User login
Picking at a Problem
An 80-year-old woman bitterly complains of itching and discomfort on the left side of her face that began several weeks ago. Her primary care provider initially diagnosed contact dermatitis and prescribed a class 6 topical steroid cream. This helped a bit with the itching but had no effect on appearance.
When a friend suggested the itch might be the result of a bug bite, the patient went directly to the emergency department and was given a two-week taper of prednisone (40 mg/d for a week, then 20 mg/d for a week). This eased the redness, but the itching returned as soon as the course was finished. Finally, she was referred to dermatology.
The patient, who has been a widow for many years, was recently “forced out” of her home of more than 50 years and into an assisted living facility; her family seldom visits, so her friend accompanies her to your office today.
EXAMINATION
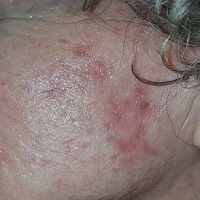
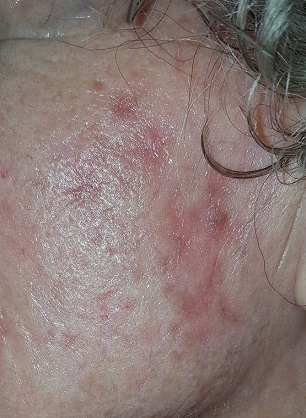
Extensive honey-colored crusting on an erythematous base is confined to the left side of the patient’s face. No nodes are palpable in the area. Her friend confirms that she has been picking at the skin.
What is the diagnosis?
DISCUSSION
This condition is impetigo—in this case “non-bullous” impetigo, a rash that almost always begins with a breach in normal skin integrity. This opens the skin to superficial invasion by staph and strep organisms, mostly emitting from the nasal passages. In this case, the patient had been picking at seborrheic keratosis (an extremely common phenomenon in someone her age) on her face.
The inclination to scratch and pick—and the inability to manage nasal secretions—make children the most likely candidates for impetigo. It is especially common in those with atopic dermatitis, who not only have poor barrier function (which manifests as eczema) but also constant nasal drainage from seasonal allergies.
The differential includes herpes simplex or zoster, eczema and contact dermatitis. But the location of the rash, the honey-colored crust on an erythematous base, and the history of skin breaches all point directly to impetigo. Lymph nodes are often palpable in the drainage area; their presence corroborates the diagnosis.
Luckily, this type of impetigo is relatively easy to treat: The patient was advised to wash the area with soap and water and apply mupirocin ointment three times a day. She was also prescribed cephalexin (500 mg tid for a week), which cleared the condition aside from a faint bit of postinflammatory pinkness.
TAKE-HOME LEARNING POINTS
• Impetigo is a superficial bacterial infection, usually on or near the face, caused by staph and strep organisms that seed the area from the nasal passages.
• These organisms generally require a break in the skin to gain entrance, often caused by picking or scratching.
• Honey-colored crust on an erythematous base, plus or minus enlarged local nodes, help to confirm the diagnosis.
• Symptoms of impetigo include itching and mild discomfort but not pain.
• Treatment can be as simple as cleaning with soap and water and applying topical mupirocin ointment. A short course of oral antibiotics may be needed to speed the clearance process.
An 80-year-old woman bitterly complains of itching and discomfort on the left side of her face that began several weeks ago. Her primary care provider initially diagnosed contact dermatitis and prescribed a class 6 topical steroid cream. This helped a bit with the itching but had no effect on appearance.
When a friend suggested the itch might be the result of a bug bite, the patient went directly to the emergency department and was given a two-week taper of prednisone (40 mg/d for a week, then 20 mg/d for a week). This eased the redness, but the itching returned as soon as the course was finished. Finally, she was referred to dermatology.
The patient, who has been a widow for many years, was recently “forced out” of her home of more than 50 years and into an assisted living facility; her family seldom visits, so her friend accompanies her to your office today.
EXAMINATION
Extensive honey-colored crusting on an erythematous base is confined to the left side of the patient’s face. No nodes are palpable in the area. Her friend confirms that she has been picking at the skin.

What is the diagnosis?
DISCUSSION
This condition is impetigo—in this case “non-bullous” impetigo, a rash that almost always begins with a breach in normal skin integrity. This opens the skin to superficial invasion by staph and strep organisms, mostly emitting from the nasal passages. In this case, the patient had been picking at seborrheic keratosis (an extremely common phenomenon in someone her age) on her face.
The inclination to scratch and pick—and the inability to manage nasal secretions—make children the most likely candidates for impetigo. It is especially common in those with atopic dermatitis, who not only have poor barrier function (which manifests as eczema) but also constant nasal drainage from seasonal allergies.
The differential includes herpes simplex or zoster, eczema and contact dermatitis. But the location of the rash, the honey-colored crust on an erythematous base, and the history of skin breaches all point directly to impetigo. Lymph nodes are often palpable in the drainage area; their presence corroborates the diagnosis.
Luckily, this type of impetigo is relatively easy to treat: The patient was advised to wash the area with soap and water and apply mupirocin ointment three times a day. She was also prescribed cephalexin (500 mg tid for a week), which cleared the condition aside from a faint bit of postinflammatory pinkness.
TAKE-HOME LEARNING POINTS
• Impetigo is a superficial bacterial infection, usually on or near the face, caused by staph and strep organisms that seed the area from the nasal passages.
• These organisms generally require a break in the skin to gain entrance, often caused by picking or scratching.
• Honey-colored crust on an erythematous base, plus or minus enlarged local nodes, help to confirm the diagnosis.
• Symptoms of impetigo include itching and mild discomfort but not pain.
• Treatment can be as simple as cleaning with soap and water and applying topical mupirocin ointment. A short course of oral antibiotics may be needed to speed the clearance process.
An 80-year-old woman bitterly complains of itching and discomfort on the left side of her face that began several weeks ago. Her primary care provider initially diagnosed contact dermatitis and prescribed a class 6 topical steroid cream. This helped a bit with the itching but had no effect on appearance.
When a friend suggested the itch might be the result of a bug bite, the patient went directly to the emergency department and was given a two-week taper of prednisone (40 mg/d for a week, then 20 mg/d for a week). This eased the redness, but the itching returned as soon as the course was finished. Finally, she was referred to dermatology.
The patient, who has been a widow for many years, was recently “forced out” of her home of more than 50 years and into an assisted living facility; her family seldom visits, so her friend accompanies her to your office today.
EXAMINATION
Extensive honey-colored crusting on an erythematous base is confined to the left side of the patient’s face. No nodes are palpable in the area. Her friend confirms that she has been picking at the skin.

What is the diagnosis?
DISCUSSION
This condition is impetigo—in this case “non-bullous” impetigo, a rash that almost always begins with a breach in normal skin integrity. This opens the skin to superficial invasion by staph and strep organisms, mostly emitting from the nasal passages. In this case, the patient had been picking at seborrheic keratosis (an extremely common phenomenon in someone her age) on her face.
The inclination to scratch and pick—and the inability to manage nasal secretions—make children the most likely candidates for impetigo. It is especially common in those with atopic dermatitis, who not only have poor barrier function (which manifests as eczema) but also constant nasal drainage from seasonal allergies.
The differential includes herpes simplex or zoster, eczema and contact dermatitis. But the location of the rash, the honey-colored crust on an erythematous base, and the history of skin breaches all point directly to impetigo. Lymph nodes are often palpable in the drainage area; their presence corroborates the diagnosis.
Luckily, this type of impetigo is relatively easy to treat: The patient was advised to wash the area with soap and water and apply mupirocin ointment three times a day. She was also prescribed cephalexin (500 mg tid for a week), which cleared the condition aside from a faint bit of postinflammatory pinkness.
TAKE-HOME LEARNING POINTS
• Impetigo is a superficial bacterial infection, usually on or near the face, caused by staph and strep organisms that seed the area from the nasal passages.
• These organisms generally require a break in the skin to gain entrance, often caused by picking or scratching.
• Honey-colored crust on an erythematous base, plus or minus enlarged local nodes, help to confirm the diagnosis.
