User login
US docs call for single-payer health reform

Photo by Matthew Lester
A group of US physicians has called for the creation of a publicly financed, single-payer national health program that would cover all Americans for all medically necessary care.
The proposal, which was drafted by a panel of 39 physicians, was announced in an editorial published in the American Journal of Public Health.
The proposal currently has more than 2000 signatures from physicians practicing in 48 states and the District of Columbia.
“Our nation is at a crossroads,” said Adam Gaffney, MD, a pulmonary disease and critical care specialist in Boston, Massachusetts, who is lead author of the editorial and co-chair of the working group that drafted the proposal.
“Despite the passage of the Affordable Care Act 6 years ago, 30 million Americans remain uninsured, an even greater number are underinsured, financial barriers to care like co-pays and deductibles are rising, bureaucracy is growing, provider networks are narrowing, and medical costs are continuing to climb.”
Dr Gaffney and his colleagues described their publicly financed, single-payer national health program (NHP) as follows.
Patients could choose to visit any doctor and hospital. Most hospitals and clinics would remain privately owned and operated, receiving a budget from the NHP to cover all operating costs. Physicians could continue to practice on a fee-for-service basis or receive salaries from group practices, hospitals, or clinics.
The program would be paid for by combining current sources of government health spending into a single fund with new taxes that would be fully offset by reductions in premiums and out-of-pocket spending. Co-pays and deductibles would be eliminated.
The single-payer program would save about $500 billion annually by eliminating the high overhead and profits of insurance firms and the paperwork they require from hospitals and doctors.
The administrative savings of the system would fully offset the costs of covering the uninsured and upgraded coverage for everyone else—eg, full coverage of prescription drugs, dental care, and long-term care. Savings would also be redirected to currently underfunded health priorities, particularly public health.
The “single payer” would be in a position to negotiate lower prices for medications and other medical supplies.
More details and documents related to the physicians’ proposal are available on the Physicians for a National Health Program website.
The Physicians for a National Health Program is a nonpartisan, nonprofit research and education organization founded in 1987. The organization had no role in funding the aforementioned proposal or editorial. ![]()

Photo by Matthew Lester
A group of US physicians has called for the creation of a publicly financed, single-payer national health program that would cover all Americans for all medically necessary care.
The proposal, which was drafted by a panel of 39 physicians, was announced in an editorial published in the American Journal of Public Health.
The proposal currently has more than 2000 signatures from physicians practicing in 48 states and the District of Columbia.
“Our nation is at a crossroads,” said Adam Gaffney, MD, a pulmonary disease and critical care specialist in Boston, Massachusetts, who is lead author of the editorial and co-chair of the working group that drafted the proposal.
“Despite the passage of the Affordable Care Act 6 years ago, 30 million Americans remain uninsured, an even greater number are underinsured, financial barriers to care like co-pays and deductibles are rising, bureaucracy is growing, provider networks are narrowing, and medical costs are continuing to climb.”
Dr Gaffney and his colleagues described their publicly financed, single-payer national health program (NHP) as follows.
Patients could choose to visit any doctor and hospital. Most hospitals and clinics would remain privately owned and operated, receiving a budget from the NHP to cover all operating costs. Physicians could continue to practice on a fee-for-service basis or receive salaries from group practices, hospitals, or clinics.
The program would be paid for by combining current sources of government health spending into a single fund with new taxes that would be fully offset by reductions in premiums and out-of-pocket spending. Co-pays and deductibles would be eliminated.
The single-payer program would save about $500 billion annually by eliminating the high overhead and profits of insurance firms and the paperwork they require from hospitals and doctors.
The administrative savings of the system would fully offset the costs of covering the uninsured and upgraded coverage for everyone else—eg, full coverage of prescription drugs, dental care, and long-term care. Savings would also be redirected to currently underfunded health priorities, particularly public health.
The “single payer” would be in a position to negotiate lower prices for medications and other medical supplies.
More details and documents related to the physicians’ proposal are available on the Physicians for a National Health Program website.
The Physicians for a National Health Program is a nonpartisan, nonprofit research and education organization founded in 1987. The organization had no role in funding the aforementioned proposal or editorial. ![]()

Photo by Matthew Lester
A group of US physicians has called for the creation of a publicly financed, single-payer national health program that would cover all Americans for all medically necessary care.
The proposal, which was drafted by a panel of 39 physicians, was announced in an editorial published in the American Journal of Public Health.
The proposal currently has more than 2000 signatures from physicians practicing in 48 states and the District of Columbia.
“Our nation is at a crossroads,” said Adam Gaffney, MD, a pulmonary disease and critical care specialist in Boston, Massachusetts, who is lead author of the editorial and co-chair of the working group that drafted the proposal.
“Despite the passage of the Affordable Care Act 6 years ago, 30 million Americans remain uninsured, an even greater number are underinsured, financial barriers to care like co-pays and deductibles are rising, bureaucracy is growing, provider networks are narrowing, and medical costs are continuing to climb.”
Dr Gaffney and his colleagues described their publicly financed, single-payer national health program (NHP) as follows.
Patients could choose to visit any doctor and hospital. Most hospitals and clinics would remain privately owned and operated, receiving a budget from the NHP to cover all operating costs. Physicians could continue to practice on a fee-for-service basis or receive salaries from group practices, hospitals, or clinics.
The program would be paid for by combining current sources of government health spending into a single fund with new taxes that would be fully offset by reductions in premiums and out-of-pocket spending. Co-pays and deductibles would be eliminated.
The single-payer program would save about $500 billion annually by eliminating the high overhead and profits of insurance firms and the paperwork they require from hospitals and doctors.
The administrative savings of the system would fully offset the costs of covering the uninsured and upgraded coverage for everyone else—eg, full coverage of prescription drugs, dental care, and long-term care. Savings would also be redirected to currently underfunded health priorities, particularly public health.
The “single payer” would be in a position to negotiate lower prices for medications and other medical supplies.
