User login
Team develops model of common infant ALL

Photo by Petr Kratochvil
After trying for nearly 2 decades, researchers have created a mouse model of t(4;11) pro-B acute lymphoblastic
leukemia (ALL).
The team said this model, described in Cancer Cell, mimics the human disease phenotypically and molecularly.
This type of ALL, which is common in infants, results from the translocation t(4;11)(q21;q23), which fuses the mixed-lineage leukemia (MLL) gene on chromosome 11 to the ALL-1 fused gene on chromosome 4 (AF4).
“For 20 years, scientists have repeatedly tried and consistently failed to make a model of MLL-AF4 pro-B acute lymphoblastic leukemia,” said study author Michael Thirman, MD, of the University of Chicago in Illinois.
“Even though we understood the basic genetic flaw, no one had been able create a mouse model that mimicked the human disease, which is crucial for evaluating potential therapies.”
That frustrated many researchers, who shifted their focus to test alternative hypotheses on the causes of t(4;11) pro-B ALL or refocused their laboratories to study different aspects of the disease.
However, Dr Thirman and his colleagues began working on this problem “years ago,” he said, and stayed with it.
The team identified 2 hurdles. The first was a problem with the retrovirus used to insert the MLL-AF4 fusion gene into mouse cells.
“We soon discovered that the virus wasn’t working,” Dr Thirman explained. “We knew that certain parts of human DNA can decrease viral titers. So we switched from the human version of AF4 to the mouse version, Af4, which is slightly different. This increased viral titers 30-fold.”
That worked, but it led to the second hurdle. The mice injected with virus transporting MLL-Af4 developed leukemia, but it was acute myeloid leukemia.
So the researchers inserted the fused MLL-Af4 gene into human CD34 cells, which were derived from cord blood or peripheral blood from volunteer donors.
The team then transferred those cells to mice, and, this time, the mice developed t(4;11) pro-B ALL.
The researchers said this model “fully recapitulates the immunophenotypic and molecular aspects” of human t(4;11) pro-B ALL and will therefore be “a valuable tool” for studying the disease. ![]()

Photo by Petr Kratochvil
After trying for nearly 2 decades, researchers have created a mouse model of t(4;11) pro-B acute lymphoblastic
leukemia (ALL).
The team said this model, described in Cancer Cell, mimics the human disease phenotypically and molecularly.
This type of ALL, which is common in infants, results from the translocation t(4;11)(q21;q23), which fuses the mixed-lineage leukemia (MLL) gene on chromosome 11 to the ALL-1 fused gene on chromosome 4 (AF4).
“For 20 years, scientists have repeatedly tried and consistently failed to make a model of MLL-AF4 pro-B acute lymphoblastic leukemia,” said study author Michael Thirman, MD, of the University of Chicago in Illinois.
“Even though we understood the basic genetic flaw, no one had been able create a mouse model that mimicked the human disease, which is crucial for evaluating potential therapies.”
That frustrated many researchers, who shifted their focus to test alternative hypotheses on the causes of t(4;11) pro-B ALL or refocused their laboratories to study different aspects of the disease.
However, Dr Thirman and his colleagues began working on this problem “years ago,” he said, and stayed with it.
The team identified 2 hurdles. The first was a problem with the retrovirus used to insert the MLL-AF4 fusion gene into mouse cells.
“We soon discovered that the virus wasn’t working,” Dr Thirman explained. “We knew that certain parts of human DNA can decrease viral titers. So we switched from the human version of AF4 to the mouse version, Af4, which is slightly different. This increased viral titers 30-fold.”
That worked, but it led to the second hurdle. The mice injected with virus transporting MLL-Af4 developed leukemia, but it was acute myeloid leukemia.
So the researchers inserted the fused MLL-Af4 gene into human CD34 cells, which were derived from cord blood or peripheral blood from volunteer donors.
The team then transferred those cells to mice, and, this time, the mice developed t(4;11) pro-B ALL.
The researchers said this model “fully recapitulates the immunophenotypic and molecular aspects” of human t(4;11) pro-B ALL and will therefore be “a valuable tool” for studying the disease. ![]()

Photo by Petr Kratochvil
After trying for nearly 2 decades, researchers have created a mouse model of t(4;11) pro-B acute lymphoblastic
leukemia (ALL).
The team said this model, described in Cancer Cell, mimics the human disease phenotypically and molecularly.
This type of ALL, which is common in infants, results from the translocation t(4;11)(q21;q23), which fuses the mixed-lineage leukemia (MLL) gene on chromosome 11 to the ALL-1 fused gene on chromosome 4 (AF4).
“For 20 years, scientists have repeatedly tried and consistently failed to make a model of MLL-AF4 pro-B acute lymphoblastic leukemia,” said study author Michael Thirman, MD, of the University of Chicago in Illinois.
“Even though we understood the basic genetic flaw, no one had been able create a mouse model that mimicked the human disease, which is crucial for evaluating potential therapies.”
That frustrated many researchers, who shifted their focus to test alternative hypotheses on the causes of t(4;11) pro-B ALL or refocused their laboratories to study different aspects of the disease.
However, Dr Thirman and his colleagues began working on this problem “years ago,” he said, and stayed with it.
The team identified 2 hurdles. The first was a problem with the retrovirus used to insert the MLL-AF4 fusion gene into mouse cells.
“We soon discovered that the virus wasn’t working,” Dr Thirman explained. “We knew that certain parts of human DNA can decrease viral titers. So we switched from the human version of AF4 to the mouse version, Af4, which is slightly different. This increased viral titers 30-fold.”
That worked, but it led to the second hurdle. The mice injected with virus transporting MLL-Af4 developed leukemia, but it was acute myeloid leukemia.
So the researchers inserted the fused MLL-Af4 gene into human CD34 cells, which were derived from cord blood or peripheral blood from volunteer donors.
The team then transferred those cells to mice, and, this time, the mice developed t(4;11) pro-B ALL.
The researchers said this model “fully recapitulates the immunophenotypic and molecular aspects” of human t(4;11) pro-B ALL and will therefore be “a valuable tool” for studying the disease. ![]()
Study shows lower bleeding risk with rivaroxaban

NEW ORLEANS—Results of the PIONEER AF-PCI trial suggest certain patients may have a lower risk of bleeding if they receive rivaroxaban rather than a vitamin K antagonist (VKA).
The study showed that patients with nonvalvular atrial fibrillation (NVAF) who underwent percutaneous coronary intervention (PCI) with stenting had a lower risk of clinically significant bleeding if they received rivaroxaban plus antiplatelet therapy rather than a VKA plus antiplatelet therapy.
However, the trial showed no significant difference between the treatment groups when it came to the risk of cardiovascular events.
These results were presented at the American Heart Association’s Scientific Sessions 2016 and simultaneously published in NEJM.
The trial was supported by Janssen Scientific Affairs LLC, and Bayer Health Care Pharmaceuticals.
“In managing the stented patient with atrial fibrillation, a pharmacologic strategy must carefully balance the risk of stent thrombosis, or blood clot, with the risk of bleeding complications,” said study investigator C. Michael Gibson, MD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts.
“This trial, which tested 2 entirely new strategies, now provides us with randomized clinical trial data demonstrating that a combination of rivaroxaban with antiplatelet therapy is successful in minimizing bleeding while preventing clotting.”
The trial included 2124 patients with NVAF who had undergone PCI with stenting. They were randomized to receive, in a 1:1:1 ratio:
- Low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor for 12 months (group 1)
- Very-low-dose rivaroxaban (2.5 mg twice daily) plus dual antiplatelet therapy (DAPT) for 1, 6, or 12 months (group 2)
- Standard therapy with a dose-adjusted VKA (once daily) plus DAPT for 1, 6, or 12 months (group 3).
Key endpoints
The study’s primary safety endpoint was clinically significant bleeding, which was a composite of major bleeding according to Thrombolysis in Myocardial Infarction (TIMI) criteria, minor bleeding according to TIMI criteria, and bleeding requiring medical attention.
At 1 year, the rate of clinically significant bleeding was significantly lower in the 2 rivaroxaban groups than in the VKA group—16.8% in group 1, 18.0% in group 2, and 26.7% in group 3.
The hazard ratio for group 1 compared to group 3 was 0.59 (P<0.001). And the hazard ratio for group 2 compared to group 3 was 0.63 (P<0.001).
The researchers said this reduction in bleeding for the 2 rivaroxaban groups was consistent across multiple subgroups of patients.
The study’s key efficacy endpoint was major adverse cardiovascular events, which was a composite of death from cardiovascular causes, myocardial infarction, and stroke.
