User login
SHARE initiative releases consensus-based JDM management recommendations
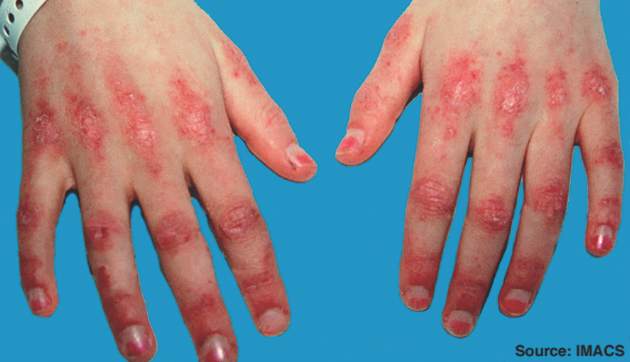
Early and aggressive therapy may prevent or stabilize organ damage and disease-related complications in patients with juvenile dermatomyositis, according to new consensus-based recommendations for the management of the disorder.
Overall, the recommendations – a project of the Single Hub and Access Point for Pediatric Rheumatology in Europe (SHARE) initiative – include 7 overarching principles, 33 recommendations on diagnosis, and 19 recommendations on therapy that were accepted with greater than 80% agreement among experts. The recommendations, which address the assessment of skin, muscle, and major organ involvement and treatment suggestions at disease onset and in refractory cases, fill a void in the area of evidence-based guidelines for this rare disease within the group of pediatric rheumatic diseases, according to Felicitas Bellutti Enders, MD, of University Medical Center Utrecht (the Netherlands) and her colleagues.
“Clear recommendations can help clinicians in the care of patients with JDM [juvenile dermatomyositis] as no international consensus regarding diagnosis and treatment is currently available and management therefore varies,” they wrote (Ann Rheum Dis. 2016 Aug 11. doi: 10.1136/annrheumdis-2016-209247).
The consensus committee, including 19 experienced pediatric rheumatologists and 2 experts in pediatric exercise physiology and physical therapy, developed recommendations based on a validated systematic literature review. The recommendations were evaluated by an online survey and then discussed at two subsequent consensus meetings.
The overarching principles accepted by more than 80% of respondents (100% in all but number 4 below), hold that:
1) All children with suspected idiopathic inflammatory myopathies should be referred to a specialized center.
2) High-risk patients need immediate/urgent referral to a specialized center.
3) Patient-/parent-reported outcomes measures are helpful when assessing disease activity and should be used at diagnosis and during disease monitoring.
4) Validated tools such as the Childhood Health Assessment Questionnaire, patient/parent visual analog scale and Juvenile Dermatomyositis Multi-dimensional Assessment Report should be used to measure health status.
5) All children with JDM should have disease activity (muscle, skin, major organ) assessed regularly in a standardized way, using tools such as the Disease Activity Score.
6) All children with JDM should have disease damage assessed at least yearly using a standardized disease damage measure, such as the Myositis Damage index.
7) All patients with JDM should have the opportunity to be registered within a research registry/repository such as the Euromyositis registry.
The consensus process also yielded both general and specific recommendations for diagnosis and management – also with 100% agreement in almost all cases – addressing investigations to consider in all those with a JDM diagnosis (muscle enzymes, full blood count, renal function and liver function tests, and infection screen – to name a few), ways that MRI can be used (both for diagnosis and disease activity monitoring), the use of muscle biopsy (use in all cases involving atypical presentation), and assessment of calcinosis and skin, lung, and cardiac involvement.
Treatment recommendations address sun protection (encourage routine use of sunblock), exercise (should be safe and appropriate and monitored by a physiotherapist), corticosteroid use (administer systematically either orally or intravenously in moderate to severe JDM and wean as the patient shows clinical improvement), use of intravenous immunoglobulin (a useful adjunct for resistant disease, especially when skin features are prominent), and the use of anti–tumor necrosis factor therapies (consider in refractory disease; infliximab or adalimumab are favored over etanercept), as well as other treatments.
The recommendations state that while there is no high-level evidence of when to stop therapy, consideration may be given to withdrawing treatment if a patient has been off steroids and in remission on methotrexate or an alternative disease-modifying antirheumatic drug [DMARD] for at least 1 year.
The management of JDM is complex and warrants a multidisciplinary approach, involving physiotherapists, specialist nurses, pediatric rheumatologists, and other specialists as needed, the authors wrote, noting that the mainstay of therapy is high-dose corticosteroids initially in combination with DMARDS.
However, the evidence base for treatment is limited and “often confined to small case-controlled studies, with the exception of two randomized controlled trials,” they said.
Similarly, there is no high-level evidence regarding when to stop immunosuppressive therapy, but the expert group suggested considering the withdrawal of methotrexate or alternative DMARD once the patient is in remission and off steroids for a minimum of 1 year.
The authors concluded that “this SHARE initiative is based on expert opinion informed by the best available evidence and provides recommendations ... with a view to improving the outcome for patients with JDM in Europe.
“It will now be important to broaden discussion and test acceptability of these to the wider community,” they wrote.
The SHARE initiative is funded by a grant from the European Agency for Health and Consumers. Dr. Enders disclosed a relationship with the Valeria e Ettore Bossi Foundation. Her coauthors reported financial or other relationships with Roche/Chugai, AbbVie, Pfizer, Novartis, Bristol-Myers Squibb, SOBI, Medac, The Myositis Association, Neopharm, GlaxoSmithKline, and/or Genzyme.
The consensus-based recommendations for the management of juvenile dermatomyositis published in Annals of Rheumatic Diseases again highlight the ability of the European and Canadian investigators to work together and pool the information from a large number of centers. Similar efforts to pool data and encourage the development of protocols to optimize care are occurring in the United States under the auspices of CARRA (the Childhood Arthritis and Rheumatology Research Alliance).
These working groups represent an important first step toward standardizing optimal care for children with rheumatic diseases. However, the protocols put forth by both groups suffer from failure to address the diversity of presentations within their diseases and a resultant lack of specificity in their recommendations. The authors of these guidelines make specific recommendations regarding diagnosis, but these fail to encompass the range of weakness that may be present initially. Is it truly appropriate to bolus every child diagnosed with dermatomyositis with high-dose corticosteroids and begin methotrexate? No mention is made of the hypertension, pancreatitis, or systemic infection that might result.
The guidelines and protocols being promulgated in pediatric rheumatology continue to suffer from the grouping of children with diverse disease presentations and probably diverse diseases under a single diagnosis. Charles Spencer and colleagues described the highly variable course of juvenile dermatomyositis and the presence of distinct subsets of patients more than 30 years ago (J Pediatr. 1984 Sep;105[3]:399-408), but this diversity is not reflected in the current protocol. Methods for better characterizing our patients based on gene activation and cytokine profiles have been developed. More effort should be placed on accurate characterization of our patients with many diseases before we attempt to treat them all the same way.
Thomas J.A. Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery, and professor of clinical pediatrics at Weill Medical Center, Cornell University, New York. He has no relevant disclosures.
The consensus-based recommendations for the management of juvenile dermatomyositis published in Annals of Rheumatic Diseases again highlight the ability of the European and Canadian investigators to work together and pool the information from a large number of centers. Similar efforts to pool data and encourage the development of protocols to optimize care are occurring in the United States under the auspices of CARRA (the Childhood Arthritis and Rheumatology Research Alliance).
These working groups represent an important first step toward standardizing optimal care for children with rheumatic diseases. However, the protocols put forth by both groups suffer from failure to address the diversity of presentations within their diseases and a resultant lack of specificity in their recommendations. The authors of these guidelines make specific recommendations regarding diagnosis, but these fail to encompass the range of weakness that may be present initially. Is it truly appropriate to bolus every child diagnosed with dermatomyositis with high-dose corticosteroids and begin methotrexate? No mention is made of the hypertension, pancreatitis, or systemic infection that might result.
The guidelines and protocols being promulgated in pediatric rheumatology continue to suffer from the grouping of children with diverse disease presentations and probably diverse diseases under a single diagnosis. Charles Spencer and colleagues described the highly variable course of juvenile dermatomyositis and the presence of distinct subsets of patients more than 30 years ago (J Pediatr. 1984 Sep;105[3]:399-408), but this diversity is not reflected in the current protocol. Methods for better characterizing our patients based on gene activation and cytokine profiles have been developed. More effort should be placed on accurate characterization of our patients with many diseases before we attempt to treat them all the same way.
Thomas J.A. Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery, and professor of clinical pediatrics at Weill Medical Center, Cornell University, New York. He has no relevant disclosures.
The consensus-based recommendations for the management of juvenile dermatomyositis published in Annals of Rheumatic Diseases again highlight the ability of the European and Canadian investigators to work together and pool the information from a large number of centers. Similar efforts to pool data and encourage the development of protocols to optimize care are occurring in the United States under the auspices of CARRA (the Childhood Arthritis and Rheumatology Research Alliance).
These working groups represent an important first step toward standardizing optimal care for children with rheumatic diseases. However, the protocols put forth by both groups suffer from failure to address the diversity of presentations within their diseases and a resultant lack of specificity in their recommendations. The authors of these guidelines make specific recommendations regarding diagnosis, but these fail to encompass the range of weakness that may be present initially. Is it truly appropriate to bolus every child diagnosed with dermatomyositis with high-dose corticosteroids and begin methotrexate? No mention is made of the hypertension, pancreatitis, or systemic infection that might result.
The guidelines and protocols being promulgated in pediatric rheumatology continue to suffer from the grouping of children with diverse disease presentations and probably diverse diseases under a single diagnosis. Charles Spencer and colleagues described the highly variable course of juvenile dermatomyositis and the presence of distinct subsets of patients more than 30 years ago (J Pediatr. 1984 Sep;105[3]:399-408), but this diversity is not reflected in the current protocol. Methods for better characterizing our patients based on gene activation and cytokine profiles have been developed. More effort should be placed on accurate characterization of our patients with many diseases before we attempt to treat them all the same way.
Thomas J.A. Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery, and professor of clinical pediatrics at Weill Medical Center, Cornell University, New York. He has no relevant disclosures.
Early and aggressive therapy may prevent or stabilize organ damage and disease-related complications in patients with juvenile dermatomyositis, according to new consensus-based recommendations for the management of the disorder.
Overall, the recommendations – a project of the Single Hub and Access Point for Pediatric Rheumatology in Europe (SHARE) initiative – include 7 overarching principles, 33 recommendations on diagnosis, and 19 recommendations on therapy that were accepted with greater than 80% agreement among experts. The recommendations, which address the assessment of skin, muscle, and major organ involvement and treatment suggestions at disease onset and in refractory cases, fill a void in the area of evidence-based guidelines for this rare disease within the group of pediatric rheumatic diseases, according to Felicitas Bellutti Enders, MD, of University Medical Center Utrecht (the Netherlands) and her colleagues.
“Clear recommendations can help clinicians in the care of patients with JDM [juvenile dermatomyositis] as no international consensus regarding diagnosis and treatment is currently available and management therefore varies,” they wrote (Ann Rheum Dis. 2016 Aug 11. doi: 10.1136/annrheumdis-2016-209247).
The consensus committee, including 19 experienced pediatric rheumatologists and 2 experts in pediatric exercise physiology and physical therapy, developed recommendations based on a validated systematic literature review. The recommendations were evaluated by an online survey and then discussed at two subsequent consensus meetings.
The overarching principles accepted by more than 80% of respondents (100% in all but number 4 below), hold that:
1) All children with suspected idiopathic inflammatory myopathies should be referred to a specialized center.
2) High-risk patients need immediate/urgent referral to a specialized center.
3) Patient-/parent-reported outcomes measures are helpful when assessing disease activity and should be used at diagnosis and during disease monitoring.
4) Validated tools such as the Childhood Health Assessment Questionnaire, patient/parent visual analog scale and Juvenile Dermatomyositis Multi-dimensional Assessment Report should be used to measure health status.
5) All children with JDM should have disease activity (muscle, skin, major organ) assessed regularly in a standardized way, using tools such as the Disease Activity Score.
6) All children with JDM should have disease damage assessed at least yearly using a standardized disease damage measure, such as the Myositis Damage index.
7) All patients with JDM should have the opportunity to be registered within a research registry/repository such as the Euromyositis registry.
The consensus process also yielded both general and specific recommendations for diagnosis and management – also with 100% agreement in almost all cases – addressing investigations to consider in all those with a JDM diagnosis (muscle enzymes, full blood count, renal function and liver function tests, and infection screen – to name a few), ways that MRI can be used (both for diagnosis and disease activity monitoring), the use of muscle biopsy (use in all cases involving atypical presentation), and assessment of calcinosis and skin, lung, and cardiac involvement.
Treatment recommendations address sun protection (encourage routine use of sunblock), exercise (should be safe and appropriate and monitored by a physiotherapist), corticosteroid use (administer systematically either orally or intravenously in moderate to severe JDM and wean as the patient shows clinical improvement), use of intravenous immunoglobulin (a useful adjunct for resistant disease, especially when skin features are prominent), and the use of anti–tumor necrosis factor therapies (consider in refractory disease; infliximab or adalimumab are favored over etanercept), as well as other treatments.
The recommendations state that while there is no high-level evidence of when to stop therapy, consideration may be given to withdrawing treatment if a patient has been off steroids and in remission on methotrexate or an alternative disease-modifying antirheumatic drug [DMARD] for at least 1 year.
The management of JDM is complex and warrants a multidisciplinary approach, involving physiotherapists, specialist nurses, pediatric rheumatologists, and other specialists as needed, the authors wrote, noting that the mainstay of therapy is high-dose corticosteroids initially in combination with DMARDS.
However, the evidence base for treatment is limited and “often confined to small case-controlled studies, with the exception of two randomized controlled trials,” they said.
Similarly, there is no high-level evidence regarding when to stop immunosuppressive therapy, but the expert group suggested considering the withdrawal of methotrexate or alternative DMARD once the patient is in remission and off steroids for a minimum of 1 year.
The authors concluded that “this SHARE initiative is based on expert opinion informed by the best available evidence and provides recommendations ... with a view to improving the outcome for patients with JDM in Europe.
“It will now be important to broaden discussion and test acceptability of these to the wider community,” they wrote.
The SHARE initiative is funded by a grant from the European Agency for Health and Consumers. Dr. Enders disclosed a relationship with the Valeria e Ettore Bossi Foundation. Her coauthors reported financial or other relationships with Roche/Chugai, AbbVie, Pfizer, Novartis, Bristol-Myers Squibb, SOBI, Medac, The Myositis Association, Neopharm, GlaxoSmithKline, and/or Genzyme.
Early and aggressive therapy may prevent or stabilize organ damage and disease-related complications in patients with juvenile dermatomyositis, according to new consensus-based recommendations for the management of the disorder.
Overall, the recommendations – a project of the Single Hub and Access Point for Pediatric Rheumatology in Europe (SHARE) initiative – include 7 overarching principles, 33 recommendations on diagnosis, and 19 recommendations on therapy that were accepted with greater than 80% agreement among experts. The recommendations, which address the assessment of skin, muscle, and major organ involvement and treatment suggestions at disease onset and in refractory cases, fill a void in the area of evidence-based guidelines for this rare disease within the group of pediatric rheumatic diseases, according to Felicitas Bellutti Enders, MD, of University Medical Center Utrecht (the Netherlands) and her colleagues.
“Clear recommendations can help clinicians in the care of patients with JDM [juvenile dermatomyositis] as no international consensus regarding diagnosis and treatment is currently available and management therefore varies,” they wrote (Ann Rheum Dis. 2016 Aug 11. doi: 10.1136/annrheumdis-2016-209247).
The consensus committee, including 19 experienced pediatric rheumatologists and 2 experts in pediatric exercise physiology and physical therapy, developed recommendations based on a validated systematic literature review. The recommendations were evaluated by an online survey and then discussed at two subsequent consensus meetings.
The overarching principles accepted by more than 80% of respondents (100% in all but number 4 below), hold that:
1) All children with suspected idiopathic inflammatory myopathies should be referred to a specialized center.
2) High-risk patients need immediate/urgent referral to a specialized center.
3) Patient-/parent-reported outcomes measures are helpful when assessing disease activity and should be used at diagnosis and during disease monitoring.
4) Validated tools such as the Childhood Health Assessment Questionnaire, patient/parent visual analog scale and Juvenile Dermatomyositis Multi-dimensional Assessment Report should be used to measure health status.
