User login
Ketamine augmentation doesn’t boost ECT outcomes
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Cognitive impairment as measured by delayed verbal recall on the Hopkins Verbal Learning Task–Revised was not reduced by the use of ketamine as an adjunctive anesthetic agent for ECT.
Data source: This randomized, multicenter trial featuring blinded assessments included 70 severely depressed patients who received either ketamine or saline in addition to standard anesthesia during their ECT sessions.
Disclosures: The Ketamine-ECT Study was funded by the U.K. National Institute for Health Research and the Medical Research Council. The presenter reported having no conflicts of interest.
Zika increase slows slightly in pregnant women
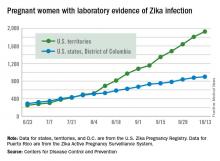
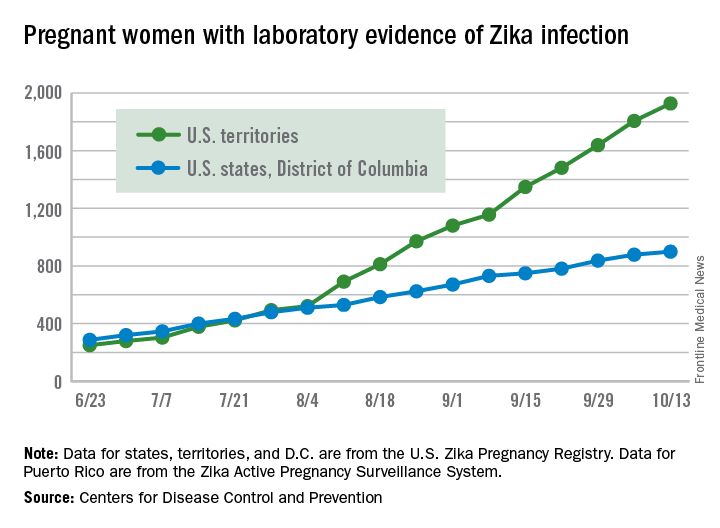
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The total number of pregnant women in the United States and its territories with laboratory evidence of Zika infection rose by 142 for the week ending Oct. 13 – the smallest increase since early September, according to the Centers for Disease Control and Prevention.
There were 121 new cases of Zika virus infection in pregnant women reported in the U.S. territories and 21 new cases in the states and the District of Columbia, bringing the corresponding totals for the year to 1,927 cases in the territories and 899 in the states/D.C. – a total of 2,826 pregnant women with Zika in the United States, the CDC reported Oct. 20.
The Zika caseload among all Americans was 31,418 as of Oct. 19. An additional 80 cases reported from Oct. 14 through Oct. 19 brought the state/D.C. total to 4,016, and 1,447 new cases for the week brings the territorial total to 27,402 for 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Webcast: Contraceptive considerations for women with headache and migraine
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
Voice recognition software errors: Often silly, sometimes serious
How much is 15%?
Not that much, on paper. With any drug, at least 15% of people will get some kind of side effect. Usually they all list dizziness and headaches at the top.
Could the same be true of a seemingly harmless technology?
Voice recognition software has become pretty commonplace in modern medicine but is far from perfect. I try to be pretty careful about proofreading my dictations, but many docs, especially those in emergency room, don’t have the time to. So VR errors slip by, persisting in 71% of notes.
Most of these errors are just silly and obvious for what they are. But a recent study at a level I ER found that 15% of dictations contained one or more errors deemed as “critical,” with the potential to adversely affect patient care (Int J Med Inform. 2016 Sep;93:70-3).
Communication among doctors, nurses, and all the other key players in the hospital environment is one of the most critical areas in modern medicine. So many people often rely on the initial dictation for an idea of what’s going on that a critical error can affect the way they think about the case from the get-go.
Another issue, sadly, in today’s hospital is that no one takes (or has) the time to get a patient’s past medical history. It’s commonplace to pull the history out of previous admission notes. (Admittedly, sometimes in a demented or unconscious patient you don’t have a choice.) As a result, errors of this sort tend to propagate down the line, from an admission, to the consults, to the discharge summary, and into the next admission.
So let’s get back to that 15%.
I have to assume that 15% of people being admitted aren’t having catastrophic events from medical errors, hopefully because the doctors and nurses handling patient care are thinking for themselves, recognizing dictation errors, and addressing them appropriately.
But even if we dial it down to a tenth of that, say 1.5%, it’s still a serious concern. Bad outcomes in medicine are never entirely avoidable. That’s the nature of the job.
But bad outcomes caused by too much trust in a still-faulty technology are avoidable.
