User login
HIPAA enforcement in 2016: Is your practice ready?
Two reports from the Office of the Inspector General (OIG) have attracted a lot of attention in recent weeks: The Office for Civil Rights (OCR), OIG said, needs to improve and expand its enforcement of the Health Insurance Portability and Accountability Act (HIPAA). In response, the OCR announced that it plans to identify a pool of potential audit targets and launch a permanent audit program this year. That, combined with the substantial fine levied against a dermatology group last year for violating one of the new rules, signals the importance of reviewing your practice’s HIPAA compliance as soon as possible.
You can compare your office’s compliance status against the recommendations listed on the OCR website, but pay particular attention to your agreements with Business Associates (BAs). Those are the individuals or businesses, other than your employees, who perform “functions or activities” on behalf of your practice that involve “creating, receiving, maintaining, or transmitting” personal health information.
First, make sure that all individuals and enterprises fitting that definition have a signed agreement in place. Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, specialty pharmacies, and record storage, microfilming, and shredding services are BAs if they must have direct access to confidential information in order to do their job.
The revised rules place additional onus on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use “reasonable diligence” in monitoring their work. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario: Previously, when protected health information (PHI) was compromised, you would have to notify only affected patients (and the government) if there was a “significant risk of financial or reputational harm,” but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to do so could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts dermatology group ran into trouble: It lost a thumb drive containing unencrypted patient records, and was forced to pay a $150,000 fine, even though there was no evidence that the information was found or exploited.
Had the lost drive been encrypted, the incident would not have been considered a breach, according to the Centers for Medicare & Medicaid Services, because its contents would not have been viewable by the finder. The biggest vulnerability in most practices is probably mobile devices carrying patient data. There is no longer any excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use.
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records (EHRs). You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 days – even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last year.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
You also should examine every part of your office where patient information is handled to identify potential violations. Examples include computer screens in your reception area that are visible to patients; laptops not locked up after hours; unencrypted emails or texts that might reveal confidential information; and documents designated for shredding that sit, unshredded, in the “to shred” bin for days.
And make sure you correct any problems you find before the OCR auditors come calling.
To view the recommendations at the OCR website so you can check your office’s compliance status, go to: www.hhs.gov/hipaa/index.html.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Two reports from the Office of the Inspector General (OIG) have attracted a lot of attention in recent weeks: The Office for Civil Rights (OCR), OIG said, needs to improve and expand its enforcement of the Health Insurance Portability and Accountability Act (HIPAA). In response, the OCR announced that it plans to identify a pool of potential audit targets and launch a permanent audit program this year. That, combined with the substantial fine levied against a dermatology group last year for violating one of the new rules, signals the importance of reviewing your practice’s HIPAA compliance as soon as possible.
You can compare your office’s compliance status against the recommendations listed on the OCR website, but pay particular attention to your agreements with Business Associates (BAs). Those are the individuals or businesses, other than your employees, who perform “functions or activities” on behalf of your practice that involve “creating, receiving, maintaining, or transmitting” personal health information.
First, make sure that all individuals and enterprises fitting that definition have a signed agreement in place. Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, specialty pharmacies, and record storage, microfilming, and shredding services are BAs if they must have direct access to confidential information in order to do their job.
The revised rules place additional onus on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use “reasonable diligence” in monitoring their work. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario: Previously, when protected health information (PHI) was compromised, you would have to notify only affected patients (and the government) if there was a “significant risk of financial or reputational harm,” but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to do so could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts dermatology group ran into trouble: It lost a thumb drive containing unencrypted patient records, and was forced to pay a $150,000 fine, even though there was no evidence that the information was found or exploited.
Had the lost drive been encrypted, the incident would not have been considered a breach, according to the Centers for Medicare & Medicaid Services, because its contents would not have been viewable by the finder. The biggest vulnerability in most practices is probably mobile devices carrying patient data. There is no longer any excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use.
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records (EHRs). You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 days – even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last year.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
You also should examine every part of your office where patient information is handled to identify potential violations. Examples include computer screens in your reception area that are visible to patients; laptops not locked up after hours; unencrypted emails or texts that might reveal confidential information; and documents designated for shredding that sit, unshredded, in the “to shred” bin for days.
And make sure you correct any problems you find before the OCR auditors come calling.
To view the recommendations at the OCR website so you can check your office’s compliance status, go to: www.hhs.gov/hipaa/index.html.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Two reports from the Office of the Inspector General (OIG) have attracted a lot of attention in recent weeks: The Office for Civil Rights (OCR), OIG said, needs to improve and expand its enforcement of the Health Insurance Portability and Accountability Act (HIPAA). In response, the OCR announced that it plans to identify a pool of potential audit targets and launch a permanent audit program this year. That, combined with the substantial fine levied against a dermatology group last year for violating one of the new rules, signals the importance of reviewing your practice’s HIPAA compliance as soon as possible.
You can compare your office’s compliance status against the recommendations listed on the OCR website, but pay particular attention to your agreements with Business Associates (BAs). Those are the individuals or businesses, other than your employees, who perform “functions or activities” on behalf of your practice that involve “creating, receiving, maintaining, or transmitting” personal health information.
First, make sure that all individuals and enterprises fitting that definition have a signed agreement in place. Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, specialty pharmacies, and record storage, microfilming, and shredding services are BAs if they must have direct access to confidential information in order to do their job.
The revised rules place additional onus on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract; you are expected to use “reasonable diligence” in monitoring their work. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is yours. Furthermore, you must now assume the worst-case scenario: Previously, when protected health information (PHI) was compromised, you would have to notify only affected patients (and the government) if there was a “significant risk of financial or reputational harm,” but now, any incident involving patient records is assumed to be a breach, and must be reported.
Failure to do so could subject your practice, as well as the contractor, to significant fines. That is where the Massachusetts dermatology group ran into trouble: It lost a thumb drive containing unencrypted patient records, and was forced to pay a $150,000 fine, even though there was no evidence that the information was found or exploited.
Had the lost drive been encrypted, the incident would not have been considered a breach, according to the Centers for Medicare & Medicaid Services, because its contents would not have been viewable by the finder. The biggest vulnerability in most practices is probably mobile devices carrying patient data. There is no longer any excuse for not encrypting HIPAA-protected information; encryption software is cheap, readily available, and easy to use.
Patients have new rights under the new rules as well; they may now restrict any PHI shared with third-party insurers and health plans, if they pay for the services themselves. They also have the right to request copies of their electronic health records (EHRs). You can bill the costs of responding to such requests. If you have EHRs, work out a system for doing this, because the response time has been decreased from 90 days to 30 days – even shorter in some states.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last year.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
You also should examine every part of your office where patient information is handled to identify potential violations. Examples include computer screens in your reception area that are visible to patients; laptops not locked up after hours; unencrypted emails or texts that might reveal confidential information; and documents designated for shredding that sit, unshredded, in the “to shred” bin for days.
And make sure you correct any problems you find before the OCR auditors come calling.
To view the recommendations at the OCR website so you can check your office’s compliance status, go to: www.hhs.gov/hipaa/index.html.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Obesity
Emily is a 15-year-old girl who was referred by her pediatrician because of cutting behavior and conflict with her parents. Her parents reported that she has had a high body weight in the obese range since early in life. She had tried various diets without success, and her parents were frustrated with the pediatrician’s emphasis on weight over the years.
Mood problems had begun when she was in the sixth grade when she began to be severely bullied about her weight. Emily said this time was so difficult that she did not have clear memories of it. She described feeling numb. She began experiencing intense anxiety about school, and she was sometimes reluctant to attend and started cutting herself as a means of managing her emotions. In middle school, she began to fight back and associated herself with a group of “mean girls” who drank. She began having increasing conflict with her parents over the drinking and the cutting.
Discussion
Obesity is an extremely complex issue without simple answers. Severe obesity is correlated with numerous health risks including not only cardiovascular disease, type 2 diabetes, hypertension, and cancer, but also psychiatric problems such as depression, anxiety, body dissatisfaction, eating disorders, and unhealthy weight control behaviors. While some of these issues relate directly to the weight itself, many of the psychiatric concerns stem from society’s extremely harsh response to obesity.
We are all aware that the percentage of overweight and obese children, teens, and adults has increased in the past 50 years, although with some recent stabilization.1 The rise in obesity is related to societal factors – the prevalence and advertising of nutrient-poor/high-calorie processed foods in the marketplace, the rise of technologies that have decreased the need for movement, increases in portion sizes in restaurants, especially fast food settings, as well as the subsidizing of unhealthy foods, limited access to and greater cost of more nutritious foods, and limited access to exercise opportunities in poorer areas. This is the “obesogenic environment.” As in numerous aspects of health, weight is also influenced by genetics. Those who are genetically more likely to gain weight are the ones who suffer most from these social changes.
The problem is that, except for bariatric surgery, the interventions prescribed for individuals with obesity don’t work for the vast majority of people in the long run. There is an assumption that if the obese would just eat and exercise the way a thin person does, then they would be thin. While there is evidence that lifestyle strategies that induce a negative energy balance through cutting calories (often by 500-1,000) and “programmed exercise” can help some people lose weight over the course of 6 months to a year, longer-term follow-up suggests that most people regain this weight in the long run, at 5 years out. Even the most optimistic estimates suggest that only about one out of five people can maintain weight losses of 10% in the long term with current standard lifestyle interventions.2
There is evidence that someone attaining a particular body mass index (BMI) through dieting is not able to consume as many calories as another person who has always been at that BMI, requiring constant dietary restraint and a very high level of exercise to maintain the weight loss.3 The great majority of people who are unable to lose the weight, or briefly succeed and then gain the weight back or more, are seen as failing by society, by many medical professionals, and by themselves. There is clearly a need to focus more of our efforts on making changes on a societal level.
There also are alternative individual approaches that take the emphasis away from dieting and weight loss and instead focus on body acceptance and self-care. These interventions go by several names including mindful eating, intuitive eating, weight neutral, and “Health at Every Size.” This approach acknowledges the environmental and genetic factors beyond personal control and discusses how society pressures people to be thin. Instead of emphasizing repeated restrictive dieting, these programs stress maximizing health through making sustainable changes to increase activity and nutrition. These programs encourage people to care for themselves now rather than focusing on dieting toward a future weight where one can start enjoying life. Enjoyment of food, taking time to savor food, and being aware of when one is hungry and when not are central. For physical activity, the emphasis is on discovering something that is pleasurable and sustainable, rather than an onerous duty, as a means to an end of weight loss.4
Management
For Emily, struggling on the individual level, there is not a neat resolution. Psychotherapy to address anxiety, trauma, and substance abuse is indicated. Psychotherapy also should address Emily’s relationship with her body, as this is at the heart of many of these issues. Acknowledging the powerful stigma that society places on the obese while tolerating and even promoting an obesogenic environment, and the reality that weight loss is in fact extremely difficult, would open the door to a discussion with Emily and her family about what she wants and all her options to find the healthiest and most enjoyable way for her to live her life.
1. Pediatr Clin North Am. 2015 Oct;62(5):1241-61.
2. Annu Rev Nutr. 2001;21:323-41.
3. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S-225S.
4. Tylka TL, Annunziato RA, Burgard D, et al. “The Weight-Inclusive versus Weight-Normative Approach to Health: Evaluating the Evidence for Prioritizing Well-Being over Weight Loss.” J Obes. 2014;2014:983495. doi: 10.1155/2014/983495.
