User login
What’s Less Noticeable: A Straight Scar or a Zigzag Scar?
One of the determinants of a successful surgical outcome is the perception, on the part of the patient, of the cosmesis of a scar. The use of Z-plasty is an accepted means by which to break a scar up into smaller geometric segments. In some instances, a Z-plasty is used for scar revision to elongate a scar that may be pulling. However, a study published online in JAMA Facial Plastic Surgery on April 7 mentions the lack of studies measuring the perception of these scars among the normal population after surgery.
Ratnarathorn et al designed a prospective Internet-based survey with a goal of 580 responses to give a power of 90%. The survey was distributed to a diverse sample of the US population. Using editing software, Ratnarathorn et al superimposed a mature linear scar and a mature zigzag scar onto the faces of standardized headshots from 4 individuals (2 males, 2 females). Each individual had 1 image of the linear scar and 1 image of the zigzag scars superimposed onto each of 3 anatomical areas—forehead (flat surface), cheek (convex surface), and temple (concave surface)—yielding 24 images for the respondents to assess.
A 24.5% (n=876) response rate was achieved with 3575 surveys distributed. Of the 876 respondents, 810 (92.5%) completed the survey (46.1% male, 53.9% female). Respondents were asked to rate the scars on a scale of 1 to 10 (1=normal skin; 10=worst scar imaginable).
Results were statistically significantly lower (better) for the linear scars compared to the zigzag scars in all 3 anatomic areas and across both male and female groups with a mean score of 2.9 versus 4.5 (P<.001). A multivariable regression model of respondent age, sex, educational level, and income showed no statistically significant effect on the rating of the scars.
What’s the issue?
This study highlights some interesting points. Coming from an academic practice, we oftentimes find ourselves teaching residents a variety of skin closure techniques to deal with defects from skin cancer excisions. It is both challenging and fun to design complex closures; however, we must keep in mind what is in the best interest of the patient. One of the points I try to emphasize is that we must understand that there are no true straight lines on the face. In fact, when scars from procedures appear as geometric shapes on the face, our eyes tend to be drawn to them. For this reason, it often is best to use curvilinear lines wherever possible. Ratnarathorn et al highlights that point exactly. More studies of this nature are needed to assess what is perceived as a successful outcome, by both physicians and patients.
As you follow your patients for the long-term, have you noticed that you perform more or fewer zigzag scars?
One of the determinants of a successful surgical outcome is the perception, on the part of the patient, of the cosmesis of a scar. The use of Z-plasty is an accepted means by which to break a scar up into smaller geometric segments. In some instances, a Z-plasty is used for scar revision to elongate a scar that may be pulling. However, a study published online in JAMA Facial Plastic Surgery on April 7 mentions the lack of studies measuring the perception of these scars among the normal population after surgery.
Ratnarathorn et al designed a prospective Internet-based survey with a goal of 580 responses to give a power of 90%. The survey was distributed to a diverse sample of the US population. Using editing software, Ratnarathorn et al superimposed a mature linear scar and a mature zigzag scar onto the faces of standardized headshots from 4 individuals (2 males, 2 females). Each individual had 1 image of the linear scar and 1 image of the zigzag scars superimposed onto each of 3 anatomical areas—forehead (flat surface), cheek (convex surface), and temple (concave surface)—yielding 24 images for the respondents to assess.
A 24.5% (n=876) response rate was achieved with 3575 surveys distributed. Of the 876 respondents, 810 (92.5%) completed the survey (46.1% male, 53.9% female). Respondents were asked to rate the scars on a scale of 1 to 10 (1=normal skin; 10=worst scar imaginable).
Results were statistically significantly lower (better) for the linear scars compared to the zigzag scars in all 3 anatomic areas and across both male and female groups with a mean score of 2.9 versus 4.5 (P<.001). A multivariable regression model of respondent age, sex, educational level, and income showed no statistically significant effect on the rating of the scars.
What’s the issue?
This study highlights some interesting points. Coming from an academic practice, we oftentimes find ourselves teaching residents a variety of skin closure techniques to deal with defects from skin cancer excisions. It is both challenging and fun to design complex closures; however, we must keep in mind what is in the best interest of the patient. One of the points I try to emphasize is that we must understand that there are no true straight lines on the face. In fact, when scars from procedures appear as geometric shapes on the face, our eyes tend to be drawn to them. For this reason, it often is best to use curvilinear lines wherever possible. Ratnarathorn et al highlights that point exactly. More studies of this nature are needed to assess what is perceived as a successful outcome, by both physicians and patients.
As you follow your patients for the long-term, have you noticed that you perform more or fewer zigzag scars?
One of the determinants of a successful surgical outcome is the perception, on the part of the patient, of the cosmesis of a scar. The use of Z-plasty is an accepted means by which to break a scar up into smaller geometric segments. In some instances, a Z-plasty is used for scar revision to elongate a scar that may be pulling. However, a study published online in JAMA Facial Plastic Surgery on April 7 mentions the lack of studies measuring the perception of these scars among the normal population after surgery.
Ratnarathorn et al designed a prospective Internet-based survey with a goal of 580 responses to give a power of 90%. The survey was distributed to a diverse sample of the US population. Using editing software, Ratnarathorn et al superimposed a mature linear scar and a mature zigzag scar onto the faces of standardized headshots from 4 individuals (2 males, 2 females). Each individual had 1 image of the linear scar and 1 image of the zigzag scars superimposed onto each of 3 anatomical areas—forehead (flat surface), cheek (convex surface), and temple (concave surface)—yielding 24 images for the respondents to assess.
A 24.5% (n=876) response rate was achieved with 3575 surveys distributed. Of the 876 respondents, 810 (92.5%) completed the survey (46.1% male, 53.9% female). Respondents were asked to rate the scars on a scale of 1 to 10 (1=normal skin; 10=worst scar imaginable).
Results were statistically significantly lower (better) for the linear scars compared to the zigzag scars in all 3 anatomic areas and across both male and female groups with a mean score of 2.9 versus 4.5 (P<.001). A multivariable regression model of respondent age, sex, educational level, and income showed no statistically significant effect on the rating of the scars.
What’s the issue?
This study highlights some interesting points. Coming from an academic practice, we oftentimes find ourselves teaching residents a variety of skin closure techniques to deal with defects from skin cancer excisions. It is both challenging and fun to design complex closures; however, we must keep in mind what is in the best interest of the patient. One of the points I try to emphasize is that we must understand that there are no true straight lines on the face. In fact, when scars from procedures appear as geometric shapes on the face, our eyes tend to be drawn to them. For this reason, it often is best to use curvilinear lines wherever possible. Ratnarathorn et al highlights that point exactly. More studies of this nature are needed to assess what is perceived as a successful outcome, by both physicians and patients.
As you follow your patients for the long-term, have you noticed that you perform more or fewer zigzag scars?
Why we should strive for a vaginal hysterectomy rate of 40%
One of the great honors of my professional career was being nominated to the presidency of the Society of Gynecologic Surgeons and being given the opportunity to deliver the presidential address at the Society’s 42nd annual scientific meeting in Palm Springs, Calif.
One of the core principles of the SGS mission statement is supporting excellence in gynecologic surgery and, to that end, the main focus of my term was to address the decline in vaginal hysterectomy rates. What follows is an excerpt from my speech explaining the rationale for vaginal hysterectomy (VH) and steps the SGS is taking to reverse the decline.
Unfortunately, what is happening in today’s practice environment is declining use of vaginal hysterectomy, with concomitant increases in endoscopic hysterectomy, mostly with the use of robotic assistance. Being the president of a society previously known as the Vaginal Surgeons Society, it would not be surprising to hear that I have been accused of being “anti-robot.”
Nothing could be further from the truth.
When we talk about the surgical treatment of patients with endometrial and cervical cancer, I do not need a randomized clinical trial to know that not making a laparotomy incision is probably a good thing when you’re treating these patients. There are benefits to using robotic techniques in this subpopulation; it is cost effective due to the reduced morbidity and straight stick laparoscopy for these patients is difficult to perform; therefore it’s not been as widely published or performed. I believe that robotic hysterectomy for these disorders should be the standard of care. In this regard, I am pro robot (Gynecologic Oncol. 2015;138[2]:457-71).
On the other hand, I also don’t need a randomized trial (even though randomized trials exist) to know that if you have a choice to make, or not make, extra incisions during surgery, it’s better to not make the extra incisions.
