User login
USPSTF: Visual skin cancer screening lacks supporting evidence
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
FROM JAMA
Measure Hospitalist Engagement with SHM’s Engagement Benchmarking Service
One of the most important questions for leaders of HM groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
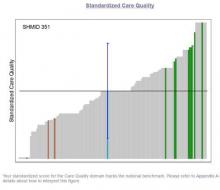
SHM has developed an Engagement Benchmarking Service to analyze engagement of hospitalists. The service evaluates relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardization score in the various domains and where it falls within the national benchmark to help you determine what is working well and identify areas for improvement in your hospital medicine group.
Recruiting ends soon. Ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
One of the most important questions for leaders of HM groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
SHM has developed an Engagement Benchmarking Service to analyze engagement of hospitalists. The service evaluates relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardization score in the various domains and where it falls within the national benchmark to help you determine what is working well and identify areas for improvement in your hospital medicine group.
Recruiting ends soon. Ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
One of the most important questions for leaders of HM groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
SHM has developed an Engagement Benchmarking Service to analyze engagement of hospitalists. The service evaluates relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardization score in the various domains and where it falls within the national benchmark to help you determine what is working well and identify areas for improvement in your hospital medicine group.
Recruiting ends soon. Ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
SHM Leadership Academy: Learning Awaits in Mastering Teamwork Course
As the SHM Leadership Academy’s course director, I always find time to visit the Mastering Teamwork course because each year, even though it’s slightly different, it’s still exciting. In past meetings, I’ve learned from talented faculty how lessons in college football relate to practice, been provided guidance on how to recognize what makes me tick, and heard firsthand perspective on large-scale medical events like 9/11, Hurricane Katrina, and even the Boston Marathon tragedy. I always learn a few new things. As they say, repetition is the mother of learning, and the Mastering Teamwork course never fails to make that learning a lot of fun.
As a professor of medicine, I’ve always liked learning. But I truly enjoy learning when it’s fun and exciting. To me, this mixture of academia and excitement is the epitome of Mastering Teamwork. When two of the faculty, Mark Williams, MD, MHM, and Amit Prachand, MEng, needed to teach about teamwork, they decided to develop an interactive session. While in Hawaii, they constructed a “river” out of cardboard and props for Mastering Teamwork participants to navigate. It was a hands-on lesson in group dynamics. It was educational and, most of all, a hoot.
Kay Cannon, MBA, taught me that the skills I used in previous job levels may not be the drivers of my success in today’s job (or tomorrow’s), and Jeffrey Wiese, MD, MHM, and Lenny Marcus, PhD, are two of the best storytellers I know and have me on the edge of my seat every time I hear them speak. Their life experiences make excellent fodder for hospitalist leadership pearls and are more riveting than Downton Abbey (or whatever drama is your favorite).
I look forward to seeing everyone at Disney’s BoardWalk Inn in Lake Buena Vista, Florida, from October 24 to 27 to experience what I know will be a memorable, enjoyable learning experience for all.
To register, visit www.shmleadershipacademy.org. TH
Dr. Howell is SHM’s senior physician advisor and course director for SHM’s Leadership Academy.
As the SHM Leadership Academy’s course director, I always find time to visit the Mastering Teamwork course because each year, even though it’s slightly different, it’s still exciting. In past meetings, I’ve learned from talented faculty how lessons in college football relate to practice, been provided guidance on how to recognize what makes me tick, and heard firsthand perspective on large-scale medical events like 9/11, Hurricane Katrina, and even the Boston Marathon tragedy. I always learn a few new things. As they say, repetition is the mother of learning, and the Mastering Teamwork course never fails to make that learning a lot of fun.
As a professor of medicine, I’ve always liked learning. But I truly enjoy learning when it’s fun and exciting. To me, this mixture of academia and excitement is the epitome of Mastering Teamwork. When two of the faculty, Mark Williams, MD, MHM, and Amit Prachand, MEng, needed to teach about teamwork, they decided to develop an interactive session. While in Hawaii, they constructed a “river” out of cardboard and props for Mastering Teamwork participants to navigate. It was a hands-on lesson in group dynamics. It was educational and, most of all, a hoot.
Kay Cannon, MBA, taught me that the skills I used in previous job levels may not be the drivers of my success in today’s job (or tomorrow’s), and Jeffrey Wiese, MD, MHM, and Lenny Marcus, PhD, are two of the best storytellers I know and have me on the edge of my seat every time I hear them speak. Their life experiences make excellent fodder for hospitalist leadership pearls and are more riveting than Downton Abbey (or whatever drama is your favorite).
I look forward to seeing everyone at Disney’s BoardWalk Inn in Lake Buena Vista, Florida, from October 24 to 27 to experience what I know will be a memorable, enjoyable learning experience for all.
To register, visit www.shmleadershipacademy.org. TH
Dr. Howell is SHM’s senior physician advisor and course director for SHM’s Leadership Academy.
As the SHM Leadership Academy’s course director, I always find time to visit the Mastering Teamwork course because each year, even though it’s slightly different, it’s still exciting. In past meetings, I’ve learned from talented faculty how lessons in college football relate to practice, been provided guidance on how to recognize what makes me tick, and heard firsthand perspective on large-scale medical events like 9/11, Hurricane Katrina, and even the Boston Marathon tragedy. I always learn a few new things. As they say, repetition is the mother of learning, and the Mastering Teamwork course never fails to make that learning a lot of fun.
As a professor of medicine, I’ve always liked learning. But I truly enjoy learning when it’s fun and exciting. To me, this mixture of academia and excitement is the epitome of Mastering Teamwork. When two of the faculty, Mark Williams, MD, MHM, and Amit Prachand, MEng, needed to teach about teamwork, they decided to develop an interactive session. While in Hawaii, they constructed a “river” out of cardboard and props for Mastering Teamwork participants to navigate. It was a hands-on lesson in group dynamics. It was educational and, most of all, a hoot.
Kay Cannon, MBA, taught me that the skills I used in previous job levels may not be the drivers of my success in today’s job (or tomorrow’s), and Jeffrey Wiese, MD, MHM, and Lenny Marcus, PhD, are two of the best storytellers I know and have me on the edge of my seat every time I hear them speak. Their life experiences make excellent fodder for hospitalist leadership pearls and are more riveting than Downton Abbey (or whatever drama is your favorite).
I look forward to seeing everyone at Disney’s BoardWalk Inn in Lake Buena Vista, Florida, from October 24 to 27 to experience what I know will be a memorable, enjoyable learning experience for all.
To register, visit www.shmleadershipacademy.org. TH
Dr. Howell is SHM’s senior physician advisor and course director for SHM’s Leadership Academy.
Long-term health burden of Hodgkin lymphoma treatment

Photo courtesy of St. Jude
Children’s Research Hospital
and Seth Dixon
New research has shown that survivors of pediatric Hodgkin lymphoma (HL) are more likely to have chronic cardiovascular conditions than adults who did not have cancer in childhood.
And cardiovascular conditions are more severe among HL survivors than the general population.
Investigators believe this research, published in The Lancet Oncology, should aid efforts to reduce and better manage the late effects of cancer treatment.
For this study, the investigators used a measurement called “cumulative burden” to better capture the distribution and magnitude of chronic disease in childhood cancer survivors.
The metric showed that, by age 50, HL survivors had more than twice as many cardiovascular problems as adults who had not had cancer as children. HL survivors were also 5 times more likely to have severe, life-threatening, or fatal heart conditions.
“With cure rates for pediatric cancer at historic highs, the question becomes, ‘What is the legacy of that cure?’” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We are doing a better job of keeping patients alive, but are we doing a better job at addressing the chronic diseases that are sometimes the price of that cure? Cumulative burden is a new tool for studying chronic illness in childhood cancer survivors or any patient population with significant morbidity, such as diabetes or HIV/AIDS.”
Unlike statistical methods that count health conditions once at diagnosis, cumulative burden tracks individuals’ multiple, recurring treatment-related health conditions.
Dr Bhakta and his colleagues focused on calculating the cumulative burden of cardiovascular disease in 670 pediatric HL survivors. The subjects were at least 18 years old and had survived at least 10 years beyond their cancer diagnoses.
The participants had been assessed for 22 chronic cardiovascular conditions, including heart attacks, hypertension, arrhythmias, and structural heart defects. Investigators used those and other clinical findings to calculate the cumulative burden by tracking the incidence and severity of cardiovascular disease.
The team also determined the cumulative burden for a comparison group of 272 community volunteers who underwent the same health assessments. The volunteers were similar in age and gender to the HL survivors but had no history of childhood cancer.
The analysis showed that the cumulative burden of cardiovascular disease, including severe and life-threatening conditions, was greater among HL survivors at 30 and 50 years of age than among the comparison group. In fact, the cumulative burden of the most serious heart problems, including heart attacks, was similar for 30-year-old HL survivors and 50-year-old community volunteers.
At age 50, 45.5% of HL survivors had developed at least one grade 3-5 cardiovascular condition, compared to 15.7% of the control subjects.
