User login
Caring for refugees requires flexibility, cultural humility
When the recent photo of a drowned Syrian toddler woke up the world to the Syrian refugee crisis more viscerally than ever before, multiple nations announced plans to take in more refugees. According to the U.S. State Department, approximately 10,000 Syrian refugees are already in processing, eventually headed to cities that may include Atlanta, San Diego, Houston, Dallas, Chicago, Boston, Boise, Nashville, Tucson, Buffalo, and Erie.
To pediatricians, that boy on the beach represents a child who might have ended up in their practice with diverse, complex needs greatly exceeding the typical needs of a U.S. child coming in for a well-child visit.
“Families are coming from a country that has been ravaged by civil war for over 4 years,” Dr. Susan S. Reines, a pediatrician with the Southeast Kaiser Permanente Medical Group and lead pediatrician for the Refugee Pediatric Clinic at DeKalb County Board of Health in Decatur, Georgia, said in an interview. “Cities have been destroyed, and millions have been forced to leave their homes and are displaced either within Syria or in neighboring countries.”
About a third of the more than 58,000 refugees admitted to the United States in 2012 were under 18 years old. Although the majority that year hailed from Bhutan, Burma, and Iraq, an increasing number of children have been coming from war-torn Syria since June 2014. The proposed ceiling for all refugees in the United States 2015 fiscal year is 70,000, a “significant number” of whom will be children with their families, according to a State Department spokesperson.
These children come with “unique medical, developmental and psychosocial needs,” noted Dr. Thomas J. Seery and fellow authors of “Caring for Refugee Children,” a Pediatrics in Review article recommended by Dr. Reines for pediatricians who may be caring for refugee children.
“The health care infrastructure of Syria is broken and many hospitals have closed, medications are difficult to obtain, and numerous doctors have fled the violence,” Dr. Reines said. She compared the anticipated health care problems of these children with those seen among Iraqi refugee children:
• Undernutrition and micronutrient deficiencies.
• Infectious diseases such as vaccine-preventable diseases like measles, but also typhoid, tuberculosis, and parasitic infections.
• Dental disease.
• Surgically amenable congenital anomalies such as congenital heart disease, myelomeningocele, and others that have not been repaired.
• Neurologic problems, such as cerebral palsy, intellectual disability, and autism.
• Hearing loss.
• Posttraumatic stress disorder (PTSD),depression, and anxiety.
• Trauma such as gunshot wounds, shrapnel injuries, and genital trauma secondary to sexual violence.
• Sequelae from illnesses that previously were easily treated, such as hearing loss and ear complications from otitis media, and rheumatic fever from inadequately treated strep throat.
• Underimmunization.
Various resources listed below, including Dr. Seery’s paper, can help guide providers in assessing and meeting these needs, and navigating paperwork and the U.S. refugee system. These resources also can help practitioners address the mental health concerns these patients and their families may face.
Mental health needs
Even children in the best physical shape will have experienced significant upheaval that could lead to depression, anxiety, and PTSD – conditions more common among refugee children than in the general population, research has shown.
“Mental health conditions will be especially present in these children uprooted from their homes and families, and exposed to the violence of war,” Dr. Francis E. Rushton Jr. of the department of pediatrics at the University of South Carolina, Columbia, and a member of the American Academy of Pediatrics Committee on Community Health Services, said in an interview. Of the four major areas of health care need he described for these children, two relate to mental health: toxic mental stress and fractured families and the lack of nurture.
One challenge pediatricians face, however, is recognizing these conditions despite cultural differences that could obscure them.
“It is not uncommon for teens and adults to deny symptoms of depression, stress, and anxiety in early encounters,” Dr. Reines said. “Many cultures stigmatize psychiatric or mental health problems, and refugees may be reluctant to admit they are having difficulties.”
One way around this obstacle is to ask patients and their parents about sleep, energy level, appetite, weight changes, and thoughts of harming one’s self, she said. Mental stress also manifests as somatic symptoms, such as headaches, stomach aches, and back pain, particularly in teens.
“Infants and toddlers are generally most adaptable as long as parents are coping well, and can provide a buffer for stress with a safe and nurturing environment,” Dr. Reines said. Children of parents with depression or PTSD, or who have lost a parent, may feel abandoned and experience depression or developmental delays.
Although school-age children may have nightmares, show anxiety, and cling to their parents, they usually transition well to their new homes. Adolescents face the biggest difficulties, especially if they have lost a parent, must care for their siblings, or have experienced sexual trauma. “They may have more vivid memories of disturbing events and a greater understanding of what their family has endured,” Dr. Reines said. Further, language and educational deficits can lead to alienation and embarrassment, yet families may rebuff behavioral health referrals.
“In these cases, it’s best to keep communication open, encourage dialogue with family, and try to find an activity or sport the refugee can participate in to improve self-esteem,” Dr. Reines said.
Avoiding cultural confusion
While cultural challenges are obvious – language barriers may necessitate translators or bicultural caseworkers – others may be more subtle. Developmental screening questions that rely on blocks, certain pictures, or other culturally specific bases, for example, may not adequately capture a child’s development.
Dr. Reines stresses a strategy for managing cultural differences that is recommended in Dr. Seery’s article: striving for cultural humility rather than cultural competence.
“It is impossible for U.S. physicians who have never practiced outside of our culture and are not bicultural or bilingual to become truly culturally competent in health care delivery for so many refugee populations,” Dr. Reines said. Instead then, cultural humility emphasizes showing respect, interest, and a willingness to learn from patients, she explained.
