User login
How Finding Mentorship Made Me Love Being a Hospitalist Again
I almost quit my job during my third year as a hospitalist. When I began my first hospitalist job out of residency, I was going to be “just a doctor” forever. After all, being at the bedside, holding my patients’ hands, making them feel better one by one was the reason I went into medicine.
Fast-forward three years. I was now a mom and still “just a doctor,” still holding my patients’ hands. Yet, somehow, it was just not enough. I pondered for a year, at times pessimistically, about my hospitalist future: the endless overnight shifts, the weekends away from family, the “spent” feeling after seven days on service. I needed to do something else. I, and many other hospitalist colleagues, went through this phase. I call this “the three-year itch.”
Fast-forward some more, I am a little better at knowing why the three-year itch occurred. For a lifelong hospitalist, it is a major milestone. It is the moment when you realize you are in love with the field of hospital medicine, want to continue for a long time, but also have this scary revelation that you cannot sustain the current status. I suppose this milestone is a natural occurrence, hardwired in our hospitalist-innovator, hospitalist-writer, hospitalist-mom, hospitalist–IT guy, hospitalist–palliative care physician, hospitalist–soon-to-be chief medical officer mind. While hospitalist groups attempt to improve job satisfaction and sustainability by hiring more nocturnists, increasing compensation, designing flexible schedules for moms, etc., I argue that, for many of us, mentoring is paramount in maintaining job satisfaction and sustainability.
Mentoring Essential
Early-career hospitalist mentoring is essential during the first three years of practice as it ensures a smooth transition and assimilation into hospital medicine. While I was surrounded by accomplished hospitalists early in my career, I never realized how essential it was for me to establish a connection with one of them until I was “attacked” by the itch. What exactly does the three-year itch involve? A Hinami et al study plotted job fit against years in current practice. An inflection point at two years of practice became apparent. These first two years, called the “assimilation period,” are when “rapid learning and attrition took place.” Perhaps some of the observed phenomenon are to be expected and unavoidable. However, providing mentorship resources during this vulnerable period would potentially decrease attrition.
I did not quit my job, but I knew I needed to find direction for myself. I spent countless hours on emails, meetings, and, yes, moping around about my future. I wished so often back then that I had a mentor to guide me. My lack of mentorship was not unique. In a survey of 222 pediatric hospitalists, only 44% said they have “adequate mentorship in their careers.”
For more than a year, I was asking the wrong question: What makes a career in hospital medicine satisfying? The Society of Hospital Medicine Career Satisfaction Task Force paper delineated 13 factors, including optimal workload, substantial pay, control over personal time, and collegiality with other physicians, that contribute to job satisfaction for hospitalists. While there are common trends, factors that affect job satisfaction are highly variable across practice models. How do you reconcile the weight of at least 13 factors that contribute to your happiness at work? Having a mentor to brainstorm ideas about job satisfaction for me would have focused my energy productively early on and, more important, could have led to more career satisfaction.
Finding a Mentor
Finding a mentor takes a lot hard work. It takes boldness, creativity, perseverance, and a bit of luck. My quest to find a mentor started at the hospital’s cafeteria with senior hospitalists. It then led me to a few meetings in the C-suite and the chiefs’ offices. I asked MDs, nurses, and quality officers the same question: “How did you get to where you are?” I emailed everyone and met with many. I suppose I was bold (and some may say ambitious), but for me, it was out of necessity. I was pleasantly surprised at the time generously given to me. The willingness to listen was bestowed even by random strangers whom I had never met. I remember very well the day I decided to email the most “famous” hospitalists in the Boston area. I heard back from all except one. I ended up having coffee on a crisp winter morning at a famous hospitalist’s house in the Boston suburbs. I almost trucked in the textbook she had written for an autograph! My path also led me to an hour-and-a-half conversation in a light-filled office in downtown Boston. Leaving at 6:30 p.m., I remember being giddy. I did not find a mentor that very specific day, but I found direction and purpose, which are what I had been looking for.
Sometimes you just have to do it yourself—build your own mentorship program from scratch. I did it at my own institution. There is a paucity of literature on this subject matter. This problem intensifies manyfold for community hospitals like mine. I was never sure of the right way to start a program. Do I start by identifying senior faculty mentors for the group, providing a list of available mentors for interested hospitalists to choose from, or creating a peer mentor network? I was certain though that doing something, even if not as well from the onset, was an improvement. This is where luck matters: I am lucky to be practicing among the most intelligent, ambitious, like-minded colleagues. We have different priorities, and each of us is blazing a separate career path. Yet I sense that we have one thing in common: We are energized and want productive careers in hospital medicine.
Starting a new program also requires leadership support. I fortunately have had unrelenting support at my hospital. Support from leadership comes in various forms: funds set aside for administrative support, assistance in networking to identify potential mentors, expertise (such as in writing and publishing), feedback on the proposed program structure. At the end of the day though, sometimes you just need to start.
While experienced mentors are desperately needed for academic hospitalist groups, a bigger need for mentors exists at community hospitals like mine compared to academic hospitals. Community hospital programs are typically smaller and more recently established, and hence, the pool of experienced and senior hospitalists typically is limited. In tertiary-care settings, mentors are needed to ensure scholarly productivity and promotion, while mentors are needed in community hospitals to ensure career satisfaction and job sustainability. Two years ago, I conducted a professional development survey of my colleagues. Of the 20 hospitalists (70% response rate) who responded, 19 (95%) answered yes to the question, “Are you professionally satisfied with your current hospitalist job?” This tracks well with the 92% of pediatric hospitalists who reported that they are “pleased with their work.” Yet burnout rate was reported to be 29.9% in 20119 and 52.3% more recently.
Why is there such a discrepancy? I think one of the clues lies in the fact that 85% of my colleagues are thinking of pursuing an interest in addition to practicing clinical hospital medicine in the next 10 years. I want to be clear that my fellow hospitalists and I are not looking to leave clinical medicine. We love it. Most of us envision our professional lives in clinical medicine. Yet we need to fulfill our “diastoles.” We also believe in the intertwined nature of a hospitalist’s life and that of a quality officer, a palliative care physician, a billing and compliance officer, etc. We know that as hospitalists, we are well-positioned to improve the care of our patients even when we are not at the bedside. As community hospital hospitalists, we are the grass-roots hospitalists with tremendous potential to impact the care of patients and the future of hospital medicine. We, as much as academic hospitalists, need a mentoring hand for our professional development.
I am “itching” now, six years after finishing residency. There are many days where the “What now?” phrase echoes in my head. Yet with the mentors who I have found, I know that I will have ready listeners when the restless voice gets loud. What troubles me is that many of the 44,000 hospitalists nationwide are suffering through the restlessness without mentors to guide them. The current call to bolster mentorship resources at academic centers, while important, is not enough. Attention, discussion, research, and definitely resources should be allocated to the development of mentorship programs for community hospitals like mine. Of course, I am interested in academic promotions, grants, and FTE support, but the journey of finding mentorship has been most significant in that it led me back my core value: I still want to be “just a doctor” forever. I just know a little more about what type of doctor I want to be. Mentorship is vital to our professional development, job satisfaction, and sustainability as community hospitalists.
References
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB. Person-job fit: an exploratory cross-sectional analysis of hospitalists. J Hosp Med. 2013;8(2):96-101.
- Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-148.
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410.
- Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318.
- Tietjen P, Griner PF. Mentoring of physicians at a community-based health system: preliminary findings. J Hosp Med. 2013;8(11):642-643.
- Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14.
- Johnson KS, Hastings SN, Purser JL, Whitson HE. The Junior Faculty Laboratory: an innovative model of peer mentoring. Acad Med. 2011;86(12):1577-1582.
- Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27.
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and outpatient general internists. J Hosp Med. 2014;9(3):176-181.
- Arora V, Fang MC, Kripalani S, Amin AN. Preparing for "diastole": advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368-377.
I almost quit my job during my third year as a hospitalist. When I began my first hospitalist job out of residency, I was going to be “just a doctor” forever. After all, being at the bedside, holding my patients’ hands, making them feel better one by one was the reason I went into medicine.
Fast-forward three years. I was now a mom and still “just a doctor,” still holding my patients’ hands. Yet, somehow, it was just not enough. I pondered for a year, at times pessimistically, about my hospitalist future: the endless overnight shifts, the weekends away from family, the “spent” feeling after seven days on service. I needed to do something else. I, and many other hospitalist colleagues, went through this phase. I call this “the three-year itch.”
Fast-forward some more, I am a little better at knowing why the three-year itch occurred. For a lifelong hospitalist, it is a major milestone. It is the moment when you realize you are in love with the field of hospital medicine, want to continue for a long time, but also have this scary revelation that you cannot sustain the current status. I suppose this milestone is a natural occurrence, hardwired in our hospitalist-innovator, hospitalist-writer, hospitalist-mom, hospitalist–IT guy, hospitalist–palliative care physician, hospitalist–soon-to-be chief medical officer mind. While hospitalist groups attempt to improve job satisfaction and sustainability by hiring more nocturnists, increasing compensation, designing flexible schedules for moms, etc., I argue that, for many of us, mentoring is paramount in maintaining job satisfaction and sustainability.
Mentoring Essential
Early-career hospitalist mentoring is essential during the first three years of practice as it ensures a smooth transition and assimilation into hospital medicine. While I was surrounded by accomplished hospitalists early in my career, I never realized how essential it was for me to establish a connection with one of them until I was “attacked” by the itch. What exactly does the three-year itch involve? A Hinami et al study plotted job fit against years in current practice. An inflection point at two years of practice became apparent. These first two years, called the “assimilation period,” are when “rapid learning and attrition took place.” Perhaps some of the observed phenomenon are to be expected and unavoidable. However, providing mentorship resources during this vulnerable period would potentially decrease attrition.
I did not quit my job, but I knew I needed to find direction for myself. I spent countless hours on emails, meetings, and, yes, moping around about my future. I wished so often back then that I had a mentor to guide me. My lack of mentorship was not unique. In a survey of 222 pediatric hospitalists, only 44% said they have “adequate mentorship in their careers.”
For more than a year, I was asking the wrong question: What makes a career in hospital medicine satisfying? The Society of Hospital Medicine Career Satisfaction Task Force paper delineated 13 factors, including optimal workload, substantial pay, control over personal time, and collegiality with other physicians, that contribute to job satisfaction for hospitalists. While there are common trends, factors that affect job satisfaction are highly variable across practice models. How do you reconcile the weight of at least 13 factors that contribute to your happiness at work? Having a mentor to brainstorm ideas about job satisfaction for me would have focused my energy productively early on and, more important, could have led to more career satisfaction.
Finding a Mentor
Finding a mentor takes a lot hard work. It takes boldness, creativity, perseverance, and a bit of luck. My quest to find a mentor started at the hospital’s cafeteria with senior hospitalists. It then led me to a few meetings in the C-suite and the chiefs’ offices. I asked MDs, nurses, and quality officers the same question: “How did you get to where you are?” I emailed everyone and met with many. I suppose I was bold (and some may say ambitious), but for me, it was out of necessity. I was pleasantly surprised at the time generously given to me. The willingness to listen was bestowed even by random strangers whom I had never met. I remember very well the day I decided to email the most “famous” hospitalists in the Boston area. I heard back from all except one. I ended up having coffee on a crisp winter morning at a famous hospitalist’s house in the Boston suburbs. I almost trucked in the textbook she had written for an autograph! My path also led me to an hour-and-a-half conversation in a light-filled office in downtown Boston. Leaving at 6:30 p.m., I remember being giddy. I did not find a mentor that very specific day, but I found direction and purpose, which are what I had been looking for.