The HL survivors had a cumulative burden of 430.6 grade 1-5 cardiovascular conditions per 100 individuals and 100.8 grade 3-5 cardiovascular conditions per 100 individuals. In comparison, controls had 227.4 grade 1-5 conditions and 17.0 grade 3-5 conditions per 100 individuals.
The investigators noted that severe, chronic heart conditions became more common with age in both groups, but serious problems accumulated more rapidly in HL survivors.
“Survivors tended to have more severe disease across the lifespan and likely need an individualized screening and treatment plan,” Dr Bhakta said.
He added that the results of this study highlight trade-offs to consider in designing future clinical trials. For example, the investigators found that reducing the dose of anthracyclines will lower the rate, but not the severity, of cardiovascular disease in pediatric and young adult HL survivors.
In contrast, lowering the heart radiation dose will not significantly lower the rate of cardiovascular disease, but it will reduce the severity.
“Cumulative burden provides us with a global view of tradeoffs between different treatment late effects that must be considered when designing new interventions,” Dr Bhakta concluded. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
and Seth Dixon
New research has shown that survivors of pediatric Hodgkin lymphoma (HL) are more likely to have chronic cardiovascular conditions than adults who did not have cancer in childhood.
And cardiovascular conditions are more severe among HL survivors than the general population.
Investigators believe this research, published in The Lancet Oncology, should aid efforts to reduce and better manage the late effects of cancer treatment.
For this study, the investigators used a measurement called “cumulative burden” to better capture the distribution and magnitude of chronic disease in childhood cancer survivors.
The metric showed that, by age 50, HL survivors had more than twice as many cardiovascular problems as adults who had not had cancer as children. HL survivors were also 5 times more likely to have severe, life-threatening, or fatal heart conditions.
“With cure rates for pediatric cancer at historic highs, the question becomes, ‘What is the legacy of that cure?’” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We are doing a better job of keeping patients alive, but are we doing a better job at addressing the chronic diseases that are sometimes the price of that cure? Cumulative burden is a new tool for studying chronic illness in childhood cancer survivors or any patient population with significant morbidity, such as diabetes or HIV/AIDS.”
Unlike statistical methods that count health conditions once at diagnosis, cumulative burden tracks individuals’ multiple, recurring treatment-related health conditions.
Dr Bhakta and his colleagues focused on calculating the cumulative burden of cardiovascular disease in 670 pediatric HL survivors. The subjects were at least 18 years old and had survived at least 10 years beyond their cancer diagnoses.
The participants had been assessed for 22 chronic cardiovascular conditions, including heart attacks, hypertension, arrhythmias, and structural heart defects. Investigators used those and other clinical findings to calculate the cumulative burden by tracking the incidence and severity of cardiovascular disease.
The team also determined the cumulative burden for a comparison group of 272 community volunteers who underwent the same health assessments. The volunteers were similar in age and gender to the HL survivors but had no history of childhood cancer.
The analysis showed that the cumulative burden of cardiovascular disease, including severe and life-threatening conditions, was greater among HL survivors at 30 and 50 years of age than among the comparison group. In fact, the cumulative burden of the most serious heart problems, including heart attacks, was similar for 30-year-old HL survivors and 50-year-old community volunteers.
At age 50, 45.5% of HL survivors had developed at least one grade 3-5 cardiovascular condition, compared to 15.7% of the control subjects.
The HL survivors had a cumulative burden of 430.6 grade 1-5 cardiovascular conditions per 100 individuals and 100.8 grade 3-5 cardiovascular conditions per 100 individuals. In comparison, controls had 227.4 grade 1-5 conditions and 17.0 grade 3-5 conditions per 100 individuals.
The investigators noted that severe, chronic heart conditions became more common with age in both groups, but serious problems accumulated more rapidly in HL survivors.
“Survivors tended to have more severe disease across the lifespan and likely need an individualized screening and treatment plan,” Dr Bhakta said.
He added that the results of this study highlight trade-offs to consider in designing future clinical trials. For example, the investigators found that reducing the dose of anthracyclines will lower the rate, but not the severity, of cardiovascular disease in pediatric and young adult HL survivors.
In contrast, lowering the heart radiation dose will not significantly lower the rate of cardiovascular disease, but it will reduce the severity.
“Cumulative burden provides us with a global view of tradeoffs between different treatment late effects that must be considered when designing new interventions,” Dr Bhakta concluded. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
and Seth Dixon
New research has shown that survivors of pediatric Hodgkin lymphoma (HL) are more likely to have chronic cardiovascular conditions than adults who did not have cancer in childhood.
And cardiovascular conditions are more severe among HL survivors than the general population.
Investigators believe this research, published in The Lancet Oncology, should aid efforts to reduce and better manage the late effects of cancer treatment.
For this study, the investigators used a measurement called “cumulative burden” to better capture the distribution and magnitude of chronic disease in childhood cancer survivors.
The metric showed that, by age 50, HL survivors had more than twice as many cardiovascular problems as adults who had not had cancer as children. HL survivors were also 5 times more likely to have severe, life-threatening, or fatal heart conditions.
“With cure rates for pediatric cancer at historic highs, the question becomes, ‘What is the legacy of that cure?’” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We are doing a better job of keeping patients alive, but are we doing a better job at addressing the chronic diseases that are sometimes the price of that cure? Cumulative burden is a new tool for studying chronic illness in childhood cancer survivors or any patient population with significant morbidity, such as diabetes or HIV/AIDS.”
Unlike statistical methods that count health conditions once at diagnosis, cumulative burden tracks individuals’ multiple, recurring treatment-related health conditions.
Dr Bhakta and his colleagues focused on calculating the cumulative burden of cardiovascular disease in 670 pediatric HL survivors. The subjects were at least 18 years old and had survived at least 10 years beyond their cancer diagnoses.
The participants had been assessed for 22 chronic cardiovascular conditions, including heart attacks, hypertension, arrhythmias, and structural heart defects. Investigators used those and other clinical findings to calculate the cumulative burden by tracking the incidence and severity of cardiovascular disease.
The team also determined the cumulative burden for a comparison group of 272 community volunteers who underwent the same health assessments. The volunteers were similar in age and gender to the HL survivors but had no history of childhood cancer.
The analysis showed that the cumulative burden of cardiovascular disease, including severe and life-threatening conditions, was greater among HL survivors at 30 and 50 years of age than among the comparison group. In fact, the cumulative burden of the most serious heart problems, including heart attacks, was similar for 30-year-old HL survivors and 50-year-old community volunteers.
At age 50, 45.5% of HL survivors had developed at least one grade 3-5 cardiovascular condition, compared to 15.7% of the control subjects.
The HL survivors had a cumulative burden of 430.6 grade 1-5 cardiovascular conditions per 100 individuals and 100.8 grade 3-5 cardiovascular conditions per 100 individuals. In comparison, controls had 227.4 grade 1-5 conditions and 17.0 grade 3-5 conditions per 100 individuals.
The investigators noted that severe, chronic heart conditions became more common with age in both groups, but serious problems accumulated more rapidly in HL survivors.
“Survivors tended to have more severe disease across the lifespan and likely need an individualized screening and treatment plan,” Dr Bhakta said.
He added that the results of this study highlight trade-offs to consider in designing future clinical trials. For example, the investigators found that reducing the dose of anthracyclines will lower the rate, but not the severity, of cardiovascular disease in pediatric and young adult HL survivors.
In contrast, lowering the heart radiation dose will not significantly lower the rate of cardiovascular disease, but it will reduce the severity.
“Cumulative burden provides us with a global view of tradeoffs between different treatment late effects that must be considered when designing new interventions,” Dr Bhakta concluded. ![]()
FDA clears kit for monitoring molecular response in CML

Photo by Juan D. Alfonso
The US Food and Drug Administration (FDA) has granted premarket clearance for the QuantideX® qPCR BCR-ABL IS Kit, a tool used to monitor molecular response (MR) in patients with chronic myeloid leukemia (CML).
The product is a quantitative polymerase chain reaction (qPCR)-based in vitro diagnostic test that quantifies BCR-ABL1 and ABL1 transcripts in total RNA from the whole blood of t(9;22)-positive CML patients expressing e13a2 and/or e14a2 fusion transcripts.
The QuantideX® qPCR BCR-ABL IS Kit is not designed to diagnose CML or monitor rare transcripts resulting from t(9;22).
The kit was cleared to run on the Applied Biosystems® 7500 Fast DX Real-Time PCR Instrument. Results are reported in International Scale (IS) values.
The QuantideX® qPCR BCR-ABL IS Kit was subjected to analytic and clinical review through the FDA’s de novo 510(k) premarket review pathway and secured clearance with a limit of detection of MR 4.7/0.002% IS (4.7 log molecular reduction from 100% IS).
The limit of detection was determined using real human RNA, not human-derived cell lines, ensuring that the assay reproducibly detects BCR-ABL1 RNA in at least 95% of patients at MR 4.7.
