User login
Team creates bone marrow on a chip
Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()

Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()

Image by Daniel E. Sabath
Engineered bone marrow grown in a microfluidic chip device mimics living bone marrow, according to research published in Tissue Engineering.
Experiments showed the engineered bone marrow responded in a way similar to living bone marrow when exposed to damaging radiation followed by treatment with compounds that aid in blood cell recovery.
The researchers said this new bone marrow-on-a-chip device holds promise for testing and developing improved radiation countermeasures.
Yu-suke Torisawa, PhD, of Kyoto University in Japan, and his colleagues conducted this research.
The team used a tissue engineering approach to induce formation of new marrow-containing bone in mice. They then surgically removed the bone, placed it in a microfluidic device, and continuously perfused it with medium in vitro.
Next, the researchers set out to determine if the device would keep the engineered bone marrow alive so they could perform tests on it.
To test the system, the team analyzed the dynamics of blood cell production and evaluated the radiation-protecting effects of granulocyte-colony stimulating factor (G-CSF) and bactericidal/permeability-increasing protein (BPI).
Experiments showed the microfluidic device could maintain hematopoietic stem and progenitor cells in normal proportions for at least 2 weeks in culture.
Over time, the researchers observed increases in the number of leukocytes and red blood cells in the microfluidic circulation. And they found that adding erythropoietin induced a significant increase in erythrocyte production.
When the researchers exposed the engineered bone marrow to gamma radiation, they saw reduced leukocyte production.
And when they treated the engineered bone marrow with G-CSF or BPI, the team saw significant increases in the number of hematopoietic stem cells and myeloid cells in the fluidic outflow.
On the other hand, BPI did not have such an effect on static bone marrow cultures. But the researchers pointed out that previous work has shown BPI can accelerate recovery from radiation-induced toxicity in vivo.
The team therefore concluded that, unlike static bone marrow cultures, engineered bone marrow grown in a microfluidic device effectively mimics the recovery response of bone marrow in the body. ![]()
Transparency doesn’t lower healthcare spending

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()

Photo by Petr Kratochvil
Providing patients with a tool that enabled them to search for healthcare prices did not decrease their spending, according to a study published in JAMA.
Researchers studied the Truven Health Analytics Treatment Cost Calculator, an online price transparency tool that tells users how much they would pay out of pocket for services such as X-rays, lab tests, outpatient surgeries, or physician office visits at different sites.
The out-of-pocket cost estimates are based on the users’ health plan benefits and on how much they have already spent on healthcare during the year.
Two large national companies offered this tool to their employees in 2011 and 2012.
The researchers compared the healthcare spending patterns of employees (n=148,655) at these companies in the year before and after the tool was introduced with patterns among employees (n=295,983) of other companies that did not offer the tool.
Overall, having access to the tool was not associated with a reduction in outpatient spending, and subjects did not switch from more expensive outpatient hospital-based care to lower-cost settings.
The average outpatient spending among employees offered the tool was $2021 in the year before the tool was introduced and $2233 in the year after. Among control subjects, average outpatient spending increased from $1985 to $2138.
The average outpatient out-of-pocket spending among employees offered the tool was $507 in the year before it was introduced and $555 in the year after. In the control group, the average outpatient out-of-pocket spending increased from $490 to $520.
After the researchers adjusted for demographic and health characteristics, being offered the tool was associated with an average $59 increase in outpatient spending and an average $18 increase in out-of-pocket spending.
When the researchers looked only at patients with higher deductibles—who would be expected to have greater price-shopping incentives—they also found no evidence of reduction in spending.
“Despite large variation in healthcare prices, prevalence of high-deductible health plans, and widespread interest in price transparency, we did not find evidence that offering price transparency to employees generated savings,” said study author Sunita Desai, PhD, of Harvard Medical School in Boston, Massachusetts.
A possible explanation for this finding is that most patients did not actually use the tool. Only 10% of the employees who were offered the tool used it at least once in the first 12 months.
When patients did use the tool, more than half the searches were for relatively expensive services of over $1000.
“For expensive care that exceeds their deductible, patients may not see any reason to switch,” said study author Ateev Mehrotra, MD, also of Harvard Medical School. “They do not save by choosing a lower-cost provider, even if the health plan does.”
Still, the researchers said the tool does provide patients with valuable information, including their expected out-of-pocket costs, their deductible, and their health plan’s provider network.