There was no significant difference between the groups with regard to this endpoint. It occurred in 6.5% of the patients in group 1, 5.6% in group 2, and 6.0% in group 3 (P>0.05 for both comparisons).
“For the first time in this population, a treatment regimen resulted in less bleeding than the current standard of care,” Dr Gibson said.
“Pairing rivaroxaban with single or dual antiplatelet therapy has the potential to transform current practice, as demonstrated in this study, with significantly less bleeding and numerically similar efficacy when compared to warfarin [VKA] with dual antiplatelet therapy.” ![]()

NEW ORLEANS—Results of the PIONEER AF-PCI trial suggest certain patients may have a lower risk of bleeding if they receive rivaroxaban rather than a vitamin K antagonist (VKA).
The study showed that patients with nonvalvular atrial fibrillation (NVAF) who underwent percutaneous coronary intervention (PCI) with stenting had a lower risk of clinically significant bleeding if they received rivaroxaban plus antiplatelet therapy rather than a VKA plus antiplatelet therapy.
However, the trial showed no significant difference between the treatment groups when it came to the risk of cardiovascular events.
These results were presented at the American Heart Association’s Scientific Sessions 2016 and simultaneously published in NEJM.
The trial was supported by Janssen Scientific Affairs LLC, and Bayer Health Care Pharmaceuticals.
“In managing the stented patient with atrial fibrillation, a pharmacologic strategy must carefully balance the risk of stent thrombosis, or blood clot, with the risk of bleeding complications,” said study investigator C. Michael Gibson, MD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts.
“This trial, which tested 2 entirely new strategies, now provides us with randomized clinical trial data demonstrating that a combination of rivaroxaban with antiplatelet therapy is successful in minimizing bleeding while preventing clotting.”
The trial included 2124 patients with NVAF who had undergone PCI with stenting. They were randomized to receive, in a 1:1:1 ratio:
- Low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor for 12 months (group 1)
- Very-low-dose rivaroxaban (2.5 mg twice daily) plus dual antiplatelet therapy (DAPT) for 1, 6, or 12 months (group 2)
- Standard therapy with a dose-adjusted VKA (once daily) plus DAPT for 1, 6, or 12 months (group 3).
Key endpoints
The study’s primary safety endpoint was clinically significant bleeding, which was a composite of major bleeding according to Thrombolysis in Myocardial Infarction (TIMI) criteria, minor bleeding according to TIMI criteria, and bleeding requiring medical attention.
At 1 year, the rate of clinically significant bleeding was significantly lower in the 2 rivaroxaban groups than in the VKA group—16.8% in group 1, 18.0% in group 2, and 26.7% in group 3.
The hazard ratio for group 1 compared to group 3 was 0.59 (P<0.001). And the hazard ratio for group 2 compared to group 3 was 0.63 (P<0.001).
The researchers said this reduction in bleeding for the 2 rivaroxaban groups was consistent across multiple subgroups of patients.
The study’s key efficacy endpoint was major adverse cardiovascular events, which was a composite of death from cardiovascular causes, myocardial infarction, and stroke.
There was no significant difference between the groups with regard to this endpoint. It occurred in 6.5% of the patients in group 1, 5.6% in group 2, and 6.0% in group 3 (P>0.05 for both comparisons).
“For the first time in this population, a treatment regimen resulted in less bleeding than the current standard of care,” Dr Gibson said.
“Pairing rivaroxaban with single or dual antiplatelet therapy has the potential to transform current practice, as demonstrated in this study, with significantly less bleeding and numerically similar efficacy when compared to warfarin [VKA] with dual antiplatelet therapy.” ![]()

NEW ORLEANS—Results of the PIONEER AF-PCI trial suggest certain patients may have a lower risk of bleeding if they receive rivaroxaban rather than a vitamin K antagonist (VKA).
The study showed that patients with nonvalvular atrial fibrillation (NVAF) who underwent percutaneous coronary intervention (PCI) with stenting had a lower risk of clinically significant bleeding if they received rivaroxaban plus antiplatelet therapy rather than a VKA plus antiplatelet therapy.
However, the trial showed no significant difference between the treatment groups when it came to the risk of cardiovascular events.
These results were presented at the American Heart Association’s Scientific Sessions 2016 and simultaneously published in NEJM.
The trial was supported by Janssen Scientific Affairs LLC, and Bayer Health Care Pharmaceuticals.
“In managing the stented patient with atrial fibrillation, a pharmacologic strategy must carefully balance the risk of stent thrombosis, or blood clot, with the risk of bleeding complications,” said study investigator C. Michael Gibson, MD, of Beth Israel Deaconess Medical Center in Boston, Massachusetts.
“This trial, which tested 2 entirely new strategies, now provides us with randomized clinical trial data demonstrating that a combination of rivaroxaban with antiplatelet therapy is successful in minimizing bleeding while preventing clotting.”
The trial included 2124 patients with NVAF who had undergone PCI with stenting. They were randomized to receive, in a 1:1:1 ratio:
- Low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor for 12 months (group 1)
- Very-low-dose rivaroxaban (2.5 mg twice daily) plus dual antiplatelet therapy (DAPT) for 1, 6, or 12 months (group 2)
- Standard therapy with a dose-adjusted VKA (once daily) plus DAPT for 1, 6, or 12 months (group 3).
Key endpoints
The study’s primary safety endpoint was clinically significant bleeding, which was a composite of major bleeding according to Thrombolysis in Myocardial Infarction (TIMI) criteria, minor bleeding according to TIMI criteria, and bleeding requiring medical attention.
At 1 year, the rate of clinically significant bleeding was significantly lower in the 2 rivaroxaban groups than in the VKA group—16.8% in group 1, 18.0% in group 2, and 26.7% in group 3.
The hazard ratio for group 1 compared to group 3 was 0.59 (P<0.001). And the hazard ratio for group 2 compared to group 3 was 0.63 (P<0.001).
The researchers said this reduction in bleeding for the 2 rivaroxaban groups was consistent across multiple subgroups of patients.
The study’s key efficacy endpoint was major adverse cardiovascular events, which was a composite of death from cardiovascular causes, myocardial infarction, and stroke.
There was no significant difference between the groups with regard to this endpoint. It occurred in 6.5% of the patients in group 1, 5.6% in group 2, and 6.0% in group 3 (P>0.05 for both comparisons).
“For the first time in this population, a treatment regimen resulted in less bleeding than the current standard of care,” Dr Gibson said.
“Pairing rivaroxaban with single or dual antiplatelet therapy has the potential to transform current practice, as demonstrated in this study, with significantly less bleeding and numerically similar efficacy when compared to warfarin [VKA] with dual antiplatelet therapy.” ![]()
Hemophilia treatment falling short, study suggests

Results of a real-world, retrospective study suggest there may be a need to improve the standard of care for hemophilia A and B in some European countries.
Hemophilia treatment practices varied widely among the 7 countries studied, as did annual bleeding rates (ABRs).
Some countries had low median ABRs in hemophilia A and B patients receiving prophylaxis—in the range of 1.0 to 2.0.
However, the median ABR was as high as 8.0 for some patients, despite receiving prophylaxis. And these were patients with moderate disease.
The study, which was sponsored by Sobi, was published in Haemophilia.
“The overall results indicate that there is a significant need to advance standard of care within hemophilia,” said Stefan Lethagen, vice president medical & clinical sciences, haemophilia at Sobi.
“Even when prophylaxis is the norm, it appears that prophylactic treatment is driven to the minimal acceptable level or even lower, which increases the risk of joint injury and limits the ability to live a full and active life.”
The study was designed to provide insight into current hemophilia treatment practice and outcomes in 7 European countries—Belgium, France, Germany, Italy, Spain, Sweden, and the UK.
The researchers analyzed data on 1346 patients with hemophilia A and 312 with hemophilia B treated in these countries.
Treatment type, dosing
Prophylaxis was, overall, the most dominating treatment for patients with severe hemophilia A. It was the most common treatment regimen among children and decreased with increasing age.
On-demand treatment was reported to be most common in moderate hemophilia A, and there was no trend across age groups.
For patients with hemophilia B, prophylaxis was the most common treatment in 4 out of the 7 countries (France, Germany, Sweden, and UK).
Overall, a majority of patients received a recombinant antihemophilic factor product as opposed to a plasma-derived product. The one exception was patients with hemophilia B in Germany. A majority of these patients (66%) received a plasma-derived product.
