User login
How hemophilia B impacts education, work

ORLANDO—Even mild or moderate hemophilia B can have a negative impact on the education and careers of patients, according to the B-HERO-S study.
Ninety-four percent of the patients studied said their disease had a negative impact on their education, and 95% said hemophilia had a negative impact on their work.
More than half of patients who had stopped working said they stopped due to hemophilia-related financial issues and/or complications.
These findings were presented in a poster at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Novo Nordisk.
The previous HERO study revealed career challenges for patients with hemophilia A or B, but it covered mostly males with moderate or severe disease.
The B-HERO-S study, on the other hand, included only patients with hemophilia B, some of whom were female and most of whom had moderate or mild disease. (This study also included caregivers of children with hemophilia, but data on those subjects will not be discussed here.)
In all, there were 299 patients, 86 of whom were women. The patients had a median age of 29 (range, 18-70). Twenty-five percent had mild hemophilia, 63% had moderate disease, and 11% had severe hemophilia.
Education
Most of the patients (71%) had at least some college education, and 17% had graduated from college.
A majority of patients (94%) said their disease had a negative impact on their education. Seventy-five percent of the patients, including those with mild or moderate disease, said hemophilia had a moderate or large impact on their education.
Patients reported difficulty concentrating at school due to bleeds or pain (69%), difficulty attending school or participating in activities due to mobility issues (44%), and hemophilia-related absences (32%).
Career
Most of the patients (81%) were working full- or part-time when surveyed. Fifty-eight percent had office-based jobs, and 39% had jobs involving manual labor. Only 10% of patients said their disease affected their career choice.
Of the 58 patients (19%) who were not working at the time of the study, 62% had never worked. Of those who worked previously, 59% said they stopped due to hemophilia-related financial issues, and 55% said they stopped due to the disease itself and/or its complications.
Nearly all patients (95%) said their disease had a negative impact on their work. Seventy percent said the impact was moderate, 20% said it was small, and 5% said it was large.
Having moderate disease, comorbidities, a higher education, and/or receiving routine infusions were all associated with a higher impact.
Thirty percent of patients said that treatment allowed them to work in most situations. This was more likely in patients with mild hemophilia (41%) and those treated on-demand (48%).
Twenty-seven percent of patients with moderate hemophilia and 28% with severe disease said they could work in most situations. Twenty-seven percent of patients receiving routine infusions said they were able to work in most situations.
“The B-HERO-S study brought to light many challenges faced by some patients with mild/moderate hemophilia B, including affected women and girls,” said Michelle Witkop, of Northern Regional Bleeding Disorder Center in Traverse City, Michigan.
“With hemophilia treatment centers (HTCs) and local hemophilia chapters focused on proactive education in people with severe hemophilia and their families, B-HERO-S data tells us we need to make sure that those with mild/moderate hemophilia B come to the HTC for routine visits so that we can proactively address issues that might come up in school, activities, and career choice.” ![]()

ORLANDO—Even mild or moderate hemophilia B can have a negative impact on the education and careers of patients, according to the B-HERO-S study.
Ninety-four percent of the patients studied said their disease had a negative impact on their education, and 95% said hemophilia had a negative impact on their work.
More than half of patients who had stopped working said they stopped due to hemophilia-related financial issues and/or complications.
These findings were presented in a poster at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Novo Nordisk.
The previous HERO study revealed career challenges for patients with hemophilia A or B, but it covered mostly males with moderate or severe disease.
The B-HERO-S study, on the other hand, included only patients with hemophilia B, some of whom were female and most of whom had moderate or mild disease. (This study also included caregivers of children with hemophilia, but data on those subjects will not be discussed here.)
In all, there were 299 patients, 86 of whom were women. The patients had a median age of 29 (range, 18-70). Twenty-five percent had mild hemophilia, 63% had moderate disease, and 11% had severe hemophilia.
Education
Most of the patients (71%) had at least some college education, and 17% had graduated from college.
A majority of patients (94%) said their disease had a negative impact on their education. Seventy-five percent of the patients, including those with mild or moderate disease, said hemophilia had a moderate or large impact on their education.
Patients reported difficulty concentrating at school due to bleeds or pain (69%), difficulty attending school or participating in activities due to mobility issues (44%), and hemophilia-related absences (32%).
Career
Most of the patients (81%) were working full- or part-time when surveyed. Fifty-eight percent had office-based jobs, and 39% had jobs involving manual labor. Only 10% of patients said their disease affected their career choice.
Of the 58 patients (19%) who were not working at the time of the study, 62% had never worked. Of those who worked previously, 59% said they stopped due to hemophilia-related financial issues, and 55% said they stopped due to the disease itself and/or its complications.
Nearly all patients (95%) said their disease had a negative impact on their work. Seventy percent said the impact was moderate, 20% said it was small, and 5% said it was large.
Having moderate disease, comorbidities, a higher education, and/or receiving routine infusions were all associated with a higher impact.
Thirty percent of patients said that treatment allowed them to work in most situations. This was more likely in patients with mild hemophilia (41%) and those treated on-demand (48%).
Twenty-seven percent of patients with moderate hemophilia and 28% with severe disease said they could work in most situations. Twenty-seven percent of patients receiving routine infusions said they were able to work in most situations.
“The B-HERO-S study brought to light many challenges faced by some patients with mild/moderate hemophilia B, including affected women and girls,” said Michelle Witkop, of Northern Regional Bleeding Disorder Center in Traverse City, Michigan.
“With hemophilia treatment centers (HTCs) and local hemophilia chapters focused on proactive education in people with severe hemophilia and their families, B-HERO-S data tells us we need to make sure that those with mild/moderate hemophilia B come to the HTC for routine visits so that we can proactively address issues that might come up in school, activities, and career choice.” ![]()

ORLANDO—Even mild or moderate hemophilia B can have a negative impact on the education and careers of patients, according to the B-HERO-S study.
Ninety-four percent of the patients studied said their disease had a negative impact on their education, and 95% said hemophilia had a negative impact on their work.
More than half of patients who had stopped working said they stopped due to hemophilia-related financial issues and/or complications.
These findings were presented in a poster at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Novo Nordisk.
The previous HERO study revealed career challenges for patients with hemophilia A or B, but it covered mostly males with moderate or severe disease.
The B-HERO-S study, on the other hand, included only patients with hemophilia B, some of whom were female and most of whom had moderate or mild disease. (This study also included caregivers of children with hemophilia, but data on those subjects will not be discussed here.)
In all, there were 299 patients, 86 of whom were women. The patients had a median age of 29 (range, 18-70). Twenty-five percent had mild hemophilia, 63% had moderate disease, and 11% had severe hemophilia.
Education
Most of the patients (71%) had at least some college education, and 17% had graduated from college.
A majority of patients (94%) said their disease had a negative impact on their education. Seventy-five percent of the patients, including those with mild or moderate disease, said hemophilia had a moderate or large impact on their education.
Patients reported difficulty concentrating at school due to bleeds or pain (69%), difficulty attending school or participating in activities due to mobility issues (44%), and hemophilia-related absences (32%).
Career
Most of the patients (81%) were working full- or part-time when surveyed. Fifty-eight percent had office-based jobs, and 39% had jobs involving manual labor. Only 10% of patients said their disease affected their career choice.
Of the 58 patients (19%) who were not working at the time of the study, 62% had never worked. Of those who worked previously, 59% said they stopped due to hemophilia-related financial issues, and 55% said they stopped due to the disease itself and/or its complications.
Nearly all patients (95%) said their disease had a negative impact on their work. Seventy percent said the impact was moderate, 20% said it was small, and 5% said it was large.
Having moderate disease, comorbidities, a higher education, and/or receiving routine infusions were all associated with a higher impact.
Thirty percent of patients said that treatment allowed them to work in most situations. This was more likely in patients with mild hemophilia (41%) and those treated on-demand (48%).
Twenty-seven percent of patients with moderate hemophilia and 28% with severe disease said they could work in most situations. Twenty-seven percent of patients receiving routine infusions said they were able to work in most situations.
“The B-HERO-S study brought to light many challenges faced by some patients with mild/moderate hemophilia B, including affected women and girls,” said Michelle Witkop, of Northern Regional Bleeding Disorder Center in Traverse City, Michigan.
“With hemophilia treatment centers (HTCs) and local hemophilia chapters focused on proactive education in people with severe hemophilia and their families, B-HERO-S data tells us we need to make sure that those with mild/moderate hemophilia B come to the HTC for routine visits so that we can proactively address issues that might come up in school, activities, and career choice.” ![]()
Light-based therapy may treat thrombocytopenia

in the bone marrow
A low-intensity type of laser therapy could provide a non-invasive, drug-free treatment option for thrombocytopenia, according to research published in Science Translational Medicine.
Researchers found that low-level laser (LLL) therapy increased the generation of platelets from megakaryocytes in vitro and had the same effect in mouse models of thrombocytopenia.