“People might use the tools more—and focus more on choosing lower-priced care options—if they are combined with additional health plan benefit features that give greater incentive to price shop,” Dr Desai said. ![]()
FDA grants priority review for blinatumomab

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()

and solution for infusion
Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application for blinatumomab (Blincyto) as a treatment for pediatric and adolescent patients with Philadelphia chromosome negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The Prescription Drug User Fee Act target action date for the supplemental biologics license application for blinatumomab is September 1, 2016.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab was previously granted breakthrough therapy and priority review designations by the FDA and is now approved in the US for the treatment of adults with Ph- relapsed or refractory B-cell precursor ALL.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials.
Blinatumomab is marketed by Amgen as Blincyto. The full US prescribing information is available at www.BLINCYTO.com.
‘205 trial
The supplemental biologics license application for blinatumomab in pediatric and adolescent patients is based on data from the phase 1/2 '205 trial.
In this study, researchers evaluated blinatumomab in patients younger than 18 years of age. The patients had B-cell precursor ALL that was refractory, had relapsed at least twice, or relapsed after an allogeneic hematopoietic stem cell transplant.
Treatment in this study has been completed, and subjects are being monitored for long-term efficacy. The data is being submitted for publication.
Preliminary data were presented at the 2014 ASH Annual Meeting (abstract 3703). ![]()
Low fitness levels linked to platelet activation in women

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()

Women with poor physical fitness may have an increased risk of thrombotic events due to increased platelet activation, according to research published in Medicine & Science in Sports & Exercise.
In a study of 62 young women, those with poor cardiorespiratory fitness (CRF) had significantly higher platelet activation than women with average to very good CRF.
However, the study also showed that women with poor CRF were able to achieve normal platelet function by increasing their physical fitness.
The women achieved platelet normalization via endurance training—running for a maximum of 40 minutes—3 times a week over a 2-month period.
“Latently activated platelets release a number of mediators that can encourage the development of atherosclerotic vascular changes,” said study author Stefan Heber, of the Medical University of Vienna in Austria.
“If poor physical fitness is accompanied by a higher level of platelet activation, one can conclude that it also has an influence upon the early stages of this pathogenesis. The training effects we’ve found here are consistent with epidemiological data, according to which fit people have an approximately 40% lower risk of cardiovascular events than those who were physically inactive.”
To uncover their findings, Heber and his colleagues analyzed different aspects of platelet function in healthy, non-smoking women with low CRF, medium CRF, and high CRF.
The team assessed CRF using maximal oxygen consumption (VO2max), which was determined by an incremental treadmill exercise test. VO2max criteria were:
- less than 45 ml/min/kg bodyweight for the low CRF group
- 45 to 55 ml/min/kg bodyweight for the medium CRF group
- more than 55 ml/min/kg bodyweight for the high CRF group.
The researchers assessed platelet activation state and platelet reactivity in these groups of women by evaluating basal and agonist-induced surface expression of CD62P and CD40L, as well as the intraplatelet amount of reactive oxygen species.
The team observed higher basal platelet activation and agonist-induced platelet reactivity in the low CRF group, when compared to the medium and high CRF groups. However, platelet activation and reactivity were roughly the same in the medium and high CRF groups.
For the low CRF group, the researchers assessed platelet function again after the women completed a supervised endurance training program that lasted 2 menstrual cycles. The team found this program was able to normalize platelet function. ![]()
The Perfect Storm: Delivery system reform and precision medicine for all
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
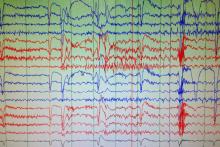
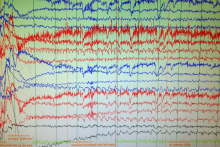
Case Study: Unclear Spells
Stephanie E. MacIver, MD
Dr. MacIver will graduate residency in July 2016 and will be starting her neurophysiology fellowship at the University of South Florida.
Selim R. Benbadis, MD
Dr. Benbadis is Professor and Director of the Comprehensive Epilepsy Program at the University of South Florida and Tampa General Hospital in Tampa, Florida.
A 35-year-old right-handed woman with anxiety has a five-year history of possible syncopal events associated with pronounced tachycardia, which occur about twice a month. The events are preceded by palpitations, anxiety, and rarely a sense of déjà vu. The patient feels that if she is able to lie down and raise her legs above her head, she can prevent a full episode from happening. If she is unable prevent the spell, then she will have altered awareness for 1 to 3 minutes and will frequently bite her lower lip. The patient also reports an isolated episode of bladder incontinence. After a spell, she may feel confused for 2 to 10 minutes. As far as she can remember, these events only occur in the daytime.