The mean prescribed prophylactic treatment ranged from 67.9 IU kg-1 per week (Belgium) to 108.4 IU kg-1 per week (Germany) for hemophilia A and 32.3 IU kg-1 per week (Belgium) to 97.7 IU kg-1 per week (France) for hemophilia B.
Most patients on prophylaxis were treated 3 or more times a week if they had hemophilia A and 2 times a week if they had hemophilia B.
ABRs across countries
For hemophilia A patients on prophylaxis, the median ABR ranged from 1.0 (Belgium, Italy, Sweden) to 4.0 (France, UK) for patients with severe disease and from 2.0 (Sweden) to 8.0 (Belgium) for patients with moderate disease.
The researchers pointed out that ABRs were higher for patients with moderate hemophilia A, but low patient numbers should be taken into account when interpreting these data.
The median ABRs for hemophilia A patients who received on-demand treatment ranged from 4.5 (Sweden) to 18.0 (UK, Belgium) for patients with severe disease and from 1.0 (Spain, Sweden) to 12.0 (UK) for patients with moderate disease.
For hemophilia B patients on prophylaxis, the median ABRs ranged from 1.0 (Germany, Sweden) to 6.0 (Belgium) for patients with severe disease and from 1.5 (Sweden) to 8.0 (Belgium) for patients with moderate disease. Again, ABRs were higher for patients with moderate disease.
The median ABRs for hemophilia B patients who received on-demand treatment ranged from 1.5 (Germany) to 14.0 (UK) for patients with severe disease and from 1.0 (Belgium, France, Germany, Italy) to 6.5 (UK) for patients with moderate disease.
The researchers said the high number of bleeds observed in some patients likely reflects insufficient therapy, inappropriate dose-interval, presence of target joints, poor adherence, or patient difficulty in correctly assessing bleeds.
The team said the overall results of the study suggest there is room for improvement of hemophilia therapy, even for patients currently on prophylactic treatment. ![]()

Results of a real-world, retrospective study suggest there may be a need to improve the standard of care for hemophilia A and B in some European countries.
Hemophilia treatment practices varied widely among the 7 countries studied, as did annual bleeding rates (ABRs).
Some countries had low median ABRs in hemophilia A and B patients receiving prophylaxis—in the range of 1.0 to 2.0.
However, the median ABR was as high as 8.0 for some patients, despite receiving prophylaxis. And these were patients with moderate disease.
The study, which was sponsored by Sobi, was published in Haemophilia.
“The overall results indicate that there is a significant need to advance standard of care within hemophilia,” said Stefan Lethagen, vice president medical & clinical sciences, haemophilia at Sobi.
“Even when prophylaxis is the norm, it appears that prophylactic treatment is driven to the minimal acceptable level or even lower, which increases the risk of joint injury and limits the ability to live a full and active life.”
The study was designed to provide insight into current hemophilia treatment practice and outcomes in 7 European countries—Belgium, France, Germany, Italy, Spain, Sweden, and the UK.
The researchers analyzed data on 1346 patients with hemophilia A and 312 with hemophilia B treated in these countries.
Treatment type, dosing
Prophylaxis was, overall, the most dominating treatment for patients with severe hemophilia A. It was the most common treatment regimen among children and decreased with increasing age.
On-demand treatment was reported to be most common in moderate hemophilia A, and there was no trend across age groups.
For patients with hemophilia B, prophylaxis was the most common treatment in 4 out of the 7 countries (France, Germany, Sweden, and UK).
Overall, a majority of patients received a recombinant antihemophilic factor product as opposed to a plasma-derived product. The one exception was patients with hemophilia B in Germany. A majority of these patients (66%) received a plasma-derived product.
The mean prescribed prophylactic treatment ranged from 67.9 IU kg-1 per week (Belgium) to 108.4 IU kg-1 per week (Germany) for hemophilia A and 32.3 IU kg-1 per week (Belgium) to 97.7 IU kg-1 per week (France) for hemophilia B.
Most patients on prophylaxis were treated 3 or more times a week if they had hemophilia A and 2 times a week if they had hemophilia B.
ABRs across countries
For hemophilia A patients on prophylaxis, the median ABR ranged from 1.0 (Belgium, Italy, Sweden) to 4.0 (France, UK) for patients with severe disease and from 2.0 (Sweden) to 8.0 (Belgium) for patients with moderate disease.
The researchers pointed out that ABRs were higher for patients with moderate hemophilia A, but low patient numbers should be taken into account when interpreting these data.
The median ABRs for hemophilia A patients who received on-demand treatment ranged from 4.5 (Sweden) to 18.0 (UK, Belgium) for patients with severe disease and from 1.0 (Spain, Sweden) to 12.0 (UK) for patients with moderate disease.
For hemophilia B patients on prophylaxis, the median ABRs ranged from 1.0 (Germany, Sweden) to 6.0 (Belgium) for patients with severe disease and from 1.5 (Sweden) to 8.0 (Belgium) for patients with moderate disease. Again, ABRs were higher for patients with moderate disease.
The median ABRs for hemophilia B patients who received on-demand treatment ranged from 1.5 (Germany) to 14.0 (UK) for patients with severe disease and from 1.0 (Belgium, France, Germany, Italy) to 6.5 (UK) for patients with moderate disease.
The researchers said the high number of bleeds observed in some patients likely reflects insufficient therapy, inappropriate dose-interval, presence of target joints, poor adherence, or patient difficulty in correctly assessing bleeds.
The team said the overall results of the study suggest there is room for improvement of hemophilia therapy, even for patients currently on prophylactic treatment. ![]()

Results of a real-world, retrospective study suggest there may be a need to improve the standard of care for hemophilia A and B in some European countries.
Hemophilia treatment practices varied widely among the 7 countries studied, as did annual bleeding rates (ABRs).
Some countries had low median ABRs in hemophilia A and B patients receiving prophylaxis—in the range of 1.0 to 2.0.
However, the median ABR was as high as 8.0 for some patients, despite receiving prophylaxis. And these were patients with moderate disease.
The study, which was sponsored by Sobi, was published in Haemophilia.
“The overall results indicate that there is a significant need to advance standard of care within hemophilia,” said Stefan Lethagen, vice president medical & clinical sciences, haemophilia at Sobi.
“Even when prophylaxis is the norm, it appears that prophylactic treatment is driven to the minimal acceptable level or even lower, which increases the risk of joint injury and limits the ability to live a full and active life.”
The study was designed to provide insight into current hemophilia treatment practice and outcomes in 7 European countries—Belgium, France, Germany, Italy, Spain, Sweden, and the UK.
The researchers analyzed data on 1346 patients with hemophilia A and 312 with hemophilia B treated in these countries.
Treatment type, dosing
Prophylaxis was, overall, the most dominating treatment for patients with severe hemophilia A. It was the most common treatment regimen among children and decreased with increasing age.
On-demand treatment was reported to be most common in moderate hemophilia A, and there was no trend across age groups.
For patients with hemophilia B, prophylaxis was the most common treatment in 4 out of the 7 countries (France, Germany, Sweden, and UK).
Overall, a majority of patients received a recombinant antihemophilic factor product as opposed to a plasma-derived product. The one exception was patients with hemophilia B in Germany. A majority of these patients (66%) received a plasma-derived product.
The mean prescribed prophylactic treatment ranged from 67.9 IU kg-1 per week (Belgium) to 108.4 IU kg-1 per week (Germany) for hemophilia A and 32.3 IU kg-1 per week (Belgium) to 97.7 IU kg-1 per week (France) for hemophilia B.
Most patients on prophylaxis were treated 3 or more times a week if they had hemophilia A and 2 times a week if they had hemophilia B.
ABRs across countries
For hemophilia A patients on prophylaxis, the median ABR ranged from 1.0 (Belgium, Italy, Sweden) to 4.0 (France, UK) for patients with severe disease and from 2.0 (Sweden) to 8.0 (Belgium) for patients with moderate disease.
The researchers pointed out that ABRs were higher for patients with moderate hemophilia A, but low patient numbers should be taken into account when interpreting these data.
The median ABRs for hemophilia A patients who received on-demand treatment ranged from 4.5 (Sweden) to 18.0 (UK, Belgium) for patients with severe disease and from 1.0 (Spain, Sweden) to 12.0 (UK) for patients with moderate disease.
For hemophilia B patients on prophylaxis, the median ABRs ranged from 1.0 (Germany, Sweden) to 6.0 (Belgium) for patients with severe disease and from 1.5 (Sweden) to 8.0 (Belgium) for patients with moderate disease. Again, ABRs were higher for patients with moderate disease.
