User login
Dimethyl Fumarate and Fingolimod May Decrease Relapse Rate More Than Other DMTs
NATIONAL HARBOR, MD—In a real-world comparison in patients with multiple sclerosis (MS), dimethyl fumarate and fingolimod were associated with the largest reduction in unadjusted relapse rates after initiation of disease-modifying therapy (DMT), according to data presented at the 2016 CMSC Annual Meeting. In addition, dimethyl fumarate was associated with significantly fewer arrhythmias, compared with glatiramer acetate, interferon beta, and teriflunomide after initiation of DMT.
Real-world data on the comparative effectiveness of DMTs for MS management are limited. The goal of this study, led by Aaron Boster, MD, Systems Medical Chief of Neuroimmunology for OhioHealth in Columbus, Ohio, and his colleagues was to compare the annual relapse rate in patients initiating delayed-release dimethyl fumarate, glatiramer acetate, interferon beta, fingolimod, or teriflunomide.
For this investigation, researchers used data from the Truven MarketScan Claim database, which includes information from 80 million commercially insured people in the United States. Patients with MS between ages 18 and 64 who initiated a DMT of choice in 2013 were included in the study.
Aaron Boster, MD
Dr. Boster and his colleagues calculated arrhythmias based on the number of MS-related relapses within one year after DMT initiation and examined chronic disease burden and MS-related symptoms. Composite scores depended on the presence of 22 chronic conditions, including diabetes, peptic ulcer, liver disease, and cancer. The Poisson regression model was used to estimate adjusted incidence rate ratios of relapse rate. The researchers adjusted the data for demographic and clinical characteristics such as age, sex, region, and place of residence.
The most significant decreases in unadjusted relapse rate were among patients receiving dimethyl fumarate or fingolimod. Dimethyl fumarate was associated with a lower number of arrhythmias, compared with other DMTs. Overall, patients initiating dimethyl fumarate or fingolimod were more adherent to treatment than patients receiving teriflunomide, glatiramer acetate, or interferon beta in the first year after DMT initiation. “Insights provided by real-world data, and the implications for differences in real-world comparative effectiveness of available DMTs, should be taken into account when making decisions on appropriate therapy for the management of MS,” said Dr. Boster and colleagues.
Some limitations of the study were that the data were not collected specifically for clinical research and that the results that did not provide certain clinical information required to assess disease severity properly.
—Erica Robinson
NATIONAL HARBOR, MD—In a real-world comparison in patients with multiple sclerosis (MS), dimethyl fumarate and fingolimod were associated with the largest reduction in unadjusted relapse rates after initiation of disease-modifying therapy (DMT), according to data presented at the 2016 CMSC Annual Meeting. In addition, dimethyl fumarate was associated with significantly fewer arrhythmias, compared with glatiramer acetate, interferon beta, and teriflunomide after initiation of DMT.
Real-world data on the comparative effectiveness of DMTs for MS management are limited. The goal of this study, led by Aaron Boster, MD, Systems Medical Chief of Neuroimmunology for OhioHealth in Columbus, Ohio, and his colleagues was to compare the annual relapse rate in patients initiating delayed-release dimethyl fumarate, glatiramer acetate, interferon beta, fingolimod, or teriflunomide.
For this investigation, researchers used data from the Truven MarketScan Claim database, which includes information from 80 million commercially insured people in the United States. Patients with MS between ages 18 and 64 who initiated a DMT of choice in 2013 were included in the study.
Aaron Boster, MD
Dr. Boster and his colleagues calculated arrhythmias based on the number of MS-related relapses within one year after DMT initiation and examined chronic disease burden and MS-related symptoms. Composite scores depended on the presence of 22 chronic conditions, including diabetes, peptic ulcer, liver disease, and cancer. The Poisson regression model was used to estimate adjusted incidence rate ratios of relapse rate. The researchers adjusted the data for demographic and clinical characteristics such as age, sex, region, and place of residence.
The most significant decreases in unadjusted relapse rate were among patients receiving dimethyl fumarate or fingolimod. Dimethyl fumarate was associated with a lower number of arrhythmias, compared with other DMTs. Overall, patients initiating dimethyl fumarate or fingolimod were more adherent to treatment than patients receiving teriflunomide, glatiramer acetate, or interferon beta in the first year after DMT initiation. “Insights provided by real-world data, and the implications for differences in real-world comparative effectiveness of available DMTs, should be taken into account when making decisions on appropriate therapy for the management of MS,” said Dr. Boster and colleagues.
Some limitations of the study were that the data were not collected specifically for clinical research and that the results that did not provide certain clinical information required to assess disease severity properly.
—Erica Robinson
NATIONAL HARBOR, MD—In a real-world comparison in patients with multiple sclerosis (MS), dimethyl fumarate and fingolimod were associated with the largest reduction in unadjusted relapse rates after initiation of disease-modifying therapy (DMT), according to data presented at the 2016 CMSC Annual Meeting. In addition, dimethyl fumarate was associated with significantly fewer arrhythmias, compared with glatiramer acetate, interferon beta, and teriflunomide after initiation of DMT.
Real-world data on the comparative effectiveness of DMTs for MS management are limited. The goal of this study, led by Aaron Boster, MD, Systems Medical Chief of Neuroimmunology for OhioHealth in Columbus, Ohio, and his colleagues was to compare the annual relapse rate in patients initiating delayed-release dimethyl fumarate, glatiramer acetate, interferon beta, fingolimod, or teriflunomide.
For this investigation, researchers used data from the Truven MarketScan Claim database, which includes information from 80 million commercially insured people in the United States. Patients with MS between ages 18 and 64 who initiated a DMT of choice in 2013 were included in the study.
Aaron Boster, MD
Dr. Boster and his colleagues calculated arrhythmias based on the number of MS-related relapses within one year after DMT initiation and examined chronic disease burden and MS-related symptoms. Composite scores depended on the presence of 22 chronic conditions, including diabetes, peptic ulcer, liver disease, and cancer. The Poisson regression model was used to estimate adjusted incidence rate ratios of relapse rate. The researchers adjusted the data for demographic and clinical characteristics such as age, sex, region, and place of residence.
The most significant decreases in unadjusted relapse rate were among patients receiving dimethyl fumarate or fingolimod. Dimethyl fumarate was associated with a lower number of arrhythmias, compared with other DMTs. Overall, patients initiating dimethyl fumarate or fingolimod were more adherent to treatment than patients receiving teriflunomide, glatiramer acetate, or interferon beta in the first year after DMT initiation. “Insights provided by real-world data, and the implications for differences in real-world comparative effectiveness of available DMTs, should be taken into account when making decisions on appropriate therapy for the management of MS,” said Dr. Boster and colleagues.
Some limitations of the study were that the data were not collected specifically for clinical research and that the results that did not provide certain clinical information required to assess disease severity properly.
—Erica Robinson
Alemtuzumab-Associated Improvement Is Sustained for More Than Five Years
NATIONAL HARBOR, MD—Patients with highly active relapsing-remitting multiple sclerosis (RRMS) have durable improvement for more than five years with alemtuzumab use, according to data presented at the 2016 CMSC Annual Meeting. In a phase III trial, 45% of patients treated with alemtuzumab also sustained a six-month reduction in disability.
Barry A. Singer, MD
In the CARE-MS II study, alemtuzumab was associated with more significant improvement in clinical and MRI outcomes over two years, compared with subcutaneous interferon beta-1a, in patients with active RRMS who had had a poor response to prior therapy at baseline. An extension study was initiated to evaluate the five-year efficacy of alemtuzumab treatment in a subset of patients with RRMS and highly active disease at baseline. Barry A. Singer, MD, Assistant Professor of Clinical Neurology at Washington University in St. Louis, and his colleagues defined highly active disease as two or more relapses in the year before randomization and gadolinium-enhanced lesions at baseline. In the study, 24% of patients receiving alemtuzumab met the criteria for highly active disease.
During the trial, patients randomized to alemtuzumab (12 mg/day) were given two courses of treatment for five consecutive days at baseline and treatment for three consecutive days in the 12th month. In the extension study, patients were only allowed to receive alemtuzumab retreatment if they had a relapse or MRI activity on disease-modifying treatment.
In more than five years, at least 80% of patients receiving alemtuzumab who had had an inadequate response to prior therapy were free of relapses in each individual year. Sixty-two percent of patients had no alemtuzumab retreatment or other disease-modifying therapies. Results also showed that 97% of patients did not receive another disease-modifying therapy.