The team also identified the probable mechanism underlying this effect.
“Our study reveals, for the first time, that low-level laser therapy enhances platelet production in animals with thrombocytopenia but not in normal controls,” said study author Mei X. Wu, PhD, of Massachusetts General Hospital in Boston.
“This result suggests that a safe, drug-free method that does not depend on donated blood products can be developed for treating or preventing thrombocytopenia.”
LLLs emit low-powered laser light that does not heat its target tissue. LLLs are known to protect the function of mitochondria, and several conditions associated with impaired platelet production are characterized by abnormalities in the mitochondria of cells that give rise to platelets.
Dr Wu and her colleagues conducted a number of experiments to investigate whether LLLs’ ability to protect mitochondrial function could mitigate several forms of thrombocytopenia.
The team found that LLL treatment of megakaryocytes increased their size, accelerated the formation of proplatelets, and doubled the production of platelets.
Infusion of LLL-treated megakaryocytes into mice led to greater platelet production than did infusion of megakaryocytes treated with normal light.
One of the keys to determining the number of platelets generated from megakaryocytes was mitochondrial production of the energy molecule ATP.
LLL treatment greatly increased mitochondrial generation in polyploid megakaryocytes, but the increase was only slight in less mature megakaryocytes with only 2 copies of each chromosome.
Whole-body LLL treatment of mice with radiation-induced thrombocytopenia spurred the rapid maturation of megakaryocytes and restored platelet levels in a light-dose-dependent fashion.
Platelets from LLL-treated mice had normal structure and function. LLL treatment of normal mice did not raise levels of either megakaryocytes or platelets.
LLL treatment also restored platelet levels in mice with the autoimmune form of thrombocytopenia or with thrombocytopenia caused by chemotherapy.
In cultured human megakaryocytes, LLL treatment at dosage levels similar to those used in mice increased ATP production and platelet generation.
Dr Wu noted that LLL’s lack of an effect in animals without thrombocytopenia indicates it would probably avoid the potential complications of current drug treatments for thrombocytopenia, which act by increasing the production of megakaryocytes from their progenitors in the bone marrow.
“Directly stimulating the differentiation of [megakaryocytes] the way all current drugs do risks clotting if platelet levels rise too high,” Dr Wu said. “LLL appears to enhance [megakaryocytes’] inherent ability to produce platelets most effectively in response to low platelet levels in the circulation, a response that stops when platelet levels are normalized.”
“The fact that treatment only has an effect in polyploid cells, which are very rare, implies that it would not increase production of mitochondria in cancer cells or other cells. In fact, while LLL has been employed in research and in clinical treatment for decades, this is the first study reporting that it can promote mitochondrial biogenesis.”
Dr Wu added that the current primary obstacle to testing LLL in humans is the lack of a device large enough to treat either the entire body or enough bones to stimulate sufficient platelet production by megakaryocytes within the bone marrow, something her team plans to address.
She also noted that, while LLL will probably be beneficial for treatment of many forms of acquired thrombocytopenia, it may not be effective when the condition is caused by inborn genetic defects. ![]()

in the bone marrow
A low-intensity type of laser therapy could provide a non-invasive, drug-free treatment option for thrombocytopenia, according to research published in Science Translational Medicine.
Researchers found that low-level laser (LLL) therapy increased the generation of platelets from megakaryocytes in vitro and had the same effect in mouse models of thrombocytopenia.
The team also identified the probable mechanism underlying this effect.
“Our study reveals, for the first time, that low-level laser therapy enhances platelet production in animals with thrombocytopenia but not in normal controls,” said study author Mei X. Wu, PhD, of Massachusetts General Hospital in Boston.
“This result suggests that a safe, drug-free method that does not depend on donated blood products can be developed for treating or preventing thrombocytopenia.”
LLLs emit low-powered laser light that does not heat its target tissue. LLLs are known to protect the function of mitochondria, and several conditions associated with impaired platelet production are characterized by abnormalities in the mitochondria of cells that give rise to platelets.
Dr Wu and her colleagues conducted a number of experiments to investigate whether LLLs’ ability to protect mitochondrial function could mitigate several forms of thrombocytopenia.
The team found that LLL treatment of megakaryocytes increased their size, accelerated the formation of proplatelets, and doubled the production of platelets.
Infusion of LLL-treated megakaryocytes into mice led to greater platelet production than did infusion of megakaryocytes treated with normal light.
One of the keys to determining the number of platelets generated from megakaryocytes was mitochondrial production of the energy molecule ATP.
LLL treatment greatly increased mitochondrial generation in polyploid megakaryocytes, but the increase was only slight in less mature megakaryocytes with only 2 copies of each chromosome.
Whole-body LLL treatment of mice with radiation-induced thrombocytopenia spurred the rapid maturation of megakaryocytes and restored platelet levels in a light-dose-dependent fashion.
Platelets from LLL-treated mice had normal structure and function. LLL treatment of normal mice did not raise levels of either megakaryocytes or platelets.
LLL treatment also restored platelet levels in mice with the autoimmune form of thrombocytopenia or with thrombocytopenia caused by chemotherapy.
In cultured human megakaryocytes, LLL treatment at dosage levels similar to those used in mice increased ATP production and platelet generation.
Dr Wu noted that LLL’s lack of an effect in animals without thrombocytopenia indicates it would probably avoid the potential complications of current drug treatments for thrombocytopenia, which act by increasing the production of megakaryocytes from their progenitors in the bone marrow.
“Directly stimulating the differentiation of [megakaryocytes] the way all current drugs do risks clotting if platelet levels rise too high,” Dr Wu said. “LLL appears to enhance [megakaryocytes’] inherent ability to produce platelets most effectively in response to low platelet levels in the circulation, a response that stops when platelet levels are normalized.”
“The fact that treatment only has an effect in polyploid cells, which are very rare, implies that it would not increase production of mitochondria in cancer cells or other cells. In fact, while LLL has been employed in research and in clinical treatment for decades, this is the first study reporting that it can promote mitochondrial biogenesis.”
Dr Wu added that the current primary obstacle to testing LLL in humans is the lack of a device large enough to treat either the entire body or enough bones to stimulate sufficient platelet production by megakaryocytes within the bone marrow, something her team plans to address.
She also noted that, while LLL will probably be beneficial for treatment of many forms of acquired thrombocytopenia, it may not be effective when the condition is caused by inborn genetic defects. ![]()

in the bone marrow
A low-intensity type of laser therapy could provide a non-invasive, drug-free treatment option for thrombocytopenia, according to research published in Science Translational Medicine.
Researchers found that low-level laser (LLL) therapy increased the generation of platelets from megakaryocytes in vitro and had the same effect in mouse models of thrombocytopenia.
The team also identified the probable mechanism underlying this effect.
“Our study reveals, for the first time, that low-level laser therapy enhances platelet production in animals with thrombocytopenia but not in normal controls,” said study author Mei X. Wu, PhD, of Massachusetts General Hospital in Boston.
“This result suggests that a safe, drug-free method that does not depend on donated blood products can be developed for treating or preventing thrombocytopenia.”
LLLs emit low-powered laser light that does not heat its target tissue. LLLs are known to protect the function of mitochondria, and several conditions associated with impaired platelet production are characterized by abnormalities in the mitochondria of cells that give rise to platelets.
Dr Wu and her colleagues conducted a number of experiments to investigate whether LLLs’ ability to protect mitochondrial function could mitigate several forms of thrombocytopenia.
The team found that LLL treatment of megakaryocytes increased their size, accelerated the formation of proplatelets, and doubled the production of platelets.
Infusion of LLL-treated megakaryocytes into mice led to greater platelet production than did infusion of megakaryocytes treated with normal light.
One of the keys to determining the number of platelets generated from megakaryocytes was mitochondrial production of the energy molecule ATP.
LLL treatment greatly increased mitochondrial generation in polyploid megakaryocytes, but the increase was only slight in less mature megakaryocytes with only 2 copies of each chromosome.
Whole-body LLL treatment of mice with radiation-induced thrombocytopenia spurred the rapid maturation of megakaryocytes and restored platelet levels in a light-dose-dependent fashion.
Platelets from LLL-treated mice had normal structure and function. LLL treatment of normal mice did not raise levels of either megakaryocytes or platelets.
LLL treatment also restored platelet levels in mice with the autoimmune form of thrombocytopenia or with thrombocytopenia caused by chemotherapy.
In cultured human megakaryocytes, LLL treatment at dosage levels similar to those used in mice increased ATP production and platelet generation.
Dr Wu noted that LLL’s lack of an effect in animals without thrombocytopenia indicates it would probably avoid the potential complications of current drug treatments for thrombocytopenia, which act by increasing the production of megakaryocytes from their progenitors in the bone marrow.
