User login
ICAAC: 2015 brings three major systematic reviews of diabetic foot infection therapy
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ICAAC 2015
CHMP recommends carfilzomib for MM

Photo courtesy of Amgen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion of the proteasome inhibitor carfilzomib (Kyprolis).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s positive opinion will be reviewed by the European Commission (EC).
The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision within 3 months. The EC’s decision will apply to the 28 member countries of the European Union (EU), as well as Iceland, Lichtenstein, and Norway.
ASPIRE trial
The CHMP’s positive opinion of carfilzomib was based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. And the median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of the patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Carfilzomib development
Carfilzomib was granted orphan drug designation in the EU in 2008. Last February, the drug’s application for EU approval was granted accelerated assessment.
Carfilzomib was approved as monotherapy in the US in July 2012 and in combination with lenalidomide and dexamethasone in July 2015. Carfilzomib is also approved for use in Argentina, Israel, Kuwait, Mexico, and Thailand.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan. For more information on the drug, visit www.kyprolis.com. ![]()

Photo courtesy of Amgen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion of the proteasome inhibitor carfilzomib (Kyprolis).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s positive opinion will be reviewed by the European Commission (EC).
The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision within 3 months. The EC’s decision will apply to the 28 member countries of the European Union (EU), as well as Iceland, Lichtenstein, and Norway.
ASPIRE trial
The CHMP’s positive opinion of carfilzomib was based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. And the median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of the patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Carfilzomib development
Carfilzomib was granted orphan drug designation in the EU in 2008. Last February, the drug’s application for EU approval was granted accelerated assessment.
Carfilzomib was approved as monotherapy in the US in July 2012 and in combination with lenalidomide and dexamethasone in July 2015. Carfilzomib is also approved for use in Argentina, Israel, Kuwait, Mexico, and Thailand.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan. For more information on the drug, visit www.kyprolis.com. ![]()

Photo courtesy of Amgen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion of the proteasome inhibitor carfilzomib (Kyprolis).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s positive opinion will be reviewed by the European Commission (EC).
The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision within 3 months. The EC’s decision will apply to the 28 member countries of the European Union (EU), as well as Iceland, Lichtenstein, and Norway.
ASPIRE trial
The CHMP’s positive opinion of carfilzomib was based on data from the phase 3 ASPIRE trial, which were presented at ASH 2014 and published in NEJM.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive carfilzomib plus lenalidomide and dexamethasone (KRd) or just lenalidomide and dexamethasone (Rd) for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
The study’s primary endpoint was progression-free survival. And the median progression-free survival was significantly longer in the KRd arm than the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69, P=0.0001).
At the time of analysis, the difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate was 87% in the KRd arm and 67% in the Rd arm. The median duration of response was 28.6 months and 21.2 months, respectively.
The rates of death due to adverse events (AEs) within 30 days of the last dose were similar between the treatment arms.
The most common causes of death not due to progressive disease occurring in patients in the KRd arm and the Rd arm, respectively, were cardiac disorders (3% vs 2%), infection (2% vs 3%), renal events (0% vs less than 1%), and other AEs (2% vs 3%).
Serious AEs were reported in 60% of the patients in the KRd arm and 54% in the Rd arm. The most common serious AEs reported in the KRd arm and the Rd arm, respectively, were pneumonia (14% vs 11%), respiratory tract infection (4% vs 2%), pyrexia (4% vs 2%), and pulmonary embolism (3% vs 2%).
Carfilzomib development
Carfilzomib was granted orphan drug designation in the EU in 2008. Last February, the drug’s application for EU approval was granted accelerated assessment.
Carfilzomib was approved as monotherapy in the US in July 2012 and in combination with lenalidomide and dexamethasone in July 2015. Carfilzomib is also approved for use in Argentina, Israel, Kuwait, Mexico, and Thailand.
Carfilzomib is a product of Onyx Pharmaceuticals, Inc., a subsidiary of Amgen that holds development and commercialization rights to the drug globally, excluding Japan. For more information on the drug, visit www.kyprolis.com. ![]()
Significant Differences in Health Care Costs for Spine Surgery
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Costs for spinal fusion surgery in the United States vary substantially by region, with costs being lowest in the Midwest and highest in the Northeast, according to a study published in the September 1 issue of Spine.