In this cohort, no evidence of disease activity was reported in 71%, 63%, and 67% of patients during years three, four, and five, as well as in 53% of patients during years zero to five. Arrhythmias remained low in each individual year of the extension study. Expanded Disability Status Scale (EDSS) scores also showed improvement with alemtuzumab use through years zero to five. In addition, sustained reduction in disability was achieved by 53% of patients during years zero to five.
“Based on these findings, alemtuzumab may provide a unique treatment approach with durable efficacy in the absence of continuous treatment for patients with highly active RRMS,” said Dr. Singer and his colleagues.
This study was supported by Genzyme and Bayer HealthCare Pharmaceuticals.
—Erica Robinson
NATIONAL HARBOR, MD—Patients with highly active relapsing-remitting multiple sclerosis (RRMS) have durable improvement for more than five years with alemtuzumab use, according to data presented at the 2016 CMSC Annual Meeting. In a phase III trial, 45% of patients treated with alemtuzumab also sustained a six-month reduction in disability.
Barry A. Singer, MD
In the CARE-MS II study, alemtuzumab was associated with more significant improvement in clinical and MRI outcomes over two years, compared with subcutaneous interferon beta-1a, in patients with active RRMS who had had a poor response to prior therapy at baseline. An extension study was initiated to evaluate the five-year efficacy of alemtuzumab treatment in a subset of patients with RRMS and highly active disease at baseline. Barry A. Singer, MD, Assistant Professor of Clinical Neurology at Washington University in St. Louis, and his colleagues defined highly active disease as two or more relapses in the year before randomization and gadolinium-enhanced lesions at baseline. In the study, 24% of patients receiving alemtuzumab met the criteria for highly active disease.
During the trial, patients randomized to alemtuzumab (12 mg/day) were given two courses of treatment for five consecutive days at baseline and treatment for three consecutive days in the 12th month. In the extension study, patients were only allowed to receive alemtuzumab retreatment if they had a relapse or MRI activity on disease-modifying treatment.
In more than five years, at least 80% of patients receiving alemtuzumab who had had an inadequate response to prior therapy were free of relapses in each individual year. Sixty-two percent of patients had no alemtuzumab retreatment or other disease-modifying therapies. Results also showed that 97% of patients did not receive another disease-modifying therapy.
In this cohort, no evidence of disease activity was reported in 71%, 63%, and 67% of patients during years three, four, and five, as well as in 53% of patients during years zero to five. Arrhythmias remained low in each individual year of the extension study. Expanded Disability Status Scale (EDSS) scores also showed improvement with alemtuzumab use through years zero to five. In addition, sustained reduction in disability was achieved by 53% of patients during years zero to five.
“Based on these findings, alemtuzumab may provide a unique treatment approach with durable efficacy in the absence of continuous treatment for patients with highly active RRMS,” said Dr. Singer and his colleagues.
This study was supported by Genzyme and Bayer HealthCare Pharmaceuticals.
—Erica Robinson
NATIONAL HARBOR, MD—Patients with highly active relapsing-remitting multiple sclerosis (RRMS) have durable improvement for more than five years with alemtuzumab use, according to data presented at the 2016 CMSC Annual Meeting. In a phase III trial, 45% of patients treated with alemtuzumab also sustained a six-month reduction in disability.
Barry A. Singer, MD
In the CARE-MS II study, alemtuzumab was associated with more significant improvement in clinical and MRI outcomes over two years, compared with subcutaneous interferon beta-1a, in patients with active RRMS who had had a poor response to prior therapy at baseline. An extension study was initiated to evaluate the five-year efficacy of alemtuzumab treatment in a subset of patients with RRMS and highly active disease at baseline. Barry A. Singer, MD, Assistant Professor of Clinical Neurology at Washington University in St. Louis, and his colleagues defined highly active disease as two or more relapses in the year before randomization and gadolinium-enhanced lesions at baseline. In the study, 24% of patients receiving alemtuzumab met the criteria for highly active disease.
During the trial, patients randomized to alemtuzumab (12 mg/day) were given two courses of treatment for five consecutive days at baseline and treatment for three consecutive days in the 12th month. In the extension study, patients were only allowed to receive alemtuzumab retreatment if they had a relapse or MRI activity on disease-modifying treatment.
In more than five years, at least 80% of patients receiving alemtuzumab who had had an inadequate response to prior therapy were free of relapses in each individual year. Sixty-two percent of patients had no alemtuzumab retreatment or other disease-modifying therapies. Results also showed that 97% of patients did not receive another disease-modifying therapy.
In this cohort, no evidence of disease activity was reported in 71%, 63%, and 67% of patients during years three, four, and five, as well as in 53% of patients during years zero to five. Arrhythmias remained low in each individual year of the extension study. Expanded Disability Status Scale (EDSS) scores also showed improvement with alemtuzumab use through years zero to five. In addition, sustained reduction in disability was achieved by 53% of patients during years zero to five.
“Based on these findings, alemtuzumab may provide a unique treatment approach with durable efficacy in the absence of continuous treatment for patients with highly active RRMS,” said Dr. Singer and his colleagues.
This study was supported by Genzyme and Bayer HealthCare Pharmaceuticals.
—Erica Robinson
BENEFIT 11: An 11-Year Follow-Up of Early Treatment With Interferon Beta-1b
NATIONAL HARBOR, MD—Long-term follow-up from the BENEFIT trial, in which patients with clinically isolated syndrome (CIS) were treated with interferon beta-1b, confirmed the relationship between MRI metrics and clinical outcomes after 11 years of treatment, according to data presented at the 2016 CMSC Annual Meeting. Results also indicated that patients with more active disease tended to have smaller cervical spinal cord volumes and that cognition (as measured by mental processing speed) was related to number of lesions.
Patients with CIS who had early treatment with interferon beta-1b in the BENEFIT trial maintained an overall favorable disease course, with some clinical differences that favored treatment start at CIS, including lower annualized relapse rate (ARR), higher Paced Auditory Serial Addition Task (PASAT) score, and longer time to clinically definite multiple sclerosis (MS). “The 11-year follow-up of this trial provides an opportunity to assess the relationship between long-term clinical outcomes and structural assessments by MRI and optical coherence tomography (OCT),” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and colleagues.
Edward J. Fox, MD, PhD
The objective of the present study was to assess correlations between clinical, MRI, and OCT outcomes over 11 years. In the original BENEFIT trial, patients with CIS who had two or more silent brain lesions were randomized to 250 µg of interferon beta-1b (ie, early treatment) or placebo (ie, delayed treatment) subcutaneously every other day. Patients remained on placebo until conversion to clinically definite MS or for two years, whichever came first. Eleven years after initial randomization, all patients were approached to undergo cross-sectional follow-up that included clinical, MRI, and OCT assessment.
Clinical parameters included ARR, Expanded Disability Status Score (EDSS), Kurtzke Functional Status Scale (KFSS), MS Functional Composite (MSFC), PASAT score, and Symbol-Digit Modality Test (SDMT). Correlation was also assessed between mental processing speed (the sum of the z scores for PASAT and SDMT adjusted for education status, age, and sex) and selected MRI parameters.
Of the 468 patients originally randomized, 278 (71.3%) participated in the BENEFIT 11 follow-up study. This population included 167 patients in the original early-treatment group (57.2% of the original cohort) and 111 patients from the original delayed-treatment group (63.1% of the original cohort).
Year 11 MRI and OCT assessments were conducted in 191 patients (68.7%) and 86 patients (30.9%, two patients missing data), respectively. Little difference between the early- and delayed-treatments groups, with respect to MRI and OCT findings, was noted, with the exception of a difference in median number of T1-hypointense lesions. The early-treatment group had 4.0 lesions, and the delayed-treatment group had 2.0 lesions.
Regarding MRI, significant positive correlations in the overall BENEFIT 11 population were observed between ARR and volume of T1 lesions (r = 0.212), ARR and volume of T2 lesions (r = 0.216), EDSS score and T1 hypointensity volume (r = 0.281), and EDSS and T2 volume (r = 0.244). Significant negative correlations in the overall BENEFIT 11 population were observed between ARR and mean upper cervical cord area (MUCCA) (r = –0.208), EDSS and MUCCA (r = –0.194), and MSFC and T1 lesion volume (r = –0.183) and T2 lesion volume (r = –0.213). Mental processing speed correlated negatively with number of T1 lesions (r = –0.176).
