Washington
Temu Brown, Wisconsin
Pablo Colon Nieves, Wisconsin
Christina Evans, PAC, Wisconsin
Swetha Karturi, MBBS, Wisconsin
Mark Babcock, DO, Wyoming
Ahmad Von Schlegell, Canada
Anand Kartha, Japan
Mohamed Sadek, Qatar
Amine Rakab, MD, Qatar
Abazar Saeed, Qatar
Joao Guerra, MD
Justin Kimsey, Alabama
Mohammed N.Y. Shah, MD, Alaska
Katharina Beeler, MD, Arizona
Khoi Nguyen, MD, Arizona
Vinay Saini, MD, Arizona
Maria Aceves, PA-C, California
Sarvenaz Alibeigi, California
Peter Cadman, MD, California
Katrina Chapman, DO, MPH, California
Cheryll Gallardo-Villena, MD, California
Sripriya Ganesan, California
Alice Gong, MD, California
Henry Kwang, MD, California
Kevin Li, California
Anthony Murphy, MD, California
Dan Nguyen, California
Daniel Oh, California
Joon Parle, California
Katie Raffel, California
Darshana Sarathchandra, MD, California
Lifang Zhang, California
Jaime Baker, MD, Colorado
Eric Johnson, PA-C, Colorado
Juan Lessing, MD, Colorado
Benjamin Ruckman, DO, Colorado
Rehaan Shaffie, MD, Colorado
Deborah Casey, MD, Connecticut
Daniel Heacock, PA-C, Connecticut
Shabana Ansari, DO, Delaware
Madhu Prattipati, MD, Delaware
Pallavi Aneja, MD, Florida
Satcha Borgella, MD, Florida
Thendrex H. Estrella, MD, Florida
Abid Hussain, MD, Florida
Daphnee Hutchinson, DO, Florida
Muhammad Jaffer, Florida
Sue Lee, ANP, Florida
Melissa Odermann, DO, Florida
Jose Guillermo Revelo Paiz, MD, Florida
Rafael J. Rolon Rivera, MD, Florida
Eleonor Rongo, Florida
Esther Roth, Florida
Shitaye Argaw, MD, Georgia
Taryn DeGrazia, Georgia
Becca Feistritzer, Georgia
Jamal Fitts, Georgia
Kristen Flint, Georgia
Zachary Hermes, Georgia
Mukesh Kumar, Georgia
Kajal Patel, Georgia
Madeline Smith, Georgia
Wade Flowers, PharmD, Idaho
Ajay Bhandare, Illinois
Kimberly Brighton, Illinois
Hristo D. Hristov, MD, Illinois
Sidney Iriana, Illinois
Aurelian Ivan, Illinois
Ming Lee, MD, Illinois
Michelle Lundholm, Illinois
Idrees Mohiuddin, MD, Illinois
Murr Murray, Illinois
Tad Nair, MD, Illinois
Shalini Reddy, MD, Illinois
Richard Rethorst, MD, Illinois
Kelly Robertshaw, Illinois
Gracelene Wegrzyn, Illinois
Evan Yates, Illinois
Lora J. Jones McClure, MD, Indiana
Carleigh Wilson, DO, Indiana
Erin Brown, ARNP, Iowa
Adam Gray, Iowa
Paul Greco, MD, Iowa
Shelly McGurk, ACNP, ARNP, Iowa
Julie Stanik-Hutt, ACNP, CNS, PhD, Iowa
Elizabeth Cozad, DO, Kansas
Roshan Pais, Kentucky
Mark Youssef, MD, Kentucky
Heather Kahn, MD, Louisiana
Danielle Parrott, PA-C, Maine
Erica Lafferty, ACNP, Maryland
Andrea Limpuangthip, Maryland
Steven Schwartz, CCM, MD, Maryland
Eisha Azhar, MBBS, Massachusetts
Badal Kalamkar, MD, MPH, Massachusetts
Bhavya Rajanna, MD, Massachusetts
Sahib Baljinder Singh, MD, Massachusetts
Kathryn Adams, Michigan
Haseeb Aslam, MD, MBBS, Michigan
Hilda Crispin, MD, Michigan
Sharmistha Dev, MD, Michigan
Tristan Feierabend, MD, Michigan
Sonal Kamalia, MD, MBBS, Michigan
Matthew Luzum, MD, Michigan
Daniel Mitzel, MD, Michigan
Richard Raad, Michigan
Mythri Ramegowda, MD, Michigan
Katie Scally, MD, Michigan
Linden Spital, MSN, NP, Michigan
Porama Koy Thanaporn, MD, Michigan
Chanteil Ulatowski, Michigan
Tingting Xiong, MD, Michigan
Adam Zahr, Michigan
Mike Beste, MD, Minnesota
Elise Haupt, PA-C, Minnesota
Lobsang Trasar, MD, Minnesota
Kari Goan, DO, Mississippi
David C. Pierre, Mississippi
Sudheer Tangella, MD, Mississippi
Tahani Atieh, Missouri
Nicholas Arnold, Missouri
Amanda Calhoun, Missouri
Jyotirmoy Das, Missouri
Umber Dube, Missouri
Daniel Gaughan, Missouri
Woojin Joo, Missouri
Khaled Jumean, MBBS, Missouri
Salma Kazmi, MBBS, MD, Missouri
Yoon Kook (Danny) Kim, Missouri
Ryan Kronen, Missouri
Alyssa Kroner, Missouri
Randy Laine, Missouri
Edward Lee, Missouri
Cerena Leung, Missouri
Patricia Lithrow, Missouri
Brandt Lydon, Missouri
