User login
Cholecystectomy Delay Linked to Substantially Increased Complication Risk
, regardless of the receipt of sphincterotomy or stenting, new research showed.
“These findings suggest an opportunity for systemic interventions, including prioritization algorithms and better perioperative coordination, to address preventable delays,” reported the authors in the study, presented at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
Choledocholithiasis can occur in up to 20% of symptomatic gallstone cases, and while guidelines recommend having a cholecystectomy concurrently with ERCP, data on the best timing is inconsistent and delays in gall bladder removal are consequently common.
One large study, for instance, the PONCHO trial conducted at 23 hospitals in Netherlands, showed complications to be significantly lower with same-admission vs interval cholecystectomy (4.7% vs 16.9%; P = .02).
Meanwhile, other research has suggested that delayed cholecystectomy is a preferred approach, allowing for removal when there is less inflammation.
Real world data meanwhile shows, despite the guidelines, the procedures are performed at the same time as ERCP only in about 41% of cases, first author Jessica El Halabi, MD, of the Johns Hopkins Hospital, Baltimore, said.
To further investigate outcomes associated with those delays, El Halabi and colleagues conducted a retrospective cohort study involving 507 patients admitted with choledocholithiasis at the hospital and community hospitals between 2005 and 2023 who had 12 months or more follow-up.
The patients had a mean age of 59 years and 59.4% were women.
Of the patients, 265 (52.3%) underwent early cholecystectomy, defined as surgery during the index admission, while 242 (47.7%) underwent delayed cholecystectomy, defined as postdischarge cholecystectomy or if cholecystectomy was not performed.
Overall, biliary complications occurred in as many as 23% of those who had delayed cholecystectomy compared with just 0.8% among those having the early cholecystectomy (P < .001).
Of patients who had delayed cholecystectomy and developed complications, 15.5% did so within 3 months, 6.5% by 6 months, and 1% by 12 months.
Among those who had ERCP with sphincterotomy, there were no significant differences in rates of biliary complications vs those who did not have sphincterotomy (26% vs 21%; P = .74), while stenting also did not reduce the risk (25% vs 27%; P = .81).
The leading reasons for delayed cholecystectomy included patients having a high surgical risk (27.3%), concurrent biliary pathology (19.2%), and physician preference (14%).
The findings underscore that “concurrent cholecystectomy is associated with the lowest risk of biliary complications,” El Halabi said.
“Delayed cholecystectomy is associated with an approximately 23% incidence of biliary complications with 1 year of initial admission, with the highest incidence occurring within 3 months,” she added. “Neither sphincterotomy nor stenting during ERCP mitigates this risk.”
“Early cholecystectomy during the index admission remains the most reliable strategy to reduce recurrent events.”
Findings Underscore Importance of Timing
Commenting on the study, Luis F. Lara, MD, division chief of digestive diseases at the University of Cincinnati, who co-moderated the session, agreed that evidence soundly supports early cholecystectomy.
“We also did a large study looking at this and there’s no doubt that doing it during the index admission has a tremendous effect on long-term outcomes,” Lara told GI & Hepatology News.
Lara noted that “part of it is people don’t show up again until they get sick again, so we don’t want to lose that opportunity the first time, during the index admission,” he said.
Lara’s previous studies have specifically documented how early cholecystectomy for acute biliary pancreatitis improves outcomes of hospitalization for cirrhosis and factors associated with early unplanned readmissions following same-admission cholecystectomy for acute biliary pancreatitis.
Akwi W. Asombang, MD, an interventional gastroenterologist at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, both in Boston, agreed that the findings are important.
“We know that if a cholecystectomy is not performed in the same admission as ERCP, the stones in the gallbladder remain and may migrate out into the bile duct, resulting in further complications as described in the study,” Asombang, also a session co-moderator, told GI & Hepatology News.
She noted that the practice can vary between institutions based on factors including the availability of physicians to perform the cholecystectomy.
Potential complications in delaying the procedure can range from inflammation and pancreatitis to obstruction of the bile duct, “which then can result in cholangitis and eventually sepsis or even death,” Asombang cautioned.
“So the timing of the procedure with ERCP is definitely significant,” she said.
El Halabi and Asombang had no disclosures to report. Lara reported a relationship with AbbVie.
A version of this article first appeared on Medscape.com.
, regardless of the receipt of sphincterotomy or stenting, new research showed.
