User login
Letters to the Editor: Rectal misoprostol for postpartum hemorrhage
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“STOP USING RECTAL MISOPROSTOL FOR THE TREATMENT OF POSTPARTUM HEMORRHAGE CAUSED BY UTERINE ATONY”
ROBERT L. BARBIERI, MD (JULY 2016)
More on rectal misoprostol for postpartum hemorrhage
We applaud Dr. Barbieri’s July Editorial urging providers to stop administering misoprostol rectally for the treatment of postpartum hemorrhage (PPH) given the well-documented evidence and pharmacokinetics that recommend the sublingual route. Confusion among providers may derive from the fact that not all international guidelines, including the American College of Obstetricians and Gynecologists clinical guidelines on the management of PPH, have been updated to reflect the latest evidence.1 Guidelines from the World Health Organization and the International Federation of Gynecology and Obstetrics reflect the latest evidence and clearly recommend the evidence-based regimen of 800 μg misoprostol sublingually for treatment of PPH,2 which has been shown to be comparable to 40 IU oxytocin intravenously in women who receive oxytocin for PPH prophylaxis.3
Although oxytocin remains the first-line treatment for PPH, evidence suggests that sublingual misoprostol should be considered a viable first alternative if oxytocin is not available or fails. There is little evidence on the benefit of methergine or carboprost over misoprostol for PPH treatment, and inclusion of these drugs in treatment guidelines and practice is based on extrapolations from studies on PPH prevention.4 As Dr. Barbieri noted, pyrexia from misoprostol has been cited in the literature; however, contrary to contraindications for methergine, for example, this rare event does not pose serious risks to women, is self-limiting, and appears to be most acute among certain populations.5
It is paramount that safe, effective, and evidence-based PPH treatments be available and known to providers both in the United States and globally in order to provide women with timely treatment. Greater discussion and research is warranted about the hierarchy of use for these drugs and the possible impact of routine use of uterotonics before and during delivery, given that overexposure to uterotonics may in fact be making PPH harder to treat.6
Gillian Burkhardt, MD, and Rasha Dabash, MPH
New York, New York
Dr. Barbieri responds
I thank Drs. Burkhardt and Dabash for sharing their expert perspective with our readers. They advocate for the use of sublingual misoprostol for the treatment of PPH “if oxytocin is not available or fails.” I agree that at a home birth, if oxytocin is not available, sublingual misoprostol would be of great benefit. I remain concerned that misoprostol has little clinical utility for the treatment of PPH in the hospital setting in which oxytocin, methergine, and carboprost are available alternatives. Misoprostol causes fever in many women, and women who develop a postpartum fever due to misoprostol will receive unnecessary antibiotic treatment. I recommend that our readers stop using misoprostol for the treatment of PPH in the hospital setting.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
- ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1048. Reaffirmed 2015.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva, Switzerland: World Health Organization; 2012.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind placebo-controlled randomized non-inferiority trial. Lancet. 2010;375(9710):217–223.
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202–210.
- Durocher J, Bynum J, León W, Barrera G, Winikoff B. High fever following postpartum administration of sublingual misoprostol. BJOG. 2010;117(7):845–852.
- Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
Product Update: JUST…Love, pjur med
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
MASSAGE AND MOISTURING OIL
Just Pure Essentials suggests that JUST…Love be used to moisturize the face and body and for facial cleansing and shaving. It has been recommended by women’s health professionals as a lubricant and moisturizer to relieve vaginal dryness, says the manufacturer.
Just Pure Essentials products are 100% vegan; chemical and preservative free; and without alcohols, additives, or phytoestrogens. The base of each product is coconut medium-chain triglyceride (MCT) oil pressed from nongenetically modified coconut oil by a steam distillation process. The oil is blended with other organic oils and botanical ingredients, including green tea see oil, French plum oil, argan, and marshmallow leaf. It does not contain dyes, perfumes, or artificial flavors. Just Pure Essentials says its formulas do not dry up and turn sticky.
FOR MORE INFORMATION, VISIT: www.justpureessentials.com
SEXUAL HEALTH AND WELLBEING
pjur med says that PREMIUM glide is specially formulated for dry or highly sensitive genital mucous membranes and is made of non–pore-blocking, high-quality silicones. SENSITIVE glide is specifically developed for those with very sensitive genital mucous membranes, according to the manufacturer. Both products are available in 3.4 fl oz bottles. pjur med suggests its products are suitable for every skin type and maintains that they are dermatologically tested and paraben-, glycerin-, and preservative-free. In addition, pjur med says its formulas have been kept as neutral as possible and the ingredients have been put together in a way that microbial growth cannot occur.