More details and documents related to the physicians’ proposal are available on the Physicians for a National Health Program website.
The Physicians for a National Health Program is a nonpartisan, nonprofit research and education organization founded in 1987. The organization had no role in funding the aforementioned proposal or editorial. ![]()
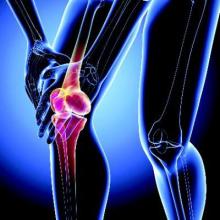
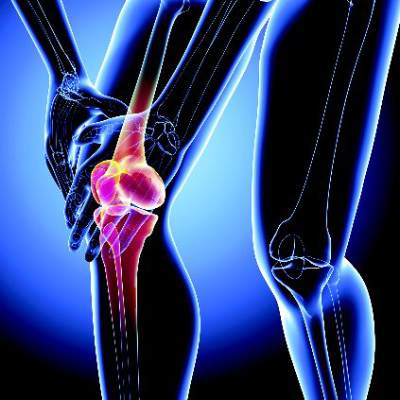
Adolescent knee pain ‘not benign,’ linked to later OA
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
AT RHEUMATOLOGY 2016
Key clinical point: Knee pain in adolescence was directly linked to later development of knee osteoarthritis.
Major finding: Adolescent knee pain increased the likelihood for developing OA, with an adjusted odds ratio of 7.5 (P = .014).
Data source: Case-control study of 190 adults with patellofemoral OA and 445 controls without patellofemoral OA who had arthroplasty.
Disclosures: Mr. Conchie and his coauthors had nothing to disclose.
Drilling down on end-of-life health care costs in heart failure
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
CHICAGO – Health care costs for heart failure patients spike dramatically in the last 6 months of life, with lack of adherence to guideline-directed outpatient medical therapy being the major modifiable factor driving the costs, Jason P. Swindle, Ph.D., reported at the annual meeting of the American College of Cardiology.
He presented a retrospective study of heart failure–related and total health care costs during the final 24 months of life for 48,026 Medicare Advantage managed care plan members with heart failure.
The researchers were interested in exploring possible racial/ethnic differences in costs, particularly in light of evidence that African Americans have a higher risk of heart failure and higher all-cause mortality. And while a first look at the data indicated racial differences in the size of end-of-life cost spikes, those differences lost their significance in multivariate analysis.
“Lack of guideline-directed outpatient heart failure therapy was a key. Also older age and the presence of coronary heart disease – those were really the big three items that were driving the spike in costs,” said Dr. Swindle of the Chicago office of Optum, a health care consulting group.
He was quick to add that, since the study was based upon administrative data, the lack of adherence to guideline-directed therapy may be unrelated to physician prescribing.
“We see the prescriptions that patients are actually filling. Patients may very well be seeing their cardiologist and being prescribed a medication, but they simply don’t fill the prescription,” he explained in an interview.
Over patients’ final 2 years of life, semiannual all-cause health care costs climbed from a baseline of roughly $10,000 during months 24-19 before death by about 4-fold during months 6-1 before death. Heart failure–related medical costs jumped 10-fold in Asians, 7.8-fold in Hispanics, 6.6-fold in African Americans, and 6.7-fold in whites. Most of the increases occurred in the final 6 months.
Zeroing in on the final 6 months of life, the mean cumulative total medical costs were $44,599, with heart failure–related medical costs accounting for $24,818 of that figure. Total medical costs averaged just under $5,000 during month 6 prior to death and rose roughly 3.5-fold over the remaining months.
This study was supported by Novartis Pharmaceuticals.
AT ACC 16
Key clinical point: Addressing lack of adherence to guideline-directed medical therapy could curb end-of-life health care costs in heart failure.
Major finding: Total monthly medical costs in heart failure patients during their final 6 months of life climbed roughly 3.5-fold.
Data source: This was a retrospective study of total and heart failure–related health care costs during the final 24 months of life for more than 48,000 patients with heart failure.
Disclosures: This study was supported by Novartis Pharmaceuticals. Dr. Swindle is an employee of Optum, which conducted the research.
8 Lessons for Hospitalists Turned Entrepreneurs
If you are a hospitalist, you are an entrepreneur almost by definition. All hospitalists are continuously engaged in improving the hospital experience for our patients. For some of us, the inner entrepreneur may grow to a point where we seriously consider a part-time or full-time commitment to an entrepreneurial dream. Combining our years of immersion in hospital patient care with an inventive streak can be a potent recipe for an innovative product or service idea.
It may be that the burgeoning startup scene in healthcare has inspired your dream. From coast to coast, there are startup incubators such as Rock Health, Healthbox, Blueprint Health, StartUp Health, Health Wildcatters, The Iron Yard, and TechSpring. These outfits support entrepreneurs with mentorship, funding, workspace, and/or information, such as how to deal with HIPAA or the FDA. Most of us have had at least a passing fascination with Steve Jobs–type characters, individuals who changed the world through their vision and force of will or who just seemed to enjoy a freedom that those who work for “The Man” will never know.
A few years ago, I caught the entrepreneurial bug. Initially, I continued with my day job and worked nights and weekends on my side project. Eventually, I made the leap to work full-time at an early-stage healthcare company. Since then, I’ve spent a lot of time trying to improve my new practice as a full-time entrepreneur, working as hard as ever, trying to be an effective innovator. Every day seems to bring new lessons—some more hard-earned than others—and there’s a lifetime of them still ahead. I’d like to share some of the insights I have learned on this journey. By the way, I still make time for patient care since that remains a priority for me.
Patience Is a Virtue, but Persistence and Positivity Count Even More
As Henry David Thoreau wrote, “Go confidently in the direction of your dreams.” Don’t postpone action indefinitely just because there are obstacles. Stop making excuses, make a start, and build momentum every day. Commit.
Becoming an entrepreneur is a long-term effort fueled by dedication and optimism, but first you have to make a start. You can’t win if you don’t play.