Cultural humility is a “lifelong process” that also demands flexibility and “allows the practitioner to release the false sense of security associated with stereotyping,” Dr. Seery and his colleagues wrote.
At the same time, pediatricians are guarding against inadvertent stereotyping; however, they can be aware of some cultural generalities that may apply to their Syrian refugee patients.
“Arab communities stress the importance of family rather than the individual and are often more modest than Westerners,” Dr. Rushton said. Further, “Arab families frequently experience discrimination on the basis of their religion in the United States, and pediatricians should be aware of ongoing traumatization even after arrival in America,” he said.
Teens may become embarrassed with discussions about sex or alcohol because few teens from the Middle East drink or become sexually active before marriage, Dr. Reines added. She noted that a Muslim male may not shake hands with females outside his family – a practice providers should respect – and that important religious holidays such as Ramadan may influence a family’s compliance with a treatment plan.
Perhaps the most important commonality, however, is one universal to most refugee families, regardless of their home country.
“The vast majority of families that we meet show incredible courage and resilience, and caring for their children is their highest priority,” Dr. Reines said. “We can learn a great deal from these families, and caring for their children is a tremendously rewarding experience.”
Other cultural resources:
Bridging Refugee Youth and Children’s Services
“The Middle of Everywhere: Helping Refugees Enter the American Community,” by Mary Pipher (Orlando: Mariner Books, 2003)
“Immigrant Medicine,” a textbook by Patricia Walker, M.D., and Elizabeth Barnett, M.D. (New York, N.Y.: Elsevier, 2007)
“Opening cultural doors: Providing culturally sensitive healthcare to Arab American and American Muslim patients” (Am J Obstet Gynecol. 2005 Oct;193]:1307-11).
When the recent photo of a drowned Syrian toddler woke up the world to the Syrian refugee crisis more viscerally than ever before, multiple nations announced plans to take in more refugees. According to the U.S. State Department, approximately 10,000 Syrian refugees are already in processing, eventually headed to cities that may include Atlanta, San Diego, Houston, Dallas, Chicago, Boston, Boise, Nashville, Tucson, Buffalo, and Erie.
To pediatricians, that boy on the beach represents a child who might have ended up in their practice with diverse, complex needs greatly exceeding the typical needs of a U.S. child coming in for a well-child visit.
“Families are coming from a country that has been ravaged by civil war for over 4 years,” Dr. Susan S. Reines, a pediatrician with the Southeast Kaiser Permanente Medical Group and lead pediatrician for the Refugee Pediatric Clinic at DeKalb County Board of Health in Decatur, Georgia, said in an interview. “Cities have been destroyed, and millions have been forced to leave their homes and are displaced either within Syria or in neighboring countries.”
About a third of the more than 58,000 refugees admitted to the United States in 2012 were under 18 years old. Although the majority that year hailed from Bhutan, Burma, and Iraq, an increasing number of children have been coming from war-torn Syria since June 2014. The proposed ceiling for all refugees in the United States 2015 fiscal year is 70,000, a “significant number” of whom will be children with their families, according to a State Department spokesperson.
These children come with “unique medical, developmental and psychosocial needs,” noted Dr. Thomas J. Seery and fellow authors of “Caring for Refugee Children,” a Pediatrics in Review article recommended by Dr. Reines for pediatricians who may be caring for refugee children.
“The health care infrastructure of Syria is broken and many hospitals have closed, medications are difficult to obtain, and numerous doctors have fled the violence,” Dr. Reines said. She compared the anticipated health care problems of these children with those seen among Iraqi refugee children:
• Undernutrition and micronutrient deficiencies.
• Infectious diseases such as vaccine-preventable diseases like measles, but also typhoid, tuberculosis, and parasitic infections.
• Dental disease.
• Surgically amenable congenital anomalies such as congenital heart disease, myelomeningocele, and others that have not been repaired.
• Neurologic problems, such as cerebral palsy, intellectual disability, and autism.
• Hearing loss.
• Posttraumatic stress disorder (PTSD),depression, and anxiety.
• Trauma such as gunshot wounds, shrapnel injuries, and genital trauma secondary to sexual violence.
• Sequelae from illnesses that previously were easily treated, such as hearing loss and ear complications from otitis media, and rheumatic fever from inadequately treated strep throat.
• Underimmunization.
Various resources listed below, including Dr. Seery’s paper, can help guide providers in assessing and meeting these needs, and navigating paperwork and the U.S. refugee system. These resources also can help practitioners address the mental health concerns these patients and their families may face.
Mental health needs
Even children in the best physical shape will have experienced significant upheaval that could lead to depression, anxiety, and PTSD – conditions more common among refugee children than in the general population, research has shown.
“Mental health conditions will be especially present in these children uprooted from their homes and families, and exposed to the violence of war,” Dr. Francis E. Rushton Jr. of the department of pediatrics at the University of South Carolina, Columbia, and a member of the American Academy of Pediatrics Committee on Community Health Services, said in an interview. Of the four major areas of health care need he described for these children, two relate to mental health: toxic mental stress and fractured families and the lack of nurture.
One challenge pediatricians face, however, is recognizing these conditions despite cultural differences that could obscure them.
“It is not uncommon for teens and adults to deny symptoms of depression, stress, and anxiety in early encounters,” Dr. Reines said. “Many cultures stigmatize psychiatric or mental health problems, and refugees may be reluctant to admit they are having difficulties.”
One way around this obstacle is to ask patients and their parents about sleep, energy level, appetite, weight changes, and thoughts of harming one’s self, she said. Mental stress also manifests as somatic symptoms, such as headaches, stomach aches, and back pain, particularly in teens.