Sometimes you just have to do it yourself—build your own mentorship program from scratch. I did it at my own institution. There is a paucity of literature on this subject matter. This problem intensifies manyfold for community hospitals like mine. I was never sure of the right way to start a program. Do I start by identifying senior faculty mentors for the group, providing a list of available mentors for interested hospitalists to choose from, or creating a peer mentor network? I was certain though that doing something, even if not as well from the onset, was an improvement. This is where luck matters: I am lucky to be practicing among the most intelligent, ambitious, like-minded colleagues. We have different priorities, and each of us is blazing a separate career path. Yet I sense that we have one thing in common: We are energized and want productive careers in hospital medicine.
Starting a new program also requires leadership support. I fortunately have had unrelenting support at my hospital. Support from leadership comes in various forms: funds set aside for administrative support, assistance in networking to identify potential mentors, expertise (such as in writing and publishing), feedback on the proposed program structure. At the end of the day though, sometimes you just need to start.
While experienced mentors are desperately needed for academic hospitalist groups, a bigger need for mentors exists at community hospitals like mine compared to academic hospitals. Community hospital programs are typically smaller and more recently established, and hence, the pool of experienced and senior hospitalists typically is limited. In tertiary-care settings, mentors are needed to ensure scholarly productivity and promotion, while mentors are needed in community hospitals to ensure career satisfaction and job sustainability. Two years ago, I conducted a professional development survey of my colleagues. Of the 20 hospitalists (70% response rate) who responded, 19 (95%) answered yes to the question, “Are you professionally satisfied with your current hospitalist job?” This tracks well with the 92% of pediatric hospitalists who reported that they are “pleased with their work.” Yet burnout rate was reported to be 29.9% in 20119 and 52.3% more recently.
Why is there such a discrepancy? I think one of the clues lies in the fact that 85% of my colleagues are thinking of pursuing an interest in addition to practicing clinical hospital medicine in the next 10 years. I want to be clear that my fellow hospitalists and I are not looking to leave clinical medicine. We love it. Most of us envision our professional lives in clinical medicine. Yet we need to fulfill our “diastoles.” We also believe in the intertwined nature of a hospitalist’s life and that of a quality officer, a palliative care physician, a billing and compliance officer, etc. We know that as hospitalists, we are well-positioned to improve the care of our patients even when we are not at the bedside. As community hospital hospitalists, we are the grass-roots hospitalists with tremendous potential to impact the care of patients and the future of hospital medicine. We, as much as academic hospitalists, need a mentoring hand for our professional development.
I am “itching” now, six years after finishing residency. There are many days where the “What now?” phrase echoes in my head. Yet with the mentors who I have found, I know that I will have ready listeners when the restless voice gets loud. What troubles me is that many of the 44,000 hospitalists nationwide are suffering through the restlessness without mentors to guide them. The current call to bolster mentorship resources at academic centers, while important, is not enough. Attention, discussion, research, and definitely resources should be allocated to the development of mentorship programs for community hospitals like mine. Of course, I am interested in academic promotions, grants, and FTE support, but the journey of finding mentorship has been most significant in that it led me back my core value: I still want to be “just a doctor” forever. I just know a little more about what type of doctor I want to be. Mentorship is vital to our professional development, job satisfaction, and sustainability as community hospitalists.
References
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB. Person-job fit: an exploratory cross-sectional analysis of hospitalists. J Hosp Med. 2013;8(2):96-101.
- Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-148.
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410.
- Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318.
- Tietjen P, Griner PF. Mentoring of physicians at a community-based health system: preliminary findings. J Hosp Med. 2013;8(11):642-643.
- Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14.
- Johnson KS, Hastings SN, Purser JL, Whitson HE. The Junior Faculty Laboratory: an innovative model of peer mentoring. Acad Med. 2011;86(12):1577-1582.
- Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27.
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and outpatient general internists. J Hosp Med. 2014;9(3):176-181.
- Arora V, Fang MC, Kripalani S, Amin AN. Preparing for "diastole": advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368-377.
I almost quit my job during my third year as a hospitalist. When I began my first hospitalist job out of residency, I was going to be “just a doctor” forever. After all, being at the bedside, holding my patients’ hands, making them feel better one by one was the reason I went into medicine.
Fast-forward three years. I was now a mom and still “just a doctor,” still holding my patients’ hands. Yet, somehow, it was just not enough. I pondered for a year, at times pessimistically, about my hospitalist future: the endless overnight shifts, the weekends away from family, the “spent” feeling after seven days on service. I needed to do something else. I, and many other hospitalist colleagues, went through this phase. I call this “the three-year itch.”
Fast-forward some more, I am a little better at knowing why the three-year itch occurred. For a lifelong hospitalist, it is a major milestone. It is the moment when you realize you are in love with the field of hospital medicine, want to continue for a long time, but also have this scary revelation that you cannot sustain the current status. I suppose this milestone is a natural occurrence, hardwired in our hospitalist-innovator, hospitalist-writer, hospitalist-mom, hospitalist–IT guy, hospitalist–palliative care physician, hospitalist–soon-to-be chief medical officer mind. While hospitalist groups attempt to improve job satisfaction and sustainability by hiring more nocturnists, increasing compensation, designing flexible schedules for moms, etc., I argue that, for many of us, mentoring is paramount in maintaining job satisfaction and sustainability.
Mentoring Essential
Early-career hospitalist mentoring is essential during the first three years of practice as it ensures a smooth transition and assimilation into hospital medicine. While I was surrounded by accomplished hospitalists early in my career, I never realized how essential it was for me to establish a connection with one of them until I was “attacked” by the itch. What exactly does the three-year itch involve? A Hinami et al study plotted job fit against years in current practice. An inflection point at two years of practice became apparent. These first two years, called the “assimilation period,” are when “rapid learning and attrition took place.” Perhaps some of the observed phenomenon are to be expected and unavoidable. However, providing mentorship resources during this vulnerable period would potentially decrease attrition.
I did not quit my job, but I knew I needed to find direction for myself. I spent countless hours on emails, meetings, and, yes, moping around about my future. I wished so often back then that I had a mentor to guide me. My lack of mentorship was not unique. In a survey of 222 pediatric hospitalists, only 44% said they have “adequate mentorship in their careers.”
For more than a year, I was asking the wrong question: What makes a career in hospital medicine satisfying? The Society of Hospital Medicine Career Satisfaction Task Force paper delineated 13 factors, including optimal workload, substantial pay, control over personal time, and collegiality with other physicians, that contribute to job satisfaction for hospitalists. While there are common trends, factors that affect job satisfaction are highly variable across practice models. How do you reconcile the weight of at least 13 factors that contribute to your happiness at work? Having a mentor to brainstorm ideas about job satisfaction for me would have focused my energy productively early on and, more important, could have led to more career satisfaction.
Finding a Mentor
Finding a mentor takes a lot hard work. It takes boldness, creativity, perseverance, and a bit of luck. My quest to find a mentor started at the hospital’s cafeteria with senior hospitalists. It then led me to a few meetings in the C-suite and the chiefs’ offices. I asked MDs, nurses, and quality officers the same question: “How did you get to where you are?” I emailed everyone and met with many. I suppose I was bold (and some may say ambitious), but for me, it was out of necessity. I was pleasantly surprised at the time generously given to me. The willingness to listen was bestowed even by random strangers whom I had never met. I remember very well the day I decided to email the most “famous” hospitalists in the Boston area. I heard back from all except one. I ended up having coffee on a crisp winter morning at a famous hospitalist’s house in the Boston suburbs. I almost trucked in the textbook she had written for an autograph! My path also led me to an hour-and-a-half conversation in a light-filled office in downtown Boston. Leaving at 6:30 p.m., I remember being giddy. I did not find a mentor that very specific day, but I found direction and purpose, which are what I had been looking for.
Sometimes you just have to do it yourself—build your own mentorship program from scratch. I did it at my own institution. There is a paucity of literature on this subject matter. This problem intensifies manyfold for community hospitals like mine. I was never sure of the right way to start a program. Do I start by identifying senior faculty mentors for the group, providing a list of available mentors for interested hospitalists to choose from, or creating a peer mentor network? I was certain though that doing something, even if not as well from the onset, was an improvement. This is where luck matters: I am lucky to be practicing among the most intelligent, ambitious, like-minded colleagues. We have different priorities, and each of us is blazing a separate career path. Yet I sense that we have one thing in common: We are energized and want productive careers in hospital medicine.
Starting a new program also requires leadership support. I fortunately have had unrelenting support at my hospital. Support from leadership comes in various forms: funds set aside for administrative support, assistance in networking to identify potential mentors, expertise (such as in writing and publishing), feedback on the proposed program structure. At the end of the day though, sometimes you just need to start.
While experienced mentors are desperately needed for academic hospitalist groups, a bigger need for mentors exists at community hospitals like mine compared to academic hospitals. Community hospital programs are typically smaller and more recently established, and hence, the pool of experienced and senior hospitalists typically is limited. In tertiary-care settings, mentors are needed to ensure scholarly productivity and promotion, while mentors are needed in community hospitals to ensure career satisfaction and job sustainability. Two years ago, I conducted a professional development survey of my colleagues. Of the 20 hospitalists (70% response rate) who responded, 19 (95%) answered yes to the question, “Are you professionally satisfied with your current hospitalist job?” This tracks well with the 92% of pediatric hospitalists who reported that they are “pleased with their work.” Yet burnout rate was reported to be 29.9% in 20119 and 52.3% more recently.
Why is there such a discrepancy? I think one of the clues lies in the fact that 85% of my colleagues are thinking of pursuing an interest in addition to practicing clinical hospital medicine in the next 10 years. I want to be clear that my fellow hospitalists and I are not looking to leave clinical medicine. We love it. Most of us envision our professional lives in clinical medicine. Yet we need to fulfill our “diastoles.” We also believe in the intertwined nature of a hospitalist’s life and that of a quality officer, a palliative care physician, a billing and compliance officer, etc. We know that as hospitalists, we are well-positioned to improve the care of our patients even when we are not at the bedside. As community hospital hospitalists, we are the grass-roots hospitalists with tremendous potential to impact the care of patients and the future of hospital medicine. We, as much as academic hospitalists, need a mentoring hand for our professional development.
I am “itching” now, six years after finishing residency. There are many days where the “What now?” phrase echoes in my head. Yet with the mentors who I have found, I know that I will have ready listeners when the restless voice gets loud. What troubles me is that many of the 44,000 hospitalists nationwide are suffering through the restlessness without mentors to guide them. The current call to bolster mentorship resources at academic centers, while important, is not enough. Attention, discussion, research, and definitely resources should be allocated to the development of mentorship programs for community hospitals like mine. Of course, I am interested in academic promotions, grants, and FTE support, but the journey of finding mentorship has been most significant in that it led me back my core value: I still want to be “just a doctor” forever. I just know a little more about what type of doctor I want to be. Mentorship is vital to our professional development, job satisfaction, and sustainability as community hospitalists.
References
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB. Person-job fit: an exploratory cross-sectional analysis of hospitalists. J Hosp Med. 2013;8(2):96-101.
- Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-148.
- Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410.
- Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318.
- Tietjen P, Griner PF. Mentoring of physicians at a community-based health system: preliminary findings. J Hosp Med. 2013;8(11):642-643.
- Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14.
- Johnson KS, Hastings SN, Purser JL, Whitson HE. The Junior Faculty Laboratory: an innovative model of peer mentoring. Acad Med. 2011;86(12):1577-1582.
- Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27.
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and outpatient general internists. J Hosp Med. 2014;9(3):176-181.
- Arora V, Fang MC, Kripalani S, Amin AN. Preparing for "diastole": advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368-377.
Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
A 46-year-old white male presented to a civilian general practitioner with an acute bilateral erythemetous, nonurticarial morbilliform rash. The patient initially presented with these symptoms (7 years prior) to a military field medical clinic, and the first presentation was seen by a nurse practitioner. The rash was evident on the lateral aspects of the thorax and on the medial portions of both arms and upper thighs. There was no evidence of involvement of hands or feet. Outside of being quite prominent, the patient reported the rash to be asymptomatic.
The patient also had concomitant symptoms of a typical upper respiratory tract infection (URTI) with fever of 100.3° F. The physical examination was unremarkable except for the pronounced rash as described and URTI-associated nasal congestion/postnasal drainage. Examination of oral mucosa of the head, ears, eyes, nose, and throat also was negative for any evidence of eruption. The patient was in excellent physical condition, took no routine medications, and had no documented underlying medical conditions. His family and social history seemed noncontributory; he reported frequent domestic airline travel associated with reserve duty as well as with his civilian job. Appropriate immunizations were all current, and regularly scheduled physical examinations had been documented in the medical record.
The patient’s rashes associated with URTI began 7 years prior at age 39 after a weeklong (fall season) operational readiness inspection (ORI), which included extended periods wearing ground crew ensemble (GCE) equipment. Protective clothing worn during that ORI included the heavily charcoal-laden over-garment, butyl rubber gloves and boots, and the then current-issue gas mask. The rashes had never appeared before age 39, and the patient reported no previous medical problems after wearing GCE equipment during 16 years of service preceding that initial presentation.
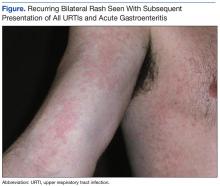
The patient’s rashes typically presented with the similar pattern and intensity at 24 to 36 hours after onset of URTI symptoms, were self-limiting, and resolved in an additional 48 to 72 hours (Figure). The rash was present with each subsequent URTI and occurred on average, about 2 times each year. Similar rashes also appeared twice during the preceding 7 years during episodes of nausea/vomiting/diarrhea consistent with acute gastroenteritis (AGE). No other household members (infant to aged 40 years) showed any similar rash during episodic URTI or AGE during the same period. However, about 2 years after the initial URTI/rash presentation, the patient’s then toddler-aged son was diagnosed with self-limiting unilateral laterothoracic exanthem (ULTE), which completely resolved in 2 to 3 weeks and never reappeared.
Discussion
The author postulated that the patient most likely experienced routine acute episodes of Picornaviridae infection. The overarching diagnosis was presumptive, as biopsy and/or serologies were not obtained on the reservist. The Picornavirus family includes genera such as Rhinovirus and Enterovirus, both significant contributors to URTI (Enterovirus for AGE). Typically, about 50% of URTIs are caused by Rhinovirus (approaches 100% in some seasonal outbreaks) with the remainder of common URTIs being caused by Enterovirus, Coronavirus, Adenovirus, Reovirus, and Parainfluenza virus.1 Unfortunately, rapid office-based or field medicine diagnostic assays to consistently confirm or differentiate these common URTI viral etiologies (excepting influenza A/B) are simply not economically feasible at this time nor are any standardized laboratory tests recommended for self-limiting URTIs in immunocompetent adults. There are multiplex polymerase chain reaction assays available for many viral URTI pathogens, but cost and the need for laboratory as well as certified medical technologist support may often preclude routine use at the point of care.
Perhaps the more intriguing aspect of this case is the consistent recurrence of the same rash presentation in a middle-aged immunocompetent adult male with every subsequent episode of typical URTI or AGE over a period of 7 years, irrespective of seasonal timing, contact history, or geographic location. There is well-documented evidence of URTI associated with Adenovirus and Coronavirus seen in otherwise healthy adults living in close quarters military environments. Particularly, Adenovirus serotypes 3, 4, 7, and 21 have been associated with respiratory disease in military troop populations.2
There are also numerous viral exanthems shown in young children with probable links to Epstein-Barr virus (EBV), Human herpesvirus (HHV) 6, and parvovirus B19.3 The patient’s then toddler-aged son experiencing ULTE subsequent to the father’s presentation raise the question of a possible link to viruses other than those associated with typical URTI/AGE. Literature review indicated ULTE may be linked to EBV, parvovirus B19, Adenovirus, Parainfluenza virus, or HHV 6 and 7.4
Adenovirus and Parainfluenza virus are causative agents for a portion of URTIs as well. However, the likelihood of all of the patient’s subsequent URTIs being caused exclusively by Adenovirus would be exceedingly low. Infections with atypical bacteria, such as mycoplasma, also are frequently associated with rash, fever, and myalgia but are more commonly linked to lower RTI.
All the above etiologies are typically self-limiting (except mycoplasma infections) and usually resolve without specific treatment. This broad differential tends to “muddy the water” when trying to tie a definitive diagnosis to what was a seemingly routine URTI in a healthy adult.
The patient’s status as a member of the reserve complicated obtaining a definitive diagnosis of the recurring rash. Even if a link between the recurring rash and earlier duty could be established, access to an acute-care appointment at a military treatment facility (in nonduty status) was/is not authorized. A timely civilian acute-care appointment for routine URTI with short-lived rash in an adult patient is difficult to book, especially if the rash appears on a weekend or holiday. Likewise, insurance reimbursement for skin biopsy or numerous serologic tests (investigating possible viral etiology) is frequently not covered for such conditions, as even a positive serology likely would not alter the current standard of supportive care.
Evaluation of any patient presenting during or immediately after field exercises or operational deployment must be conducted in full consideration of such conditions. A thorough history relating to geographic location, immunization status, season, environmental or chemical contacts, animal/insect exposure, living conditions, other ill unit personnel, sanitation/hygiene and food/water quality is of paramount importance. Likewise, there may be value in at least having a unit briefing/follow-up with a medical provider 2 to 6 months after deployment/field training activities. A general follow-up such as this may help find any emerging trends or common concerns pertaining to unit members’ health.
Reservists present a unique health maintenance challenge as they may complete a duty tour and drop out of military health system visibility for weeks to months. The inherent design of some reserve component programs makes even the discovery of a case such as this difficult at best. A structured follow-up (be that a group briefing or even a survey-type questionnaire) becomes highly useful for monitoring emerging health trends in reservists.
Conclusion
This patient’s initial field presentation at age 39 was clinically explained and documented in the official medical record as routine URTI. The diagnosis was based on clinical picture, occurrence during fall season, and close quarters/field living conditions. A definitive diagnosis of the recurring rash was never documented in the patient’s military medical records.
Differing somewhat from a typical case report, the purpose of this presentation was to offer “field medicine” points and systematic considerations when faced with the presentation of a seemingly common ailment, not to provide readers with a definitive diagnosis. These considerations along with the need for follow-up health maintenance strategies are especially highlighted by the unique situations presented by reservists. In addition, this case demonstrates the validity of the often forgotten history and physical question of “Has this ever happened before?” These are at least issues that should be considered before signing the disposition as “URTI, OTC meds PRN, quarters for 24 hours, then return to duty.”
1. Galagas ME, Mylonakis E. Gorbach’s 5-Minute Infectious Diseases Consult. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
2. Levinson W, ed. DNA nonenveloped viruses. In: Review of Medical Microbiology and Immunology. 12th ed. New York, NY: McGraw-Hill; 2012:291-292.
3. Lam JM. Characterizing viral exanthems. Pediatr Health. 2010;4(6):623-635.
4. Scheinfeld N. Unilateral laterothoracis exanthema with evidence of Epstein Barr virus reactivation: exploration of a possible link. Dermatol Online J. 2007;13(3):13.
A 46-year-old white male presented to a civilian general practitioner with an acute bilateral erythemetous, nonurticarial morbilliform rash. The patient initially presented with these symptoms (7 years prior) to a military field medical clinic, and the first presentation was seen by a nurse practitioner. The rash was evident on the lateral aspects of the thorax and on the medial portions of both arms and upper thighs. There was no evidence of involvement of hands or feet. Outside of being quite prominent, the patient reported the rash to be asymptomatic.
The patient also had concomitant symptoms of a typical upper respiratory tract infection (URTI) with fever of 100.3° F. The physical examination was unremarkable except for the pronounced rash as described and URTI-associated nasal congestion/postnasal drainage. Examination of oral mucosa of the head, ears, eyes, nose, and throat also was negative for any evidence of eruption. The patient was in excellent physical condition, took no routine medications, and had no documented underlying medical conditions. His family and social history seemed noncontributory; he reported frequent domestic airline travel associated with reserve duty as well as with his civilian job. Appropriate immunizations were all current, and regularly scheduled physical examinations had been documented in the medical record.
The patient’s rashes associated with URTI began 7 years prior at age 39 after a weeklong (fall season) operational readiness inspection (ORI), which included extended periods wearing ground crew ensemble (GCE) equipment. Protective clothing worn during that ORI included the heavily charcoal-laden over-garment, butyl rubber gloves and boots, and the then current-issue gas mask. The rashes had never appeared before age 39, and the patient reported no previous medical problems after wearing GCE equipment during 16 years of service preceding that initial presentation.
The patient’s rashes typically presented with the similar pattern and intensity at 24 to 36 hours after onset of URTI symptoms, were self-limiting, and resolved in an additional 48 to 72 hours (Figure). The rash was present with each subsequent URTI and occurred on average, about 2 times each year. Similar rashes also appeared twice during the preceding 7 years during episodes of nausea/vomiting/diarrhea consistent with acute gastroenteritis (AGE). No other household members (infant to aged 40 years) showed any similar rash during episodic URTI or AGE during the same period. However, about 2 years after the initial URTI/rash presentation, the patient’s then toddler-aged son was diagnosed with self-limiting unilateral laterothoracic exanthem (ULTE), which completely resolved in 2 to 3 weeks and never reappeared.
Discussion
The author postulated that the patient most likely experienced routine acute episodes of Picornaviridae infection. The overarching diagnosis was presumptive, as biopsy and/or serologies were not obtained on the reservist. The Picornavirus family includes genera such as Rhinovirus and Enterovirus, both significant contributors to URTI (Enterovirus for AGE). Typically, about 50% of URTIs are caused by Rhinovirus (approaches 100% in some seasonal outbreaks) with the remainder of common URTIs being caused by Enterovirus, Coronavirus, Adenovirus, Reovirus, and Parainfluenza virus.1 Unfortunately, rapid office-based or field medicine diagnostic assays to consistently confirm or differentiate these common URTI viral etiologies (excepting influenza A/B) are simply not economically feasible at this time nor are any standardized laboratory tests recommended for self-limiting URTIs in immunocompetent adults. There are multiplex polymerase chain reaction assays available for many viral URTI pathogens, but cost and the need for laboratory as well as certified medical technologist support may often preclude routine use at the point of care.
