User login
MRI detects early stages of MF in mice

Magnetic resonance imaging (MRI) can effectively detect myelofibrosis (MF) in a mouse model, according to research published in the journal Blood Cancer.
In fact, researchers found that MRI could detect early and late stages of primary MF.
The researchers believe this discovery could potentially change the way MF is diagnosed, as MRI might be used to help physicians decide if or where to biopsy.
Katya Ravid, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues conducted this research, aiming to determine whether T2-weighted MRI could detect bone marrow fibrosis in a mouse model of primary MF.
The team looked specifically at how effectively MRI could detect MF during the pre-fibrotic stage (when mice were less than 16 weeks old), when the mice had early MF (16 to 36 weeks old), and once the mice had overt MF (older than 36 weeks).
The researchers found that MRI could detect MF at the pre-fibrotic stage as well as detecting progressive MF.
The team said they observed a clear, bright signal that allowed them to differentiate mice with MF from healthy control mice.
The researchers proposed that the abundance of large megakaryocytes contributed to the bright signal they observed, since, in T2-weighted MR images, increased water/proton content, as in increased cellularity, yields high MR-signal intensity.
The team said this study provides proof of concept that T2-weighted MRI can detect primary MF in the early and late stages. ![]()

Magnetic resonance imaging (MRI) can effectively detect myelofibrosis (MF) in a mouse model, according to research published in the journal Blood Cancer.
In fact, researchers found that MRI could detect early and late stages of primary MF.
The researchers believe this discovery could potentially change the way MF is diagnosed, as MRI might be used to help physicians decide if or where to biopsy.
Katya Ravid, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues conducted this research, aiming to determine whether T2-weighted MRI could detect bone marrow fibrosis in a mouse model of primary MF.
The team looked specifically at how effectively MRI could detect MF during the pre-fibrotic stage (when mice were less than 16 weeks old), when the mice had early MF (16 to 36 weeks old), and once the mice had overt MF (older than 36 weeks).
The researchers found that MRI could detect MF at the pre-fibrotic stage as well as detecting progressive MF.
The team said they observed a clear, bright signal that allowed them to differentiate mice with MF from healthy control mice.
The researchers proposed that the abundance of large megakaryocytes contributed to the bright signal they observed, since, in T2-weighted MR images, increased water/proton content, as in increased cellularity, yields high MR-signal intensity.
The team said this study provides proof of concept that T2-weighted MRI can detect primary MF in the early and late stages. ![]()

Magnetic resonance imaging (MRI) can effectively detect myelofibrosis (MF) in a mouse model, according to research published in the journal Blood Cancer.
In fact, researchers found that MRI could detect early and late stages of primary MF.
The researchers believe this discovery could potentially change the way MF is diagnosed, as MRI might be used to help physicians decide if or where to biopsy.
Katya Ravid, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues conducted this research, aiming to determine whether T2-weighted MRI could detect bone marrow fibrosis in a mouse model of primary MF.
The team looked specifically at how effectively MRI could detect MF during the pre-fibrotic stage (when mice were less than 16 weeks old), when the mice had early MF (16 to 36 weeks old), and once the mice had overt MF (older than 36 weeks).
The researchers found that MRI could detect MF at the pre-fibrotic stage as well as detecting progressive MF.
The team said they observed a clear, bright signal that allowed them to differentiate mice with MF from healthy control mice.
The researchers proposed that the abundance of large megakaryocytes contributed to the bright signal they observed, since, in T2-weighted MR images, increased water/proton content, as in increased cellularity, yields high MR-signal intensity.
The team said this study provides proof of concept that T2-weighted MRI can detect primary MF in the early and late stages. ![]()
Antiplatelet drugs produce similar results in PAD

Photo from AstraZeneca
NEW ORLEANS—Results of the EUCLID trial suggest ticagrelor does not a provide a benefit over clopidogrel in patients with symptomatic peripheral artery disease (PAD).
The incidence of atherothrombotic events was similar in patients who received ticagrelor and those who received clopidogrel.
Likewise, there was no significant difference between the treatment arms with regard to major bleeding.
Manesh R. Patel, MD, of Duke University Medical Center in Durham, North Carolina, presented results from the EUCLID trial at the American Heart Association Scientific Sessions.
Results were also published in NEJM. The trial was supported by AstraZeneca.
EUCLID included 13,885 patients with symptomatic PAD. They had median age of 66, and 72% were male.
The patients were randomized to receive ticagrelor at 90 mg twice daily or clopidogrel at 75 mg once daily.
The study’s primary efficacy endpoint was a composite of adjudicated cardiovascular death, myocardial infarction, and ischemic stroke.
At a median follow-up of 30 months, the primary efficacy endpoint had occurred in 10.8% (751/6930) of patients in the ticagrelor arm and 10.6% (740/6955) in the clopidogrel arm (P=0.65).
When the researchers assessed each of the components of the primary endpoint alone, they found a significant difference between the treatment groups in the incidence of ischemic stroke but not cardiovascular death or myocardial infarction.
Cardiovascular death occurred in 5.2% of patients in the ticagrelor arm and 4.9% of those in the clopidogrel arm (P=0.40). Myocardial infarction occurred in 5% and 4.8%, respectively (P=0.48). And ischemic stroke occurred in 1.9% and 2.4%, respectively (P=0.03).
The study’s primary safety endpoint was major bleeding, which occurred in 1.6% of patients in both treatment arms (P=0.49).
Fatal bleeding occurred in 0.1% of patients in the ticagrelor arm and 0.3% of patients in the clopidogrel arm (P=0.10). And intracranial bleeding occurred in 0.5% of patients in both arms (P=0.82).
However, significantly more patients discontinued ticagrelor due to bleeding—2.4%, compared to 1.6% of patients who discontinued clopidogrel due to bleeding (P<0.001).
Significantly more patients discontinued ticagrelor due to dyspnea as well—4.8% vs 0.8% (P<0.001).
In all, 30.1% of patients in the ticagrelor arm and 25.9% of those in the clopidogrel arm prematurely discontinued treatment. This includes patients who discontinued due to adverse events, meeting the primary efficacy endpoint, and death. ![]()

Photo from AstraZeneca
NEW ORLEANS—Results of the EUCLID trial suggest ticagrelor does not a provide a benefit over clopidogrel in patients with symptomatic peripheral artery disease (PAD).
The incidence of atherothrombotic events was similar in patients who received ticagrelor and those who received clopidogrel.
Likewise, there was no significant difference between the treatment arms with regard to major bleeding.
Manesh R. Patel, MD, of Duke University Medical Center in Durham, North Carolina, presented results from the EUCLID trial at the American Heart Association Scientific Sessions.
Results were also published in NEJM. The trial was supported by AstraZeneca.
EUCLID included 13,885 patients with symptomatic PAD. They had median age of 66, and 72% were male.
The patients were randomized to receive ticagrelor at 90 mg twice daily or clopidogrel at 75 mg once daily.
The study’s primary efficacy endpoint was a composite of adjudicated cardiovascular death, myocardial infarction, and ischemic stroke.
At a median follow-up of 30 months, the primary efficacy endpoint had occurred in 10.8% (751/6930) of patients in the ticagrelor arm and 10.6% (740/6955) in the clopidogrel arm (P=0.65).
When the researchers assessed each of the components of the primary endpoint alone, they found a significant difference between the treatment groups in the incidence of ischemic stroke but not cardiovascular death or myocardial infarction.
Cardiovascular death occurred in 5.2% of patients in the ticagrelor arm and 4.9% of those in the clopidogrel arm (P=0.40). Myocardial infarction occurred in 5% and 4.8%, respectively (P=0.48). And ischemic stroke occurred in 1.9% and 2.4%, respectively (P=0.03).
The study’s primary safety endpoint was major bleeding, which occurred in 1.6% of patients in both treatment arms (P=0.49).
