User login
The Current State of PHM Fellowships
Pediatric hospital medicine (PHM) fellowship programs came into existence approximately 20 years ago in Canada,[1] and since that time the number of programs in North America has grown dramatically. The first 3 PHM fellowship programs in the United States were initiated in 2003, and by 2008 there were 7 active programs. Just 5 years later in 2013, there were 20 fellowship programs in existence. Now, in 2015, there are over 30 programs, with several more in development. The goal of postresidency training in PHM is to improve the care of hospitalized children by training future hospitalists to provide high‐quality, evidence‐based clinical care and to generate new knowledge and scholarship in areas such as clinical research, patient safety and quality improvement, medical education, practice management, and patient outcomes.[2] Many pediatric hospitalists want to be able to perform research or quality improvement, but feel that they lack the time, skills, resources, and mentorship to do so.[3] To date, fellowship‐trained hospitalists have a demonstrated track record of contributing to the body of literature that is shaping the care of hospitalized children.[4, 5]
At present, PHM is not a recognized subspecialty of the American Board of Pediatrics (ABP) and therefore does not fall under the purview of the Accreditation Council of Graduate Medical Education (ACGME), leading to concern from some about the variability in depth and breadth of training across programs.[1] The development and publication of the PHM Core Competencies in 2010 helped define the scope of practice of pediatric hospitalists and provide guidelines for training programs, specifically with respect to clinical and nonclinical areas for assessment of competency.[6] Furthermore, studies of early career hospitalists have identified areas for future fellowship curriculum development, such as core procedural skills, quality improvement, and practice management.[7]
In an effort to address training variability across programs, PHM fellowship directors (FDs) have come together as an organized group, first meeting in 2008, with the primary goal of defining training standards and sharing curricular resources. Annual meetings of the FDs, sponsored by the American Academy of Pediatrics Section on Hospital Medicine (AAP‐SOHM), began in 2012. A key objective of this annual meeting has been to develop a standardized fellowship curriculum for use across programs as well as to determine gaps in training that need to be addressed. During this process, we have received input from key stakeholders including community hospitalists, internal medicine‐pediatrics hospitalists, and the PHM Certification Steering Committee, which organized the application for subspecialty certification to the ABP. To inform this process of curriculum standardization, we fielded a survey of PHM fellowship directors. The purpose of this article is to summarize the current curricula, operations, and logistics of PHM fellowship programs.
METHODS
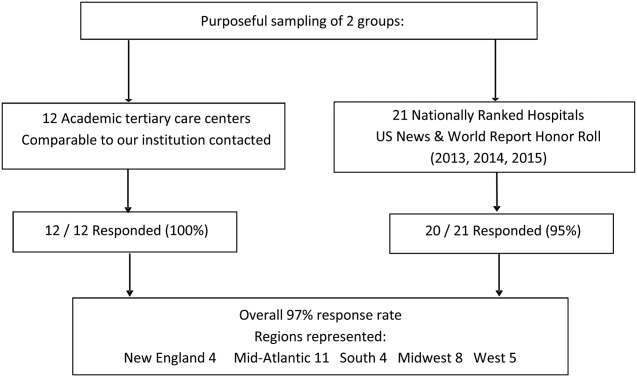
This was a cross‐sectional study of 31 PHM fellowship programs across the United States and Canada in April 2014. Inclusion criteria included all pediatric fellowships that were self‐identified to the AAP‐SOHM as providing a hospital medicine fellowship option. This included both PHM fellowships as well as academic general pediatric fellowships with a hospitalist track. A web‐based survey (SurveyMonkey, Inc.) was distributed by e‐mail to the FDs at the 31 training programs (see Supporting Information in the online version of this article). To enhance content validity of survey responses, survey questions were designed using an iterative consensus process among the authors, who included junior and senior FDs and represented the 2014 annual FD meeting planning committee. Items were created to gather feedback on the following key areas of PHM fellowships: program demographics, types of required and elective clinical rotations, graduate coursework offerings, amount of time spent in clinical activities, fellow billing practices, and description of fellows' research activities. The survey consisted of 30 multiple‐choice and short‐answer questions. Follow‐up e‐mail reminders were sent to all FDs 2 weeks and 4 weeks after the initial request was sent. Survey completion was voluntary, and no incentives were offered. The study was determined to be exempt by the Stanford University Institutional Review Board. Data were summarized using frequency distributions. No subgroup comparisons were made.
RESULTS
Program directors from 27/31 (87%) PHM fellowship programs responded to the survey; 25 were active programs, and 2 were under development. Responding programs represented all 4 major regions of the country and Canada, with varying program initiation dates, ranging from 1997 to 2013.
Program Demographics
The duration of most programs (17/27) was 2 years (63%), with 6 (22%) 1‐year programs and 4 (15%) 3‐year programs making up the remainder. Four programs described variable lengths, which could be tailored based on the fellow's individual interest. Two of the programs are 2 years in length, but offer a 1‐year option for fellows who wish to focus on enhancing clinical skills without an academic focus. The other 2 programs are 2 years in length, but will offer an extension to a third year for those pursuing a graduate degree.
Fellow Clinical Activities
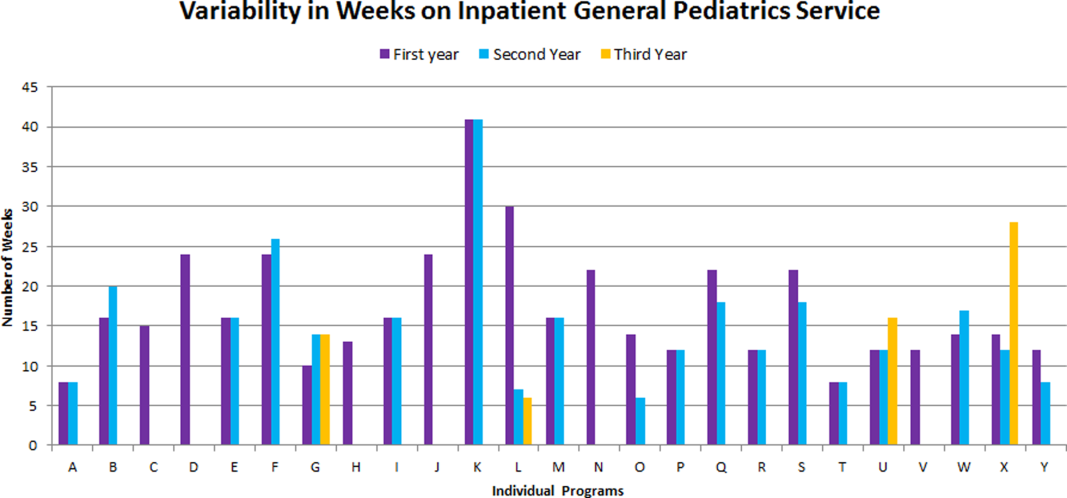
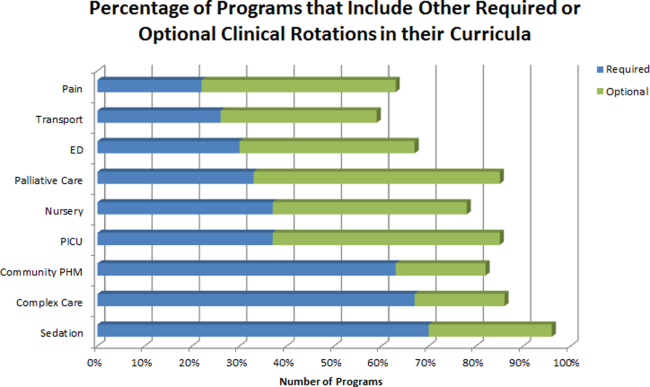
The average amount of total clinical time (weeks on service) across responding programs was 50% (range, 20%65%). When looking specifically at time on the inpatient general pediatric service, number of weeks varied by year of training and by institution, with 12 to 41 weeks in the first year of fellowship, 6 to 41 weeks in the second year of fellowship, and 6 to 28 weeks in the third year of fellowship (Figure 1). Though the range is large, on average, fellows spend 17 weeks on inpatient general pediatrics service during each year of training. Of note, the median number of weeks on inpatient general pediatrics service by year of training was 15 weeks, 16 weeks, and 16.5 weeks, respectively. In addition to inpatient general pediatrics service time, most programs require other clinical rotations, with sedation, complex care, and inpatient pediatrics at community sites being the most frequent (Figure 2). Of the 6 responding 1‐year programs, 5 (83%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 15 responding 2‐year programs, 11 (73%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 4 responding 3‐year programs, 2 (50%) allow their fellows to bill/generate clinical revenue at some point during their training.
Fellow Scholarly Activities
With respect to time dedicated to research, the majority of programs offer coursework such as courses for credit, noncredit courses, or certificate courses. In addition, 11 programs offer fellows a masters' degree in areas including public health, clinical science, epidemiology, education, academic sciences, healthcare quality, clinical and translational research, or health services administration. The majority of these degrees are paid for by departmental funds, with tuition reimbursement, university support, training grants, and personal funds making up the remainder. Twenty‐one (81%) programs provide a scholarship oversight committee for their fellows. Current fellows' (n = 63) primary areas of research are varied and include clinical research (36%), quality‐improvement research (22%), medical education research (20%), health services research (16%), and other areas (6%).
DISCUSSION
This is the most comprehensive description of pediatric hospital medicine fellowship curricula to date. Understanding the scope of these programs is an important first step in developing a standardized curriculum that can be used by all. The results of this survey indicate that although there is variability among PHM fellowship curricular content, several common themes exist.
The number of clinical weeks on the inpatient general pediatrics service varied from program to program, though the majority of programs require fellows to spend 15 to 16 weeks each year of training. The variability may be due in part to the way in which respondents defined the term week on clinical service. For example, if the fellow is primarily on a shift schedule, then he/she may only work 2 to 3 shifts in 1 week, which may have been viewed similarly to daily presence on a more traditional inpatient teaching service with 5 to 7 consecutive days of service. The current study did not explore the details of inpatient general pediatric clinical activities or exposure to opportunities to hone procedural skills, areas that are worth investigating as we move forward to better understand the needs of trainees.
Most residency training programs in general pediatrics require a significant amount of inpatient clinical time, specifically a minimum of 10 units or months, though only half of this time is required to be in inpatient general pediatrics.[8] Although nonfellowship trained early career hospitalists may feel adequately prepared to manage the clinical care of some hospitalized children, perceived competency is significantly lower than their fellowship‐trained colleagues with regard to care of the child with medical complexity and technology‐dependence, and with regard to provision of sedation for procedures.[7] The majority of FDs surveyed in our study indicated that additional clinical experience with sedation, complex care, and inpatient pediatrics at community sites were required of their fellows. Of note, many of these rotations are not commonly required in pediatric residency training programs; however, the PHM core competencies suggest that hospitalists should demonstrate proficiency in these areas to provide optimal care for hospitalized children. Our results suggest that current PHM fellowship curricula help address these clinical gaps. The requirement of these particular specialized experiences may reflect the clinical scope of practice that is expected from potential employers or may be related to staffing needs. It is well documented that the inpatient demographic of large pediatric tertiary care referral centers has changed over the past decade, with an increasing prevalence of children with medical complexity.[9, 10] In both tertiary referral centers and community hospitals, the expansion of the role of the hospitalist in providing specialized clinical services, such as sedation or surgical comanagement, has been significantly driven by financial factors, though a more recent focus on improvement of efficiency and quality of care within the hospital system has relied heavily on hospitalist input.[11, 12, 13] Important next steps in curriculum standardization include ensuring that training programs allow for adequate clinical exposure and proper assessment of competency in these areas, and determining the full complement of clinical training experiences that will produce hospitalists with a well‐defined scope of practice that adequately addresses the needs of hospitalized children.
Most fellowship‐trained hospitalists work primarily in university‐affiliated institutions with expectations for scholarly productivity.[5, 7] Fellowship‐trained hospitalists have made large contributions to the growing body of PHM literature, specifically in the realms of medical education, healthcare quality, clinical pediatrics, and healthcare outcomes.[4] Many PHM fellowship‐trained hospitalists have educational or administrative leadership roles.[2] Our results indicate that current PHM fellows continue to be active in a variety of research activities. In addition, FDs reported that the vast majority of programs included scholarship oversight committees, which ensure a mentored and structured research experience. Finally, most programs require or offer additional coursework, and many programs with university affiliations allow for attainment of graduate degrees. Inclusion of robust research training and infrastructure in all programs is a paramount goal of PHM fellowship training. This will allow graduates to be successful researchers, generating new knowledge and supporting the provision of high‐quality, evidence‐based, and value‐driven care for hospitalized children.
A unique feature of several PHM fellowship programs is that fellows are allowed to bill for clinical encounters. Many programs rely on clinical revenue to support fellow salaries.[14] For some programs, a portion of this clinical revenue comes from fellows billing for clinical encounters.[15] Programs that allow fellows to bill/generate clinical revenue have fellows working in attending roles without direct supervision, whereas nonbilling fellows have direct supervision by an attending.[15] In the current ABP training model, subspecialty fellows cannot independently bill for clinical encounters within their own subspecialty, though they can moonlight as long as they meet the duty hour requirements set forth by the ACGME.[16] FDs will need to consider the impact of this requirement on fellow autonomy and on financial revenue for funding fellow salaries if the field achieves ABP subspecialty status.
Regardless of whether or not PHM becomes a designated subspecialty of the ABP, FDs will continue to work together to develop a standard core curriculum that incorporates elements of clinical and nonclinical training to ensure that graduates not only provide high‐quality care for hospitalized children, but also generate new knowledge that advances the field in care delivery and quality of care in any setting. The results of this study will not only help to inform curriculum standardization, but also assessment and evaluation methods. Currently, PHM FDs meet annually and are nearing consensus on a standard 2‐year curriculum based on the PHM Core Competencies that incorporates core clinical, systems, and scholarly domains. We continue to solicit the input of stakeholders, including new FDs, community hospitalist leaders, internal medicine‐pediatrics hospitalist leaders, the Joint Council of Pediatric Hospital Medicine, and leaders of national organizations, such as the American Academy of Pediatrics, Academic Pediatrics Association, and Society of Hospital Medicine. Additional work around standardizing the fellowship application and recruitment process has resulted in our recent acceptance into the Fall Subspecialty Match through the National Residency Match Program, as well as development and implementation of a common fellowship application form. The FD group has recently formalized, voting into place an executive steering committee, which is responsible for the development and execution of long‐term goals that include finalizing a standardized curriculum, refining program and fellow assessment methods through critical evaluation of fellow metrics and outcomes, and standardization of evaluation methods.
Adopting a standard 2‐year curriculum may affect some programs, specifically those that are currently 1 year in duration. These programs would need to extend the length of their fellowship to allow for the breadth of experiences expected with a standardized 2‐year curriculum. This could result in significant financial challenges, effectively increasing the cost to administer the program. In addition, at present, programs have the flexibility to highlight individual areas of strength to attract candidates, allowing fellows to gain an in‐depth experience in domains such as clinical research, quality improvement, medical education, or health services research. With a standardized curriculum, some programs may have to assemble specific clinical and nonclinical experiences to meet the agreed‐upon expectations for PHM fellowship training. If these resources are not available, programs may need to seek relationships with other institutions to complete their offerings, a possibility that is being actively explored by this group. FDs continue to work with each other to share resources, identify training opportunities, and partner with each other to ensure that the requirements of a standard curriculum can be met.
This study has several limitations. First, it was a voluntary survey of program directors, and though we captured over 80% of programs at the time of the survey, there are currently more programs that have come into existence and more still that are in the development stage, leading to potential sampling error. Second, variable effort or accuracy by participants may have led to some degree of response error, such as content error or nonreporting error. Third, the survey questions focused on high‐level information, making it difficult to make nuanced comparisons between curricular elements or determine best curricular practice. In addition, this survey did not explore medical education and quality improvement activities of fellows, 2 major areas in which hospitalists play a major role in the inpatient setting.[1, 17, 18, 19, 20]
CONCLUSION
PHM fellowship programs have grown and continue to grow at a rapid rate. Variability in training is evident, both in clinical experiences and research experiences, though several common elements were identified in this study. The majority of programs are 2 years, and clinical experience comprises approximately 50% of training time, often including key rotations such as sedation, complex care, and rotations at community hospitals. Future directions include standardizing clinical training and expectations for scholarship, formulating appropriate methods for assessment of competency that can be used across programs, and seeking sustainable sources of funding.
Disclosure
Nothing to report.
- , . Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157–163.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , . Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38–44.
- , , , . Pediatric hospital medicine fellowships: outcomes and future directions. Paper presented at: Pediatric Hospital Medicine 2014; July 26, 2014; Orlando, FL.
- , , . Pediatric hospitalist research productivity: predictors of success at presenting abstracts and publishing peer‐reviewed manuscripts among pediatric hospitalists. Hosp Pediatr. 2012;2(3):149–160.
- , , . Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5:339–343.
- , , , . Perceived core competency achievements of fellowship and non‐fellowship early career pediatric hospitalists. J Hosp Med. 2015;10(6):373–389.
- Accreditation Council of Graduate Medical Education. ACGME program requirements for graduate medical education in pediatrics. Available at: https://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Published September 30, 2012. Accessed July 7, 2015.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , . The expanding role of hospitalists in the United States. Swiss Med Wkly. 2006;136:591–596.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , , , , . Development of a pediatric hospitalist sedation service: training and implementation. J Hosp Med. 2012;7(4):335–339.
- , . Sources of funding and support for pediatric hospital medicine fellowship programs. Poster presented at: Pediatric Hospital Medicine 2014; July 27, 2014; Orlando, FL.
- Council of Pediatric Hospital Medicine Fellowship Directors. Pediatric Hospital Medicine Fellowship Directors Annual Meeting: funding and return on investment. July 24, 2014.
- Accreditation Council of Graduate Medical Education. Frequently asked questions: ACGME common duty hour requirements. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/dh‐faqs2011.pdf. Updated June 18, 2014. Accessed July 7, 2015.
- , . Pediatric hospitalists: training, current practice and career goals. J Hosp Med. 2009;4(3):179–186.
- , . The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103:473–477.
- . Pediatric hospitalists and medical education. Pediatr Ann. 2014;43(7):e151–e156
- , , , et al. Quality improvement research in pediatric hospital medicine and the role of the Pediatric Research in Inpatient Settings (PRIS) network. Acad Pediatr. 2013;13(6):S54–S60.
Pediatric hospital medicine (PHM) fellowship programs came into existence approximately 20 years ago in Canada,[1] and since that time the number of programs in North America has grown dramatically. The first 3 PHM fellowship programs in the United States were initiated in 2003, and by 2008 there were 7 active programs. Just 5 years later in 2013, there were 20 fellowship programs in existence. Now, in 2015, there are over 30 programs, with several more in development. The goal of postresidency training in PHM is to improve the care of hospitalized children by training future hospitalists to provide high‐quality, evidence‐based clinical care and to generate new knowledge and scholarship in areas such as clinical research, patient safety and quality improvement, medical education, practice management, and patient outcomes.[2] Many pediatric hospitalists want to be able to perform research or quality improvement, but feel that they lack the time, skills, resources, and mentorship to do so.[3] To date, fellowship‐trained hospitalists have a demonstrated track record of contributing to the body of literature that is shaping the care of hospitalized children.[4, 5]
At present, PHM is not a recognized subspecialty of the American Board of Pediatrics (ABP) and therefore does not fall under the purview of the Accreditation Council of Graduate Medical Education (ACGME), leading to concern from some about the variability in depth and breadth of training across programs.[1] The development and publication of the PHM Core Competencies in 2010 helped define the scope of practice of pediatric hospitalists and provide guidelines for training programs, specifically with respect to clinical and nonclinical areas for assessment of competency.[6] Furthermore, studies of early career hospitalists have identified areas for future fellowship curriculum development, such as core procedural skills, quality improvement, and practice management.[7]
In an effort to address training variability across programs, PHM fellowship directors (FDs) have come together as an organized group, first meeting in 2008, with the primary goal of defining training standards and sharing curricular resources. Annual meetings of the FDs, sponsored by the American Academy of Pediatrics Section on Hospital Medicine (AAP‐SOHM), began in 2012. A key objective of this annual meeting has been to develop a standardized fellowship curriculum for use across programs as well as to determine gaps in training that need to be addressed. During this process, we have received input from key stakeholders including community hospitalists, internal medicine‐pediatrics hospitalists, and the PHM Certification Steering Committee, which organized the application for subspecialty certification to the ABP. To inform this process of curriculum standardization, we fielded a survey of PHM fellowship directors. The purpose of this article is to summarize the current curricula, operations, and logistics of PHM fellowship programs.
METHODS
This was a cross‐sectional study of 31 PHM fellowship programs across the United States and Canada in April 2014. Inclusion criteria included all pediatric fellowships that were self‐identified to the AAP‐SOHM as providing a hospital medicine fellowship option. This included both PHM fellowships as well as academic general pediatric fellowships with a hospitalist track. A web‐based survey (SurveyMonkey, Inc.) was distributed by e‐mail to the FDs at the 31 training programs (see Supporting Information in the online version of this article). To enhance content validity of survey responses, survey questions were designed using an iterative consensus process among the authors, who included junior and senior FDs and represented the 2014 annual FD meeting planning committee. Items were created to gather feedback on the following key areas of PHM fellowships: program demographics, types of required and elective clinical rotations, graduate coursework offerings, amount of time spent in clinical activities, fellow billing practices, and description of fellows' research activities. The survey consisted of 30 multiple‐choice and short‐answer questions. Follow‐up e‐mail reminders were sent to all FDs 2 weeks and 4 weeks after the initial request was sent. Survey completion was voluntary, and no incentives were offered. The study was determined to be exempt by the Stanford University Institutional Review Board. Data were summarized using frequency distributions. No subgroup comparisons were made.
RESULTS
Program directors from 27/31 (87%) PHM fellowship programs responded to the survey; 25 were active programs, and 2 were under development. Responding programs represented all 4 major regions of the country and Canada, with varying program initiation dates, ranging from 1997 to 2013.
Program Demographics
The duration of most programs (17/27) was 2 years (63%), with 6 (22%) 1‐year programs and 4 (15%) 3‐year programs making up the remainder. Four programs described variable lengths, which could be tailored based on the fellow's individual interest. Two of the programs are 2 years in length, but offer a 1‐year option for fellows who wish to focus on enhancing clinical skills without an academic focus. The other 2 programs are 2 years in length, but will offer an extension to a third year for those pursuing a graduate degree.
Fellow Clinical Activities
The average amount of total clinical time (weeks on service) across responding programs was 50% (range, 20%65%). When looking specifically at time on the inpatient general pediatric service, number of weeks varied by year of training and by institution, with 12 to 41 weeks in the first year of fellowship, 6 to 41 weeks in the second year of fellowship, and 6 to 28 weeks in the third year of fellowship (Figure 1). Though the range is large, on average, fellows spend 17 weeks on inpatient general pediatrics service during each year of training. Of note, the median number of weeks on inpatient general pediatrics service by year of training was 15 weeks, 16 weeks, and 16.5 weeks, respectively. In addition to inpatient general pediatrics service time, most programs require other clinical rotations, with sedation, complex care, and inpatient pediatrics at community sites being the most frequent (Figure 2). Of the 6 responding 1‐year programs, 5 (83%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 15 responding 2‐year programs, 11 (73%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 4 responding 3‐year programs, 2 (50%) allow their fellows to bill/generate clinical revenue at some point during their training.


Fellow Scholarly Activities
With respect to time dedicated to research, the majority of programs offer coursework such as courses for credit, noncredit courses, or certificate courses. In addition, 11 programs offer fellows a masters' degree in areas including public health, clinical science, epidemiology, education, academic sciences, healthcare quality, clinical and translational research, or health services administration. The majority of these degrees are paid for by departmental funds, with tuition reimbursement, university support, training grants, and personal funds making up the remainder. Twenty‐one (81%) programs provide a scholarship oversight committee for their fellows. Current fellows' (n = 63) primary areas of research are varied and include clinical research (36%), quality‐improvement research (22%), medical education research (20%), health services research (16%), and other areas (6%).
DISCUSSION
This is the most comprehensive description of pediatric hospital medicine fellowship curricula to date. Understanding the scope of these programs is an important first step in developing a standardized curriculum that can be used by all. The results of this survey indicate that although there is variability among PHM fellowship curricular content, several common themes exist.
The number of clinical weeks on the inpatient general pediatrics service varied from program to program, though the majority of programs require fellows to spend 15 to 16 weeks each year of training. The variability may be due in part to the way in which respondents defined the term week on clinical service. For example, if the fellow is primarily on a shift schedule, then he/she may only work 2 to 3 shifts in 1 week, which may have been viewed similarly to daily presence on a more traditional inpatient teaching service with 5 to 7 consecutive days of service. The current study did not explore the details of inpatient general pediatric clinical activities or exposure to opportunities to hone procedural skills, areas that are worth investigating as we move forward to better understand the needs of trainees.
Most residency training programs in general pediatrics require a significant amount of inpatient clinical time, specifically a minimum of 10 units or months, though only half of this time is required to be in inpatient general pediatrics.[8] Although nonfellowship trained early career hospitalists may feel adequately prepared to manage the clinical care of some hospitalized children, perceived competency is significantly lower than their fellowship‐trained colleagues with regard to care of the child with medical complexity and technology‐dependence, and with regard to provision of sedation for procedures.[7] The majority of FDs surveyed in our study indicated that additional clinical experience with sedation, complex care, and inpatient pediatrics at community sites were required of their fellows. Of note, many of these rotations are not commonly required in pediatric residency training programs; however, the PHM core competencies suggest that hospitalists should demonstrate proficiency in these areas to provide optimal care for hospitalized children. Our results suggest that current PHM fellowship curricula help address these clinical gaps. The requirement of these particular specialized experiences may reflect the clinical scope of practice that is expected from potential employers or may be related to staffing needs. It is well documented that the inpatient demographic of large pediatric tertiary care referral centers has changed over the past decade, with an increasing prevalence of children with medical complexity.[9, 10] In both tertiary referral centers and community hospitals, the expansion of the role of the hospitalist in providing specialized clinical services, such as sedation or surgical comanagement, has been significantly driven by financial factors, though a more recent focus on improvement of efficiency and quality of care within the hospital system has relied heavily on hospitalist input.[11, 12, 13] Important next steps in curriculum standardization include ensuring that training programs allow for adequate clinical exposure and proper assessment of competency in these areas, and determining the full complement of clinical training experiences that will produce hospitalists with a well‐defined scope of practice that adequately addresses the needs of hospitalized children.
Most fellowship‐trained hospitalists work primarily in university‐affiliated institutions with expectations for scholarly productivity.[5, 7] Fellowship‐trained hospitalists have made large contributions to the growing body of PHM literature, specifically in the realms of medical education, healthcare quality, clinical pediatrics, and healthcare outcomes.[4] Many PHM fellowship‐trained hospitalists have educational or administrative leadership roles.[2] Our results indicate that current PHM fellows continue to be active in a variety of research activities. In addition, FDs reported that the vast majority of programs included scholarship oversight committees, which ensure a mentored and structured research experience. Finally, most programs require or offer additional coursework, and many programs with university affiliations allow for attainment of graduate degrees. Inclusion of robust research training and infrastructure in all programs is a paramount goal of PHM fellowship training. This will allow graduates to be successful researchers, generating new knowledge and supporting the provision of high‐quality, evidence‐based, and value‐driven care for hospitalized children.