“Directly stimulating the differentiation of [megakaryocytes] the way all current drugs do risks clotting if platelet levels rise too high,” Dr Wu said. “LLL appears to enhance [megakaryocytes’] inherent ability to produce platelets most effectively in response to low platelet levels in the circulation, a response that stops when platelet levels are normalized.”
“The fact that treatment only has an effect in polyploid cells, which are very rare, implies that it would not increase production of mitochondria in cancer cells or other cells. In fact, while LLL has been employed in research and in clinical treatment for decades, this is the first study reporting that it can promote mitochondrial biogenesis.”
Dr Wu added that the current primary obstacle to testing LLL in humans is the lack of a device large enough to treat either the entire body or enough bones to stimulate sufficient platelet production by megakaryocytes within the bone marrow, something her team plans to address.
She also noted that, while LLL will probably be beneficial for treatment of many forms of acquired thrombocytopenia, it may not be effective when the condition is caused by inborn genetic defects. ![]()
Compounds can fight lymphoma, other cancers

Image by Ed Uthman
Preclinical research suggests a novel class of compounds are effective against pediatric and adult cancers, including lymphoma.
The compounds are amphiphilic amines (RCn) based on a tricyclic amine hydrophilic head and a hydrophobic linear alkyl tail of variable length.
The RCn compounds proved cytotoxic in a range of cancer cell lines, including the Burkitt lymphoma cell line Ramos.
The lead compound, RC16, exhibited antitumor effects in vivo and enhanced the in vitro activity of 3 other anticancer agents.
Timothy Cripe, MD, PhD, of Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues reported these results in Pharmaceutical Research.
The investigators first evaluated the RCn compounds for cytotoxicity and mechanism of cell death in several adult and pediatric cancer cell lines.
“We tested RCn’s tumor killing efficacy in cell lines of numerous cancers, including sarcomas, lymphoma, and neuroblastoma,” Dr Cripe said. “We observed anticancer activity of the RCn amines in all the cancer cell lines analyzed.”
The investigators also found that RC16 was 10 times more effective in harming tumor cells than normal cells, including keratinocytes, fibroblasts, and umbilical vein endothelial cells.
In addition, RC16 demonstrated “significant antitumor effects” in several mouse models of malignancy, both when given intravenously and orally.
Because of the amphiphilic molecular structure of RC16, it self-assembled into micelles in water. This chemical structure allowed complexation of the anticancer drugs doxorubicin, etoposide, and paclitaxel.
The micelles significantly improved the in vitro antitumor activity of these drugs by enhancing their solubility in water.
“The antitumor activity of lipophilic amines was interesting because of its action on the mitochondria and lysosomes of cells,” said study author Isabella Orienti, PhD, of the University of Bologna in Italy.
“Moreover, their amphiphilic character improves their bioavailability. We correctly hypothesized these amphiphilic amines would have high antitumor activity and high bioavailability.”
“We are in the process of determining our next steps with testing this new drug,” Dr Cripe added. “This is a promising new therapy for adult and pediatric cancers, and we look forward to further testing its merits.” ![]()

Image by Ed Uthman
Preclinical research suggests a novel class of compounds are effective against pediatric and adult cancers, including lymphoma.
The compounds are amphiphilic amines (RCn) based on a tricyclic amine hydrophilic head and a hydrophobic linear alkyl tail of variable length.
The RCn compounds proved cytotoxic in a range of cancer cell lines, including the Burkitt lymphoma cell line Ramos.
The lead compound, RC16, exhibited antitumor effects in vivo and enhanced the in vitro activity of 3 other anticancer agents.
Timothy Cripe, MD, PhD, of Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues reported these results in Pharmaceutical Research.
The investigators first evaluated the RCn compounds for cytotoxicity and mechanism of cell death in several adult and pediatric cancer cell lines.
“We tested RCn’s tumor killing efficacy in cell lines of numerous cancers, including sarcomas, lymphoma, and neuroblastoma,” Dr Cripe said. “We observed anticancer activity of the RCn amines in all the cancer cell lines analyzed.”
The investigators also found that RC16 was 10 times more effective in harming tumor cells than normal cells, including keratinocytes, fibroblasts, and umbilical vein endothelial cells.
In addition, RC16 demonstrated “significant antitumor effects” in several mouse models of malignancy, both when given intravenously and orally.
Because of the amphiphilic molecular structure of RC16, it self-assembled into micelles in water. This chemical structure allowed complexation of the anticancer drugs doxorubicin, etoposide, and paclitaxel.
The micelles significantly improved the in vitro antitumor activity of these drugs by enhancing their solubility in water.
“The antitumor activity of lipophilic amines was interesting because of its action on the mitochondria and lysosomes of cells,” said study author Isabella Orienti, PhD, of the University of Bologna in Italy.
“Moreover, their amphiphilic character improves their bioavailability. We correctly hypothesized these amphiphilic amines would have high antitumor activity and high bioavailability.”
“We are in the process of determining our next steps with testing this new drug,” Dr Cripe added. “This is a promising new therapy for adult and pediatric cancers, and we look forward to further testing its merits.” ![]()

Image by Ed Uthman
Preclinical research suggests a novel class of compounds are effective against pediatric and adult cancers, including lymphoma.
The compounds are amphiphilic amines (RCn) based on a tricyclic amine hydrophilic head and a hydrophobic linear alkyl tail of variable length.
The RCn compounds proved cytotoxic in a range of cancer cell lines, including the Burkitt lymphoma cell line Ramos.
The lead compound, RC16, exhibited antitumor effects in vivo and enhanced the in vitro activity of 3 other anticancer agents.
Timothy Cripe, MD, PhD, of Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues reported these results in Pharmaceutical Research.
The investigators first evaluated the RCn compounds for cytotoxicity and mechanism of cell death in several adult and pediatric cancer cell lines.
“We tested RCn’s tumor killing efficacy in cell lines of numerous cancers, including sarcomas, lymphoma, and neuroblastoma,” Dr Cripe said. “We observed anticancer activity of the RCn amines in all the cancer cell lines analyzed.”
The investigators also found that RC16 was 10 times more effective in harming tumor cells than normal cells, including keratinocytes, fibroblasts, and umbilical vein endothelial cells.
In addition, RC16 demonstrated “significant antitumor effects” in several mouse models of malignancy, both when given intravenously and orally.
Because of the amphiphilic molecular structure of RC16, it self-assembled into micelles in water. This chemical structure allowed complexation of the anticancer drugs doxorubicin, etoposide, and paclitaxel.
The micelles significantly improved the in vitro antitumor activity of these drugs by enhancing their solubility in water.
“The antitumor activity of lipophilic amines was interesting because of its action on the mitochondria and lysosomes of cells,” said study author Isabella Orienti, PhD, of the University of Bologna in Italy.
“Moreover, their amphiphilic character improves their bioavailability. We correctly hypothesized these amphiphilic amines would have high antitumor activity and high bioavailability.”
“We are in the process of determining our next steps with testing this new drug,” Dr Cripe added. “This is a promising new therapy for adult and pediatric cancers, and we look forward to further testing its merits.” ![]()
PBC can present asymptomatically in elderly patients
An 83-year-old woman who was admitted to an emergency department for unsteadiness and dizziness was eventually diagnosed with primary biliary cirrhosis (PBC), according to a case report by Patrice Baptiste, MBBS, and F. Akinshipo.
Although admitted for unsteadiness, the patient denied falling over, loss of consciousness, chest pain, palpitations, and shortness of breath. Background conditions included hypertension, hypercholesterolemia, atrial fibrillation, a transient ischemic attack, varicose veins, and tinnitus. There was nothing significant in family history, and the patient was an occasional drinker but had never smoked.
An additional review of symptoms before investigation began found coryzal symptoms, dysuria, urinary frequency, and pruritus. After an initial investigation, viral hepatitis was suspected, but additional investigations and a series of negative test results for viral hepatitis led to a diagnosis of PBC. The patient was prescribed ursodeoxycholic acid and discharged from the emergency department.
“There were little findings in this patient’s presentation to suggest PBC; the revelation of pruritus was the only clue before the investigations were conducted,” the investigators wrote. “It is well known that patients can present with nonspecific symptoms or no symptoms at all. Therefore, PBC is an important differential to consider in elderly patients, especially when we know a large proportion of over 65-year-olds are diagnosed with PBC.”
Find the full case report in European Geriatric Medicine (2016 Mar 31. doi: 10.1016/j.eurger.2016.03.002).
An 83-year-old woman who was admitted to an emergency department for unsteadiness and dizziness was eventually diagnosed with primary biliary cirrhosis (PBC), according to a case report by Patrice Baptiste, MBBS, and F. Akinshipo.
Although admitted for unsteadiness, the patient denied falling over, loss of consciousness, chest pain, palpitations, and shortness of breath. Background conditions included hypertension, hypercholesterolemia, atrial fibrillation, a transient ischemic attack, varicose veins, and tinnitus. There was nothing significant in family history, and the patient was an occasional drinker but had never smoked.
