User login
Motivational interviewing for HPV vaccination well accepted by doctors
BALTIMORE – Motivational interviewing (MI) was well accepted by providers as part of a communication tool kit to improve human papillomavirus vaccine uptake, according to results of an eight-site study.
Overall, most of the 107 medical providers who participated in the cluster-randomized trial found MI to be a “somewhat useful” (47%) or “very useful” (31%) tactic to use when discussing human papillomavirus (HPV) vaccination with parents of adolescents. The overall amount of time that providers spent discussing vaccinations actually decreased after implementing MI; at the same time, providers felt that they had more power to influence parental decision-making when using MI techniques.
“Primary care providers given the Physician Communication Toolkit used MI frequently, and this use was generally sustained over time,” said lead author Amanda Dempsey, MD, who presented the findings during a poster symposium at the annual meeting of the Pediatric Academic Societies.
Motivational interviewing, an open-ended, nonjudgmental listening and communication style, was taught to providers in one 30-minute webinar and two 1-hour in-person role-playing sessions. Participants were able to practice using MI both in circumstances where parents were accepting of vaccination, and with vaccine-hesitant families.
Participating providers were surveyed pretraining and at 4, 7, and 10 months after the training to assess their practices in the preceding month. The two primary outcome measures assessed, and compared from baseline, were the estimated time spent discussing HPV vaccination with both vaccine-hesitant and nonhesitant families, and the providers’ perceived abilities to influence decisions about HPV. Dr. Dempsey and her colleagues also asked whether practitioners were actually using MI techniques with vaccine-hesitant parents, and whether they found the techniques useful in HPV vaccination discussions.
Dr. Dempsey, associate professor of pediatrics at Children’s Hospital Colorado in Aurora, said that uptake of MI was initially high and remained so. Three months after the intervention, 85% of providers reported they were using MI; at 9 months after the intervention, the figure was 72%.
Previous research has shown that providers generally do not communicate strong recommendations about HPV vaccination. “Providers often feel the parents will argue with them about it, and sometimes don’t even bring it up,” Dr. Dempsey said in an interview. “Anecdotally, providers found MI a useful way to frame the conversation, and they found it less confrontational.”
Overall, about three-quarters of providers responding to the sequential surveys were physicians, another 15%-20% were physician assistants, and the remainder were nurse practitioners. About one in four respondents were male. The pediatric and family medicine practices were approximately evenly divided between public and private clinics.
Although participation in the training and the subsequent surveys was voluntary, uptake was fairly high at participating clinics. The training was offered for 25 MOC (maintenance of certification) part 4 credits, which probably helped participation rates, said Dr. Dempsey.
The small sample size of the study, said Dr. Dempsey, limits the generalizability of the findings. However, the eight sites chosen represented a wide range of socioeconomic and cultural demographics in the patients served. Also, self-report of MI use may be subject to some bias. Finally, because this was a naturalistic study that allowed providers full discretion in using the various components of the Physician Communication Toolkit, it was not possible to perform a completely independent analysis of the effects of using MI apart from the other toolkit components.
“Use of MI did not appear to lengthen the time of clinical visits, and in some cases may actually shorten them,” said Dr. Dempsey. In addition to analyzing whether MI and other components of the toolkit increased HPV vaccine uptake rates, Dr. Dempsey and her colleagues also plan to explore whether MI would be an effective approach to use when discussing immunizations with parents of infants and younger children.
The study was funded by the National Center for Immunization and Respiratory Diseases and the Centers for Disease Control and Prevention, with survey administration supported by the National Institutes of Health. Dr. Dempsey reported no conflicts of interest.
On Twitter @karioakes
BALTIMORE – Motivational interviewing (MI) was well accepted by providers as part of a communication tool kit to improve human papillomavirus vaccine uptake, according to results of an eight-site study.
Overall, most of the 107 medical providers who participated in the cluster-randomized trial found MI to be a “somewhat useful” (47%) or “very useful” (31%) tactic to use when discussing human papillomavirus (HPV) vaccination with parents of adolescents. The overall amount of time that providers spent discussing vaccinations actually decreased after implementing MI; at the same time, providers felt that they had more power to influence parental decision-making when using MI techniques.
“Primary care providers given the Physician Communication Toolkit used MI frequently, and this use was generally sustained over time,” said lead author Amanda Dempsey, MD, who presented the findings during a poster symposium at the annual meeting of the Pediatric Academic Societies.
Motivational interviewing, an open-ended, nonjudgmental listening and communication style, was taught to providers in one 30-minute webinar and two 1-hour in-person role-playing sessions. Participants were able to practice using MI both in circumstances where parents were accepting of vaccination, and with vaccine-hesitant families.
Participating providers were surveyed pretraining and at 4, 7, and 10 months after the training to assess their practices in the preceding month. The two primary outcome measures assessed, and compared from baseline, were the estimated time spent discussing HPV vaccination with both vaccine-hesitant and nonhesitant families, and the providers’ perceived abilities to influence decisions about HPV. Dr. Dempsey and her colleagues also asked whether practitioners were actually using MI techniques with vaccine-hesitant parents, and whether they found the techniques useful in HPV vaccination discussions.