5) All children with JDM should have disease activity (muscle, skin, major organ) assessed regularly in a standardized way, using tools such as the Disease Activity Score.
6) All children with JDM should have disease damage assessed at least yearly using a standardized disease damage measure, such as the Myositis Damage index.
7) All patients with JDM should have the opportunity to be registered within a research registry/repository such as the Euromyositis registry.
The consensus process also yielded both general and specific recommendations for diagnosis and management – also with 100% agreement in almost all cases – addressing investigations to consider in all those with a JDM diagnosis (muscle enzymes, full blood count, renal function and liver function tests, and infection screen – to name a few), ways that MRI can be used (both for diagnosis and disease activity monitoring), the use of muscle biopsy (use in all cases involving atypical presentation), and assessment of calcinosis and skin, lung, and cardiac involvement.
Treatment recommendations address sun protection (encourage routine use of sunblock), exercise (should be safe and appropriate and monitored by a physiotherapist), corticosteroid use (administer systematically either orally or intravenously in moderate to severe JDM and wean as the patient shows clinical improvement), use of intravenous immunoglobulin (a useful adjunct for resistant disease, especially when skin features are prominent), and the use of anti–tumor necrosis factor therapies (consider in refractory disease; infliximab or adalimumab are favored over etanercept), as well as other treatments.
The recommendations state that while there is no high-level evidence of when to stop therapy, consideration may be given to withdrawing treatment if a patient has been off steroids and in remission on methotrexate or an alternative disease-modifying antirheumatic drug [DMARD] for at least 1 year.
The management of JDM is complex and warrants a multidisciplinary approach, involving physiotherapists, specialist nurses, pediatric rheumatologists, and other specialists as needed, the authors wrote, noting that the mainstay of therapy is high-dose corticosteroids initially in combination with DMARDS.
However, the evidence base for treatment is limited and “often confined to small case-controlled studies, with the exception of two randomized controlled trials,” they said.
Similarly, there is no high-level evidence regarding when to stop immunosuppressive therapy, but the expert group suggested considering the withdrawal of methotrexate or alternative DMARD once the patient is in remission and off steroids for a minimum of 1 year.
The authors concluded that “this SHARE initiative is based on expert opinion informed by the best available evidence and provides recommendations ... with a view to improving the outcome for patients with JDM in Europe.
“It will now be important to broaden discussion and test acceptability of these to the wider community,” they wrote.
The SHARE initiative is funded by a grant from the European Agency for Health and Consumers. Dr. Enders disclosed a relationship with the Valeria e Ettore Bossi Foundation. Her coauthors reported financial or other relationships with Roche/Chugai, AbbVie, Pfizer, Novartis, Bristol-Myers Squibb, SOBI, Medac, The Myositis Association, Neopharm, GlaxoSmithKline, and/or Genzyme.
FROM ANNALS OF THE RHEUMATIC DISEASES
Symptomatic Management of Dementia
VANCOUVER—Medications can help manage symptoms and prolong function in patients with dementia, even if the effects are modest and do not affect the underlying disease, according to an overview of dementia management provided at the 68th Annual Meeting of the American Academy of Neurology. Neurologists should bear in mind drug indications and patient diagnoses because certain drugs are indicated for Alzheimer’s disease dementia only, and it is unclear if they provide benefit in other forms of dementia.
Psychotropic medications also may play a role in treating behavioral and psychiatric symptoms, although it is important to first identify underlying causes that could trigger these symptoms, said Gregory S. Day, MD, MSc, Instructor in Neurology at Washington University in St. Louis.
Two Drug Types
Two types of medications are FDA-approved for the treatment of dementia: acetylcholinesterase inhibitors (eg, donepezil, rivastigmine, galantamine) and memantine, a partial NMDA-receptor antagonist. The FDA first approved an acetylcholinesterase inhibitor to treat dementia in 1993. Memantine was approved in 2003.
More recently, new formulations and dosages of those four drugs have been approved by the FDA, including a rivastigmine transdermal patch, a once-daily dose of memantine, and a donepezil and memantine combination pill.
Each medication requires a dose titration schedule. Donepezil is taken once daily; rivastigmine, galantamine, and memantine have once- and twice-daily formulations.
The acetylcholinesterase inhibitors are approved for the treatment of dementia due to Alzheimer’s disease. Rivastigmine also is FDA-approved for Parkinson’s disease dementia. Each drug also is used off label for the treatment of other forms of dementia, particularly dementia with Lewy bodies, Parkinson’s disease dementia, and vascular dementia, although it is unclear if off-label use in other forms of dementia provides benefit, Dr. Day said.
Donepezil and rivastigmine are approved for all stages of dementia, whereas galantamine is not approved for severe dementia because a controlled trial was never conducted in that patient population. A systematic review found no measurable effect of acetylcholinesterase inhibitors in patients with undifferentiated mild cognitive impairment.
In clinical trials, individuals with mild to moderate dementia due to Alzheimer’s disease taking acetylcholinesterase inhibitors had a modest benefit in function and cognition, compared with those taking placebo. “That benefit is relatively sustained throughout the course of the trial, but it is modest at best,” he said.
Adverse Events
Side effects of acetylcholinesterase inhibitors occur in 10% to 20% of patients. Gastrointestinal side effects (eg, nausea, vomiting, diarrhea, or anorexia) are the most common. Starting patients at a lower dose, varying the time of day the medication is taken, and taking the medication with food may alleviate some of these symptoms. Side effects may subside after one or two weeks, so it may be worth continuing the medication if the side effects are not severe, Dr. Day said.
Serious side effects can include syncope, convulsive seizures, loss of consciousness, and rhabdomyolysis. Less common side effects can include changes in cognitive or psychiatric comportment and cardiac complications. Rivastigmine in the transdermal formulation has an additional caveat because the patches can cause hypersensitivity reactions on the skin.
A 2015 review of drug prescribing records in North America found an increased risk of death in individuals taking acetylcholinesterase inhibitors. “Digging a little deeper, we see that this risk is almost entirely accounted for by rivastigmine,” Dr. Day said. “If anything, we could argue that people prescribed donepezil and galantamine have a lower risk of death than those not on these medications.”
Various factors could account for the elevated risk with rivastigmine. The drug has a slightly different mechanism of action and a longer half-life. Perhaps more importantly, as the only medication that can be delivered via transdermal patch, it may be prescribed to people with more severe forms of dementia who are no longer able to take medications by mouth, or who have agitation or psychoses. In addition, patients may forget to remove a patch and end up wearing multiple patches. Finally, rivastigmine is FDA-approved for Parkinson’s disease dementia, and the risk of death may be higher in those patients, Dr. Day said.
Memantine Alone and in Combination
Memantine is FDA-approved for patients with moderate to severe dementia due to Alzheimer’s disease. Pooled data indicate a small beneficial effect on cognition, activities of daily living, and behavior in this population. Despite multiple trials, there is no evidence of benefit of memantine in patients with mild dementia due to Alzheimer’s disease, and there is no convincing evidence to support the use of memantine in other forms of dementia.
Memantine is associated with fewer side effects than acetylcholinesterase inhibitors. Side effects can include dizziness, headache, and confusion. In clinical trials, more people taking placebo experienced side effects than those taking memantine. In patients with moderate to severe Alzheimer’s disease dementia receiving donepezil, the addition of memantine resulted in significantly better cognitive outcomes, function, and behavior. The addition of memantine is well tolerated in patients who are stable on an acetylcholinesterase inhibitor, said Dr. Day.
When to Stop Medication
Long-term observational data suggest a persistent effect of acetylcholinesterase inhibitors in Alzheimer’s disease dementia, with about five years of benefit in some trials. In a study that used admission to a nursing home as an outcome, the median time to admission in individuals not prescribed medication was around four to five years. The median time to admission in patients taking an acetylcholinesterase inhibitor was between eight and 10 years. There were not enough data for patients receiving combination therapy, but they may stay out of nursing homes a little longer. “There is this suggestion that use of these medications may delay admission to a nursing home,” he said.
Recommendations regarding stopping treatment vary. Some recommendations suggest that it is reasonable to discontinue these medications in patients who have transitioned to a severe dementia stage, while other recommendations suggest continuing the drugs indefinitely.
The American College of Physicians and American Academy of Family Physicians argue that if slowing decline is no longer a goal of treatment, memantine or acetylcholinesterase inhibitors are no longer appropriate.
If the family or patient’s “focus is shifting away from, ‘Let’s keep them as good as possible for as long as possible,’ to more, ‘Let’s keep them comfortable,’ then maybe removing a medication is part of that. I think it is very reasonable to consider,” Dr. Day said.
When considering stopping treatment, patients can stop medication for two weeks, and the patient and a caregiver can assess perceived cognition, function, and behaviors. If there is not a noticeable change, there is no need to restart the medication. “If they do perceive a decline, I think it is reasonable in that case to restart therapy if that is what they would like to do,” Dr. Day said. “Restarting, you simply revert back to the titration schedule that we would start whenever we’re prescribing these medications.”
Behavioral and Psychiatric Symptoms
Behavioral and psychiatric symptoms may occur in 60% to 90% of patients with dementia. These symptoms are distressing, add to caregiver burden, and are a major reason for institutionalization, Dr. Day said. Symptoms fall into four main categories: agitation, depression, apathy, and psychoses.
First, neurologists should screen for and treat any provoking causes, Dr. Day said. If a patient with a mild dementia syndrome suddenly presents with new agitation, delusions, or hallucinations, neurologists should investigate for oral ulceration, tooth problems, skin breakdown, urinary tract infection, dehydration, or visual or auditory impairment, which may contribute to or trigger these symptoms.
Otherwise, first-line treatment of agitation and psychoses entails atypical antipsychotic medication, although these treatments are not FDA-approved in patients with dementia. In patients with agitation alone, first-line therapy is behavioral interventions, although antipsychotic medications, mood stabilizers, or serotonergic compounds also can be considered. Depression and apathy may respond well to serotonergic compounds.
Black Box Warning
“Anytime we are prescribing antipsychotic medications to our patients with dementia, we need to be cognizant of the fact that these medications are prescribed off label and that they come with a black box warning,” Dr. Day said. Patients with dementia treated with antipsychotic drugs are at an increased risk of death. The risk of death appears to be greatest in those who are continued on antipsychotic medications across a three-month period. In addition, before prescribing antipsychotic medication, “you need to ask yourself—and maybe a nurse and a medical student—do you think that this patient could have dementia with Lewy bodies?” Patients with cortical Lewy bodies can experience significant morbidity or mortality due to severe neuroleptic sensitivity reactions.
Behavioral modification techniques are as effective as antipsychotic and antidepressant drugs, with fewer adverse effects, although they can be challenging to implement in a household environment.
Other Recommendations
When patients ask what else they can do to slow the progression of dementia, Dr. Day refers to seven modifiable risk factors identified in a 2014 Lancet Neurology article. These modifiable risk factors include physical inactivity, depression, midlife hypertension, midlife obesity, smoking, low educational attainment, and diabetes. In promoting physical activity, the CDC recommends a weekly exercise schedule of 150 minutes of moderate intensity activity or 75 minutes of more strenuous activity for individuals 65 and older. “That is a reasonable place to start,” Dr. Day said.
There is no clear demonstration of benefit of cognitive training, nor is there evidence to suggest that one cognitive training strategy is better than another. “If [patients] like doing sudoku or they like doing puzzles, Scrabble, or cards, that is what I am going to emphasize,” he said. “It is very reasonable to recommend that our patients remain cognitively and socially engaged, whatever that means for the individual patient.”
In addition, a Mediterranean-type diet high in olive oil, fresh vegetables, and fish may be worth recommending to patients and their caregivers, as it has been associated in studies with a lower risk of Alzheimer’s disease and mild cognitive impairment. Furthermore, neurologists should screen patients for sleep dysfunction, excessive alcohol intake, thyroid dysfunction, vitamin B12 deficiency, stroke risk factors, and medications that may impair cognition.
Other potential dementia therapies, including numerous interventions promoted online, have little or no evidence to support them. “We have a responsibility, in my opinion, to step in and provide some counseling regarding that to save our patients from committing to potentially expensive treatments with questionable efficacy,” said Dr. Day.
—Jake Remaly
Suggested Reading
Ali TB, Schleret TR, Reilly BM, et al. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS One. 2015;10(12):e0144337.
Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer’s disease progression. Alzheimers Dement. 2013;9(3):338-345.
VANCOUVER—Medications can help manage symptoms and prolong function in patients with dementia, even if the effects are modest and do not affect the underlying disease, according to an overview of dementia management provided at the 68th Annual Meeting of the American Academy of Neurology. Neurologists should bear in mind drug indications and patient diagnoses because certain drugs are indicated for Alzheimer’s disease dementia only, and it is unclear if they provide benefit in other forms of dementia.
Psychotropic medications also may play a role in treating behavioral and psychiatric symptoms, although it is important to first identify underlying causes that could trigger these symptoms, said Gregory S. Day, MD, MSc, Instructor in Neurology at Washington University in St. Louis.
Two Drug Types
Two types of medications are FDA-approved for the treatment of dementia: acetylcholinesterase inhibitors (eg, donepezil, rivastigmine, galantamine) and memantine, a partial NMDA-receptor antagonist. The FDA first approved an acetylcholinesterase inhibitor to treat dementia in 1993. Memantine was approved in 2003.
More recently, new formulations and dosages of those four drugs have been approved by the FDA, including a rivastigmine transdermal patch, a once-daily dose of memantine, and a donepezil and memantine combination pill.
Each medication requires a dose titration schedule. Donepezil is taken once daily; rivastigmine, galantamine, and memantine have once- and twice-daily formulations.
The acetylcholinesterase inhibitors are approved for the treatment of dementia due to Alzheimer’s disease. Rivastigmine also is FDA-approved for Parkinson’s disease dementia. Each drug also is used off label for the treatment of other forms of dementia, particularly dementia with Lewy bodies, Parkinson’s disease dementia, and vascular dementia, although it is unclear if off-label use in other forms of dementia provides benefit, Dr. Day said.
Donepezil and rivastigmine are approved for all stages of dementia, whereas galantamine is not approved for severe dementia because a controlled trial was never conducted in that patient population. A systematic review found no measurable effect of acetylcholinesterase inhibitors in patients with undifferentiated mild cognitive impairment.
In clinical trials, individuals with mild to moderate dementia due to Alzheimer’s disease taking acetylcholinesterase inhibitors had a modest benefit in function and cognition, compared with those taking placebo. “That benefit is relatively sustained throughout the course of the trial, but it is modest at best,” he said.
Adverse Events
Side effects of acetylcholinesterase inhibitors occur in 10% to 20% of patients. Gastrointestinal side effects (eg, nausea, vomiting, diarrhea, or anorexia) are the most common. Starting patients at a lower dose, varying the time of day the medication is taken, and taking the medication with food may alleviate some of these symptoms. Side effects may subside after one or two weeks, so it may be worth continuing the medication if the side effects are not severe, Dr. Day said.
Serious side effects can include syncope, convulsive seizures, loss of consciousness, and rhabdomyolysis. Less common side effects can include changes in cognitive or psychiatric comportment and cardiac complications. Rivastigmine in the transdermal formulation has an additional caveat because the patches can cause hypersensitivity reactions on the skin.
A 2015 review of drug prescribing records in North America found an increased risk of death in individuals taking acetylcholinesterase inhibitors. “Digging a little deeper, we see that this risk is almost entirely accounted for by rivastigmine,” Dr. Day said. “If anything, we could argue that people prescribed donepezil and galantamine have a lower risk of death than those not on these medications.”
Various factors could account for the elevated risk with rivastigmine. The drug has a slightly different mechanism of action and a longer half-life. Perhaps more importantly, as the only medication that can be delivered via transdermal patch, it may be prescribed to people with more severe forms of dementia who are no longer able to take medications by mouth, or who have agitation or psychoses. In addition, patients may forget to remove a patch and end up wearing multiple patches. Finally, rivastigmine is FDA-approved for Parkinson’s disease dementia, and the risk of death may be higher in those patients, Dr. Day said.
Memantine Alone and in Combination
Memantine is FDA-approved for patients with moderate to severe dementia due to Alzheimer’s disease. Pooled data indicate a small beneficial effect on cognition, activities of daily living, and behavior in this population. Despite multiple trials, there is no evidence of benefit of memantine in patients with mild dementia due to Alzheimer’s disease, and there is no convincing evidence to support the use of memantine in other forms of dementia.