If 15% of people had a serious outcome from a medication, you’d be very cautious about using it. We need to treat these technological gadgets with the same concerns we extend to drugs and procedures. Avoidable bad outcomes, regardless of cause, are never good.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much is 15%?
Not that much, on paper. With any drug, at least 15% of people will get some kind of side effect. Usually they all list dizziness and headaches at the top.
Could the same be true of a seemingly harmless technology?
Voice recognition software has become pretty commonplace in modern medicine but is far from perfect. I try to be pretty careful about proofreading my dictations, but many docs, especially those in emergency room, don’t have the time to. So VR errors slip by, persisting in 71% of notes.
Most of these errors are just silly and obvious for what they are. But a recent study at a level I ER found that 15% of dictations contained one or more errors deemed as “critical,” with the potential to adversely affect patient care (Int J Med Inform. 2016 Sep;93:70-3).
Communication among doctors, nurses, and all the other key players in the hospital environment is one of the most critical areas in modern medicine. So many people often rely on the initial dictation for an idea of what’s going on that a critical error can affect the way they think about the case from the get-go.
Another issue, sadly, in today’s hospital is that no one takes (or has) the time to get a patient’s past medical history. It’s commonplace to pull the history out of previous admission notes. (Admittedly, sometimes in a demented or unconscious patient you don’t have a choice.) As a result, errors of this sort tend to propagate down the line, from an admission, to the consults, to the discharge summary, and into the next admission.
So let’s get back to that 15%.
I have to assume that 15% of people being admitted aren’t having catastrophic events from medical errors, hopefully because the doctors and nurses handling patient care are thinking for themselves, recognizing dictation errors, and addressing them appropriately.
But even if we dial it down to a tenth of that, say 1.5%, it’s still a serious concern. Bad outcomes in medicine are never entirely avoidable. That’s the nature of the job.
But bad outcomes caused by too much trust in a still-faulty technology are avoidable.
If 15% of people had a serious outcome from a medication, you’d be very cautious about using it. We need to treat these technological gadgets with the same concerns we extend to drugs and procedures. Avoidable bad outcomes, regardless of cause, are never good.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much is 15%?
Not that much, on paper. With any drug, at least 15% of people will get some kind of side effect. Usually they all list dizziness and headaches at the top.
Could the same be true of a seemingly harmless technology?
Voice recognition software has become pretty commonplace in modern medicine but is far from perfect. I try to be pretty careful about proofreading my dictations, but many docs, especially those in emergency room, don’t have the time to. So VR errors slip by, persisting in 71% of notes.
Most of these errors are just silly and obvious for what they are. But a recent study at a level I ER found that 15% of dictations contained one or more errors deemed as “critical,” with the potential to adversely affect patient care (Int J Med Inform. 2016 Sep;93:70-3).
Communication among doctors, nurses, and all the other key players in the hospital environment is one of the most critical areas in modern medicine. So many people often rely on the initial dictation for an idea of what’s going on that a critical error can affect the way they think about the case from the get-go.
Another issue, sadly, in today’s hospital is that no one takes (or has) the time to get a patient’s past medical history. It’s commonplace to pull the history out of previous admission notes. (Admittedly, sometimes in a demented or unconscious patient you don’t have a choice.) As a result, errors of this sort tend to propagate down the line, from an admission, to the consults, to the discharge summary, and into the next admission.
So let’s get back to that 15%.
I have to assume that 15% of people being admitted aren’t having catastrophic events from medical errors, hopefully because the doctors and nurses handling patient care are thinking for themselves, recognizing dictation errors, and addressing them appropriately.
But even if we dial it down to a tenth of that, say 1.5%, it’s still a serious concern. Bad outcomes in medicine are never entirely avoidable. That’s the nature of the job.
But bad outcomes caused by too much trust in a still-faulty technology are avoidable.
If 15% of people had a serious outcome from a medication, you’d be very cautious about using it. We need to treat these technological gadgets with the same concerns we extend to drugs and procedures. Avoidable bad outcomes, regardless of cause, are never good.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
NAMS hormone therapy guidelines stress individualized treatment
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Experts outline phenotype approach to rosacea
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Rosacea diagnosis, severity grading, and management should be based on disease phenotypes, which can span more than one of the currently recognized subtypes.
Major finding: Persistent centrofacial erythema with periodic intensification, and phymatous changes, are two phenotypes independently diagnostic of rosacea
Data source: An expert panel of 17 dermatologists from North America, Europe, Asia, Africa, and South America.
Disclosures: Galderma sponsored the study, for which all authors received honoraria; 12 disclosed additional funding from Galderma or other manufacturers.