She was initially evaluated by a cardiologist who suspected a diagnosis of either orthostatic hypotension or postural orthostatic tachycardia syndrome (POTS). Her tilt-table testing was inconclusive. After she failed to respond to midodrine and metoprolol, she had an implantable loop recorder placed and was referred to a neurologist. Her routine EEG and MRI were normal. She presented no other risk factors for epilepsy; however she was started on a trial dose of Keppra (levetiracetam) 500 mg twice daily. The patient was unsure if the addition of Keppra helped, so she was referred to an epilepsy monitoring unit for further clarification.
While she was hospitalized, Keppra was withheld, which lead to her typical event. She endorsed an anxiety-provoking sensation and palpitations. On the single-lead EKG her pulse went from the 60s to the 130s. She tried to stop the event by lying down and propping her feet on the railing at the foot of the bed. Initially, she was able to respond to nursing staff promptly, but as the duration of the event continued, her responses slowed yet remained appropriate. The total duration of the entire episode was 1 minute and 14 seconds. EEG demonstrated an electrographic seizure with focal onset in the right posterior hemisphere.
EEG at the onset of her seizure:
EEG evolution:
At discharge, this patient was started on the higher dose of Keppra 750 mg twice daily with the plan to increase until the events are controlled. Her cardiologist removed the loop recorder, titrated her off midodrine, and is considering reduction of metoprolol.
The correct diagnosis for this patient is focal epilepsy (right posterior hemisphere).
Stephanie E. MacIver, MD
Dr. MacIver will graduate residency in July 2016 and will be starting her neurophysiology fellowship at the University of South Florida.
Selim R. Benbadis, MD
Dr. Benbadis is Professor and Director of the Comprehensive Epilepsy Program at the University of South Florida and Tampa General Hospital in Tampa, Florida.
A 35-year-old right-handed woman with anxiety has a five-year history of possible syncopal events associated with pronounced tachycardia, which occur about twice a month. The events are preceded by palpitations, anxiety, and rarely a sense of déjà vu. The patient feels that if she is able to lie down and raise her legs above her head, she can prevent a full episode from happening. If she is unable prevent the spell, then she will have altered awareness for 1 to 3 minutes and will frequently bite her lower lip. The patient also reports an isolated episode of bladder incontinence. After a spell, she may feel confused for 2 to 10 minutes. As far as she can remember, these events only occur in the daytime.
She was initially evaluated by a cardiologist who suspected a diagnosis of either orthostatic hypotension or postural orthostatic tachycardia syndrome (POTS). Her tilt-table testing was inconclusive. After she failed to respond to midodrine and metoprolol, she had an implantable loop recorder placed and was referred to a neurologist. Her routine EEG and MRI were normal. She presented no other risk factors for epilepsy; however she was started on a trial dose of Keppra (levetiracetam) 500 mg twice daily. The patient was unsure if the addition of Keppra helped, so she was referred to an epilepsy monitoring unit for further clarification.
While she was hospitalized, Keppra was withheld, which lead to her typical event. She endorsed an anxiety-provoking sensation and palpitations. On the single-lead EKG her pulse went from the 60s to the 130s. She tried to stop the event by lying down and propping her feet on the railing at the foot of the bed. Initially, she was able to respond to nursing staff promptly, but as the duration of the event continued, her responses slowed yet remained appropriate. The total duration of the entire episode was 1 minute and 14 seconds. EEG demonstrated an electrographic seizure with focal onset in the right posterior hemisphere.
EEG at the onset of her seizure:
EEG evolution:
At discharge, this patient was started on the higher dose of Keppra 750 mg twice daily with the plan to increase until the events are controlled. Her cardiologist removed the loop recorder, titrated her off midodrine, and is considering reduction of metoprolol.
The correct diagnosis for this patient is focal epilepsy (right posterior hemisphere).
Stephanie E. MacIver, MD
Dr. MacIver will graduate residency in July 2016 and will be starting her neurophysiology fellowship at the University of South Florida.
Selim R. Benbadis, MD
Dr. Benbadis is Professor and Director of the Comprehensive Epilepsy Program at the University of South Florida and Tampa General Hospital in Tampa, Florida.
A 35-year-old right-handed woman with anxiety has a five-year history of possible syncopal events associated with pronounced tachycardia, which occur about twice a month. The events are preceded by palpitations, anxiety, and rarely a sense of déjà vu. The patient feels that if she is able to lie down and raise her legs above her head, she can prevent a full episode from happening. If she is unable prevent the spell, then she will have altered awareness for 1 to 3 minutes and will frequently bite her lower lip. The patient also reports an isolated episode of bladder incontinence. After a spell, she may feel confused for 2 to 10 minutes. As far as she can remember, these events only occur in the daytime.