• Symptoms of impetigo include itching and mild discomfort but not pain.
• Treatment can be as simple as cleaning with soap and water and applying topical mupirocin ointment. A short course of oral antibiotics may be needed to speed the clearance process.
Ozanimod has lasting effect on ulcerative colitis
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
AT ACG 2016
Key clinical point:
Major finding: Ozanimod maintains efficacy in ulcerative colitis out to 1 year, with 90% of patients having little or no evidence of active disease.
Data source: Open-label extension study following a phase II clinical trial.
Disclosures: Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
Experts: Fewer opioids, more treatment laws mean nothing without better access to care
WASHINGTON – Pressure on physicians to prescribe fewer opioids could have unintended consequences in the absence of adequate access to treatment, according to experts.
“There is mixed evidence that, when medication-assisted treatment is lacking, there are higher rates of transition from prescription opioids to heroin,” Gary Tsai, MD, said during a presentation at the American Psychiatric Association’s Institute on Psychiatric Services.
“As we constrict our prescribing, we want to make sure that there is ready access to these interventions, so that those who are already dependent on opioids can transition to something safer,” said Dr. Tsai, medical director and science officer of Substance Abuse Prevention and Control, a division of Los Angeles County’s public health department.
Medication-assisted treatment (MAT) uses methadone, buprenorphine, or naltrexone in combination with appropriate behavioral and other other psychosocial therapies to help achieve opioid abstinence. Despite MAT’s well-established superiority to either pharmacotherapy or psychosocial interventions alone, the use of MAT has, in some cases, declined. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), MAT was used in 35% of heroin-related treatment admissions in 2002, compared with 28% in 2010.
Reasons for MAT’s difficult path to acceptance are manifold, ranging from lack of certified facilities to administer the medications to misunderstanding about how the medications work, Dr. Tsai said.
A law passed earlier this year and the issuance of a final federal rule that increases the legal patient load that certified MAT providers can treat annually were designed to expand access to MAT. These, however, are only partial solutions, according to Margaret Chaplin, MD, a psychiatrist and program director of Community Mental Health Affiliates in New Britain, Conn.
“Can you imagine if endocrinologists were the only doctors who were certified to prescribe insulin and that each of them was only limited to prescribing to 100 patients?” Dr. Chaplin said in an interview. The final rule brought the number from 100 to 275 patients per year that a certified addiction specialist can treat. This might expand access to care, but “it sends a message that either the people with [addiction] don’t deserve treatment or that they don’t have a legitimate illness,” said Dr. Chaplin, who also was a presenter at the meeting.
Viewing people with opioid addiction through a lens of moral failing only compounds the nation’s addiction crisis, Dr. Chaplin believes. “Not to say that a person with a substance use disorder doesn’t have a responsibility to take care of their illness, [but] our [leaders] haven’t been well educated on the scientific evidence that addiction is a brain disease.”
It is true that, until the Comprehensive Addiction and Recovery Act was signed into law over the summer, nurse practitioners and physician assistants could have prescribed controlled substances such as acetaminophen/oxycontin but not the far less dangerous – and potentially life-saving – partial opioid agonist buprenorphine. Under the new law, those health care professions now have the same buprenorphine prescribing rights as physicians.
New legislation does not guarantee access to treatment, however. “Funding for MAT programs varies throughout the states, and the availability of these medications on formularies often determines how readily accessible MAT interventions are,” said Dr. Tsai, who emphasized the role of collaboration in ensuring the laws take hold.
“Addiction specialists comprise a minority of the work force. To scale MAT up, we need to engage other prescribers from other systems, including those in primary care and mental health,” Dr. Tai said. To wit, the three primary MAT facilities in Los Angeles County offer learning collaboratives with primary care clinicians who want to incorporate these services into their practice, even if they are not certified addiction specialists themselves. This helps increase referrals to the treatment facilities, he explained.
Overcoming resistance to offering MAT ultimately will depend on educating leaders about the costs of not doing so, Dr. Tsai and Dr. Chaplin said.
“Our system has been slow to adopt a disease model of addiction,” Dr. Chaplin said. “Buprenorphine and methadone are life-saving medical treatments that are regulated in ways that you don’t see for any other medical condition.”
SAMHSA currently is requesting comments through Nov. 1, 2016, on what should be required of MAT providers under the new law.
Neither Dr. Tsai nor Dr. Chaplin had any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – Pressure on physicians to prescribe fewer opioids could have unintended consequences in the absence of adequate access to treatment, according to experts.
“There is mixed evidence that, when medication-assisted treatment is lacking, there are higher rates of transition from prescription opioids to heroin,” Gary Tsai, MD, said during a presentation at the American Psychiatric Association’s Institute on Psychiatric Services.
“As we constrict our prescribing, we want to make sure that there is ready access to these interventions, so that those who are already dependent on opioids can transition to something safer,” said Dr. Tsai, medical director and science officer of Substance Abuse Prevention and Control, a division of Los Angeles County’s public health department.
Medication-assisted treatment (MAT) uses methadone, buprenorphine, or naltrexone in combination with appropriate behavioral and other other psychosocial therapies to help achieve opioid abstinence. Despite MAT’s well-established superiority to either pharmacotherapy or psychosocial interventions alone, the use of MAT has, in some cases, declined. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), MAT was used in 35% of heroin-related treatment admissions in 2002, compared with 28% in 2010.
Reasons for MAT’s difficult path to acceptance are manifold, ranging from lack of certified facilities to administer the medications to misunderstanding about how the medications work, Dr. Tsai said.
A law passed earlier this year and the issuance of a final federal rule that increases the legal patient load that certified MAT providers can treat annually were designed to expand access to MAT. These, however, are only partial solutions, according to Margaret Chaplin, MD, a psychiatrist and program director of Community Mental Health Affiliates in New Britain, Conn.
“Can you imagine if endocrinologists were the only doctors who were certified to prescribe insulin and that each of them was only limited to prescribing to 100 patients?” Dr. Chaplin said in an interview. The final rule brought the number from 100 to 275 patients per year that a certified addiction specialist can treat. This might expand access to care, but “it sends a message that either the people with [addiction] don’t deserve treatment or that they don’t have a legitimate illness,” said Dr. Chaplin, who also was a presenter at the meeting.
Viewing people with opioid addiction through a lens of moral failing only compounds the nation’s addiction crisis, Dr. Chaplin believes. “Not to say that a person with a substance use disorder doesn’t have a responsibility to take care of their illness, [but] our [leaders] haven’t been well educated on the scientific evidence that addiction is a brain disease.”
It is true that, until the Comprehensive Addiction and Recovery Act was signed into law over the summer, nurse practitioners and physician assistants could have prescribed controlled substances such as acetaminophen/oxycontin but not the far less dangerous – and potentially life-saving – partial opioid agonist buprenorphine. Under the new law, those health care professions now have the same buprenorphine prescribing rights as physicians.
New legislation does not guarantee access to treatment, however. “Funding for MAT programs varies throughout the states, and the availability of these medications on formularies often determines how readily accessible MAT interventions are,” said Dr. Tsai, who emphasized the role of collaboration in ensuring the laws take hold.
“Addiction specialists comprise a minority of the work force. To scale MAT up, we need to engage other prescribers from other systems, including those in primary care and mental health,” Dr. Tai said. To wit, the three primary MAT facilities in Los Angeles County offer learning collaboratives with primary care clinicians who want to incorporate these services into their practice, even if they are not certified addiction specialists themselves. This helps increase referrals to the treatment facilities, he explained.
Overcoming resistance to offering MAT ultimately will depend on educating leaders about the costs of not doing so, Dr. Tsai and Dr. Chaplin said.
“Our system has been slow to adopt a disease model of addiction,” Dr. Chaplin said. “Buprenorphine and methadone are life-saving medical treatments that are regulated in ways that you don’t see for any other medical condition.”
SAMHSA currently is requesting comments through Nov. 1, 2016, on what should be required of MAT providers under the new law.
Neither Dr. Tsai nor Dr. Chaplin had any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – Pressure on physicians to prescribe fewer opioids could have unintended consequences in the absence of adequate access to treatment, according to experts.
