User login
Maternal vaccination against pertussis can protect premature infants
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
FROM PEDIATRICS
Become an SHM Ambassador for a Chance at Free HM17 Registration
Now through December 31, all active SHM members can earn 2017–2018 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members. Active members will be eligible for:
- A $35 credit toward 2017–2018 dues when recruiting 1 new member
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to HM17 in Las Vegas. To be counted as a referral, the new member must:
- Be a brand-new member to SHM. (Past members whose membership has lapsed do not qualify.)
- Register as a physician, physician assistant, nurse practitioner, pharmacist, or affiliate member.
- Include an active member’s name in the “referred by” field on a printed application or the online join form.
- Join before December 31, 2016.
Note: SHM members are not eligible for dues credits through the Membership Ambassador Program for member referrals attributed to free memberships received as a result of HM17 registrations.
Now through December 31, all active SHM members can earn 2017–2018 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members. Active members will be eligible for:
- A $35 credit toward 2017–2018 dues when recruiting 1 new member
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to HM17 in Las Vegas. To be counted as a referral, the new member must:
- Be a brand-new member to SHM. (Past members whose membership has lapsed do not qualify.)
- Register as a physician, physician assistant, nurse practitioner, pharmacist, or affiliate member.
- Include an active member’s name in the “referred by” field on a printed application or the online join form.
- Join before December 31, 2016.
Note: SHM members are not eligible for dues credits through the Membership Ambassador Program for member referrals attributed to free memberships received as a result of HM17 registrations.
Now through December 31, all active SHM members can earn 2017–2018 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members. Active members will be eligible for:
- A $35 credit toward 2017–2018 dues when recruiting 1 new member
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to HM17 in Las Vegas. To be counted as a referral, the new member must:
- Be a brand-new member to SHM. (Past members whose membership has lapsed do not qualify.)
- Register as a physician, physician assistant, nurse practitioner, pharmacist, or affiliate member.
- Include an active member’s name in the “referred by” field on a printed application or the online join form.
- Join before December 31, 2016.
Note: SHM members are not eligible for dues credits through the Membership Ambassador Program for member referrals attributed to free memberships received as a result of HM17 registrations.
Hospitalist Chief Finds Value in SHM’s Hospitalist Engagement Benchmarking Service
Rachel Lovins, MD, SFHM, CPE, is the chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. In 2015, she read about the Hospitalist Engagement Benchmarking Service, a new offering from SHM that assesses the engagement level of approximately 1,500 hospitalists nationwide. Soon thereafter, she enrolled her hospital medicine group.
The service provides a snapshot and benchmark comparison of physician attitudes toward a wide range of aspects, including organizational climate, care quality, effective motivation, burnout risk, and more.
Dr. Lovins recently shared her thoughts on the survey with The Hospitalist and explained how she and her team are using the results of the survey to improve the engagement of their hospitalist group. More than 80% of survey respondents indicated they will utilize the service again and plan to recommend the service to a colleague. Learn more and join the second cohort at www.hospitalmedicine.org/pmad3.
Question: How did you become aware of the Hospitalist Engagement Benchmarking Service?
Answer: Last year, I read a blog post written by practice management expert Leslie Flores, MHA, SFHM, about happiness. In the post, she shared information about the country of Bhutan and its Gross National Happiness Index. She proceeded to relate it to practice management, stressing the importance of “paying deliberate attention to hospitalist personal and professional well-being” to ensure sustainability in our field.
As she reflected on the implications of Bhutan’s happiness index and its relation to hospital medicine, she suggested having hospital medicine groups complete SHM’s Hospitalist Engagement Benchmarking Survey to know where they stood with their own happiness indices. As the chief of hospital medicine in my hospital, it truly resonated with me. (As an aside, I often joke that I do whatever Leslie says—because she is pretty much always right!)
Q: What factors inspired you to enroll your group in the service?
A: I’m a total believer in the philosophy of Leslie and her consulting partner, John Nelson, MD, MHM, that a healthy hospital medicine group needs a culture of ownership. If members don’t feel engaged, burnout and isolation are not far behind. Hospitalist work is not easy, and the hours can be long. If you don’t feel empowered, safe, and engaged, it’s going to be unhappy work and an unhappy group.
The leadership team in my program sincerely wants our members to feel satisfied professionally and personally at work. In addition to having a high-performing group, we want people to feel like they belong and that they have some control over what goes on in their daily practice.
Q: How would you describe your experience throughout the survey, including findings and follow-up?
A: I found the survey very easy. I supplied the emails of the participating hospitalists to SHM, and their team took care of the rest, including consistent follow-up. A few months after our group completed the survey, I received the results, which were extremely helpful. It was particularly interesting to see where we scored compared to other hospitalist groups.
Q: What were the main findings upon completion for your team at Middlesex Hospital? How did you implement the takeaways/changes following the service? What were/are the results?
A: I was happy to see that our group felt like they made a difference to our patients and the hospital and that the leadership provided good support. I was, however, discouraged that there were issues with perceived fairness in patient distribution and that our percentages for folks looking forward to and being excited by their jobs were somewhat low.
These two issues—and risk for burnout specifically—are part of our strategic plan moving forward. We need to find ways to make patient distribution more transparent and make people feel happier about coming to work, partially through quarterly “think tanks,” which we just started this year. Because of the results of this survey and another hospital survey, we created an anonymous internal survey to get more specific information. Through that, I was able to target some very specific issues and to reach out to members of the group to try and resolve them.
This is an ongoing process, and we have to keep working on it. It’s like a marriage; you can’t just sit back and assume a relationship will work out on its own. You have to constantly reassess your partner’s needs and be concerned about their happiness as well as your own. We certainly don’t do a perfect job meeting everyone’s needs, but we strive to do so. Having a tool that is validated and easy to use is extremely beneficial to us, and I will definitely use it again. I’d recommend it to anyone who manages a hospitalist group. TH
Brett Radler is SHM’s communications coordinator.
Rachel Lovins, MD, SFHM, CPE, is the chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. In 2015, she read about the Hospitalist Engagement Benchmarking Service, a new offering from SHM that assesses the engagement level of approximately 1,500 hospitalists nationwide. Soon thereafter, she enrolled her hospital medicine group.
The service provides a snapshot and benchmark comparison of physician attitudes toward a wide range of aspects, including organizational climate, care quality, effective motivation, burnout risk, and more.
Dr. Lovins recently shared her thoughts on the survey with The Hospitalist and explained how she and her team are using the results of the survey to improve the engagement of their hospitalist group. More than 80% of survey respondents indicated they will utilize the service again and plan to recommend the service to a colleague. Learn more and join the second cohort at www.hospitalmedicine.org/pmad3.
Question: How did you become aware of the Hospitalist Engagement Benchmarking Service?
Answer: Last year, I read a blog post written by practice management expert Leslie Flores, MHA, SFHM, about happiness. In the post, she shared information about the country of Bhutan and its Gross National Happiness Index. She proceeded to relate it to practice management, stressing the importance of “paying deliberate attention to hospitalist personal and professional well-being” to ensure sustainability in our field.
As she reflected on the implications of Bhutan’s happiness index and its relation to hospital medicine, she suggested having hospital medicine groups complete SHM’s Hospitalist Engagement Benchmarking Survey to know where they stood with their own happiness indices. As the chief of hospital medicine in my hospital, it truly resonated with me. (As an aside, I often joke that I do whatever Leslie says—because she is pretty much always right!)