The researchers analyzed 2012 Medicare data on the costs of anterior cervical discectomy and fusion (ACDF) and posterior lumbar fusion (PLF). For comparison, the costs of total knee arthroplasty (TKA) also were assessed. The analysis focused on direct costs, defined as the amount reimbursed to health care providers by Medicare or other payers. Most previous economic analyses of spinal surgery have focused on the amount billed by providers to payers.
Average national costs were about $14,000 for a single-level ACDF procedure and $26,000 for a single-level PLF. These total figures reflected combined professional and facility costs. Average cost for TKA was about $13,000, increasing to $22,000 for TKA in patients with other accompanying major medical conditions.
“Each procedure had a significant range in cost across the country,” said William Ryan Spiker, MD, Assistant Professor at the University of Utah in Salt Lake City. “This data sheds light on the actual cost of common surgeries throughout the United States, and will allow further progress towards the development of cost effective, value driven care.”
Costs for ACDF ranged from about $11,000 to $25,000, while PLF costs ranged from $20,000 to $37,000. For TKA patients without major medical conditions, the range was from about $11,000 to $19,000.
All procedures except ACDF also showed significant variations on the regional level, with the lowest costs in the Midwest and highest costs in the Northeast. For PLF, costs ranged from $24,000 in the Midwest to $28,000 in the Northeast. The figures were $12,000 versus $14,000 for primary TKA, and $21,000 versus $25,000 for TKA with major medical conditions.
On the state level, total costs for all 4 procedures were significantly correlated with the state’s cost of living index, but not with state population.
Spinal fusion procedures such as ACDF and PLF are a major source of costs for Medicare and other payers. From 2001 to 2010, an estimated 3.6 million spinal fusions were performed in the United States, with total charges of more than $287 billion.
The study does not show what’s behind the variations in cost, although state cost-of-living index is one related factor. Dr. Spiker and coauthors said, “In the pursuit of cost optimization, and the broader pursuit of value driven health care, it may prove valuable to study the factors that allow these states to deliver care at a lower cost.”
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Suggested Reading
Goz V, Rane A, Abtahi AM, et al. Geographic variations in the cost of spine surgery. Spine (Phila Pa 1976). 2015;40(17):1380-1389.
Are Knee and Hip Replacements Bad For the Heart?
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Topical fluorouracil shows long-term benefit for actinic keratoses
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
FROM JAMA DERMATOLOGY
Key clinical point:One course of topical fluorouracil cream, 5%, decreased the need for localized treatment and the number of actinic keratoses long term.
Major finding: Participants whose AKs were treated with a course of fluorouracil cream, 5%, had significantly fewer AKs and required fewer treatments, compared with the control group, for over 2 years.
Data source: The randomized, double-blind study compared the effect of a course of topical fluorouracil cream with a vehicle cream on the number of AKs and other measures, over a mean follow-up of 2.6 years, in 932 patients treated at 12 VA dermatology clinics.
Disclosures: The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others had no disclosures.
Impact of Demographic and Health System Variables on Survival in Early Stage (Stage I and II) Non-Small Cell Lung Carcinoma: A National Cancer Database Analysis
Background: Non-small cell lung carcinoma (NSCLC) is the most common type of lung cancer. According to a Surveillance, Epidemiology, and End Results (SEER) database analysis, the 5-year survival rates for clinical stages IA, IB, IIA, and IIB NSCLC are 50%, 43%, 36%, and 25%, respectively. Even with advances in therapies in both the surgical and medical fields, patient outcomes remain suboptimal. Our aim was to assess the role of various demographic and insurance characteristics on patient survival in early stage (stage I and II) NSCLC.
Methods: This is a retrospective study of patients diagnosed with stage I and stage II NSCLC between 1998 and 2012 utilizing the National Cancer Database (NCDB) participant user file (PUF). The NCDB is a nationwide oncology outcomes database for more than 1,500 American College of Surgeons Commission on Cancer-accredited cancer programs. The impact of various factors on survival was analyzed using the Cox proportional hazards model.
Results: A total of 304,092 patients with early stage NSCLC were analyzed for this study. On multivariate analysis, the factors associated with decreased survival were male (hazard ratio [HR] 1.32, P < .0001) compared with female, increasing age (HR 1.036, P < .0001), African American (HR 1.15, P < .0001) compared with white, and rural residency (HR 1.146, P < .0001) compared with metro areas. Privately insured patients had better survival when compared with uninsured patients (HR 0.674, P < .0001), whereas Medicaid patients had the worst survival (HR 1.076, P < .0001). Also, the patients who were diagnosed between 2008 and 2012 had a higher survival than those diagnosed earlier (HR 0.645, P < .0001).