“These findings suggest an opportunity for systemic interventions, including prioritization algorithms and better perioperative coordination, to address preventable delays,” reported the authors in the study, presented at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
Choledocholithiasis can occur in up to 20% of symptomatic gallstone cases, and while guidelines recommend having a cholecystectomy concurrently with ERCP, data on the best timing is inconsistent and delays in gall bladder removal are consequently common.
One large study, for instance, the PONCHO trial conducted at 23 hospitals in Netherlands, showed complications to be significantly lower with same-admission vs interval cholecystectomy (4.7% vs 16.9%; P = .02).
Meanwhile, other research has suggested that delayed cholecystectomy is a preferred approach, allowing for removal when there is less inflammation.
Real world data meanwhile shows, despite the guidelines, the procedures are performed at the same time as ERCP only in about 41% of cases, first author Jessica El Halabi, MD, of the Johns Hopkins Hospital, Baltimore, said.
To further investigate outcomes associated with those delays, El Halabi and colleagues conducted a retrospective cohort study involving 507 patients admitted with choledocholithiasis at the hospital and community hospitals between 2005 and 2023 who had 12 months or more follow-up.
The patients had a mean age of 59 years and 59.4% were women.
Of the patients, 265 (52.3%) underwent early cholecystectomy, defined as surgery during the index admission, while 242 (47.7%) underwent delayed cholecystectomy, defined as postdischarge cholecystectomy or if cholecystectomy was not performed.
Overall, biliary complications occurred in as many as 23% of those who had delayed cholecystectomy compared with just 0.8% among those having the early cholecystectomy (P < .001).
Of patients who had delayed cholecystectomy and developed complications, 15.5% did so within 3 months, 6.5% by 6 months, and 1% by 12 months.
Among those who had ERCP with sphincterotomy, there were no significant differences in rates of biliary complications vs those who did not have sphincterotomy (26% vs 21%; P = .74), while stenting also did not reduce the risk (25% vs 27%; P = .81).
The leading reasons for delayed cholecystectomy included patients having a high surgical risk (27.3%), concurrent biliary pathology (19.2%), and physician preference (14%).
The findings underscore that “concurrent cholecystectomy is associated with the lowest risk of biliary complications,” El Halabi said.
“Delayed cholecystectomy is associated with an approximately 23% incidence of biliary complications with 1 year of initial admission, with the highest incidence occurring within 3 months,” she added. “Neither sphincterotomy nor stenting during ERCP mitigates this risk.”
“Early cholecystectomy during the index admission remains the most reliable strategy to reduce recurrent events.”
Findings Underscore Importance of Timing
Commenting on the study, Luis F. Lara, MD, division chief of digestive diseases at the University of Cincinnati, who co-moderated the session, agreed that evidence soundly supports early cholecystectomy.
“We also did a large study looking at this and there’s no doubt that doing it during the index admission has a tremendous effect on long-term outcomes,” Lara told GI & Hepatology News.
Lara noted that “part of it is people don’t show up again until they get sick again, so we don’t want to lose that opportunity the first time, during the index admission,” he said.
Lara’s previous studies have specifically documented how early cholecystectomy for acute biliary pancreatitis improves outcomes of hospitalization for cirrhosis and factors associated with early unplanned readmissions following same-admission cholecystectomy for acute biliary pancreatitis.
Akwi W. Asombang, MD, an interventional gastroenterologist at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, both in Boston, agreed that the findings are important.
“We know that if a cholecystectomy is not performed in the same admission as ERCP, the stones in the gallbladder remain and may migrate out into the bile duct, resulting in further complications as described in the study,” Asombang, also a session co-moderator, told GI & Hepatology News.
She noted that the practice can vary between institutions based on factors including the availability of physicians to perform the cholecystectomy.
Potential complications in delaying the procedure can range from inflammation and pancreatitis to obstruction of the bile duct, “which then can result in cholangitis and eventually sepsis or even death,” Asombang cautioned.
“So the timing of the procedure with ERCP is definitely significant,” she said.
El Halabi and Asombang had no disclosures to report. Lara reported a relationship with AbbVie.
A version of this article first appeared on Medscape.com.
, regardless of the receipt of sphincterotomy or stenting, new research showed.