A unique feature of several PHM fellowship programs is that fellows are allowed to bill for clinical encounters. Many programs rely on clinical revenue to support fellow salaries.[14] For some programs, a portion of this clinical revenue comes from fellows billing for clinical encounters.[15] Programs that allow fellows to bill/generate clinical revenue have fellows working in attending roles without direct supervision, whereas nonbilling fellows have direct supervision by an attending.[15] In the current ABP training model, subspecialty fellows cannot independently bill for clinical encounters within their own subspecialty, though they can moonlight as long as they meet the duty hour requirements set forth by the ACGME.[16] FDs will need to consider the impact of this requirement on fellow autonomy and on financial revenue for funding fellow salaries if the field achieves ABP subspecialty status.
Regardless of whether or not PHM becomes a designated subspecialty of the ABP, FDs will continue to work together to develop a standard core curriculum that incorporates elements of clinical and nonclinical training to ensure that graduates not only provide high‐quality care for hospitalized children, but also generate new knowledge that advances the field in care delivery and quality of care in any setting. The results of this study will not only help to inform curriculum standardization, but also assessment and evaluation methods. Currently, PHM FDs meet annually and are nearing consensus on a standard 2‐year curriculum based on the PHM Core Competencies that incorporates core clinical, systems, and scholarly domains. We continue to solicit the input of stakeholders, including new FDs, community hospitalist leaders, internal medicine‐pediatrics hospitalist leaders, the Joint Council of Pediatric Hospital Medicine, and leaders of national organizations, such as the American Academy of Pediatrics, Academic Pediatrics Association, and Society of Hospital Medicine. Additional work around standardizing the fellowship application and recruitment process has resulted in our recent acceptance into the Fall Subspecialty Match through the National Residency Match Program, as well as development and implementation of a common fellowship application form. The FD group has recently formalized, voting into place an executive steering committee, which is responsible for the development and execution of long‐term goals that include finalizing a standardized curriculum, refining program and fellow assessment methods through critical evaluation of fellow metrics and outcomes, and standardization of evaluation methods.
Adopting a standard 2‐year curriculum may affect some programs, specifically those that are currently 1 year in duration. These programs would need to extend the length of their fellowship to allow for the breadth of experiences expected with a standardized 2‐year curriculum. This could result in significant financial challenges, effectively increasing the cost to administer the program. In addition, at present, programs have the flexibility to highlight individual areas of strength to attract candidates, allowing fellows to gain an in‐depth experience in domains such as clinical research, quality improvement, medical education, or health services research. With a standardized curriculum, some programs may have to assemble specific clinical and nonclinical experiences to meet the agreed‐upon expectations for PHM fellowship training. If these resources are not available, programs may need to seek relationships with other institutions to complete their offerings, a possibility that is being actively explored by this group. FDs continue to work with each other to share resources, identify training opportunities, and partner with each other to ensure that the requirements of a standard curriculum can be met.
This study has several limitations. First, it was a voluntary survey of program directors, and though we captured over 80% of programs at the time of the survey, there are currently more programs that have come into existence and more still that are in the development stage, leading to potential sampling error. Second, variable effort or accuracy by participants may have led to some degree of response error, such as content error or nonreporting error. Third, the survey questions focused on high‐level information, making it difficult to make nuanced comparisons between curricular elements or determine best curricular practice. In addition, this survey did not explore medical education and quality improvement activities of fellows, 2 major areas in which hospitalists play a major role in the inpatient setting.[1, 17, 18, 19, 20]
CONCLUSION
PHM fellowship programs have grown and continue to grow at a rapid rate. Variability in training is evident, both in clinical experiences and research experiences, though several common elements were identified in this study. The majority of programs are 2 years, and clinical experience comprises approximately 50% of training time, often including key rotations such as sedation, complex care, and rotations at community hospitals. Future directions include standardizing clinical training and expectations for scholarship, formulating appropriate methods for assessment of competency that can be used across programs, and seeking sustainable sources of funding.
Disclosure
Nothing to report.
Pediatric hospital medicine (PHM) fellowship programs came into existence approximately 20 years ago in Canada,[1] and since that time the number of programs in North America has grown dramatically. The first 3 PHM fellowship programs in the United States were initiated in 2003, and by 2008 there were 7 active programs. Just 5 years later in 2013, there were 20 fellowship programs in existence. Now, in 2015, there are over 30 programs, with several more in development. The goal of postresidency training in PHM is to improve the care of hospitalized children by training future hospitalists to provide high‐quality, evidence‐based clinical care and to generate new knowledge and scholarship in areas such as clinical research, patient safety and quality improvement, medical education, practice management, and patient outcomes.[2] Many pediatric hospitalists want to be able to perform research or quality improvement, but feel that they lack the time, skills, resources, and mentorship to do so.[3] To date, fellowship‐trained hospitalists have a demonstrated track record of contributing to the body of literature that is shaping the care of hospitalized children.[4, 5]
At present, PHM is not a recognized subspecialty of the American Board of Pediatrics (ABP) and therefore does not fall under the purview of the Accreditation Council of Graduate Medical Education (ACGME), leading to concern from some about the variability in depth and breadth of training across programs.[1] The development and publication of the PHM Core Competencies in 2010 helped define the scope of practice of pediatric hospitalists and provide guidelines for training programs, specifically with respect to clinical and nonclinical areas for assessment of competency.[6] Furthermore, studies of early career hospitalists have identified areas for future fellowship curriculum development, such as core procedural skills, quality improvement, and practice management.[7]
In an effort to address training variability across programs, PHM fellowship directors (FDs) have come together as an organized group, first meeting in 2008, with the primary goal of defining training standards and sharing curricular resources. Annual meetings of the FDs, sponsored by the American Academy of Pediatrics Section on Hospital Medicine (AAP‐SOHM), began in 2012. A key objective of this annual meeting has been to develop a standardized fellowship curriculum for use across programs as well as to determine gaps in training that need to be addressed. During this process, we have received input from key stakeholders including community hospitalists, internal medicine‐pediatrics hospitalists, and the PHM Certification Steering Committee, which organized the application for subspecialty certification to the ABP. To inform this process of curriculum standardization, we fielded a survey of PHM fellowship directors. The purpose of this article is to summarize the current curricula, operations, and logistics of PHM fellowship programs.
METHODS
This was a cross‐sectional study of 31 PHM fellowship programs across the United States and Canada in April 2014. Inclusion criteria included all pediatric fellowships that were self‐identified to the AAP‐SOHM as providing a hospital medicine fellowship option. This included both PHM fellowships as well as academic general pediatric fellowships with a hospitalist track. A web‐based survey (SurveyMonkey, Inc.) was distributed by e‐mail to the FDs at the 31 training programs (see Supporting Information in the online version of this article). To enhance content validity of survey responses, survey questions were designed using an iterative consensus process among the authors, who included junior and senior FDs and represented the 2014 annual FD meeting planning committee. Items were created to gather feedback on the following key areas of PHM fellowships: program demographics, types of required and elective clinical rotations, graduate coursework offerings, amount of time spent in clinical activities, fellow billing practices, and description of fellows' research activities. The survey consisted of 30 multiple‐choice and short‐answer questions. Follow‐up e‐mail reminders were sent to all FDs 2 weeks and 4 weeks after the initial request was sent. Survey completion was voluntary, and no incentives were offered. The study was determined to be exempt by the Stanford University Institutional Review Board. Data were summarized using frequency distributions. No subgroup comparisons were made.
RESULTS
Program directors from 27/31 (87%) PHM fellowship programs responded to the survey; 25 were active programs, and 2 were under development. Responding programs represented all 4 major regions of the country and Canada, with varying program initiation dates, ranging from 1997 to 2013.
Program Demographics
The duration of most programs (17/27) was 2 years (63%), with 6 (22%) 1‐year programs and 4 (15%) 3‐year programs making up the remainder. Four programs described variable lengths, which could be tailored based on the fellow's individual interest. Two of the programs are 2 years in length, but offer a 1‐year option for fellows who wish to focus on enhancing clinical skills without an academic focus. The other 2 programs are 2 years in length, but will offer an extension to a third year for those pursuing a graduate degree.
Fellow Clinical Activities
The average amount of total clinical time (weeks on service) across responding programs was 50% (range, 20%65%). When looking specifically at time on the inpatient general pediatric service, number of weeks varied by year of training and by institution, with 12 to 41 weeks in the first year of fellowship, 6 to 41 weeks in the second year of fellowship, and 6 to 28 weeks in the third year of fellowship (Figure 1). Though the range is large, on average, fellows spend 17 weeks on inpatient general pediatrics service during each year of training. Of note, the median number of weeks on inpatient general pediatrics service by year of training was 15 weeks, 16 weeks, and 16.5 weeks, respectively. In addition to inpatient general pediatrics service time, most programs require other clinical rotations, with sedation, complex care, and inpatient pediatrics at community sites being the most frequent (Figure 2). Of the 6 responding 1‐year programs, 5 (83%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 15 responding 2‐year programs, 11 (73%) allow fellows to bill/generate clinical revenue at some point during their training. Of the 4 responding 3‐year programs, 2 (50%) allow their fellows to bill/generate clinical revenue at some point during their training.


Fellow Scholarly Activities
With respect to time dedicated to research, the majority of programs offer coursework such as courses for credit, noncredit courses, or certificate courses. In addition, 11 programs offer fellows a masters' degree in areas including public health, clinical science, epidemiology, education, academic sciences, healthcare quality, clinical and translational research, or health services administration. The majority of these degrees are paid for by departmental funds, with tuition reimbursement, university support, training grants, and personal funds making up the remainder. Twenty‐one (81%) programs provide a scholarship oversight committee for their fellows. Current fellows' (n = 63) primary areas of research are varied and include clinical research (36%), quality‐improvement research (22%), medical education research (20%), health services research (16%), and other areas (6%).
DISCUSSION
This is the most comprehensive description of pediatric hospital medicine fellowship curricula to date. Understanding the scope of these programs is an important first step in developing a standardized curriculum that can be used by all. The results of this survey indicate that although there is variability among PHM fellowship curricular content, several common themes exist.
The number of clinical weeks on the inpatient general pediatrics service varied from program to program, though the majority of programs require fellows to spend 15 to 16 weeks each year of training. The variability may be due in part to the way in which respondents defined the term week on clinical service. For example, if the fellow is primarily on a shift schedule, then he/she may only work 2 to 3 shifts in 1 week, which may have been viewed similarly to daily presence on a more traditional inpatient teaching service with 5 to 7 consecutive days of service. The current study did not explore the details of inpatient general pediatric clinical activities or exposure to opportunities to hone procedural skills, areas that are worth investigating as we move forward to better understand the needs of trainees.
Most residency training programs in general pediatrics require a significant amount of inpatient clinical time, specifically a minimum of 10 units or months, though only half of this time is required to be in inpatient general pediatrics.[8] Although nonfellowship trained early career hospitalists may feel adequately prepared to manage the clinical care of some hospitalized children, perceived competency is significantly lower than their fellowship‐trained colleagues with regard to care of the child with medical complexity and technology‐dependence, and with regard to provision of sedation for procedures.[7] The majority of FDs surveyed in our study indicated that additional clinical experience with sedation, complex care, and inpatient pediatrics at community sites were required of their fellows. Of note, many of these rotations are not commonly required in pediatric residency training programs; however, the PHM core competencies suggest that hospitalists should demonstrate proficiency in these areas to provide optimal care for hospitalized children. Our results suggest that current PHM fellowship curricula help address these clinical gaps. The requirement of these particular specialized experiences may reflect the clinical scope of practice that is expected from potential employers or may be related to staffing needs. It is well documented that the inpatient demographic of large pediatric tertiary care referral centers has changed over the past decade, with an increasing prevalence of children with medical complexity.[9, 10] In both tertiary referral centers and community hospitals, the expansion of the role of the hospitalist in providing specialized clinical services, such as sedation or surgical comanagement, has been significantly driven by financial factors, though a more recent focus on improvement of efficiency and quality of care within the hospital system has relied heavily on hospitalist input.[11, 12, 13] Important next steps in curriculum standardization include ensuring that training programs allow for adequate clinical exposure and proper assessment of competency in these areas, and determining the full complement of clinical training experiences that will produce hospitalists with a well‐defined scope of practice that adequately addresses the needs of hospitalized children.
Most fellowship‐trained hospitalists work primarily in university‐affiliated institutions with expectations for scholarly productivity.[5, 7] Fellowship‐trained hospitalists have made large contributions to the growing body of PHM literature, specifically in the realms of medical education, healthcare quality, clinical pediatrics, and healthcare outcomes.[4] Many PHM fellowship‐trained hospitalists have educational or administrative leadership roles.[2] Our results indicate that current PHM fellows continue to be active in a variety of research activities. In addition, FDs reported that the vast majority of programs included scholarship oversight committees, which ensure a mentored and structured research experience. Finally, most programs require or offer additional coursework, and many programs with university affiliations allow for attainment of graduate degrees. Inclusion of robust research training and infrastructure in all programs is a paramount goal of PHM fellowship training. This will allow graduates to be successful researchers, generating new knowledge and supporting the provision of high‐quality, evidence‐based, and value‐driven care for hospitalized children.
A unique feature of several PHM fellowship programs is that fellows are allowed to bill for clinical encounters. Many programs rely on clinical revenue to support fellow salaries.[14] For some programs, a portion of this clinical revenue comes from fellows billing for clinical encounters.[15] Programs that allow fellows to bill/generate clinical revenue have fellows working in attending roles without direct supervision, whereas nonbilling fellows have direct supervision by an attending.[15] In the current ABP training model, subspecialty fellows cannot independently bill for clinical encounters within their own subspecialty, though they can moonlight as long as they meet the duty hour requirements set forth by the ACGME.[16] FDs will need to consider the impact of this requirement on fellow autonomy and on financial revenue for funding fellow salaries if the field achieves ABP subspecialty status.
Regardless of whether or not PHM becomes a designated subspecialty of the ABP, FDs will continue to work together to develop a standard core curriculum that incorporates elements of clinical and nonclinical training to ensure that graduates not only provide high‐quality care for hospitalized children, but also generate new knowledge that advances the field in care delivery and quality of care in any setting. The results of this study will not only help to inform curriculum standardization, but also assessment and evaluation methods. Currently, PHM FDs meet annually and are nearing consensus on a standard 2‐year curriculum based on the PHM Core Competencies that incorporates core clinical, systems, and scholarly domains. We continue to solicit the input of stakeholders, including new FDs, community hospitalist leaders, internal medicine‐pediatrics hospitalist leaders, the Joint Council of Pediatric Hospital Medicine, and leaders of national organizations, such as the American Academy of Pediatrics, Academic Pediatrics Association, and Society of Hospital Medicine. Additional work around standardizing the fellowship application and recruitment process has resulted in our recent acceptance into the Fall Subspecialty Match through the National Residency Match Program, as well as development and implementation of a common fellowship application form. The FD group has recently formalized, voting into place an executive steering committee, which is responsible for the development and execution of long‐term goals that include finalizing a standardized curriculum, refining program and fellow assessment methods through critical evaluation of fellow metrics and outcomes, and standardization of evaluation methods.
Adopting a standard 2‐year curriculum may affect some programs, specifically those that are currently 1 year in duration. These programs would need to extend the length of their fellowship to allow for the breadth of experiences expected with a standardized 2‐year curriculum. This could result in significant financial challenges, effectively increasing the cost to administer the program. In addition, at present, programs have the flexibility to highlight individual areas of strength to attract candidates, allowing fellows to gain an in‐depth experience in domains such as clinical research, quality improvement, medical education, or health services research. With a standardized curriculum, some programs may have to assemble specific clinical and nonclinical experiences to meet the agreed‐upon expectations for PHM fellowship training. If these resources are not available, programs may need to seek relationships with other institutions to complete their offerings, a possibility that is being actively explored by this group. FDs continue to work with each other to share resources, identify training opportunities, and partner with each other to ensure that the requirements of a standard curriculum can be met.
This study has several limitations. First, it was a voluntary survey of program directors, and though we captured over 80% of programs at the time of the survey, there are currently more programs that have come into existence and more still that are in the development stage, leading to potential sampling error. Second, variable effort or accuracy by participants may have led to some degree of response error, such as content error or nonreporting error. Third, the survey questions focused on high‐level information, making it difficult to make nuanced comparisons between curricular elements or determine best curricular practice. In addition, this survey did not explore medical education and quality improvement activities of fellows, 2 major areas in which hospitalists play a major role in the inpatient setting.[1, 17, 18, 19, 20]
CONCLUSION
PHM fellowship programs have grown and continue to grow at a rapid rate. Variability in training is evident, both in clinical experiences and research experiences, though several common elements were identified in this study. The majority of programs are 2 years, and clinical experience comprises approximately 50% of training time, often including key rotations such as sedation, complex care, and rotations at community hospitals. Future directions include standardizing clinical training and expectations for scholarship, formulating appropriate methods for assessment of competency that can be used across programs, and seeking sustainable sources of funding.
Disclosure
Nothing to report.
- , . Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157–163.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , . Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38–44.
- , , , . Pediatric hospital medicine fellowships: outcomes and future directions. Paper presented at: Pediatric Hospital Medicine 2014; July 26, 2014; Orlando, FL.
- , , . Pediatric hospitalist research productivity: predictors of success at presenting abstracts and publishing peer‐reviewed manuscripts among pediatric hospitalists. Hosp Pediatr. 2012;2(3):149–160.
- , , . Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5:339–343.
- , , , . Perceived core competency achievements of fellowship and non‐fellowship early career pediatric hospitalists. J Hosp Med. 2015;10(6):373–389.
- Accreditation Council of Graduate Medical Education. ACGME program requirements for graduate medical education in pediatrics. Available at: https://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Published September 30, 2012. Accessed July 7, 2015.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , . The expanding role of hospitalists in the United States. Swiss Med Wkly. 2006;136:591–596.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , , , , . Development of a pediatric hospitalist sedation service: training and implementation. J Hosp Med. 2012;7(4):335–339.
- , . Sources of funding and support for pediatric hospital medicine fellowship programs. Poster presented at: Pediatric Hospital Medicine 2014; July 27, 2014; Orlando, FL.
- Council of Pediatric Hospital Medicine Fellowship Directors. Pediatric Hospital Medicine Fellowship Directors Annual Meeting: funding and return on investment. July 24, 2014.
- Accreditation Council of Graduate Medical Education. Frequently asked questions: ACGME common duty hour requirements. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/dh‐faqs2011.pdf. Updated June 18, 2014. Accessed July 7, 2015.
- , . Pediatric hospitalists: training, current practice and career goals. J Hosp Med. 2009;4(3):179–186.
- , . The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103:473–477.
- . Pediatric hospitalists and medical education. Pediatr Ann. 2014;43(7):e151–e156
- , , , et al. Quality improvement research in pediatric hospital medicine and the role of the Pediatric Research in Inpatient Settings (PRIS) network. Acad Pediatr. 2013;13(6):S54–S60.
- , . Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157–163.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , . Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38–44.
- , , , . Pediatric hospital medicine fellowships: outcomes and future directions. Paper presented at: Pediatric Hospital Medicine 2014; July 26, 2014; Orlando, FL.
- , , . Pediatric hospitalist research productivity: predictors of success at presenting abstracts and publishing peer‐reviewed manuscripts among pediatric hospitalists. Hosp Pediatr. 2012;2(3):149–160.
- , , . Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5:339–343.
- , , , . Perceived core competency achievements of fellowship and non‐fellowship early career pediatric hospitalists. J Hosp Med. 2015;10(6):373–389.
- Accreditation Council of Graduate Medical Education. ACGME program requirements for graduate medical education in pediatrics. Available at: https://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Published September 30, 2012. Accessed July 7, 2015.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , . The expanding role of hospitalists in the United States. Swiss Med Wkly. 2006;136:591–596.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , , , , . Development of a pediatric hospitalist sedation service: training and implementation. J Hosp Med. 2012;7(4):335–339.
- , . Sources of funding and support for pediatric hospital medicine fellowship programs. Poster presented at: Pediatric Hospital Medicine 2014; July 27, 2014; Orlando, FL.
- Council of Pediatric Hospital Medicine Fellowship Directors. Pediatric Hospital Medicine Fellowship Directors Annual Meeting: funding and return on investment. July 24, 2014.
- Accreditation Council of Graduate Medical Education. Frequently asked questions: ACGME common duty hour requirements. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/dh‐faqs2011.pdf. Updated June 18, 2014. Accessed July 7, 2015.
- , . Pediatric hospitalists: training, current practice and career goals. J Hosp Med. 2009;4(3):179–186.
- , . The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103:473–477.
- . Pediatric hospitalists and medical education. Pediatr Ann. 2014;43(7):e151–e156
- , , , et al. Quality improvement research in pediatric hospital medicine and the role of the Pediatric Research in Inpatient Settings (PRIS) network. Acad Pediatr. 2013;13(6):S54–S60.
© 2016 Society of Hospital Medicine
Antibiotics in Persons Who Inject Drugs
In the United States, there are an estimated 744,000 individuals who have engaged in recent injection drug use (IDU) and 6.6 million individuals who have ever injected a drug.[1] The practice of IDU predisposes individuals to serious bacterial and fungal infections that often require long‐term intravenous antibiotics. In individuals without IDU, these serious infections are often treated with outpatient parenteral antibiotic therapy (OPAT). However, a different standard exists for many persons who inject drugs (PWID)the mandated completion of antibiotics in an inpatient setting.
Though mandating inpatient antibiotic therapy for PWID is a widely adopted standard, this practice is not evidence based and may increase overall costs to the healthcare system. In 2012, in a quality‐improvement initiative, UKHealthCare established a protocol for treating appropriate PWID with OPAT.[2] They found very few inpatient providers willing to discharge PWID on OPAT, even with an established protocol.
To better understand the reasons for the low adoption of this protocol, Fanucchi and colleagues developed a survey designed to assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT.[2] The results of this survey are reported in this issue of the Journal of Hospital Medicine.
The study found that 95% of inpatient providers use OPAT for patients without IDU, but only 29% would even consider OPAT in PWID. The most common barriers to discharging a patient with IDU on OPAT were socioeconomic factors, willingness of infectious diseases physicians to follow as an outpatient, and concerns for misuse of peripherally inserted central catheters and adherence with antibiotic treatment.
At first glance, these reservations seem very reasonable. The presence of socioeconomic factors such as homelessness or lack of infectious diseases specialist follow‐up would make the risks of discharge on OPAT significant. The concerns for misuse of peripherally inserted central catheters and adherence to antibiotic treatment suggest that inpatient providers have an overall goal of reducing drug misuse and improving treatment outcomes.
Unfortunately, there are no data to suggest that completion of antibiotics in an inpatient setting reduces drug misuse or improves adherence to antibiotic treatments. Studies have found that at least 16% of PWID will misuse drugs during their hospitalization,[3] and 25% to 30% will be discharged against medical advice.[3, 4] This may be in large part due to the fact that inpatient providers are historically poor at addressing substance use disorders, even in patients with serious infections associated with IDU.[5] Yet the provision of methadone during hospitalization has been associated with a significant reduction in discharges against medical advice.[4] Rather than focusing on placing restrictions on individuals with risky behaviors, patients may benefit more from minimizing these risks through prompt recognition and management of substance use disorder.
Although limited, there is also evidence to support the feasibility of safe and effective OPAT in some PWID. A study by Ho et al. used OPAT to treat 29 PWID hospitalized with serious infections.[6] The study population had adequate housing, a reliable guardian, and signed a contract agreeing to abstain from drug misuse. In addition, all patients received substance use counseling and novel tamper‐proof security seals to prevent misuse of peripherally inserted central catheters, and antibiotics were delivered daily at an infusion center. They found no evidence of line tampering, excess readmissions, or excess line infections. Of note, the study population included 2 patients who were discharged against medical advice but successfully completed OPAT without issue. Although we do not believe that all individuals are appropriate for OPAT, this study suggests that OPAT can be considered in select PWID.
The study by Fanucchi et al. also reinforces the importance of making individualized risk assessments of persons with a history of IDU rather than assuming uniformity among the population. Of particular note is the lack of agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months). The idea that individuals with a decade of sobriety could be subject to the same restrictions as a patient injecting multiple times a day speaks to providers' discomfort with assessing the individual risk of a person who has suffered from substance use disorder. Further, the fact that so few providers felt substance use disorder treatment was a critical component of a decision to allow OPAT raises concerns that providers are not aware of effective means to treat addiction. In particular, it is crucial for providers to understand that medication‐assisted treatment, such as methadone or buprenorphine for opioid use disorder, has significant evidence to support efficacy in decreasing drug misuse and improving outcomes.
This study suggests more work will need to be done before inpatient providers will be comfortable discharging any PWID with OPAT. This includes improved outpatient services (enhanced case management and home health services, and better access to outpatient physicians including infectious diseases specialists), the development of tamper‐evident devices to deter misuse of peripherally inserted central catheters, and defined legal protection for providers.
In addition, more research needs to be done on this population to objectively stratify risk for PWID and assess outcomes for PWID treated with OPAT versus the current standard of care. This research should have a particular focus on the long‐term financial and societal costs associated with PWID leaving against medical advice or receiving potentially unnecessary inpatient services. Minimizing the length of stay may defray inpatient costs and afford investment into more robust, effective outpatient services. It is essential that we develop a system to provide antibiotics in a way that optimizes outcomes and is cost‐effective.
Regardless of the decision to mandate antibiotic treatment in an inpatient setting or to discharge with OPAT, it is clear that more needs to be done to address addiction in hospitalized patients. All hospitalized PWID should receive safe injection education and a referral to a substance use disorder specialist. In addition, individuals with opioid‐misuse or opioid use disorder should receive opioid overdose education and naloxone distribution. Hospitalizations serve as important opportunities to engage individuals in the treatment of their addiction. It is essential that hospitalists begin utilizing these opportunities.
Disclosures: Nothing to report.
- , , , et al. Estimating the number of persons who inject drugs in the United States by meta‐analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9:e97596.
- , , , . Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581–582.
- , , , et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66:95–102.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35:56–59.
- , , , , . Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis [published online November 18, 2015]. Am J Med. doi: 10.1016/j.amjmed.2015.09.024.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
In the United States, there are an estimated 744,000 individuals who have engaged in recent injection drug use (IDU) and 6.6 million individuals who have ever injected a drug.[1] The practice of IDU predisposes individuals to serious bacterial and fungal infections that often require long‐term intravenous antibiotics. In individuals without IDU, these serious infections are often treated with outpatient parenteral antibiotic therapy (OPAT). However, a different standard exists for many persons who inject drugs (PWID)the mandated completion of antibiotics in an inpatient setting.
Though mandating inpatient antibiotic therapy for PWID is a widely adopted standard, this practice is not evidence based and may increase overall costs to the healthcare system. In 2012, in a quality‐improvement initiative, UKHealthCare established a protocol for treating appropriate PWID with OPAT.[2] They found very few inpatient providers willing to discharge PWID on OPAT, even with an established protocol.
To better understand the reasons for the low adoption of this protocol, Fanucchi and colleagues developed a survey designed to assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT.[2] The results of this survey are reported in this issue of the Journal of Hospital Medicine.
The study found that 95% of inpatient providers use OPAT for patients without IDU, but only 29% would even consider OPAT in PWID. The most common barriers to discharging a patient with IDU on OPAT were socioeconomic factors, willingness of infectious diseases physicians to follow as an outpatient, and concerns for misuse of peripherally inserted central catheters and adherence with antibiotic treatment.
At first glance, these reservations seem very reasonable. The presence of socioeconomic factors such as homelessness or lack of infectious diseases specialist follow‐up would make the risks of discharge on OPAT significant. The concerns for misuse of peripherally inserted central catheters and adherence to antibiotic treatment suggest that inpatient providers have an overall goal of reducing drug misuse and improving treatment outcomes.