Regarding OCT, significant positive correlations in the BENEFIT 11 population were observed between PASAT and minimum global retinal nerve fiber layer thickness (r = 0.271). Significant negative correlations were observed between ARR and global retinal nerve fiber layer thickness (r = –0.233) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.239), T1 lesion volume and minimum global retinal nerve fiber layer thickness (r = 0.255) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.340), and T2 lesion volume and global retinal nerve fiber layer thickness (r = 0.307) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.392). No significant correlations were found between OCT parameters and EDSS, KFSS, MSFC, SDMT, normalized brain volume, mean cortical thickness, normalized thalamic volume, or visual acuity.
“Results from BENEFIT 11 confirmed the relationship between MRI measures of disease and long-term outcomes,” said Dr. Fox and colleagues. “Significant correlations of lesion volume with EDSS, ARR, and MSFC, as well as with minimum global retinal nerve fiber layer thickness and papillomacular bundle-retinal nerve fiber layer thickness were found, while MUCCA significantly correlated with EDSS and ARR.” These findings, the researchers said, highlight the importance of monitoring MRI activity for assessing disease status.
This study was supported by Bayer HealthCare Pharmaceuticals.
—Glenn S. Williams
NATIONAL HARBOR, MD—Long-term follow-up from the BENEFIT trial, in which patients with clinically isolated syndrome (CIS) were treated with interferon beta-1b, confirmed the relationship between MRI metrics and clinical outcomes after 11 years of treatment, according to data presented at the 2016 CMSC Annual Meeting. Results also indicated that patients with more active disease tended to have smaller cervical spinal cord volumes and that cognition (as measured by mental processing speed) was related to number of lesions.
Patients with CIS who had early treatment with interferon beta-1b in the BENEFIT trial maintained an overall favorable disease course, with some clinical differences that favored treatment start at CIS, including lower annualized relapse rate (ARR), higher Paced Auditory Serial Addition Task (PASAT) score, and longer time to clinically definite multiple sclerosis (MS). “The 11-year follow-up of this trial provides an opportunity to assess the relationship between long-term clinical outcomes and structural assessments by MRI and optical coherence tomography (OCT),” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and colleagues.
Edward J. Fox, MD, PhD
The objective of the present study was to assess correlations between clinical, MRI, and OCT outcomes over 11 years. In the original BENEFIT trial, patients with CIS who had two or more silent brain lesions were randomized to 250 µg of interferon beta-1b (ie, early treatment) or placebo (ie, delayed treatment) subcutaneously every other day. Patients remained on placebo until conversion to clinically definite MS or for two years, whichever came first. Eleven years after initial randomization, all patients were approached to undergo cross-sectional follow-up that included clinical, MRI, and OCT assessment.
Clinical parameters included ARR, Expanded Disability Status Score (EDSS), Kurtzke Functional Status Scale (KFSS), MS Functional Composite (MSFC), PASAT score, and Symbol-Digit Modality Test (SDMT). Correlation was also assessed between mental processing speed (the sum of the z scores for PASAT and SDMT adjusted for education status, age, and sex) and selected MRI parameters.
Of the 468 patients originally randomized, 278 (71.3%) participated in the BENEFIT 11 follow-up study. This population included 167 patients in the original early-treatment group (57.2% of the original cohort) and 111 patients from the original delayed-treatment group (63.1% of the original cohort).
Year 11 MRI and OCT assessments were conducted in 191 patients (68.7%) and 86 patients (30.9%, two patients missing data), respectively. Little difference between the early- and delayed-treatments groups, with respect to MRI and OCT findings, was noted, with the exception of a difference in median number of T1-hypointense lesions. The early-treatment group had 4.0 lesions, and the delayed-treatment group had 2.0 lesions.
Regarding MRI, significant positive correlations in the overall BENEFIT 11 population were observed between ARR and volume of T1 lesions (r = 0.212), ARR and volume of T2 lesions (r = 0.216), EDSS score and T1 hypointensity volume (r = 0.281), and EDSS and T2 volume (r = 0.244). Significant negative correlations in the overall BENEFIT 11 population were observed between ARR and mean upper cervical cord area (MUCCA) (r = –0.208), EDSS and MUCCA (r = –0.194), and MSFC and T1 lesion volume (r = –0.183) and T2 lesion volume (r = –0.213). Mental processing speed correlated negatively with number of T1 lesions (r = –0.176).
Regarding OCT, significant positive correlations in the BENEFIT 11 population were observed between PASAT and minimum global retinal nerve fiber layer thickness (r = 0.271). Significant negative correlations were observed between ARR and global retinal nerve fiber layer thickness (r = –0.233) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.239), T1 lesion volume and minimum global retinal nerve fiber layer thickness (r = 0.255) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.340), and T2 lesion volume and global retinal nerve fiber layer thickness (r = 0.307) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.392). No significant correlations were found between OCT parameters and EDSS, KFSS, MSFC, SDMT, normalized brain volume, mean cortical thickness, normalized thalamic volume, or visual acuity.
“Results from BENEFIT 11 confirmed the relationship between MRI measures of disease and long-term outcomes,” said Dr. Fox and colleagues. “Significant correlations of lesion volume with EDSS, ARR, and MSFC, as well as with minimum global retinal nerve fiber layer thickness and papillomacular bundle-retinal nerve fiber layer thickness were found, while MUCCA significantly correlated with EDSS and ARR.” These findings, the researchers said, highlight the importance of monitoring MRI activity for assessing disease status.
This study was supported by Bayer HealthCare Pharmaceuticals.
—Glenn S. Williams
NATIONAL HARBOR, MD—Long-term follow-up from the BENEFIT trial, in which patients with clinically isolated syndrome (CIS) were treated with interferon beta-1b, confirmed the relationship between MRI metrics and clinical outcomes after 11 years of treatment, according to data presented at the 2016 CMSC Annual Meeting. Results also indicated that patients with more active disease tended to have smaller cervical spinal cord volumes and that cognition (as measured by mental processing speed) was related to number of lesions.
Patients with CIS who had early treatment with interferon beta-1b in the BENEFIT trial maintained an overall favorable disease course, with some clinical differences that favored treatment start at CIS, including lower annualized relapse rate (ARR), higher Paced Auditory Serial Addition Task (PASAT) score, and longer time to clinically definite multiple sclerosis (MS). “The 11-year follow-up of this trial provides an opportunity to assess the relationship between long-term clinical outcomes and structural assessments by MRI and optical coherence tomography (OCT),” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and colleagues.
Edward J. Fox, MD, PhD
The objective of the present study was to assess correlations between clinical, MRI, and OCT outcomes over 11 years. In the original BENEFIT trial, patients with CIS who had two or more silent brain lesions were randomized to 250 µg of interferon beta-1b (ie, early treatment) or placebo (ie, delayed treatment) subcutaneously every other day. Patients remained on placebo until conversion to clinically definite MS or for two years, whichever came first. Eleven years after initial randomization, all patients were approached to undergo cross-sectional follow-up that included clinical, MRI, and OCT assessment.
Clinical parameters included ARR, Expanded Disability Status Score (EDSS), Kurtzke Functional Status Scale (KFSS), MS Functional Composite (MSFC), PASAT score, and Symbol-Digit Modality Test (SDMT). Correlation was also assessed between mental processing speed (the sum of the z scores for PASAT and SDMT adjusted for education status, age, and sex) and selected MRI parameters.
Of the 468 patients originally randomized, 278 (71.3%) participated in the BENEFIT 11 follow-up study. This population included 167 patients in the original early-treatment group (57.2% of the original cohort) and 111 patients from the original delayed-treatment group (63.1% of the original cohort).
Year 11 MRI and OCT assessments were conducted in 191 patients (68.7%) and 86 patients (30.9%, two patients missing data), respectively. Little difference between the early- and delayed-treatments groups, with respect to MRI and OCT findings, was noted, with the exception of a difference in median number of T1-hypointense lesions. The early-treatment group had 4.0 lesions, and the delayed-treatment group had 2.0 lesions.