Molecular subtypes of Crohn’s may shape treatment approach
Researchers have identified two distinct molecular subclasses of Crohn’s disease, based on gene expression and phenotype, which could help with better tailoring of treatment.
Crohn’s disease is a chronic inflammatory disorder with a diverse clinical presentation and pattern of progression, yet the molecular and genetic factors underlying the disease are not well understood.
This complicates treatment – which currently relies on doctors’ subjective clinical classification – and makes it difficult to develop an evidence-based personalized approach.
Writing in the Oct. 14 online edition of Gut, Matthew Weiser, a graduate student in the genetics department at the University of North Carolina at Chapel Hill and his coauthors report the results of a genetic and molecular analysis of noninflamed colon tissue from a cohort of 21 adult patients with Crohn’s disease and from healthy controls.
This analysis revealed two distinct molecular phenotypes in colon tissue, which they labeled as “colon-like” and “ileum-like.” They found significant differences in cellular metabolism and immune pathways (Gut. 2016 Oct 14. doi: 10.1136/gutjnl-2016-312518).
In the colon-like subtype, the tissue samples – which were all taken from the colon – showed gene expression patterns that were typical of cells from the colon. However, in the ileum-like subtype, the gene expression patterns in these colon cells more closely resembled the gene expression of ileum cells.
They also saw significant differences in other molecular traits, such as chromatin accessibility, and pathways involved in lipid and xenobiotic metabolism.
“Furthermore, chromatin accessibility data suggest these subclasses exist due to stable molecular transformations of the genomic architecture in colon tissue cells, and not transient differences due to external cellular signaling,” researchers reported.
To rule out the possibility that these differences might be from treatment history, researchers conducted a similar analysis using samples from 201 treatment-naive pediatric patients with Crohn’s disease and found the same molecular and genetic patterns.
“Together, these data strongly suggest that the colon-like and ileum-like molecular signatures define two forms of [Crohn’s disease] present, regardless of tissue sampling location, patient age, or treatment status.”
The researchers also examined the impact the two subtypes might have on the clinical disease and therefore the therapeutic approach taken. In the cohort of treatment-naive pediatric patients, they found that patients with the more colon-like Crohn’s disease were more likely to have both colon and ileum involvement, have deep ulcers, and show more macroscopic inflammation than those with more ileum-like Crohn’s disease.
They also found that adults in the colon-like disease subclass were the only ones who were likely to have rectal disease or require a colectomy, although they noted that the sample size was small.
More patients with the ileum-like disease showed no inflammation and were also more likely to have involvement of the colon only.
“Although our sample size is small, these data suggest that molecular subtypes of [Crohn’s disease] can stratify patients into clinically distinct and relevant subgroups and may prospectively identify those more likely to require intensive medical therapy,” the authors wrote.
“As a first step, molecular stratifications of archived patient tissue and serum from major clinical trials could be performed in the context of response to biological and microbial therapies for [Crohn’s disease].”
The authors pointed out that they did not study samples from both intestinal regions in each patient but suggested that their findings should motivate future studies in larger cohorts using matched tissue samples from both the colon and ileum.
“These data emphasize the need to continue and expand these studies over time to incorporate the evolving clinical phenotype in both adult and pediatric patients, and the need to study both tissues in the same patient.”
The study was supported by the National Institute of Environmental Health Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Gastroenterological Association, Broad Medical Research Program, Crohn’s and Colitis Foundation of America, International Human Microbiome Consortium, UNC Team Translational Science Award, and Helmsley Charitable Trust SHARE 2, Project 3. No conflicts of interest were declared.
Researchers have identified two distinct molecular subclasses of Crohn’s disease, based on gene expression and phenotype, which could help with better tailoring of treatment.
Crohn’s disease is a chronic inflammatory disorder with a diverse clinical presentation and pattern of progression, yet the molecular and genetic factors underlying the disease are not well understood.
This complicates treatment – which currently relies on doctors’ subjective clinical classification – and makes it difficult to develop an evidence-based personalized approach.
Writing in the Oct. 14 online edition of Gut, Matthew Weiser, a graduate student in the genetics department at the University of North Carolina at Chapel Hill and his coauthors report the results of a genetic and molecular analysis of noninflamed colon tissue from a cohort of 21 adult patients with Crohn’s disease and from healthy controls.
This analysis revealed two distinct molecular phenotypes in colon tissue, which they labeled as “colon-like” and “ileum-like.” They found significant differences in cellular metabolism and immune pathways (Gut. 2016 Oct 14. doi: 10.1136/gutjnl-2016-312518).