Discussion: The above data suggest that there is a disparity in outcomes among patients with early stage NSCLC based on various demographic and health system factors. Despite an overall increase in survival due to improved therapies from 1998 to 2012, significant differences exist in terms of patient age, gender, race, residency, and insurance status. This could be secondary to decreased receipt of appropriate treatment in certain subgroups or due to a difference in cancer biology in some of these groups. Nevertheless, this study suggests room for improvement in health care delivery to all patients for optimal outcomes.
Background: Non-small cell lung carcinoma (NSCLC) is the most common type of lung cancer. According to a Surveillance, Epidemiology, and End Results (SEER) database analysis, the 5-year survival rates for clinical stages IA, IB, IIA, and IIB NSCLC are 50%, 43%, 36%, and 25%, respectively. Even with advances in therapies in both the surgical and medical fields, patient outcomes remain suboptimal. Our aim was to assess the role of various demographic and insurance characteristics on patient survival in early stage (stage I and II) NSCLC.
Methods: This is a retrospective study of patients diagnosed with stage I and stage II NSCLC between 1998 and 2012 utilizing the National Cancer Database (NCDB) participant user file (PUF). The NCDB is a nationwide oncology outcomes database for more than 1,500 American College of Surgeons Commission on Cancer-accredited cancer programs. The impact of various factors on survival was analyzed using the Cox proportional hazards model.
Results: A total of 304,092 patients with early stage NSCLC were analyzed for this study. On multivariate analysis, the factors associated with decreased survival were male (hazard ratio [HR] 1.32, P < .0001) compared with female, increasing age (HR 1.036, P < .0001), African American (HR 1.15, P < .0001) compared with white, and rural residency (HR 1.146, P < .0001) compared with metro areas. Privately insured patients had better survival when compared with uninsured patients (HR 0.674, P < .0001), whereas Medicaid patients had the worst survival (HR 1.076, P < .0001). Also, the patients who were diagnosed between 2008 and 2012 had a higher survival than those diagnosed earlier (HR 0.645, P < .0001).
Discussion: The above data suggest that there is a disparity in outcomes among patients with early stage NSCLC based on various demographic and health system factors. Despite an overall increase in survival due to improved therapies from 1998 to 2012, significant differences exist in terms of patient age, gender, race, residency, and insurance status. This could be secondary to decreased receipt of appropriate treatment in certain subgroups or due to a difference in cancer biology in some of these groups. Nevertheless, this study suggests room for improvement in health care delivery to all patients for optimal outcomes.
Background: Non-small cell lung carcinoma (NSCLC) is the most common type of lung cancer. According to a Surveillance, Epidemiology, and End Results (SEER) database analysis, the 5-year survival rates for clinical stages IA, IB, IIA, and IIB NSCLC are 50%, 43%, 36%, and 25%, respectively. Even with advances in therapies in both the surgical and medical fields, patient outcomes remain suboptimal. Our aim was to assess the role of various demographic and insurance characteristics on patient survival in early stage (stage I and II) NSCLC.
Methods: This is a retrospective study of patients diagnosed with stage I and stage II NSCLC between 1998 and 2012 utilizing the National Cancer Database (NCDB) participant user file (PUF). The NCDB is a nationwide oncology outcomes database for more than 1,500 American College of Surgeons Commission on Cancer-accredited cancer programs. The impact of various factors on survival was analyzed using the Cox proportional hazards model.
Results: A total of 304,092 patients with early stage NSCLC were analyzed for this study. On multivariate analysis, the factors associated with decreased survival were male (hazard ratio [HR] 1.32, P < .0001) compared with female, increasing age (HR 1.036, P < .0001), African American (HR 1.15, P < .0001) compared with white, and rural residency (HR 1.146, P < .0001) compared with metro areas. Privately insured patients had better survival when compared with uninsured patients (HR 0.674, P < .0001), whereas Medicaid patients had the worst survival (HR 1.076, P < .0001). Also, the patients who were diagnosed between 2008 and 2012 had a higher survival than those diagnosed earlier (HR 0.645, P < .0001).
Discussion: The above data suggest that there is a disparity in outcomes among patients with early stage NSCLC based on various demographic and health system factors. Despite an overall increase in survival due to improved therapies from 1998 to 2012, significant differences exist in terms of patient age, gender, race, residency, and insurance status. This could be secondary to decreased receipt of appropriate treatment in certain subgroups or due to a difference in cancer biology in some of these groups. Nevertheless, this study suggests room for improvement in health care delivery to all patients for optimal outcomes.