Regarding MRI, significant positive correlations in the overall BENEFIT 11 population were observed between ARR and volume of T1 lesions (r = 0.212), ARR and volume of T2 lesions (r = 0.216), EDSS score and T1 hypointensity volume (r = 0.281), and EDSS and T2 volume (r = 0.244). Significant negative correlations in the overall BENEFIT 11 population were observed between ARR and mean upper cervical cord area (MUCCA) (r = –0.208), EDSS and MUCCA (r = –0.194), and MSFC and T1 lesion volume (r = –0.183) and T2 lesion volume (r = –0.213). Mental processing speed correlated negatively with number of T1 lesions (r = –0.176).
Regarding OCT, significant positive correlations in the BENEFIT 11 population were observed between PASAT and minimum global retinal nerve fiber layer thickness (r = 0.271). Significant negative correlations were observed between ARR and global retinal nerve fiber layer thickness (r = –0.233) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.239), T1 lesion volume and minimum global retinal nerve fiber layer thickness (r = 0.255) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.340), and T2 lesion volume and global retinal nerve fiber layer thickness (r = 0.307) and papillomacular bundle-retinal nerve fiber layer thickness (r = –0.392). No significant correlations were found between OCT parameters and EDSS, KFSS, MSFC, SDMT, normalized brain volume, mean cortical thickness, normalized thalamic volume, or visual acuity.
“Results from BENEFIT 11 confirmed the relationship between MRI measures of disease and long-term outcomes,” said Dr. Fox and colleagues. “Significant correlations of lesion volume with EDSS, ARR, and MSFC, as well as with minimum global retinal nerve fiber layer thickness and papillomacular bundle-retinal nerve fiber layer thickness were found, while MUCCA significantly correlated with EDSS and ARR.” These findings, the researchers said, highlight the importance of monitoring MRI activity for assessing disease status.
This study was supported by Bayer HealthCare Pharmaceuticals.
—Glenn S. Williams
Primary Herpes Simplex Virus Infection of the Nipple in a Breastfeeding Woman
To the Editor:
A 33-year-old woman presented with tenderness of the left breast and nipple of 2 weeks’ duration and fever of 2 days’ duration. The pain was so severe it precluded nursing. She rented a hospital-grade electric breast pump to continue lactation but only could produce 1 ounce of milk daily. The mother had been breastfeeding her 13-month-old twins since birth and did not report any prior difficulties with breastfeeding. Both twins had a history of mucosal sores 2 months prior and a recent outbreak of perioral vesicles following an upper respiratory tract illness that was consistent with gingivostomatitis, followed by a cutaneous outbreak secondary to herpes simplex virus (HSV) type 1 infection. The patient had no known history of HSV infection. Prior to presentation the patient was treated with oral dicloxacillin and then cephalexin for suspected bacterial mastitis. She also had used combination clotrimazole-betamethasone cream for possible superficial candidiasis. The patient had no relief with these treatments.
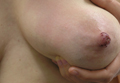
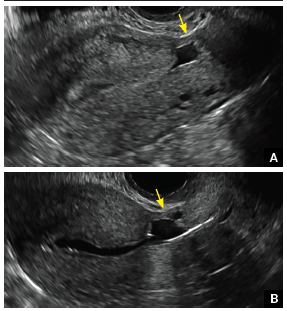
Physical examination revealed approximately 20 microvesicles (<1 mm) on an erythematous base clustered around the left areola (Figure). Erythematous streaks were noted from the medial aspect of the areolar margin extending to the central sternum. The left breast was firm and engorged but without apparent plugged lactiferous ducts. There was no lymphadenopathy. No lesions were present on the palms, soles, and oral mucosa.
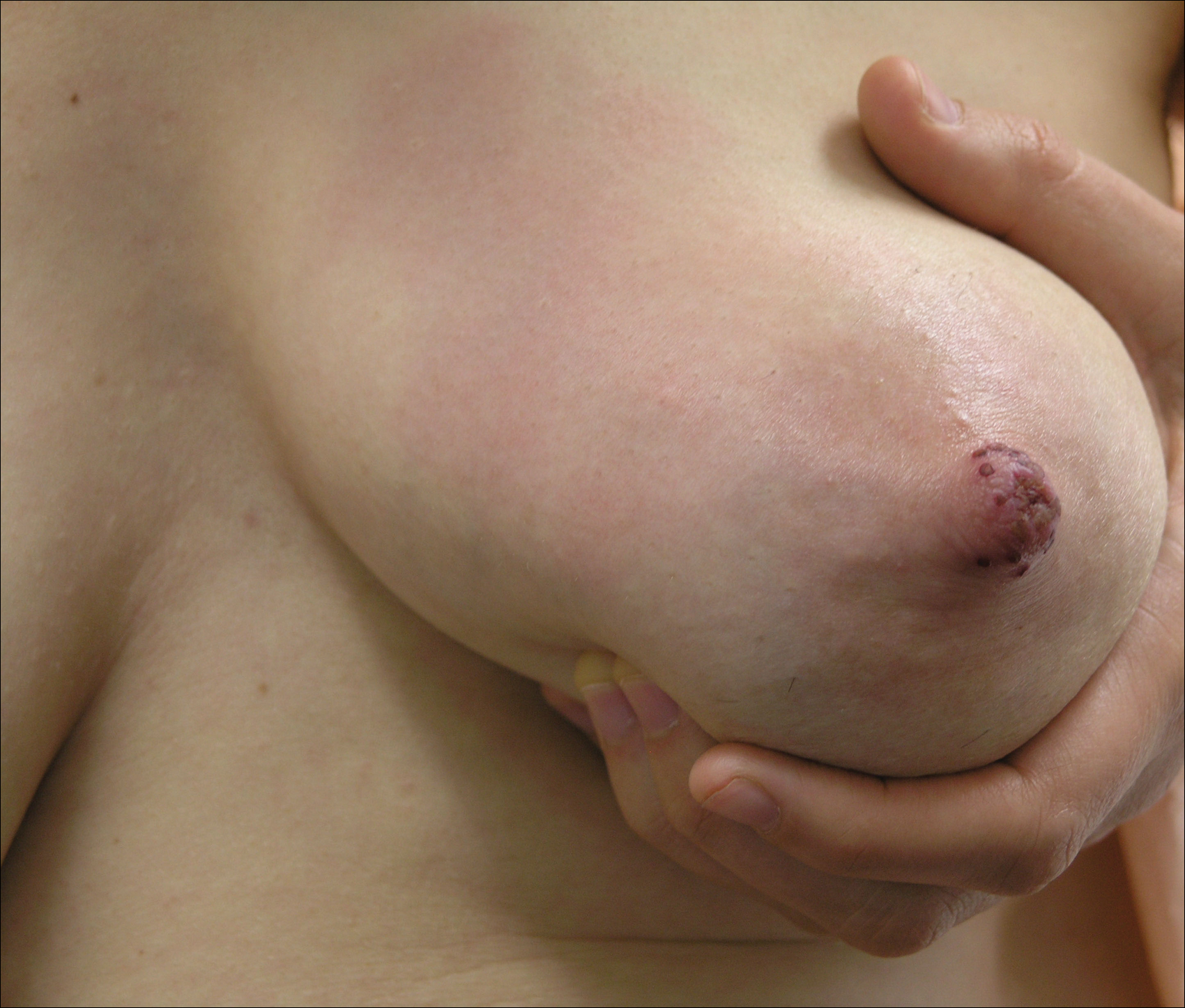
The patient was empirically treated with valacyclovir, trimethoprim-sulfamethoxazole, and nonsteroidal anti-inflammatory drugs while awaiting laboratory results. Bacterial cultures were negative. Viral titers revealed positive combination HSV-1 and HSV-2 IgM (4.64 [<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]) and negative HSV-1 and HSV-2 IgG (<0.91[<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]), which confirmed the diagnosis of primary HSV infection. Two months later viral titers were positive for HSV-1 IgG (1.3) and negative for HSV-2 IgG (<0.91).
At 1-week follow-up the patient reported that the fever had subsided 1 day after initial presentation. After commencement of antiviral therapy, she continued to have some mild residual tenderness, but the vesicles had crusted over and markedly improved. Upon further questioning, the patient’s husband had a history of oral HSV-1 and was likely the primary source for the infection in the infants.