Fatal bleeding occurred in 0.1% of patients in the ticagrelor arm and 0.3% of patients in the clopidogrel arm (P=0.10). And intracranial bleeding occurred in 0.5% of patients in both arms (P=0.82).
However, significantly more patients discontinued ticagrelor due to bleeding—2.4%, compared to 1.6% of patients who discontinued clopidogrel due to bleeding (P<0.001).
Significantly more patients discontinued ticagrelor due to dyspnea as well—4.8% vs 0.8% (P<0.001).
In all, 30.1% of patients in the ticagrelor arm and 25.9% of those in the clopidogrel arm prematurely discontinued treatment. This includes patients who discontinued due to adverse events, meeting the primary efficacy endpoint, and death. ![]()

Photo from AstraZeneca
NEW ORLEANS—Results of the EUCLID trial suggest ticagrelor does not a provide a benefit over clopidogrel in patients with symptomatic peripheral artery disease (PAD).
The incidence of atherothrombotic events was similar in patients who received ticagrelor and those who received clopidogrel.
Likewise, there was no significant difference between the treatment arms with regard to major bleeding.
Manesh R. Patel, MD, of Duke University Medical Center in Durham, North Carolina, presented results from the EUCLID trial at the American Heart Association Scientific Sessions.
Results were also published in NEJM. The trial was supported by AstraZeneca.
EUCLID included 13,885 patients with symptomatic PAD. They had median age of 66, and 72% were male.
The patients were randomized to receive ticagrelor at 90 mg twice daily or clopidogrel at 75 mg once daily.
The study’s primary efficacy endpoint was a composite of adjudicated cardiovascular death, myocardial infarction, and ischemic stroke.
At a median follow-up of 30 months, the primary efficacy endpoint had occurred in 10.8% (751/6930) of patients in the ticagrelor arm and 10.6% (740/6955) in the clopidogrel arm (P=0.65).
When the researchers assessed each of the components of the primary endpoint alone, they found a significant difference between the treatment groups in the incidence of ischemic stroke but not cardiovascular death or myocardial infarction.
Cardiovascular death occurred in 5.2% of patients in the ticagrelor arm and 4.9% of those in the clopidogrel arm (P=0.40). Myocardial infarction occurred in 5% and 4.8%, respectively (P=0.48). And ischemic stroke occurred in 1.9% and 2.4%, respectively (P=0.03).
The study’s primary safety endpoint was major bleeding, which occurred in 1.6% of patients in both treatment arms (P=0.49).
Fatal bleeding occurred in 0.1% of patients in the ticagrelor arm and 0.3% of patients in the clopidogrel arm (P=0.10). And intracranial bleeding occurred in 0.5% of patients in both arms (P=0.82).
However, significantly more patients discontinued ticagrelor due to bleeding—2.4%, compared to 1.6% of patients who discontinued clopidogrel due to bleeding (P<0.001).
Significantly more patients discontinued ticagrelor due to dyspnea as well—4.8% vs 0.8% (P<0.001).
In all, 30.1% of patients in the ticagrelor arm and 25.9% of those in the clopidogrel arm prematurely discontinued treatment. This includes patients who discontinued due to adverse events, meeting the primary efficacy endpoint, and death. ![]()
FDA grants priority review for midostaurin
The US Food and Drug Administration (FDA) has granted priority review for the new drug application for midostaurin (PKC412) as a treatment for advanced systemic mastocytosis (SM) and newly diagnosed, FLT3-mutated acute myeloid leukemia (AML).
The FDA has also accepted for review the premarket approval application for the midostaurin FLT3 companion diagnostic, which is designed to help identify patients who may have a FLT3 mutation and could potentially benefit from treatment with midostaurin.
Midostaurin is being developed by Novartis. The companion diagnostic is being developed by Novartis and Invivoscribe Technologies, Inc.
About priority review
The FDA grants priority review to applications for therapies that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it. The goal in the standard review process is to take action within 10 months.
About midostaurin
Midostaurin is an oral, multi-targeted kinase inhibitor. The drug was granted breakthrough therapy designation by the FDA earlier this year for newly diagnosed, FLT3-mutated AML.
According to Novartis, the new drug application submission for midostaurin includes data from the largest clinical trials conducted to date in advanced SM and newly diagnosed, FLT3-mutated AML.
Midostaurin in AML
In the phase 3 RATIFY trial, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in adults younger than 60 with FLT3-mutated AML. Results from this trial were presented at the 2015 ASH Annual Meeting.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.0074).
There was no significant difference in the overall rate of grade 3 or higher hematologic and non-hematologic adverse events in midostaurin arm and the placebo arm. Similarly, there was no significant difference in treatment-related deaths between the arms.
Midostaurin in SM
Data from the phase 2 study of midostaurin in patients with advanced SM were published in NEJM in June.
The drug produced a 60% overall response rate, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose of midostaurin.
Access to midostaurin
Since midostaurin remains investigational, both within the US and globally, Novartis opened a Global Individual Patient Program (compassionate use program) and, in the US, an Expanded Treatment Protocol, to provide access to midostaurin for eligible patients with newly diagnosed AML and advanced SM.
Physicians who want to request midostaurin for eligible patients can contact a Novartis medical representative in their respective countries. In the US, physicians can call 1-888-NOW-NOVA (1-888-669-6682) for more information. ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application for midostaurin (PKC412) as a treatment for advanced systemic mastocytosis (SM) and newly diagnosed, FLT3-mutated acute myeloid leukemia (AML).
The FDA has also accepted for review the premarket approval application for the midostaurin FLT3 companion diagnostic, which is designed to help identify patients who may have a FLT3 mutation and could potentially benefit from treatment with midostaurin.
Midostaurin is being developed by Novartis. The companion diagnostic is being developed by Novartis and Invivoscribe Technologies, Inc.
About priority review
The FDA grants priority review to applications for therapies that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it. The goal in the standard review process is to take action within 10 months.
About midostaurin
Midostaurin is an oral, multi-targeted kinase inhibitor. The drug was granted breakthrough therapy designation by the FDA earlier this year for newly diagnosed, FLT3-mutated AML.
According to Novartis, the new drug application submission for midostaurin includes data from the largest clinical trials conducted to date in advanced SM and newly diagnosed, FLT3-mutated AML.
Midostaurin in AML
In the phase 3 RATIFY trial, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in adults younger than 60 with FLT3-mutated AML. Results from this trial were presented at the 2015 ASH Annual Meeting.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.0074).
There was no significant difference in the overall rate of grade 3 or higher hematologic and non-hematologic adverse events in midostaurin arm and the placebo arm. Similarly, there was no significant difference in treatment-related deaths between the arms.
Midostaurin in SM
Data from the phase 2 study of midostaurin in patients with advanced SM were published in NEJM in June.
The drug produced a 60% overall response rate, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose of midostaurin.
Access to midostaurin
Since midostaurin remains investigational, both within the US and globally, Novartis opened a Global Individual Patient Program (compassionate use program) and, in the US, an Expanded Treatment Protocol, to provide access to midostaurin for eligible patients with newly diagnosed AML and advanced SM.
Physicians who want to request midostaurin for eligible patients can contact a Novartis medical representative in their respective countries. In the US, physicians can call 1-888-NOW-NOVA (1-888-669-6682) for more information. ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application for midostaurin (PKC412) as a treatment for advanced systemic mastocytosis (SM) and newly diagnosed, FLT3-mutated acute myeloid leukemia (AML).
The FDA has also accepted for review the premarket approval application for the midostaurin FLT3 companion diagnostic, which is designed to help identify patients who may have a FLT3 mutation and could potentially benefit from treatment with midostaurin.
Midostaurin is being developed by Novartis. The companion diagnostic is being developed by Novartis and Invivoscribe Technologies, Inc.
About priority review
The FDA grants priority review to applications for therapies that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it. The goal in the standard review process is to take action within 10 months.
