User login
Your significant role in modifying risk factors for coronary artery disease and managing problems subsequently
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
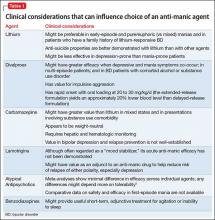
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
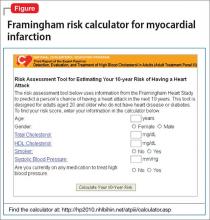
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
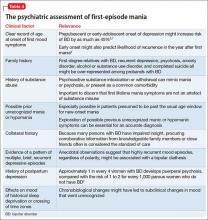
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
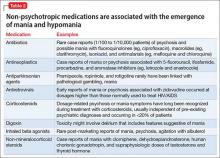
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
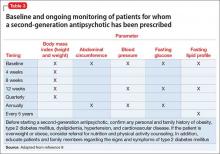
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
Malnourished and psychotic, and found incompetent to stand trial
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
What to do when your depressed patient develops mania
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
Obstructive sleep apnea: Evaluation and management
Patients with undiagnosed and untreated obstructive sleep apnea (OSA) are at increased risk for excessive daytime sleepiness, as well as cardiovascular and cerebrovascular complications. This review describes how best to address the symptoms and complications that make OSA a public health concern. To read the full article, go to Clinician Reviews: http://www.clinicianreviews.com/cecme/cecme-activities/article/obstructive-sleep-apnea-evaluation-management/b511b960cab855040da9165a39ab5eb8.html?tx_ttnews%5BsViewPointer%5D=1.
Patients with undiagnosed and untreated obstructive sleep apnea (OSA) are at increased risk for excessive daytime sleepiness, as well as cardiovascular and cerebrovascular complications. This review describes how best to address the symptoms and complications that make OSA a public health concern. To read the full article, go to Clinician Reviews: http://www.clinicianreviews.com/cecme/cecme-activities/article/obstructive-sleep-apnea-evaluation-management/b511b960cab855040da9165a39ab5eb8.html?tx_ttnews%5BsViewPointer%5D=1.
Patients with undiagnosed and untreated obstructive sleep apnea (OSA) are at increased risk for excessive daytime sleepiness, as well as cardiovascular and cerebrovascular complications. This review describes how best to address the symptoms and complications that make OSA a public health concern. To read the full article, go to Clinician Reviews: http://www.clinicianreviews.com/cecme/cecme-activities/article/obstructive-sleep-apnea-evaluation-management/b511b960cab855040da9165a39ab5eb8.html?tx_ttnews%5BsViewPointer%5D=1.
The Use of a Telehealth Clinic to Support Patients Receiving Radiation Therapy at a Site Distant From Their PCP
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these 48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these 48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these 48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Urine drug screens: When might a test result be false-positive?
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
Needed: A biopsychosocial ‘therapeutic placenta’ for people with schizophrenia
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
Online Refill Reduces Time Without Statins in Diabetes Patients
NEW YORK - Diabetes patients in the Kaiser Health System spent more days with their prescribed statins on hand if they used Kaiser's online refill tool, a study shows.
The researchers looked specifically at racial and ethnic minorities and found that while these groups had poorer medication adherence than white patients before using the online refills, using the online refills conferred the same benefit for every group.
"Many other systems are implementing online portals other than Kaiser," said lead author Dr. Courtney Lyles in a phone interview. "The key message that our study puts forward is that providing tools to help with medication adherence is critical."
Lyles, an affiliate investigator at the Kaiser Permanente Division of Research and assistant professor at the University of California, San Francisco, and her colleagues studied patients in the type 2 diabetes registry in Kaiser Permanente Northern California between 2006 and 2012.
All had access to online patient tools that allow for viewing medical history and visit summaries, viewing laboratory results, scheduling appointments, sending and receiving secure email messages with providers, and requesting prescription refills.
Patients could request a prescription refill online and receive the medication by mail or pick it up in person at the pharmacy.
The researchers compared diabetes patients with statin prescriptions who used the online refill tool to those who did not, and compared individual patients before and after they started using the online refills.
According to pharmacy data, at baseline, whites on average spent about 12% of the time without having the medicine on hand, compared to about 16% of the time for blacks and Latinos, and about 13% of the time for Asians and Filipinos.
But when patients switched from other refill habits to exclusively online refills, they reduced their time without statins by more than 3%, the researchers reported in an article online September 2 in the Journal of the American Medical Informatics Association.
Racial and ethnic minority patients are less likely to use online portals, even after adjusting for Internet access or use of Internet in everyday life, Lyles said.