Mary Morgan Scott, Missouri
Jay Patel, Missouri
Justin Porter, Missouri
Danelle Reagin, FNP-C, Missouri
Amanda Reis, Missouri
Awik Som, Missouri
Abby Sung, Missouri
Mary Sutherland, Missouri
Maggie Wang, Missouri
Noah Wasserman, Missouri
Alexis Webber, Missouri
Ryan White, Missouri
Amy Xu, Missouri
Ran Xu, Missouri
Michael Yang, Missouri
Christopher Dietrich, MD, Montana
Jason Kunz, DO, Montana
Jodi Cantrell, MD, Nebraska
Steven Hart, MD, Nebraska
Kurt Kapels, MD, Nebraska
Brian Keegan, MD, Nebraska
Shaun Jang, MD, Nevada
Gurpinder Singh, MD, New Hampshire
Pragati Banda, MD, New Jersey
Sahai Donaldson, MBBS, New Jersey
Ashesha Mechineni, MD, New Jersey
Alisa Clark, New Mexico
Prajit Arora, MBBS, New Mexico
Crystal Cardwell, New Mexico
Landon Casaus, New Mexico
Tapuwa Mupfumira, MD, New Mexico
Eric Rightley, New Mexico
David S. Anderson, New York
Joan Bosco, MD, New York
Jessica Caro, New York
Anna Dewan, New York
Amrita Dhillon, MBBS, New York
Julia Frydman, New York
Radhika Gali, MBBS, MDS, New York
Allison Guttmann, MD, New York
Aryles Hedjar, MD, New York
Peter Janes, New York
Nadine Kalavazoff, New York
Jeffrey Lach, DO, New York
Keron Lezama, MD, New York
Yingheng Liu, New York
Taimur Mirza, New York
Cyrus Nensey, MD, New York
Nekee Pandya, MD, New York
Thushara Paul, MD, New York
Yu Sung, New York
Joel Boggan, MD, North Carolina
Angela Fletcher, North Carolina
Rebecca Gimpert, PA-C, North Carolina
Samantha Levering, PA-C, North Carolina
Nancy Martin, North Carolina
Richard Sherwood, North Carolina
Kranthi K. Sitammagari, MD, North Carolina
Aaron Swedberg, MPAS, PA-C, North Carolina
Yih-Cherng Tsai, North Carolina
Richard Bakker, MD, PhD, Ohio
Matthew Broderick, MD, Ohio
Subbaraju Budharaju, MD, MS, Ohio
Steven Bumb, MD, Ohio
Ahmed Eltelbany, MD, Ohio
Tracey Hardin, MS, Ohio
Patricia Hardman, APRN, Ohio
Michael Lewis, MD, Ohio
Volodymyr Manko, Ohio
Rebecca Stone, Ohio
Chaitanya Valluri, Ohio
Holly Wierzbicki, CNP, Ohio
Jamie Yockey, APRN, CNP, Ohio
Mahdi Mussa, MD, Oklahoma
Monica Saemz, DO, Oklahoma
Peter Ganter, MD, Oregon
Bethany Roy, MD, Oregon
Mary Clare Bohnett, Oregon
Molly Rabinowitz, Oregon
Abdullateef Abdulkareem, MD, MPH, Pennsylvania
David Ahamba, MD, MPH, Pennsylvania
David Chin, MD, Pennsylvania
Thomas Conlon, Pennsylvania
Dan Giesler, MD, Pennsylvania
Umair Randhawa, MD, Pennsylvania
Syed Yusuf, MBBS, Pennsylvania
Michael Rigatti, Pennsylvania
Thaylon Barreto, Rhode Island
Jessica Cook, MD, South Carolina
Robin Malik, MD, South Carolina
John Busigin, Tennessee
Shefali Paranjape, MD, Tennessee
Thai Dang, MD, Texas
Matthew Glover, MD, Texas
Snigdha Jain, MD, Texas
David Kellenberger, Texas
Sumeet Kumar, Texas
Kyle McClendon, PA-C, Texas
Sowjanya Mohan, Texas
Akhil D. Vats, MD, Texas
Samatha Vellanki, Texas
Lee-Anna Burgess, MD, Vermont
Rick Hildebrant, MD, Vermont
Matthew Backens, MD, Virginia
Megan Coe, Virginia
Kevin Dehaan, Virginia
Stephen Fox, Virginia
Amber Inofuentes, MD, Virginia
Jessica Keiser, MD, Virginia
Joseph Perez, MD, FAAFP, MBA, Virginia
Kanwapreet S. Saini, MD, Virginia
Erin Vipler, MD, Virginia
Naveen Voore, MBBS, Virginia
Abhishek Agarwal, MD, MBBS, Washington
Robert Cooney, MD, Washington
Cynthia Horton, MD, Washington
Rich A. Kukreja, MD, Washington
Ji Young Nam, MD, Washington
Kai Wilhelm, MD, Washington
In Kyu Yoo, Washington
Temu Brown, Wisconsin
Pablo Colon Nieves, Wisconsin
Christina Evans, PAC, Wisconsin
Swetha Karturi, MBBS, Wisconsin
Mark Babcock, DO, Wyoming
Ahmad Von Schlegell, Canada
Anand Kartha, Japan
Mohamed Sadek, Qatar
Amine Rakab, MD, Qatar
Abazar Saeed, Qatar
Joao Guerra, MD
VIDEO: HIV PrEP effective, but adoption lags among high-risk patients
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
What’s in store for CMS under Seema Verma?