“There is mixed evidence that, when medication-assisted treatment is lacking, there are higher rates of transition from prescription opioids to heroin,” Gary Tsai, MD, said during a presentation at the American Psychiatric Association’s Institute on Psychiatric Services.
“As we constrict our prescribing, we want to make sure that there is ready access to these interventions, so that those who are already dependent on opioids can transition to something safer,” said Dr. Tsai, medical director and science officer of Substance Abuse Prevention and Control, a division of Los Angeles County’s public health department.
Medication-assisted treatment (MAT) uses methadone, buprenorphine, or naltrexone in combination with appropriate behavioral and other other psychosocial therapies to help achieve opioid abstinence. Despite MAT’s well-established superiority to either pharmacotherapy or psychosocial interventions alone, the use of MAT has, in some cases, declined. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), MAT was used in 35% of heroin-related treatment admissions in 2002, compared with 28% in 2010.
Reasons for MAT’s difficult path to acceptance are manifold, ranging from lack of certified facilities to administer the medications to misunderstanding about how the medications work, Dr. Tsai said.
A law passed earlier this year and the issuance of a final federal rule that increases the legal patient load that certified MAT providers can treat annually were designed to expand access to MAT. These, however, are only partial solutions, according to Margaret Chaplin, MD, a psychiatrist and program director of Community Mental Health Affiliates in New Britain, Conn.
“Can you imagine if endocrinologists were the only doctors who were certified to prescribe insulin and that each of them was only limited to prescribing to 100 patients?” Dr. Chaplin said in an interview. The final rule brought the number from 100 to 275 patients per year that a certified addiction specialist can treat. This might expand access to care, but “it sends a message that either the people with [addiction] don’t deserve treatment or that they don’t have a legitimate illness,” said Dr. Chaplin, who also was a presenter at the meeting.
Viewing people with opioid addiction through a lens of moral failing only compounds the nation’s addiction crisis, Dr. Chaplin believes. “Not to say that a person with a substance use disorder doesn’t have a responsibility to take care of their illness, [but] our [leaders] haven’t been well educated on the scientific evidence that addiction is a brain disease.”
It is true that, until the Comprehensive Addiction and Recovery Act was signed into law over the summer, nurse practitioners and physician assistants could have prescribed controlled substances such as acetaminophen/oxycontin but not the far less dangerous – and potentially life-saving – partial opioid agonist buprenorphine. Under the new law, those health care professions now have the same buprenorphine prescribing rights as physicians.
New legislation does not guarantee access to treatment, however. “Funding for MAT programs varies throughout the states, and the availability of these medications on formularies often determines how readily accessible MAT interventions are,” said Dr. Tsai, who emphasized the role of collaboration in ensuring the laws take hold.
“Addiction specialists comprise a minority of the work force. To scale MAT up, we need to engage other prescribers from other systems, including those in primary care and mental health,” Dr. Tai said. To wit, the three primary MAT facilities in Los Angeles County offer learning collaboratives with primary care clinicians who want to incorporate these services into their practice, even if they are not certified addiction specialists themselves. This helps increase referrals to the treatment facilities, he explained.
Overcoming resistance to offering MAT ultimately will depend on educating leaders about the costs of not doing so, Dr. Tsai and Dr. Chaplin said.
“Our system has been slow to adopt a disease model of addiction,” Dr. Chaplin said. “Buprenorphine and methadone are life-saving medical treatments that are regulated in ways that you don’t see for any other medical condition.”
SAMHSA currently is requesting comments through Nov. 1, 2016, on what should be required of MAT providers under the new law.
Neither Dr. Tsai nor Dr. Chaplin had any relevant disclosures.
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM INSTITUTE ON PSYCHIATRIC SERVICES
SAVR for radiation-induced aortic stenosis has high late mortality
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
ROME – Radiation-induced aortic stenosis is associated with markedly worse long-term outcome after surgical aortic valve replacement than when the operation is performed in patients without a history of radiotherapy, Milind Y. Desai, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, the Society of Thoracic Surgeons (STS) score isn’t good at risk-stratifying patients with radiation-induced aortic stenosis who are under consideration for surgical aortic valve replacement (SAVR).
Radiation-induced heart disease is a late complication of thoracic radiotherapy. It’s particularly common in patients who got radiation for lymphomas or breast cancer. It can affect any cardiac structure, including the myocardium, pericardium, valves, coronary arteries, and the conduction system.
Aortic stenosis is the most common valvular manifestation, present in roughly 80% of patients with radiation-induced heart disease. At the Cleveland Clinic, the average time from radiotherapy to development of radiation-induced aortic stenosis (RIAS) is about 20 years. The condition is characterized by thickening of the junction between the base of the anterior mitral leaflet and aortic root, known as the aortomitral curtain, Dr. Desai explained.
He presented a retrospective observational cohort study involving 172 patients who underwent SAVR for RIAS and an equal number of SAVR patients with no such history. The groups were matched by age, sex, aortic valve area, and type and timing of SAVR. Of note, the group with RIAS had a mean preoperative STS score of 11, and the control group averaged a similar score of 10.
The key finding: During a mean follow-up of 6 years, the all-cause mortality rate was a hefty 48% in patients with RIAS, compared with just 7% in matched controls. Only about 5% of deaths in the group with RIAS were from recurrent malignancy. The low figure is not surprising given the average 20-year lag between radiotherapy and development of radiation-induced heart disease.
“In our experience, most of these patients develop a recurrent pleural effusion and nasty cardiopulmonary issues that result in their death,” according to Dr. Desai.
In a multivariate Cox proportional hazards analysis, a history of chest radiation therapy was by far the strongest predictor of all-cause mortality, conferring an 8.5-fold increase in risk.
The only other statistically significant predictor of mortality during follow-up in multivariate analysis was a high STS score, with an associated weak albeit statistically significant 1.15-fold increased risk. A total of 30 of 78 (39%) RIAS patients with an STS score below 4 died during follow-up, compared with none of 91 controls.
Thirty-four of 92 (37%) RIAS patients under age 65 died during follow-up, whereas none of 83 control SAVR patients did so.
Having coronary artery bypass surgery or other cardiac surgery at the time of SAVR was not associated with significantly increased risk of mortality compared with solo SAVR.
In-hospital outcomes were consistently worse after SAVR in the RIAS group. Half of the RIAS patients experienced in-hospital atrial fibrillation and 29% developed persistent atrial fibrillation, compared with 30% and 24% of controls. About 22% of RIAS patients were readmitted within 3 months after surgery, as were only 8% of controls. In-hospital mortality occurred in 2% of SAVR patients with RIAS; none of the matched controls did.
Dr. Desai reported having no financial interests relative to this study.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: All-cause mortality occurred in 48% of 172 patients with radiation-induced severe aortic stenosis during a mean follow-up of 6 years after surgical aortic valve replacement, compared with just 7% of matched controls.
Data source: This was a retrospective observational study involving 172 closely matched pairs of surgical aortic valve replacement patients.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study.
Frailty stratifies pediatric liver disease severity
MONTREAL – A newly devised measurement of frailty in children effectively determined the severity of liver disease in pediatric patients and might serve as a useful, independent predictor of outcomes following liver transplantations in children and adolescents.
The adapted pediatric frailty assessment formula is a “very valid, feasible, and valuable tool” for assessing children with chronic liver disease, Eberhard Lurz, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. “Frailty captures an additional marker of ill health that is independent of the MELD-Na [Model for End-Stage Liver Disease–Na] and PELD,” [Pediatric End-Stage Liver Disease] said Dr. Lurz, a pediatric gastroenterologist at the Hospital for Sick Children in Toronto.
The idea of frailty assessment of children with liver disease sprang from a 2014 report that showed a five-item frailty index could predict mortality in adults with liver disease who were listed for liver transplantation and that this predictive power was independent of the patients’ MELD scores (Am J Transplant. 2014 Aug;14[8]:1870-9). That study used a five-item frailty index developed for adults (J Gerontol A Biol Sci Med Sci. 2001;56[3]:M146-57).