"There is a concern that if particular populations are less likely to use these portals, then perhaps the benefits will be differential," she said. "But we found that racial minorities are less likely to use it overall, but have the same benefit in adherence."
Online portals do appear to make prescription refills more convenient, and people using the portals reduced the number of days without medication by 10 or 15 days per year, according to Dr. Jessica S. Ancker, a health care policy researcher at Weill Cornell Medical College in New York City, who was not part of the new study.
Healthcare organizations started offering online portals as a "leap of faith," assuming they would improve patient experiences, but without any concrete proof, she said.
"A new wave of research measures whether it's improving things," she said.
The Agency for Healthcare Research and Quality funded this research.
NEW YORK - Diabetes patients in the Kaiser Health System spent more days with their prescribed statins on hand if they used Kaiser's online refill tool, a study shows.
The researchers looked specifically at racial and ethnic minorities and found that while these groups had poorer medication adherence than white patients before using the online refills, using the online refills conferred the same benefit for every group.
"Many other systems are implementing online portals other than Kaiser," said lead author Dr. Courtney Lyles in a phone interview. "The key message that our study puts forward is that providing tools to help with medication adherence is critical."
Lyles, an affiliate investigator at the Kaiser Permanente Division of Research and assistant professor at the University of California, San Francisco, and her colleagues studied patients in the type 2 diabetes registry in Kaiser Permanente Northern California between 2006 and 2012.
All had access to online patient tools that allow for viewing medical history and visit summaries, viewing laboratory results, scheduling appointments, sending and receiving secure email messages with providers, and requesting prescription refills.
Patients could request a prescription refill online and receive the medication by mail or pick it up in person at the pharmacy.
The researchers compared diabetes patients with statin prescriptions who used the online refill tool to those who did not, and compared individual patients before and after they started using the online refills.
According to pharmacy data, at baseline, whites on average spent about 12% of the time without having the medicine on hand, compared to about 16% of the time for blacks and Latinos, and about 13% of the time for Asians and Filipinos.
But when patients switched from other refill habits to exclusively online refills, they reduced their time without statins by more than 3%, the researchers reported in an article online September 2 in the Journal of the American Medical Informatics Association.
Racial and ethnic minority patients are less likely to use online portals, even after adjusting for Internet access or use of Internet in everyday life, Lyles said.
"There is a concern that if particular populations are less likely to use these portals, then perhaps the benefits will be differential," she said. "But we found that racial minorities are less likely to use it overall, but have the same benefit in adherence."
Online portals do appear to make prescription refills more convenient, and people using the portals reduced the number of days without medication by 10 or 15 days per year, according to Dr. Jessica S. Ancker, a health care policy researcher at Weill Cornell Medical College in New York City, who was not part of the new study.
Healthcare organizations started offering online portals as a "leap of faith," assuming they would improve patient experiences, but without any concrete proof, she said.
"A new wave of research measures whether it's improving things," she said.
The Agency for Healthcare Research and Quality funded this research.
NEW YORK - Diabetes patients in the Kaiser Health System spent more days with their prescribed statins on hand if they used Kaiser's online refill tool, a study shows.
The researchers looked specifically at racial and ethnic minorities and found that while these groups had poorer medication adherence than white patients before using the online refills, using the online refills conferred the same benefit for every group.
"Many other systems are implementing online portals other than Kaiser," said lead author Dr. Courtney Lyles in a phone interview. "The key message that our study puts forward is that providing tools to help with medication adherence is critical."
Lyles, an affiliate investigator at the Kaiser Permanente Division of Research and assistant professor at the University of California, San Francisco, and her colleagues studied patients in the type 2 diabetes registry in Kaiser Permanente Northern California between 2006 and 2012.
All had access to online patient tools that allow for viewing medical history and visit summaries, viewing laboratory results, scheduling appointments, sending and receiving secure email messages with providers, and requesting prescription refills.
Patients could request a prescription refill online and receive the medication by mail or pick it up in person at the pharmacy.
The researchers compared diabetes patients with statin prescriptions who used the online refill tool to those who did not, and compared individual patients before and after they started using the online refills.
According to pharmacy data, at baseline, whites on average spent about 12% of the time without having the medicine on hand, compared to about 16% of the time for blacks and Latinos, and about 13% of the time for Asians and Filipinos.
But when patients switched from other refill habits to exclusively online refills, they reduced their time without statins by more than 3%, the researchers reported in an article online September 2 in the Journal of the American Medical Informatics Association.