It’s certainly not rocket science to know that a Zeppelin or Heaney clamp is orders of magnitude cheaper than equipment required to perform an endoscopic hysterectomy – $22.25 USD for instrument and $3.19 USD to process per case (Am J Obstet Gynecol. 2016;214[4]:S461-2]).
Level I evidence demonstrates that when compared to other minimally invasive hysterectomy techniques, vaginal hysterectomy is cheaper, the convalescence is stable or reduced, and the complication rates are lower (Cochrane Database Syst Rev. 2015 Aug. 12;8:CD003677).
Moreover, if you don’t place a port, you can’t get a port site complication (these complications are rare, but potentially serious when they occur). You can’t perforate the common iliac vein. You can’t put a Veress needle through the small bowel. You can’t get a Richter’s hernia. And finally, while you can get cuff dehiscence with vaginal hysterectomy, I’ve never seen it, and this is a real issue with the endoscopic approaches (Cochrane Database Syst Rev. 2012 Feb. 15;2:CD006583 ).
This isn’t just my opinion. Every major surgical society has recommended vaginal hysterectomy when technically feasible.
Of course, “technical feasibility” is the kicker and it’s important to ask what this means.
First, we have to look at what I call the hysterectomy continuum. There are the young, sexually-active women with uterovaginal procidentia where an endoscopic approach for sacral colpopexy might be considered. Then you have patients who are vaginally parous, have a mobile uterus less than 12 weeks in size, and have a basic gynecologic condition such as dysfunctional uterine bleeding, cervical intraepithelial neoplasia, or painful menses (this is about 40%-50% of patients when I reviewed internal North Valley Permanente Group data in 2012); these patients are certainly excellent candidates for vaginal hysterectomy. Then there are patients with 30-week-size fibroid uterus, three prior C-sections, and known stage 4 endometriosis (where an open or robotic approach would be justified).
Second, we have to address the contradictory data presented in the literature regarding vaginal hysterectomy rates. On one hand, we have data from large case series and randomized, controlled trials which demonstrate that it’s feasible to perform a high percentage of vaginal hysterectomies (Obstet Gynecol. 2004;103[6]:1321-5and Arch Gynecol Obstet. 2014;290[3]:485-91). On the other hand, 40 years of population data show the opposite (Obstet Gynecol. 2009;114[5]:1041-8).
In the pre-endoscopic era, 80% and 20% of hysterectomies were performed via the abdominal and vaginal routes, respectively. During the laparoscopic era, 64%, 22%, and 14% of hysterectomies were performed via the abdominal, vaginal, and laparoscopic routes, respectively. And during the current robotic era, it is now 32%, 16%, 28%, and 25% performed via the abdominal, vaginal, laparoscopic, and robotic routes, respectively.
During this 40-year time frame, despite data and recommendations that support vaginal hysterectomy, there has never been an obvious incentive to perform this procedure (e.g. to my knowledge, no one has ever been paid more to do a vaginal hysterectomy, or been prominently featured on a hospital’s website regarding his or her ability to perform an “incision-less” hysterectomy (Am J Obstet Gynecol. 2012;207[3]:174.e1-174.e7). Why weren’t and why aren’t we outraged about this? I have always been under the impression that cheaper and safer is better!
The first thing I hear to explain this – mostly from robotic surgeons and from the robotic surgery device sales representatives – is that the decline in the proportion of vaginal hysterectomies is irrelevant in that it has taken the robot to meaningfully reduce open hysterectomy rates. The other argument I hear – mostly from the laparoscopic surgeons – is that vaginal hysterectomy rates have not changed because most gynecologists cannot and will never be able to perform the procedure. So, what is the point of even discussing solutions?
I disagree with the laparoscopic and robotic surgeons. We should be outraged and do something to effect change. Vaginal hysterectomy offers better value (for surgeons who aren’t thinking about value right now, I suggest that you start. Value-based reimbursement is coming soon) and we know that a high percentage of vaginal hysterectomies are feasible in general gynecologic populations. Surgeons who perform vaginal hysterectomy are not magicians or better surgeons, just differently trained. We have to recognize that many, or even most, patients are candidates for vaginal hysterectomy.
Finally, when we look at robotics for benign disease, we spend more money than on other minimally invasive hysterectomy techniques but we don’t get better outcomes (J Minim Invasive Gynecol. 2010;17[6]:730-8and Eur J Obstet Gynecol Reprod Biol. 150[1]:92-6). Yet surgeons currently use robotics for 25% or more of benign hysterectomies.
What are we thinking and how can we afford to continue this?
We need to counsel our patients (and ourselves) that a total hysterectomy requires an incision in the vagina, and there can be a need for additional abdominal incisions of varying size and number. Fully informed consent must include a discussion of all types of hysterectomy including both patient and surgeon factors associated with the recommended route. Ultimately, the route of hysterectomy should be based on the patient and not the surgeon (Obstet Gynecol. 2014;124[3]:585-8).
It is easy to say, and supported by the evidence, that we should do more vaginal hysterectomies. It is also easy to note that the rate of vaginal hysterectomy has been stable to declining over the last 4 decades and that there are significant issues with residency training in gynecologic surgery (serious issues, but beyond the scope of this editorial).
So, what are we at SGS doing to support increased rates of vaginal hysterectomy? Every December we sponsor a postgraduate course on vaginal hysterectomy techniques. This is an excellent learning opportunity. (Visit www.sgsonline.org for more information regarding dates and costs). We’re starting partnerships with the American College of Obstetricians and Gynecologists (ACOG), the Foundation for Exxcellence in Women’s Health and others, to begin a “train the trainer” program to teach junior faculty how to do and teach vaginal hysterectomy. We’ve developed CREOG (Council on Resident Education in Obstetrics and Gynecology) modules to educate residents about the procedure, and we are in the process of communicating with residency and fellowship program directors about what else we can do to assist them with vaginal hysterectomy teaching. Other goals are to work with ACOG to develop quality metrics for hysterectomy and to develop physician-focused alternative payment models that recognize the value of vaginal hysterectomy.
I believe that in this country we should train for, incentivize, and insist upon a vaginal hysterectomy rate of at least 40% (this albeit arbitrary percentage is based upon the majority of vaginally parous women with uteri less than 12 weeks in size and a minority of the more difficult patients getting a vaginal hysterectomy). And before you say “it’s never been 40%,” please consider the famous quotation by Dr. William Mayo: “The best interest of the patient is the only interest to be considered.” Clearly, the best interest of the patient, if she is a candidate, is to have a vaginal hysterectomy. Our mission at SGS is to facilitate surgical education to make more patients candidates for vaginal hysterectomy so that we can achieve the 40% goal.
Dr. Walter is director of urogynecology and pelvic pain at The Permanente Medical Group, Roseville, Calif. He is also the immediate past president of the Society of Gynecologic Surgeons. He reported having no financial disclosures.
One of the great honors of my professional career was being nominated to the presidency of the Society of Gynecologic Surgeons and being given the opportunity to deliver the presidential address at the Society’s 42nd annual scientific meeting in Palm Springs, Calif.
One of the core principles of the SGS mission statement is supporting excellence in gynecologic surgery and, to that end, the main focus of my term was to address the decline in vaginal hysterectomy rates. What follows is an excerpt from my speech explaining the rationale for vaginal hysterectomy (VH) and steps the SGS is taking to reverse the decline.
Unfortunately, what is happening in today’s practice environment is declining use of vaginal hysterectomy, with concomitant increases in endoscopic hysterectomy, mostly with the use of robotic assistance. Being the president of a society previously known as the Vaginal Surgeons Society, it would not be surprising to hear that I have been accused of being “anti-robot.”
Nothing could be further from the truth.
When we talk about the surgical treatment of patients with endometrial and cervical cancer, I do not need a randomized clinical trial to know that not making a laparotomy incision is probably a good thing when you’re treating these patients. There are benefits to using robotic techniques in this subpopulation; it is cost effective due to the reduced morbidity and straight stick laparoscopy for these patients is difficult to perform; therefore it’s not been as widely published or performed. I believe that robotic hysterectomy for these disorders should be the standard of care. In this regard, I am pro robot (Gynecologic Oncol. 2015;138[2]:457-71).