The median ABRs for hemophilia B patients who received on-demand treatment ranged from 1.5 (Germany) to 14.0 (UK) for patients with severe disease and from 1.0 (Belgium, France, Germany, Italy) to 6.5 (UK) for patients with moderate disease.
The researchers said the high number of bleeds observed in some patients likely reflects insufficient therapy, inappropriate dose-interval, presence of target joints, poor adherence, or patient difficulty in correctly assessing bleeds.
The team said the overall results of the study suggest there is room for improvement of hemophilia therapy, even for patients currently on prophylactic treatment. ![]()
Company withdraws application for eryaspase in ALL
ERYTECH Pharma has announced its decision to withdraw the European marketing authorization application (MAA) for eryaspase (GRASPA®) as a treatment for acute lymphoblastic leukemia (ALL).
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) asked for additional data on eryaspase, but ERYTECH said the time allowed by the EMA’s approval process is not sufficient to provide the data requested.
Therefore, the company decided to withdraw the MAA and resubmit it next year.
About eryaspase
Eryaspase consists of L-asparaginase encapsulated inside donor-derived red blood cells. These enzyme-loaded red blood cells function as bioreactors to eliminate circulating asparagine and “starve” cancer cells, thereby inducing their death.
Research has suggested this delivery system provides improved pharmacodynamics, protecting L-asparaginase from circulating proteolytic enzymes and preventing early liver or renal clearance.
The system also appears to reduce the risk of adverse events compared to native L-asparaginase.
The EMA and the US Food and Drug Administration have granted orphan drug designations for eryaspase for the treatment of ALL, acute myeloid
leukemia, and pancreatic cancer.
About the MAA
ERYTECH submitted an MAA for eryaspase in September 2015, based on positive results from a phase 2/3 study in which researchers compared eryaspase and native L-asparaginase in patients with relapsed and refractory ALL.
One year later (September 2016), the company received the CHMP’s Day 180 List of Outstanding Issues, which highlighted the need for additional data.
Specifically, the CHMP asked for data regarding the comparability between the old and new form of asparaginase encapsulated in eryaspase and the development of a new immunogenicity assay, as well as the pharmacodynamic effects of eryaspase.
Given the fact that the generation of these data will require more time than allowed in the EMA’s approval procedures, ERYTECH has notified the CHMP of the withdrawal of the MAA.
The company intends to resubmit the MAA in mid-2017, as soon as the newly generated data are available.
ERYTECH stressed that there have been no safety issues with eryaspase, and the withdrawal of this MAA will not affect any ongoing trials.
“We are committed to pursuing regulatory approval for GRASPA and intend to work closely with our investigators and advisors to generate the additional information requested and to resubmit an MAA next year,” said Iman El-Hariry, chief medical officer of ERYTECH.
“We believe we have generated strong clinical data in our different programs of eryaspase, and we continue to execute our plans towards making the product available to patients with aggressive forms of cancer, such as acute lymphoblastic leukemia, acute myeloid leukemia, and pancreatic cancer,” added Gil Beyen, ERYTECH’s chairman and chief executive officer. ![]()
ERYTECH Pharma has announced its decision to withdraw the European marketing authorization application (MAA) for eryaspase (GRASPA®) as a treatment for acute lymphoblastic leukemia (ALL).
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) asked for additional data on eryaspase, but ERYTECH said the time allowed by the EMA’s approval process is not sufficient to provide the data requested.
Therefore, the company decided to withdraw the MAA and resubmit it next year.
About eryaspase
Eryaspase consists of L-asparaginase encapsulated inside donor-derived red blood cells. These enzyme-loaded red blood cells function as bioreactors to eliminate circulating asparagine and “starve” cancer cells, thereby inducing their death.
Research has suggested this delivery system provides improved pharmacodynamics, protecting L-asparaginase from circulating proteolytic enzymes and preventing early liver or renal clearance.
The system also appears to reduce the risk of adverse events compared to native L-asparaginase.
The EMA and the US Food and Drug Administration have granted orphan drug designations for eryaspase for the treatment of ALL, acute myeloid
leukemia, and pancreatic cancer.
About the MAA
ERYTECH submitted an MAA for eryaspase in September 2015, based on positive results from a phase 2/3 study in which researchers compared eryaspase and native L-asparaginase in patients with relapsed and refractory ALL.
One year later (September 2016), the company received the CHMP’s Day 180 List of Outstanding Issues, which highlighted the need for additional data.
Specifically, the CHMP asked for data regarding the comparability between the old and new form of asparaginase encapsulated in eryaspase and the development of a new immunogenicity assay, as well as the pharmacodynamic effects of eryaspase.
Given the fact that the generation of these data will require more time than allowed in the EMA’s approval procedures, ERYTECH has notified the CHMP of the withdrawal of the MAA.
The company intends to resubmit the MAA in mid-2017, as soon as the newly generated data are available.
ERYTECH stressed that there have been no safety issues with eryaspase, and the withdrawal of this MAA will not affect any ongoing trials.
“We are committed to pursuing regulatory approval for GRASPA and intend to work closely with our investigators and advisors to generate the additional information requested and to resubmit an MAA next year,” said Iman El-Hariry, chief medical officer of ERYTECH.
“We believe we have generated strong clinical data in our different programs of eryaspase, and we continue to execute our plans towards making the product available to patients with aggressive forms of cancer, such as acute lymphoblastic leukemia, acute myeloid leukemia, and pancreatic cancer,” added Gil Beyen, ERYTECH’s chairman and chief executive officer. ![]()
ERYTECH Pharma has announced its decision to withdraw the European marketing authorization application (MAA) for eryaspase (GRASPA®) as a treatment for acute lymphoblastic leukemia (ALL).
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) asked for additional data on eryaspase, but ERYTECH said the time allowed by the EMA’s approval process is not sufficient to provide the data requested.
Therefore, the company decided to withdraw the MAA and resubmit it next year.
About eryaspase
Eryaspase consists of L-asparaginase encapsulated inside donor-derived red blood cells. These enzyme-loaded red blood cells function as bioreactors to eliminate circulating asparagine and “starve” cancer cells, thereby inducing their death.
Research has suggested this delivery system provides improved pharmacodynamics, protecting L-asparaginase from circulating proteolytic enzymes and preventing early liver or renal clearance.
The system also appears to reduce the risk of adverse events compared to native L-asparaginase.
The EMA and the US Food and Drug Administration have granted orphan drug designations for eryaspase for the treatment of ALL, acute myeloid
leukemia, and pancreatic cancer.
About the MAA
ERYTECH submitted an MAA for eryaspase in September 2015, based on positive results from a phase 2/3 study in which researchers compared eryaspase and native L-asparaginase in patients with relapsed and refractory ALL.
One year later (September 2016), the company received the CHMP’s Day 180 List of Outstanding Issues, which highlighted the need for additional data.
Specifically, the CHMP asked for data regarding the comparability between the old and new form of asparaginase encapsulated in eryaspase and the development of a new immunogenicity assay, as well as the pharmacodynamic effects of eryaspase.
Given the fact that the generation of these data will require more time than allowed in the EMA’s approval procedures, ERYTECH has notified the CHMP of the withdrawal of the MAA.
The company intends to resubmit the MAA in mid-2017, as soon as the newly generated data are available.
ERYTECH stressed that there have been no safety issues with eryaspase, and the withdrawal of this MAA will not affect any ongoing trials.
“We are committed to pursuing regulatory approval for GRASPA and intend to work closely with our investigators and advisors to generate the additional information requested and to resubmit an MAA next year,” said Iman El-Hariry, chief medical officer of ERYTECH.
“We believe we have generated strong clinical data in our different programs of eryaspase, and we continue to execute our plans towards making the product available to patients with aggressive forms of cancer, such as acute lymphoblastic leukemia, acute myeloid leukemia, and pancreatic cancer,” added Gil Beyen, ERYTECH’s chairman and chief executive officer. ![]()
Taking Steps to Reduce Arthritis Pain
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Kidney Disease & “Bad Teeth”
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11
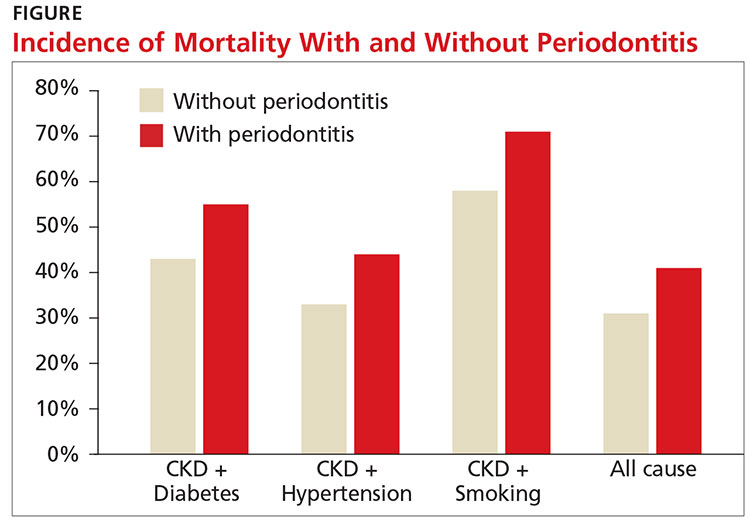
Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Abatacept makes inroads in psoriatic arthritis
WASHINGTON – Abatacept achieved promising results in patients with psoriatic arthritis through 24 weeks of treatment in a placebo-controlled, phase III trial.