Dr. Hall is an assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington.
Emily is a 15-year-old girl who was referred by her pediatrician because of cutting behavior and conflict with her parents. Her parents reported that she has had a high body weight in the obese range since early in life. She had tried various diets without success, and her parents were frustrated with the pediatrician’s emphasis on weight over the years.
Mood problems had begun when she was in the sixth grade when she began to be severely bullied about her weight. Emily said this time was so difficult that she did not have clear memories of it. She described feeling numb. She began experiencing intense anxiety about school, and she was sometimes reluctant to attend and started cutting herself as a means of managing her emotions. In middle school, she began to fight back and associated herself with a group of “mean girls” who drank. She began having increasing conflict with her parents over the drinking and the cutting.
Discussion
Obesity is an extremely complex issue without simple answers. Severe obesity is correlated with numerous health risks including not only cardiovascular disease, type 2 diabetes, hypertension, and cancer, but also psychiatric problems such as depression, anxiety, body dissatisfaction, eating disorders, and unhealthy weight control behaviors. While some of these issues relate directly to the weight itself, many of the psychiatric concerns stem from society’s extremely harsh response to obesity.
We are all aware that the percentage of overweight and obese children, teens, and adults has increased in the past 50 years, although with some recent stabilization.1 The rise in obesity is related to societal factors – the prevalence and advertising of nutrient-poor/high-calorie processed foods in the marketplace, the rise of technologies that have decreased the need for movement, increases in portion sizes in restaurants, especially fast food settings, as well as the subsidizing of unhealthy foods, limited access to and greater cost of more nutritious foods, and limited access to exercise opportunities in poorer areas. This is the “obesogenic environment.” As in numerous aspects of health, weight is also influenced by genetics. Those who are genetically more likely to gain weight are the ones who suffer most from these social changes.
The problem is that, except for bariatric surgery, the interventions prescribed for individuals with obesity don’t work for the vast majority of people in the long run. There is an assumption that if the obese would just eat and exercise the way a thin person does, then they would be thin. While there is evidence that lifestyle strategies that induce a negative energy balance through cutting calories (often by 500-1,000) and “programmed exercise” can help some people lose weight over the course of 6 months to a year, longer-term follow-up suggests that most people regain this weight in the long run, at 5 years out. Even the most optimistic estimates suggest that only about one out of five people can maintain weight losses of 10% in the long term with current standard lifestyle interventions.2
There is evidence that someone attaining a particular body mass index (BMI) through dieting is not able to consume as many calories as another person who has always been at that BMI, requiring constant dietary restraint and a very high level of exercise to maintain the weight loss.3 The great majority of people who are unable to lose the weight, or briefly succeed and then gain the weight back or more, are seen as failing by society, by many medical professionals, and by themselves. There is clearly a need to focus more of our efforts on making changes on a societal level.
There also are alternative individual approaches that take the emphasis away from dieting and weight loss and instead focus on body acceptance and self-care. These interventions go by several names including mindful eating, intuitive eating, weight neutral, and “Health at Every Size.” This approach acknowledges the environmental and genetic factors beyond personal control and discusses how society pressures people to be thin. Instead of emphasizing repeated restrictive dieting, these programs stress maximizing health through making sustainable changes to increase activity and nutrition. These programs encourage people to care for themselves now rather than focusing on dieting toward a future weight where one can start enjoying life. Enjoyment of food, taking time to savor food, and being aware of when one is hungry and when not are central. For physical activity, the emphasis is on discovering something that is pleasurable and sustainable, rather than an onerous duty, as a means to an end of weight loss.4
Management
For Emily, struggling on the individual level, there is not a neat resolution. Psychotherapy to address anxiety, trauma, and substance abuse is indicated. Psychotherapy also should address Emily’s relationship with her body, as this is at the heart of many of these issues. Acknowledging the powerful stigma that society places on the obese while tolerating and even promoting an obesogenic environment, and the reality that weight loss is in fact extremely difficult, would open the door to a discussion with Emily and her family about what she wants and all her options to find the healthiest and most enjoyable way for her to live her life.
1. Pediatr Clin North Am. 2015 Oct;62(5):1241-61.
2. Annu Rev Nutr. 2001;21:323-41.
3. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S-225S.
4. Tylka TL, Annunziato RA, Burgard D, et al. “The Weight-Inclusive versus Weight-Normative Approach to Health: Evaluating the Evidence for Prioritizing Well-Being over Weight Loss.” J Obes. 2014;2014:983495. doi: 10.1155/2014/983495.
Dr. Hall is an assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington.
Emily is a 15-year-old girl who was referred by her pediatrician because of cutting behavior and conflict with her parents. Her parents reported that she has had a high body weight in the obese range since early in life. She had tried various diets without success, and her parents were frustrated with the pediatrician’s emphasis on weight over the years.
Mood problems had begun when she was in the sixth grade when she began to be severely bullied about her weight. Emily said this time was so difficult that she did not have clear memories of it. She described feeling numb. She began experiencing intense anxiety about school, and she was sometimes reluctant to attend and started cutting herself as a means of managing her emotions. In middle school, she began to fight back and associated herself with a group of “mean girls” who drank. She began having increasing conflict with her parents over the drinking and the cutting.
Discussion
Obesity is an extremely complex issue without simple answers. Severe obesity is correlated with numerous health risks including not only cardiovascular disease, type 2 diabetes, hypertension, and cancer, but also psychiatric problems such as depression, anxiety, body dissatisfaction, eating disorders, and unhealthy weight control behaviors. While some of these issues relate directly to the weight itself, many of the psychiatric concerns stem from society’s extremely harsh response to obesity.
We are all aware that the percentage of overweight and obese children, teens, and adults has increased in the past 50 years, although with some recent stabilization.1 The rise in obesity is related to societal factors – the prevalence and advertising of nutrient-poor/high-calorie processed foods in the marketplace, the rise of technologies that have decreased the need for movement, increases in portion sizes in restaurants, especially fast food settings, as well as the subsidizing of unhealthy foods, limited access to and greater cost of more nutritious foods, and limited access to exercise opportunities in poorer areas. This is the “obesogenic environment.” As in numerous aspects of health, weight is also influenced by genetics. Those who are genetically more likely to gain weight are the ones who suffer most from these social changes.
The problem is that, except for bariatric surgery, the interventions prescribed for individuals with obesity don’t work for the vast majority of people in the long run. There is an assumption that if the obese would just eat and exercise the way a thin person does, then they would be thin. While there is evidence that lifestyle strategies that induce a negative energy balance through cutting calories (often by 500-1,000) and “programmed exercise” can help some people lose weight over the course of 6 months to a year, longer-term follow-up suggests that most people regain this weight in the long run, at 5 years out. Even the most optimistic estimates suggest that only about one out of five people can maintain weight losses of 10% in the long term with current standard lifestyle interventions.2
There is evidence that someone attaining a particular body mass index (BMI) through dieting is not able to consume as many calories as another person who has always been at that BMI, requiring constant dietary restraint and a very high level of exercise to maintain the weight loss.3 The great majority of people who are unable to lose the weight, or briefly succeed and then gain the weight back or more, are seen as failing by society, by many medical professionals, and by themselves. There is clearly a need to focus more of our efforts on making changes on a societal level.
There also are alternative individual approaches that take the emphasis away from dieting and weight loss and instead focus on body acceptance and self-care. These interventions go by several names including mindful eating, intuitive eating, weight neutral, and “Health at Every Size.” This approach acknowledges the environmental and genetic factors beyond personal control and discusses how society pressures people to be thin. Instead of emphasizing repeated restrictive dieting, these programs stress maximizing health through making sustainable changes to increase activity and nutrition. These programs encourage people to care for themselves now rather than focusing on dieting toward a future weight where one can start enjoying life. Enjoyment of food, taking time to savor food, and being aware of when one is hungry and when not are central. For physical activity, the emphasis is on discovering something that is pleasurable and sustainable, rather than an onerous duty, as a means to an end of weight loss.4
Management
For Emily, struggling on the individual level, there is not a neat resolution. Psychotherapy to address anxiety, trauma, and substance abuse is indicated. Psychotherapy also should address Emily’s relationship with her body, as this is at the heart of many of these issues. Acknowledging the powerful stigma that society places on the obese while tolerating and even promoting an obesogenic environment, and the reality that weight loss is in fact extremely difficult, would open the door to a discussion with Emily and her family about what she wants and all her options to find the healthiest and most enjoyable way for her to live her life.
1. Pediatr Clin North Am. 2015 Oct;62(5):1241-61.
2. Annu Rev Nutr. 2001;21:323-41.
3. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S-225S.
4. Tylka TL, Annunziato RA, Burgard D, et al. “The Weight-Inclusive versus Weight-Normative Approach to Health: Evaluating the Evidence for Prioritizing Well-Being over Weight Loss.” J Obes. 2014;2014:983495. doi: 10.1155/2014/983495.
Dr. Hall is an assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington.
Make Room on Your Shelves
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
As orthopedic surgeons, we’ve made a commitment to lifelong learning. I can’t think of a single surgery that I perform the same way I did when I was in training. With rapidly evolving technology, continuously advancing procedures, and ever-increasing documentation requirements, it’s hard to stay on top of it all. We know your time is precious and that you have less of it than ever before. What little time you have that is not dedicated to work is reserved for your family or your hobbies. There’s no time to read every orthopedic journal, many filled with articles that have no practical value to your practice. That’s why we’ve created the new AJO. Our goal, as an editorial staff, is to provide a journal where every article, column, and feature contains information that directly benefits your practice, your patients, or your bottom line, and keeps you informed of the latest techniques, procedures, and products. We will help surgeons “work smarter, not harder,” implement new technologies into their practices, and find creative revenue streams that are both legal and compliant.
We’ve assembled a team of talented editors to accomplish this task, and will introduce them throughout the coming year. In this issue, you will meet our Deputy Editors-in-Chief and some of our new Associate Editors who’ve collaborated to bring you the “new AJO”.
At this year’s Academy, the AJO launched an extensive rebranding. We have a new look, a new logo, and a new creative directive. The journal will now feature new columns, invited articles, and innovative surgical techniques. We will publish 5 issues for the remainder of 2016. Our March/April issue is a special edition dedicated to baseball. In time for Spring Training/Opening Day, this issue includes articles from Major League Baseball’s physicians and trainers, a “Codes to Know” segment, “Tips of the Trade,” and a “Tools of the Trade” feature. “The Baseball Issue” will set the tone for what readers can expect from the “new AJO”.
Our first feature article, written by Jed Kuhn, takes a philosophical look at the evolution of the throwing shoulder, and invites the reader to help unlock some of the great shoulder anatomy mysteries by viewing them from a time when throwing was an activity of daily living. In ancient times, if you couldn’t throw, you couldn’t eat. We know that children who play baseball remodel their shoulder to allow for increased external rotation. Read Dr. Kuhn’s article and imagine when a shoulder optimized for throwing was a competitive advantage for survival.
Our second feature article is written by Stan Conte, a legend of the game and longtime trainer for the Los Angeles Dodgers. Dr. Conte studied injury trends in baseball over the past 18 seasons and provides an analysis of the staggering cost of placing players on the disabled list.
A baseball issue could not be complete without an article on Tommy John surgery. In this issue, AJO shares a revolutionary new technique for treating players with MUCL tears by author Jeffrey Dugas. Named the “Internal Brace”, Dr. Dugas shares his technique for augmenting the injured MUCL and we are proud to bring it to you first.