Memantine is associated with fewer side effects than acetylcholinesterase inhibitors. Side effects can include dizziness, headache, and confusion. In clinical trials, more people taking placebo experienced side effects than those taking memantine. In patients with moderate to severe Alzheimer’s disease dementia receiving donepezil, the addition of memantine resulted in significantly better cognitive outcomes, function, and behavior. The addition of memantine is well tolerated in patients who are stable on an acetylcholinesterase inhibitor, said Dr. Day.
When to Stop Medication
Long-term observational data suggest a persistent effect of acetylcholinesterase inhibitors in Alzheimer’s disease dementia, with about five years of benefit in some trials. In a study that used admission to a nursing home as an outcome, the median time to admission in individuals not prescribed medication was around four to five years. The median time to admission in patients taking an acetylcholinesterase inhibitor was between eight and 10 years. There were not enough data for patients receiving combination therapy, but they may stay out of nursing homes a little longer. “There is this suggestion that use of these medications may delay admission to a nursing home,” he said.
Recommendations regarding stopping treatment vary. Some recommendations suggest that it is reasonable to discontinue these medications in patients who have transitioned to a severe dementia stage, while other recommendations suggest continuing the drugs indefinitely.
The American College of Physicians and American Academy of Family Physicians argue that if slowing decline is no longer a goal of treatment, memantine or acetylcholinesterase inhibitors are no longer appropriate.
If the family or patient’s “focus is shifting away from, ‘Let’s keep them as good as possible for as long as possible,’ to more, ‘Let’s keep them comfortable,’ then maybe removing a medication is part of that. I think it is very reasonable to consider,” Dr. Day said.
When considering stopping treatment, patients can stop medication for two weeks, and the patient and a caregiver can assess perceived cognition, function, and behaviors. If there is not a noticeable change, there is no need to restart the medication. “If they do perceive a decline, I think it is reasonable in that case to restart therapy if that is what they would like to do,” Dr. Day said. “Restarting, you simply revert back to the titration schedule that we would start whenever we’re prescribing these medications.”
Behavioral and Psychiatric Symptoms
Behavioral and psychiatric symptoms may occur in 60% to 90% of patients with dementia. These symptoms are distressing, add to caregiver burden, and are a major reason for institutionalization, Dr. Day said. Symptoms fall into four main categories: agitation, depression, apathy, and psychoses.
First, neurologists should screen for and treat any provoking causes, Dr. Day said. If a patient with a mild dementia syndrome suddenly presents with new agitation, delusions, or hallucinations, neurologists should investigate for oral ulceration, tooth problems, skin breakdown, urinary tract infection, dehydration, or visual or auditory impairment, which may contribute to or trigger these symptoms.
Otherwise, first-line treatment of agitation and psychoses entails atypical antipsychotic medication, although these treatments are not FDA-approved in patients with dementia. In patients with agitation alone, first-line therapy is behavioral interventions, although antipsychotic medications, mood stabilizers, or serotonergic compounds also can be considered. Depression and apathy may respond well to serotonergic compounds.
Black Box Warning
“Anytime we are prescribing antipsychotic medications to our patients with dementia, we need to be cognizant of the fact that these medications are prescribed off label and that they come with a black box warning,” Dr. Day said. Patients with dementia treated with antipsychotic drugs are at an increased risk of death. The risk of death appears to be greatest in those who are continued on antipsychotic medications across a three-month period. In addition, before prescribing antipsychotic medication, “you need to ask yourself—and maybe a nurse and a medical student—do you think that this patient could have dementia with Lewy bodies?” Patients with cortical Lewy bodies can experience significant morbidity or mortality due to severe neuroleptic sensitivity reactions.
Behavioral modification techniques are as effective as antipsychotic and antidepressant drugs, with fewer adverse effects, although they can be challenging to implement in a household environment.
Other Recommendations
When patients ask what else they can do to slow the progression of dementia, Dr. Day refers to seven modifiable risk factors identified in a 2014 Lancet Neurology article. These modifiable risk factors include physical inactivity, depression, midlife hypertension, midlife obesity, smoking, low educational attainment, and diabetes. In promoting physical activity, the CDC recommends a weekly exercise schedule of 150 minutes of moderate intensity activity or 75 minutes of more strenuous activity for individuals 65 and older. “That is a reasonable place to start,” Dr. Day said.
There is no clear demonstration of benefit of cognitive training, nor is there evidence to suggest that one cognitive training strategy is better than another. “If [patients] like doing sudoku or they like doing puzzles, Scrabble, or cards, that is what I am going to emphasize,” he said. “It is very reasonable to recommend that our patients remain cognitively and socially engaged, whatever that means for the individual patient.”
In addition, a Mediterranean-type diet high in olive oil, fresh vegetables, and fish may be worth recommending to patients and their caregivers, as it has been associated in studies with a lower risk of Alzheimer’s disease and mild cognitive impairment. Furthermore, neurologists should screen patients for sleep dysfunction, excessive alcohol intake, thyroid dysfunction, vitamin B12 deficiency, stroke risk factors, and medications that may impair cognition.
Other potential dementia therapies, including numerous interventions promoted online, have little or no evidence to support them. “We have a responsibility, in my opinion, to step in and provide some counseling regarding that to save our patients from committing to potentially expensive treatments with questionable efficacy,” said Dr. Day.
—Jake Remaly
VANCOUVER—Medications can help manage symptoms and prolong function in patients with dementia, even if the effects are modest and do not affect the underlying disease, according to an overview of dementia management provided at the 68th Annual Meeting of the American Academy of Neurology. Neurologists should bear in mind drug indications and patient diagnoses because certain drugs are indicated for Alzheimer’s disease dementia only, and it is unclear if they provide benefit in other forms of dementia.
Psychotropic medications also may play a role in treating behavioral and psychiatric symptoms, although it is important to first identify underlying causes that could trigger these symptoms, said Gregory S. Day, MD, MSc, Instructor in Neurology at Washington University in St. Louis.
Two Drug Types
Two types of medications are FDA-approved for the treatment of dementia: acetylcholinesterase inhibitors (eg, donepezil, rivastigmine, galantamine) and memantine, a partial NMDA-receptor antagonist. The FDA first approved an acetylcholinesterase inhibitor to treat dementia in 1993. Memantine was approved in 2003.
More recently, new formulations and dosages of those four drugs have been approved by the FDA, including a rivastigmine transdermal patch, a once-daily dose of memantine, and a donepezil and memantine combination pill.
Each medication requires a dose titration schedule. Donepezil is taken once daily; rivastigmine, galantamine, and memantine have once- and twice-daily formulations.
The acetylcholinesterase inhibitors are approved for the treatment of dementia due to Alzheimer’s disease. Rivastigmine also is FDA-approved for Parkinson’s disease dementia. Each drug also is used off label for the treatment of other forms of dementia, particularly dementia with Lewy bodies, Parkinson’s disease dementia, and vascular dementia, although it is unclear if off-label use in other forms of dementia provides benefit, Dr. Day said.
Donepezil and rivastigmine are approved for all stages of dementia, whereas galantamine is not approved for severe dementia because a controlled trial was never conducted in that patient population. A systematic review found no measurable effect of acetylcholinesterase inhibitors in patients with undifferentiated mild cognitive impairment.
In clinical trials, individuals with mild to moderate dementia due to Alzheimer’s disease taking acetylcholinesterase inhibitors had a modest benefit in function and cognition, compared with those taking placebo. “That benefit is relatively sustained throughout the course of the trial, but it is modest at best,” he said.
Adverse Events
Side effects of acetylcholinesterase inhibitors occur in 10% to 20% of patients. Gastrointestinal side effects (eg, nausea, vomiting, diarrhea, or anorexia) are the most common. Starting patients at a lower dose, varying the time of day the medication is taken, and taking the medication with food may alleviate some of these symptoms. Side effects may subside after one or two weeks, so it may be worth continuing the medication if the side effects are not severe, Dr. Day said.
Serious side effects can include syncope, convulsive seizures, loss of consciousness, and rhabdomyolysis. Less common side effects can include changes in cognitive or psychiatric comportment and cardiac complications. Rivastigmine in the transdermal formulation has an additional caveat because the patches can cause hypersensitivity reactions on the skin.
A 2015 review of drug prescribing records in North America found an increased risk of death in individuals taking acetylcholinesterase inhibitors. “Digging a little deeper, we see that this risk is almost entirely accounted for by rivastigmine,” Dr. Day said. “If anything, we could argue that people prescribed donepezil and galantamine have a lower risk of death than those not on these medications.”
Various factors could account for the elevated risk with rivastigmine. The drug has a slightly different mechanism of action and a longer half-life. Perhaps more importantly, as the only medication that can be delivered via transdermal patch, it may be prescribed to people with more severe forms of dementia who are no longer able to take medications by mouth, or who have agitation or psychoses. In addition, patients may forget to remove a patch and end up wearing multiple patches. Finally, rivastigmine is FDA-approved for Parkinson’s disease dementia, and the risk of death may be higher in those patients, Dr. Day said.
Memantine Alone and in Combination
Memantine is FDA-approved for patients with moderate to severe dementia due to Alzheimer’s disease. Pooled data indicate a small beneficial effect on cognition, activities of daily living, and behavior in this population. Despite multiple trials, there is no evidence of benefit of memantine in patients with mild dementia due to Alzheimer’s disease, and there is no convincing evidence to support the use of memantine in other forms of dementia.
Memantine is associated with fewer side effects than acetylcholinesterase inhibitors. Side effects can include dizziness, headache, and confusion. In clinical trials, more people taking placebo experienced side effects than those taking memantine. In patients with moderate to severe Alzheimer’s disease dementia receiving donepezil, the addition of memantine resulted in significantly better cognitive outcomes, function, and behavior. The addition of memantine is well tolerated in patients who are stable on an acetylcholinesterase inhibitor, said Dr. Day.
When to Stop Medication
Long-term observational data suggest a persistent effect of acetylcholinesterase inhibitors in Alzheimer’s disease dementia, with about five years of benefit in some trials. In a study that used admission to a nursing home as an outcome, the median time to admission in individuals not prescribed medication was around four to five years. The median time to admission in patients taking an acetylcholinesterase inhibitor was between eight and 10 years. There were not enough data for patients receiving combination therapy, but they may stay out of nursing homes a little longer. “There is this suggestion that use of these medications may delay admission to a nursing home,” he said.
Recommendations regarding stopping treatment vary. Some recommendations suggest that it is reasonable to discontinue these medications in patients who have transitioned to a severe dementia stage, while other recommendations suggest continuing the drugs indefinitely.
The American College of Physicians and American Academy of Family Physicians argue that if slowing decline is no longer a goal of treatment, memantine or acetylcholinesterase inhibitors are no longer appropriate.
If the family or patient’s “focus is shifting away from, ‘Let’s keep them as good as possible for as long as possible,’ to more, ‘Let’s keep them comfortable,’ then maybe removing a medication is part of that. I think it is very reasonable to consider,” Dr. Day said.
When considering stopping treatment, patients can stop medication for two weeks, and the patient and a caregiver can assess perceived cognition, function, and behaviors. If there is not a noticeable change, there is no need to restart the medication. “If they do perceive a decline, I think it is reasonable in that case to restart therapy if that is what they would like to do,” Dr. Day said. “Restarting, you simply revert back to the titration schedule that we would start whenever we’re prescribing these medications.”
Behavioral and Psychiatric Symptoms
Behavioral and psychiatric symptoms may occur in 60% to 90% of patients with dementia. These symptoms are distressing, add to caregiver burden, and are a major reason for institutionalization, Dr. Day said. Symptoms fall into four main categories: agitation, depression, apathy, and psychoses.
First, neurologists should screen for and treat any provoking causes, Dr. Day said. If a patient with a mild dementia syndrome suddenly presents with new agitation, delusions, or hallucinations, neurologists should investigate for oral ulceration, tooth problems, skin breakdown, urinary tract infection, dehydration, or visual or auditory impairment, which may contribute to or trigger these symptoms.
Otherwise, first-line treatment of agitation and psychoses entails atypical antipsychotic medication, although these treatments are not FDA-approved in patients with dementia. In patients with agitation alone, first-line therapy is behavioral interventions, although antipsychotic medications, mood stabilizers, or serotonergic compounds also can be considered. Depression and apathy may respond well to serotonergic compounds.
Black Box Warning
“Anytime we are prescribing antipsychotic medications to our patients with dementia, we need to be cognizant of the fact that these medications are prescribed off label and that they come with a black box warning,” Dr. Day said. Patients with dementia treated with antipsychotic drugs are at an increased risk of death. The risk of death appears to be greatest in those who are continued on antipsychotic medications across a three-month period. In addition, before prescribing antipsychotic medication, “you need to ask yourself—and maybe a nurse and a medical student—do you think that this patient could have dementia with Lewy bodies?” Patients with cortical Lewy bodies can experience significant morbidity or mortality due to severe neuroleptic sensitivity reactions.
Behavioral modification techniques are as effective as antipsychotic and antidepressant drugs, with fewer adverse effects, although they can be challenging to implement in a household environment.
Other Recommendations
When patients ask what else they can do to slow the progression of dementia, Dr. Day refers to seven modifiable risk factors identified in a 2014 Lancet Neurology article. These modifiable risk factors include physical inactivity, depression, midlife hypertension, midlife obesity, smoking, low educational attainment, and diabetes. In promoting physical activity, the CDC recommends a weekly exercise schedule of 150 minutes of moderate intensity activity or 75 minutes of more strenuous activity for individuals 65 and older. “That is a reasonable place to start,” Dr. Day said.
There is no clear demonstration of benefit of cognitive training, nor is there evidence to suggest that one cognitive training strategy is better than another. “If [patients] like doing sudoku or they like doing puzzles, Scrabble, or cards, that is what I am going to emphasize,” he said. “It is very reasonable to recommend that our patients remain cognitively and socially engaged, whatever that means for the individual patient.”
In addition, a Mediterranean-type diet high in olive oil, fresh vegetables, and fish may be worth recommending to patients and their caregivers, as it has been associated in studies with a lower risk of Alzheimer’s disease and mild cognitive impairment. Furthermore, neurologists should screen patients for sleep dysfunction, excessive alcohol intake, thyroid dysfunction, vitamin B12 deficiency, stroke risk factors, and medications that may impair cognition.
Other potential dementia therapies, including numerous interventions promoted online, have little or no evidence to support them. “We have a responsibility, in my opinion, to step in and provide some counseling regarding that to save our patients from committing to potentially expensive treatments with questionable efficacy,” said Dr. Day.
—Jake Remaly
Suggested Reading
Ali TB, Schleret TR, Reilly BM, et al. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS One. 2015;10(12):e0144337.
Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer’s disease progression. Alzheimers Dement. 2013;9(3):338-345.
Suggested Reading
Ali TB, Schleret TR, Reilly BM, et al. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS One. 2015;10(12):e0144337.
Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer’s disease progression. Alzheimers Dement. 2013;9(3):338-345.
Expanded Indication for DBS Sets Stage for Trial in Early Parkinson’s Disease
HILTON HEAD, SC—An expanded indication for deep brain stimulation (DBS) in mid-stage Parkinson’s disease, approved by the FDA in November 2015, is “a big step” toward use of DBS earlier in the disease, said David Charles, MD, Professor and Vice-Chair of Neurology at Vanderbilt University in Nashville and Chief Medical Officer of the Vanderbilt Neuroscience Institute.
Results from a pilot trial of DBS in early Parkinson’s disease suggest that early treatment may slow the progression of tremor, a finding discovered by Mallory Hacker, PhD, Assistant Professor of Neurology at Vanderbilt University. “If this finding holds up in a pivotal trial, this would be the first therapy shown to slow the progression of anything in Parkinson’s disease,” Dr. Charles said.
Expanded Indication
Since 2002, DBS has been indicated for advanced Parkinson’s disease when symptoms are no longer adequately controlled with medicine. This year, Dublin-based Medtronic announced that the FDA had approved an expanded indication for its DBS therapy. Bilateral stimulation of the subthalamic nucleus or internal globus pallidus gained an indication for adjunctive therapy in patients with Parkinson’s disease duration of at least four years and at least four months of motor complications.
This expanded indication was based on results from the EARLYSTIM clinical trial published in the New England Journal of Medicine in 2013. The trial enrolled 251 participants with disease duration of at least four years and the presence of dyskinesias or other motor fluctuations. Patients treated with DBS and medical therapy reported an average 26% improvement in disease-related quality of life at two years, compared with a 1% decline in patients treated with medical therapy alone.