Perhaps the more intriguing aspect of this case is the consistent recurrence of the same rash presentation in a middle-aged immunocompetent adult male with every subsequent episode of typical URTI or AGE over a period of 7 years, irrespective of seasonal timing, contact history, or geographic location. There is well-documented evidence of URTI associated with Adenovirus and Coronavirus seen in otherwise healthy adults living in close quarters military environments. Particularly, Adenovirus serotypes 3, 4, 7, and 21 have been associated with respiratory disease in military troop populations.2
There are also numerous viral exanthems shown in young children with probable links to Epstein-Barr virus (EBV), Human herpesvirus (HHV) 6, and parvovirus B19.3 The patient’s then toddler-aged son experiencing ULTE subsequent to the father’s presentation raise the question of a possible link to viruses other than those associated with typical URTI/AGE. Literature review indicated ULTE may be linked to EBV, parvovirus B19, Adenovirus, Parainfluenza virus, or HHV 6 and 7.4
Adenovirus and Parainfluenza virus are causative agents for a portion of URTIs as well. However, the likelihood of all of the patient’s subsequent URTIs being caused exclusively by Adenovirus would be exceedingly low. Infections with atypical bacteria, such as mycoplasma, also are frequently associated with rash, fever, and myalgia but are more commonly linked to lower RTI.
All the above etiologies are typically self-limiting (except mycoplasma infections) and usually resolve without specific treatment. This broad differential tends to “muddy the water” when trying to tie a definitive diagnosis to what was a seemingly routine URTI in a healthy adult.
The patient’s status as a member of the reserve complicated obtaining a definitive diagnosis of the recurring rash. Even if a link between the recurring rash and earlier duty could be established, access to an acute-care appointment at a military treatment facility (in nonduty status) was/is not authorized. A timely civilian acute-care appointment for routine URTI with short-lived rash in an adult patient is difficult to book, especially if the rash appears on a weekend or holiday. Likewise, insurance reimbursement for skin biopsy or numerous serologic tests (investigating possible viral etiology) is frequently not covered for such conditions, as even a positive serology likely would not alter the current standard of supportive care.
Evaluation of any patient presenting during or immediately after field exercises or operational deployment must be conducted in full consideration of such conditions. A thorough history relating to geographic location, immunization status, season, environmental or chemical contacts, animal/insect exposure, living conditions, other ill unit personnel, sanitation/hygiene and food/water quality is of paramount importance. Likewise, there may be value in at least having a unit briefing/follow-up with a medical provider 2 to 6 months after deployment/field training activities. A general follow-up such as this may help find any emerging trends or common concerns pertaining to unit members’ health.
Reservists present a unique health maintenance challenge as they may complete a duty tour and drop out of military health system visibility for weeks to months. The inherent design of some reserve component programs makes even the discovery of a case such as this difficult at best. A structured follow-up (be that a group briefing or even a survey-type questionnaire) becomes highly useful for monitoring emerging health trends in reservists.
Conclusion
This patient’s initial field presentation at age 39 was clinically explained and documented in the official medical record as routine URTI. The diagnosis was based on clinical picture, occurrence during fall season, and close quarters/field living conditions. A definitive diagnosis of the recurring rash was never documented in the patient’s military medical records.
Differing somewhat from a typical case report, the purpose of this presentation was to offer “field medicine” points and systematic considerations when faced with the presentation of a seemingly common ailment, not to provide readers with a definitive diagnosis. These considerations along with the need for follow-up health maintenance strategies are especially highlighted by the unique situations presented by reservists. In addition, this case demonstrates the validity of the often forgotten history and physical question of “Has this ever happened before?” These are at least issues that should be considered before signing the disposition as “URTI, OTC meds PRN, quarters for 24 hours, then return to duty.”
A 46-year-old white male presented to a civilian general practitioner with an acute bilateral erythemetous, nonurticarial morbilliform rash. The patient initially presented with these symptoms (7 years prior) to a military field medical clinic, and the first presentation was seen by a nurse practitioner. The rash was evident on the lateral aspects of the thorax and on the medial portions of both arms and upper thighs. There was no evidence of involvement of hands or feet. Outside of being quite prominent, the patient reported the rash to be asymptomatic.
The patient also had concomitant symptoms of a typical upper respiratory tract infection (URTI) with fever of 100.3° F. The physical examination was unremarkable except for the pronounced rash as described and URTI-associated nasal congestion/postnasal drainage. Examination of oral mucosa of the head, ears, eyes, nose, and throat also was negative for any evidence of eruption. The patient was in excellent physical condition, took no routine medications, and had no documented underlying medical conditions. His family and social history seemed noncontributory; he reported frequent domestic airline travel associated with reserve duty as well as with his civilian job. Appropriate immunizations were all current, and regularly scheduled physical examinations had been documented in the medical record.
The patient’s rashes associated with URTI began 7 years prior at age 39 after a weeklong (fall season) operational readiness inspection (ORI), which included extended periods wearing ground crew ensemble (GCE) equipment. Protective clothing worn during that ORI included the heavily charcoal-laden over-garment, butyl rubber gloves and boots, and the then current-issue gas mask. The rashes had never appeared before age 39, and the patient reported no previous medical problems after wearing GCE equipment during 16 years of service preceding that initial presentation.
The patient’s rashes typically presented with the similar pattern and intensity at 24 to 36 hours after onset of URTI symptoms, were self-limiting, and resolved in an additional 48 to 72 hours (Figure). The rash was present with each subsequent URTI and occurred on average, about 2 times each year. Similar rashes also appeared twice during the preceding 7 years during episodes of nausea/vomiting/diarrhea consistent with acute gastroenteritis (AGE). No other household members (infant to aged 40 years) showed any similar rash during episodic URTI or AGE during the same period. However, about 2 years after the initial URTI/rash presentation, the patient’s then toddler-aged son was diagnosed with self-limiting unilateral laterothoracic exanthem (ULTE), which completely resolved in 2 to 3 weeks and never reappeared.
Discussion
The author postulated that the patient most likely experienced routine acute episodes of Picornaviridae infection. The overarching diagnosis was presumptive, as biopsy and/or serologies were not obtained on the reservist. The Picornavirus family includes genera such as Rhinovirus and Enterovirus, both significant contributors to URTI (Enterovirus for AGE). Typically, about 50% of URTIs are caused by Rhinovirus (approaches 100% in some seasonal outbreaks) with the remainder of common URTIs being caused by Enterovirus, Coronavirus, Adenovirus, Reovirus, and Parainfluenza virus.1 Unfortunately, rapid office-based or field medicine diagnostic assays to consistently confirm or differentiate these common URTI viral etiologies (excepting influenza A/B) are simply not economically feasible at this time nor are any standardized laboratory tests recommended for self-limiting URTIs in immunocompetent adults. There are multiplex polymerase chain reaction assays available for many viral URTI pathogens, but cost and the need for laboratory as well as certified medical technologist support may often preclude routine use at the point of care.
Perhaps the more intriguing aspect of this case is the consistent recurrence of the same rash presentation in a middle-aged immunocompetent adult male with every subsequent episode of typical URTI or AGE over a period of 7 years, irrespective of seasonal timing, contact history, or geographic location. There is well-documented evidence of URTI associated with Adenovirus and Coronavirus seen in otherwise healthy adults living in close quarters military environments. Particularly, Adenovirus serotypes 3, 4, 7, and 21 have been associated with respiratory disease in military troop populations.2
There are also numerous viral exanthems shown in young children with probable links to Epstein-Barr virus (EBV), Human herpesvirus (HHV) 6, and parvovirus B19.3 The patient’s then toddler-aged son experiencing ULTE subsequent to the father’s presentation raise the question of a possible link to viruses other than those associated with typical URTI/AGE. Literature review indicated ULTE may be linked to EBV, parvovirus B19, Adenovirus, Parainfluenza virus, or HHV 6 and 7.4
Adenovirus and Parainfluenza virus are causative agents for a portion of URTIs as well. However, the likelihood of all of the patient’s subsequent URTIs being caused exclusively by Adenovirus would be exceedingly low. Infections with atypical bacteria, such as mycoplasma, also are frequently associated with rash, fever, and myalgia but are more commonly linked to lower RTI.
All the above etiologies are typically self-limiting (except mycoplasma infections) and usually resolve without specific treatment. This broad differential tends to “muddy the water” when trying to tie a definitive diagnosis to what was a seemingly routine URTI in a healthy adult.
The patient’s status as a member of the reserve complicated obtaining a definitive diagnosis of the recurring rash. Even if a link between the recurring rash and earlier duty could be established, access to an acute-care appointment at a military treatment facility (in nonduty status) was/is not authorized. A timely civilian acute-care appointment for routine URTI with short-lived rash in an adult patient is difficult to book, especially if the rash appears on a weekend or holiday. Likewise, insurance reimbursement for skin biopsy or numerous serologic tests (investigating possible viral etiology) is frequently not covered for such conditions, as even a positive serology likely would not alter the current standard of supportive care.
Evaluation of any patient presenting during or immediately after field exercises or operational deployment must be conducted in full consideration of such conditions. A thorough history relating to geographic location, immunization status, season, environmental or chemical contacts, animal/insect exposure, living conditions, other ill unit personnel, sanitation/hygiene and food/water quality is of paramount importance. Likewise, there may be value in at least having a unit briefing/follow-up with a medical provider 2 to 6 months after deployment/field training activities. A general follow-up such as this may help find any emerging trends or common concerns pertaining to unit members’ health.
Reservists present a unique health maintenance challenge as they may complete a duty tour and drop out of military health system visibility for weeks to months. The inherent design of some reserve component programs makes even the discovery of a case such as this difficult at best. A structured follow-up (be that a group briefing or even a survey-type questionnaire) becomes highly useful for monitoring emerging health trends in reservists.
Conclusion
This patient’s initial field presentation at age 39 was clinically explained and documented in the official medical record as routine URTI. The diagnosis was based on clinical picture, occurrence during fall season, and close quarters/field living conditions. A definitive diagnosis of the recurring rash was never documented in the patient’s military medical records.
Differing somewhat from a typical case report, the purpose of this presentation was to offer “field medicine” points and systematic considerations when faced with the presentation of a seemingly common ailment, not to provide readers with a definitive diagnosis. These considerations along with the need for follow-up health maintenance strategies are especially highlighted by the unique situations presented by reservists. In addition, this case demonstrates the validity of the often forgotten history and physical question of “Has this ever happened before?” These are at least issues that should be considered before signing the disposition as “URTI, OTC meds PRN, quarters for 24 hours, then return to duty.”
1. Galagas ME, Mylonakis E. Gorbach’s 5-Minute Infectious Diseases Consult. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
2. Levinson W, ed. DNA nonenveloped viruses. In: Review of Medical Microbiology and Immunology. 12th ed. New York, NY: McGraw-Hill; 2012:291-292.
3. Lam JM. Characterizing viral exanthems. Pediatr Health. 2010;4(6):623-635.
4. Scheinfeld N. Unilateral laterothoracis exanthema with evidence of Epstein Barr virus reactivation: exploration of a possible link. Dermatol Online J. 2007;13(3):13.
1. Galagas ME, Mylonakis E. Gorbach’s 5-Minute Infectious Diseases Consult. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
2. Levinson W, ed. DNA nonenveloped viruses. In: Review of Medical Microbiology and Immunology. 12th ed. New York, NY: McGraw-Hill; 2012:291-292.
3. Lam JM. Characterizing viral exanthems. Pediatr Health. 2010;4(6):623-635.
4. Scheinfeld N. Unilateral laterothoracis exanthema with evidence of Epstein Barr virus reactivation: exploration of a possible link. Dermatol Online J. 2007;13(3):13.
Drug fails to meet endpoint in phase 3 ITP trial

The SYK inhibitor fostamatinib did not meet the primary endpoint in a phase 3 study of adults with chronic/persistent immune thrombocytopenia (ITP), according to Rigel Pharmaceuticals, Inc., the company developing the drug.