She was initially evaluated by a cardiologist who suspected a diagnosis of either orthostatic hypotension or postural orthostatic tachycardia syndrome (POTS). Her tilt-table testing was inconclusive. After she failed to respond to midodrine and metoprolol, she had an implantable loop recorder placed and was referred to a neurologist. Her routine EEG and MRI were normal. She presented no other risk factors for epilepsy; however she was started on a trial dose of Keppra (levetiracetam) 500 mg twice daily. The patient was unsure if the addition of Keppra helped, so she was referred to an epilepsy monitoring unit for further clarification.
While she was hospitalized, Keppra was withheld, which lead to her typical event. She endorsed an anxiety-provoking sensation and palpitations. On the single-lead EKG her pulse went from the 60s to the 130s. She tried to stop the event by lying down and propping her feet on the railing at the foot of the bed. Initially, she was able to respond to nursing staff promptly, but as the duration of the event continued, her responses slowed yet remained appropriate. The total duration of the entire episode was 1 minute and 14 seconds. EEG demonstrated an electrographic seizure with focal onset in the right posterior hemisphere.
EEG at the onset of her seizure:
EEG evolution:
At discharge, this patient was started on the higher dose of Keppra 750 mg twice daily with the plan to increase until the events are controlled. Her cardiologist removed the loop recorder, titrated her off midodrine, and is considering reduction of metoprolol.
The correct diagnosis for this patient is focal epilepsy (right posterior hemisphere).
Paperwork snarls stand between kids and at-school asthma medications
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
AT THE PAS ANNUAL MEETING
Key clinical point: Four out of five high-risk elementary school children lacked proper orders for at-school asthma medication administration.
Major finding: The average number of elementary school children with asthma medication orders on file was 21% at year’s end.
Data source: Yearlong study of 10 inner-city Milwaukee elementary schools; enrollees with asthma ranged from about 40 to nearly 200.
Disclosures: The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
Clinical Pearls for the Extended Focused Assessment With Sonography for Trauma Examination
The extended focused assessment with sonography for trauma (EFAST) examination provides rapid point-of-care (POC) evaluation of patients with thoracoabdominal trauma. This article offers essential clinical pearls to ensure an accurate and thorough examination, including tips on proper gain adjustment, correct probe fanning, shadow removal, visualization of the paracolic gutters, seeking the “spine sign” to determine effusion, and assessing effusion or consolidation of the lung.
Turning Down the Gain
Too much gain (signal amplification) will wash out the ultrasound image, making it challenging to detect small quantities of free fluid. This is especially true in the pelvic windows. Sound waves travel easily through the fluid-filled bladder and a posterior acoustic enhancement artifact will make the far field of the image appear too bright, obscuring small quantities of fluid (Figure 1). To correct this issue without changing the gain of the entire image, the far-field gain can be adjusted on most ultrasound devices by using the time-gain compensation bar or a far field gain knob.
Fanning Is Key
With the probe placed at a single location on the skin, one can dramatically change the structures visualized by fanning (tilting the probe). The image visualized on the ultrasound screen represents only a single slice of the anatomy—one that is about the thickness of a credit card. A single image therefore can only show structures that are within that thin beam of the probe. Just as one would not make a clinical decision based on a single-slice computed tomography (CT) scan image, the same is true of ultrasound. By fanning the probe toward the anterior and posterior abdomen, the clinician will catch smaller quantities of free fluid within each quadrant. A good rule of thumb is to scan through the entire organ of interest from edge-to-edge (eg, the entire bladder when imaging the pelvic window; the entire kidney in the right upper quadrant (RUQ) window; the entire spleen in the left upper quadrant [LUQ]).
Get Rid of the Rib Shadows
The RUQ and LUQ windows can be difficult to visualize when the view is obscured by rib shadows. To minimize/remove rib shadows, some clinicians prefer to use the phased array probe, which has a small footprint that fits easily in the intercostal space. Clinicians who prefer using the curvilinear probe should place the probe at an oblique angle (Figure 2); this probe will fit between the ribs and remove shadowing artifacts.
Remember the Paracolic
In some patients, the paracolic gutters are the most dependent portion of the abdomen and the first place where free fluid collects. When evaluating the RUQ, the clinician should first identify Morrison’s pouch, which is the interface between the liver and the kidney. After this pouch has been identified, the clinician should slide the probe toward the patient’s feet, paying close attention to the area around the inferior tip of the liver, and continue sliding the probe down to the inferior tip of the kidney, looking for fluid layering above the kidney or the psoas muscle (Figure 3). The same holds true for the LUQ technique. Once one has looked between the spleen and the diaphragm for free fluid, the probe should be moved down to the flank to evaluate the inferior tip of the spleen and the region anterior to the kidney.