“Infants and toddlers are generally most adaptable as long as parents are coping well, and can provide a buffer for stress with a safe and nurturing environment,” Dr. Reines said. Children of parents with depression or PTSD, or who have lost a parent, may feel abandoned and experience depression or developmental delays.
Although school-age children may have nightmares, show anxiety, and cling to their parents, they usually transition well to their new homes. Adolescents face the biggest difficulties, especially if they have lost a parent, must care for their siblings, or have experienced sexual trauma. “They may have more vivid memories of disturbing events and a greater understanding of what their family has endured,” Dr. Reines said. Further, language and educational deficits can lead to alienation and embarrassment, yet families may rebuff behavioral health referrals.
“In these cases, it’s best to keep communication open, encourage dialogue with family, and try to find an activity or sport the refugee can participate in to improve self-esteem,” Dr. Reines said.
Avoiding cultural confusion
While cultural challenges are obvious – language barriers may necessitate translators or bicultural caseworkers – others may be more subtle. Developmental screening questions that rely on blocks, certain pictures, or other culturally specific bases, for example, may not adequately capture a child’s development.
Dr. Reines stresses a strategy for managing cultural differences that is recommended in Dr. Seery’s article: striving for cultural humility rather than cultural competence.
“It is impossible for U.S. physicians who have never practiced outside of our culture and are not bicultural or bilingual to become truly culturally competent in health care delivery for so many refugee populations,” Dr. Reines said. Instead then, cultural humility emphasizes showing respect, interest, and a willingness to learn from patients, she explained.
Cultural humility is a “lifelong process” that also demands flexibility and “allows the practitioner to release the false sense of security associated with stereotyping,” Dr. Seery and his colleagues wrote.
At the same time, pediatricians are guarding against inadvertent stereotyping; however, they can be aware of some cultural generalities that may apply to their Syrian refugee patients.
“Arab communities stress the importance of family rather than the individual and are often more modest than Westerners,” Dr. Rushton said. Further, “Arab families frequently experience discrimination on the basis of their religion in the United States, and pediatricians should be aware of ongoing traumatization even after arrival in America,” he said.
Teens may become embarrassed with discussions about sex or alcohol because few teens from the Middle East drink or become sexually active before marriage, Dr. Reines added. She noted that a Muslim male may not shake hands with females outside his family – a practice providers should respect – and that important religious holidays such as Ramadan may influence a family’s compliance with a treatment plan.
Perhaps the most important commonality, however, is one universal to most refugee families, regardless of their home country.
“The vast majority of families that we meet show incredible courage and resilience, and caring for their children is their highest priority,” Dr. Reines said. “We can learn a great deal from these families, and caring for their children is a tremendously rewarding experience.”
Other cultural resources:
Bridging Refugee Youth and Children’s Services
“The Middle of Everywhere: Helping Refugees Enter the American Community,” by Mary Pipher (Orlando: Mariner Books, 2003)
“Immigrant Medicine,” a textbook by Patricia Walker, M.D., and Elizabeth Barnett, M.D. (New York, N.Y.: Elsevier, 2007)
“Opening cultural doors: Providing culturally sensitive healthcare to Arab American and American Muslim patients” (Am J Obstet Gynecol. 2005 Oct;193]:1307-11).
When the recent photo of a drowned Syrian toddler woke up the world to the Syrian refugee crisis more viscerally than ever before, multiple nations announced plans to take in more refugees. According to the U.S. State Department, approximately 10,000 Syrian refugees are already in processing, eventually headed to cities that may include Atlanta, San Diego, Houston, Dallas, Chicago, Boston, Boise, Nashville, Tucson, Buffalo, and Erie.
To pediatricians, that boy on the beach represents a child who might have ended up in their practice with diverse, complex needs greatly exceeding the typical needs of a U.S. child coming in for a well-child visit.
“Families are coming from a country that has been ravaged by civil war for over 4 years,” Dr. Susan S. Reines, a pediatrician with the Southeast Kaiser Permanente Medical Group and lead pediatrician for the Refugee Pediatric Clinic at DeKalb County Board of Health in Decatur, Georgia, said in an interview. “Cities have been destroyed, and millions have been forced to leave their homes and are displaced either within Syria or in neighboring countries.”
About a third of the more than 58,000 refugees admitted to the United States in 2012 were under 18 years old. Although the majority that year hailed from Bhutan, Burma, and Iraq, an increasing number of children have been coming from war-torn Syria since June 2014. The proposed ceiling for all refugees in the United States 2015 fiscal year is 70,000, a “significant number” of whom will be children with their families, according to a State Department spokesperson.
These children come with “unique medical, developmental and psychosocial needs,” noted Dr. Thomas J. Seery and fellow authors of “Caring for Refugee Children,” a Pediatrics in Review article recommended by Dr. Reines for pediatricians who may be caring for refugee children.
“The health care infrastructure of Syria is broken and many hospitals have closed, medications are difficult to obtain, and numerous doctors have fled the violence,” Dr. Reines said. She compared the anticipated health care problems of these children with those seen among Iraqi refugee children:
• Undernutrition and micronutrient deficiencies.
• Infectious diseases such as vaccine-preventable diseases like measles, but also typhoid, tuberculosis, and parasitic infections.
• Dental disease.
• Surgically amenable congenital anomalies such as congenital heart disease, myelomeningocele, and others that have not been repaired.
• Neurologic problems, such as cerebral palsy, intellectual disability, and autism.
• Hearing loss.
• Posttraumatic stress disorder (PTSD),depression, and anxiety.
• Trauma such as gunshot wounds, shrapnel injuries, and genital trauma secondary to sexual violence.