Based on these results of the trial, called ASTRAEA, the manufacturer of abatacept (Orencia) and sponsor of the study, Bristol-Myers Squibb, has submitted a Supplemental Biologics Application with the Food and Drug Administration as well as a Variation Application with the European Medicines Agency for an extended indication that includes the treatment of adult psoriatic arthritis (PsA).
ASTRAEA (Efficacy and Safety of Subcutaneous Abatacept in Adults With Active Psoriatic Arthritis) enrolled 424 patients who met ACR PsA criteria and had evidence of active arthritis and psoriasis, plus inadequate response or intolerance to at least one nonbiologic disease-modifying antirheumatic drug (DMARD). The patients, of whom 60% failed prior tumor necrosis factor inhibitor (TNFi) treatment, were randomized to abatacept subcutaneous injection 125 mg/week or placebo. Median age was around 50 years, and about 50% were males. A total of 60% were on concomitant methotrexate.
“This was an active disease population, with about two-thirds having elevated C-reactive protein,” Dr. Mease noted at the annual meeting of the American College of Rheumatology.
A total of 39.4% of abatacept-treated patients achieved the primary endpoint of 24-week ACR20 response versus 22.3% of placebo patients (P less than .001). Improvement on ACR20 continued out to week 44.
Patients who were TNFi naive had better ACR20 responses than did those previously exposed to TNFi, he said. An ACR20 response occurred in 44% of TNFi-naive patients on abatacept, compared with 36.4% of TNFi-exposed patients. The same pattern was observed for ACR50 and ACR70 response rates.
A linear relationship was seen between response to abatacept and CRP baseline level, with a greater degree of response in those with elevated CRP.
Numerical trends favored abatacept for 28-joint Disease Activity Score (DAS28), Health Assessment Questionnaire (HAQ) responses, and X-ray assessment of structural damage. “We saw complete resolution of enthesitis and dactylitis at week 24 in 30%-40% of patients taking abatacept, and responses improved over time,” Dr. Mease told listeners. “Skin responses on the PASI 50 and PASI 75 were modest and not as good as we see with other agents,” Dr. Mease said.
Few serious adverse events were reported. There were three serious infections in the abatacept group and two in the placebo group at week 24.
“These data are consistent with phase II data. Overall, we saw beneficial trends with abatacept for all key endpoints, and the benefits were observed in both TNFi-exposed and TNFi-naive patients subgroups. Abatacept may be an appropriate option for PsA patients to try, especially those with musculoskeletal manifestations [given the modest responses in skin],” Dr. Mease said.
All of the ASTRAEA investigators had industry relationships, including some with Bristol-Myers Squibb. Two were employees of the company.
WASHINGTON – Abatacept achieved promising results in patients with psoriatic arthritis through 24 weeks of treatment in a placebo-controlled, phase III trial.
Based on these results of the trial, called ASTRAEA, the manufacturer of abatacept (Orencia) and sponsor of the study, Bristol-Myers Squibb, has submitted a Supplemental Biologics Application with the Food and Drug Administration as well as a Variation Application with the European Medicines Agency for an extended indication that includes the treatment of adult psoriatic arthritis (PsA).
ASTRAEA (Efficacy and Safety of Subcutaneous Abatacept in Adults With Active Psoriatic Arthritis) enrolled 424 patients who met ACR PsA criteria and had evidence of active arthritis and psoriasis, plus inadequate response or intolerance to at least one nonbiologic disease-modifying antirheumatic drug (DMARD). The patients, of whom 60% failed prior tumor necrosis factor inhibitor (TNFi) treatment, were randomized to abatacept subcutaneous injection 125 mg/week or placebo. Median age was around 50 years, and about 50% were males. A total of 60% were on concomitant methotrexate.
“This was an active disease population, with about two-thirds having elevated C-reactive protein,” Dr. Mease noted at the annual meeting of the American College of Rheumatology.
A total of 39.4% of abatacept-treated patients achieved the primary endpoint of 24-week ACR20 response versus 22.3% of placebo patients (P less than .001). Improvement on ACR20 continued out to week 44.
Patients who were TNFi naive had better ACR20 responses than did those previously exposed to TNFi, he said. An ACR20 response occurred in 44% of TNFi-naive patients on abatacept, compared with 36.4% of TNFi-exposed patients. The same pattern was observed for ACR50 and ACR70 response rates.
A linear relationship was seen between response to abatacept and CRP baseline level, with a greater degree of response in those with elevated CRP.
Numerical trends favored abatacept for 28-joint Disease Activity Score (DAS28), Health Assessment Questionnaire (HAQ) responses, and X-ray assessment of structural damage. “We saw complete resolution of enthesitis and dactylitis at week 24 in 30%-40% of patients taking abatacept, and responses improved over time,” Dr. Mease told listeners. “Skin responses on the PASI 50 and PASI 75 were modest and not as good as we see with other agents,” Dr. Mease said.
Few serious adverse events were reported. There were three serious infections in the abatacept group and two in the placebo group at week 24.
“These data are consistent with phase II data. Overall, we saw beneficial trends with abatacept for all key endpoints, and the benefits were observed in both TNFi-exposed and TNFi-naive patients subgroups. Abatacept may be an appropriate option for PsA patients to try, especially those with musculoskeletal manifestations [given the modest responses in skin],” Dr. Mease said.
All of the ASTRAEA investigators had industry relationships, including some with Bristol-Myers Squibb. Two were employees of the company.
WASHINGTON – Abatacept achieved promising results in patients with psoriatic arthritis through 24 weeks of treatment in a placebo-controlled, phase III trial.
Based on these results of the trial, called ASTRAEA, the manufacturer of abatacept (Orencia) and sponsor of the study, Bristol-Myers Squibb, has submitted a Supplemental Biologics Application with the Food and Drug Administration as well as a Variation Application with the European Medicines Agency for an extended indication that includes the treatment of adult psoriatic arthritis (PsA).
ASTRAEA (Efficacy and Safety of Subcutaneous Abatacept in Adults With Active Psoriatic Arthritis) enrolled 424 patients who met ACR PsA criteria and had evidence of active arthritis and psoriasis, plus inadequate response or intolerance to at least one nonbiologic disease-modifying antirheumatic drug (DMARD). The patients, of whom 60% failed prior tumor necrosis factor inhibitor (TNFi) treatment, were randomized to abatacept subcutaneous injection 125 mg/week or placebo. Median age was around 50 years, and about 50% were males. A total of 60% were on concomitant methotrexate.
“This was an active disease population, with about two-thirds having elevated C-reactive protein,” Dr. Mease noted at the annual meeting of the American College of Rheumatology.
A total of 39.4% of abatacept-treated patients achieved the primary endpoint of 24-week ACR20 response versus 22.3% of placebo patients (P less than .001). Improvement on ACR20 continued out to week 44.
Patients who were TNFi naive had better ACR20 responses than did those previously exposed to TNFi, he said. An ACR20 response occurred in 44% of TNFi-naive patients on abatacept, compared with 36.4% of TNFi-exposed patients. The same pattern was observed for ACR50 and ACR70 response rates.
A linear relationship was seen between response to abatacept and CRP baseline level, with a greater degree of response in those with elevated CRP.
Numerical trends favored abatacept for 28-joint Disease Activity Score (DAS28), Health Assessment Questionnaire (HAQ) responses, and X-ray assessment of structural damage. “We saw complete resolution of enthesitis and dactylitis at week 24 in 30%-40% of patients taking abatacept, and responses improved over time,” Dr. Mease told listeners. “Skin responses on the PASI 50 and PASI 75 were modest and not as good as we see with other agents,” Dr. Mease said.