Dr. Lurz came up with a pediatric version of this frailty score using pediatric-oriented measures for each of the five items. To measure exhaustion he used the PedsQL (Pediatric Quality of Life Inventory) Multidimensional Fatigue Scale; for slowness he used a 6-minute walk test; for weakness he measured grip strength; for shrinkage he measured triceps skinfold thickness; and for diminished activity he used an age-appropriate physical activity questionnaire. He prespecified that a patient’s scores for each of these five measures are calculated by comparing their test results against age-specific norms. A patient with a value that fell more than one standard deviation below the normal range scores one point for the item and those with values more than two standard deviations below the normal range score two points. Hence the maximum score for all five items is 10.
Researchers at the collaborating centers completed full assessments for 71 of 85 pediatric patients with chronic liver disease in their clinics, and each full assessment took a median of 60 minutes. The patients ranged from 8-16 years old, with an average age of 13. The cohort included 36 patients with compensated chronic liver disease (CCLD) and 35 with end-stage liver disease (ESLD) who were listed for liver transplantation.
The median frailty score of the CCLD patients was 3 and the median score for those with ESLD was 5, a statistically significant difference that was largely driven by between-group differences in fatigue scores and physical activity scores. A receiver operating characteristic curve analysis by area under the curve showed that the frailty score accounted for 83% of the difference between patients with CCLD and ESLD, comparable to the distinguishing power of the MELD-Na score. Using a cutoff on the score of 6 or greater identified patients with ESLD with 47% sensitivity and 98% specificity, and this diagnostic capability was independent of a patient’s MELD-Na or PELD score.
The five elements that contribute to this pediatric frailty score could be the focus for targeted interventions to improve the outcomes of patients scheduled to undergo liver transplantation, Dr. Lurz said.
Dr. Lurz had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
MONTREAL – A newly devised measurement of frailty in children effectively determined the severity of liver disease in pediatric patients and might serve as a useful, independent predictor of outcomes following liver transplantations in children and adolescents.
The adapted pediatric frailty assessment formula is a “very valid, feasible, and valuable tool” for assessing children with chronic liver disease, Eberhard Lurz, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. “Frailty captures an additional marker of ill health that is independent of the MELD-Na [Model for End-Stage Liver Disease–Na] and PELD,” [Pediatric End-Stage Liver Disease] said Dr. Lurz, a pediatric gastroenterologist at the Hospital for Sick Children in Toronto.
The idea of frailty assessment of children with liver disease sprang from a 2014 report that showed a five-item frailty index could predict mortality in adults with liver disease who were listed for liver transplantation and that this predictive power was independent of the patients’ MELD scores (Am J Transplant. 2014 Aug;14[8]:1870-9). That study used a five-item frailty index developed for adults (J Gerontol A Biol Sci Med Sci. 2001;56[3]:M146-57).
Dr. Lurz came up with a pediatric version of this frailty score using pediatric-oriented measures for each of the five items. To measure exhaustion he used the PedsQL (Pediatric Quality of Life Inventory) Multidimensional Fatigue Scale; for slowness he used a 6-minute walk test; for weakness he measured grip strength; for shrinkage he measured triceps skinfold thickness; and for diminished activity he used an age-appropriate physical activity questionnaire. He prespecified that a patient’s scores for each of these five measures are calculated by comparing their test results against age-specific norms. A patient with a value that fell more than one standard deviation below the normal range scores one point for the item and those with values more than two standard deviations below the normal range score two points. Hence the maximum score for all five items is 10.
Researchers at the collaborating centers completed full assessments for 71 of 85 pediatric patients with chronic liver disease in their clinics, and each full assessment took a median of 60 minutes. The patients ranged from 8-16 years old, with an average age of 13. The cohort included 36 patients with compensated chronic liver disease (CCLD) and 35 with end-stage liver disease (ESLD) who were listed for liver transplantation.
The median frailty score of the CCLD patients was 3 and the median score for those with ESLD was 5, a statistically significant difference that was largely driven by between-group differences in fatigue scores and physical activity scores. A receiver operating characteristic curve analysis by area under the curve showed that the frailty score accounted for 83% of the difference between patients with CCLD and ESLD, comparable to the distinguishing power of the MELD-Na score. Using a cutoff on the score of 6 or greater identified patients with ESLD with 47% sensitivity and 98% specificity, and this diagnostic capability was independent of a patient’s MELD-Na or PELD score.
The five elements that contribute to this pediatric frailty score could be the focus for targeted interventions to improve the outcomes of patients scheduled to undergo liver transplantation, Dr. Lurz said.
Dr. Lurz had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
MONTREAL – A newly devised measurement of frailty in children effectively determined the severity of liver disease in pediatric patients and might serve as a useful, independent predictor of outcomes following liver transplantations in children and adolescents.
The adapted pediatric frailty assessment formula is a “very valid, feasible, and valuable tool” for assessing children with chronic liver disease, Eberhard Lurz, MD, said at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. “Frailty captures an additional marker of ill health that is independent of the MELD-Na [Model for End-Stage Liver Disease–Na] and PELD,” [Pediatric End-Stage Liver Disease] said Dr. Lurz, a pediatric gastroenterologist at the Hospital for Sick Children in Toronto.
The idea of frailty assessment of children with liver disease sprang from a 2014 report that showed a five-item frailty index could predict mortality in adults with liver disease who were listed for liver transplantation and that this predictive power was independent of the patients’ MELD scores (Am J Transplant. 2014 Aug;14[8]:1870-9). That study used a five-item frailty index developed for adults (J Gerontol A Biol Sci Med Sci. 2001;56[3]:M146-57).
Dr. Lurz came up with a pediatric version of this frailty score using pediatric-oriented measures for each of the five items. To measure exhaustion he used the PedsQL (Pediatric Quality of Life Inventory) Multidimensional Fatigue Scale; for slowness he used a 6-minute walk test; for weakness he measured grip strength; for shrinkage he measured triceps skinfold thickness; and for diminished activity he used an age-appropriate physical activity questionnaire. He prespecified that a patient’s scores for each of these five measures are calculated by comparing their test results against age-specific norms. A patient with a value that fell more than one standard deviation below the normal range scores one point for the item and those with values more than two standard deviations below the normal range score two points. Hence the maximum score for all five items is 10.
Researchers at the collaborating centers completed full assessments for 71 of 85 pediatric patients with chronic liver disease in their clinics, and each full assessment took a median of 60 minutes. The patients ranged from 8-16 years old, with an average age of 13. The cohort included 36 patients with compensated chronic liver disease (CCLD) and 35 with end-stage liver disease (ESLD) who were listed for liver transplantation.
The median frailty score of the CCLD patients was 3 and the median score for those with ESLD was 5, a statistically significant difference that was largely driven by between-group differences in fatigue scores and physical activity scores. A receiver operating characteristic curve analysis by area under the curve showed that the frailty score accounted for 83% of the difference between patients with CCLD and ESLD, comparable to the distinguishing power of the MELD-Na score. Using a cutoff on the score of 6 or greater identified patients with ESLD with 47% sensitivity and 98% specificity, and this diagnostic capability was independent of a patient’s MELD-Na or PELD score.
The five elements that contribute to this pediatric frailty score could be the focus for targeted interventions to improve the outcomes of patients scheduled to undergo liver transplantation, Dr. Lurz said.
Dr. Lurz had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT WCPGHAN 2016
Key clinical point:
Major finding: The pediatric frailty score identified patients with end-stage liver disease with sensitivity of 47% and specificity of 98%.
Data source: A series of 71 pediatric patients with liver disease compiled from 17 U.S. and Canadian centers.
Disclosures: Dr. Lurz had no relevant financial disclosures.
High resting heart rate may signal exacerbation risk in COPD patients
LOS ANGELES – Higher resting heart rate may predict future risk of exacerbation in patients with recent chronic obstructive pulmonary disease (COPD) exacerbation, results from a multicenter study suggest.
“Resting heart [rate] is often a readily available clinical data,” lead study author Ahmad Ismail, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “Its significance is often overlooked in daily clinical practice until tachycardia or bradycardia happens. In COPD patients, it has been shown that the resting heart rate can predict mortality. However, there is a lack of data showing its association with the rates of exacerbations, the major player in determining overall outcome in patients with COPD.”