In the colon-like subtype, the tissue samples – which were all taken from the colon – showed gene expression patterns that were typical of cells from the colon. However, in the ileum-like subtype, the gene expression patterns in these colon cells more closely resembled the gene expression of ileum cells.
They also saw significant differences in other molecular traits, such as chromatin accessibility, and pathways involved in lipid and xenobiotic metabolism.
“Furthermore, chromatin accessibility data suggest these subclasses exist due to stable molecular transformations of the genomic architecture in colon tissue cells, and not transient differences due to external cellular signaling,” researchers reported.
To rule out the possibility that these differences might be from treatment history, researchers conducted a similar analysis using samples from 201 treatment-naive pediatric patients with Crohn’s disease and found the same molecular and genetic patterns.
“Together, these data strongly suggest that the colon-like and ileum-like molecular signatures define two forms of [Crohn’s disease] present, regardless of tissue sampling location, patient age, or treatment status.”
The researchers also examined the impact the two subtypes might have on the clinical disease and therefore the therapeutic approach taken. In the cohort of treatment-naive pediatric patients, they found that patients with the more colon-like Crohn’s disease were more likely to have both colon and ileum involvement, have deep ulcers, and show more macroscopic inflammation than those with more ileum-like Crohn’s disease.
They also found that adults in the colon-like disease subclass were the only ones who were likely to have rectal disease or require a colectomy, although they noted that the sample size was small.
More patients with the ileum-like disease showed no inflammation and were also more likely to have involvement of the colon only.
“Although our sample size is small, these data suggest that molecular subtypes of [Crohn’s disease] can stratify patients into clinically distinct and relevant subgroups and may prospectively identify those more likely to require intensive medical therapy,” the authors wrote.
“As a first step, molecular stratifications of archived patient tissue and serum from major clinical trials could be performed in the context of response to biological and microbial therapies for [Crohn’s disease].”
The authors pointed out that they did not study samples from both intestinal regions in each patient but suggested that their findings should motivate future studies in larger cohorts using matched tissue samples from both the colon and ileum.
“These data emphasize the need to continue and expand these studies over time to incorporate the evolving clinical phenotype in both adult and pediatric patients, and the need to study both tissues in the same patient.”
The study was supported by the National Institute of Environmental Health Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Gastroenterological Association, Broad Medical Research Program, Crohn’s and Colitis Foundation of America, International Human Microbiome Consortium, UNC Team Translational Science Award, and Helmsley Charitable Trust SHARE 2, Project 3. No conflicts of interest were declared.
Researchers have identified two distinct molecular subclasses of Crohn’s disease, based on gene expression and phenotype, which could help with better tailoring of treatment.
Crohn’s disease is a chronic inflammatory disorder with a diverse clinical presentation and pattern of progression, yet the molecular and genetic factors underlying the disease are not well understood.
This complicates treatment – which currently relies on doctors’ subjective clinical classification – and makes it difficult to develop an evidence-based personalized approach.
Writing in the Oct. 14 online edition of Gut, Matthew Weiser, a graduate student in the genetics department at the University of North Carolina at Chapel Hill and his coauthors report the results of a genetic and molecular analysis of noninflamed colon tissue from a cohort of 21 adult patients with Crohn’s disease and from healthy controls.
This analysis revealed two distinct molecular phenotypes in colon tissue, which they labeled as “colon-like” and “ileum-like.” They found significant differences in cellular metabolism and immune pathways (Gut. 2016 Oct 14. doi: 10.1136/gutjnl-2016-312518).
In the colon-like subtype, the tissue samples – which were all taken from the colon – showed gene expression patterns that were typical of cells from the colon. However, in the ileum-like subtype, the gene expression patterns in these colon cells more closely resembled the gene expression of ileum cells.
They also saw significant differences in other molecular traits, such as chromatin accessibility, and pathways involved in lipid and xenobiotic metabolism.
“Furthermore, chromatin accessibility data suggest these subclasses exist due to stable molecular transformations of the genomic architecture in colon tissue cells, and not transient differences due to external cellular signaling,” researchers reported.
To rule out the possibility that these differences might be from treatment history, researchers conducted a similar analysis using samples from 201 treatment-naive pediatric patients with Crohn’s disease and found the same molecular and genetic patterns.
“Together, these data strongly suggest that the colon-like and ileum-like molecular signatures define two forms of [Crohn’s disease] present, regardless of tissue sampling location, patient age, or treatment status.”