Racial and ethnic minority patients are less likely to use online portals, even after adjusting for Internet access or use of Internet in everyday life, Lyles said.
"There is a concern that if particular populations are less likely to use these portals, then perhaps the benefits will be differential," she said. "But we found that racial minorities are less likely to use it overall, but have the same benefit in adherence."
Online portals do appear to make prescription refills more convenient, and people using the portals reduced the number of days without medication by 10 or 15 days per year, according to Dr. Jessica S. Ancker, a health care policy researcher at Weill Cornell Medical College in New York City, who was not part of the new study.
Healthcare organizations started offering online portals as a "leap of faith," assuming they would improve patient experiences, but without any concrete proof, she said.
"A new wave of research measures whether it's improving things," she said.
The Agency for Healthcare Research and Quality funded this research.
Pregnant cancer patients: Start treatment ASAP

Photo by Nina Matthews
VIENNA—Women who are pregnant when diagnosed with cancer should carry their child to term but start cancer treatment immediately, according to researchers.
A study of young children suggested that exposure to cancer treatment in utero did not have detrimental effects on a child’s mental development or heart function.
Premature delivery, on the other hand, was associated with delayed cognitive development.
“Our results show that fear of cancer treatment is no reason to terminate a pregnancy, that maternal treatment should not be delayed, and that chemotherapy can be given,” said Frederic Amant, MD, PhD, of University Hospitals Leuven in Belgium.
“The study also shows that children suffer more from prematurity than from chemotherapy, so avoiding prematurity is more important than avoiding chemotherapy.”
Dr Amant presented these findings at the 2015 European Cancer Congress. The study was also published in NEJM.
The study included 129 children born to mothers with cancer, matched with 129 children of the same gestational age who were born to mothers unaffected by cancer.
The most common malignancies were breast (n=69) and hematologic cancers. This included acute myeloid leukemia (n=4), acute lymphoblastic leukemia (n=1), chronic myeloid leukemia (n=1), Hodgkin lymphoma (n=8), and non-Hodgkin lymphoma (n=6).
The researchers assessed the children’s general health and mental development when they were 18 months and 3 years old. At the age of 3, 47 of the children also had their heart function checked with electrocardiograms and echocardiography.
Ninety-six children (74.4%) were exposed to chemotherapy (alone or in combination with other treatment) before birth, 11 children (8.5%) were exposed to radiotherapy (alone or in combination), 13 (10.1%) were exposed to surgery alone, and 2 (1.6%) were exposed to drugs other than chemotherapeutic agents. Fourteen (10.9%) mothers did not receive cancer treatment during pregnancy.
Mental development
“Compared to the control group of children, we found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment,” Dr Amant said. “Nor was the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, related to the outcome of the children.”
To measure cognitive development, the researchers used the Bayley Scales of Infant Development. The median score was 101 (range, 56-145) in children exposed to cancer treatment and 100 (range, 50-145) in unexposed children.
When compared to controls, there was no significant difference in Bayley II or III score for all children born to mothers with cancer (P=0.08), children exposed to any chemotherapy (P=0.43), children exposed to anthracyclines (P=0.43), children exposed to taxanes (P=0.57), children exposed to platinum derivatives (P=0.95), children exposed to radiotherapy (P=0.69), children exposed to surgery alone (P=0.13), and children whose mothers did not undergo treatment (P=0.08).
Premature birth
Conversely, Bayley scores tended to increase by an average of 2.9 points for every week in gestational age. This was after the researchers controlled for a child’s age, gender, country, ethnicity, and parental education level.
“Delayed development of mental processes appeared to be related to premature birth,” Dr Amant said.
Premature birth was more frequent among children born to mothers with cancer, regardless of whether or not they received prenatal treatment, than in the general population in the countries participating in this study (Belgium, The Netherlands, Italy, and the Czech Republic).
The children born to mothers with cancer had a median gestational age of 36 weeks, ranging from 27 to 41 weeks. Seventy-nine (61.2%) children were born preterm, compared to 7% to 8% in the general population.
“In most cases, they were born prematurely due to a medical decision to induce preterm so as to continue cancer treatment after the delivery,” Dr Amant said.
“In some cases, preterm delivery was spontaneous, and it is possible that cancer treatment plays a role in this. But we do not know what exactly triggers preterm delivery. It could be that chemotherapy induces preterm contractions or vaginal inflammation with preterm rupture of the membranes.”
Cardiac function
The researchers assessed cardiac function in 47 three-year-olds whose mothers had cancer and 47 control children.