The Left Upper Quadrant—Do Not Let the Stomach Fake You Out
A fluid-filled stomach can be a fake-out for free fluid appearing black on ultrasound (Figure 4). Remember, free fluid in the LUQ window will typically appear between the spleen and the diaphragm or at either pole of the spleen, so the clinician should pay particular attention to these areas. When evaluating the LUQ, a good rule of thumb is to place one’s hand on the patient’s bed while holding the probe; this will ensure that the scan is sufficiently posterior. The probe may also need to be fanned toward the bed to identify the kidneys in the retroperitoneum.
Look in the Chest and Remember the Spine Sign
Rapidly identifying a hemothorax can be a critical finding on the EFAST examination. Therefore, it is important to remember that air in the lungs scatters sound waves, so one does not normally visualize distinct structures that are deep to the pleural line. This is why the spine is not typically visible in the chest above the level of the diaphragm. When pathology is present, however, the sound waves are not blocked by air-filled lungs and one can see the “spine sign,” which suggests the presence of either effusion or consolidation of the lung (Figure 5).
Tough Cardiac Window? Try These Tips
A subxiphoid window is typically used to assess for pericardial effusion. To obtain this view, the clinician usually needs to increase the depth setting by a few centimeters (typically to around 18 cm). When the patient is able to do so, he or she may assist in the examination by bending his or her knees or taking a deep breath to help bring the heart into view. Despite these efforts, however, in some patients, it is technically impossible to obtain a subxiphoid view. In such cases, switching to an alternate view, such as the parasternal window, may be successful in visualizing the subxiphoid region.
Summary
Proper gain adjustment, thorough scanning of the thoracoabdominal region, and knowledge of common artifacts and signs are essential to ensuring an accurate and thorough POC EFAST examination.
The extended focused assessment with sonography for trauma (EFAST) examination provides rapid point-of-care (POC) evaluation of patients with thoracoabdominal trauma. This article offers essential clinical pearls to ensure an accurate and thorough examination, including tips on proper gain adjustment, correct probe fanning, shadow removal, visualization of the paracolic gutters, seeking the “spine sign” to determine effusion, and assessing effusion or consolidation of the lung.
Turning Down the Gain
Too much gain (signal amplification) will wash out the ultrasound image, making it challenging to detect small quantities of free fluid. This is especially true in the pelvic windows. Sound waves travel easily through the fluid-filled bladder and a posterior acoustic enhancement artifact will make the far field of the image appear too bright, obscuring small quantities of fluid (Figure 1). To correct this issue without changing the gain of the entire image, the far-field gain can be adjusted on most ultrasound devices by using the time-gain compensation bar or a far field gain knob.
Fanning Is Key
With the probe placed at a single location on the skin, one can dramatically change the structures visualized by fanning (tilting the probe). The image visualized on the ultrasound screen represents only a single slice of the anatomy—one that is about the thickness of a credit card. A single image therefore can only show structures that are within that thin beam of the probe. Just as one would not make a clinical decision based on a single-slice computed tomography (CT) scan image, the same is true of ultrasound. By fanning the probe toward the anterior and posterior abdomen, the clinician will catch smaller quantities of free fluid within each quadrant. A good rule of thumb is to scan through the entire organ of interest from edge-to-edge (eg, the entire bladder when imaging the pelvic window; the entire kidney in the right upper quadrant (RUQ) window; the entire spleen in the left upper quadrant [LUQ]).
Get Rid of the Rib Shadows
The RUQ and LUQ windows can be difficult to visualize when the view is obscured by rib shadows. To minimize/remove rib shadows, some clinicians prefer to use the phased array probe, which has a small footprint that fits easily in the intercostal space. Clinicians who prefer using the curvilinear probe should place the probe at an oblique angle (Figure 2); this probe will fit between the ribs and remove shadowing artifacts.
Remember the Paracolic
In some patients, the paracolic gutters are the most dependent portion of the abdomen and the first place where free fluid collects. When evaluating the RUQ, the clinician should first identify Morrison’s pouch, which is the interface between the liver and the kidney. After this pouch has been identified, the clinician should slide the probe toward the patient’s feet, paying close attention to the area around the inferior tip of the liver, and continue sliding the probe down to the inferior tip of the kidney, looking for fluid layering above the kidney or the psoas muscle (Figure 3). The same holds true for the LUQ technique. Once one has looked between the spleen and the diaphragm for free fluid, the probe should be moved down to the flank to evaluate the inferior tip of the spleen and the region anterior to the kidney.