Few serious adverse events were reported. There were three serious infections in the abatacept group and two in the placebo group at week 24.
“These data are consistent with phase II data. Overall, we saw beneficial trends with abatacept for all key endpoints, and the benefits were observed in both TNFi-exposed and TNFi-naive patients subgroups. Abatacept may be an appropriate option for PsA patients to try, especially those with musculoskeletal manifestations [given the modest responses in skin],” Dr. Mease said.
All of the ASTRAEA investigators had industry relationships, including some with Bristol-Myers Squibb. Two were employees of the company.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Week 24 ACR20 response rates were 39.4% for abatacept vs. 22.3% for placebo (P less than .001).
Data source: International, randomized, double-blind, multicenter phase III study of 424 patients with active psoriatic arthritis.
Disclosures: Bristol-Myers Squibb funded the study. All of the investigators had industry relationships, including some with Bristol-Myers Squibb. Two were employees of the company.
Imaging Markers Predict Neuropsychologic Outcome After Pediatric TBI
VANCOUVER—Early reductions in N-acetylaspartate (NAA) after pediatric traumatic brain injury (TBI) predict neuropsychologic outcomes one year later, according to a study presented at the 45th Annual Meeting of the Child Neurology Society.
Researchers at Loma Linda University in California conducted a prospective study that looked at NAA levels. In a separate but related study, they found that hemorrhagic MRI brain lesions after pediatric TBI are associated with neurologic and neuropsychologic outcomes at one year.
NAA Levels
Barbara Holshouser, PhD, Professor of Radiology at Loma Linda University, and colleagues used MR spectroscopic imaging (MRSI) to assess NAA levels in 69 children with TBI. Patients were ages 4 to 18, had a Glasgow Coma Scale (GCS) score of 13 to 15, and had hemorrhage or contusion on imaging. Initial scans to assess NAA levels were conducted an average of 11.5 days after injury. Follow-up scans were conducted at one year. The researchers obtained mean NAA/creatine, NAA/choline, and choline/creatine ratios for each brain region. They also scanned 75 controls with no history of head injury.
Patients in the TBI group (n = 69) had an average age of 11.8, and 19 patients were female. Seventeen patients were injured in motor vehicle accidents, 22 patients were hit by a motor vehicle, and one patient was injured in a fight. The other patients were injured in accidents that involved all-terrain vehicles (six patients), falls (16 patients), sports (six patients), and boating (one patient). Patients in the control group (n = 75) had an average age of 12.5, and 39 were female.
Patients with TBI had significant decreases of NAA/creatine and NAA/choline in all brain regions, compared with controls. Patients with TBI were dichotomized by those with a 12-month Pediatric Cerebral Performance Category Scale (PCPCS) score of 1 (ie, normal) and those with a PCPCS score 2 to 5 (ie, with disability).
A logistic regression analysis using total and regional NAA/creatine ratios predicted dichotomized PCPCS, full-scale IQ, general memory, and general attention scores at one year.
“A reduction of NAA in the subcortical region, consisting of the basal ganglia, corpus callosum, and thalamus, showed the strongest, most significant correlations” with tests of visual spatial processing, attention, general memory, and immediate and delayed visual memory. “At the subacute stage, a reduction of NAA caused by neuronal loss or dysfunction is a sensitive marker of injury that can be used to predict long-term (12-month) neurologic and neuropsychologic outcomes,” the researchers concluded.
Hemorrhagic Lesions
Stephen Ashwal, MD, Professor of Pediatric Neurology at Loma Linda University, and colleagues presented the results of a related study that found that, among children with moderate or severe TBI or complicated mild TBI, hemorrhagic MRI brain lesions are associated with neurologic and neuropsychologic outcomes at one year.
Susceptibility weighted imaging (SWI) has improved the ability of MRI to detect and quantify micro- and macro-hemorrhagic lesions after TBI. Studies in children, however, had not included repeated long-term MRI combined with neurologic and neuropsychologic measures. Dr. Ashwal and colleagues conducted a study to assess the relationship of acute lesions with one-year neurologic and neuropsychologic outcomes.
The researchers included 74 patients with moderate or severe TBI (ie, GCS score of less than 13) or complicated mild TBI (ie, with hemorrhagic intracranial injury on CT). Patients underwent MRI at six to 18 days after injury and at one year to determine the number and volume of hemorrhagic brain lesions.
Patients had an average age of 11.4, and 53 were male. Injury mechanisms were assault (one patient), sports (six patients), falls (20 patients), and vehicular (47 patients). Initial median GCS score was 9. Mean initial SWI lesion number was 84.3, and mean initial SWI lesion volume was 10,810.6 cm3.
Thirty-six patients had severe TBI (ie, GCS score of 3 to 8). Patients with severe TBI had higher mean SWI lesion numbers and volumes and lower scores on neuropsychologic tests at 12 months. SWI lesions correlated with general 12-month outcome scores on the PCPCS, King’s Outcome Scale for Childhood Head Injury, and Barthel Activities of Daily Living Index.
Initial SWI lesions correlated with measures of general memory (Children’s Memory Scale) and attention (Test of Everyday Attention for Children), but not IQ. In addition, SWI lesion volume in the occipital lobe correlated with visual immediate memory and visual delayed memory scores. Lesions in the temporal lobe also correlated with visual delayed memory scores.
Total lesion number and volume decreased by approximately 50% over 12 months regardless of initial GCS score, and improvement in lesions was associated with improved neurologic outcomes, Dr. Ashwal and colleagues said.
—Jake Remaly
VANCOUVER—Early reductions in N-acetylaspartate (NAA) after pediatric traumatic brain injury (TBI) predict neuropsychologic outcomes one year later, according to a study presented at the 45th Annual Meeting of the Child Neurology Society.
Researchers at Loma Linda University in California conducted a prospective study that looked at NAA levels. In a separate but related study, they found that hemorrhagic MRI brain lesions after pediatric TBI are associated with neurologic and neuropsychologic outcomes at one year.
NAA Levels
Barbara Holshouser, PhD, Professor of Radiology at Loma Linda University, and colleagues used MR spectroscopic imaging (MRSI) to assess NAA levels in 69 children with TBI. Patients were ages 4 to 18, had a Glasgow Coma Scale (GCS) score of 13 to 15, and had hemorrhage or contusion on imaging. Initial scans to assess NAA levels were conducted an average of 11.5 days after injury. Follow-up scans were conducted at one year. The researchers obtained mean NAA/creatine, NAA/choline, and choline/creatine ratios for each brain region. They also scanned 75 controls with no history of head injury.
Patients in the TBI group (n = 69) had an average age of 11.8, and 19 patients were female. Seventeen patients were injured in motor vehicle accidents, 22 patients were hit by a motor vehicle, and one patient was injured in a fight. The other patients were injured in accidents that involved all-terrain vehicles (six patients), falls (16 patients), sports (six patients), and boating (one patient). Patients in the control group (n = 75) had an average age of 12.5, and 39 were female.
Patients with TBI had significant decreases of NAA/creatine and NAA/choline in all brain regions, compared with controls. Patients with TBI were dichotomized by those with a 12-month Pediatric Cerebral Performance Category Scale (PCPCS) score of 1 (ie, normal) and those with a PCPCS score 2 to 5 (ie, with disability).
A logistic regression analysis using total and regional NAA/creatine ratios predicted dichotomized PCPCS, full-scale IQ, general memory, and general attention scores at one year.
“A reduction of NAA in the subcortical region, consisting of the basal ganglia, corpus callosum, and thalamus, showed the strongest, most significant correlations” with tests of visual spatial processing, attention, general memory, and immediate and delayed visual memory. “At the subacute stage, a reduction of NAA caused by neuronal loss or dysfunction is a sensitive marker of injury that can be used to predict long-term (12-month) neurologic and neuropsychologic outcomes,” the researchers concluded.
Hemorrhagic Lesions
Stephen Ashwal, MD, Professor of Pediatric Neurology at Loma Linda University, and colleagues presented the results of a related study that found that, among children with moderate or severe TBI or complicated mild TBI, hemorrhagic MRI brain lesions are associated with neurologic and neuropsychologic outcomes at one year.
Susceptibility weighted imaging (SWI) has improved the ability of MRI to detect and quantify micro- and macro-hemorrhagic lesions after TBI. Studies in children, however, had not included repeated long-term MRI combined with neurologic and neuropsychologic measures. Dr. Ashwal and colleagues conducted a study to assess the relationship of acute lesions with one-year neurologic and neuropsychologic outcomes.