On the other hand, I also don’t need a randomized trial (even though randomized trials exist) to know that if you have a choice to make, or not make, extra incisions during surgery, it’s better to not make the extra incisions.
It’s certainly not rocket science to know that a Zeppelin or Heaney clamp is orders of magnitude cheaper than equipment required to perform an endoscopic hysterectomy – $22.25 USD for instrument and $3.19 USD to process per case (Am J Obstet Gynecol. 2016;214[4]:S461-2]).
Level I evidence demonstrates that when compared to other minimally invasive hysterectomy techniques, vaginal hysterectomy is cheaper, the convalescence is stable or reduced, and the complication rates are lower (Cochrane Database Syst Rev. 2015 Aug. 12;8:CD003677).
Moreover, if you don’t place a port, you can’t get a port site complication (these complications are rare, but potentially serious when they occur). You can’t perforate the common iliac vein. You can’t put a Veress needle through the small bowel. You can’t get a Richter’s hernia. And finally, while you can get cuff dehiscence with vaginal hysterectomy, I’ve never seen it, and this is a real issue with the endoscopic approaches (Cochrane Database Syst Rev. 2012 Feb. 15;2:CD006583 ).
This isn’t just my opinion. Every major surgical society has recommended vaginal hysterectomy when technically feasible.
Of course, “technical feasibility” is the kicker and it’s important to ask what this means.
First, we have to look at what I call the hysterectomy continuum. There are the young, sexually-active women with uterovaginal procidentia where an endoscopic approach for sacral colpopexy might be considered. Then you have patients who are vaginally parous, have a mobile uterus less than 12 weeks in size, and have a basic gynecologic condition such as dysfunctional uterine bleeding, cervical intraepithelial neoplasia, or painful menses (this is about 40%-50% of patients when I reviewed internal North Valley Permanente Group data in 2012); these patients are certainly excellent candidates for vaginal hysterectomy. Then there are patients with 30-week-size fibroid uterus, three prior C-sections, and known stage 4 endometriosis (where an open or robotic approach would be justified).
Second, we have to address the contradictory data presented in the literature regarding vaginal hysterectomy rates. On one hand, we have data from large case series and randomized, controlled trials which demonstrate that it’s feasible to perform a high percentage of vaginal hysterectomies (Obstet Gynecol. 2004;103[6]:1321-5and Arch Gynecol Obstet. 2014;290[3]:485-91). On the other hand, 40 years of population data show the opposite (Obstet Gynecol. 2009;114[5]:1041-8).
In the pre-endoscopic era, 80% and 20% of hysterectomies were performed via the abdominal and vaginal routes, respectively. During the laparoscopic era, 64%, 22%, and 14% of hysterectomies were performed via the abdominal, vaginal, and laparoscopic routes, respectively. And during the current robotic era, it is now 32%, 16%, 28%, and 25% performed via the abdominal, vaginal, laparoscopic, and robotic routes, respectively.
During this 40-year time frame, despite data and recommendations that support vaginal hysterectomy, there has never been an obvious incentive to perform this procedure (e.g. to my knowledge, no one has ever been paid more to do a vaginal hysterectomy, or been prominently featured on a hospital’s website regarding his or her ability to perform an “incision-less” hysterectomy (Am J Obstet Gynecol. 2012;207[3]:174.e1-174.e7). Why weren’t and why aren’t we outraged about this? I have always been under the impression that cheaper and safer is better!
The first thing I hear to explain this – mostly from robotic surgeons and from the robotic surgery device sales representatives – is that the decline in the proportion of vaginal hysterectomies is irrelevant in that it has taken the robot to meaningfully reduce open hysterectomy rates. The other argument I hear – mostly from the laparoscopic surgeons – is that vaginal hysterectomy rates have not changed because most gynecologists cannot and will never be able to perform the procedure. So, what is the point of even discussing solutions?
I disagree with the laparoscopic and robotic surgeons. We should be outraged and do something to effect change. Vaginal hysterectomy offers better value (for surgeons who aren’t thinking about value right now, I suggest that you start. Value-based reimbursement is coming soon) and we know that a high percentage of vaginal hysterectomies are feasible in general gynecologic populations. Surgeons who perform vaginal hysterectomy are not magicians or better surgeons, just differently trained. We have to recognize that many, or even most, patients are candidates for vaginal hysterectomy.
Finally, when we look at robotics for benign disease, we spend more money than on other minimally invasive hysterectomy techniques but we don’t get better outcomes (J Minim Invasive Gynecol. 2010;17[6]:730-8and Eur J Obstet Gynecol Reprod Biol. 150[1]:92-6). Yet surgeons currently use robotics for 25% or more of benign hysterectomies.
What are we thinking and how can we afford to continue this?
We need to counsel our patients (and ourselves) that a total hysterectomy requires an incision in the vagina, and there can be a need for additional abdominal incisions of varying size and number. Fully informed consent must include a discussion of all types of hysterectomy including both patient and surgeon factors associated with the recommended route. Ultimately, the route of hysterectomy should be based on the patient and not the surgeon (Obstet Gynecol. 2014;124[3]:585-8).
It is easy to say, and supported by the evidence, that we should do more vaginal hysterectomies. It is also easy to note that the rate of vaginal hysterectomy has been stable to declining over the last 4 decades and that there are significant issues with residency training in gynecologic surgery (serious issues, but beyond the scope of this editorial).
So, what are we at SGS doing to support increased rates of vaginal hysterectomy? Every December we sponsor a postgraduate course on vaginal hysterectomy techniques. This is an excellent learning opportunity. (Visit www.sgsonline.org for more information regarding dates and costs). We’re starting partnerships with the American College of Obstetricians and Gynecologists (ACOG), the Foundation for Exxcellence in Women’s Health and others, to begin a “train the trainer” program to teach junior faculty how to do and teach vaginal hysterectomy. We’ve developed CREOG (Council on Resident Education in Obstetrics and Gynecology) modules to educate residents about the procedure, and we are in the process of communicating with residency and fellowship program directors about what else we can do to assist them with vaginal hysterectomy teaching. Other goals are to work with ACOG to develop quality metrics for hysterectomy and to develop physician-focused alternative payment models that recognize the value of vaginal hysterectomy.
I believe that in this country we should train for, incentivize, and insist upon a vaginal hysterectomy rate of at least 40% (this albeit arbitrary percentage is based upon the majority of vaginally parous women with uteri less than 12 weeks in size and a minority of the more difficult patients getting a vaginal hysterectomy). And before you say “it’s never been 40%,” please consider the famous quotation by Dr. William Mayo: “The best interest of the patient is the only interest to be considered.” Clearly, the best interest of the patient, if she is a candidate, is to have a vaginal hysterectomy. Our mission at SGS is to facilitate surgical education to make more patients candidates for vaginal hysterectomy so that we can achieve the 40% goal.
Dr. Walter is director of urogynecology and pelvic pain at The Permanente Medical Group, Roseville, Calif. He is also the immediate past president of the Society of Gynecologic Surgeons. He reported having no financial disclosures.
One of the great honors of my professional career was being nominated to the presidency of the Society of Gynecologic Surgeons and being given the opportunity to deliver the presidential address at the Society’s 42nd annual scientific meeting in Palm Springs, Calif.
One of the core principles of the SGS mission statement is supporting excellence in gynecologic surgery and, to that end, the main focus of my term was to address the decline in vaginal hysterectomy rates. What follows is an excerpt from my speech explaining the rationale for vaginal hysterectomy (VH) and steps the SGS is taking to reverse the decline.
Unfortunately, what is happening in today’s practice environment is declining use of vaginal hysterectomy, with concomitant increases in endoscopic hysterectomy, mostly with the use of robotic assistance. Being the president of a society previously known as the Vaginal Surgeons Society, it would not be surprising to hear that I have been accused of being “anti-robot.”
Nothing could be further from the truth.
When we talk about the surgical treatment of patients with endometrial and cervical cancer, I do not need a randomized clinical trial to know that not making a laparotomy incision is probably a good thing when you’re treating these patients. There are benefits to using robotic techniques in this subpopulation; it is cost effective due to the reduced morbidity and straight stick laparoscopy for these patients is difficult to perform; therefore it’s not been as widely published or performed. I believe that robotic hysterectomy for these disorders should be the standard of care. In this regard, I am pro robot (Gynecologic Oncol. 2015;138[2]:457-71).