Q: What factors inspired you to enroll your group in the service?
A: I’m a total believer in the philosophy of Leslie and her consulting partner, John Nelson, MD, MHM, that a healthy hospital medicine group needs a culture of ownership. If members don’t feel engaged, burnout and isolation are not far behind. Hospitalist work is not easy, and the hours can be long. If you don’t feel empowered, safe, and engaged, it’s going to be unhappy work and an unhappy group.
The leadership team in my program sincerely wants our members to feel satisfied professionally and personally at work. In addition to having a high-performing group, we want people to feel like they belong and that they have some control over what goes on in their daily practice.
Q: How would you describe your experience throughout the survey, including findings and follow-up?
A: I found the survey very easy. I supplied the emails of the participating hospitalists to SHM, and their team took care of the rest, including consistent follow-up. A few months after our group completed the survey, I received the results, which were extremely helpful. It was particularly interesting to see where we scored compared to other hospitalist groups.
Q: What were the main findings upon completion for your team at Middlesex Hospital? How did you implement the takeaways/changes following the service? What were/are the results?
A: I was happy to see that our group felt like they made a difference to our patients and the hospital and that the leadership provided good support. I was, however, discouraged that there were issues with perceived fairness in patient distribution and that our percentages for folks looking forward to and being excited by their jobs were somewhat low.
These two issues—and risk for burnout specifically—are part of our strategic plan moving forward. We need to find ways to make patient distribution more transparent and make people feel happier about coming to work, partially through quarterly “think tanks,” which we just started this year. Because of the results of this survey and another hospital survey, we created an anonymous internal survey to get more specific information. Through that, I was able to target some very specific issues and to reach out to members of the group to try and resolve them.
This is an ongoing process, and we have to keep working on it. It’s like a marriage; you can’t just sit back and assume a relationship will work out on its own. You have to constantly reassess your partner’s needs and be concerned about their happiness as well as your own. We certainly don’t do a perfect job meeting everyone’s needs, but we strive to do so. Having a tool that is validated and easy to use is extremely beneficial to us, and I will definitely use it again. I’d recommend it to anyone who manages a hospitalist group. TH
Brett Radler is SHM’s communications coordinator.
Rachel Lovins, MD, SFHM, CPE, is the chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. In 2015, she read about the Hospitalist Engagement Benchmarking Service, a new offering from SHM that assesses the engagement level of approximately 1,500 hospitalists nationwide. Soon thereafter, she enrolled her hospital medicine group.
The service provides a snapshot and benchmark comparison of physician attitudes toward a wide range of aspects, including organizational climate, care quality, effective motivation, burnout risk, and more.
Dr. Lovins recently shared her thoughts on the survey with The Hospitalist and explained how she and her team are using the results of the survey to improve the engagement of their hospitalist group. More than 80% of survey respondents indicated they will utilize the service again and plan to recommend the service to a colleague. Learn more and join the second cohort at www.hospitalmedicine.org/pmad3.
Question: How did you become aware of the Hospitalist Engagement Benchmarking Service?
Answer: Last year, I read a blog post written by practice management expert Leslie Flores, MHA, SFHM, about happiness. In the post, she shared information about the country of Bhutan and its Gross National Happiness Index. She proceeded to relate it to practice management, stressing the importance of “paying deliberate attention to hospitalist personal and professional well-being” to ensure sustainability in our field.
As she reflected on the implications of Bhutan’s happiness index and its relation to hospital medicine, she suggested having hospital medicine groups complete SHM’s Hospitalist Engagement Benchmarking Survey to know where they stood with their own happiness indices. As the chief of hospital medicine in my hospital, it truly resonated with me. (As an aside, I often joke that I do whatever Leslie says—because she is pretty much always right!)
Q: What factors inspired you to enroll your group in the service?
A: I’m a total believer in the philosophy of Leslie and her consulting partner, John Nelson, MD, MHM, that a healthy hospital medicine group needs a culture of ownership. If members don’t feel engaged, burnout and isolation are not far behind. Hospitalist work is not easy, and the hours can be long. If you don’t feel empowered, safe, and engaged, it’s going to be unhappy work and an unhappy group.
The leadership team in my program sincerely wants our members to feel satisfied professionally and personally at work. In addition to having a high-performing group, we want people to feel like they belong and that they have some control over what goes on in their daily practice.
Q: How would you describe your experience throughout the survey, including findings and follow-up?
A: I found the survey very easy. I supplied the emails of the participating hospitalists to SHM, and their team took care of the rest, including consistent follow-up. A few months after our group completed the survey, I received the results, which were extremely helpful. It was particularly interesting to see where we scored compared to other hospitalist groups.
Q: What were the main findings upon completion for your team at Middlesex Hospital? How did you implement the takeaways/changes following the service? What were/are the results?
A: I was happy to see that our group felt like they made a difference to our patients and the hospital and that the leadership provided good support. I was, however, discouraged that there were issues with perceived fairness in patient distribution and that our percentages for folks looking forward to and being excited by their jobs were somewhat low.
These two issues—and risk for burnout specifically—are part of our strategic plan moving forward. We need to find ways to make patient distribution more transparent and make people feel happier about coming to work, partially through quarterly “think tanks,” which we just started this year. Because of the results of this survey and another hospital survey, we created an anonymous internal survey to get more specific information. Through that, I was able to target some very specific issues and to reach out to members of the group to try and resolve them.
This is an ongoing process, and we have to keep working on it. It’s like a marriage; you can’t just sit back and assume a relationship will work out on its own. You have to constantly reassess your partner’s needs and be concerned about their happiness as well as your own. We certainly don’t do a perfect job meeting everyone’s needs, but we strive to do so. Having a tool that is validated and easy to use is extremely beneficial to us, and I will definitely use it again. I’d recommend it to anyone who manages a hospitalist group. TH
Brett Radler is SHM’s communications coordinator.
Growths on face and scalp
The FP diagnosed molluscum contagiosum and recognized that some of the larger lesions on the scalp were related to the patient’s altered immune status. Molluscum contagiosum is a viral skin infection that produces pearly papules that often have central umbilication. This skin infection is most commonly seen in children, but can also be transmitted sexually among adults. The number of cases in adults increased in the 1980s in the United States, probably as a result of the HIV/AIDS epidemic. Since the introduction of highly active antiretroviral therapy (HAART), the number of molluscum contagiosum cases in HIV/AIDS patients has decreased substantially. However, the prevalence of molluscum contagiosum in patients who are HIV-positive may still be as high as 5% to 18%.
The FP encouraged the patient to take her antiretroviral medication as prescribed and suggested that she return to her HIV specialist to see if her therapeutic regimen required any adjustments. He also offered her cryotherapy, with a follow-up appointment one month later. The patient agreed to the cryotherapy, which was performed with a cryogun using a bent tip spray. The patient’s eye was protected using a tongue depressor, while her eyelid was sprayed with liquid nitrogen.