Action and Learning Matter More than Ideation
Start with your idea and a rough plan, but above all, believe in yourself, especially your ability to problem-solve. Many of the qualities that have fueled our success as physicians—precision, thoughtfulness, error aversion, and compulsiveness—might be constraints in a startup environment. Startups are hostile places for perfectionists and those who require complete information before proceeding. Have a bias for action and become comfortable with ambiguity. Entrepreneurs turn little things into big things by making progress every day.
Perhaps contrary to what we learn as physicians, entrepreneurs understand progress is measured more by authentic learning than by getting particular results. Entrepreneurs must quickly learn how to fail. In fact, progress often resembles multiple experiments that allow you to fail (and learn) faster. For entrepreneurs, perfection truly is the enemy of the good.
Learn, make adjustments, and progress will follow.
Guidance Is More Valuable than Money
Commercializing an idea is a challenging proposition. First-timers need advice, support, and help. For advice, find a mentor who has successfully launched a startup. Most of the successful people I know have had the wisdom or good fortune to have a mentor to provide guidance.
Startup incubators can be another source of support. Nearly all large cities and many medium and small cities now have business incubators or accelerators. Attend an event and get involved. They will provide many of the tools you will need to get started.
There are lots of opportunities for innovation in healthcare. But commercializing an idea will be one of the most challenging things you’ll ever do. Surround yourself with people who have skills that complement yours. Physician entrepreneurs need to be part of a viable team.
Sell, Sell, Sell
In business, as in life, “we’re all in sales.” We sell our ideas, our work product, ourselves. Even as physicians we have to sell patients and colleagues on our thought processes to be successful. Successful entrepreneurs are comfortable selling and put their best foot forward when trying to recruit a resource or persuade a potential customer.
Conflicts of Interest
“There is no interest without conflict.” If you look hard enough, you’ll see that we all have conflicts of interest. The key is to recognize them and disclose them. Of course, there are certain conflicts that are deal breakers. They must be avoided. If you remain employed, most of them are spelled out in your employer’s conflict of interest and intellectual property policies.
HIPAA Is an Innovation Killer
If your idea involves technology or services that address protected health information, become a HIPAA savant as soon as possible. The good news is that if you can effectively navigate the HIPAA challenge, you will have an advantage over your competitors.
Pure ‘Tech’ Plays Are Difficult
If you want to try to build the next killer app for healthcare and hope it will go viral, good luck. Based on my experience, it is difficult to get market traction with a pure technology offering. The strategy with a higher likelihood of success is to provide services with a technology platform that supports those services. As a provider of a service, you can provide immediate value to the customer and become “sticky” as you build your business (and software).
Enjoy the Journey, No Matter What
At first, you will be propelled by irrational exuberance and a passion for the greatness of your idea. That’s not only a good thing, it’s a requirement. But becoming a successful entrepreneur is a heavy haul down a long road of hard work and execution. Enjoying the journey is crucial since, beyond that, there are no guarantees. But life is short, so maybe you also value a career with no regrets. Take a chance and enjoy the ride.
Being a physician entrepreneur is not for everyone. But for those who take the plunge, it can be one of the most fulfilling, exciting, and meaningful journeys one could imagine. TH
Author note: I’d like to thank Dr. Jason Stein and Joe Miller for their helpful comments on this column.

If you are a hospitalist, you are an entrepreneur almost by definition. All hospitalists are continuously engaged in improving the hospital experience for our patients. For some of us, the inner entrepreneur may grow to a point where we seriously consider a part-time or full-time commitment to an entrepreneurial dream. Combining our years of immersion in hospital patient care with an inventive streak can be a potent recipe for an innovative product or service idea.
It may be that the burgeoning startup scene in healthcare has inspired your dream. From coast to coast, there are startup incubators such as Rock Health, Healthbox, Blueprint Health, StartUp Health, Health Wildcatters, The Iron Yard, and TechSpring. These outfits support entrepreneurs with mentorship, funding, workspace, and/or information, such as how to deal with HIPAA or the FDA. Most of us have had at least a passing fascination with Steve Jobs–type characters, individuals who changed the world through their vision and force of will or who just seemed to enjoy a freedom that those who work for “The Man” will never know.
A few years ago, I caught the entrepreneurial bug. Initially, I continued with my day job and worked nights and weekends on my side project. Eventually, I made the leap to work full-time at an early-stage healthcare company. Since then, I’ve spent a lot of time trying to improve my new practice as a full-time entrepreneur, working as hard as ever, trying to be an effective innovator. Every day seems to bring new lessons—some more hard-earned than others—and there’s a lifetime of them still ahead. I’d like to share some of the insights I have learned on this journey. By the way, I still make time for patient care since that remains a priority for me.
Patience Is a Virtue, but Persistence and Positivity Count Even More
As Henry David Thoreau wrote, “Go confidently in the direction of your dreams.” Don’t postpone action indefinitely just because there are obstacles. Stop making excuses, make a start, and build momentum every day. Commit.
Becoming an entrepreneur is a long-term effort fueled by dedication and optimism, but first you have to make a start. You can’t win if you don’t play.
Action and Learning Matter More than Ideation
Start with your idea and a rough plan, but above all, believe in yourself, especially your ability to problem-solve. Many of the qualities that have fueled our success as physicians—precision, thoughtfulness, error aversion, and compulsiveness—might be constraints in a startup environment. Startups are hostile places for perfectionists and those who require complete information before proceeding. Have a bias for action and become comfortable with ambiguity. Entrepreneurs turn little things into big things by making progress every day.
Perhaps contrary to what we learn as physicians, entrepreneurs understand progress is measured more by authentic learning than by getting particular results. Entrepreneurs must quickly learn how to fail. In fact, progress often resembles multiple experiments that allow you to fail (and learn) faster. For entrepreneurs, perfection truly is the enemy of the good.
Learn, make adjustments, and progress will follow.
Guidance Is More Valuable than Money
Commercializing an idea is a challenging proposition. First-timers need advice, support, and help. For advice, find a mentor who has successfully launched a startup. Most of the successful people I know have had the wisdom or good fortune to have a mentor to provide guidance.