Dr. Dempsey, associate professor of pediatrics at Children’s Hospital Colorado in Aurora, said that uptake of MI was initially high and remained so. Three months after the intervention, 85% of providers reported they were using MI; at 9 months after the intervention, the figure was 72%.
Previous research has shown that providers generally do not communicate strong recommendations about HPV vaccination. “Providers often feel the parents will argue with them about it, and sometimes don’t even bring it up,” Dr. Dempsey said in an interview. “Anecdotally, providers found MI a useful way to frame the conversation, and they found it less confrontational.”
Overall, about three-quarters of providers responding to the sequential surveys were physicians, another 15%-20% were physician assistants, and the remainder were nurse practitioners. About one in four respondents were male. The pediatric and family medicine practices were approximately evenly divided between public and private clinics.
Although participation in the training and the subsequent surveys was voluntary, uptake was fairly high at participating clinics. The training was offered for 25 MOC (maintenance of certification) part 4 credits, which probably helped participation rates, said Dr. Dempsey.
The small sample size of the study, said Dr. Dempsey, limits the generalizability of the findings. However, the eight sites chosen represented a wide range of socioeconomic and cultural demographics in the patients served. Also, self-report of MI use may be subject to some bias. Finally, because this was a naturalistic study that allowed providers full discretion in using the various components of the Physician Communication Toolkit, it was not possible to perform a completely independent analysis of the effects of using MI apart from the other toolkit components.
“Use of MI did not appear to lengthen the time of clinical visits, and in some cases may actually shorten them,” said Dr. Dempsey. In addition to analyzing whether MI and other components of the toolkit increased HPV vaccine uptake rates, Dr. Dempsey and her colleagues also plan to explore whether MI would be an effective approach to use when discussing immunizations with parents of infants and younger children.
The study was funded by the National Center for Immunization and Respiratory Diseases and the Centers for Disease Control and Prevention, with survey administration supported by the National Institutes of Health. Dr. Dempsey reported no conflicts of interest.
On Twitter @karioakes
BALTIMORE – Motivational interviewing (MI) was well accepted by providers as part of a communication tool kit to improve human papillomavirus vaccine uptake, according to results of an eight-site study.
Overall, most of the 107 medical providers who participated in the cluster-randomized trial found MI to be a “somewhat useful” (47%) or “very useful” (31%) tactic to use when discussing human papillomavirus (HPV) vaccination with parents of adolescents. The overall amount of time that providers spent discussing vaccinations actually decreased after implementing MI; at the same time, providers felt that they had more power to influence parental decision-making when using MI techniques.
“Primary care providers given the Physician Communication Toolkit used MI frequently, and this use was generally sustained over time,” said lead author Amanda Dempsey, MD, who presented the findings during a poster symposium at the annual meeting of the Pediatric Academic Societies.
Motivational interviewing, an open-ended, nonjudgmental listening and communication style, was taught to providers in one 30-minute webinar and two 1-hour in-person role-playing sessions. Participants were able to practice using MI both in circumstances where parents were accepting of vaccination, and with vaccine-hesitant families.
Participating providers were surveyed pretraining and at 4, 7, and 10 months after the training to assess their practices in the preceding month. The two primary outcome measures assessed, and compared from baseline, were the estimated time spent discussing HPV vaccination with both vaccine-hesitant and nonhesitant families, and the providers’ perceived abilities to influence decisions about HPV. Dr. Dempsey and her colleagues also asked whether practitioners were actually using MI techniques with vaccine-hesitant parents, and whether they found the techniques useful in HPV vaccination discussions.
Dr. Dempsey, associate professor of pediatrics at Children’s Hospital Colorado in Aurora, said that uptake of MI was initially high and remained so. Three months after the intervention, 85% of providers reported they were using MI; at 9 months after the intervention, the figure was 72%.
Previous research has shown that providers generally do not communicate strong recommendations about HPV vaccination. “Providers often feel the parents will argue with them about it, and sometimes don’t even bring it up,” Dr. Dempsey said in an interview. “Anecdotally, providers found MI a useful way to frame the conversation, and they found it less confrontational.”
Overall, about three-quarters of providers responding to the sequential surveys were physicians, another 15%-20% were physician assistants, and the remainder were nurse practitioners. About one in four respondents were male. The pediatric and family medicine practices were approximately evenly divided between public and private clinics.
Although participation in the training and the subsequent surveys was voluntary, uptake was fairly high at participating clinics. The training was offered for 25 MOC (maintenance of certification) part 4 credits, which probably helped participation rates, said Dr. Dempsey.
The small sample size of the study, said Dr. Dempsey, limits the generalizability of the findings. However, the eight sites chosen represented a wide range of socioeconomic and cultural demographics in the patients served. Also, self-report of MI use may be subject to some bias. Finally, because this was a naturalistic study that allowed providers full discretion in using the various components of the Physician Communication Toolkit, it was not possible to perform a completely independent analysis of the effects of using MI apart from the other toolkit components.
“Use of MI did not appear to lengthen the time of clinical visits, and in some cases may actually shorten them,” said Dr. Dempsey. In addition to analyzing whether MI and other components of the toolkit increased HPV vaccine uptake rates, Dr. Dempsey and her colleagues also plan to explore whether MI would be an effective approach to use when discussing immunizations with parents of infants and younger children.
The study was funded by the National Center for Immunization and Respiratory Diseases and the Centers for Disease Control and Prevention, with survey administration supported by the National Institutes of Health. Dr. Dempsey reported no conflicts of interest.
On Twitter @karioakes
AT THE PAS ANNUAL MEETING
Key clinical point: Seventy-eight percent of providers found motivational interviewing (MI) useful for HPV vaccine counseling.
Major finding: Nine months after MI training, 72% of providers were still using the technique in HPV vaccine counseling.
Data source: Pilot study of 107 medical providers at eight clinics who received training to use MI for HPV vaccine counseling.
Disclosures: The study was funded by the National Center for Immunization and Respiratory Diseases and the Centers for Disease Control and Prevention, with survey administration supported by the National Institutes of Health. Dr. Dempsey reported no conflicts of interest.
Gestational Diabetes Ups Risk for Infantile Hemangioma
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
AT THE SPD ANNUAL MEETING
Gestational diabetes ups risk for infantile hemangiomas
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
On Twitter @karioakes
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
On Twitter @karioakes
MINNEAPOLIS – Gestational diabetes and prenatal progesterone use were among the maternal factors associated with increased risk of infantile hemangioma, a benign vascular neoplasm whose incidence has been steadily rising over the past several decades.
Data from a large longitudinal epidemiology study were used to explore the association of a number of maternal risk factors with infantile hemangiomas, said Jennifer Schoch, MD, who presented these findings in a poster session at the annual meeting of the Society for Pediatric Dermatology.
After adjusting for gestational age and multiple gestations, the researchers found that infants born to mothers with gestational diabetes were more likely to have an infantile hemangioma (odds ratio, 1.79; P = .029). Maternal preeclampsia was even more strongly associated with infantile hemangioma (OR, 3.43, P = .017), as was prenatal progesterone use (OR, 2.25; P less than .001). Forceps-assisted vaginal delivery also increased the likelihood of infantile hemangioma (OR, 1.45; P = .035).
Low birth weight, prematurity, and being female and of non-Hispanic white race are some of the infant risk factors known to be associated with infantile hemangioma, but maternal risk factors in the development of infantile hemangioma are less clear, according to the researchers from the Mayo Clinic, Rochester, Minn. Some previous work has suggested that placental abnormalities and invasive procedures carried out during pregnancy, as well as the use of progesterone and corticosteroids during pregnancy, may increase the risk of infantile hemangiomas.
Using a retrospective case-control approach, the researchers used data from the 50-year-old Rochester Epidemiology Project. A chart review identified 869 mother-infant pairs with infantile hemangiomas and 869 age- and sex-matched control maternal-infant pairs whose infants did not have the condition. More than half (65%) of the infants in aggregate were girls (n = 561). Multivariable analysis was used to adjust for gestational age and multiple gestations.
Looking at the trends over time revealed that the rates of gestational diabetes, assisted reproduction techniques, and progesterone use during pregnancy have all increased during the same 35-year period of increased infantile hemangioma incidence, Dr. Schoch said in an interview.
Some earlier work suggests that infantile hemangiomas may arise from fetal placental progenitor cells. Since gestational diabetes can be associated with degradation of the placenta in late pregnancy, Dr. Schoch said that these effects on the placenta may have some connection to the increased risk of infantile hemangiomas in infants whose mothers have gestational diabetes.
Dr. Schoch, who is now professor of dermatology at the University of Florida, Gainesville, also noted that the study, completed during her fellowship at the Mayo Clinic, was limited by the low ethnic diversity of the study population, which draws from several counties in Minnesota and Wisconsin.
The Rochester Epidemiology Project is supported by the National Institutes of Health. The researchers reported having no financial disclosures.
On Twitter @karioakes
AT THE SPD ANNUAL MEETING
Key clinical point: Gestational diabetes and other maternal factors may increase the risk for infantile hemangioma.
Major finding: Infants born to mothers with gestational diabetes had an odds ratio of 1.79 for infantile hemangioma (P = .029).
Data source: Retrospective control-matched study of 865 infants with infantile hemangioma.
Disclosures: The researchers reported having no financial disclosures.
FDA updates warning label for systemic fluoroquinolones
The Food and Drug Administration has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ alarming potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that, for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched onto a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox; Cipro, both standard and extended release; Factive; Levaquin; and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use. Their active ingredients are moxifloxacin, ciprofloxacin, gemifloxacin, levofloxacin, and ofloxacin, respectively.
Additional side effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, diarrhea, as well as aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA also added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
The Food and Drug Administration has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ alarming potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that, for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched onto a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox; Cipro, both standard and extended release; Factive; Levaquin; and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use. Their active ingredients are moxifloxacin, ciprofloxacin, gemifloxacin, levofloxacin, and ofloxacin, respectively.
Additional side effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, diarrhea, as well as aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA also added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
The Food and Drug Administration has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ alarming potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that, for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched onto a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox; Cipro, both standard and extended release; Factive; Levaquin; and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use. Their active ingredients are moxifloxacin, ciprofloxacin, gemifloxacin, levofloxacin, and ofloxacin, respectively.
Additional side effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, diarrhea, as well as aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA also added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
Shortness of breath: Looking beyond the usual suspects
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
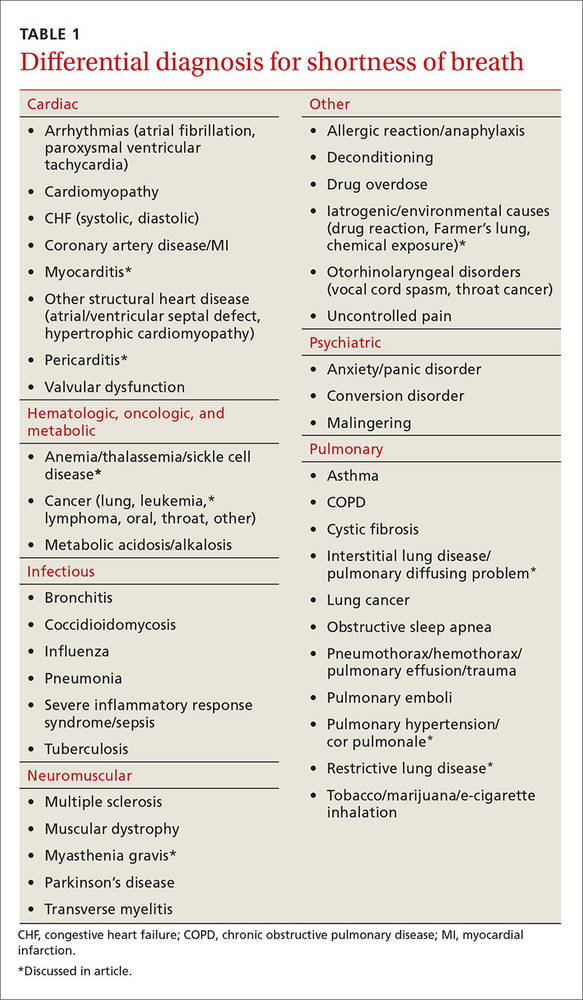
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.
Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
Medicare annual wellness visit remains underused
TORONTO – The annual Medicare wellness visit offers patients and their doctors the chance to review a variety of health concerns, and includes a cognitive health checkup. It’s free for patients and fully reimbursable for physicians. But the visit is far underused, averaging about 14% uptake each year across the United States, according to Pamela Mink, PhD.
Dr. Mink and her colleagues at Minneapolis, Minn.–based Allina Health examined which patients used the visit during 2011-2015 in the Allina Health system. They found annual increases in usage, but by the end of the 5-year period it had been used at least once by only 44% of eligible patients despite some considerable outreach efforts. Those who did were most often white or Asian women who lived in urban areas and were younger than 74 years.
“There’s a real opportunity for tracking and identifying cognitive health issues early here, and it’s being missed,” Dr. Mink said during a press briefing at the Alzheimer’s Association International Conference 2016. “Improving this situation is going to require leadership from health systems, the clinicians administering the visit, and the patients themselves. It’s basic education: Clinicians need to know it’s reimbursable. Patients need to know it’s free, and that it’s a valuable opportunity to address health issues – including any cognitive impairment that may be developing – early.”
Dr. Mink is employed by Allina Health as a senior scientist.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – The annual Medicare wellness visit offers patients and their doctors the chance to review a variety of health concerns, and includes a cognitive health checkup. It’s free for patients and fully reimbursable for physicians. But the visit is far underused, averaging about 14% uptake each year across the United States, according to Pamela Mink, PhD.
Dr. Mink and her colleagues at Minneapolis, Minn.–based Allina Health examined which patients used the visit during 2011-2015 in the Allina Health system. They found annual increases in usage, but by the end of the 5-year period it had been used at least once by only 44% of eligible patients despite some considerable outreach efforts. Those who did were most often white or Asian women who lived in urban areas and were younger than 74 years.
“There’s a real opportunity for tracking and identifying cognitive health issues early here, and it’s being missed,” Dr. Mink said during a press briefing at the Alzheimer’s Association International Conference 2016. “Improving this situation is going to require leadership from health systems, the clinicians administering the visit, and the patients themselves. It’s basic education: Clinicians need to know it’s reimbursable. Patients need to know it’s free, and that it’s a valuable opportunity to address health issues – including any cognitive impairment that may be developing – early.”
Dr. Mink is employed by Allina Health as a senior scientist.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – The annual Medicare wellness visit offers patients and their doctors the chance to review a variety of health concerns, and includes a cognitive health checkup. It’s free for patients and fully reimbursable for physicians. But the visit is far underused, averaging about 14% uptake each year across the United States, according to Pamela Mink, PhD.
Dr. Mink and her colleagues at Minneapolis, Minn.–based Allina Health examined which patients used the visit during 2011-2015 in the Allina Health system. They found annual increases in usage, but by the end of the 5-year period it had been used at least once by only 44% of eligible patients despite some considerable outreach efforts. Those who did were most often white or Asian women who lived in urban areas and were younger than 74 years.
“There’s a real opportunity for tracking and identifying cognitive health issues early here, and it’s being missed,” Dr. Mink said during a press briefing at the Alzheimer’s Association International Conference 2016. “Improving this situation is going to require leadership from health systems, the clinicians administering the visit, and the patients themselves. It’s basic education: Clinicians need to know it’s reimbursable. Patients need to know it’s free, and that it’s a valuable opportunity to address health issues – including any cognitive impairment that may be developing – early.”
Dr. Mink is employed by Allina Health as a senior scientist.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2016
Congenital craniofacial deformities
1. This youngster is being followed for symptoms related to his right-sided cranial asymmetry noted at birth, including neurologic impairment (sensorineural hearing loss and visual problems including strabismus) and breathing problems; developmental delay and/or intellectual disability; and hydrocephalus. The hands and feet are normal.
Source: By Michael L. Kaufman at the English language Wikipedia, CC BY-SA 3.0
Diagnosis: Craniosynostosis, also known as acrocephalosyndactyly, is a condition in which the skull sutures close prematurely. This can cause pressure to build up inside the head and skull or facial bones, leading to a misshapen head and distinctive facial features—ocular hypertelorism, proptosis, midface hypoplasia, small beaked nose, and prognathism—and occasionally abnormal fingers and toes.
Mutations of the FGFR gene, which regulates the fibroblast growth factor receptor protein, are responsible for the eight disorders comprising the FGFR-related craniosynostosis spectrum. The protein plays an important role in bone growth, particularly during embryonic development. For example, this protein signals certain immature cells in the developing embryo to become bone cells in the head, hands, feet, and other tissues.
For more information, see Robin NH, MD, Falk MJ, Haldeman-Englert CR. FGFR-related craniosynostosis syndromes. In: GeneReviews® [Internet]. Pagon RA, Adam MP, Ardinger HH, et al, eds. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
2. This infant presents with a misshapen cranium, most notably the flattened area on the posterior right side. His history includes limited passive neck rotation at birth, preferential head orientation, supine sleep position, and lower activity level.
Source: Wikimedia Commons; By Medical advises - http://larece.ru/?p=27115, CC BY-SA 3.0
Diagnosis: Plagiocephaly, literally means "oblique head" (from the Greek "plagio" for oblique and "cephale" for head). The incidence in infants at 7 to 12 weeks of age is estimated to be 46.6%. Of all infants with plagiocephaly, 63.2% are affected on the right side and 78.3% have a mild form.
For more information, see “Helmets for Positional Skull Deformities: A Good Idea or Not?” Clin Rev. 2015;25(2):16,18.
For the next photograph, proceed to the next page >>
3. An adolescent patient presents with marked facial asymmetry, present since birth. The auricular tissue on the left side is malformed; on the right side, low set. The right midface is underdeveloped with micrognathia and chin deviation, resulting in malocclusion.
Source: Wikimedia Commons
Diagnosis: Craniofacial microsomia, possibly caused by bleeding in the temporal area during gestation, describes a spectrum of cranial and facial abnormalities. After Down syndrome, it is the most common congenital syndrome, occurring in approximately 1 in 3,500 to 4,500 live births. In about two-thirds of cases, abnormalities differ from one side to the other.
These individuals typically demonstrate one-sided maxillary or mandibular hypoplasia, which can cause dental problems and difficulties with feeding and speech. In cases of severe mandibular hypoplasia, breathing may also be affected.
Abnormalities affecting one or both ears may range from preauricular tags to an underdeveloped or absent external ear or a closed or absent ear canal; these patients may experience hearing loss. Microphthalmia, occurring less frequently, may result in vision loss.
For more information, see Heike CL, Luquetti DV, Hing AV. Craniofacial microsomia overview. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
4. A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” on his forehead that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
Diagnosis: Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors. They are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck and more commonly in females. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also manifest in extracutaneous areas (eg, the liver); these are usually detected via imaging.
For more information, see “A New Approach to “Birthmarks” Clin Rev. 2016;26(3):W1.
RELATED ARTICLE:
Infantile Hemangiomas Increasing, Linked to Prematurity, Low Birth Weight Bruce Jancin, Family Practice News Digital Network
1. This youngster is being followed for symptoms related to his right-sided cranial asymmetry noted at birth, including neurologic impairment (sensorineural hearing loss and visual problems including strabismus) and breathing problems; developmental delay and/or intellectual disability; and hydrocephalus. The hands and feet are normal.
Source: By Michael L. Kaufman at the English language Wikipedia, CC BY-SA 3.0
Diagnosis: Craniosynostosis, also known as acrocephalosyndactyly, is a condition in which the skull sutures close prematurely. This can cause pressure to build up inside the head and skull or facial bones, leading to a misshapen head and distinctive facial features—ocular hypertelorism, proptosis, midface hypoplasia, small beaked nose, and prognathism—and occasionally abnormal fingers and toes.
Mutations of the FGFR gene, which regulates the fibroblast growth factor receptor protein, are responsible for the eight disorders comprising the FGFR-related craniosynostosis spectrum. The protein plays an important role in bone growth, particularly during embryonic development. For example, this protein signals certain immature cells in the developing embryo to become bone cells in the head, hands, feet, and other tissues.
For more information, see Robin NH, MD, Falk MJ, Haldeman-Englert CR. FGFR-related craniosynostosis syndromes. In: GeneReviews® [Internet]. Pagon RA, Adam MP, Ardinger HH, et al, eds. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
2. This infant presents with a misshapen cranium, most notably the flattened area on the posterior right side. His history includes limited passive neck rotation at birth, preferential head orientation, supine sleep position, and lower activity level.
Source: Wikimedia Commons; By Medical advises - http://larece.ru/?p=27115, CC BY-SA 3.0
Diagnosis: Plagiocephaly, literally means "oblique head" (from the Greek "plagio" for oblique and "cephale" for head). The incidence in infants at 7 to 12 weeks of age is estimated to be 46.6%. Of all infants with plagiocephaly, 63.2% are affected on the right side and 78.3% have a mild form.
For more information, see “Helmets for Positional Skull Deformities: A Good Idea or Not?” Clin Rev. 2015;25(2):16,18.
For the next photograph, proceed to the next page >>
3. An adolescent patient presents with marked facial asymmetry, present since birth. The auricular tissue on the left side is malformed; on the right side, low set. The right midface is underdeveloped with micrognathia and chin deviation, resulting in malocclusion.
Source: Wikimedia Commons
Diagnosis: Craniofacial microsomia, possibly caused by bleeding in the temporal area during gestation, describes a spectrum of cranial and facial abnormalities. After Down syndrome, it is the most common congenital syndrome, occurring in approximately 1 in 3,500 to 4,500 live births. In about two-thirds of cases, abnormalities differ from one side to the other.
These individuals typically demonstrate one-sided maxillary or mandibular hypoplasia, which can cause dental problems and difficulties with feeding and speech. In cases of severe mandibular hypoplasia, breathing may also be affected.
Abnormalities affecting one or both ears may range from preauricular tags to an underdeveloped or absent external ear or a closed or absent ear canal; these patients may experience hearing loss. Microphthalmia, occurring less frequently, may result in vision loss.
For more information, see Heike CL, Luquetti DV, Hing AV. Craniofacial microsomia overview. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
4. A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” on his forehead that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
Diagnosis: Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors. They are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck and more commonly in females. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also manifest in extracutaneous areas (eg, the liver); these are usually detected via imaging.
For more information, see “A New Approach to “Birthmarks” Clin Rev. 2016;26(3):W1.
RELATED ARTICLE:
Infantile Hemangiomas Increasing, Linked to Prematurity, Low Birth Weight Bruce Jancin, Family Practice News Digital Network
1. This youngster is being followed for symptoms related to his right-sided cranial asymmetry noted at birth, including neurologic impairment (sensorineural hearing loss and visual problems including strabismus) and breathing problems; developmental delay and/or intellectual disability; and hydrocephalus. The hands and feet are normal.
Source: By Michael L. Kaufman at the English language Wikipedia, CC BY-SA 3.0
Diagnosis: Craniosynostosis, also known as acrocephalosyndactyly, is a condition in which the skull sutures close prematurely. This can cause pressure to build up inside the head and skull or facial bones, leading to a misshapen head and distinctive facial features—ocular hypertelorism, proptosis, midface hypoplasia, small beaked nose, and prognathism—and occasionally abnormal fingers and toes.
Mutations of the FGFR gene, which regulates the fibroblast growth factor receptor protein, are responsible for the eight disorders comprising the FGFR-related craniosynostosis spectrum. The protein plays an important role in bone growth, particularly during embryonic development. For example, this protein signals certain immature cells in the developing embryo to become bone cells in the head, hands, feet, and other tissues.
For more information, see Robin NH, MD, Falk MJ, Haldeman-Englert CR. FGFR-related craniosynostosis syndromes. In: GeneReviews® [Internet]. Pagon RA, Adam MP, Ardinger HH, et al, eds. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
2. This infant presents with a misshapen cranium, most notably the flattened area on the posterior right side. His history includes limited passive neck rotation at birth, preferential head orientation, supine sleep position, and lower activity level.
Source: Wikimedia Commons; By Medical advises - http://larece.ru/?p=27115, CC BY-SA 3.0
Diagnosis: Plagiocephaly, literally means "oblique head" (from the Greek "plagio" for oblique and "cephale" for head). The incidence in infants at 7 to 12 weeks of age is estimated to be 46.6%. Of all infants with plagiocephaly, 63.2% are affected on the right side and 78.3% have a mild form.
For more information, see “Helmets for Positional Skull Deformities: A Good Idea or Not?” Clin Rev. 2015;25(2):16,18.
For the next photograph, proceed to the next page >>
3. An adolescent patient presents with marked facial asymmetry, present since birth. The auricular tissue on the left side is malformed; on the right side, low set. The right midface is underdeveloped with micrognathia and chin deviation, resulting in malocclusion.
Source: Wikimedia Commons
Diagnosis: Craniofacial microsomia, possibly caused by bleeding in the temporal area during gestation, describes a spectrum of cranial and facial abnormalities. After Down syndrome, it is the most common congenital syndrome, occurring in approximately 1 in 3,500 to 4,500 live births. In about two-thirds of cases, abnormalities differ from one side to the other.
These individuals typically demonstrate one-sided maxillary or mandibular hypoplasia, which can cause dental problems and difficulties with feeding and speech. In cases of severe mandibular hypoplasia, breathing may also be affected.
Abnormalities affecting one or both ears may range from preauricular tags to an underdeveloped or absent external ear or a closed or absent ear canal; these patients may experience hearing loss. Microphthalmia, occurring less frequently, may result in vision loss.
For more information, see Heike CL, Luquetti DV, Hing AV. Craniofacial microsomia overview. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2016.
For the next photograph, proceed to the next page >>
4. A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” on his forehead that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
Diagnosis: Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors. They are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck and more commonly in females. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also manifest in extracutaneous areas (eg, the liver); these are usually detected via imaging.
For more information, see “A New Approach to “Birthmarks” Clin Rev. 2016;26(3):W1.
RELATED ARTICLE:
Infantile Hemangiomas Increasing, Linked to Prematurity, Low Birth Weight Bruce Jancin, Family Practice News Digital Network
Gay and Bisexual Male High Schoolers Have High Injected Drug Use
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.
She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
AT AIDS 2016
Gay and bisexual male high schoolers have high injected drug use
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.
She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
AT AIDS 2016
Key clinical point: Male U.S. students in grades 9-12 who identify themselves as sexual minorities have markedly higher rates of illicit drug use than their heterosexual peers.
Major finding: A total of 4.4% of U.S. male high school students identify themselves as gay or bisexual, and they are about sevenfold more likely than heterosexual students to have ever injected illegal drugs.
Data source: A national population-based survey of 15,624 male students in grades 9-12.
Disclosures: The presenter is an employee of the Centers for Disease Control and Prevention, which sponsored the annual Youth Risk Behavior Survey.
VIDEO: Medicare pays billions for potentially avoidable hospital admissions in dementia patients
TORONTO – In 2013, Medicare spent $2.6 billion on potentially avoidable hospitalizations for patients with Alzheimer’s disease and other dementias.
About half of these hospital visits – each of which cost Medicare around $7,000 – were for conditions that could have been effectively managed in primary care settings, Pei-Jung Lin, PhD, said at the Alzheimer’s Association International Conference 2016.
Half of the admissions were for chronic illnesses such as diabetes, hypertension, and asthma that are often poorly controlled as dementia progresses. The other half was for acute illnesses, said Dr. Lin, director of the Center for the Evaluation of Value and Risk in Health at Tufts University, Boston. But these admissions for acute conditions involved problems that could be treated on an outpatient basis if they’d been caught early – urinary tract infections, pneumonias, and dehydration.
“In 2013, 1 in 10 Alzheimer’s disease patients had at least one potentially avoidable hospitalization, and 1 in 7 hospital admissions among these patients (14%) was for a potentially avoidable condition,” she said during a press briefing.
Although the financial finding is striking, it isn’t the whole story, Dr. Lin said. Hospitalizations put patients at increased risk for infections, confusion, and delirium – any of which can initiate a downward clinical spiral that results in more intervention, more stress, and more cost.
“Not only are these incidences expensive, they can be dangerous for our patients. Being in the hospital is stressful for someone with little grasp of reality. Patients can become even more confused and disoriented. They’re at risk for hospital-acquired infections, which increase costs and caregiver stress, and decrease patient quality of life,” she said.
Dr. Lin and her team looked for potentially avoidable hospitalizations filed in 2013 among 2.75 million patients with Alzheimer’s and other dementias. The acute conditions included in the analysis were bacterial pneumonia, urinary tract infection, and dehydration, and chronic conditions were diabetes, cardiovascular diseases, and respiratory conditions.
About half these admissions (188,870; 47%) were for acute conditions, while the rest were for chronic conditions. About half of all the admissions also occurred in patients with late-stage disease, and these were the most expensive ones. Admissions for these late-stage patients accounted for 59% of the expenditures and cost Medicare $1.53 billion in 2013.
Claims data don’t offer the kind of details that allow a case-by-case review of how the admissions could have been prevented. But one message comes through loud and clear, Dr. Lin said.
“Comorbidity management is simply not optimal among many of our patients with Alzheimer’s disease. Many of these admissions, and much of this cost, could have been prevented with better ambulatory care and early, effective treatment.”
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on these issues in a video interview.
Dr. Lin had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – In 2013, Medicare spent $2.6 billion on potentially avoidable hospitalizations for patients with Alzheimer’s disease and other dementias.
About half of these hospital visits – each of which cost Medicare around $7,000 – were for conditions that could have been effectively managed in primary care settings, Pei-Jung Lin, PhD, said at the Alzheimer’s Association International Conference 2016.
Half of the admissions were for chronic illnesses such as diabetes, hypertension, and asthma that are often poorly controlled as dementia progresses. The other half was for acute illnesses, said Dr. Lin, director of the Center for the Evaluation of Value and Risk in Health at Tufts University, Boston. But these admissions for acute conditions involved problems that could be treated on an outpatient basis if they’d been caught early – urinary tract infections, pneumonias, and dehydration.
“In 2013, 1 in 10 Alzheimer’s disease patients had at least one potentially avoidable hospitalization, and 1 in 7 hospital admissions among these patients (14%) was for a potentially avoidable condition,” she said during a press briefing.
Although the financial finding is striking, it isn’t the whole story, Dr. Lin said. Hospitalizations put patients at increased risk for infections, confusion, and delirium – any of which can initiate a downward clinical spiral that results in more intervention, more stress, and more cost.
“Not only are these incidences expensive, they can be dangerous for our patients. Being in the hospital is stressful for someone with little grasp of reality. Patients can become even more confused and disoriented. They’re at risk for hospital-acquired infections, which increase costs and caregiver stress, and decrease patient quality of life,” she said.
Dr. Lin and her team looked for potentially avoidable hospitalizations filed in 2013 among 2.75 million patients with Alzheimer’s and other dementias. The acute conditions included in the analysis were bacterial pneumonia, urinary tract infection, and dehydration, and chronic conditions were diabetes, cardiovascular diseases, and respiratory conditions.
About half these admissions (188,870; 47%) were for acute conditions, while the rest were for chronic conditions. About half of all the admissions also occurred in patients with late-stage disease, and these were the most expensive ones. Admissions for these late-stage patients accounted for 59% of the expenditures and cost Medicare $1.53 billion in 2013.
Claims data don’t offer the kind of details that allow a case-by-case review of how the admissions could have been prevented. But one message comes through loud and clear, Dr. Lin said.
“Comorbidity management is simply not optimal among many of our patients with Alzheimer’s disease. Many of these admissions, and much of this cost, could have been prevented with better ambulatory care and early, effective treatment.”
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on these issues in a video interview.
Dr. Lin had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – In 2013, Medicare spent $2.6 billion on potentially avoidable hospitalizations for patients with Alzheimer’s disease and other dementias.
About half of these hospital visits – each of which cost Medicare around $7,000 – were for conditions that could have been effectively managed in primary care settings, Pei-Jung Lin, PhD, said at the Alzheimer’s Association International Conference 2016.
Half of the admissions were for chronic illnesses such as diabetes, hypertension, and asthma that are often poorly controlled as dementia progresses. The other half was for acute illnesses, said Dr. Lin, director of the Center for the Evaluation of Value and Risk in Health at Tufts University, Boston. But these admissions for acute conditions involved problems that could be treated on an outpatient basis if they’d been caught early – urinary tract infections, pneumonias, and dehydration.
“In 2013, 1 in 10 Alzheimer’s disease patients had at least one potentially avoidable hospitalization, and 1 in 7 hospital admissions among these patients (14%) was for a potentially avoidable condition,” she said during a press briefing.
Although the financial finding is striking, it isn’t the whole story, Dr. Lin said. Hospitalizations put patients at increased risk for infections, confusion, and delirium – any of which can initiate a downward clinical spiral that results in more intervention, more stress, and more cost.
“Not only are these incidences expensive, they can be dangerous for our patients. Being in the hospital is stressful for someone with little grasp of reality. Patients can become even more confused and disoriented. They’re at risk for hospital-acquired infections, which increase costs and caregiver stress, and decrease patient quality of life,” she said.
Dr. Lin and her team looked for potentially avoidable hospitalizations filed in 2013 among 2.75 million patients with Alzheimer’s and other dementias. The acute conditions included in the analysis were bacterial pneumonia, urinary tract infection, and dehydration, and chronic conditions were diabetes, cardiovascular diseases, and respiratory conditions.
About half these admissions (188,870; 47%) were for acute conditions, while the rest were for chronic conditions. About half of all the admissions also occurred in patients with late-stage disease, and these were the most expensive ones. Admissions for these late-stage patients accounted for 59% of the expenditures and cost Medicare $1.53 billion in 2013.
Claims data don’t offer the kind of details that allow a case-by-case review of how the admissions could have been prevented. But one message comes through loud and clear, Dr. Lin said.
“Comorbidity management is simply not optimal among many of our patients with Alzheimer’s disease. Many of these admissions, and much of this cost, could have been prevented with better ambulatory care and early, effective treatment.”
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on these issues in a video interview.
Dr. Lin had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2016
Key clinical point: Billions are being spent on potentially avoidable hospitalizations for people with Alzheimer’s disease and other dementias.
Major finding: In 2013, Medicare spent $2.6 billion on possibly avoidable hospitalizations for acute and chronic illnesses that could have been prevented with better primary care.
Data source: The study included 2013 Medicare claims data on 2.75 million patients with Alzheimer’s and other dementias.
Disclosures: Dr. Lin had no financial disclosures.