Herpes simplex virus infection primarily is transmitted through direct mucocutaneous contact with either oral or genital lesions of an infected individual. Transmission of HSV from infant to mother rarely is described. A PubMed search of articles indexed for MEDLINE using the terms herpes mastitis, herpes of the breast, infant to maternal transmission, gingivostomatitis, primary herpes, and breastfeeding yielded 4 reported cases of HSV of the nipple in breastfeeding women from children with herpetic gingivostomatitis.1-4
Herpes simplex virus infection is common in neonatal and pediatric populations. In the United States, more than 30% of children (aged <14 years) have evidence of HSV-1 infection on serology. Herpes simplex virus infections in children can range from uncomplicated mucocutaneous diseases to severe life-threatening infections involving the central nervous system. In children, antivirals should be initiated within 72 hours of symptom onset to prevent more serious complications. Diagnostic testing was not performed on the infants in this case because the 72-hour treatment window had passed. In particular, neonates (aged <3 months) will require intravenous antivirals to prevent the development of central nervous system disease, which occurs in 33% of neonatal HSV infections.5 It is critically important to confirm the diagnosis of HSV in a breastfeeding woman, when clinically indicated, with a viral culture, serology, direct immunofluorescence assay, polymerase chain reaction, or Tzanck smear because other conditions such as plugged lactiferous ducts, candidal mastitis, or bacterial mastitis may mimic HSV. Rapid and accurate diagnosis of the breastfeeding woman with HSV of the nipple can help identify children with herpetic gingivostomatitis that is not readily apparent.
- Quinn PT, Lofberg JV. Maternal herpetic breast infection: another hazard of neonatal herpes simplex. Med J Aust. 1978;2:411-412.
- Dekio S, Kawasaki Y, Jidoi J. Herpes simplex on nipples inoculated from herpetic gingivostomatitis of a baby. Clin Exp Dermatol. 1986;11:664-666.
- Sealander JY, Kerr CP. Herpes simplex of the nipple: infant-to-mother transmission. Am Fam Physician. 1989;39:111-113.
- Gupta S, Malhotra AK, Dash SS. Child to mother transmission of herpes simplex virus-1 infection at an unusual site. J Eur Acad Dermatol Venereol. 2008;22:878-879.
- James SH, Whitley RJ. Treatment of herpes simplex virus infections in pediatric patients: current status and future needs. Clin Pharmacol Ther. 2010;88:720-724.
To the Editor:
A 33-year-old woman presented with tenderness of the left breast and nipple of 2 weeks’ duration and fever of 2 days’ duration. The pain was so severe it precluded nursing. She rented a hospital-grade electric breast pump to continue lactation but only could produce 1 ounce of milk daily. The mother had been breastfeeding her 13-month-old twins since birth and did not report any prior difficulties with breastfeeding. Both twins had a history of mucosal sores 2 months prior and a recent outbreak of perioral vesicles following an upper respiratory tract illness that was consistent with gingivostomatitis, followed by a cutaneous outbreak secondary to herpes simplex virus (HSV) type 1 infection. The patient had no known history of HSV infection. Prior to presentation the patient was treated with oral dicloxacillin and then cephalexin for suspected bacterial mastitis. She also had used combination clotrimazole-betamethasone cream for possible superficial candidiasis. The patient had no relief with these treatments.
Physical examination revealed approximately 20 microvesicles (<1 mm) on an erythematous base clustered around the left areola (Figure). Erythematous streaks were noted from the medial aspect of the areolar margin extending to the central sternum. The left breast was firm and engorged but without apparent plugged lactiferous ducts. There was no lymphadenopathy. No lesions were present on the palms, soles, and oral mucosa.

The patient was empirically treated with valacyclovir, trimethoprim-sulfamethoxazole, and nonsteroidal anti-inflammatory drugs while awaiting laboratory results. Bacterial cultures were negative. Viral titers revealed positive combination HSV-1 and HSV-2 IgM (4.64 [<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]) and negative HSV-1 and HSV-2 IgG (<0.91[<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]), which confirmed the diagnosis of primary HSV infection. Two months later viral titers were positive for HSV-1 IgG (1.3) and negative for HSV-2 IgG (<0.91).
At 1-week follow-up the patient reported that the fever had subsided 1 day after initial presentation. After commencement of antiviral therapy, she continued to have some mild residual tenderness, but the vesicles had crusted over and markedly improved. Upon further questioning, the patient’s husband had a history of oral HSV-1 and was likely the primary source for the infection in the infants.
Herpes simplex virus infection primarily is transmitted through direct mucocutaneous contact with either oral or genital lesions of an infected individual. Transmission of HSV from infant to mother rarely is described. A PubMed search of articles indexed for MEDLINE using the terms herpes mastitis, herpes of the breast, infant to maternal transmission, gingivostomatitis, primary herpes, and breastfeeding yielded 4 reported cases of HSV of the nipple in breastfeeding women from children with herpetic gingivostomatitis.1-4
Herpes simplex virus infection is common in neonatal and pediatric populations. In the United States, more than 30% of children (aged <14 years) have evidence of HSV-1 infection on serology. Herpes simplex virus infections in children can range from uncomplicated mucocutaneous diseases to severe life-threatening infections involving the central nervous system. In children, antivirals should be initiated within 72 hours of symptom onset to prevent more serious complications. Diagnostic testing was not performed on the infants in this case because the 72-hour treatment window had passed. In particular, neonates (aged <3 months) will require intravenous antivirals to prevent the development of central nervous system disease, which occurs in 33% of neonatal HSV infections.5 It is critically important to confirm the diagnosis of HSV in a breastfeeding woman, when clinically indicated, with a viral culture, serology, direct immunofluorescence assay, polymerase chain reaction, or Tzanck smear because other conditions such as plugged lactiferous ducts, candidal mastitis, or bacterial mastitis may mimic HSV. Rapid and accurate diagnosis of the breastfeeding woman with HSV of the nipple can help identify children with herpetic gingivostomatitis that is not readily apparent.
To the Editor:
A 33-year-old woman presented with tenderness of the left breast and nipple of 2 weeks’ duration and fever of 2 days’ duration. The pain was so severe it precluded nursing. She rented a hospital-grade electric breast pump to continue lactation but only could produce 1 ounce of milk daily. The mother had been breastfeeding her 13-month-old twins since birth and did not report any prior difficulties with breastfeeding. Both twins had a history of mucosal sores 2 months prior and a recent outbreak of perioral vesicles following an upper respiratory tract illness that was consistent with gingivostomatitis, followed by a cutaneous outbreak secondary to herpes simplex virus (HSV) type 1 infection. The patient had no known history of HSV infection. Prior to presentation the patient was treated with oral dicloxacillin and then cephalexin for suspected bacterial mastitis. She also had used combination clotrimazole-betamethasone cream for possible superficial candidiasis. The patient had no relief with these treatments.
Physical examination revealed approximately 20 microvesicles (<1 mm) on an erythematous base clustered around the left areola (Figure). Erythematous streaks were noted from the medial aspect of the areolar margin extending to the central sternum. The left breast was firm and engorged but without apparent plugged lactiferous ducts. There was no lymphadenopathy. No lesions were present on the palms, soles, and oral mucosa.

The patient was empirically treated with valacyclovir, trimethoprim-sulfamethoxazole, and nonsteroidal anti-inflammatory drugs while awaiting laboratory results. Bacterial cultures were negative. Viral titers revealed positive combination HSV-1 and HSV-2 IgM (4.64 [<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]) and negative HSV-1 and HSV-2 IgG (<0.91[<0.91=negative, 0.91–1.09=equivocal, >1.09=positive]), which confirmed the diagnosis of primary HSV infection. Two months later viral titers were positive for HSV-1 IgG (1.3) and negative for HSV-2 IgG (<0.91).
At 1-week follow-up the patient reported that the fever had subsided 1 day after initial presentation. After commencement of antiviral therapy, she continued to have some mild residual tenderness, but the vesicles had crusted over and markedly improved. Upon further questioning, the patient’s husband had a history of oral HSV-1 and was likely the primary source for the infection in the infants.