However, fostamatinib did meet that endpoint—a significantly higher incidence of stable platelet response compared to placebo—in an identical phase 3 study.
The combined data from both studies—known as FIT 1 and FIT 2—suggest fostamatinib confers a benefit over placebo.
Therefore, Rigel Pharmaceuticals is still planning to submit a new drug application for fostamatinib to the US Food and Drug Administration (FDA) next year, pending feedback from the agency.
“We believe that the totality and consistency of data from the FIT phase 3 program . . . strongly supports a clear treatment effect, with a sustained clinical benefit of fostamatinib,” said Raul Rodriguez, president and chief executive officer of Rigel Pharmaceuticals.
“We are encouraged by these results and believe that the risk/benefit ratio for fostamatinib is positive for patients with chronic/persistent ITP . . . . As a result, we will continue to pursue this opportunity. Our next step is to seek feedback from the FDA.”
About the FIT studies
Rigel’s FIT program consists of 2 identical, multicenter, randomized, double-blind studies of approximately 75 adults each—FIT 1 (Study 047) and FIT 2 (Study 048).
The patients enrolled in each study had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL of blood.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Patients were subsequently given the opportunity to enroll in an open-label, long-term, phase 3 extension study (Study 049), which is ongoing.
Patient characteristics
In FIT 1, 51 patients were randomized to fostamatinib and 25 to placebo. The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 100%), rituximab (51% and 44%), thrombopoietic agents (50% and 60%), and splenectomy (39% and 40%).
The median platelet count at baseline was 15,000/uL in the fostamatinib arm and 16,000/uL in the placebo arm.
In FIT 2, 50 patients were randomized to fostamatinib and 24 to placebo. The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 92%), rituximab (16% and 13%), thrombopoietic agents (40% and 42%), and splenectomy (28% and 38%).
The median platelet count at baseline was 16,000/uL in the fostamatinib arm and 21,000/uL in the placebo arm.
Efficacy
The primary efficacy endpoint in both studies is a stable platelet response, which is defined as achieving platelet counts greater than 50,000/uL of blood for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In FIT 1, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
In FIT 2, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
When the data from FIT 1 and FIT 2 are combined, the response rate is significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
The response rate is significantly better in the fostamatinib arm across all subgroups, regardless of whether patients had prior splenectomy, prior exposure to thrombopoietic agents, or baseline platelet counts above or below 15,000/uL.
In the combined dataset, patients who met the primary endpoint had their platelet counts increase from a median of 18,500/uL at baseline to more than 100,000/uL at week 24 of treatment.
In addition, patients who met the primary endpoint had a timely platelet response, and that response was enduring, according to James B. Bussel, MD, a professor at Weill Cornell Medicine in New York, New York, principal investigator on the FIT phase 3 program, and a member of Rigel’s advisory/scientific board.
“The FIT phase 3 studies have both demonstrated that fostamatinib provided a robust and enduring benefit for those patients who responded to the drug,” he said.
Safety
In FIT 1, the overall incidence of adverse events (AEs) was 96% in the fostamatinib arm and 76% in the placebo arm. The incidence of serious AEs was 16% and 20%, respectively. And the incidence of treatment-related AEs was 77% and 28%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (nausea, diarrhea, vomiting, abdominal pain; 61% and 20%), nausea (29% and 4%), diarrhea (45% and 16%), infection (33% and 20%), hypertension during visit (35% and 8%), and transaminase elevation (22% and 0%).
In FIT 2, the overall incidence of AEs was 71% in the fostamatinib arm and 78% in the placebo arm. The incidence of serious AEs was 10% and 26%, respectively. And the incidence of treatment-related AEs was 39% and 26%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (22% in both arms), nausea (8% and 13%), diarrhea (18% and 13%), infection (22% in both arms), hypertension during visit (20% and 17%), and transaminase elevation (6% and 0%). ![]()

The SYK inhibitor fostamatinib did not meet the primary endpoint in a phase 3 study of adults with chronic/persistent immune thrombocytopenia (ITP), according to Rigel Pharmaceuticals, Inc., the company developing the drug.
However, fostamatinib did meet that endpoint—a significantly higher incidence of stable platelet response compared to placebo—in an identical phase 3 study.
The combined data from both studies—known as FIT 1 and FIT 2—suggest fostamatinib confers a benefit over placebo.
Therefore, Rigel Pharmaceuticals is still planning to submit a new drug application for fostamatinib to the US Food and Drug Administration (FDA) next year, pending feedback from the agency.
“We believe that the totality and consistency of data from the FIT phase 3 program . . . strongly supports a clear treatment effect, with a sustained clinical benefit of fostamatinib,” said Raul Rodriguez, president and chief executive officer of Rigel Pharmaceuticals.
“We are encouraged by these results and believe that the risk/benefit ratio for fostamatinib is positive for patients with chronic/persistent ITP . . . . As a result, we will continue to pursue this opportunity. Our next step is to seek feedback from the FDA.”
About the FIT studies
Rigel’s FIT program consists of 2 identical, multicenter, randomized, double-blind studies of approximately 75 adults each—FIT 1 (Study 047) and FIT 2 (Study 048).
The patients enrolled in each study had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL of blood.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Patients were subsequently given the opportunity to enroll in an open-label, long-term, phase 3 extension study (Study 049), which is ongoing.
Patient characteristics
In FIT 1, 51 patients were randomized to fostamatinib and 25 to placebo. The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 100%), rituximab (51% and 44%), thrombopoietic agents (50% and 60%), and splenectomy (39% and 40%).
The median platelet count at baseline was 15,000/uL in the fostamatinib arm and 16,000/uL in the placebo arm.
In FIT 2, 50 patients were randomized to fostamatinib and 24 to placebo. The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 92%), rituximab (16% and 13%), thrombopoietic agents (40% and 42%), and splenectomy (28% and 38%).
The median platelet count at baseline was 16,000/uL in the fostamatinib arm and 21,000/uL in the placebo arm.
Efficacy
The primary efficacy endpoint in both studies is a stable platelet response, which is defined as achieving platelet counts greater than 50,000/uL of blood for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In FIT 1, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
In FIT 2, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
When the data from FIT 1 and FIT 2 are combined, the response rate is significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
The response rate is significantly better in the fostamatinib arm across all subgroups, regardless of whether patients had prior splenectomy, prior exposure to thrombopoietic agents, or baseline platelet counts above or below 15,000/uL.
In the combined dataset, patients who met the primary endpoint had their platelet counts increase from a median of 18,500/uL at baseline to more than 100,000/uL at week 24 of treatment.
In addition, patients who met the primary endpoint had a timely platelet response, and that response was enduring, according to James B. Bussel, MD, a professor at Weill Cornell Medicine in New York, New York, principal investigator on the FIT phase 3 program, and a member of Rigel’s advisory/scientific board.
“The FIT phase 3 studies have both demonstrated that fostamatinib provided a robust and enduring benefit for those patients who responded to the drug,” he said.
Safety
In FIT 1, the overall incidence of adverse events (AEs) was 96% in the fostamatinib arm and 76% in the placebo arm. The incidence of serious AEs was 16% and 20%, respectively. And the incidence of treatment-related AEs was 77% and 28%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (nausea, diarrhea, vomiting, abdominal pain; 61% and 20%), nausea (29% and 4%), diarrhea (45% and 16%), infection (33% and 20%), hypertension during visit (35% and 8%), and transaminase elevation (22% and 0%).
In FIT 2, the overall incidence of AEs was 71% in the fostamatinib arm and 78% in the placebo arm. The incidence of serious AEs was 10% and 26%, respectively. And the incidence of treatment-related AEs was 39% and 26%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (22% in both arms), nausea (8% and 13%), diarrhea (18% and 13%), infection (22% in both arms), hypertension during visit (20% and 17%), and transaminase elevation (6% and 0%). ![]()

The SYK inhibitor fostamatinib did not meet the primary endpoint in a phase 3 study of adults with chronic/persistent immune thrombocytopenia (ITP), according to Rigel Pharmaceuticals, Inc., the company developing the drug.
However, fostamatinib did meet that endpoint—a significantly higher incidence of stable platelet response compared to placebo—in an identical phase 3 study.
The combined data from both studies—known as FIT 1 and FIT 2—suggest fostamatinib confers a benefit over placebo.
Therefore, Rigel Pharmaceuticals is still planning to submit a new drug application for fostamatinib to the US Food and Drug Administration (FDA) next year, pending feedback from the agency.
“We believe that the totality and consistency of data from the FIT phase 3 program . . . strongly supports a clear treatment effect, with a sustained clinical benefit of fostamatinib,” said Raul Rodriguez, president and chief executive officer of Rigel Pharmaceuticals.
“We are encouraged by these results and believe that the risk/benefit ratio for fostamatinib is positive for patients with chronic/persistent ITP . . . . As a result, we will continue to pursue this opportunity. Our next step is to seek feedback from the FDA.”
About the FIT studies
Rigel’s FIT program consists of 2 identical, multicenter, randomized, double-blind studies of approximately 75 adults each—FIT 1 (Study 047) and FIT 2 (Study 048).
The patients enrolled in each study had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL of blood.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Patients were subsequently given the opportunity to enroll in an open-label, long-term, phase 3 extension study (Study 049), which is ongoing.
Patient characteristics
In FIT 1, 51 patients were randomized to fostamatinib and 25 to placebo. The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 100%), rituximab (51% and 44%), thrombopoietic agents (50% and 60%), and splenectomy (39% and 40%).
The median platelet count at baseline was 15,000/uL in the fostamatinib arm and 16,000/uL in the placebo arm.
In FIT 2, 50 patients were randomized to fostamatinib and 24 to placebo. The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
Prior treatments (in the fostamatinib and placebo arms, respectively) included steroids (90% and 92%), rituximab (16% and 13%), thrombopoietic agents (40% and 42%), and splenectomy (28% and 38%).
The median platelet count at baseline was 16,000/uL in the fostamatinib arm and 21,000/uL in the placebo arm.
Efficacy
The primary efficacy endpoint in both studies is a stable platelet response, which is defined as achieving platelet counts greater than 50,000/uL of blood for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In FIT 1, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
In FIT 2, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
When the data from FIT 1 and FIT 2 are combined, the response rate is significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
The response rate is significantly better in the fostamatinib arm across all subgroups, regardless of whether patients had prior splenectomy, prior exposure to thrombopoietic agents, or baseline platelet counts above or below 15,000/uL.
In the combined dataset, patients who met the primary endpoint had their platelet counts increase from a median of 18,500/uL at baseline to more than 100,000/uL at week 24 of treatment.
In addition, patients who met the primary endpoint had a timely platelet response, and that response was enduring, according to James B. Bussel, MD, a professor at Weill Cornell Medicine in New York, New York, principal investigator on the FIT phase 3 program, and a member of Rigel’s advisory/scientific board.
“The FIT phase 3 studies have both demonstrated that fostamatinib provided a robust and enduring benefit for those patients who responded to the drug,” he said.
Safety
In FIT 1, the overall incidence of adverse events (AEs) was 96% in the fostamatinib arm and 76% in the placebo arm. The incidence of serious AEs was 16% and 20%, respectively. And the incidence of treatment-related AEs was 77% and 28%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (nausea, diarrhea, vomiting, abdominal pain; 61% and 20%), nausea (29% and 4%), diarrhea (45% and 16%), infection (33% and 20%), hypertension during visit (35% and 8%), and transaminase elevation (22% and 0%).