FOR MORE INFORMATION, VISIT: www.pjurmed.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Vaccine/PD-1 inhibitor combo shows early promise against mCRPC
NATIONAL HARBOR, MD. – To date, checkpoint inhibitors have shown little clinical activity as single agents against metastatic, castration-resistant prostate cancer, but a combination of a DNA vaccine and a programmed-death 1 inhibitor shows promise for enhancing anti-tumor immune responses, report investigators in a phase I trial.
“If you vaccinate animals, PD-1 expression transiently goes up, and if you block it at that point you get a better anti-tumor response, and that was in models where anti PD-1 therapy alone didn’t do anything. So we thought this could be a good approach for prostate cancer,” Douglas G. McNeel, MD, PhD, of the University of Wisconsin, Madison, said in an interview at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. McNeel and colleagues are exploring the therapeutic potential of combining the PD-1 inhibitor pembrolizumab (Keytruda) with an investigational DNA vaccine targeted against prostatic acid phosphatase (PAP), the same antigen targeted by sipuleucel-T (Provenge).
They presented data in a scientific poster from a pilot study of the combination in patients with metastatic, castration-resistant prostate cancer (mCRPC).
Vaccine ramps up PD-1 expression
The investigators had previously shown that patients immunized with a DNA vaccine encoding PAP (pTVG-HP; currently in phase II clinical trials) developed PD-1-regulated, PAP-specific T cells and had increased PD-L1 expression in circulating tumor cells. They also demonstrated in preclinical studies with mouse models that increased PD-1 expression on vaccine-induced CD8-positive T cells led to inferior anti-tumor immune responses, and that blocking PD-1 at the time of T-cell activation with vaccine improved anti-tumor responses.
“The current pilot trial was designed to evaluate whether delivery of PD-1 blockade after immunization (when PD-1-regulated T cells are elicited and when PD-L1 expression is induced with vaccination on tumor cells) or with immunization (when PD-1 expression increases on antigen-specific CD8+ T cells with activation) results in anti-tumor responses in patients with advanced, metastatic, castration-resistant prostate cancer,” Dr. McNeel and his colleagues wrote.
They reported preliminary impressions of the efficacy and safety of the combination in 12 patients with mCRPC. The patients all had evidence of progressive disease and were at least 6 months out from chemotherapy. Patients could have previously been treated with enzalutamide (Xtandi) or abiraterone (Zytiga), but were excluded if they had received sipuleucel-T vaccine.
Patients in the ongoing trial are randomized to receive six doses of pTVG-HP over 10 weeks with either four doses of concurrent pembrolizumab, or four doses of pembrolizumab delivered every 3 weeks beginning 2 weeks after the last vaccine dose.
The authors reported that treatment with the combination evokes changes in serum prostate-specific antigen (PSA) that are associated with objective radiographic changes, and “elicits robust and persistent PAP-specific Th1-based immunity.”
They also found evidence to suggest that concurrent administration of the vaccine and the PD-1 inhibitor may be more effective than sequential administration, based on differences in serum PSA and PAP.
Adverse events were generally mild and similar to those seen in other studies of pembrolizumab and DNA vaccine, with only three grade 3 events and no grade 4 events.
The investigators hope to expand the pilot study beyond the 12 weeks originally scheduled, and plan to explore potential biomarkers for vaccine response based on T-cell proliferation in tumors and in regional lymph nodes as seen on imaging modalities.
The study is funded by a 2014 Movember Prostate Cancer Foundation Challenge Award and Madison Vaccines Inc. Dr. McNeel reports an ownership interest and funding support from Madison Vaccines. All other coauthors reported no conflicts of interest.
NATIONAL HARBOR, MD. – To date, checkpoint inhibitors have shown little clinical activity as single agents against metastatic, castration-resistant prostate cancer, but a combination of a DNA vaccine and a programmed-death 1 inhibitor shows promise for enhancing anti-tumor immune responses, report investigators in a phase I trial.