Unfortunately, there are no data to suggest that completion of antibiotics in an inpatient setting reduces drug misuse or improves adherence to antibiotic treatments. Studies have found that at least 16% of PWID will misuse drugs during their hospitalization,[3] and 25% to 30% will be discharged against medical advice.[3, 4] This may be in large part due to the fact that inpatient providers are historically poor at addressing substance use disorders, even in patients with serious infections associated with IDU.[5] Yet the provision of methadone during hospitalization has been associated with a significant reduction in discharges against medical advice.[4] Rather than focusing on placing restrictions on individuals with risky behaviors, patients may benefit more from minimizing these risks through prompt recognition and management of substance use disorder.
Although limited, there is also evidence to support the feasibility of safe and effective OPAT in some PWID. A study by Ho et al. used OPAT to treat 29 PWID hospitalized with serious infections.[6] The study population had adequate housing, a reliable guardian, and signed a contract agreeing to abstain from drug misuse. In addition, all patients received substance use counseling and novel tamper‐proof security seals to prevent misuse of peripherally inserted central catheters, and antibiotics were delivered daily at an infusion center. They found no evidence of line tampering, excess readmissions, or excess line infections. Of note, the study population included 2 patients who were discharged against medical advice but successfully completed OPAT without issue. Although we do not believe that all individuals are appropriate for OPAT, this study suggests that OPAT can be considered in select PWID.
The study by Fanucchi et al. also reinforces the importance of making individualized risk assessments of persons with a history of IDU rather than assuming uniformity among the population. Of particular note is the lack of agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months). The idea that individuals with a decade of sobriety could be subject to the same restrictions as a patient injecting multiple times a day speaks to providers' discomfort with assessing the individual risk of a person who has suffered from substance use disorder. Further, the fact that so few providers felt substance use disorder treatment was a critical component of a decision to allow OPAT raises concerns that providers are not aware of effective means to treat addiction. In particular, it is crucial for providers to understand that medication‐assisted treatment, such as methadone or buprenorphine for opioid use disorder, has significant evidence to support efficacy in decreasing drug misuse and improving outcomes.
This study suggests more work will need to be done before inpatient providers will be comfortable discharging any PWID with OPAT. This includes improved outpatient services (enhanced case management and home health services, and better access to outpatient physicians including infectious diseases specialists), the development of tamper‐evident devices to deter misuse of peripherally inserted central catheters, and defined legal protection for providers.
In addition, more research needs to be done on this population to objectively stratify risk for PWID and assess outcomes for PWID treated with OPAT versus the current standard of care. This research should have a particular focus on the long‐term financial and societal costs associated with PWID leaving against medical advice or receiving potentially unnecessary inpatient services. Minimizing the length of stay may defray inpatient costs and afford investment into more robust, effective outpatient services. It is essential that we develop a system to provide antibiotics in a way that optimizes outcomes and is cost‐effective.
Regardless of the decision to mandate antibiotic treatment in an inpatient setting or to discharge with OPAT, it is clear that more needs to be done to address addiction in hospitalized patients. All hospitalized PWID should receive safe injection education and a referral to a substance use disorder specialist. In addition, individuals with opioid‐misuse or opioid use disorder should receive opioid overdose education and naloxone distribution. Hospitalizations serve as important opportunities to engage individuals in the treatment of their addiction. It is essential that hospitalists begin utilizing these opportunities.
Disclosures: Nothing to report.
In the United States, there are an estimated 744,000 individuals who have engaged in recent injection drug use (IDU) and 6.6 million individuals who have ever injected a drug.[1] The practice of IDU predisposes individuals to serious bacterial and fungal infections that often require long‐term intravenous antibiotics. In individuals without IDU, these serious infections are often treated with outpatient parenteral antibiotic therapy (OPAT). However, a different standard exists for many persons who inject drugs (PWID)the mandated completion of antibiotics in an inpatient setting.
Though mandating inpatient antibiotic therapy for PWID is a widely adopted standard, this practice is not evidence based and may increase overall costs to the healthcare system. In 2012, in a quality‐improvement initiative, UKHealthCare established a protocol for treating appropriate PWID with OPAT.[2] They found very few inpatient providers willing to discharge PWID on OPAT, even with an established protocol.
To better understand the reasons for the low adoption of this protocol, Fanucchi and colleagues developed a survey designed to assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT.[2] The results of this survey are reported in this issue of the Journal of Hospital Medicine.
The study found that 95% of inpatient providers use OPAT for patients without IDU, but only 29% would even consider OPAT in PWID. The most common barriers to discharging a patient with IDU on OPAT were socioeconomic factors, willingness of infectious diseases physicians to follow as an outpatient, and concerns for misuse of peripherally inserted central catheters and adherence with antibiotic treatment.
At first glance, these reservations seem very reasonable. The presence of socioeconomic factors such as homelessness or lack of infectious diseases specialist follow‐up would make the risks of discharge on OPAT significant. The concerns for misuse of peripherally inserted central catheters and adherence to antibiotic treatment suggest that inpatient providers have an overall goal of reducing drug misuse and improving treatment outcomes.
Unfortunately, there are no data to suggest that completion of antibiotics in an inpatient setting reduces drug misuse or improves adherence to antibiotic treatments. Studies have found that at least 16% of PWID will misuse drugs during their hospitalization,[3] and 25% to 30% will be discharged against medical advice.[3, 4] This may be in large part due to the fact that inpatient providers are historically poor at addressing substance use disorders, even in patients with serious infections associated with IDU.[5] Yet the provision of methadone during hospitalization has been associated with a significant reduction in discharges against medical advice.[4] Rather than focusing on placing restrictions on individuals with risky behaviors, patients may benefit more from minimizing these risks through prompt recognition and management of substance use disorder.
Although limited, there is also evidence to support the feasibility of safe and effective OPAT in some PWID. A study by Ho et al. used OPAT to treat 29 PWID hospitalized with serious infections.[6] The study population had adequate housing, a reliable guardian, and signed a contract agreeing to abstain from drug misuse. In addition, all patients received substance use counseling and novel tamper‐proof security seals to prevent misuse of peripherally inserted central catheters, and antibiotics were delivered daily at an infusion center. They found no evidence of line tampering, excess readmissions, or excess line infections. Of note, the study population included 2 patients who were discharged against medical advice but successfully completed OPAT without issue. Although we do not believe that all individuals are appropriate for OPAT, this study suggests that OPAT can be considered in select PWID.
The study by Fanucchi et al. also reinforces the importance of making individualized risk assessments of persons with a history of IDU rather than assuming uniformity among the population. Of particular note is the lack of agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months). The idea that individuals with a decade of sobriety could be subject to the same restrictions as a patient injecting multiple times a day speaks to providers' discomfort with assessing the individual risk of a person who has suffered from substance use disorder. Further, the fact that so few providers felt substance use disorder treatment was a critical component of a decision to allow OPAT raises concerns that providers are not aware of effective means to treat addiction. In particular, it is crucial for providers to understand that medication‐assisted treatment, such as methadone or buprenorphine for opioid use disorder, has significant evidence to support efficacy in decreasing drug misuse and improving outcomes.
This study suggests more work will need to be done before inpatient providers will be comfortable discharging any PWID with OPAT. This includes improved outpatient services (enhanced case management and home health services, and better access to outpatient physicians including infectious diseases specialists), the development of tamper‐evident devices to deter misuse of peripherally inserted central catheters, and defined legal protection for providers.
In addition, more research needs to be done on this population to objectively stratify risk for PWID and assess outcomes for PWID treated with OPAT versus the current standard of care. This research should have a particular focus on the long‐term financial and societal costs associated with PWID leaving against medical advice or receiving potentially unnecessary inpatient services. Minimizing the length of stay may defray inpatient costs and afford investment into more robust, effective outpatient services. It is essential that we develop a system to provide antibiotics in a way that optimizes outcomes and is cost‐effective.
Regardless of the decision to mandate antibiotic treatment in an inpatient setting or to discharge with OPAT, it is clear that more needs to be done to address addiction in hospitalized patients. All hospitalized PWID should receive safe injection education and a referral to a substance use disorder specialist. In addition, individuals with opioid‐misuse or opioid use disorder should receive opioid overdose education and naloxone distribution. Hospitalizations serve as important opportunities to engage individuals in the treatment of their addiction. It is essential that hospitalists begin utilizing these opportunities.
Disclosures: Nothing to report.
- , , , et al. Estimating the number of persons who inject drugs in the United States by meta‐analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9:e97596.
- , , , . Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581–582.
- , , , et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66:95–102.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35:56–59.
- , , , , . Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis [published online November 18, 2015]. Am J Med. doi: 10.1016/j.amjmed.2015.09.024.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
- , , , et al. Estimating the number of persons who inject drugs in the United States by meta‐analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9:e97596.
- , , , . Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581–582.
- , , , et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66:95–102.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35:56–59.
- , , , , . Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis [published online November 18, 2015]. Am J Med. doi: 10.1016/j.amjmed.2015.09.024.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
Caught red‐handed
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.
The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
A previously healthy 58‐year‐old man presented to a community hospital's emergency department 1 day after the sudden onset of a severe headache, fever, diffuse abdominal pain, nausea, vomiting, and disorientation. The patient had a history of allergic rhinitis and his only medication was a daily multivitamin.
Key features of this patient's presentation include the abrupt onset of severe headache, disorientation, fever, and abdominal pain. The list of entities likely to make a previously healthy individual this ill this quickly is typically circumscribed. His presentation raises the possibility of bacterial meningitis (including Listeria, given his age), viral encephalitis, or other extraneural etiologies of sepsis. Noninfectious explanations seem much less likely given the rapid tempo of illness.
His proclivity for gardening and apparent tick exposure raise the question of tick‐borne illnesses. This would constitute a rather explosive onset for any of these; however, babesiosis, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and anaplasmosis could present this abruptly, with dog exposure linked to RMSF.
The potential causes of fever and rash are myriad, although the severity and acuity of this patient's illness narrow the differential considerably, likely to an infectious cause. Diagnoses that typically include a generalized exanthem involving the palms and soles are meningococcal meningitis, overwhelming Staphylococcus aureus sepsis, RMSF (realizing that this disease is not common in the upper Midwest), and toxic shock syndrome. The rash described is not the classic and/or fully developed rash typical of any of these; subsequent evolution to a petechial appearance would lend further support to the first 3 diagnoses. Ehrlichiosis is still a possibility, although the palm and sole involvement would be unusual. The presence of a rash makes anaplasmosis very unlikely, although not entirely excluded. The finding of modest splenomegaly does not help further distinguish between these possibilities.
Empiric antimicrobials should be immediately administered after blood cultures, a complete blood count, and coagulation studies are obtained. Doxycycline would be appropriate to treat the possible tick‐borne diseases already mentioned, whereas antimicrobials appropriate to cover community‐acquired bacterial meningitis in a 58‐year‐old (ie, vancomycin, ampicillin, and a third‐generation cephalosporin) should also be empirically administered. Given the patient's altered mentation, a brain computed tomography (CT) should be urgently obtained. Provided this did not show evidence of increased intracranial pressure and that coagulation studies and a platelet count did not suggest a contraindication, a lumbar puncture should then be performed promptly. The patient should be placed in droplet precautions until meningococcal disease is excluded. Although most patients with bacterial meningitis will exhibit meningismus, a substantial minority will not.
These laboratory results do not significantly affect the differential diagnosis. Although nonspecific, moderate thrombocytopenia and modest elevation of hepatic transaminases are typical for tick‐borne diseases, whereas leukocytosis is somewhat atypical for these entities. Marked elevation of the C‐reactive protein with a less striking increase in the erythrocyte sedimentation rate, along with significant hypoalbuminemia, are commonly encountered early in the course of critical infectious illnesses. The elevated troponin likely reflects severe sepsis and demand ischemia, and is associated with a less favorable prognosis; an electrocardiogram and serial cardiac biomarkers are appropriate to help exclude an acute coronary syndrome. As already noted, blood cultures need to be obtained and a lumbar puncture should be performed, provided this can be safely accomplished.
Results of the lumbar puncture exclude bacterial meningitis as the explanation of this patient's illness; the mildly elevated protein is nonspecific. These studies do not otherwise change the differential diagnosis.
Supporting data for a diagnosis of pneumonia, such as pulmonary infiltrates or supplemental oxygen requirement, are lacking. Given his critical illness, broad spectrum antimicrobial coverage is indicated, and as a primary central nervous system (CNS) infection now appears unlikely, piperacillin/tazobactam (which does not have adequate CNS penetration) and vancomycin are reasonable. Empiric treatment for RMSF is appropriate, and should have been initiated earlier in the patient's course, despite the upper Midwest being out of the typical range for this disease. Doxycycline will also provide excellent coverage for ehrlichiosis and anaplasmosis.
Given the patient's deterioration, it is important to stop and reconsider the differential diagnosis in an attempt to avoid anchoring bias and premature closure. The patient's illness is almost certainly infectious in nature, and the differential is not substantially altered by the most recent information. A skin biopsy should be performed in an attempt to secure the diagnosis.
The patient's overall course, including rapid onset of severe illness and especially the apparent dramatic response to doxycycline, make tick‐borne illness very likely. Completing a course of doxycycline is certainly appropriate, typically for 7 to 14 days. The acute serologies drawn prior to discharge may well reveal the causative agent, but convalescent serology should also be obtained at the time of an outpatient follow‐up visit as immunoglobulin G has a delayed rise. Without hyponatremia or respiratory symptoms, Legionella seems unlikely.

The appearance of late desquamation of the palms and soles is an unexpected and important sign. Desquamation in this pattern following an illness of this nature strongly suggests a diagnosis of staphylococcal toxic shock syndrome (TSS), and in conjunction with the negative serologies, argues that tick‐borne disease is unlikely. The list of other entities that might lead to desquamation in this setting is very short, namely adult Kawasaki disease and drug reaction. The former seems reasonably excluded based on details of the case, whereas a doxycycline‐related drug reaction, although not entirely implausible, seems quite unlikely as this medication was started after the onset of the initial rash. This patient most likely had staphylococcal TSS secondary to a minor and unappreciated skin lesion.
DISCUSSION
TSS is a systemic illness resulting in multiorgan dysfunction.[1] Infection by S aureus or Streptococcus pyogenes causes TSS by stimulating maladaptive T‐cell proliferation and cytokine release resulting in shock.[1, 2] A definitive diagnosis requires fever, a diffuse macular erythematous rash (often resembling a sunburn), with subsequent desquamation, hypotension, and involvement of at least 3 organ systems. Blood cultures, cerebrospinal cultures, and serologies for other organisms should be negative; although Staphylococcus and Streptococcus species may be isolated, they frequently are not (Table 1).[3]
| Diagnostic Criteria* | This Case |
|---|---|
| |
| Fever: Temperature 102.0F | Fever: 105.3F on admission |
| Rash: Diffuse macular erythroderma | Diffuse morbilliform rash with progression to confluent erythroderma |
| Desquamation of rash: occurs 12 weeks following rash onset | Desquamation 12 days after discharge |
| Hypotension: SBP 90 mm Hg for adults | Intermittent |
| Multisystem involvement, 3 of the following: | 4 organ systems definitively involved |
| GI: vomiting or diarrhea at disease onset | Vomiting and abdominal pain |
| Muscular: severe myalgias, or creatine phosphokinase >2 times the upper limit of normal | |
| Mucous membranes: vaginal, oropharyngeal, or conjunctival hyperemia | |
| Renal: BUN or Cr >2 times the upper limit of normal, or pyuria without evidence of infection | |
| Hepatic: total bilirubin, AST, or ALT levels >2 times the upper limit of normal | AST and ALT peaked at 128IU/L and 94 IU/L |
| Hematologic: platelets 100,000/mm3 | Platelet nadir of 80,000/mm3 |
| CNS: disorientation or altered consciousness without focal neurologic signs | Disorientation and somnolence |
| Probable case: 4 out of 5 clinical criteria present | |
| Confirmed case: 5 out of 5 clinical criteria present, or patient dies before desquamation can occur | |
A rare cause of shock, TSS is most associated with a surge of menstruation‐related cases linked to tampon use in young women in the 1980s.[4] However, in Centers for Disease Control and Prevention (CDC) surveillance between 1987 and 1996, only 59% of the 1069 cases identified were noted to be menstruation‐related, as compared to nearly 80% of all cases earlier in the decade.[4, 5] Today, the syndrome is more likely to present after musculoskeletal and cutaneous trauma, oropharyngeal infections, surgical procedures, and device implantation.[1, 6] Despite the disease's evolving epidemiology, the illness script used by physicians likely continues to focus on young women as the primary at risk population for TSS, causing physicians to neglect the diagnosis in other populations.[1, 6, 7, 8, 9] Given this change in risk factors, it is imperative that clinicians rewrite their scripts and recognize the early signs of TSS in all patients to enable quick and effective treatment.
In addition to its shifting epidemiology and rarity, the diagnosis of TSS vexes clinicians for several reasons. First, TSS cannot be quickly and definitively diagnosed because 2 diagnostic criteria cannot be fulfilled during the acute illness. The disease's hallmarka desquamative rashoccurs only if the patient survives.[3] Serologies often take weeks to return, further delaying diagnosis. During this period of diagnostic delay, the illness has usually already resolved or resulted in death. In addition, the presenting symptoms of rash, fever, and shock are nonspecific. Alternative etiologies include meningococcal meningitis, which can also present dramatically as with this patient; RMSF, which can occasionally have a fulminant presentation; bacterial sepsis, usually from Staphylococcus or Streptococcus species; acute viral syndromes; and severe drug reactions.[6, 10, 11, 12] Palmoplantar desquamation, as in this case, can further narrow the differential as this presentation is uncommon but characteristic of TSS, RMSF, and secondary syphilis.[11] Other diagnostic clues offered by the pattern of the rash may be limited by physician discomfort with diagnosing and describing rashes. Because of this lack of a definitive diagnostic test in the acute setting, it is imperative that the clinician include TSS in the differential of fever, shock, and rash, as mortality from TSS can exceed 20% in patients who are untreated.[13]
Treatment of TSS is straightforward once considered and includes the administration of antibiotics that cover both Staphylococcus and Streptococcus species, in addition to aggressive hydration and supportive care.[14] The final critical detail in this case was the appropriate arrangement of follow‐up. Given the patient's drastic improvement, the complicated process of arranging follow‐up for a transferred patient, and the current model where the hospitalists providing inpatient care do not typically follow their patients in clinic, patients such as these can easily be lost to follow‐up. Had this occurred, the desquamation would have been missed, and the patient's diagnosis would have been incomplete.
This patient was eventually diagnosed with TSS by fulfilling all 5 CDC criteria (Table 1).[3] He made a full recovery, likely aided by the administration of broad‐spectrum antibiotics (followed by doxycycline, which provided community‐acquired methicillin‐resistant S aureus coverage) and his lack of serious comorbidities. This case should serve as a reminder to hospitalists that with a discerning eye, a careful assessment of the clinical facts, and appropriate follow‐up, perhaps the next case of TSS can be caught red‐handed.
KEY POINTS
- When presented with a patient with fever, rash, and shock, hospitalists should consider meningococcal meningitis, RMSF bacterial sepsis, acute viral illness, severe drug reaction, and TSS.
- TSS, caused by S aureus or S pyogenes, is no longer predominantly associated with tampon use. Postsurgical infection and cutaneous trauma have become important present‐day risk factors.
- The initial presentation of TSS is nonspecific. Definitive diagnosis requires proper follow‐up, allowing time for infectious serologies to return negative and for the disease's hallmark desquamation to occur.
Disclosure
Nothing to report.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
- . Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29:651–675.
- . The toxic shock syndromes. Infect Dis Clin North Am. 1996;10(4):727–746.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Toxic shock syndrome (other than Streptococcal) (TSS) 2011 Case Definition. Available at: http://wwwn.cdc.gov/nndss/conditions/toxic‐shock‐syndrome‐other‐than‐streptococcal/case‐definition/2011. Accessed June 4, 2015.
- Centers for Disease Control and Prevention. Update: toxic‐shock syndrome—United States. MMWR Morb Mortal Wkly Rep. 1983;32(30):398–400.
- , , , , , . Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5(6):807–810.
- . Fever and rash. Infect Dis Clin North Am. 1996;10(1):101–110.
- , , , et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
- , , , et al. Toxic‐shock syndrome in menstruating women: association with tampon use and staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303(25):1436–1442.
- , , , . Toxic‐shock syndrome—epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435.
- , . Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62(4):804–816.
- . Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131–136.
- , , , . Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009;80(1):72–77.
- , , , et al. One in five mortality in non‐menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbio Infect Dis. 2008;27(1):37–43.
- , . Gram‐positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290.
Outpatient Parenteral Therapy in PWID
Injection drug use (IDU) is a major public health problem leading to increased morbidity, mortality, and healthcare expenditures.[1, 2, 3] Persons who inject drugs (PWID) are often hospitalized with severe infections, such as endocarditis,[4, 5] which typically require prolonged courses of intravenous (IV) antibiotics. Outpatient parenteral antibiotic therapy (OPAT) via a peripherally inserted central catheter (PICC) is the standard of care for continuing IV medications once patients are medically stable and ready for discharge.[6] PWID have been excluded from OPAT studies,[6] leaving little evidence to guide care.[7] Furthermore, likely due to fears of ongoing IDU, PWID are often kept in the hospital for the full duration of their antibiotic courses. This practice is costly and may not be optimal, especially considering that hospitalized PWID have high rates of discharges against medical advice.[8, 9]
In 2012, as part of a quality‐improvement effort focused on hospitalized PWID requiring long courses of IV antibiotics, UKHealthCare in Lexington, Kentucky, established a protocol for OPAT in PWID meeting specific criteria. As this protocol was not widely adopted, we sought to formally assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT to inform future efforts. This study was approved by the University of Kentucky (UK) Institutional Review Board.
METHODS
A 14‐item survey (see Supporting Information, Appendix, in the online version of this article) with multiple‐choice and open‐ended response items was developed based on the existing protocol, and themes were confirmed through semistructured interviews with 10 attending physicians in hospital medicine (HM) and infectious disease (ID). Questions were designed to elucidate the role that IDU played in the decision to discharge patients on OPAT, identify barriers to discharging PWID on OPAT, as well as elicit recommendations for requisite services or programs. The first question excluded providers not caring for patients requiring long‐term IV antibiotics. Questions that allowed for open‐ended responses were categorized thematically initially by 1 researcher (L.F.), then refined and confirmed by another team member (J.L.). The survey was distributed over email through Qualtrics (Provo, Utah) software to attending physicians in HM, ID, cardiology, and surgery at UK. Qualtrics software was used to generate descriptive statistics.
RESULTS
In January 2015, the survey was emailed to 66 physicians, and the response rate was 83%, with 91% reporting caring for patients requiring long‐term IV antibiotics. Of those, 41 (82%) completed all items; 66% of completers were in HM, 12% ID, 10% surgery, and 2% cardiology. Sixty percent were male and in practice an average of 7.2 years. Thirty‐nine (95%) use OPAT for patients without IDU, but only 12 (29%) would consider OPAT in PWID. If the patient has a remote history of IDU, then 33 (79%) would consider OPAT. There was no agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months).
The most common physician‐identified barriers to discharging PWID on OPAT, as well as recommendations for services or processes to be in place to allow PWID to be discharged with OPAT, are listed in Table 1.
| Identified Barriers to Discharging PWID on OPAT (41 Responses) | % (No.) |
|---|---|
| |
| Socioeconomic factors (stable housing, transportation, living with responsible adult) | 66 (27) |
| Potential risk of the patient misusing PICC line for IDU | 66 (27) |
| Willingness of ID physician to follow the patient as an outpatient | 59 (24) |
| Potential risk of not completing IV antibiotic therapy | 49 (20) |
| Positive urine drug screen on admission | 44 (18) |
| Patient willingness to sign behavioral contract* | 39 (16) |
| Patient willingness to enter mental health or substance use disorder treatment | 39 (16) |
| Lack of a tamper‐evident mechanism that discourages misuse of the PICC line | 27 (11) |
| Lack of data on outcomes for OPAT in PWID | 24 (10) |
| Potential risk of being sued by a patient or family | 20 (8) |
| Other | |
| Recommendations for services or processes among providers who do not currently consider discharging PWID on OPAT (28 responses) | |
| Outpatient or ID follow‐up | 32 (9) |
| Monitoring mechanism including random urine drug screens | |
| Substance use disorder and mental health services and treatment | |
| Home health services | |
| Institutional placement (eg, inpatient rehab, extended‐care facility) | |
| More explicit legal protection | |
| Screening criteria to identify high risk for PICC line misuse | |
| Designated coordinator for this patient population | |
DISCUSSION
This survey illustrates the extremely complex barriers present when treating hospitalized PWID requiring long courses of IV antibiotics, and supports the anecdotal evidence that physicians often keep PWID in the hospital for weeks to administer IV antibiotics. The majority of our sample of physicians believe that the largest barriers to OPAT in PWID are socioeconomic factors and the potential risk of the patient misusing the PICC line. Although the overall response rate of our physician survey was robust,[10] our results reflect the opinions of HM and ID physicians at a single site. The low response rate among cardiologists in particular limits the generalizability of this survey. We suspect, however, that our results pertain to HM in other US hospitals, as nearly three‐fourths of 37 HM physicians surveyed at the University of California, Irvine were very concerned about PWIDs potentially misusing the PICC line, and approximately half reported they usually or always kept PWID in the hospital for prolonged treatment due to concern of substance use (personal and email communication: Lloyd Rucker, MD, unpublished data, November 6, 2015).
We were surprised that fewer than half of respondents identified substance use disorder (SUD) treatment as essential to the OPAT decision. The reasons that may explain this observation are likely multifactorial, and may include gaps in knowledge about and resources to provide evidence‐based addiction medicine. Further research is warranted to explore this observation, including the effect of enrollment into medication‐assisted treatment programs (eg, methadone, buprenorphine).
This survey suggests that although there is variability, OPAT may be an option in PWID, if outpatient follow‐up and ancillary services (ie, home health and possibly intensive case management) were well established. We believe the comorbid SUD must be also addressed. Based on the survey results and recommendations, we have begun relationships with community SUD treatment providers willing to monitor IV antibiotics with PICC lines, and dedicated additional case management staff to this population. We are evaluating these programs with the goal of contributing to an evidence base for this high‐risk population.
Acknowledgements
The authors thank Inski Yu, MD, for assistance with survey development, and Lloyd Rucker, MD, for data sharing.
Disclosure: Nothing to report.
- , , , et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458.
- , , , . Increases in drug and opioid overdose deaths—United States, 2000‐2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382.
- , , , , , . Understanding patterns of high‐cost health care use across different substance user groups. Health Aff (Millwood). 2016;35(1):12–19.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , . Bacterial infections in drug users. N Engl J Med. 2005;353(18):1945–1954.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004 2004;38(12):1651–1671.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.
- , , , . Hospitals as a ‘risk environment’: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59–66.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , . Do additional recontacts to increase response rate improve physician survey data quality? Med Care. 2013;51(10):945–948.
Injection drug use (IDU) is a major public health problem leading to increased morbidity, mortality, and healthcare expenditures.[1, 2, 3] Persons who inject drugs (PWID) are often hospitalized with severe infections, such as endocarditis,[4, 5] which typically require prolonged courses of intravenous (IV) antibiotics. Outpatient parenteral antibiotic therapy (OPAT) via a peripherally inserted central catheter (PICC) is the standard of care for continuing IV medications once patients are medically stable and ready for discharge.[6] PWID have been excluded from OPAT studies,[6] leaving little evidence to guide care.[7] Furthermore, likely due to fears of ongoing IDU, PWID are often kept in the hospital for the full duration of their antibiotic courses. This practice is costly and may not be optimal, especially considering that hospitalized PWID have high rates of discharges against medical advice.[8, 9]
In 2012, as part of a quality‐improvement effort focused on hospitalized PWID requiring long courses of IV antibiotics, UKHealthCare in Lexington, Kentucky, established a protocol for OPAT in PWID meeting specific criteria. As this protocol was not widely adopted, we sought to formally assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT to inform future efforts. This study was approved by the University of Kentucky (UK) Institutional Review Board.