“In evaluating the QuantideX® qPCR BCR-ABL IS Kit, we confirmed the high level of sensitivity achieved for human clinical samples measured in our laboratory at MR 4.7 (0.002% IS),” said Y. Lynn. Wang, MD, PhD, of the University of Chicago Comprehensive Cancer Center.
“The configuration of the assay—multiplexed, single-lot reagents, efficient workflow, and direct IS reporting—provided the robustness, sensitivity, and data quality we believe to be unprecedented in the market today. The high level of sensitivity will contribute to the assessment of the depth and duration of clinical response to [tyrosine kinase inhibitors] and experimental therapies.”
The QuantideX® qPCR BCR-ABL IS Kit is now available for order in the US and Europe. The kit is a product of Asuragen, Inc. ![]()

Photo by Juan D. Alfonso
The US Food and Drug Administration (FDA) has granted premarket clearance for the QuantideX® qPCR BCR-ABL IS Kit, a tool used to monitor molecular response (MR) in patients with chronic myeloid leukemia (CML).
The product is a quantitative polymerase chain reaction (qPCR)-based in vitro diagnostic test that quantifies BCR-ABL1 and ABL1 transcripts in total RNA from the whole blood of t(9;22)-positive CML patients expressing e13a2 and/or e14a2 fusion transcripts.
The QuantideX® qPCR BCR-ABL IS Kit is not designed to diagnose CML or monitor rare transcripts resulting from t(9;22).
The kit was cleared to run on the Applied Biosystems® 7500 Fast DX Real-Time PCR Instrument. Results are reported in International Scale (IS) values.
The QuantideX® qPCR BCR-ABL IS Kit was subjected to analytic and clinical review through the FDA’s de novo 510(k) premarket review pathway and secured clearance with a limit of detection of MR 4.7/0.002% IS (4.7 log molecular reduction from 100% IS).
The limit of detection was determined using real human RNA, not human-derived cell lines, ensuring that the assay reproducibly detects BCR-ABL1 RNA in at least 95% of patients at MR 4.7.
“In evaluating the QuantideX® qPCR BCR-ABL IS Kit, we confirmed the high level of sensitivity achieved for human clinical samples measured in our laboratory at MR 4.7 (0.002% IS),” said Y. Lynn. Wang, MD, PhD, of the University of Chicago Comprehensive Cancer Center.
“The configuration of the assay—multiplexed, single-lot reagents, efficient workflow, and direct IS reporting—provided the robustness, sensitivity, and data quality we believe to be unprecedented in the market today. The high level of sensitivity will contribute to the assessment of the depth and duration of clinical response to [tyrosine kinase inhibitors] and experimental therapies.”
The QuantideX® qPCR BCR-ABL IS Kit is now available for order in the US and Europe. The kit is a product of Asuragen, Inc. ![]()

Photo by Juan D. Alfonso
The US Food and Drug Administration (FDA) has granted premarket clearance for the QuantideX® qPCR BCR-ABL IS Kit, a tool used to monitor molecular response (MR) in patients with chronic myeloid leukemia (CML).
The product is a quantitative polymerase chain reaction (qPCR)-based in vitro diagnostic test that quantifies BCR-ABL1 and ABL1 transcripts in total RNA from the whole blood of t(9;22)-positive CML patients expressing e13a2 and/or e14a2 fusion transcripts.
The QuantideX® qPCR BCR-ABL IS Kit is not designed to diagnose CML or monitor rare transcripts resulting from t(9;22).
The kit was cleared to run on the Applied Biosystems® 7500 Fast DX Real-Time PCR Instrument. Results are reported in International Scale (IS) values.
The QuantideX® qPCR BCR-ABL IS Kit was subjected to analytic and clinical review through the FDA’s de novo 510(k) premarket review pathway and secured clearance with a limit of detection of MR 4.7/0.002% IS (4.7 log molecular reduction from 100% IS).
The limit of detection was determined using real human RNA, not human-derived cell lines, ensuring that the assay reproducibly detects BCR-ABL1 RNA in at least 95% of patients at MR 4.7.
“In evaluating the QuantideX® qPCR BCR-ABL IS Kit, we confirmed the high level of sensitivity achieved for human clinical samples measured in our laboratory at MR 4.7 (0.002% IS),” said Y. Lynn. Wang, MD, PhD, of the University of Chicago Comprehensive Cancer Center.
“The configuration of the assay—multiplexed, single-lot reagents, efficient workflow, and direct IS reporting—provided the robustness, sensitivity, and data quality we believe to be unprecedented in the market today. The high level of sensitivity will contribute to the assessment of the depth and duration of clinical response to [tyrosine kinase inhibitors] and experimental therapies.”
The QuantideX® qPCR BCR-ABL IS Kit is now available for order in the US and Europe. The kit is a product of Asuragen, Inc. ![]()
Chickens may protect humans from malaria

Photo by Geri Glastra
Research published in Malaria Journal indicates that malaria-transmitting mosquitoes use their sense of smell to avoid feeding on chickens.
Investigators therefore believe that odors emitted by chickens and other animals could provide protection for humans at risk of mosquito-transmitted diseases.
The study showed that Anopheles arabiensis, one of the predominant species of mosquitoes transmitting malaria in sub-Saharan Africa, avoids chickens when looking for hosts to feed on.
And the mosquitoes can distinguish chickens from other animals using their sense of smell.
“We were surprised to find that malaria mosquitoes are repelled by the odors emitted by chickens,” said study author Rickard Ignell, PhD, of the Swedish University of Agricultural Sciences in Alnarp, Sweden.
“This study shows, for the first time, that malaria mosquitoes actively avoid feeding on certain animal species and that this behavior is regulated through odor cues.”
To find out which species the mosquitoes prefer, Dr Ignell and his colleagues collected data on the population of human and domestic animals in 3 Ethiopian villages. People living in these villages share their living quarters with their livestock.
The investigators also collected blood-fed mosquitoes to test for the source of the blood the mosquitoes had consumed.
The team found that An arabiensis strongly prefers human over animal blood when seeking hosts indoors and randomly feeds on cattle, goats, and sheep when outdoors. However, the mosquitoes avoid chickens in both settings, despite their relatively high abundance.
Since mosquitoes select and discriminate between their hosts mainly based on their sense of smell, the investigators collected hair, wool, and feathers from potential host and non-host species to analyze the odor compounds present in them.
Identifying certain compounds that were only present in chicken feathers, the team used these and other compounds obtained from all species to test their ability to repel mosquitoes from mosquito traps.
The traps were set up in 11 thatched houses in one of the villages for a total of 11 days. In each of the houses, a single volunteer between ages 27 and 36 slept under an untreated bed net.
The investigators found that significantly fewer mosquitoes were caught in traps baited with chicken compounds than in control traps. Suspending a living chicken in a cage next to a trap had a similar repellent effect.
Because it feeds indoors and outdoors on various host species, An arabiensis is difficult to control with existing methods, previous research has shown. The results of the current study suggest that, in combination with established control methods, the odors emitted by chickens and other non-host species could prove useful in controlling An arabiensis.
“People in sub-Saharan Africa have suffered considerably under the burden of malaria over an extended period of time, and mosquitoes are becoming increasingly physiologically resistant to pesticides, while also changing their feeding habits, for example, by moving from indoors to outdoors,” Dr Ignell said.
“For this reason, there is a need to develop novel control methods. In our study, we have been able to identify a number of natural odor compounds which could repel host-seeking malaria mosquitoes and prevent them from getting in contact with people.” ![]()

Photo by Geri Glastra
Research published in Malaria Journal indicates that malaria-transmitting mosquitoes use their sense of smell to avoid feeding on chickens.
Investigators therefore believe that odors emitted by chickens and other animals could provide protection for humans at risk of mosquito-transmitted diseases.
The study showed that Anopheles arabiensis, one of the predominant species of mosquitoes transmitting malaria in sub-Saharan Africa, avoids chickens when looking for hosts to feed on.
And the mosquitoes can distinguish chickens from other animals using their sense of smell.
“We were surprised to find that malaria mosquitoes are repelled by the odors emitted by chickens,” said study author Rickard Ignell, PhD, of the Swedish University of Agricultural Sciences in Alnarp, Sweden.
“This study shows, for the first time, that malaria mosquitoes actively avoid feeding on certain animal species and that this behavior is regulated through odor cues.”
To find out which species the mosquitoes prefer, Dr Ignell and his colleagues collected data on the population of human and domestic animals in 3 Ethiopian villages. People living in these villages share their living quarters with their livestock.
The investigators also collected blood-fed mosquitoes to test for the source of the blood the mosquitoes had consumed.
The team found that An arabiensis strongly prefers human over animal blood when seeking hosts indoors and randomly feeds on cattle, goats, and sheep when outdoors. However, the mosquitoes avoid chickens in both settings, despite their relatively high abundance.
Since mosquitoes select and discriminate between their hosts mainly based on their sense of smell, the investigators collected hair, wool, and feathers from potential host and non-host species to analyze the odor compounds present in them.
Identifying certain compounds that were only present in chicken feathers, the team used these and other compounds obtained from all species to test their ability to repel mosquitoes from mosquito traps.