Researchers have hypothesized that early treatment with DBS might slow the progression of Parkinson’s disease, and preclinical studies suggest a possible mechanism by which DBS could have this effect, said Dr. Charles. Caryl Sortwell, PhD, Professor of Translational Science and Molecular Medicine at Michigan State University in Grand Rapids, and colleagues have shown in animal models that brain-derived neurotrophic growth factor (BDNF) is upregulated in the substantia nigra when the subthalamic nucleus undergoes stimulation. This increased production of BDNF may be therapeutic and also neuroprotective, Dr. Charles said.
Four Patients in Pilot Study Improved
In 2006, researchers at Vanderbilt University initiated a pilot study in 30 patients with early Parkinson’s disease to evaluate the safety and tolerability of DBS. Participants were between the ages of 50 and 75, had received medication for at least six months but not more than four years, and had no history of dyskinesias or other motor fluctuations. Participants had an average age of 60 and had been on medication for an average of about two years. Half of the participants received DBS plus medicine, and half received standard medication only. They were followed for 24 months. Researchers observed a trend toward better motor outcomes in the DBS group, compared with the medication-only group, but the differences were not statistically significant. In addition, the DBS group took less medication than the medication-only group at every time point. At two years, the medication-only group began to experience complications of therapy (eg, fluctuations or dyskinesias), whereas the DBS group did not.
Every six months during the study, patients were admitted to the hospital and stopped all medicine (and stimulation, if present) for a week. Motor scores after the washout periods in the DBS group trended better than those in the medicine group at 24 months. When researchers evaluated patients’ motor scores after each washout period individually, all 14 patients in the medication-only group worsened over two years. In the DBS group, five of 14 patients did not worsen; four patients actually improved. “That should not happen,” Dr. Charles said. These results from the pilot study suggest “a nearly fourfold increase in the chance of not worsening if you give DBS plus medicine early,” he said.
In addition, when researchers examined patients’ tremor after the seven-day washout, tremor scores in the DBS group did not worsen, whereas tremor scores in patients who received medication only slowly worsened over time as expected.
Planned Pivotal Trial
In the phase III trial of DBS in early Parkinson’s disease, investigators plan to enroll 280 patients at 18 centers. Eligible patients will have received medication for Parkinson’s disease for at least one year but less than four years and have no history of motor fluctuations. All participants will undergo DBS surgery and be randomized to have their stimulator kept off and continue on standard medical therapy, or to have their stimulator turned on in addition to receiving medical therapy. Researchers will follow the participants for two years.
The primary end point for the trial will be a composite measure that assesses whether, at 24 months, a patient worsened or not. Worsening will be defined as a three-point increase in the Unified Parkinson’s Disease Rating Scale (UPDRS) III on-treatment motor score, combined with a one-point increase in the UPDRS IV complications of therapy score, Dr. Charles said.
—Jake Remaly
Suggested Reading
Hacker ML, Tonascia J, Turchan M, et al. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(10):1177-1183.
Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610-622.
Spieles-Engemann AL, Steece-Collier K, Behbehani MM, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis. 2011;1(1):123-136.
HILTON HEAD, SC—An expanded indication for deep brain stimulation (DBS) in mid-stage Parkinson’s disease, approved by the FDA in November 2015, is “a big step” toward use of DBS earlier in the disease, said David Charles, MD, Professor and Vice-Chair of Neurology at Vanderbilt University in Nashville and Chief Medical Officer of the Vanderbilt Neuroscience Institute.
Results from a pilot trial of DBS in early Parkinson’s disease suggest that early treatment may slow the progression of tremor, a finding discovered by Mallory Hacker, PhD, Assistant Professor of Neurology at Vanderbilt University. “If this finding holds up in a pivotal trial, this would be the first therapy shown to slow the progression of anything in Parkinson’s disease,” Dr. Charles said.
Expanded Indication
Since 2002, DBS has been indicated for advanced Parkinson’s disease when symptoms are no longer adequately controlled with medicine. This year, Dublin-based Medtronic announced that the FDA had approved an expanded indication for its DBS therapy. Bilateral stimulation of the subthalamic nucleus or internal globus pallidus gained an indication for adjunctive therapy in patients with Parkinson’s disease duration of at least four years and at least four months of motor complications.
This expanded indication was based on results from the EARLYSTIM clinical trial published in the New England Journal of Medicine in 2013. The trial enrolled 251 participants with disease duration of at least four years and the presence of dyskinesias or other motor fluctuations. Patients treated with DBS and medical therapy reported an average 26% improvement in disease-related quality of life at two years, compared with a 1% decline in patients treated with medical therapy alone.
Researchers have hypothesized that early treatment with DBS might slow the progression of Parkinson’s disease, and preclinical studies suggest a possible mechanism by which DBS could have this effect, said Dr. Charles. Caryl Sortwell, PhD, Professor of Translational Science and Molecular Medicine at Michigan State University in Grand Rapids, and colleagues have shown in animal models that brain-derived neurotrophic growth factor (BDNF) is upregulated in the substantia nigra when the subthalamic nucleus undergoes stimulation. This increased production of BDNF may be therapeutic and also neuroprotective, Dr. Charles said.
Four Patients in Pilot Study Improved
In 2006, researchers at Vanderbilt University initiated a pilot study in 30 patients with early Parkinson’s disease to evaluate the safety and tolerability of DBS. Participants were between the ages of 50 and 75, had received medication for at least six months but not more than four years, and had no history of dyskinesias or other motor fluctuations. Participants had an average age of 60 and had been on medication for an average of about two years. Half of the participants received DBS plus medicine, and half received standard medication only. They were followed for 24 months. Researchers observed a trend toward better motor outcomes in the DBS group, compared with the medication-only group, but the differences were not statistically significant. In addition, the DBS group took less medication than the medication-only group at every time point. At two years, the medication-only group began to experience complications of therapy (eg, fluctuations or dyskinesias), whereas the DBS group did not.
Every six months during the study, patients were admitted to the hospital and stopped all medicine (and stimulation, if present) for a week. Motor scores after the washout periods in the DBS group trended better than those in the medicine group at 24 months. When researchers evaluated patients’ motor scores after each washout period individually, all 14 patients in the medication-only group worsened over two years. In the DBS group, five of 14 patients did not worsen; four patients actually improved. “That should not happen,” Dr. Charles said. These results from the pilot study suggest “a nearly fourfold increase in the chance of not worsening if you give DBS plus medicine early,” he said.
In addition, when researchers examined patients’ tremor after the seven-day washout, tremor scores in the DBS group did not worsen, whereas tremor scores in patients who received medication only slowly worsened over time as expected.
Planned Pivotal Trial
In the phase III trial of DBS in early Parkinson’s disease, investigators plan to enroll 280 patients at 18 centers. Eligible patients will have received medication for Parkinson’s disease for at least one year but less than four years and have no history of motor fluctuations. All participants will undergo DBS surgery and be randomized to have their stimulator kept off and continue on standard medical therapy, or to have their stimulator turned on in addition to receiving medical therapy. Researchers will follow the participants for two years.
The primary end point for the trial will be a composite measure that assesses whether, at 24 months, a patient worsened or not. Worsening will be defined as a three-point increase in the Unified Parkinson’s Disease Rating Scale (UPDRS) III on-treatment motor score, combined with a one-point increase in the UPDRS IV complications of therapy score, Dr. Charles said.
—Jake Remaly
HILTON HEAD, SC—An expanded indication for deep brain stimulation (DBS) in mid-stage Parkinson’s disease, approved by the FDA in November 2015, is “a big step” toward use of DBS earlier in the disease, said David Charles, MD, Professor and Vice-Chair of Neurology at Vanderbilt University in Nashville and Chief Medical Officer of the Vanderbilt Neuroscience Institute.
Results from a pilot trial of DBS in early Parkinson’s disease suggest that early treatment may slow the progression of tremor, a finding discovered by Mallory Hacker, PhD, Assistant Professor of Neurology at Vanderbilt University. “If this finding holds up in a pivotal trial, this would be the first therapy shown to slow the progression of anything in Parkinson’s disease,” Dr. Charles said.
Expanded Indication
Since 2002, DBS has been indicated for advanced Parkinson’s disease when symptoms are no longer adequately controlled with medicine. This year, Dublin-based Medtronic announced that the FDA had approved an expanded indication for its DBS therapy. Bilateral stimulation of the subthalamic nucleus or internal globus pallidus gained an indication for adjunctive therapy in patients with Parkinson’s disease duration of at least four years and at least four months of motor complications.
This expanded indication was based on results from the EARLYSTIM clinical trial published in the New England Journal of Medicine in 2013. The trial enrolled 251 participants with disease duration of at least four years and the presence of dyskinesias or other motor fluctuations. Patients treated with DBS and medical therapy reported an average 26% improvement in disease-related quality of life at two years, compared with a 1% decline in patients treated with medical therapy alone.
Researchers have hypothesized that early treatment with DBS might slow the progression of Parkinson’s disease, and preclinical studies suggest a possible mechanism by which DBS could have this effect, said Dr. Charles. Caryl Sortwell, PhD, Professor of Translational Science and Molecular Medicine at Michigan State University in Grand Rapids, and colleagues have shown in animal models that brain-derived neurotrophic growth factor (BDNF) is upregulated in the substantia nigra when the subthalamic nucleus undergoes stimulation. This increased production of BDNF may be therapeutic and also neuroprotective, Dr. Charles said.
Four Patients in Pilot Study Improved
In 2006, researchers at Vanderbilt University initiated a pilot study in 30 patients with early Parkinson’s disease to evaluate the safety and tolerability of DBS. Participants were between the ages of 50 and 75, had received medication for at least six months but not more than four years, and had no history of dyskinesias or other motor fluctuations. Participants had an average age of 60 and had been on medication for an average of about two years. Half of the participants received DBS plus medicine, and half received standard medication only. They were followed for 24 months. Researchers observed a trend toward better motor outcomes in the DBS group, compared with the medication-only group, but the differences were not statistically significant. In addition, the DBS group took less medication than the medication-only group at every time point. At two years, the medication-only group began to experience complications of therapy (eg, fluctuations or dyskinesias), whereas the DBS group did not.
Every six months during the study, patients were admitted to the hospital and stopped all medicine (and stimulation, if present) for a week. Motor scores after the washout periods in the DBS group trended better than those in the medicine group at 24 months. When researchers evaluated patients’ motor scores after each washout period individually, all 14 patients in the medication-only group worsened over two years. In the DBS group, five of 14 patients did not worsen; four patients actually improved. “That should not happen,” Dr. Charles said. These results from the pilot study suggest “a nearly fourfold increase in the chance of not worsening if you give DBS plus medicine early,” he said.
In addition, when researchers examined patients’ tremor after the seven-day washout, tremor scores in the DBS group did not worsen, whereas tremor scores in patients who received medication only slowly worsened over time as expected.
Planned Pivotal Trial
In the phase III trial of DBS in early Parkinson’s disease, investigators plan to enroll 280 patients at 18 centers. Eligible patients will have received medication for Parkinson’s disease for at least one year but less than four years and have no history of motor fluctuations. All participants will undergo DBS surgery and be randomized to have their stimulator kept off and continue on standard medical therapy, or to have their stimulator turned on in addition to receiving medical therapy. Researchers will follow the participants for two years.
The primary end point for the trial will be a composite measure that assesses whether, at 24 months, a patient worsened or not. Worsening will be defined as a three-point increase in the Unified Parkinson’s Disease Rating Scale (UPDRS) III on-treatment motor score, combined with a one-point increase in the UPDRS IV complications of therapy score, Dr. Charles said.
—Jake Remaly
Suggested Reading
Hacker ML, Tonascia J, Turchan M, et al. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(10):1177-1183.
Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610-622.
Spieles-Engemann AL, Steece-Collier K, Behbehani MM, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis. 2011;1(1):123-136.
Suggested Reading
Hacker ML, Tonascia J, Turchan M, et al. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(10):1177-1183.
Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610-622.
Spieles-Engemann AL, Steece-Collier K, Behbehani MM, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis. 2011;1(1):123-136.
Is burnout on the rise and what are the signs ObGyns should be on the lookout for?
In a peer-to-peer audiocast, Ms. DiVenere probed Dr. Smith for the problem areas that appear in the early to late stages of burnout. He spoke to the stressors that residents experience in the learning situation and that ObGyns must deal with in practice, as well as described strategies to deal with that stress.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Have you read "ObGyn burnout: ACOG takes aim," by Lucia DiVenere, MA (September 2016)?
In a peer-to-peer audiocast, Ms. DiVenere probed Dr. Smith for the problem areas that appear in the early to late stages of burnout. He spoke to the stressors that residents experience in the learning situation and that ObGyns must deal with in practice, as well as described strategies to deal with that stress.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Have you read "ObGyn burnout: ACOG takes aim," by Lucia DiVenere, MA (September 2016)?
In a peer-to-peer audiocast, Ms. DiVenere probed Dr. Smith for the problem areas that appear in the early to late stages of burnout. He spoke to the stressors that residents experience in the learning situation and that ObGyns must deal with in practice, as well as described strategies to deal with that stress.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Have you read "ObGyn burnout: ACOG takes aim," by Lucia DiVenere, MA (September 2016)?
Checklist May Capture Predementia Diagnosis of Mild Behavioral Impairment
TORONTO—A behavioral syndrome called mild behavioral impairment (MBI) may be a forerunner of Alzheimer’s disease and other neurodegenerative diseases, according to research described at the Alzheimer’s Association International Conference. Investigators have developed a tool for diagnosing it.
MBI, according to the researchers, is a syndrome characterized by new-onset neuropsychiatric symptoms that are sustained for at least six months in patients without dementia who are older than 50. Symptoms can occur in any of the following five domains: motivation, mood, impulse control, social appropriateness, and psychosis.
Zahinoor Ismail, MD, Assistant Professor of Psychiatry and Neurology at the Hotchkiss Brain Institute of the University of Calgary in Canada, described MBI at the conference and unveiled the MBI Checklist (MBI-C), a two-page screen that identifies and scores these symptoms. The MBI-C is a project of the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART).
Family members or informants rate the presence and severity of their symptoms on a three-point scale. The researchers who developed the MBI-C chose age 50 as the lower age limit because symptoms that emerge at that age can herald the onset of frontotemporal dementia. While the MBI-C is available for clinical and research use, the scoring risk stratification is still being validated. At present, the MBI-C identifies symptoms of concern and monitors change over time, said Dr. Ismail.
Changes in personality are often the earliest signs of an emerging neurocognitive disorder and appear well before any problems with memory or cognition. The MBI-C is intended to identify and track these changes in patients.
“We can now describe this preclinical dementia phenotype and use this tool to diagnose it and to capture change over time,” said Dr. Ismail.
Checklist May Aid Research
In addition to being clinically useful, the checklist will aid research, he added. It could identify a population at greatest risk for neurocognitive decline at a time when any nonpharmacological interventions or future disease-modifying drugs could be most beneficial.
“We all know that dementia is much more than memory or cognitive impairment alone. The neuropsychiatric symptoms of dementia are associated with functional impairment, caregiver burden, institutionalization, accelerated rates of progression, and a greater burden of plaques and tangles. There is a great need to identify people early on, people in whom we might be able to change the course of illness. These patients who present with early neuropsychiatric symptoms may be a population we can examine to see if that is possible,” said Dr. Ismail.
Previous studies have linked new-onset neuropsychiatric symptoms with neurocognitive disease, particularly frontotemporal dementia. One study included about 500 subjects enrolled in the ongoing Mayo Clinic Study of Aging. Investigators followed these participants for five years. This study found that the emergence of neuropsychiatric symptoms in cognitively normal older adults was associated with significant increases in the risk of developing mild cognitive impairment (MCI). Agitation conferred the highest risk (hazard ratio [HR], 3.06), followed by apathy (HR, 2.6), anxiety (HR, 1.87), irritability (HR, 1.84), and depression (HR, 1.63).
New-onset neuropsychiatric symptoms are common by the time patients enter care for memory concerns. Dr. Ismail presented data on a group of about 300 patients with MCI who attended a memory clinic. About 82% of patients endorsed at least one neuropsychiatric symptom. As measured by the MBI-C, 78% of patients reported mood symptoms, 64% reported impulse-control symptoms, 52% reported apathy, 28% reported social appropriateness, and 9% reported psychotic symptoms.