Startup incubators can be another source of support. Nearly all large cities and many medium and small cities now have business incubators or accelerators. Attend an event and get involved. They will provide many of the tools you will need to get started.
There are lots of opportunities for innovation in healthcare. But commercializing an idea will be one of the most challenging things you’ll ever do. Surround yourself with people who have skills that complement yours. Physician entrepreneurs need to be part of a viable team.
Sell, Sell, Sell
In business, as in life, “we’re all in sales.” We sell our ideas, our work product, ourselves. Even as physicians we have to sell patients and colleagues on our thought processes to be successful. Successful entrepreneurs are comfortable selling and put their best foot forward when trying to recruit a resource or persuade a potential customer.
Conflicts of Interest
“There is no interest without conflict.” If you look hard enough, you’ll see that we all have conflicts of interest. The key is to recognize them and disclose them. Of course, there are certain conflicts that are deal breakers. They must be avoided. If you remain employed, most of them are spelled out in your employer’s conflict of interest and intellectual property policies.
HIPAA Is an Innovation Killer
If your idea involves technology or services that address protected health information, become a HIPAA savant as soon as possible. The good news is that if you can effectively navigate the HIPAA challenge, you will have an advantage over your competitors.
Pure ‘Tech’ Plays Are Difficult
If you want to try to build the next killer app for healthcare and hope it will go viral, good luck. Based on my experience, it is difficult to get market traction with a pure technology offering. The strategy with a higher likelihood of success is to provide services with a technology platform that supports those services. As a provider of a service, you can provide immediate value to the customer and become “sticky” as you build your business (and software).
Enjoy the Journey, No Matter What
At first, you will be propelled by irrational exuberance and a passion for the greatness of your idea. That’s not only a good thing, it’s a requirement. But becoming a successful entrepreneur is a heavy haul down a long road of hard work and execution. Enjoying the journey is crucial since, beyond that, there are no guarantees. But life is short, so maybe you also value a career with no regrets. Take a chance and enjoy the ride.
Being a physician entrepreneur is not for everyone. But for those who take the plunge, it can be one of the most fulfilling, exciting, and meaningful journeys one could imagine. TH
Author note: I’d like to thank Dr. Jason Stein and Joe Miller for their helpful comments on this column.

If you are a hospitalist, you are an entrepreneur almost by definition. All hospitalists are continuously engaged in improving the hospital experience for our patients. For some of us, the inner entrepreneur may grow to a point where we seriously consider a part-time or full-time commitment to an entrepreneurial dream. Combining our years of immersion in hospital patient care with an inventive streak can be a potent recipe for an innovative product or service idea.
It may be that the burgeoning startup scene in healthcare has inspired your dream. From coast to coast, there are startup incubators such as Rock Health, Healthbox, Blueprint Health, StartUp Health, Health Wildcatters, The Iron Yard, and TechSpring. These outfits support entrepreneurs with mentorship, funding, workspace, and/or information, such as how to deal with HIPAA or the FDA. Most of us have had at least a passing fascination with Steve Jobs–type characters, individuals who changed the world through their vision and force of will or who just seemed to enjoy a freedom that those who work for “The Man” will never know.
A few years ago, I caught the entrepreneurial bug. Initially, I continued with my day job and worked nights and weekends on my side project. Eventually, I made the leap to work full-time at an early-stage healthcare company. Since then, I’ve spent a lot of time trying to improve my new practice as a full-time entrepreneur, working as hard as ever, trying to be an effective innovator. Every day seems to bring new lessons—some more hard-earned than others—and there’s a lifetime of them still ahead. I’d like to share some of the insights I have learned on this journey. By the way, I still make time for patient care since that remains a priority for me.
Patience Is a Virtue, but Persistence and Positivity Count Even More
As Henry David Thoreau wrote, “Go confidently in the direction of your dreams.” Don’t postpone action indefinitely just because there are obstacles. Stop making excuses, make a start, and build momentum every day. Commit.
Becoming an entrepreneur is a long-term effort fueled by dedication and optimism, but first you have to make a start. You can’t win if you don’t play.
Action and Learning Matter More than Ideation
Start with your idea and a rough plan, but above all, believe in yourself, especially your ability to problem-solve. Many of the qualities that have fueled our success as physicians—precision, thoughtfulness, error aversion, and compulsiveness—might be constraints in a startup environment. Startups are hostile places for perfectionists and those who require complete information before proceeding. Have a bias for action and become comfortable with ambiguity. Entrepreneurs turn little things into big things by making progress every day.
Perhaps contrary to what we learn as physicians, entrepreneurs understand progress is measured more by authentic learning than by getting particular results. Entrepreneurs must quickly learn how to fail. In fact, progress often resembles multiple experiments that allow you to fail (and learn) faster. For entrepreneurs, perfection truly is the enemy of the good.
Learn, make adjustments, and progress will follow.
Guidance Is More Valuable than Money
Commercializing an idea is a challenging proposition. First-timers need advice, support, and help. For advice, find a mentor who has successfully launched a startup. Most of the successful people I know have had the wisdom or good fortune to have a mentor to provide guidance.
Startup incubators can be another source of support. Nearly all large cities and many medium and small cities now have business incubators or accelerators. Attend an event and get involved. They will provide many of the tools you will need to get started.
There are lots of opportunities for innovation in healthcare. But commercializing an idea will be one of the most challenging things you’ll ever do. Surround yourself with people who have skills that complement yours. Physician entrepreneurs need to be part of a viable team.
Sell, Sell, Sell
In business, as in life, “we’re all in sales.” We sell our ideas, our work product, ourselves. Even as physicians we have to sell patients and colleagues on our thought processes to be successful. Successful entrepreneurs are comfortable selling and put their best foot forward when trying to recruit a resource or persuade a potential customer.