The traps were set up in 11 thatched houses in one of the villages for a total of 11 days. In each of the houses, a single volunteer between ages 27 and 36 slept under an untreated bed net.
The investigators found that significantly fewer mosquitoes were caught in traps baited with chicken compounds than in control traps. Suspending a living chicken in a cage next to a trap had a similar repellent effect.
Because it feeds indoors and outdoors on various host species, An arabiensis is difficult to control with existing methods, previous research has shown. The results of the current study suggest that, in combination with established control methods, the odors emitted by chickens and other non-host species could prove useful in controlling An arabiensis.
“People in sub-Saharan Africa have suffered considerably under the burden of malaria over an extended period of time, and mosquitoes are becoming increasingly physiologically resistant to pesticides, while also changing their feeding habits, for example, by moving from indoors to outdoors,” Dr Ignell said.
“For this reason, there is a need to develop novel control methods. In our study, we have been able to identify a number of natural odor compounds which could repel host-seeking malaria mosquitoes and prevent them from getting in contact with people.” ![]()

Photo by Geri Glastra
Research published in Malaria Journal indicates that malaria-transmitting mosquitoes use their sense of smell to avoid feeding on chickens.
Investigators therefore believe that odors emitted by chickens and other animals could provide protection for humans at risk of mosquito-transmitted diseases.
The study showed that Anopheles arabiensis, one of the predominant species of mosquitoes transmitting malaria in sub-Saharan Africa, avoids chickens when looking for hosts to feed on.
And the mosquitoes can distinguish chickens from other animals using their sense of smell.
“We were surprised to find that malaria mosquitoes are repelled by the odors emitted by chickens,” said study author Rickard Ignell, PhD, of the Swedish University of Agricultural Sciences in Alnarp, Sweden.
“This study shows, for the first time, that malaria mosquitoes actively avoid feeding on certain animal species and that this behavior is regulated through odor cues.”
To find out which species the mosquitoes prefer, Dr Ignell and his colleagues collected data on the population of human and domestic animals in 3 Ethiopian villages. People living in these villages share their living quarters with their livestock.
The investigators also collected blood-fed mosquitoes to test for the source of the blood the mosquitoes had consumed.
The team found that An arabiensis strongly prefers human over animal blood when seeking hosts indoors and randomly feeds on cattle, goats, and sheep when outdoors. However, the mosquitoes avoid chickens in both settings, despite their relatively high abundance.
Since mosquitoes select and discriminate between their hosts mainly based on their sense of smell, the investigators collected hair, wool, and feathers from potential host and non-host species to analyze the odor compounds present in them.
Identifying certain compounds that were only present in chicken feathers, the team used these and other compounds obtained from all species to test their ability to repel mosquitoes from mosquito traps.
The traps were set up in 11 thatched houses in one of the villages for a total of 11 days. In each of the houses, a single volunteer between ages 27 and 36 slept under an untreated bed net.
The investigators found that significantly fewer mosquitoes were caught in traps baited with chicken compounds than in control traps. Suspending a living chicken in a cage next to a trap had a similar repellent effect.
Because it feeds indoors and outdoors on various host species, An arabiensis is difficult to control with existing methods, previous research has shown. The results of the current study suggest that, in combination with established control methods, the odors emitted by chickens and other non-host species could prove useful in controlling An arabiensis.
“People in sub-Saharan Africa have suffered considerably under the burden of malaria over an extended period of time, and mosquitoes are becoming increasingly physiologically resistant to pesticides, while also changing their feeding habits, for example, by moving from indoors to outdoors,” Dr Ignell said.
“For this reason, there is a need to develop novel control methods. In our study, we have been able to identify a number of natural odor compounds which could repel host-seeking malaria mosquitoes and prevent them from getting in contact with people.” ![]()
CDC updates recommendations regarding Zika virus

Photo by Nina Matthews
The US Centers for Disease Control and Prevention (CDC) has updated some of its recommendations regarding the Zika virus.
The agency issued an updated interim guidance for healthcare providers caring for pregnant women with possible exposure to Zika virus and an updated interim guidance on preventing sexual transmission of the virus.
The CDC issued these updates based on the accumulating evidence, expert opinion, and knowledge about the risk associated with other viral infections. The CDC said it will continue to make updates as new information becomes available.
Guidance for pregnant women
This updated guidance expands the timeframe during which pregnant women can be tested for Zika virus—with an rRT-PCR test—from 7 days to 14 days after symptoms start. The CDC said this expansion will provide a definite diagnosis for more pregnant women infected with the Zika virus.
Scientists previously thought the virus stays in the blood for about a week after symptoms start. So the first week of illness was thought to be the best time to find evidence of the virus in blood using a Zika-specific test (rRT-PCR).
For patients who visited a healthcare provider more than a week after symptoms started and those who were possibly exposed to Zika but never developed symptoms, healthcare providers could perform Zika virus IgM testing. However, this test might not provide a definite diagnosis, as it can also detect related viruses.
New information has indicated that some infected pregnant women can have evidence of the Zika virus in their blood for longer than 7 days after symptoms begin, and even pregnant women without symptoms can have evidence of the virus in their blood and urine.
Therefore, the updated guidance expands the use of Zika-specific blood testing for a longer period, up to 14 days, in pregnant women with symptoms and advises that pregnant women with possible Zika exposure but no symptoms receive this testing as well.
In addition, if pregnant women visit their healthcare provider after the 14-day testing window and test positive with the IgM test, rRT-PCR testing can now be offered to potentially provide a definite diagnosis.
The CDC’s new guidance also includes recommendations to help healthcare providers better care for their pregnant patients with confirmed or possible Zika infection.
Guidance for sexual transmission
This updated guidance is based on a recently reported case of female-to-male sexual transmission of the Zika virus in New York City and limited human and non-human primate data indicating that Zika virus RNA can be detected in vaginal secretions.
The guidance expands the CDC’s definition of sexual exposure to Zika to include sex without a barrier method (including male or female condoms, among other methods) with any person—male or female—who has traveled to or lives in an area with active Zika virus transmission.
The updated recommendations for pregnant couples include pregnant women with female sex partners who are potentially infected with Zika. The recommendations also provide advice for potentially infected women on how to reduce their risk of sexually transmitting the virus to partners.
Specifically, the CDC recommends that all pregnant women with sex partners who live in or traveled to an area with active Zika virus transmission use condoms during sex or abstain from sex for the remainder of their pregnancy.
All other couples in which a partner has been in an area with active Zika virus transmission can also reduce the risk of sexual transmission by using condoms or abstaining from sex. Sex includes vaginal, anal, and oral sex, and may also include the sharing of sex toys.
Healthcare providers should test all pregnant women who may have been exposed to Zika sexually. Providers should also test patients if they develop symptoms of the Zika virus and report potential sexual exposure to a partner who lives in or traveled to an area with active Zika virus transmission.
The CDC encourages local and state health departments to report potential cases of sexually transmitted Zika virus infection. ![]()

Photo by Nina Matthews
The US Centers for Disease Control and Prevention (CDC) has updated some of its recommendations regarding the Zika virus.
The agency issued an updated interim guidance for healthcare providers caring for pregnant women with possible exposure to Zika virus and an updated interim guidance on preventing sexual transmission of the virus.
The CDC issued these updates based on the accumulating evidence, expert opinion, and knowledge about the risk associated with other viral infections. The CDC said it will continue to make updates as new information becomes available.
Guidance for pregnant women
This updated guidance expands the timeframe during which pregnant women can be tested for Zika virus—with an rRT-PCR test—from 7 days to 14 days after symptoms start. The CDC said this expansion will provide a definite diagnosis for more pregnant women infected with the Zika virus.
Scientists previously thought the virus stays in the blood for about a week after symptoms start. So the first week of illness was thought to be the best time to find evidence of the virus in blood using a Zika-specific test (rRT-PCR).
For patients who visited a healthcare provider more than a week after symptoms started and those who were possibly exposed to Zika but never developed symptoms, healthcare providers could perform Zika virus IgM testing. However, this test might not provide a definite diagnosis, as it can also detect related viruses.
New information has indicated that some infected pregnant women can have evidence of the Zika virus in their blood for longer than 7 days after symptoms begin, and even pregnant women without symptoms can have evidence of the virus in their blood and urine.
Therefore, the updated guidance expands the use of Zika-specific blood testing for a longer period, up to 14 days, in pregnant women with symptoms and advises that pregnant women with possible Zika exposure but no symptoms receive this testing as well.
In addition, if pregnant women visit their healthcare provider after the 14-day testing window and test positive with the IgM test, rRT-PCR testing can now be offered to potentially provide a definite diagnosis.
The CDC’s new guidance also includes recommendations to help healthcare providers better care for their pregnant patients with confirmed or possible Zika infection.
Guidance for sexual transmission
This updated guidance is based on a recently reported case of female-to-male sexual transmission of the Zika virus in New York City and limited human and non-human primate data indicating that Zika virus RNA can be detected in vaginal secretions.