An additional review of symptoms before investigation began found coryzal symptoms, dysuria, urinary frequency, and pruritus. After an initial investigation, viral hepatitis was suspected, but additional investigations and a series of negative test results for viral hepatitis led to a diagnosis of PBC. The patient was prescribed ursodeoxycholic acid and discharged from the emergency department.
“There were little findings in this patient’s presentation to suggest PBC; the revelation of pruritus was the only clue before the investigations were conducted,” the investigators wrote. “It is well known that patients can present with nonspecific symptoms or no symptoms at all. Therefore, PBC is an important differential to consider in elderly patients, especially when we know a large proportion of over 65-year-olds are diagnosed with PBC.”
Find the full case report in European Geriatric Medicine (2016 Mar 31. doi: 10.1016/j.eurger.2016.03.002).
An 83-year-old woman who was admitted to an emergency department for unsteadiness and dizziness was eventually diagnosed with primary biliary cirrhosis (PBC), according to a case report by Patrice Baptiste, MBBS, and F. Akinshipo.
Although admitted for unsteadiness, the patient denied falling over, loss of consciousness, chest pain, palpitations, and shortness of breath. Background conditions included hypertension, hypercholesterolemia, atrial fibrillation, a transient ischemic attack, varicose veins, and tinnitus. There was nothing significant in family history, and the patient was an occasional drinker but had never smoked.
An additional review of symptoms before investigation began found coryzal symptoms, dysuria, urinary frequency, and pruritus. After an initial investigation, viral hepatitis was suspected, but additional investigations and a series of negative test results for viral hepatitis led to a diagnosis of PBC. The patient was prescribed ursodeoxycholic acid and discharged from the emergency department.
“There were little findings in this patient’s presentation to suggest PBC; the revelation of pruritus was the only clue before the investigations were conducted,” the investigators wrote. “It is well known that patients can present with nonspecific symptoms or no symptoms at all. Therefore, PBC is an important differential to consider in elderly patients, especially when we know a large proportion of over 65-year-olds are diagnosed with PBC.”
Find the full case report in European Geriatric Medicine (2016 Mar 31. doi: 10.1016/j.eurger.2016.03.002).
FROM EUROPEAN GERIATRIC MEDICINE
Common Medication Provides Insight Into Brain Abnormalities in Dystonia
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Sixteen patients with idiopathic cervical dystonia were compared using a 3-T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity, compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in the middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Roxana G. Burciu, PhD
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Sixteen patients with idiopathic cervical dystonia were compared using a 3-T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity, compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in the middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Roxana G. Burciu, PhD
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Sixteen patients with idiopathic cervical dystonia were compared using a 3-T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity, compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in the middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Roxana G. Burciu, PhD
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”
Which Factors Predict Response to Treatment for Episodic Migraine?
SAN DIEGO—Sustained 24-hour pain relief is common among migraineurs who achieve pain freedom two hours after treatment, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Factors that may predict recurrent headache after two-hour pain freedom include high headache frequency, allodynia, depression, and medication overuse.
Patients with migraine report that rapid pain relief without recurrence is an important outcome. Sagar Munjal, MD, Senior Director at Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues examined data from the American Migraine Prevalence and Prevention Study to identify predictors of two-hour pain freedom and 24-hour sustained response to treatment.
Sagar Munjal, MD
The researchers specifically analyzed responses to two questions from the 2006 survey. The first question was, “After taking your migraine medication, are you pain-free within two hours for most attacks?” The second question was, “Does one dose usually relieve your headache and keep it away for at least 24 hours?” Participants who reported two-hour pain freedom and responded “half the time or more” to the second question were considered to have adequate 24-hour sustained pain relief. Participants who reported two-hour pain freedom and responded “never,” “rarely,” or “less than half the time” to the second question were considered to have inadequate sustained relief.
Dr. Munjal and colleagues used separate binary logistic regression models to evaluate sociodemographics, lifestyle characteristics, headache features, and treatment patterns. They removed variables that were not associated with the outcome from the final model. The factors that remained in the final model were age, gender, marital status, smoking status, allodynia, monthly headache frequency, migraine symptom severity, depression, and medication overuse.
The investigators examined data for 8,333 migraineurs age 18 or older. Approximately 44% of participants reported two-hour pain freedom. Of this population, 82% were female, and the mean age was 47. Among participants with two-hour pain freedom, about 74% reported 24-hour sustained pain relief.
Average headache pain intensity was the main variable that predicted two-hour pain freedom. Allodynia, depression, preventive migraine medication, BMI, female sex, and marital status also predicted two-hour pain freedom. The results indicate the desirability of a fast-acting medication that patients can take early or before pain intensity becomes severe, said Dr. Munjal.
In descending order of significance, predictors of inadequate sustained response were allodynia (odds ratio [OR], 1.55), depression (OR, 1.48), medication overuse (OR, 1.29), and higher monthly headache frequency (OR, 1.06). Insurance status, BMI, number of alcoholic beverages consumed per week, and headache pain severity were removed from the model for nonsignificance.
“These results underscore that unmet need exists for acute migraine treatment in the United States, especially among people with certain sociodemographic and headache characteristics,” said Dr. Munjal. “These data also supplement recent findings that poor treatment optimization is associated with increased risk of chronic migraine onset among people with episodic migraine.”
—Erik Greb
SAN DIEGO—Sustained 24-hour pain relief is common among migraineurs who achieve pain freedom two hours after treatment, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Factors that may predict recurrent headache after two-hour pain freedom include high headache frequency, allodynia, depression, and medication overuse.
Patients with migraine report that rapid pain relief without recurrence is an important outcome. Sagar Munjal, MD, Senior Director at Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues examined data from the American Migraine Prevalence and Prevention Study to identify predictors of two-hour pain freedom and 24-hour sustained response to treatment.
Sagar Munjal, MD
The researchers specifically analyzed responses to two questions from the 2006 survey. The first question was, “After taking your migraine medication, are you pain-free within two hours for most attacks?” The second question was, “Does one dose usually relieve your headache and keep it away for at least 24 hours?” Participants who reported two-hour pain freedom and responded “half the time or more” to the second question were considered to have adequate 24-hour sustained pain relief. Participants who reported two-hour pain freedom and responded “never,” “rarely,” or “less than half the time” to the second question were considered to have inadequate sustained relief.
Dr. Munjal and colleagues used separate binary logistic regression models to evaluate sociodemographics, lifestyle characteristics, headache features, and treatment patterns. They removed variables that were not associated with the outcome from the final model. The factors that remained in the final model were age, gender, marital status, smoking status, allodynia, monthly headache frequency, migraine symptom severity, depression, and medication overuse.
The investigators examined data for 8,333 migraineurs age 18 or older. Approximately 44% of participants reported two-hour pain freedom. Of this population, 82% were female, and the mean age was 47. Among participants with two-hour pain freedom, about 74% reported 24-hour sustained pain relief.
Average headache pain intensity was the main variable that predicted two-hour pain freedom. Allodynia, depression, preventive migraine medication, BMI, female sex, and marital status also predicted two-hour pain freedom. The results indicate the desirability of a fast-acting medication that patients can take early or before pain intensity becomes severe, said Dr. Munjal.
In descending order of significance, predictors of inadequate sustained response were allodynia (odds ratio [OR], 1.55), depression (OR, 1.48), medication overuse (OR, 1.29), and higher monthly headache frequency (OR, 1.06). Insurance status, BMI, number of alcoholic beverages consumed per week, and headache pain severity were removed from the model for nonsignificance.
“These results underscore that unmet need exists for acute migraine treatment in the United States, especially among people with certain sociodemographic and headache characteristics,” said Dr. Munjal. “These data also supplement recent findings that poor treatment optimization is associated with increased risk of chronic migraine onset among people with episodic migraine.”
—Erik Greb
SAN DIEGO—Sustained 24-hour pain relief is common among migraineurs who achieve pain freedom two hours after treatment, according to research presented at the 58th Annual Scientific Meeting of the American Headache Society. Factors that may predict recurrent headache after two-hour pain freedom include high headache frequency, allodynia, depression, and medication overuse.
Patients with migraine report that rapid pain relief without recurrence is an important outcome. Sagar Munjal, MD, Senior Director at Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories in Princeton, New Jersey, and colleagues examined data from the American Migraine Prevalence and Prevention Study to identify predictors of two-hour pain freedom and 24-hour sustained response to treatment.
Sagar Munjal, MD
The researchers specifically analyzed responses to two questions from the 2006 survey. The first question was, “After taking your migraine medication, are you pain-free within two hours for most attacks?” The second question was, “Does one dose usually relieve your headache and keep it away for at least 24 hours?” Participants who reported two-hour pain freedom and responded “half the time or more” to the second question were considered to have adequate 24-hour sustained pain relief. Participants who reported two-hour pain freedom and responded “never,” “rarely,” or “less than half the time” to the second question were considered to have inadequate sustained relief.