Lesion Sprang Up Under His Nose (Well, to One Side, Actually …)
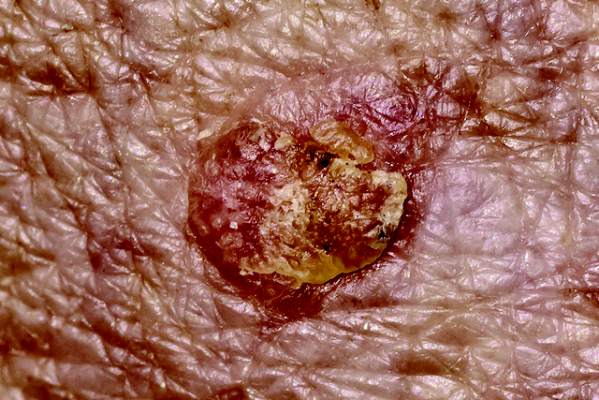
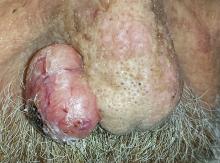
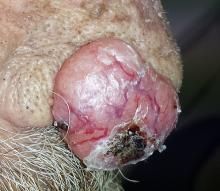
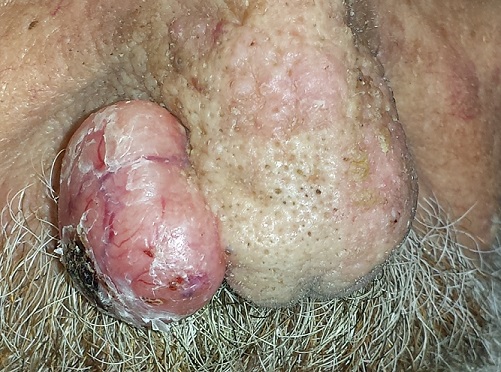
An 80-year-old man is brought in by family for evaluation of a lesion on his nose. It manifested several years ago, at a smaller size, but has recently and abruptly grown. Although asymptomatic, the lesion is disturbing to the patient, who can now see it out of the corner of his eye.
The patient worked all of his adult life in the outdoors, as a farm and ranch hand. He has an extensive history of nonmelanoma skin cancer; several lesions have been removed from his face and arm.
Since the patient lives alone and rarely has visitors, it has been months since anyone has seen him. But as soon as his son-in-law saw the patient, he was sufficiently alarmed by the lesion to insist that care be sought.
EXAMINATION
The patient’s facial skin shows abundant evidence of chronic, severe sun damage: a whitish, spongy look to the skin on his forehead and upper cheeks and a great deal of discoloration and scaling.
The lesion in question is a 3 x 1.5–cm, round, bulbous, smooth mass covering the right alar bulb. The surface is glassy-looking, with multiple telangiectasias. It is very firm but nontender on palpation. Shave biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results confirmed the suspicion of basal cell carcinoma (BCC). BCCs typically grow very slowly, often taking years to become noticeable, although not every BCC follows the rules. Some are more aggressive than others, both in terms of growth and clinical behavior.
It’s quite likely that in this case, the patient’s social isolation created the impression that his lesion grew abruptly and dramatically. (A subsequent eye exam revealed a number of problems, including severe presbyopia and advanced cataracts, so the patient himself might not have noticed the lesion for a while.) However, due to the large size and aggressive nature of the lesion—and the fact that the patient lives more than two hours from the nearest city, rendering his other treatment option, radiation, impractical—he was referred for Mohs surgery.
This process will establish clear surgical margins and provide acceptable closure. The latter may require reconstruction of the nose, depending on the depth of the cancer. Mohs surgeons often co-manage such cases with their counterparts in ENT or plastic surgery.
The differential for this lesion included keratoacanthoma , squamous cell carcinoma, and cyst.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is typically very slow growing, but there are exceptions.
• Social isolation can allow lesions and conditions to advance before they’re detected.
• Shave biopsy is indicated only for possible nonmelanoma skin cancers. Possible melanomas require excision, multiple punches, or deep shave to establish depth (a key prognostic factor).
• Rapid growth of BCCs suggests more aggressive clinical behavior, which in turn suggests the need for controlled margins to ensure complete removal.
An 80-year-old man is brought in by family for evaluation of a lesion on his nose. It manifested several years ago, at a smaller size, but has recently and abruptly grown. Although asymptomatic, the lesion is disturbing to the patient, who can now see it out of the corner of his eye.