The guidance expands the CDC’s definition of sexual exposure to Zika to include sex without a barrier method (including male or female condoms, among other methods) with any person—male or female—who has traveled to or lives in an area with active Zika virus transmission.
The updated recommendations for pregnant couples include pregnant women with female sex partners who are potentially infected with Zika. The recommendations also provide advice for potentially infected women on how to reduce their risk of sexually transmitting the virus to partners.
Specifically, the CDC recommends that all pregnant women with sex partners who live in or traveled to an area with active Zika virus transmission use condoms during sex or abstain from sex for the remainder of their pregnancy.
All other couples in which a partner has been in an area with active Zika virus transmission can also reduce the risk of sexual transmission by using condoms or abstaining from sex. Sex includes vaginal, anal, and oral sex, and may also include the sharing of sex toys.
Healthcare providers should test all pregnant women who may have been exposed to Zika sexually. Providers should also test patients if they develop symptoms of the Zika virus and report potential sexual exposure to a partner who lives in or traveled to an area with active Zika virus transmission.
The CDC encourages local and state health departments to report potential cases of sexually transmitted Zika virus infection. ![]()

Photo by Nina Matthews
The US Centers for Disease Control and Prevention (CDC) has updated some of its recommendations regarding the Zika virus.
The agency issued an updated interim guidance for healthcare providers caring for pregnant women with possible exposure to Zika virus and an updated interim guidance on preventing sexual transmission of the virus.
The CDC issued these updates based on the accumulating evidence, expert opinion, and knowledge about the risk associated with other viral infections. The CDC said it will continue to make updates as new information becomes available.
Guidance for pregnant women
This updated guidance expands the timeframe during which pregnant women can be tested for Zika virus—with an rRT-PCR test—from 7 days to 14 days after symptoms start. The CDC said this expansion will provide a definite diagnosis for more pregnant women infected with the Zika virus.
Scientists previously thought the virus stays in the blood for about a week after symptoms start. So the first week of illness was thought to be the best time to find evidence of the virus in blood using a Zika-specific test (rRT-PCR).
For patients who visited a healthcare provider more than a week after symptoms started and those who were possibly exposed to Zika but never developed symptoms, healthcare providers could perform Zika virus IgM testing. However, this test might not provide a definite diagnosis, as it can also detect related viruses.
New information has indicated that some infected pregnant women can have evidence of the Zika virus in their blood for longer than 7 days after symptoms begin, and even pregnant women without symptoms can have evidence of the virus in their blood and urine.
Therefore, the updated guidance expands the use of Zika-specific blood testing for a longer period, up to 14 days, in pregnant women with symptoms and advises that pregnant women with possible Zika exposure but no symptoms receive this testing as well.
In addition, if pregnant women visit their healthcare provider after the 14-day testing window and test positive with the IgM test, rRT-PCR testing can now be offered to potentially provide a definite diagnosis.
The CDC’s new guidance also includes recommendations to help healthcare providers better care for their pregnant patients with confirmed or possible Zika infection.
Guidance for sexual transmission
This updated guidance is based on a recently reported case of female-to-male sexual transmission of the Zika virus in New York City and limited human and non-human primate data indicating that Zika virus RNA can be detected in vaginal secretions.
The guidance expands the CDC’s definition of sexual exposure to Zika to include sex without a barrier method (including male or female condoms, among other methods) with any person—male or female—who has traveled to or lives in an area with active Zika virus transmission.
The updated recommendations for pregnant couples include pregnant women with female sex partners who are potentially infected with Zika. The recommendations also provide advice for potentially infected women on how to reduce their risk of sexually transmitting the virus to partners.
Specifically, the CDC recommends that all pregnant women with sex partners who live in or traveled to an area with active Zika virus transmission use condoms during sex or abstain from sex for the remainder of their pregnancy.
All other couples in which a partner has been in an area with active Zika virus transmission can also reduce the risk of sexual transmission by using condoms or abstaining from sex. Sex includes vaginal, anal, and oral sex, and may also include the sharing of sex toys.
Healthcare providers should test all pregnant women who may have been exposed to Zika sexually. Providers should also test patients if they develop symptoms of the Zika virus and report potential sexual exposure to a partner who lives in or traveled to an area with active Zika virus transmission.
The CDC encourages local and state health departments to report potential cases of sexually transmitted Zika virus infection. ![]()
Extreme Athlete, 18, With Worsening Cough
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
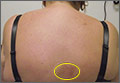
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
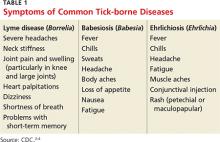
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
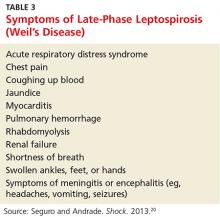
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
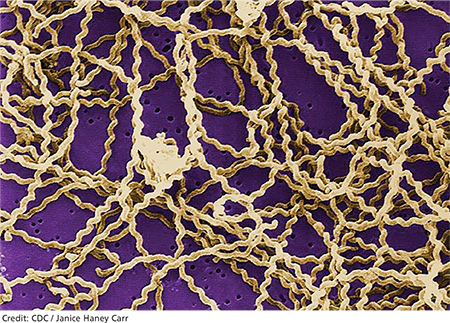
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
Pruritic hyperpigmented patch on back
A 60-year-old woman visited our clinic complaining of an area on the right side of her middle back that was itchy, and had been bothering her for the past 10 years. She said her symptoms began without a trigger, and that a darkened area had appeared in the location of the itch. She had already been prescribed topical corticosteroids and antifungals and had tried over-the counter aids, but nothing relieved the itch. The patient had a history of cervical radiculopathy and was morbidly obese at the time of the visit.
On examination, the pruritic area consisted of a hyperpigmented, non-infiltrated 7-cm patch that was lateral to the vertebral column and within the dermatomes T4 to T6 (FIGURE). The patient also had hyperesthesia to light touch in this region and scratch abrasions.
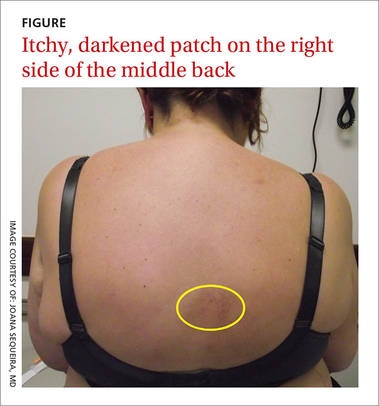
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Notalgia paresthetica
The location of the pruritic area and the patient’s clinical presentation led us to diagnose notalgia paresthetica. NP is a common dermatologic complaint characterized by unilateral pruritus that is medial or inferior to the scapula with dermatomal distribution.
The etiology of NP remains unknown, although it is thought to be a neuropathic itch caused by afferent nerve entrapment. The dorsal rami of the thoracic spinal nerves T2 to T6 are considered to be responsible for these symptoms. NP is not only a skin disease, but a cutaneous sign of an underlying spinal condition, including degenerative cervical spine disease.1-3
NP is a clinical diagnosis. There is typically a history of localized pruritus on the unilateral infrascapula area and there are few or no visible signs of disease. Patients frequently report a spider-bite sensation, prickly feelings, and/or an indescribable itch sensation. In addition, they may experience dysesthesia with diffuse mild burning, some surface numbness, and “under the skin” discomfort.
On physical examination, the patient may have a unilateral and ill-defined tan, pink, or hyperpigmented nonindurated patch on the infrascapular back that is a result of long-time scratching. Secondary skin changes such as lichenification, excoriations, eczema, xerosis, and infection often occur. Mild sensory alterations to light touch, vibration, and pin pricks may round out the clinical picture.1-3 Atypical forms of NP include localized pruritus on the upper back, neck, scalp, or shoulder.
Pruritus without other skin lesions can help pinpoint the Dx
The differential diagnosis for NP includes atopic dermatitis, contact dermatitis, drug eruptions, herpes zoster, idiopathic pruritus and systemic disease (such as renal, cholestatic, or hematologic pruritus, or pruritus associated with malignancy), tinea corporis, tinea versicolor, and xerosis.