“These findings suggest an opportunity for systemic interventions, including prioritization algorithms and better perioperative coordination, to address preventable delays,” reported the authors in the study, presented at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
Choledocholithiasis can occur in up to 20% of symptomatic gallstone cases, and while guidelines recommend having a cholecystectomy concurrently with ERCP, data on the best timing is inconsistent and delays in gall bladder removal are consequently common.
One large study, for instance, the PONCHO trial conducted at 23 hospitals in Netherlands, showed complications to be significantly lower with same-admission vs interval cholecystectomy (4.7% vs 16.9%; P = .02).
Meanwhile, other research has suggested that delayed cholecystectomy is a preferred approach, allowing for removal when there is less inflammation.
Real world data meanwhile shows, despite the guidelines, the procedures are performed at the same time as ERCP only in about 41% of cases, first author Jessica El Halabi, MD, of the Johns Hopkins Hospital, Baltimore, said.
To further investigate outcomes associated with those delays, El Halabi and colleagues conducted a retrospective cohort study involving 507 patients admitted with choledocholithiasis at the hospital and community hospitals between 2005 and 2023 who had 12 months or more follow-up.
The patients had a mean age of 59 years and 59.4% were women.
Of the patients, 265 (52.3%) underwent early cholecystectomy, defined as surgery during the index admission, while 242 (47.7%) underwent delayed cholecystectomy, defined as postdischarge cholecystectomy or if cholecystectomy was not performed.
Overall, biliary complications occurred in as many as 23% of those who had delayed cholecystectomy compared with just 0.8% among those having the early cholecystectomy (P < .001).
Of patients who had delayed cholecystectomy and developed complications, 15.5% did so within 3 months, 6.5% by 6 months, and 1% by 12 months.
Among those who had ERCP with sphincterotomy, there were no significant differences in rates of biliary complications vs those who did not have sphincterotomy (26% vs 21%; P = .74), while stenting also did not reduce the risk (25% vs 27%; P = .81).
The leading reasons for delayed cholecystectomy included patients having a high surgical risk (27.3%), concurrent biliary pathology (19.2%), and physician preference (14%).
The findings underscore that “concurrent cholecystectomy is associated with the lowest risk of biliary complications,” El Halabi said.
“Delayed cholecystectomy is associated with an approximately 23% incidence of biliary complications with 1 year of initial admission, with the highest incidence occurring within 3 months,” she added. “Neither sphincterotomy nor stenting during ERCP mitigates this risk.”
“Early cholecystectomy during the index admission remains the most reliable strategy to reduce recurrent events.”
Findings Underscore Importance of Timing
Commenting on the study, Luis F. Lara, MD, division chief of digestive diseases at the University of Cincinnati, who co-moderated the session, agreed that evidence soundly supports early cholecystectomy.
“We also did a large study looking at this and there’s no doubt that doing it during the index admission has a tremendous effect on long-term outcomes,” Lara told GI & Hepatology News.
Lara noted that “part of it is people don’t show up again until they get sick again, so we don’t want to lose that opportunity the first time, during the index admission,” he said.
Lara’s previous studies have specifically documented how early cholecystectomy for acute biliary pancreatitis improves outcomes of hospitalization for cirrhosis and factors associated with early unplanned readmissions following same-admission cholecystectomy for acute biliary pancreatitis.
Akwi W. Asombang, MD, an interventional gastroenterologist at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, both in Boston, agreed that the findings are important.
“We know that if a cholecystectomy is not performed in the same admission as ERCP, the stones in the gallbladder remain and may migrate out into the bile duct, resulting in further complications as described in the study,” Asombang, also a session co-moderator, told GI & Hepatology News.
She noted that the practice can vary between institutions based on factors including the availability of physicians to perform the cholecystectomy.
Potential complications in delaying the procedure can range from inflammation and pancreatitis to obstruction of the bile duct, “which then can result in cholangitis and eventually sepsis or even death,” Asombang cautioned.
“So the timing of the procedure with ERCP is definitely significant,” she said.
El Halabi and Asombang had no disclosures to report. Lara reported a relationship with AbbVie.
A version of this article first appeared on Medscape.com.
FROM ACG 2025
New Drug Eases Side Effects of Weight-Loss Meds
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2025
Approach to Weight Management in GI Practice
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
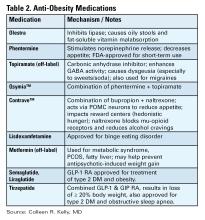
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
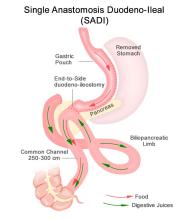
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
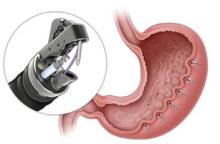
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Resmetirom Reduces Liver Stiffness in MASH Cirrhosis
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
FROM ACG 2025
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).