Validation of MBI-C Is Ongoing
“Our study suggests that this concept of MBI may be a common and clinically relevant syndrome, particularly given that neuropsychiatric symptoms are associated with greater caregiver burden,” said Dr. Ismail.
“This idea of symptoms persisting for at least six months is important,” he continued. “What we’re talking about is a sustained change from baseline personality. But these are still patients without dementia. Function is maintained. Independent activities of daily living are intact.”
The most comprehensive domain encompasses impulse control, agitation, and reward. “This [domain] captures a lot of function with regard to agitation in dementia, new-onset substance abuse, irritability, new-onset road rage … things we might not otherwise capture,” said Dr. Ismail.
The social appropriateness domain examines symptoms like a loss of the ability to share appropriately, acting out sexually, and loss of social judgment. The psychosis domain inquires about feelings of aggrandizement, persecution, and suspicion, as well as auditory and visual hallucinations.
The mood domain asks about new-onset anxiety, panic, and depression. The motivation domain asks about the development of apathy or disinterest in family, friends, and activities.
Validation studies in large cohorts are ongoing, as are studies that Dr. Ismail hopes will link these early behavioral changes to well-established Alzheimer’s disease biomarkers. The checklist is availablefor free by request emailed to [email protected].
—Michele G. Sullivan
Suggested Reading
Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493-498.
Geda YE, Roberts RO, Mielke MM et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572-581.
Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202.
Mirza SS, Wolters FJ, Swanson SA, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3(7):628-635.
TORONTO—A behavioral syndrome called mild behavioral impairment (MBI) may be a forerunner of Alzheimer’s disease and other neurodegenerative diseases, according to research described at the Alzheimer’s Association International Conference. Investigators have developed a tool for diagnosing it.
MBI, according to the researchers, is a syndrome characterized by new-onset neuropsychiatric symptoms that are sustained for at least six months in patients without dementia who are older than 50. Symptoms can occur in any of the following five domains: motivation, mood, impulse control, social appropriateness, and psychosis.
Zahinoor Ismail, MD, Assistant Professor of Psychiatry and Neurology at the Hotchkiss Brain Institute of the University of Calgary in Canada, described MBI at the conference and unveiled the MBI Checklist (MBI-C), a two-page screen that identifies and scores these symptoms. The MBI-C is a project of the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART).
Family members or informants rate the presence and severity of their symptoms on a three-point scale. The researchers who developed the MBI-C chose age 50 as the lower age limit because symptoms that emerge at that age can herald the onset of frontotemporal dementia. While the MBI-C is available for clinical and research use, the scoring risk stratification is still being validated. At present, the MBI-C identifies symptoms of concern and monitors change over time, said Dr. Ismail.
Changes in personality are often the earliest signs of an emerging neurocognitive disorder and appear well before any problems with memory or cognition. The MBI-C is intended to identify and track these changes in patients.
“We can now describe this preclinical dementia phenotype and use this tool to diagnose it and to capture change over time,” said Dr. Ismail.
Checklist May Aid Research
In addition to being clinically useful, the checklist will aid research, he added. It could identify a population at greatest risk for neurocognitive decline at a time when any nonpharmacological interventions or future disease-modifying drugs could be most beneficial.
“We all know that dementia is much more than memory or cognitive impairment alone. The neuropsychiatric symptoms of dementia are associated with functional impairment, caregiver burden, institutionalization, accelerated rates of progression, and a greater burden of plaques and tangles. There is a great need to identify people early on, people in whom we might be able to change the course of illness. These patients who present with early neuropsychiatric symptoms may be a population we can examine to see if that is possible,” said Dr. Ismail.
Previous studies have linked new-onset neuropsychiatric symptoms with neurocognitive disease, particularly frontotemporal dementia. One study included about 500 subjects enrolled in the ongoing Mayo Clinic Study of Aging. Investigators followed these participants for five years. This study found that the emergence of neuropsychiatric symptoms in cognitively normal older adults was associated with significant increases in the risk of developing mild cognitive impairment (MCI). Agitation conferred the highest risk (hazard ratio [HR], 3.06), followed by apathy (HR, 2.6), anxiety (HR, 1.87), irritability (HR, 1.84), and depression (HR, 1.63).
New-onset neuropsychiatric symptoms are common by the time patients enter care for memory concerns. Dr. Ismail presented data on a group of about 300 patients with MCI who attended a memory clinic. About 82% of patients endorsed at least one neuropsychiatric symptom. As measured by the MBI-C, 78% of patients reported mood symptoms, 64% reported impulse-control symptoms, 52% reported apathy, 28% reported social appropriateness, and 9% reported psychotic symptoms.
Validation of MBI-C Is Ongoing
“Our study suggests that this concept of MBI may be a common and clinically relevant syndrome, particularly given that neuropsychiatric symptoms are associated with greater caregiver burden,” said Dr. Ismail.
“This idea of symptoms persisting for at least six months is important,” he continued. “What we’re talking about is a sustained change from baseline personality. But these are still patients without dementia. Function is maintained. Independent activities of daily living are intact.”
The most comprehensive domain encompasses impulse control, agitation, and reward. “This [domain] captures a lot of function with regard to agitation in dementia, new-onset substance abuse, irritability, new-onset road rage … things we might not otherwise capture,” said Dr. Ismail.
The social appropriateness domain examines symptoms like a loss of the ability to share appropriately, acting out sexually, and loss of social judgment. The psychosis domain inquires about feelings of aggrandizement, persecution, and suspicion, as well as auditory and visual hallucinations.
The mood domain asks about new-onset anxiety, panic, and depression. The motivation domain asks about the development of apathy or disinterest in family, friends, and activities.
Validation studies in large cohorts are ongoing, as are studies that Dr. Ismail hopes will link these early behavioral changes to well-established Alzheimer’s disease biomarkers. The checklist is availablefor free by request emailed to [email protected].
—Michele G. Sullivan
TORONTO—A behavioral syndrome called mild behavioral impairment (MBI) may be a forerunner of Alzheimer’s disease and other neurodegenerative diseases, according to research described at the Alzheimer’s Association International Conference. Investigators have developed a tool for diagnosing it.
MBI, according to the researchers, is a syndrome characterized by new-onset neuropsychiatric symptoms that are sustained for at least six months in patients without dementia who are older than 50. Symptoms can occur in any of the following five domains: motivation, mood, impulse control, social appropriateness, and psychosis.
Zahinoor Ismail, MD, Assistant Professor of Psychiatry and Neurology at the Hotchkiss Brain Institute of the University of Calgary in Canada, described MBI at the conference and unveiled the MBI Checklist (MBI-C), a two-page screen that identifies and scores these symptoms. The MBI-C is a project of the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART).
Family members or informants rate the presence and severity of their symptoms on a three-point scale. The researchers who developed the MBI-C chose age 50 as the lower age limit because symptoms that emerge at that age can herald the onset of frontotemporal dementia. While the MBI-C is available for clinical and research use, the scoring risk stratification is still being validated. At present, the MBI-C identifies symptoms of concern and monitors change over time, said Dr. Ismail.
Changes in personality are often the earliest signs of an emerging neurocognitive disorder and appear well before any problems with memory or cognition. The MBI-C is intended to identify and track these changes in patients.
“We can now describe this preclinical dementia phenotype and use this tool to diagnose it and to capture change over time,” said Dr. Ismail.
Checklist May Aid Research
In addition to being clinically useful, the checklist will aid research, he added. It could identify a population at greatest risk for neurocognitive decline at a time when any nonpharmacological interventions or future disease-modifying drugs could be most beneficial.
“We all know that dementia is much more than memory or cognitive impairment alone. The neuropsychiatric symptoms of dementia are associated with functional impairment, caregiver burden, institutionalization, accelerated rates of progression, and a greater burden of plaques and tangles. There is a great need to identify people early on, people in whom we might be able to change the course of illness. These patients who present with early neuropsychiatric symptoms may be a population we can examine to see if that is possible,” said Dr. Ismail.
Previous studies have linked new-onset neuropsychiatric symptoms with neurocognitive disease, particularly frontotemporal dementia. One study included about 500 subjects enrolled in the ongoing Mayo Clinic Study of Aging. Investigators followed these participants for five years. This study found that the emergence of neuropsychiatric symptoms in cognitively normal older adults was associated with significant increases in the risk of developing mild cognitive impairment (MCI). Agitation conferred the highest risk (hazard ratio [HR], 3.06), followed by apathy (HR, 2.6), anxiety (HR, 1.87), irritability (HR, 1.84), and depression (HR, 1.63).
New-onset neuropsychiatric symptoms are common by the time patients enter care for memory concerns. Dr. Ismail presented data on a group of about 300 patients with MCI who attended a memory clinic. About 82% of patients endorsed at least one neuropsychiatric symptom. As measured by the MBI-C, 78% of patients reported mood symptoms, 64% reported impulse-control symptoms, 52% reported apathy, 28% reported social appropriateness, and 9% reported psychotic symptoms.
Validation of MBI-C Is Ongoing
“Our study suggests that this concept of MBI may be a common and clinically relevant syndrome, particularly given that neuropsychiatric symptoms are associated with greater caregiver burden,” said Dr. Ismail.
“This idea of symptoms persisting for at least six months is important,” he continued. “What we’re talking about is a sustained change from baseline personality. But these are still patients without dementia. Function is maintained. Independent activities of daily living are intact.”
The most comprehensive domain encompasses impulse control, agitation, and reward. “This [domain] captures a lot of function with regard to agitation in dementia, new-onset substance abuse, irritability, new-onset road rage … things we might not otherwise capture,” said Dr. Ismail.
The social appropriateness domain examines symptoms like a loss of the ability to share appropriately, acting out sexually, and loss of social judgment. The psychosis domain inquires about feelings of aggrandizement, persecution, and suspicion, as well as auditory and visual hallucinations.
The mood domain asks about new-onset anxiety, panic, and depression. The motivation domain asks about the development of apathy or disinterest in family, friends, and activities.
Validation studies in large cohorts are ongoing, as are studies that Dr. Ismail hopes will link these early behavioral changes to well-established Alzheimer’s disease biomarkers. The checklist is availablefor free by request emailed to [email protected].
—Michele G. Sullivan
Suggested Reading
Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493-498.
Geda YE, Roberts RO, Mielke MM et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572-581.
Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202.
Mirza SS, Wolters FJ, Swanson SA, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3(7):628-635.
Suggested Reading
Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493-498.
Geda YE, Roberts RO, Mielke MM et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572-581.
Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202.
Mirza SS, Wolters FJ, Swanson SA, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3(7):628-635.
What Are the Next-Morning Effects of Hypnotic Drugs?
DENVER—Sleep medication, when administered in recommended dosages at bedtime, can significantly impair next-morning short- and long-term memory, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Additionally, the medication significantly impairs psychomotor speed in healthy adults, but not in the elderly.
Joris C. Verster, PhD, Associate Professor in the Division of Pharmacology at Utrecht University in the Netherlands, and colleagues conducted two meta-analyses on the next-morning effects of hypnotic drugs. In one analysis, they looked at the drugs’ effects on short- and long-term memory functioning in healthy adults and in the elderly. In another study, they examined the next-morning effects on psychomotor speed and motor control in the same two age groups. For these meta-analyses, Dr. Verster and colleagues identified 33,969 potentially relevant articles.
Morning-After Effects on Memory
Studies were included if they assessed next-morning short- and long-term memory after bedtime administration of recommended dosages of hypnotic drugs and were double-blind, placebo-controlled, and conducted in healthy volunteers, and if sufficient data were reported. Separate analyses were performed for adults (ages 18 to 65) and for elderly healthy volunteers (age 65 or older).
In adults, eight studies assessing next-morning short-term memory (after bedtime administration of nitrazepam, triazolam, temazepam, flurazepam, melatonin, zaleplon, lormetazepam, or zolpidem) and five studies assessing long-term memory (after bedtime administration of triazolam, nitrazepam, zopiclone, flurazepam, or zolpidem) were included in the meta-analysis. The analysis revealed that next-morning short- and long-term memory were significantly impaired.
In the elderly, three studies assessing next-morning short-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) and three studies assessing long-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) were included in the meta-analysis. This analysis revealed that in the elderly, next-morning short-term memory was significantly impaired, but no significant impairment was detected in long-term memory.
Morning-After Effects on Psychomotor Speed and Motor Control
Studies were included in the analysis if they assessed next-morning effects on psychomotor speed or motor control after bedtime administration of recommended dosages of hypnotic drugs. As in the meta-analyses described above, studies also had to be double-blind, placebo-controlled, and conducted in healthy subjects, and to provide sufficient data.
In adults, 15 studies assessing next-morning psychomotor speed and five studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that next-morning psychomotor speed was significantly impaired, whereas next-morning motor control was not.
In the elderly, six studies assessing next-morning psychomotor speed and three studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that in the elderly, next-morning psychomotor speed and motor control were not significantly impaired. Taken together, the analyses show that skills and abilities that are relevant to daily activities such as driving a car or job performance may be impaired the day after using hypnotic drugs.
—Glenn S. Williams
DENVER—Sleep medication, when administered in recommended dosages at bedtime, can significantly impair next-morning short- and long-term memory, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Additionally, the medication significantly impairs psychomotor speed in healthy adults, but not in the elderly.
Joris C. Verster, PhD, Associate Professor in the Division of Pharmacology at Utrecht University in the Netherlands, and colleagues conducted two meta-analyses on the next-morning effects of hypnotic drugs. In one analysis, they looked at the drugs’ effects on short- and long-term memory functioning in healthy adults and in the elderly. In another study, they examined the next-morning effects on psychomotor speed and motor control in the same two age groups. For these meta-analyses, Dr. Verster and colleagues identified 33,969 potentially relevant articles.
Morning-After Effects on Memory
Studies were included if they assessed next-morning short- and long-term memory after bedtime administration of recommended dosages of hypnotic drugs and were double-blind, placebo-controlled, and conducted in healthy volunteers, and if sufficient data were reported. Separate analyses were performed for adults (ages 18 to 65) and for elderly healthy volunteers (age 65 or older).
In adults, eight studies assessing next-morning short-term memory (after bedtime administration of nitrazepam, triazolam, temazepam, flurazepam, melatonin, zaleplon, lormetazepam, or zolpidem) and five studies assessing long-term memory (after bedtime administration of triazolam, nitrazepam, zopiclone, flurazepam, or zolpidem) were included in the meta-analysis. The analysis revealed that next-morning short- and long-term memory were significantly impaired.
In the elderly, three studies assessing next-morning short-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) and three studies assessing long-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) were included in the meta-analysis. This analysis revealed that in the elderly, next-morning short-term memory was significantly impaired, but no significant impairment was detected in long-term memory.
Morning-After Effects on Psychomotor Speed and Motor Control
Studies were included in the analysis if they assessed next-morning effects on psychomotor speed or motor control after bedtime administration of recommended dosages of hypnotic drugs. As in the meta-analyses described above, studies also had to be double-blind, placebo-controlled, and conducted in healthy subjects, and to provide sufficient data.
In adults, 15 studies assessing next-morning psychomotor speed and five studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that next-morning psychomotor speed was significantly impaired, whereas next-morning motor control was not.
In the elderly, six studies assessing next-morning psychomotor speed and three studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that in the elderly, next-morning psychomotor speed and motor control were not significantly impaired. Taken together, the analyses show that skills and abilities that are relevant to daily activities such as driving a car or job performance may be impaired the day after using hypnotic drugs.
—Glenn S. Williams
DENVER—Sleep medication, when administered in recommended dosages at bedtime, can significantly impair next-morning short- and long-term memory, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Additionally, the medication significantly impairs psychomotor speed in healthy adults, but not in the elderly.
Joris C. Verster, PhD, Associate Professor in the Division of Pharmacology at Utrecht University in the Netherlands, and colleagues conducted two meta-analyses on the next-morning effects of hypnotic drugs. In one analysis, they looked at the drugs’ effects on short- and long-term memory functioning in healthy adults and in the elderly. In another study, they examined the next-morning effects on psychomotor speed and motor control in the same two age groups. For these meta-analyses, Dr. Verster and colleagues identified 33,969 potentially relevant articles.