The mean age of the study population was 67 years, and 77% of them had higher resting heart rates, defined as one that exceeded 80 beats per minute (BPM). The mean resting heart rate in the higher resting heart rate group was 92, compared with a mean of 70 BPM in the lower resting heart rate group. Dr. Ismail reported that at month 3, patients with higher resting heart rates had significantly higher proportion of exacerbations, compared with those who had a lower resting heart rates (54% vs. 27%; P = .013). The trend was followed through until month 9. There was also a statistically significant moderate strength linear correlation between resting heart rate and exacerbation frequency at 3, 6, and 9 months (r = 0.400; P less than .001: r = 0.440; P less than .001: and r = 0.416; P = .004, respectively). The mean exacerbation frequency was also significantly higher in the higher resting heart rate group at month 3 and month 6 (2.00 vs. 0.48; P less than .001: and 3.42 vs. 1.14; P = .004).
“Higher resting heart rate may predict future risk of exacerbation in patients with recent COPD exacerbation,” Dr. Ismail concluded. “Further study however is required to determine the effect of lowering resting heart rate on the future risk of exacerbation.” He acknowledged certain limitations of the study, including the fact that it excluded patients who were on beta-blockers or any rate-modifying drugs, and those with history of cardiac failure and ischemic heart disease, and that there was no baseline echocardiogram performed to ensure the absence of ischemic heart disease and other possible causes of the higher resting heart rates. “We also had slightly higher than expected dropouts giving a nonsignificant result at 12 months follow-up, though the trend follows the overall results of the study,” he said.
The study was funded by a grant from the Malaysian Thoracic Society. Dr. Ismail reported having no financial disclosures.
LOS ANGELES – Higher resting heart rate may predict future risk of exacerbation in patients with recent chronic obstructive pulmonary disease (COPD) exacerbation, results from a multicenter study suggest.
“Resting heart [rate] is often a readily available clinical data,” lead study author Ahmad Ismail, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “Its significance is often overlooked in daily clinical practice until tachycardia or bradycardia happens. In COPD patients, it has been shown that the resting heart rate can predict mortality. However, there is a lack of data showing its association with the rates of exacerbations, the major player in determining overall outcome in patients with COPD.”
The mean age of the study population was 67 years, and 77% of them had higher resting heart rates, defined as one that exceeded 80 beats per minute (BPM). The mean resting heart rate in the higher resting heart rate group was 92, compared with a mean of 70 BPM in the lower resting heart rate group. Dr. Ismail reported that at month 3, patients with higher resting heart rates had significantly higher proportion of exacerbations, compared with those who had a lower resting heart rates (54% vs. 27%; P = .013). The trend was followed through until month 9. There was also a statistically significant moderate strength linear correlation between resting heart rate and exacerbation frequency at 3, 6, and 9 months (r = 0.400; P less than .001: r = 0.440; P less than .001: and r = 0.416; P = .004, respectively). The mean exacerbation frequency was also significantly higher in the higher resting heart rate group at month 3 and month 6 (2.00 vs. 0.48; P less than .001: and 3.42 vs. 1.14; P = .004).
“Higher resting heart rate may predict future risk of exacerbation in patients with recent COPD exacerbation,” Dr. Ismail concluded. “Further study however is required to determine the effect of lowering resting heart rate on the future risk of exacerbation.” He acknowledged certain limitations of the study, including the fact that it excluded patients who were on beta-blockers or any rate-modifying drugs, and those with history of cardiac failure and ischemic heart disease, and that there was no baseline echocardiogram performed to ensure the absence of ischemic heart disease and other possible causes of the higher resting heart rates. “We also had slightly higher than expected dropouts giving a nonsignificant result at 12 months follow-up, though the trend follows the overall results of the study,” he said.
The study was funded by a grant from the Malaysian Thoracic Society. Dr. Ismail reported having no financial disclosures.
LOS ANGELES – Higher resting heart rate may predict future risk of exacerbation in patients with recent chronic obstructive pulmonary disease (COPD) exacerbation, results from a multicenter study suggest.
“Resting heart [rate] is often a readily available clinical data,” lead study author Ahmad Ismail, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “Its significance is often overlooked in daily clinical practice until tachycardia or bradycardia happens. In COPD patients, it has been shown that the resting heart rate can predict mortality. However, there is a lack of data showing its association with the rates of exacerbations, the major player in determining overall outcome in patients with COPD.”
The mean age of the study population was 67 years, and 77% of them had higher resting heart rates, defined as one that exceeded 80 beats per minute (BPM). The mean resting heart rate in the higher resting heart rate group was 92, compared with a mean of 70 BPM in the lower resting heart rate group. Dr. Ismail reported that at month 3, patients with higher resting heart rates had significantly higher proportion of exacerbations, compared with those who had a lower resting heart rates (54% vs. 27%; P = .013). The trend was followed through until month 9. There was also a statistically significant moderate strength linear correlation between resting heart rate and exacerbation frequency at 3, 6, and 9 months (r = 0.400; P less than .001: r = 0.440; P less than .001: and r = 0.416; P = .004, respectively). The mean exacerbation frequency was also significantly higher in the higher resting heart rate group at month 3 and month 6 (2.00 vs. 0.48; P less than .001: and 3.42 vs. 1.14; P = .004).
“Higher resting heart rate may predict future risk of exacerbation in patients with recent COPD exacerbation,” Dr. Ismail concluded. “Further study however is required to determine the effect of lowering resting heart rate on the future risk of exacerbation.” He acknowledged certain limitations of the study, including the fact that it excluded patients who were on beta-blockers or any rate-modifying drugs, and those with history of cardiac failure and ischemic heart disease, and that there was no baseline echocardiogram performed to ensure the absence of ischemic heart disease and other possible causes of the higher resting heart rates. “We also had slightly higher than expected dropouts giving a nonsignificant result at 12 months follow-up, though the trend follows the overall results of the study,” he said.
The study was funded by a grant from the Malaysian Thoracic Society. Dr. Ismail reported having no financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: At month 3, patients with higher resting heart rates had significantly higher proportion of exacerbations, compared with those who had a lower resting heart rates (54% vs. 27%; P = .013).
Data source: An evaluation of 147 COPD patients at 10 centers who were hospitalized for acute exacerbation of COPD between April 2012 and September 2015.
Disclosures: The study was funded by a grant from the Malaysian Thoracic Society. Dr. Ismail reported having no financial disclosures.
What to Know about CMS’s New Emergency Preparedness Requirements
Are you ready?
The Centers for Medicare & Medicaid Services (CMS) recently released new emergency preparedness requirements to ensure that providers and suppliers are duly prepared to adequately serve their community during disasters or emergencies. These requirements were stimulated by unexpected and catastrophic events, such as the September 11 terrorist attacks, the 2009 H1N1 pandemic, and innumerable natural disasters (tornados, floods, and hurricanes, to name a few). The CMS final rule issued “requirements that establish a comprehensive, consistent, flexible, and dynamic regulatory approach to emergency preparedness and response that incorporates the lessons learned from the past, combined with the proven best practices of the present.” In the rule, CMS outlines three essential guiding principles that any healthcare facility or supplier would need to preserve in the event of a disaster:
- Safeguard human resources.
- Maintain business continuity.
- Protect physical resources.
4 Ways to Be Prepared
What does having a comprehensive disaster preparedness program mean for hospitalists, regardless of site of practice? CMS recommends having four key elements for an adequate program:
1. Perform a risk assessment that focuses on the capacities and capabilities that are critical for a full spectrum of types of emergencies or disasters. This risk assessment should take into consideration the type and location of the facility as well as the disasters that are most likely to occur in its area. It should include at a minimum “care-related emergencies; equipment and power failures; interruptions in communications, including cyber attacks; loss of a portion or all of a facility; and interruptions in the normal supply of essentials, such as water and food.”
2. Develop and implement policies and procedures that support the emergency plan. Hospitalists should know about organizational policies and procedures that support the implementation of the emergency plan and how their team is factored into that plan.