WASHINGTON – Big changes could be in store for the Medicaid program under the potential leadership of Seema Verma as administrator of the Centers for Medicare & Medicaid Services.
In nominating Ms. Verma, President Trump called her a leading expert on Medicaid and Medicare who will help “transform our health care system for the benefit of all Americans.”
A relative unknown, Ms. Verma is a health policy consultant who has spent 20 years quietly designing policy projects involving Medicaid, including the crafting of Indiana’s Medicaid expansion under the Affordable Care Act in which she worked closely with Vice President Pence.
How the savvy consultant could alter the Medicaid program during her potential CMS tenure has been the source of much speculation. President Trump and Rep. Tom Price (R-Ga.), Health & Human Services secretary–designee, have called for the restructuring of Medicaid, including the possibility of block grants that would set limits on total annual spending regardless of enrollment or caps that would limit average spending per enrollee.
Ms. Verma’s role in the revamp will largely depend on what Congress allows, said Mark Polston, a Washington, D.C.–based health law attorney and former associate general counsel for litigation in the HHS Office of General Counsel, CMS division, under President George W. Bush.
“We don’t know what cards she has in her hands – let alone what’s in the deck for her to deal from,” Mr. Polston said at a meeting sponsored by the American Bar Association. “That’s really going to be set by Congress.”
“From a CMS perspective, the Medicaid program is in large part run by the states,” Mr. Blum said at the ABA meeting. “What the new nominee says to me is that there is going to be a very high priority placed upon working with states, working with governors to modify Medicaid programs and to shape them to meet different state priorities.”
Look no further than the Healthy Indiana Plan 2.0 to get an idea of what such plans could look like, said Leslie V. Norwalk, a Washington, D.C.–based health law attorney and a former acting CMS administrator under President George W. Bush. The Indiana plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Ms. Verma could work with governors to develop similar model waivers, Ms. Norwalk said.
Mr. Blum said he believes a top priority for Ms. Verma will be limiting the disruption that could come if the ACA is repealed and finding ways to cover those who lose coverage.
“What the next team really needs to really realize [is] that those who sign up for coverage – with the exchanges or through the new state expansions – are sicker on average, have lower income on average, and they’re going to have to think very carefully about the transitions going on,” Mr. Blum said at the meeting. “How you think about the transitions, how you think about continuity of care, how you think about disruptions – those will be very real and very tangible for the next team.”
“If she’s smart, she’ll find a deputy who’s very strong on Medicare. She’ll probably also try to find a former Hill staffer who can help her on the Hill,” Mr. Scully said. “The best news for her is that they picked her really early, so she’s got a head start.”
Hiring a deputy with a strong insurance background will also be key, Ms. Norwalk said in an interview. Even if the exchanges are repealed, other CMS programs, such as Medicare Part D and Medicare Advantage rely heavily on insurers, she said.
Ms. Norwalk noted that Ms. Verma may be surprised to find that much of the CMS agenda is controlled by the requirements of Medicare’s regulatory cycle. “[It] will take up a lot of her time, perhaps more than she might anticipate,” she said. “But in addition, you’ll have governors coming in wanting to do waivers. ... The third component will be how much time she spends on Capitol Hill working on whether repeal is done or not, and certainly the replace function and how that works will be a critical component of what she does. [If she’s confirmed,] she’ll be a very busy lady.”
[email protected]
On Twitter @legal_med
WASHINGTON – Big changes could be in store for the Medicaid program under the potential leadership of Seema Verma as administrator of the Centers for Medicare & Medicaid Services.
In nominating Ms. Verma, President Trump called her a leading expert on Medicaid and Medicare who will help “transform our health care system for the benefit of all Americans.”
A relative unknown, Ms. Verma is a health policy consultant who has spent 20 years quietly designing policy projects involving Medicaid, including the crafting of Indiana’s Medicaid expansion under the Affordable Care Act in which she worked closely with Vice President Pence.
How the savvy consultant could alter the Medicaid program during her potential CMS tenure has been the source of much speculation. President Trump and Rep. Tom Price (R-Ga.), Health & Human Services secretary–designee, have called for the restructuring of Medicaid, including the possibility of block grants that would set limits on total annual spending regardless of enrollment or caps that would limit average spending per enrollee.
Ms. Verma’s role in the revamp will largely depend on what Congress allows, said Mark Polston, a Washington, D.C.–based health law attorney and former associate general counsel for litigation in the HHS Office of General Counsel, CMS division, under President George W. Bush.
“We don’t know what cards she has in her hands – let alone what’s in the deck for her to deal from,” Mr. Polston said at a meeting sponsored by the American Bar Association. “That’s really going to be set by Congress.”
“From a CMS perspective, the Medicaid program is in large part run by the states,” Mr. Blum said at the ABA meeting. “What the new nominee says to me is that there is going to be a very high priority placed upon working with states, working with governors to modify Medicaid programs and to shape them to meet different state priorities.”
Look no further than the Healthy Indiana Plan 2.0 to get an idea of what such plans could look like, said Leslie V. Norwalk, a Washington, D.C.–based health law attorney and a former acting CMS administrator under President George W. Bush. The Indiana plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Ms. Verma could work with governors to develop similar model waivers, Ms. Norwalk said.
Mr. Blum said he believes a top priority for Ms. Verma will be limiting the disruption that could come if the ACA is repealed and finding ways to cover those who lose coverage.