There were no significant differences between the exposed and control children for most measures of cardiac function, such as heart rate, ejection fraction, fractional shortening, global longitudinal strain, and circumferential strain.
The only exceptions were diastolic blood pressure, which was higher among exposed children (P=0.001), and tissue Doppler imaging measurements of the basal segment of the interventricular septum. There were higher mean peak systolic and early diastolic velocities in the control group than the exposed group (P=0.003 for both comparisons).
The researchers noted, however, that the differences in tissue Doppler velocities were not present when comparing the control group and the 26 children who were exposed to anthracyclines.
Next steps
Last year, Dr Amant reported similarly favorable results in 54 children exposed to chemotherapy or radiation in utero. The new report is a continuation of this work.
“These latest results are, again, reassuring,” Dr Amant said. “But given that we have a larger group of children . . . , the current data are much more robust.”
However, he also pointed out that this study has some limitations.
“Our data include many types of chemotherapy, but we cannot guarantee that all types of chemotherapy are safe,” Dr Amant said. “We need to look at larger numbers of children and larger numbers exposed to each drug in order to be able to document the potential effects of individual drugs.”
“In addition, we cannot extrapolate to newer drugs, including targeted drugs. We need longer follow-up to see if there are any long-term toxic effects in cases where cisplatin was administered before birth.”
“For these reasons, we will continue to follow these children until the age of 18 years, and we will enlarge the group. This will allow us to document longer-term effects and to draw conclusions for specific drugs. In addition, we will investigate to what extent anticancer drugs are diluted in the body during pregnancy and also [examine] the psycho-emotional needs of mothers and their partners.” ![]()

Photo by Nina Matthews
VIENNA—Women who are pregnant when diagnosed with cancer should carry their child to term but start cancer treatment immediately, according to researchers.
A study of young children suggested that exposure to cancer treatment in utero did not have detrimental effects on a child’s mental development or heart function.
Premature delivery, on the other hand, was associated with delayed cognitive development.
“Our results show that fear of cancer treatment is no reason to terminate a pregnancy, that maternal treatment should not be delayed, and that chemotherapy can be given,” said Frederic Amant, MD, PhD, of University Hospitals Leuven in Belgium.
“The study also shows that children suffer more from prematurity than from chemotherapy, so avoiding prematurity is more important than avoiding chemotherapy.”
Dr Amant presented these findings at the 2015 European Cancer Congress. The study was also published in NEJM.
The study included 129 children born to mothers with cancer, matched with 129 children of the same gestational age who were born to mothers unaffected by cancer.
The most common malignancies were breast (n=69) and hematologic cancers. This included acute myeloid leukemia (n=4), acute lymphoblastic leukemia (n=1), chronic myeloid leukemia (n=1), Hodgkin lymphoma (n=8), and non-Hodgkin lymphoma (n=6).
The researchers assessed the children’s general health and mental development when they were 18 months and 3 years old. At the age of 3, 47 of the children also had their heart function checked with electrocardiograms and echocardiography.
Ninety-six children (74.4%) were exposed to chemotherapy (alone or in combination with other treatment) before birth, 11 children (8.5%) were exposed to radiotherapy (alone or in combination), 13 (10.1%) were exposed to surgery alone, and 2 (1.6%) were exposed to drugs other than chemotherapeutic agents. Fourteen (10.9%) mothers did not receive cancer treatment during pregnancy.
Mental development
“Compared to the control group of children, we found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment,” Dr Amant said. “Nor was the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, related to the outcome of the children.”
To measure cognitive development, the researchers used the Bayley Scales of Infant Development. The median score was 101 (range, 56-145) in children exposed to cancer treatment and 100 (range, 50-145) in unexposed children.
When compared to controls, there was no significant difference in Bayley II or III score for all children born to mothers with cancer (P=0.08), children exposed to any chemotherapy (P=0.43), children exposed to anthracyclines (P=0.43), children exposed to taxanes (P=0.57), children exposed to platinum derivatives (P=0.95), children exposed to radiotherapy (P=0.69), children exposed to surgery alone (P=0.13), and children whose mothers did not undergo treatment (P=0.08).
Premature birth
Conversely, Bayley scores tended to increase by an average of 2.9 points for every week in gestational age. This was after the researchers controlled for a child’s age, gender, country, ethnicity, and parental education level.
“Delayed development of mental processes appeared to be related to premature birth,” Dr Amant said.