The patient worked all of his adult life in the outdoors, as a farm and ranch hand. He has an extensive history of nonmelanoma skin cancer; several lesions have been removed from his face and arm.
Since the patient lives alone and rarely has visitors, it has been months since anyone has seen him. But as soon as his son-in-law saw the patient, he was sufficiently alarmed by the lesion to insist that care be sought.
EXAMINATION
The patient’s facial skin shows abundant evidence of chronic, severe sun damage: a whitish, spongy look to the skin on his forehead and upper cheeks and a great deal of discoloration and scaling.
The lesion in question is a 3 x 1.5–cm, round, bulbous, smooth mass covering the right alar bulb. The surface is glassy-looking, with multiple telangiectasias. It is very firm but nontender on palpation. Shave biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results confirmed the suspicion of basal cell carcinoma (BCC). BCCs typically grow very slowly, often taking years to become noticeable, although not every BCC follows the rules. Some are more aggressive than others, both in terms of growth and clinical behavior.
It’s quite likely that in this case, the patient’s social isolation created the impression that his lesion grew abruptly and dramatically. (A subsequent eye exam revealed a number of problems, including severe presbyopia and advanced cataracts, so the patient himself might not have noticed the lesion for a while.) However, due to the large size and aggressive nature of the lesion—and the fact that the patient lives more than two hours from the nearest city, rendering his other treatment option, radiation, impractical—he was referred for Mohs surgery.
This process will establish clear surgical margins and provide acceptable closure. The latter may require reconstruction of the nose, depending on the depth of the cancer. Mohs surgeons often co-manage such cases with their counterparts in ENT or plastic surgery.
The differential for this lesion included keratoacanthoma , squamous cell carcinoma, and cyst.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is typically very slow growing, but there are exceptions.
• Social isolation can allow lesions and conditions to advance before they’re detected.
• Shave biopsy is indicated only for possible nonmelanoma skin cancers. Possible melanomas require excision, multiple punches, or deep shave to establish depth (a key prognostic factor).
• Rapid growth of BCCs suggests more aggressive clinical behavior, which in turn suggests the need for controlled margins to ensure complete removal.
An 80-year-old man is brought in by family for evaluation of a lesion on his nose. It manifested several years ago, at a smaller size, but has recently and abruptly grown. Although asymptomatic, the lesion is disturbing to the patient, who can now see it out of the corner of his eye.
The patient worked all of his adult life in the outdoors, as a farm and ranch hand. He has an extensive history of nonmelanoma skin cancer; several lesions have been removed from his face and arm.
Since the patient lives alone and rarely has visitors, it has been months since anyone has seen him. But as soon as his son-in-law saw the patient, he was sufficiently alarmed by the lesion to insist that care be sought.
EXAMINATION
The patient’s facial skin shows abundant evidence of chronic, severe sun damage: a whitish, spongy look to the skin on his forehead and upper cheeks and a great deal of discoloration and scaling.
The lesion in question is a 3 x 1.5–cm, round, bulbous, smooth mass covering the right alar bulb. The surface is glassy-looking, with multiple telangiectasias. It is very firm but nontender on palpation. Shave biopsy is performed.
What is the diagnosis?
DISCUSSION
The biopsy results confirmed the suspicion of basal cell carcinoma (BCC). BCCs typically grow very slowly, often taking years to become noticeable, although not every BCC follows the rules. Some are more aggressive than others, both in terms of growth and clinical behavior.
It’s quite likely that in this case, the patient’s social isolation created the impression that his lesion grew abruptly and dramatically. (A subsequent eye exam revealed a number of problems, including severe presbyopia and advanced cataracts, so the patient himself might not have noticed the lesion for a while.) However, due to the large size and aggressive nature of the lesion—and the fact that the patient lives more than two hours from the nearest city, rendering his other treatment option, radiation, impractical—he was referred for Mohs surgery.
This process will establish clear surgical margins and provide acceptable closure. The latter may require reconstruction of the nose, depending on the depth of the cancer. Mohs surgeons often co-manage such cases with their counterparts in ENT or plastic surgery.
The differential for this lesion included keratoacanthoma , squamous cell carcinoma, and cyst.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is typically very slow growing, but there are exceptions.
• Social isolation can allow lesions and conditions to advance before they’re detected.
• Shave biopsy is indicated only for possible nonmelanoma skin cancers. Possible melanomas require excision, multiple punches, or deep shave to establish depth (a key prognostic factor).