About midostaurin
Midostaurin is an oral, multi-targeted kinase inhibitor. The drug was granted breakthrough therapy designation by the FDA earlier this year for newly diagnosed, FLT3-mutated AML.
According to Novartis, the new drug application submission for midostaurin includes data from the largest clinical trials conducted to date in advanced SM and newly diagnosed, FLT3-mutated AML.
Midostaurin in AML
In the phase 3 RATIFY trial, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in adults younger than 60 with FLT3-mutated AML. Results from this trial were presented at the 2015 ASH Annual Meeting.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.0074).
There was no significant difference in the overall rate of grade 3 or higher hematologic and non-hematologic adverse events in midostaurin arm and the placebo arm. Similarly, there was no significant difference in treatment-related deaths between the arms.
Midostaurin in SM
Data from the phase 2 study of midostaurin in patients with advanced SM were published in NEJM in June.
The drug produced a 60% overall response rate, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose of midostaurin.
Access to midostaurin
Since midostaurin remains investigational, both within the US and globally, Novartis opened a Global Individual Patient Program (compassionate use program) and, in the US, an Expanded Treatment Protocol, to provide access to midostaurin for eligible patients with newly diagnosed AML and advanced SM.
Physicians who want to request midostaurin for eligible patients can contact a Novartis medical representative in their respective countries. In the US, physicians can call 1-888-NOW-NOVA (1-888-669-6682) for more information. ![]()
EMA recommends orphan status for drug in AML

The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has recommended that BP1001 receive orphan designation as a treatment for acute myeloid leukemia (AML).
BP1001 (liposomal Grb2 antisense) is a neutral-charge, liposome-incorporated, antisense drug designed to inhibit protein synthesis of growth factor receptor bound protein 2 (Grb2).
BP1001 is being developed by Bio-Path Holdings, Inc.
According to Bio-Path, inhibition of Grb2 by BP1001 represents a significant advance in treating cancers with activated tyrosine kinases using a target not druggable with small molecule inhibitors.
Research has suggested that Grb2 plays an essential role in cancer cell activation via the RAS pathway. Grb2 bridges signals between activated and mutated tyrosine kinases, such as Flt3, c-Kit, and Bcr-Abl, and the Ras pathway, leading to activation of the ERK and AKT proteins.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
Trials of BP1001
Bio-Path has completed a phase 1 trial of BP1001 in patients with relapsed/refractory AML, chronic myeloid leukemia, and myelodysplastic syndromes.
The company has also completed the safety segment of a phase 2 trial in which BP1001 is being investigated in combination with low-dose ara-C to treat AML.
Bio-Path recently released data from these studies.
The phase 1 study included patients who had received an average of 6 prior therapies.
The patients received 8 doses of BP1001 over 4 weeks, escalating to a maximum dose of 90 mg/m2. There were no dose-limiting toxicities, and Bio-Path said the drug was well tolerated.
Of the 18 evaluable patients with circulating blasts, 83% responded to BP1001. The average reduction in circulating blasts was 67%.
The phase 2 trial included patients with relapsed/refractory AML. There were 3 evaluable patients in each of 2 dosing cohorts—60 mg/m2 and 90 mg/m2. Patients received BP1001 twice a week for 4 weeks.
Five of the patients responded—3 with a complete response and 2 with a partial response. There were no adverse events attributed to BP1001, and the maximum-tolerated dose was not reached. ![]()

The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has recommended that BP1001 receive orphan designation as a treatment for acute myeloid leukemia (AML).
BP1001 (liposomal Grb2 antisense) is a neutral-charge, liposome-incorporated, antisense drug designed to inhibit protein synthesis of growth factor receptor bound protein 2 (Grb2).
BP1001 is being developed by Bio-Path Holdings, Inc.
According to Bio-Path, inhibition of Grb2 by BP1001 represents a significant advance in treating cancers with activated tyrosine kinases using a target not druggable with small molecule inhibitors.
Research has suggested that Grb2 plays an essential role in cancer cell activation via the RAS pathway. Grb2 bridges signals between activated and mutated tyrosine kinases, such as Flt3, c-Kit, and Bcr-Abl, and the Ras pathway, leading to activation of the ERK and AKT proteins.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
Trials of BP1001
Bio-Path has completed a phase 1 trial of BP1001 in patients with relapsed/refractory AML, chronic myeloid leukemia, and myelodysplastic syndromes.
The company has also completed the safety segment of a phase 2 trial in which BP1001 is being investigated in combination with low-dose ara-C to treat AML.
Bio-Path recently released data from these studies.
The phase 1 study included patients who had received an average of 6 prior therapies.
The patients received 8 doses of BP1001 over 4 weeks, escalating to a maximum dose of 90 mg/m2. There were no dose-limiting toxicities, and Bio-Path said the drug was well tolerated.
Of the 18 evaluable patients with circulating blasts, 83% responded to BP1001. The average reduction in circulating blasts was 67%.
The phase 2 trial included patients with relapsed/refractory AML. There were 3 evaluable patients in each of 2 dosing cohorts—60 mg/m2 and 90 mg/m2. Patients received BP1001 twice a week for 4 weeks.
Five of the patients responded—3 with a complete response and 2 with a partial response. There were no adverse events attributed to BP1001, and the maximum-tolerated dose was not reached. ![]()

The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has recommended that BP1001 receive orphan designation as a treatment for acute myeloid leukemia (AML).
BP1001 (liposomal Grb2 antisense) is a neutral-charge, liposome-incorporated, antisense drug designed to inhibit protein synthesis of growth factor receptor bound protein 2 (Grb2).
BP1001 is being developed by Bio-Path Holdings, Inc.
According to Bio-Path, inhibition of Grb2 by BP1001 represents a significant advance in treating cancers with activated tyrosine kinases using a target not druggable with small molecule inhibitors.
Research has suggested that Grb2 plays an essential role in cancer cell activation via the RAS pathway. Grb2 bridges signals between activated and mutated tyrosine kinases, such as Flt3, c-Kit, and Bcr-Abl, and the Ras pathway, leading to activation of the ERK and AKT proteins.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
Trials of BP1001
Bio-Path has completed a phase 1 trial of BP1001 in patients with relapsed/refractory AML, chronic myeloid leukemia, and myelodysplastic syndromes.
The company has also completed the safety segment of a phase 2 trial in which BP1001 is being investigated in combination with low-dose ara-C to treat AML.
Bio-Path recently released data from these studies.
The phase 1 study included patients who had received an average of 6 prior therapies.
The patients received 8 doses of BP1001 over 4 weeks, escalating to a maximum dose of 90 mg/m2. There were no dose-limiting toxicities, and Bio-Path said the drug was well tolerated.
Of the 18 evaluable patients with circulating blasts, 83% responded to BP1001. The average reduction in circulating blasts was 67%.
The phase 2 trial included patients with relapsed/refractory AML. There were 3 evaluable patients in each of 2 dosing cohorts—60 mg/m2 and 90 mg/m2. Patients received BP1001 twice a week for 4 weeks.
Five of the patients responded—3 with a complete response and 2 with a partial response. There were no adverse events attributed to BP1001, and the maximum-tolerated dose was not reached. ![]()
Patients Know About Diabetic Retinopathy Risk—But Don’t Get Screened
Patients may understand that diabetes can lead to eye disease, and they may receive a recommendation for screening for diabetic retinopathy—but that doesn’t mean they’ll get screened. Researchers from Harbor-UCLA Medical Center surveyed 101 patients with diabetes and 44 providers and staffers at a clinic where annual screening rates for diabetic retinopathy were low. They found that 93% of patients understood the potential risk, but only 55% were getting screened.
The study goal, however, wasn’t to measure understanding of risk but to find out what patients considered barriers to screening and whether health care providers (HCPs) understood those barriers. And the researchers found a gap between the 2 groups.