In FIT 2, the overall incidence of AEs was 71% in the fostamatinib arm and 78% in the placebo arm. The incidence of serious AEs was 10% and 26%, respectively. And the incidence of treatment-related AEs was 39% and 26%, respectively.
AEs (in the fostamatinib and placebo arms, respectively) included gastrointestinal complaints (22% in both arms), nausea (8% and 13%), diarrhea (18% and 13%), infection (22% in both arms), hypertension during visit (20% and 17%), and transaminase elevation (6% and 0%). ![]()
Combos produce ‘encouraging’ responses in MM

LEIPZIG, GERMANY—Combination therapies including the investigational drug MOR202 have produced “encouraging and long-lasting responses” in a phase 1/2 trial of patients with relapsed/refractory multiple myeloma (MM), according to researchers.
MOR202 is an antibody targeting CD38. In this trial, researchers are testing the drug in combination with dexamethasone (Dex), lenalidomide (Len) and Dex, or pomalidomide (Pom) and Dex.
Results thus far suggest the combinations are well-tolerated and can produce durable responses in heavily pretreated patients with relapsed/refractory MM.
The results were presented in a poster at the 2016 annual meeting of the German, Austrian and Swiss Societies for Hematology and Medical Oncology (abstract P235).
The study, which is ongoing, is sponsored by MorphoSys AG. The poster is available for download from the company’s website.
Treatment
This 3+3 dose-escalation study started with MOR202 monotherapy given at 0.01 mg/kg every 2 weeks, and the dose was escalated up to 16 mg/kg every 2 weeks and 8 mg/kg every week.
Once the dose-escalation phase was complete, researchers began testing MOR202 in combination with Dex, Pom and Dex, or Len and Dex.
Patients
As of September 05, 2016, 73 patients had been treated with MOR202, but the researchers only reported data on the 38 patients who received MOR202 as part of combination therapy.
Eighteen patients had received MOR202 plus Dex, 7 had received MOR202 plus Pom and Dex, and 13 had received MOR202 plus Len and Dex. The patients’ median age was 67, 64, and 64, respectively.
The median number of prior therapies was 3, 4, and 2, respectively. And the percentage of patients who were refractory to their last therapy was 78%, 100%, and 56%, respectively.
Safety
The researchers said the maximum-tolerated dose of MOR202, alone or in combination, has not yet been reached. However, the data suggest MOR202 can be safely administered as a 2-hour intravenous infusion at doses up to 16 mg/kg.
All 38 patients who received MOR202 in combination were evaluable for safety—18 in the Dex arm, 7 in the Pom/Dex arm, and 13 in the Len/Dex arm.
The most frequent grade 3 or higher adverse events were hematologic in nature. These included (in the Dex, Pom/Dex, and Len/Dex arms, respectively) leukopenia (11%, 57%, 31%), lymphopenia (39%, 29%, 54%), neutropenia (22%, 71%, 39%), thrombocytopenia (17%, 43%, 8%), and anemia (11%, 14%, 15%).
Infusion-related reactions occurred in 3 (8%) patients and were mainly limited to the first infusion. One patient developed a transient anti-MOR202 antibody response.
One patient discontinued treatment due to an adverse event that may have been caused by MOR202. However, researchers also said the event (decrease in platelet count) may have been caused by Dex.
There were no treatment-related deaths.
Efficacy
Efficacy data were available for 31 of the 38 patients. Fifteen patients responded, and 12 of these responses are ongoing for up to 56 weeks.
In all, there were 10 partial responses (PRs), 3 very good partial responses (VGPRs), and 2 complete responses (CRs).
There were 5 responses in the Dex arm—3 PRs and 2 VGPRs. There were 3 responses in the Pom/Dex arm—2 CRs and 1 PR. And there were 7 responses in the Len/Dex arm—6 PRs (3 unconfirmed) and 1 VGPR.
“We are very pleased that these results are consistent with earlier data from the ongoing trial and show further improved responses with more patients being evaluable for efficacy and safety assessment,” said Arndt Schottelius, MD, PhD, chief development officer of MorphoSys AG.
“In addition to the very short infusion time, we are particularly interested in the efficacy results in patients treated with MOR202 plus pomalidomide, who had a median of 4 prior therapies. The dose-escalation study is ongoing as planned, currently focusing on the highest dose cohorts of 16 mg/kg MOR202 in combination with pomalidomide and lenalidomide.” ![]()

LEIPZIG, GERMANY—Combination therapies including the investigational drug MOR202 have produced “encouraging and long-lasting responses” in a phase 1/2 trial of patients with relapsed/refractory multiple myeloma (MM), according to researchers.
MOR202 is an antibody targeting CD38. In this trial, researchers are testing the drug in combination with dexamethasone (Dex), lenalidomide (Len) and Dex, or pomalidomide (Pom) and Dex.
Results thus far suggest the combinations are well-tolerated and can produce durable responses in heavily pretreated patients with relapsed/refractory MM.
The results were presented in a poster at the 2016 annual meeting of the German, Austrian and Swiss Societies for Hematology and Medical Oncology (abstract P235).
The study, which is ongoing, is sponsored by MorphoSys AG. The poster is available for download from the company’s website.
Treatment
This 3+3 dose-escalation study started with MOR202 monotherapy given at 0.01 mg/kg every 2 weeks, and the dose was escalated up to 16 mg/kg every 2 weeks and 8 mg/kg every week.
Once the dose-escalation phase was complete, researchers began testing MOR202 in combination with Dex, Pom and Dex, or Len and Dex.
Patients
As of September 05, 2016, 73 patients had been treated with MOR202, but the researchers only reported data on the 38 patients who received MOR202 as part of combination therapy.
Eighteen patients had received MOR202 plus Dex, 7 had received MOR202 plus Pom and Dex, and 13 had received MOR202 plus Len and Dex. The patients’ median age was 67, 64, and 64, respectively.
The median number of prior therapies was 3, 4, and 2, respectively. And the percentage of patients who were refractory to their last therapy was 78%, 100%, and 56%, respectively.
Safety
The researchers said the maximum-tolerated dose of MOR202, alone or in combination, has not yet been reached. However, the data suggest MOR202 can be safely administered as a 2-hour intravenous infusion at doses up to 16 mg/kg.
All 38 patients who received MOR202 in combination were evaluable for safety—18 in the Dex arm, 7 in the Pom/Dex arm, and 13 in the Len/Dex arm.
The most frequent grade 3 or higher adverse events were hematologic in nature. These included (in the Dex, Pom/Dex, and Len/Dex arms, respectively) leukopenia (11%, 57%, 31%), lymphopenia (39%, 29%, 54%), neutropenia (22%, 71%, 39%), thrombocytopenia (17%, 43%, 8%), and anemia (11%, 14%, 15%).
Infusion-related reactions occurred in 3 (8%) patients and were mainly limited to the first infusion. One patient developed a transient anti-MOR202 antibody response.
One patient discontinued treatment due to an adverse event that may have been caused by MOR202. However, researchers also said the event (decrease in platelet count) may have been caused by Dex.
There were no treatment-related deaths.
Efficacy
Efficacy data were available for 31 of the 38 patients. Fifteen patients responded, and 12 of these responses are ongoing for up to 56 weeks.
In all, there were 10 partial responses (PRs), 3 very good partial responses (VGPRs), and 2 complete responses (CRs).
There were 5 responses in the Dex arm—3 PRs and 2 VGPRs. There were 3 responses in the Pom/Dex arm—2 CRs and 1 PR. And there were 7 responses in the Len/Dex arm—6 PRs (3 unconfirmed) and 1 VGPR.
“We are very pleased that these results are consistent with earlier data from the ongoing trial and show further improved responses with more patients being evaluable for efficacy and safety assessment,” said Arndt Schottelius, MD, PhD, chief development officer of MorphoSys AG.
“In addition to the very short infusion time, we are particularly interested in the efficacy results in patients treated with MOR202 plus pomalidomide, who had a median of 4 prior therapies. The dose-escalation study is ongoing as planned, currently focusing on the highest dose cohorts of 16 mg/kg MOR202 in combination with pomalidomide and lenalidomide.” ![]()

LEIPZIG, GERMANY—Combination therapies including the investigational drug MOR202 have produced “encouraging and long-lasting responses” in a phase 1/2 trial of patients with relapsed/refractory multiple myeloma (MM), according to researchers.
MOR202 is an antibody targeting CD38. In this trial, researchers are testing the drug in combination with dexamethasone (Dex), lenalidomide (Len) and Dex, or pomalidomide (Pom) and Dex.
Results thus far suggest the combinations are well-tolerated and can produce durable responses in heavily pretreated patients with relapsed/refractory MM.
The results were presented in a poster at the 2016 annual meeting of the German, Austrian and Swiss Societies for Hematology and Medical Oncology (abstract P235).
The study, which is ongoing, is sponsored by MorphoSys AG. The poster is available for download from the company’s website.
Treatment
This 3+3 dose-escalation study started with MOR202 monotherapy given at 0.01 mg/kg every 2 weeks, and the dose was escalated up to 16 mg/kg every 2 weeks and 8 mg/kg every week.
Once the dose-escalation phase was complete, researchers began testing MOR202 in combination with Dex, Pom and Dex, or Len and Dex.
Patients
As of September 05, 2016, 73 patients had been treated with MOR202, but the researchers only reported data on the 38 patients who received MOR202 as part of combination therapy.
Eighteen patients had received MOR202 plus Dex, 7 had received MOR202 plus Pom and Dex, and 13 had received MOR202 plus Len and Dex. The patients’ median age was 67, 64, and 64, respectively.
The median number of prior therapies was 3, 4, and 2, respectively. And the percentage of patients who were refractory to their last therapy was 78%, 100%, and 56%, respectively.
Safety
The researchers said the maximum-tolerated dose of MOR202, alone or in combination, has not yet been reached. However, the data suggest MOR202 can be safely administered as a 2-hour intravenous infusion at doses up to 16 mg/kg.
All 38 patients who received MOR202 in combination were evaluable for safety—18 in the Dex arm, 7 in the Pom/Dex arm, and 13 in the Len/Dex arm.
The most frequent grade 3 or higher adverse events were hematologic in nature. These included (in the Dex, Pom/Dex, and Len/Dex arms, respectively) leukopenia (11%, 57%, 31%), lymphopenia (39%, 29%, 54%), neutropenia (22%, 71%, 39%), thrombocytopenia (17%, 43%, 8%), and anemia (11%, 14%, 15%).
Infusion-related reactions occurred in 3 (8%) patients and were mainly limited to the first infusion. One patient developed a transient anti-MOR202 antibody response.
One patient discontinued treatment due to an adverse event that may have been caused by MOR202. However, researchers also said the event (decrease in platelet count) may have been caused by Dex.
There were no treatment-related deaths.
Efficacy
Efficacy data were available for 31 of the 38 patients. Fifteen patients responded, and 12 of these responses are ongoing for up to 56 weeks.
In all, there were 10 partial responses (PRs), 3 very good partial responses (VGPRs), and 2 complete responses (CRs).
There were 5 responses in the Dex arm—3 PRs and 2 VGPRs. There were 3 responses in the Pom/Dex arm—2 CRs and 1 PR. And there were 7 responses in the Len/Dex arm—6 PRs (3 unconfirmed) and 1 VGPR.
“We are very pleased that these results are consistent with earlier data from the ongoing trial and show further improved responses with more patients being evaluable for efficacy and safety assessment,” said Arndt Schottelius, MD, PhD, chief development officer of MorphoSys AG.