METHODS
A 14‐item survey (see Supporting Information, Appendix, in the online version of this article) with multiple‐choice and open‐ended response items was developed based on the existing protocol, and themes were confirmed through semistructured interviews with 10 attending physicians in hospital medicine (HM) and infectious disease (ID). Questions were designed to elucidate the role that IDU played in the decision to discharge patients on OPAT, identify barriers to discharging PWID on OPAT, as well as elicit recommendations for requisite services or programs. The first question excluded providers not caring for patients requiring long‐term IV antibiotics. Questions that allowed for open‐ended responses were categorized thematically initially by 1 researcher (L.F.), then refined and confirmed by another team member (J.L.). The survey was distributed over email through Qualtrics (Provo, Utah) software to attending physicians in HM, ID, cardiology, and surgery at UK. Qualtrics software was used to generate descriptive statistics.
RESULTS
In January 2015, the survey was emailed to 66 physicians, and the response rate was 83%, with 91% reporting caring for patients requiring long‐term IV antibiotics. Of those, 41 (82%) completed all items; 66% of completers were in HM, 12% ID, 10% surgery, and 2% cardiology. Sixty percent were male and in practice an average of 7.2 years. Thirty‐nine (95%) use OPAT for patients without IDU, but only 12 (29%) would consider OPAT in PWID. If the patient has a remote history of IDU, then 33 (79%) would consider OPAT. There was no agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months).
The most common physician‐identified barriers to discharging PWID on OPAT, as well as recommendations for services or processes to be in place to allow PWID to be discharged with OPAT, are listed in Table 1.
| Identified Barriers to Discharging PWID on OPAT (41 Responses) | % (No.) |
|---|---|
| |
| Socioeconomic factors (stable housing, transportation, living with responsible adult) | 66 (27) |
| Potential risk of the patient misusing PICC line for IDU | 66 (27) |
| Willingness of ID physician to follow the patient as an outpatient | 59 (24) |
| Potential risk of not completing IV antibiotic therapy | 49 (20) |
| Positive urine drug screen on admission | 44 (18) |
| Patient willingness to sign behavioral contract* | 39 (16) |
| Patient willingness to enter mental health or substance use disorder treatment | 39 (16) |
| Lack of a tamper‐evident mechanism that discourages misuse of the PICC line | 27 (11) |
| Lack of data on outcomes for OPAT in PWID | 24 (10) |
| Potential risk of being sued by a patient or family | 20 (8) |
| Other | |
| Recommendations for services or processes among providers who do not currently consider discharging PWID on OPAT (28 responses) | |
| Outpatient or ID follow‐up | 32 (9) |
| Monitoring mechanism including random urine drug screens | |
| Substance use disorder and mental health services and treatment | |
| Home health services | |
| Institutional placement (eg, inpatient rehab, extended‐care facility) | |
| More explicit legal protection | |
| Screening criteria to identify high risk for PICC line misuse | |
| Designated coordinator for this patient population | |
DISCUSSION
This survey illustrates the extremely complex barriers present when treating hospitalized PWID requiring long courses of IV antibiotics, and supports the anecdotal evidence that physicians often keep PWID in the hospital for weeks to administer IV antibiotics. The majority of our sample of physicians believe that the largest barriers to OPAT in PWID are socioeconomic factors and the potential risk of the patient misusing the PICC line. Although the overall response rate of our physician survey was robust,[10] our results reflect the opinions of HM and ID physicians at a single site. The low response rate among cardiologists in particular limits the generalizability of this survey. We suspect, however, that our results pertain to HM in other US hospitals, as nearly three‐fourths of 37 HM physicians surveyed at the University of California, Irvine were very concerned about PWIDs potentially misusing the PICC line, and approximately half reported they usually or always kept PWID in the hospital for prolonged treatment due to concern of substance use (personal and email communication: Lloyd Rucker, MD, unpublished data, November 6, 2015).
We were surprised that fewer than half of respondents identified substance use disorder (SUD) treatment as essential to the OPAT decision. The reasons that may explain this observation are likely multifactorial, and may include gaps in knowledge about and resources to provide evidence‐based addiction medicine. Further research is warranted to explore this observation, including the effect of enrollment into medication‐assisted treatment programs (eg, methadone, buprenorphine).
This survey suggests that although there is variability, OPAT may be an option in PWID, if outpatient follow‐up and ancillary services (ie, home health and possibly intensive case management) were well established. We believe the comorbid SUD must be also addressed. Based on the survey results and recommendations, we have begun relationships with community SUD treatment providers willing to monitor IV antibiotics with PICC lines, and dedicated additional case management staff to this population. We are evaluating these programs with the goal of contributing to an evidence base for this high‐risk population.
Acknowledgements
The authors thank Inski Yu, MD, for assistance with survey development, and Lloyd Rucker, MD, for data sharing.
Disclosure: Nothing to report.
Injection drug use (IDU) is a major public health problem leading to increased morbidity, mortality, and healthcare expenditures.[1, 2, 3] Persons who inject drugs (PWID) are often hospitalized with severe infections, such as endocarditis,[4, 5] which typically require prolonged courses of intravenous (IV) antibiotics. Outpatient parenteral antibiotic therapy (OPAT) via a peripherally inserted central catheter (PICC) is the standard of care for continuing IV medications once patients are medically stable and ready for discharge.[6] PWID have been excluded from OPAT studies,[6] leaving little evidence to guide care.[7] Furthermore, likely due to fears of ongoing IDU, PWID are often kept in the hospital for the full duration of their antibiotic courses. This practice is costly and may not be optimal, especially considering that hospitalized PWID have high rates of discharges against medical advice.[8, 9]
In 2012, as part of a quality‐improvement effort focused on hospitalized PWID requiring long courses of IV antibiotics, UKHealthCare in Lexington, Kentucky, established a protocol for OPAT in PWID meeting specific criteria. As this protocol was not widely adopted, we sought to formally assess attitudes, practices, and mediating factors impacting the decision making about discharging PWID on OPAT to inform future efforts. This study was approved by the University of Kentucky (UK) Institutional Review Board.
METHODS
A 14‐item survey (see Supporting Information, Appendix, in the online version of this article) with multiple‐choice and open‐ended response items was developed based on the existing protocol, and themes were confirmed through semistructured interviews with 10 attending physicians in hospital medicine (HM) and infectious disease (ID). Questions were designed to elucidate the role that IDU played in the decision to discharge patients on OPAT, identify barriers to discharging PWID on OPAT, as well as elicit recommendations for requisite services or programs. The first question excluded providers not caring for patients requiring long‐term IV antibiotics. Questions that allowed for open‐ended responses were categorized thematically initially by 1 researcher (L.F.), then refined and confirmed by another team member (J.L.). The survey was distributed over email through Qualtrics (Provo, Utah) software to attending physicians in HM, ID, cardiology, and surgery at UK. Qualtrics software was used to generate descriptive statistics.
RESULTS
In January 2015, the survey was emailed to 66 physicians, and the response rate was 83%, with 91% reporting caring for patients requiring long‐term IV antibiotics. Of those, 41 (82%) completed all items; 66% of completers were in HM, 12% ID, 10% surgery, and 2% cardiology. Sixty percent were male and in practice an average of 7.2 years. Thirty‐nine (95%) use OPAT for patients without IDU, but only 12 (29%) would consider OPAT in PWID. If the patient has a remote history of IDU, then 33 (79%) would consider OPAT. There was no agreed‐upon definition of remote history of IDU (range, 2120 months; median, 12 months).
The most common physician‐identified barriers to discharging PWID on OPAT, as well as recommendations for services or processes to be in place to allow PWID to be discharged with OPAT, are listed in Table 1.
| Identified Barriers to Discharging PWID on OPAT (41 Responses) | % (No.) |
|---|---|
| |
| Socioeconomic factors (stable housing, transportation, living with responsible adult) | 66 (27) |
| Potential risk of the patient misusing PICC line for IDU | 66 (27) |
| Willingness of ID physician to follow the patient as an outpatient | 59 (24) |
| Potential risk of not completing IV antibiotic therapy | 49 (20) |
| Positive urine drug screen on admission | 44 (18) |
| Patient willingness to sign behavioral contract* | 39 (16) |
| Patient willingness to enter mental health or substance use disorder treatment | 39 (16) |
| Lack of a tamper‐evident mechanism that discourages misuse of the PICC line | 27 (11) |
| Lack of data on outcomes for OPAT in PWID | 24 (10) |
| Potential risk of being sued by a patient or family | 20 (8) |
| Other | |
| Recommendations for services or processes among providers who do not currently consider discharging PWID on OPAT (28 responses) | |
| Outpatient or ID follow‐up | 32 (9) |
| Monitoring mechanism including random urine drug screens | |
| Substance use disorder and mental health services and treatment | |
| Home health services | |
| Institutional placement (eg, inpatient rehab, extended‐care facility) | |
| More explicit legal protection | |
| Screening criteria to identify high risk for PICC line misuse | |
| Designated coordinator for this patient population | |
DISCUSSION
This survey illustrates the extremely complex barriers present when treating hospitalized PWID requiring long courses of IV antibiotics, and supports the anecdotal evidence that physicians often keep PWID in the hospital for weeks to administer IV antibiotics. The majority of our sample of physicians believe that the largest barriers to OPAT in PWID are socioeconomic factors and the potential risk of the patient misusing the PICC line. Although the overall response rate of our physician survey was robust,[10] our results reflect the opinions of HM and ID physicians at a single site. The low response rate among cardiologists in particular limits the generalizability of this survey. We suspect, however, that our results pertain to HM in other US hospitals, as nearly three‐fourths of 37 HM physicians surveyed at the University of California, Irvine were very concerned about PWIDs potentially misusing the PICC line, and approximately half reported they usually or always kept PWID in the hospital for prolonged treatment due to concern of substance use (personal and email communication: Lloyd Rucker, MD, unpublished data, November 6, 2015).
We were surprised that fewer than half of respondents identified substance use disorder (SUD) treatment as essential to the OPAT decision. The reasons that may explain this observation are likely multifactorial, and may include gaps in knowledge about and resources to provide evidence‐based addiction medicine. Further research is warranted to explore this observation, including the effect of enrollment into medication‐assisted treatment programs (eg, methadone, buprenorphine).
This survey suggests that although there is variability, OPAT may be an option in PWID, if outpatient follow‐up and ancillary services (ie, home health and possibly intensive case management) were well established. We believe the comorbid SUD must be also addressed. Based on the survey results and recommendations, we have begun relationships with community SUD treatment providers willing to monitor IV antibiotics with PICC lines, and dedicated additional case management staff to this population. We are evaluating these programs with the goal of contributing to an evidence base for this high‐risk population.
Acknowledgements
The authors thank Inski Yu, MD, for assistance with survey development, and Lloyd Rucker, MD, for data sharing.
Disclosure: Nothing to report.
- , , , et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458.
- , , , . Increases in drug and opioid overdose deaths—United States, 2000‐2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382.
- , , , , , . Understanding patterns of high‐cost health care use across different substance user groups. Health Aff (Millwood). 2016;35(1):12–19.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , . Bacterial infections in drug users. N Engl J Med. 2005;353(18):1945–1954.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004 2004;38(12):1651–1671.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.
- , , , . Hospitals as a ‘risk environment’: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59–66.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , . Do additional recontacts to increase response rate improve physician survey data quality? Med Care. 2013;51(10):945–948.
- , , , et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458.
- , , , . Increases in drug and opioid overdose deaths—United States, 2000‐2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382.
- , , , , , . Understanding patterns of high‐cost health care use across different substance user groups. Health Aff (Millwood). 2016;35(1):12–19.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , . Bacterial infections in drug users. N Engl J Med. 2005;353(18):1945–1954.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004 2004;38(12):1651–1671.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.
- , , , . Hospitals as a ‘risk environment’: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59–66.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , . Do additional recontacts to increase response rate improve physician survey data quality? Med Care. 2013;51(10):945–948.
AMI and Heavy Drinking
Moderate alcohol consumption has been associated with lower risk of coronary heart disease death.[1, 2, 3] This benefit has been shown across all age groups, both sexes, in low‐risk patients (without prior cardiovascular disease [CVD], diabetics and even in patients with established CVD.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] The relationship between the dose of alcohol and total mortality has been depicted in many observational studies as a J‐shaped curve, attributed to a combined effect of both benefits and harms.[3, 4, 13] Unlike moderate drinking, heavy drinking and particularly binge drinking may have net negative cardiovascular effects. For example, higher levels of intake of alcohol were associated with increased mortality in men with previous myocardial infarction,[14] whereas some reports suggest a continued beneficial association with acute myocardial infarction (AMI).[15, 16, 17] In other studies, the association between AMI and binge or chronic heavy drinking is inconsistent or lacks enough power to report the risk/benefit estimates.[3] Data are sparse on the effects of alcoholism on outcomes in patients hospitalized due to an AMI. Therefore, we sought to investigate the prevalence and association of alcohol‐related diagnoses with in‐hospital mortality in patients presenting with AMI in the United States.
METHODS
This study was a cross‐sectional analysis of the 2011 Nationwide Inpatient Sample (NIS). The NIS is a publicly available deidentified database of hospital discharges in the United States.[18] It contains data from approximately 8 million hospital stays that were selected using a complex probability sampling design and weighting scheme intended to represent all discharges from nonfederal hospitals in the United States. Each record includes 1 primary diagnosis and up to 24 secondary diagnoses.
Analysis was conducted for all patients aged 21 years and greater with a primary discharge diagnosis of AMI based on International Classification of Diseases, 9th Revision (ICD‐9) codes. ST‐elevation myocardial infarction (STEMI) and nonST‐elevation myocardial infarction (NSTEMI) were recorded when the principal diagnosis included the appropriate ICD‐9 codes (see Supporting Table 1 in the online version of this article). Alcohol‐related diagnosis was categorized as the presence of alcohol use disorders or other chronic conditions caused by heavy drinking such as alcoholic cardiomyopathy and alcoholic liver disease among others. Variables reflecting acute effects and chronic effects of alcohol use were created for analytic purposes. Acute effects that increase the risk for acute withdrawal syndrome and hemodynamic instability (and may thereby effect mortality) were characterized by alcohol withdrawal, acute alcoholic hepatitis, alcoholic gastritis, or acute alcohol intoxication. Chronic effects of alcohol were characterized by alcohol dependence, alcoholic polyneuropathy, alcoholic cardiomyopathy, or alcoholic liver damage other than acute hepatitis. A number of comorbidities were generated from ICD‐9 codes including smoking, chronic liver disease, peripheral vascular disease, hypertension, diabetes, renal failure, drug abuse, arrhythmia, and gastrointestinal bleeding using Clinical Classification Software codes provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality[19] (see Supporting Table 1 in the online version of this article).
The risk for alcohol‐related diagnoses in AMI patients adjusting for age and sex was estimated using all adult discharge records. All other analyses included only AMI discharges. The principal outcome measure was in‐hospital mortality. Secondary outcomes included having a cardiac procedure (diagnostic catheterization, percutaneous coronary angioplasty, or coronary bypass grafting), and length of stay.
All statistical analyses were performed using Statistical Analysis Software version 9.4 (SAS Inc., Cary, NC). Logistic regression methods appropriate for the NIS sample design were utilized to predict AMI mortality risk associated with alcohol‐related diagnoses (overall and separately for acute and chronic alcohol‐related diagnoses). Mortality risk was evaluated in all AMI discharges and again for STEMI and NSTEMI discharges. To control for factors frequently associated with alcoholism, adjustment was made for age, sex, liver disease, hypertension, diabetes, renal failure, peripheral vascular disease, arrhythmias, drug abuse, gastrointestinal bleed, and smoking. For secondary outcomes, odds ratios were calculated for having a cardiac procedure performed during the hospital admission and length of stay above the median.
RESULTS
Table 1 lists characteristics of AMI patients stratified by in‐hospital mortality. In 2011, AMI accounted for 610,963 (1.9%) of overall adult hospital admissions, with an in‐hospital mortality of 5.3%. Thirty‐two percent were STEMI admissions and 68% were NSTEMI admissions with in‐hospital mortality of 8.5% and 3.8%, respectively. Patients with alcohol‐related diagnoses comprised 18,684 (3.1%) of all AMI admissions. This prevalence was significantly lower relative to non‐AMI admissions (4.9%), even after age and sex adjustment (adjusted odds ratio [OR]: 0.7, 95% confidence interval [CI]: 0.6‐0.7, P 0.001).
| Variables | AMI, In‐hospital Death | AMI, Alive at Discharge | P Value |
|---|---|---|---|
| |||
| No. | 32,399 (5.3) | 578,564 (94.7) | 0.0001 |
| Age, y (SD) | 76 (7577) | 67 (6668) | |
| Sex | |||
| Males | 17,483 (54) | 352,943 (61) | 0.0001 |
| Females | 14,916 (46) | 225,621 (39) | 0.0001 |
| Race | |||
| White | 22,517 (70) | 387,816 (67) | 0.0001 |
| Black | 2,580 (7.9) | 56,735 (9.8) | 0.0001 |
| Hispanic | 2,002 (6.1) | 41,399 (7.2) | 0.0001 |
| Asian | 685 (2) | 11,160 (1.9) | 0.0001 |
| Native American | 146 (0.3) | 2,240 (0.4) | 0.0001 |
| Others | 991 (3) | 17,711 (3.2) | 0.0001 |
| Unspecified | 3,478 (10.7) | 61,503 (10.5) | 0.0001 |
| STEMI | 16,437 (50.7) | 177,240 (30.6) | 0.0001 |
| NSTEMI | 15,962 (49.3) | 401,324 (69.4) | 0.0001 |
| Alcohol diagnoses | |||
| Acute drinking | 110 (0.3) | 2,615 (0.5) | 0.1389 |
| Chronic drinking | 816 (2.5) | 15,143 (2.6) | 0.2473 |
| Comorbidities | |||
| Diabetes mellitus | 11,497 (35.5) | 211,321 (36.5) | 0.5963 |
| Hypertension | 20,068 (61.9) | 411,853 (71.2) | 0.0001 |
| Peripheral vascular disease | 4,962 (15.3) | 70,024 (12.1) | 0.0001 |
| Renal failure | 9,929 (30.6) | 113,714 (19.7) | 0.0001 |
| Drug abuse | 330 (1.0) | 13,263 (2.3) | 0.0001 |
| Arrhythmias | 14,977 (46.2) | 167,286 (28.9) | 0.0001 |
| Liver disease | 442 (1.4) | 6,493 (1.1) | 0.0753 |
| Smoking history | 6,736 (20.8) | 210,205 (36.3) | 0.0001 |
| Gastrointestinal bleed | 1,982 (6.1) | 12,086 (2.1) | 0.0001 |
Table 2 lists the characteristics of AMI patients stratified by alcohol status. Patients with alcohol‐related disorders presenting with AMI were younger, overwhelmingly male, and had a higher prevalence of the following comorbid conditions: drug abuse, liver disease, gastrointestinal bleeding, and smoking history. They had a lower prevalence of diabetes, hypertension, and renal failure.
| Variables | Alcohol‐Related Diagnoses | No Alcohol‐Related Diagnoses | P Value |
|---|---|---|---|
| |||
| No. | 18,684 (3.1) | 592,279 (96.9) | 0.0001 |
| Age, y, mean | 59 (5860) | 68 (6769) | 0.0001 |
| Sex | |||
| Males | 16,315 (87.3) | 354,051 (59.8) | 0.0001 |
| Females | 2,369 (12.7) | 238,228 (40.2) | 0.0001 |
| Race | |||
| White | 11,917 (63.8) | 398,766 (67.2) | 0.0001 |
| Black | 2,613 (13.9) | 56,723 (9.6) | 0.0001 |
| Hispanic | 1,400 (7.5) | 42,052 (7.1) | 0.0001 |
| Asian | 125 (0.7) | 11,724 (1.9) | 0.0001 |
| Native American | 165 (0.9) | 2,221 (0.4) | 0.0001 |
| Others | 570 (2.9) | 18,139 (3.2) | 0.0001 |
| Unspecified | 1,894 (10.1) | 62,654 (10.6) | 0.0001 |
| STEMI | 6,541 (35.1) | 187,136 (31.2) | 0.0001 |
| NSTEMI | 12,143 (64.9) | 405,143 (68.8) | 0.0001 |
| Died | 881 (4.7) | 31,518 (5.3) | 0.1312 |
| Comorbidities | |||
| Diabetes mellitus | 4,663 (24.9) | 218,446 (36.8) | 0.0001 |
| Hypertension | 12,501 (66.8) | 420,001 (70.8) | 0.0001 |
| Peripheral vascular disease | 2,269 (12.1) | 72,773 (12.3) | 0.7987 |
| Renal failure | 1,937 (10.4) | 121,925 (20.6) | 0.0001 |
| Drug abuse | 2,894 (15.5) | 10,708 (1.8) | 0.0001 |
| Arrhythmias | 5,476 (29.3) | 177,088 (29.9) | 0.4076 |
| Liver disease | 887 (4.7) | 6,053 (1.0) | 0.0001 |
| Smoking history | 12,771 (68.3) | 204,390 (34.5) | 0.0001 |
| Gastrointestinal bleed | 730 (3.9) | 13,347 (2.3) | 0.0001 |
Among AMI patients, unadjusted in‐hospital mortality was observed to be similar in the alcohol use disorder group (4.7% vs 5.3%, P = 0.131), STEMI hospitalizations (7.9% vs 8.5%, P = 0.475), and lower in NSTEMI hospitalizations (3% vs 3.9%, P = 0.035). However, as shown in Table 2, there were a number of factors that may have influenced death in AMI patients that differed between those with and without alcohol diagnoses. Table 3 shows the adjusted risk for death and each secondary outcome. After adjusting for factors associated with alcoholism, including age, sex, liver disease, hypertension, diabetes, renal failure, drug abuse, gastrointestinal bleed, and smoking, alcohol‐related diagnoses were associated with increased mortality in AMI hospitalizations (adjusted OR: 1.5, 95% CI: 1.2‐1.7, P 0.001). Contrary to our expectations, however, acute alcohol‐related diagnoses were not independently associated with mortality. The association with alcohol‐related diagnoses was significant in both STEMI (adjusted OR: 1.7, 95% CI: 1.4‐2.2, P 0.001) and NSTEMI patients (adjusted OR: 1.3, 95% CI: 1.0‐1.7, P = 0.025).
| Adjusted Odds Ratio* | 95% Confidence Intervals | P Value | |
|---|---|---|---|
| |||
| Primary outcome: death | |||
| AMI | |||
| Alcohol diagnoses | 1.5 | 1.21.7 | 0.001 |
| Acute alcohol diagnoses | 1.0 | 0.71.5 | 0.886 |
| Chronic alcohol diagnoses | 1.5 | 1.21.8 | 0.001 |
| STEMI | |||
| Alcohol diagnoses | 1.7 | 1.42.2 | 0.001 |
| Acute alcohol diagnoses | 1.1 | 0.61.9 | 0.835 |
| Chronic alcohol diagnoses | 1.6 | 1.22.1 | 0.001 |
| NSTEMI | |||
| Alcohol diagnoses | 1.3 | 1.01.7 | 0.025 |
| Acute alcohol diagnoses | 1.2 | 0.72.1 | 0.581 |
| Chronic alcohol diagnoses | 1.4 | 1.11.9 | 0.022 |
| Secondary outcomes | |||
| AMI | |||
| Length of stay | 1.5 | 1.31.6 | 0.001 |
| All cardiac procedures | 0.6 | 0.60.7 | 0.001 |
| CABG | 1.2 | 1.01.3 | 0.008 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
| STEMI | |||
| Length of stay | 1.2 | 1.11.4 | 0.001 |
| All cardiac procedures | 0.6 | 0.50.7 | 0.001 |
| CABG | 1.2 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.50.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.9 | 0.001 |
| NSTEMI | |||
| Length of stay | 1.6 | 1.51.8 | 0.001 |
| All cardiac procedures | 0.7 | 0.60.8 | 0.001 |
| CABG | 1.1 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
Regarding secondary outcomes, alcohol‐related diagnoses were associated with an increased length of stay, fewer diagnostic catheterizations and angioplasties, but higher coronary artery bypass grafting (CABG) procedures (Table 3).
DISCUSSION
In this analysis of AMI discharges, a modestly increased risk of in‐hospital mortality was found for patients with alcohol‐related diagnoses, although AMI patients were less likely to have a diagnosis related to alcohol. This increased risk of in‐hospital mortality was present in both STEMI and NSTEMI patients with alcohol‐related diagnoses, and was present in patients with chronic alcohol‐related diagnoses but not with withdrawal or intoxication. In addition to mortality differences, AMI patients with alcohol‐related diagnoses had a higher length of stay, but were less likely to have a cardiac procedure.
The association of alcohol‐related diagnoses with cardiovascular outcomes is not as well defined as the beneficial association between coronary heart disease and moderate alcohol use. Heavy drinking has been associated with greater risk of sudden cardiac death in subjects with preexisting coronary heart disease.[20, 21] Data from the Nurses Health Study demonstrated a U‐shaped curve between alcohol use and sudden cardiac death, but with limited power for assessing heavy drinking patterns.[22] In the Physicians Health Study, there was no significant increase in the risk of sudden cardiac death in men with higher intake of alcohol (2 drinks/day), but again with limited power for evaluating truly heavy drinking.[23] More recently, as shown by Mukamal et al., there was a trend toward higher overall cardiovascular deaths (OR: 1.07, 95% CI: 0.94‐1.22) but lower coronary heart disease mortality (OR: 0.80, 95% CI: 0.61‐1.05) in heavy drinkers, but results were not statistically significant even after adjusting for age, sex, and race.[3] One study demonstrated that heavy episodic drinking within the preceding 24 hours was associated with an increased risk of myocardial infarction (OR: 1.4, 95% confidence interval: 1.1‐1.9), particularly in the elderly (>65 years old) (OR: 5.3, 95% CI: 1.6‐18),[24] but the study did not consider mortality. The more recent study done by Mostofsky et al. has shown higher incidence of AMI onset within 1 hour after alcohol consumption among people who are not daily drinkers,[25] but the study did not consider mortality outcomes.
As an extension of knowledge regarding the association of alcohol‐related diagnoses with cardiovascular outcomes, we believe that our analysis of the NIS is the first to show a statistically significant positive age‐adjusted association of in‐hospital mortality with alcohol‐related diagnoses in AMI patients. Episodic or binge drinking has been noted to have proarrhythmogenic effects leading to sudden cardiac death.[26] This would often occur prior to hospitalization, but once hospitalized the presence of rhythm abnormalities was not associated with alcohol diagnoses. Alcohol effects might also be expected to lead to increased AMI mortality due to autonomic instability, gastrointestinal bleeding, or liver disease, but intoxication, withdrawal, gastrointestinal bleeding, liver disease, or comorbid tobacco or drug abuse did not account for excess alcohol‐associated AMI mortality in this study. Additional research will be required to determine the reasons underlying the increased age‐adjusted mortality.