On the other hand, I also don’t need a randomized trial (even though randomized trials exist) to know that if you have a choice to make, or not make, extra incisions during surgery, it’s better to not make the extra incisions.
It’s certainly not rocket science to know that a Zeppelin or Heaney clamp is orders of magnitude cheaper than equipment required to perform an endoscopic hysterectomy – $22.25 USD for instrument and $3.19 USD to process per case (Am J Obstet Gynecol. 2016;214[4]:S461-2]).
Level I evidence demonstrates that when compared to other minimally invasive hysterectomy techniques, vaginal hysterectomy is cheaper, the convalescence is stable or reduced, and the complication rates are lower (Cochrane Database Syst Rev. 2015 Aug. 12;8:CD003677).
Moreover, if you don’t place a port, you can’t get a port site complication (these complications are rare, but potentially serious when they occur). You can’t perforate the common iliac vein. You can’t put a Veress needle through the small bowel. You can’t get a Richter’s hernia. And finally, while you can get cuff dehiscence with vaginal hysterectomy, I’ve never seen it, and this is a real issue with the endoscopic approaches (Cochrane Database Syst Rev. 2012 Feb. 15;2:CD006583 ).
This isn’t just my opinion. Every major surgical society has recommended vaginal hysterectomy when technically feasible.
Of course, “technical feasibility” is the kicker and it’s important to ask what this means.
First, we have to look at what I call the hysterectomy continuum. There are the young, sexually-active women with uterovaginal procidentia where an endoscopic approach for sacral colpopexy might be considered. Then you have patients who are vaginally parous, have a mobile uterus less than 12 weeks in size, and have a basic gynecologic condition such as dysfunctional uterine bleeding, cervical intraepithelial neoplasia, or painful menses (this is about 40%-50% of patients when I reviewed internal North Valley Permanente Group data in 2012); these patients are certainly excellent candidates for vaginal hysterectomy. Then there are patients with 30-week-size fibroid uterus, three prior C-sections, and known stage 4 endometriosis (where an open or robotic approach would be justified).
Second, we have to address the contradictory data presented in the literature regarding vaginal hysterectomy rates. On one hand, we have data from large case series and randomized, controlled trials which demonstrate that it’s feasible to perform a high percentage of vaginal hysterectomies (Obstet Gynecol. 2004;103[6]:1321-5and Arch Gynecol Obstet. 2014;290[3]:485-91). On the other hand, 40 years of population data show the opposite (Obstet Gynecol. 2009;114[5]:1041-8).
In the pre-endoscopic era, 80% and 20% of hysterectomies were performed via the abdominal and vaginal routes, respectively. During the laparoscopic era, 64%, 22%, and 14% of hysterectomies were performed via the abdominal, vaginal, and laparoscopic routes, respectively. And during the current robotic era, it is now 32%, 16%, 28%, and 25% performed via the abdominal, vaginal, laparoscopic, and robotic routes, respectively.
During this 40-year time frame, despite data and recommendations that support vaginal hysterectomy, there has never been an obvious incentive to perform this procedure (e.g. to my knowledge, no one has ever been paid more to do a vaginal hysterectomy, or been prominently featured on a hospital’s website regarding his or her ability to perform an “incision-less” hysterectomy (Am J Obstet Gynecol. 2012;207[3]:174.e1-174.e7). Why weren’t and why aren’t we outraged about this? I have always been under the impression that cheaper and safer is better!
The first thing I hear to explain this – mostly from robotic surgeons and from the robotic surgery device sales representatives – is that the decline in the proportion of vaginal hysterectomies is irrelevant in that it has taken the robot to meaningfully reduce open hysterectomy rates. The other argument I hear – mostly from the laparoscopic surgeons – is that vaginal hysterectomy rates have not changed because most gynecologists cannot and will never be able to perform the procedure. So, what is the point of even discussing solutions?
I disagree with the laparoscopic and robotic surgeons. We should be outraged and do something to effect change. Vaginal hysterectomy offers better value (for surgeons who aren’t thinking about value right now, I suggest that you start. Value-based reimbursement is coming soon) and we know that a high percentage of vaginal hysterectomies are feasible in general gynecologic populations. Surgeons who perform vaginal hysterectomy are not magicians or better surgeons, just differently trained. We have to recognize that many, or even most, patients are candidates for vaginal hysterectomy.
Finally, when we look at robotics for benign disease, we spend more money than on other minimally invasive hysterectomy techniques but we don’t get better outcomes (J Minim Invasive Gynecol. 2010;17[6]:730-8and Eur J Obstet Gynecol Reprod Biol. 150[1]:92-6). Yet surgeons currently use robotics for 25% or more of benign hysterectomies.
What are we thinking and how can we afford to continue this?
We need to counsel our patients (and ourselves) that a total hysterectomy requires an incision in the vagina, and there can be a need for additional abdominal incisions of varying size and number. Fully informed consent must include a discussion of all types of hysterectomy including both patient and surgeon factors associated with the recommended route. Ultimately, the route of hysterectomy should be based on the patient and not the surgeon (Obstet Gynecol. 2014;124[3]:585-8).
It is easy to say, and supported by the evidence, that we should do more vaginal hysterectomies. It is also easy to note that the rate of vaginal hysterectomy has been stable to declining over the last 4 decades and that there are significant issues with residency training in gynecologic surgery (serious issues, but beyond the scope of this editorial).
So, what are we at SGS doing to support increased rates of vaginal hysterectomy? Every December we sponsor a postgraduate course on vaginal hysterectomy techniques. This is an excellent learning opportunity. (Visit www.sgsonline.org for more information regarding dates and costs). We’re starting partnerships with the American College of Obstetricians and Gynecologists (ACOG), the Foundation for Exxcellence in Women’s Health and others, to begin a “train the trainer” program to teach junior faculty how to do and teach vaginal hysterectomy. We’ve developed CREOG (Council on Resident Education in Obstetrics and Gynecology) modules to educate residents about the procedure, and we are in the process of communicating with residency and fellowship program directors about what else we can do to assist them with vaginal hysterectomy teaching. Other goals are to work with ACOG to develop quality metrics for hysterectomy and to develop physician-focused alternative payment models that recognize the value of vaginal hysterectomy.
I believe that in this country we should train for, incentivize, and insist upon a vaginal hysterectomy rate of at least 40% (this albeit arbitrary percentage is based upon the majority of vaginally parous women with uteri less than 12 weeks in size and a minority of the more difficult patients getting a vaginal hysterectomy). And before you say “it’s never been 40%,” please consider the famous quotation by Dr. William Mayo: “The best interest of the patient is the only interest to be considered.” Clearly, the best interest of the patient, if she is a candidate, is to have a vaginal hysterectomy. Our mission at SGS is to facilitate surgical education to make more patients candidates for vaginal hysterectomy so that we can achieve the 40% goal.
Dr. Walter is director of urogynecology and pelvic pain at The Permanente Medical Group, Roseville, Calif. He is also the immediate past president of the Society of Gynecologic Surgeons. He reported having no financial disclosures.
USDA to release more funds for antibiotic resistance research
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
Novel device therapy shows continued benefits in pediatric peanut allergy
LOS ANGELES – A peanut protein–bearing skin patch known as the Viaskin Peanut gave a continued strong performance for treatment of peanut allergy in children during the second year of an international study of this novel form of epicutaneous immunotherapy.
The clinical response rate in 6- to 11-year-olds after 1 year of treatment with the 250-mcg dose of peanut protein in the medical device was 57% in the phase IIb, double-blind, 22-site, international VIPES trial, as reported last year.
After an additional year of treatment with the 250-mcg Viaskin Peanut in the open-label extension study known as OLFUS-VIPES, this rate climbed to 80%. Safety and tolerability of the device therapy remained excellent, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In adolescents and adults, however, the clinical response – while significantly better than placebo in VIPES – was less robust than in children, and it remained stable from year 1 to year 2. This is believed to be due to the greater plasticity of the immune system in children, observed Dr. Sampson, director of the Jaffe Food Allergy Institute at Kravis Children’s Hospital at Mount Sinai in New York and chief scientific officer at DBV Technologies, which is developing the Viaskin Peanut.
The ongoing phase III trial uses the 250-mcg dose of peanut protein – the highest of several doses studied in VIPES and OLFUS-VIPES – and is restricted to peanut-allergic children ages 4-11 years. Doses of peanut protein greater than 250 mcg will be explored in separate studies of adolescents and adults.