“In addition to the very short infusion time, we are particularly interested in the efficacy results in patients treated with MOR202 plus pomalidomide, who had a median of 4 prior therapies. The dose-escalation study is ongoing as planned, currently focusing on the highest dose cohorts of 16 mg/kg MOR202 in combination with pomalidomide and lenalidomide.” ![]()
FDA issues warning about radiation therapy devices

to receive radiation
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has warned healthcare providers to stop using radiation therapy devices manufactured and sold by Multidata Systems International Corporation.
The FDA said the company has distributed at least 2 devices in the US that were never reviewed by or registered with the FDA—(1) accessories to radiation therapy devices including the Real Time Dosimetry Waterphantom System and (2) the Dual Channel Electrometer.
Since 2003, Multidata has been under a decree that prohibits the company from designing, manufacturing, processing, and distributing medical devices, among other restrictions.
However, the FDA learned that Multidata manufactured and distributed medical devices in violation of the decree, including repairing and exchanging Waterphantom devices for newer design models.
As a result, on March 3, 2016, the FDA sent a letter to Multidata ordering the firm to stop designing, manufacturing, processing, packing, repacking, labeling, installing, holding for sale, and distributing any medical device. The company has permanently ceased operations and will be dissolving.
The FDA said it doesn’t know how many devices manufactured by Multidata are in use throughout the US or if any of these devices have caused adverse events since the decree was issued.
The agency has urged healthcare providers to stop using and dispose of any devices manufactured by Multidata.
Instead, providers should use accessories to radiation therapy devices and radiation treatment planning software that have been cleared by the FDA. Registered manufacturers of such products are listed under the IYE product code in the FDA’s Registration and Listing Database.
The FDA has also recommended that providers report any adverse events related to Multidata devices. Reports can be submitted through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. ![]()

to receive radiation
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has warned healthcare providers to stop using radiation therapy devices manufactured and sold by Multidata Systems International Corporation.
The FDA said the company has distributed at least 2 devices in the US that were never reviewed by or registered with the FDA—(1) accessories to radiation therapy devices including the Real Time Dosimetry Waterphantom System and (2) the Dual Channel Electrometer.
Since 2003, Multidata has been under a decree that prohibits the company from designing, manufacturing, processing, and distributing medical devices, among other restrictions.
However, the FDA learned that Multidata manufactured and distributed medical devices in violation of the decree, including repairing and exchanging Waterphantom devices for newer design models.
As a result, on March 3, 2016, the FDA sent a letter to Multidata ordering the firm to stop designing, manufacturing, processing, packing, repacking, labeling, installing, holding for sale, and distributing any medical device. The company has permanently ceased operations and will be dissolving.
The FDA said it doesn’t know how many devices manufactured by Multidata are in use throughout the US or if any of these devices have caused adverse events since the decree was issued.
The agency has urged healthcare providers to stop using and dispose of any devices manufactured by Multidata.
Instead, providers should use accessories to radiation therapy devices and radiation treatment planning software that have been cleared by the FDA. Registered manufacturers of such products are listed under the IYE product code in the FDA’s Registration and Listing Database.
The FDA has also recommended that providers report any adverse events related to Multidata devices. Reports can be submitted through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. ![]()

to receive radiation
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has warned healthcare providers to stop using radiation therapy devices manufactured and sold by Multidata Systems International Corporation.
The FDA said the company has distributed at least 2 devices in the US that were never reviewed by or registered with the FDA—(1) accessories to radiation therapy devices including the Real Time Dosimetry Waterphantom System and (2) the Dual Channel Electrometer.
Since 2003, Multidata has been under a decree that prohibits the company from designing, manufacturing, processing, and distributing medical devices, among other restrictions.
However, the FDA learned that Multidata manufactured and distributed medical devices in violation of the decree, including repairing and exchanging Waterphantom devices for newer design models.
As a result, on March 3, 2016, the FDA sent a letter to Multidata ordering the firm to stop designing, manufacturing, processing, packing, repacking, labeling, installing, holding for sale, and distributing any medical device. The company has permanently ceased operations and will be dissolving.
The FDA said it doesn’t know how many devices manufactured by Multidata are in use throughout the US or if any of these devices have caused adverse events since the decree was issued.
The agency has urged healthcare providers to stop using and dispose of any devices manufactured by Multidata.
Instead, providers should use accessories to radiation therapy devices and radiation treatment planning software that have been cleared by the FDA. Registered manufacturers of such products are listed under the IYE product code in the FDA’s Registration and Listing Database.
The FDA has also recommended that providers report any adverse events related to Multidata devices. Reports can be submitted through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. ![]()
Psych issues adding to pediatric hospitalization costs
Although pediatric hospitalizations are driven mostly by medical costs, comorbid psychiatric problems are increasingly adding to the expense, according to a review of 3,114,099 hospitalizations at 33 children’s hospitals in the United States from 2005 to 2014.
For pediatric hospitalizations without a psychiatric diagnosis, costs rose from $3,696 to $4,150 per day over the past decade. For medical stays with a psychiatric diagnosis, the price rose from $2,694 to $3,393 per day, a higher percent change. Overall, the cost of hospitalizing sick children with comorbid psychiatric diagnoses increased from $671 million to $1.6 billion.
The “strategic planning to meet the rising demand for psychiatric care in tertiary care children’s hospitals should place high priority on the needs of children with a primary medical condition and co-occurring psychiatric disorders,” said investigators led by Bonnie Zima, MD, from the University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior (Pediatrics. 2016 Oct 21. doi: 10.1542/peds.2016-0909).
“From 2005 through 2014 ... there was significant cumulative percent increase in total number of hospitalizations, hospital days, and hospital costs for all patients. Of the total hospitalizations, 18.3% (568,449) were associated with a psychiatric disorder, either primary or secondary,” they noted.
Meanwhile, hospitalizations for psychiatric-only diagnoses were minimal, just 3.6% of the total with the most common problems again being depression, anxiety, and suicide and self-injury. “Hospital resource use for only psychiatric disorders declined, consistent with the national shift to managed care for behavioral health services,” the investigators said.
“Collectively, these data will further guide strategic planning by children’s hospitals as they strive to integrate mental health care into their health care systems. Together, these findings … underscore the need for quality improvement interventions that target improving linkage with community mental health care after pediatric hospitalization,” they said.
The National Institutes of Health funded the study. Dr. Zima was supported by the National Institute of Mental Health and Behavioral Health Centers of Excellence for California. Dr. Naomi S. Bardach and Dr. Tumaini R. Coker were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Jay G. Berry was supported by the Agency for Healthcare Research and Quality. The authors had no conflicts of interest.
Although pediatric hospitalizations are driven mostly by medical costs, comorbid psychiatric problems are increasingly adding to the expense, according to a review of 3,114,099 hospitalizations at 33 children’s hospitals in the United States from 2005 to 2014.
For pediatric hospitalizations without a psychiatric diagnosis, costs rose from $3,696 to $4,150 per day over the past decade. For medical stays with a psychiatric diagnosis, the price rose from $2,694 to $3,393 per day, a higher percent change. Overall, the cost of hospitalizing sick children with comorbid psychiatric diagnoses increased from $671 million to $1.6 billion.
The “strategic planning to meet the rising demand for psychiatric care in tertiary care children’s hospitals should place high priority on the needs of children with a primary medical condition and co-occurring psychiatric disorders,” said investigators led by Bonnie Zima, MD, from the University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior (Pediatrics. 2016 Oct 21. doi: 10.1542/peds.2016-0909).
“From 2005 through 2014 ... there was significant cumulative percent increase in total number of hospitalizations, hospital days, and hospital costs for all patients. Of the total hospitalizations, 18.3% (568,449) were associated with a psychiatric disorder, either primary or secondary,” they noted.
Meanwhile, hospitalizations for psychiatric-only diagnoses were minimal, just 3.6% of the total with the most common problems again being depression, anxiety, and suicide and self-injury. “Hospital resource use for only psychiatric disorders declined, consistent with the national shift to managed care for behavioral health services,” the investigators said.
“Collectively, these data will further guide strategic planning by children’s hospitals as they strive to integrate mental health care into their health care systems. Together, these findings … underscore the need for quality improvement interventions that target improving linkage with community mental health care after pediatric hospitalization,” they said.
The National Institutes of Health funded the study. Dr. Zima was supported by the National Institute of Mental Health and Behavioral Health Centers of Excellence for California. Dr. Naomi S. Bardach and Dr. Tumaini R. Coker were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Jay G. Berry was supported by the Agency for Healthcare Research and Quality. The authors had no conflicts of interest.
Although pediatric hospitalizations are driven mostly by medical costs, comorbid psychiatric problems are increasingly adding to the expense, according to a review of 3,114,099 hospitalizations at 33 children’s hospitals in the United States from 2005 to 2014.
For pediatric hospitalizations without a psychiatric diagnosis, costs rose from $3,696 to $4,150 per day over the past decade. For medical stays with a psychiatric diagnosis, the price rose from $2,694 to $3,393 per day, a higher percent change. Overall, the cost of hospitalizing sick children with comorbid psychiatric diagnoses increased from $671 million to $1.6 billion.
The “strategic planning to meet the rising demand for psychiatric care in tertiary care children’s hospitals should place high priority on the needs of children with a primary medical condition and co-occurring psychiatric disorders,” said investigators led by Bonnie Zima, MD, from the University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior (Pediatrics. 2016 Oct 21. doi: 10.1542/peds.2016-0909).
“From 2005 through 2014 ... there was significant cumulative percent increase in total number of hospitalizations, hospital days, and hospital costs for all patients. Of the total hospitalizations, 18.3% (568,449) were associated with a psychiatric disorder, either primary or secondary,” they noted.
Meanwhile, hospitalizations for psychiatric-only diagnoses were minimal, just 3.6% of the total with the most common problems again being depression, anxiety, and suicide and self-injury. “Hospital resource use for only psychiatric disorders declined, consistent with the national shift to managed care for behavioral health services,” the investigators said.
“Collectively, these data will further guide strategic planning by children’s hospitals as they strive to integrate mental health care into their health care systems. Together, these findings … underscore the need for quality improvement interventions that target improving linkage with community mental health care after pediatric hospitalization,” they said.
The National Institutes of Health funded the study. Dr. Zima was supported by the National Institute of Mental Health and Behavioral Health Centers of Excellence for California. Dr. Naomi S. Bardach and Dr. Tumaini R. Coker were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Jay G. Berry was supported by the Agency for Healthcare Research and Quality. The authors had no conflicts of interest.
FROM PEDIATRICS
Key clinical point:
Major finding: For pediatric hospitalizations without a psychiatric diagnosis, costs rose during the past decade from $3,696 to $4,150 per day. For medical stays with a psychiatric diagnosis, the price rose from $2,694 to $3,393 per day.
Data source: Review of 3,114,099 hospitalizations at 33 children’s hospitals in the United States during 2005-2014
Disclosures: The National Institutes of Health funded the study. Dr. Zima was supported by the National Institute of Mental Health and Behavioral Health Centers of Excellence for California. Dr. Naomi S. Bardach and Dr. Tumaini R. Coker were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Jay G. Berry was supported by the Agency for Healthcare Research and Quality. The authors had no conflicts of interest.
Medicare subsidies eliminate disparities in adherence to hormonal
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.
Texas medical board drops appeal against Teladoc
The Texas Medical Board (TMB) has dropped an appeal that had challenged whether national telemedicine company Teladoc could sue over telemedicine restrictions enacted by the board. Both parties will now prepare to argue the merits behind the case in U.S. District Court in Austin, Texas.