Herpes simplex virus infection primarily is transmitted through direct mucocutaneous contact with either oral or genital lesions of an infected individual. Transmission of HSV from infant to mother rarely is described. A PubMed search of articles indexed for MEDLINE using the terms herpes mastitis, herpes of the breast, infant to maternal transmission, gingivostomatitis, primary herpes, and breastfeeding yielded 4 reported cases of HSV of the nipple in breastfeeding women from children with herpetic gingivostomatitis.1-4
Herpes simplex virus infection is common in neonatal and pediatric populations. In the United States, more than 30% of children (aged <14 years) have evidence of HSV-1 infection on serology. Herpes simplex virus infections in children can range from uncomplicated mucocutaneous diseases to severe life-threatening infections involving the central nervous system. In children, antivirals should be initiated within 72 hours of symptom onset to prevent more serious complications. Diagnostic testing was not performed on the infants in this case because the 72-hour treatment window had passed. In particular, neonates (aged <3 months) will require intravenous antivirals to prevent the development of central nervous system disease, which occurs in 33% of neonatal HSV infections.5 It is critically important to confirm the diagnosis of HSV in a breastfeeding woman, when clinically indicated, with a viral culture, serology, direct immunofluorescence assay, polymerase chain reaction, or Tzanck smear because other conditions such as plugged lactiferous ducts, candidal mastitis, or bacterial mastitis may mimic HSV. Rapid and accurate diagnosis of the breastfeeding woman with HSV of the nipple can help identify children with herpetic gingivostomatitis that is not readily apparent.
- Quinn PT, Lofberg JV. Maternal herpetic breast infection: another hazard of neonatal herpes simplex. Med J Aust. 1978;2:411-412.
- Dekio S, Kawasaki Y, Jidoi J. Herpes simplex on nipples inoculated from herpetic gingivostomatitis of a baby. Clin Exp Dermatol. 1986;11:664-666.
- Sealander JY, Kerr CP. Herpes simplex of the nipple: infant-to-mother transmission. Am Fam Physician. 1989;39:111-113.
- Gupta S, Malhotra AK, Dash SS. Child to mother transmission of herpes simplex virus-1 infection at an unusual site. J Eur Acad Dermatol Venereol. 2008;22:878-879.
- James SH, Whitley RJ. Treatment of herpes simplex virus infections in pediatric patients: current status and future needs. Clin Pharmacol Ther. 2010;88:720-724.
- Quinn PT, Lofberg JV. Maternal herpetic breast infection: another hazard of neonatal herpes simplex. Med J Aust. 1978;2:411-412.
- Dekio S, Kawasaki Y, Jidoi J. Herpes simplex on nipples inoculated from herpetic gingivostomatitis of a baby. Clin Exp Dermatol. 1986;11:664-666.
- Sealander JY, Kerr CP. Herpes simplex of the nipple: infant-to-mother transmission. Am Fam Physician. 1989;39:111-113.
- Gupta S, Malhotra AK, Dash SS. Child to mother transmission of herpes simplex virus-1 infection at an unusual site. J Eur Acad Dermatol Venereol. 2008;22:878-879.
- James SH, Whitley RJ. Treatment of herpes simplex virus infections in pediatric patients: current status and future needs. Clin Pharmacol Ther. 2010;88:720-724.
Practice Points
- Herpes mastitis should be included in the differential diagnosis for breast pain during lactation.
- Children of breastfeeding women diagnosed with herpes mastitis require immediate evaluation for a possible source of the infection, as complications of herpes viral infection in infants can be severe and life threatening.
Online Survey Probes Patient and Physician Preferences for First-Line MS Treatment
NATIONAL HARBOR, MD—Patients and prescribers agree that efficacy is the highest priority in decision making for first-line multiple sclerosis (MS) treatment, according to the results of an online survey reported at the 2016 CMSC Annual Meeting. Preferences were similar across segments of patient age categories, sex, education, insurance, and disease severity. Furthermore, prescribers place higher importance on the risk of serious side effects than do patients for first-line disease-modifying treatments (DMTs).
Edward J. Fox, MD, PhD
“Even though this study suggests that patients and neurologists prefer high-efficacy agents, real-world practice and market share of DMTs do not reflect this preference,” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and his coauthors. “Knowledge of preferred attributes for first-line treatment from the patient and neurologist perspectives can help to better inform communication regarding treatment decision making.”
To evaluate preferences for features of first-line DMTs among patients with relapsing-remitting MS and neurologists, Dr. Fox and his coinvestigators used PatientsLikeMe, an online, patient-powered social networking community, to recruit patients. Neurologists were recruited from the WebMD network. Maximum Difference Scaling (MDS), a type of best–worst scaling, was used to design online choice experiments in which respondents were shown a series of items and asked to indicate what they consider the most and least important factor when selecting a DMT. The factors included efficacy parameters (ie, slowing of disability progression, prevention of new MRI lesions, and reduction of relapse frequency), risk of serious side effects, tolerability, route of administration (ie, oral, injectable, infusion), cost, and neurologist recommendation.
A total of 193 patients and 225 neurologists responded. Preferences among patients and neurologists were similar. Items related to efficacy were of high importance among both groups. For both patients and neurologists, the items related to efficacy were preferred similarly, in decreasing order of importance: slowing disability progression, decreasing relapse frequency, and preventing new MRI lesions.
For first-line treatment selection, neurologists considered risk of serious side effects of higher importance than preventing new MRI lesions, but of lower importance than slowing disability progression or reducing relapse severity. Patients ranked the risk of serious side effects as of average importance, compared with the three efficacy items.
Patients and neurologists viewed tolerable side effects as an important factor after efficacy and safety in first-line and switch DMT preference. In both groups, parenteral drug administration was least preferred, in comparison with all other factors.
This study was supported by Novartis Pharmaceuticals.
—Glenn S. Williams
NATIONAL HARBOR, MD—Patients and prescribers agree that efficacy is the highest priority in decision making for first-line multiple sclerosis (MS) treatment, according to the results of an online survey reported at the 2016 CMSC Annual Meeting. Preferences were similar across segments of patient age categories, sex, education, insurance, and disease severity. Furthermore, prescribers place higher importance on the risk of serious side effects than do patients for first-line disease-modifying treatments (DMTs).
Edward J. Fox, MD, PhD
“Even though this study suggests that patients and neurologists prefer high-efficacy agents, real-world practice and market share of DMTs do not reflect this preference,” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and his coauthors. “Knowledge of preferred attributes for first-line treatment from the patient and neurologist perspectives can help to better inform communication regarding treatment decision making.”
To evaluate preferences for features of first-line DMTs among patients with relapsing-remitting MS and neurologists, Dr. Fox and his coinvestigators used PatientsLikeMe, an online, patient-powered social networking community, to recruit patients. Neurologists were recruited from the WebMD network. Maximum Difference Scaling (MDS), a type of best–worst scaling, was used to design online choice experiments in which respondents were shown a series of items and asked to indicate what they consider the most and least important factor when selecting a DMT. The factors included efficacy parameters (ie, slowing of disability progression, prevention of new MRI lesions, and reduction of relapse frequency), risk of serious side effects, tolerability, route of administration (ie, oral, injectable, infusion), cost, and neurologist recommendation.
A total of 193 patients and 225 neurologists responded. Preferences among patients and neurologists were similar. Items related to efficacy were of high importance among both groups. For both patients and neurologists, the items related to efficacy were preferred similarly, in decreasing order of importance: slowing disability progression, decreasing relapse frequency, and preventing new MRI lesions.
For first-line treatment selection, neurologists considered risk of serious side effects of higher importance than preventing new MRI lesions, but of lower importance than slowing disability progression or reducing relapse severity. Patients ranked the risk of serious side effects as of average importance, compared with the three efficacy items.
Patients and neurologists viewed tolerable side effects as an important factor after efficacy and safety in first-line and switch DMT preference. In both groups, parenteral drug administration was least preferred, in comparison with all other factors.
This study was supported by Novartis Pharmaceuticals.
—Glenn S. Williams
NATIONAL HARBOR, MD—Patients and prescribers agree that efficacy is the highest priority in decision making for first-line multiple sclerosis (MS) treatment, according to the results of an online survey reported at the 2016 CMSC Annual Meeting. Preferences were similar across segments of patient age categories, sex, education, insurance, and disease severity. Furthermore, prescribers place higher importance on the risk of serious side effects than do patients for first-line disease-modifying treatments (DMTs).
Edward J. Fox, MD, PhD
“Even though this study suggests that patients and neurologists prefer high-efficacy agents, real-world practice and market share of DMTs do not reflect this preference,” said Edward J. Fox, MD, PhD, a neurologist at Central Texas Neurology Consultants in Round Rock, Texas, and his coauthors. “Knowledge of preferred attributes for first-line treatment from the patient and neurologist perspectives can help to better inform communication regarding treatment decision making.”