A recurring feature in the new AJO will be a section we refer to as “Codes to Know.” In partnership with Karen Zupko, AJO will present little-known coding secrets and proper coding techniques to help you get reimbursed appropriately for your work. This month, in the first article of a 3-part series, Alan Hirahara teaches us how to properly code for a diagnostic ultrasound examination of the shoulder. The article includes templates available for download to assist you with proper documentation. Parts 2 and 3 will provide a tutorial on the proper technique for examinations and injections.
While shoulder and elbow injuries get more attention, Major League Baseball’s Injury Panel has produced a look at the staggering amount of knee injuries over the 2011-2014 seasons, inspiring us to feature the knee in our 2 “Trade” Columns.
The “Tips of the Trade” column will continue, featuring this month a guide to identifying and treating meniscal root tears. A new segment, referred to as “Tools of the Trade,” reviews the latest products for all-inside meniscal repair. Our “Tools” section will feature announcements and reviews of the hottest new products, with a buying guide and surgical pearls from the surgeons who know them best.
While we are discussing the lower extremity, we should point out that we plan to do the “leg work” for you. Each AJO issue will have handouts that can be downloaded from our website and utilized in your practice. Read Robin West’s article entitled “Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation,” and download Return to Throwing and Hitting programs your patients and therapists can use.
Finally, I’d like to thank our previous Editor-in-Chief Dr. Peter McCann for his stewardship the last 10 years and recognize him for his dedication to the journal.
Thank you for reading AJO and for continuing to do so in the future. I know that collectively, we can turn AJO into a product worthy of its title. We know our past reputation. We are no longer that journal. Spend some time to get to know the “new AJO”, and make some room on your shelves, because the information between the covers will provide a template to implement new technologies and revenue streams into your practice and help fulfill your commitment to learning.
Piebaldism in Children
Case Report
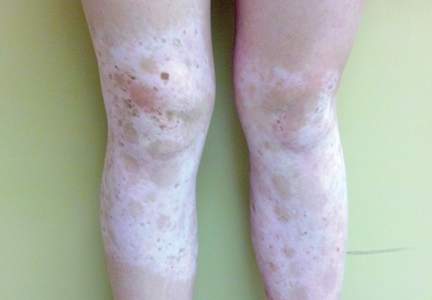
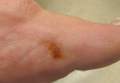
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
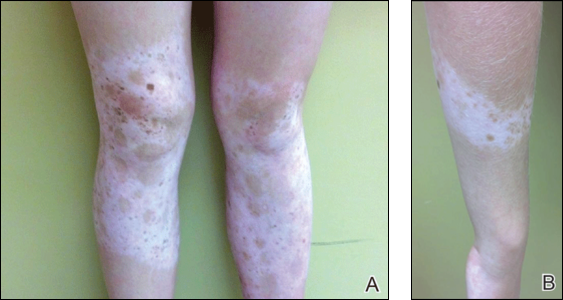
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.
A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
Case Report
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.

A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
Case Report
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.

A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
Practice Points
- Poliosis circumscripta (or white forelock) is commonly the only manifestation of piebaldism in children.
- Affected areas of leukoderma in piebaldism are classically distributed on the central forehead, anterior trunk, and mid extremities.
- The presence of congenital leukoderma should prompt a thorough skin examination and review of the patient’s medical history for evidence of ocular, auditory, and/or neurologic abnormalities.
Poverty promotes flu hospitalizations
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
FROM MMWR
What Is Your Diagnosis? Stinkbug Staining
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
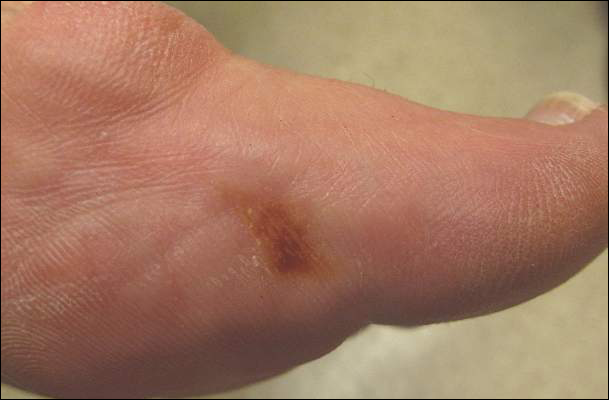
A 56-year-old woman presented at the clinic for a total-body skin examination. A pigmented lesion was found on the medial aspect of the left first toe during the examination. The patient did not recognize this spot as a long-standing nevus. The area was scrubbed vigorously with an alcohol swab, which did not change the pigment. Clinically the lesion was concerning for an atypical nevus. Dermoscopic examination showed an unusual pattern with pigment deposition in ridges and on furrows.
Rare Neurological Disease Special Report
Click here to visit the digital edition.
Click here to visit the digital edition.
Click here to visit the digital edition.
Overuse of Antibiotics for Acne Vulgaris: Too Much of a Good Thing
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
Polycystic Ovary Syndrome in Adolescents
From the Department of Pediatrics, Section of Endocrinology & Diabetes, Medical College of Wisconsin, Milwaukee, WI.
Abstract
- Objective: To review the diagnosis and management of polycystic ovary syndrome (PCOS) in adolescent patients.
- Methods: Review of the literature.
- Results: PCOS is a complex, heterogeneous disorder that frequently manifests during puberty. The symptoms of PCOS (ie, menstrual irregularities, hirsutism, and acne) tend to overlap with normal pubertal changes. Diagnostic criteria for PCOS in the adolescent age-group is still lacking. Current practice is to utilize adult diagnostic criteria, which raises the concern for misdiagnosis. The underlying etiology for the disorder is still unclear, but insulin resistance is present in both obese and non-obese PCOS patients. Although recognizing adolescents with PCOS is challenging, evaluating and managing patients for hyperandrogenemia and metabolic syndrome is imperative to prevent long-term reproductive and metabolic complications.
- Conclusion: PCOS is increasingly encountered during adolescence. Recognizing adolescent girls with PCOS is a challenge but important for preventing long-term adverse health outcomes.
Polycystic ovary syndrome (PCOS) is a complex disorder most commonly characterized by chronic anovulation and clinical and biochemical features of hyperandrogenemia. It affects 4% to 12% of reproductive-aged women [1,2]. In adolescents, the exact prevalence is unknown, but in a recent study the prevalence of a confirmed diagnosis of PCOS in adolescents aged 15 to 19 years was 0.56%, which increased to 1.14% when undiagnosed cases with documented symptoms qualifying for PCOS according to NIH criteria were included [3]. The primary underlying defect in PCOS remains unknown, but key features include insulin resistance, impaired gonadotropin dynamics, and androgen excess.
 Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Case Study
Initial Presentation
A 16-year-old female patient presents to the PCOS clinic for evaluation of obesity and amenorrhea.
History
The patient, who is otherwise healthy, began gaining weight at age 7. During this period, her weight increased from the 15th to (currently) the 90th percentile; her height remained constant (75th percentile). Menarche was at 12 years of age. Menstrual periods have been irregular since the onset of menarche and she has had no periods for the past 5 months. She noticed excessive hair growth on her face, chin, and neck soon after the onset of menarche. She has been shaving her facial hair once every 2–3 days.
The patient’s detailed diet history included eating 3 meals daily and snacks in-between meals. The patient was consuming sweet beverages regularly. There was minimal intake fruits and vegetables. The portion sizes for each meal were large. The patient had minimal physical activity and screen time was more than 2 hours daily.
Family history is significant for obesity and type 2 diabetes in her mother and maternal grandmother and is negative for PCOS.
Physical Examination
Vital signs were within normal limits. She was 5 ft 6 in tall and weighed 242 lb, with a body mass index (BMI) of 40 (99th percentile; Z-score 2.41). Physical examination showed coarse hair extending from the sideburns to the chin as well as from pubis symphysis to navel with evidence of hair removal. She had acanthosis nigricans on her neck, mild acne, and evidence of central obesity with pink striae marks on the abdomen. She was Tanner stage 5 for breast and pubic hair and there was no evidence of virilization (clitoral hypertrophy, deepening of the voice, severe hirsutism, male pattern baldness, and masculine habitus). Other physical examination findings were within normal limits.
What physical findings in this patient are suggestive of clinical hyperandrogenemia?
Physiologic irregular menstruation is a well known phenomenon in adolescent girls and is generally due to anovulatory cycles [9–12]. Menstrual cycles shorter than 19 days or longer than 90 days at any stage after menarche are considered abnormal. The menstrual irregularity that is commonly seen within the first 2–3 years after the first menarche can last up to 5 years [5]. However, the majority of girls establish 20- to 45-day cycles within the first 2 years [13].
Androgen excess, defined by the presence of clinical and/or biochemical hyperandrogenemia, should be considered in any adolescent girl who is 2 to 3 years’ post-menarche and presenting with irregular menstrual periods, coarse terminal hair in a male distribution pattern (hirsutism), or moderate to severe inflammatory acne. Hirsutism is androgen dependent [14–16] and must be distinguished from hypertrichosis, which is generalized excessive vellus hair growth present all over the body. Clinical hyperandrogenemia, which includes hirsutism, acne vulgaris, as well as androgenetic alopecia, is well correlated with elevated androgen levels; however, the severity of hirsutism does not correlate well with circulating androgen levels [17,18]. Mild hirsutism is often not associated with hyperandrogenemia in otherwise asymptomatic individuals,but it may be a sign of hyperandrogenemia in adolescents when associated with other features of PCOS, ie, menstrual irregularity [14–16, 19–22]. Defining hirsutism in early adolescence may be difficult since the sexual hair may still be developing, and laboratory evaluation should be considered (see below), especially in an overweight/obese adolescent girl presenting with oligomenorrhea. Ethnic variation due to decreased skin sensitivity to androgens can result in minimal hirsutism despite elevated plasma androgen levels and must be considered among certain Asian women. Women with PCOS from China, Japan, Thailand, and East and Southeast Asian countries tend to have low scores on hirsutism rating scales even with elevated plasma androgens levels [16,23].
Although having acne during puberty is not considered as a marker for hyperandrogenemia, patients with moderate to severe inflammatory acne that is poorly responsive to topical treatment should be evaluated for underlying hyperandrogenemia [19,24,25].
What laboratory tests should be obtained to when there is clinical suspicion of hyperandrogenemia?
Case Continued
The patient underwent laboratory assessment that included total and free testosterone levels, lipid panel, thyroid studies, prolactin level, comprehensive metabolic panel (CMP) and hemoglobin A1c (HbA1c). Due to lack of virilization, she was not tested for PCOS-like syndromes. Her total and free testosterone were 90 ng/dL (normal, < 41) and 24.7 pg/mL (normal, 0.5–3.9) respectively. Thyroid-stimulating hormone and prolactin levels were normal. She had normal lipid levels and CMP but HbA1c was 5.9% (pre-diabetic range). The results of a 2-hour oral glucose tolerance test revealed a level of 160 mg/dL, indicative of impaired glucose tolerance.
What is the pathophysiology and diagnostic criteria for PCOS in adolescents?
PCOS has diverse etiology and has been linked to both genetic and environmental factors affecting ovarian steroidogenesis [13,30]. While the familial clustering strongly supports the role of genetic factors, variability in phenotypic features within the same or different families indicates the importance of environmental contribution [31–34].