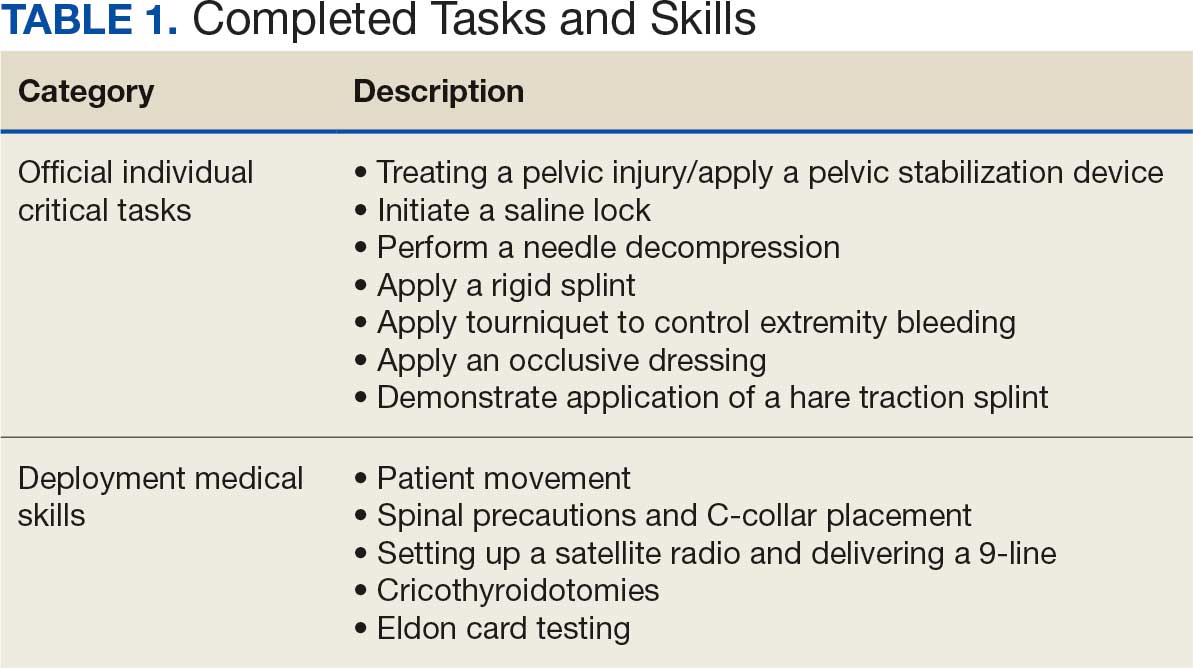
Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.
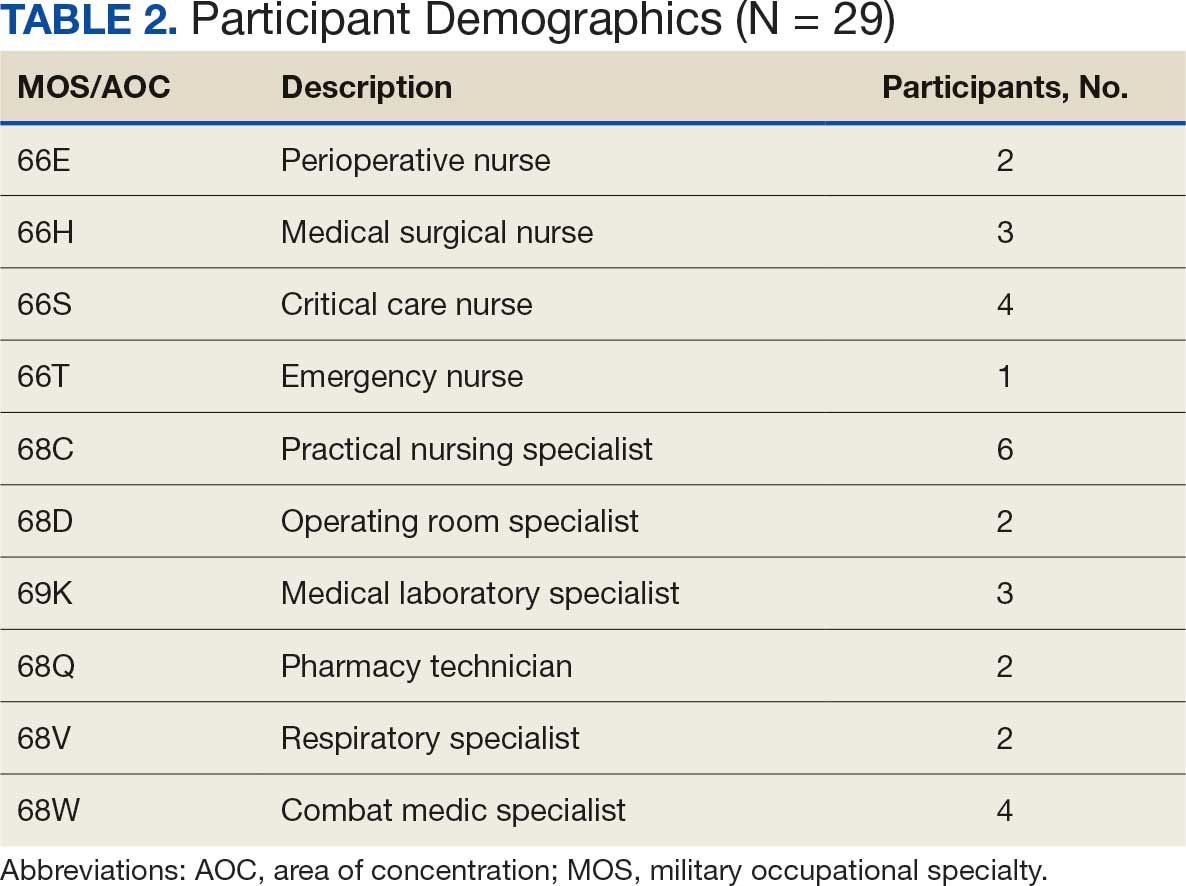
Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