Clues in the history. The chronic evolution of pruritus without other skin lesions, like vesicles or squamous areas, and the location of a hyperpigmented patch near the scapula region in a midlife patient, should prompt you to consider NP. A biopsy may show signs of post-inflammatory infiltrate of the papillary dermis with dermal melanophages.4,5
Although imaging tests are not required for a diagnosis of NP, basic cervical and possibly thoracic radiographs or magnetic resonance imaging (MRI) may be helpful in patients with symptoms of spine pain, tenderness, spasms, decreased range of motion, or any history of spinal trauma or injury. The images may reveal spinal disorders, including osteoarthritic lesions such as kyphosis, kyphoscoliosis, and vertebral hyperostosis.4
The exact cause of NP is unclear, but the evidence suggests that it results from damage to the cutaneous branches of the posterior divisions of the spinal nerves. This can occur by either impingement from degenerative changes in the spine or by spasms in the paraspinal musculature.2
The itch is neuropathic; antihistamines, steroids won’t help
It is difficult to treat NP without treating the underlying disease, which is usually spinal damage.4 Little has been published on the treatment of NP, and most of the literature on the subject involves case reports. Because the pruritus in NP is neuropathic, antihistamines and topical steroids are ineffective.4
The most commonly used treatment for NP among dermatologists is capsaicin as a 0.025% cream or 8% patch. One study with 20 patients reported improvement of pruritus in 70% of patients at 2 weeks, with some relapsing in about a month.6
Another treatment that has been used is cutaneous botulinum toxin type A injections, but its use is controversial. This strategy was proposed by Weinfeld7 after successful treatment of 2 patients. However, other studies have had variable outcomes with no resolution of pruritus.8
Other treatments include gabapentin,9 transcutaneous electrical nerve stimulation,10 and narrow-band ultraviolet-B.11 It is appropriate to consider surgical decompression or neurolysis of the nerve when other forms of treatment fail.12
Our patient was treated with topical capsaicin cream 0.25 mg/g, which lessened the intensity of her itching. After 2 months, the patient reported improvement of her symptoms.
CORRESPONDENCE
Joana Sequeira, MD, Estrada da Mata nº56, Leiria, Portugal; [email protected].
1. Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26:104-109.
2. Ellis C. Notalgia paresthetica: the unreachable itch. Dermatol Pract Concept. 2013;3:3-6.
3. Bolognia JL, Jorizzo JL, Schaffer JV, eds. Pruritus and dysesthesia. In: Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders;2012:121.
4. Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
5. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
6. Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995;32:287-289.
7. Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
8. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Notalgia paresthetica: treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014;105:74-77.
9. Maciel AA, Cunha PR, Laraia IO, et al. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with nostalgia paresthetica. An Bras Dermatol. 2014;89:570-575.
10. Savk E, Savk O, Sendur F. Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J Dermatol. 2007;34:315-319.
11. Pérez-Pérez L, Allegue F, Fabeiro JM, et al. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol. 2010;24:730-732.
12. Williams EH, Rosson GD, Elsamanoudi I, et al. Surgical decompression for notalgia paresthetica: a case report. Microsurgery. 2010;30:70-72.
A 60-year-old woman visited our clinic complaining of an area on the right side of her middle back that was itchy, and had been bothering her for the past 10 years. She said her symptoms began without a trigger, and that a darkened area had appeared in the location of the itch. She had already been prescribed topical corticosteroids and antifungals and had tried over-the counter aids, but nothing relieved the itch. The patient had a history of cervical radiculopathy and was morbidly obese at the time of the visit.
On examination, the pruritic area consisted of a hyperpigmented, non-infiltrated 7-cm patch that was lateral to the vertebral column and within the dermatomes T4 to T6 (FIGURE). The patient also had hyperesthesia to light touch in this region and scratch abrasions.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Notalgia paresthetica
The location of the pruritic area and the patient’s clinical presentation led us to diagnose notalgia paresthetica. NP is a common dermatologic complaint characterized by unilateral pruritus that is medial or inferior to the scapula with dermatomal distribution.
The etiology of NP remains unknown, although it is thought to be a neuropathic itch caused by afferent nerve entrapment. The dorsal rami of the thoracic spinal nerves T2 to T6 are considered to be responsible for these symptoms. NP is not only a skin disease, but a cutaneous sign of an underlying spinal condition, including degenerative cervical spine disease.1-3
NP is a clinical diagnosis. There is typically a history of localized pruritus on the unilateral infrascapula area and there are few or no visible signs of disease. Patients frequently report a spider-bite sensation, prickly feelings, and/or an indescribable itch sensation. In addition, they may experience dysesthesia with diffuse mild burning, some surface numbness, and “under the skin” discomfort.
On physical examination, the patient may have a unilateral and ill-defined tan, pink, or hyperpigmented nonindurated patch on the infrascapular back that is a result of long-time scratching. Secondary skin changes such as lichenification, excoriations, eczema, xerosis, and infection often occur. Mild sensory alterations to light touch, vibration, and pin pricks may round out the clinical picture.1-3 Atypical forms of NP include localized pruritus on the upper back, neck, scalp, or shoulder.
Pruritus without other skin lesions can help pinpoint the Dx
The differential diagnosis for NP includes atopic dermatitis, contact dermatitis, drug eruptions, herpes zoster, idiopathic pruritus and systemic disease (such as renal, cholestatic, or hematologic pruritus, or pruritus associated with malignancy), tinea corporis, tinea versicolor, and xerosis.
Clues in the history. The chronic evolution of pruritus without other skin lesions, like vesicles or squamous areas, and the location of a hyperpigmented patch near the scapula region in a midlife patient, should prompt you to consider NP. A biopsy may show signs of post-inflammatory infiltrate of the papillary dermis with dermal melanophages.4,5
Although imaging tests are not required for a diagnosis of NP, basic cervical and possibly thoracic radiographs or magnetic resonance imaging (MRI) may be helpful in patients with symptoms of spine pain, tenderness, spasms, decreased range of motion, or any history of spinal trauma or injury. The images may reveal spinal disorders, including osteoarthritic lesions such as kyphosis, kyphoscoliosis, and vertebral hyperostosis.4
The exact cause of NP is unclear, but the evidence suggests that it results from damage to the cutaneous branches of the posterior divisions of the spinal nerves. This can occur by either impingement from degenerative changes in the spine or by spasms in the paraspinal musculature.2
The itch is neuropathic; antihistamines, steroids won’t help
It is difficult to treat NP without treating the underlying disease, which is usually spinal damage.4 Little has been published on the treatment of NP, and most of the literature on the subject involves case reports. Because the pruritus in NP is neuropathic, antihistamines and topical steroids are ineffective.4
The most commonly used treatment for NP among dermatologists is capsaicin as a 0.025% cream or 8% patch. One study with 20 patients reported improvement of pruritus in 70% of patients at 2 weeks, with some relapsing in about a month.6
Another treatment that has been used is cutaneous botulinum toxin type A injections, but its use is controversial. This strategy was proposed by Weinfeld7 after successful treatment of 2 patients. However, other studies have had variable outcomes with no resolution of pruritus.8
Other treatments include gabapentin,9 transcutaneous electrical nerve stimulation,10 and narrow-band ultraviolet-B.11 It is appropriate to consider surgical decompression or neurolysis of the nerve when other forms of treatment fail.12
Our patient was treated with topical capsaicin cream 0.25 mg/g, which lessened the intensity of her itching. After 2 months, the patient reported improvement of her symptoms.
CORRESPONDENCE
Joana Sequeira, MD, Estrada da Mata nº56, Leiria, Portugal; [email protected].
A 60-year-old woman visited our clinic complaining of an area on the right side of her middle back that was itchy, and had been bothering her for the past 10 years. She said her symptoms began without a trigger, and that a darkened area had appeared in the location of the itch. She had already been prescribed topical corticosteroids and antifungals and had tried over-the counter aids, but nothing relieved the itch. The patient had a history of cervical radiculopathy and was morbidly obese at the time of the visit.
On examination, the pruritic area consisted of a hyperpigmented, non-infiltrated 7-cm patch that was lateral to the vertebral column and within the dermatomes T4 to T6 (FIGURE). The patient also had hyperesthesia to light touch in this region and scratch abrasions.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Notalgia paresthetica
The location of the pruritic area and the patient’s clinical presentation led us to diagnose notalgia paresthetica. NP is a common dermatologic complaint characterized by unilateral pruritus that is medial or inferior to the scapula with dermatomal distribution.
The etiology of NP remains unknown, although it is thought to be a neuropathic itch caused by afferent nerve entrapment. The dorsal rami of the thoracic spinal nerves T2 to T6 are considered to be responsible for these symptoms. NP is not only a skin disease, but a cutaneous sign of an underlying spinal condition, including degenerative cervical spine disease.1-3
NP is a clinical diagnosis. There is typically a history of localized pruritus on the unilateral infrascapula area and there are few or no visible signs of disease. Patients frequently report a spider-bite sensation, prickly feelings, and/or an indescribable itch sensation. In addition, they may experience dysesthesia with diffuse mild burning, some surface numbness, and “under the skin” discomfort.
On physical examination, the patient may have a unilateral and ill-defined tan, pink, or hyperpigmented nonindurated patch on the infrascapular back that is a result of long-time scratching. Secondary skin changes such as lichenification, excoriations, eczema, xerosis, and infection often occur. Mild sensory alterations to light touch, vibration, and pin pricks may round out the clinical picture.1-3 Atypical forms of NP include localized pruritus on the upper back, neck, scalp, or shoulder.
Pruritus without other skin lesions can help pinpoint the Dx
The differential diagnosis for NP includes atopic dermatitis, contact dermatitis, drug eruptions, herpes zoster, idiopathic pruritus and systemic disease (such as renal, cholestatic, or hematologic pruritus, or pruritus associated with malignancy), tinea corporis, tinea versicolor, and xerosis.