The clinical response rate in children on the 250-mcg Viaskin Peanut rose from 57% after 1 year to 80% – that is, 16 of 20 subjects – after 2 years. A clinical response in VIPES and OLFUS-VIPES was defined as nonreactivity to a dose of at least 1,000 mg of peanut protein – the equivalent of four peanuts – during a formal double-blind food challenge or at least a tenfold increase in the eliciting dose, compared to the original eliciting dose.
In VIPES, one-third of children on the 250-mcg device therapy for 1 year could tolerate at least 1,000 mg of peanut protein; after an additional year of open-label therapy, 60% of children were able to do so.
Among 6- to 11-year-olds, the median cumulative reactive dose of peanut protein was 44 mg at baseline, 444 mg after 12 months of using the 250-mcg Viaskin Peanut, and 1,444 mg at 2 years.
The children’s immunologic response to the Viaskin Peanut was impressive: A 40% reduction from baseline in peanut IgE at 2 years, along with a ninefold increase in protective peanut-specific IgG4.
The skin patch consists of a dried allergen – in this case, peanut protein – which is made electrostatically adherent to a membrane on a Band-Aid–like chamber. A set of patches is placed on noneczematous skin on a child’s back and on the inner upper arm of older patients. Moisture emitted from the skin gradually causes the protein allergen to solubilize and get absorbed into the outer layer of the skin. It is then picked up by antigen-presenting Langerhans cells and transported to regional lymph nodes for deactivation. The patches are changed daily.
“It appears that we need to look at the skin as a tolerogenic organ when it’s uninflamed,” Dr. Sampson observed.
Compliance with treatment was in excess of 96% in both VIPES and OLFUS-VIPES. There have been no serious treatment-related adverse events and no need for the use of epinephrine. Side effects have been limited to occasional mild to moderate application site reactions easily managed with antihistamines and/or topical steroids, according to Dr. Sampson.
The double-blind VIPES study included 207 subjects with documented peanut allergy. OLFUS-VIPES, which will continue for 1 additional year of open-label therapy, includes 171 of the original 207, including 97 children, 49 adolescents, and 25 adults up to age 55 years.
“We’ll see if there’s continued improvement in children through the third year or it levels off, but based upon the immunologic parameters I think it’s having continued effect,” the pediatric allergist said.
LOS ANGELES – A peanut protein–bearing skin patch known as the Viaskin Peanut gave a continued strong performance for treatment of peanut allergy in children during the second year of an international study of this novel form of epicutaneous immunotherapy.
The clinical response rate in 6- to 11-year-olds after 1 year of treatment with the 250-mcg dose of peanut protein in the medical device was 57% in the phase IIb, double-blind, 22-site, international VIPES trial, as reported last year.
After an additional year of treatment with the 250-mcg Viaskin Peanut in the open-label extension study known as OLFUS-VIPES, this rate climbed to 80%. Safety and tolerability of the device therapy remained excellent, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In adolescents and adults, however, the clinical response – while significantly better than placebo in VIPES – was less robust than in children, and it remained stable from year 1 to year 2. This is believed to be due to the greater plasticity of the immune system in children, observed Dr. Sampson, director of the Jaffe Food Allergy Institute at Kravis Children’s Hospital at Mount Sinai in New York and chief scientific officer at DBV Technologies, which is developing the Viaskin Peanut.
The ongoing phase III trial uses the 250-mcg dose of peanut protein – the highest of several doses studied in VIPES and OLFUS-VIPES – and is restricted to peanut-allergic children ages 4-11 years. Doses of peanut protein greater than 250 mcg will be explored in separate studies of adolescents and adults.
The clinical response rate in children on the 250-mcg Viaskin Peanut rose from 57% after 1 year to 80% – that is, 16 of 20 subjects – after 2 years. A clinical response in VIPES and OLFUS-VIPES was defined as nonreactivity to a dose of at least 1,000 mg of peanut protein – the equivalent of four peanuts – during a formal double-blind food challenge or at least a tenfold increase in the eliciting dose, compared to the original eliciting dose.
In VIPES, one-third of children on the 250-mcg device therapy for 1 year could tolerate at least 1,000 mg of peanut protein; after an additional year of open-label therapy, 60% of children were able to do so.
Among 6- to 11-year-olds, the median cumulative reactive dose of peanut protein was 44 mg at baseline, 444 mg after 12 months of using the 250-mcg Viaskin Peanut, and 1,444 mg at 2 years.
The children’s immunologic response to the Viaskin Peanut was impressive: A 40% reduction from baseline in peanut IgE at 2 years, along with a ninefold increase in protective peanut-specific IgG4.
The skin patch consists of a dried allergen – in this case, peanut protein – which is made electrostatically adherent to a membrane on a Band-Aid–like chamber. A set of patches is placed on noneczematous skin on a child’s back and on the inner upper arm of older patients. Moisture emitted from the skin gradually causes the protein allergen to solubilize and get absorbed into the outer layer of the skin. It is then picked up by antigen-presenting Langerhans cells and transported to regional lymph nodes for deactivation. The patches are changed daily.
“It appears that we need to look at the skin as a tolerogenic organ when it’s uninflamed,” Dr. Sampson observed.
Compliance with treatment was in excess of 96% in both VIPES and OLFUS-VIPES. There have been no serious treatment-related adverse events and no need for the use of epinephrine. Side effects have been limited to occasional mild to moderate application site reactions easily managed with antihistamines and/or topical steroids, according to Dr. Sampson.
The double-blind VIPES study included 207 subjects with documented peanut allergy. OLFUS-VIPES, which will continue for 1 additional year of open-label therapy, includes 171 of the original 207, including 97 children, 49 adolescents, and 25 adults up to age 55 years.
“We’ll see if there’s continued improvement in children through the third year or it levels off, but based upon the immunologic parameters I think it’s having continued effect,” the pediatric allergist said.
LOS ANGELES – A peanut protein–bearing skin patch known as the Viaskin Peanut gave a continued strong performance for treatment of peanut allergy in children during the second year of an international study of this novel form of epicutaneous immunotherapy.
The clinical response rate in 6- to 11-year-olds after 1 year of treatment with the 250-mcg dose of peanut protein in the medical device was 57% in the phase IIb, double-blind, 22-site, international VIPES trial, as reported last year.
After an additional year of treatment with the 250-mcg Viaskin Peanut in the open-label extension study known as OLFUS-VIPES, this rate climbed to 80%. Safety and tolerability of the device therapy remained excellent, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In adolescents and adults, however, the clinical response – while significantly better than placebo in VIPES – was less robust than in children, and it remained stable from year 1 to year 2. This is believed to be due to the greater plasticity of the immune system in children, observed Dr. Sampson, director of the Jaffe Food Allergy Institute at Kravis Children’s Hospital at Mount Sinai in New York and chief scientific officer at DBV Technologies, which is developing the Viaskin Peanut.
The ongoing phase III trial uses the 250-mcg dose of peanut protein – the highest of several doses studied in VIPES and OLFUS-VIPES – and is restricted to peanut-allergic children ages 4-11 years. Doses of peanut protein greater than 250 mcg will be explored in separate studies of adolescents and adults.
The clinical response rate in children on the 250-mcg Viaskin Peanut rose from 57% after 1 year to 80% – that is, 16 of 20 subjects – after 2 years. A clinical response in VIPES and OLFUS-VIPES was defined as nonreactivity to a dose of at least 1,000 mg of peanut protein – the equivalent of four peanuts – during a formal double-blind food challenge or at least a tenfold increase in the eliciting dose, compared to the original eliciting dose.
In VIPES, one-third of children on the 250-mcg device therapy for 1 year could tolerate at least 1,000 mg of peanut protein; after an additional year of open-label therapy, 60% of children were able to do so.
Among 6- to 11-year-olds, the median cumulative reactive dose of peanut protein was 44 mg at baseline, 444 mg after 12 months of using the 250-mcg Viaskin Peanut, and 1,444 mg at 2 years.
The children’s immunologic response to the Viaskin Peanut was impressive: A 40% reduction from baseline in peanut IgE at 2 years, along with a ninefold increase in protective peanut-specific IgG4.