• Rapid growth of BCCs suggests more aggressive clinical behavior, which in turn suggests the need for controlled margins to ensure complete removal.
Bipolar type I patients’ relatives lack brain connectivity disruptions
Anatomical connectivity in discreet frontal regions of the brain is disrupted in bipolar I disorder patients, but not in mentally healthy relatives of such patients, according to a study.
The researchers looked for connectivity abnormalities in the brains of multiply affected bipolar I disorder families “to assess the utility of dysconnectivity as a biomarker and its endophenotypic potential.” Tractography was done on magnetic resonance diffusion images of the brains of 19 bipolar I patients in remission, 21 of the patients’ first-degree relatives who did not have bipolar I, and 18 unrelated controls who also did not have bipolar I. A connectivity matrix was generated for each patient, and the Brain Connectivity Toolbox was used to extract neural network metrics.
“Whole brain analysis revealed no differences between groups,” according to Natalie J. Forde, a PhD candidate at the University Medical Centre Gronigen (the Netherlands) and her colleagues. “Analysis of specific mainly frontal regions, previously implicated as potentially endophenotypic by functional magnetic resonance imaging analysis of the same cohort, revealed a significant effect of group in the right medial superior frontal gyrus and left middle frontal gyrus driven by reduced [organization] in [bipolar I] patients, compared with controls.”
Read the full study in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.08.004).
Anatomical connectivity in discreet frontal regions of the brain is disrupted in bipolar I disorder patients, but not in mentally healthy relatives of such patients, according to a study.
The researchers looked for connectivity abnormalities in the brains of multiply affected bipolar I disorder families “to assess the utility of dysconnectivity as a biomarker and its endophenotypic potential.” Tractography was done on magnetic resonance diffusion images of the brains of 19 bipolar I patients in remission, 21 of the patients’ first-degree relatives who did not have bipolar I, and 18 unrelated controls who also did not have bipolar I. A connectivity matrix was generated for each patient, and the Brain Connectivity Toolbox was used to extract neural network metrics.
“Whole brain analysis revealed no differences between groups,” according to Natalie J. Forde, a PhD candidate at the University Medical Centre Gronigen (the Netherlands) and her colleagues. “Analysis of specific mainly frontal regions, previously implicated as potentially endophenotypic by functional magnetic resonance imaging analysis of the same cohort, revealed a significant effect of group in the right medial superior frontal gyrus and left middle frontal gyrus driven by reduced [organization] in [bipolar I] patients, compared with controls.”
Read the full study in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.08.004).
Anatomical connectivity in discreet frontal regions of the brain is disrupted in bipolar I disorder patients, but not in mentally healthy relatives of such patients, according to a study.
The researchers looked for connectivity abnormalities in the brains of multiply affected bipolar I disorder families “to assess the utility of dysconnectivity as a biomarker and its endophenotypic potential.” Tractography was done on magnetic resonance diffusion images of the brains of 19 bipolar I patients in remission, 21 of the patients’ first-degree relatives who did not have bipolar I, and 18 unrelated controls who also did not have bipolar I. A connectivity matrix was generated for each patient, and the Brain Connectivity Toolbox was used to extract neural network metrics.
“Whole brain analysis revealed no differences between groups,” according to Natalie J. Forde, a PhD candidate at the University Medical Centre Gronigen (the Netherlands) and her colleagues. “Analysis of specific mainly frontal regions, previously implicated as potentially endophenotypic by functional magnetic resonance imaging analysis of the same cohort, revealed a significant effect of group in the right medial superior frontal gyrus and left middle frontal gyrus driven by reduced [organization] in [bipolar I] patients, compared with controls.”
Read the full study in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.08.004).
FROM PSYCHIATRY RESEARCH: NEUROIMAGING
Reducing side effects of CAR T-cell therapy

Photo courtesy of UCSF
Researchers have reported progress in developing an “on/off switch” to temper the over-active immune response and severe toxicities that can result from chimeric antigen receptor (CAR) T-cell therapy.
The team created CAR T cells that are “off” by default, homing to CD19-expressing cancer cells but remaining inactive until a small molecule is administered.
This system effectively targeted leukemia and lymphoma cells in preclinical experiments.
But the researchers said it’s not ready for clinical testing, as the small-molecule “trigger” is expensive and lasts only 4 hours.
Still, the team believes this type of CAR T-cell therapy could eventually help doctors gradually increase the immune response to treatment and therefore avoid toxicities such as cytokine release syndrome and tumor lysis syndrome.