Clues in the history. The chronic evolution of pruritus without other skin lesions, like vesicles or squamous areas, and the location of a hyperpigmented patch near the scapula region in a midlife patient, should prompt you to consider NP. A biopsy may show signs of post-inflammatory infiltrate of the papillary dermis with dermal melanophages.4,5
Although imaging tests are not required for a diagnosis of NP, basic cervical and possibly thoracic radiographs or magnetic resonance imaging (MRI) may be helpful in patients with symptoms of spine pain, tenderness, spasms, decreased range of motion, or any history of spinal trauma or injury. The images may reveal spinal disorders, including osteoarthritic lesions such as kyphosis, kyphoscoliosis, and vertebral hyperostosis.4
The exact cause of NP is unclear, but the evidence suggests that it results from damage to the cutaneous branches of the posterior divisions of the spinal nerves. This can occur by either impingement from degenerative changes in the spine or by spasms in the paraspinal musculature.2
The itch is neuropathic; antihistamines, steroids won’t help
It is difficult to treat NP without treating the underlying disease, which is usually spinal damage.4 Little has been published on the treatment of NP, and most of the literature on the subject involves case reports. Because the pruritus in NP is neuropathic, antihistamines and topical steroids are ineffective.4
The most commonly used treatment for NP among dermatologists is capsaicin as a 0.025% cream or 8% patch. One study with 20 patients reported improvement of pruritus in 70% of patients at 2 weeks, with some relapsing in about a month.6
Another treatment that has been used is cutaneous botulinum toxin type A injections, but its use is controversial. This strategy was proposed by Weinfeld7 after successful treatment of 2 patients. However, other studies have had variable outcomes with no resolution of pruritus.8
Other treatments include gabapentin,9 transcutaneous electrical nerve stimulation,10 and narrow-band ultraviolet-B.11 It is appropriate to consider surgical decompression or neurolysis of the nerve when other forms of treatment fail.12
Our patient was treated with topical capsaicin cream 0.25 mg/g, which lessened the intensity of her itching. After 2 months, the patient reported improvement of her symptoms.
CORRESPONDENCE
Joana Sequeira, MD, Estrada da Mata nº56, Leiria, Portugal; [email protected].
1. Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26:104-109.
2. Ellis C. Notalgia paresthetica: the unreachable itch. Dermatol Pract Concept. 2013;3:3-6.
3. Bolognia JL, Jorizzo JL, Schaffer JV, eds. Pruritus and dysesthesia. In: Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders;2012:121.
4. Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
5. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
6. Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995;32:287-289.
7. Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
8. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Notalgia paresthetica: treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014;105:74-77.
9. Maciel AA, Cunha PR, Laraia IO, et al. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with nostalgia paresthetica. An Bras Dermatol. 2014;89:570-575.
10. Savk E, Savk O, Sendur F. Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J Dermatol. 2007;34:315-319.
11. Pérez-Pérez L, Allegue F, Fabeiro JM, et al. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol. 2010;24:730-732.
12. Williams EH, Rosson GD, Elsamanoudi I, et al. Surgical decompression for notalgia paresthetica: a case report. Microsurgery. 2010;30:70-72.
1. Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26:104-109.
2. Ellis C. Notalgia paresthetica: the unreachable itch. Dermatol Pract Concept. 2013;3:3-6.
3. Bolognia JL, Jorizzo JL, Schaffer JV, eds. Pruritus and dysesthesia. In: Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders;2012:121.
4. Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
5. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
6. Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995;32:287-289.
7. Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
8. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Notalgia paresthetica: treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014;105:74-77.
9. Maciel AA, Cunha PR, Laraia IO, et al. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with nostalgia paresthetica. An Bras Dermatol. 2014;89:570-575.
10. Savk E, Savk O, Sendur F. Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J Dermatol. 2007;34:315-319.
11. Pérez-Pérez L, Allegue F, Fabeiro JM, et al. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol. 2010;24:730-732.
12. Williams EH, Rosson GD, Elsamanoudi I, et al. Surgical decompression for notalgia paresthetica: a case report. Microsurgery. 2010;30:70-72.
CMS proposes bundled payments for AMI, CABG
The Centers for Medicare & Medicaid Services is proposing new bundled payment models for acute myocardial infarction and coronary artery bypass grafting, and a separate payment to incentivize the use of cardiac rehabilitation.
As part of the proposal, CMS also is developing a pathway that would allow the bundle to be recognized as an advanced alternative payment model under the Medicare Access and CHIP Reauthorization Act and qualify the physicians and clinicians being paid through the model for the 5% incentive payment.
The proposed bundled payment model would place patient care accountability for 90 days after discharge on the hospital where acute myocardial infarction care or coronary artery bypass grafting occurred. Beginning July 1, 2017, hospitals in 98 randomly selected metropolitan statistical areas would be placed under this model and monitored for a 5-year period to test whether the model leads to improved outcomes and generates cost savings.
The proposed rule can be seen here and an advanced notice is expected to be published on the Federal Register website on July 26. CMS will be accepting comments on the proposal for 60 days following official publication in the Federal Register.
“In 2014, more than 200,000 Medicare beneficiaries were hospitalized for heart attack treatment or underwent bypass surgery, costing Medicare over $6 billion. But the cost of treating patients varied by 50% across hospitals, and the share of patients readmitted to the hospitals within 30 days varied by more than 50%. And patient experience also varies,” CMS Acting Principal Deputy Administrator and Chief Medical Officer Patrick Conway, MD, said during a July 25 press teleconference introducing the proposal. “In some cases, hospitals, doctors, and rehabilitation facilities work together to support a patient from heart attack or surgery all the way through recovery. But in other cases, coordination breaks down, especially when a patient leaves the hospital. By structuring a payment around a patient’s total experience of care, bundled payments support better care coordination and ultimately better outcomes for patients.”
The hospital would be paid a fixed target price for each care episode, with hospitals delivering higher-quality care receiving a higher target price. The hospital would either keep the savings achieved or, if the costs exceeded the target pricing, have to repay Medicare the difference.
Target prices will be based on historical cost data beginning with hospitalization and extending out 90 days following discharge and adjusted based on the complexity of treatment required. For the 18 months of the program (July 1, 2017, through Dec. 31, 2018) target prices would be based on a blend of two-thirds participant-specific data and one-third regional data. In the third performance year (2019), the mix would move to one-third participant data and two-thirds regional data. Beginning in 2020, only regional data would be used to set target prices.
For heart attacks, the following quality measures are being proposed: Hospital 30-day, all-cause, risk-standardized mortality following acute myocardial infarction hospitalization; excess days in acute care after hospitalization for acute myocardial infarction; Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores; and voluntary hybrid hospital 30-day, all-cause, risk-standardized mortality eMeasure data submission.
For bypass surgery, the quality measures will be the hospital 30-day all-cause, risk-standardized mortality rate following coronary artery bypass graft; and HCAHPS survey scores.
“CMS’s evaluation ... will examine quality during the episode period, after the episode period ends, and for longer durations such as 1-year mortality rates,” the agency said in a fact sheet describing the proposal. “CMS will examine outcomes and patient experience measures such as mortality, readmissions, complications, and other clinically relevant outcomes.”
Separately, the agency is proposing to test a cardiac rehabilitation incentive payment. The two-part cardiac rehabilitation incentive payment would be paid retrospectively based on the total cardiac rehabilitation use of beneficiaries attributable to participant hospitals.
“Currently, only 15% of heart attack patients receive cardiac rehabilitation, even though completing a rehabilitation program can lower the risk of the second heart attack or death,” Dr. Conway said. “Patients who receive cardiac rehabilitation are assigned a team of health care professionals such as cardiologists, dietitians, and physical therapists who help the patient to recover and regain cardiovascular fitness.”
The initial payment would be $25 per cardiac rehabilitation service for each of the first 11 services paid for by Medicare during the 90-day care period for a heart attack or bypass surgery. After 11 services, the payment would increase to $175 during the care period.
The number of sessions would be limited to two 1-hour sessions per day up to 36 sessions over up to 36 weeks, with the option for an additional 36 sessions over an extended period if approved by the local Medicare contractor. Intensive cardiac rehabilitation program sessions would be limited to 72 1-hour sessions, up to six sessions per day, over 18 weeks.
While officials from the American College of Cardiology said that the organization supports the concepts of value-based care, “it is important that bundled care models be carried out in such a way that clinicians are given the time and tools to truly impact patient care in the best ways possible. Changes in payment structures in health care can pose significant challenges to clinicians and must be driven by clinical practices that improve patient outcomes,” ACC President Richard A. Chazal, MD, said in a statement. “We are optimistic that CMS will listen to comments, incorporate feedback from clinicians, and provide ample time for implementation of these new payment models. Our ultimate goal is to improve patient care and to improve heart health.”
The Centers for Medicare & Medicaid Services is proposing new bundled payment models for acute myocardial infarction and coronary artery bypass grafting, and a separate payment to incentivize the use of cardiac rehabilitation.
As part of the proposal, CMS also is developing a pathway that would allow the bundle to be recognized as an advanced alternative payment model under the Medicare Access and CHIP Reauthorization Act and qualify the physicians and clinicians being paid through the model for the 5% incentive payment.