The skin patch consists of a dried allergen – in this case, peanut protein – which is made electrostatically adherent to a membrane on a Band-Aid–like chamber. A set of patches is placed on noneczematous skin on a child’s back and on the inner upper arm of older patients. Moisture emitted from the skin gradually causes the protein allergen to solubilize and get absorbed into the outer layer of the skin. It is then picked up by antigen-presenting Langerhans cells and transported to regional lymph nodes for deactivation. The patches are changed daily.
“It appears that we need to look at the skin as a tolerogenic organ when it’s uninflamed,” Dr. Sampson observed.
Compliance with treatment was in excess of 96% in both VIPES and OLFUS-VIPES. There have been no serious treatment-related adverse events and no need for the use of epinephrine. Side effects have been limited to occasional mild to moderate application site reactions easily managed with antihistamines and/or topical steroids, according to Dr. Sampson.
The double-blind VIPES study included 207 subjects with documented peanut allergy. OLFUS-VIPES, which will continue for 1 additional year of open-label therapy, includes 171 of the original 207, including 97 children, 49 adolescents, and 25 adults up to age 55 years.
“We’ll see if there’s continued improvement in children through the third year or it levels off, but based upon the immunologic parameters I think it’s having continued effect,” the pediatric allergist said.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: A peanut protein–bearing skin patch shows favorable efficacy and safety as a treatment for peanut allergy, especially in children.
Major finding: After 1 year using the Viaskin Peanut device at the 250-mcg dose, one-third of formerly peanut-allergic children tolerated at least 1,000 mg of peanut protein in an oral food challenge; after 2 years using the patch, the rate increased to 60%.
Data source: Ongoing 2-year, open-label extension of the yearlong, double-blind, randomized VIPES trial of 171 peanut-allergic subjects aged 6-55 years.
Disclosures: The study was funded by DBV Technologies and presented by the company’s chief scientific officer.
Regional Lymphomatoid Papulosis of the Breast Restricted to an Area of Prior Radiotherapy
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.
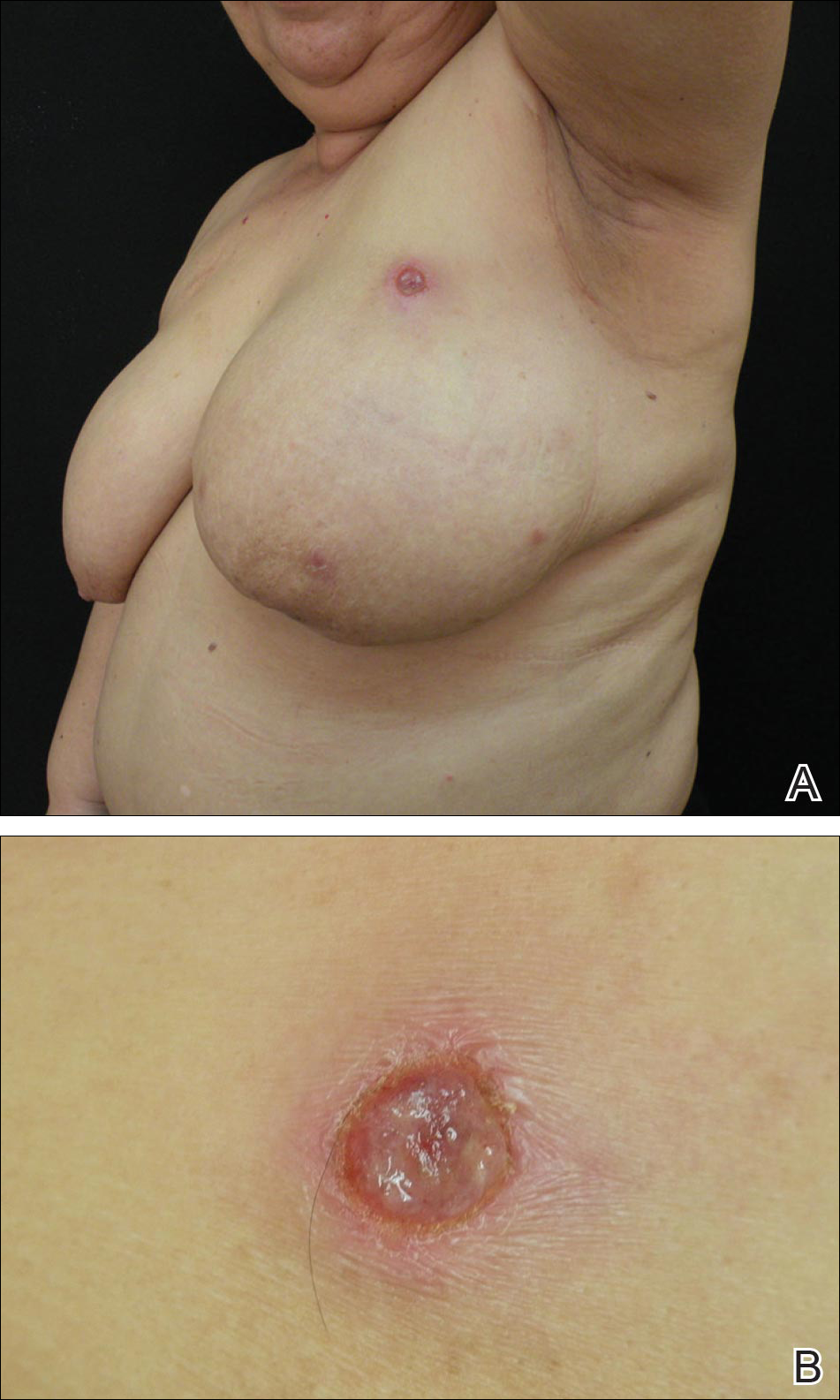
Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.

Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.

Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
Practice Points
- Cutaneous lesions of lymphomatoid papulosis (LyP) sometimes are confined to only one area of the skin, which is known as regional LyP.
- Patients with regional LyP have the same prognosis as those with widespread LyP, and no specific association has been reported with this clinical variant.
- Lesions of regional LyP respond to the same treatments as widespread LyP.
Onychomadesis Following Hand-foot-and-mouth Disease
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.
The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.


The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.


The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
Practice Points
- Onychomadesis is a late complication of hand-foot-and-mouth disease (HFMD) with a latency period of 1 to 2 months.
- Although the mechanism between onychomadesis and HFMD is still unclear, we propose that it is caused by the viral infection itself rather than severe systemic disease.
Opioid-based therapies reduce TKA needs for OA patients, but not costs
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
FROM ARTHRITIS CARE AND RESEARCH
Animal model recapitulates human MM, other neoplasms

Researchers say they have developed an animal model that allows them to better understand the mechanisms leading to multiple myeloma (MM) and other plasma cell neoplasms.
The group described this model in Scientific Reports.
“So far, there have not been animal models of malignant plasma cell diseases that allow us to study their stepwise progression and fully understand the complex cellular mechanisms,” said study author Stephen D. Nimer, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami in Florida.
“Now that we have a proper model of the disease, we’ll be able to more effectively study multiple myeloma as well as potential treatments.”
To create this model, the researchers crossed 2 genetically modified mice: mice lacking the Mef gene and mice with a Rad50 gene mutation (Rad50s).
Mef, also called Elf4, is a transcription factor known to both promote and suppress cancer formation. Rad50 is a component of a sensor of DNA damage induced by various stresses, and it regulates the DNA damage response pathways in cells.
The researchers found that Mef−/−Rad50s/s mice initially had abnormal plasma cell proliferation and monoclonal protein production.
Then, they developed anemia and a decreased bone mineral density. And 70% of these mice died from MM or other plasma cell neoplasms.
“We also found that the phenotype of these mice is not linked to activation of a specific oncogene or inactivation of a specific tumor suppressor, other than Mef,” said study author Takashi Asai, MD, PhD, also of the Sylvester Comprehensive Cancer Center.
Considering their findings together, the researchers said this model recapitulates the systemic manifestations of human MM and other plasma cell neoplasms. And their work suggests the Rad50s and Mef/Elf4 pathways cooperate to initiate myelomagenic mutations that promote plasma cell transformation.
“Although outcomes for multiple myeloma patients have greatly improved, it remains an incurable disease, despite the availability of newer treatments,” Dr Nimer noted.