The important strength of the present study includes the use of a large national database that allowed us to link alcohol‐related diagnoses to AMI death in the hospital, and to explore potential confounders of this association (eg, gastrointestinal bleeding, withdrawal, liver disease). However, a number of limitations merit consideration. The NIS sampling frame is limited to hospital discharges. As such, we have no data on prehospital AMI death and alcohol use pattern immediately preceding hospitalization. Similarly, we were unable to consider mortality immediately beyond the hospital discharge. Other important predictors that are not recorded in the NIS are details regarding a patient's physical activity and medications such as statins and ‐blockers that could affect survivorship in AMI patients. Another potential limitation of our analysis is the lack of differentiating between type 2 myocardial infarction, occurring from sepsis or acute kidney injury, from a true NSTEMI. However, we included only primary discharge diagnoses of AMI, and results for STEMI and NSTEMI discharges were similar. Regarding the cross‐sectional study design, we are unable to establish a cause and effect relationship between in‐hospital AMI mortality and alcohol‐related diagnoses. The NIS data were abstracted from administrative databases that may lack important details on alcohol‐related problems. In particular, it seems likely that heavy drinkers with less obvious alcohol‐related problems would be underidentified in clinical settings, and this may have biased our results toward an overestimation of the alcohol‐associated risk. Due to these limitations, AMI mortality will need to be evaluated in other samples to definitively evaluate associations with diagnoses related to heavy drinking and determine the reasons underlying the association. The increased death and CABG despite decreased angiography and angioplasty suggests that these patients presentations may be with more severe coronary heart disease, which is a question requiring further study. Finally, an alcohol user who presents with an AMI is less likely to have cardiac risk factors like diabetes, renal failure, and possibly hypertension. Rather, alcohol diagnoses in AMI patients associate with tobacco and drug abuse, liver disease, and higher age‐adjusted risk for death. It is important for a practicing hospitalist to have a high index of suspicion for these atypical AMI patients.
CONCLUSION
Although alcohol‐related diagnoses are less commonly documented in AMI patients relative to other admission diagnoses, results of this study suggest that they independently predict in‐hospital mortality. More research is needed to definitively measure the risk of such death attributable to alcohol and determine the mechanisms underlying the association.
Disclosure
Nothing to report.
- , , , , . Alcohol and coronary heart disease: a meta‐analysis. Addiction. 2000;95(10):1505–1523.
- , , , et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944.
- , , , . Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55(13):1328–1335.
- , , , , , . Alcohol dosing and total mortality in men and women: an updated meta‐analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445.
- , , , et al. Alcohol intake and risk of coronary heart disease in younger, middle‐aged, and older adults. Circulation. 2010;121(14):1589–1597.
- , , , . Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112(25):3839–3845.
- , , , et al. Comparison of outcomes among moderate alcohol drinkers before acute myocardial infarction to effect of continued versus discontinuing alcohol intake after the infarct. Am J Cardiol. 2010;105(12):1651–1654.
- , , , , . Alcohol consumption and mortality in patients with cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2010;55(13):1339–1347.
- , , , , . Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285(15):1965–1970.
- , , , , . Lifestyle, social factors, and survival after age 75: population based study. BMJ. 2012;345:e5568.
- , , , et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–981.
- , , , , . Meta‐analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49(4):648–652.
- , , , , . Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc. 2014;89(3):382–393.
- , . Alcohol intake and mortality in middle aged men with diagnosed coronary heart disease. Heart. 2000;83(4):394–399.
- , , , et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart. 2010;96(2):124–130.
- , , . Does recent alcohol consumption reduce the risk of acute myocardial infarction and coronary death in regular drinkers? Am J Epidemiol. 1992;136(7):819–824.
- , . How much alcohol and how often? Population based case‐control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314(7088):1159–1164.
- HCUP Nationwide Inpatient Sample. Healthcare Cost and Utilization Project. Rockville, MD; Agency for Healthcare Research and Quality, 2011. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp.
- HCUP Clinical Classifications Software for Services and Procedures. Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp. Accessed May 10th, 2014.
- , . Drinking habits and cardiovascular disease: the Framingham Study. Am Heart J. 1983;105(4):667–673.
- , . Alcohol and sudden cardiac death. Br Heart J. 1992;68(5):443–448.
- , , , et al. Light‐to‐moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7(10):1374–1380.
- , , , , , . Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100(9):944–950.
- , , , et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case‐control study. Circulation. 2014;130(5):390–398.
- , , , et al. Risk of myocardial infarction immediately after alcohol consumption. Epidemiology. 2015;26(2):143–150.
- , , , , , . Drinking habits and coronary heart disease: the Yugoslavia cardiovascular disease study. Am J Epidemiol. 1982;116(5):748–758.
Moderate alcohol consumption has been associated with lower risk of coronary heart disease death.[1, 2, 3] This benefit has been shown across all age groups, both sexes, in low‐risk patients (without prior cardiovascular disease [CVD], diabetics and even in patients with established CVD.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] The relationship between the dose of alcohol and total mortality has been depicted in many observational studies as a J‐shaped curve, attributed to a combined effect of both benefits and harms.[3, 4, 13] Unlike moderate drinking, heavy drinking and particularly binge drinking may have net negative cardiovascular effects. For example, higher levels of intake of alcohol were associated with increased mortality in men with previous myocardial infarction,[14] whereas some reports suggest a continued beneficial association with acute myocardial infarction (AMI).[15, 16, 17] In other studies, the association between AMI and binge or chronic heavy drinking is inconsistent or lacks enough power to report the risk/benefit estimates.[3] Data are sparse on the effects of alcoholism on outcomes in patients hospitalized due to an AMI. Therefore, we sought to investigate the prevalence and association of alcohol‐related diagnoses with in‐hospital mortality in patients presenting with AMI in the United States.
METHODS
This study was a cross‐sectional analysis of the 2011 Nationwide Inpatient Sample (NIS). The NIS is a publicly available deidentified database of hospital discharges in the United States.[18] It contains data from approximately 8 million hospital stays that were selected using a complex probability sampling design and weighting scheme intended to represent all discharges from nonfederal hospitals in the United States. Each record includes 1 primary diagnosis and up to 24 secondary diagnoses.
Analysis was conducted for all patients aged 21 years and greater with a primary discharge diagnosis of AMI based on International Classification of Diseases, 9th Revision (ICD‐9) codes. ST‐elevation myocardial infarction (STEMI) and nonST‐elevation myocardial infarction (NSTEMI) were recorded when the principal diagnosis included the appropriate ICD‐9 codes (see Supporting Table 1 in the online version of this article). Alcohol‐related diagnosis was categorized as the presence of alcohol use disorders or other chronic conditions caused by heavy drinking such as alcoholic cardiomyopathy and alcoholic liver disease among others. Variables reflecting acute effects and chronic effects of alcohol use were created for analytic purposes. Acute effects that increase the risk for acute withdrawal syndrome and hemodynamic instability (and may thereby effect mortality) were characterized by alcohol withdrawal, acute alcoholic hepatitis, alcoholic gastritis, or acute alcohol intoxication. Chronic effects of alcohol were characterized by alcohol dependence, alcoholic polyneuropathy, alcoholic cardiomyopathy, or alcoholic liver damage other than acute hepatitis. A number of comorbidities were generated from ICD‐9 codes including smoking, chronic liver disease, peripheral vascular disease, hypertension, diabetes, renal failure, drug abuse, arrhythmia, and gastrointestinal bleeding using Clinical Classification Software codes provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality[19] (see Supporting Table 1 in the online version of this article).
The risk for alcohol‐related diagnoses in AMI patients adjusting for age and sex was estimated using all adult discharge records. All other analyses included only AMI discharges. The principal outcome measure was in‐hospital mortality. Secondary outcomes included having a cardiac procedure (diagnostic catheterization, percutaneous coronary angioplasty, or coronary bypass grafting), and length of stay.
All statistical analyses were performed using Statistical Analysis Software version 9.4 (SAS Inc., Cary, NC). Logistic regression methods appropriate for the NIS sample design were utilized to predict AMI mortality risk associated with alcohol‐related diagnoses (overall and separately for acute and chronic alcohol‐related diagnoses). Mortality risk was evaluated in all AMI discharges and again for STEMI and NSTEMI discharges. To control for factors frequently associated with alcoholism, adjustment was made for age, sex, liver disease, hypertension, diabetes, renal failure, peripheral vascular disease, arrhythmias, drug abuse, gastrointestinal bleed, and smoking. For secondary outcomes, odds ratios were calculated for having a cardiac procedure performed during the hospital admission and length of stay above the median.
RESULTS
Table 1 lists characteristics of AMI patients stratified by in‐hospital mortality. In 2011, AMI accounted for 610,963 (1.9%) of overall adult hospital admissions, with an in‐hospital mortality of 5.3%. Thirty‐two percent were STEMI admissions and 68% were NSTEMI admissions with in‐hospital mortality of 8.5% and 3.8%, respectively. Patients with alcohol‐related diagnoses comprised 18,684 (3.1%) of all AMI admissions. This prevalence was significantly lower relative to non‐AMI admissions (4.9%), even after age and sex adjustment (adjusted odds ratio [OR]: 0.7, 95% confidence interval [CI]: 0.6‐0.7, P 0.001).
| Variables | AMI, In‐hospital Death | AMI, Alive at Discharge | P Value |
|---|---|---|---|
| |||
| No. | 32,399 (5.3) | 578,564 (94.7) | 0.0001 |
| Age, y (SD) | 76 (7577) | 67 (6668) | |
| Sex | |||
| Males | 17,483 (54) | 352,943 (61) | 0.0001 |
| Females | 14,916 (46) | 225,621 (39) | 0.0001 |
| Race | |||
| White | 22,517 (70) | 387,816 (67) | 0.0001 |
| Black | 2,580 (7.9) | 56,735 (9.8) | 0.0001 |
| Hispanic | 2,002 (6.1) | 41,399 (7.2) | 0.0001 |
| Asian | 685 (2) | 11,160 (1.9) | 0.0001 |
| Native American | 146 (0.3) | 2,240 (0.4) | 0.0001 |
| Others | 991 (3) | 17,711 (3.2) | 0.0001 |
| Unspecified | 3,478 (10.7) | 61,503 (10.5) | 0.0001 |
| STEMI | 16,437 (50.7) | 177,240 (30.6) | 0.0001 |
| NSTEMI | 15,962 (49.3) | 401,324 (69.4) | 0.0001 |
| Alcohol diagnoses | |||
| Acute drinking | 110 (0.3) | 2,615 (0.5) | 0.1389 |
| Chronic drinking | 816 (2.5) | 15,143 (2.6) | 0.2473 |
| Comorbidities | |||
| Diabetes mellitus | 11,497 (35.5) | 211,321 (36.5) | 0.5963 |
| Hypertension | 20,068 (61.9) | 411,853 (71.2) | 0.0001 |
| Peripheral vascular disease | 4,962 (15.3) | 70,024 (12.1) | 0.0001 |
| Renal failure | 9,929 (30.6) | 113,714 (19.7) | 0.0001 |
| Drug abuse | 330 (1.0) | 13,263 (2.3) | 0.0001 |
| Arrhythmias | 14,977 (46.2) | 167,286 (28.9) | 0.0001 |
| Liver disease | 442 (1.4) | 6,493 (1.1) | 0.0753 |
| Smoking history | 6,736 (20.8) | 210,205 (36.3) | 0.0001 |
| Gastrointestinal bleed | 1,982 (6.1) | 12,086 (2.1) | 0.0001 |
Table 2 lists the characteristics of AMI patients stratified by alcohol status. Patients with alcohol‐related disorders presenting with AMI were younger, overwhelmingly male, and had a higher prevalence of the following comorbid conditions: drug abuse, liver disease, gastrointestinal bleeding, and smoking history. They had a lower prevalence of diabetes, hypertension, and renal failure.
| Variables | Alcohol‐Related Diagnoses | No Alcohol‐Related Diagnoses | P Value |
|---|---|---|---|
| |||
| No. | 18,684 (3.1) | 592,279 (96.9) | 0.0001 |
| Age, y, mean | 59 (5860) | 68 (6769) | 0.0001 |
| Sex | |||
| Males | 16,315 (87.3) | 354,051 (59.8) | 0.0001 |
| Females | 2,369 (12.7) | 238,228 (40.2) | 0.0001 |
| Race | |||
| White | 11,917 (63.8) | 398,766 (67.2) | 0.0001 |
| Black | 2,613 (13.9) | 56,723 (9.6) | 0.0001 |
| Hispanic | 1,400 (7.5) | 42,052 (7.1) | 0.0001 |
| Asian | 125 (0.7) | 11,724 (1.9) | 0.0001 |
| Native American | 165 (0.9) | 2,221 (0.4) | 0.0001 |
| Others | 570 (2.9) | 18,139 (3.2) | 0.0001 |
| Unspecified | 1,894 (10.1) | 62,654 (10.6) | 0.0001 |
| STEMI | 6,541 (35.1) | 187,136 (31.2) | 0.0001 |
| NSTEMI | 12,143 (64.9) | 405,143 (68.8) | 0.0001 |
| Died | 881 (4.7) | 31,518 (5.3) | 0.1312 |
| Comorbidities | |||
| Diabetes mellitus | 4,663 (24.9) | 218,446 (36.8) | 0.0001 |
| Hypertension | 12,501 (66.8) | 420,001 (70.8) | 0.0001 |
| Peripheral vascular disease | 2,269 (12.1) | 72,773 (12.3) | 0.7987 |
| Renal failure | 1,937 (10.4) | 121,925 (20.6) | 0.0001 |
| Drug abuse | 2,894 (15.5) | 10,708 (1.8) | 0.0001 |
| Arrhythmias | 5,476 (29.3) | 177,088 (29.9) | 0.4076 |
| Liver disease | 887 (4.7) | 6,053 (1.0) | 0.0001 |
| Smoking history | 12,771 (68.3) | 204,390 (34.5) | 0.0001 |
| Gastrointestinal bleed | 730 (3.9) | 13,347 (2.3) | 0.0001 |
Among AMI patients, unadjusted in‐hospital mortality was observed to be similar in the alcohol use disorder group (4.7% vs 5.3%, P = 0.131), STEMI hospitalizations (7.9% vs 8.5%, P = 0.475), and lower in NSTEMI hospitalizations (3% vs 3.9%, P = 0.035). However, as shown in Table 2, there were a number of factors that may have influenced death in AMI patients that differed between those with and without alcohol diagnoses. Table 3 shows the adjusted risk for death and each secondary outcome. After adjusting for factors associated with alcoholism, including age, sex, liver disease, hypertension, diabetes, renal failure, drug abuse, gastrointestinal bleed, and smoking, alcohol‐related diagnoses were associated with increased mortality in AMI hospitalizations (adjusted OR: 1.5, 95% CI: 1.2‐1.7, P 0.001). Contrary to our expectations, however, acute alcohol‐related diagnoses were not independently associated with mortality. The association with alcohol‐related diagnoses was significant in both STEMI (adjusted OR: 1.7, 95% CI: 1.4‐2.2, P 0.001) and NSTEMI patients (adjusted OR: 1.3, 95% CI: 1.0‐1.7, P = 0.025).
| Adjusted Odds Ratio* | 95% Confidence Intervals | P Value | |
|---|---|---|---|
| |||
| Primary outcome: death | |||
| AMI | |||
| Alcohol diagnoses | 1.5 | 1.21.7 | 0.001 |
| Acute alcohol diagnoses | 1.0 | 0.71.5 | 0.886 |
| Chronic alcohol diagnoses | 1.5 | 1.21.8 | 0.001 |
| STEMI | |||
| Alcohol diagnoses | 1.7 | 1.42.2 | 0.001 |
| Acute alcohol diagnoses | 1.1 | 0.61.9 | 0.835 |
| Chronic alcohol diagnoses | 1.6 | 1.22.1 | 0.001 |
| NSTEMI | |||
| Alcohol diagnoses | 1.3 | 1.01.7 | 0.025 |
| Acute alcohol diagnoses | 1.2 | 0.72.1 | 0.581 |
| Chronic alcohol diagnoses | 1.4 | 1.11.9 | 0.022 |
| Secondary outcomes | |||
| AMI | |||
| Length of stay | 1.5 | 1.31.6 | 0.001 |
| All cardiac procedures | 0.6 | 0.60.7 | 0.001 |
| CABG | 1.2 | 1.01.3 | 0.008 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
| STEMI | |||
| Length of stay | 1.2 | 1.11.4 | 0.001 |
| All cardiac procedures | 0.6 | 0.50.7 | 0.001 |
| CABG | 1.2 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.50.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.9 | 0.001 |
| NSTEMI | |||
| Length of stay | 1.6 | 1.51.8 | 0.001 |
| All cardiac procedures | 0.7 | 0.60.8 | 0.001 |
| CABG | 1.1 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
Regarding secondary outcomes, alcohol‐related diagnoses were associated with an increased length of stay, fewer diagnostic catheterizations and angioplasties, but higher coronary artery bypass grafting (CABG) procedures (Table 3).
DISCUSSION
In this analysis of AMI discharges, a modestly increased risk of in‐hospital mortality was found for patients with alcohol‐related diagnoses, although AMI patients were less likely to have a diagnosis related to alcohol. This increased risk of in‐hospital mortality was present in both STEMI and NSTEMI patients with alcohol‐related diagnoses, and was present in patients with chronic alcohol‐related diagnoses but not with withdrawal or intoxication. In addition to mortality differences, AMI patients with alcohol‐related diagnoses had a higher length of stay, but were less likely to have a cardiac procedure.
The association of alcohol‐related diagnoses with cardiovascular outcomes is not as well defined as the beneficial association between coronary heart disease and moderate alcohol use. Heavy drinking has been associated with greater risk of sudden cardiac death in subjects with preexisting coronary heart disease.[20, 21] Data from the Nurses Health Study demonstrated a U‐shaped curve between alcohol use and sudden cardiac death, but with limited power for assessing heavy drinking patterns.[22] In the Physicians Health Study, there was no significant increase in the risk of sudden cardiac death in men with higher intake of alcohol (2 drinks/day), but again with limited power for evaluating truly heavy drinking.[23] More recently, as shown by Mukamal et al., there was a trend toward higher overall cardiovascular deaths (OR: 1.07, 95% CI: 0.94‐1.22) but lower coronary heart disease mortality (OR: 0.80, 95% CI: 0.61‐1.05) in heavy drinkers, but results were not statistically significant even after adjusting for age, sex, and race.[3] One study demonstrated that heavy episodic drinking within the preceding 24 hours was associated with an increased risk of myocardial infarction (OR: 1.4, 95% confidence interval: 1.1‐1.9), particularly in the elderly (>65 years old) (OR: 5.3, 95% CI: 1.6‐18),[24] but the study did not consider mortality. The more recent study done by Mostofsky et al. has shown higher incidence of AMI onset within 1 hour after alcohol consumption among people who are not daily drinkers,[25] but the study did not consider mortality outcomes.
As an extension of knowledge regarding the association of alcohol‐related diagnoses with cardiovascular outcomes, we believe that our analysis of the NIS is the first to show a statistically significant positive age‐adjusted association of in‐hospital mortality with alcohol‐related diagnoses in AMI patients. Episodic or binge drinking has been noted to have proarrhythmogenic effects leading to sudden cardiac death.[26] This would often occur prior to hospitalization, but once hospitalized the presence of rhythm abnormalities was not associated with alcohol diagnoses. Alcohol effects might also be expected to lead to increased AMI mortality due to autonomic instability, gastrointestinal bleeding, or liver disease, but intoxication, withdrawal, gastrointestinal bleeding, liver disease, or comorbid tobacco or drug abuse did not account for excess alcohol‐associated AMI mortality in this study. Additional research will be required to determine the reasons underlying the increased age‐adjusted mortality.
The important strength of the present study includes the use of a large national database that allowed us to link alcohol‐related diagnoses to AMI death in the hospital, and to explore potential confounders of this association (eg, gastrointestinal bleeding, withdrawal, liver disease). However, a number of limitations merit consideration. The NIS sampling frame is limited to hospital discharges. As such, we have no data on prehospital AMI death and alcohol use pattern immediately preceding hospitalization. Similarly, we were unable to consider mortality immediately beyond the hospital discharge. Other important predictors that are not recorded in the NIS are details regarding a patient's physical activity and medications such as statins and ‐blockers that could affect survivorship in AMI patients. Another potential limitation of our analysis is the lack of differentiating between type 2 myocardial infarction, occurring from sepsis or acute kidney injury, from a true NSTEMI. However, we included only primary discharge diagnoses of AMI, and results for STEMI and NSTEMI discharges were similar. Regarding the cross‐sectional study design, we are unable to establish a cause and effect relationship between in‐hospital AMI mortality and alcohol‐related diagnoses. The NIS data were abstracted from administrative databases that may lack important details on alcohol‐related problems. In particular, it seems likely that heavy drinkers with less obvious alcohol‐related problems would be underidentified in clinical settings, and this may have biased our results toward an overestimation of the alcohol‐associated risk. Due to these limitations, AMI mortality will need to be evaluated in other samples to definitively evaluate associations with diagnoses related to heavy drinking and determine the reasons underlying the association. The increased death and CABG despite decreased angiography and angioplasty suggests that these patients presentations may be with more severe coronary heart disease, which is a question requiring further study. Finally, an alcohol user who presents with an AMI is less likely to have cardiac risk factors like diabetes, renal failure, and possibly hypertension. Rather, alcohol diagnoses in AMI patients associate with tobacco and drug abuse, liver disease, and higher age‐adjusted risk for death. It is important for a practicing hospitalist to have a high index of suspicion for these atypical AMI patients.
CONCLUSION
Although alcohol‐related diagnoses are less commonly documented in AMI patients relative to other admission diagnoses, results of this study suggest that they independently predict in‐hospital mortality. More research is needed to definitively measure the risk of such death attributable to alcohol and determine the mechanisms underlying the association.
Disclosure
Nothing to report.
Moderate alcohol consumption has been associated with lower risk of coronary heart disease death.[1, 2, 3] This benefit has been shown across all age groups, both sexes, in low‐risk patients (without prior cardiovascular disease [CVD], diabetics and even in patients with established CVD.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] The relationship between the dose of alcohol and total mortality has been depicted in many observational studies as a J‐shaped curve, attributed to a combined effect of both benefits and harms.[3, 4, 13] Unlike moderate drinking, heavy drinking and particularly binge drinking may have net negative cardiovascular effects. For example, higher levels of intake of alcohol were associated with increased mortality in men with previous myocardial infarction,[14] whereas some reports suggest a continued beneficial association with acute myocardial infarction (AMI).[15, 16, 17] In other studies, the association between AMI and binge or chronic heavy drinking is inconsistent or lacks enough power to report the risk/benefit estimates.[3] Data are sparse on the effects of alcoholism on outcomes in patients hospitalized due to an AMI. Therefore, we sought to investigate the prevalence and association of alcohol‐related diagnoses with in‐hospital mortality in patients presenting with AMI in the United States.
METHODS
This study was a cross‐sectional analysis of the 2011 Nationwide Inpatient Sample (NIS). The NIS is a publicly available deidentified database of hospital discharges in the United States.[18] It contains data from approximately 8 million hospital stays that were selected using a complex probability sampling design and weighting scheme intended to represent all discharges from nonfederal hospitals in the United States. Each record includes 1 primary diagnosis and up to 24 secondary diagnoses.
Analysis was conducted for all patients aged 21 years and greater with a primary discharge diagnosis of AMI based on International Classification of Diseases, 9th Revision (ICD‐9) codes. ST‐elevation myocardial infarction (STEMI) and nonST‐elevation myocardial infarction (NSTEMI) were recorded when the principal diagnosis included the appropriate ICD‐9 codes (see Supporting Table 1 in the online version of this article). Alcohol‐related diagnosis was categorized as the presence of alcohol use disorders or other chronic conditions caused by heavy drinking such as alcoholic cardiomyopathy and alcoholic liver disease among others. Variables reflecting acute effects and chronic effects of alcohol use were created for analytic purposes. Acute effects that increase the risk for acute withdrawal syndrome and hemodynamic instability (and may thereby effect mortality) were characterized by alcohol withdrawal, acute alcoholic hepatitis, alcoholic gastritis, or acute alcohol intoxication. Chronic effects of alcohol were characterized by alcohol dependence, alcoholic polyneuropathy, alcoholic cardiomyopathy, or alcoholic liver damage other than acute hepatitis. A number of comorbidities were generated from ICD‐9 codes including smoking, chronic liver disease, peripheral vascular disease, hypertension, diabetes, renal failure, drug abuse, arrhythmia, and gastrointestinal bleeding using Clinical Classification Software codes provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality[19] (see Supporting Table 1 in the online version of this article).
The risk for alcohol‐related diagnoses in AMI patients adjusting for age and sex was estimated using all adult discharge records. All other analyses included only AMI discharges. The principal outcome measure was in‐hospital mortality. Secondary outcomes included having a cardiac procedure (diagnostic catheterization, percutaneous coronary angioplasty, or coronary bypass grafting), and length of stay.
All statistical analyses were performed using Statistical Analysis Software version 9.4 (SAS Inc., Cary, NC). Logistic regression methods appropriate for the NIS sample design were utilized to predict AMI mortality risk associated with alcohol‐related diagnoses (overall and separately for acute and chronic alcohol‐related diagnoses). Mortality risk was evaluated in all AMI discharges and again for STEMI and NSTEMI discharges. To control for factors frequently associated with alcoholism, adjustment was made for age, sex, liver disease, hypertension, diabetes, renal failure, peripheral vascular disease, arrhythmias, drug abuse, gastrointestinal bleed, and smoking. For secondary outcomes, odds ratios were calculated for having a cardiac procedure performed during the hospital admission and length of stay above the median.