“What the next team really needs to really realize [is] that those who sign up for coverage – with the exchanges or through the new state expansions – are sicker on average, have lower income on average, and they’re going to have to think very carefully about the transitions going on,” Mr. Blum said at the meeting. “How you think about the transitions, how you think about continuity of care, how you think about disruptions – those will be very real and very tangible for the next team.”
“If she’s smart, she’ll find a deputy who’s very strong on Medicare. She’ll probably also try to find a former Hill staffer who can help her on the Hill,” Mr. Scully said. “The best news for her is that they picked her really early, so she’s got a head start.”
Hiring a deputy with a strong insurance background will also be key, Ms. Norwalk said in an interview. Even if the exchanges are repealed, other CMS programs, such as Medicare Part D and Medicare Advantage rely heavily on insurers, she said.
Ms. Norwalk noted that Ms. Verma may be surprised to find that much of the CMS agenda is controlled by the requirements of Medicare’s regulatory cycle. “[It] will take up a lot of her time, perhaps more than she might anticipate,” she said. “But in addition, you’ll have governors coming in wanting to do waivers. ... The third component will be how much time she spends on Capitol Hill working on whether repeal is done or not, and certainly the replace function and how that works will be a critical component of what she does. [If she’s confirmed,] she’ll be a very busy lady.”
[email protected]
On Twitter @legal_med
WASHINGTON – Big changes could be in store for the Medicaid program under the potential leadership of Seema Verma as administrator of the Centers for Medicare & Medicaid Services.
In nominating Ms. Verma, President Trump called her a leading expert on Medicaid and Medicare who will help “transform our health care system for the benefit of all Americans.”
A relative unknown, Ms. Verma is a health policy consultant who has spent 20 years quietly designing policy projects involving Medicaid, including the crafting of Indiana’s Medicaid expansion under the Affordable Care Act in which she worked closely with Vice President Pence.
How the savvy consultant could alter the Medicaid program during her potential CMS tenure has been the source of much speculation. President Trump and Rep. Tom Price (R-Ga.), Health & Human Services secretary–designee, have called for the restructuring of Medicaid, including the possibility of block grants that would set limits on total annual spending regardless of enrollment or caps that would limit average spending per enrollee.
Ms. Verma’s role in the revamp will largely depend on what Congress allows, said Mark Polston, a Washington, D.C.–based health law attorney and former associate general counsel for litigation in the HHS Office of General Counsel, CMS division, under President George W. Bush.
“We don’t know what cards she has in her hands – let alone what’s in the deck for her to deal from,” Mr. Polston said at a meeting sponsored by the American Bar Association. “That’s really going to be set by Congress.”
“From a CMS perspective, the Medicaid program is in large part run by the states,” Mr. Blum said at the ABA meeting. “What the new nominee says to me is that there is going to be a very high priority placed upon working with states, working with governors to modify Medicaid programs and to shape them to meet different state priorities.”
Look no further than the Healthy Indiana Plan 2.0 to get an idea of what such plans could look like, said Leslie V. Norwalk, a Washington, D.C.–based health law attorney and a former acting CMS administrator under President George W. Bush. The Indiana plan requires patients to pay a small amount to receive health coverage and includes a lockout period if payments are missed. Ms. Verma could work with governors to develop similar model waivers, Ms. Norwalk said.
Mr. Blum said he believes a top priority for Ms. Verma will be limiting the disruption that could come if the ACA is repealed and finding ways to cover those who lose coverage.
“What the next team really needs to really realize [is] that those who sign up for coverage – with the exchanges or through the new state expansions – are sicker on average, have lower income on average, and they’re going to have to think very carefully about the transitions going on,” Mr. Blum said at the meeting. “How you think about the transitions, how you think about continuity of care, how you think about disruptions – those will be very real and very tangible for the next team.”
“If she’s smart, she’ll find a deputy who’s very strong on Medicare. She’ll probably also try to find a former Hill staffer who can help her on the Hill,” Mr. Scully said. “The best news for her is that they picked her really early, so she’s got a head start.”
Hiring a deputy with a strong insurance background will also be key, Ms. Norwalk said in an interview. Even if the exchanges are repealed, other CMS programs, such as Medicare Part D and Medicare Advantage rely heavily on insurers, she said.
Ms. Norwalk noted that Ms. Verma may be surprised to find that much of the CMS agenda is controlled by the requirements of Medicare’s regulatory cycle. “[It] will take up a lot of her time, perhaps more than she might anticipate,” she said. “But in addition, you’ll have governors coming in wanting to do waivers. ... The third component will be how much time she spends on Capitol Hill working on whether repeal is done or not, and certainly the replace function and how that works will be a critical component of what she does. [If she’s confirmed,] she’ll be a very busy lady.”
[email protected]
On Twitter @legal_med
AT THE AMERICAN BAR ASSOCIATION HEALTH LAW SUMMIT
Half of newly detected antimicrobial antibodies do not lead to PBC
Nearly half of newly detected antimitochondrial antibodies (AMAs) in clinical practice do not lead to a diagnosis of primary biliary cholangitis (PBC), according to a prospective study.
Geraldine Dahlqvist, MD, and her associates examined 720 patients whose AMA tests were registered during a 1-year census period. They were divided into groups according to whether they were newly diagnosed (275), were previously diagnosed (216), or had a nonestablished diagnosis (229) of PBC. Results showed the prevalence of AMA-positive patients without evidence of PBC was 16.1 per 100,000 inhabitants. It was four (all AMA-positive patients) to six (PBC patients) times higher in women than in men. The median age was 58 years, with the median AMA titer at 1:16. Normal serum alkaline phosphatases (ALP) were 74%, and were 1.5 times above the upper limit of normal in 13% of patients, while cirrhosis was found in 6%. Among the patients with normal ALP and no evidence of cirrhosis, the 5-year incidence rate of PBC was 16%.