3. Develop and maintain a communication plan that also complies with state and federal law. All the preparations in the world can be crippled without a robust and clear communication plan. The facility must have primary and backup mechanisms to contact providers, staff, and personnel in a timely fashion; this should include mechanisms to repeatedly update providers as the event evolves so that everyone knows what they are supposed to be doing and when.
4. Develop and maintain a training and testing program for all personnel. This includes onboarding and annual refreshers, including drills and exercises that test the plan and identify any gaps in performance. Hospitalists will undoubtedly be key members in developing, implementing, and receiving such critical training.
Expectations
There isn’t a single U.S. healthcare facility or provider that will not be affected by these provisions. An estimated 72,000 healthcare providers and suppliers (from nursing homes to dialysis facilities to home health agencies) will be expected to comply with these requirements within about a year.
In addition to hospitals, CMS also extended the requirements to many types of facilities and suppliers so that such providers can more likely stay open and provide care during disasters and emergencies, or at least can resume operations as soon as possible, to provide the very best ongoing care to the affected community. In most of these scenarios, the need for complex and varied care goes up, not down, further exacerbating gaps in basic care if ambulatory facilities and home care providers are unavailable.
CMS does acknowledge that these requirements will be more difficult to execute in facilities that previously did not have requirements or in smaller facilities with more limited resources. It also acknowledges that the cost of implementation could reach up to $279 million, which some argue is actually an underestimation. Despite these challenges, it is hard to argue against basic disaster preparedness for any healthcare facility or provider as a standard and positive business practice. While most acute-care hospitals have long had disaster preparedness plans and programs, gaps in these programs have become readily apparent during natural disasters such as Hurricane Katrina and Superstorm Sandy. CMS also stresses the need for a community approach to planning and implementation and that there is no reason during planning, or during an actual event, that facilities should operate in isolation but rather train and respond together as a community.
As hospitalists, regardless of site of practice, we should all be involved in at least understanding, if not developing and implementing, these basic requirements in our facilities. It is without a doubt that hospitalists will be a core group of physicians who will be called upon to serve within or outside healthcare facilities in the event of a disaster or emergency. In fact, in most recent disasters, we already have. It is better, of course, to be prepared and ready to serve than unprepared and regretful.
Reference
- The Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Emergency Preparedness Requirements for Medicare and Medicaid Participating Providers and Suppliers. Federal Register website. Accessed October 6, 2016.

Are you ready?
The Centers for Medicare & Medicaid Services (CMS) recently released new emergency preparedness requirements to ensure that providers and suppliers are duly prepared to adequately serve their community during disasters or emergencies. These requirements were stimulated by unexpected and catastrophic events, such as the September 11 terrorist attacks, the 2009 H1N1 pandemic, and innumerable natural disasters (tornados, floods, and hurricanes, to name a few). The CMS final rule issued “requirements that establish a comprehensive, consistent, flexible, and dynamic regulatory approach to emergency preparedness and response that incorporates the lessons learned from the past, combined with the proven best practices of the present.” In the rule, CMS outlines three essential guiding principles that any healthcare facility or supplier would need to preserve in the event of a disaster:
- Safeguard human resources.
- Maintain business continuity.
- Protect physical resources.
4 Ways to Be Prepared
What does having a comprehensive disaster preparedness program mean for hospitalists, regardless of site of practice? CMS recommends having four key elements for an adequate program:
1. Perform a risk assessment that focuses on the capacities and capabilities that are critical for a full spectrum of types of emergencies or disasters. This risk assessment should take into consideration the type and location of the facility as well as the disasters that are most likely to occur in its area. It should include at a minimum “care-related emergencies; equipment and power failures; interruptions in communications, including cyber attacks; loss of a portion or all of a facility; and interruptions in the normal supply of essentials, such as water and food.”
2. Develop and implement policies and procedures that support the emergency plan. Hospitalists should know about organizational policies and procedures that support the implementation of the emergency plan and how their team is factored into that plan.
3. Develop and maintain a communication plan that also complies with state and federal law. All the preparations in the world can be crippled without a robust and clear communication plan. The facility must have primary and backup mechanisms to contact providers, staff, and personnel in a timely fashion; this should include mechanisms to repeatedly update providers as the event evolves so that everyone knows what they are supposed to be doing and when.
4. Develop and maintain a training and testing program for all personnel. This includes onboarding and annual refreshers, including drills and exercises that test the plan and identify any gaps in performance. Hospitalists will undoubtedly be key members in developing, implementing, and receiving such critical training.
Expectations
There isn’t a single U.S. healthcare facility or provider that will not be affected by these provisions. An estimated 72,000 healthcare providers and suppliers (from nursing homes to dialysis facilities to home health agencies) will be expected to comply with these requirements within about a year.
In addition to hospitals, CMS also extended the requirements to many types of facilities and suppliers so that such providers can more likely stay open and provide care during disasters and emergencies, or at least can resume operations as soon as possible, to provide the very best ongoing care to the affected community. In most of these scenarios, the need for complex and varied care goes up, not down, further exacerbating gaps in basic care if ambulatory facilities and home care providers are unavailable.
CMS does acknowledge that these requirements will be more difficult to execute in facilities that previously did not have requirements or in smaller facilities with more limited resources. It also acknowledges that the cost of implementation could reach up to $279 million, which some argue is actually an underestimation. Despite these challenges, it is hard to argue against basic disaster preparedness for any healthcare facility or provider as a standard and positive business practice. While most acute-care hospitals have long had disaster preparedness plans and programs, gaps in these programs have become readily apparent during natural disasters such as Hurricane Katrina and Superstorm Sandy. CMS also stresses the need for a community approach to planning and implementation and that there is no reason during planning, or during an actual event, that facilities should operate in isolation but rather train and respond together as a community.
As hospitalists, regardless of site of practice, we should all be involved in at least understanding, if not developing and implementing, these basic requirements in our facilities. It is without a doubt that hospitalists will be a core group of physicians who will be called upon to serve within or outside healthcare facilities in the event of a disaster or emergency. In fact, in most recent disasters, we already have. It is better, of course, to be prepared and ready to serve than unprepared and regretful.
Reference
- The Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Emergency Preparedness Requirements for Medicare and Medicaid Participating Providers and Suppliers. Federal Register website. Accessed October 6, 2016.

Are you ready?
The Centers for Medicare & Medicaid Services (CMS) recently released new emergency preparedness requirements to ensure that providers and suppliers are duly prepared to adequately serve their community during disasters or emergencies. These requirements were stimulated by unexpected and catastrophic events, such as the September 11 terrorist attacks, the 2009 H1N1 pandemic, and innumerable natural disasters (tornados, floods, and hurricanes, to name a few). The CMS final rule issued “requirements that establish a comprehensive, consistent, flexible, and dynamic regulatory approach to emergency preparedness and response that incorporates the lessons learned from the past, combined with the proven best practices of the present.” In the rule, CMS outlines three essential guiding principles that any healthcare facility or supplier would need to preserve in the event of a disaster:
- Safeguard human resources.
- Maintain business continuity.
- Protect physical resources.
4 Ways to Be Prepared
What does having a comprehensive disaster preparedness program mean for hospitalists, regardless of site of practice? CMS recommends having four key elements for an adequate program:
1. Perform a risk assessment that focuses on the capacities and capabilities that are critical for a full spectrum of types of emergencies or disasters. This risk assessment should take into consideration the type and location of the facility as well as the disasters that are most likely to occur in its area. It should include at a minimum “care-related emergencies; equipment and power failures; interruptions in communications, including cyber attacks; loss of a portion or all of a facility; and interruptions in the normal supply of essentials, such as water and food.”
2. Develop and implement policies and procedures that support the emergency plan. Hospitalists should know about organizational policies and procedures that support the implementation of the emergency plan and how their team is factored into that plan.
3. Develop and maintain a communication plan that also complies with state and federal law. All the preparations in the world can be crippled without a robust and clear communication plan. The facility must have primary and backup mechanisms to contact providers, staff, and personnel in a timely fashion; this should include mechanisms to repeatedly update providers as the event evolves so that everyone knows what they are supposed to be doing and when.