RESULTS
Table 1 lists characteristics of AMI patients stratified by in‐hospital mortality. In 2011, AMI accounted for 610,963 (1.9%) of overall adult hospital admissions, with an in‐hospital mortality of 5.3%. Thirty‐two percent were STEMI admissions and 68% were NSTEMI admissions with in‐hospital mortality of 8.5% and 3.8%, respectively. Patients with alcohol‐related diagnoses comprised 18,684 (3.1%) of all AMI admissions. This prevalence was significantly lower relative to non‐AMI admissions (4.9%), even after age and sex adjustment (adjusted odds ratio [OR]: 0.7, 95% confidence interval [CI]: 0.6‐0.7, P 0.001).
| Variables | AMI, In‐hospital Death | AMI, Alive at Discharge | P Value |
|---|---|---|---|
| |||
| No. | 32,399 (5.3) | 578,564 (94.7) | 0.0001 |
| Age, y (SD) | 76 (7577) | 67 (6668) | |
| Sex | |||
| Males | 17,483 (54) | 352,943 (61) | 0.0001 |
| Females | 14,916 (46) | 225,621 (39) | 0.0001 |
| Race | |||
| White | 22,517 (70) | 387,816 (67) | 0.0001 |
| Black | 2,580 (7.9) | 56,735 (9.8) | 0.0001 |
| Hispanic | 2,002 (6.1) | 41,399 (7.2) | 0.0001 |
| Asian | 685 (2) | 11,160 (1.9) | 0.0001 |
| Native American | 146 (0.3) | 2,240 (0.4) | 0.0001 |
| Others | 991 (3) | 17,711 (3.2) | 0.0001 |
| Unspecified | 3,478 (10.7) | 61,503 (10.5) | 0.0001 |
| STEMI | 16,437 (50.7) | 177,240 (30.6) | 0.0001 |
| NSTEMI | 15,962 (49.3) | 401,324 (69.4) | 0.0001 |
| Alcohol diagnoses | |||
| Acute drinking | 110 (0.3) | 2,615 (0.5) | 0.1389 |
| Chronic drinking | 816 (2.5) | 15,143 (2.6) | 0.2473 |
| Comorbidities | |||
| Diabetes mellitus | 11,497 (35.5) | 211,321 (36.5) | 0.5963 |
| Hypertension | 20,068 (61.9) | 411,853 (71.2) | 0.0001 |
| Peripheral vascular disease | 4,962 (15.3) | 70,024 (12.1) | 0.0001 |
| Renal failure | 9,929 (30.6) | 113,714 (19.7) | 0.0001 |
| Drug abuse | 330 (1.0) | 13,263 (2.3) | 0.0001 |
| Arrhythmias | 14,977 (46.2) | 167,286 (28.9) | 0.0001 |
| Liver disease | 442 (1.4) | 6,493 (1.1) | 0.0753 |
| Smoking history | 6,736 (20.8) | 210,205 (36.3) | 0.0001 |
| Gastrointestinal bleed | 1,982 (6.1) | 12,086 (2.1) | 0.0001 |
Table 2 lists the characteristics of AMI patients stratified by alcohol status. Patients with alcohol‐related disorders presenting with AMI were younger, overwhelmingly male, and had a higher prevalence of the following comorbid conditions: drug abuse, liver disease, gastrointestinal bleeding, and smoking history. They had a lower prevalence of diabetes, hypertension, and renal failure.
| Variables | Alcohol‐Related Diagnoses | No Alcohol‐Related Diagnoses | P Value |
|---|---|---|---|
| |||
| No. | 18,684 (3.1) | 592,279 (96.9) | 0.0001 |
| Age, y, mean | 59 (5860) | 68 (6769) | 0.0001 |
| Sex | |||
| Males | 16,315 (87.3) | 354,051 (59.8) | 0.0001 |
| Females | 2,369 (12.7) | 238,228 (40.2) | 0.0001 |
| Race | |||
| White | 11,917 (63.8) | 398,766 (67.2) | 0.0001 |
| Black | 2,613 (13.9) | 56,723 (9.6) | 0.0001 |
| Hispanic | 1,400 (7.5) | 42,052 (7.1) | 0.0001 |
| Asian | 125 (0.7) | 11,724 (1.9) | 0.0001 |
| Native American | 165 (0.9) | 2,221 (0.4) | 0.0001 |
| Others | 570 (2.9) | 18,139 (3.2) | 0.0001 |
| Unspecified | 1,894 (10.1) | 62,654 (10.6) | 0.0001 |
| STEMI | 6,541 (35.1) | 187,136 (31.2) | 0.0001 |
| NSTEMI | 12,143 (64.9) | 405,143 (68.8) | 0.0001 |
| Died | 881 (4.7) | 31,518 (5.3) | 0.1312 |
| Comorbidities | |||
| Diabetes mellitus | 4,663 (24.9) | 218,446 (36.8) | 0.0001 |
| Hypertension | 12,501 (66.8) | 420,001 (70.8) | 0.0001 |
| Peripheral vascular disease | 2,269 (12.1) | 72,773 (12.3) | 0.7987 |
| Renal failure | 1,937 (10.4) | 121,925 (20.6) | 0.0001 |
| Drug abuse | 2,894 (15.5) | 10,708 (1.8) | 0.0001 |
| Arrhythmias | 5,476 (29.3) | 177,088 (29.9) | 0.4076 |
| Liver disease | 887 (4.7) | 6,053 (1.0) | 0.0001 |
| Smoking history | 12,771 (68.3) | 204,390 (34.5) | 0.0001 |
| Gastrointestinal bleed | 730 (3.9) | 13,347 (2.3) | 0.0001 |
Among AMI patients, unadjusted in‐hospital mortality was observed to be similar in the alcohol use disorder group (4.7% vs 5.3%, P = 0.131), STEMI hospitalizations (7.9% vs 8.5%, P = 0.475), and lower in NSTEMI hospitalizations (3% vs 3.9%, P = 0.035). However, as shown in Table 2, there were a number of factors that may have influenced death in AMI patients that differed between those with and without alcohol diagnoses. Table 3 shows the adjusted risk for death and each secondary outcome. After adjusting for factors associated with alcoholism, including age, sex, liver disease, hypertension, diabetes, renal failure, drug abuse, gastrointestinal bleed, and smoking, alcohol‐related diagnoses were associated with increased mortality in AMI hospitalizations (adjusted OR: 1.5, 95% CI: 1.2‐1.7, P 0.001). Contrary to our expectations, however, acute alcohol‐related diagnoses were not independently associated with mortality. The association with alcohol‐related diagnoses was significant in both STEMI (adjusted OR: 1.7, 95% CI: 1.4‐2.2, P 0.001) and NSTEMI patients (adjusted OR: 1.3, 95% CI: 1.0‐1.7, P = 0.025).
| Adjusted Odds Ratio* | 95% Confidence Intervals | P Value | |
|---|---|---|---|
| |||
| Primary outcome: death | |||
| AMI | |||
| Alcohol diagnoses | 1.5 | 1.21.7 | 0.001 |
| Acute alcohol diagnoses | 1.0 | 0.71.5 | 0.886 |
| Chronic alcohol diagnoses | 1.5 | 1.21.8 | 0.001 |
| STEMI | |||
| Alcohol diagnoses | 1.7 | 1.42.2 | 0.001 |
| Acute alcohol diagnoses | 1.1 | 0.61.9 | 0.835 |
| Chronic alcohol diagnoses | 1.6 | 1.22.1 | 0.001 |
| NSTEMI | |||
| Alcohol diagnoses | 1.3 | 1.01.7 | 0.025 |
| Acute alcohol diagnoses | 1.2 | 0.72.1 | 0.581 |
| Chronic alcohol diagnoses | 1.4 | 1.11.9 | 0.022 |
| Secondary outcomes | |||
| AMI | |||
| Length of stay | 1.5 | 1.31.6 | 0.001 |
| All cardiac procedures | 0.6 | 0.60.7 | 0.001 |
| CABG | 1.2 | 1.01.3 | 0.008 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
| STEMI | |||
| Length of stay | 1.2 | 1.11.4 | 0.001 |
| All cardiac procedures | 0.6 | 0.50.7 | 0.001 |
| CABG | 1.2 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.50.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.9 | 0.001 |
| NSTEMI | |||
| Length of stay | 1.6 | 1.51.8 | 0.001 |
| All cardiac procedures | 0.7 | 0.60.8 | 0.001 |
| CABG | 1.1 | 0.91.5 | 0.125 |
| Angioplasty | 0.6 | 0.60.7 | 0.001 |
| Diagnostic angiogram | 0.7 | 0.60.8 | 0.001 |
Regarding secondary outcomes, alcohol‐related diagnoses were associated with an increased length of stay, fewer diagnostic catheterizations and angioplasties, but higher coronary artery bypass grafting (CABG) procedures (Table 3).
DISCUSSION
In this analysis of AMI discharges, a modestly increased risk of in‐hospital mortality was found for patients with alcohol‐related diagnoses, although AMI patients were less likely to have a diagnosis related to alcohol. This increased risk of in‐hospital mortality was present in both STEMI and NSTEMI patients with alcohol‐related diagnoses, and was present in patients with chronic alcohol‐related diagnoses but not with withdrawal or intoxication. In addition to mortality differences, AMI patients with alcohol‐related diagnoses had a higher length of stay, but were less likely to have a cardiac procedure.
The association of alcohol‐related diagnoses with cardiovascular outcomes is not as well defined as the beneficial association between coronary heart disease and moderate alcohol use. Heavy drinking has been associated with greater risk of sudden cardiac death in subjects with preexisting coronary heart disease.[20, 21] Data from the Nurses Health Study demonstrated a U‐shaped curve between alcohol use and sudden cardiac death, but with limited power for assessing heavy drinking patterns.[22] In the Physicians Health Study, there was no significant increase in the risk of sudden cardiac death in men with higher intake of alcohol (2 drinks/day), but again with limited power for evaluating truly heavy drinking.[23] More recently, as shown by Mukamal et al., there was a trend toward higher overall cardiovascular deaths (OR: 1.07, 95% CI: 0.94‐1.22) but lower coronary heart disease mortality (OR: 0.80, 95% CI: 0.61‐1.05) in heavy drinkers, but results were not statistically significant even after adjusting for age, sex, and race.[3] One study demonstrated that heavy episodic drinking within the preceding 24 hours was associated with an increased risk of myocardial infarction (OR: 1.4, 95% confidence interval: 1.1‐1.9), particularly in the elderly (>65 years old) (OR: 5.3, 95% CI: 1.6‐18),[24] but the study did not consider mortality. The more recent study done by Mostofsky et al. has shown higher incidence of AMI onset within 1 hour after alcohol consumption among people who are not daily drinkers,[25] but the study did not consider mortality outcomes.
As an extension of knowledge regarding the association of alcohol‐related diagnoses with cardiovascular outcomes, we believe that our analysis of the NIS is the first to show a statistically significant positive age‐adjusted association of in‐hospital mortality with alcohol‐related diagnoses in AMI patients. Episodic or binge drinking has been noted to have proarrhythmogenic effects leading to sudden cardiac death.[26] This would often occur prior to hospitalization, but once hospitalized the presence of rhythm abnormalities was not associated with alcohol diagnoses. Alcohol effects might also be expected to lead to increased AMI mortality due to autonomic instability, gastrointestinal bleeding, or liver disease, but intoxication, withdrawal, gastrointestinal bleeding, liver disease, or comorbid tobacco or drug abuse did not account for excess alcohol‐associated AMI mortality in this study. Additional research will be required to determine the reasons underlying the increased age‐adjusted mortality.
The important strength of the present study includes the use of a large national database that allowed us to link alcohol‐related diagnoses to AMI death in the hospital, and to explore potential confounders of this association (eg, gastrointestinal bleeding, withdrawal, liver disease). However, a number of limitations merit consideration. The NIS sampling frame is limited to hospital discharges. As such, we have no data on prehospital AMI death and alcohol use pattern immediately preceding hospitalization. Similarly, we were unable to consider mortality immediately beyond the hospital discharge. Other important predictors that are not recorded in the NIS are details regarding a patient's physical activity and medications such as statins and ‐blockers that could affect survivorship in AMI patients. Another potential limitation of our analysis is the lack of differentiating between type 2 myocardial infarction, occurring from sepsis or acute kidney injury, from a true NSTEMI. However, we included only primary discharge diagnoses of AMI, and results for STEMI and NSTEMI discharges were similar. Regarding the cross‐sectional study design, we are unable to establish a cause and effect relationship between in‐hospital AMI mortality and alcohol‐related diagnoses. The NIS data were abstracted from administrative databases that may lack important details on alcohol‐related problems. In particular, it seems likely that heavy drinkers with less obvious alcohol‐related problems would be underidentified in clinical settings, and this may have biased our results toward an overestimation of the alcohol‐associated risk. Due to these limitations, AMI mortality will need to be evaluated in other samples to definitively evaluate associations with diagnoses related to heavy drinking and determine the reasons underlying the association. The increased death and CABG despite decreased angiography and angioplasty suggests that these patients presentations may be with more severe coronary heart disease, which is a question requiring further study. Finally, an alcohol user who presents with an AMI is less likely to have cardiac risk factors like diabetes, renal failure, and possibly hypertension. Rather, alcohol diagnoses in AMI patients associate with tobacco and drug abuse, liver disease, and higher age‐adjusted risk for death. It is important for a practicing hospitalist to have a high index of suspicion for these atypical AMI patients.
CONCLUSION
Although alcohol‐related diagnoses are less commonly documented in AMI patients relative to other admission diagnoses, results of this study suggest that they independently predict in‐hospital mortality. More research is needed to definitively measure the risk of such death attributable to alcohol and determine the mechanisms underlying the association.
Disclosure
Nothing to report.
- , , , , . Alcohol and coronary heart disease: a meta‐analysis. Addiction. 2000;95(10):1505–1523.
- , , , et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944.
- , , , . Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55(13):1328–1335.
- , , , , , . Alcohol dosing and total mortality in men and women: an updated meta‐analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445.
- , , , et al. Alcohol intake and risk of coronary heart disease in younger, middle‐aged, and older adults. Circulation. 2010;121(14):1589–1597.
- , , , . Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112(25):3839–3845.
- , , , et al. Comparison of outcomes among moderate alcohol drinkers before acute myocardial infarction to effect of continued versus discontinuing alcohol intake after the infarct. Am J Cardiol. 2010;105(12):1651–1654.
- , , , , . Alcohol consumption and mortality in patients with cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2010;55(13):1339–1347.
- , , , , . Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285(15):1965–1970.
- , , , , . Lifestyle, social factors, and survival after age 75: population based study. BMJ. 2012;345:e5568.
- , , , et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–981.
- , , , , . Meta‐analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49(4):648–652.
- , , , , . Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc. 2014;89(3):382–393.
- , . Alcohol intake and mortality in middle aged men with diagnosed coronary heart disease. Heart. 2000;83(4):394–399.
- , , , et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart. 2010;96(2):124–130.
- , , . Does recent alcohol consumption reduce the risk of acute myocardial infarction and coronary death in regular drinkers? Am J Epidemiol. 1992;136(7):819–824.
- , . How much alcohol and how often? Population based case‐control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314(7088):1159–1164.
- HCUP Nationwide Inpatient Sample. Healthcare Cost and Utilization Project. Rockville, MD; Agency for Healthcare Research and Quality, 2011. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp.
- HCUP Clinical Classifications Software for Services and Procedures. Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp. Accessed May 10th, 2014.
- , . Drinking habits and cardiovascular disease: the Framingham Study. Am Heart J. 1983;105(4):667–673.
- , . Alcohol and sudden cardiac death. Br Heart J. 1992;68(5):443–448.
- , , , et al. Light‐to‐moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7(10):1374–1380.
- , , , , , . Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100(9):944–950.
- , , , et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case‐control study. Circulation. 2014;130(5):390–398.
- , , , et al. Risk of myocardial infarction immediately after alcohol consumption. Epidemiology. 2015;26(2):143–150.
- , , , , , . Drinking habits and coronary heart disease: the Yugoslavia cardiovascular disease study. Am J Epidemiol. 1982;116(5):748–758.
- , , , , . Alcohol and coronary heart disease: a meta‐analysis. Addiction. 2000;95(10):1505–1523.
- , , , et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944.
- , , , . Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55(13):1328–1335.
- , , , , , . Alcohol dosing and total mortality in men and women: an updated meta‐analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445.
- , , , et al. Alcohol intake and risk of coronary heart disease in younger, middle‐aged, and older adults. Circulation. 2010;121(14):1589–1597.
- , , , . Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112(25):3839–3845.
- , , , et al. Comparison of outcomes among moderate alcohol drinkers before acute myocardial infarction to effect of continued versus discontinuing alcohol intake after the infarct. Am J Cardiol. 2010;105(12):1651–1654.
- , , , , . Alcohol consumption and mortality in patients with cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2010;55(13):1339–1347.
- , , , , . Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285(15):1965–1970.
- , , , , . Lifestyle, social factors, and survival after age 75: population based study. BMJ. 2012;345:e5568.
- , , , et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–981.
- , , , , . Meta‐analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49(4):648–652.
- , , , , . Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc. 2014;89(3):382–393.
- , . Alcohol intake and mortality in middle aged men with diagnosed coronary heart disease. Heart. 2000;83(4):394–399.
- , , , et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart. 2010;96(2):124–130.
- , , . Does recent alcohol consumption reduce the risk of acute myocardial infarction and coronary death in regular drinkers? Am J Epidemiol. 1992;136(7):819–824.
- , . How much alcohol and how often? Population based case‐control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314(7088):1159–1164.
- HCUP Nationwide Inpatient Sample. Healthcare Cost and Utilization Project. Rockville, MD; Agency for Healthcare Research and Quality, 2011. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp.
- HCUP Clinical Classifications Software for Services and Procedures. Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp. Accessed May 10th, 2014.
- , . Drinking habits and cardiovascular disease: the Framingham Study. Am Heart J. 1983;105(4):667–673.
- , . Alcohol and sudden cardiac death. Br Heart J. 1992;68(5):443–448.
- , , , et al. Light‐to‐moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7(10):1374–1380.
- , , , , , . Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100(9):944–950.
- , , , et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case‐control study. Circulation. 2014;130(5):390–398.
- , , , et al. Risk of myocardial infarction immediately after alcohol consumption. Epidemiology. 2015;26(2):143–150.
- , , , , , . Drinking habits and coronary heart disease: the Yugoslavia cardiovascular disease study. Am J Epidemiol. 1982;116(5):748–758.
Interhospital Transfer Handoff Practices
Transitions of care are major sources of preventable medical errors. Incomplete or inaccurate communication during handoffs is the root cause of many adverse events.[1] In a prospective study, adverse events were found to occur during interhospital transfer up to 30% of the time.[2] Furthermore, patients subject to interhospital transfer have longer length of stay and higher inpatient mortality, even after adjusting for mortality risk predictors.[3] Standardizing intrahospital handoff structures and communication practices has been shown to reduce medical errors.[4, 5, 6] Interhospital transfer is an understudied area among the transitions of care literature. Little is known about institutional variations in the process of information transfer and its association with patient outcomes. Although it is challenging to ascertain the total burden of transferred patients, it has been estimated that 1.6 million inpatients originated at another facility.[7] Additionally, approximately 5.9% of admissions to a representative sample of US intensive care units (ICU) originated from other hospitals.[8] Patients are transferred between hospitals for multiple reasons beyond medical necessity, for example, to adjust for patient preferences, bed availability, and hospital staffing patterns. This creates a setting in which complex and often critically ill patients are subject to variable and sometimes ambiguous handoff processes.[9]
This survey of 32 tertiary care centers in the United States was undertaken to identify common practices in communication and documentation during interhospital patient transfers. Additional goals were to understand the structure of the handoff process, the role of the transfer center, and how electronic medical records (EMR) and interhospital communication play a role in this care transition. Subsequently, common challenges in coordinating interhospital transfers were identified to provide a conceptual framework for process improvement.
METHODS
Survey Process
The survey was initiated in September 2013 and concluded in September 2015, and was designed to quantify patient volume and identify common as well as unique practices to improve communication across the transfer process. The respondents were transfer center directors or managers, typically with a nursing background. Mass e‐mail generated a very poor response rate and did not allow for discussion and clarification of responses. The strategy was then modified to contact individual institutions directly. The survey was performed via phone whenever possible. Figure 1 represents purposeful sampling conducted on 2 different groups of hospitals. These hospitals represent a convenience sample of institutions from a nationally ranked list of hospitals as well as others comparable to our own institutions. Hospitals were selected based on status as academic tertiary care centers with roughly similar bed sizes (600). Several were selected based on similar EMR capabilities. Geographic diversity was also taken into account. Thirty‐two academic tertiary care centers were ultimately included in the survey. Data were entered into a survey form and deidentified. The RutgersRobert Wood Johnson Medical School Institutional Review Board approved this study.
Survey Content
Qualitative and quantitative data were collected by the study team. Data included number and origin of transfers (including those from inpatient facilities and emergency departments), staff characteristics, transfer process, documentation received prior to transfer, EMR access and type, outcomes, and clinical status tracking (see Supporting Figure 1 in the online version of this article for the complete survey tool).
Measurement and Data Analysis
Descriptive statistics are presented in unweighted fashion as a number and percentage for dichotomous variables, or a numeric range for ordinal variables. When a range was given by survey participants, the lower end of the range was used to calculate the population median. Several institutions surveyed were unable to provide specific numeric values, but instead cited how many requests for transfer they received either daily or monthly; these were omitted from the demographics analysis.
Respondents also provided a description of their overall triage and acceptance process for qualitative analysis. Unique strategies were identified by the study personnel at the time of each interview and amassed at the end of the interview period. These strategies were then discussed by the study team, and separated into categories that addressed the main challenges associated with interhospital transfers. Five general tenants of the transfer process were identified: acceptance and transport, need for clinical updates, provider handoffs and coordination of care, information availability, and feedback.
RESULTS
Based on a survey question asking respondents to estimate the total number of interhospital transfers received per month, the annual burden of patients transferred into these 32 hospitals represented approximately 247,000 patients yearly. The median number of patients transferred per month, based on a point estimate if given or the lower end of the range if a range was provided, was 700 (range, 2502500). On average, 28% (range, 10%50%) were transferred directly to an ICU, representing approximately 69,000 critically ill patients. A majority of hospitals polled (65%) received patients from more than 100 referring institutions, and a minority (23%) identified EMR interoperability for more than a quarter of the sending facilities. The overall acceptance rate ranged from 50% to 95%.
Table 1 represents common transition elements of participating institutions. Thirty‐eight percent of hospitals utilize a critical caretrained registered nurse as the initial triage point of contact. The process and quality controls for coordinating transfers from outside hospitals were highly variable. Although clinical updates from acceptance to arrival were required in a majority of hospitals (81%), the acceptable time interval was inconsistent, varying from 2 to 4 hours (13%) to 24 hours (38%). A mandatory 3‐way recorded discussion (between transfer center staff, and referring and accepting physician) was nearly uniform. Objective clinical information to assist the handoff (ie, current labs, radiology images, history and physical, progress notes, or discharge summary) was available in only 29% of hospitals. Only 23% of hospitals also recorded a 3‐way nursing handoff (bedside‐to‐bedside nursing report). A minority of hospitals utilized their principal EMR to document the transfer process and share incoming clinical information among providers (32%).
| Survey Question | Survey Response | N (%) |
|---|---|---|
| ||
| What is the training background of the staff member who takes the initial call and triages patients in your transfer center? | Critical care experienced RN | 12/32 (38%) |
| Other clinical background (EMT, RN) | 13/32 (41%) | |
| Nonclinical personnel | 7/32 (22%) | |
| Prior to the patient's arrival, do you require any documentation to be transmitted from the transferring institution? | Objective clinical data required | 9/32 (28%) |
| Objective clinical data not required | 23/32 (72%) | |
| Is a 3‐way recorded conversation facilitated by the transfer center required? | Initial physician‐to‐physician acceptance discussion | 27/32 (84%) |
| RN‐to‐RN report | 6/26 (23%) | |
| Are clinical status updates required? | Updates required every 24 hours | 12/32 (38%) |
| Updates required every 812 hours | 7/32 (22%) | |
| Updates required every 24 hours | 4/32 (13%) | |
| Updates required but timing not specified | 3/32 (9%) | |
| Clinical status updates not required | 6/32 (19%) | |
| Is any clinical information obtained by the transfer center available to the patient's providers in real time on your EMR system? | Yes | 10/31 (32%) |
| No | 21/31 (68%) | |
| Do you track the outcomes of patients you accept from outside hospitals? | Yes | 14/24 (58%) |
| No | 10/24 (42%) | |
Descriptions of the transfer process were conceptually evaluated by the study team, then divided into 5 common themes: acceptance and transport, clinical updates, coordination of care, information availability, and quality improvement (Table 2). Institutions devised novel approaches including providing high bed priority to expedite transit, a dedicated quarterback physician to coordinate safe transfer and uninterrupted communication, electronic transfer notes to share communication with all providers, and a standardized system of feedback to referring hospitals. Several institutions relied on an expect note, which could be a free‐text document or a form document in the EMR. This preserves verbal handoff information that may otherwise be lost if the accepting physician at the time of transfer is not the physician receiving the handoff.
| Challenges | Innovative Practices |
|---|---|
| |
| Expedited acceptance and transport | Automatic acceptance for certain diagnoses (ie, neurosurgical indication for transfer) |
| Transferred patients prioritized for hospital beds over all patients except codes | |
| Hospital controls transportation units, allowing for immediate dispatch and patient retrieval | |
| Outsourcing of transfer center and interfacility transfer to third party | |
| Timeliness of clinical updates | Transfer center communicates with bedside RN for clinical updates at the time of transfer |
| Clinical status updates every 24 hours for critical patients | |
| Daily reevaluation of clinical status | |
| Accepting physician alerted of changes in clinical status | |
| Handoff and coordination of care | Physician accept tool in EMR |
| Quarterback physician who triages and accepts all patients during a given time period | |
| Critical patients are accepted into a critical care resuscitation unit, an all‐purpose intensive care unit staffed by an intensivist who shares decision making with the referring provider and is involved in all communications regarding the transferred patient | |
| Availability of protected clinical information | Scribed physician handoff imported into EMR |
| Expect note in EMR: summary of clinical information documented by accepting physician | |
| PACS radiology cloud networks for hospital systems or statewide | |
| EMR interoperability: Care Everywhere module in Epic EMR | |
| Health and information management department responsible for obtaining and scanning outside records into EMR | |
| Feedback and quality improvement | Automatic review if patient upgraded to ICU within 4 hours of arrival |
| Departmental chair review of physician verbal handoff if poor outcome or difficulty with transfer | |
| Outcomes and quality of handoff reported back to referring hospital | |
| Discharge summary sent to referring hospital | |
| Referring hospital able to view patient's chart for 1 year | |
Quality improvement occurred via both internal and external feedback at several institutions. There were two notable mechanisms of internal feedback. Review of recorded physician verbal handoff by department chair occurred if an adverse event involved a transferred patient. An automatic internal review was triggered if a patient was upgraded to a higher level of care within 4 hours of arrival. These advanced mechanisms require vigilance and dedication on the part of the transfer center and physicians involved in the transfer process. External feedback was provided to referring hospitals through both active and passive mechanisms. One advanced health system allowed referring providers to access the patient's inpatient medical record for 1 year and sent a discharge summary to all referring hospitals. Another hospital maintained a sophisticated scorecard, with key measures shared with internal stakeholders and referring hospitals. Some of the metrics tracked included: denials due to insufficient bed capacity, change in bed status within 12 hours of transfer, and duration of stay in the postanesthesia care unit or emergency department awaiting an inpatient bed. This organization also performed site visits to referring hospitals, addressing handoff quality improvement.
DISCUSSION
Standardizing intrahospital handoffs has been shown to decrease preventable medical errors and reduce possible near‐miss events.[6, 10] Interhospital care transitions are inherently more complex due to increased acuity and decreased continuity; yet, there is no universal standardization of these handovers. We found that practices vary widely among tertiary care centers, and the level of transfer center involvement in the verbal and written handoff is inconsistent.
Evidence‐based frameworks to improve healthcare delivery, such as TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), first require an organizational assessment to identify barriers to effective communication.[11] Interhospital transfers offer multiple unique barriers to continuity: physical distance, uncertainty in timing, incongruent treatment goals, disparate information sources, and distractions. This study provides the first step in conceptualizing the unique aspects of interhospital transfers, as well as highlights strategies to improve care coordination (Table 2).
A tailored intervention needs not only to overcome the typical barriers to handoffs such as time constraints, information sharing, and ambiguity in provider roles, but also to overcome multiple systems barriers. Bed management systems add another time‐related variable due to fixed and frequently overburdened bed capacity. Prioritization of transfers depends upon an accurate clinical depiction of patient acuity as well as organizational strategies. For example, neurologic diagnoses are commonly a top priority and are triaged as such, sometimes instead of higher‐acuity patients with other principal diagnoses. The complexity of this process may lead to delays in high‐acuity transfers, and is contingent upon accurate and updated clinical information. Coordinating handovers amidst complex provider schedules is another systems barrier. The commonly adopted 7 on, 7 off model for hospitalists, and shift work for intensivists, may increase the possibility that a transfer occurs across multiple provider changes. Patient follow‐up and closed‐loop feedback are important components of intrahospital handovers, but are much more challenging to implement for interhospital handovers with incongruent information systems and providers.
Programs to improve intrahospital handovers (eg, IPASS) emphasize creating an accurate clinical depiction of a patient using both verbal and written handoffs.[12] This is arguably more difficult over the phone without a concurrent written handoff. Recording of 3‐way physician and nurse handoffs is common, but reviews of recorded conversations are often unavailable or cumbersome in real time. EMR documentation of verbal information exchanged during the handoff is a possible solution. However, there may be legal implications for a transcribed verbal handoff. Furthermore, transfer centers often work with a software program separate from the principal EMR, and documentation in real time is challenging. EMR integration could help reinforce a patient‐centered shared mental model by allowing visualization of lab trends, radiology, vitals, and other documentation during and after the verbal handoff.