• Sequelae from illnesses that previously were easily treated, such as hearing loss and ear complications from otitis media, and rheumatic fever from inadequately treated strep throat.
• Underimmunization.
Various resources listed below, including Dr. Seery’s paper, can help guide providers in assessing and meeting these needs, and navigating paperwork and the U.S. refugee system. These resources also can help practitioners address the mental health concerns these patients and their families may face.
Mental health needs
Even children in the best physical shape will have experienced significant upheaval that could lead to depression, anxiety, and PTSD – conditions more common among refugee children than in the general population, research has shown.
“Mental health conditions will be especially present in these children uprooted from their homes and families, and exposed to the violence of war,” Dr. Francis E. Rushton Jr. of the department of pediatrics at the University of South Carolina, Columbia, and a member of the American Academy of Pediatrics Committee on Community Health Services, said in an interview. Of the four major areas of health care need he described for these children, two relate to mental health: toxic mental stress and fractured families and the lack of nurture.
One challenge pediatricians face, however, is recognizing these conditions despite cultural differences that could obscure them.
“It is not uncommon for teens and adults to deny symptoms of depression, stress, and anxiety in early encounters,” Dr. Reines said. “Many cultures stigmatize psychiatric or mental health problems, and refugees may be reluctant to admit they are having difficulties.”
One way around this obstacle is to ask patients and their parents about sleep, energy level, appetite, weight changes, and thoughts of harming one’s self, she said. Mental stress also manifests as somatic symptoms, such as headaches, stomach aches, and back pain, particularly in teens.
“Infants and toddlers are generally most adaptable as long as parents are coping well, and can provide a buffer for stress with a safe and nurturing environment,” Dr. Reines said. Children of parents with depression or PTSD, or who have lost a parent, may feel abandoned and experience depression or developmental delays.
Although school-age children may have nightmares, show anxiety, and cling to their parents, they usually transition well to their new homes. Adolescents face the biggest difficulties, especially if they have lost a parent, must care for their siblings, or have experienced sexual trauma. “They may have more vivid memories of disturbing events and a greater understanding of what their family has endured,” Dr. Reines said. Further, language and educational deficits can lead to alienation and embarrassment, yet families may rebuff behavioral health referrals.
“In these cases, it’s best to keep communication open, encourage dialogue with family, and try to find an activity or sport the refugee can participate in to improve self-esteem,” Dr. Reines said.
Avoiding cultural confusion
While cultural challenges are obvious – language barriers may necessitate translators or bicultural caseworkers – others may be more subtle. Developmental screening questions that rely on blocks, certain pictures, or other culturally specific bases, for example, may not adequately capture a child’s development.
Dr. Reines stresses a strategy for managing cultural differences that is recommended in Dr. Seery’s article: striving for cultural humility rather than cultural competence.
“It is impossible for U.S. physicians who have never practiced outside of our culture and are not bicultural or bilingual to become truly culturally competent in health care delivery for so many refugee populations,” Dr. Reines said. Instead then, cultural humility emphasizes showing respect, interest, and a willingness to learn from patients, she explained.
Cultural humility is a “lifelong process” that also demands flexibility and “allows the practitioner to release the false sense of security associated with stereotyping,” Dr. Seery and his colleagues wrote.
At the same time, pediatricians are guarding against inadvertent stereotyping; however, they can be aware of some cultural generalities that may apply to their Syrian refugee patients.
“Arab communities stress the importance of family rather than the individual and are often more modest than Westerners,” Dr. Rushton said. Further, “Arab families frequently experience discrimination on the basis of their religion in the United States, and pediatricians should be aware of ongoing traumatization even after arrival in America,” he said.
Teens may become embarrassed with discussions about sex or alcohol because few teens from the Middle East drink or become sexually active before marriage, Dr. Reines added. She noted that a Muslim male may not shake hands with females outside his family – a practice providers should respect – and that important religious holidays such as Ramadan may influence a family’s compliance with a treatment plan.
Perhaps the most important commonality, however, is one universal to most refugee families, regardless of their home country.
“The vast majority of families that we meet show incredible courage and resilience, and caring for their children is their highest priority,” Dr. Reines said. “We can learn a great deal from these families, and caring for their children is a tremendously rewarding experience.”
Other cultural resources:
Bridging Refugee Youth and Children’s Services
“The Middle of Everywhere: Helping Refugees Enter the American Community,” by Mary Pipher (Orlando: Mariner Books, 2003)
“Immigrant Medicine,” a textbook by Patricia Walker, M.D., and Elizabeth Barnett, M.D. (New York, N.Y.: Elsevier, 2007)
“Opening cultural doors: Providing culturally sensitive healthcare to Arab American and American Muslim patients” (Am J Obstet Gynecol. 2005 Oct;193]:1307-11).