Premature birth was more frequent among children born to mothers with cancer, regardless of whether or not they received prenatal treatment, than in the general population in the countries participating in this study (Belgium, The Netherlands, Italy, and the Czech Republic).
The children born to mothers with cancer had a median gestational age of 36 weeks, ranging from 27 to 41 weeks. Seventy-nine (61.2%) children were born preterm, compared to 7% to 8% in the general population.
“In most cases, they were born prematurely due to a medical decision to induce preterm so as to continue cancer treatment after the delivery,” Dr Amant said.
“In some cases, preterm delivery was spontaneous, and it is possible that cancer treatment plays a role in this. But we do not know what exactly triggers preterm delivery. It could be that chemotherapy induces preterm contractions or vaginal inflammation with preterm rupture of the membranes.”
Cardiac function
The researchers assessed cardiac function in 47 three-year-olds whose mothers had cancer and 47 control children.
There were no significant differences between the exposed and control children for most measures of cardiac function, such as heart rate, ejection fraction, fractional shortening, global longitudinal strain, and circumferential strain.
The only exceptions were diastolic blood pressure, which was higher among exposed children (P=0.001), and tissue Doppler imaging measurements of the basal segment of the interventricular septum. There were higher mean peak systolic and early diastolic velocities in the control group than the exposed group (P=0.003 for both comparisons).
The researchers noted, however, that the differences in tissue Doppler velocities were not present when comparing the control group and the 26 children who were exposed to anthracyclines.
Next steps
Last year, Dr Amant reported similarly favorable results in 54 children exposed to chemotherapy or radiation in utero. The new report is a continuation of this work.
“These latest results are, again, reassuring,” Dr Amant said. “But given that we have a larger group of children . . . , the current data are much more robust.”
However, he also pointed out that this study has some limitations.
“Our data include many types of chemotherapy, but we cannot guarantee that all types of chemotherapy are safe,” Dr Amant said. “We need to look at larger numbers of children and larger numbers exposed to each drug in order to be able to document the potential effects of individual drugs.”
“In addition, we cannot extrapolate to newer drugs, including targeted drugs. We need longer follow-up to see if there are any long-term toxic effects in cases where cisplatin was administered before birth.”
“For these reasons, we will continue to follow these children until the age of 18 years, and we will enlarge the group. This will allow us to document longer-term effects and to draw conclusions for specific drugs. In addition, we will investigate to what extent anticancer drugs are diluted in the body during pregnancy and also [examine] the psycho-emotional needs of mothers and their partners.” ![]()

Photo by Nina Matthews
VIENNA—Women who are pregnant when diagnosed with cancer should carry their child to term but start cancer treatment immediately, according to researchers.
A study of young children suggested that exposure to cancer treatment in utero did not have detrimental effects on a child’s mental development or heart function.
Premature delivery, on the other hand, was associated with delayed cognitive development.
“Our results show that fear of cancer treatment is no reason to terminate a pregnancy, that maternal treatment should not be delayed, and that chemotherapy can be given,” said Frederic Amant, MD, PhD, of University Hospitals Leuven in Belgium.
“The study also shows that children suffer more from prematurity than from chemotherapy, so avoiding prematurity is more important than avoiding chemotherapy.”
Dr Amant presented these findings at the 2015 European Cancer Congress. The study was also published in NEJM.
The study included 129 children born to mothers with cancer, matched with 129 children of the same gestational age who were born to mothers unaffected by cancer.
The most common malignancies were breast (n=69) and hematologic cancers. This included acute myeloid leukemia (n=4), acute lymphoblastic leukemia (n=1), chronic myeloid leukemia (n=1), Hodgkin lymphoma (n=8), and non-Hodgkin lymphoma (n=6).
The researchers assessed the children’s general health and mental development when they were 18 months and 3 years old. At the age of 3, 47 of the children also had their heart function checked with electrocardiograms and echocardiography.
Ninety-six children (74.4%) were exposed to chemotherapy (alone or in combination with other treatment) before birth, 11 children (8.5%) were exposed to radiotherapy (alone or in combination), 13 (10.1%) were exposed to surgery alone, and 2 (1.6%) were exposed to drugs other than chemotherapeutic agents. Fourteen (10.9%) mothers did not receive cancer treatment during pregnancy.
Mental development
“Compared to the control group of children, we found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment,” Dr Amant said. “Nor was the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, related to the outcome of the children.”
To measure cognitive development, the researchers used the Bayley Scales of Infant Development. The median score was 101 (range, 56-145) in children exposed to cancer treatment and 100 (range, 50-145) in unexposed children.