Dr. Munjal and colleagues used separate binary logistic regression models to evaluate sociodemographics, lifestyle characteristics, headache features, and treatment patterns. They removed variables that were not associated with the outcome from the final model. The factors that remained in the final model were age, gender, marital status, smoking status, allodynia, monthly headache frequency, migraine symptom severity, depression, and medication overuse.
The investigators examined data for 8,333 migraineurs age 18 or older. Approximately 44% of participants reported two-hour pain freedom. Of this population, 82% were female, and the mean age was 47. Among participants with two-hour pain freedom, about 74% reported 24-hour sustained pain relief.
Average headache pain intensity was the main variable that predicted two-hour pain freedom. Allodynia, depression, preventive migraine medication, BMI, female sex, and marital status also predicted two-hour pain freedom. The results indicate the desirability of a fast-acting medication that patients can take early or before pain intensity becomes severe, said Dr. Munjal.
In descending order of significance, predictors of inadequate sustained response were allodynia (odds ratio [OR], 1.55), depression (OR, 1.48), medication overuse (OR, 1.29), and higher monthly headache frequency (OR, 1.06). Insurance status, BMI, number of alcoholic beverages consumed per week, and headache pain severity were removed from the model for nonsignificance.
“These results underscore that unmet need exists for acute migraine treatment in the United States, especially among people with certain sociodemographic and headache characteristics,” said Dr. Munjal. “These data also supplement recent findings that poor treatment optimization is associated with increased risk of chronic migraine onset among people with episodic migraine.”
—Erik Greb
Outpatient parenteral antimicrobial therapy for homeless patients saves lives, cuts costs
Prolonged hospitalizations for complicated patients with severe infections who need long courses of intravenous antibiotics, are common in many institutions.
Outpatient parenteral antimicrobial therapy (OPAT) is a safe and cost-effective way to administer intravenous (IV) antimicrobial therapy to patients with the potential to decrease hospital length of stay (LOS). OPAT programs train motivated patients in self-administration of IV medications at home, in a stable environment. Ideally, infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy.
A potential barrier to successful utilization of OPAT programs is the need for stable housing at discharge for home infusion services.
Challenge facing homeless patients
There is very little published data regarding the use of OPAT at a medical respite facility for homeless patients. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, active substance abuse, specifically injection drug use (IDU), is a significant problem.
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatient for the duration of their course, which can cause significant inpatient discharge delays and increased LOS. Recommending long-term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased health care costs and contribute to conflicts between patients and staff.
Our study, recently published in the Journal of Hospital Medicine (J Hosp Med. 2016 Apr 27. doi: 10.1002/jhm.2597), aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population (including those with homelessness, mental illness, substance abuse) in which OPAT was received at medical respite.
Our rate of adverse events was 7%, similar to other OPAT studies in the published literature. Our total readmission rate of 30% was similar to what current literature suggests. Our data suggest that providing OPAT to homeless patients is feasible at a medical respite facility with care coordination between members of a multidisciplinary team, including nursing, home infusion pharmacist, and ID clinic.
Partnering with medical respite programs is important, as home infusion services are not available otherwise to homeless patients. The recommendation for ID consultation is beneficial to determine candidacy for OPAT, including close scrutiny of social behaviors in the OPAT patient selection process, and can assist with transitions in care from inpatient to outpatient setting.
Homeless IDU patients remain a challenging population to treat with long term IV antibiotics. However, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors are needed. Furthermore, an evaluation of the patient’s willingness to comply with care agreements while inpatient and at medical respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/non-adherence should be encouraged.
Medical respite programs
Treating homeless IDU patients with OPAT is possible under close supervision at medical respite. Our patients sign an agreement to refrain from using their IV access for drug use. Security seals are used on all connections and tubing to prevent tampering. The IV access sites are inspected daily, and ID providers are contacted to discuss any patients suspicious of tampering with their IV to determine plan of care – either readmission or transition to oral antibiotics.
Medical respite programs are gaining in popularity in the United States. Medical respite can help engage patients in follow-up care and provide linkage to housing, mental health, and chemical dependency services. Many programs support harm reduction IDU practices and offer referrals for substance abuse treatment programs, which are not typically offered during inpatient admission in most hospitals.
Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There are ongoing challenges for housed IDU patients who require OPAT, as medical respite placement and home infusion are generally not options, and skilled nursing facility placement can be difficult. Careful review of substance abuse history; evaluation of psychosocial factors, such as housing status; mental health history; and outpatient support systems are needed.
Again, ID consultation is highly recommended to determine appropriate IV therapy, and if possible, early transition to oral antimicrobial therapy, as well as duration of treatment for specific illnesses on a case-by-case basis. Close follow-up is needed to ensure patient compliance with prescribed antimicrobial regimen, sometimes requiring weekly visits.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidates – particularly among homeless and IDU patients. OPAT can be successful in a closely monitored medical respite setting for homeless patients with multiple comorbidities, with the help of a multidisciplinary team. Medical respite OPAT can decrease LOS in patients who would otherwise require long hospitalizations, resulting in overall cost savings.
Shireesha Dhanireddy, MD, is medical director of the infectious disease clinic at Harborview Medical Center, Seattle. Alison Beieler, PA-C, MPAS, runs the OPAT program in the infectious disease clinic at Harborview Medical Center.
Prolonged hospitalizations for complicated patients with severe infections who need long courses of intravenous antibiotics, are common in many institutions.
Outpatient parenteral antimicrobial therapy (OPAT) is a safe and cost-effective way to administer intravenous (IV) antimicrobial therapy to patients with the potential to decrease hospital length of stay (LOS). OPAT programs train motivated patients in self-administration of IV medications at home, in a stable environment. Ideally, infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy.
A potential barrier to successful utilization of OPAT programs is the need for stable housing at discharge for home infusion services.
Challenge facing homeless patients
There is very little published data regarding the use of OPAT at a medical respite facility for homeless patients. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, active substance abuse, specifically injection drug use (IDU), is a significant problem.
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatient for the duration of their course, which can cause significant inpatient discharge delays and increased LOS. Recommending long-term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased health care costs and contribute to conflicts between patients and staff.
Our study, recently published in the Journal of Hospital Medicine (J Hosp Med. 2016 Apr 27. doi: 10.1002/jhm.2597), aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population (including those with homelessness, mental illness, substance abuse) in which OPAT was received at medical respite.
Our rate of adverse events was 7%, similar to other OPAT studies in the published literature. Our total readmission rate of 30% was similar to what current literature suggests. Our data suggest that providing OPAT to homeless patients is feasible at a medical respite facility with care coordination between members of a multidisciplinary team, including nursing, home infusion pharmacist, and ID clinic.
Partnering with medical respite programs is important, as home infusion services are not available otherwise to homeless patients. The recommendation for ID consultation is beneficial to determine candidacy for OPAT, including close scrutiny of social behaviors in the OPAT patient selection process, and can assist with transitions in care from inpatient to outpatient setting.
Homeless IDU patients remain a challenging population to treat with long term IV antibiotics. However, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors are needed. Furthermore, an evaluation of the patient’s willingness to comply with care agreements while inpatient and at medical respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/non-adherence should be encouraged.
Medical respite programs
Treating homeless IDU patients with OPAT is possible under close supervision at medical respite. Our patients sign an agreement to refrain from using their IV access for drug use. Security seals are used on all connections and tubing to prevent tampering. The IV access sites are inspected daily, and ID providers are contacted to discuss any patients suspicious of tampering with their IV to determine plan of care – either readmission or transition to oral antibiotics.
Medical respite programs are gaining in popularity in the United States. Medical respite can help engage patients in follow-up care and provide linkage to housing, mental health, and chemical dependency services. Many programs support harm reduction IDU practices and offer referrals for substance abuse treatment programs, which are not typically offered during inpatient admission in most hospitals.
Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There are ongoing challenges for housed IDU patients who require OPAT, as medical respite placement and home infusion are generally not options, and skilled nursing facility placement can be difficult. Careful review of substance abuse history; evaluation of psychosocial factors, such as housing status; mental health history; and outpatient support systems are needed.
Again, ID consultation is highly recommended to determine appropriate IV therapy, and if possible, early transition to oral antimicrobial therapy, as well as duration of treatment for specific illnesses on a case-by-case basis. Close follow-up is needed to ensure patient compliance with prescribed antimicrobial regimen, sometimes requiring weekly visits.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidates – particularly among homeless and IDU patients. OPAT can be successful in a closely monitored medical respite setting for homeless patients with multiple comorbidities, with the help of a multidisciplinary team. Medical respite OPAT can decrease LOS in patients who would otherwise require long hospitalizations, resulting in overall cost savings.
Shireesha Dhanireddy, MD, is medical director of the infectious disease clinic at Harborview Medical Center, Seattle. Alison Beieler, PA-C, MPAS, runs the OPAT program in the infectious disease clinic at Harborview Medical Center.