The proposed bundled payment model would place patient care accountability for 90 days after discharge on the hospital where acute myocardial infarction care or coronary artery bypass grafting occurred. Beginning July 1, 2017, hospitals in 98 randomly selected metropolitan statistical areas would be placed under this model and monitored for a 5-year period to test whether the model leads to improved outcomes and generates cost savings.
The proposed rule can be seen here and an advanced notice is expected to be published on the Federal Register website on July 26. CMS will be accepting comments on the proposal for 60 days following official publication in the Federal Register.
“In 2014, more than 200,000 Medicare beneficiaries were hospitalized for heart attack treatment or underwent bypass surgery, costing Medicare over $6 billion. But the cost of treating patients varied by 50% across hospitals, and the share of patients readmitted to the hospitals within 30 days varied by more than 50%. And patient experience also varies,” CMS Acting Principal Deputy Administrator and Chief Medical Officer Patrick Conway, MD, said during a July 25 press teleconference introducing the proposal. “In some cases, hospitals, doctors, and rehabilitation facilities work together to support a patient from heart attack or surgery all the way through recovery. But in other cases, coordination breaks down, especially when a patient leaves the hospital. By structuring a payment around a patient’s total experience of care, bundled payments support better care coordination and ultimately better outcomes for patients.”
The hospital would be paid a fixed target price for each care episode, with hospitals delivering higher-quality care receiving a higher target price. The hospital would either keep the savings achieved or, if the costs exceeded the target pricing, have to repay Medicare the difference.
Target prices will be based on historical cost data beginning with hospitalization and extending out 90 days following discharge and adjusted based on the complexity of treatment required. For the 18 months of the program (July 1, 2017, through Dec. 31, 2018) target prices would be based on a blend of two-thirds participant-specific data and one-third regional data. In the third performance year (2019), the mix would move to one-third participant data and two-thirds regional data. Beginning in 2020, only regional data would be used to set target prices.
For heart attacks, the following quality measures are being proposed: Hospital 30-day, all-cause, risk-standardized mortality following acute myocardial infarction hospitalization; excess days in acute care after hospitalization for acute myocardial infarction; Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores; and voluntary hybrid hospital 30-day, all-cause, risk-standardized mortality eMeasure data submission.
For bypass surgery, the quality measures will be the hospital 30-day all-cause, risk-standardized mortality rate following coronary artery bypass graft; and HCAHPS survey scores.
“CMS’s evaluation ... will examine quality during the episode period, after the episode period ends, and for longer durations such as 1-year mortality rates,” the agency said in a fact sheet describing the proposal. “CMS will examine outcomes and patient experience measures such as mortality, readmissions, complications, and other clinically relevant outcomes.”
Separately, the agency is proposing to test a cardiac rehabilitation incentive payment. The two-part cardiac rehabilitation incentive payment would be paid retrospectively based on the total cardiac rehabilitation use of beneficiaries attributable to participant hospitals.
“Currently, only 15% of heart attack patients receive cardiac rehabilitation, even though completing a rehabilitation program can lower the risk of the second heart attack or death,” Dr. Conway said. “Patients who receive cardiac rehabilitation are assigned a team of health care professionals such as cardiologists, dietitians, and physical therapists who help the patient to recover and regain cardiovascular fitness.”
The initial payment would be $25 per cardiac rehabilitation service for each of the first 11 services paid for by Medicare during the 90-day care period for a heart attack or bypass surgery. After 11 services, the payment would increase to $175 during the care period.
The number of sessions would be limited to two 1-hour sessions per day up to 36 sessions over up to 36 weeks, with the option for an additional 36 sessions over an extended period if approved by the local Medicare contractor. Intensive cardiac rehabilitation program sessions would be limited to 72 1-hour sessions, up to six sessions per day, over 18 weeks.
While officials from the American College of Cardiology said that the organization supports the concepts of value-based care, “it is important that bundled care models be carried out in such a way that clinicians are given the time and tools to truly impact patient care in the best ways possible. Changes in payment structures in health care can pose significant challenges to clinicians and must be driven by clinical practices that improve patient outcomes,” ACC President Richard A. Chazal, MD, said in a statement. “We are optimistic that CMS will listen to comments, incorporate feedback from clinicians, and provide ample time for implementation of these new payment models. Our ultimate goal is to improve patient care and to improve heart health.”
The Centers for Medicare & Medicaid Services is proposing new bundled payment models for acute myocardial infarction and coronary artery bypass grafting, and a separate payment to incentivize the use of cardiac rehabilitation.
As part of the proposal, CMS also is developing a pathway that would allow the bundle to be recognized as an advanced alternative payment model under the Medicare Access and CHIP Reauthorization Act and qualify the physicians and clinicians being paid through the model for the 5% incentive payment.
The proposed bundled payment model would place patient care accountability for 90 days after discharge on the hospital where acute myocardial infarction care or coronary artery bypass grafting occurred. Beginning July 1, 2017, hospitals in 98 randomly selected metropolitan statistical areas would be placed under this model and monitored for a 5-year period to test whether the model leads to improved outcomes and generates cost savings.
The proposed rule can be seen here and an advanced notice is expected to be published on the Federal Register website on July 26. CMS will be accepting comments on the proposal for 60 days following official publication in the Federal Register.
“In 2014, more than 200,000 Medicare beneficiaries were hospitalized for heart attack treatment or underwent bypass surgery, costing Medicare over $6 billion. But the cost of treating patients varied by 50% across hospitals, and the share of patients readmitted to the hospitals within 30 days varied by more than 50%. And patient experience also varies,” CMS Acting Principal Deputy Administrator and Chief Medical Officer Patrick Conway, MD, said during a July 25 press teleconference introducing the proposal. “In some cases, hospitals, doctors, and rehabilitation facilities work together to support a patient from heart attack or surgery all the way through recovery. But in other cases, coordination breaks down, especially when a patient leaves the hospital. By structuring a payment around a patient’s total experience of care, bundled payments support better care coordination and ultimately better outcomes for patients.”
The hospital would be paid a fixed target price for each care episode, with hospitals delivering higher-quality care receiving a higher target price. The hospital would either keep the savings achieved or, if the costs exceeded the target pricing, have to repay Medicare the difference.
Target prices will be based on historical cost data beginning with hospitalization and extending out 90 days following discharge and adjusted based on the complexity of treatment required. For the 18 months of the program (July 1, 2017, through Dec. 31, 2018) target prices would be based on a blend of two-thirds participant-specific data and one-third regional data. In the third performance year (2019), the mix would move to one-third participant data and two-thirds regional data. Beginning in 2020, only regional data would be used to set target prices.
For heart attacks, the following quality measures are being proposed: Hospital 30-day, all-cause, risk-standardized mortality following acute myocardial infarction hospitalization; excess days in acute care after hospitalization for acute myocardial infarction; Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores; and voluntary hybrid hospital 30-day, all-cause, risk-standardized mortality eMeasure data submission.
For bypass surgery, the quality measures will be the hospital 30-day all-cause, risk-standardized mortality rate following coronary artery bypass graft; and HCAHPS survey scores.
“CMS’s evaluation ... will examine quality during the episode period, after the episode period ends, and for longer durations such as 1-year mortality rates,” the agency said in a fact sheet describing the proposal. “CMS will examine outcomes and patient experience measures such as mortality, readmissions, complications, and other clinically relevant outcomes.”
Separately, the agency is proposing to test a cardiac rehabilitation incentive payment. The two-part cardiac rehabilitation incentive payment would be paid retrospectively based on the total cardiac rehabilitation use of beneficiaries attributable to participant hospitals.
“Currently, only 15% of heart attack patients receive cardiac rehabilitation, even though completing a rehabilitation program can lower the risk of the second heart attack or death,” Dr. Conway said. “Patients who receive cardiac rehabilitation are assigned a team of health care professionals such as cardiologists, dietitians, and physical therapists who help the patient to recover and regain cardiovascular fitness.”
The initial payment would be $25 per cardiac rehabilitation service for each of the first 11 services paid for by Medicare during the 90-day care period for a heart attack or bypass surgery. After 11 services, the payment would increase to $175 during the care period.
The number of sessions would be limited to two 1-hour sessions per day up to 36 sessions over up to 36 weeks, with the option for an additional 36 sessions over an extended period if approved by the local Medicare contractor. Intensive cardiac rehabilitation program sessions would be limited to 72 1-hour sessions, up to six sessions per day, over 18 weeks.
While officials from the American College of Cardiology said that the organization supports the concepts of value-based care, “it is important that bundled care models be carried out in such a way that clinicians are given the time and tools to truly impact patient care in the best ways possible. Changes in payment structures in health care can pose significant challenges to clinicians and must be driven by clinical practices that improve patient outcomes,” ACC President Richard A. Chazal, MD, said in a statement. “We are optimistic that CMS will listen to comments, incorporate feedback from clinicians, and provide ample time for implementation of these new payment models. Our ultimate goal is to improve patient care and to improve heart health.”