FROM ACG 2025
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).
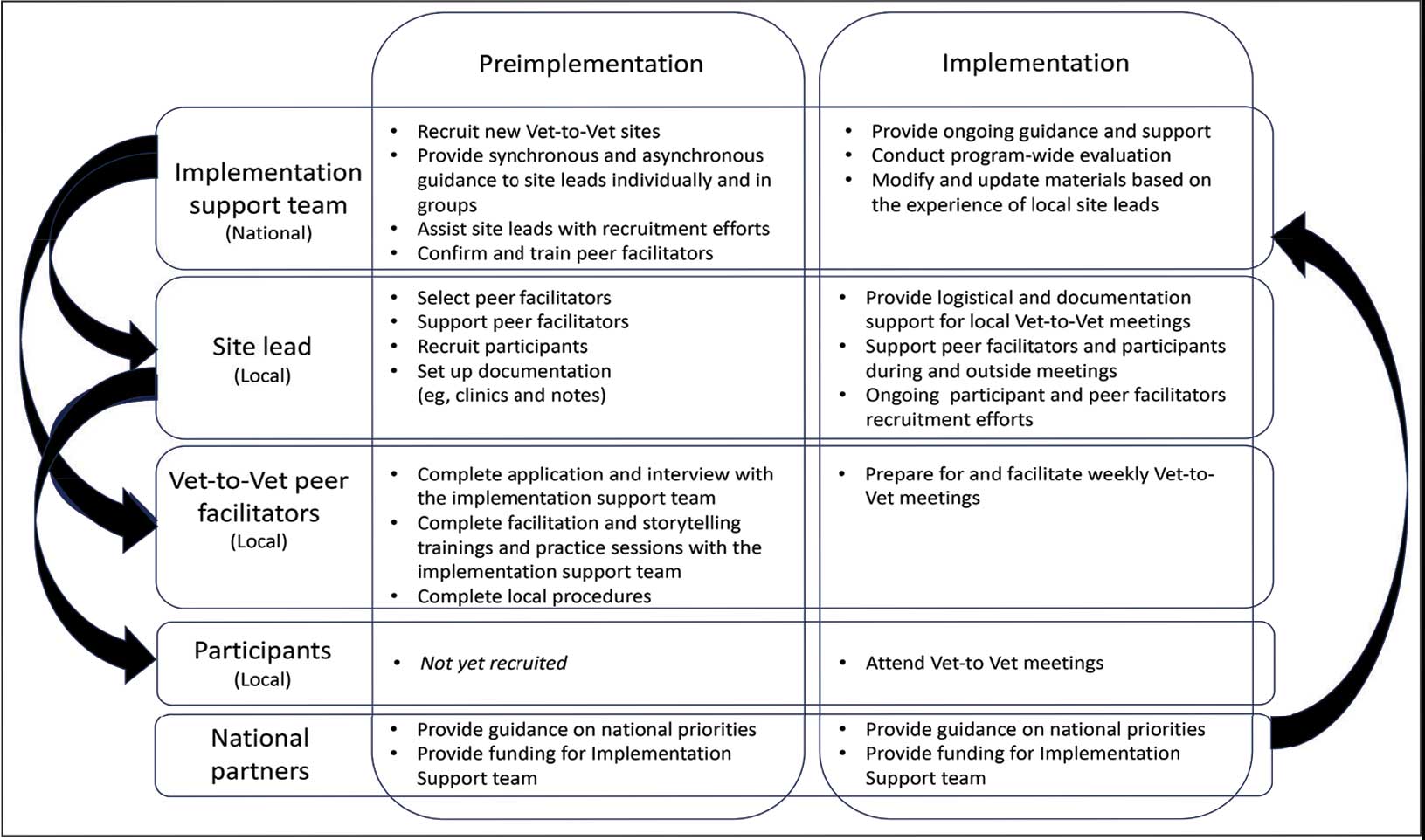
Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).
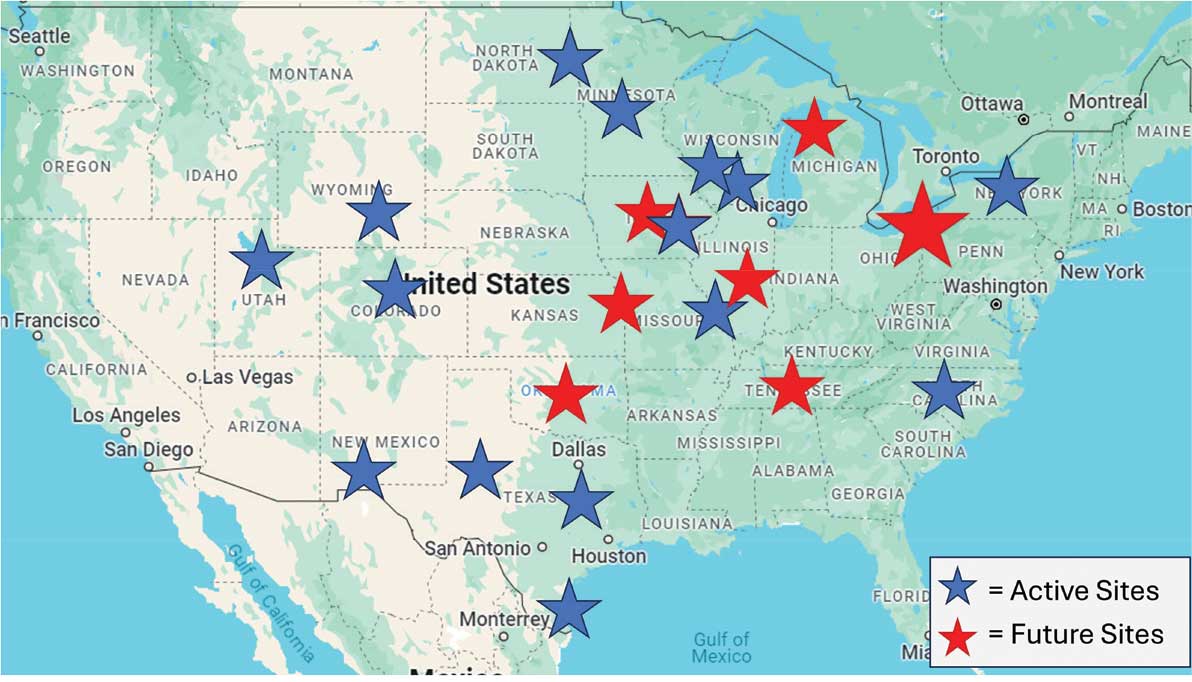
Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
Needle-Knife Fistulotomy is Safe During ERCP, Even for Trainees
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY