User login
Severe Insomnia Linked to Epilepsy and Poor Quality of Life
Among 207 patients with epilepsy, 43% reported clinically significant insomnia, while 51% had at least mild insomnia according to investigators from the University of Virginia. Their results, derived from an Insomnia Severity Index survey, also found that younger patients, those with a shorter duration of epilepsy, and patients taking sedatives or hypnotics, reported more severe insomnia. Similarly, patients with delayed sleep timing and depression were more likely to experience more severe insomnia. However, even after researchers factored out these covariates, they found that more severe insomnia was significantly associated with seizures and poorer quality of life.
Quigg M, Gharai S, Ruland J, et al. Insomnia in epilepsy is associated with continuing seizures and worse quality of life. Epilepsy Res. 2016;122:91-96.
Among 207 patients with epilepsy, 43% reported clinically significant insomnia, while 51% had at least mild insomnia according to investigators from the University of Virginia. Their results, derived from an Insomnia Severity Index survey, also found that younger patients, those with a shorter duration of epilepsy, and patients taking sedatives or hypnotics, reported more severe insomnia. Similarly, patients with delayed sleep timing and depression were more likely to experience more severe insomnia. However, even after researchers factored out these covariates, they found that more severe insomnia was significantly associated with seizures and poorer quality of life.
Quigg M, Gharai S, Ruland J, et al. Insomnia in epilepsy is associated with continuing seizures and worse quality of life. Epilepsy Res. 2016;122:91-96.
Among 207 patients with epilepsy, 43% reported clinically significant insomnia, while 51% had at least mild insomnia according to investigators from the University of Virginia. Their results, derived from an Insomnia Severity Index survey, also found that younger patients, those with a shorter duration of epilepsy, and patients taking sedatives or hypnotics, reported more severe insomnia. Similarly, patients with delayed sleep timing and depression were more likely to experience more severe insomnia. However, even after researchers factored out these covariates, they found that more severe insomnia was significantly associated with seizures and poorer quality of life.
Quigg M, Gharai S, Ruland J, et al. Insomnia in epilepsy is associated with continuing seizures and worse quality of life. Epilepsy Res. 2016;122:91-96.
Point/Counterpoint: Do pharmacist-prescribing laws provide adequate access to contraception?
YES: Immediate and safe patient access
Pharmacist-prescribed contraception laws are an opportunity to safely and easily improve access to contraception for women. While over-the-counter access remains an important goal, there are many practical considerations that must be addressed prior to implementation.
For example, in order to change the status of a medication to OTC, each product’s manufacturer needs to apply to the Food and Drug Administration for the change. This application includes studies that demonstrate their product can be safely used by the general public, without guidance from a health professional. The American College of Obstetricians and Gynecologists has already weighed in on the overall safety of oral contraceptives, and some studies have been done that show women can accurately complete a health questionnaire related to their OC eligibility. While these are good steps toward demonstrating safe use, they do not satisfy FDA requirements for OTC status. These initial studies never went to the next step of having women interpret the questions in an “actual use study,” an FDA requirement.
On the other hand, there have been studies demonstrating that pharmacists can safely provide hormonal contraception (J Am Pharm Assoc. 2008 Mar-Apr;48[2]:212-21). State laws can’t overrule the FDA and make hormonal contraception OTC, but they can change the scope of what pharmacists are permitted to do. In Oregon and California, specific laws allowing pharmacists to prescribe hormonal contraception have already been implemented. Speaking from our experience in Oregon, this change has solved many concerns that have been raised about hormonal contraception going OTC.
Maintaining insurance coverage
Since pharmacists are able to write prescriptions, they can bill insurance plans for the product in the same way as for any other prescription. The majority of OTC medications are not covered by insurance and if a future law required insurance to cover contraception obtained OTC, pharmacists would still be involved in billing.
Patients will continue to get counseling
Pharmacists need to undergo training/certification in order to prescribe hormonal contraception. In Oregon, the certification was developed by pharmacists and physicians with input from the Oregon Health Authority and the Oregon Board of Pharmacy. Having a trained health care professional involved in counseling means that accurate and important information is still conveyed, including ensuring women are aware that hormonal contraception is not going to protect against sexually transmitted infections, and teaching women about the importance of adherence, but also how to deal with missed pills and problems. The importance of accessing other preventive health services can also be stressed.
Patients will be appropriately screened for their eligibility
Women who are unable to safely use hormonal contraceptives will be referred for more appropriate methods.
We have the opportunity to act now to prevent unintended pregnancy through pharmacist-prescribed hormonal contraceptives. In addition, allowing pharmacists to directly prescribe hormonal contraceptives provides the opportunity to evaluate the safety, efficacy, and acceptability of the practice. This evidence can then be used to look at the feasibility and possible mechanisms that could be undertaken to safely move hormonal contraceptives to OTC, which helps the OTC movement rather than distracting from it.
Dr. Anderson is an instructor at the Oregon State University College of Pharmacy in Corvallis. Dr. Rodriguez is an assistant professor of ob.gyn. at Oregon Health & Science University in Portland. Dr. Edelman is a professor of ob.gyn. and director of the Oregon Family Planning Fellowship at OHSU. They reported having no relevant financial disclosures.
NO: Exchanging one barrier for another
Birth control is an essential part of women’s health care and the value of contraception has been proven time and again. Not only do oral contraceptives provide women with the ability to choose if, and when, they want to become pregnant, they allow women to have more control over their lives by timing pregnancy around education, careers, and other life goals. Nearly 90% of women in the United States between the ages of 15 and 44 will use a highly effective, reversible method of contraception, such as oral contraceptives, injected contraceptives, cervical rings, contraceptive implants, or intrauterine devices.
Ob.gyns. know from evidence and experience that oral contraceptives are safe enough for OTC access. In fact, oral contraceptives are safer than many other medications that are already available OTC, such as acetaminophen. Of note, thromboembolism, the most common serious risk associated with oral contraceptives, is not only exceedingly low but is also much lower than the risk for thromboembolism during pregnancy and the postpartum period. After all, no diagnosis is required to prescribe oral contraceptives. Of course, ob.gyns. and other women’s health care providers do screen for easily recognizable risk factors, such as smoking. However, research shows that women are very adept at self-screening for any potential risks.
For these reasons, ACOG has long been a public supporter of OTC access to oral contraceptives, as it already is in many countries around the world. ACOG supports efforts to increase affordable, reliable access for American women to the contraceptives they need, when they need them.
However, the pharmacist-prescribing laws do not go far enough in expanding access to contraception. A pharmacist’s prescription simply exchanges one barrier – a physician’s prescription – for another.
Women still need to arrange a consultation with a pharmacist during pharmacy hours and any potential cost associated with that consultation would add out-of-pocket expenses. This will not allow us to reach women who remain underserved by the current prescribing requirements. For example, this may prevent women who are uninsured or underinsured from accessing contraception. For some, the need to visit a health care provider prevents access to contraception.
ACOG continues to stand by full, no-copay coverage of all FDA-approved methods of birth control, under the Affordable Care Act, as we recognize that OTC access to birth control is not a blanket solution. We also continue to strongly advocate for access to long-acting reversible contraceptive methods, the most effective method of preventing pregnancy.
Pharmacists and ob.gyns. alike share a commitment to quality patient care. However, if our shared goal is increasing access to safe, effective contraception, women should be trusted to control their reproductive lives and should be able to do so without barriers.
Dr. DeFrancesco is an ob.gyn. in Cheshire, Conn., and the president of the American College of Obstetricians and Gynecologists. He reported having no relevant financial disclosures.
YES: Immediate and safe patient access
Pharmacist-prescribed contraception laws are an opportunity to safely and easily improve access to contraception for women. While over-the-counter access remains an important goal, there are many practical considerations that must be addressed prior to implementation.
For example, in order to change the status of a medication to OTC, each product’s manufacturer needs to apply to the Food and Drug Administration for the change. This application includes studies that demonstrate their product can be safely used by the general public, without guidance from a health professional. The American College of Obstetricians and Gynecologists has already weighed in on the overall safety of oral contraceptives, and some studies have been done that show women can accurately complete a health questionnaire related to their OC eligibility. While these are good steps toward demonstrating safe use, they do not satisfy FDA requirements for OTC status. These initial studies never went to the next step of having women interpret the questions in an “actual use study,” an FDA requirement.
On the other hand, there have been studies demonstrating that pharmacists can safely provide hormonal contraception (J Am Pharm Assoc. 2008 Mar-Apr;48[2]:212-21). State laws can’t overrule the FDA and make hormonal contraception OTC, but they can change the scope of what pharmacists are permitted to do. In Oregon and California, specific laws allowing pharmacists to prescribe hormonal contraception have already been implemented. Speaking from our experience in Oregon, this change has solved many concerns that have been raised about hormonal contraception going OTC.
Maintaining insurance coverage
Since pharmacists are able to write prescriptions, they can bill insurance plans for the product in the same way as for any other prescription. The majority of OTC medications are not covered by insurance and if a future law required insurance to cover contraception obtained OTC, pharmacists would still be involved in billing.
Patients will continue to get counseling
Pharmacists need to undergo training/certification in order to prescribe hormonal contraception. In Oregon, the certification was developed by pharmacists and physicians with input from the Oregon Health Authority and the Oregon Board of Pharmacy. Having a trained health care professional involved in counseling means that accurate and important information is still conveyed, including ensuring women are aware that hormonal contraception is not going to protect against sexually transmitted infections, and teaching women about the importance of adherence, but also how to deal with missed pills and problems. The importance of accessing other preventive health services can also be stressed.
Patients will be appropriately screened for their eligibility
Women who are unable to safely use hormonal contraceptives will be referred for more appropriate methods.
We have the opportunity to act now to prevent unintended pregnancy through pharmacist-prescribed hormonal contraceptives. In addition, allowing pharmacists to directly prescribe hormonal contraceptives provides the opportunity to evaluate the safety, efficacy, and acceptability of the practice. This evidence can then be used to look at the feasibility and possible mechanisms that could be undertaken to safely move hormonal contraceptives to OTC, which helps the OTC movement rather than distracting from it.
Dr. Anderson is an instructor at the Oregon State University College of Pharmacy in Corvallis. Dr. Rodriguez is an assistant professor of ob.gyn. at Oregon Health & Science University in Portland. Dr. Edelman is a professor of ob.gyn. and director of the Oregon Family Planning Fellowship at OHSU. They reported having no relevant financial disclosures.
NO: Exchanging one barrier for another
Birth control is an essential part of women’s health care and the value of contraception has been proven time and again. Not only do oral contraceptives provide women with the ability to choose if, and when, they want to become pregnant, they allow women to have more control over their lives by timing pregnancy around education, careers, and other life goals. Nearly 90% of women in the United States between the ages of 15 and 44 will use a highly effective, reversible method of contraception, such as oral contraceptives, injected contraceptives, cervical rings, contraceptive implants, or intrauterine devices.
Ob.gyns. know from evidence and experience that oral contraceptives are safe enough for OTC access. In fact, oral contraceptives are safer than many other medications that are already available OTC, such as acetaminophen. Of note, thromboembolism, the most common serious risk associated with oral contraceptives, is not only exceedingly low but is also much lower than the risk for thromboembolism during pregnancy and the postpartum period. After all, no diagnosis is required to prescribe oral contraceptives. Of course, ob.gyns. and other women’s health care providers do screen for easily recognizable risk factors, such as smoking. However, research shows that women are very adept at self-screening for any potential risks.
For these reasons, ACOG has long been a public supporter of OTC access to oral contraceptives, as it already is in many countries around the world. ACOG supports efforts to increase affordable, reliable access for American women to the contraceptives they need, when they need them.
However, the pharmacist-prescribing laws do not go far enough in expanding access to contraception. A pharmacist’s prescription simply exchanges one barrier – a physician’s prescription – for another.
Women still need to arrange a consultation with a pharmacist during pharmacy hours and any potential cost associated with that consultation would add out-of-pocket expenses. This will not allow us to reach women who remain underserved by the current prescribing requirements. For example, this may prevent women who are uninsured or underinsured from accessing contraception. For some, the need to visit a health care provider prevents access to contraception.
ACOG continues to stand by full, no-copay coverage of all FDA-approved methods of birth control, under the Affordable Care Act, as we recognize that OTC access to birth control is not a blanket solution. We also continue to strongly advocate for access to long-acting reversible contraceptive methods, the most effective method of preventing pregnancy.
Pharmacists and ob.gyns. alike share a commitment to quality patient care. However, if our shared goal is increasing access to safe, effective contraception, women should be trusted to control their reproductive lives and should be able to do so without barriers.
Dr. DeFrancesco is an ob.gyn. in Cheshire, Conn., and the president of the American College of Obstetricians and Gynecologists. He reported having no relevant financial disclosures.
YES: Immediate and safe patient access
Pharmacist-prescribed contraception laws are an opportunity to safely and easily improve access to contraception for women. While over-the-counter access remains an important goal, there are many practical considerations that must be addressed prior to implementation.
For example, in order to change the status of a medication to OTC, each product’s manufacturer needs to apply to the Food and Drug Administration for the change. This application includes studies that demonstrate their product can be safely used by the general public, without guidance from a health professional. The American College of Obstetricians and Gynecologists has already weighed in on the overall safety of oral contraceptives, and some studies have been done that show women can accurately complete a health questionnaire related to their OC eligibility. While these are good steps toward demonstrating safe use, they do not satisfy FDA requirements for OTC status. These initial studies never went to the next step of having women interpret the questions in an “actual use study,” an FDA requirement.
On the other hand, there have been studies demonstrating that pharmacists can safely provide hormonal contraception (J Am Pharm Assoc. 2008 Mar-Apr;48[2]:212-21). State laws can’t overrule the FDA and make hormonal contraception OTC, but they can change the scope of what pharmacists are permitted to do. In Oregon and California, specific laws allowing pharmacists to prescribe hormonal contraception have already been implemented. Speaking from our experience in Oregon, this change has solved many concerns that have been raised about hormonal contraception going OTC.
Maintaining insurance coverage
Since pharmacists are able to write prescriptions, they can bill insurance plans for the product in the same way as for any other prescription. The majority of OTC medications are not covered by insurance and if a future law required insurance to cover contraception obtained OTC, pharmacists would still be involved in billing.
Patients will continue to get counseling
Pharmacists need to undergo training/certification in order to prescribe hormonal contraception. In Oregon, the certification was developed by pharmacists and physicians with input from the Oregon Health Authority and the Oregon Board of Pharmacy. Having a trained health care professional involved in counseling means that accurate and important information is still conveyed, including ensuring women are aware that hormonal contraception is not going to protect against sexually transmitted infections, and teaching women about the importance of adherence, but also how to deal with missed pills and problems. The importance of accessing other preventive health services can also be stressed.
Patients will be appropriately screened for their eligibility
Women who are unable to safely use hormonal contraceptives will be referred for more appropriate methods.
We have the opportunity to act now to prevent unintended pregnancy through pharmacist-prescribed hormonal contraceptives. In addition, allowing pharmacists to directly prescribe hormonal contraceptives provides the opportunity to evaluate the safety, efficacy, and acceptability of the practice. This evidence can then be used to look at the feasibility and possible mechanisms that could be undertaken to safely move hormonal contraceptives to OTC, which helps the OTC movement rather than distracting from it.
Dr. Anderson is an instructor at the Oregon State University College of Pharmacy in Corvallis. Dr. Rodriguez is an assistant professor of ob.gyn. at Oregon Health & Science University in Portland. Dr. Edelman is a professor of ob.gyn. and director of the Oregon Family Planning Fellowship at OHSU. They reported having no relevant financial disclosures.
NO: Exchanging one barrier for another
Birth control is an essential part of women’s health care and the value of contraception has been proven time and again. Not only do oral contraceptives provide women with the ability to choose if, and when, they want to become pregnant, they allow women to have more control over their lives by timing pregnancy around education, careers, and other life goals. Nearly 90% of women in the United States between the ages of 15 and 44 will use a highly effective, reversible method of contraception, such as oral contraceptives, injected contraceptives, cervical rings, contraceptive implants, or intrauterine devices.
Ob.gyns. know from evidence and experience that oral contraceptives are safe enough for OTC access. In fact, oral contraceptives are safer than many other medications that are already available OTC, such as acetaminophen. Of note, thromboembolism, the most common serious risk associated with oral contraceptives, is not only exceedingly low but is also much lower than the risk for thromboembolism during pregnancy and the postpartum period. After all, no diagnosis is required to prescribe oral contraceptives. Of course, ob.gyns. and other women’s health care providers do screen for easily recognizable risk factors, such as smoking. However, research shows that women are very adept at self-screening for any potential risks.
For these reasons, ACOG has long been a public supporter of OTC access to oral contraceptives, as it already is in many countries around the world. ACOG supports efforts to increase affordable, reliable access for American women to the contraceptives they need, when they need them.
However, the pharmacist-prescribing laws do not go far enough in expanding access to contraception. A pharmacist’s prescription simply exchanges one barrier – a physician’s prescription – for another.
Women still need to arrange a consultation with a pharmacist during pharmacy hours and any potential cost associated with that consultation would add out-of-pocket expenses. This will not allow us to reach women who remain underserved by the current prescribing requirements. For example, this may prevent women who are uninsured or underinsured from accessing contraception. For some, the need to visit a health care provider prevents access to contraception.
ACOG continues to stand by full, no-copay coverage of all FDA-approved methods of birth control, under the Affordable Care Act, as we recognize that OTC access to birth control is not a blanket solution. We also continue to strongly advocate for access to long-acting reversible contraceptive methods, the most effective method of preventing pregnancy.
Pharmacists and ob.gyns. alike share a commitment to quality patient care. However, if our shared goal is increasing access to safe, effective contraception, women should be trusted to control their reproductive lives and should be able to do so without barriers.
Dr. DeFrancesco is an ob.gyn. in Cheshire, Conn., and the president of the American College of Obstetricians and Gynecologists. He reported having no relevant financial disclosures.
ED visits due to anaphylaxis doubled at Canadian children’s hospital
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Key clinical point: The percentage of ED visits due to anaphylaxis more than doubled at one Canadian children’s hospital between 2011 and 2015.
Major finding: The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41% during the 4-year study.
Data source: A single-center longitudinal analysis of 965 ED visits for anaphylaxis.
Disclosures: The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
Dragon’s funniest progress notes
Voice recognition software has come a long way. I tried it 15 years ago, but gave up in frustration. Recently, I took another look and found that technology has advanced a lot. Although I can now dictate fast and see the words pour out with impressive accuracy, there are still plenty of errors – some of them quite … interesting. Below is a case summary made up of examples from my growing collection of things the computer seems to have thought I said.
The moral: If you dictate with voice recognition software, proofread!
Chief Complaint
This 43-year-old woman presents with chief complaints of “facial and skin issues.”
Past Medical History
She reports that he has been engaged in a prolonged bottle with acne. It has lasted 18 months, urinary half. A previous physician prescribed an oral antibiotic, either doxycycline or minor cycling. She reports recurrent tachycardia on the forehead and cheeks. Although her outbreaks are not always hormonal, she sometimes gets worse around her. I said around her.! Her menstrual.!! Oh, never mind. It’s good that her name isn’t Cohen, because the computer hears that as :
She has scalp itch and here loss, and is convinced she has pediculosis capitis because of head Lausten chief found. There is a remote history of localized baldness, but not of alopecia universe Alice.
Though free of eczema in recent years, she recalls ataxic childhood.
She was recently exposed to chicken pox but did not contract Maricella. Her history of viral illnesses includes Molested contagiosum.
When she goes in the sun, she is not able to get a 10. She takes no narcotic analgesics, especially Oxyclean.
Occasional scaly rashes have been treated with both antifungal and antibiotic East creams, and sometimes with topical spheroids.
The patient has undergone various medical anesthetic procedures.
Personal and social history
The last 4 digits of her Social Security number are 1/6/09.
She is Director of Marketing for an appetizing agency. Her uncle is a scientist who won the No Bell Prize.
Hobbies: Skiing in Aspirin, Colorado. Competitive barbecue in dialysis, Texas.
Physical Examination
Eyes: There is a cystic lesion on the right I. This is a she’ll lazy on.
Face: Her breakouts are popular. The facial lesions are robbed because of Washington with vigor. Several are just above the nose on the club Ella. There is also sun damage: She has to saltwater keratoses on the 4 head.
Neck: Shotty notes. There is dark thickening typical of a cantholysis Neighbor can’t. There are firm lesions on the occipital scalp consistent with folliculitis Keloid Dallas. The redness on both sides of her neck represents Poikilokderma of survived.
Hands: Xerosis, aggravated by frequent hand washing with puerile. Nails demonstrate the partial separation of cholelithiasis.
Torso: There is a lichenoid rash that sometimes loses. This rash is all over and is very expensive. It is lichenoid and shows a violent color. She has many demented lesions. All are B9.
Upper extremities: There are four systolic keratoses on the vulvar forearm.
Lower extremities: There is a bleeding red lesion of recent onset on the left thigh. It is a high and Janet granuloma.
Groin: Her penile wart is not visible.
Assessment and plan
I will desiccate her high and Janet granuloma.
Will treat her losing rash with Burro solution soaks, followed by topical spheroids.
She was worried that she has precancerous cemented lesions, but I see no indication that she dies.
She will clean her hands less often, otherwise he will have Cirrhosis from Washington. She will moisturize with Aqua 4.
While outdoors, she will protect herself from the son.
For acne, recommended isotretinoin, enrolled patient in iPledge program. She cannot have a fasting blood test today, as she 8 this morning. He will obtain a pregnancy test, and I will confirm patient’s cuddling.
My staph will send a report of today’s visit to the patient’s Coronary Physician.
Dr. Rockoff practices dermatology in Brookline, Mass. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Voice recognition software has come a long way. I tried it 15 years ago, but gave up in frustration. Recently, I took another look and found that technology has advanced a lot. Although I can now dictate fast and see the words pour out with impressive accuracy, there are still plenty of errors – some of them quite … interesting. Below is a case summary made up of examples from my growing collection of things the computer seems to have thought I said.
The moral: If you dictate with voice recognition software, proofread!
Chief Complaint
This 43-year-old woman presents with chief complaints of “facial and skin issues.”
Past Medical History
She reports that he has been engaged in a prolonged bottle with acne. It has lasted 18 months, urinary half. A previous physician prescribed an oral antibiotic, either doxycycline or minor cycling. She reports recurrent tachycardia on the forehead and cheeks. Although her outbreaks are not always hormonal, she sometimes gets worse around her. I said around her.! Her menstrual.!! Oh, never mind. It’s good that her name isn’t Cohen, because the computer hears that as :
She has scalp itch and here loss, and is convinced she has pediculosis capitis because of head Lausten chief found. There is a remote history of localized baldness, but not of alopecia universe Alice.
Though free of eczema in recent years, she recalls ataxic childhood.
She was recently exposed to chicken pox but did not contract Maricella. Her history of viral illnesses includes Molested contagiosum.
When she goes in the sun, she is not able to get a 10. She takes no narcotic analgesics, especially Oxyclean.
Occasional scaly rashes have been treated with both antifungal and antibiotic East creams, and sometimes with topical spheroids.
The patient has undergone various medical anesthetic procedures.
Personal and social history
The last 4 digits of her Social Security number are 1/6/09.
She is Director of Marketing for an appetizing agency. Her uncle is a scientist who won the No Bell Prize.
Hobbies: Skiing in Aspirin, Colorado. Competitive barbecue in dialysis, Texas.
Physical Examination
Eyes: There is a cystic lesion on the right I. This is a she’ll lazy on.
Face: Her breakouts are popular. The facial lesions are robbed because of Washington with vigor. Several are just above the nose on the club Ella. There is also sun damage: She has to saltwater keratoses on the 4 head.
Neck: Shotty notes. There is dark thickening typical of a cantholysis Neighbor can’t. There are firm lesions on the occipital scalp consistent with folliculitis Keloid Dallas. The redness on both sides of her neck represents Poikilokderma of survived.
Hands: Xerosis, aggravated by frequent hand washing with puerile. Nails demonstrate the partial separation of cholelithiasis.
Torso: There is a lichenoid rash that sometimes loses. This rash is all over and is very expensive. It is lichenoid and shows a violent color. She has many demented lesions. All are B9.
Upper extremities: There are four systolic keratoses on the vulvar forearm.
Lower extremities: There is a bleeding red lesion of recent onset on the left thigh. It is a high and Janet granuloma.
Groin: Her penile wart is not visible.
Assessment and plan
I will desiccate her high and Janet granuloma.
Will treat her losing rash with Burro solution soaks, followed by topical spheroids.
She was worried that she has precancerous cemented lesions, but I see no indication that she dies.
She will clean her hands less often, otherwise he will have Cirrhosis from Washington. She will moisturize with Aqua 4.
While outdoors, she will protect herself from the son.
For acne, recommended isotretinoin, enrolled patient in iPledge program. She cannot have a fasting blood test today, as she 8 this morning. He will obtain a pregnancy test, and I will confirm patient’s cuddling.
My staph will send a report of today’s visit to the patient’s Coronary Physician.
Dr. Rockoff practices dermatology in Brookline, Mass. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Voice recognition software has come a long way. I tried it 15 years ago, but gave up in frustration. Recently, I took another look and found that technology has advanced a lot. Although I can now dictate fast and see the words pour out with impressive accuracy, there are still plenty of errors – some of them quite … interesting. Below is a case summary made up of examples from my growing collection of things the computer seems to have thought I said.
The moral: If you dictate with voice recognition software, proofread!
Chief Complaint
This 43-year-old woman presents with chief complaints of “facial and skin issues.”
Past Medical History
She reports that he has been engaged in a prolonged bottle with acne. It has lasted 18 months, urinary half. A previous physician prescribed an oral antibiotic, either doxycycline or minor cycling. She reports recurrent tachycardia on the forehead and cheeks. Although her outbreaks are not always hormonal, she sometimes gets worse around her. I said around her.! Her menstrual.!! Oh, never mind. It’s good that her name isn’t Cohen, because the computer hears that as :
She has scalp itch and here loss, and is convinced she has pediculosis capitis because of head Lausten chief found. There is a remote history of localized baldness, but not of alopecia universe Alice.
Though free of eczema in recent years, she recalls ataxic childhood.
She was recently exposed to chicken pox but did not contract Maricella. Her history of viral illnesses includes Molested contagiosum.
When she goes in the sun, she is not able to get a 10. She takes no narcotic analgesics, especially Oxyclean.
Occasional scaly rashes have been treated with both antifungal and antibiotic East creams, and sometimes with topical spheroids.
The patient has undergone various medical anesthetic procedures.
Personal and social history
The last 4 digits of her Social Security number are 1/6/09.
She is Director of Marketing for an appetizing agency. Her uncle is a scientist who won the No Bell Prize.
Hobbies: Skiing in Aspirin, Colorado. Competitive barbecue in dialysis, Texas.
Physical Examination
Eyes: There is a cystic lesion on the right I. This is a she’ll lazy on.
Face: Her breakouts are popular. The facial lesions are robbed because of Washington with vigor. Several are just above the nose on the club Ella. There is also sun damage: She has to saltwater keratoses on the 4 head.
Neck: Shotty notes. There is dark thickening typical of a cantholysis Neighbor can’t. There are firm lesions on the occipital scalp consistent with folliculitis Keloid Dallas. The redness on both sides of her neck represents Poikilokderma of survived.
Hands: Xerosis, aggravated by frequent hand washing with puerile. Nails demonstrate the partial separation of cholelithiasis.
Torso: There is a lichenoid rash that sometimes loses. This rash is all over and is very expensive. It is lichenoid and shows a violent color. She has many demented lesions. All are B9.
Upper extremities: There are four systolic keratoses on the vulvar forearm.
Lower extremities: There is a bleeding red lesion of recent onset on the left thigh. It is a high and Janet granuloma.
Groin: Her penile wart is not visible.
Assessment and plan
I will desiccate her high and Janet granuloma.
Will treat her losing rash with Burro solution soaks, followed by topical spheroids.
She was worried that she has precancerous cemented lesions, but I see no indication that she dies.
She will clean her hands less often, otherwise he will have Cirrhosis from Washington. She will moisturize with Aqua 4.
While outdoors, she will protect herself from the son.
For acne, recommended isotretinoin, enrolled patient in iPledge program. She cannot have a fasting blood test today, as she 8 this morning. He will obtain a pregnancy test, and I will confirm patient’s cuddling.
My staph will send a report of today’s visit to the patient’s Coronary Physician.
Dr. Rockoff practices dermatology in Brookline, Mass. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
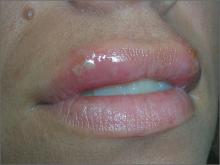
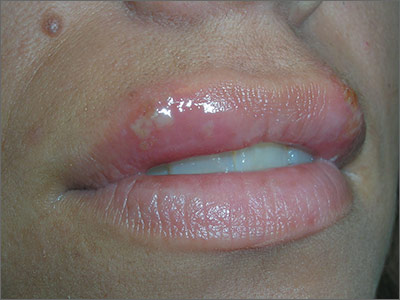
Ulcers on upper lip
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed recurrent orolabial herpes. Orolabial herpes typically takes the form of painful vesicles and ulcerative erosions on the tongue, palate, gingiva, buccal mucosa, and lips. Treatment for primary orolabial herpes includes oral acyclovir (200 mg 5 times daily for 5 days), which accelerates healing by one day and can reduce the mean duration of pain by 36%. Alternatively, valacyclovir can be given 2000 mg orally every 12 hours for one day and, like acyclovir, should be started as soon as possible after the onset of symptoms.
Docosanol cream is available without a prescription for oral herpes. One randomized controlled trial (RCT) of 743 patients with herpes labialis showed a faster healing time in patients treated with docosanol 10% cream when compared with placebo cream (4.1 vs 4.8 days), as well as reduced duration of pain symptoms (2.2 vs 2.7 days).1 More than 90% of patients in both groups healed completely within 10 days.1 Treatment with docosanol cream, when applied 5 times per day and within 12 hours of episode onset until symptoms are relieved, is safe and somewhat effective.
Suppression for patients with frequent recurrences may be provided with valacyclovir 500 mg daily or acyclovir 400 mg twice daily.
Suppression was not indicated for this patient since she had infrequent episodes. She was told that the best prevention would be to use a high-potency sunscreen on her lips and face when out in the sun and to use protective clothing such as a broad brim hat. To prevent skin cancers and recurrent oral labial herpes, she was told to avoid the midday sun whenever possible.
The FP also explained that it was too late now to start oral antiviral treatment, but that she might want to use topical petrolatum for symptom relief. He also offered her a prescription for an oral antiviral medicine that she could fill at the start of symptoms during a future outbreak. He explained that there is some benefit to the over-the-counter topical medicine, docosanol.
1. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
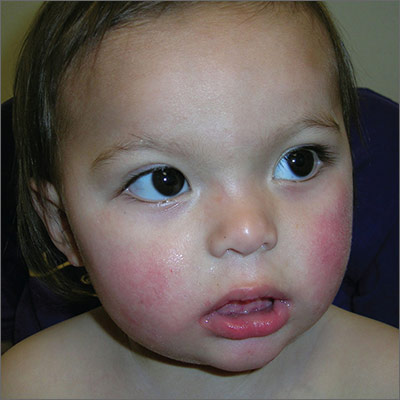
Child with malar rash
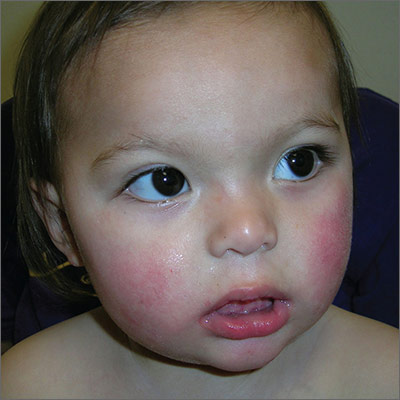
The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The patient’s “slapped-cheek” appearance led to the diagnosis: fifth disease (erythema infectiosum). The name of the diagnosis derives from the fact that it represents the fifth of 6 common childhood viral exanthems. Transmission of the causative parvovirus B19 occurs through respiratory secretions (possibly through fomites), parenterally via vertical transmission from mother to fetus, or by transfusion of blood or blood products.
Fifth disease is very contagious via the respiratory route and occurs more frequently between late winter and early summer. Up to 60% of the population is seropositive for antiparvovirus B19 immunoglobulin G (IgG) by age 20. Thirty to 40% of pregnant women lack measurable IgG to the infecting agent and are therefore presumed to be susceptible to infection. Infection during pregnancy can, in some cases, lead to fetal death.
Fifth disease is usually a biphasic illness, starting with upper respiratory tract symptoms that may include headache, fever, sore throat, pruritus, coryza, abdominal pain, diarrhea, and/or arthralgias. These constitutional symptoms coincide with the onset of viremia and usually resolve (for about a week) before the next stage begins. The second stage is characterized by a classic erythematous malar rash with relative circumoral pallor (the “slapped-cheek” appearance in children). This is followed by a “lace-like” erythematous rash on the trunk and extremities.
Pregnant women who are exposed to or have symptoms of parvovirus infection should have serologic testing. Fortunately in this case, the mother and the day care providers were not pregnant. The parents were reassured that this would go away on its own. They were told that their son should avoid excessive heat and sunlight, which can cause the rash to flare up. Children who present with the classic skin findings of fifth disease are past the infectious state and can attend school and day care. The child in this case returned to day care the next day with a note from the FP.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Fifth disease. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:728-731.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
From The Journal of Family Practice | 2016;65(5).
New Hospitalist Billing Code Should Benefit Hospitalists, Patients
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
The Centers for Medicare & Medicaid Services (CMS) recently announced that within the year hospitalists will be assigned their own specialty designation code.
Up to 85% of hospitalists are currently designated internal medicine, says Ron Greeno, MD, MHM, founding member of SHM and chair of SHM’s Public Policy Committee, but when it comes to quality metrics—and resulting penalties and bonuses—without a way to distinguish themselves from their clinic-based peers, hospitalists have been disadvantaged.
“It is almost impossible to look good when compared to a world of mostly outpatient physicians,” says Dr. Greeno, chief strategy officer at IPC Healthcare, based in North Hollywood, Calif., and SHM’s president-elect.
Today, hospitalists get lumped together with their office-based internal medicine or primary care counterparts, says Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians, based in Tacoma, Wash. Yet, he says, “The quality metrics should be different because it’s a different scope of practice.”
For example, with the Physician Quality Reporting System (PQRS) in recent years, hospitalists have been evaluated based on their patients’ HbA1c, a measure of their diabetic control over the three months prior to admission. But diabetic patients admitted to the hospital are there because they are sick and much less likely to have been well-managed.
“Hospitalists have had no control over their patients’ outpatient diabetes management during the time leading up to admissions, yet these admitted patients are compared to those in an outpatient setting, where their physicians do have control,” Dr. Sears says.
“[This] skews the data and real reporting patterns that are part of that specialty,” says Raemarie Jimenez, CPC, vice president of certifications and member development at AAPC, a professional organization for medical coders and more. “CMS wants the data it is using to be meaningful.”
Once the code is established, the choice to identify as a hospitalist will fall to individual physicians, hospitals, or hospitalist groups, Dr. Greeno says. The designation is noteworthy since hospital medicine does not have a board certification. Today, there are more than 48,000 hospitalists in the U.S., and the announcement comes as hospitalists celebrate 20 years as a specialty. SHM is calling 2016 the “Year of the Hospitalist.”
The decision to seek a hospitalist-specific billing code first arose at SHM several years ago, Dr. Greeno says, with discussions about the advantages, disadvantages, and possible unintended consequences of pursuing it. At the time, SHM chose to hold off, but that changed recently.
“A lot of thought was put into it, and two and a half years later, it’s very clear we made the right decision,” he says. “More and more depends on your data and a lot of different value-based measures. … The Public Policy Committee decided the benefits probably outweigh the potential risks.”
The billing code should make it easier to compare apples to apples, both for hospitalists and hospitals, and Dr. Sears says it should also enable patients to compare hospitalist performance to make better-informed healthcare decisions.
“When you have three or four hospitals in your community, you can compare inpatient hospitalist performance to determine who is providing the most consistent high-quality outcomes,” he says.
It may also enhance reimbursement, says Jimenez. Multiple providers often see patients in the hospital and handle their care. Two providers with the same designation may round on a patient on the same day and appear to CMS and private payors to deliver the same services.
“If a specialist is called in, or their family medicine provider is also seeing the patient, they will not be of the same designation, and that might help with some denials of payments that family or internal medicine physicians are getting,” she says.
Dr. Greeno also says the code may more effectively demonstrate to CMS that hospitalists do not have enough PQRS metrics to adequately qualify for value-based purchasing.
Yet challenges will remain that a specialty code cannot address. “A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears says. “I don’t think it’s an end-all, be-all, but it’s a place to start.”
SHM will continue to actively push CMS to implement the code, Dr. Greeno says, and it will develop strategies for educating members to help them make the decision that is right for them or their group.
Jimenez believes SHM will be capable of doing much more with the data that emerge through robust use of the code.
“Right now, in the industry, big data is it, and the more you can segregate or report on the specifics of data, the better you are at identifying trends,” she says. “We don’t even know yet about clinical outcomes: Are hospitalists’ patients seeing a better outcome of patient experience versus waiting all day to see a family physician? Are there shorter admission times? Trying to improve patient outcomes and reduce costs are two things CMS is desperately interested in.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Free Webinars Aim to Help Reduce Admissions, Optimize Glycemic Control
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Last month, SHM presented live webinars on two of its signature mentored implementation programs that continue to change the way hospitals manage readmissions and glycemic control.
Find out how Project BOOST can help your hospital reduce preventable readmissions, decrease average length of stay, and improve patient satisfaction with one year of individualized mentoring from a physician leader. Watch the webinar at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide; added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls, and more. View the free webinar to learn more at www.hospitalmedicine.org/gc.
Register for Pediatric Hospital Medicine 2016
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Register now. Pediatric Hospital Medicine (PHM) 2016 is the premier educational conference for pediatric hospitalists and other clinicians involved in the care of hospitalized children. This year, PHM 2016 will be held at the Hyatt Regency Chicago in Illinois from July 28 to 31. For the latest information, visit www.phmmeeting.org.
Older Patients with Rosacea are Likely to be Diagnosed with Dementia
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.
NEW YORK (Reuters Health) - Older patients with the inflammatory skin disorder rosacea appear significantly more likely to be diagnosed with dementia, according to Danish researchers.
As Dr. Alexander Egeberg told Reuters Health by email, "We found an increased risk of dementia, in particular Alzheimer's disease (AD), in patients with rosacea. The risk was only increased in patients older than 60 years, however."
"Emerging data," he added, "suggest a link between rosacea and neurological disorders. Yet, it is important for patients to remember that the absolute risk is still low."
In an April 28 online paper in Annals of Neurology, Dr. Egeberg, of the University of Copenhagen, and colleagues note that in rosacea there's upregulation of various inflammatory mediators, for example, cytokines, antimicrobial peptides (AMPs), chemokines, and matrix metalloproteinases (MMPs). Similar processes appear to be at work in certain neurodegenerative disorders.
To examine the possible relationship, the team studied data from 1997 to 2012 on almost 5.6 million Danes. Of these, 82,439 had rosacea at baseline. Over a maximum follow-up of 16 years, 99,040 developed dementia, of whom 29,193 were diagnosed with AD.
After adjustment, patients with rosacea were at significantly increased risk of dementia (hazard ratio 1.07) and of AD (HR 1.25). Women were at greater risk of AD (HR 1.28) than men (HR 1.16).
However, stratification by age at study entry showed that the risk of AD was significantly increased only in those enrolled at the age of 60 or more (HR 1.20). When analyses were limited to patients with a hospital dermatologist diagnosis of rosacea, the HR for dementia was 1.42 and for AD it was 1.92.
The current sum of evidence, conclude the investigators,"suggests that certain forms of dementia, in particular AD, have prominent inflammatory components, and MMPs and AMPs may provide mechanistic links for the observed association between rosacea and dementia."
"Increased focus on symptoms of cognitive dysfunction in patients with rosacea may be warranted," they say.
The authors reported no funding or disclosures.