Related: Long-Acting Insulin Analogs: Effects on Diabetic Retinopathy
Patients were mostly low-income Hispanics and African Americans. The survey asked participants to rate any given barrier that would delay or prevent getting screened. Health care providers and staff were asked to rate the importance of addressing the barriers.
Most of the patients (26%) reported at least 1 barrier to screening, most commonly depression (22%) and financial problems (26%); others reported language issues, lack of transportation, and lack of time.
When surveying HCPs, though, the researchers found “markedly divergent perceptions” between the 2 groups. For instance, only small numbers of patients said transportation, language issues, denial, fear, or cultural beliefs were barriers—yet most HCPs and staff thought those were “very” or “extremely important.”
Related: Diabetic Macular Edema: Is Your Patient Going Blind?
By contrast, the barriers the patients did think were important—financial burdens and depression—were rated as less important than other barriers by the HCPs and staff.
The differences in opinions suggest “a lack of high-quality patient-provider communication,” the researchers say. They suggest that more effective patient education as well as heightened awareness of depression and its impact are key to getting more patients screened.
Patients may understand that diabetes can lead to eye disease, and they may receive a recommendation for screening for diabetic retinopathy—but that doesn’t mean they’ll get screened. Researchers from Harbor-UCLA Medical Center surveyed 101 patients with diabetes and 44 providers and staffers at a clinic where annual screening rates for diabetic retinopathy were low. They found that 93% of patients understood the potential risk, but only 55% were getting screened.
The study goal, however, wasn’t to measure understanding of risk but to find out what patients considered barriers to screening and whether health care providers (HCPs) understood those barriers. And the researchers found a gap between the 2 groups.
Related: Long-Acting Insulin Analogs: Effects on Diabetic Retinopathy
Patients were mostly low-income Hispanics and African Americans. The survey asked participants to rate any given barrier that would delay or prevent getting screened. Health care providers and staff were asked to rate the importance of addressing the barriers.
Most of the patients (26%) reported at least 1 barrier to screening, most commonly depression (22%) and financial problems (26%); others reported language issues, lack of transportation, and lack of time.
When surveying HCPs, though, the researchers found “markedly divergent perceptions” between the 2 groups. For instance, only small numbers of patients said transportation, language issues, denial, fear, or cultural beliefs were barriers—yet most HCPs and staff thought those were “very” or “extremely important.”
Related: Diabetic Macular Edema: Is Your Patient Going Blind?
By contrast, the barriers the patients did think were important—financial burdens and depression—were rated as less important than other barriers by the HCPs and staff.
The differences in opinions suggest “a lack of high-quality patient-provider communication,” the researchers say. They suggest that more effective patient education as well as heightened awareness of depression and its impact are key to getting more patients screened.
Patients may understand that diabetes can lead to eye disease, and they may receive a recommendation for screening for diabetic retinopathy—but that doesn’t mean they’ll get screened. Researchers from Harbor-UCLA Medical Center surveyed 101 patients with diabetes and 44 providers and staffers at a clinic where annual screening rates for diabetic retinopathy were low. They found that 93% of patients understood the potential risk, but only 55% were getting screened.
The study goal, however, wasn’t to measure understanding of risk but to find out what patients considered barriers to screening and whether health care providers (HCPs) understood those barriers. And the researchers found a gap between the 2 groups.
Related: Long-Acting Insulin Analogs: Effects on Diabetic Retinopathy
Patients were mostly low-income Hispanics and African Americans. The survey asked participants to rate any given barrier that would delay or prevent getting screened. Health care providers and staff were asked to rate the importance of addressing the barriers.
Most of the patients (26%) reported at least 1 barrier to screening, most commonly depression (22%) and financial problems (26%); others reported language issues, lack of transportation, and lack of time.
When surveying HCPs, though, the researchers found “markedly divergent perceptions” between the 2 groups. For instance, only small numbers of patients said transportation, language issues, denial, fear, or cultural beliefs were barriers—yet most HCPs and staff thought those were “very” or “extremely important.”
Related: Diabetic Macular Edema: Is Your Patient Going Blind?
By contrast, the barriers the patients did think were important—financial burdens and depression—were rated as less important than other barriers by the HCPs and staff.
The differences in opinions suggest “a lack of high-quality patient-provider communication,” the researchers say. They suggest that more effective patient education as well as heightened awareness of depression and its impact are key to getting more patients screened.
Preschool ADHD diagnoses plateaued after 2011 AAP guideline
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
Key clinical point:
Major finding: The rate of ADHD diagnoses was 0.7% before the guidelines and 0.9% after, while stimulant prescriptions remained constant at 0.4% across the study period.
Data source: An analysis of electronic health record data from 143,881 children across 63 primary care practice from January 2008 to July 2014.
Disclosures: The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
Monitoring Home BP Readings Just Got Easier

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
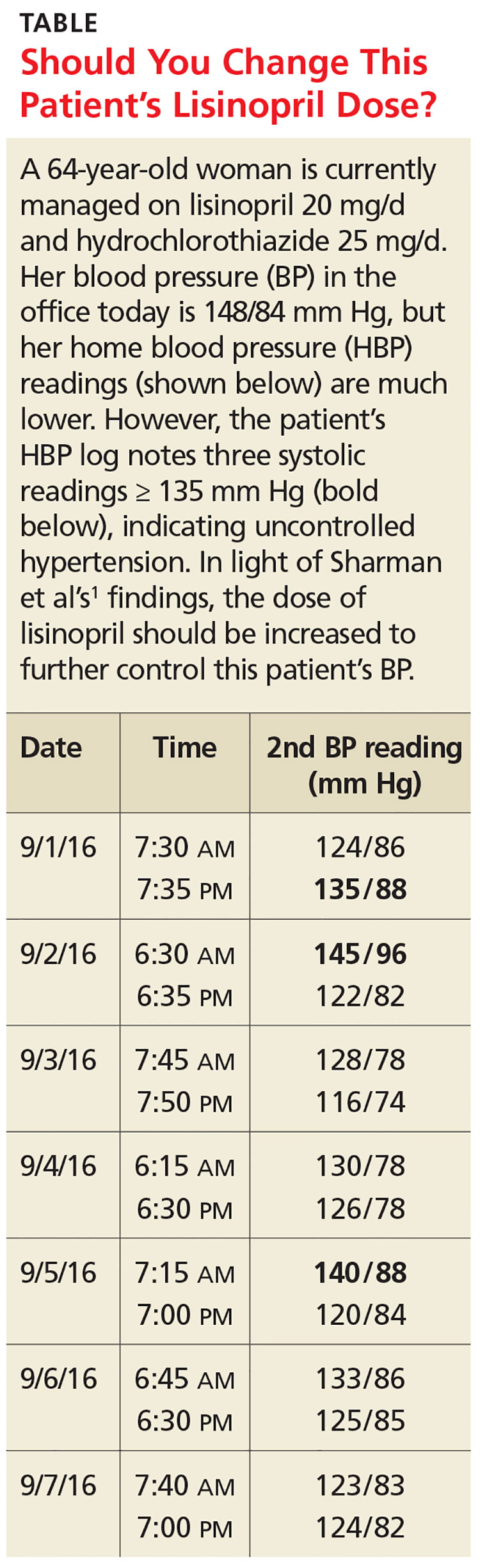
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5

HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5

HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Release of the MACRA Final Rule
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
From the Editors: Querencia
In the flood of emails, periodicals, Twitter, Facebook, Doximity, Medscape, and other information that washes over surgeons every day, why should they use their precious time to read ACS Surgery News? That question is foremost in the minds of the editors of this publication as we consider news stories and commentaries for inclusion. Is this an article our readers are going to find informative, pertinent, and stimulating? We want ACS Surgery News to be a querencia: a source of reliable, vetted information that gives surgeons a place of intellectual security along the information highway.