Wendell Lim, PhD, of University of California, San Francisco, and his colleagues described this work in Science.
“T cells are really powerful beasts, and they can be lethal when they’re activated,” Dr Lim said. “We’ve needed a remote control system that retains the power of these engineered T cells but allows us to communicate specifically with them and manage them while they’re in the body.”
To that end, he and his colleagues created a CAR that requires both an antigen and a small molecule for activation. They dubbed it the “ON-switch CAR.”
ON-switch CAR
The researchers explained that the ON-switch CAR consists of 2 parts that assemble in a small molecule-dependent manner.
Part 1 consists of a CD8α signal sequence, Myc epitope, anti-CD19 single-chain variable fragment, CD8α hinge and transmembrane domain, 4-1BB costimulatory motif, and FK506 Binding Protein (FKBP) domain for heterodimerization.
Part 2 consists of the ectodomain of DNAX-activating protein 10 (DAP10) for homodimerization, CD8α transmembrane domain for membrane anchoring, 4-1BB costimulatory motif, T2089L mutant of FKBP-rapamycin binding (FRB*) domain, T-cell receptor CD3ζ signaling chain, and mCherry tag.
The FKBP and FRB* domains heterodimerize in the presence of the rapamycin analog AP21967, referred to as the “rapalog.”
The researchers conducted in vitro experiments with this ON-switch CAR in cells expressing CD19 (K562, Raji, and Daudi).
The ON-switch CAR T cells homed to CD19-expressing cells but did nothing else until the rapalog was added. Once the rapalog was added, CD19-expressing cells were killed off in a dose-dependent manner.
The team observed similar results in mice with leukemia. Leukemia cells (K562) were selectively eliminated by the ON-switch CARs only after the rapalog had been administered.
Dr Lim stressed that this work should be considered a proof of principle, as the rapalog has too short a half-life to be clinically useful. Nevertheless, he believes the research provides the foundation for practical remote control of CAR T cells.
Members of his lab are exploring other techniques to accomplish this goal, such as controlling CAR T-cell activation with light.
The team is also working to reduce side effects of CAR T-cell therapy by introducing multiple CARs into T cells so the cells will respond to multiple characteristics that are distinctive to an individual patient’s tumor, rather than to a single protein that may also be found on normal cells.
“That we can engineer CAR T cells to have slightly different, quite powerful effects—even if for a subset of patients or for certain types of cancer—is really remarkable,” Dr Lim said. “And this is just the tip of the iceberg.” ![]()

Photo courtesy of UCSF
Researchers have reported progress in developing an “on/off switch” to temper the over-active immune response and severe toxicities that can result from chimeric antigen receptor (CAR) T-cell therapy.
The team created CAR T cells that are “off” by default, homing to CD19-expressing cancer cells but remaining inactive until a small molecule is administered.
This system effectively targeted leukemia and lymphoma cells in preclinical experiments.
But the researchers said it’s not ready for clinical testing, as the small-molecule “trigger” is expensive and lasts only 4 hours.
Still, the team believes this type of CAR T-cell therapy could eventually help doctors gradually increase the immune response to treatment and therefore avoid toxicities such as cytokine release syndrome and tumor lysis syndrome.
Wendell Lim, PhD, of University of California, San Francisco, and his colleagues described this work in Science.
“T cells are really powerful beasts, and they can be lethal when they’re activated,” Dr Lim said. “We’ve needed a remote control system that retains the power of these engineered T cells but allows us to communicate specifically with them and manage them while they’re in the body.”
To that end, he and his colleagues created a CAR that requires both an antigen and a small molecule for activation. They dubbed it the “ON-switch CAR.”
ON-switch CAR
The researchers explained that the ON-switch CAR consists of 2 parts that assemble in a small molecule-dependent manner.
Part 1 consists of a CD8α signal sequence, Myc epitope, anti-CD19 single-chain variable fragment, CD8α hinge and transmembrane domain, 4-1BB costimulatory motif, and FK506 Binding Protein (FKBP) domain for heterodimerization.
Part 2 consists of the ectodomain of DNAX-activating protein 10 (DAP10) for homodimerization, CD8α transmembrane domain for membrane anchoring, 4-1BB costimulatory motif, T2089L mutant of FKBP-rapamycin binding (FRB*) domain, T-cell receptor CD3ζ signaling chain, and mCherry tag.
The FKBP and FRB* domains heterodimerize in the presence of the rapamycin analog AP21967, referred to as the “rapalog.”