“Several animal models of multiple myeloma have been reported, including models of human myeloma cells. However, these models imperfectly mimic the human disease. Developing more reliable and accurate animal models that help us better understand myeloma and test new treatments will take us to the next level on the long and challenging road to a cure.” ![]()

Researchers say they have developed an animal model that allows them to better understand the mechanisms leading to multiple myeloma (MM) and other plasma cell neoplasms.
The group described this model in Scientific Reports.
“So far, there have not been animal models of malignant plasma cell diseases that allow us to study their stepwise progression and fully understand the complex cellular mechanisms,” said study author Stephen D. Nimer, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami in Florida.
“Now that we have a proper model of the disease, we’ll be able to more effectively study multiple myeloma as well as potential treatments.”
To create this model, the researchers crossed 2 genetically modified mice: mice lacking the Mef gene and mice with a Rad50 gene mutation (Rad50s).
Mef, also called Elf4, is a transcription factor known to both promote and suppress cancer formation. Rad50 is a component of a sensor of DNA damage induced by various stresses, and it regulates the DNA damage response pathways in cells.
The researchers found that Mef−/−Rad50s/s mice initially had abnormal plasma cell proliferation and monoclonal protein production.
Then, they developed anemia and a decreased bone mineral density. And 70% of these mice died from MM or other plasma cell neoplasms.
“We also found that the phenotype of these mice is not linked to activation of a specific oncogene or inactivation of a specific tumor suppressor, other than Mef,” said study author Takashi Asai, MD, PhD, also of the Sylvester Comprehensive Cancer Center.
Considering their findings together, the researchers said this model recapitulates the systemic manifestations of human MM and other plasma cell neoplasms. And their work suggests the Rad50s and Mef/Elf4 pathways cooperate to initiate myelomagenic mutations that promote plasma cell transformation.
“Although outcomes for multiple myeloma patients have greatly improved, it remains an incurable disease, despite the availability of newer treatments,” Dr Nimer noted.
“Several animal models of multiple myeloma have been reported, including models of human myeloma cells. However, these models imperfectly mimic the human disease. Developing more reliable and accurate animal models that help us better understand myeloma and test new treatments will take us to the next level on the long and challenging road to a cure.” ![]()

Researchers say they have developed an animal model that allows them to better understand the mechanisms leading to multiple myeloma (MM) and other plasma cell neoplasms.
The group described this model in Scientific Reports.
“So far, there have not been animal models of malignant plasma cell diseases that allow us to study their stepwise progression and fully understand the complex cellular mechanisms,” said study author Stephen D. Nimer, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami in Florida.
“Now that we have a proper model of the disease, we’ll be able to more effectively study multiple myeloma as well as potential treatments.”
To create this model, the researchers crossed 2 genetically modified mice: mice lacking the Mef gene and mice with a Rad50 gene mutation (Rad50s).
Mef, also called Elf4, is a transcription factor known to both promote and suppress cancer formation. Rad50 is a component of a sensor of DNA damage induced by various stresses, and it regulates the DNA damage response pathways in cells.
The researchers found that Mef−/−Rad50s/s mice initially had abnormal plasma cell proliferation and monoclonal protein production.
Then, they developed anemia and a decreased bone mineral density. And 70% of these mice died from MM or other plasma cell neoplasms.
“We also found that the phenotype of these mice is not linked to activation of a specific oncogene or inactivation of a specific tumor suppressor, other than Mef,” said study author Takashi Asai, MD, PhD, also of the Sylvester Comprehensive Cancer Center.
Considering their findings together, the researchers said this model recapitulates the systemic manifestations of human MM and other plasma cell neoplasms. And their work suggests the Rad50s and Mef/Elf4 pathways cooperate to initiate myelomagenic mutations that promote plasma cell transformation.
“Although outcomes for multiple myeloma patients have greatly improved, it remains an incurable disease, despite the availability of newer treatments,” Dr Nimer noted.
“Several animal models of multiple myeloma have been reported, including models of human myeloma cells. However, these models imperfectly mimic the human disease. Developing more reliable and accurate animal models that help us better understand myeloma and test new treatments will take us to the next level on the long and challenging road to a cure.” ![]()
Not All EDs Adopt Interventions to Improve Flow, Decrease Crowding
Clinical question: What is the relationship between crowding in the ED and the number of interventions adopted by the ED to address this?
Background: ED crowding results in long waits, prolonged lengths of stay, and delays in providing treatments, which can result in adverse events. Numerous interventions, including bedside registration, ED observation units, fast track, bed czar, surgical schedule smoothing, and pooled nursing, have been implemented to reduce crowding.
Study design: Retrospective, cross-sectional analysis.
Setting: U.S. hospitals in the National Hospital Ambulatory Medical Care Survey (NHAMCS).
Synopsis: From 2007 to 2010, an average of 341 hospitals per year were analyzed from the NHAMCS, representing 139,502 patient encounters. This study evaluated the adoption of nine crowding interventions at the emergency department level (bedside registration, electronic dashboard, RFID tracking, etc.) and eight crowding interventions at the hospital level (bed czar, pooled nursing, full-capacity protocol, board patients in inpatient hallways, etc.).
Bedside registration, electronic dashboard, RFID tracking, bed census, pooled nursing, full-capacity protocol, and boarding patients in the hallway had the highest statistically significant increases in adoption over the study period.
The average number of interventions adopted increased to 6.6 from 5.2, and more-crowded EDs adopted a greater number of interventions than less-crowded EDs. However, in the most-crowded quartile of EDs, 19% did not use bedside registration, and 94% did not use surgical schedule smoothing.
Given that this study is a retrospective, cross-sectional study, it is difficult to determine causality.
Bottom line: More interventions are being adopted by EDs and hospitals to decrease ED crowding, but several of the busiest EDs and hospitals have room for improvement.
Citation: Warner LS, Pines JM, Chambers JG, Schuur JD. The most crowded US hospital emergency departments did not adopt effective interventions to improve flow, 2007–10. Health Aff. 2015;34(12):2151-2159.
Clinical question: What is the relationship between crowding in the ED and the number of interventions adopted by the ED to address this?
Background: ED crowding results in long waits, prolonged lengths of stay, and delays in providing treatments, which can result in adverse events. Numerous interventions, including bedside registration, ED observation units, fast track, bed czar, surgical schedule smoothing, and pooled nursing, have been implemented to reduce crowding.
Study design: Retrospective, cross-sectional analysis.
Setting: U.S. hospitals in the National Hospital Ambulatory Medical Care Survey (NHAMCS).
Synopsis: From 2007 to 2010, an average of 341 hospitals per year were analyzed from the NHAMCS, representing 139,502 patient encounters. This study evaluated the adoption of nine crowding interventions at the emergency department level (bedside registration, electronic dashboard, RFID tracking, etc.) and eight crowding interventions at the hospital level (bed czar, pooled nursing, full-capacity protocol, board patients in inpatient hallways, etc.).
Bedside registration, electronic dashboard, RFID tracking, bed census, pooled nursing, full-capacity protocol, and boarding patients in the hallway had the highest statistically significant increases in adoption over the study period.
The average number of interventions adopted increased to 6.6 from 5.2, and more-crowded EDs adopted a greater number of interventions than less-crowded EDs. However, in the most-crowded quartile of EDs, 19% did not use bedside registration, and 94% did not use surgical schedule smoothing.
Given that this study is a retrospective, cross-sectional study, it is difficult to determine causality.
Bottom line: More interventions are being adopted by EDs and hospitals to decrease ED crowding, but several of the busiest EDs and hospitals have room for improvement.
Citation: Warner LS, Pines JM, Chambers JG, Schuur JD. The most crowded US hospital emergency departments did not adopt effective interventions to improve flow, 2007–10. Health Aff. 2015;34(12):2151-2159.
Clinical question: What is the relationship between crowding in the ED and the number of interventions adopted by the ED to address this?
Background: ED crowding results in long waits, prolonged lengths of stay, and delays in providing treatments, which can result in adverse events. Numerous interventions, including bedside registration, ED observation units, fast track, bed czar, surgical schedule smoothing, and pooled nursing, have been implemented to reduce crowding.
Study design: Retrospective, cross-sectional analysis.
Setting: U.S. hospitals in the National Hospital Ambulatory Medical Care Survey (NHAMCS).