The editors of ACS Surgery News understand surgery from the scrub sink up. While our mission includes keeping our readers informed about these looming thunderstorms, we are also privileged to report progress and innovations that keep coming no matter how the forces of red tape and commerce play against our profession. Bringing news of both challenges and beacons of hope for our profession with commentary and perspective from our colleagues is our objective. For the editors, this is both a mission and a pleasure. Since most of the editors and our Editorial Advisory Board (EAB), like our readers, must focus primarily on our jobs as surgeons, teachers, and researchers, we cannot read every journal or attend every meeting. The role of ACS Surgery News is to find the relevant news of interest and importance to surgeons, wherever it may be found, and to report it succinctly and accurately in a readable form. Before an article appears in ACS Surgery News, it is reviewed by the author of the paper or presentation for accuracy and reviewed by the most appropriate member of the EAB as well as by both Co-Editors for importance and relevance to our surgeon readers. We do not want to shy away from controversial topics, but endeavor to present such topics with balance and sensitivity, just as the ACS itself always attempts to do: to shed light, rather than merely heat, on all subjects that we cover in our pages.
The editors of ACS Surgery News hope that in the months and years to come, this publication can be a querencia for the surgeon: a safe and secure place to engage all the forces that a surgeon must confront to be successful. In these pages we hope you will find knowledge, wisdom, camaraderie, and support for your practice, whatever that may be.
Surgery is a life of great joy and great sorrow, sometimes happening all within the same hour. We hope to be part of the joy and to soften the sorrow by being a publication you look forward to reading and wherein you find those things that contribute to your being a great surgeon and human being.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Co-Editor of ACS Surgery News.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
In the flood of emails, periodicals, Twitter, Facebook, Doximity, Medscape, and other information that washes over surgeons every day, why should they use their precious time to read ACS Surgery News? That question is foremost in the minds of the editors of this publication as we consider news stories and commentaries for inclusion. Is this an article our readers are going to find informative, pertinent, and stimulating? We want ACS Surgery News to be a querencia: a source of reliable, vetted information that gives surgeons a place of intellectual security along the information highway.
The editors of ACS Surgery News understand surgery from the scrub sink up. While our mission includes keeping our readers informed about these looming thunderstorms, we are also privileged to report progress and innovations that keep coming no matter how the forces of red tape and commerce play against our profession. Bringing news of both challenges and beacons of hope for our profession with commentary and perspective from our colleagues is our objective. For the editors, this is both a mission and a pleasure. Since most of the editors and our Editorial Advisory Board (EAB), like our readers, must focus primarily on our jobs as surgeons, teachers, and researchers, we cannot read every journal or attend every meeting. The role of ACS Surgery News is to find the relevant news of interest and importance to surgeons, wherever it may be found, and to report it succinctly and accurately in a readable form. Before an article appears in ACS Surgery News, it is reviewed by the author of the paper or presentation for accuracy and reviewed by the most appropriate member of the EAB as well as by both Co-Editors for importance and relevance to our surgeon readers. We do not want to shy away from controversial topics, but endeavor to present such topics with balance and sensitivity, just as the ACS itself always attempts to do: to shed light, rather than merely heat, on all subjects that we cover in our pages.
The editors of ACS Surgery News hope that in the months and years to come, this publication can be a querencia for the surgeon: a safe and secure place to engage all the forces that a surgeon must confront to be successful. In these pages we hope you will find knowledge, wisdom, camaraderie, and support for your practice, whatever that may be.
Surgery is a life of great joy and great sorrow, sometimes happening all within the same hour. We hope to be part of the joy and to soften the sorrow by being a publication you look forward to reading and wherein you find those things that contribute to your being a great surgeon and human being.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Co-Editor of ACS Surgery News.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
In the flood of emails, periodicals, Twitter, Facebook, Doximity, Medscape, and other information that washes over surgeons every day, why should they use their precious time to read ACS Surgery News? That question is foremost in the minds of the editors of this publication as we consider news stories and commentaries for inclusion. Is this an article our readers are going to find informative, pertinent, and stimulating? We want ACS Surgery News to be a querencia: a source of reliable, vetted information that gives surgeons a place of intellectual security along the information highway.
The editors of ACS Surgery News understand surgery from the scrub sink up. While our mission includes keeping our readers informed about these looming thunderstorms, we are also privileged to report progress and innovations that keep coming no matter how the forces of red tape and commerce play against our profession. Bringing news of both challenges and beacons of hope for our profession with commentary and perspective from our colleagues is our objective. For the editors, this is both a mission and a pleasure. Since most of the editors and our Editorial Advisory Board (EAB), like our readers, must focus primarily on our jobs as surgeons, teachers, and researchers, we cannot read every journal or attend every meeting. The role of ACS Surgery News is to find the relevant news of interest and importance to surgeons, wherever it may be found, and to report it succinctly and accurately in a readable form. Before an article appears in ACS Surgery News, it is reviewed by the author of the paper or presentation for accuracy and reviewed by the most appropriate member of the EAB as well as by both Co-Editors for importance and relevance to our surgeon readers. We do not want to shy away from controversial topics, but endeavor to present such topics with balance and sensitivity, just as the ACS itself always attempts to do: to shed light, rather than merely heat, on all subjects that we cover in our pages.
The editors of ACS Surgery News hope that in the months and years to come, this publication can be a querencia for the surgeon: a safe and secure place to engage all the forces that a surgeon must confront to be successful. In these pages we hope you will find knowledge, wisdom, camaraderie, and support for your practice, whatever that may be.
Surgery is a life of great joy and great sorrow, sometimes happening all within the same hour. We hope to be part of the joy and to soften the sorrow by being a publication you look forward to reading and wherein you find those things that contribute to your being a great surgeon and human being.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the Co-Editor of ACS Surgery News.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Courtney M. Townsend, Jr., MD, FACS, installed as 97th President of the ACS
Courtney M. Townsend, Jr., MD, FACS, a general surgeon from Galveston, TX, was installed as the 97th President of the American College of Surgeons (ACS) during the Convocation on October 16 at the Walter E. Washington Convention Center, Washington, DC.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery; professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB.
Dr. Townsend earned his bachelor’s degree in history and English from the University of Texas, Austin. He then earned his medical degree and completed his internship and general surgery training at UTMB. Dr. Townsend completed a surgical oncology fellowship at the University of California, Los Angeles (UCLA), and was a McLaughlin Fellow twice, a Jeane B. Kempner Fellow, an American Cancer Society clinical fellow, and an NIH postdoctoral fellow.
Dr. Townsend’s first teaching position was as an adjunct assistant professor of surgery, division of oncology, department of surgery, at UCLA (1974–1976). He then served in the U.S. Navy from 1976 to 1978 as a staff surgeon and surgical director in the intensive care unit at the National Naval Medical Center, Bethesda, MD.
In 1978, Dr. Townsend returned to UTMB as an associate professor in the department of surgery. In 1981, he was promoted to Robertson-Poth Associate Professor of Surgery, and the next year he became director of the surgical research laboratory at UTMB. From 1983 to 1995, Dr. Townsend was the Robertson-Poth Professor of Surgery, and from 1987 to 1995, he served as interim director of the UTMB Cancer Center. He assumed his current roles as professor of physician assistant studies in 1989, as graduate faculty in the cell biology program in 2001, and as Robertson-Poth Distinguished Chair in General Surgery in 2009. Dr. Townsend also served as John Woods Harris Distinguished Chairman from 1995 to 2013.
Dr. Townsend has served in many leadership roles at the College, including ACS Secretary (2006–2015). He held prominent positions on the Board of Governors (B/G), including Chair (2004–2005), B/G Executive Committee Member (1999–2003); and ACS Governor from the Society for Surgery of the Alimentary Tract (1986–1992). He has also served in various capacities on the Commission on Cancer (CoC) and on other ACS committees. Dr. Townsend served on the CoC Committee on Approvals (1989–1994), the CoC National Cancer Data Committee and the National Cancer Data Base Governing Board (1989–1995), the ACS Committee for the Forum on Fundamental Surgical Problems and the Committee on Special Issues (both 1991–1994), the Committee on Papers (2000–2003), the Member Services Liaison Committee (2003–2004), and the Nominating Committee of the Fellows (2000–2002).