Prolonged hospitalizations for complicated patients with severe infections who need long courses of intravenous antibiotics, are common in many institutions.
Outpatient parenteral antimicrobial therapy (OPAT) is a safe and cost-effective way to administer intravenous (IV) antimicrobial therapy to patients with the potential to decrease hospital length of stay (LOS). OPAT programs train motivated patients in self-administration of IV medications at home, in a stable environment. Ideally, infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy.
A potential barrier to successful utilization of OPAT programs is the need for stable housing at discharge for home infusion services.
Challenge facing homeless patients
There is very little published data regarding the use of OPAT at a medical respite facility for homeless patients. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, active substance abuse, specifically injection drug use (IDU), is a significant problem.
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatient for the duration of their course, which can cause significant inpatient discharge delays and increased LOS. Recommending long-term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased health care costs and contribute to conflicts between patients and staff.
Our study, recently published in the Journal of Hospital Medicine (J Hosp Med. 2016 Apr 27. doi: 10.1002/jhm.2597), aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population (including those with homelessness, mental illness, substance abuse) in which OPAT was received at medical respite.
Our rate of adverse events was 7%, similar to other OPAT studies in the published literature. Our total readmission rate of 30% was similar to what current literature suggests. Our data suggest that providing OPAT to homeless patients is feasible at a medical respite facility with care coordination between members of a multidisciplinary team, including nursing, home infusion pharmacist, and ID clinic.
Partnering with medical respite programs is important, as home infusion services are not available otherwise to homeless patients. The recommendation for ID consultation is beneficial to determine candidacy for OPAT, including close scrutiny of social behaviors in the OPAT patient selection process, and can assist with transitions in care from inpatient to outpatient setting.
Homeless IDU patients remain a challenging population to treat with long term IV antibiotics. However, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors are needed. Furthermore, an evaluation of the patient’s willingness to comply with care agreements while inpatient and at medical respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/non-adherence should be encouraged.
Medical respite programs
Treating homeless IDU patients with OPAT is possible under close supervision at medical respite. Our patients sign an agreement to refrain from using their IV access for drug use. Security seals are used on all connections and tubing to prevent tampering. The IV access sites are inspected daily, and ID providers are contacted to discuss any patients suspicious of tampering with their IV to determine plan of care – either readmission or transition to oral antibiotics.
Medical respite programs are gaining in popularity in the United States. Medical respite can help engage patients in follow-up care and provide linkage to housing, mental health, and chemical dependency services. Many programs support harm reduction IDU practices and offer referrals for substance abuse treatment programs, which are not typically offered during inpatient admission in most hospitals.
Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There are ongoing challenges for housed IDU patients who require OPAT, as medical respite placement and home infusion are generally not options, and skilled nursing facility placement can be difficult. Careful review of substance abuse history; evaluation of psychosocial factors, such as housing status; mental health history; and outpatient support systems are needed.
Again, ID consultation is highly recommended to determine appropriate IV therapy, and if possible, early transition to oral antimicrobial therapy, as well as duration of treatment for specific illnesses on a case-by-case basis. Close follow-up is needed to ensure patient compliance with prescribed antimicrobial regimen, sometimes requiring weekly visits.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidates – particularly among homeless and IDU patients. OPAT can be successful in a closely monitored medical respite setting for homeless patients with multiple comorbidities, with the help of a multidisciplinary team. Medical respite OPAT can decrease LOS in patients who would otherwise require long hospitalizations, resulting in overall cost savings.
Shireesha Dhanireddy, MD, is medical director of the infectious disease clinic at Harborview Medical Center, Seattle. Alison Beieler, PA-C, MPAS, runs the OPAT program in the infectious disease clinic at Harborview Medical Center.
Poor Physical Performance May Be an Early Sign of Late-Age Dementia
Poor physical performance was linked with an increased risk of dementia in a study of individuals age 90 and older who were followed for an average of 2.6 years. After controlling for various factors, poor standing balance had the strongest association with dementia, followed by poor performance in a four-meter walk test and a handgrip test. The study findings were published online ahead of print July 5 in the Journal of the American Geriatrics Society.
“The oldest old, people aged 90 and older, represent the fastest-growing segment of society with the highest rates of dementia; however, many of the traditional risk factors of dementia lose or change their effect in this age group. Therefore, it is crucial that we identify age-specific risk and protective factors for late-age dementia,” said lead author Szofia S. Bullain, MD, an Assistant Professor of Neurology at the University of California, Irvine. “The fact that we were able to detect impairment in physical performance two to three years before the onset of dementia suggests that poor physical performance may be a risk factor for, or an early sign of, developing late-age dementia.”
Dr. Bullain and colleagues conducted a population-based, longitudinal study to examine the relationship between physical performance and dementia in individuals age 90 and older without dementia. They enrolled 176 men and 402 women without dementia from the 90+ Study. Among the total cohort of 578, the mean age was 93.3. At baseline, 54% of the participants were cognitively normal, and 46% had cognitive impairment, but no dementia.
Szofia S. Bullain, MD
Physical performance measures included a four-meter walk, five chair stands, handgrip, and standing balance. Measures were scored from zero (unable to perform) to four (best performance). The outcome was dementia, diagnosed according to DSM-IV criteria. Hazard ratios (HRs) for dementia in relation to baseline physical performance were estimated using Cox regression after adjustment for potential confounders.
Poor physical performance in most measures was associated with greater risk of incident dementia over a mean follow-up of 2.6 years (range, seven months to nine years). After controlling for potential confounders, standing balance had the strongest association with incident dementia (HR, 1.9 to 2.5), followed by four-meter walk (HR, 1.1 to 1.8) and handgrip (HR, 1.0 to 2.0). The association with five chair stands was not significant.
The researchers next plan to examine the underlying pathologic processes, which may provide clues to new preventive and treatment strategies for late-age dementia.
—Glenn S. Williams
Suggested Reading
Bullain SS, Corrada MM, Perry SM, Kawas CH. Sound body sound mind? physical performance and the risk of dementia in the oldest old: the 90+ study. J Am Geriatr Soc. 2016 July 5 [Epub ahead of print].
Poor physical performance was linked with an increased risk of dementia in a study of individuals age 90 and older who were followed for an average of 2.6 years. After controlling for various factors, poor standing balance had the strongest association with dementia, followed by poor performance in a four-meter walk test and a handgrip test. The study findings were published online ahead of print July 5 in the Journal of the American Geriatrics Society.
“The oldest old, people aged 90 and older, represent the fastest-growing segment of society with the highest rates of dementia; however, many of the traditional risk factors of dementia lose or change their effect in this age group. Therefore, it is crucial that we identify age-specific risk and protective factors for late-age dementia,” said lead author Szofia S. Bullain, MD, an Assistant Professor of Neurology at the University of California, Irvine. “The fact that we were able to detect impairment in physical performance two to three years before the onset of dementia suggests that poor physical performance may be a risk factor for, or an early sign of, developing late-age dementia.”
Dr. Bullain and colleagues conducted a population-based, longitudinal study to examine the relationship between physical performance and dementia in individuals age 90 and older without dementia. They enrolled 176 men and 402 women without dementia from the 90+ Study. Among the total cohort of 578, the mean age was 93.3. At baseline, 54% of the participants were cognitively normal, and 46% had cognitive impairment, but no dementia.
Szofia S. Bullain, MD
Physical performance measures included a four-meter walk, five chair stands, handgrip, and standing balance. Measures were scored from zero (unable to perform) to four (best performance). The outcome was dementia, diagnosed according to DSM-IV criteria. Hazard ratios (HRs) for dementia in relation to baseline physical performance were estimated using Cox regression after adjustment for potential confounders.
Poor physical performance in most measures was associated with greater risk of incident dementia over a mean follow-up of 2.6 years (range, seven months to nine years). After controlling for potential confounders, standing balance had the strongest association with incident dementia (HR, 1.9 to 2.5), followed by four-meter walk (HR, 1.1 to 1.8) and handgrip (HR, 1.0 to 2.0). The association with five chair stands was not significant.
The researchers next plan to examine the underlying pathologic processes, which may provide clues to new preventive and treatment strategies for late-age dementia.
—Glenn S. Williams
Poor physical performance was linked with an increased risk of dementia in a study of individuals age 90 and older who were followed for an average of 2.6 years. After controlling for various factors, poor standing balance had the strongest association with dementia, followed by poor performance in a four-meter walk test and a handgrip test. The study findings were published online ahead of print July 5 in the Journal of the American Geriatrics Society.
“The oldest old, people aged 90 and older, represent the fastest-growing segment of society with the highest rates of dementia; however, many of the traditional risk factors of dementia lose or change their effect in this age group. Therefore, it is crucial that we identify age-specific risk and protective factors for late-age dementia,” said lead author Szofia S. Bullain, MD, an Assistant Professor of Neurology at the University of California, Irvine. “The fact that we were able to detect impairment in physical performance two to three years before the onset of dementia suggests that poor physical performance may be a risk factor for, or an early sign of, developing late-age dementia.”