“If you vaccinate animals, PD-1 expression transiently goes up, and if you block it at that point you get a better anti-tumor response, and that was in models where anti PD-1 therapy alone didn’t do anything. So we thought this could be a good approach for prostate cancer,” Douglas G. McNeel, MD, PhD, of the University of Wisconsin, Madison, said in an interview at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. McNeel and colleagues are exploring the therapeutic potential of combining the PD-1 inhibitor pembrolizumab (Keytruda) with an investigational DNA vaccine targeted against prostatic acid phosphatase (PAP), the same antigen targeted by sipuleucel-T (Provenge).
They presented data in a scientific poster from a pilot study of the combination in patients with metastatic, castration-resistant prostate cancer (mCRPC).
Vaccine ramps up PD-1 expression
The investigators had previously shown that patients immunized with a DNA vaccine encoding PAP (pTVG-HP; currently in phase II clinical trials) developed PD-1-regulated, PAP-specific T cells and had increased PD-L1 expression in circulating tumor cells. They also demonstrated in preclinical studies with mouse models that increased PD-1 expression on vaccine-induced CD8-positive T cells led to inferior anti-tumor immune responses, and that blocking PD-1 at the time of T-cell activation with vaccine improved anti-tumor responses.
“The current pilot trial was designed to evaluate whether delivery of PD-1 blockade after immunization (when PD-1-regulated T cells are elicited and when PD-L1 expression is induced with vaccination on tumor cells) or with immunization (when PD-1 expression increases on antigen-specific CD8+ T cells with activation) results in anti-tumor responses in patients with advanced, metastatic, castration-resistant prostate cancer,” Dr. McNeel and his colleagues wrote.
They reported preliminary impressions of the efficacy and safety of the combination in 12 patients with mCRPC. The patients all had evidence of progressive disease and were at least 6 months out from chemotherapy. Patients could have previously been treated with enzalutamide (Xtandi) or abiraterone (Zytiga), but were excluded if they had received sipuleucel-T vaccine.
Patients in the ongoing trial are randomized to receive six doses of pTVG-HP over 10 weeks with either four doses of concurrent pembrolizumab, or four doses of pembrolizumab delivered every 3 weeks beginning 2 weeks after the last vaccine dose.
The authors reported that treatment with the combination evokes changes in serum prostate-specific antigen (PSA) that are associated with objective radiographic changes, and “elicits robust and persistent PAP-specific Th1-based immunity.”
They also found evidence to suggest that concurrent administration of the vaccine and the PD-1 inhibitor may be more effective than sequential administration, based on differences in serum PSA and PAP.
Adverse events were generally mild and similar to those seen in other studies of pembrolizumab and DNA vaccine, with only three grade 3 events and no grade 4 events.
The investigators hope to expand the pilot study beyond the 12 weeks originally scheduled, and plan to explore potential biomarkers for vaccine response based on T-cell proliferation in tumors and in regional lymph nodes as seen on imaging modalities.
The study is funded by a 2014 Movember Prostate Cancer Foundation Challenge Award and Madison Vaccines Inc. Dr. McNeel reports an ownership interest and funding support from Madison Vaccines. All other coauthors reported no conflicts of interest.
NATIONAL HARBOR, MD. – To date, checkpoint inhibitors have shown little clinical activity as single agents against metastatic, castration-resistant prostate cancer, but a combination of a DNA vaccine and a programmed-death 1 inhibitor shows promise for enhancing anti-tumor immune responses, report investigators in a phase I trial.
“If you vaccinate animals, PD-1 expression transiently goes up, and if you block it at that point you get a better anti-tumor response, and that was in models where anti PD-1 therapy alone didn’t do anything. So we thought this could be a good approach for prostate cancer,” Douglas G. McNeel, MD, PhD, of the University of Wisconsin, Madison, said in an interview at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. McNeel and colleagues are exploring the therapeutic potential of combining the PD-1 inhibitor pembrolizumab (Keytruda) with an investigational DNA vaccine targeted against prostatic acid phosphatase (PAP), the same antigen targeted by sipuleucel-T (Provenge).
They presented data in a scientific poster from a pilot study of the combination in patients with metastatic, castration-resistant prostate cancer (mCRPC).
Vaccine ramps up PD-1 expression
The investigators had previously shown that patients immunized with a DNA vaccine encoding PAP (pTVG-HP; currently in phase II clinical trials) developed PD-1-regulated, PAP-specific T cells and had increased PD-L1 expression in circulating tumor cells. They also demonstrated in preclinical studies with mouse models that increased PD-1 expression on vaccine-induced CD8-positive T cells led to inferior anti-tumor immune responses, and that blocking PD-1 at the time of T-cell activation with vaccine improved anti-tumor responses.