It was noted that no patients died officially from PBC in this study. The 1-, 3-, and 5-year rates of survival were 95%, 90%, and 75% (95% CI, 63-87), respectively, compared with 90% in the control group.
“The younger age and lower autoantibody titer of these patients, together with the frequent mild abnormalities of their biochemical liver tests, supports a very early, presymptomatic precholestatic stage of the disease,” Dr. Dahlqvist, of Catholic University of Louvain (Belgium), and her colleagues noted. “The incidence of clinical manifestations of PBC seems, however, much lower than previously reported.”
Find the full story in Hepatology (doi: 10.1002/hep.28559).
Nearly half of newly detected antimitochondrial antibodies (AMAs) in clinical practice do not lead to a diagnosis of primary biliary cholangitis (PBC), according to a prospective study.
Geraldine Dahlqvist, MD, and her associates examined 720 patients whose AMA tests were registered during a 1-year census period. They were divided into groups according to whether they were newly diagnosed (275), were previously diagnosed (216), or had a nonestablished diagnosis (229) of PBC. Results showed the prevalence of AMA-positive patients without evidence of PBC was 16.1 per 100,000 inhabitants. It was four (all AMA-positive patients) to six (PBC patients) times higher in women than in men. The median age was 58 years, with the median AMA titer at 1:16. Normal serum alkaline phosphatases (ALP) were 74%, and were 1.5 times above the upper limit of normal in 13% of patients, while cirrhosis was found in 6%. Among the patients with normal ALP and no evidence of cirrhosis, the 5-year incidence rate of PBC was 16%.
It was noted that no patients died officially from PBC in this study. The 1-, 3-, and 5-year rates of survival were 95%, 90%, and 75% (95% CI, 63-87), respectively, compared with 90% in the control group.
“The younger age and lower autoantibody titer of these patients, together with the frequent mild abnormalities of their biochemical liver tests, supports a very early, presymptomatic precholestatic stage of the disease,” Dr. Dahlqvist, of Catholic University of Louvain (Belgium), and her colleagues noted. “The incidence of clinical manifestations of PBC seems, however, much lower than previously reported.”
Find the full story in Hepatology (doi: 10.1002/hep.28559).
Nearly half of newly detected antimitochondrial antibodies (AMAs) in clinical practice do not lead to a diagnosis of primary biliary cholangitis (PBC), according to a prospective study.
Geraldine Dahlqvist, MD, and her associates examined 720 patients whose AMA tests were registered during a 1-year census period. They were divided into groups according to whether they were newly diagnosed (275), were previously diagnosed (216), or had a nonestablished diagnosis (229) of PBC. Results showed the prevalence of AMA-positive patients without evidence of PBC was 16.1 per 100,000 inhabitants. It was four (all AMA-positive patients) to six (PBC patients) times higher in women than in men. The median age was 58 years, with the median AMA titer at 1:16. Normal serum alkaline phosphatases (ALP) were 74%, and were 1.5 times above the upper limit of normal in 13% of patients, while cirrhosis was found in 6%. Among the patients with normal ALP and no evidence of cirrhosis, the 5-year incidence rate of PBC was 16%.
It was noted that no patients died officially from PBC in this study. The 1-, 3-, and 5-year rates of survival were 95%, 90%, and 75% (95% CI, 63-87), respectively, compared with 90% in the control group.
“The younger age and lower autoantibody titer of these patients, together with the frequent mild abnormalities of their biochemical liver tests, supports a very early, presymptomatic precholestatic stage of the disease,” Dr. Dahlqvist, of Catholic University of Louvain (Belgium), and her colleagues noted. “The incidence of clinical manifestations of PBC seems, however, much lower than previously reported.”
Find the full story in Hepatology (doi: 10.1002/hep.28559).
FROM HEPATOLOGY
When to discontinue contact precautions for patients with MRSA
Methicillin-resistant Staphylococcus aureus (MRSA) is a common hospital-acquired infection with significant morbidity and mortality. The CDC currently recommends contact precautions as a mainstay to prevent transmission of MRSA in health care settings. Most hospitals routinely screen patients for MRSA and use contact precautions for those who screen positive. The duration of these precautions vary across hospitals and no standard recommendation exists.
A recent study of members of the Society for Healthcare Epidemiology of America (SHEA) research network indicated that the majority of physicians (94%) and nurses (76%) dislike contact precautions (CP) and most (63%) were in favor of implementing CP in a different way than current practice.1 Patients also report less satisfaction and increased isolation.1
My colleagues and I recently published a study2 in the American Journal of Infection Control to explore the necessary duration of contact precautions for hospitalized patients with MRSA. Our goal was to maintain contact precautions as long as necessary to prevent undesired MRSA infections and colonization but minimize unnecessary days in contact isolation. We also sought to figure out whether patients with positive MRSA surveillance cultures should always remain in isolation and, if not, at what point they could be considered for rescreening and removal of precautions if culture negative.
Our hospital has been performing active surveillance cultures weekly to screen for MRSA among our hospitalized patients for many years; however from 2010 to 2014, we began screening patients who were previously known to be positive for MRSA colonization or infection for at least 1 year. We then assessed medical and demographic factors associated with persistent carriage of MRSA.