When compared to controls, there was no significant difference in Bayley II or III score for all children born to mothers with cancer (P=0.08), children exposed to any chemotherapy (P=0.43), children exposed to anthracyclines (P=0.43), children exposed to taxanes (P=0.57), children exposed to platinum derivatives (P=0.95), children exposed to radiotherapy (P=0.69), children exposed to surgery alone (P=0.13), and children whose mothers did not undergo treatment (P=0.08).
Premature birth
Conversely, Bayley scores tended to increase by an average of 2.9 points for every week in gestational age. This was after the researchers controlled for a child’s age, gender, country, ethnicity, and parental education level.
“Delayed development of mental processes appeared to be related to premature birth,” Dr Amant said.
Premature birth was more frequent among children born to mothers with cancer, regardless of whether or not they received prenatal treatment, than in the general population in the countries participating in this study (Belgium, The Netherlands, Italy, and the Czech Republic).
The children born to mothers with cancer had a median gestational age of 36 weeks, ranging from 27 to 41 weeks. Seventy-nine (61.2%) children were born preterm, compared to 7% to 8% in the general population.
“In most cases, they were born prematurely due to a medical decision to induce preterm so as to continue cancer treatment after the delivery,” Dr Amant said.
“In some cases, preterm delivery was spontaneous, and it is possible that cancer treatment plays a role in this. But we do not know what exactly triggers preterm delivery. It could be that chemotherapy induces preterm contractions or vaginal inflammation with preterm rupture of the membranes.”
Cardiac function
The researchers assessed cardiac function in 47 three-year-olds whose mothers had cancer and 47 control children.
There were no significant differences between the exposed and control children for most measures of cardiac function, such as heart rate, ejection fraction, fractional shortening, global longitudinal strain, and circumferential strain.
The only exceptions were diastolic blood pressure, which was higher among exposed children (P=0.001), and tissue Doppler imaging measurements of the basal segment of the interventricular septum. There were higher mean peak systolic and early diastolic velocities in the control group than the exposed group (P=0.003 for both comparisons).
The researchers noted, however, that the differences in tissue Doppler velocities were not present when comparing the control group and the 26 children who were exposed to anthracyclines.
Next steps
Last year, Dr Amant reported similarly favorable results in 54 children exposed to chemotherapy or radiation in utero. The new report is a continuation of this work.
“These latest results are, again, reassuring,” Dr Amant said. “But given that we have a larger group of children . . . , the current data are much more robust.”
However, he also pointed out that this study has some limitations.
“Our data include many types of chemotherapy, but we cannot guarantee that all types of chemotherapy are safe,” Dr Amant said. “We need to look at larger numbers of children and larger numbers exposed to each drug in order to be able to document the potential effects of individual drugs.”
“In addition, we cannot extrapolate to newer drugs, including targeted drugs. We need longer follow-up to see if there are any long-term toxic effects in cases where cisplatin was administered before birth.”
“For these reasons, we will continue to follow these children until the age of 18 years, and we will enlarge the group. This will allow us to document longer-term effects and to draw conclusions for specific drugs. In addition, we will investigate to what extent anticancer drugs are diluted in the body during pregnancy and also [examine] the psycho-emotional needs of mothers and their partners.” ![]()
Combo demonstrates potential in MM

ROME—Early results of a pilot study indicate that a 3-agent combination regimen can produce responses in patients with relapsed or refractory multiple myeloma (MM).
The treatment consists of carfilzomib, dexamethasone, and pelareorep (Reolysin), a proprietary isolate of human reovirus (Type 3 Dearing strain).
All 8 evaluable patients treated with this regimen experienced an objective response, although 1 patient later progressed.
The investigators said the regimen has been relatively well tolerated, but most patients experience flu-like symptoms over the first week of treatment. And cytopenias, especially thrombocytopenia, are common.
Douglas Sborov, MD, of The Ohio State University in Columbus, and his colleagues presented this research at the 15th International Myeloma Workshop (poster #379).
The investigators noted that this is the first time a pelareorep-based combination has been tested in relapsed MM patients. A previous single-agent study indicated that pelareorep was well tolerated, and preclinical research has shown that reovirus and carfilzomib synergize to kill MM cells.
To expand upon these results, Dr Sborov and colleagues began testing pelareorep with carfilzomib and dexamethasone in patients with relapsed/refractory MM. The ongoing study, known as NCI-9603, is sponsored by the National Cancer Institute.