“The current pilot trial was designed to evaluate whether delivery of PD-1 blockade after immunization (when PD-1-regulated T cells are elicited and when PD-L1 expression is induced with vaccination on tumor cells) or with immunization (when PD-1 expression increases on antigen-specific CD8+ T cells with activation) results in anti-tumor responses in patients with advanced, metastatic, castration-resistant prostate cancer,” Dr. McNeel and his colleagues wrote.
They reported preliminary impressions of the efficacy and safety of the combination in 12 patients with mCRPC. The patients all had evidence of progressive disease and were at least 6 months out from chemotherapy. Patients could have previously been treated with enzalutamide (Xtandi) or abiraterone (Zytiga), but were excluded if they had received sipuleucel-T vaccine.
Patients in the ongoing trial are randomized to receive six doses of pTVG-HP over 10 weeks with either four doses of concurrent pembrolizumab, or four doses of pembrolizumab delivered every 3 weeks beginning 2 weeks after the last vaccine dose.
The authors reported that treatment with the combination evokes changes in serum prostate-specific antigen (PSA) that are associated with objective radiographic changes, and “elicits robust and persistent PAP-specific Th1-based immunity.”
They also found evidence to suggest that concurrent administration of the vaccine and the PD-1 inhibitor may be more effective than sequential administration, based on differences in serum PSA and PAP.
Adverse events were generally mild and similar to those seen in other studies of pembrolizumab and DNA vaccine, with only three grade 3 events and no grade 4 events.
The investigators hope to expand the pilot study beyond the 12 weeks originally scheduled, and plan to explore potential biomarkers for vaccine response based on T-cell proliferation in tumors and in regional lymph nodes as seen on imaging modalities.
The study is funded by a 2014 Movember Prostate Cancer Foundation Challenge Award and Madison Vaccines Inc. Dr. McNeel reports an ownership interest and funding support from Madison Vaccines. All other coauthors reported no conflicts of interest.
AT SITC 2016
Key clinical point:. Adding the PD-1 inhibitor pembrolizumab to a DNA vaccine may enhance vaccine-induced immunity against metastatic castration-resistant prostate cancer (mCRPC).
Major finding: Treatment with a vaccine targeted to prostatic acid phosphatase and pembrolizumab elicited changes in PAP that are associated with objective radiographic responses.
Data source: Ongoing open-label, randomized pilot study in men with mCRPC.
Disclosures: The study is funded by a 2014 Movember Prostate Cancer Foundation Challenge Award and Madison Vaccines Inc. Dr. McNeel reports an ownership interest and funding support from Madison Vaccines. All other coauthors reported no conflicts of interest.
The art of persuasion
With the advent of the Internet, many parents and teen patients come in armed with information and sometimes even a diagnosis. Much of our time is spent dispelling falsehoods that were posted on the Internet or clarifying information that was misinterpreted. Although generally more information is a good thing, too much false information can result in limiting health care.
Vaccine administration has suffered significantly because of this. With a simple Google search, you can find articles that do everything just short of proving that vaccines are harmful, and tear-jerking stories about children who were harmed by the administration of vaccines. Many sites – Vaxtruth.org, healthwyze.org, naturalnews.com – all present convincing data that would scare any concerned parent to not vaccinate their child. So how do medical professionals regain the trust of their parents and/or patients?
The strategies put forth by the Centers for Disease Control and Prevention for talking to parents about vaccines begin with listening.1 Many parents come with fears that are unfounded and unrealistic that can simply be discussed and resolved. Others present with information from the Internet that discourages vaccines or life experiences such as another family member who was thought to be harmed by vaccines; this discussion is more complex.
It is imperative to become familiar with the most popular information sources on the Internet so that you can speak directly to the validity of the source. As well, countering with a more reliable source will substantiate your position. Healthychildren.org2 is an excellent reference for the AAP recommendations and further references. Vaccinesafety.edu is an independent source that reviews vaccine safety and current research.
Being proactive also builds trust. Provide families with the list of ingredients (vaccinesafety.edu), what their role is in keeping vaccines safe (tell them to go to cdc.gov and search under “vaccines for parents”), and help them understand how vaccines work. Parents then see that you are well informed and are passionate about the health of their children. The AAP provides physicians with a tool kit for the HPV vaccine, and the CDC has an HPV tipsheet entitled “Addressing Parents’ Top Questions about HPV Vaccine” that gives suggestions for what you can say or that can save you time if you provide it while the family waits to be seen.