The researchers also examined the impact the two subtypes might have on the clinical disease and therefore the therapeutic approach taken. In the cohort of treatment-naive pediatric patients, they found that patients with the more colon-like Crohn’s disease were more likely to have both colon and ileum involvement, have deep ulcers, and show more macroscopic inflammation than those with more ileum-like Crohn’s disease.
They also found that adults in the colon-like disease subclass were the only ones who were likely to have rectal disease or require a colectomy, although they noted that the sample size was small.
More patients with the ileum-like disease showed no inflammation and were also more likely to have involvement of the colon only.
“Although our sample size is small, these data suggest that molecular subtypes of [Crohn’s disease] can stratify patients into clinically distinct and relevant subgroups and may prospectively identify those more likely to require intensive medical therapy,” the authors wrote.
“As a first step, molecular stratifications of archived patient tissue and serum from major clinical trials could be performed in the context of response to biological and microbial therapies for [Crohn’s disease].”
The authors pointed out that they did not study samples from both intestinal regions in each patient but suggested that their findings should motivate future studies in larger cohorts using matched tissue samples from both the colon and ileum.
“These data emphasize the need to continue and expand these studies over time to incorporate the evolving clinical phenotype in both adult and pediatric patients, and the need to study both tissues in the same patient.”
The study was supported by the National Institute of Environmental Health Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Gastroenterological Association, Broad Medical Research Program, Crohn’s and Colitis Foundation of America, International Human Microbiome Consortium, UNC Team Translational Science Award, and Helmsley Charitable Trust SHARE 2, Project 3. No conflicts of interest were declared.
FROM GUT
Key clinical point: Researchers have identified two distinct molecular subclasses of Crohn’s disease, based on gene expression and phenotype, which could help with better tailoring of treatment.
Major finding: Molecular and genetic analysis has revealed a “colon-like” subtype and “ileum-like” subtype of Crohn’s disease, with distinct molecular profiles that correspond with clinical features.
Data source: Molecular and genetic analysis of samples from 21 adults with Crohn’s disease and 201 treatment-naive pediatric Crohn’s disease patients.
Disclosures: The study was supported by the National Institute of Environmental Health Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Gastroenterological Association, Broad Medical Research Program, Crohn’s and Colitis Foundation of America, International Human Microbiome Consortium, UNC Team Translational Science Award, and Helmsley Charitable Trust SHARE 2, Project 3. No conflicts of interest were declared.
IBD medical homes dramatically cut ED visits, hospitalizations
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: ED visits were cut by 51.9% and hospitalizations by 53.1% compared to the previous year.
Data source: Observational study.
Disclosures: Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
Lip Augmentation With Juvéderm Ultra XC



October 2016 Digital Edition
Click here to access the October 2016 Digital Edition.
Table of Contents
- Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
- FDA Black Box, VA Red Ink? A Successful Service-Connected Disability Claim for Chronic Neuropsychiatric Adverse Effects From Mefloquine
- Epinephrine-Induced Takotsubo Cardiomyopathy
- Tooth Decay Is the Most Prevalent Disease
- Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
- Solving the VA Physician Shortage Problem: The Right Thing to Do
- An Electronic Template to Improve Psychotropic Medication Review and Gradual Dose-Reduction Documentation
- Evaluation of a Dementia Resource Fair for Veterans, Caregivers, and Staff
- FDA Boxed Warnings

Click here to access the October 2016 Digital Edition.
Table of Contents
- Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
- FDA Black Box, VA Red Ink? A Successful Service-Connected Disability Claim for Chronic Neuropsychiatric Adverse Effects From Mefloquine
- Epinephrine-Induced Takotsubo Cardiomyopathy
- Tooth Decay Is the Most Prevalent Disease
- Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
- Solving the VA Physician Shortage Problem: The Right Thing to Do
- An Electronic Template to Improve Psychotropic Medication Review and Gradual Dose-Reduction Documentation
- Evaluation of a Dementia Resource Fair for Veterans, Caregivers, and Staff
- FDA Boxed Warnings

Click here to access the October 2016 Digital Edition.
Table of Contents
- Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
- FDA Black Box, VA Red Ink? A Successful Service-Connected Disability Claim for Chronic Neuropsychiatric Adverse Effects From Mefloquine
- Epinephrine-Induced Takotsubo Cardiomyopathy
- Tooth Decay Is the Most Prevalent Disease
- Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
- Solving the VA Physician Shortage Problem: The Right Thing to Do
- An Electronic Template to Improve Psychotropic Medication Review and Gradual Dose-Reduction Documentation
- Evaluation of a Dementia Resource Fair for Veterans, Caregivers, and Staff
- FDA Boxed Warnings