Dr. Bullain and colleagues conducted a population-based, longitudinal study to examine the relationship between physical performance and dementia in individuals age 90 and older without dementia. They enrolled 176 men and 402 women without dementia from the 90+ Study. Among the total cohort of 578, the mean age was 93.3. At baseline, 54% of the participants were cognitively normal, and 46% had cognitive impairment, but no dementia.
Szofia S. Bullain, MD
Physical performance measures included a four-meter walk, five chair stands, handgrip, and standing balance. Measures were scored from zero (unable to perform) to four (best performance). The outcome was dementia, diagnosed according to DSM-IV criteria. Hazard ratios (HRs) for dementia in relation to baseline physical performance were estimated using Cox regression after adjustment for potential confounders.
Poor physical performance in most measures was associated with greater risk of incident dementia over a mean follow-up of 2.6 years (range, seven months to nine years). After controlling for potential confounders, standing balance had the strongest association with incident dementia (HR, 1.9 to 2.5), followed by four-meter walk (HR, 1.1 to 1.8) and handgrip (HR, 1.0 to 2.0). The association with five chair stands was not significant.
The researchers next plan to examine the underlying pathologic processes, which may provide clues to new preventive and treatment strategies for late-age dementia.
—Glenn S. Williams
Suggested Reading
Bullain SS, Corrada MM, Perry SM, Kawas CH. Sound body sound mind? physical performance and the risk of dementia in the oldest old: the 90+ study. J Am Geriatr Soc. 2016 July 5 [Epub ahead of print].
Suggested Reading
Bullain SS, Corrada MM, Perry SM, Kawas CH. Sound body sound mind? physical performance and the risk of dementia in the oldest old: the 90+ study. J Am Geriatr Soc. 2016 July 5 [Epub ahead of print].
Can an Online Screening Tool Identify People With Early Parkinson’s Disease?
VANCOUVER—An algorithm that estimates a person’s risk of Parkinson’s disease based on responses to an online questionnaire may help identify people with the earliest stages of the disease, according to research described at the 68th Annual Meeting of the American Academy of Neurology. People with the highest risk scores have poorer smell, increased rates of REM sleep behavior disorder, and slower finger tapping—intermediate markers of Parkinson’s disease—compared with those with the lowest risk scores. In addition, researchers observed a significant relationship between baseline risk score and incident Parkinson’s disease at three years.
The researchers plan to evaluate the screening tool in a larger cohort, which will allow them to observe more incident cases of Parkinson’s disease and modify the algorithm to improve its strength, said Alastair Noyce, MRCP, PhD, a Parkinson’s UK Research Fellow at University College London Institute of Neurology.
Calculating Risk
Dr. Noyce and his research colleagues developed a prediction algorithm based on a systematic review of Parkinson’s disease risk factors. Factors that increase risk include family history of the disease, constipation, anxiety, depression, pesticide exposure, and head injury. Factors that decrease risk include smoking, coffee and alcohol intake, use of calcium channel blockers, and hypertension.
To evaluate the algorithm, they initiated the longitudinal PREDICT-PD study. Approximately 1,500 people enrolled, and about 1,300 of them were eligible for the study, meaning they were between the ages of 60 and 80, lived in the United Kingdom, and did not have Parkinson’s disease, movement disorders, dementia, stroke, or motor neuron disease, and did not take drugs that can cause parkinsonism. Participants answered questionnaires about motor and nonmotor features and risk factors.
Three prominent features in the Parkinson’s disease prodrome—poor smell, REM sleep behavior disorder, and slow finger tapping—were not included in the prediction algorithm. Instead, the researchers considered those features intermediate markers of Parkinson’s disease and used them to assess at baseline whether the risk stratification process was working. The investigators hypothesized that, compared with the 100 lowest-risk participants, the 100 highest-risk participants would have deficits in smell, as measured by the University of Pennsylvania Smell Identification Test (UPSIT), higher rates of REM sleep behavior disorder (RBD), as measured by the RBD Screening Questionnaire, and slower finger tapping, as measured by the bradykinesia akinesia incoordination test. “That’s exactly what we saw,” Dr. Noyce said. The differences between groups were small but statistically significant.
Participants were asked to complete the questionnaire again each year, and their risk scores were recalculated. As fewer participants completed the survey in subsequent years, researchers compared the 15% of participants with the highest risk scores versus the 15% of participants with the lowest risk scores. When they evaluated intermediate markers in year three, they again observed small but statistically significant differences between the high- and low-risk groups.
In addition, Dr. Noyce recorded video of high-, low-, and intermediate-risk participants performing motor tests in their homes. Researchers blinded to participants’ risk estimates scored them on the Unified Parkinson’s Disease Rating Scale. Depending on the definition of mild parkinsonian signs used, 20% to 30% of the high-risk group had mild parkinsonian signs, compared with 5% of the low-risk group.
Conversion to Parkinson’s Disease
Investigators plan to see if high-risk participants are more likely to convert to Parkinson’s disease over time. Seven participants so far have received independent diagnoses of Parkinson’s disease. Prior to diagnosis, the participants had heterogeneous performance on the various intermediate markers, Dr. Noyce said. For example, two of the participants had normal UPSIT scores, one participant had a borderline UPSIT score, and the remaining four had abnormal UPSIT scores. The finger tapping score “seems to be particularly useful,” he said. Several of the participants who later were diagnosed with Parkinson’s disease had low finger tapping scores.
Using Cox regression analysis, the researchers found a significant relationship between baseline risk score and incident Parkinson’s disease at three years, with a hazard ratio of 4.4. The analysis was based on a small number of incident cases, however, and the hazard ratio had wide confidence intervals, Dr. Noyce noted.
—Jake Remaly
Suggested Reading
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors in Parkinson disease. Ann Neurol. 2012;72(6):893-901.
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. PREDICT-PD: identifying risk of Parkinson’s disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2014;85(1):31-37.
Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(8):871-878.
Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):637-648.
VANCOUVER—An algorithm that estimates a person’s risk of Parkinson’s disease based on responses to an online questionnaire may help identify people with the earliest stages of the disease, according to research described at the 68th Annual Meeting of the American Academy of Neurology. People with the highest risk scores have poorer smell, increased rates of REM sleep behavior disorder, and slower finger tapping—intermediate markers of Parkinson’s disease—compared with those with the lowest risk scores. In addition, researchers observed a significant relationship between baseline risk score and incident Parkinson’s disease at three years.
The researchers plan to evaluate the screening tool in a larger cohort, which will allow them to observe more incident cases of Parkinson’s disease and modify the algorithm to improve its strength, said Alastair Noyce, MRCP, PhD, a Parkinson’s UK Research Fellow at University College London Institute of Neurology.
Calculating Risk
Dr. Noyce and his research colleagues developed a prediction algorithm based on a systematic review of Parkinson’s disease risk factors. Factors that increase risk include family history of the disease, constipation, anxiety, depression, pesticide exposure, and head injury. Factors that decrease risk include smoking, coffee and alcohol intake, use of calcium channel blockers, and hypertension.
To evaluate the algorithm, they initiated the longitudinal PREDICT-PD study. Approximately 1,500 people enrolled, and about 1,300 of them were eligible for the study, meaning they were between the ages of 60 and 80, lived in the United Kingdom, and did not have Parkinson’s disease, movement disorders, dementia, stroke, or motor neuron disease, and did not take drugs that can cause parkinsonism. Participants answered questionnaires about motor and nonmotor features and risk factors.
Three prominent features in the Parkinson’s disease prodrome—poor smell, REM sleep behavior disorder, and slow finger tapping—were not included in the prediction algorithm. Instead, the researchers considered those features intermediate markers of Parkinson’s disease and used them to assess at baseline whether the risk stratification process was working. The investigators hypothesized that, compared with the 100 lowest-risk participants, the 100 highest-risk participants would have deficits in smell, as measured by the University of Pennsylvania Smell Identification Test (UPSIT), higher rates of REM sleep behavior disorder (RBD), as measured by the RBD Screening Questionnaire, and slower finger tapping, as measured by the bradykinesia akinesia incoordination test. “That’s exactly what we saw,” Dr. Noyce said. The differences between groups were small but statistically significant.
Participants were asked to complete the questionnaire again each year, and their risk scores were recalculated. As fewer participants completed the survey in subsequent years, researchers compared the 15% of participants with the highest risk scores versus the 15% of participants with the lowest risk scores. When they evaluated intermediate markers in year three, they again observed small but statistically significant differences between the high- and low-risk groups.