Most recently, Dr. Townsend served on the ACS Surgical Research and Education Committee, which he chaired for two years (1998–2000). At the local level, he has served on the Southern Texas District #1 Committee on Applicants (1996–1999) and as President of the ACS South Texas Chapter (1988–1989).
Dr. Townsend has assumed leadership roles in several other medical organizations as well. He is past-director and chair of the American Board of Surgery (2000–2007); served on the Accreditation Council for Graduate Medical Education Residency Review Committee for Surgery (1994–1999); American Pancreatic Association president (1992−1993); American Surgical Association president (2007–2008); Southern Surgical Association president (2004); and Texas Surgical Society council member (1997–1999). He is an honorary member of the Society of Black Academic Surgeons and the Association of Women Surgeons (AWS) and is a recipient of UTMB’s John P. McGovern Lifetime Achievement Award in Oslerian Medicine.
Dr. Townsend has been editor-in-chief of the Sabiston Textbook for Surgery: The Biological Basis of Modern Surgical Practice since 2000 and was the editor of Surgical Oncology (1992−1999). He has served on the editorial board of the Journal of the American College of Surgeons (JACS), Surgery, and The American Journal of Surgery.
Vice-Presidents
In addition, during the Convocation, Hilary Sanfey, MB, BCh, MHPE, FACS, FRCSI, FRCS, was installed as ACS First Vice-President, and Mary C. McCarthy, MD, FACS, was installed as ACS Second Vice-President.
Dr. Sanfey is professor of surgery and vice-chair for educational affairs, department of surgery, and associate director, Academy for Scholarship and Education, Southern Illinois University (SIU) School of Medicine, Springfield. Dr. Sanfey, who hails from Ireland, graduated from Trinity College Dublin School of Medicine in 1976. She trained at the Royal College of Surgeons in Ireland (RCSI), spent three years as a research fellow at Johns Hopkins University, Baltimore, MD, and worked as a consultant transplant surgeon at the Royal Infirmary of Edinburgh for four years before moving to the University of Virginia, Charlottesville, in 1996. She remained on the clinical faculty at the University of Virginia, starting as an assistant professor of hepatobiliary surgery in 1991 and leaving in 2008 for SIU as a tenured professor of surgery. In 2009, she received a master’s degree in health professions education from the University of Illinois, Chicago.
Dr. Sanfey is the immediate past-president of the International Society of Surgery, U.S. chapter, and a member of the American Surgical Association. Dr. Sanfey serves as faculty for the ACS Residents as Teachers and Leaders Program and has served as a specialist advisor in postgraduate surgical training and education in the department of surgical affairs, RCSI.
An ACS Fellow since 2001, Dr. Sanfey served as the ACS Liaison to the American Medical Association (AMA) Women Physicians Congress (2006–2009) and an ACS Governor (2006–2012). As a Governor, she chaired the B/G Committee on Chapters Subcommittee on Diversity (2009–2011) and the Nominating Committee (2010–2012). In addition, she served on the Executive Committee of the Committee on Medical Student Education (2005–2011) and as a liaison to the Program Committee. She presently serves on the Executive Committee of the Scholarship Committee.
She has been active on the Women in Surgery Committee since 2005. She has held high-ranking positions in other prestigious surgical organizations as well, including the AWS (president, 2005–2006) and the U.S. chapter of the International Surgical Society (president, 2013–2015). In addition, she has served on key committees of the Association of Program Directors in Surgery, the Association for Surgical Education, and the American Society of Transplant Surgeons.
Dr. Sanfey is on the editorial boards of the Association for Surgical Education, Journal of the Royal Colleges of Edinburgh and Ireland, and JACS. She is an accomplished surgical investigator, has contributed to more than 100 peer-reviewed papers and 24 book chapters, and has been a frequent guest lecturer and visiting professor at international symposia and workshops.
She is the recipient of many awards in surgical education. The AWS in 2010 renamed its Outstanding Woman Resident Award as the Hilary Sanfey Outstanding Resident Award, and in 2013 and 2014, respectively, Dr. Sanfey was honored with the AWS Olga Jonasson Distinguished Member Award and Nina Starr Braunwald Award.
Dr. McCarthy is the Elizabeth Berry Gray Chair and Professor, department of surgery, Boonshoft School of Medicine, and adjunct graduate faculty, School of Engineering, Wright State University (WSU); and an acute care surgeon at Miami Valley Hospital, Dayton, OH. Before moving to WSU, she was assistant professor of surgery (1983–1988) at Indiana University (IU) School of Medicine, Indianapolis.
An ACS Fellow since 1986, Dr. McCarthy has served in a number of leadership positions within the organization, including as an ACS Governor (1995–2001). As a Specialty Society Governor for the AWS, she served on the Nominating Committee (member, 1996–1997, and Vice-Chair, 1997–1998); the Governors Committee on Chapter Activities (1995–2001), chairing the committee’s Subcommittee on Chapter Membership Recruitment, Retention, and Diversification (1998–2001); and Advisor to the Governors Committee on Chapter Activities Executive Committee (1995).
Dr. McCarthy also served on the ACS Advisory Council for General Surgery and is a current member of the Committee on Trauma. She has served on the Surgical Education and Self-Assessment Program (SESAP®) Committee, including as Co-Chair for SESAP XII, 1999; the Committee on Continuing Education (Member, 1994–1999, and Vice-Chair, 1995–1997); the Committee on Applicants for District 6 (present); and the Clinical Congress Abstract Selection Committee (2007–2009). While at IU, she was active in the Indiana Chapter, and she remains active in the Ohio chapter, having served on the Executive Committee (1995–2001) and the Ohio Committee on Trauma (1991–present).
She is a past-president of the AWS (1990–1992) and has served in prominent positions in the Association for Surgical Education, Eastern Association for the Surgery of Trauma, Halsted Surgical Society, Midwest Surgical Association, Parkland Surgical Society, and Society of Critical Care Medicine.
She is the recipient of numerous professional awards, including the American Hospital Association Nova Award, and AWS Distinguished Member, Olga Jonasson Award, and Nina Starr Braunwald Awards. She is a prolific author of peer-reviewed publications, book chapters, and abstracts on trauma and critical care.
Courtney M. Townsend, Jr., MD, FACS, a general surgeon from Galveston, TX, was installed as the 97th President of the American College of Surgeons (ACS) during the Convocation on October 16 at the Walter E. Washington Convention Center, Washington, DC.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery; professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB.
Dr. Townsend earned his bachelor’s degree in history and English from the University of Texas, Austin. He then earned his medical degree and completed his internship and general surgery training at UTMB. Dr. Townsend completed a surgical oncology fellowship at the University of California, Los Angeles (UCLA), and was a McLaughlin Fellow twice, a Jeane B. Kempner Fellow, an American Cancer Society clinical fellow, and an NIH postdoctoral fellow.
Dr. Townsend’s first teaching position was as an adjunct assistant professor of surgery, division of oncology, department of surgery, at UCLA (1974–1976). He then served in the U.S. Navy from 1976 to 1978 as a staff surgeon and surgical director in the intensive care unit at the National Naval Medical Center, Bethesda, MD.
In 1978, Dr. Townsend returned to UTMB as an associate professor in the department of surgery. In 1981, he was promoted to Robertson-Poth Associate Professor of Surgery, and the next year he became director of the surgical research laboratory at UTMB. From 1983 to 1995, Dr. Townsend was the Robertson-Poth Professor of Surgery, and from 1987 to 1995, he served as interim director of the UTMB Cancer Center. He assumed his current roles as professor of physician assistant studies in 1989, as graduate faculty in the cell biology program in 2001, and as Robertson-Poth Distinguished Chair in General Surgery in 2009. Dr. Townsend also served as John Woods Harris Distinguished Chairman from 1995 to 2013.
Dr. Townsend has served in many leadership roles at the College, including ACS Secretary (2006–2015). He held prominent positions on the Board of Governors (B/G), including Chair (2004–2005), B/G Executive Committee Member (1999–2003); and ACS Governor from the Society for Surgery of the Alimentary Tract (1986–1992). He has also served in various capacities on the Commission on Cancer (CoC) and on other ACS committees. Dr. Townsend served on the CoC Committee on Approvals (1989–1994), the CoC National Cancer Data Committee and the National Cancer Data Base Governing Board (1989–1995), the ACS Committee for the Forum on Fundamental Surgical Problems and the Committee on Special Issues (both 1991–1994), the Committee on Papers (2000–2003), the Member Services Liaison Committee (2003–2004), and the Nominating Committee of the Fellows (2000–2002).
Most recently, Dr. Townsend served on the ACS Surgical Research and Education Committee, which he chaired for two years (1998–2000). At the local level, he has served on the Southern Texas District #1 Committee on Applicants (1996–1999) and as President of the ACS South Texas Chapter (1988–1989).
Dr. Townsend has assumed leadership roles in several other medical organizations as well. He is past-director and chair of the American Board of Surgery (2000–2007); served on the Accreditation Council for Graduate Medical Education Residency Review Committee for Surgery (1994–1999); American Pancreatic Association president (1992−1993); American Surgical Association president (2007–2008); Southern Surgical Association president (2004); and Texas Surgical Society council member (1997–1999). He is an honorary member of the Society of Black Academic Surgeons and the Association of Women Surgeons (AWS) and is a recipient of UTMB’s John P. McGovern Lifetime Achievement Award in Oslerian Medicine.
Dr. Townsend has been editor-in-chief of the Sabiston Textbook for Surgery: The Biological Basis of Modern Surgical Practice since 2000 and was the editor of Surgical Oncology (1992−1999). He has served on the editorial board of the Journal of the American College of Surgeons (JACS), Surgery, and The American Journal of Surgery.
Vice-Presidents
In addition, during the Convocation, Hilary Sanfey, MB, BCh, MHPE, FACS, FRCSI, FRCS, was installed as ACS First Vice-President, and Mary C. McCarthy, MD, FACS, was installed as ACS Second Vice-President.
Dr. Sanfey is professor of surgery and vice-chair for educational affairs, department of surgery, and associate director, Academy for Scholarship and Education, Southern Illinois University (SIU) School of Medicine, Springfield. Dr. Sanfey, who hails from Ireland, graduated from Trinity College Dublin School of Medicine in 1976. She trained at the Royal College of Surgeons in Ireland (RCSI), spent three years as a research fellow at Johns Hopkins University, Baltimore, MD, and worked as a consultant transplant surgeon at the Royal Infirmary of Edinburgh for four years before moving to the University of Virginia, Charlottesville, in 1996. She remained on the clinical faculty at the University of Virginia, starting as an assistant professor of hepatobiliary surgery in 1991 and leaving in 2008 for SIU as a tenured professor of surgery. In 2009, she received a master’s degree in health professions education from the University of Illinois, Chicago.
Dr. Sanfey is the immediate past-president of the International Society of Surgery, U.S. chapter, and a member of the American Surgical Association. Dr. Sanfey serves as faculty for the ACS Residents as Teachers and Leaders Program and has served as a specialist advisor in postgraduate surgical training and education in the department of surgical affairs, RCSI.
An ACS Fellow since 2001, Dr. Sanfey served as the ACS Liaison to the American Medical Association (AMA) Women Physicians Congress (2006–2009) and an ACS Governor (2006–2012). As a Governor, she chaired the B/G Committee on Chapters Subcommittee on Diversity (2009–2011) and the Nominating Committee (2010–2012). In addition, she served on the Executive Committee of the Committee on Medical Student Education (2005–2011) and as a liaison to the Program Committee. She presently serves on the Executive Committee of the Scholarship Committee.
She has been active on the Women in Surgery Committee since 2005. She has held high-ranking positions in other prestigious surgical organizations as well, including the AWS (president, 2005–2006) and the U.S. chapter of the International Surgical Society (president, 2013–2015). In addition, she has served on key committees of the Association of Program Directors in Surgery, the Association for Surgical Education, and the American Society of Transplant Surgeons.
Dr. Sanfey is on the editorial boards of the Association for Surgical Education, Journal of the Royal Colleges of Edinburgh and Ireland, and JACS. She is an accomplished surgical investigator, has contributed to more than 100 peer-reviewed papers and 24 book chapters, and has been a frequent guest lecturer and visiting professor at international symposia and workshops.
She is the recipient of many awards in surgical education. The AWS in 2010 renamed its Outstanding Woman Resident Award as the Hilary Sanfey Outstanding Resident Award, and in 2013 and 2014, respectively, Dr. Sanfey was honored with the AWS Olga Jonasson Distinguished Member Award and Nina Starr Braunwald Award.
Dr. McCarthy is the Elizabeth Berry Gray Chair and Professor, department of surgery, Boonshoft School of Medicine, and adjunct graduate faculty, School of Engineering, Wright State University (WSU); and an acute care surgeon at Miami Valley Hospital, Dayton, OH. Before moving to WSU, she was assistant professor of surgery (1983–1988) at Indiana University (IU) School of Medicine, Indianapolis.
An ACS Fellow since 1986, Dr. McCarthy has served in a number of leadership positions within the organization, including as an ACS Governor (1995–2001). As a Specialty Society Governor for the AWS, she served on the Nominating Committee (member, 1996–1997, and Vice-Chair, 1997–1998); the Governors Committee on Chapter Activities (1995–2001), chairing the committee’s Subcommittee on Chapter Membership Recruitment, Retention, and Diversification (1998–2001); and Advisor to the Governors Committee on Chapter Activities Executive Committee (1995).
Dr. McCarthy also served on the ACS Advisory Council for General Surgery and is a current member of the Committee on Trauma. She has served on the Surgical Education and Self-Assessment Program (SESAP®) Committee, including as Co-Chair for SESAP XII, 1999; the Committee on Continuing Education (Member, 1994–1999, and Vice-Chair, 1995–1997); the Committee on Applicants for District 6 (present); and the Clinical Congress Abstract Selection Committee (2007–2009). While at IU, she was active in the Indiana Chapter, and she remains active in the Ohio chapter, having served on the Executive Committee (1995–2001) and the Ohio Committee on Trauma (1991–present).
She is a past-president of the AWS (1990–1992) and has served in prominent positions in the Association for Surgical Education, Eastern Association for the Surgery of Trauma, Halsted Surgical Society, Midwest Surgical Association, Parkland Surgical Society, and Society of Critical Care Medicine.
She is the recipient of numerous professional awards, including the American Hospital Association Nova Award, and AWS Distinguished Member, Olga Jonasson Award, and Nina Starr Braunwald Awards. She is a prolific author of peer-reviewed publications, book chapters, and abstracts on trauma and critical care.
Courtney M. Townsend, Jr., MD, FACS, a general surgeon from Galveston, TX, was installed as the 97th President of the American College of Surgeons (ACS) during the Convocation on October 16 at the Walter E. Washington Convention Center, Washington, DC.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery; professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB.
Dr. Townsend earned his bachelor’s degree in history and English from the University of Texas, Austin. He then earned his medical degree and completed his internship and general surgery training at UTMB. Dr. Townsend completed a surgical oncology fellowship at the University of California, Los Angeles (UCLA), and was a McLaughlin Fellow twice, a Jeane B. Kempner Fellow, an American Cancer Society clinical fellow, and an NIH postdoctoral fellow.