Characteristics Associated With Active Defects in Juvenile Spondylolysis
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
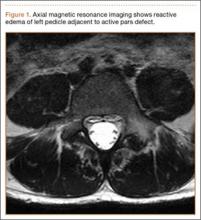
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
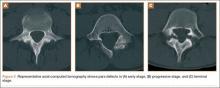
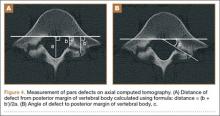
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
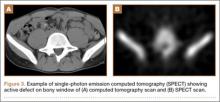
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
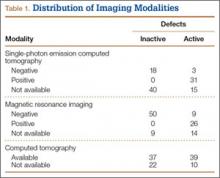
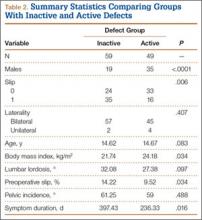
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
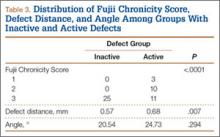
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Current Evidence Does Not Support Medicare’s 3-Day Rule in Primary Total Joint Arthroplasty
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
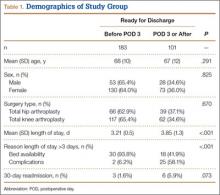
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
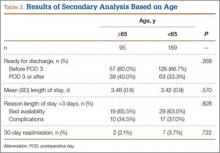
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Dementia risk is 2-fold in type 1 diabetes mellitus patients
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Nursing Home Residents Likely to Suffer Fractures
NEW YORK - Older adults living in long-term care are more than twice as likely as their peers living at home to suffer a fracture, and a new guideline endorsed by the Scientific Advisory Council of Osteoporosis Canada explains how to reduce their risk.
Residents of long-term care tend to be frailer and have more health problems than similar people who live on their own, which explains the higher risk of fractures in long-term care facilities, said lead author Dr. Alexandra Papaioannou of McMaster University and Hamilton Health Sciences in Hamilton, Ontario.
"Up to a third of seniors in long-term care suffer a fracture," often of the hip or spine, she said. For these residents, "long-term care is their home, the nurses know them, and acute care can be a frightening traumatic experience for residents."
The authors developed the new guideline based on input from older people and their families, who most wanted to avoid pain, loss of activity, and hospitalization, Papaioannou said. They also studied published literature on the risks and benefits of strategies to prevent fracture.
The guideline strongly recommends calcium supplementation of 1200 mg or three servings of dairy daily for people older than 70. These calcium levels reduce hip fracture risk and slightly reduce the risk of other fractures, but they may also cause gastrointestinal side effects. For residents who want to avoid these, supplementation may not be a good option, the authors write.
Residents at high risk of fracture, i.e., those with prior fracture of the hip or spine, more than one prior fracture, or one prior fracture and recent use of glucocorticoids, should also take daily vitamin D3 supplements, which are more affordable than vitamin D2, the authors wrote online September 14 in CMAJ.
They also recommend that high-risk residents take alendronate weekly, or risedronate weekly or monthly, as first-line therapy to prevent fractures, as long as they do not have difficulty swallowing and can remain upright for 30 minutes after administration, and they do not have severe renal insufficiency.
For residents who are at high risk of fractures and who have difficulty taking oral medications, they recommend zoledronic acid or denosumab as first-line therapy.
High-risk residents who are mobile should wear hip-protectors, which can protect against fracture in the event of a fall. Low-risk residents who are mobile may wear the devices, depending on their values and preferences.
Balance, strength, and functional exercise can help prevent falls for low-risk residents, and may be useful for high-risk residents, but the exercise itself increases the risk of fall slightly.
Lastly, the authors recommend that all residents have "multifactorial interventions" that are tailored to each individual and include medication reviews, environmental hazard assessment, assistive device use, exercise, and educational interventions for staff.
"Many residents have multiple medical conditions and we need to make sure that they include their lifespan and goals of care in the assessment," Papaioannou said. "The goals of those with short lifespans may be very different from those with longer lifespans."
These recommendations are similar to those for residential care facilities in Australia and the ones made by the Society for Post-Acute and Long-Term Care Medicine in the U.S., the authors point out.
"The document is an excellent guide on how to identify patients at risk, who should be treated and how," said Dr. Gustavo Duque, director of the Musculoskeletal Ageing Research Program at the University of Sydney in Australia.
Doctors often reduce medications for residents who are admitted to nursing homes without realizing that those with osteoporosis or previous fractures are more likely to suffer a fracture at their new residence than in the community, Duque, who was not involved in writing the new guideline, said by email.
"Ceasing osteoporosis treatment has demonstrated to increase the risk of fractures," Duque said. "Unfortunately we see that situation every day."
NEW YORK - Older adults living in long-term care are more than twice as likely as their peers living at home to suffer a fracture, and a new guideline endorsed by the Scientific Advisory Council of Osteoporosis Canada explains how to reduce their risk.
Residents of long-term care tend to be frailer and have more health problems than similar people who live on their own, which explains the higher risk of fractures in long-term care facilities, said lead author Dr. Alexandra Papaioannou of McMaster University and Hamilton Health Sciences in Hamilton, Ontario.
"Up to a third of seniors in long-term care suffer a fracture," often of the hip or spine, she said. For these residents, "long-term care is their home, the nurses know them, and acute care can be a frightening traumatic experience for residents."
The authors developed the new guideline based on input from older people and their families, who most wanted to avoid pain, loss of activity, and hospitalization, Papaioannou said. They also studied published literature on the risks and benefits of strategies to prevent fracture.
The guideline strongly recommends calcium supplementation of 1200 mg or three servings of dairy daily for people older than 70. These calcium levels reduce hip fracture risk and slightly reduce the risk of other fractures, but they may also cause gastrointestinal side effects. For residents who want to avoid these, supplementation may not be a good option, the authors write.
Residents at high risk of fracture, i.e., those with prior fracture of the hip or spine, more than one prior fracture, or one prior fracture and recent use of glucocorticoids, should also take daily vitamin D3 supplements, which are more affordable than vitamin D2, the authors wrote online September 14 in CMAJ.
They also recommend that high-risk residents take alendronate weekly, or risedronate weekly or monthly, as first-line therapy to prevent fractures, as long as they do not have difficulty swallowing and can remain upright for 30 minutes after administration, and they do not have severe renal insufficiency.