The Left Upper Quadrant—Do Not Let the Stomach Fake You Out
A fluid-filled stomach can be a fake-out for free fluid appearing black on ultrasound (Figure 4). Remember, free fluid in the LUQ window will typically appear between the spleen and the diaphragm or at either pole of the spleen, so the clinician should pay particular attention to these areas. When evaluating the LUQ, a good rule of thumb is to place one’s hand on the patient’s bed while holding the probe; this will ensure that the scan is sufficiently posterior. The probe may also need to be fanned toward the bed to identify the kidneys in the retroperitoneum.
Look in the Chest and Remember the Spine Sign
Rapidly identifying a hemothorax can be a critical finding on the EFAST examination. Therefore, it is important to remember that air in the lungs scatters sound waves, so one does not normally visualize distinct structures that are deep to the pleural line. This is why the spine is not typically visible in the chest above the level of the diaphragm. When pathology is present, however, the sound waves are not blocked by air-filled lungs and one can see the “spine sign,” which suggests the presence of either effusion or consolidation of the lung (Figure 5).
Tough Cardiac Window? Try These Tips
A subxiphoid window is typically used to assess for pericardial effusion. To obtain this view, the clinician usually needs to increase the depth setting by a few centimeters (typically to around 18 cm). When the patient is able to do so, he or she may assist in the examination by bending his or her knees or taking a deep breath to help bring the heart into view. Despite these efforts, however, in some patients, it is technically impossible to obtain a subxiphoid view. In such cases, switching to an alternate view, such as the parasternal window, may be successful in visualizing the subxiphoid region.
Summary
Proper gain adjustment, thorough scanning of the thoracoabdominal region, and knowledge of common artifacts and signs are essential to ensuring an accurate and thorough POC EFAST examination.
The extended focused assessment with sonography for trauma (EFAST) examination provides rapid point-of-care (POC) evaluation of patients with thoracoabdominal trauma. This article offers essential clinical pearls to ensure an accurate and thorough examination, including tips on proper gain adjustment, correct probe fanning, shadow removal, visualization of the paracolic gutters, seeking the “spine sign” to determine effusion, and assessing effusion or consolidation of the lung.
Turning Down the Gain
Too much gain (signal amplification) will wash out the ultrasound image, making it challenging to detect small quantities of free fluid. This is especially true in the pelvic windows. Sound waves travel easily through the fluid-filled bladder and a posterior acoustic enhancement artifact will make the far field of the image appear too bright, obscuring small quantities of fluid (Figure 1). To correct this issue without changing the gain of the entire image, the far-field gain can be adjusted on most ultrasound devices by using the time-gain compensation bar or a far field gain knob.
Fanning Is Key
With the probe placed at a single location on the skin, one can dramatically change the structures visualized by fanning (tilting the probe). The image visualized on the ultrasound screen represents only a single slice of the anatomy—one that is about the thickness of a credit card. A single image therefore can only show structures that are within that thin beam of the probe. Just as one would not make a clinical decision based on a single-slice computed tomography (CT) scan image, the same is true of ultrasound. By fanning the probe toward the anterior and posterior abdomen, the clinician will catch smaller quantities of free fluid within each quadrant. A good rule of thumb is to scan through the entire organ of interest from edge-to-edge (eg, the entire bladder when imaging the pelvic window; the entire kidney in the right upper quadrant (RUQ) window; the entire spleen in the left upper quadrant [LUQ]).
Get Rid of the Rib Shadows
The RUQ and LUQ windows can be difficult to visualize when the view is obscured by rib shadows. To minimize/remove rib shadows, some clinicians prefer to use the phased array probe, which has a small footprint that fits easily in the intercostal space. Clinicians who prefer using the curvilinear probe should place the probe at an oblique angle (Figure 2); this probe will fit between the ribs and remove shadowing artifacts.
Remember the Paracolic
In some patients, the paracolic gutters are the most dependent portion of the abdomen and the first place where free fluid collects. When evaluating the RUQ, the clinician should first identify Morrison’s pouch, which is the interface between the liver and the kidney. After this pouch has been identified, the clinician should slide the probe toward the patient’s feet, paying close attention to the area around the inferior tip of the liver, and continue sliding the probe down to the inferior tip of the kidney, looking for fluid layering above the kidney or the psoas muscle (Figure 3). The same holds true for the LUQ technique. Once one has looked between the spleen and the diaphragm for free fluid, the probe should be moved down to the flank to evaluate the inferior tip of the spleen and the region anterior to the kidney.
The Left Upper Quadrant—Do Not Let the Stomach Fake You Out
A fluid-filled stomach can be a fake-out for free fluid appearing black on ultrasound (Figure 4). Remember, free fluid in the LUQ window will typically appear between the spleen and the diaphragm or at either pole of the spleen, so the clinician should pay particular attention to these areas. When evaluating the LUQ, a good rule of thumb is to place one’s hand on the patient’s bed while holding the probe; this will ensure that the scan is sufficiently posterior. The probe may also need to be fanned toward the bed to identify the kidneys in the retroperitoneum.