In addition, Dr. Noyce recorded video of high-, low-, and intermediate-risk participants performing motor tests in their homes. Researchers blinded to participants’ risk estimates scored them on the Unified Parkinson’s Disease Rating Scale. Depending on the definition of mild parkinsonian signs used, 20% to 30% of the high-risk group had mild parkinsonian signs, compared with 5% of the low-risk group.
Conversion to Parkinson’s Disease
Investigators plan to see if high-risk participants are more likely to convert to Parkinson’s disease over time. Seven participants so far have received independent diagnoses of Parkinson’s disease. Prior to diagnosis, the participants had heterogeneous performance on the various intermediate markers, Dr. Noyce said. For example, two of the participants had normal UPSIT scores, one participant had a borderline UPSIT score, and the remaining four had abnormal UPSIT scores. The finger tapping score “seems to be particularly useful,” he said. Several of the participants who later were diagnosed with Parkinson’s disease had low finger tapping scores.
Using Cox regression analysis, the researchers found a significant relationship between baseline risk score and incident Parkinson’s disease at three years, with a hazard ratio of 4.4. The analysis was based on a small number of incident cases, however, and the hazard ratio had wide confidence intervals, Dr. Noyce noted.
—Jake Remaly
VANCOUVER—An algorithm that estimates a person’s risk of Parkinson’s disease based on responses to an online questionnaire may help identify people with the earliest stages of the disease, according to research described at the 68th Annual Meeting of the American Academy of Neurology. People with the highest risk scores have poorer smell, increased rates of REM sleep behavior disorder, and slower finger tapping—intermediate markers of Parkinson’s disease—compared with those with the lowest risk scores. In addition, researchers observed a significant relationship between baseline risk score and incident Parkinson’s disease at three years.
The researchers plan to evaluate the screening tool in a larger cohort, which will allow them to observe more incident cases of Parkinson’s disease and modify the algorithm to improve its strength, said Alastair Noyce, MRCP, PhD, a Parkinson’s UK Research Fellow at University College London Institute of Neurology.
Calculating Risk
Dr. Noyce and his research colleagues developed a prediction algorithm based on a systematic review of Parkinson’s disease risk factors. Factors that increase risk include family history of the disease, constipation, anxiety, depression, pesticide exposure, and head injury. Factors that decrease risk include smoking, coffee and alcohol intake, use of calcium channel blockers, and hypertension.
To evaluate the algorithm, they initiated the longitudinal PREDICT-PD study. Approximately 1,500 people enrolled, and about 1,300 of them were eligible for the study, meaning they were between the ages of 60 and 80, lived in the United Kingdom, and did not have Parkinson’s disease, movement disorders, dementia, stroke, or motor neuron disease, and did not take drugs that can cause parkinsonism. Participants answered questionnaires about motor and nonmotor features and risk factors.
Three prominent features in the Parkinson’s disease prodrome—poor smell, REM sleep behavior disorder, and slow finger tapping—were not included in the prediction algorithm. Instead, the researchers considered those features intermediate markers of Parkinson’s disease and used them to assess at baseline whether the risk stratification process was working. The investigators hypothesized that, compared with the 100 lowest-risk participants, the 100 highest-risk participants would have deficits in smell, as measured by the University of Pennsylvania Smell Identification Test (UPSIT), higher rates of REM sleep behavior disorder (RBD), as measured by the RBD Screening Questionnaire, and slower finger tapping, as measured by the bradykinesia akinesia incoordination test. “That’s exactly what we saw,” Dr. Noyce said. The differences between groups were small but statistically significant.
Participants were asked to complete the questionnaire again each year, and their risk scores were recalculated. As fewer participants completed the survey in subsequent years, researchers compared the 15% of participants with the highest risk scores versus the 15% of participants with the lowest risk scores. When they evaluated intermediate markers in year three, they again observed small but statistically significant differences between the high- and low-risk groups.
In addition, Dr. Noyce recorded video of high-, low-, and intermediate-risk participants performing motor tests in their homes. Researchers blinded to participants’ risk estimates scored them on the Unified Parkinson’s Disease Rating Scale. Depending on the definition of mild parkinsonian signs used, 20% to 30% of the high-risk group had mild parkinsonian signs, compared with 5% of the low-risk group.
Conversion to Parkinson’s Disease
Investigators plan to see if high-risk participants are more likely to convert to Parkinson’s disease over time. Seven participants so far have received independent diagnoses of Parkinson’s disease. Prior to diagnosis, the participants had heterogeneous performance on the various intermediate markers, Dr. Noyce said. For example, two of the participants had normal UPSIT scores, one participant had a borderline UPSIT score, and the remaining four had abnormal UPSIT scores. The finger tapping score “seems to be particularly useful,” he said. Several of the participants who later were diagnosed with Parkinson’s disease had low finger tapping scores.
Using Cox regression analysis, the researchers found a significant relationship between baseline risk score and incident Parkinson’s disease at three years, with a hazard ratio of 4.4. The analysis was based on a small number of incident cases, however, and the hazard ratio had wide confidence intervals, Dr. Noyce noted.
—Jake Remaly
Suggested Reading
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors in Parkinson disease. Ann Neurol. 2012;72(6):893-901.
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. PREDICT-PD: identifying risk of Parkinson’s disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2014;85(1):31-37.
Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(8):871-878.
Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):637-648.
Suggested Reading
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors in Parkinson disease. Ann Neurol. 2012;72(6):893-901.
Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. PREDICT-PD: identifying risk of Parkinson’s disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2014;85(1):31-37.
Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(8):871-878.
Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):637-648.
Sport-related concussion: How best to help young athletes
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
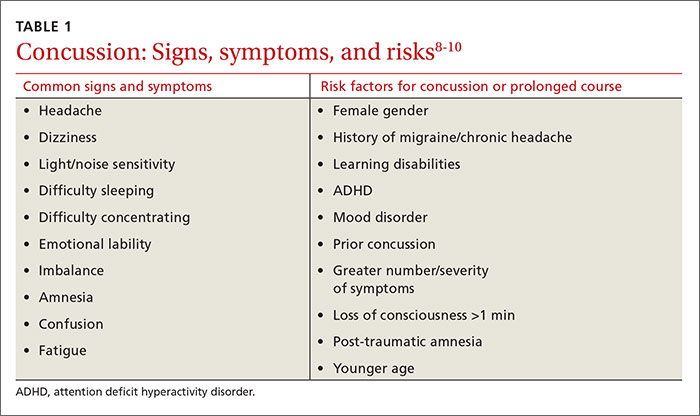
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
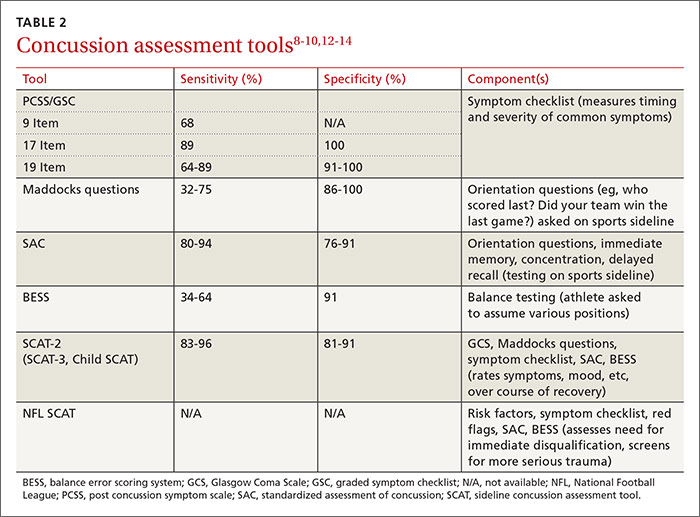
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
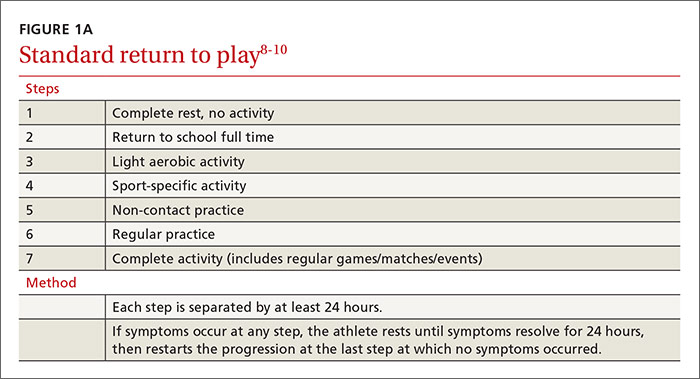
Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
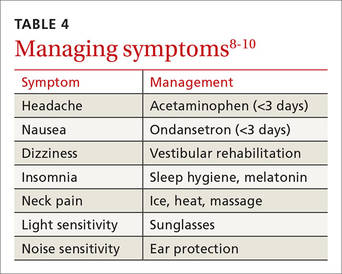
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
From The Journal of Family Practice | 2016;65(8):538-544,546.