In our study, more than 400 patients with known MRSA were rescreened with an active surveillance culture at a subsequent hospital admission. Ultimately 20% of the patients remained MRSA positive on the active surveillance culture. Most patients who were culture positive for MRSA were found on the first active surveillance culture (16.4%) but the remaining positive cultures were found on a second active surveillance culture or a clinical culture.
The amount of time that passed since the patient was culture positive was significantly associated with a lower risk of a positive culture at screening. This continued to drop over time with only 12.5% of patients remaining active surveillance culture positive for MRSA at 5 years after the original positive culture.
Two factors were found to significantly impact the MRSA culture on the multivariate analysis: (1) Female sex reduced the risk of positivity, and (2) Presence of a foreign body increased the risk of positivity.
Most patients who remained positive for an MRSA culture were found with the first active surveillance culture, less than 4% were detected subsequently with a repeat surveillance or clinical culture and this percentage also decreased over time. This indicates that in the absence of a positive active surveillance culture it may be reasonable to discontinue contact precautions, which could result in a substantial cost savings for the hospital and improved patient and provider satisfaction without increasing the risk of MRSA transmission.
We concluded that in the absence of a foreign body and with at least a year from the last known positive culture, patients with known MRSA should be rescreened and, if negative on an active surveillance culture, should be removed from contact precautions.
Lauren Richey, MD, MPH, is assistant professor in the infectious diseases division at the Medical University of South Carolina.
References
1. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: A review of the literature. Am J Infect Control. 2009 Mar;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257.
2. Richey LE, Oh Y, Tchamba DM, Engle M, Formby L, Salgado CD. When should contact precautions be discontinued for patients with Methicillin-resistant Staphylococcus aureus? Am J Infect Control. 2016 Aug 30. doi: 10.1016/j.ajic.2016.05.030.
Methicillin-resistant Staphylococcus aureus (MRSA) is a common hospital-acquired infection with significant morbidity and mortality. The CDC currently recommends contact precautions as a mainstay to prevent transmission of MRSA in health care settings. Most hospitals routinely screen patients for MRSA and use contact precautions for those who screen positive. The duration of these precautions vary across hospitals and no standard recommendation exists.
A recent study of members of the Society for Healthcare Epidemiology of America (SHEA) research network indicated that the majority of physicians (94%) and nurses (76%) dislike contact precautions (CP) and most (63%) were in favor of implementing CP in a different way than current practice.1 Patients also report less satisfaction and increased isolation.1
My colleagues and I recently published a study2 in the American Journal of Infection Control to explore the necessary duration of contact precautions for hospitalized patients with MRSA. Our goal was to maintain contact precautions as long as necessary to prevent undesired MRSA infections and colonization but minimize unnecessary days in contact isolation. We also sought to figure out whether patients with positive MRSA surveillance cultures should always remain in isolation and, if not, at what point they could be considered for rescreening and removal of precautions if culture negative.
Our hospital has been performing active surveillance cultures weekly to screen for MRSA among our hospitalized patients for many years; however from 2010 to 2014, we began screening patients who were previously known to be positive for MRSA colonization or infection for at least 1 year. We then assessed medical and demographic factors associated with persistent carriage of MRSA.
In our study, more than 400 patients with known MRSA were rescreened with an active surveillance culture at a subsequent hospital admission. Ultimately 20% of the patients remained MRSA positive on the active surveillance culture. Most patients who were culture positive for MRSA were found on the first active surveillance culture (16.4%) but the remaining positive cultures were found on a second active surveillance culture or a clinical culture.
The amount of time that passed since the patient was culture positive was significantly associated with a lower risk of a positive culture at screening. This continued to drop over time with only 12.5% of patients remaining active surveillance culture positive for MRSA at 5 years after the original positive culture.
Two factors were found to significantly impact the MRSA culture on the multivariate analysis: (1) Female sex reduced the risk of positivity, and (2) Presence of a foreign body increased the risk of positivity.
Most patients who remained positive for an MRSA culture were found with the first active surveillance culture, less than 4% were detected subsequently with a repeat surveillance or clinical culture and this percentage also decreased over time. This indicates that in the absence of a positive active surveillance culture it may be reasonable to discontinue contact precautions, which could result in a substantial cost savings for the hospital and improved patient and provider satisfaction without increasing the risk of MRSA transmission.
We concluded that in the absence of a foreign body and with at least a year from the last known positive culture, patients with known MRSA should be rescreened and, if negative on an active surveillance culture, should be removed from contact precautions.
Lauren Richey, MD, MPH, is assistant professor in the infectious diseases division at the Medical University of South Carolina.
References
1. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: A review of the literature. Am J Infect Control. 2009 Mar;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257.
2. Richey LE, Oh Y, Tchamba DM, Engle M, Formby L, Salgado CD. When should contact precautions be discontinued for patients with Methicillin-resistant Staphylococcus aureus? Am J Infect Control. 2016 Aug 30. doi: 10.1016/j.ajic.2016.05.030.
Methicillin-resistant Staphylococcus aureus (MRSA) is a common hospital-acquired infection with significant morbidity and mortality. The CDC currently recommends contact precautions as a mainstay to prevent transmission of MRSA in health care settings. Most hospitals routinely screen patients for MRSA and use contact precautions for those who screen positive. The duration of these precautions vary across hospitals and no standard recommendation exists.