The researchers included 74 patients with moderate or severe TBI (ie, GCS score of less than 13) or complicated mild TBI (ie, with hemorrhagic intracranial injury on CT). Patients underwent MRI at six to 18 days after injury and at one year to determine the number and volume of hemorrhagic brain lesions.
Patients had an average age of 11.4, and 53 were male. Injury mechanisms were assault (one patient), sports (six patients), falls (20 patients), and vehicular (47 patients). Initial median GCS score was 9. Mean initial SWI lesion number was 84.3, and mean initial SWI lesion volume was 10,810.6 cm3.
Thirty-six patients had severe TBI (ie, GCS score of 3 to 8). Patients with severe TBI had higher mean SWI lesion numbers and volumes and lower scores on neuropsychologic tests at 12 months. SWI lesions correlated with general 12-month outcome scores on the PCPCS, King’s Outcome Scale for Childhood Head Injury, and Barthel Activities of Daily Living Index.
Initial SWI lesions correlated with measures of general memory (Children’s Memory Scale) and attention (Test of Everyday Attention for Children), but not IQ. In addition, SWI lesion volume in the occipital lobe correlated with visual immediate memory and visual delayed memory scores. Lesions in the temporal lobe also correlated with visual delayed memory scores.
Total lesion number and volume decreased by approximately 50% over 12 months regardless of initial GCS score, and improvement in lesions was associated with improved neurologic outcomes, Dr. Ashwal and colleagues said.
—Jake Remaly
VANCOUVER—Early reductions in N-acetylaspartate (NAA) after pediatric traumatic brain injury (TBI) predict neuropsychologic outcomes one year later, according to a study presented at the 45th Annual Meeting of the Child Neurology Society.
Researchers at Loma Linda University in California conducted a prospective study that looked at NAA levels. In a separate but related study, they found that hemorrhagic MRI brain lesions after pediatric TBI are associated with neurologic and neuropsychologic outcomes at one year.
NAA Levels
Barbara Holshouser, PhD, Professor of Radiology at Loma Linda University, and colleagues used MR spectroscopic imaging (MRSI) to assess NAA levels in 69 children with TBI. Patients were ages 4 to 18, had a Glasgow Coma Scale (GCS) score of 13 to 15, and had hemorrhage or contusion on imaging. Initial scans to assess NAA levels were conducted an average of 11.5 days after injury. Follow-up scans were conducted at one year. The researchers obtained mean NAA/creatine, NAA/choline, and choline/creatine ratios for each brain region. They also scanned 75 controls with no history of head injury.
Patients in the TBI group (n = 69) had an average age of 11.8, and 19 patients were female. Seventeen patients were injured in motor vehicle accidents, 22 patients were hit by a motor vehicle, and one patient was injured in a fight. The other patients were injured in accidents that involved all-terrain vehicles (six patients), falls (16 patients), sports (six patients), and boating (one patient). Patients in the control group (n = 75) had an average age of 12.5, and 39 were female.
Patients with TBI had significant decreases of NAA/creatine and NAA/choline in all brain regions, compared with controls. Patients with TBI were dichotomized by those with a 12-month Pediatric Cerebral Performance Category Scale (PCPCS) score of 1 (ie, normal) and those with a PCPCS score 2 to 5 (ie, with disability).
A logistic regression analysis using total and regional NAA/creatine ratios predicted dichotomized PCPCS, full-scale IQ, general memory, and general attention scores at one year.
“A reduction of NAA in the subcortical region, consisting of the basal ganglia, corpus callosum, and thalamus, showed the strongest, most significant correlations” with tests of visual spatial processing, attention, general memory, and immediate and delayed visual memory. “At the subacute stage, a reduction of NAA caused by neuronal loss or dysfunction is a sensitive marker of injury that can be used to predict long-term (12-month) neurologic and neuropsychologic outcomes,” the researchers concluded.
Hemorrhagic Lesions
Stephen Ashwal, MD, Professor of Pediatric Neurology at Loma Linda University, and colleagues presented the results of a related study that found that, among children with moderate or severe TBI or complicated mild TBI, hemorrhagic MRI brain lesions are associated with neurologic and neuropsychologic outcomes at one year.
Susceptibility weighted imaging (SWI) has improved the ability of MRI to detect and quantify micro- and macro-hemorrhagic lesions after TBI. Studies in children, however, had not included repeated long-term MRI combined with neurologic and neuropsychologic measures. Dr. Ashwal and colleagues conducted a study to assess the relationship of acute lesions with one-year neurologic and neuropsychologic outcomes.
The researchers included 74 patients with moderate or severe TBI (ie, GCS score of less than 13) or complicated mild TBI (ie, with hemorrhagic intracranial injury on CT). Patients underwent MRI at six to 18 days after injury and at one year to determine the number and volume of hemorrhagic brain lesions.
Patients had an average age of 11.4, and 53 were male. Injury mechanisms were assault (one patient), sports (six patients), falls (20 patients), and vehicular (47 patients). Initial median GCS score was 9. Mean initial SWI lesion number was 84.3, and mean initial SWI lesion volume was 10,810.6 cm3.
Thirty-six patients had severe TBI (ie, GCS score of 3 to 8). Patients with severe TBI had higher mean SWI lesion numbers and volumes and lower scores on neuropsychologic tests at 12 months. SWI lesions correlated with general 12-month outcome scores on the PCPCS, King’s Outcome Scale for Childhood Head Injury, and Barthel Activities of Daily Living Index.
Initial SWI lesions correlated with measures of general memory (Children’s Memory Scale) and attention (Test of Everyday Attention for Children), but not IQ. In addition, SWI lesion volume in the occipital lobe correlated with visual immediate memory and visual delayed memory scores. Lesions in the temporal lobe also correlated with visual delayed memory scores.
Total lesion number and volume decreased by approximately 50% over 12 months regardless of initial GCS score, and improvement in lesions was associated with improved neurologic outcomes, Dr. Ashwal and colleagues said.
—Jake Remaly
Neonatal Sleep Measures Predict Neurodevelopmental Outcomes
VANCOUVER—Among newborns at risk of neurologic dysfunction, measures of neonatal sleep help predict 18-month neurodevelopmental outcomes, according to research presented at the 45th Annual Meeting of the Child Neurology Society.
Studies suggest that abnormal sleep has neurocognitive consequences for older infants and children and that polysomnogram data are associated with brain function in newborns who require neonatal intensive care. “Although sleep is a highly sophisticated brain function, it is not typically included in the newborn clinical neurological assessment,” said Renée A. Shellhaas, MD, MS, Assistant Professor of Pediatrics and Communicable Diseases at the University of Michigan in Ann Arbor, and colleagues.
To evaluate how polysomnography measures may add to standard predictors of neurodevelopmental outcome for newborns who require intensive care and are at risk for neurologic dysfunction, Dr. Shellhaas and colleagues conducted a longitudinal study of 29 newborns. Patients had a gestational age of 35 weeks or more, were cared for in a neonatal intensive care unit, and were clinically determined to be at risk of seizures. Researchers excluded patients with congenital anomalies or syndromes known to affect neurodevelopmental outcome or predispose patients to sleep-disordered breathing. They also excluded patients who had severely abnormal EEG without sleep–wake cycling.
Once a newborn was medically stable, researchers conducted a 12-hour attended, bedside polysomnogram. Polysomnograms were scored by a polysomnography technologist and reviewed by a sleep-medicine physician. Researchers calculated the proportion of each sleep–wake stage, entropy of the sequence of sleep–wake state transitions, and power spectra of the EEG portion of the polysomnogram.
Researchers evaluated neurodevelopmental outcome at 18 months to 22 months using the third edition of the Bayley Scales of Infant Development (BSID). They assessed associations between polysomnogram results and neurodevelopmental outcomes using regression techniques that de-emphasized outliers. Patients’ mean gestational age was 39.6 weeks. Seventeen of the 29 patients were male. Mean birth weight was 3.42 kg, and median five-minute Apgar score was 8.
In univariate analysis, increased time in quiet sleep predicted lower 18-month cognitive, language, and motor BSID scores. Higher entropy of sleep–wake transitions predicted lower motor scores. Increased low-frequency EEG power during quiet sleep predicted higher motor and language BSID scores. Gestational age and illness severity were not predictive of BSID results. A more abnormal neonatal neurologic exam score (ie, Thompson score) predicted lower cognitive and motor BSID scores.