The exact underlying mechanism leading to disruption of ovulation is still unclear; however, hyperinsulinemia augmenting ovarian androgen production has been well recognized [35–37]. Insulin resistance is a characteristic finding in PCOS and occurs both in obese and lean patients [38,39]. Obesity further exacerbates the insulin resistance state in PCOS patients. Therefore, obese patients with PCOS have more severe hyperandrogenemia and consequences from it (hirsutism, menstrual abnormalities, and metabolic derangements) than normal-weight PCOS patients [40,41]. Similar to LH, insulin can stimulate ovarian theca cells directly and cause increased production of androgens [42]. Elevated androgen levels cause the irregular menstrual periods as well as clinical signs of hyperandrogenemia, such as hirsutism and acne.
Altered gonadotropin dynamics is another possible etiological factor that is linked with PCOS. Hyperinsulinemia affects the regulation of gonadotropin-releasing hormone (GnRH) pulse generator, causing hypersecretion of LH [43]. Obese peripubertal girls have been identified having altered LH secretion [44,45]. This results in increased LH levels relative to FSH. Normal FSH is required to stimulate ovarian folliculognensis; insufficient FSH levels cause anovulation and menstrual irregularities. Abnormal LH secretion and fasting insulin levels have been identified the independent predictors for hyperandrogenemia in some peripubertal obese girls [46].
In 2010 Carmina et al published new criteria to diagnose PCOS in adolescents [27].They recommended that in diagnosing PCOS in adolescents, all 3 previously mentioned criteria should be present: hyperandrogenemia, chronic anovulation, and polycystic ovaries. With the exception of worsening hirsutism, the new recommendations greatly emphasized biochemical hyperandrogenemia (elevated free testosterone levels using sensitive assays). Chronic anovulation was defined as persistence of menstrual irregularities 2 years post-menarche and pelvic ultrasound (USG) showing increased ovarian size (> 10 cm3). Normal physiological variations unrelated to hyperandrogenemia are common in adolescent ovaries and limits the usefulness of pelvic USG as a diagnostic criterion for PCOS [13,47,48]. Also, the prevalence of increased ovarian size in hyperandrogenemic adolescent patients was reported to be low, and its utility as a criterion for diagnosis needs to be further explored [49]. In our current practice we do not rely on pelvic USG findings to make a PCOS diagnosis.
Due to longstanding controversies and lack of consensus surrounding the accurate diagnostic criteria, a recent guideline was developed by experts in pediatric endocrinology and adolescent medicine invited by the Pediatric Endocrine Society to address these issues [13].The guideline committee assessed the literature in order to define which criteria have sufficient evidence to be used for diagnosis of PCOS in adolescents. They recommend that PCOS should be considered in an adolescent girl presenting with unexplained menstrual irregularities, moderate to severe hirsutism or acne, and elevated levels of serum androgens (total and free testosterone) using reliable assay with well-defined ranges. Although intrinsic insulin resistance unique to PCOS is well known, none of the current guidelines either for adolescent and adult women include it as part of the diagnostic criteria. Since longitudinal studies focusing on the natural history of PCOS in this age-group are lacking, the current recommendations focus on timely screening and treatment in symptomatic adolescent girls suspected of having PCOS.
When there are PCOS features but menstrual irregularity has not been present for at least 2 years, one can defer the diagnostic label and instead use the term at-risk for PCOS. Such patients should have frequent longitudinal re-evaluations and should be offered treatment for their symptoms [13].
How should adolescents with PCOS be managed?
The treatment of PCOS is symptom-directed and should be tailored according to the complaints of the individual patient. However, it also must focus on the core dysfunctions: anovulation, hyperandrogenemia, obesity, and insulin resistance. It also requires bridging patient expectations of regulating menses, lessening the troublesome clinical signs of hyperandrogenemia (hirsutism, acne), and obesity management with the health care provider’s goals of preventing endometrial hyperplasia and cancer, diabetes mellitus, and cardiovascular disease.
Regulating menstruation and reducing cutaneous manifestations of hyperandrogenemia is the priority for any adolescent with PCOS. Combined oral contraceptive pills (COCs) are the first line of medical treatment for most adolescents. COCs restore endometrial cycling and suppress androgen levels, and are therefore optimal in treating abnormal uterine bleeding, protecting against endometrial carcinoma, and alleviating cutaneous manifestations of hyperandrogenemia (hirsutism and acne). Progestin monotherapy is considered an alternative therapy in individuals with contraindications to COCs (ie, thromboembolic risk). Although it is not effective in lowering androgen levels thus does not help reduce hair growth and acne, progestin monotherapy protects the endometrium and reduces the risk of endometrial cancer [50].
The majority of patients with PCOS are overweight or obese. Regardless of BMI, patients with PCOS have profound intrinsic insulin resistance that gets worse with overweight or obesity. Weight reduction by restricting caloric intake and increasing physical exercise is vital and has shown to be effective in regulating menstrual cycles, but is difficult to achieve [51–53]. Metformin can regulate menstrual cycles and decrease androgen levels by improving insulin sensitivity [54,55]. The use of metformin in PCOS patients is still controversial and abnormal glucose tolerance is the only approved indication [61]. However, combing metformin with COCs and lifestyle modification in obese PCOS patients has been shown to be used more frequently in pediatric endocrine clinics [56]. COCs are the only agents that can lower testosterone levels and improve ovulation and hirsutism; these effects are seen less frequently with lifestyle modification or metformin, either used alone or in combination.
COC monotherapy is first-line therapy to treat hirsutism. Consider anti-androgen treatment for hirsutism if there is no improvement after 6–9 months of hormonal treatment [57]. Antiandrogens reduce hirsutism by decreasing androgen production and binding the androgen receptors in target tissue. Spironolactone is the most commonly used antiandrogen therapy in adolescent girls with PCOS. Given the risk of teratogenicity with antiandrogens if pregnancy occurs, it is recommended to use it in combination with COCs [57]. Cosmetic measures including direct hair removal and electrolysis should be discussed with patients as other options for treatment of hirsutism.
Obese patients with PCOS are at higher risk for metabolic syndrome, a constellation of features including glucose intolerance, central obesity, hypertension, and dyslipidemia. Hyperandrogenemia and insulin resistance are linked with metabolic syndrome in PCOS. Reducing hyperandrogenemia and insulin resistance could reverse metabolic derangements and further reduce the risk of cardiovascular disease [58].
Worsening insulin resistance with COCs in PCOS has raised the concern of long-term metabolic derangements and cardiovascular adverse effects. COCs tend to increase total cholesterol, triglyceride, and high-sensitivity C-reactive protein levels [59]. However, the long-term implications of these findings are not well understood, attributable to the lack of longitudinal studies, especially in women with PCOS receiving COCs. Newer COCs containing less androgenic progestin may have less deleterious effect on insulin resistance and lipid profile. Due to insufficient use in adolescent patients, a definitive conclusion about their long-term safety cannot be drawn. Thus, there remains a theoretical risk of COCs exacerbating the underlying metabolic derangements in PCOS that can lead to subsequent adverse cardiovascular events.
Adolescent girls with PCOS are also at an increased risk for depression and anxiety disorders. The 2013 Endocrine Society clinical practice guideline suggests that adolescent girls with PCOS should be screened for depression and anxiety by history [51].If symptoms are present, patients should receive appropriate psychological referral and treatment.
Case Continued
As she had no contraindications to COCs, the patient was started on COC therapy to regulate her menstrual periods and alleviate the symptoms of hirsutism. Due to impaired glucose tolerance test results and increased risk for type 2 diabetes, treatment with metformin was also initiated. The patient met with a dietician, who offered recommendations for adopting a healthy lifestyle and introduced her to the “3,2,1,0, blast off” model: 3 consistent meals, 2 hours or less of screen time, 1 hour or more of physical activity, and 0 sweetened beverages a day. The patient was also advised to increase daily consumption of fruits and vegetables. Results of the 2-item Patient Health Questionnaire (PHQ-2) for depression were negative.
At a follow-up visit 6 months later, the patient reported that her menstrual periods were regular. There was some improvement in hirsutism, requiring less shaving, and there was no increase in weight. Repeat laboratory evaluations showed normal free testosterone level, decreased HbA1c (5.2%), and improved random blood glucose (130 mg/dL). The patient was seen regularly and treatment results monitored. No side effects were seen over a 4.5-year period. As PCOS is a lifelong condition, at the age of 21 the patient was referred to an adult endocrine clinic for further management.
Corresponding author: Alvina R. Kansra, MD, Medical College of Wisconsin, 8701 Watertown Plank Rd., Wauwatosa, WI 53226, [email protected].
Financial disclosures: None.
1. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome inthe Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999v;84:4006–11.
2. Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9.
3. Christensen SB, Black MH, Smith N, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100:470–7.
4. Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab 2013;98:3572–83.
5. Venturoli S, Porcu E, Fabbri R, et al. Menstrual irregularities in adolescents: hormonal pattern and ovarian morphology. Horm Res 1986;24:269–79.
6. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston: Blackwell Scientific; 1992.
7. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
8. Azziz R, Carmina E, Dewailly D, et al; Androgen Excess Society. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–45.
9. Treloar A, Boynton R, Benn B, Brown B. Variation of human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126.
10. Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol 1977;7:1–193.
11. Diaz A, Laufer MR, Breech LL; American Academy of Pediatrics Committee on Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Menstruation in girls and adolescents:using the menstrual cycle as a vital sign. Pediatrics 2006;118:2245–50.
12. Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol 1983;97:213–9.
13. Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015 Apr 1.
14. Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000;21:363–92.
15. Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
16. Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2012;18:146–70.
17. Rosenfield RL. The polycystic ovary morphology-polycystic ovary syndrome spectrum. J Pediatr Adolesc Gynecol 2015;28:412–9.
18. Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010;16:51–64.
19. Chen WC, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol 2009;1:81–6.
20. Hawryluk EB, English JC 3rd. Female adolescent hair disorders. J Pediatr Adolesc Gynecol 2009;22:271–81.
21. Souter I, Sanchez A, Perez M, et al. The prevalence of androgen excess among patients with minimal unwanted hair growth. Am J Obstet Gynecol 2004;191:1914–20.
22. Di Fede G, Mansueto P, Pepe G, et al. High prevalence of polycystic ovary syndrome in women with mild hirsutism and no other significant clinical symptoms. Fertil Steril 2010; 94:194–7.
23. Chan CNJ, Haines CJ, Chow CCF, et al. Polycystic ovarian syndrome in Hong Kong Chinese women: patient characteristics and diagnostic criteria. Hong Kong Med J 2005;11:336–41.
24. Lucky AW, Biro FM, Simbartl LA, et al. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr 1997;130:30–9.
25. Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics 2013;131:S163–S186.
26. Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003;88:4682–8.
27. Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201.e1–5.
28. Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics 2015;136:1154–65.
29. Cho LW, Jayagopal V, Kilpatrick ES, Holding S. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann Clin Biochem 2006;43(Pt 3):217–9.
30. Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Sperling M, editor. Pediatric Endocrinology. 4th ed. Philadelphia: Elsevier; 2014:569–663.
31. Givens JR. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am 1988;17:771–83.
32. Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 1998;95:14956–60.
33. Amato P, Simpson JL. The genetics of polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2005;18:707–18.
34. Prapas N, Karkanaki A, Prapas I, et al. Genetics of polycystic ovary syndrome. Hippokratia 2009;13:216–23.
35. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980;50:113–6.
36. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030.
37. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800.
38. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983;57:356–9.
39. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–74.
40. Gambineri A, Pelusi C, Vicennati V, et al. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–96.
41. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr 2001;138:38–44.
42. Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998;83:2001–5.
43. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12:351–61.
44. McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714–22.
45. McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;92:430–6.
46. Knudsen KL, Blank SK, Burt Solorzano C, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring) 2010;18:2118–24.
47. Venturoli S, Porcu E, Fabbri R, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80.
48. Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 2006;91:3786–90.
49. Fruzzetti F, Campagna AM, Perini D, Carmina E. Ovarian volume in normal and hyperandrogenic adolescent women. Fertil Steril 2015;104:196–9.
50. Fearnley EJ, Marquart L, Spurdle AB, et al, the Australian Ovarian Cancer Study Group and the Australian National Endometrial Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Austrialian case-control study. Cancer Causes Control 2010;21:2303–8.
51. Legro RS, Arslanian SA, Ehrmann DA, et al; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565-92.
52. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab 2013;98:4655.
53. Lass N, Kleber M, Winkel K, et al. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab 2011;96:3533.
54. Costello M, Eden J. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003;79:1–13.
55. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951–3.
56. Auble B, Elder D, Gross A, Hillman JB. Differences in the management of adolescents with polycystic ovary syndrome across pediatric specialties. J Pediatr Adolesc Gynecol 2013;26:234–8.
57. Martin KA, Chang J, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clincal practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
58. Geller DH, Pacaud D, Gordon CM, Misra M, for the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: emerging therapies: the use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS). Int J Ped Endocrinol 2011;2011:9.
59. Tfayli H, Ulnach JW, Lee S, et al. Drospirenon/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal and cardiovascular risk factors. J Clin Endocrinol Metab 2011;96:1311–9.
From the Department of Pediatrics, Section of Endocrinology & Diabetes, Medical College of Wisconsin, Milwaukee, WI.
Abstract
- Objective: To review the diagnosis and management of polycystic ovary syndrome (PCOS) in adolescent patients.
- Methods: Review of the literature.
- Results: PCOS is a complex, heterogeneous disorder that frequently manifests during puberty. The symptoms of PCOS (ie, menstrual irregularities, hirsutism, and acne) tend to overlap with normal pubertal changes. Diagnostic criteria for PCOS in the adolescent age-group is still lacking. Current practice is to utilize adult diagnostic criteria, which raises the concern for misdiagnosis. The underlying etiology for the disorder is still unclear, but insulin resistance is present in both obese and non-obese PCOS patients. Although recognizing adolescents with PCOS is challenging, evaluating and managing patients for hyperandrogenemia and metabolic syndrome is imperative to prevent long-term reproductive and metabolic complications.
- Conclusion: PCOS is increasingly encountered during adolescence. Recognizing adolescent girls with PCOS is a challenge but important for preventing long-term adverse health outcomes.
Polycystic ovary syndrome (PCOS) is a complex disorder most commonly characterized by chronic anovulation and clinical and biochemical features of hyperandrogenemia. It affects 4% to 12% of reproductive-aged women [1,2]. In adolescents, the exact prevalence is unknown, but in a recent study the prevalence of a confirmed diagnosis of PCOS in adolescents aged 15 to 19 years was 0.56%, which increased to 1.14% when undiagnosed cases with documented symptoms qualifying for PCOS according to NIH criteria were included [3]. The primary underlying defect in PCOS remains unknown, but key features include insulin resistance, impaired gonadotropin dynamics, and androgen excess.
 Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Case Study
Initial Presentation
A 16-year-old female patient presents to the PCOS clinic for evaluation of obesity and amenorrhea.
History
The patient, who is otherwise healthy, began gaining weight at age 7. During this period, her weight increased from the 15th to (currently) the 90th percentile; her height remained constant (75th percentile). Menarche was at 12 years of age. Menstrual periods have been irregular since the onset of menarche and she has had no periods for the past 5 months. She noticed excessive hair growth on her face, chin, and neck soon after the onset of menarche. She has been shaving her facial hair once every 2–3 days.
The patient’s detailed diet history included eating 3 meals daily and snacks in-between meals. The patient was consuming sweet beverages regularly. There was minimal intake fruits and vegetables. The portion sizes for each meal were large. The patient had minimal physical activity and screen time was more than 2 hours daily.
Family history is significant for obesity and type 2 diabetes in her mother and maternal grandmother and is negative for PCOS.
Physical Examination
Vital signs were within normal limits. She was 5 ft 6 in tall and weighed 242 lb, with a body mass index (BMI) of 40 (99th percentile; Z-score 2.41). Physical examination showed coarse hair extending from the sideburns to the chin as well as from pubis symphysis to navel with evidence of hair removal. She had acanthosis nigricans on her neck, mild acne, and evidence of central obesity with pink striae marks on the abdomen. She was Tanner stage 5 for breast and pubic hair and there was no evidence of virilization (clitoral hypertrophy, deepening of the voice, severe hirsutism, male pattern baldness, and masculine habitus). Other physical examination findings were within normal limits.
What physical findings in this patient are suggestive of clinical hyperandrogenemia?
Physiologic irregular menstruation is a well known phenomenon in adolescent girls and is generally due to anovulatory cycles [9–12]. Menstrual cycles shorter than 19 days or longer than 90 days at any stage after menarche are considered abnormal. The menstrual irregularity that is commonly seen within the first 2–3 years after the first menarche can last up to 5 years [5]. However, the majority of girls establish 20- to 45-day cycles within the first 2 years [13].
Androgen excess, defined by the presence of clinical and/or biochemical hyperandrogenemia, should be considered in any adolescent girl who is 2 to 3 years’ post-menarche and presenting with irregular menstrual periods, coarse terminal hair in a male distribution pattern (hirsutism), or moderate to severe inflammatory acne. Hirsutism is androgen dependent [14–16] and must be distinguished from hypertrichosis, which is generalized excessive vellus hair growth present all over the body. Clinical hyperandrogenemia, which includes hirsutism, acne vulgaris, as well as androgenetic alopecia, is well correlated with elevated androgen levels; however, the severity of hirsutism does not correlate well with circulating androgen levels [17,18]. Mild hirsutism is often not associated with hyperandrogenemia in otherwise asymptomatic individuals,but it may be a sign of hyperandrogenemia in adolescents when associated with other features of PCOS, ie, menstrual irregularity [14–16, 19–22]. Defining hirsutism in early adolescence may be difficult since the sexual hair may still be developing, and laboratory evaluation should be considered (see below), especially in an overweight/obese adolescent girl presenting with oligomenorrhea. Ethnic variation due to decreased skin sensitivity to androgens can result in minimal hirsutism despite elevated plasma androgen levels and must be considered among certain Asian women. Women with PCOS from China, Japan, Thailand, and East and Southeast Asian countries tend to have low scores on hirsutism rating scales even with elevated plasma androgens levels [16,23].
Although having acne during puberty is not considered as a marker for hyperandrogenemia, patients with moderate to severe inflammatory acne that is poorly responsive to topical treatment should be evaluated for underlying hyperandrogenemia [19,24,25].
What laboratory tests should be obtained to when there is clinical suspicion of hyperandrogenemia?
Case Continued
The patient underwent laboratory assessment that included total and free testosterone levels, lipid panel, thyroid studies, prolactin level, comprehensive metabolic panel (CMP) and hemoglobin A1c (HbA1c). Due to lack of virilization, she was not tested for PCOS-like syndromes. Her total and free testosterone were 90 ng/dL (normal, < 41) and 24.7 pg/mL (normal, 0.5–3.9) respectively. Thyroid-stimulating hormone and prolactin levels were normal. She had normal lipid levels and CMP but HbA1c was 5.9% (pre-diabetic range). The results of a 2-hour oral glucose tolerance test revealed a level of 160 mg/dL, indicative of impaired glucose tolerance.
What is the pathophysiology and diagnostic criteria for PCOS in adolescents?
PCOS has diverse etiology and has been linked to both genetic and environmental factors affecting ovarian steroidogenesis [13,30]. While the familial clustering strongly supports the role of genetic factors, variability in phenotypic features within the same or different families indicates the importance of environmental contribution [31–34].
The exact underlying mechanism leading to disruption of ovulation is still unclear; however, hyperinsulinemia augmenting ovarian androgen production has been well recognized [35–37]. Insulin resistance is a characteristic finding in PCOS and occurs both in obese and lean patients [38,39]. Obesity further exacerbates the insulin resistance state in PCOS patients. Therefore, obese patients with PCOS have more severe hyperandrogenemia and consequences from it (hirsutism, menstrual abnormalities, and metabolic derangements) than normal-weight PCOS patients [40,41]. Similar to LH, insulin can stimulate ovarian theca cells directly and cause increased production of androgens [42]. Elevated androgen levels cause the irregular menstrual periods as well as clinical signs of hyperandrogenemia, such as hirsutism and acne.
Altered gonadotropin dynamics is another possible etiological factor that is linked with PCOS. Hyperinsulinemia affects the regulation of gonadotropin-releasing hormone (GnRH) pulse generator, causing hypersecretion of LH [43]. Obese peripubertal girls have been identified having altered LH secretion [44,45]. This results in increased LH levels relative to FSH. Normal FSH is required to stimulate ovarian folliculognensis; insufficient FSH levels cause anovulation and menstrual irregularities. Abnormal LH secretion and fasting insulin levels have been identified the independent predictors for hyperandrogenemia in some peripubertal obese girls [46].
In 2010 Carmina et al published new criteria to diagnose PCOS in adolescents [27].They recommended that in diagnosing PCOS in adolescents, all 3 previously mentioned criteria should be present: hyperandrogenemia, chronic anovulation, and polycystic ovaries. With the exception of worsening hirsutism, the new recommendations greatly emphasized biochemical hyperandrogenemia (elevated free testosterone levels using sensitive assays). Chronic anovulation was defined as persistence of menstrual irregularities 2 years post-menarche and pelvic ultrasound (USG) showing increased ovarian size (> 10 cm3). Normal physiological variations unrelated to hyperandrogenemia are common in adolescent ovaries and limits the usefulness of pelvic USG as a diagnostic criterion for PCOS [13,47,48]. Also, the prevalence of increased ovarian size in hyperandrogenemic adolescent patients was reported to be low, and its utility as a criterion for diagnosis needs to be further explored [49]. In our current practice we do not rely on pelvic USG findings to make a PCOS diagnosis.
Due to longstanding controversies and lack of consensus surrounding the accurate diagnostic criteria, a recent guideline was developed by experts in pediatric endocrinology and adolescent medicine invited by the Pediatric Endocrine Society to address these issues [13].The guideline committee assessed the literature in order to define which criteria have sufficient evidence to be used for diagnosis of PCOS in adolescents. They recommend that PCOS should be considered in an adolescent girl presenting with unexplained menstrual irregularities, moderate to severe hirsutism or acne, and elevated levels of serum androgens (total and free testosterone) using reliable assay with well-defined ranges. Although intrinsic insulin resistance unique to PCOS is well known, none of the current guidelines either for adolescent and adult women include it as part of the diagnostic criteria. Since longitudinal studies focusing on the natural history of PCOS in this age-group are lacking, the current recommendations focus on timely screening and treatment in symptomatic adolescent girls suspected of having PCOS.