Conflicts of Interest
“There is no interest without conflict.” If you look hard enough, you’ll see that we all have conflicts of interest. The key is to recognize them and disclose them. Of course, there are certain conflicts that are deal breakers. They must be avoided. If you remain employed, most of them are spelled out in your employer’s conflict of interest and intellectual property policies.
HIPAA Is an Innovation Killer
If your idea involves technology or services that address protected health information, become a HIPAA savant as soon as possible. The good news is that if you can effectively navigate the HIPAA challenge, you will have an advantage over your competitors.
Pure ‘Tech’ Plays Are Difficult
If you want to try to build the next killer app for healthcare and hope it will go viral, good luck. Based on my experience, it is difficult to get market traction with a pure technology offering. The strategy with a higher likelihood of success is to provide services with a technology platform that supports those services. As a provider of a service, you can provide immediate value to the customer and become “sticky” as you build your business (and software).
Enjoy the Journey, No Matter What
At first, you will be propelled by irrational exuberance and a passion for the greatness of your idea. That’s not only a good thing, it’s a requirement. But becoming a successful entrepreneur is a heavy haul down a long road of hard work and execution. Enjoying the journey is crucial since, beyond that, there are no guarantees. But life is short, so maybe you also value a career with no regrets. Take a chance and enjoy the ride.
Being a physician entrepreneur is not for everyone. But for those who take the plunge, it can be one of the most fulfilling, exciting, and meaningful journeys one could imagine. TH
Author note: I’d like to thank Dr. Jason Stein and Joe Miller for their helpful comments on this column.

Tackling the Readmissions Problem
Virtually every hospital system in the country deals with the challenge of readmissions, especially 30-day readmissions, and it’s only getting worse. “With the changes in healthcare and length of stay becoming shorter, patients are being discharged sicker than they used to be,” says Kevin Tolliver, MD, FACP, of Sidney & Lois Eskenazi Hospital Outpatient Care Center. “At our large public hospital system in Indianapolis, we designed an Internal Medicine Transitional Care Practice with the goal of decreasing readmission rates.”
Since October 2015, patients without a primary care doctor and those with a high LACE score have been referred to the new Transitional Care clinic. The first step: While still hospitalized, they meet briefly with Dr. Tolliver, who tells them, “‘You’re a candidate for this hospital follow-up clinic; this is why we think you would benefit.’ Patients, universally, are very thankful and eager to come.” The patients have their follow-up appointment scheduled before they are discharged.
At that appointment, the goal is to head off anything that would put them at risk for readmission. “We have a pharmacy, social workers, substance abuse counselors, diabetes educators—it’s one-stop shopping to address their needs,” Dr. Tolliver says. “Once we ensure that they’re not at risk for readmission, we help them get back to their primary care doctor or help them get one.”
Data for the clinic’s first four months show those patients who met with Dr. Tolliver before leaving the hospital were 50% more likely to keep their hospital follow-up visit. “That’s significant, particularly for us, because we take care of an indigent population; the no-show rate is usually our biggest challenge,” he says. Patients who were seen had a 30-day readmission rate of about 13.9%, while those who qualified but weren’t seen had a readmission rate of 21.8%.
“That has all kinds of positive consequences: less frustration for providers and patients and huge financial implications for the hospital system as well,” Dr. Tolliver says. “That there are these new models of post-discharge clinics out there and that there’s data suggesting that they work, particularly for a high-risk group of people, I think is worth knowing.”
Virtually every hospital system in the country deals with the challenge of readmissions, especially 30-day readmissions, and it’s only getting worse. “With the changes in healthcare and length of stay becoming shorter, patients are being discharged sicker than they used to be,” says Kevin Tolliver, MD, FACP, of Sidney & Lois Eskenazi Hospital Outpatient Care Center. “At our large public hospital system in Indianapolis, we designed an Internal Medicine Transitional Care Practice with the goal of decreasing readmission rates.”
Since October 2015, patients without a primary care doctor and those with a high LACE score have been referred to the new Transitional Care clinic. The first step: While still hospitalized, they meet briefly with Dr. Tolliver, who tells them, “‘You’re a candidate for this hospital follow-up clinic; this is why we think you would benefit.’ Patients, universally, are very thankful and eager to come.” The patients have their follow-up appointment scheduled before they are discharged.
At that appointment, the goal is to head off anything that would put them at risk for readmission. “We have a pharmacy, social workers, substance abuse counselors, diabetes educators—it’s one-stop shopping to address their needs,” Dr. Tolliver says. “Once we ensure that they’re not at risk for readmission, we help them get back to their primary care doctor or help them get one.”
Data for the clinic’s first four months show those patients who met with Dr. Tolliver before leaving the hospital were 50% more likely to keep their hospital follow-up visit. “That’s significant, particularly for us, because we take care of an indigent population; the no-show rate is usually our biggest challenge,” he says. Patients who were seen had a 30-day readmission rate of about 13.9%, while those who qualified but weren’t seen had a readmission rate of 21.8%.
“That has all kinds of positive consequences: less frustration for providers and patients and huge financial implications for the hospital system as well,” Dr. Tolliver says. “That there are these new models of post-discharge clinics out there and that there’s data suggesting that they work, particularly for a high-risk group of people, I think is worth knowing.”
Virtually every hospital system in the country deals with the challenge of readmissions, especially 30-day readmissions, and it’s only getting worse. “With the changes in healthcare and length of stay becoming shorter, patients are being discharged sicker than they used to be,” says Kevin Tolliver, MD, FACP, of Sidney & Lois Eskenazi Hospital Outpatient Care Center. “At our large public hospital system in Indianapolis, we designed an Internal Medicine Transitional Care Practice with the goal of decreasing readmission rates.”
Since October 2015, patients without a primary care doctor and those with a high LACE score have been referred to the new Transitional Care clinic. The first step: While still hospitalized, they meet briefly with Dr. Tolliver, who tells them, “‘You’re a candidate for this hospital follow-up clinic; this is why we think you would benefit.’ Patients, universally, are very thankful and eager to come.” The patients have their follow-up appointment scheduled before they are discharged.