At the follow-up visit, the molluscum lesions had improved and a second round of cryotherapy was performed. Although it was not offered to this patient, topical imiquimod is another treatment option for molluscum contagiosum. This treatment has not, however, been approved by the Food and Drug Administration for this diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed molluscum contagiosum and recognized that some of the larger lesions on the scalp were related to the patient’s altered immune status. Molluscum contagiosum is a viral skin infection that produces pearly papules that often have central umbilication. This skin infection is most commonly seen in children, but can also be transmitted sexually among adults. The number of cases in adults increased in the 1980s in the United States, probably as a result of the HIV/AIDS epidemic. Since the introduction of highly active antiretroviral therapy (HAART), the number of molluscum contagiosum cases in HIV/AIDS patients has decreased substantially. However, the prevalence of molluscum contagiosum in patients who are HIV-positive may still be as high as 5% to 18%.
The FP encouraged the patient to take her antiretroviral medication as prescribed and suggested that she return to her HIV specialist to see if her therapeutic regimen required any adjustments. He also offered her cryotherapy, with a follow-up appointment one month later. The patient agreed to the cryotherapy, which was performed with a cryogun using a bent tip spray. The patient’s eye was protected using a tongue depressor, while her eyelid was sprayed with liquid nitrogen.
At the follow-up visit, the molluscum lesions had improved and a second round of cryotherapy was performed. Although it was not offered to this patient, topical imiquimod is another treatment option for molluscum contagiosum. This treatment has not, however, been approved by the Food and Drug Administration for this diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed molluscum contagiosum and recognized that some of the larger lesions on the scalp were related to the patient’s altered immune status. Molluscum contagiosum is a viral skin infection that produces pearly papules that often have central umbilication. This skin infection is most commonly seen in children, but can also be transmitted sexually among adults. The number of cases in adults increased in the 1980s in the United States, probably as a result of the HIV/AIDS epidemic. Since the introduction of highly active antiretroviral therapy (HAART), the number of molluscum contagiosum cases in HIV/AIDS patients has decreased substantially. However, the prevalence of molluscum contagiosum in patients who are HIV-positive may still be as high as 5% to 18%.
The FP encouraged the patient to take her antiretroviral medication as prescribed and suggested that she return to her HIV specialist to see if her therapeutic regimen required any adjustments. He also offered her cryotherapy, with a follow-up appointment one month later. The patient agreed to the cryotherapy, which was performed with a cryogun using a bent tip spray. The patient’s eye was protected using a tongue depressor, while her eyelid was sprayed with liquid nitrogen.
At the follow-up visit, the molluscum lesions had improved and a second round of cryotherapy was performed. Although it was not offered to this patient, topical imiquimod is another treatment option for molluscum contagiosum. This treatment has not, however, been approved by the Food and Drug Administration for this diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Molluscum contagiosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:743-748.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Two compounds show promise against Zika virus

Photo courtesy of
Muhammad Mahdi Karim
Two compounds have shown activity against the Zika virus, according to Biotech Biotron, a company that develops compounds to fight viral diseases such as HIV and hepatitis C. The two compounds from its library killed the Zika virus in vitro, as determined by an independent USA laboratory facility.
“These early results are encouraging,” Michelle Miller, PhD, of Biotron, said. “Identification of these active compounds in our library is a starting point for designing potent drugs against Zika.”
At present, there is no approved vaccine or treatment for Zika virus, whose common symptoms include fever, rash, joint pain, and conjunctivitis.
While the symptoms are generally mild, Zika infection during pregnancy has been associated with microcephaly and other severe brain defects in the newborn.
In addition, Zika infection may be associated with an increased risk of Guillain-Barré syndrome, which is being investigated by the Centers for Disease Control and Prevention.
Biotron is planning to carry out more tests on the Zika virus to determine whether the compounds are likely to be safe and effective in humans.
The Zika virus is primarily spread by infected mosquitoes. But exposure to an infected person’s blood or other body fluids may also result in transmission. ![]()

Photo courtesy of
Muhammad Mahdi Karim
Two compounds have shown activity against the Zika virus, according to Biotech Biotron, a company that develops compounds to fight viral diseases such as HIV and hepatitis C. The two compounds from its library killed the Zika virus in vitro, as determined by an independent USA laboratory facility.
“These early results are encouraging,” Michelle Miller, PhD, of Biotron, said. “Identification of these active compounds in our library is a starting point for designing potent drugs against Zika.”
At present, there is no approved vaccine or treatment for Zika virus, whose common symptoms include fever, rash, joint pain, and conjunctivitis.
While the symptoms are generally mild, Zika infection during pregnancy has been associated with microcephaly and other severe brain defects in the newborn.
In addition, Zika infection may be associated with an increased risk of Guillain-Barré syndrome, which is being investigated by the Centers for Disease Control and Prevention.
Biotron is planning to carry out more tests on the Zika virus to determine whether the compounds are likely to be safe and effective in humans.
The Zika virus is primarily spread by infected mosquitoes. But exposure to an infected person’s blood or other body fluids may also result in transmission. ![]()

Photo courtesy of
Muhammad Mahdi Karim
Two compounds have shown activity against the Zika virus, according to Biotech Biotron, a company that develops compounds to fight viral diseases such as HIV and hepatitis C. The two compounds from its library killed the Zika virus in vitro, as determined by an independent USA laboratory facility.
“These early results are encouraging,” Michelle Miller, PhD, of Biotron, said. “Identification of these active compounds in our library is a starting point for designing potent drugs against Zika.”
At present, there is no approved vaccine or treatment for Zika virus, whose common symptoms include fever, rash, joint pain, and conjunctivitis.
While the symptoms are generally mild, Zika infection during pregnancy has been associated with microcephaly and other severe brain defects in the newborn.
In addition, Zika infection may be associated with an increased risk of Guillain-Barré syndrome, which is being investigated by the Centers for Disease Control and Prevention.
Biotron is planning to carry out more tests on the Zika virus to determine whether the compounds are likely to be safe and effective in humans.
The Zika virus is primarily spread by infected mosquitoes. But exposure to an infected person’s blood or other body fluids may also result in transmission. ![]()
Platelet transfusions do not reduce IVH risk in VLBW infants
A retrospective, multicenter study of 972 very-low-birth-weight (VLBW) infants treated in 6 US neonatal intensive care units (NICUs) has shown that platelet transfusions do not significantly affect the incidence of intraventricular hemorrhage (IVH).
Thrombocytopenia is a risk factor for IVH, but investigators found no correlation between its severity and risk for IVH. Nor did they find platelet transfusions to have a significant effect on the incidence of IVH.
To describe platelet transfusion practices in US NICUs, senior author Martha Sola-Visner, MD, of Boston Children’s Hospital in Massachusetts, and colleagues studied NICU admissions from January 1, 2006, to December 31, 2007. They collected the last data on December 4, 2008.
Of the 972 VLBW infants, 231 (23.8%) received at least 1 platelet transfusion. And more males received transfusions (61%) than females.
Infants who received transfusions were more premature at 26.3 weeks’ gestation age compared with 28.8 weeks for those who did not receive transfusions, P<0.001.
Transfused infants were also smaller, with a mean birth weight of 805 g compared with 1113 g in the group that did not receive a transfusion, P<0.001.
Platelet transfusions
The 231 transfused infants received a total of 1002 platelet transfusions, with a mean of 4.3 per infant (range 1 to 63 transfusions).
Forty-one percent of infants had transfusions during the first 7 days of life only, amounting to 281 transfusions; 32.9% had transfusions after the first 7 days only, and 26.4% had transfusions during both periods. Seven hundred twenty-one transfusions were administered after day 7.
Almost two thirds of the transfusions, 65.4% or 653 of 998 transfusions, were given to infants who had a pre-transfusion platelet count of at least 50,000/μL.
The investigators poined out that this finding “was in contrast to UK NICUs,” where transfusions are administered at a median platelet count of 27,000/μL.
Illness severity
The investigators found significant differences among NICU sites in terms of clinical markers for transfusions.
Overall, 189 VLBW infants had platelet counts less than 100,000/μL in the first 7 days of life for a total of 402 days. And at least 1 platelet transfusion was given on 212 of those days. Of these, 198 transfusions (93.4%) had a marker of severe illness or bleeding.
On the other hand, of the 190 patient days without a transfusion, 113 (59.5%) had at least 1 of these markers (P<0.001).
Thrombocytopenia and IVH risk
The investigators evaluated the risk for IVH based on the lowest platelet count before the diagnosis of IVH was made.
They found that infants with thrombocytopenia were at higher risk for IVH, with a hazard ratio of 2.17 for any platelet count less than 150,000/μL (P<0.001).
Nevertheless, for the 314 infants with at least 1 platelet count less than 150,000/μL during the first 7 days of life, they found no association between severity of thrombocytopenia and the risk for subsequent IVH (P=0.70).
Transfusion and IVH risk
To determine whether platelet transfusions protected VLBW infants from IVH during their first 7 days of life, the investigators performed a Cox regression analysis in 756 infants.
They found that 134 infants (17.7%) had an IVH, including 62 (8.2%) with grade III or IV. So in the unadjusted model, they found a significant association between platelet transfusion and subsequent IVH, P=0.004.
However, when they adjusted the model for clinical covariates, only infants with grade III or IV IVH had a significantly greater risk with platelet transfusion, P=0.01.
Clinical covariates included sex, gestational age less than 28 weeks, 5-minute Apgar score less than 7, antenatal corticosteroid treatment, and pregnancy-induced hypertension as an indication for delivery.
The investigators also adjusted the model for clinical covariates and nadir platelet count of less than 15,000/μL. In this model, platelet transfusion became nonsignificant, even for IVH of grade III or IV.
The investigators noted that the degree to which their results are generalizable to infants with more severe thrombocytopenia is unclear, since infants in this analysis often had transfusions at platelet levels between 50,000/μL and 150,000/μL. They also collected the data approximately 8 years ago, and transfusion practices may have changed since then.
The 6 NICU study sites included Boston Children’s Hospital, Boston, Massachusetts; University of Iowa Children’s Hospital, Iowa City, Iowa; and 4 NICUs affiliated with Intermountain Health Care in Utah.
The investigators published their findings in JAMA Pediatrics. ![]()

A retrospective, multicenter study of 972 very-low-birth-weight (VLBW) infants treated in 6 US neonatal intensive care units (NICUs) has shown that platelet transfusions do not significantly affect the incidence of intraventricular hemorrhage (IVH).
Thrombocytopenia is a risk factor for IVH, but investigators found no correlation between its severity and risk for IVH. Nor did they find platelet transfusions to have a significant effect on the incidence of IVH.
To describe platelet transfusion practices in US NICUs, senior author Martha Sola-Visner, MD, of Boston Children’s Hospital in Massachusetts, and colleagues studied NICU admissions from January 1, 2006, to December 31, 2007. They collected the last data on December 4, 2008.
Of the 972 VLBW infants, 231 (23.8%) received at least 1 platelet transfusion. And more males received transfusions (61%) than females.
Infants who received transfusions were more premature at 26.3 weeks’ gestation age compared with 28.8 weeks for those who did not receive transfusions, P<0.001.
Transfused infants were also smaller, with a mean birth weight of 805 g compared with 1113 g in the group that did not receive a transfusion, P<0.001.
Platelet transfusions
The 231 transfused infants received a total of 1002 platelet transfusions, with a mean of 4.3 per infant (range 1 to 63 transfusions).
Forty-one percent of infants had transfusions during the first 7 days of life only, amounting to 281 transfusions; 32.9% had transfusions after the first 7 days only, and 26.4% had transfusions during both periods. Seven hundred twenty-one transfusions were administered after day 7.
Almost two thirds of the transfusions, 65.4% or 653 of 998 transfusions, were given to infants who had a pre-transfusion platelet count of at least 50,000/μL.
The investigators poined out that this finding “was in contrast to UK NICUs,” where transfusions are administered at a median platelet count of 27,000/μL.
Illness severity
The investigators found significant differences among NICU sites in terms of clinical markers for transfusions.
Overall, 189 VLBW infants had platelet counts less than 100,000/μL in the first 7 days of life for a total of 402 days. And at least 1 platelet transfusion was given on 212 of those days. Of these, 198 transfusions (93.4%) had a marker of severe illness or bleeding.
On the other hand, of the 190 patient days without a transfusion, 113 (59.5%) had at least 1 of these markers (P<0.001).
Thrombocytopenia and IVH risk
The investigators evaluated the risk for IVH based on the lowest platelet count before the diagnosis of IVH was made.
They found that infants with thrombocytopenia were at higher risk for IVH, with a hazard ratio of 2.17 for any platelet count less than 150,000/μL (P<0.001).
Nevertheless, for the 314 infants with at least 1 platelet count less than 150,000/μL during the first 7 days of life, they found no association between severity of thrombocytopenia and the risk for subsequent IVH (P=0.70).
Transfusion and IVH risk
To determine whether platelet transfusions protected VLBW infants from IVH during their first 7 days of life, the investigators performed a Cox regression analysis in 756 infants.
They found that 134 infants (17.7%) had an IVH, including 62 (8.2%) with grade III or IV. So in the unadjusted model, they found a significant association between platelet transfusion and subsequent IVH, P=0.004.
However, when they adjusted the model for clinical covariates, only infants with grade III or IV IVH had a significantly greater risk with platelet transfusion, P=0.01.
Clinical covariates included sex, gestational age less than 28 weeks, 5-minute Apgar score less than 7, antenatal corticosteroid treatment, and pregnancy-induced hypertension as an indication for delivery.
The investigators also adjusted the model for clinical covariates and nadir platelet count of less than 15,000/μL. In this model, platelet transfusion became nonsignificant, even for IVH of grade III or IV.
The investigators noted that the degree to which their results are generalizable to infants with more severe thrombocytopenia is unclear, since infants in this analysis often had transfusions at platelet levels between 50,000/μL and 150,000/μL. They also collected the data approximately 8 years ago, and transfusion practices may have changed since then.
The 6 NICU study sites included Boston Children’s Hospital, Boston, Massachusetts; University of Iowa Children’s Hospital, Iowa City, Iowa; and 4 NICUs affiliated with Intermountain Health Care in Utah.
The investigators published their findings in JAMA Pediatrics. ![]()

A retrospective, multicenter study of 972 very-low-birth-weight (VLBW) infants treated in 6 US neonatal intensive care units (NICUs) has shown that platelet transfusions do not significantly affect the incidence of intraventricular hemorrhage (IVH).
Thrombocytopenia is a risk factor for IVH, but investigators found no correlation between its severity and risk for IVH. Nor did they find platelet transfusions to have a significant effect on the incidence of IVH.
To describe platelet transfusion practices in US NICUs, senior author Martha Sola-Visner, MD, of Boston Children’s Hospital in Massachusetts, and colleagues studied NICU admissions from January 1, 2006, to December 31, 2007. They collected the last data on December 4, 2008.
Of the 972 VLBW infants, 231 (23.8%) received at least 1 platelet transfusion. And more males received transfusions (61%) than females.
Infants who received transfusions were more premature at 26.3 weeks’ gestation age compared with 28.8 weeks for those who did not receive transfusions, P<0.001.
Transfused infants were also smaller, with a mean birth weight of 805 g compared with 1113 g in the group that did not receive a transfusion, P<0.001.
Platelet transfusions
The 231 transfused infants received a total of 1002 platelet transfusions, with a mean of 4.3 per infant (range 1 to 63 transfusions).
Forty-one percent of infants had transfusions during the first 7 days of life only, amounting to 281 transfusions; 32.9% had transfusions after the first 7 days only, and 26.4% had transfusions during both periods. Seven hundred twenty-one transfusions were administered after day 7.
Almost two thirds of the transfusions, 65.4% or 653 of 998 transfusions, were given to infants who had a pre-transfusion platelet count of at least 50,000/μL.
The investigators poined out that this finding “was in contrast to UK NICUs,” where transfusions are administered at a median platelet count of 27,000/μL.
Illness severity
The investigators found significant differences among NICU sites in terms of clinical markers for transfusions.
Overall, 189 VLBW infants had platelet counts less than 100,000/μL in the first 7 days of life for a total of 402 days. And at least 1 platelet transfusion was given on 212 of those days. Of these, 198 transfusions (93.4%) had a marker of severe illness or bleeding.
On the other hand, of the 190 patient days without a transfusion, 113 (59.5%) had at least 1 of these markers (P<0.001).
Thrombocytopenia and IVH risk
The investigators evaluated the risk for IVH based on the lowest platelet count before the diagnosis of IVH was made.
They found that infants with thrombocytopenia were at higher risk for IVH, with a hazard ratio of 2.17 for any platelet count less than 150,000/μL (P<0.001).
Nevertheless, for the 314 infants with at least 1 platelet count less than 150,000/μL during the first 7 days of life, they found no association between severity of thrombocytopenia and the risk for subsequent IVH (P=0.70).
Transfusion and IVH risk
To determine whether platelet transfusions protected VLBW infants from IVH during their first 7 days of life, the investigators performed a Cox regression analysis in 756 infants.
They found that 134 infants (17.7%) had an IVH, including 62 (8.2%) with grade III or IV. So in the unadjusted model, they found a significant association between platelet transfusion and subsequent IVH, P=0.004.
However, when they adjusted the model for clinical covariates, only infants with grade III or IV IVH had a significantly greater risk with platelet transfusion, P=0.01.
Clinical covariates included sex, gestational age less than 28 weeks, 5-minute Apgar score less than 7, antenatal corticosteroid treatment, and pregnancy-induced hypertension as an indication for delivery.
The investigators also adjusted the model for clinical covariates and nadir platelet count of less than 15,000/μL. In this model, platelet transfusion became nonsignificant, even for IVH of grade III or IV.
The investigators noted that the degree to which their results are generalizable to infants with more severe thrombocytopenia is unclear, since infants in this analysis often had transfusions at platelet levels between 50,000/μL and 150,000/μL. They also collected the data approximately 8 years ago, and transfusion practices may have changed since then.
The 6 NICU study sites included Boston Children’s Hospital, Boston, Massachusetts; University of Iowa Children’s Hospital, Iowa City, Iowa; and 4 NICUs affiliated with Intermountain Health Care in Utah.
The investigators published their findings in JAMA Pediatrics. ![]()
Mortality Risk and Patient Experience
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
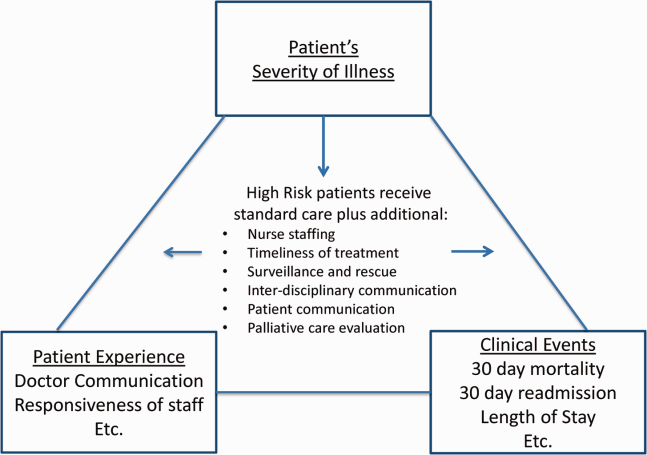
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.
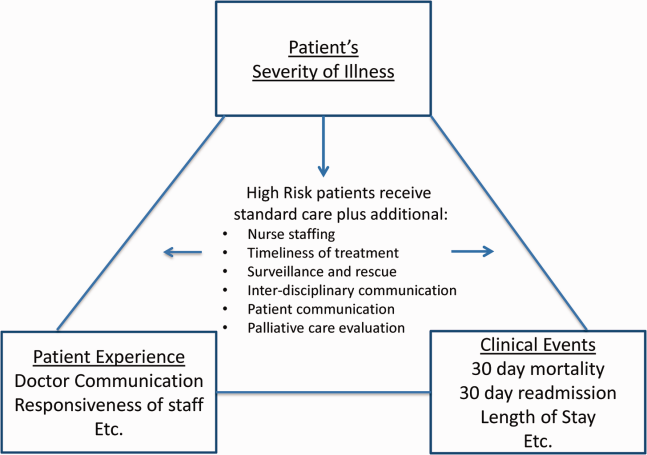
METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | 0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | 0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | 0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | 0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | 0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | 0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | 0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | 0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | 0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | 0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | 0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | 0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | 0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | 0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | 0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | 0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | 0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | 0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | 0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | 0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | 0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | 0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | 0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | 0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | 0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | 0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | 0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | 0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P 0.0001) than their low‐risk counterparts.
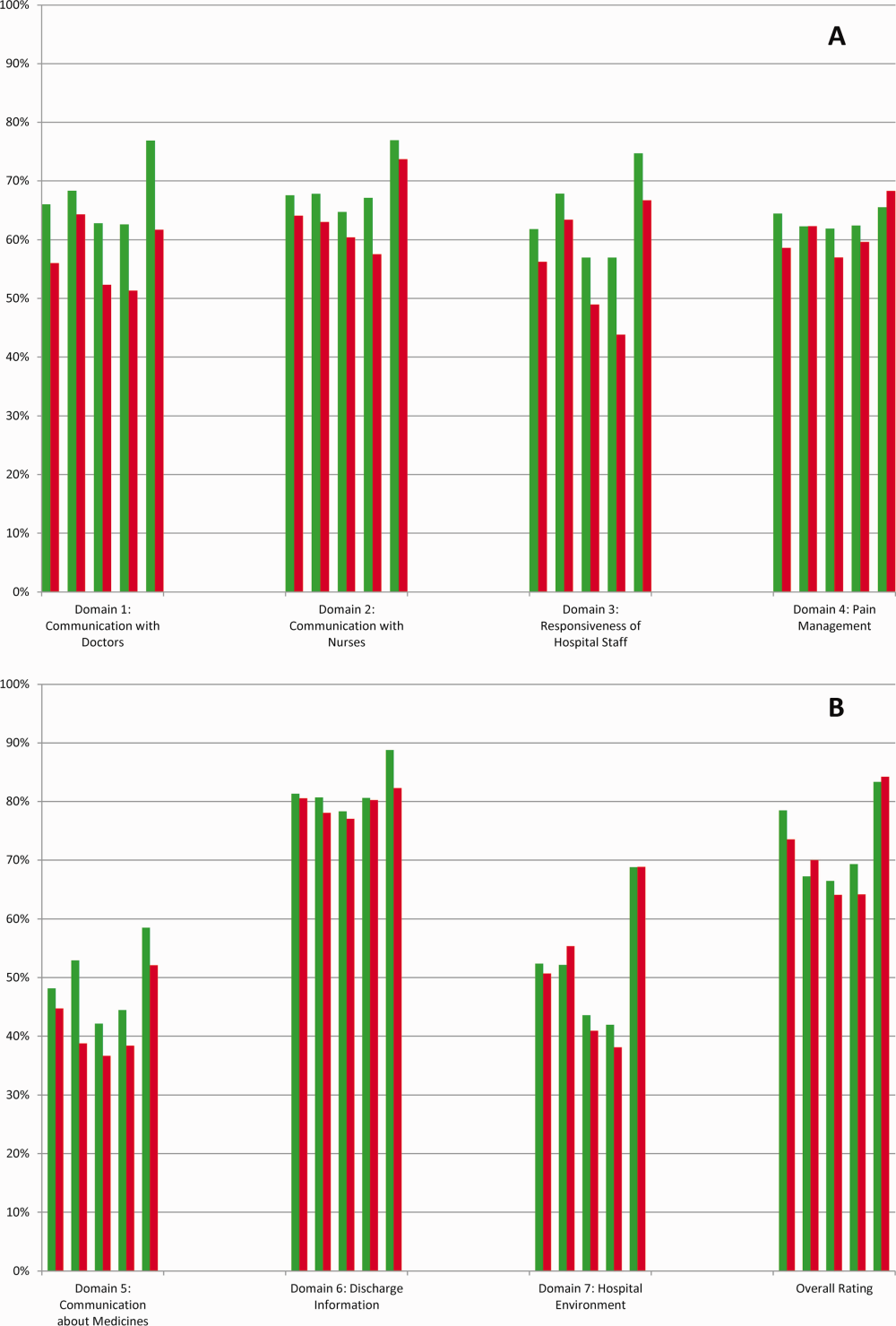
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).
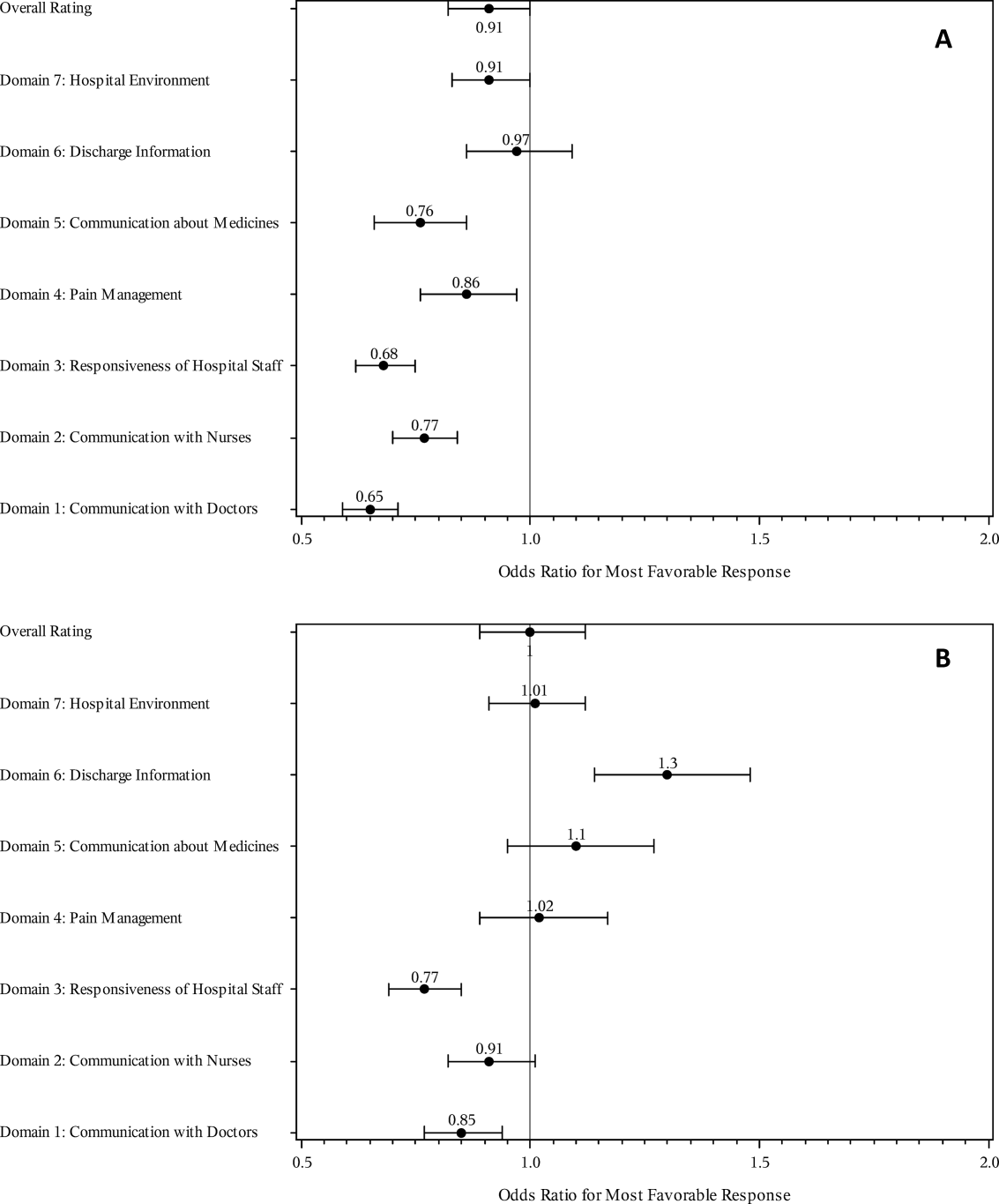
The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.

METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | 0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | 0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | 0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | 0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | 0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | 0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | 0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | 0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | 0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | 0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | 0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | 0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | 0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | 0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | 0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | 0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | 0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | 0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | 0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | 0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | 0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | 0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | 0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | 0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | 0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | 0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | 0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | 0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P 0.0001) than their low‐risk counterparts.
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).


The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.

METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | 0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | 0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | 0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | 0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | 0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | 0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | 0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | 0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | 0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | 0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | 0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | 0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | 0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | 0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | 0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | 0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | 0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | 0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | 0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | 0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | 0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | 0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | 0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | 0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | 0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | 0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | 0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | 0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P 0.0001) than their low‐risk counterparts.
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).


The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
It's a matter of respect
Serious illnesses challenge patients, their families, clinicians, and the health systems that care for them. In this issue of the Journal of Hospital Medicine, Cowen and coauthors shed light on the experience of inpatients on medical and surgical services with a high risk of mortality on admission, as measured by Hospital Consumer Assessment of Healthcare Providers and Systems Surveys (HCAHPS).[1] In their study population, even after adjustment for some confounders, these patients tended to rate responsiveness of hospital staff and communication by doctors lower than patients with a low risk of mortality on admission.
A more generalizable frame than admission risk of mortality is to consider the patients they identified as high risk to be patients with serious illness. Using this frame will be helpful in understanding the implications of their results, but it is important to acknowledge that for several reasons, the data in this study may not represent the entire population of seriously ill patients. First, there may be patients at lower risk of mortality who would qualify as having a serious illness. Second, the study's data were from only a few hospitals in 1 healthcare system. Third, 93% of patients at high risk of mortality on admission did not return surveys. Despite these significant limitations, there are still important insights to be gleaned from their work.
Before exploring what they found, it is also important to note that it can be challenging to know what to make of HCAHPS scores. For instance, patients with higher HCAHPS scores have been found to have higher costs of care and higher mortality.[2] Satisfied patients are not clearly better off. However, what if, for purposes of learning, the scores serve as a window into the seriously ill patient's experience, helping inform an understanding of the challenges and opportunities for improvement?
One of the key findings of this study was that seriously ill patients rated responsiveness by hospital staff worse than those who were not as ill. Patients were asked 2 questions as part of the composite measure: During this hospital stay, after you pressed the call button, how often did you get help as soon as you wanted it? How often did you get help in getting to the bathroom or in using a bedpan as soon as you wanted?
It is not difficult to imagine how seriously ill patients might have more intense care needs that would result in more requests for help, nor is it difficult to imagine how some proportion of those requests might not be handled in a timely fashion. Objective research shows higher rates of call button requests have been associated with slower response times, and it appears there is a complex relationship with staffing levels and the intensity of work on the floor.[3] Certainly there may be times that patients want a quick response after pressing a call button, but do not need one, and a lot of time could be spent discussing these quandaries. However, there are also times when a patient describes having called for help, really needing it, yet no one came. At least some of the time, responsiveness is a matter of respect, especially considering the vulnerability of seriously ill patients and the issue of dignity around toileting.
Another key finding was about communication by doctors, and the questions patients answered were: During this hospital stay, how often did doctors treat you with courtesy and respect? During this hospital stay, how often did doctors listen carefully to you? During this hospital stay, how often did doctors explain things in a way you could understand?
There is a growing and important body of literature about communication with seriously ill patients.[4] Consider some of the data about patients with advanced cancer. Evidence suggests the majority of such patients want to know their prognosis, and that when it is discussed it does not worsen the patient‐physician relationship, sadness, or anxiety.[5] Despite this, among physicians who have formulated a prognosis for patients with advanced cancer, even if they were asked directly by those patients about their prognosis, 23% of the time they would communicate no prognosis. Forty percent of the time they would communicate a different prognosis than what they had formulated, with 70% of those being optimistically discrepant.[6] Although data are more limited, there is evidence that hospitalists are similarly wary to acknowledge when patients are at risk of dying.[7]
Although certainly other aspects of communication by doctors with seriously ill patients contributed to this study's findings, this issue of acknowledging and discussing the serious illness itself is important to highlight. Healthcare professionals have an ethical obligation to respect patients' autonomy by helping them make informed decisions about their care. Having these conversations can be challenging, but training programs and conversation guides are showing promise.[8] If health professionals do not try to ensure that seriously ill patients understand their diagnosis, prognosis, and full range of treatment options in patient‐centered ways, then by definition patients cannot be making informed decisions. It is a matter of respect.
This study's most important contribution is how it focuses attention on the domains of responsiveness by hospital staff and communication by doctors, encouraging a deeper dive to consider what else is known about these topics. Allowing that the lower scores from seriously ill patients might reflect more than just poor satisfaction reveals that at least some proportion of the time, these patients are experiencing disrespect. The work then becomes clear: What are the ways in which health professionals should reliably be demonstrating respect toward patients, especially those who are seriously ill? It is there, in the process of developing a reliable practice of respect, that consensus about how to improve the patient experience is most likely to be found.
Disclosure
Nothing to report.
- , , , , , . The risk‐outcome‐experience triad: mortality risk and the Hospital Consumer Assessment of Healthcare Providers and Systems Survey. J Hosp Med. 2016;11(9):628–635.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , . Exploring the relationship between patient call‐light use rate and nurse call‐light response time in acute care settings. Comput Inform Nurs. 2011;29(3):138–143.
- , ; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , , . Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809–3816.
- , . Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134(12):1096–1105.
- , , . Dancing around death: hospitalist‐patient communication about serious illness. Qual Health Res. 2013;23(1):3–13.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
Serious illnesses challenge patients, their families, clinicians, and the health systems that care for them. In this issue of the Journal of Hospital Medicine, Cowen and coauthors shed light on the experience of inpatients on medical and surgical services with a high risk of mortality on admission, as measured by Hospital Consumer Assessment of Healthcare Providers and Systems Surveys (HCAHPS).[1] In their study population, even after adjustment for some confounders, these patients tended to rate responsiveness of hospital staff and communication by doctors lower than patients with a low risk of mortality on admission.
A more generalizable frame than admission risk of mortality is to consider the patients they identified as high risk to be patients with serious illness. Using this frame will be helpful in understanding the implications of their results, but it is important to acknowledge that for several reasons, the data in this study may not represent the entire population of seriously ill patients. First, there may be patients at lower risk of mortality who would qualify as having a serious illness. Second, the study's data were from only a few hospitals in 1 healthcare system. Third, 93% of patients at high risk of mortality on admission did not return surveys. Despite these significant limitations, there are still important insights to be gleaned from their work.
Before exploring what they found, it is also important to note that it can be challenging to know what to make of HCAHPS scores. For instance, patients with higher HCAHPS scores have been found to have higher costs of care and higher mortality.[2] Satisfied patients are not clearly better off. However, what if, for purposes of learning, the scores serve as a window into the seriously ill patient's experience, helping inform an understanding of the challenges and opportunities for improvement?
One of the key findings of this study was that seriously ill patients rated responsiveness by hospital staff worse than those who were not as ill. Patients were asked 2 questions as part of the composite measure: During this hospital stay, after you pressed the call button, how often did you get help as soon as you wanted it? How often did you get help in getting to the bathroom or in using a bedpan as soon as you wanted?
It is not difficult to imagine how seriously ill patients might have more intense care needs that would result in more requests for help, nor is it difficult to imagine how some proportion of those requests might not be handled in a timely fashion. Objective research shows higher rates of call button requests have been associated with slower response times, and it appears there is a complex relationship with staffing levels and the intensity of work on the floor.[3] Certainly there may be times that patients want a quick response after pressing a call button, but do not need one, and a lot of time could be spent discussing these quandaries. However, there are also times when a patient describes having called for help, really needing it, yet no one came. At least some of the time, responsiveness is a matter of respect, especially considering the vulnerability of seriously ill patients and the issue of dignity around toileting.
Another key finding was about communication by doctors, and the questions patients answered were: During this hospital stay, how often did doctors treat you with courtesy and respect? During this hospital stay, how often did doctors listen carefully to you? During this hospital stay, how often did doctors explain things in a way you could understand?
There is a growing and important body of literature about communication with seriously ill patients.[4] Consider some of the data about patients with advanced cancer. Evidence suggests the majority of such patients want to know their prognosis, and that when it is discussed it does not worsen the patient‐physician relationship, sadness, or anxiety.[5] Despite this, among physicians who have formulated a prognosis for patients with advanced cancer, even if they were asked directly by those patients about their prognosis, 23% of the time they would communicate no prognosis. Forty percent of the time they would communicate a different prognosis than what they had formulated, with 70% of those being optimistically discrepant.[6] Although data are more limited, there is evidence that hospitalists are similarly wary to acknowledge when patients are at risk of dying.[7]
Although certainly other aspects of communication by doctors with seriously ill patients contributed to this study's findings, this issue of acknowledging and discussing the serious illness itself is important to highlight. Healthcare professionals have an ethical obligation to respect patients' autonomy by helping them make informed decisions about their care. Having these conversations can be challenging, but training programs and conversation guides are showing promise.[8] If health professionals do not try to ensure that seriously ill patients understand their diagnosis, prognosis, and full range of treatment options in patient‐centered ways, then by definition patients cannot be making informed decisions. It is a matter of respect.
This study's most important contribution is how it focuses attention on the domains of responsiveness by hospital staff and communication by doctors, encouraging a deeper dive to consider what else is known about these topics. Allowing that the lower scores from seriously ill patients might reflect more than just poor satisfaction reveals that at least some proportion of the time, these patients are experiencing disrespect. The work then becomes clear: What are the ways in which health professionals should reliably be demonstrating respect toward patients, especially those who are seriously ill? It is there, in the process of developing a reliable practice of respect, that consensus about how to improve the patient experience is most likely to be found.
Disclosure
Nothing to report.
Serious illnesses challenge patients, their families, clinicians, and the health systems that care for them. In this issue of the Journal of Hospital Medicine, Cowen and coauthors shed light on the experience of inpatients on medical and surgical services with a high risk of mortality on admission, as measured by Hospital Consumer Assessment of Healthcare Providers and Systems Surveys (HCAHPS).[1] In their study population, even after adjustment for some confounders, these patients tended to rate responsiveness of hospital staff and communication by doctors lower than patients with a low risk of mortality on admission.
A more generalizable frame than admission risk of mortality is to consider the patients they identified as high risk to be patients with serious illness. Using this frame will be helpful in understanding the implications of their results, but it is important to acknowledge that for several reasons, the data in this study may not represent the entire population of seriously ill patients. First, there may be patients at lower risk of mortality who would qualify as having a serious illness. Second, the study's data were from only a few hospitals in 1 healthcare system. Third, 93% of patients at high risk of mortality on admission did not return surveys. Despite these significant limitations, there are still important insights to be gleaned from their work.
Before exploring what they found, it is also important to note that it can be challenging to know what to make of HCAHPS scores. For instance, patients with higher HCAHPS scores have been found to have higher costs of care and higher mortality.[2] Satisfied patients are not clearly better off. However, what if, for purposes of learning, the scores serve as a window into the seriously ill patient's experience, helping inform an understanding of the challenges and opportunities for improvement?
One of the key findings of this study was that seriously ill patients rated responsiveness by hospital staff worse than those who were not as ill. Patients were asked 2 questions as part of the composite measure: During this hospital stay, after you pressed the call button, how often did you get help as soon as you wanted it? How often did you get help in getting to the bathroom or in using a bedpan as soon as you wanted?
It is not difficult to imagine how seriously ill patients might have more intense care needs that would result in more requests for help, nor is it difficult to imagine how some proportion of those requests might not be handled in a timely fashion. Objective research shows higher rates of call button requests have been associated with slower response times, and it appears there is a complex relationship with staffing levels and the intensity of work on the floor.[3] Certainly there may be times that patients want a quick response after pressing a call button, but do not need one, and a lot of time could be spent discussing these quandaries. However, there are also times when a patient describes having called for help, really needing it, yet no one came. At least some of the time, responsiveness is a matter of respect, especially considering the vulnerability of seriously ill patients and the issue of dignity around toileting.
Another key finding was about communication by doctors, and the questions patients answered were: During this hospital stay, how often did doctors treat you with courtesy and respect? During this hospital stay, how often did doctors listen carefully to you? During this hospital stay, how often did doctors explain things in a way you could understand?
There is a growing and important body of literature about communication with seriously ill patients.[4] Consider some of the data about patients with advanced cancer. Evidence suggests the majority of such patients want to know their prognosis, and that when it is discussed it does not worsen the patient‐physician relationship, sadness, or anxiety.[5] Despite this, among physicians who have formulated a prognosis for patients with advanced cancer, even if they were asked directly by those patients about their prognosis, 23% of the time they would communicate no prognosis. Forty percent of the time they would communicate a different prognosis than what they had formulated, with 70% of those being optimistically discrepant.[6] Although data are more limited, there is evidence that hospitalists are similarly wary to acknowledge when patients are at risk of dying.[7]
Although certainly other aspects of communication by doctors with seriously ill patients contributed to this study's findings, this issue of acknowledging and discussing the serious illness itself is important to highlight. Healthcare professionals have an ethical obligation to respect patients' autonomy by helping them make informed decisions about their care. Having these conversations can be challenging, but training programs and conversation guides are showing promise.[8] If health professionals do not try to ensure that seriously ill patients understand their diagnosis, prognosis, and full range of treatment options in patient‐centered ways, then by definition patients cannot be making informed decisions. It is a matter of respect.
This study's most important contribution is how it focuses attention on the domains of responsiveness by hospital staff and communication by doctors, encouraging a deeper dive to consider what else is known about these topics. Allowing that the lower scores from seriously ill patients might reflect more than just poor satisfaction reveals that at least some proportion of the time, these patients are experiencing disrespect. The work then becomes clear: What are the ways in which health professionals should reliably be demonstrating respect toward patients, especially those who are seriously ill? It is there, in the process of developing a reliable practice of respect, that consensus about how to improve the patient experience is most likely to be found.
Disclosure
Nothing to report.
- , , , , , . The risk‐outcome‐experience triad: mortality risk and the Hospital Consumer Assessment of Healthcare Providers and Systems Survey. J Hosp Med. 2016;11(9):628–635.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , . Exploring the relationship between patient call‐light use rate and nurse call‐light response time in acute care settings. Comput Inform Nurs. 2011;29(3):138–143.
- , ; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , , . Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809–3816.
- , . Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134(12):1096–1105.
- , , . Dancing around death: hospitalist‐patient communication about serious illness. Qual Health Res. 2013;23(1):3–13.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , , , . The risk‐outcome‐experience triad: mortality risk and the Hospital Consumer Assessment of Healthcare Providers and Systems Survey. J Hosp Med. 2016;11(9):628–635.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , . Exploring the relationship between patient call‐light use rate and nurse call‐light response time in acute care settings. Comput Inform Nurs. 2011;29(3):138–143.
- , ; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , , . Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809–3816.
- , . Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134(12):1096–1105.
- , , . Dancing around death: hospitalist‐patient communication about serious illness. Qual Health Res. 2013;23(1):3–13.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
Shellfish Allergies and CT Scans
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
© 2015 Society of Hospital Medicine
Early results positive for treating high-grade gliomas with virus-based therapy
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A phase I trial indicates that Toca 511 is safe and shows activity in treating patients with high-grade or recurrent glioblastoma.
Major finding: Median overall survival was 13.6 months (95% CI, 10.8-20.0) for patients with high-grade glioblastoma (n = 43), almost twice as long as that of similar patients from a separate trial treated with standard therapy (hazard ratio, 0.45; P = .003).
Data source: A phase I trial of 45 patients with glioblastoma, compared with an external control group.
Disclosures: This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.