Probably the most important strategy is believing in what you’re doing. No matter what you’re promoting, if you truly believe in it, then you will encourage others to believe in it as well. This requires educating yourself on current research and recommendations, as well as what is being reported in the news so you can be armed with factual data when parents have questions.
Today, health care is a partnership, and we must embrace our role as educators to empower patients to make good choices for themselves as well as their families.
References
1. http://www.cdc.gov/vaccines/hcp/conversations/conv-materials.html
2. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/Vaccine-Safety-The-Facts.aspx
3. http://www.immunize.org
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
With the advent of the Internet, many parents and teen patients come in armed with information and sometimes even a diagnosis. Much of our time is spent dispelling falsehoods that were posted on the Internet or clarifying information that was misinterpreted. Although generally more information is a good thing, too much false information can result in limiting health care.
Vaccine administration has suffered significantly because of this. With a simple Google search, you can find articles that do everything just short of proving that vaccines are harmful, and tear-jerking stories about children who were harmed by the administration of vaccines. Many sites – Vaxtruth.org, healthwyze.org, naturalnews.com – all present convincing data that would scare any concerned parent to not vaccinate their child. So how do medical professionals regain the trust of their parents and/or patients?
The strategies put forth by the Centers for Disease Control and Prevention for talking to parents about vaccines begin with listening.1 Many parents come with fears that are unfounded and unrealistic that can simply be discussed and resolved. Others present with information from the Internet that discourages vaccines or life experiences such as another family member who was thought to be harmed by vaccines; this discussion is more complex.
It is imperative to become familiar with the most popular information sources on the Internet so that you can speak directly to the validity of the source. As well, countering with a more reliable source will substantiate your position. Healthychildren.org2 is an excellent reference for the AAP recommendations and further references. Vaccinesafety.edu is an independent source that reviews vaccine safety and current research.
Being proactive also builds trust. Provide families with the list of ingredients (vaccinesafety.edu), what their role is in keeping vaccines safe (tell them to go to cdc.gov and search under “vaccines for parents”), and help them understand how vaccines work. Parents then see that you are well informed and are passionate about the health of their children. The AAP provides physicians with a tool kit for the HPV vaccine, and the CDC has an HPV tipsheet entitled “Addressing Parents’ Top Questions about HPV Vaccine” that gives suggestions for what you can say or that can save you time if you provide it while the family waits to be seen.
Probably the most important strategy is believing in what you’re doing. No matter what you’re promoting, if you truly believe in it, then you will encourage others to believe in it as well. This requires educating yourself on current research and recommendations, as well as what is being reported in the news so you can be armed with factual data when parents have questions.
Today, health care is a partnership, and we must embrace our role as educators to empower patients to make good choices for themselves as well as their families.
References
1. http://www.cdc.gov/vaccines/hcp/conversations/conv-materials.html
2. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/Vaccine-Safety-The-Facts.aspx
3. http://www.immunize.org
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
With the advent of the Internet, many parents and teen patients come in armed with information and sometimes even a diagnosis. Much of our time is spent dispelling falsehoods that were posted on the Internet or clarifying information that was misinterpreted. Although generally more information is a good thing, too much false information can result in limiting health care.
Vaccine administration has suffered significantly because of this. With a simple Google search, you can find articles that do everything just short of proving that vaccines are harmful, and tear-jerking stories about children who were harmed by the administration of vaccines. Many sites – Vaxtruth.org, healthwyze.org, naturalnews.com – all present convincing data that would scare any concerned parent to not vaccinate their child. So how do medical professionals regain the trust of their parents and/or patients?
The strategies put forth by the Centers for Disease Control and Prevention for talking to parents about vaccines begin with listening.1 Many parents come with fears that are unfounded and unrealistic that can simply be discussed and resolved. Others present with information from the Internet that discourages vaccines or life experiences such as another family member who was thought to be harmed by vaccines; this discussion is more complex.
It is imperative to become familiar with the most popular information sources on the Internet so that you can speak directly to the validity of the source. As well, countering with a more reliable source will substantiate your position. Healthychildren.org2 is an excellent reference for the AAP recommendations and further references. Vaccinesafety.edu is an independent source that reviews vaccine safety and current research.