Look in the Chest and Remember the Spine Sign
Rapidly identifying a hemothorax can be a critical finding on the EFAST examination. Therefore, it is important to remember that air in the lungs scatters sound waves, so one does not normally visualize distinct structures that are deep to the pleural line. This is why the spine is not typically visible in the chest above the level of the diaphragm. When pathology is present, however, the sound waves are not blocked by air-filled lungs and one can see the “spine sign,” which suggests the presence of either effusion or consolidation of the lung (Figure 5).
Tough Cardiac Window? Try These Tips
A subxiphoid window is typically used to assess for pericardial effusion. To obtain this view, the clinician usually needs to increase the depth setting by a few centimeters (typically to around 18 cm). When the patient is able to do so, he or she may assist in the examination by bending his or her knees or taking a deep breath to help bring the heart into view. Despite these efforts, however, in some patients, it is technically impossible to obtain a subxiphoid view. In such cases, switching to an alternate view, such as the parasternal window, may be successful in visualizing the subxiphoid region.
Summary
Proper gain adjustment, thorough scanning of the thoracoabdominal region, and knowledge of common artifacts and signs are essential to ensuring an accurate and thorough POC EFAST examination.
Thrower’s Fracture of the Humerus
Case
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
Case
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
Case
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
The Things You Do (but Don’t) See Every Day …
Fifty-year-old twin sisters are seen for what appears to be the same problem: changes in the color and texture of their neck skin, recently noted by a family member. Both deny ever noticing it before and further deny experiencing any associated symptoms.
The sisters are accompanying their mother, who is being treated for basal cell carcinoma. All three women grew up living and working on the family farm, spending a great deal of time in the sun from an early age.
EXAMINATION
Both patients have type III skin, meaning that they seldom burn, tan fairly easily, and are able to keep that tan. The patients have identical, obvious hypo- and hyperpigmentation covering the sides of their faces and necks, sparing a well-defined oval area on the submental anterior neck.
The affected areas appear hyperemic, displaying a reddish brown tinge, but fail to blanch with digital pressure. The changes are completely macular.
What is the diagnosis?
DISCUSSION
The changes seen with poikiloderma of Civatte (PC) are obvious to the observer (see photograph) but appear so gradually that the patient often fails to notice them until they are pointed out. Both patients in this case almost certainly have had the condition for years.
PC was first described by Achille Civatte in France in 1923, around the same time that sunbathing became popular. Prior to that time, most women carefully protected their skin from the sun.
Civatte and others found that virtually all PC patients had a history of chronic overexposure to the sun, and that the clinical features of the condition—especially the characteristic sparing of the anterior neck skin (due to shading by the chin)—supported this hypothesis. They were also able to detect histologic changes (eg, solar elastosis) on biopsy, which further corroborated this theory.
There is little doubt that chronic overexposure to UV radiation is the cause, though other factors appear to be involved, as this case illustrates. For example, PC affects far more women than men, which suggests a possible role for hormones. It also appears that, in some cases, the tendency to develop PC is due to the genetic inheritance of an increased susceptibility to UV light. The mother of the two women in this case also had PC.
The range of presentations of PC includes involvement of the chest, face, and posterior neck. It should also be noted that somewhat similar patterns can be seen in other diseases, such as lupus, dermatomyositis, and early cutaneous T-cell lymphoma. Careful history-taking and biopsy, when indicated, will serve to distinguish between these diagnoses.
PC is usually left untreated, although lasers have been successfully used on motivated patients.
TAKE-HOME LEARNING POINTS
• Poikiloderma of Civatte (PC) is quite common and is seen more in women than men.
• Chronic overexposure to UV light is the primary cause of PC, but other factors—such as gender and heredity—appear to be involved.
• The term poikiloderma refers to the variability of the red, brown, and white color changes, as well as the solar atrophy seen with this condition.
• The differential for PC includes lupus, dermatomyositis, and early cutaneous T-cell lymphoma.
Fifty-year-old twin sisters are seen for what appears to be the same problem: changes in the color and texture of their neck skin, recently noted by a family member. Both deny ever noticing it before and further deny experiencing any associated symptoms.
The sisters are accompanying their mother, who is being treated for basal cell carcinoma. All three women grew up living and working on the family farm, spending a great deal of time in the sun from an early age.
EXAMINATION
Both patients have type III skin, meaning that they seldom burn, tan fairly easily, and are able to keep that tan. The patients have identical, obvious hypo- and hyperpigmentation covering the sides of their faces and necks, sparing a well-defined oval area on the submental anterior neck.