At that appointment, the goal is to head off anything that would put them at risk for readmission. “We have a pharmacy, social workers, substance abuse counselors, diabetes educators—it’s one-stop shopping to address their needs,” Dr. Tolliver says. “Once we ensure that they’re not at risk for readmission, we help them get back to their primary care doctor or help them get one.”
Data for the clinic’s first four months show those patients who met with Dr. Tolliver before leaving the hospital were 50% more likely to keep their hospital follow-up visit. “That’s significant, particularly for us, because we take care of an indigent population; the no-show rate is usually our biggest challenge,” he says. Patients who were seen had a 30-day readmission rate of about 13.9%, while those who qualified but weren’t seen had a readmission rate of 21.8%.
“That has all kinds of positive consequences: less frustration for providers and patients and huge financial implications for the hospital system as well,” Dr. Tolliver says. “That there are these new models of post-discharge clinics out there and that there’s data suggesting that they work, particularly for a high-risk group of people, I think is worth knowing.”
A new paper-based test for the Zika virus
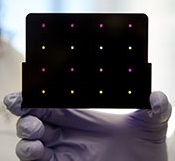
based test for Zika virus.
Purple dots indicate samples
infected with Zika, and yellow
dots indicate Zika-free samples.
Photo courtesy of the Wyss
Institute at Harvard University
A new paper-based test can diagnose Zika virus infection within a few hours, according to research published in Cell.
The test is based on technology previously developed to detect the Ebola virus.
In October 2014, researchers demonstrated that they could create synthetic gene networks and embed them on small discs of paper.
These gene networks can be programmed to detect a particular genetic sequence, which causes the paper to change color.
Upon learning about the Zika outbreak, the researchers decided to try adapting this technology to diagnose Zika.
“In a small number of weeks, we developed and validated a relatively rapid, inexpensive Zika diagnostic platform,” said study author James Collins, PhD, of the Massachusetts Institute of Technology in Cambridge.
Dr Collins and his colleagues developed sensors, embedded in the paper discs, that can detect 24 different RNA sequences found in the Zika viral genome. When the target RNA sequence is present, it initiates a series of interactions that turns the paper from yellow to purple.
This color change can be seen with the naked eye, but the researchers also developed an electronic reader that makes it easier to quantify the change, especially in cases where the sensor is detecting more than one RNA sequence.
All of the cellular components necessary for this process—including proteins, nucleic acids, and ribosomes—can be extracted from living cells and freeze-dried onto paper.
These paper discs can be stored at room temperature, making it easy to ship them to any location. Once rehydrated, all of the components function just as they would inside a living cell.
The researchers also incorporated a step that boosts the amount of viral RNA in the blood sample before exposing it to the sensor, using a system called nucleic acid sequence based amplification (NASBA). This amplification step, which takes 1 to 2 hours, increases the test’s sensitivity 1 million-fold.
The team tested this diagnostic platform using synthesized RNA sequences corresponding to the Zika genome, which were then added to human blood serum.
They found the test could detect very low viral RNA concentrations in those samples and could also distinguish Zika from dengue.
The researchers then tested samples taken from monkeys infected with the Zika virus. (Samples from humans affected by the current Zika outbreak were too difficult to obtain.)
The team found that, in these samples, the test could detect viral RNA concentrations as low as 2 or 3 parts per quadrillion.
The researchers believe this approach could also be adapted to other viruses that may emerge in the future. Dr Collins hopes to team up with other scientists to further develop the technology for diagnosing Zika.
“Here, we’ve done a nice proof-of-principle demonstration, but more work and additional testing would be needed to ensure safety and efficacy before actual deployment,” he said. “We’re not far off.” ![]()

based test for Zika virus.
Purple dots indicate samples
infected with Zika, and yellow
dots indicate Zika-free samples.
Photo courtesy of the Wyss
Institute at Harvard University
A new paper-based test can diagnose Zika virus infection within a few hours, according to research published in Cell.
The test is based on technology previously developed to detect the Ebola virus.
In October 2014, researchers demonstrated that they could create synthetic gene networks and embed them on small discs of paper.
These gene networks can be programmed to detect a particular genetic sequence, which causes the paper to change color.
Upon learning about the Zika outbreak, the researchers decided to try adapting this technology to diagnose Zika.
“In a small number of weeks, we developed and validated a relatively rapid, inexpensive Zika diagnostic platform,” said study author James Collins, PhD, of the Massachusetts Institute of Technology in Cambridge.
Dr Collins and his colleagues developed sensors, embedded in the paper discs, that can detect 24 different RNA sequences found in the Zika viral genome. When the target RNA sequence is present, it initiates a series of interactions that turns the paper from yellow to purple.
This color change can be seen with the naked eye, but the researchers also developed an electronic reader that makes it easier to quantify the change, especially in cases where the sensor is detecting more than one RNA sequence.
All of the cellular components necessary for this process—including proteins, nucleic acids, and ribosomes—can be extracted from living cells and freeze-dried onto paper.
These paper discs can be stored at room temperature, making it easy to ship them to any location. Once rehydrated, all of the components function just as they would inside a living cell.
The researchers also incorporated a step that boosts the amount of viral RNA in the blood sample before exposing it to the sensor, using a system called nucleic acid sequence based amplification (NASBA). This amplification step, which takes 1 to 2 hours, increases the test’s sensitivity 1 million-fold.
The team tested this diagnostic platform using synthesized RNA sequences corresponding to the Zika genome, which were then added to human blood serum.
They found the test could detect very low viral RNA concentrations in those samples and could also distinguish Zika from dengue.
The researchers then tested samples taken from monkeys infected with the Zika virus. (Samples from humans affected by the current Zika outbreak were too difficult to obtain.)
The team found that, in these samples, the test could detect viral RNA concentrations as low as 2 or 3 parts per quadrillion.
The researchers believe this approach could also be adapted to other viruses that may emerge in the future. Dr Collins hopes to team up with other scientists to further develop the technology for diagnosing Zika.