Being proactive also builds trust. Provide families with the list of ingredients (vaccinesafety.edu), what their role is in keeping vaccines safe (tell them to go to cdc.gov and search under “vaccines for parents”), and help them understand how vaccines work. Parents then see that you are well informed and are passionate about the health of their children. The AAP provides physicians with a tool kit for the HPV vaccine, and the CDC has an HPV tipsheet entitled “Addressing Parents’ Top Questions about HPV Vaccine” that gives suggestions for what you can say or that can save you time if you provide it while the family waits to be seen.
Probably the most important strategy is believing in what you’re doing. No matter what you’re promoting, if you truly believe in it, then you will encourage others to believe in it as well. This requires educating yourself on current research and recommendations, as well as what is being reported in the news so you can be armed with factual data when parents have questions.
Today, health care is a partnership, and we must embrace our role as educators to empower patients to make good choices for themselves as well as their families.
References
1. http://www.cdc.gov/vaccines/hcp/conversations/conv-materials.html
2. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/Vaccine-Safety-The-Facts.aspx
3. http://www.immunize.org
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
HCV patients with early-stage hepatocellular carcinoma can achieve SVR
BOSTON – Among patients with hepatocellular carcinoma (HCC), rates of sustained viral response (SVR) to direct-acting regimens for hepatitis C virus were 79% for genotype 1, 69% for genotype 2, and 47% for genotype 3 infections, reported George N. Ioannou, MD.
“These rates are lower than in patients who do not have hepatocellular carcinoma [HCC], but are still remarkably high,” Dr. Ioannou said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases. “Antiviral therapy should be considered in patients with early-stage hepatocellular carcinoma, ideally after adequate locoregional treatments.”
The study included Veterans Affairs Health Care System data on 17,487 recipients of direct-acting anti-HCV regimens. When patients did not have HCC, SVR rates were 93% for genotype 1 infection, 87% for genotype 2 (GT2), and 76% for GT3. Among the 624 (3.6%) patients with a history of HCC, 142 underwent antiviral treatment after transplantation and 482 received other types of cancer therapy.
Why HCC is associated with lower SVR in HCV patients remains unclear, Dr. Ioannou noted. Age does not seem to explain the effect, and neither does sex, race, or ethnicity; cirrhosis or decompensated cirrhosis; renal disease; diabetes; HCV viral load; genotype or subgenotype; HCV regimen; or treatment experience, he said.
Dr. Ioannou noted several study limitations. Nine percent of patients lacked data on SVR, and the imputation to correct for this lowered SVR rates by about 1%-2%. The dataset also did not include information on HCC tumor size or number, and the researchers have not yet examined how antiviral therapy affects the likelihood of de novo HCC, recurrent HCC, or progression of cirrhosis and liver dysfunction.
The Veterans Affairs Office of Research and Development sponsored the study. Dr. Ioannou had no disclosures.
BOSTON – Among patients with hepatocellular carcinoma (HCC), rates of sustained viral response (SVR) to direct-acting regimens for hepatitis C virus were 79% for genotype 1, 69% for genotype 2, and 47% for genotype 3 infections, reported George N. Ioannou, MD.
“These rates are lower than in patients who do not have hepatocellular carcinoma [HCC], but are still remarkably high,” Dr. Ioannou said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases. “Antiviral therapy should be considered in patients with early-stage hepatocellular carcinoma, ideally after adequate locoregional treatments.”
The study included Veterans Affairs Health Care System data on 17,487 recipients of direct-acting anti-HCV regimens. When patients did not have HCC, SVR rates were 93% for genotype 1 infection, 87% for genotype 2 (GT2), and 76% for GT3. Among the 624 (3.6%) patients with a history of HCC, 142 underwent antiviral treatment after transplantation and 482 received other types of cancer therapy.
Why HCC is associated with lower SVR in HCV patients remains unclear, Dr. Ioannou noted. Age does not seem to explain the effect, and neither does sex, race, or ethnicity; cirrhosis or decompensated cirrhosis; renal disease; diabetes; HCV viral load; genotype or subgenotype; HCV regimen; or treatment experience, he said.
Dr. Ioannou noted several study limitations. Nine percent of patients lacked data on SVR, and the imputation to correct for this lowered SVR rates by about 1%-2%. The dataset also did not include information on HCC tumor size or number, and the researchers have not yet examined how antiviral therapy affects the likelihood of de novo HCC, recurrent HCC, or progression of cirrhosis and liver dysfunction.