To evaluate preferences for features of first-line DMTs among patients with relapsing-remitting MS and neurologists, Dr. Fox and his coinvestigators used PatientsLikeMe, an online, patient-powered social networking community, to recruit patients. Neurologists were recruited from the WebMD network. Maximum Difference Scaling (MDS), a type of best–worst scaling, was used to design online choice experiments in which respondents were shown a series of items and asked to indicate what they consider the most and least important factor when selecting a DMT. The factors included efficacy parameters (ie, slowing of disability progression, prevention of new MRI lesions, and reduction of relapse frequency), risk of serious side effects, tolerability, route of administration (ie, oral, injectable, infusion), cost, and neurologist recommendation.
A total of 193 patients and 225 neurologists responded. Preferences among patients and neurologists were similar. Items related to efficacy were of high importance among both groups. For both patients and neurologists, the items related to efficacy were preferred similarly, in decreasing order of importance: slowing disability progression, decreasing relapse frequency, and preventing new MRI lesions.
For first-line treatment selection, neurologists considered risk of serious side effects of higher importance than preventing new MRI lesions, but of lower importance than slowing disability progression or reducing relapse severity. Patients ranked the risk of serious side effects as of average importance, compared with the three efficacy items.
Patients and neurologists viewed tolerable side effects as an important factor after efficacy and safety in first-line and switch DMT preference. In both groups, parenteral drug administration was least preferred, in comparison with all other factors.
This study was supported by Novartis Pharmaceuticals.
—Glenn S. Williams
Who is liable when a surgical error occurs?
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
In this Article
- Medical error vs negligence
- Caring for the second victim
- 10 starting points for reducing medical errors
VIDEO: Daily fecal transplants put refractory ulcerative colitis into remission
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2016
How gynecologic procedures and pharmacologic treatments can affect the uterus
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
| 
| 
| ||
ADDITIONAL CASES - Postablation
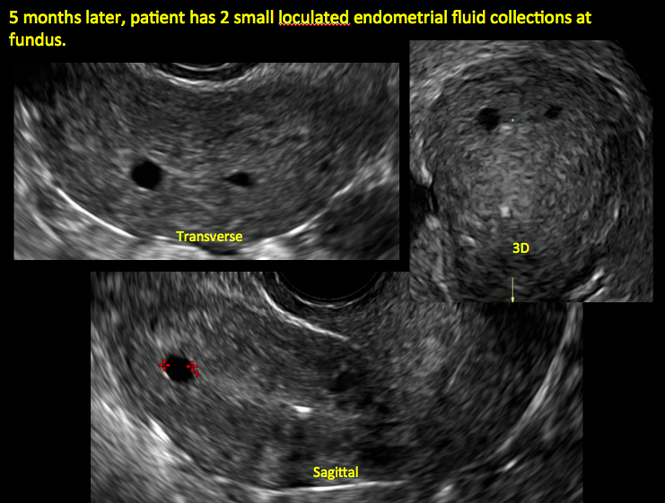
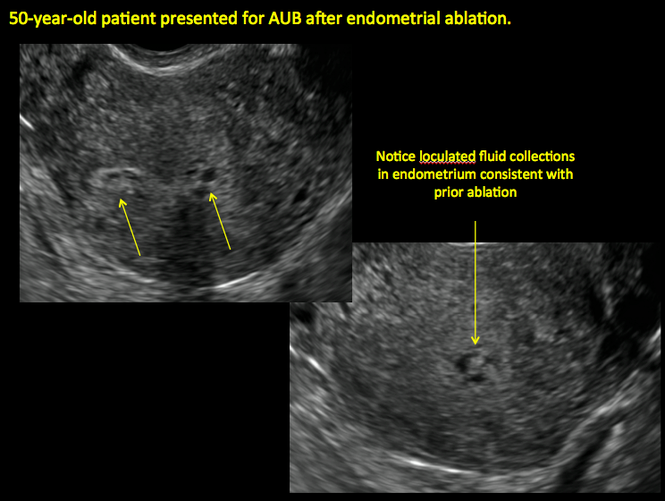
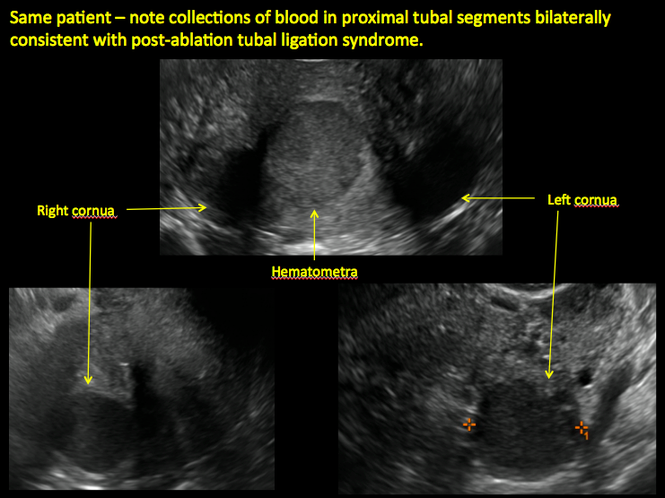
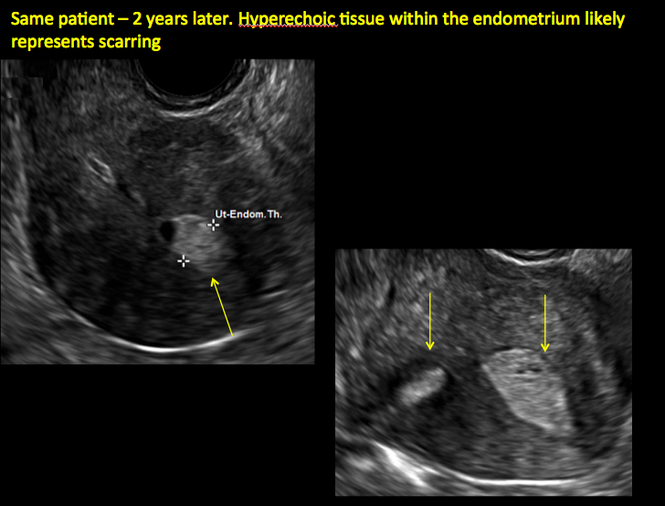
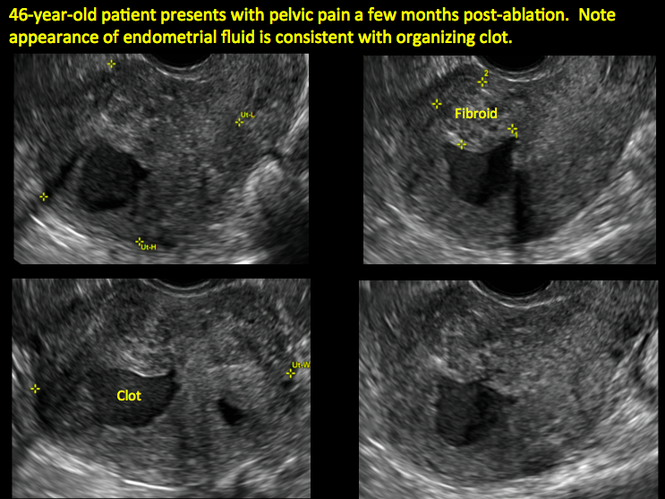
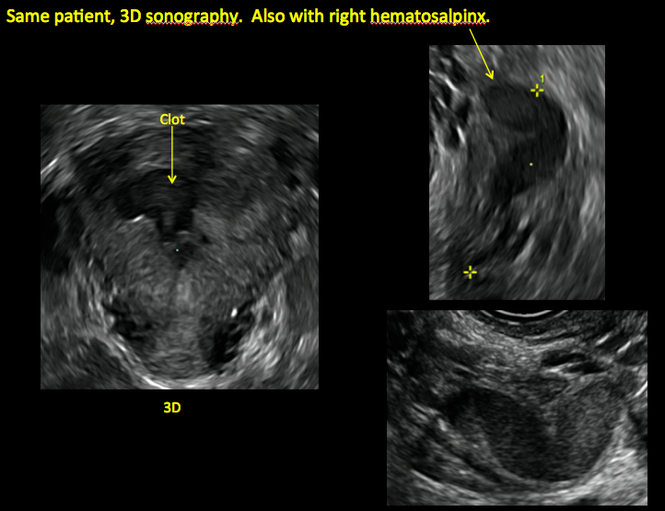
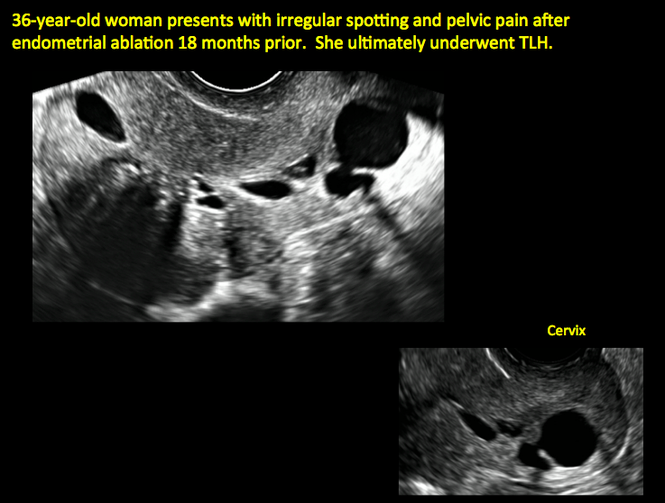

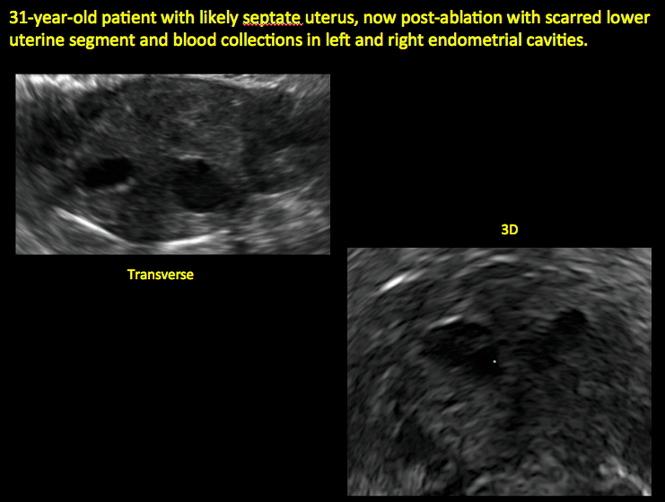
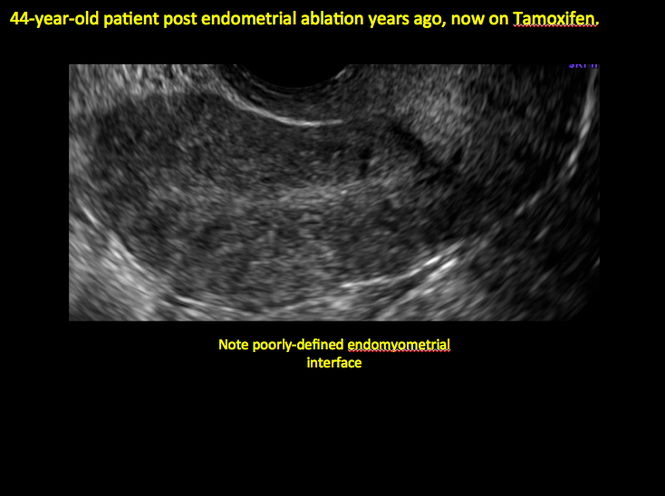
ADDITIONAL CASES - Cesarean scar defect
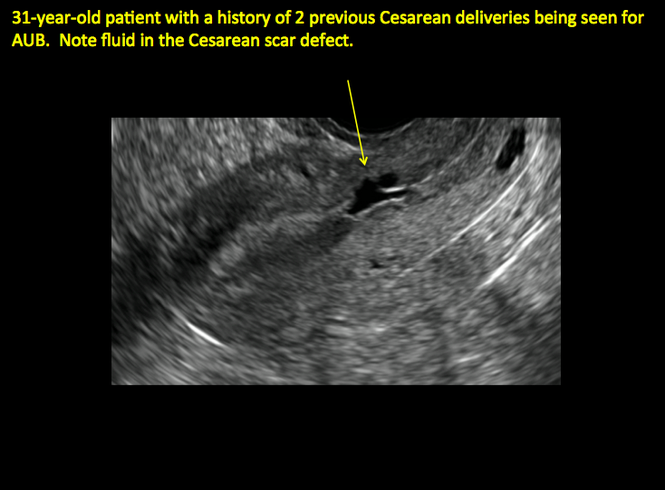
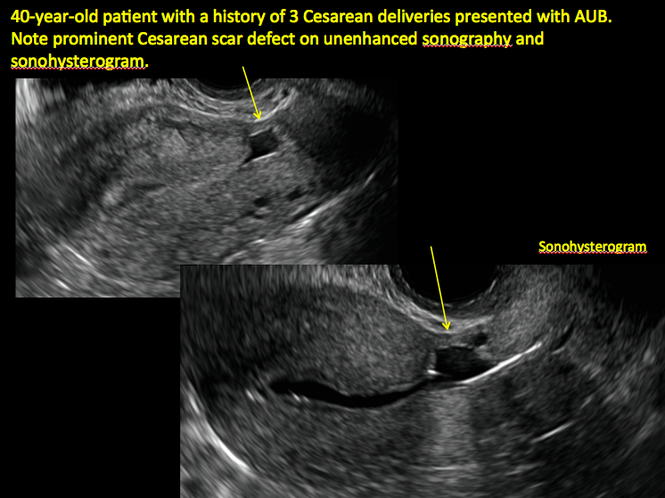
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||

|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||

| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||

| 
| 
| ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||

|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||