Physician‐driven checklist accept tools are another solution. Usually the responsibility of the accepting attending or fellow, this type of document is most useful as a modifiable document in the EMR. Accept tools, such as the one created by Malpass et al., have demonstrated successful shared decision making, and have resulted in fewer emergent procedures and fewer antibiotic changes on arrival.[13] One of the challenges with this approach is the frequency of utilization. In the aforementioned study, the adoption rate of the accept tool was about 70% in a closed university medical ICU, where these types of interventions may be viewed favorably by providers instead of burdensome.[13]
The most consistent finding of this survey was the lack of common processes to improve outcomes. Simple interventions, such as regular clinical updates, documentation of the handoff process, and obtaining objective information early in the process, were inconsistently adopted. Outcomes tracking and feedback are necessary components of team‐based quality improvement. Approximately half of the hospitals surveyed specifically tracked outcomes of transferred patients, and a minority had systems in place to provide feedback to referring centers.
Improving care delivery requires buy‐in from all participants, necessitating engagement of referring hospitals. Interventions such as frequent status updates and providing early documentation have the potential to increase the burden on referring providers when feedback or incentives are not commonplace. Moreover, the referring provider has the option of transferring a patient to a hospital with reduced handoff requirements, creating a disincentive for quality improvement. Quality metrics that incorporate outcomes of transferred patients may be necessary to better align the goals of sending and receiving physicians.
This study was intended to be a qualitative investigation and has some limitations. Any verbal qualitative study has the possibility of misinterpretation of information given by transfer center personnel. A single investigator performed most of the discussions and was able to clarify when needed, providing a degree of consistency, but may also be a source of bias. Categorical answers and a team‐based approach to conceptualizing responses likely minimized this potential bias.
We selected hospitals from the U.S. News and World Report Honor Roll plus additional hospitals chosen based on similarity to our home institutions. This may be a skewed sample and may not represent other major US hospitals and networks. However, we chose to interview large academic tertiary care centers, many accepting more than 1000 patients monthly, as these are likely to be the most proficient at performing transfers, and responses may be generalizable.
CONCLUSIONS
Standardization of information exchange during interhospital transfers does not currently exist. Practices vary widely amongst academic tertiary care centers. There is a paucity of data to support the association of specific processes with patient outcomes. Ultimately, a multicenter study examining the impact of improved information transfer on patient outcomes is warranted, utilizing tracking resources already in place. Optimizing and aligning practices between sending and receiving hospitals may improve interhospital handover efficiency and patient safety.
Disclosures
Dr. Usher is supported by a National Institutes of Health Clinical and Translational Science Award at the University of Minnesota: UL1TR000114. Dr. Steinberg has received support from Arena Pharmaceuticals and Major League Baseball. Drs. Herrigel, Parikh, Fanning, and Carroll have no disclosures. A prior version of this article was presented as an abstract at the Society of General Internal Medicine Mid‐Atlantic Regional Meeting in April 2014 in New York, New York.
- , , , . Doctors' handovers in hospitals: a literature review. BMJ Qual Saf. 2011;20(2):128–133.
- , , , et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446–R451.
- , , , , . Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes [published online November 20, 2015]. J Hosp Med. doi: 10.1002/jhm.2515.
- , , , et al. Evaluation of postoperative handover using a tool to assess information transfer and teamwork. Ann Surg. 2011;253(4):831–837.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed 26 May 2015.
- , , , , , . Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447–455.
- , , . Reasons underlying inter‐hospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , et al. Validation of a teamwork perceptions measure to increase patient safety. BMJ Qual Saf. 2014;23(9):718–726.
- , , , et al. Development, implementation, and dissemination of the I‐PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876–884.
- , , , . The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30(6):351–357.
Transitions of care are major sources of preventable medical errors. Incomplete or inaccurate communication during handoffs is the root cause of many adverse events.[1] In a prospective study, adverse events were found to occur during interhospital transfer up to 30% of the time.[2] Furthermore, patients subject to interhospital transfer have longer length of stay and higher inpatient mortality, even after adjusting for mortality risk predictors.[3] Standardizing intrahospital handoff structures and communication practices has been shown to reduce medical errors.[4, 5, 6] Interhospital transfer is an understudied area among the transitions of care literature. Little is known about institutional variations in the process of information transfer and its association with patient outcomes. Although it is challenging to ascertain the total burden of transferred patients, it has been estimated that 1.6 million inpatients originated at another facility.[7] Additionally, approximately 5.9% of admissions to a representative sample of US intensive care units (ICU) originated from other hospitals.[8] Patients are transferred between hospitals for multiple reasons beyond medical necessity, for example, to adjust for patient preferences, bed availability, and hospital staffing patterns. This creates a setting in which complex and often critically ill patients are subject to variable and sometimes ambiguous handoff processes.[9]
This survey of 32 tertiary care centers in the United States was undertaken to identify common practices in communication and documentation during interhospital patient transfers. Additional goals were to understand the structure of the handoff process, the role of the transfer center, and how electronic medical records (EMR) and interhospital communication play a role in this care transition. Subsequently, common challenges in coordinating interhospital transfers were identified to provide a conceptual framework for process improvement.
METHODS
Survey Process
The survey was initiated in September 2013 and concluded in September 2015, and was designed to quantify patient volume and identify common as well as unique practices to improve communication across the transfer process. The respondents were transfer center directors or managers, typically with a nursing background. Mass e‐mail generated a very poor response rate and did not allow for discussion and clarification of responses. The strategy was then modified to contact individual institutions directly. The survey was performed via phone whenever possible. Figure 1 represents purposeful sampling conducted on 2 different groups of hospitals. These hospitals represent a convenience sample of institutions from a nationally ranked list of hospitals as well as others comparable to our own institutions. Hospitals were selected based on status as academic tertiary care centers with roughly similar bed sizes (600). Several were selected based on similar EMR capabilities. Geographic diversity was also taken into account. Thirty‐two academic tertiary care centers were ultimately included in the survey. Data were entered into a survey form and deidentified. The RutgersRobert Wood Johnson Medical School Institutional Review Board approved this study.

Survey Content
Qualitative and quantitative data were collected by the study team. Data included number and origin of transfers (including those from inpatient facilities and emergency departments), staff characteristics, transfer process, documentation received prior to transfer, EMR access and type, outcomes, and clinical status tracking (see Supporting Figure 1 in the online version of this article for the complete survey tool).
Measurement and Data Analysis
Descriptive statistics are presented in unweighted fashion as a number and percentage for dichotomous variables, or a numeric range for ordinal variables. When a range was given by survey participants, the lower end of the range was used to calculate the population median. Several institutions surveyed were unable to provide specific numeric values, but instead cited how many requests for transfer they received either daily or monthly; these were omitted from the demographics analysis.
Respondents also provided a description of their overall triage and acceptance process for qualitative analysis. Unique strategies were identified by the study personnel at the time of each interview and amassed at the end of the interview period. These strategies were then discussed by the study team, and separated into categories that addressed the main challenges associated with interhospital transfers. Five general tenants of the transfer process were identified: acceptance and transport, need for clinical updates, provider handoffs and coordination of care, information availability, and feedback.
RESULTS
Based on a survey question asking respondents to estimate the total number of interhospital transfers received per month, the annual burden of patients transferred into these 32 hospitals represented approximately 247,000 patients yearly. The median number of patients transferred per month, based on a point estimate if given or the lower end of the range if a range was provided, was 700 (range, 2502500). On average, 28% (range, 10%50%) were transferred directly to an ICU, representing approximately 69,000 critically ill patients. A majority of hospitals polled (65%) received patients from more than 100 referring institutions, and a minority (23%) identified EMR interoperability for more than a quarter of the sending facilities. The overall acceptance rate ranged from 50% to 95%.
Table 1 represents common transition elements of participating institutions. Thirty‐eight percent of hospitals utilize a critical caretrained registered nurse as the initial triage point of contact. The process and quality controls for coordinating transfers from outside hospitals were highly variable. Although clinical updates from acceptance to arrival were required in a majority of hospitals (81%), the acceptable time interval was inconsistent, varying from 2 to 4 hours (13%) to 24 hours (38%). A mandatory 3‐way recorded discussion (between transfer center staff, and referring and accepting physician) was nearly uniform. Objective clinical information to assist the handoff (ie, current labs, radiology images, history and physical, progress notes, or discharge summary) was available in only 29% of hospitals. Only 23% of hospitals also recorded a 3‐way nursing handoff (bedside‐to‐bedside nursing report). A minority of hospitals utilized their principal EMR to document the transfer process and share incoming clinical information among providers (32%).
| Survey Question | Survey Response | N (%) |
|---|---|---|
| ||
| What is the training background of the staff member who takes the initial call and triages patients in your transfer center? | Critical care experienced RN | 12/32 (38%) |
| Other clinical background (EMT, RN) | 13/32 (41%) | |
| Nonclinical personnel | 7/32 (22%) | |
| Prior to the patient's arrival, do you require any documentation to be transmitted from the transferring institution? | Objective clinical data required | 9/32 (28%) |
| Objective clinical data not required | 23/32 (72%) | |
| Is a 3‐way recorded conversation facilitated by the transfer center required? | Initial physician‐to‐physician acceptance discussion | 27/32 (84%) |
| RN‐to‐RN report | 6/26 (23%) | |
| Are clinical status updates required? | Updates required every 24 hours | 12/32 (38%) |
| Updates required every 812 hours | 7/32 (22%) | |
| Updates required every 24 hours | 4/32 (13%) | |
| Updates required but timing not specified | 3/32 (9%) | |
| Clinical status updates not required | 6/32 (19%) | |
| Is any clinical information obtained by the transfer center available to the patient's providers in real time on your EMR system? | Yes | 10/31 (32%) |
| No | 21/31 (68%) | |
| Do you track the outcomes of patients you accept from outside hospitals? | Yes | 14/24 (58%) |
| No | 10/24 (42%) | |
Descriptions of the transfer process were conceptually evaluated by the study team, then divided into 5 common themes: acceptance and transport, clinical updates, coordination of care, information availability, and quality improvement (Table 2). Institutions devised novel approaches including providing high bed priority to expedite transit, a dedicated quarterback physician to coordinate safe transfer and uninterrupted communication, electronic transfer notes to share communication with all providers, and a standardized system of feedback to referring hospitals. Several institutions relied on an expect note, which could be a free‐text document or a form document in the EMR. This preserves verbal handoff information that may otherwise be lost if the accepting physician at the time of transfer is not the physician receiving the handoff.
| Challenges | Innovative Practices |
|---|---|
| |
| Expedited acceptance and transport | Automatic acceptance for certain diagnoses (ie, neurosurgical indication for transfer) |
| Transferred patients prioritized for hospital beds over all patients except codes | |
| Hospital controls transportation units, allowing for immediate dispatch and patient retrieval | |
| Outsourcing of transfer center and interfacility transfer to third party | |
| Timeliness of clinical updates | Transfer center communicates with bedside RN for clinical updates at the time of transfer |
| Clinical status updates every 24 hours for critical patients | |
| Daily reevaluation of clinical status | |
| Accepting physician alerted of changes in clinical status | |
| Handoff and coordination of care | Physician accept tool in EMR |
| Quarterback physician who triages and accepts all patients during a given time period | |
| Critical patients are accepted into a critical care resuscitation unit, an all‐purpose intensive care unit staffed by an intensivist who shares decision making with the referring provider and is involved in all communications regarding the transferred patient | |
| Availability of protected clinical information | Scribed physician handoff imported into EMR |
| Expect note in EMR: summary of clinical information documented by accepting physician | |
| PACS radiology cloud networks for hospital systems or statewide | |
| EMR interoperability: Care Everywhere module in Epic EMR | |
| Health and information management department responsible for obtaining and scanning outside records into EMR | |
| Feedback and quality improvement | Automatic review if patient upgraded to ICU within 4 hours of arrival |
| Departmental chair review of physician verbal handoff if poor outcome or difficulty with transfer | |
| Outcomes and quality of handoff reported back to referring hospital | |
| Discharge summary sent to referring hospital | |
| Referring hospital able to view patient's chart for 1 year | |
Quality improvement occurred via both internal and external feedback at several institutions. There were two notable mechanisms of internal feedback. Review of recorded physician verbal handoff by department chair occurred if an adverse event involved a transferred patient. An automatic internal review was triggered if a patient was upgraded to a higher level of care within 4 hours of arrival. These advanced mechanisms require vigilance and dedication on the part of the transfer center and physicians involved in the transfer process. External feedback was provided to referring hospitals through both active and passive mechanisms. One advanced health system allowed referring providers to access the patient's inpatient medical record for 1 year and sent a discharge summary to all referring hospitals. Another hospital maintained a sophisticated scorecard, with key measures shared with internal stakeholders and referring hospitals. Some of the metrics tracked included: denials due to insufficient bed capacity, change in bed status within 12 hours of transfer, and duration of stay in the postanesthesia care unit or emergency department awaiting an inpatient bed. This organization also performed site visits to referring hospitals, addressing handoff quality improvement.
DISCUSSION
Standardizing intrahospital handoffs has been shown to decrease preventable medical errors and reduce possible near‐miss events.[6, 10] Interhospital care transitions are inherently more complex due to increased acuity and decreased continuity; yet, there is no universal standardization of these handovers. We found that practices vary widely among tertiary care centers, and the level of transfer center involvement in the verbal and written handoff is inconsistent.
Evidence‐based frameworks to improve healthcare delivery, such as TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), first require an organizational assessment to identify barriers to effective communication.[11] Interhospital transfers offer multiple unique barriers to continuity: physical distance, uncertainty in timing, incongruent treatment goals, disparate information sources, and distractions. This study provides the first step in conceptualizing the unique aspects of interhospital transfers, as well as highlights strategies to improve care coordination (Table 2).
A tailored intervention needs not only to overcome the typical barriers to handoffs such as time constraints, information sharing, and ambiguity in provider roles, but also to overcome multiple systems barriers. Bed management systems add another time‐related variable due to fixed and frequently overburdened bed capacity. Prioritization of transfers depends upon an accurate clinical depiction of patient acuity as well as organizational strategies. For example, neurologic diagnoses are commonly a top priority and are triaged as such, sometimes instead of higher‐acuity patients with other principal diagnoses. The complexity of this process may lead to delays in high‐acuity transfers, and is contingent upon accurate and updated clinical information. Coordinating handovers amidst complex provider schedules is another systems barrier. The commonly adopted 7 on, 7 off model for hospitalists, and shift work for intensivists, may increase the possibility that a transfer occurs across multiple provider changes. Patient follow‐up and closed‐loop feedback are important components of intrahospital handovers, but are much more challenging to implement for interhospital handovers with incongruent information systems and providers.
Programs to improve intrahospital handovers (eg, IPASS) emphasize creating an accurate clinical depiction of a patient using both verbal and written handoffs.[12] This is arguably more difficult over the phone without a concurrent written handoff. Recording of 3‐way physician and nurse handoffs is common, but reviews of recorded conversations are often unavailable or cumbersome in real time. EMR documentation of verbal information exchanged during the handoff is a possible solution. However, there may be legal implications for a transcribed verbal handoff. Furthermore, transfer centers often work with a software program separate from the principal EMR, and documentation in real time is challenging. EMR integration could help reinforce a patient‐centered shared mental model by allowing visualization of lab trends, radiology, vitals, and other documentation during and after the verbal handoff.
Physician‐driven checklist accept tools are another solution. Usually the responsibility of the accepting attending or fellow, this type of document is most useful as a modifiable document in the EMR. Accept tools, such as the one created by Malpass et al., have demonstrated successful shared decision making, and have resulted in fewer emergent procedures and fewer antibiotic changes on arrival.[13] One of the challenges with this approach is the frequency of utilization. In the aforementioned study, the adoption rate of the accept tool was about 70% in a closed university medical ICU, where these types of interventions may be viewed favorably by providers instead of burdensome.[13]
The most consistent finding of this survey was the lack of common processes to improve outcomes. Simple interventions, such as regular clinical updates, documentation of the handoff process, and obtaining objective information early in the process, were inconsistently adopted. Outcomes tracking and feedback are necessary components of team‐based quality improvement. Approximately half of the hospitals surveyed specifically tracked outcomes of transferred patients, and a minority had systems in place to provide feedback to referring centers.
Improving care delivery requires buy‐in from all participants, necessitating engagement of referring hospitals. Interventions such as frequent status updates and providing early documentation have the potential to increase the burden on referring providers when feedback or incentives are not commonplace. Moreover, the referring provider has the option of transferring a patient to a hospital with reduced handoff requirements, creating a disincentive for quality improvement. Quality metrics that incorporate outcomes of transferred patients may be necessary to better align the goals of sending and receiving physicians.
This study was intended to be a qualitative investigation and has some limitations. Any verbal qualitative study has the possibility of misinterpretation of information given by transfer center personnel. A single investigator performed most of the discussions and was able to clarify when needed, providing a degree of consistency, but may also be a source of bias. Categorical answers and a team‐based approach to conceptualizing responses likely minimized this potential bias.
We selected hospitals from the U.S. News and World Report Honor Roll plus additional hospitals chosen based on similarity to our home institutions. This may be a skewed sample and may not represent other major US hospitals and networks. However, we chose to interview large academic tertiary care centers, many accepting more than 1000 patients monthly, as these are likely to be the most proficient at performing transfers, and responses may be generalizable.
CONCLUSIONS
Standardization of information exchange during interhospital transfers does not currently exist. Practices vary widely amongst academic tertiary care centers. There is a paucity of data to support the association of specific processes with patient outcomes. Ultimately, a multicenter study examining the impact of improved information transfer on patient outcomes is warranted, utilizing tracking resources already in place. Optimizing and aligning practices between sending and receiving hospitals may improve interhospital handover efficiency and patient safety.
Disclosures
Dr. Usher is supported by a National Institutes of Health Clinical and Translational Science Award at the University of Minnesota: UL1TR000114. Dr. Steinberg has received support from Arena Pharmaceuticals and Major League Baseball. Drs. Herrigel, Parikh, Fanning, and Carroll have no disclosures. A prior version of this article was presented as an abstract at the Society of General Internal Medicine Mid‐Atlantic Regional Meeting in April 2014 in New York, New York.
Transitions of care are major sources of preventable medical errors. Incomplete or inaccurate communication during handoffs is the root cause of many adverse events.[1] In a prospective study, adverse events were found to occur during interhospital transfer up to 30% of the time.[2] Furthermore, patients subject to interhospital transfer have longer length of stay and higher inpatient mortality, even after adjusting for mortality risk predictors.[3] Standardizing intrahospital handoff structures and communication practices has been shown to reduce medical errors.[4, 5, 6] Interhospital transfer is an understudied area among the transitions of care literature. Little is known about institutional variations in the process of information transfer and its association with patient outcomes. Although it is challenging to ascertain the total burden of transferred patients, it has been estimated that 1.6 million inpatients originated at another facility.[7] Additionally, approximately 5.9% of admissions to a representative sample of US intensive care units (ICU) originated from other hospitals.[8] Patients are transferred between hospitals for multiple reasons beyond medical necessity, for example, to adjust for patient preferences, bed availability, and hospital staffing patterns. This creates a setting in which complex and often critically ill patients are subject to variable and sometimes ambiguous handoff processes.[9]
This survey of 32 tertiary care centers in the United States was undertaken to identify common practices in communication and documentation during interhospital patient transfers. Additional goals were to understand the structure of the handoff process, the role of the transfer center, and how electronic medical records (EMR) and interhospital communication play a role in this care transition. Subsequently, common challenges in coordinating interhospital transfers were identified to provide a conceptual framework for process improvement.
METHODS
Survey Process
The survey was initiated in September 2013 and concluded in September 2015, and was designed to quantify patient volume and identify common as well as unique practices to improve communication across the transfer process. The respondents were transfer center directors or managers, typically with a nursing background. Mass e‐mail generated a very poor response rate and did not allow for discussion and clarification of responses. The strategy was then modified to contact individual institutions directly. The survey was performed via phone whenever possible. Figure 1 represents purposeful sampling conducted on 2 different groups of hospitals. These hospitals represent a convenience sample of institutions from a nationally ranked list of hospitals as well as others comparable to our own institutions. Hospitals were selected based on status as academic tertiary care centers with roughly similar bed sizes (600). Several were selected based on similar EMR capabilities. Geographic diversity was also taken into account. Thirty‐two academic tertiary care centers were ultimately included in the survey. Data were entered into a survey form and deidentified. The RutgersRobert Wood Johnson Medical School Institutional Review Board approved this study.

Survey Content
Qualitative and quantitative data were collected by the study team. Data included number and origin of transfers (including those from inpatient facilities and emergency departments), staff characteristics, transfer process, documentation received prior to transfer, EMR access and type, outcomes, and clinical status tracking (see Supporting Figure 1 in the online version of this article for the complete survey tool).
Measurement and Data Analysis
Descriptive statistics are presented in unweighted fashion as a number and percentage for dichotomous variables, or a numeric range for ordinal variables. When a range was given by survey participants, the lower end of the range was used to calculate the population median. Several institutions surveyed were unable to provide specific numeric values, but instead cited how many requests for transfer they received either daily or monthly; these were omitted from the demographics analysis.
Respondents also provided a description of their overall triage and acceptance process for qualitative analysis. Unique strategies were identified by the study personnel at the time of each interview and amassed at the end of the interview period. These strategies were then discussed by the study team, and separated into categories that addressed the main challenges associated with interhospital transfers. Five general tenants of the transfer process were identified: acceptance and transport, need for clinical updates, provider handoffs and coordination of care, information availability, and feedback.
RESULTS
Based on a survey question asking respondents to estimate the total number of interhospital transfers received per month, the annual burden of patients transferred into these 32 hospitals represented approximately 247,000 patients yearly. The median number of patients transferred per month, based on a point estimate if given or the lower end of the range if a range was provided, was 700 (range, 2502500). On average, 28% (range, 10%50%) were transferred directly to an ICU, representing approximately 69,000 critically ill patients. A majority of hospitals polled (65%) received patients from more than 100 referring institutions, and a minority (23%) identified EMR interoperability for more than a quarter of the sending facilities. The overall acceptance rate ranged from 50% to 95%.
Table 1 represents common transition elements of participating institutions. Thirty‐eight percent of hospitals utilize a critical caretrained registered nurse as the initial triage point of contact. The process and quality controls for coordinating transfers from outside hospitals were highly variable. Although clinical updates from acceptance to arrival were required in a majority of hospitals (81%), the acceptable time interval was inconsistent, varying from 2 to 4 hours (13%) to 24 hours (38%). A mandatory 3‐way recorded discussion (between transfer center staff, and referring and accepting physician) was nearly uniform. Objective clinical information to assist the handoff (ie, current labs, radiology images, history and physical, progress notes, or discharge summary) was available in only 29% of hospitals. Only 23% of hospitals also recorded a 3‐way nursing handoff (bedside‐to‐bedside nursing report). A minority of hospitals utilized their principal EMR to document the transfer process and share incoming clinical information among providers (32%).
| Survey Question | Survey Response | N (%) |
|---|---|---|
| ||
| What is the training background of the staff member who takes the initial call and triages patients in your transfer center? | Critical care experienced RN | 12/32 (38%) |
| Other clinical background (EMT, RN) | 13/32 (41%) | |
| Nonclinical personnel | 7/32 (22%) | |
| Prior to the patient's arrival, do you require any documentation to be transmitted from the transferring institution? | Objective clinical data required | 9/32 (28%) |
| Objective clinical data not required | 23/32 (72%) | |
| Is a 3‐way recorded conversation facilitated by the transfer center required? | Initial physician‐to‐physician acceptance discussion | 27/32 (84%) |
| RN‐to‐RN report | 6/26 (23%) | |
| Are clinical status updates required? | Updates required every 24 hours | 12/32 (38%) |
| Updates required every 812 hours | 7/32 (22%) | |
| Updates required every 24 hours | 4/32 (13%) | |
| Updates required but timing not specified | 3/32 (9%) | |
| Clinical status updates not required | 6/32 (19%) | |
| Is any clinical information obtained by the transfer center available to the patient's providers in real time on your EMR system? | Yes | 10/31 (32%) |
| No | 21/31 (68%) | |
| Do you track the outcomes of patients you accept from outside hospitals? | Yes | 14/24 (58%) |
| No | 10/24 (42%) | |
Descriptions of the transfer process were conceptually evaluated by the study team, then divided into 5 common themes: acceptance and transport, clinical updates, coordination of care, information availability, and quality improvement (Table 2). Institutions devised novel approaches including providing high bed priority to expedite transit, a dedicated quarterback physician to coordinate safe transfer and uninterrupted communication, electronic transfer notes to share communication with all providers, and a standardized system of feedback to referring hospitals. Several institutions relied on an expect note, which could be a free‐text document or a form document in the EMR. This preserves verbal handoff information that may otherwise be lost if the accepting physician at the time of transfer is not the physician receiving the handoff.
| Challenges | Innovative Practices |
|---|---|
| |
| Expedited acceptance and transport | Automatic acceptance for certain diagnoses (ie, neurosurgical indication for transfer) |
| Transferred patients prioritized for hospital beds over all patients except codes | |
| Hospital controls transportation units, allowing for immediate dispatch and patient retrieval | |
| Outsourcing of transfer center and interfacility transfer to third party | |
| Timeliness of clinical updates | Transfer center communicates with bedside RN for clinical updates at the time of transfer |
| Clinical status updates every 24 hours for critical patients | |
| Daily reevaluation of clinical status | |
| Accepting physician alerted of changes in clinical status | |
| Handoff and coordination of care | Physician accept tool in EMR |
| Quarterback physician who triages and accepts all patients during a given time period | |
| Critical patients are accepted into a critical care resuscitation unit, an all‐purpose intensive care unit staffed by an intensivist who shares decision making with the referring provider and is involved in all communications regarding the transferred patient | |
| Availability of protected clinical information | Scribed physician handoff imported into EMR |
| Expect note in EMR: summary of clinical information documented by accepting physician | |
| PACS radiology cloud networks for hospital systems or statewide | |
| EMR interoperability: Care Everywhere module in Epic EMR | |
| Health and information management department responsible for obtaining and scanning outside records into EMR | |
| Feedback and quality improvement | Automatic review if patient upgraded to ICU within 4 hours of arrival |
| Departmental chair review of physician verbal handoff if poor outcome or difficulty with transfer | |
| Outcomes and quality of handoff reported back to referring hospital | |
| Discharge summary sent to referring hospital | |
| Referring hospital able to view patient's chart for 1 year | |
Quality improvement occurred via both internal and external feedback at several institutions. There were two notable mechanisms of internal feedback. Review of recorded physician verbal handoff by department chair occurred if an adverse event involved a transferred patient. An automatic internal review was triggered if a patient was upgraded to a higher level of care within 4 hours of arrival. These advanced mechanisms require vigilance and dedication on the part of the transfer center and physicians involved in the transfer process. External feedback was provided to referring hospitals through both active and passive mechanisms. One advanced health system allowed referring providers to access the patient's inpatient medical record for 1 year and sent a discharge summary to all referring hospitals. Another hospital maintained a sophisticated scorecard, with key measures shared with internal stakeholders and referring hospitals. Some of the metrics tracked included: denials due to insufficient bed capacity, change in bed status within 12 hours of transfer, and duration of stay in the postanesthesia care unit or emergency department awaiting an inpatient bed. This organization also performed site visits to referring hospitals, addressing handoff quality improvement.
DISCUSSION
Standardizing intrahospital handoffs has been shown to decrease preventable medical errors and reduce possible near‐miss events.[6, 10] Interhospital care transitions are inherently more complex due to increased acuity and decreased continuity; yet, there is no universal standardization of these handovers. We found that practices vary widely among tertiary care centers, and the level of transfer center involvement in the verbal and written handoff is inconsistent.
Evidence‐based frameworks to improve healthcare delivery, such as TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), first require an organizational assessment to identify barriers to effective communication.[11] Interhospital transfers offer multiple unique barriers to continuity: physical distance, uncertainty in timing, incongruent treatment goals, disparate information sources, and distractions. This study provides the first step in conceptualizing the unique aspects of interhospital transfers, as well as highlights strategies to improve care coordination (Table 2).