Of the 8 patients enrolled thus far, 5 had intermediate- or high-risk disease at baseline, and 1 patient was dialysis-dependent. The median age was 63 (range, 43-70).
Patients had received a median of 2 prior lines of therapy (range, 1-6) and a median of 4 prior treatments (range, 2-8). All of the patients had received lenalidomide, 1 had received carfilzomib, and 4 were refractory to bortezomib.
For the current study, patients were assigned to receive dexamethasone, carfilzomib over 10 minutes, and pelareorep over 60 minutes on days 1, 2, 8, 9, 15, and 16. Treatment is set to repeat every 28 days in the absence of disease progression or unacceptable toxicity.
Patients who achieve a minimal response or better may reduce treatment to days 1, 8, and 15 after 4 courses and to days 1 and 15 after 12 courses.
Treatment results
The investigators said the treatment produced a significant (P=0.005) increase in caspase-3, a marker associated with apoptotic cell death.
The combination was also associated with an increased infiltration of CD8+ T cells and the significant (P=0.005) upregulation of PD-L1, suggesting the addition of a PD-1 or PD-L1 inhibitor might further optimize the regimen.
All 8 patients experienced an objective response to treatment, as measured by changes in blood monoclonal protein. Two patients had a very good partial response, 3 had a partial response, and 3 had a minor response.
Five of the patients remain on study. The dialysis-dependent patient discontinued treatment due to progression after 3 treatment cycles. And 2 patients discontinued treatment due to dose-limiting toxicities after 2 doses.
One of the patients with dose-limiting toxicities had grade 4 myocarditis, grade 3 left ventricular dysfunction, and grade 4 respiratory failure possibly attributable to pelareorep and carfilzomib. The other patient had lower gastrointestinal bleeding that was not attributed to treatment.
Adverse events in cycle 1 that were possibly, likely, or definitely attributed to treatment included hypertension (n=5), thrombocytopenia (n=4), anemia (n=4), dyspnea on exertion (n=4), fatigue (n=4), myalgia (n=3), fever (n=2), leukopenia (n=2), lymphopenia (n=2), nausea (n=2), and diarrhea (n=2).
For more details, visit the Oncolytics Biotech Inc. website to view the poster. Oncolytics is the company developing pelareorep. ![]()

ROME—Early results of a pilot study indicate that a 3-agent combination regimen can produce responses in patients with relapsed or refractory multiple myeloma (MM).
The treatment consists of carfilzomib, dexamethasone, and pelareorep (Reolysin), a proprietary isolate of human reovirus (Type 3 Dearing strain).
All 8 evaluable patients treated with this regimen experienced an objective response, although 1 patient later progressed.
The investigators said the regimen has been relatively well tolerated, but most patients experience flu-like symptoms over the first week of treatment. And cytopenias, especially thrombocytopenia, are common.
Douglas Sborov, MD, of The Ohio State University in Columbus, and his colleagues presented this research at the 15th International Myeloma Workshop (poster #379).
The investigators noted that this is the first time a pelareorep-based combination has been tested in relapsed MM patients. A previous single-agent study indicated that pelareorep was well tolerated, and preclinical research has shown that reovirus and carfilzomib synergize to kill MM cells.
To expand upon these results, Dr Sborov and colleagues began testing pelareorep with carfilzomib and dexamethasone in patients with relapsed/refractory MM. The ongoing study, known as NCI-9603, is sponsored by the National Cancer Institute.
Of the 8 patients enrolled thus far, 5 had intermediate- or high-risk disease at baseline, and 1 patient was dialysis-dependent. The median age was 63 (range, 43-70).
Patients had received a median of 2 prior lines of therapy (range, 1-6) and a median of 4 prior treatments (range, 2-8). All of the patients had received lenalidomide, 1 had received carfilzomib, and 4 were refractory to bortezomib.
For the current study, patients were assigned to receive dexamethasone, carfilzomib over 10 minutes, and pelareorep over 60 minutes on days 1, 2, 8, 9, 15, and 16. Treatment is set to repeat every 28 days in the absence of disease progression or unacceptable toxicity.
Patients who achieve a minimal response or better may reduce treatment to days 1, 8, and 15 after 4 courses and to days 1 and 15 after 12 courses.
Treatment results
The investigators said the treatment produced a significant (P=0.005) increase in caspase-3, a marker associated with apoptotic cell death.
The combination was also associated with an increased infiltration of CD8+ T cells and the significant (P=0.005) upregulation of PD-L1, suggesting the addition of a PD-1 or PD-L1 inhibitor might further optimize the regimen.