The Veterans Affairs Office of Research and Development sponsored the study. Dr. Ioannou had no disclosures.
BOSTON – Among patients with hepatocellular carcinoma (HCC), rates of sustained viral response (SVR) to direct-acting regimens for hepatitis C virus were 79% for genotype 1, 69% for genotype 2, and 47% for genotype 3 infections, reported George N. Ioannou, MD.
“These rates are lower than in patients who do not have hepatocellular carcinoma [HCC], but are still remarkably high,” Dr. Ioannou said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases. “Antiviral therapy should be considered in patients with early-stage hepatocellular carcinoma, ideally after adequate locoregional treatments.”
The study included Veterans Affairs Health Care System data on 17,487 recipients of direct-acting anti-HCV regimens. When patients did not have HCC, SVR rates were 93% for genotype 1 infection, 87% for genotype 2 (GT2), and 76% for GT3. Among the 624 (3.6%) patients with a history of HCC, 142 underwent antiviral treatment after transplantation and 482 received other types of cancer therapy.
Why HCC is associated with lower SVR in HCV patients remains unclear, Dr. Ioannou noted. Age does not seem to explain the effect, and neither does sex, race, or ethnicity; cirrhosis or decompensated cirrhosis; renal disease; diabetes; HCV viral load; genotype or subgenotype; HCV regimen; or treatment experience, he said.
Dr. Ioannou noted several study limitations. Nine percent of patients lacked data on SVR, and the imputation to correct for this lowered SVR rates by about 1%-2%. The dataset also did not include information on HCC tumor size or number, and the researchers have not yet examined how antiviral therapy affects the likelihood of de novo HCC, recurrent HCC, or progression of cirrhosis and liver dysfunction.
The Veterans Affairs Office of Research and Development sponsored the study. Dr. Ioannou had no disclosures.
AT THE LIVER MEETING
Key clinical point: Consider direct-acting antiviral therapy in HCV-infected patients with early-stage HCC.
Major finding: Rates of sustained viral response were 79% in HCC patients with GT1 HCV infection, 69% in GT2 patients, and 47% in GT3 patients.
Data source: An analysis of Veterans Affairs Health Care System data on 17,487 recipients of direct-acting antiviral regimens, including 624 patients with HCC.
Disclosures: The Veterans Affairs Office of Research and Development sponsored the study.
Early TIPS effective in high-risk cirrhosis patients, but still underutilized
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
[email protected]
On Twitter @whitneymcknight
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
[email protected]
On Twitter @whitneymcknight
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
[email protected]
On Twitter @whitneymcknight
AT THE LIVER MEETING
Key clinical point:
Major finding: At 1 year post procedure, early TIPS was associated with better rates of survival and lower rates of adverse events, compared with those who did not receive early TIPS.
Data source: Multicenter, international observational study between 2011 and 2015 of 671 high-risk patients with cirrhosis managed according to current guidelines.
Disclosures: Dr. Hernandez-Gea did not have any relevant disclosures.
Texas reports local Zika transmission
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
Survey finds high rate of misdiagnosed fungal infections
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
FROM JAAD
Key clinical point: Fungal infections may often be missed or misdiagnosed by dermatologists.
Major finding: In 1 of 13 cases did 90% of an audience of dermatologists correctly categorize the condition.
Data source: A survey of board-certified dermatologists, asked whether or not 13 clinical images were a fungal infection or not, during a session on fungal infections at a dermatology meeting.
Disclosures: The research team had no relevant financial conflicts to disclose.
Comparing Cost, Efficacy, and Safety of Intravenous and Topical Tranexamic Acid in Total Hip and Knee Arthroplasty
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
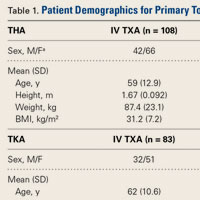
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
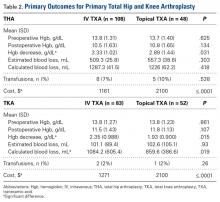
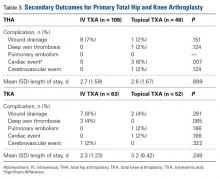
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
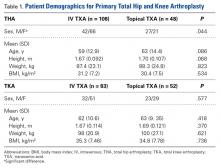
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Kratom: A New Product in an Expanding Substance Abuse Market
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.