When there are PCOS features but menstrual irregularity has not been present for at least 2 years, one can defer the diagnostic label and instead use the term at-risk for PCOS. Such patients should have frequent longitudinal re-evaluations and should be offered treatment for their symptoms [13].
How should adolescents with PCOS be managed?
The treatment of PCOS is symptom-directed and should be tailored according to the complaints of the individual patient. However, it also must focus on the core dysfunctions: anovulation, hyperandrogenemia, obesity, and insulin resistance. It also requires bridging patient expectations of regulating menses, lessening the troublesome clinical signs of hyperandrogenemia (hirsutism, acne), and obesity management with the health care provider’s goals of preventing endometrial hyperplasia and cancer, diabetes mellitus, and cardiovascular disease.
Regulating menstruation and reducing cutaneous manifestations of hyperandrogenemia is the priority for any adolescent with PCOS. Combined oral contraceptive pills (COCs) are the first line of medical treatment for most adolescents. COCs restore endometrial cycling and suppress androgen levels, and are therefore optimal in treating abnormal uterine bleeding, protecting against endometrial carcinoma, and alleviating cutaneous manifestations of hyperandrogenemia (hirsutism and acne). Progestin monotherapy is considered an alternative therapy in individuals with contraindications to COCs (ie, thromboembolic risk). Although it is not effective in lowering androgen levels thus does not help reduce hair growth and acne, progestin monotherapy protects the endometrium and reduces the risk of endometrial cancer [50].
The majority of patients with PCOS are overweight or obese. Regardless of BMI, patients with PCOS have profound intrinsic insulin resistance that gets worse with overweight or obesity. Weight reduction by restricting caloric intake and increasing physical exercise is vital and has shown to be effective in regulating menstrual cycles, but is difficult to achieve [51–53]. Metformin can regulate menstrual cycles and decrease androgen levels by improving insulin sensitivity [54,55]. The use of metformin in PCOS patients is still controversial and abnormal glucose tolerance is the only approved indication [61]. However, combing metformin with COCs and lifestyle modification in obese PCOS patients has been shown to be used more frequently in pediatric endocrine clinics [56]. COCs are the only agents that can lower testosterone levels and improve ovulation and hirsutism; these effects are seen less frequently with lifestyle modification or metformin, either used alone or in combination.
COC monotherapy is first-line therapy to treat hirsutism. Consider anti-androgen treatment for hirsutism if there is no improvement after 6–9 months of hormonal treatment [57]. Antiandrogens reduce hirsutism by decreasing androgen production and binding the androgen receptors in target tissue. Spironolactone is the most commonly used antiandrogen therapy in adolescent girls with PCOS. Given the risk of teratogenicity with antiandrogens if pregnancy occurs, it is recommended to use it in combination with COCs [57]. Cosmetic measures including direct hair removal and electrolysis should be discussed with patients as other options for treatment of hirsutism.
Obese patients with PCOS are at higher risk for metabolic syndrome, a constellation of features including glucose intolerance, central obesity, hypertension, and dyslipidemia. Hyperandrogenemia and insulin resistance are linked with metabolic syndrome in PCOS. Reducing hyperandrogenemia and insulin resistance could reverse metabolic derangements and further reduce the risk of cardiovascular disease [58].
Worsening insulin resistance with COCs in PCOS has raised the concern of long-term metabolic derangements and cardiovascular adverse effects. COCs tend to increase total cholesterol, triglyceride, and high-sensitivity C-reactive protein levels [59]. However, the long-term implications of these findings are not well understood, attributable to the lack of longitudinal studies, especially in women with PCOS receiving COCs. Newer COCs containing less androgenic progestin may have less deleterious effect on insulin resistance and lipid profile. Due to insufficient use in adolescent patients, a definitive conclusion about their long-term safety cannot be drawn. Thus, there remains a theoretical risk of COCs exacerbating the underlying metabolic derangements in PCOS that can lead to subsequent adverse cardiovascular events.
Adolescent girls with PCOS are also at an increased risk for depression and anxiety disorders. The 2013 Endocrine Society clinical practice guideline suggests that adolescent girls with PCOS should be screened for depression and anxiety by history [51].If symptoms are present, patients should receive appropriate psychological referral and treatment.
Case Continued
As she had no contraindications to COCs, the patient was started on COC therapy to regulate her menstrual periods and alleviate the symptoms of hirsutism. Due to impaired glucose tolerance test results and increased risk for type 2 diabetes, treatment with metformin was also initiated. The patient met with a dietician, who offered recommendations for adopting a healthy lifestyle and introduced her to the “3,2,1,0, blast off” model: 3 consistent meals, 2 hours or less of screen time, 1 hour or more of physical activity, and 0 sweetened beverages a day. The patient was also advised to increase daily consumption of fruits and vegetables. Results of the 2-item Patient Health Questionnaire (PHQ-2) for depression were negative.
At a follow-up visit 6 months later, the patient reported that her menstrual periods were regular. There was some improvement in hirsutism, requiring less shaving, and there was no increase in weight. Repeat laboratory evaluations showed normal free testosterone level, decreased HbA1c (5.2%), and improved random blood glucose (130 mg/dL). The patient was seen regularly and treatment results monitored. No side effects were seen over a 4.5-year period. As PCOS is a lifelong condition, at the age of 21 the patient was referred to an adult endocrine clinic for further management.
Corresponding author: Alvina R. Kansra, MD, Medical College of Wisconsin, 8701 Watertown Plank Rd., Wauwatosa, WI 53226, [email protected].
Financial disclosures: None.
From the Department of Pediatrics, Section of Endocrinology & Diabetes, Medical College of Wisconsin, Milwaukee, WI.
Abstract
- Objective: To review the diagnosis and management of polycystic ovary syndrome (PCOS) in adolescent patients.
- Methods: Review of the literature.
- Results: PCOS is a complex, heterogeneous disorder that frequently manifests during puberty. The symptoms of PCOS (ie, menstrual irregularities, hirsutism, and acne) tend to overlap with normal pubertal changes. Diagnostic criteria for PCOS in the adolescent age-group is still lacking. Current practice is to utilize adult diagnostic criteria, which raises the concern for misdiagnosis. The underlying etiology for the disorder is still unclear, but insulin resistance is present in both obese and non-obese PCOS patients. Although recognizing adolescents with PCOS is challenging, evaluating and managing patients for hyperandrogenemia and metabolic syndrome is imperative to prevent long-term reproductive and metabolic complications.
- Conclusion: PCOS is increasingly encountered during adolescence. Recognizing adolescent girls with PCOS is a challenge but important for preventing long-term adverse health outcomes.
Polycystic ovary syndrome (PCOS) is a complex disorder most commonly characterized by chronic anovulation and clinical and biochemical features of hyperandrogenemia. It affects 4% to 12% of reproductive-aged women [1,2]. In adolescents, the exact prevalence is unknown, but in a recent study the prevalence of a confirmed diagnosis of PCOS in adolescents aged 15 to 19 years was 0.56%, which increased to 1.14% when undiagnosed cases with documented symptoms qualifying for PCOS according to NIH criteria were included [3]. The primary underlying defect in PCOS remains unknown, but key features include insulin resistance, impaired gonadotropin dynamics, and androgen excess.
 Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Profound functional variations in the hypothalamic-pituitary-ovarian axis commonly seen during normal puberty may result in clinical and biochemical changes that mimic some of the features of PCOS. During the early stages of puberty, adolescent girls tend to have anovulatory menstrual cycles, higher androgen levels, and polycystic ovaries [4,5]. Thus, the clinical signs of hyperandrogenemia commonly seen in adults are less reliable in the adolescent age-group. Diagnostic criteria have been developed for adults and are based upon the various combinations of oligomenorrhea, unexplained hyperandrogenemia, and polycystic ovaries on imaging (Table 1) [6–8]. Applying these adult criteria in adolescent patients with suspected PCOS has always raised the concern of misdiagnosis as some of the changes seen in this age-group may likely be due to normal pubertal development. However, due to the paucity of data, the current practice is to utilize the adult diagnostic criteria. Because of the heterogeneous nature of the disorder, recognizing adolescents with PCOS may be challenging. However, early recognition and management is important to prevent some of the long-term reproductive and metabolic complications associated with this syndrome.
Case Study
Initial Presentation
A 16-year-old female patient presents to the PCOS clinic for evaluation of obesity and amenorrhea.
History
The patient, who is otherwise healthy, began gaining weight at age 7. During this period, her weight increased from the 15th to (currently) the 90th percentile; her height remained constant (75th percentile). Menarche was at 12 years of age. Menstrual periods have been irregular since the onset of menarche and she has had no periods for the past 5 months. She noticed excessive hair growth on her face, chin, and neck soon after the onset of menarche. She has been shaving her facial hair once every 2–3 days.
The patient’s detailed diet history included eating 3 meals daily and snacks in-between meals. The patient was consuming sweet beverages regularly. There was minimal intake fruits and vegetables. The portion sizes for each meal were large. The patient had minimal physical activity and screen time was more than 2 hours daily.
Family history is significant for obesity and type 2 diabetes in her mother and maternal grandmother and is negative for PCOS.
Physical Examination
Vital signs were within normal limits. She was 5 ft 6 in tall and weighed 242 lb, with a body mass index (BMI) of 40 (99th percentile; Z-score 2.41). Physical examination showed coarse hair extending from the sideburns to the chin as well as from pubis symphysis to navel with evidence of hair removal. She had acanthosis nigricans on her neck, mild acne, and evidence of central obesity with pink striae marks on the abdomen. She was Tanner stage 5 for breast and pubic hair and there was no evidence of virilization (clitoral hypertrophy, deepening of the voice, severe hirsutism, male pattern baldness, and masculine habitus). Other physical examination findings were within normal limits.
What physical findings in this patient are suggestive of clinical hyperandrogenemia?
Physiologic irregular menstruation is a well known phenomenon in adolescent girls and is generally due to anovulatory cycles [9–12]. Menstrual cycles shorter than 19 days or longer than 90 days at any stage after menarche are considered abnormal. The menstrual irregularity that is commonly seen within the first 2–3 years after the first menarche can last up to 5 years [5]. However, the majority of girls establish 20- to 45-day cycles within the first 2 years [13].
Androgen excess, defined by the presence of clinical and/or biochemical hyperandrogenemia, should be considered in any adolescent girl who is 2 to 3 years’ post-menarche and presenting with irregular menstrual periods, coarse terminal hair in a male distribution pattern (hirsutism), or moderate to severe inflammatory acne. Hirsutism is androgen dependent [14–16] and must be distinguished from hypertrichosis, which is generalized excessive vellus hair growth present all over the body. Clinical hyperandrogenemia, which includes hirsutism, acne vulgaris, as well as androgenetic alopecia, is well correlated with elevated androgen levels; however, the severity of hirsutism does not correlate well with circulating androgen levels [17,18]. Mild hirsutism is often not associated with hyperandrogenemia in otherwise asymptomatic individuals,but it may be a sign of hyperandrogenemia in adolescents when associated with other features of PCOS, ie, menstrual irregularity [14–16, 19–22]. Defining hirsutism in early adolescence may be difficult since the sexual hair may still be developing, and laboratory evaluation should be considered (see below), especially in an overweight/obese adolescent girl presenting with oligomenorrhea. Ethnic variation due to decreased skin sensitivity to androgens can result in minimal hirsutism despite elevated plasma androgen levels and must be considered among certain Asian women. Women with PCOS from China, Japan, Thailand, and East and Southeast Asian countries tend to have low scores on hirsutism rating scales even with elevated plasma androgens levels [16,23].