The affected areas appear hyperemic, displaying a reddish brown tinge, but fail to blanch with digital pressure. The changes are completely macular.
What is the diagnosis?
DISCUSSION
The changes seen with poikiloderma of Civatte (PC) are obvious to the observer (see photograph) but appear so gradually that the patient often fails to notice them until they are pointed out. Both patients in this case almost certainly have had the condition for years.
PC was first described by Achille Civatte in France in 1923, around the same time that sunbathing became popular. Prior to that time, most women carefully protected their skin from the sun.
Civatte and others found that virtually all PC patients had a history of chronic overexposure to the sun, and that the clinical features of the condition—especially the characteristic sparing of the anterior neck skin (due to shading by the chin)—supported this hypothesis. They were also able to detect histologic changes (eg, solar elastosis) on biopsy, which further corroborated this theory.
There is little doubt that chronic overexposure to UV radiation is the cause, though other factors appear to be involved, as this case illustrates. For example, PC affects far more women than men, which suggests a possible role for hormones. It also appears that, in some cases, the tendency to develop PC is due to the genetic inheritance of an increased susceptibility to UV light. The mother of the two women in this case also had PC.
The range of presentations of PC includes involvement of the chest, face, and posterior neck. It should also be noted that somewhat similar patterns can be seen in other diseases, such as lupus, dermatomyositis, and early cutaneous T-cell lymphoma. Careful history-taking and biopsy, when indicated, will serve to distinguish between these diagnoses.
PC is usually left untreated, although lasers have been successfully used on motivated patients.
TAKE-HOME LEARNING POINTS
• Poikiloderma of Civatte (PC) is quite common and is seen more in women than men.
• Chronic overexposure to UV light is the primary cause of PC, but other factors—such as gender and heredity—appear to be involved.
• The term poikiloderma refers to the variability of the red, brown, and white color changes, as well as the solar atrophy seen with this condition.
• The differential for PC includes lupus, dermatomyositis, and early cutaneous T-cell lymphoma.
Fifty-year-old twin sisters are seen for what appears to be the same problem: changes in the color and texture of their neck skin, recently noted by a family member. Both deny ever noticing it before and further deny experiencing any associated symptoms.
The sisters are accompanying their mother, who is being treated for basal cell carcinoma. All three women grew up living and working on the family farm, spending a great deal of time in the sun from an early age.
EXAMINATION
Both patients have type III skin, meaning that they seldom burn, tan fairly easily, and are able to keep that tan. The patients have identical, obvious hypo- and hyperpigmentation covering the sides of their faces and necks, sparing a well-defined oval area on the submental anterior neck.
The affected areas appear hyperemic, displaying a reddish brown tinge, but fail to blanch with digital pressure. The changes are completely macular.
What is the diagnosis?
DISCUSSION
The changes seen with poikiloderma of Civatte (PC) are obvious to the observer (see photograph) but appear so gradually that the patient often fails to notice them until they are pointed out. Both patients in this case almost certainly have had the condition for years.
PC was first described by Achille Civatte in France in 1923, around the same time that sunbathing became popular. Prior to that time, most women carefully protected their skin from the sun.
Civatte and others found that virtually all PC patients had a history of chronic overexposure to the sun, and that the clinical features of the condition—especially the characteristic sparing of the anterior neck skin (due to shading by the chin)—supported this hypothesis. They were also able to detect histologic changes (eg, solar elastosis) on biopsy, which further corroborated this theory.
There is little doubt that chronic overexposure to UV radiation is the cause, though other factors appear to be involved, as this case illustrates. For example, PC affects far more women than men, which suggests a possible role for hormones. It also appears that, in some cases, the tendency to develop PC is due to the genetic inheritance of an increased susceptibility to UV light. The mother of the two women in this case also had PC.
The range of presentations of PC includes involvement of the chest, face, and posterior neck. It should also be noted that somewhat similar patterns can be seen in other diseases, such as lupus, dermatomyositis, and early cutaneous T-cell lymphoma. Careful history-taking and biopsy, when indicated, will serve to distinguish between these diagnoses.
PC is usually left untreated, although lasers have been successfully used on motivated patients.
TAKE-HOME LEARNING POINTS
• Poikiloderma of Civatte (PC) is quite common and is seen more in women than men.
• Chronic overexposure to UV light is the primary cause of PC, but other factors—such as gender and heredity—appear to be involved.
• The term poikiloderma refers to the variability of the red, brown, and white color changes, as well as the solar atrophy seen with this condition.
• The differential for PC includes lupus, dermatomyositis, and early cutaneous T-cell lymphoma.