In analyses adjusted for Thompson score, higher EEG power during neonatal quiet sleep was associated with better 18-month motor and language scores. In addition, increased time in neonatal quiet sleep was associated with lower 18-month cognitive and motor scores. “Notably, Thompson score was not an independent predictor of outcome when the sleep data were included in the bivariate models,” Dr. Shellhaas and colleagues said.
“Our results suggest that inefficient neonatal quiet sleep—more time in quiet sleep and lower delta frequency power during that stage—predicts lower 18-month neurodevelopmental outcome scores,” the researchers concluded. “Importantly, these novel measures of brain functional integrity were robust predictors even after adjusting for the neonatal neurologic examination score.”
—Jake Remaly
Suggested Reading
Shellhaas RA, Burns JW, Barks JD, Chervin RD. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology. 2014;82(5):390-395.
Shellhaas RA, Burns JW, Wiggins SA, et al. Sleep-wake cycling and cerebral oxygen metabolism among critically ill neonates. J Child Neurol. 2014;29(4):530-533.
VANCOUVER—Among newborns at risk of neurologic dysfunction, measures of neonatal sleep help predict 18-month neurodevelopmental outcomes, according to research presented at the 45th Annual Meeting of the Child Neurology Society.
Studies suggest that abnormal sleep has neurocognitive consequences for older infants and children and that polysomnogram data are associated with brain function in newborns who require neonatal intensive care. “Although sleep is a highly sophisticated brain function, it is not typically included in the newborn clinical neurological assessment,” said Renée A. Shellhaas, MD, MS, Assistant Professor of Pediatrics and Communicable Diseases at the University of Michigan in Ann Arbor, and colleagues.
To evaluate how polysomnography measures may add to standard predictors of neurodevelopmental outcome for newborns who require intensive care and are at risk for neurologic dysfunction, Dr. Shellhaas and colleagues conducted a longitudinal study of 29 newborns. Patients had a gestational age of 35 weeks or more, were cared for in a neonatal intensive care unit, and were clinically determined to be at risk of seizures. Researchers excluded patients with congenital anomalies or syndromes known to affect neurodevelopmental outcome or predispose patients to sleep-disordered breathing. They also excluded patients who had severely abnormal EEG without sleep–wake cycling.
Once a newborn was medically stable, researchers conducted a 12-hour attended, bedside polysomnogram. Polysomnograms were scored by a polysomnography technologist and reviewed by a sleep-medicine physician. Researchers calculated the proportion of each sleep–wake stage, entropy of the sequence of sleep–wake state transitions, and power spectra of the EEG portion of the polysomnogram.
Researchers evaluated neurodevelopmental outcome at 18 months to 22 months using the third edition of the Bayley Scales of Infant Development (BSID). They assessed associations between polysomnogram results and neurodevelopmental outcomes using regression techniques that de-emphasized outliers. Patients’ mean gestational age was 39.6 weeks. Seventeen of the 29 patients were male. Mean birth weight was 3.42 kg, and median five-minute Apgar score was 8.
In univariate analysis, increased time in quiet sleep predicted lower 18-month cognitive, language, and motor BSID scores. Higher entropy of sleep–wake transitions predicted lower motor scores. Increased low-frequency EEG power during quiet sleep predicted higher motor and language BSID scores. Gestational age and illness severity were not predictive of BSID results. A more abnormal neonatal neurologic exam score (ie, Thompson score) predicted lower cognitive and motor BSID scores.
In analyses adjusted for Thompson score, higher EEG power during neonatal quiet sleep was associated with better 18-month motor and language scores. In addition, increased time in neonatal quiet sleep was associated with lower 18-month cognitive and motor scores. “Notably, Thompson score was not an independent predictor of outcome when the sleep data were included in the bivariate models,” Dr. Shellhaas and colleagues said.
“Our results suggest that inefficient neonatal quiet sleep—more time in quiet sleep and lower delta frequency power during that stage—predicts lower 18-month neurodevelopmental outcome scores,” the researchers concluded. “Importantly, these novel measures of brain functional integrity were robust predictors even after adjusting for the neonatal neurologic examination score.”
—Jake Remaly
Suggested Reading
Shellhaas RA, Burns JW, Barks JD, Chervin RD. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology. 2014;82(5):390-395.
Shellhaas RA, Burns JW, Wiggins SA, et al. Sleep-wake cycling and cerebral oxygen metabolism among critically ill neonates. J Child Neurol. 2014;29(4):530-533.
VANCOUVER—Among newborns at risk of neurologic dysfunction, measures of neonatal sleep help predict 18-month neurodevelopmental outcomes, according to research presented at the 45th Annual Meeting of the Child Neurology Society.
Studies suggest that abnormal sleep has neurocognitive consequences for older infants and children and that polysomnogram data are associated with brain function in newborns who require neonatal intensive care. “Although sleep is a highly sophisticated brain function, it is not typically included in the newborn clinical neurological assessment,” said Renée A. Shellhaas, MD, MS, Assistant Professor of Pediatrics and Communicable Diseases at the University of Michigan in Ann Arbor, and colleagues.
To evaluate how polysomnography measures may add to standard predictors of neurodevelopmental outcome for newborns who require intensive care and are at risk for neurologic dysfunction, Dr. Shellhaas and colleagues conducted a longitudinal study of 29 newborns. Patients had a gestational age of 35 weeks or more, were cared for in a neonatal intensive care unit, and were clinically determined to be at risk of seizures. Researchers excluded patients with congenital anomalies or syndromes known to affect neurodevelopmental outcome or predispose patients to sleep-disordered breathing. They also excluded patients who had severely abnormal EEG without sleep–wake cycling.
Once a newborn was medically stable, researchers conducted a 12-hour attended, bedside polysomnogram. Polysomnograms were scored by a polysomnography technologist and reviewed by a sleep-medicine physician. Researchers calculated the proportion of each sleep–wake stage, entropy of the sequence of sleep–wake state transitions, and power spectra of the EEG portion of the polysomnogram.
Researchers evaluated neurodevelopmental outcome at 18 months to 22 months using the third edition of the Bayley Scales of Infant Development (BSID). They assessed associations between polysomnogram results and neurodevelopmental outcomes using regression techniques that de-emphasized outliers. Patients’ mean gestational age was 39.6 weeks. Seventeen of the 29 patients were male. Mean birth weight was 3.42 kg, and median five-minute Apgar score was 8.
In univariate analysis, increased time in quiet sleep predicted lower 18-month cognitive, language, and motor BSID scores. Higher entropy of sleep–wake transitions predicted lower motor scores. Increased low-frequency EEG power during quiet sleep predicted higher motor and language BSID scores. Gestational age and illness severity were not predictive of BSID results. A more abnormal neonatal neurologic exam score (ie, Thompson score) predicted lower cognitive and motor BSID scores.
In analyses adjusted for Thompson score, higher EEG power during neonatal quiet sleep was associated with better 18-month motor and language scores. In addition, increased time in neonatal quiet sleep was associated with lower 18-month cognitive and motor scores. “Notably, Thompson score was not an independent predictor of outcome when the sleep data were included in the bivariate models,” Dr. Shellhaas and colleagues said.
“Our results suggest that inefficient neonatal quiet sleep—more time in quiet sleep and lower delta frequency power during that stage—predicts lower 18-month neurodevelopmental outcome scores,” the researchers concluded. “Importantly, these novel measures of brain functional integrity were robust predictors even after adjusting for the neonatal neurologic examination score.”
—Jake Remaly
Suggested Reading
Shellhaas RA, Burns JW, Barks JD, Chervin RD. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology. 2014;82(5):390-395.
Shellhaas RA, Burns JW, Wiggins SA, et al. Sleep-wake cycling and cerebral oxygen metabolism among critically ill neonates. J Child Neurol. 2014;29(4):530-533.
Diffuse Rash With Associated Ulceration
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.
Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1
Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.

Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1

Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.

Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1

Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
A 72-year-old woman who was admitted for pneumonia and acute hypoxic respiratory failure was seen for an inpatient consultation for a diffuse rash with associated ulceration. She reported a rash of 20 months' duration that began on the legs and then spread to the trunk, arms, head, and neck with minimal pruritus and no pain or photosensitivity. She had been treated with hydroxychloroquine, mycophenolate mofetil, and prednisone without improvement. The patient noted recent ulceration on the rash. Physical examination revealed violaceous patches, plaques, nodules, and tumors with rare ulceration involving the face, trunk, and extremities. Biopsy showed a diffuse infiltration of the dermis with medium-sized atypical lymphocytes with scant cytoplasm and round to irregular hyperchromatic nuclei with clumped chromatin. Epidermotropism with small collections of atypical lymphocytes also was present within the epidermis.