Morning-After Effects on Memory
Studies were included if they assessed next-morning short- and long-term memory after bedtime administration of recommended dosages of hypnotic drugs and were double-blind, placebo-controlled, and conducted in healthy volunteers, and if sufficient data were reported. Separate analyses were performed for adults (ages 18 to 65) and for elderly healthy volunteers (age 65 or older).
In adults, eight studies assessing next-morning short-term memory (after bedtime administration of nitrazepam, triazolam, temazepam, flurazepam, melatonin, zaleplon, lormetazepam, or zolpidem) and five studies assessing long-term memory (after bedtime administration of triazolam, nitrazepam, zopiclone, flurazepam, or zolpidem) were included in the meta-analysis. The analysis revealed that next-morning short- and long-term memory were significantly impaired.
In the elderly, three studies assessing next-morning short-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) and three studies assessing long-term memory (after bedtime administration of flurazepam, zolpidem, or temazepam) were included in the meta-analysis. This analysis revealed that in the elderly, next-morning short-term memory was significantly impaired, but no significant impairment was detected in long-term memory.
Morning-After Effects on Psychomotor Speed and Motor Control
Studies were included in the analysis if they assessed next-morning effects on psychomotor speed or motor control after bedtime administration of recommended dosages of hypnotic drugs. As in the meta-analyses described above, studies also had to be double-blind, placebo-controlled, and conducted in healthy subjects, and to provide sufficient data.
In adults, 15 studies assessing next-morning psychomotor speed and five studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that next-morning psychomotor speed was significantly impaired, whereas next-morning motor control was not.
In the elderly, six studies assessing next-morning psychomotor speed and three studies assessing next-morning motor control were included in the meta-analysis. This analysis revealed that in the elderly, next-morning psychomotor speed and motor control were not significantly impaired. Taken together, the analyses show that skills and abilities that are relevant to daily activities such as driving a car or job performance may be impaired the day after using hypnotic drugs.
—Glenn S. Williams
Nonpharmacologic AD therapy: Strongest evidence supports moisturizers
BOSTON – Moisturizers are “a cornerstone” of therapy for children with atopic dermatitis, according to Julie V. Schaffer, MD.
Moisturizers improve skin hydration, increase the time between flares, and reduce xerosis and pruritus, Dr. Schaffer of Hackensack (N.J.) University Medical Group said at the American Academy of Dermatology summer meeting.
In 2014, the AAD released guidelines that “very strongly” recommended moisturizers as an important nonpharmacologic intervention for patients with AD, stating that moisturizer use decreases disease severity and can reduce the need for pharmacologic intervention, she said.
In fact, the recommendation for moisturizer was based on “strength A, level 1 evidence,” she noted.
The role of bathing is a bit less clear; bathing is suggested as part of treatment and maintenance, but no standard exists with respect to frequency or duration for those with AD (evidence level: III, strength of recommendation: C). In general, the AAD recommends daily or less frequent bathing in warm water for 5-10 minutes, but surveys suggest that bathing recommendations vary widely among specialists and primary care providers, Dr. Schaffer said.
She noted that she sometimes sees children who have been told to bathe only once a week.
“They will come in just covered with disgusting gunk and it can’t be good for them,” she said. Bathing, especially if they have crusting and scaling, removes irritants and potential allergens, and provides hydration. It can also improve penetration of topical medications, as well as tolerance of those medications so that they burn less.
“So I give a thumbs up to daily bathing,” she said.
It is generally agreed that moisturizers should be applied soon after bathing (after applying medication) to improve skin hydration in patients with AD, Dr. Schaffer said.
The AAD says that moisturizers should be applied liberally and frequently, but the ideal frequency and type of moisturizer remains “a bit of an art form rather than a precise science,” she added.
The ideal moisturizer is one that is safe, effective, and free of fragrance, irritants, and potential sensitizers, she said, noting that “an individualized approach to moisturizer and vehicle selection can be very helpful.”
For young children, it is important that the product doesn’t sting; an ointment may be preferable in this population. Preteens and teenagers may dislike greasiness, so that is an important consideration, she said.
Dr. Schaffer pointed out that lotion formulations typically have water content that is too high to be helpful for patients with substantial xerosis. Creams or ointments may be a better bet, but take care to avoid contamination in large jars of such products, she advised.
“I’ve had a couple times when patients were getting recurrent infections, and we traced it down to a nasty jar that had a little too much bacteria in it,” she said, noting that using a clean scoop or pump can help prevent contamination.
As for cleansers, the “pretty clear winner” is a nonsoap cleanser, Dr. Schaffer said.
The AAD recommends limited use of hypoallergenic, fragrance-free, nonsoap cleansers with neutral to low pH, but the evidence is insufficient for recommending the addition of bath oils, emollients, oatmeal, and most other additives to bath water, as well as for the use of acidic spring water, she said (evidence level: III, strength of recommendation: C). An exception is bleach baths, as adding a small amount of bleach to bath water has been shown to improve symptoms, but the other products have not been shown to be beneficial.
The AAD notes that wet wrap therapy, either with or without a topical corticosteroid, can be recommended for patients with moderate to severe AD, as this can decrease disease severity and water loss during flares (evidence level: II, strength of recommendation: B).
Use moisturizer in newborns at risk for AD
Moisturizers don’t just help improve atopic dermatitis in children, they may also prevent the condition in at risk newborns.
Parents of a child with eczema who are concerned about the condition developing in their next child may find hope in the findings from two studies published in 2014, Dr. Schaffer said.
In a study of 124 newborns at high risk for AD who were randomized to daily emollient therapy or usual infant skin care started by age 3 weeks, the incidence of AD over 6 months was 43% in the control group, vs. 22% in the emollient group, a relative risk reduction of 50% (J Allergy Clin Immunol. 2014 Oct;134[4]:818-23). Parents in the emollient therapy group were allowed to choose between sunflower oil, Cetaphil cream, or Aquaphor Healing Ointment.
In a similar Japanese study of 118 high risk infants who were randomized to daily treatment with an emulsion-type emollient or usual skin care starting the first week of life, the AD/eczema rates at 32 weeks were 47% and 32% in the control and emollient groups, respectively (J Allergy Clin Immunol. 2014 Oct;134[4], 824-30). Both groups were allowed to use petroleum jelly.
“So that is something you can potentially make a recommendation for,” she said.
Dr. Schaffer reported having no conflicts of interest.
BOSTON – Moisturizers are “a cornerstone” of therapy for children with atopic dermatitis, according to Julie V. Schaffer, MD.
Moisturizers improve skin hydration, increase the time between flares, and reduce xerosis and pruritus, Dr. Schaffer of Hackensack (N.J.) University Medical Group said at the American Academy of Dermatology summer meeting.
In 2014, the AAD released guidelines that “very strongly” recommended moisturizers as an important nonpharmacologic intervention for patients with AD, stating that moisturizer use decreases disease severity and can reduce the need for pharmacologic intervention, she said.
In fact, the recommendation for moisturizer was based on “strength A, level 1 evidence,” she noted.
The role of bathing is a bit less clear; bathing is suggested as part of treatment and maintenance, but no standard exists with respect to frequency or duration for those with AD (evidence level: III, strength of recommendation: C). In general, the AAD recommends daily or less frequent bathing in warm water for 5-10 minutes, but surveys suggest that bathing recommendations vary widely among specialists and primary care providers, Dr. Schaffer said.
She noted that she sometimes sees children who have been told to bathe only once a week.
“They will come in just covered with disgusting gunk and it can’t be good for them,” she said. Bathing, especially if they have crusting and scaling, removes irritants and potential allergens, and provides hydration. It can also improve penetration of topical medications, as well as tolerance of those medications so that they burn less.
“So I give a thumbs up to daily bathing,” she said.
It is generally agreed that moisturizers should be applied soon after bathing (after applying medication) to improve skin hydration in patients with AD, Dr. Schaffer said.
The AAD says that moisturizers should be applied liberally and frequently, but the ideal frequency and type of moisturizer remains “a bit of an art form rather than a precise science,” she added.
The ideal moisturizer is one that is safe, effective, and free of fragrance, irritants, and potential sensitizers, she said, noting that “an individualized approach to moisturizer and vehicle selection can be very helpful.”
For young children, it is important that the product doesn’t sting; an ointment may be preferable in this population. Preteens and teenagers may dislike greasiness, so that is an important consideration, she said.
Dr. Schaffer pointed out that lotion formulations typically have water content that is too high to be helpful for patients with substantial xerosis. Creams or ointments may be a better bet, but take care to avoid contamination in large jars of such products, she advised.
“I’ve had a couple times when patients were getting recurrent infections, and we traced it down to a nasty jar that had a little too much bacteria in it,” she said, noting that using a clean scoop or pump can help prevent contamination.
As for cleansers, the “pretty clear winner” is a nonsoap cleanser, Dr. Schaffer said.
The AAD recommends limited use of hypoallergenic, fragrance-free, nonsoap cleansers with neutral to low pH, but the evidence is insufficient for recommending the addition of bath oils, emollients, oatmeal, and most other additives to bath water, as well as for the use of acidic spring water, she said (evidence level: III, strength of recommendation: C). An exception is bleach baths, as adding a small amount of bleach to bath water has been shown to improve symptoms, but the other products have not been shown to be beneficial.
The AAD notes that wet wrap therapy, either with or without a topical corticosteroid, can be recommended for patients with moderate to severe AD, as this can decrease disease severity and water loss during flares (evidence level: II, strength of recommendation: B).
Use moisturizer in newborns at risk for AD
Moisturizers don’t just help improve atopic dermatitis in children, they may also prevent the condition in at risk newborns.
Parents of a child with eczema who are concerned about the condition developing in their next child may find hope in the findings from two studies published in 2014, Dr. Schaffer said.
In a study of 124 newborns at high risk for AD who were randomized to daily emollient therapy or usual infant skin care started by age 3 weeks, the incidence of AD over 6 months was 43% in the control group, vs. 22% in the emollient group, a relative risk reduction of 50% (J Allergy Clin Immunol. 2014 Oct;134[4]:818-23). Parents in the emollient therapy group were allowed to choose between sunflower oil, Cetaphil cream, or Aquaphor Healing Ointment.
In a similar Japanese study of 118 high risk infants who were randomized to daily treatment with an emulsion-type emollient or usual skin care starting the first week of life, the AD/eczema rates at 32 weeks were 47% and 32% in the control and emollient groups, respectively (J Allergy Clin Immunol. 2014 Oct;134[4], 824-30). Both groups were allowed to use petroleum jelly.
“So that is something you can potentially make a recommendation for,” she said.
Dr. Schaffer reported having no conflicts of interest.
BOSTON – Moisturizers are “a cornerstone” of therapy for children with atopic dermatitis, according to Julie V. Schaffer, MD.
Moisturizers improve skin hydration, increase the time between flares, and reduce xerosis and pruritus, Dr. Schaffer of Hackensack (N.J.) University Medical Group said at the American Academy of Dermatology summer meeting.
In 2014, the AAD released guidelines that “very strongly” recommended moisturizers as an important nonpharmacologic intervention for patients with AD, stating that moisturizer use decreases disease severity and can reduce the need for pharmacologic intervention, she said.
In fact, the recommendation for moisturizer was based on “strength A, level 1 evidence,” she noted.
The role of bathing is a bit less clear; bathing is suggested as part of treatment and maintenance, but no standard exists with respect to frequency or duration for those with AD (evidence level: III, strength of recommendation: C). In general, the AAD recommends daily or less frequent bathing in warm water for 5-10 minutes, but surveys suggest that bathing recommendations vary widely among specialists and primary care providers, Dr. Schaffer said.
She noted that she sometimes sees children who have been told to bathe only once a week.
“They will come in just covered with disgusting gunk and it can’t be good for them,” she said. Bathing, especially if they have crusting and scaling, removes irritants and potential allergens, and provides hydration. It can also improve penetration of topical medications, as well as tolerance of those medications so that they burn less.
“So I give a thumbs up to daily bathing,” she said.
It is generally agreed that moisturizers should be applied soon after bathing (after applying medication) to improve skin hydration in patients with AD, Dr. Schaffer said.
The AAD says that moisturizers should be applied liberally and frequently, but the ideal frequency and type of moisturizer remains “a bit of an art form rather than a precise science,” she added.
The ideal moisturizer is one that is safe, effective, and free of fragrance, irritants, and potential sensitizers, she said, noting that “an individualized approach to moisturizer and vehicle selection can be very helpful.”
For young children, it is important that the product doesn’t sting; an ointment may be preferable in this population. Preteens and teenagers may dislike greasiness, so that is an important consideration, she said.
Dr. Schaffer pointed out that lotion formulations typically have water content that is too high to be helpful for patients with substantial xerosis. Creams or ointments may be a better bet, but take care to avoid contamination in large jars of such products, she advised.
“I’ve had a couple times when patients were getting recurrent infections, and we traced it down to a nasty jar that had a little too much bacteria in it,” she said, noting that using a clean scoop or pump can help prevent contamination.
As for cleansers, the “pretty clear winner” is a nonsoap cleanser, Dr. Schaffer said.
The AAD recommends limited use of hypoallergenic, fragrance-free, nonsoap cleansers with neutral to low pH, but the evidence is insufficient for recommending the addition of bath oils, emollients, oatmeal, and most other additives to bath water, as well as for the use of acidic spring water, she said (evidence level: III, strength of recommendation: C). An exception is bleach baths, as adding a small amount of bleach to bath water has been shown to improve symptoms, but the other products have not been shown to be beneficial.
The AAD notes that wet wrap therapy, either with or without a topical corticosteroid, can be recommended for patients with moderate to severe AD, as this can decrease disease severity and water loss during flares (evidence level: II, strength of recommendation: B).
Use moisturizer in newborns at risk for AD
Moisturizers don’t just help improve atopic dermatitis in children, they may also prevent the condition in at risk newborns.
Parents of a child with eczema who are concerned about the condition developing in their next child may find hope in the findings from two studies published in 2014, Dr. Schaffer said.
In a study of 124 newborns at high risk for AD who were randomized to daily emollient therapy or usual infant skin care started by age 3 weeks, the incidence of AD over 6 months was 43% in the control group, vs. 22% in the emollient group, a relative risk reduction of 50% (J Allergy Clin Immunol. 2014 Oct;134[4]:818-23). Parents in the emollient therapy group were allowed to choose between sunflower oil, Cetaphil cream, or Aquaphor Healing Ointment.
In a similar Japanese study of 118 high risk infants who were randomized to daily treatment with an emulsion-type emollient or usual skin care starting the first week of life, the AD/eczema rates at 32 weeks were 47% and 32% in the control and emollient groups, respectively (J Allergy Clin Immunol. 2014 Oct;134[4], 824-30). Both groups were allowed to use petroleum jelly.
“So that is something you can potentially make a recommendation for,” she said.
Dr. Schaffer reported having no conflicts of interest.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2016
Native Israelis have higher risk of Hodgkin lymphoma

Photo from Hebrew
University of Jerusalem
A study of 2.3 million Jewish subjects suggests that being born in Israel increases a person’s risk of developing Hodgkin lymphoma (HL).
Native Israelis had a higher risk of HL than subjects who immigrated to Israel from Europe, Asia, or North Africa.
Researchers said this finding, published in Leukemia & Lymphoma, suggests that exposure to as-yet-unidentified elements of Israel’s environment increases the risk of HL.
“While we still need further studies to identify the specific causes of the high rates of Hodgkin lymphoma among native Israelis, our findings direct us to search for possible environmental causes in Israel and the neighboring countries,” said study author Hagai Levine, MD, of Hebrew University of Jerusalem in Israel.
“These causes could be not only environmental exposures but also diet, climate, social environment, and stress that may be related to chronic regional conflict.”
Dr Levine and his colleagues noted that the incidence of HL in Israel is among the highest in the world. Based on GLOBOCAN estimates for 2012, Israeli females have the highest age-standardized incidence rate of HL worldwide, and Israeli males have the second highest. From 1960 through 2005, Israel experienced a sharp rise in HL incidence among Israeli-born individuals of both sexes.
Despite evidence for an environmental etiology, very few risk factors have been identified. Studying immigrant populations provides a means of investigating the relative importance of genetic factors as compared to environmental factors in disease development.
With all that in mind, Dr Levine and his colleagues conducted the current study. They included Jewish men and women, ages 16 to 19 at examination, with no history of a cancer diagnosis.
Nationwide data on 2,285,009 adolescents, collected from 1967 through 2011, were linked to Israel’s Cancer Registry to obtain the incidence of HL until 2012.
During 47 million person-years of follow-up, there were 2093 cases of HL detected.
Multivariate analysis suggested native Israelis had a significantly higher risk of developing HL than individuals who immigrated to the country (hazard ratio=1.29, P<0.001). This increased risk was driven largely by an elevated risk for the nodular sclerosis subtype of HL (hazard ratio=1.59, P<0.001).
The researchers noted that the elevated risk appeared within one generation. The low incidence of HL observed for immigrants from Western Asia was no longer evident among Israeli-born individuals of Asian origin. Among immigrants, there was no difference by age at migration.
Therefore, the researchers suggested that immigration from a low-risk to a high-risk location, mostly to locales with a modern lifestyle and environment, is associated with an increase in HL incidence (mainly the nodular sclerosis subtype) within short periods, making genetic drift unlikely as a causal explanation.
On the other hand, the Israeli lifestyle and environment, either independently or by gene-environment interaction, may pose exceptional risks for HL.
“There is increasing evidence for developmental origins of health and disease, with different possible mechanisms, including epigenetic changes or endocrine disruption,” Dr Levine said.
“There is also increasing evidence on the role of prenatal stress in offspring development, including cancer development, especially for hematological malignancies. These data suggest that risk of HL (the nodular sclerosis subtype) is possibly increased due to preconception, prenatal, or early life exposures to a changing lifestyle and environment and its interaction with susceptibility genes.”
Dr Levine and his colleagues also found a higher risk of HL for women, subjects born in more recent years, those with a higher body mass index, and subjects of taller stature. ![]()

Photo from Hebrew
University of Jerusalem
A study of 2.3 million Jewish subjects suggests that being born in Israel increases a person’s risk of developing Hodgkin lymphoma (HL).
Native Israelis had a higher risk of HL than subjects who immigrated to Israel from Europe, Asia, or North Africa.
Researchers said this finding, published in Leukemia & Lymphoma, suggests that exposure to as-yet-unidentified elements of Israel’s environment increases the risk of HL.
“While we still need further studies to identify the specific causes of the high rates of Hodgkin lymphoma among native Israelis, our findings direct us to search for possible environmental causes in Israel and the neighboring countries,” said study author Hagai Levine, MD, of Hebrew University of Jerusalem in Israel.
“These causes could be not only environmental exposures but also diet, climate, social environment, and stress that may be related to chronic regional conflict.”
Dr Levine and his colleagues noted that the incidence of HL in Israel is among the highest in the world. Based on GLOBOCAN estimates for 2012, Israeli females have the highest age-standardized incidence rate of HL worldwide, and Israeli males have the second highest. From 1960 through 2005, Israel experienced a sharp rise in HL incidence among Israeli-born individuals of both sexes.
Despite evidence for an environmental etiology, very few risk factors have been identified. Studying immigrant populations provides a means of investigating the relative importance of genetic factors as compared to environmental factors in disease development.
With all that in mind, Dr Levine and his colleagues conducted the current study. They included Jewish men and women, ages 16 to 19 at examination, with no history of a cancer diagnosis.
Nationwide data on 2,285,009 adolescents, collected from 1967 through 2011, were linked to Israel’s Cancer Registry to obtain the incidence of HL until 2012.
During 47 million person-years of follow-up, there were 2093 cases of HL detected.
Multivariate analysis suggested native Israelis had a significantly higher risk of developing HL than individuals who immigrated to the country (hazard ratio=1.29, P<0.001). This increased risk was driven largely by an elevated risk for the nodular sclerosis subtype of HL (hazard ratio=1.59, P<0.001).
The researchers noted that the elevated risk appeared within one generation. The low incidence of HL observed for immigrants from Western Asia was no longer evident among Israeli-born individuals of Asian origin. Among immigrants, there was no difference by age at migration.
Therefore, the researchers suggested that immigration from a low-risk to a high-risk location, mostly to locales with a modern lifestyle and environment, is associated with an increase in HL incidence (mainly the nodular sclerosis subtype) within short periods, making genetic drift unlikely as a causal explanation.
On the other hand, the Israeli lifestyle and environment, either independently or by gene-environment interaction, may pose exceptional risks for HL.
“There is increasing evidence for developmental origins of health and disease, with different possible mechanisms, including epigenetic changes or endocrine disruption,” Dr Levine said.
“There is also increasing evidence on the role of prenatal stress in offspring development, including cancer development, especially for hematological malignancies. These data suggest that risk of HL (the nodular sclerosis subtype) is possibly increased due to preconception, prenatal, or early life exposures to a changing lifestyle and environment and its interaction with susceptibility genes.”
Dr Levine and his colleagues also found a higher risk of HL for women, subjects born in more recent years, those with a higher body mass index, and subjects of taller stature. ![]()

Photo from Hebrew
University of Jerusalem
A study of 2.3 million Jewish subjects suggests that being born in Israel increases a person’s risk of developing Hodgkin lymphoma (HL).
Native Israelis had a higher risk of HL than subjects who immigrated to Israel from Europe, Asia, or North Africa.
Researchers said this finding, published in Leukemia & Lymphoma, suggests that exposure to as-yet-unidentified elements of Israel’s environment increases the risk of HL.
“While we still need further studies to identify the specific causes of the high rates of Hodgkin lymphoma among native Israelis, our findings direct us to search for possible environmental causes in Israel and the neighboring countries,” said study author Hagai Levine, MD, of Hebrew University of Jerusalem in Israel.
“These causes could be not only environmental exposures but also diet, climate, social environment, and stress that may be related to chronic regional conflict.”
Dr Levine and his colleagues noted that the incidence of HL in Israel is among the highest in the world. Based on GLOBOCAN estimates for 2012, Israeli females have the highest age-standardized incidence rate of HL worldwide, and Israeli males have the second highest. From 1960 through 2005, Israel experienced a sharp rise in HL incidence among Israeli-born individuals of both sexes.
Despite evidence for an environmental etiology, very few risk factors have been identified. Studying immigrant populations provides a means of investigating the relative importance of genetic factors as compared to environmental factors in disease development.
With all that in mind, Dr Levine and his colleagues conducted the current study. They included Jewish men and women, ages 16 to 19 at examination, with no history of a cancer diagnosis.
Nationwide data on 2,285,009 adolescents, collected from 1967 through 2011, were linked to Israel’s Cancer Registry to obtain the incidence of HL until 2012.
During 47 million person-years of follow-up, there were 2093 cases of HL detected.
Multivariate analysis suggested native Israelis had a significantly higher risk of developing HL than individuals who immigrated to the country (hazard ratio=1.29, P<0.001). This increased risk was driven largely by an elevated risk for the nodular sclerosis subtype of HL (hazard ratio=1.59, P<0.001).
The researchers noted that the elevated risk appeared within one generation. The low incidence of HL observed for immigrants from Western Asia was no longer evident among Israeli-born individuals of Asian origin. Among immigrants, there was no difference by age at migration.
Therefore, the researchers suggested that immigration from a low-risk to a high-risk location, mostly to locales with a modern lifestyle and environment, is associated with an increase in HL incidence (mainly the nodular sclerosis subtype) within short periods, making genetic drift unlikely as a causal explanation.
On the other hand, the Israeli lifestyle and environment, either independently or by gene-environment interaction, may pose exceptional risks for HL.
“There is increasing evidence for developmental origins of health and disease, with different possible mechanisms, including epigenetic changes or endocrine disruption,” Dr Levine said.
“There is also increasing evidence on the role of prenatal stress in offspring development, including cancer development, especially for hematological malignancies. These data suggest that risk of HL (the nodular sclerosis subtype) is possibly increased due to preconception, prenatal, or early life exposures to a changing lifestyle and environment and its interaction with susceptibility genes.”
Dr Levine and his colleagues also found a higher risk of HL for women, subjects born in more recent years, those with a higher body mass index, and subjects of taller stature. ![]()
Gastroesophageal cancers continue to make their mark globally
CHICAGO – Severe intestinal metaplasia can progress to adenocarcinoma in a small number of patients over 10 years, whether it’s in the esophagus or in the stomach.
Studies emerging from around the world find the same patterns and similar rates of progression in both diseases: 2% for esophageal and 3%-5% for gastric cancers over 10 years, no matter where the studies are conducted, Ernst Kuipers, MD, PhD, said at the meeting sponsored by the American Gastroenterological Association.
“It doesn’t matter if you’re in an area with a high rate or a low rate,” of gastroesophageal cancer, said Dr. Kuipers, professor of gastroenterology and hepatology at Erasmus University Medical Center, Rotterdam, the Netherlands. “The risk is about the same.”
On the flip side, global data also confirm that surveillance and treatment mitigate the risks. Following at-risk patients endoscopically means tumors are found earlier. And when a severely dysplastic lesion is removed, the risk of recurrence is very low – less than 1%.
These findings of a relatively constant rate of progression from gastroesophageal dysplasia to adenocarcinomas somewhat contradict the idea that the cancers are more of a concern in Asian countries, and fading away in Western countries. There has indeed been a dramatic decrease in stomach cancer in the last century – in the early 1900s, Dr. Kuipers said, up to 40% of cancers reported in Germany were gastric. The reasons for the decrease are many: improved diet, improved hygiene, and widespread use of antibiotics are factors. But the disease does still exist, especially among some ethnic/racial groups.
“The U.S. Surveillance, Epidemiology, and End Results (SEER) database shows that there is still a lot of it out there, and there’s huge disparity within groups, so we have to look at this from a broader perspective.”
Overall, the U.S. rates of esophageal and gastric cancer are about 8 and 10/100,000, respectively. In whites, those rates are about 8 and 9/100,000, but much higher in blacks, Asians, Native Americans, and Hispanics, with gastric cancer hovering around 14/100,000.
A 2010 meta-analysis found that Barrett’s metaplasia progressed to esophageal adenocarcinoma at a rate of 6.3/1,000 patients per year, but that number in particular came from analysis of tertiary care cohorts (Clin Gastroenterol Hepatol. 2010. doi: 10.1016/j.cgh.2009.10.010).
A 2015 analysis found lower rates of progression – about 2/1,000 patients per year in patients with short-segment Barrett’s, and about 3/1,000 patients per year in long-segment Barrett’s patients that have no dysplasia. “That means if you’re following 300 patients, one of these will convert to cancer every year,” Dr. Kuipers said (Gut. 2015. doi: 10.1136/gutjnl-2013-305506).
The risk appears much higher for patients with dysplastic Barrett’s, although the data vary widely. “Some report low progression rates, but some report these patients have a 50% or higher risk of progression within a few years. This variation depends on how selective one is in diagnosing low-grade dysplasia.”
A Dutch nationwide study of 42,200 patients with Barrett’s found that 4% progressed to adenocarcinoma over 10 years, for an annual progression rate of 0.4%. But among the small group of those with low-grade dysplasia, more than 10% had progressed by 10 years – a 1% annual progression rate (Gut. 2010. doi: 10.1136/gut.2009.176701).
An Irish national study found strikingly similar results. The annual progression risk in patients with metaplasia was 1.6/1,000 patients per year overall, but 2.7/1,000 per year in those with intestinal metaplasia (J Natl Cancer Inst. 2011. doi: 10.1093/jnci/djr203).
“So, if we have some idea of progression rate, is there evidence that we could identify and treat these cancers earlier if our patients are under surveillance?” Dr. Kuipers said. “Well the answer is ‘yes.’ ”
He cited a very recent study by his colleagues at Erasmus University (Gut. 2016. doi: 10.1136/gutjnl-2014-308802). Investigators determined that in Barrett’s patients who were followed endoscopically, esophageal cancers were identified at much earlier stages than among the general population; 66% of the neoplasias were identified at the high-grade dysplasia stage, 26% at stage 1. The remainder were stage 2; there were no stage 3 or 4 cancers. In the general population, numbers were reversed: 45% were stage 4 when identified, 25% stage 3, 18% stage 2, and only a few at stages 1 or high-grade dysplasia.
Gastric cancer shows the same consistency of incidence and relation to baseline premalignant severity. A Dutch study with 98,000 cases found the annual incidence of gastric cancer was 0.1% for patients with atrophic gastritis, 0.25% for intestinal metaplasia, 0.6% for mild to moderate dysplasia, and 6% for severe dysplasia within 5 years after diagnosis (Gastroenterology. 2008. doi: 10.1053/j.gastro.2008.01.071).
A Swedish study last year found a progression rate of 3% over 10 years for patients with extensive intestinal metaplasia. (BMJ. 2015. doi: 10.1136/bmj.h3867).
“And this year, from Los Angeles, we saw a study of 4,300 patients with extensive intestinal metaplasia and a very similar progression rate of close to 5% in 10 years (Am J Gastro. 2016. doi: 10.1038/ajg.2016.188). It’s the same findings, everywhere,” he said, adding that a team from Iran presented almost identical numbers for gastric cancer incidence at this year’s Digestive Disease Week.
Again, follow-up improves outcomes. A gastric cancer endoscopy screening program for high-risk people improved survival of gastric cancer significantly over community-identified patients (80% vs. 60% at 60 months after diagnosis) (J Gastro Hepatol. 2014. doi: 10.1111/jgh.12387).
These data contribute strongly to recent guidelines for managing patients with premalignant stomach conditions. The European Society for Gastroenterological Endoscopy recommends that those with extensive intestinal metaplasia undergo endoscopy every 3 years (Endoscopy. 2012. doi: 10.1055/s-0031-1291491). Last year, the Kyoto global consensus report on Helicobacter pylori gastritis (Gut. 2015. doi: 10.1136/gutjnl-2015-309252) recommended that patients with extensive gastric atrophy be offered endoscopic surveillance.
There is no place for general population screening, Dr. Kuipers said in an interview. “I would not advocate actual population screening – i.e., offer the general population or risk groups a first screening endoscopy. There is at present no indication [for] this. This is different, however, from surveillance of patients who happen to be diagnosed with advanced intestinal metaplasia of the stomach, or long-segment Barrett’s esophagus, because this allows us to early detect development of neoplasia (high-grade dysplasia and cancer), which then allows for less invasive treatment, and better outcomes.”
He had no financial disclosures.
CHICAGO – Severe intestinal metaplasia can progress to adenocarcinoma in a small number of patients over 10 years, whether it’s in the esophagus or in the stomach.
Studies emerging from around the world find the same patterns and similar rates of progression in both diseases: 2% for esophageal and 3%-5% for gastric cancers over 10 years, no matter where the studies are conducted, Ernst Kuipers, MD, PhD, said at the meeting sponsored by the American Gastroenterological Association.
“It doesn’t matter if you’re in an area with a high rate or a low rate,” of gastroesophageal cancer, said Dr. Kuipers, professor of gastroenterology and hepatology at Erasmus University Medical Center, Rotterdam, the Netherlands. “The risk is about the same.”
On the flip side, global data also confirm that surveillance and treatment mitigate the risks. Following at-risk patients endoscopically means tumors are found earlier. And when a severely dysplastic lesion is removed, the risk of recurrence is very low – less than 1%.
These findings of a relatively constant rate of progression from gastroesophageal dysplasia to adenocarcinomas somewhat contradict the idea that the cancers are more of a concern in Asian countries, and fading away in Western countries. There has indeed been a dramatic decrease in stomach cancer in the last century – in the early 1900s, Dr. Kuipers said, up to 40% of cancers reported in Germany were gastric. The reasons for the decrease are many: improved diet, improved hygiene, and widespread use of antibiotics are factors. But the disease does still exist, especially among some ethnic/racial groups.
“The U.S. Surveillance, Epidemiology, and End Results (SEER) database shows that there is still a lot of it out there, and there’s huge disparity within groups, so we have to look at this from a broader perspective.”
Overall, the U.S. rates of esophageal and gastric cancer are about 8 and 10/100,000, respectively. In whites, those rates are about 8 and 9/100,000, but much higher in blacks, Asians, Native Americans, and Hispanics, with gastric cancer hovering around 14/100,000.
A 2010 meta-analysis found that Barrett’s metaplasia progressed to esophageal adenocarcinoma at a rate of 6.3/1,000 patients per year, but that number in particular came from analysis of tertiary care cohorts (Clin Gastroenterol Hepatol. 2010. doi: 10.1016/j.cgh.2009.10.010).
A 2015 analysis found lower rates of progression – about 2/1,000 patients per year in patients with short-segment Barrett’s, and about 3/1,000 patients per year in long-segment Barrett’s patients that have no dysplasia. “That means if you’re following 300 patients, one of these will convert to cancer every year,” Dr. Kuipers said (Gut. 2015. doi: 10.1136/gutjnl-2013-305506).