For residents who are at high risk of fractures and who have difficulty taking oral medications, they recommend zoledronic acid or denosumab as first-line therapy.
High-risk residents who are mobile should wear hip-protectors, which can protect against fracture in the event of a fall. Low-risk residents who are mobile may wear the devices, depending on their values and preferences.
Balance, strength, and functional exercise can help prevent falls for low-risk residents, and may be useful for high-risk residents, but the exercise itself increases the risk of fall slightly.
Lastly, the authors recommend that all residents have "multifactorial interventions" that are tailored to each individual and include medication reviews, environmental hazard assessment, assistive device use, exercise, and educational interventions for staff.
"Many residents have multiple medical conditions and we need to make sure that they include their lifespan and goals of care in the assessment," Papaioannou said. "The goals of those with short lifespans may be very different from those with longer lifespans."
These recommendations are similar to those for residential care facilities in Australia and the ones made by the Society for Post-Acute and Long-Term Care Medicine in the U.S., the authors point out.
"The document is an excellent guide on how to identify patients at risk, who should be treated and how," said Dr. Gustavo Duque, director of the Musculoskeletal Ageing Research Program at the University of Sydney in Australia.
Doctors often reduce medications for residents who are admitted to nursing homes without realizing that those with osteoporosis or previous fractures are more likely to suffer a fracture at their new residence than in the community, Duque, who was not involved in writing the new guideline, said by email.
"Ceasing osteoporosis treatment has demonstrated to increase the risk of fractures," Duque said. "Unfortunately we see that situation every day."
NEW YORK - Older adults living in long-term care are more than twice as likely as their peers living at home to suffer a fracture, and a new guideline endorsed by the Scientific Advisory Council of Osteoporosis Canada explains how to reduce their risk.
Residents of long-term care tend to be frailer and have more health problems than similar people who live on their own, which explains the higher risk of fractures in long-term care facilities, said lead author Dr. Alexandra Papaioannou of McMaster University and Hamilton Health Sciences in Hamilton, Ontario.
"Up to a third of seniors in long-term care suffer a fracture," often of the hip or spine, she said. For these residents, "long-term care is their home, the nurses know them, and acute care can be a frightening traumatic experience for residents."
The authors developed the new guideline based on input from older people and their families, who most wanted to avoid pain, loss of activity, and hospitalization, Papaioannou said. They also studied published literature on the risks and benefits of strategies to prevent fracture.
The guideline strongly recommends calcium supplementation of 1200 mg or three servings of dairy daily for people older than 70. These calcium levels reduce hip fracture risk and slightly reduce the risk of other fractures, but they may also cause gastrointestinal side effects. For residents who want to avoid these, supplementation may not be a good option, the authors write.
Residents at high risk of fracture, i.e., those with prior fracture of the hip or spine, more than one prior fracture, or one prior fracture and recent use of glucocorticoids, should also take daily vitamin D3 supplements, which are more affordable than vitamin D2, the authors wrote online September 14 in CMAJ.
They also recommend that high-risk residents take alendronate weekly, or risedronate weekly or monthly, as first-line therapy to prevent fractures, as long as they do not have difficulty swallowing and can remain upright for 30 minutes after administration, and they do not have severe renal insufficiency.
For residents who are at high risk of fractures and who have difficulty taking oral medications, they recommend zoledronic acid or denosumab as first-line therapy.
High-risk residents who are mobile should wear hip-protectors, which can protect against fracture in the event of a fall. Low-risk residents who are mobile may wear the devices, depending on their values and preferences.
Balance, strength, and functional exercise can help prevent falls for low-risk residents, and may be useful for high-risk residents, but the exercise itself increases the risk of fall slightly.
Lastly, the authors recommend that all residents have "multifactorial interventions" that are tailored to each individual and include medication reviews, environmental hazard assessment, assistive device use, exercise, and educational interventions for staff.
"Many residents have multiple medical conditions and we need to make sure that they include their lifespan and goals of care in the assessment," Papaioannou said. "The goals of those with short lifespans may be very different from those with longer lifespans."
These recommendations are similar to those for residential care facilities in Australia and the ones made by the Society for Post-Acute and Long-Term Care Medicine in the U.S., the authors point out.
"The document is an excellent guide on how to identify patients at risk, who should be treated and how," said Dr. Gustavo Duque, director of the Musculoskeletal Ageing Research Program at the University of Sydney in Australia.
Doctors often reduce medications for residents who are admitted to nursing homes without realizing that those with osteoporosis or previous fractures are more likely to suffer a fracture at their new residence than in the community, Duque, who was not involved in writing the new guideline, said by email.
"Ceasing osteoporosis treatment has demonstrated to increase the risk of fractures," Duque said. "Unfortunately we see that situation every day."
ACP guidelines aim to improve PE diagnosis
Photo by Angela Mary Butler
Clinicians should stratify patients with suspected acute pulmonary embolism (PE) to ensure use of the appropriate diagnostic strategy, according to guidelines from the American College of Physicians (ACP).
The ACP’s guidelines, published in Annals of Internal Medicine, are designed to help clinicians identify patients who should undergo diagnostic testing for PE—D-dimer and imaging—and those who should not.
“The use of computed tomography (CT) for the evaluation of patients with suspected pulmonary embolism is increasing, despite no evidence that this increased use has led to improved patient outcomes, while exposing patients to unnecessary risks and expense,” said ACP President Wayne J. Riley, MD.
“ACP’s advice is designed to help physicians identify patients for whom a PE is so unlikely that they need no further testing, for whom plasma D-dimer testing can provide additional risk stratification, and for whom imaging is indicated because of their high risk and clinical presentation.”