4. Develop and maintain a training and testing program for all personnel. This includes onboarding and annual refreshers, including drills and exercises that test the plan and identify any gaps in performance. Hospitalists will undoubtedly be key members in developing, implementing, and receiving such critical training.
Expectations
There isn’t a single U.S. healthcare facility or provider that will not be affected by these provisions. An estimated 72,000 healthcare providers and suppliers (from nursing homes to dialysis facilities to home health agencies) will be expected to comply with these requirements within about a year.
In addition to hospitals, CMS also extended the requirements to many types of facilities and suppliers so that such providers can more likely stay open and provide care during disasters and emergencies, or at least can resume operations as soon as possible, to provide the very best ongoing care to the affected community. In most of these scenarios, the need for complex and varied care goes up, not down, further exacerbating gaps in basic care if ambulatory facilities and home care providers are unavailable.
CMS does acknowledge that these requirements will be more difficult to execute in facilities that previously did not have requirements or in smaller facilities with more limited resources. It also acknowledges that the cost of implementation could reach up to $279 million, which some argue is actually an underestimation. Despite these challenges, it is hard to argue against basic disaster preparedness for any healthcare facility or provider as a standard and positive business practice. While most acute-care hospitals have long had disaster preparedness plans and programs, gaps in these programs have become readily apparent during natural disasters such as Hurricane Katrina and Superstorm Sandy. CMS also stresses the need for a community approach to planning and implementation and that there is no reason during planning, or during an actual event, that facilities should operate in isolation but rather train and respond together as a community.
As hospitalists, regardless of site of practice, we should all be involved in at least understanding, if not developing and implementing, these basic requirements in our facilities. It is without a doubt that hospitalists will be a core group of physicians who will be called upon to serve within or outside healthcare facilities in the event of a disaster or emergency. In fact, in most recent disasters, we already have. It is better, of course, to be prepared and ready to serve than unprepared and regretful.
Reference
- The Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Emergency Preparedness Requirements for Medicare and Medicaid Participating Providers and Suppliers. Federal Register website. Accessed October 6, 2016.

EBV-CTLs accepted into EMA’s PRIME program

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()
Burden of cancer varies by cancer type, race

Photo by Rhoda Baer
A new study suggests that leukemia and non-Hodgkin lymphoma (NHL) are among the top 10 cancers with the greatest burden (most years of healthy life lost) in the US.
The research also showed that the burden of different cancer types varied between patients belonging to different racial/ethnic groups.
For example, the contribution of leukemia to the overall cancer burden was twice as high in Hispanics as it was in non-Hispanic blacks. The same was true for NHL.
Joannie Lortet-Tieulent, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in the American Journal of Preventive Medicine.
The researchers calculated the burden of cancer in the US in 2011 for 24 cancer types. They calculated burden using disability-adjusted life years (DALYs), which combine cancer incidence, mortality, survival, and quality of life into a summary indicator.
The results suggested the burden of cancer in 2011 was over 9.8 million DALYs, which was equally shared between men and women—4.9 million DALYs for each sex.
DALYs lost to cancer were mostly related to premature death due to cancer (91%). The remaining 9% were related to impaired quality of life because of the disease or its treatment, or other disease-related issues.
Top 10 contributors
The researchers calculated the proportion of DALYs lost for each of the cancer types. And they found that lung cancer was the largest contributor to the loss of healthy years, accounting for 24% of the burden (2.4 million DALYs).
The second biggest contributor to the loss of healthy years was breast cancer (10%), followed by colorectal cancer (9%), pancreatic cancer (6%), prostate cancer (5%), leukemia (4%), liver cancer (4%), brain cancer (3%), NHL (3%), and ovarian cancer (3%).
The researchers also calculated the proportion of DALYs lost from the top 10 cancer types according to race/ethnicity.
They found the contribution of leukemia to the loss of healthy years was greatest for Hispanics (6%), followed by non-Hispanic Asians (5%), non-Hispanic whites (4%), and non-Hispanic blacks (3%).
The contribution of NHL to the loss of healthy years was greatest for Hispanics (4%), followed by non-Hispanic Asians/non-Hispanic whites (3% for both), and non-Hispanic blacks (2%).
DALYs by race/ethnicity
The researchers found that, overall, the cancer burden was highest in non-Hispanic blacks, followed by non-Hispanic whites, Hispanics, and non-Hispanic Asians. However, this pattern was not consistent across the different cancer types.
Age-standardized DALYs lost (per 100,000 individuals) were as follows:
All cancers combined
3588 for non-Hispanic blacks
2898 for non-Hispanic whites
1978 for Hispanics
1798 for non-Hispanic Asians.
Leukemia
115 for non-Hispanic blacks and non-Hispanic whites
98 for Hispanics
82 for non-Hispanic Asians.
NHL
93 for non-Hispanic whites
86 for non-Hispanic blacks
78 for Hispanics
60 for non-Hispanic Asians.
Hodgkin lymphoma
11 for non-Hispanic blacks
10 for non-Hispanic whites and Hispanics
3 for non-Hispanic Asians.
Myeloma
93 for non-Hispanic blacks
43 for non-Hispanic whites
42 for Hispanics
26 for non-Hispanic Asians.
The researchers noted that, despite these differences, the cancer burden in all races/ethnicities was driven by years of life lost. They said this highlights the need to prevent deaths by improving prevention, early detection, and treatment of cancers. ![]()

Photo by Rhoda Baer
A new study suggests that leukemia and non-Hodgkin lymphoma (NHL) are among the top 10 cancers with the greatest burden (most years of healthy life lost) in the US.
The research also showed that the burden of different cancer types varied between patients belonging to different racial/ethnic groups.
For example, the contribution of leukemia to the overall cancer burden was twice as high in Hispanics as it was in non-Hispanic blacks. The same was true for NHL.
Joannie Lortet-Tieulent, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in the American Journal of Preventive Medicine.
The researchers calculated the burden of cancer in the US in 2011 for 24 cancer types. They calculated burden using disability-adjusted life years (DALYs), which combine cancer incidence, mortality, survival, and quality of life into a summary indicator.
The results suggested the burden of cancer in 2011 was over 9.8 million DALYs, which was equally shared between men and women—4.9 million DALYs for each sex.
DALYs lost to cancer were mostly related to premature death due to cancer (91%). The remaining 9% were related to impaired quality of life because of the disease or its treatment, or other disease-related issues.
Top 10 contributors
The researchers calculated the proportion of DALYs lost for each of the cancer types. And they found that lung cancer was the largest contributor to the loss of healthy years, accounting for 24% of the burden (2.4 million DALYs).
The second biggest contributor to the loss of healthy years was breast cancer (10%), followed by colorectal cancer (9%), pancreatic cancer (6%), prostate cancer (5%), leukemia (4%), liver cancer (4%), brain cancer (3%), NHL (3%), and ovarian cancer (3%).
The researchers also calculated the proportion of DALYs lost from the top 10 cancer types according to race/ethnicity.
They found the contribution of leukemia to the loss of healthy years was greatest for Hispanics (6%), followed by non-Hispanic Asians (5%), non-Hispanic whites (4%), and non-Hispanic blacks (3%).
The contribution of NHL to the loss of healthy years was greatest for Hispanics (4%), followed by non-Hispanic Asians/non-Hispanic whites (3% for both), and non-Hispanic blacks (2%).
DALYs by race/ethnicity
The researchers found that, overall, the cancer burden was highest in non-Hispanic blacks, followed by non-Hispanic whites, Hispanics, and non-Hispanic Asians. However, this pattern was not consistent across the different cancer types.
Age-standardized DALYs lost (per 100,000 individuals) were as follows:
All cancers combined
3588 for non-Hispanic blacks
2898 for non-Hispanic whites
1978 for Hispanics
1798 for non-Hispanic Asians.
Leukemia
115 for non-Hispanic blacks and non-Hispanic whites
98 for Hispanics
82 for non-Hispanic Asians.
NHL
93 for non-Hispanic whites
86 for non-Hispanic blacks
78 for Hispanics
60 for non-Hispanic Asians.