Dr. Townsend’s first teaching position was as an adjunct assistant professor of surgery, division of oncology, department of surgery, at UCLA (1974–1976). He then served in the U.S. Navy from 1976 to 1978 as a staff surgeon and surgical director in the intensive care unit at the National Naval Medical Center, Bethesda, MD.
In 1978, Dr. Townsend returned to UTMB as an associate professor in the department of surgery. In 1981, he was promoted to Robertson-Poth Associate Professor of Surgery, and the next year he became director of the surgical research laboratory at UTMB. From 1983 to 1995, Dr. Townsend was the Robertson-Poth Professor of Surgery, and from 1987 to 1995, he served as interim director of the UTMB Cancer Center. He assumed his current roles as professor of physician assistant studies in 1989, as graduate faculty in the cell biology program in 2001, and as Robertson-Poth Distinguished Chair in General Surgery in 2009. Dr. Townsend also served as John Woods Harris Distinguished Chairman from 1995 to 2013.
Dr. Townsend has served in many leadership roles at the College, including ACS Secretary (2006–2015). He held prominent positions on the Board of Governors (B/G), including Chair (2004–2005), B/G Executive Committee Member (1999–2003); and ACS Governor from the Society for Surgery of the Alimentary Tract (1986–1992). He has also served in various capacities on the Commission on Cancer (CoC) and on other ACS committees. Dr. Townsend served on the CoC Committee on Approvals (1989–1994), the CoC National Cancer Data Committee and the National Cancer Data Base Governing Board (1989–1995), the ACS Committee for the Forum on Fundamental Surgical Problems and the Committee on Special Issues (both 1991–1994), the Committee on Papers (2000–2003), the Member Services Liaison Committee (2003–2004), and the Nominating Committee of the Fellows (2000–2002).
Most recently, Dr. Townsend served on the ACS Surgical Research and Education Committee, which he chaired for two years (1998–2000). At the local level, he has served on the Southern Texas District #1 Committee on Applicants (1996–1999) and as President of the ACS South Texas Chapter (1988–1989).
Dr. Townsend has assumed leadership roles in several other medical organizations as well. He is past-director and chair of the American Board of Surgery (2000–2007); served on the Accreditation Council for Graduate Medical Education Residency Review Committee for Surgery (1994–1999); American Pancreatic Association president (1992−1993); American Surgical Association president (2007–2008); Southern Surgical Association president (2004); and Texas Surgical Society council member (1997–1999). He is an honorary member of the Society of Black Academic Surgeons and the Association of Women Surgeons (AWS) and is a recipient of UTMB’s John P. McGovern Lifetime Achievement Award in Oslerian Medicine.
Dr. Townsend has been editor-in-chief of the Sabiston Textbook for Surgery: The Biological Basis of Modern Surgical Practice since 2000 and was the editor of Surgical Oncology (1992−1999). He has served on the editorial board of the Journal of the American College of Surgeons (JACS), Surgery, and The American Journal of Surgery.
Vice-Presidents
In addition, during the Convocation, Hilary Sanfey, MB, BCh, MHPE, FACS, FRCSI, FRCS, was installed as ACS First Vice-President, and Mary C. McCarthy, MD, FACS, was installed as ACS Second Vice-President.
Dr. Sanfey is professor of surgery and vice-chair for educational affairs, department of surgery, and associate director, Academy for Scholarship and Education, Southern Illinois University (SIU) School of Medicine, Springfield. Dr. Sanfey, who hails from Ireland, graduated from Trinity College Dublin School of Medicine in 1976. She trained at the Royal College of Surgeons in Ireland (RCSI), spent three years as a research fellow at Johns Hopkins University, Baltimore, MD, and worked as a consultant transplant surgeon at the Royal Infirmary of Edinburgh for four years before moving to the University of Virginia, Charlottesville, in 1996. She remained on the clinical faculty at the University of Virginia, starting as an assistant professor of hepatobiliary surgery in 1991 and leaving in 2008 for SIU as a tenured professor of surgery. In 2009, she received a master’s degree in health professions education from the University of Illinois, Chicago.
Dr. Sanfey is the immediate past-president of the International Society of Surgery, U.S. chapter, and a member of the American Surgical Association. Dr. Sanfey serves as faculty for the ACS Residents as Teachers and Leaders Program and has served as a specialist advisor in postgraduate surgical training and education in the department of surgical affairs, RCSI.
An ACS Fellow since 2001, Dr. Sanfey served as the ACS Liaison to the American Medical Association (AMA) Women Physicians Congress (2006–2009) and an ACS Governor (2006–2012). As a Governor, she chaired the B/G Committee on Chapters Subcommittee on Diversity (2009–2011) and the Nominating Committee (2010–2012). In addition, she served on the Executive Committee of the Committee on Medical Student Education (2005–2011) and as a liaison to the Program Committee. She presently serves on the Executive Committee of the Scholarship Committee.
She has been active on the Women in Surgery Committee since 2005. She has held high-ranking positions in other prestigious surgical organizations as well, including the AWS (president, 2005–2006) and the U.S. chapter of the International Surgical Society (president, 2013–2015). In addition, she has served on key committees of the Association of Program Directors in Surgery, the Association for Surgical Education, and the American Society of Transplant Surgeons.
Dr. Sanfey is on the editorial boards of the Association for Surgical Education, Journal of the Royal Colleges of Edinburgh and Ireland, and JACS. She is an accomplished surgical investigator, has contributed to more than 100 peer-reviewed papers and 24 book chapters, and has been a frequent guest lecturer and visiting professor at international symposia and workshops.
She is the recipient of many awards in surgical education. The AWS in 2010 renamed its Outstanding Woman Resident Award as the Hilary Sanfey Outstanding Resident Award, and in 2013 and 2014, respectively, Dr. Sanfey was honored with the AWS Olga Jonasson Distinguished Member Award and Nina Starr Braunwald Award.
Dr. McCarthy is the Elizabeth Berry Gray Chair and Professor, department of surgery, Boonshoft School of Medicine, and adjunct graduate faculty, School of Engineering, Wright State University (WSU); and an acute care surgeon at Miami Valley Hospital, Dayton, OH. Before moving to WSU, she was assistant professor of surgery (1983–1988) at Indiana University (IU) School of Medicine, Indianapolis.
An ACS Fellow since 1986, Dr. McCarthy has served in a number of leadership positions within the organization, including as an ACS Governor (1995–2001). As a Specialty Society Governor for the AWS, she served on the Nominating Committee (member, 1996–1997, and Vice-Chair, 1997–1998); the Governors Committee on Chapter Activities (1995–2001), chairing the committee’s Subcommittee on Chapter Membership Recruitment, Retention, and Diversification (1998–2001); and Advisor to the Governors Committee on Chapter Activities Executive Committee (1995).
Dr. McCarthy also served on the ACS Advisory Council for General Surgery and is a current member of the Committee on Trauma. She has served on the Surgical Education and Self-Assessment Program (SESAP®) Committee, including as Co-Chair for SESAP XII, 1999; the Committee on Continuing Education (Member, 1994–1999, and Vice-Chair, 1995–1997); the Committee on Applicants for District 6 (present); and the Clinical Congress Abstract Selection Committee (2007–2009). While at IU, she was active in the Indiana Chapter, and she remains active in the Ohio chapter, having served on the Executive Committee (1995–2001) and the Ohio Committee on Trauma (1991–present).
She is a past-president of the AWS (1990–1992) and has served in prominent positions in the Association for Surgical Education, Eastern Association for the Surgery of Trauma, Halsted Surgical Society, Midwest Surgical Association, Parkland Surgical Society, and Society of Critical Care Medicine.
She is the recipient of numerous professional awards, including the American Hospital Association Nova Award, and AWS Distinguished Member, Olga Jonasson Award, and Nina Starr Braunwald Awards. She is a prolific author of peer-reviewed publications, book chapters, and abstracts on trauma and critical care.