“Here, we’ve done a nice proof-of-principle demonstration, but more work and additional testing would be needed to ensure safety and efficacy before actual deployment,” he said. “We’re not far off.” ![]()

based test for Zika virus.
Purple dots indicate samples
infected with Zika, and yellow
dots indicate Zika-free samples.
Photo courtesy of the Wyss
Institute at Harvard University
A new paper-based test can diagnose Zika virus infection within a few hours, according to research published in Cell.
The test is based on technology previously developed to detect the Ebola virus.
In October 2014, researchers demonstrated that they could create synthetic gene networks and embed them on small discs of paper.
These gene networks can be programmed to detect a particular genetic sequence, which causes the paper to change color.
Upon learning about the Zika outbreak, the researchers decided to try adapting this technology to diagnose Zika.
“In a small number of weeks, we developed and validated a relatively rapid, inexpensive Zika diagnostic platform,” said study author James Collins, PhD, of the Massachusetts Institute of Technology in Cambridge.
Dr Collins and his colleagues developed sensors, embedded in the paper discs, that can detect 24 different RNA sequences found in the Zika viral genome. When the target RNA sequence is present, it initiates a series of interactions that turns the paper from yellow to purple.
This color change can be seen with the naked eye, but the researchers also developed an electronic reader that makes it easier to quantify the change, especially in cases where the sensor is detecting more than one RNA sequence.
All of the cellular components necessary for this process—including proteins, nucleic acids, and ribosomes—can be extracted from living cells and freeze-dried onto paper.
These paper discs can be stored at room temperature, making it easy to ship them to any location. Once rehydrated, all of the components function just as they would inside a living cell.
The researchers also incorporated a step that boosts the amount of viral RNA in the blood sample before exposing it to the sensor, using a system called nucleic acid sequence based amplification (NASBA). This amplification step, which takes 1 to 2 hours, increases the test’s sensitivity 1 million-fold.
The team tested this diagnostic platform using synthesized RNA sequences corresponding to the Zika genome, which were then added to human blood serum.
They found the test could detect very low viral RNA concentrations in those samples and could also distinguish Zika from dengue.
The researchers then tested samples taken from monkeys infected with the Zika virus. (Samples from humans affected by the current Zika outbreak were too difficult to obtain.)
The team found that, in these samples, the test could detect viral RNA concentrations as low as 2 or 3 parts per quadrillion.
The researchers believe this approach could also be adapted to other viruses that may emerge in the future. Dr Collins hopes to team up with other scientists to further develop the technology for diagnosing Zika.
“Here, we’ve done a nice proof-of-principle demonstration, but more work and additional testing would be needed to ensure safety and efficacy before actual deployment,” he said. “We’re not far off.” ![]()
NCI: Use of dug wells in New England linked with risk of bladder cancer
Increased risk of bladder cancer in New England may be partly due to drinking water from private wells, particularly dug wells established during the first half of the 20th century, according to researchers from the National Cancer Institute. Bladder cancer mortality rates have been elevated in northern New England for at least 5 decades. Incidence rates in Maine, New Hampshire, and Vermont are about 20% higher than in the United States overall, researchers said in a press release.
To explore reasons for the elevated risk, NCI investigators and colleagues in New England compared well water consumption, smoking, occupation, ancestry, use of wood-burning stoves, and consumption of various foods for 1,213 people in New England who were newly diagnosed with bladder cancer, and 1,418 people without bladder cancer who were matched by geographic area.
The amount of arsenic ingested through drinking water was estimated based on current levels and historical information. Increasing cumulative exposure was associated with an increasing risk of bladder cancer. Among people who used private wells, people who drank the most water had twice the risk of those who drank the least. Highest risk was seen among those who drank water from dug wells established before 1960, when the use of arsenic-based pesticides was common.
“Arsenic is an established cause of bladder cancer, largely based on observations from earlier studies in highly exposed populations,” said Debra Silverman, Sc.D., chief of the Occupational and Environmental Epidemiology Branch of the NCI, Rockville, Md., and senior author on the study. “However, emerging evidence suggests that low to moderate levels of exposure may also increase risk,” she said in the press release.
“Although smoking and employment in high-risk occupations both showed their expected associations with bladder cancer risk in this population, they were similar to those found in other populations,” Dr. Silverman said. “This suggests that neither risk factor explains the excess occurrence of bladder cancer in northern New England.”
These study results indicate historical consumption of water from private wells, particularly dug wells in an era when arsenic-based pesticides were widely used, may have contributed to the excess rate in New England residents, Dr. Silverman and colleagues concluded.
The study was published in the Journal of the National Cancer Institute (2016;108[9]:djw099).
On Twitter @NikolaidesLaura
Increased risk of bladder cancer in New England may be partly due to drinking water from private wells, particularly dug wells established during the first half of the 20th century, according to researchers from the National Cancer Institute. Bladder cancer mortality rates have been elevated in northern New England for at least 5 decades. Incidence rates in Maine, New Hampshire, and Vermont are about 20% higher than in the United States overall, researchers said in a press release.
To explore reasons for the elevated risk, NCI investigators and colleagues in New England compared well water consumption, smoking, occupation, ancestry, use of wood-burning stoves, and consumption of various foods for 1,213 people in New England who were newly diagnosed with bladder cancer, and 1,418 people without bladder cancer who were matched by geographic area.
The amount of arsenic ingested through drinking water was estimated based on current levels and historical information. Increasing cumulative exposure was associated with an increasing risk of bladder cancer. Among people who used private wells, people who drank the most water had twice the risk of those who drank the least. Highest risk was seen among those who drank water from dug wells established before 1960, when the use of arsenic-based pesticides was common.
“Arsenic is an established cause of bladder cancer, largely based on observations from earlier studies in highly exposed populations,” said Debra Silverman, Sc.D., chief of the Occupational and Environmental Epidemiology Branch of the NCI, Rockville, Md., and senior author on the study. “However, emerging evidence suggests that low to moderate levels of exposure may also increase risk,” she said in the press release.