Hodgkin lymphoma
11 for non-Hispanic blacks
10 for non-Hispanic whites and Hispanics
3 for non-Hispanic Asians.
Myeloma
93 for non-Hispanic blacks
43 for non-Hispanic whites
42 for Hispanics
26 for non-Hispanic Asians.
The researchers noted that, despite these differences, the cancer burden in all races/ethnicities was driven by years of life lost. They said this highlights the need to prevent deaths by improving prevention, early detection, and treatment of cancers. ![]()

Photo by Rhoda Baer
A new study suggests that leukemia and non-Hodgkin lymphoma (NHL) are among the top 10 cancers with the greatest burden (most years of healthy life lost) in the US.
The research also showed that the burden of different cancer types varied between patients belonging to different racial/ethnic groups.
For example, the contribution of leukemia to the overall cancer burden was twice as high in Hispanics as it was in non-Hispanic blacks. The same was true for NHL.
Joannie Lortet-Tieulent, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in the American Journal of Preventive Medicine.
The researchers calculated the burden of cancer in the US in 2011 for 24 cancer types. They calculated burden using disability-adjusted life years (DALYs), which combine cancer incidence, mortality, survival, and quality of life into a summary indicator.
The results suggested the burden of cancer in 2011 was over 9.8 million DALYs, which was equally shared between men and women—4.9 million DALYs for each sex.
DALYs lost to cancer were mostly related to premature death due to cancer (91%). The remaining 9% were related to impaired quality of life because of the disease or its treatment, or other disease-related issues.
Top 10 contributors
The researchers calculated the proportion of DALYs lost for each of the cancer types. And they found that lung cancer was the largest contributor to the loss of healthy years, accounting for 24% of the burden (2.4 million DALYs).
The second biggest contributor to the loss of healthy years was breast cancer (10%), followed by colorectal cancer (9%), pancreatic cancer (6%), prostate cancer (5%), leukemia (4%), liver cancer (4%), brain cancer (3%), NHL (3%), and ovarian cancer (3%).
The researchers also calculated the proportion of DALYs lost from the top 10 cancer types according to race/ethnicity.
They found the contribution of leukemia to the loss of healthy years was greatest for Hispanics (6%), followed by non-Hispanic Asians (5%), non-Hispanic whites (4%), and non-Hispanic blacks (3%).
The contribution of NHL to the loss of healthy years was greatest for Hispanics (4%), followed by non-Hispanic Asians/non-Hispanic whites (3% for both), and non-Hispanic blacks (2%).
DALYs by race/ethnicity
The researchers found that, overall, the cancer burden was highest in non-Hispanic blacks, followed by non-Hispanic whites, Hispanics, and non-Hispanic Asians. However, this pattern was not consistent across the different cancer types.
Age-standardized DALYs lost (per 100,000 individuals) were as follows:
All cancers combined
3588 for non-Hispanic blacks
2898 for non-Hispanic whites
1978 for Hispanics
1798 for non-Hispanic Asians.
Leukemia
115 for non-Hispanic blacks and non-Hispanic whites
98 for Hispanics
82 for non-Hispanic Asians.
NHL
93 for non-Hispanic whites
86 for non-Hispanic blacks
78 for Hispanics
60 for non-Hispanic Asians.
Hodgkin lymphoma
11 for non-Hispanic blacks
10 for non-Hispanic whites and Hispanics
3 for non-Hispanic Asians.
Myeloma
93 for non-Hispanic blacks
43 for non-Hispanic whites
42 for Hispanics
26 for non-Hispanic Asians.
The researchers noted that, despite these differences, the cancer burden in all races/ethnicities was driven by years of life lost. They said this highlights the need to prevent deaths by improving prevention, early detection, and treatment of cancers. ![]()
Single-cell findings could inform CLL treatment

Researchers say they have found a better way to examine individual cells, and this tool provided insight that could inform the treatment of leukemia.
The team used a technique called microarrayed single-cell sequencing (MASC-seq) to examine individual cells in samples from patients with chronic lymphocytic leukemia (CLL).
This revealed a number of CLL subclones within each sample that exhibited different gene expression.
“With this new, highly cost-effective technology, we can now get a whole new view of this complexity within the blood cancer sample,” said study author Joakim Lundeberg, PhD, of KTH Royal Institute of Technology in Stockholm, Sweden.
“Molecular resolution of single cells is likely to become a more widely used therapy option.”
Dr Lundeberg and his colleagues described this work in Nature Communications.
The researchers said current methods of single-cell analysis don’t allow for the combination of cell imaging and transcriptome profiling, exhibit low-throughput by analyzing a single cell at a time, or require expensive droplet instrumentation for high-throughput analysis.
MASC-seq, on the other hand, can image cells to provide information on morphology and profile the expression of thousands of single cells per day at a cost of $0.13 USD per cell.
Dr Lundeberg and his colleagues tested MASC-seq by analyzing samples from 3 patients with different subtypes of CLL.
The team found clear differences in the average gene expression levels of cells from the different CLL subtypes, but they also found subtle differences between single cells within each of the subtypes.
The researchers therefore concluded that MASC-seq has the potential to accelerate the study of subtle clonal dynamics and help provide insight into the development of CLL and other diseases. ![]()

Researchers say they have found a better way to examine individual cells, and this tool provided insight that could inform the treatment of leukemia.
The team used a technique called microarrayed single-cell sequencing (MASC-seq) to examine individual cells in samples from patients with chronic lymphocytic leukemia (CLL).
This revealed a number of CLL subclones within each sample that exhibited different gene expression.
“With this new, highly cost-effective technology, we can now get a whole new view of this complexity within the blood cancer sample,” said study author Joakim Lundeberg, PhD, of KTH Royal Institute of Technology in Stockholm, Sweden.
“Molecular resolution of single cells is likely to become a more widely used therapy option.”
Dr Lundeberg and his colleagues described this work in Nature Communications.
The researchers said current methods of single-cell analysis don’t allow for the combination of cell imaging and transcriptome profiling, exhibit low-throughput by analyzing a single cell at a time, or require expensive droplet instrumentation for high-throughput analysis.
MASC-seq, on the other hand, can image cells to provide information on morphology and profile the expression of thousands of single cells per day at a cost of $0.13 USD per cell.
Dr Lundeberg and his colleagues tested MASC-seq by analyzing samples from 3 patients with different subtypes of CLL.
The team found clear differences in the average gene expression levels of cells from the different CLL subtypes, but they also found subtle differences between single cells within each of the subtypes.
The researchers therefore concluded that MASC-seq has the potential to accelerate the study of subtle clonal dynamics and help provide insight into the development of CLL and other diseases. ![]()

Researchers say they have found a better way to examine individual cells, and this tool provided insight that could inform the treatment of leukemia.
The team used a technique called microarrayed single-cell sequencing (MASC-seq) to examine individual cells in samples from patients with chronic lymphocytic leukemia (CLL).
This revealed a number of CLL subclones within each sample that exhibited different gene expression.
“With this new, highly cost-effective technology, we can now get a whole new view of this complexity within the blood cancer sample,” said study author Joakim Lundeberg, PhD, of KTH Royal Institute of Technology in Stockholm, Sweden.
“Molecular resolution of single cells is likely to become a more widely used therapy option.”
Dr Lundeberg and his colleagues described this work in Nature Communications.
The researchers said current methods of single-cell analysis don’t allow for the combination of cell imaging and transcriptome profiling, exhibit low-throughput by analyzing a single cell at a time, or require expensive droplet instrumentation for high-throughput analysis.
MASC-seq, on the other hand, can image cells to provide information on morphology and profile the expression of thousands of single cells per day at a cost of $0.13 USD per cell.
Dr Lundeberg and his colleagues tested MASC-seq by analyzing samples from 3 patients with different subtypes of CLL.
The team found clear differences in the average gene expression levels of cells from the different CLL subtypes, but they also found subtle differences between single cells within each of the subtypes.
The researchers therefore concluded that MASC-seq has the potential to accelerate the study of subtle clonal dynamics and help provide insight into the development of CLL and other diseases. ![]()