A recent study of members of the Society for Healthcare Epidemiology of America (SHEA) research network indicated that the majority of physicians (94%) and nurses (76%) dislike contact precautions (CP) and most (63%) were in favor of implementing CP in a different way than current practice.1 Patients also report less satisfaction and increased isolation.1
My colleagues and I recently published a study2 in the American Journal of Infection Control to explore the necessary duration of contact precautions for hospitalized patients with MRSA. Our goal was to maintain contact precautions as long as necessary to prevent undesired MRSA infections and colonization but minimize unnecessary days in contact isolation. We also sought to figure out whether patients with positive MRSA surveillance cultures should always remain in isolation and, if not, at what point they could be considered for rescreening and removal of precautions if culture negative.
Our hospital has been performing active surveillance cultures weekly to screen for MRSA among our hospitalized patients for many years; however from 2010 to 2014, we began screening patients who were previously known to be positive for MRSA colonization or infection for at least 1 year. We then assessed medical and demographic factors associated with persistent carriage of MRSA.
In our study, more than 400 patients with known MRSA were rescreened with an active surveillance culture at a subsequent hospital admission. Ultimately 20% of the patients remained MRSA positive on the active surveillance culture. Most patients who were culture positive for MRSA were found on the first active surveillance culture (16.4%) but the remaining positive cultures were found on a second active surveillance culture or a clinical culture.
The amount of time that passed since the patient was culture positive was significantly associated with a lower risk of a positive culture at screening. This continued to drop over time with only 12.5% of patients remaining active surveillance culture positive for MRSA at 5 years after the original positive culture.
Two factors were found to significantly impact the MRSA culture on the multivariate analysis: (1) Female sex reduced the risk of positivity, and (2) Presence of a foreign body increased the risk of positivity.
Most patients who remained positive for an MRSA culture were found with the first active surveillance culture, less than 4% were detected subsequently with a repeat surveillance or clinical culture and this percentage also decreased over time. This indicates that in the absence of a positive active surveillance culture it may be reasonable to discontinue contact precautions, which could result in a substantial cost savings for the hospital and improved patient and provider satisfaction without increasing the risk of MRSA transmission.
We concluded that in the absence of a foreign body and with at least a year from the last known positive culture, patients with known MRSA should be rescreened and, if negative on an active surveillance culture, should be removed from contact precautions.
Lauren Richey, MD, MPH, is assistant professor in the infectious diseases division at the Medical University of South Carolina.
References
1. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: A review of the literature. Am J Infect Control. 2009 Mar;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257.
2. Richey LE, Oh Y, Tchamba DM, Engle M, Formby L, Salgado CD. When should contact precautions be discontinued for patients with Methicillin-resistant Staphylococcus aureus? Am J Infect Control. 2016 Aug 30. doi: 10.1016/j.ajic.2016.05.030.
AKT inhibition not superior to everolimus for RCC
The AKT inhibitor MK-2206 was not superior to everolimus (Afinitor) for patients with metastatic renal cell carcinoma refractory to vascular endothelial growth factor inhibitors, according to a phase II trial from the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival was 3.68 months in the 29 patients randomized to MK-2206, versus 5.98 months in the 14 randomized to everolimus, leading to closure of the study, reported Eric Jonasch, MD, of the department of genitourinary medical oncology at MD Anderson, and his associates.
However, dichotomous response rate profiles were seen in the MK-2206 arm with one complete response and three partial responses in the MK-2206 arm versus none in the everolimus arm.
“Whereas patients treated with everolimus for the large part had minimal changes in tumor size, MK-2206 induced a fairly dichotomous response dynamic, with [a few] patients demonstrating profound response, [but] a number of patients exhibiting rapid growth,” Dr. Jonasch and associates said (Ann Oncol. 2017 Jan 3. pii: mdw676. doi: 10.1093/annonc/mdw676).
Several studies have shown that upregulation of the PI3K/AKT pathway is associated with poor prognosis in renal cell carcinoma (RCC), making the pathway an attractive target for therapeutic intervention. The trial “results indicate that potential exists for effective blockade of the PI3K pathway in patients with RCC, but considerable work is required to better understand the nuances of this pathway before we can consistently modulate it to benefit patients with RCC,” the investigators said.
Molecular analysis failed to find a biomarker for response, but did demonstrate that deleterious tumor protein 53 or ataxia telangiectasia mutations or deletions were associated with poor prognosis. Among patients who progressed, 57.1% had TP53 or ATM aberrations; TP53 and ATM defects were absent in patients who did not progress.
Malfunction of DNA repair driven by TP53 and ATM gene modifications, the group said, “are associated with early disease progression, indicating that dysregulation of DNA repair is associated with a more aggressive tumor phenotype in RCC ... This subcategory of patients clearly needs new approaches based on our emerging understanding of the significance of TP53 mutations in RCC biology.”
MK-2206 induced significantly more rash and pruritus than did everolimus, with dose reduction in 37.9% of MK-2206 versus 21.4% of everolimus patients.
Subjects were a median of 63.5 years old in the everolimus group and 59 years in the MK-2206 group. The majority of patients were white men. More than 65% of the patients had performance status 1 and around 60% were in the Memorial Sloan Kettering Cancer Center intermediate risk group. The majority of patients in both treatment arms had clear cell histology; 57.1% (8) in the everolimus group and 82.8% (24) in the MK-2206 group had lung metastasis; half of the everolimus and 59% (17) of MK-2206 subjects were previously treated with sunitinib (Sutent).
The National Institutes of Health funded the work. The authors reported no conflicts of interest.