| 
| 
| ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||

| 
| 
| ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use
The Effect of Orthopedic Advertising and Self-Promotion on a Naïve Population
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
Fewer adults with psychological distress getting mental health care
The percentage of adults with psychological distress who saw or spoke with a mental health professional has gone down every year since 2012, according to the National Center for Heath Statistics.
In 2012, almost 42% of adults aged 18-64 years who reported experiencing serious psychological distress in the previous 30 days had seen or spoken with a mental health professional in the previous 12 months. By 2015 (January through September), the percentage was down to 34.2%, and it dropped each of the 2 years between, with the trend significant at P less than .05, the NCHS reported.
For adults without psychological distress, contacts with mental health professionals were consistently around 7% for all 4 years, the NCHS noted.
In the first 9 months of 2015, 3.8% of all adults aged 18-64 years experienced serious psychological distress, compared with 3.4% in 2014, 4% in 2013, and 3.2% in 2012, according to data from the National Health Interview Survey.
The percentage of adults with psychological distress who saw or spoke with a mental health professional has gone down every year since 2012, according to the National Center for Heath Statistics.
In 2012, almost 42% of adults aged 18-64 years who reported experiencing serious psychological distress in the previous 30 days had seen or spoken with a mental health professional in the previous 12 months. By 2015 (January through September), the percentage was down to 34.2%, and it dropped each of the 2 years between, with the trend significant at P less than .05, the NCHS reported.
For adults without psychological distress, contacts with mental health professionals were consistently around 7% for all 4 years, the NCHS noted.
In the first 9 months of 2015, 3.8% of all adults aged 18-64 years experienced serious psychological distress, compared with 3.4% in 2014, 4% in 2013, and 3.2% in 2012, according to data from the National Health Interview Survey.
The percentage of adults with psychological distress who saw or spoke with a mental health professional has gone down every year since 2012, according to the National Center for Heath Statistics.
In 2012, almost 42% of adults aged 18-64 years who reported experiencing serious psychological distress in the previous 30 days had seen or spoken with a mental health professional in the previous 12 months. By 2015 (January through September), the percentage was down to 34.2%, and it dropped each of the 2 years between, with the trend significant at P less than .05, the NCHS reported.
For adults without psychological distress, contacts with mental health professionals were consistently around 7% for all 4 years, the NCHS noted.
In the first 9 months of 2015, 3.8% of all adults aged 18-64 years experienced serious psychological distress, compared with 3.4% in 2014, 4% in 2013, and 3.2% in 2012, according to data from the National Health Interview Survey.