The risk appears much higher for patients with dysplastic Barrett’s, although the data vary widely. “Some report low progression rates, but some report these patients have a 50% or higher risk of progression within a few years. This variation depends on how selective one is in diagnosing low-grade dysplasia.”
A Dutch nationwide study of 42,200 patients with Barrett’s found that 4% progressed to adenocarcinoma over 10 years, for an annual progression rate of 0.4%. But among the small group of those with low-grade dysplasia, more than 10% had progressed by 10 years – a 1% annual progression rate (Gut. 2010. doi: 10.1136/gut.2009.176701).
An Irish national study found strikingly similar results. The annual progression risk in patients with metaplasia was 1.6/1,000 patients per year overall, but 2.7/1,000 per year in those with intestinal metaplasia (J Natl Cancer Inst. 2011. doi: 10.1093/jnci/djr203).
“So, if we have some idea of progression rate, is there evidence that we could identify and treat these cancers earlier if our patients are under surveillance?” Dr. Kuipers said. “Well the answer is ‘yes.’ ”
He cited a very recent study by his colleagues at Erasmus University (Gut. 2016. doi: 10.1136/gutjnl-2014-308802). Investigators determined that in Barrett’s patients who were followed endoscopically, esophageal cancers were identified at much earlier stages than among the general population; 66% of the neoplasias were identified at the high-grade dysplasia stage, 26% at stage 1. The remainder were stage 2; there were no stage 3 or 4 cancers. In the general population, numbers were reversed: 45% were stage 4 when identified, 25% stage 3, 18% stage 2, and only a few at stages 1 or high-grade dysplasia.
Gastric cancer shows the same consistency of incidence and relation to baseline premalignant severity. A Dutch study with 98,000 cases found the annual incidence of gastric cancer was 0.1% for patients with atrophic gastritis, 0.25% for intestinal metaplasia, 0.6% for mild to moderate dysplasia, and 6% for severe dysplasia within 5 years after diagnosis (Gastroenterology. 2008. doi: 10.1053/j.gastro.2008.01.071).
A Swedish study last year found a progression rate of 3% over 10 years for patients with extensive intestinal metaplasia. (BMJ. 2015. doi: 10.1136/bmj.h3867).
“And this year, from Los Angeles, we saw a study of 4,300 patients with extensive intestinal metaplasia and a very similar progression rate of close to 5% in 10 years (Am J Gastro. 2016. doi: 10.1038/ajg.2016.188). It’s the same findings, everywhere,” he said, adding that a team from Iran presented almost identical numbers for gastric cancer incidence at this year’s Digestive Disease Week.
Again, follow-up improves outcomes. A gastric cancer endoscopy screening program for high-risk people improved survival of gastric cancer significantly over community-identified patients (80% vs. 60% at 60 months after diagnosis) (J Gastro Hepatol. 2014. doi: 10.1111/jgh.12387).
These data contribute strongly to recent guidelines for managing patients with premalignant stomach conditions. The European Society for Gastroenterological Endoscopy recommends that those with extensive intestinal metaplasia undergo endoscopy every 3 years (Endoscopy. 2012. doi: 10.1055/s-0031-1291491). Last year, the Kyoto global consensus report on Helicobacter pylori gastritis (Gut. 2015. doi: 10.1136/gutjnl-2015-309252) recommended that patients with extensive gastric atrophy be offered endoscopic surveillance.
There is no place for general population screening, Dr. Kuipers said in an interview. “I would not advocate actual population screening – i.e., offer the general population or risk groups a first screening endoscopy. There is at present no indication [for] this. This is different, however, from surveillance of patients who happen to be diagnosed with advanced intestinal metaplasia of the stomach, or long-segment Barrett’s esophagus, because this allows us to early detect development of neoplasia (high-grade dysplasia and cancer), which then allows for less invasive treatment, and better outcomes.”
He had no financial disclosures.
CHICAGO – Severe intestinal metaplasia can progress to adenocarcinoma in a small number of patients over 10 years, whether it’s in the esophagus or in the stomach.
Studies emerging from around the world find the same patterns and similar rates of progression in both diseases: 2% for esophageal and 3%-5% for gastric cancers over 10 years, no matter where the studies are conducted, Ernst Kuipers, MD, PhD, said at the meeting sponsored by the American Gastroenterological Association.
“It doesn’t matter if you’re in an area with a high rate or a low rate,” of gastroesophageal cancer, said Dr. Kuipers, professor of gastroenterology and hepatology at Erasmus University Medical Center, Rotterdam, the Netherlands. “The risk is about the same.”
On the flip side, global data also confirm that surveillance and treatment mitigate the risks. Following at-risk patients endoscopically means tumors are found earlier. And when a severely dysplastic lesion is removed, the risk of recurrence is very low – less than 1%.
These findings of a relatively constant rate of progression from gastroesophageal dysplasia to adenocarcinomas somewhat contradict the idea that the cancers are more of a concern in Asian countries, and fading away in Western countries. There has indeed been a dramatic decrease in stomach cancer in the last century – in the early 1900s, Dr. Kuipers said, up to 40% of cancers reported in Germany were gastric. The reasons for the decrease are many: improved diet, improved hygiene, and widespread use of antibiotics are factors. But the disease does still exist, especially among some ethnic/racial groups.
“The U.S. Surveillance, Epidemiology, and End Results (SEER) database shows that there is still a lot of it out there, and there’s huge disparity within groups, so we have to look at this from a broader perspective.”
Overall, the U.S. rates of esophageal and gastric cancer are about 8 and 10/100,000, respectively. In whites, those rates are about 8 and 9/100,000, but much higher in blacks, Asians, Native Americans, and Hispanics, with gastric cancer hovering around 14/100,000.
A 2010 meta-analysis found that Barrett’s metaplasia progressed to esophageal adenocarcinoma at a rate of 6.3/1,000 patients per year, but that number in particular came from analysis of tertiary care cohorts (Clin Gastroenterol Hepatol. 2010. doi: 10.1016/j.cgh.2009.10.010).
A 2015 analysis found lower rates of progression – about 2/1,000 patients per year in patients with short-segment Barrett’s, and about 3/1,000 patients per year in long-segment Barrett’s patients that have no dysplasia. “That means if you’re following 300 patients, one of these will convert to cancer every year,” Dr. Kuipers said (Gut. 2015. doi: 10.1136/gutjnl-2013-305506).
The risk appears much higher for patients with dysplastic Barrett’s, although the data vary widely. “Some report low progression rates, but some report these patients have a 50% or higher risk of progression within a few years. This variation depends on how selective one is in diagnosing low-grade dysplasia.”
A Dutch nationwide study of 42,200 patients with Barrett’s found that 4% progressed to adenocarcinoma over 10 years, for an annual progression rate of 0.4%. But among the small group of those with low-grade dysplasia, more than 10% had progressed by 10 years – a 1% annual progression rate (Gut. 2010. doi: 10.1136/gut.2009.176701).
An Irish national study found strikingly similar results. The annual progression risk in patients with metaplasia was 1.6/1,000 patients per year overall, but 2.7/1,000 per year in those with intestinal metaplasia (J Natl Cancer Inst. 2011. doi: 10.1093/jnci/djr203).
“So, if we have some idea of progression rate, is there evidence that we could identify and treat these cancers earlier if our patients are under surveillance?” Dr. Kuipers said. “Well the answer is ‘yes.’ ”
He cited a very recent study by his colleagues at Erasmus University (Gut. 2016. doi: 10.1136/gutjnl-2014-308802). Investigators determined that in Barrett’s patients who were followed endoscopically, esophageal cancers were identified at much earlier stages than among the general population; 66% of the neoplasias were identified at the high-grade dysplasia stage, 26% at stage 1. The remainder were stage 2; there were no stage 3 or 4 cancers. In the general population, numbers were reversed: 45% were stage 4 when identified, 25% stage 3, 18% stage 2, and only a few at stages 1 or high-grade dysplasia.
Gastric cancer shows the same consistency of incidence and relation to baseline premalignant severity. A Dutch study with 98,000 cases found the annual incidence of gastric cancer was 0.1% for patients with atrophic gastritis, 0.25% for intestinal metaplasia, 0.6% for mild to moderate dysplasia, and 6% for severe dysplasia within 5 years after diagnosis (Gastroenterology. 2008. doi: 10.1053/j.gastro.2008.01.071).
A Swedish study last year found a progression rate of 3% over 10 years for patients with extensive intestinal metaplasia. (BMJ. 2015. doi: 10.1136/bmj.h3867).
“And this year, from Los Angeles, we saw a study of 4,300 patients with extensive intestinal metaplasia and a very similar progression rate of close to 5% in 10 years (Am J Gastro. 2016. doi: 10.1038/ajg.2016.188). It’s the same findings, everywhere,” he said, adding that a team from Iran presented almost identical numbers for gastric cancer incidence at this year’s Digestive Disease Week.
Again, follow-up improves outcomes. A gastric cancer endoscopy screening program for high-risk people improved survival of gastric cancer significantly over community-identified patients (80% vs. 60% at 60 months after diagnosis) (J Gastro Hepatol. 2014. doi: 10.1111/jgh.12387).
These data contribute strongly to recent guidelines for managing patients with premalignant stomach conditions. The European Society for Gastroenterological Endoscopy recommends that those with extensive intestinal metaplasia undergo endoscopy every 3 years (Endoscopy. 2012. doi: 10.1055/s-0031-1291491). Last year, the Kyoto global consensus report on Helicobacter pylori gastritis (Gut. 2015. doi: 10.1136/gutjnl-2015-309252) recommended that patients with extensive gastric atrophy be offered endoscopic surveillance.
There is no place for general population screening, Dr. Kuipers said in an interview. “I would not advocate actual population screening – i.e., offer the general population or risk groups a first screening endoscopy. There is at present no indication [for] this. This is different, however, from surveillance of patients who happen to be diagnosed with advanced intestinal metaplasia of the stomach, or long-segment Barrett’s esophagus, because this allows us to early detect development of neoplasia (high-grade dysplasia and cancer), which then allows for less invasive treatment, and better outcomes.”
He had no financial disclosures.
AT THE 2016 JAMES W. FRESTON CONFERENCE
Ties to industry among NCCN guideline authors
A study published in JAMA Oncology has quantified financial ties to the pharmaceutical industry among authors of National Comprehensive Cancer Network (NCCN) guidelines.
In 2014, the authors studied received more money in research payments than “general” payments (for things like consulting, meals, and lodging)—$29 million vs $1.25 million.
But more of the authors received general payments than research payments—84% vs 47%.
Study investigators said this finding may mean that some of the guideline authors are receiving general payments unconnected to research. However, because the study only included 1 year of data, these results may not tell the full story.
“Understanding the extent to which guideline authors have financial relationships with the pharmaceutical industry—and the types of financial arrangements that they have—is useful for the NCCN and for the public,” said investigator Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“As we learn more about the role of industry payments in shaping prescribing and practice, it is best to proceed with caution and continue to encourage transparency.”
For this study, Dr Dusetzina and her colleagues analyzed financial conflicts of interest (FCOIs) for 125 panelists who worked on setting the NCCN guidelines for lung, breast, prostate, and colorectal cancer (the cancers with the highest incidence in the US).
Eighty-six percent (n=108) of the guideline authors reported at least 1 FCOI in 2014. The total value of FCOIs was $30,287,549, which included $29,036,127 in research payments and $1,251,422 in general payments.
Eighty-four percent of the authors (n=105) received general payments, and 47% (n=59) received research payments.
The authors received an average of $10,011 in general payments and an average of $236,066 in research payments.
The majority of the payments received were within the limits set by the NCCN, but 8 authors (6%) exceeded them. The NCCN says guideline authors cannot receive $20,000 or more from a single company or $50,000 or more in total.
Dr Dusetzina and her colleagues noted that this study was not designed to explore whether the payments influenced the guideline authors’ clinical practice or the recommendations they made in the guidelines.
However, finding a high prevalence of financial relationships with industry among guideline authors lays the foundation for future studies to investigate the impact of such relationships.
“It is not a given that industry funding leads to undue influence,” Dr Dusetzina said, “but it is important to analyze these relationships and the potential impact they have on care guidelines because they do influence patient care decisions and the cost of providing patient care.” ![]()
A study published in JAMA Oncology has quantified financial ties to the pharmaceutical industry among authors of National Comprehensive Cancer Network (NCCN) guidelines.
In 2014, the authors studied received more money in research payments than “general” payments (for things like consulting, meals, and lodging)—$29 million vs $1.25 million.
But more of the authors received general payments than research payments—84% vs 47%.
Study investigators said this finding may mean that some of the guideline authors are receiving general payments unconnected to research. However, because the study only included 1 year of data, these results may not tell the full story.
“Understanding the extent to which guideline authors have financial relationships with the pharmaceutical industry—and the types of financial arrangements that they have—is useful for the NCCN and for the public,” said investigator Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“As we learn more about the role of industry payments in shaping prescribing and practice, it is best to proceed with caution and continue to encourage transparency.”
For this study, Dr Dusetzina and her colleagues analyzed financial conflicts of interest (FCOIs) for 125 panelists who worked on setting the NCCN guidelines for lung, breast, prostate, and colorectal cancer (the cancers with the highest incidence in the US).
Eighty-six percent (n=108) of the guideline authors reported at least 1 FCOI in 2014. The total value of FCOIs was $30,287,549, which included $29,036,127 in research payments and $1,251,422 in general payments.
Eighty-four percent of the authors (n=105) received general payments, and 47% (n=59) received research payments.
The authors received an average of $10,011 in general payments and an average of $236,066 in research payments.
The majority of the payments received were within the limits set by the NCCN, but 8 authors (6%) exceeded them. The NCCN says guideline authors cannot receive $20,000 or more from a single company or $50,000 or more in total.
Dr Dusetzina and her colleagues noted that this study was not designed to explore whether the payments influenced the guideline authors’ clinical practice or the recommendations they made in the guidelines.
However, finding a high prevalence of financial relationships with industry among guideline authors lays the foundation for future studies to investigate the impact of such relationships.
“It is not a given that industry funding leads to undue influence,” Dr Dusetzina said, “but it is important to analyze these relationships and the potential impact they have on care guidelines because they do influence patient care decisions and the cost of providing patient care.” ![]()
A study published in JAMA Oncology has quantified financial ties to the pharmaceutical industry among authors of National Comprehensive Cancer Network (NCCN) guidelines.
In 2014, the authors studied received more money in research payments than “general” payments (for things like consulting, meals, and lodging)—$29 million vs $1.25 million.
But more of the authors received general payments than research payments—84% vs 47%.
Study investigators said this finding may mean that some of the guideline authors are receiving general payments unconnected to research. However, because the study only included 1 year of data, these results may not tell the full story.
“Understanding the extent to which guideline authors have financial relationships with the pharmaceutical industry—and the types of financial arrangements that they have—is useful for the NCCN and for the public,” said investigator Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“As we learn more about the role of industry payments in shaping prescribing and practice, it is best to proceed with caution and continue to encourage transparency.”
For this study, Dr Dusetzina and her colleagues analyzed financial conflicts of interest (FCOIs) for 125 panelists who worked on setting the NCCN guidelines for lung, breast, prostate, and colorectal cancer (the cancers with the highest incidence in the US).
Eighty-six percent (n=108) of the guideline authors reported at least 1 FCOI in 2014. The total value of FCOIs was $30,287,549, which included $29,036,127 in research payments and $1,251,422 in general payments.
Eighty-four percent of the authors (n=105) received general payments, and 47% (n=59) received research payments.
The authors received an average of $10,011 in general payments and an average of $236,066 in research payments.
The majority of the payments received were within the limits set by the NCCN, but 8 authors (6%) exceeded them. The NCCN says guideline authors cannot receive $20,000 or more from a single company or $50,000 or more in total.
Dr Dusetzina and her colleagues noted that this study was not designed to explore whether the payments influenced the guideline authors’ clinical practice or the recommendations they made in the guidelines.
However, finding a high prevalence of financial relationships with industry among guideline authors lays the foundation for future studies to investigate the impact of such relationships.
“It is not a given that industry funding leads to undue influence,” Dr Dusetzina said, “but it is important to analyze these relationships and the potential impact they have on care guidelines because they do influence patient care decisions and the cost of providing patient care.” ![]()