Synopsis: From 2007 to 2010, an average of 341 hospitals per year were analyzed from the NHAMCS, representing 139,502 patient encounters. This study evaluated the adoption of nine crowding interventions at the emergency department level (bedside registration, electronic dashboard, RFID tracking, etc.) and eight crowding interventions at the hospital level (bed czar, pooled nursing, full-capacity protocol, board patients in inpatient hallways, etc.).
Bedside registration, electronic dashboard, RFID tracking, bed census, pooled nursing, full-capacity protocol, and boarding patients in the hallway had the highest statistically significant increases in adoption over the study period.
The average number of interventions adopted increased to 6.6 from 5.2, and more-crowded EDs adopted a greater number of interventions than less-crowded EDs. However, in the most-crowded quartile of EDs, 19% did not use bedside registration, and 94% did not use surgical schedule smoothing.
Given that this study is a retrospective, cross-sectional study, it is difficult to determine causality.
Bottom line: More interventions are being adopted by EDs and hospitals to decrease ED crowding, but several of the busiest EDs and hospitals have room for improvement.
Citation: Warner LS, Pines JM, Chambers JG, Schuur JD. The most crowded US hospital emergency departments did not adopt effective interventions to improve flow, 2007–10. Health Aff. 2015;34(12):2151-2159.
Close INR Monitoring Might Prevent Adverse Events
Clinical question: What is the appropriate frequency of INR monitoring in the hospital and its relationship to the risk of over-anticoagulation and warfarin-related adverse events?
Background: Warfarin use is a common cause of adverse drug events in hospitalized patients due to narrow therapeutic windows, drug interactions, and variability of metabolism. Current guidelines, including those by the American College of Chest Physicians, do not provide recommendations on how often to monitor INR or adjust warfarin dosing in the hospital.
Study design: Retrospective cohort.
Setting: Hospitalized patients included in the Medicare Patient Safety Monitoring System.
Synopsis: The study included 14,217 adult patients ≥18 years of age from the Medicare Patient Safety Monitoring System admitted from 2009 to 2013 with pneumonia, acute cardiac disease (myocardial infarction or congestive heart failure), or surgery and taking warfarin. Of those, 1,055 (7.4%) developed a warfarin-associated adverse event (bleeding, drop in hematocrit ≥3, hematoma, death, intracranial bleeding, or cardiac arrest). Patients admitted for acute cardiac disease (acute myocardial infarction or heart failure) or surgery on warfarin for ≥3 days but not monitored for ≥2 days had more warfarin-associated adverse events (OR 1.48; 95% CI, 1.02–2.17), but this association was not true in pneumonia patients. Cardiac and pneumonia patients with ≥1 day without INR being measured had higher rates of INR ≥6.0 (OR 1.61; 95% CI, 1.07–2.41, and OR 1.92, 95% CI, 1.36–2.71, respectively). A single-day rise in INR ≥0.9 had a likelihood ratio of 4.2 in predicting subsequent INR ≥6.0.
Bottom line: Frequent monitoring of INR may decrease warfarin-associated adverse events in hospitalized patients.
Citation: Metersky ML, Eldridge N, Wang Y, et al. Predictors of warfarin-associated adverse events in hospitalized patients: opportunities to prevent harm. J Hosp Med. 2016;11(4):276-282.
Short Take
CDC Guidelines on Prescribing Opioids
New CDC guidelines for chronic pain management stress the importance of non-pharmacologic (physical therapy, etc.) and non-opioid therapy (NSAIDs, etc.), using opioid therapy only if the expected benefits outweigh the risks.
Citation: CDC. CDC guideline for prescribing opioids for chronic pain. Available at: http://www.cdc.gov/drugoverdose/prescribing/guideline.html. Published March 16, 2016. Accessed April 8, 2016.
Clinical question: What is the appropriate frequency of INR monitoring in the hospital and its relationship to the risk of over-anticoagulation and warfarin-related adverse events?
Background: Warfarin use is a common cause of adverse drug events in hospitalized patients due to narrow therapeutic windows, drug interactions, and variability of metabolism. Current guidelines, including those by the American College of Chest Physicians, do not provide recommendations on how often to monitor INR or adjust warfarin dosing in the hospital.
Study design: Retrospective cohort.
Setting: Hospitalized patients included in the Medicare Patient Safety Monitoring System.
Synopsis: The study included 14,217 adult patients ≥18 years of age from the Medicare Patient Safety Monitoring System admitted from 2009 to 2013 with pneumonia, acute cardiac disease (myocardial infarction or congestive heart failure), or surgery and taking warfarin. Of those, 1,055 (7.4%) developed a warfarin-associated adverse event (bleeding, drop in hematocrit ≥3, hematoma, death, intracranial bleeding, or cardiac arrest). Patients admitted for acute cardiac disease (acute myocardial infarction or heart failure) or surgery on warfarin for ≥3 days but not monitored for ≥2 days had more warfarin-associated adverse events (OR 1.48; 95% CI, 1.02–2.17), but this association was not true in pneumonia patients. Cardiac and pneumonia patients with ≥1 day without INR being measured had higher rates of INR ≥6.0 (OR 1.61; 95% CI, 1.07–2.41, and OR 1.92, 95% CI, 1.36–2.71, respectively). A single-day rise in INR ≥0.9 had a likelihood ratio of 4.2 in predicting subsequent INR ≥6.0.
Bottom line: Frequent monitoring of INR may decrease warfarin-associated adverse events in hospitalized patients.
Citation: Metersky ML, Eldridge N, Wang Y, et al. Predictors of warfarin-associated adverse events in hospitalized patients: opportunities to prevent harm. J Hosp Med. 2016;11(4):276-282.
Short Take
CDC Guidelines on Prescribing Opioids
New CDC guidelines for chronic pain management stress the importance of non-pharmacologic (physical therapy, etc.) and non-opioid therapy (NSAIDs, etc.), using opioid therapy only if the expected benefits outweigh the risks.
Citation: CDC. CDC guideline for prescribing opioids for chronic pain. Available at: http://www.cdc.gov/drugoverdose/prescribing/guideline.html. Published March 16, 2016. Accessed April 8, 2016.
Clinical question: What is the appropriate frequency of INR monitoring in the hospital and its relationship to the risk of over-anticoagulation and warfarin-related adverse events?
Background: Warfarin use is a common cause of adverse drug events in hospitalized patients due to narrow therapeutic windows, drug interactions, and variability of metabolism. Current guidelines, including those by the American College of Chest Physicians, do not provide recommendations on how often to monitor INR or adjust warfarin dosing in the hospital.
Study design: Retrospective cohort.
Setting: Hospitalized patients included in the Medicare Patient Safety Monitoring System.
Synopsis: The study included 14,217 adult patients ≥18 years of age from the Medicare Patient Safety Monitoring System admitted from 2009 to 2013 with pneumonia, acute cardiac disease (myocardial infarction or congestive heart failure), or surgery and taking warfarin. Of those, 1,055 (7.4%) developed a warfarin-associated adverse event (bleeding, drop in hematocrit ≥3, hematoma, death, intracranial bleeding, or cardiac arrest). Patients admitted for acute cardiac disease (acute myocardial infarction or heart failure) or surgery on warfarin for ≥3 days but not monitored for ≥2 days had more warfarin-associated adverse events (OR 1.48; 95% CI, 1.02–2.17), but this association was not true in pneumonia patients. Cardiac and pneumonia patients with ≥1 day without INR being measured had higher rates of INR ≥6.0 (OR 1.61; 95% CI, 1.07–2.41, and OR 1.92, 95% CI, 1.36–2.71, respectively). A single-day rise in INR ≥0.9 had a likelihood ratio of 4.2 in predicting subsequent INR ≥6.0.
Bottom line: Frequent monitoring of INR may decrease warfarin-associated adverse events in hospitalized patients.
Citation: Metersky ML, Eldridge N, Wang Y, et al. Predictors of warfarin-associated adverse events in hospitalized patients: opportunities to prevent harm. J Hosp Med. 2016;11(4):276-282.
Short Take
CDC Guidelines on Prescribing Opioids
New CDC guidelines for chronic pain management stress the importance of non-pharmacologic (physical therapy, etc.) and non-opioid therapy (NSAIDs, etc.), using opioid therapy only if the expected benefits outweigh the risks.
Citation: CDC. CDC guideline for prescribing opioids for chronic pain. Available at: http://www.cdc.gov/drugoverdose/prescribing/guideline.html. Published March 16, 2016. Accessed April 8, 2016.