The researchers conducted in vitro experiments with this ON-switch CAR in cells expressing CD19 (K562, Raji, and Daudi).
The ON-switch CAR T cells homed to CD19-expressing cells but did nothing else until the rapalog was added. Once the rapalog was added, CD19-expressing cells were killed off in a dose-dependent manner.
The team observed similar results in mice with leukemia. Leukemia cells (K562) were selectively eliminated by the ON-switch CARs only after the rapalog had been administered.
Dr Lim stressed that this work should be considered a proof of principle, as the rapalog has too short a half-life to be clinically useful. Nevertheless, he believes the research provides the foundation for practical remote control of CAR T cells.
Members of his lab are exploring other techniques to accomplish this goal, such as controlling CAR T-cell activation with light.
The team is also working to reduce side effects of CAR T-cell therapy by introducing multiple CARs into T cells so the cells will respond to multiple characteristics that are distinctive to an individual patient’s tumor, rather than to a single protein that may also be found on normal cells.
“That we can engineer CAR T cells to have slightly different, quite powerful effects—even if for a subset of patients or for certain types of cancer—is really remarkable,” Dr Lim said. “And this is just the tip of the iceberg.” ![]()

Photo courtesy of UCSF
Researchers have reported progress in developing an “on/off switch” to temper the over-active immune response and severe toxicities that can result from chimeric antigen receptor (CAR) T-cell therapy.
The team created CAR T cells that are “off” by default, homing to CD19-expressing cancer cells but remaining inactive until a small molecule is administered.
This system effectively targeted leukemia and lymphoma cells in preclinical experiments.
But the researchers said it’s not ready for clinical testing, as the small-molecule “trigger” is expensive and lasts only 4 hours.
Still, the team believes this type of CAR T-cell therapy could eventually help doctors gradually increase the immune response to treatment and therefore avoid toxicities such as cytokine release syndrome and tumor lysis syndrome.
Wendell Lim, PhD, of University of California, San Francisco, and his colleagues described this work in Science.
“T cells are really powerful beasts, and they can be lethal when they’re activated,” Dr Lim said. “We’ve needed a remote control system that retains the power of these engineered T cells but allows us to communicate specifically with them and manage them while they’re in the body.”
To that end, he and his colleagues created a CAR that requires both an antigen and a small molecule for activation. They dubbed it the “ON-switch CAR.”
ON-switch CAR
The researchers explained that the ON-switch CAR consists of 2 parts that assemble in a small molecule-dependent manner.
Part 1 consists of a CD8α signal sequence, Myc epitope, anti-CD19 single-chain variable fragment, CD8α hinge and transmembrane domain, 4-1BB costimulatory motif, and FK506 Binding Protein (FKBP) domain for heterodimerization.
Part 2 consists of the ectodomain of DNAX-activating protein 10 (DAP10) for homodimerization, CD8α transmembrane domain for membrane anchoring, 4-1BB costimulatory motif, T2089L mutant of FKBP-rapamycin binding (FRB*) domain, T-cell receptor CD3ζ signaling chain, and mCherry tag.
The FKBP and FRB* domains heterodimerize in the presence of the rapamycin analog AP21967, referred to as the “rapalog.”
The researchers conducted in vitro experiments with this ON-switch CAR in cells expressing CD19 (K562, Raji, and Daudi).
The ON-switch CAR T cells homed to CD19-expressing cells but did nothing else until the rapalog was added. Once the rapalog was added, CD19-expressing cells were killed off in a dose-dependent manner.
The team observed similar results in mice with leukemia. Leukemia cells (K562) were selectively eliminated by the ON-switch CARs only after the rapalog had been administered.
Dr Lim stressed that this work should be considered a proof of principle, as the rapalog has too short a half-life to be clinically useful. Nevertheless, he believes the research provides the foundation for practical remote control of CAR T cells.
Members of his lab are exploring other techniques to accomplish this goal, such as controlling CAR T-cell activation with light.
The team is also working to reduce side effects of CAR T-cell therapy by introducing multiple CARs into T cells so the cells will respond to multiple characteristics that are distinctive to an individual patient’s tumor, rather than to a single protein that may also be found on normal cells.
“That we can engineer CAR T cells to have slightly different, quite powerful effects—even if for a subset of patients or for certain types of cancer—is really remarkable,” Dr Lim said. “And this is just the tip of the iceberg.” ![]()
Studies raise concerns about drug approval process

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()