The guidelines say the first step for clinicians evaluating patients with suspected acute PE is to use a validated clinical prediction rule to estimate the patients’ pre-test probability of PE. The Wells and Geneva rules have been validated and are considered equally accurate in predicting the probability of PE.
In patients who have a low pre-test probability of PE, clinicians should apply the Pulmonary Embolism Rule-Out Criteria (PERC) rule. Clinicians should not obtain D-dimer tests or imaging studies in patients with a low pre-test probability of PE and who meet all 8 PERC.
Patients who have an intermediate pre-test probability of PE or patients with low pre-test probability of PE who do not meet all PERC should have a high sensitivity D-dimer test as the initial step in diagnosis.
Clinicians should not use imaging as the initial test in patients who have a low or intermediate pre-test probability of PE.
Since normal D-dimer levels increase with age, clinicians should use age-adjusted D-dimer thresholds (age times 10 ng/mL rather than a generic 500 ng/mL) in patients older than 50 years to determine whether imaging is warranted.
Clinicians should not use imaging in low- or intermediate-risk patients with a D-dimer below the age-adjusted cutoff.
“While highly sensitive, plasma D-dimer testing is nonspecific, and false-positives can lead to unnecessary imaging,” said guideline author Ali S. Raja, MD, of Massachusetts General Hospital in Boston.
“The use of an age-adjusted threshold resulted in maintenance of sensitivities with improved specificities in all age groups.”
Patients with high pre-test probability of PE should undergo imaging with CT pulmonary angiography. Clinicians should reserve V/Q scans for patients who have a contraindication for CT pulmonary angiography or if CT pulmonary angiography is not available.
Clinicians should avoid obtaining a D-dimer measurement in patients with a high pre-test probability of PE. ![]()

Photo by Angela Mary Butler
Clinicians should stratify patients with suspected acute pulmonary embolism (PE) to ensure use of the appropriate diagnostic strategy, according to guidelines from the American College of Physicians (ACP).
The ACP’s guidelines, published in Annals of Internal Medicine, are designed to help clinicians identify patients who should undergo diagnostic testing for PE—D-dimer and imaging—and those who should not.
“The use of computed tomography (CT) for the evaluation of patients with suspected pulmonary embolism is increasing, despite no evidence that this increased use has led to improved patient outcomes, while exposing patients to unnecessary risks and expense,” said ACP President Wayne J. Riley, MD.
“ACP’s advice is designed to help physicians identify patients for whom a PE is so unlikely that they need no further testing, for whom plasma D-dimer testing can provide additional risk stratification, and for whom imaging is indicated because of their high risk and clinical presentation.”
The guidelines say the first step for clinicians evaluating patients with suspected acute PE is to use a validated clinical prediction rule to estimate the patients’ pre-test probability of PE. The Wells and Geneva rules have been validated and are considered equally accurate in predicting the probability of PE.
In patients who have a low pre-test probability of PE, clinicians should apply the Pulmonary Embolism Rule-Out Criteria (PERC) rule. Clinicians should not obtain D-dimer tests or imaging studies in patients with a low pre-test probability of PE and who meet all 8 PERC.
Patients who have an intermediate pre-test probability of PE or patients with low pre-test probability of PE who do not meet all PERC should have a high sensitivity D-dimer test as the initial step in diagnosis.
Clinicians should not use imaging as the initial test in patients who have a low or intermediate pre-test probability of PE.
Since normal D-dimer levels increase with age, clinicians should use age-adjusted D-dimer thresholds (age times 10 ng/mL rather than a generic 500 ng/mL) in patients older than 50 years to determine whether imaging is warranted.
Clinicians should not use imaging in low- or intermediate-risk patients with a D-dimer below the age-adjusted cutoff.
“While highly sensitive, plasma D-dimer testing is nonspecific, and false-positives can lead to unnecessary imaging,” said guideline author Ali S. Raja, MD, of Massachusetts General Hospital in Boston.
“The use of an age-adjusted threshold resulted in maintenance of sensitivities with improved specificities in all age groups.”
Patients with high pre-test probability of PE should undergo imaging with CT pulmonary angiography. Clinicians should reserve V/Q scans for patients who have a contraindication for CT pulmonary angiography or if CT pulmonary angiography is not available.
Clinicians should avoid obtaining a D-dimer measurement in patients with a high pre-test probability of PE. ![]()

Photo by Angela Mary Butler
Clinicians should stratify patients with suspected acute pulmonary embolism (PE) to ensure use of the appropriate diagnostic strategy, according to guidelines from the American College of Physicians (ACP).
The ACP’s guidelines, published in Annals of Internal Medicine, are designed to help clinicians identify patients who should undergo diagnostic testing for PE—D-dimer and imaging—and those who should not.
“The use of computed tomography (CT) for the evaluation of patients with suspected pulmonary embolism is increasing, despite no evidence that this increased use has led to improved patient outcomes, while exposing patients to unnecessary risks and expense,” said ACP President Wayne J. Riley, MD.
“ACP’s advice is designed to help physicians identify patients for whom a PE is so unlikely that they need no further testing, for whom plasma D-dimer testing can provide additional risk stratification, and for whom imaging is indicated because of their high risk and clinical presentation.”
The guidelines say the first step for clinicians evaluating patients with suspected acute PE is to use a validated clinical prediction rule to estimate the patients’ pre-test probability of PE. The Wells and Geneva rules have been validated and are considered equally accurate in predicting the probability of PE.