TMB plans to vigorously defend its telemedicine rules in court, said Scott Freshour, interim executive director. He did not elaborate on the reasons behind the board’s Oct. 14 vote to withdraw its appeal in the case.
TMB withdrew its appeal because it didn’t want to suffer another loss to Teladoc in the courts, said Adam Vandervoort, Teladoc’s chief legal counsel. In a public meeting, a TMB official called the decision to withdraw “purely strategic,” according to Mr. Vandervoort.
This “raises troubling questions about the TMB’s motives in both filing, and subsequently retracting, the appeal,” Mr. Vandervoort said in a statement. “Teladoc and its amicus parties expended substantial resources on defending the appeal, which now will not result in a decision.”
The dispute stems from a medical board rule that requires Texas physicians to conduct a “face-to-face” evaluation before treating a patient via telemedicine. The face-to-face visit can be conducted through telemedicine at an established medical site, but it may not be established through an online questionnaire, e-mail, text, chat, or telephonic evaluation or consultation. In addition, the TMB requires that distant site providers establish a physician-patient relationship, which at a minimum includes: establishing that the person requesting the treatment is in fact who the person claims to be, establishing a diagnosis through the use of acceptable medical practices, discussing with the patient the risks and benefits of various treatment options, and ensuring the availability of the distant site provider or coverage of the patient for appropriate follow-up care.
Teladoc sued the medical board in April 2015 claiming the face-to-face rule violates federal antitrust laws. Teladoc provides access to medical care via phone or interactive video and treats patients for nonemergency conditions. A judge halted the rule’s enforcement until the litigation is resolved.
The TMB requested that a judge throw out the suit, arguing that the board is immune from antitrust liability as a state agency. A district court disagreed and allowed the case to proceed. The board then appealed to 5th U.S. Circuit Court of Appeals to overturn the district court’s decision.
It’s likely that the board’s decision not to pursue the appeal was affected by the recent backing of Teladoc by the U.S. Department of Justice (DOJ) and the Federal Trade Commission (FTC) regarding the application of the state action doctrine, said Paul W. Pitts, a San Francisco health law attorney who has closely followed the case. The doctrine protects the deliberate policy choices of sovereign states to displace competition with regulation or monopoly public service.
In a brief to the 5th Circuit, the DOJ and the FTC urged the appeals court to dismiss the board’s appeal. The agencies called TMB’s telemedicine rules anticompetitive and said the board was not protected by the state action doctrine because requirements under the doctrine were not satisfied. However, the America Medical Association and the Texas Medical Association sided with the TMB, telling the court the entity should be immune from federal antitrust liability.
Aside from the AMA and Texas Medical Association, the Texas board had few allies in the appeals dispute,” Mr. Pitts said in an interview.
“Many interested parties were lining up on the side of Teledoc by filing amici curiae arguing that the 5th Circuit lacked jurisdiction to hear this appeal and that the state action doctrine is not applicable,” he said. “As the market for telemedicine grows rapidly, there is an increasing number of parties with something at stake in this case.”
Now that the appeal has ended, the district court can get back to the primary issue at hand: whether the rule requiring a face-to-face exam can be justified or whether it’s just a means of protecting the traditional physician practice, Mr. Pitts said. The ultimate ruling in the case has broad implications for the practice of telemedicine in Texas and beyond.
“Medical boards in other states are revising their rules to be more accommodating of telemedicine as the use of this approach grows in acceptance around the country.” he said. “The Texas Medical Board is increasingly an outlier in this space. If Teledoc prevails, you can expect to see investors taking another look at the telemedicine space as the Texas market opens up and the role of the state medical board is diminished.”
[email protected]
On Twitter @legal_med
The Texas Medical Board (TMB) has dropped an appeal that had challenged whether national telemedicine company Teladoc could sue over telemedicine restrictions enacted by the board. Both parties will now prepare to argue the merits behind the case in U.S. District Court in Austin, Texas.
TMB plans to vigorously defend its telemedicine rules in court, said Scott Freshour, interim executive director. He did not elaborate on the reasons behind the board’s Oct. 14 vote to withdraw its appeal in the case.
TMB withdrew its appeal because it didn’t want to suffer another loss to Teladoc in the courts, said Adam Vandervoort, Teladoc’s chief legal counsel. In a public meeting, a TMB official called the decision to withdraw “purely strategic,” according to Mr. Vandervoort.
This “raises troubling questions about the TMB’s motives in both filing, and subsequently retracting, the appeal,” Mr. Vandervoort said in a statement. “Teladoc and its amicus parties expended substantial resources on defending the appeal, which now will not result in a decision.”
The dispute stems from a medical board rule that requires Texas physicians to conduct a “face-to-face” evaluation before treating a patient via telemedicine. The face-to-face visit can be conducted through telemedicine at an established medical site, but it may not be established through an online questionnaire, e-mail, text, chat, or telephonic evaluation or consultation. In addition, the TMB requires that distant site providers establish a physician-patient relationship, which at a minimum includes: establishing that the person requesting the treatment is in fact who the person claims to be, establishing a diagnosis through the use of acceptable medical practices, discussing with the patient the risks and benefits of various treatment options, and ensuring the availability of the distant site provider or coverage of the patient for appropriate follow-up care.
Teladoc sued the medical board in April 2015 claiming the face-to-face rule violates federal antitrust laws. Teladoc provides access to medical care via phone or interactive video and treats patients for nonemergency conditions. A judge halted the rule’s enforcement until the litigation is resolved.
The TMB requested that a judge throw out the suit, arguing that the board is immune from antitrust liability as a state agency. A district court disagreed and allowed the case to proceed. The board then appealed to 5th U.S. Circuit Court of Appeals to overturn the district court’s decision.
It’s likely that the board’s decision not to pursue the appeal was affected by the recent backing of Teladoc by the U.S. Department of Justice (DOJ) and the Federal Trade Commission (FTC) regarding the application of the state action doctrine, said Paul W. Pitts, a San Francisco health law attorney who has closely followed the case. The doctrine protects the deliberate policy choices of sovereign states to displace competition with regulation or monopoly public service.
In a brief to the 5th Circuit, the DOJ and the FTC urged the appeals court to dismiss the board’s appeal. The agencies called TMB’s telemedicine rules anticompetitive and said the board was not protected by the state action doctrine because requirements under the doctrine were not satisfied. However, the America Medical Association and the Texas Medical Association sided with the TMB, telling the court the entity should be immune from federal antitrust liability.
Aside from the AMA and Texas Medical Association, the Texas board had few allies in the appeals dispute,” Mr. Pitts said in an interview.
“Many interested parties were lining up on the side of Teledoc by filing amici curiae arguing that the 5th Circuit lacked jurisdiction to hear this appeal and that the state action doctrine is not applicable,” he said. “As the market for telemedicine grows rapidly, there is an increasing number of parties with something at stake in this case.”
Now that the appeal has ended, the district court can get back to the primary issue at hand: whether the rule requiring a face-to-face exam can be justified or whether it’s just a means of protecting the traditional physician practice, Mr. Pitts said. The ultimate ruling in the case has broad implications for the practice of telemedicine in Texas and beyond.
“Medical boards in other states are revising their rules to be more accommodating of telemedicine as the use of this approach grows in acceptance around the country.” he said. “The Texas Medical Board is increasingly an outlier in this space. If Teledoc prevails, you can expect to see investors taking another look at the telemedicine space as the Texas market opens up and the role of the state medical board is diminished.”
[email protected]
On Twitter @legal_med
The Texas Medical Board (TMB) has dropped an appeal that had challenged whether national telemedicine company Teladoc could sue over telemedicine restrictions enacted by the board. Both parties will now prepare to argue the merits behind the case in U.S. District Court in Austin, Texas.
TMB plans to vigorously defend its telemedicine rules in court, said Scott Freshour, interim executive director. He did not elaborate on the reasons behind the board’s Oct. 14 vote to withdraw its appeal in the case.
TMB withdrew its appeal because it didn’t want to suffer another loss to Teladoc in the courts, said Adam Vandervoort, Teladoc’s chief legal counsel. In a public meeting, a TMB official called the decision to withdraw “purely strategic,” according to Mr. Vandervoort.
This “raises troubling questions about the TMB’s motives in both filing, and subsequently retracting, the appeal,” Mr. Vandervoort said in a statement. “Teladoc and its amicus parties expended substantial resources on defending the appeal, which now will not result in a decision.”
The dispute stems from a medical board rule that requires Texas physicians to conduct a “face-to-face” evaluation before treating a patient via telemedicine. The face-to-face visit can be conducted through telemedicine at an established medical site, but it may not be established through an online questionnaire, e-mail, text, chat, or telephonic evaluation or consultation. In addition, the TMB requires that distant site providers establish a physician-patient relationship, which at a minimum includes: establishing that the person requesting the treatment is in fact who the person claims to be, establishing a diagnosis through the use of acceptable medical practices, discussing with the patient the risks and benefits of various treatment options, and ensuring the availability of the distant site provider or coverage of the patient for appropriate follow-up care.
Teladoc sued the medical board in April 2015 claiming the face-to-face rule violates federal antitrust laws. Teladoc provides access to medical care via phone or interactive video and treats patients for nonemergency conditions. A judge halted the rule’s enforcement until the litigation is resolved.
The TMB requested that a judge throw out the suit, arguing that the board is immune from antitrust liability as a state agency. A district court disagreed and allowed the case to proceed. The board then appealed to 5th U.S. Circuit Court of Appeals to overturn the district court’s decision.
It’s likely that the board’s decision not to pursue the appeal was affected by the recent backing of Teladoc by the U.S. Department of Justice (DOJ) and the Federal Trade Commission (FTC) regarding the application of the state action doctrine, said Paul W. Pitts, a San Francisco health law attorney who has closely followed the case. The doctrine protects the deliberate policy choices of sovereign states to displace competition with regulation or monopoly public service.
In a brief to the 5th Circuit, the DOJ and the FTC urged the appeals court to dismiss the board’s appeal. The agencies called TMB’s telemedicine rules anticompetitive and said the board was not protected by the state action doctrine because requirements under the doctrine were not satisfied. However, the America Medical Association and the Texas Medical Association sided with the TMB, telling the court the entity should be immune from federal antitrust liability.
Aside from the AMA and Texas Medical Association, the Texas board had few allies in the appeals dispute,” Mr. Pitts said in an interview.
“Many interested parties were lining up on the side of Teledoc by filing amici curiae arguing that the 5th Circuit lacked jurisdiction to hear this appeal and that the state action doctrine is not applicable,” he said. “As the market for telemedicine grows rapidly, there is an increasing number of parties with something at stake in this case.”
Now that the appeal has ended, the district court can get back to the primary issue at hand: whether the rule requiring a face-to-face exam can be justified or whether it’s just a means of protecting the traditional physician practice, Mr. Pitts said. The ultimate ruling in the case has broad implications for the practice of telemedicine in Texas and beyond.
“Medical boards in other states are revising their rules to be more accommodating of telemedicine as the use of this approach grows in acceptance around the country.” he said. “The Texas Medical Board is increasingly an outlier in this space. If Teledoc prevails, you can expect to see investors taking another look at the telemedicine space as the Texas market opens up and the role of the state medical board is diminished.”
[email protected]
On Twitter @legal_med
Public speaking fundamentals. Presentation follow-up: What to do after the last slide is shown

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Analysis: CMS expects no MACRA pay cut for most small practices
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.