“Although smoking and employment in high-risk occupations both showed their expected associations with bladder cancer risk in this population, they were similar to those found in other populations,” Dr. Silverman said. “This suggests that neither risk factor explains the excess occurrence of bladder cancer in northern New England.”
These study results indicate historical consumption of water from private wells, particularly dug wells in an era when arsenic-based pesticides were widely used, may have contributed to the excess rate in New England residents, Dr. Silverman and colleagues concluded.
The study was published in the Journal of the National Cancer Institute (2016;108[9]:djw099).
On Twitter @NikolaidesLaura
Increased risk of bladder cancer in New England may be partly due to drinking water from private wells, particularly dug wells established during the first half of the 20th century, according to researchers from the National Cancer Institute. Bladder cancer mortality rates have been elevated in northern New England for at least 5 decades. Incidence rates in Maine, New Hampshire, and Vermont are about 20% higher than in the United States overall, researchers said in a press release.
To explore reasons for the elevated risk, NCI investigators and colleagues in New England compared well water consumption, smoking, occupation, ancestry, use of wood-burning stoves, and consumption of various foods for 1,213 people in New England who were newly diagnosed with bladder cancer, and 1,418 people without bladder cancer who were matched by geographic area.
The amount of arsenic ingested through drinking water was estimated based on current levels and historical information. Increasing cumulative exposure was associated with an increasing risk of bladder cancer. Among people who used private wells, people who drank the most water had twice the risk of those who drank the least. Highest risk was seen among those who drank water from dug wells established before 1960, when the use of arsenic-based pesticides was common.
“Arsenic is an established cause of bladder cancer, largely based on observations from earlier studies in highly exposed populations,” said Debra Silverman, Sc.D., chief of the Occupational and Environmental Epidemiology Branch of the NCI, Rockville, Md., and senior author on the study. “However, emerging evidence suggests that low to moderate levels of exposure may also increase risk,” she said in the press release.
“Although smoking and employment in high-risk occupations both showed their expected associations with bladder cancer risk in this population, they were similar to those found in other populations,” Dr. Silverman said. “This suggests that neither risk factor explains the excess occurrence of bladder cancer in northern New England.”
These study results indicate historical consumption of water from private wells, particularly dug wells in an era when arsenic-based pesticides were widely used, may have contributed to the excess rate in New England residents, Dr. Silverman and colleagues concluded.
The study was published in the Journal of the National Cancer Institute (2016;108[9]:djw099).
On Twitter @NikolaidesLaura
Amaal J. Starling, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Brodalumab effective for rare, severe types of psoriasis
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The interluekin-17 inhibitor brodalumab significantly improved symptoms of generalized pustular psoriasis and psoriatic erythroderma in a small, Japanese open label study.
Major finding: Almost all brodalumab-treated patients with generalized pustular psoriasis (GPP) or psoriatic erythroderma (PsE) showed high levels of clinical improvement and low levels of adverse events.
Data sources: The phase III open-label multicenter study evaluated the safety and efficacy of brodalumab in 30 adults with GPP or PsE over 52 weeks.
Disclosures: Funding was provided by Kyowa Hakko Kirin. All authors disclosed ties to industry sources, including the funding source.
Reassurance on cardiovascular safety of Breo Ellipta for COPD
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
CHICAGO – Using an inhaled long-acting beta agonist and corticosteroid to treat patients with moderate chronic obstructive pulmonary disease who have known cardiovascular disease had no impact on the cardiovascular event rate in the landmark SUMMIT trial, Dr. David E. Newby reported at the annual meeting of the American College of Cardiology.
SUMMIT (the Study to Understand Mortality and Morbidity) was a randomized, double-blind, placebo-controlled, 43-country, four-arm clinical trial in which 16,485 patients with moderate COPD were placed on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg by dry powder inhaler (Breo Ellipta), either drug alone, or placebo for an average of 2 years. Two-thirds of participants had overt cardiovascular disease; the rest were at high risk as defined by age greater than 60 years plus the presence of two or more cardiovascular risk factors.
This was the first large prospective clinical trial to investigate the impact of respiratory therapy on survival in patients with two commonly comorbid conditions. The incidence of major adverse cardiovascular events – a prespecified secondary endpoint – was of major interest because some other long-acting beta agonists have been linked to increased cardiovascular risk, explained Dr. Newby of the University of Edinburgh.
The primary endpoint in SUMMIT was all-cause mortality. The 12.2% relative risk reduction in the fluticasone furoate/vilanterol group, compared with placebo, didn’t achieve statistical significance. Neither did the 7.4% reduction in the secondary composite endpoint of cardiovascular death, MI, stroke, unstable angina, or TIA seen in the corticosteroid/LABA group, which is reassuring from a safety standpoint, he noted.
A positive result was seen for another secondary endpoint: the rate of lung function decline as measured by forced expiratory volume in 1 second. The rate of decline was 8 mL/year less with fluticasone furoate/vilanterol, compared with placebo.
The Breo Ellipta group also had significantly lower rates of moderate or severe COPD exacerbations, hospitalization for exacerbations, and improved quality of life as measured by the St. George’s Respiratory Questionnaire – COPD total score at 12 months.
The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.
AT ACC 16
Key clinical point: Once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg to treat moderate COPD in patients with comorbid cardiovascular disease did not increase their risk of cardiovascular events.
Major finding: The major adverse cardiovascular event rate in patients on once-daily inhaled fluticasone furoate/vilanterol 100/25 mcg was 7.4% less than with placebo, a nonsignificant difference.
Data source: This was a randomized, double-blind, placebo-controlled, 2-year clinical trial including 16,485 patients with moderate COPD and overt cardiovascular disease or at high risk for it.
Disclosures: The SUMMIT trial was sponsored by GlaxoSmithKline. The presenter is a consultant to GSK and eight other pharmaceutical companies.