All 8 patients experienced an objective response to treatment, as measured by changes in blood monoclonal protein. Two patients had a very good partial response, 3 had a partial response, and 3 had a minor response.
Five of the patients remain on study. The dialysis-dependent patient discontinued treatment due to progression after 3 treatment cycles. And 2 patients discontinued treatment due to dose-limiting toxicities after 2 doses.
One of the patients with dose-limiting toxicities had grade 4 myocarditis, grade 3 left ventricular dysfunction, and grade 4 respiratory failure possibly attributable to pelareorep and carfilzomib. The other patient had lower gastrointestinal bleeding that was not attributed to treatment.
Adverse events in cycle 1 that were possibly, likely, or definitely attributed to treatment included hypertension (n=5), thrombocytopenia (n=4), anemia (n=4), dyspnea on exertion (n=4), fatigue (n=4), myalgia (n=3), fever (n=2), leukopenia (n=2), lymphopenia (n=2), nausea (n=2), and diarrhea (n=2).
For more details, visit the Oncolytics Biotech Inc. website to view the poster. Oncolytics is the company developing pelareorep. ![]()

ROME—Early results of a pilot study indicate that a 3-agent combination regimen can produce responses in patients with relapsed or refractory multiple myeloma (MM).
The treatment consists of carfilzomib, dexamethasone, and pelareorep (Reolysin), a proprietary isolate of human reovirus (Type 3 Dearing strain).
All 8 evaluable patients treated with this regimen experienced an objective response, although 1 patient later progressed.
The investigators said the regimen has been relatively well tolerated, but most patients experience flu-like symptoms over the first week of treatment. And cytopenias, especially thrombocytopenia, are common.
Douglas Sborov, MD, of The Ohio State University in Columbus, and his colleagues presented this research at the 15th International Myeloma Workshop (poster #379).
The investigators noted that this is the first time a pelareorep-based combination has been tested in relapsed MM patients. A previous single-agent study indicated that pelareorep was well tolerated, and preclinical research has shown that reovirus and carfilzomib synergize to kill MM cells.
To expand upon these results, Dr Sborov and colleagues began testing pelareorep with carfilzomib and dexamethasone in patients with relapsed/refractory MM. The ongoing study, known as NCI-9603, is sponsored by the National Cancer Institute.
Of the 8 patients enrolled thus far, 5 had intermediate- or high-risk disease at baseline, and 1 patient was dialysis-dependent. The median age was 63 (range, 43-70).
Patients had received a median of 2 prior lines of therapy (range, 1-6) and a median of 4 prior treatments (range, 2-8). All of the patients had received lenalidomide, 1 had received carfilzomib, and 4 were refractory to bortezomib.
For the current study, patients were assigned to receive dexamethasone, carfilzomib over 10 minutes, and pelareorep over 60 minutes on days 1, 2, 8, 9, 15, and 16. Treatment is set to repeat every 28 days in the absence of disease progression or unacceptable toxicity.
Patients who achieve a minimal response or better may reduce treatment to days 1, 8, and 15 after 4 courses and to days 1 and 15 after 12 courses.
Treatment results
The investigators said the treatment produced a significant (P=0.005) increase in caspase-3, a marker associated with apoptotic cell death.
The combination was also associated with an increased infiltration of CD8+ T cells and the significant (P=0.005) upregulation of PD-L1, suggesting the addition of a PD-1 or PD-L1 inhibitor might further optimize the regimen.
All 8 patients experienced an objective response to treatment, as measured by changes in blood monoclonal protein. Two patients had a very good partial response, 3 had a partial response, and 3 had a minor response.
Five of the patients remain on study. The dialysis-dependent patient discontinued treatment due to progression after 3 treatment cycles. And 2 patients discontinued treatment due to dose-limiting toxicities after 2 doses.
One of the patients with dose-limiting toxicities had grade 4 myocarditis, grade 3 left ventricular dysfunction, and grade 4 respiratory failure possibly attributable to pelareorep and carfilzomib. The other patient had lower gastrointestinal bleeding that was not attributed to treatment.
Adverse events in cycle 1 that were possibly, likely, or definitely attributed to treatment included hypertension (n=5), thrombocytopenia (n=4), anemia (n=4), dyspnea on exertion (n=4), fatigue (n=4), myalgia (n=3), fever (n=2), leukopenia (n=2), lymphopenia (n=2), nausea (n=2), and diarrhea (n=2).
For more details, visit the Oncolytics Biotech Inc. website to view the poster. Oncolytics is the company developing pelareorep. ![]()