The AKT inhibitor MK-2206 was not superior to everolimus (Afinitor) for patients with metastatic renal cell carcinoma refractory to vascular endothelial growth factor inhibitors, according to a phase II trial from the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival was 3.68 months in the 29 patients randomized to MK-2206, versus 5.98 months in the 14 randomized to everolimus, leading to closure of the study, reported Eric Jonasch, MD, of the department of genitourinary medical oncology at MD Anderson, and his associates.
However, dichotomous response rate profiles were seen in the MK-2206 arm with one complete response and three partial responses in the MK-2206 arm versus none in the everolimus arm.
“Whereas patients treated with everolimus for the large part had minimal changes in tumor size, MK-2206 induced a fairly dichotomous response dynamic, with [a few] patients demonstrating profound response, [but] a number of patients exhibiting rapid growth,” Dr. Jonasch and associates said (Ann Oncol. 2017 Jan 3. pii: mdw676. doi: 10.1093/annonc/mdw676).
Several studies have shown that upregulation of the PI3K/AKT pathway is associated with poor prognosis in renal cell carcinoma (RCC), making the pathway an attractive target for therapeutic intervention. The trial “results indicate that potential exists for effective blockade of the PI3K pathway in patients with RCC, but considerable work is required to better understand the nuances of this pathway before we can consistently modulate it to benefit patients with RCC,” the investigators said.
Molecular analysis failed to find a biomarker for response, but did demonstrate that deleterious tumor protein 53 or ataxia telangiectasia mutations or deletions were associated with poor prognosis. Among patients who progressed, 57.1% had TP53 or ATM aberrations; TP53 and ATM defects were absent in patients who did not progress.
Malfunction of DNA repair driven by TP53 and ATM gene modifications, the group said, “are associated with early disease progression, indicating that dysregulation of DNA repair is associated with a more aggressive tumor phenotype in RCC ... This subcategory of patients clearly needs new approaches based on our emerging understanding of the significance of TP53 mutations in RCC biology.”
MK-2206 induced significantly more rash and pruritus than did everolimus, with dose reduction in 37.9% of MK-2206 versus 21.4% of everolimus patients.
Subjects were a median of 63.5 years old in the everolimus group and 59 years in the MK-2206 group. The majority of patients were white men. More than 65% of the patients had performance status 1 and around 60% were in the Memorial Sloan Kettering Cancer Center intermediate risk group. The majority of patients in both treatment arms had clear cell histology; 57.1% (8) in the everolimus group and 82.8% (24) in the MK-2206 group had lung metastasis; half of the everolimus and 59% (17) of MK-2206 subjects were previously treated with sunitinib (Sutent).
The National Institutes of Health funded the work. The authors reported no conflicts of interest.
The AKT inhibitor MK-2206 was not superior to everolimus (Afinitor) for patients with metastatic renal cell carcinoma refractory to vascular endothelial growth factor inhibitors, according to a phase II trial from the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival was 3.68 months in the 29 patients randomized to MK-2206, versus 5.98 months in the 14 randomized to everolimus, leading to closure of the study, reported Eric Jonasch, MD, of the department of genitourinary medical oncology at MD Anderson, and his associates.
However, dichotomous response rate profiles were seen in the MK-2206 arm with one complete response and three partial responses in the MK-2206 arm versus none in the everolimus arm.
“Whereas patients treated with everolimus for the large part had minimal changes in tumor size, MK-2206 induced a fairly dichotomous response dynamic, with [a few] patients demonstrating profound response, [but] a number of patients exhibiting rapid growth,” Dr. Jonasch and associates said (Ann Oncol. 2017 Jan 3. pii: mdw676. doi: 10.1093/annonc/mdw676).
Several studies have shown that upregulation of the PI3K/AKT pathway is associated with poor prognosis in renal cell carcinoma (RCC), making the pathway an attractive target for therapeutic intervention. The trial “results indicate that potential exists for effective blockade of the PI3K pathway in patients with RCC, but considerable work is required to better understand the nuances of this pathway before we can consistently modulate it to benefit patients with RCC,” the investigators said.
Molecular analysis failed to find a biomarker for response, but did demonstrate that deleterious tumor protein 53 or ataxia telangiectasia mutations or deletions were associated with poor prognosis. Among patients who progressed, 57.1% had TP53 or ATM aberrations; TP53 and ATM defects were absent in patients who did not progress.
Malfunction of DNA repair driven by TP53 and ATM gene modifications, the group said, “are associated with early disease progression, indicating that dysregulation of DNA repair is associated with a more aggressive tumor phenotype in RCC ... This subcategory of patients clearly needs new approaches based on our emerging understanding of the significance of TP53 mutations in RCC biology.”
MK-2206 induced significantly more rash and pruritus than did everolimus, with dose reduction in 37.9% of MK-2206 versus 21.4% of everolimus patients.
Subjects were a median of 63.5 years old in the everolimus group and 59 years in the MK-2206 group. The majority of patients were white men. More than 65% of the patients had performance status 1 and around 60% were in the Memorial Sloan Kettering Cancer Center intermediate risk group. The majority of patients in both treatment arms had clear cell histology; 57.1% (8) in the everolimus group and 82.8% (24) in the MK-2206 group had lung metastasis; half of the everolimus and 59% (17) of MK-2206 subjects were previously treated with sunitinib (Sutent).
The National Institutes of Health funded the work. The authors reported no conflicts of interest.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: Progression-free survival was a median of 3.68 months in the 29 patients randomized to MK-2206, versus 5.98 months in the 14 randomized to everolimus.
Data source: Phase II trial with 43 patients.
Disclosures: The National Institutes of Health funded the work. The authors reported no conflicts of interest.