Although having acne during puberty is not considered as a marker for hyperandrogenemia, patients with moderate to severe inflammatory acne that is poorly responsive to topical treatment should be evaluated for underlying hyperandrogenemia [19,24,25].
What laboratory tests should be obtained to when there is clinical suspicion of hyperandrogenemia?
Case Continued
The patient underwent laboratory assessment that included total and free testosterone levels, lipid panel, thyroid studies, prolactin level, comprehensive metabolic panel (CMP) and hemoglobin A1c (HbA1c). Due to lack of virilization, she was not tested for PCOS-like syndromes. Her total and free testosterone were 90 ng/dL (normal, < 41) and 24.7 pg/mL (normal, 0.5–3.9) respectively. Thyroid-stimulating hormone and prolactin levels were normal. She had normal lipid levels and CMP but HbA1c was 5.9% (pre-diabetic range). The results of a 2-hour oral glucose tolerance test revealed a level of 160 mg/dL, indicative of impaired glucose tolerance.
What is the pathophysiology and diagnostic criteria for PCOS in adolescents?
PCOS has diverse etiology and has been linked to both genetic and environmental factors affecting ovarian steroidogenesis [13,30]. While the familial clustering strongly supports the role of genetic factors, variability in phenotypic features within the same or different families indicates the importance of environmental contribution [31–34].
The exact underlying mechanism leading to disruption of ovulation is still unclear; however, hyperinsulinemia augmenting ovarian androgen production has been well recognized [35–37]. Insulin resistance is a characteristic finding in PCOS and occurs both in obese and lean patients [38,39]. Obesity further exacerbates the insulin resistance state in PCOS patients. Therefore, obese patients with PCOS have more severe hyperandrogenemia and consequences from it (hirsutism, menstrual abnormalities, and metabolic derangements) than normal-weight PCOS patients [40,41]. Similar to LH, insulin can stimulate ovarian theca cells directly and cause increased production of androgens [42]. Elevated androgen levels cause the irregular menstrual periods as well as clinical signs of hyperandrogenemia, such as hirsutism and acne.
Altered gonadotropin dynamics is another possible etiological factor that is linked with PCOS. Hyperinsulinemia affects the regulation of gonadotropin-releasing hormone (GnRH) pulse generator, causing hypersecretion of LH [43]. Obese peripubertal girls have been identified having altered LH secretion [44,45]. This results in increased LH levels relative to FSH. Normal FSH is required to stimulate ovarian folliculognensis; insufficient FSH levels cause anovulation and menstrual irregularities. Abnormal LH secretion and fasting insulin levels have been identified the independent predictors for hyperandrogenemia in some peripubertal obese girls [46].
In 2010 Carmina et al published new criteria to diagnose PCOS in adolescents [27].They recommended that in diagnosing PCOS in adolescents, all 3 previously mentioned criteria should be present: hyperandrogenemia, chronic anovulation, and polycystic ovaries. With the exception of worsening hirsutism, the new recommendations greatly emphasized biochemical hyperandrogenemia (elevated free testosterone levels using sensitive assays). Chronic anovulation was defined as persistence of menstrual irregularities 2 years post-menarche and pelvic ultrasound (USG) showing increased ovarian size (> 10 cm3). Normal physiological variations unrelated to hyperandrogenemia are common in adolescent ovaries and limits the usefulness of pelvic USG as a diagnostic criterion for PCOS [13,47,48]. Also, the prevalence of increased ovarian size in hyperandrogenemic adolescent patients was reported to be low, and its utility as a criterion for diagnosis needs to be further explored [49]. In our current practice we do not rely on pelvic USG findings to make a PCOS diagnosis.
Due to longstanding controversies and lack of consensus surrounding the accurate diagnostic criteria, a recent guideline was developed by experts in pediatric endocrinology and adolescent medicine invited by the Pediatric Endocrine Society to address these issues [13].The guideline committee assessed the literature in order to define which criteria have sufficient evidence to be used for diagnosis of PCOS in adolescents. They recommend that PCOS should be considered in an adolescent girl presenting with unexplained menstrual irregularities, moderate to severe hirsutism or acne, and elevated levels of serum androgens (total and free testosterone) using reliable assay with well-defined ranges. Although intrinsic insulin resistance unique to PCOS is well known, none of the current guidelines either for adolescent and adult women include it as part of the diagnostic criteria. Since longitudinal studies focusing on the natural history of PCOS in this age-group are lacking, the current recommendations focus on timely screening and treatment in symptomatic adolescent girls suspected of having PCOS.
When there are PCOS features but menstrual irregularity has not been present for at least 2 years, one can defer the diagnostic label and instead use the term at-risk for PCOS. Such patients should have frequent longitudinal re-evaluations and should be offered treatment for their symptoms [13].
How should adolescents with PCOS be managed?
The treatment of PCOS is symptom-directed and should be tailored according to the complaints of the individual patient. However, it also must focus on the core dysfunctions: anovulation, hyperandrogenemia, obesity, and insulin resistance. It also requires bridging patient expectations of regulating menses, lessening the troublesome clinical signs of hyperandrogenemia (hirsutism, acne), and obesity management with the health care provider’s goals of preventing endometrial hyperplasia and cancer, diabetes mellitus, and cardiovascular disease.
Regulating menstruation and reducing cutaneous manifestations of hyperandrogenemia is the priority for any adolescent with PCOS. Combined oral contraceptive pills (COCs) are the first line of medical treatment for most adolescents. COCs restore endometrial cycling and suppress androgen levels, and are therefore optimal in treating abnormal uterine bleeding, protecting against endometrial carcinoma, and alleviating cutaneous manifestations of hyperandrogenemia (hirsutism and acne). Progestin monotherapy is considered an alternative therapy in individuals with contraindications to COCs (ie, thromboembolic risk). Although it is not effective in lowering androgen levels thus does not help reduce hair growth and acne, progestin monotherapy protects the endometrium and reduces the risk of endometrial cancer [50].
The majority of patients with PCOS are overweight or obese. Regardless of BMI, patients with PCOS have profound intrinsic insulin resistance that gets worse with overweight or obesity. Weight reduction by restricting caloric intake and increasing physical exercise is vital and has shown to be effective in regulating menstrual cycles, but is difficult to achieve [51–53]. Metformin can regulate menstrual cycles and decrease androgen levels by improving insulin sensitivity [54,55]. The use of metformin in PCOS patients is still controversial and abnormal glucose tolerance is the only approved indication [61]. However, combing metformin with COCs and lifestyle modification in obese PCOS patients has been shown to be used more frequently in pediatric endocrine clinics [56]. COCs are the only agents that can lower testosterone levels and improve ovulation and hirsutism; these effects are seen less frequently with lifestyle modification or metformin, either used alone or in combination.
COC monotherapy is first-line therapy to treat hirsutism. Consider anti-androgen treatment for hirsutism if there is no improvement after 6–9 months of hormonal treatment [57]. Antiandrogens reduce hirsutism by decreasing androgen production and binding the androgen receptors in target tissue. Spironolactone is the most commonly used antiandrogen therapy in adolescent girls with PCOS. Given the risk of teratogenicity with antiandrogens if pregnancy occurs, it is recommended to use it in combination with COCs [57]. Cosmetic measures including direct hair removal and electrolysis should be discussed with patients as other options for treatment of hirsutism.
Obese patients with PCOS are at higher risk for metabolic syndrome, a constellation of features including glucose intolerance, central obesity, hypertension, and dyslipidemia. Hyperandrogenemia and insulin resistance are linked with metabolic syndrome in PCOS. Reducing hyperandrogenemia and insulin resistance could reverse metabolic derangements and further reduce the risk of cardiovascular disease [58].
Worsening insulin resistance with COCs in PCOS has raised the concern of long-term metabolic derangements and cardiovascular adverse effects. COCs tend to increase total cholesterol, triglyceride, and high-sensitivity C-reactive protein levels [59]. However, the long-term implications of these findings are not well understood, attributable to the lack of longitudinal studies, especially in women with PCOS receiving COCs. Newer COCs containing less androgenic progestin may have less deleterious effect on insulin resistance and lipid profile. Due to insufficient use in adolescent patients, a definitive conclusion about their long-term safety cannot be drawn. Thus, there remains a theoretical risk of COCs exacerbating the underlying metabolic derangements in PCOS that can lead to subsequent adverse cardiovascular events.
Adolescent girls with PCOS are also at an increased risk for depression and anxiety disorders. The 2013 Endocrine Society clinical practice guideline suggests that adolescent girls with PCOS should be screened for depression and anxiety by history [51].If symptoms are present, patients should receive appropriate psychological referral and treatment.
Case Continued
As she had no contraindications to COCs, the patient was started on COC therapy to regulate her menstrual periods and alleviate the symptoms of hirsutism. Due to impaired glucose tolerance test results and increased risk for type 2 diabetes, treatment with metformin was also initiated. The patient met with a dietician, who offered recommendations for adopting a healthy lifestyle and introduced her to the “3,2,1,0, blast off” model: 3 consistent meals, 2 hours or less of screen time, 1 hour or more of physical activity, and 0 sweetened beverages a day. The patient was also advised to increase daily consumption of fruits and vegetables. Results of the 2-item Patient Health Questionnaire (PHQ-2) for depression were negative.
At a follow-up visit 6 months later, the patient reported that her menstrual periods were regular. There was some improvement in hirsutism, requiring less shaving, and there was no increase in weight. Repeat laboratory evaluations showed normal free testosterone level, decreased HbA1c (5.2%), and improved random blood glucose (130 mg/dL). The patient was seen regularly and treatment results monitored. No side effects were seen over a 4.5-year period. As PCOS is a lifelong condition, at the age of 21 the patient was referred to an adult endocrine clinic for further management.
Corresponding author: Alvina R. Kansra, MD, Medical College of Wisconsin, 8701 Watertown Plank Rd., Wauwatosa, WI 53226, [email protected].
Financial disclosures: None.
1. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome inthe Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999v;84:4006–11.
2. Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9.
3. Christensen SB, Black MH, Smith N, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100:470–7.
4. Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab 2013;98:3572–83.
5. Venturoli S, Porcu E, Fabbri R, et al. Menstrual irregularities in adolescents: hormonal pattern and ovarian morphology. Horm Res 1986;24:269–79.
6. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston: Blackwell Scientific; 1992.
7. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
8. Azziz R, Carmina E, Dewailly D, et al; Androgen Excess Society. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–45.
9. Treloar A, Boynton R, Benn B, Brown B. Variation of human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126.
10. Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol 1977;7:1–193.
11. Diaz A, Laufer MR, Breech LL; American Academy of Pediatrics Committee on Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Menstruation in girls and adolescents:using the menstrual cycle as a vital sign. Pediatrics 2006;118:2245–50.
12. Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol 1983;97:213–9.
13. Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015 Apr 1.
14. Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000;21:363–92.
15. Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
16. Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2012;18:146–70.
17. Rosenfield RL. The polycystic ovary morphology-polycystic ovary syndrome spectrum. J Pediatr Adolesc Gynecol 2015;28:412–9.
18. Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010;16:51–64.