A tailored intervention needs not only to overcome the typical barriers to handoffs such as time constraints, information sharing, and ambiguity in provider roles, but also to overcome multiple systems barriers. Bed management systems add another time‐related variable due to fixed and frequently overburdened bed capacity. Prioritization of transfers depends upon an accurate clinical depiction of patient acuity as well as organizational strategies. For example, neurologic diagnoses are commonly a top priority and are triaged as such, sometimes instead of higher‐acuity patients with other principal diagnoses. The complexity of this process may lead to delays in high‐acuity transfers, and is contingent upon accurate and updated clinical information. Coordinating handovers amidst complex provider schedules is another systems barrier. The commonly adopted 7 on, 7 off model for hospitalists, and shift work for intensivists, may increase the possibility that a transfer occurs across multiple provider changes. Patient follow‐up and closed‐loop feedback are important components of intrahospital handovers, but are much more challenging to implement for interhospital handovers with incongruent information systems and providers.
Programs to improve intrahospital handovers (eg, IPASS) emphasize creating an accurate clinical depiction of a patient using both verbal and written handoffs.[12] This is arguably more difficult over the phone without a concurrent written handoff. Recording of 3‐way physician and nurse handoffs is common, but reviews of recorded conversations are often unavailable or cumbersome in real time. EMR documentation of verbal information exchanged during the handoff is a possible solution. However, there may be legal implications for a transcribed verbal handoff. Furthermore, transfer centers often work with a software program separate from the principal EMR, and documentation in real time is challenging. EMR integration could help reinforce a patient‐centered shared mental model by allowing visualization of lab trends, radiology, vitals, and other documentation during and after the verbal handoff.
Physician‐driven checklist accept tools are another solution. Usually the responsibility of the accepting attending or fellow, this type of document is most useful as a modifiable document in the EMR. Accept tools, such as the one created by Malpass et al., have demonstrated successful shared decision making, and have resulted in fewer emergent procedures and fewer antibiotic changes on arrival.[13] One of the challenges with this approach is the frequency of utilization. In the aforementioned study, the adoption rate of the accept tool was about 70% in a closed university medical ICU, where these types of interventions may be viewed favorably by providers instead of burdensome.[13]
The most consistent finding of this survey was the lack of common processes to improve outcomes. Simple interventions, such as regular clinical updates, documentation of the handoff process, and obtaining objective information early in the process, were inconsistently adopted. Outcomes tracking and feedback are necessary components of team‐based quality improvement. Approximately half of the hospitals surveyed specifically tracked outcomes of transferred patients, and a minority had systems in place to provide feedback to referring centers.
Improving care delivery requires buy‐in from all participants, necessitating engagement of referring hospitals. Interventions such as frequent status updates and providing early documentation have the potential to increase the burden on referring providers when feedback or incentives are not commonplace. Moreover, the referring provider has the option of transferring a patient to a hospital with reduced handoff requirements, creating a disincentive for quality improvement. Quality metrics that incorporate outcomes of transferred patients may be necessary to better align the goals of sending and receiving physicians.
This study was intended to be a qualitative investigation and has some limitations. Any verbal qualitative study has the possibility of misinterpretation of information given by transfer center personnel. A single investigator performed most of the discussions and was able to clarify when needed, providing a degree of consistency, but may also be a source of bias. Categorical answers and a team‐based approach to conceptualizing responses likely minimized this potential bias.
We selected hospitals from the U.S. News and World Report Honor Roll plus additional hospitals chosen based on similarity to our home institutions. This may be a skewed sample and may not represent other major US hospitals and networks. However, we chose to interview large academic tertiary care centers, many accepting more than 1000 patients monthly, as these are likely to be the most proficient at performing transfers, and responses may be generalizable.
CONCLUSIONS
Standardization of information exchange during interhospital transfers does not currently exist. Practices vary widely amongst academic tertiary care centers. There is a paucity of data to support the association of specific processes with patient outcomes. Ultimately, a multicenter study examining the impact of improved information transfer on patient outcomes is warranted, utilizing tracking resources already in place. Optimizing and aligning practices between sending and receiving hospitals may improve interhospital handover efficiency and patient safety.
Disclosures
Dr. Usher is supported by a National Institutes of Health Clinical and Translational Science Award at the University of Minnesota: UL1TR000114. Dr. Steinberg has received support from Arena Pharmaceuticals and Major League Baseball. Drs. Herrigel, Parikh, Fanning, and Carroll have no disclosures. A prior version of this article was presented as an abstract at the Society of General Internal Medicine Mid‐Atlantic Regional Meeting in April 2014 in New York, New York.
- , , , . Doctors' handovers in hospitals: a literature review. BMJ Qual Saf. 2011;20(2):128–133.
- , , , et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446–R451.
- , , , , . Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes [published online November 20, 2015]. J Hosp Med. doi: 10.1002/jhm.2515.
- , , , et al. Evaluation of postoperative handover using a tool to assess information transfer and teamwork. Ann Surg. 2011;253(4):831–837.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed 26 May 2015.
- , , , , , . Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447–455.
- , , . Reasons underlying inter‐hospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , et al. Validation of a teamwork perceptions measure to increase patient safety. BMJ Qual Saf. 2014;23(9):718–726.
- , , , et al. Development, implementation, and dissemination of the I‐PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876–884.
- , , , . The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30(6):351–357.
- , , , . Doctors' handovers in hospitals: a literature review. BMJ Qual Saf. 2011;20(2):128–133.
- , , , et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446–R451.
- , , , , . Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes [published online November 20, 2015]. J Hosp Med. doi: 10.1002/jhm.2515.
- , , , et al. Evaluation of postoperative handover using a tool to assess information transfer and teamwork. Ann Surg. 2011;253(4):831–837.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed 26 May 2015.
- , , , , , . Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447–455.
- , , . Reasons underlying inter‐hospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , et al. Validation of a teamwork perceptions measure to increase patient safety. BMJ Qual Saf. 2014;23(9):718–726.
- , , , et al. Development, implementation, and dissemination of the I‐PASS handoff curriculum: a multisite educational intervention to improve patient handoffs. Acad Med. 2014;89(6):876–884.
- , , , . The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30(6):351–357.
© 2016 Society of Hospital Medicine
The crushing of innovation for treating female pelvic floor disorders: A story of “lead or be led”
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
Janelle Yates: Author, editor, women’s health expert
As readers of OBG Management, you are very familiar with the name Janelle Yates. Janelle was an editor and writer for the journal for more than 15 years. Her byline has graced articles on, among other topics, obstetrics, liability, menopause, and tissue extraction. You may recall the 3-part series on endometriosis she authored beginning in April 2015.1 She worked with several expert surgeons to deliver an in-depth look at diagnosis, treatment, and related infertility. She interviewed presidents of the American Congress of Obstetricians and Gynecologists (ACOG)2 and worked with ACOG staff, including Lucia DiVenere, MA, on legislative articles for OBG Management, helping to bring new policies and practice changes to the forefront for readers.3,4
One topic that was of particular, personal interest to Janelle was breast cancer. She lived on and off with, but always under the shadow of, breast cancer for more than 8 years. This past December, Janelle developed a rare and incurable cancer of the nervous system, which took her life in January.
Janelle’s contributions to OBG Management Janelle worked closely with Robert L. Barbieri, MD, and the OBG Management Board of Editors on writing and editing projects. In fact, Janelle began as Senior Associate Editor with the journal in 2000, only a few months after Dr. Barbieri was inaugurated as Editor in Chief.
“Janelle was exceptionally skillful in polishing a rough manuscript into a superbly crafted article,” says Dr. Barbieri. “The physician authors with whom she collaborated were in awe of her talent and recognized the value of her contributions to advancing women’s health care.”
“Janelle approached the craft of writing like an artist, always searching for an additional layer of deeper meaning and insight. Her strength of character and myriad life experiences gave her unique skills in exploring, questioning, and improving the content that was brought forth.”
“She and I had an ongoing conversation on the pros and cons of being concise,” says Dr. Barbieri. “I would ask her the hypothetical, ‘If an author could effectively deliver his or her core message in a 1-page article, why take 3 pages to do so?’ As a counterpoint, her perspective was that if you could concisely make your point in 3 pages, it might be even better for an author to expand their article to 9 pages to help the reader achieve a deeper level of understanding and insight.”
In May 2013, Barbara S. Levy, MD, after serving for 17 years as an OBG Management Board of Editors member, assumed the position of Vice President for Health Policy at ACOG, and resigned her position on the journal’s editorial board. Janelle collaborated with Dr. Levy on an article commemorating her lifetime of service to women.5 Dr. Levy recalls that article, and the numerous others she partnered on with Janelle:
“She was the quintessential professional. We are professional doctors, but Janelle was a professional writer and editor. She had an ability to, when speaking with us, get the best out of us, and take what we said and translate that into a cogent, crisp presentation that was really meaningful to readers. Having her perspective as a partner in writing helped me reach a core in readers that I believe I otherwise would not have been able to reach.”
Andrew M. Kaunitz, MD, Board of Editors member since 2006, describes Janelle as “a wonderful colleague and person.”
“My early perceptions of Janelle,” he says, “relate to her tremendous skills as a medical writer. Over time, however, I recognized that, in addition to her wonderful talents as a writer, she brought what I can only call a sense of grace to each interaction that I had with her. I continue to find it hard to imagine a world without her.”
“During my 15-year excursion as the Editor in Chief of OBG Management,” says Dr. Barbieri, “Janelle was the perfect guide and travel companion. She will always be in my thoughts and heart.”
Janelle’s memory enduresJanelle’s colleagues at the journal office and the Board of Editors honor her dedication to OBG Management, and truly to women’s health in general, in a permanent way in the pages of OBG Management. Janelle’s name has been added to the journal’s staff masthead with the title, “Editor Emeritus.” We feel this is a small but sincere gesture from those of us who have had the immense pleasure and incredible honor to work with Janelle over the years.
John Baranowski, who served as Editor of OBG Management from 2008 until mid-2012, eloquently states: “As her supervisor for several years, I was the initial recipient of tens of thousands of her well-ordered, well-chosen, and insightful words. At this time, it is hard for me to find words to offer on her behalf. Her outsized skill, her easeful manner, and her certain success at improving the care that physicians provide—those are humbling, silencing remembrances; things of such great value that words just do not work as tribute.”
Dianne Reynolds, Publisher of OBG Management, has known Janelle since 2008 and says that she feels blessed and fortunate to have had Janelle in her life on more than just a professional level. “I will cherish her memory forever,” she says.
OBG Management Managing Editor Deborah Reale joined the journal staff in 2010. “Words connected Janelle and I not only in our editorial work but also personally, through our shared love of poetry,” she says. Unbeknownst to many, Janelle was a published poet. She also wrote two biographies, of Woodie Guthrie and Zora Neale Hurston, while working many years ago as an editor for Ward Hill Press.
Like so many others, I feel privileged to have worked with Janelle. Her work was fearless. Whether it was an audio interview with gynecologic oncologist Eva Chalas, MD, on preserving minimally invasive approaches to gynecologic surgery6 or a Q&A article on liability claims in obstetrics,7 Janelle did it brilliantly. She applied her years of knowledge and experience to each piece she wrote or edited, always bringing an expert’s best voice forward. In fact, Dianne Reynolds says, “Janelle once told me that she had dreamed of being a doctor, and she imagined herself pushing through hospital doors on her way to treat patients. Instead, Janelle used her acquired medical knowledge in women’s health and writing acumen to assist physicians in explaining their techniques for the benefit of their colleagues.”
Janelle’s siblings Diana and Kent Yates, and her daughter Adrienne Cano, say they have been aware of how supportive the OBG Management extended team has been to Janelle. In speaking with Board of Editors member, Cheryl B. Iglesia, MD, Diana says, “This was such a blessing in her life. It was a rare thing, the kind of relationship she had with all of you. We appreciate so very much your nurturing of her talents and of her as a person.”
Janelle meant a great deal to her colleagues, of course because of her superb work and wise perspective, but also because of her warmth and gentle ease. Simply put, Janelle was a wonderful person to be near.
“She was absolutely amazing and will be greatly missed both personally and professionally,” says 10-year OBG Management board member JoAnn V. Pinkerton, MD.
Dr. Iglesia asserts, “Janelle never will be forgotten. She truly has left a legacy of very important articles for many generations.”
Janelle, we can only hope that with your name on the masthead, readers of OBG Management in future generations will have the privilege of your touch. May that touch bring them the gift of journalistic accuracy, professional integrity, spot-on syntax and, above all, compelling reading.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Yates J. Endometriosis: expert answers to 7 crucial questions on diagnosis. OBG Manag. 2015;27(4):39–40, 42–46.
- Yates J. ACOG presidents highlight their visions for the College at the 2015 clinical meeting. OBG Manag. 2015;27(5).
- Yates J, DiVenere L. It's a Republican majority following midterm election results. How will that affect the ACA and women's health? OBG Manag. 2014(26):12.
- DiVenere L. The well-woman visit comes of age: what it offers, how we got here. OBG Manag. 2016;28(1):25–29.
- Yates J. A lifetime of service to women and their health--the career of Barbara S. Levy, MD. OBG Manag. 2015;25(5):17–20.
- Yates J. 46 experts pen open letter to the FDA on uterine power morcellation. An interview with Eva Chalas, MD. OBG Manag. 2015;12.
- Yates J. A survey of liability claims against obstetric providers highlights major areas of contention. OBG Manag. 2015; 27(8):40–42.
As readers of OBG Management, you are very familiar with the name Janelle Yates. Janelle was an editor and writer for the journal for more than 15 years. Her byline has graced articles on, among other topics, obstetrics, liability, menopause, and tissue extraction. You may recall the 3-part series on endometriosis she authored beginning in April 2015.1 She worked with several expert surgeons to deliver an in-depth look at diagnosis, treatment, and related infertility. She interviewed presidents of the American Congress of Obstetricians and Gynecologists (ACOG)2 and worked with ACOG staff, including Lucia DiVenere, MA, on legislative articles for OBG Management, helping to bring new policies and practice changes to the forefront for readers.3,4
One topic that was of particular, personal interest to Janelle was breast cancer. She lived on and off with, but always under the shadow of, breast cancer for more than 8 years. This past December, Janelle developed a rare and incurable cancer of the nervous system, which took her life in January.
Janelle’s contributions to OBG Management Janelle worked closely with Robert L. Barbieri, MD, and the OBG Management Board of Editors on writing and editing projects. In fact, Janelle began as Senior Associate Editor with the journal in 2000, only a few months after Dr. Barbieri was inaugurated as Editor in Chief.
“Janelle was exceptionally skillful in polishing a rough manuscript into a superbly crafted article,” says Dr. Barbieri. “The physician authors with whom she collaborated were in awe of her talent and recognized the value of her contributions to advancing women’s health care.”
“Janelle approached the craft of writing like an artist, always searching for an additional layer of deeper meaning and insight. Her strength of character and myriad life experiences gave her unique skills in exploring, questioning, and improving the content that was brought forth.”
“She and I had an ongoing conversation on the pros and cons of being concise,” says Dr. Barbieri. “I would ask her the hypothetical, ‘If an author could effectively deliver his or her core message in a 1-page article, why take 3 pages to do so?’ As a counterpoint, her perspective was that if you could concisely make your point in 3 pages, it might be even better for an author to expand their article to 9 pages to help the reader achieve a deeper level of understanding and insight.”
In May 2013, Barbara S. Levy, MD, after serving for 17 years as an OBG Management Board of Editors member, assumed the position of Vice President for Health Policy at ACOG, and resigned her position on the journal’s editorial board. Janelle collaborated with Dr. Levy on an article commemorating her lifetime of service to women.5 Dr. Levy recalls that article, and the numerous others she partnered on with Janelle:
“She was the quintessential professional. We are professional doctors, but Janelle was a professional writer and editor. She had an ability to, when speaking with us, get the best out of us, and take what we said and translate that into a cogent, crisp presentation that was really meaningful to readers. Having her perspective as a partner in writing helped me reach a core in readers that I believe I otherwise would not have been able to reach.”
Andrew M. Kaunitz, MD, Board of Editors member since 2006, describes Janelle as “a wonderful colleague and person.”
“My early perceptions of Janelle,” he says, “relate to her tremendous skills as a medical writer. Over time, however, I recognized that, in addition to her wonderful talents as a writer, she brought what I can only call a sense of grace to each interaction that I had with her. I continue to find it hard to imagine a world without her.”
“During my 15-year excursion as the Editor in Chief of OBG Management,” says Dr. Barbieri, “Janelle was the perfect guide and travel companion. She will always be in my thoughts and heart.”
Janelle’s memory enduresJanelle’s colleagues at the journal office and the Board of Editors honor her dedication to OBG Management, and truly to women’s health in general, in a permanent way in the pages of OBG Management. Janelle’s name has been added to the journal’s staff masthead with the title, “Editor Emeritus.” We feel this is a small but sincere gesture from those of us who have had the immense pleasure and incredible honor to work with Janelle over the years.
John Baranowski, who served as Editor of OBG Management from 2008 until mid-2012, eloquently states: “As her supervisor for several years, I was the initial recipient of tens of thousands of her well-ordered, well-chosen, and insightful words. At this time, it is hard for me to find words to offer on her behalf. Her outsized skill, her easeful manner, and her certain success at improving the care that physicians provide—those are humbling, silencing remembrances; things of such great value that words just do not work as tribute.”
Dianne Reynolds, Publisher of OBG Management, has known Janelle since 2008 and says that she feels blessed and fortunate to have had Janelle in her life on more than just a professional level. “I will cherish her memory forever,” she says.
OBG Management Managing Editor Deborah Reale joined the journal staff in 2010. “Words connected Janelle and I not only in our editorial work but also personally, through our shared love of poetry,” she says. Unbeknownst to many, Janelle was a published poet. She also wrote two biographies, of Woodie Guthrie and Zora Neale Hurston, while working many years ago as an editor for Ward Hill Press.
Like so many others, I feel privileged to have worked with Janelle. Her work was fearless. Whether it was an audio interview with gynecologic oncologist Eva Chalas, MD, on preserving minimally invasive approaches to gynecologic surgery6 or a Q&A article on liability claims in obstetrics,7 Janelle did it brilliantly. She applied her years of knowledge and experience to each piece she wrote or edited, always bringing an expert’s best voice forward. In fact, Dianne Reynolds says, “Janelle once told me that she had dreamed of being a doctor, and she imagined herself pushing through hospital doors on her way to treat patients. Instead, Janelle used her acquired medical knowledge in women’s health and writing acumen to assist physicians in explaining their techniques for the benefit of their colleagues.”
Janelle’s siblings Diana and Kent Yates, and her daughter Adrienne Cano, say they have been aware of how supportive the OBG Management extended team has been to Janelle. In speaking with Board of Editors member, Cheryl B. Iglesia, MD, Diana says, “This was such a blessing in her life. It was a rare thing, the kind of relationship she had with all of you. We appreciate so very much your nurturing of her talents and of her as a person.”
Janelle meant a great deal to her colleagues, of course because of her superb work and wise perspective, but also because of her warmth and gentle ease. Simply put, Janelle was a wonderful person to be near.
“She was absolutely amazing and will be greatly missed both personally and professionally,” says 10-year OBG Management board member JoAnn V. Pinkerton, MD.
Dr. Iglesia asserts, “Janelle never will be forgotten. She truly has left a legacy of very important articles for many generations.”
Janelle, we can only hope that with your name on the masthead, readers of OBG Management in future generations will have the privilege of your touch. May that touch bring them the gift of journalistic accuracy, professional integrity, spot-on syntax and, above all, compelling reading.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As readers of OBG Management, you are very familiar with the name Janelle Yates. Janelle was an editor and writer for the journal for more than 15 years. Her byline has graced articles on, among other topics, obstetrics, liability, menopause, and tissue extraction. You may recall the 3-part series on endometriosis she authored beginning in April 2015.1 She worked with several expert surgeons to deliver an in-depth look at diagnosis, treatment, and related infertility. She interviewed presidents of the American Congress of Obstetricians and Gynecologists (ACOG)2 and worked with ACOG staff, including Lucia DiVenere, MA, on legislative articles for OBG Management, helping to bring new policies and practice changes to the forefront for readers.3,4
One topic that was of particular, personal interest to Janelle was breast cancer. She lived on and off with, but always under the shadow of, breast cancer for more than 8 years. This past December, Janelle developed a rare and incurable cancer of the nervous system, which took her life in January.
Janelle’s contributions to OBG Management Janelle worked closely with Robert L. Barbieri, MD, and the OBG Management Board of Editors on writing and editing projects. In fact, Janelle began as Senior Associate Editor with the journal in 2000, only a few months after Dr. Barbieri was inaugurated as Editor in Chief.
“Janelle was exceptionally skillful in polishing a rough manuscript into a superbly crafted article,” says Dr. Barbieri. “The physician authors with whom she collaborated were in awe of her talent and recognized the value of her contributions to advancing women’s health care.”
“Janelle approached the craft of writing like an artist, always searching for an additional layer of deeper meaning and insight. Her strength of character and myriad life experiences gave her unique skills in exploring, questioning, and improving the content that was brought forth.”
“She and I had an ongoing conversation on the pros and cons of being concise,” says Dr. Barbieri. “I would ask her the hypothetical, ‘If an author could effectively deliver his or her core message in a 1-page article, why take 3 pages to do so?’ As a counterpoint, her perspective was that if you could concisely make your point in 3 pages, it might be even better for an author to expand their article to 9 pages to help the reader achieve a deeper level of understanding and insight.”
In May 2013, Barbara S. Levy, MD, after serving for 17 years as an OBG Management Board of Editors member, assumed the position of Vice President for Health Policy at ACOG, and resigned her position on the journal’s editorial board. Janelle collaborated with Dr. Levy on an article commemorating her lifetime of service to women.5 Dr. Levy recalls that article, and the numerous others she partnered on with Janelle:
“She was the quintessential professional. We are professional doctors, but Janelle was a professional writer and editor. She had an ability to, when speaking with us, get the best out of us, and take what we said and translate that into a cogent, crisp presentation that was really meaningful to readers. Having her perspective as a partner in writing helped me reach a core in readers that I believe I otherwise would not have been able to reach.”
Andrew M. Kaunitz, MD, Board of Editors member since 2006, describes Janelle as “a wonderful colleague and person.”
“My early perceptions of Janelle,” he says, “relate to her tremendous skills as a medical writer. Over time, however, I recognized that, in addition to her wonderful talents as a writer, she brought what I can only call a sense of grace to each interaction that I had with her. I continue to find it hard to imagine a world without her.”
“During my 15-year excursion as the Editor in Chief of OBG Management,” says Dr. Barbieri, “Janelle was the perfect guide and travel companion. She will always be in my thoughts and heart.”
Janelle’s memory enduresJanelle’s colleagues at the journal office and the Board of Editors honor her dedication to OBG Management, and truly to women’s health in general, in a permanent way in the pages of OBG Management. Janelle’s name has been added to the journal’s staff masthead with the title, “Editor Emeritus.” We feel this is a small but sincere gesture from those of us who have had the immense pleasure and incredible honor to work with Janelle over the years.
John Baranowski, who served as Editor of OBG Management from 2008 until mid-2012, eloquently states: “As her supervisor for several years, I was the initial recipient of tens of thousands of her well-ordered, well-chosen, and insightful words. At this time, it is hard for me to find words to offer on her behalf. Her outsized skill, her easeful manner, and her certain success at improving the care that physicians provide—those are humbling, silencing remembrances; things of such great value that words just do not work as tribute.”
Dianne Reynolds, Publisher of OBG Management, has known Janelle since 2008 and says that she feels blessed and fortunate to have had Janelle in her life on more than just a professional level. “I will cherish her memory forever,” she says.
OBG Management Managing Editor Deborah Reale joined the journal staff in 2010. “Words connected Janelle and I not only in our editorial work but also personally, through our shared love of poetry,” she says. Unbeknownst to many, Janelle was a published poet. She also wrote two biographies, of Woodie Guthrie and Zora Neale Hurston, while working many years ago as an editor for Ward Hill Press.
Like so many others, I feel privileged to have worked with Janelle. Her work was fearless. Whether it was an audio interview with gynecologic oncologist Eva Chalas, MD, on preserving minimally invasive approaches to gynecologic surgery6 or a Q&A article on liability claims in obstetrics,7 Janelle did it brilliantly. She applied her years of knowledge and experience to each piece she wrote or edited, always bringing an expert’s best voice forward. In fact, Dianne Reynolds says, “Janelle once told me that she had dreamed of being a doctor, and she imagined herself pushing through hospital doors on her way to treat patients. Instead, Janelle used her acquired medical knowledge in women’s health and writing acumen to assist physicians in explaining their techniques for the benefit of their colleagues.”
Janelle’s siblings Diana and Kent Yates, and her daughter Adrienne Cano, say they have been aware of how supportive the OBG Management extended team has been to Janelle. In speaking with Board of Editors member, Cheryl B. Iglesia, MD, Diana says, “This was such a blessing in her life. It was a rare thing, the kind of relationship she had with all of you. We appreciate so very much your nurturing of her talents and of her as a person.”
Janelle meant a great deal to her colleagues, of course because of her superb work and wise perspective, but also because of her warmth and gentle ease. Simply put, Janelle was a wonderful person to be near.
“She was absolutely amazing and will be greatly missed both personally and professionally,” says 10-year OBG Management board member JoAnn V. Pinkerton, MD.
Dr. Iglesia asserts, “Janelle never will be forgotten. She truly has left a legacy of very important articles for many generations.”
Janelle, we can only hope that with your name on the masthead, readers of OBG Management in future generations will have the privilege of your touch. May that touch bring them the gift of journalistic accuracy, professional integrity, spot-on syntax and, above all, compelling reading.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Yates J. Endometriosis: expert answers to 7 crucial questions on diagnosis. OBG Manag. 2015;27(4):39–40, 42–46.
- Yates J. ACOG presidents highlight their visions for the College at the 2015 clinical meeting. OBG Manag. 2015;27(5).
- Yates J, DiVenere L. It's a Republican majority following midterm election results. How will that affect the ACA and women's health? OBG Manag. 2014(26):12.
- DiVenere L. The well-woman visit comes of age: what it offers, how we got here. OBG Manag. 2016;28(1):25–29.
- Yates J. A lifetime of service to women and their health--the career of Barbara S. Levy, MD. OBG Manag. 2015;25(5):17–20.
- Yates J. 46 experts pen open letter to the FDA on uterine power morcellation. An interview with Eva Chalas, MD. OBG Manag. 2015;12.
- Yates J. A survey of liability claims against obstetric providers highlights major areas of contention. OBG Manag. 2015; 27(8):40–42.
- Yates J. Endometriosis: expert answers to 7 crucial questions on diagnosis. OBG Manag. 2015;27(4):39–40, 42–46.
- Yates J. ACOG presidents highlight their visions for the College at the 2015 clinical meeting. OBG Manag. 2015;27(5).
- Yates J, DiVenere L. It's a Republican majority following midterm election results. How will that affect the ACA and women's health? OBG Manag. 2014(26):12.
- DiVenere L. The well-woman visit comes of age: what it offers, how we got here. OBG Manag. 2016;28(1):25–29.
- Yates J. A lifetime of service to women and their health--the career of Barbara S. Levy, MD. OBG Manag. 2015;25(5):17–20.
- Yates J. 46 experts pen open letter to the FDA on uterine power morcellation. An interview with Eva Chalas, MD. OBG Manag. 2015;12.
- Yates J. A survey of liability claims against obstetric providers highlights major areas of contention. OBG Manag. 2015; 27(8):40–42.
Reader reactions to the problem of inadequate contraception for high-risk women
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Do antidepressants really cause autism?
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.