19. Chen WC, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol 2009;1:81–6.
20. Hawryluk EB, English JC 3rd. Female adolescent hair disorders. J Pediatr Adolesc Gynecol 2009;22:271–81.
21. Souter I, Sanchez A, Perez M, et al. The prevalence of androgen excess among patients with minimal unwanted hair growth. Am J Obstet Gynecol 2004;191:1914–20.
22. Di Fede G, Mansueto P, Pepe G, et al. High prevalence of polycystic ovary syndrome in women with mild hirsutism and no other significant clinical symptoms. Fertil Steril 2010; 94:194–7.
23. Chan CNJ, Haines CJ, Chow CCF, et al. Polycystic ovarian syndrome in Hong Kong Chinese women: patient characteristics and diagnostic criteria. Hong Kong Med J 2005;11:336–41.
24. Lucky AW, Biro FM, Simbartl LA, et al. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr 1997;130:30–9.
25. Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics 2013;131:S163–S186.
26. Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003;88:4682–8.
27. Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201.e1–5.
28. Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics 2015;136:1154–65.
29. Cho LW, Jayagopal V, Kilpatrick ES, Holding S. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann Clin Biochem 2006;43(Pt 3):217–9.
30. Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Sperling M, editor. Pediatric Endocrinology. 4th ed. Philadelphia: Elsevier; 2014:569–663.
31. Givens JR. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am 1988;17:771–83.
32. Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 1998;95:14956–60.
33. Amato P, Simpson JL. The genetics of polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2005;18:707–18.
34. Prapas N, Karkanaki A, Prapas I, et al. Genetics of polycystic ovary syndrome. Hippokratia 2009;13:216–23.
35. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980;50:113–6.
36. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030.
37. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800.
38. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983;57:356–9.
39. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–74.
40. Gambineri A, Pelusi C, Vicennati V, et al. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–96.
41. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr 2001;138:38–44.
42. Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998;83:2001–5.
43. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12:351–61.
44. McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714–22.
45. McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;92:430–6.
46. Knudsen KL, Blank SK, Burt Solorzano C, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring) 2010;18:2118–24.
47. Venturoli S, Porcu E, Fabbri R, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80.
48. Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 2006;91:3786–90.
49. Fruzzetti F, Campagna AM, Perini D, Carmina E. Ovarian volume in normal and hyperandrogenic adolescent women. Fertil Steril 2015;104:196–9.
50. Fearnley EJ, Marquart L, Spurdle AB, et al, the Australian Ovarian Cancer Study Group and the Australian National Endometrial Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Austrialian case-control study. Cancer Causes Control 2010;21:2303–8.
51. Legro RS, Arslanian SA, Ehrmann DA, et al; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565-92.
52. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab 2013;98:4655.
53. Lass N, Kleber M, Winkel K, et al. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab 2011;96:3533.
54. Costello M, Eden J. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003;79:1–13.
55. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951–3.
56. Auble B, Elder D, Gross A, Hillman JB. Differences in the management of adolescents with polycystic ovary syndrome across pediatric specialties. J Pediatr Adolesc Gynecol 2013;26:234–8.
57. Martin KA, Chang J, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clincal practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
58. Geller DH, Pacaud D, Gordon CM, Misra M, for the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: emerging therapies: the use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS). Int J Ped Endocrinol 2011;2011:9.
59. Tfayli H, Ulnach JW, Lee S, et al. Drospirenon/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal and cardiovascular risk factors. J Clin Endocrinol Metab 2011;96:1311–9.
1. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome inthe Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999v;84:4006–11.
2. Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9.
3. Christensen SB, Black MH, Smith N, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100:470–7.
4. Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab 2013;98:3572–83.
5. Venturoli S, Porcu E, Fabbri R, et al. Menstrual irregularities in adolescents: hormonal pattern and ovarian morphology. Horm Res 1986;24:269–79.
6. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston: Blackwell Scientific; 1992.
7. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
8. Azziz R, Carmina E, Dewailly D, et al; Androgen Excess Society. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–45.
9. Treloar A, Boynton R, Benn B, Brown B. Variation of human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126.
10. Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol 1977;7:1–193.
11. Diaz A, Laufer MR, Breech LL; American Academy of Pediatrics Committee on Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Menstruation in girls and adolescents:using the menstrual cycle as a vital sign. Pediatrics 2006;118:2245–50.
12. Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol 1983;97:213–9.
13. Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015 Apr 1.
14. Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000;21:363–92.
15. Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
16. Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2012;18:146–70.
17. Rosenfield RL. The polycystic ovary morphology-polycystic ovary syndrome spectrum. J Pediatr Adolesc Gynecol 2015;28:412–9.
18. Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010;16:51–64.
19. Chen WC, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol 2009;1:81–6.
20. Hawryluk EB, English JC 3rd. Female adolescent hair disorders. J Pediatr Adolesc Gynecol 2009;22:271–81.
21. Souter I, Sanchez A, Perez M, et al. The prevalence of androgen excess among patients with minimal unwanted hair growth. Am J Obstet Gynecol 2004;191:1914–20.
22. Di Fede G, Mansueto P, Pepe G, et al. High prevalence of polycystic ovary syndrome in women with mild hirsutism and no other significant clinical symptoms. Fertil Steril 2010; 94:194–7.
23. Chan CNJ, Haines CJ, Chow CCF, et al. Polycystic ovarian syndrome in Hong Kong Chinese women: patient characteristics and diagnostic criteria. Hong Kong Med J 2005;11:336–41.
24. Lucky AW, Biro FM, Simbartl LA, et al. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr 1997;130:30–9.
25. Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics 2013;131:S163–S186.
26. Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003;88:4682–8.
27. Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201.e1–5.
28. Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics 2015;136:1154–65.
29. Cho LW, Jayagopal V, Kilpatrick ES, Holding S. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann Clin Biochem 2006;43(Pt 3):217–9.
30. Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Sperling M, editor. Pediatric Endocrinology. 4th ed. Philadelphia: Elsevier; 2014:569–663.
31. Givens JR. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am 1988;17:771–83.
32. Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 1998;95:14956–60.
33. Amato P, Simpson JL. The genetics of polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2005;18:707–18.
34. Prapas N, Karkanaki A, Prapas I, et al. Genetics of polycystic ovary syndrome. Hippokratia 2009;13:216–23.
35. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980;50:113–6.
36. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030.
37. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800.
38. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983;57:356–9.
39. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–74.
40. Gambineri A, Pelusi C, Vicennati V, et al. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–96.
41. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr 2001;138:38–44.
42. Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998;83:2001–5.
43. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12:351–61.
44. McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714–22.
45. McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;92:430–6.
46. Knudsen KL, Blank SK, Burt Solorzano C, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring) 2010;18:2118–24.
47. Venturoli S, Porcu E, Fabbri R, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80.
48. Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 2006;91:3786–90.
49. Fruzzetti F, Campagna AM, Perini D, Carmina E. Ovarian volume in normal and hyperandrogenic adolescent women. Fertil Steril 2015;104:196–9.
50. Fearnley EJ, Marquart L, Spurdle AB, et al, the Australian Ovarian Cancer Study Group and the Australian National Endometrial Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Austrialian case-control study. Cancer Causes Control 2010;21:2303–8.
51. Legro RS, Arslanian SA, Ehrmann DA, et al; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565-92.
52. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab 2013;98:4655.
53. Lass N, Kleber M, Winkel K, et al. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab 2011;96:3533.
54. Costello M, Eden J. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003;79:1–13.
55. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951–3.
56. Auble B, Elder D, Gross A, Hillman JB. Differences in the management of adolescents with polycystic ovary syndrome across pediatric specialties. J Pediatr Adolesc Gynecol 2013;26:234–8.
57. Martin KA, Chang J, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clincal practice guideline. J Clin Endocrinol Metab 2008;93:1105–20.
58. Geller DH, Pacaud D, Gordon CM, Misra M, for the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: emerging therapies: the use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS). Int J Ped Endocrinol 2011;2011:9.
59. Tfayli H, Ulnach JW, Lee S, et al. Drospirenon/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal and cardiovascular risk factors. J Clin Endocrinol Metab 2011;96:1311–9.
Robert E. Burke, MD, MS, Earns 2016 SHM Junior Investigator Award
The Society of Hospital Medicine (SHM) proudly names Robert E. Burke, MD, MS, assistant chief of hospital medicine at Denver VA Medical Center, as the recipient of the 2016 Junior Investigator Award. He will receive the award at HM16 in San Diego.
Dr. Burke’s research focuses on improving transitional care outcomes for older adults. An academic hospitalist and health services researcher, Dr. Burke is working toward becoming a nationally recognized outcomes researcher and implementation scientist working in hospitals and post-acute-care (PAC) facilities.
In addition to this award, Dr. Burke also received the 2015 Career Development Award from SHM, the Alliance for Academic Internal Medicine, and the Association of Specialty Professors in support of the Grants for Early Medical/Surgical Subspecialists’ Transition to Aging Research Program (GEMSSTAR).
The Junior Investigator Award recognizes talented early-stage investigators in the first five years of a faculty position. Criteria for selection include the impact the research may have on hospital medicine, career achievements and milestones (e.g., abstracts, peer-reviewed publications, intra- and extramural grant funding), and engagement with SHM. TH
Brett Radler is SHM’s communications coordinator.
The Society of Hospital Medicine (SHM) proudly names Robert E. Burke, MD, MS, assistant chief of hospital medicine at Denver VA Medical Center, as the recipient of the 2016 Junior Investigator Award. He will receive the award at HM16 in San Diego.
Dr. Burke’s research focuses on improving transitional care outcomes for older adults. An academic hospitalist and health services researcher, Dr. Burke is working toward becoming a nationally recognized outcomes researcher and implementation scientist working in hospitals and post-acute-care (PAC) facilities.
In addition to this award, Dr. Burke also received the 2015 Career Development Award from SHM, the Alliance for Academic Internal Medicine, and the Association of Specialty Professors in support of the Grants for Early Medical/Surgical Subspecialists’ Transition to Aging Research Program (GEMSSTAR).
The Junior Investigator Award recognizes talented early-stage investigators in the first five years of a faculty position. Criteria for selection include the impact the research may have on hospital medicine, career achievements and milestones (e.g., abstracts, peer-reviewed publications, intra- and extramural grant funding), and engagement with SHM. TH
Brett Radler is SHM’s communications coordinator.
The Society of Hospital Medicine (SHM) proudly names Robert E. Burke, MD, MS, assistant chief of hospital medicine at Denver VA Medical Center, as the recipient of the 2016 Junior Investigator Award. He will receive the award at HM16 in San Diego.
Dr. Burke’s research focuses on improving transitional care outcomes for older adults. An academic hospitalist and health services researcher, Dr. Burke is working toward becoming a nationally recognized outcomes researcher and implementation scientist working in hospitals and post-acute-care (PAC) facilities.
In addition to this award, Dr. Burke also received the 2015 Career Development Award from SHM, the Alliance for Academic Internal Medicine, and the Association of Specialty Professors in support of the Grants for Early Medical/Surgical Subspecialists’ Transition to Aging Research Program (GEMSSTAR).
The Junior Investigator Award recognizes talented early-stage investigators in the first five years of a faculty position. Criteria for selection include the impact the research may have on hospital medicine, career achievements and milestones (e.g., abstracts, peer-reviewed publications, intra- and extramural grant funding), and engagement with SHM. TH
Brett Radler is SHM’s communications coordinator.