In patients who have a low pre-test probability of PE, clinicians should apply the Pulmonary Embolism Rule-Out Criteria (PERC) rule. Clinicians should not obtain D-dimer tests or imaging studies in patients with a low pre-test probability of PE and who meet all 8 PERC.
Patients who have an intermediate pre-test probability of PE or patients with low pre-test probability of PE who do not meet all PERC should have a high sensitivity D-dimer test as the initial step in diagnosis.
Clinicians should not use imaging as the initial test in patients who have a low or intermediate pre-test probability of PE.
Since normal D-dimer levels increase with age, clinicians should use age-adjusted D-dimer thresholds (age times 10 ng/mL rather than a generic 500 ng/mL) in patients older than 50 years to determine whether imaging is warranted.
Clinicians should not use imaging in low- or intermediate-risk patients with a D-dimer below the age-adjusted cutoff.
“While highly sensitive, plasma D-dimer testing is nonspecific, and false-positives can lead to unnecessary imaging,” said guideline author Ali S. Raja, MD, of Massachusetts General Hospital in Boston.
“The use of an age-adjusted threshold resulted in maintenance of sensitivities with improved specificities in all age groups.”
Patients with high pre-test probability of PE should undergo imaging with CT pulmonary angiography. Clinicians should reserve V/Q scans for patients who have a contraindication for CT pulmonary angiography or if CT pulmonary angiography is not available.
Clinicians should avoid obtaining a D-dimer measurement in patients with a high pre-test probability of PE. ![]()
Nonviral gene transfer of CARs tested in humans

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()
Problems in pediatric cancer care in Europe
Photo by Logan Tuttle
VIENNA—Despite progress made in recent years, there are “major problems” in pediatric oncology care in Europe, according to a report from the European Society for Paediatric Oncology (SIOPE).
Cancer is still the first cause of death by disease in children age 1 and older in Europe, and more than 300,000 European citizens are pediatric cancer survivors.
These individuals have a higher risk of death at 5 years after diagnosis than that of the general population.
“This is a serious problem for patients, their families, and for health services, with major inequalities existing across Europe,” said SIOPE President Gilles Vassal, MD, PhD, of the Institut Gustave Roussy in Villejuif, France.
“Add to this the fact that 35% of such cancers normally occur before the child is 5 years old and that many pediatric cancers are difficult to treat, and you will understand why we thought it essential to try to tackle this problem in a practical way.”
The resulting report, “The SIOPE Strategic Plan: A European Cancer Plan for Children and Adolescents,” was recently presented at the 2015 European Cancer Congress.
Problem-solving
The report was drawn up after widespread consultation, including discussions with parents, patients, and survivors. It sets out existing problems and proposes solutions to tackle them.
Among these problems are poor access to new drugs for pediatric patients; lack of funding; disparities across Europe in access to treatment and, hence, survival; and the fact that pediatric oncology has been relatively isolated from the adult oncology community.
With the goal of fixing these problems, the report sets out a number of goals and lists the key factors that will be necessary in order to achieve them.
These include a commitment of all funding bodies to finance projects and structures of relevance to pediatric oncology; a strong partnership with patients, parents, and survivors, including better communication and dissemination of information; better collaboration with adult oncology; and transparent partnerships with industry.
Understanding biology
“One of the most important objectives focuses on increasing our knowledge of the biology of pediatric tumors,” said SIOPE President-Elect Martin Schrappe, MD, of the University of Kiel, Germany.
“Cancers in adults result from a multistep process, usually after exposure to external carcinogens such as tobacco, alcohol, and diet, and often progress over many years. Pediatric malignancies develop early in life and over a much shorter time period. This suggests that fewer and stronger events are required for them to progress. Compared with adult cancers, most of them show fewer genetic defects and have a lower genetic complexity.”
“Major progress has been made in understanding pediatric tumor biology, and this has led to the discovery of some unique cancer hallmarks. Now, we need to use modern, innovative technologies to further decipher the mechanisms of pediatric tumor development, progression, and relapse, and speed up its translation to the clinic.”
To do this effectively and fairly, according to the report, interactions need to be strengthened at several levels—between networks of basic research teams, between basic scientists and clinical researchers, and by increasing the involvement of patients and parents in the search for personalized treatments. SIOPE plans to monitor progress through research into outcomes.
Improving quality of life
Another important issue for SIOPE is improving the quality of life for survivors.
“We believe that, in 2020, there will be nearly half a million European pediatric cancer survivors, and many of them will have side effects that are severe enough to affect their daily lives,” Dr Schrappe said. “While the fact that so many survive is a cause for rejoicing, we have a duty to provide them with optimal long-term care so that the rest of their lives may be as normal as possible.”
“One way of doing this would be the creation of a ‘survivorship passport’ for each child and adolescent cured of a cancer. This would contain a history of their disease and treatment, together with relevant follow-up measures aimed at improving their quality of life and a database for storing the clinical data [that would] facilitate monitoring and research.” ![]()

Photo by Logan Tuttle
VIENNA—Despite progress made in recent years, there are “major problems” in pediatric oncology care in Europe, according to a report from the European Society for Paediatric Oncology (SIOPE).
Cancer is still the first cause of death by disease in children age 1 and older in Europe, and more than 300,000 European citizens are pediatric cancer survivors.
These individuals have a higher risk of death at 5 years after diagnosis than that of the general population.
“This is a serious problem for patients, their families, and for health services, with major inequalities existing across Europe,” said SIOPE President Gilles Vassal, MD, PhD, of the Institut Gustave Roussy in Villejuif, France.
“Add to this the fact that 35% of such cancers normally occur before the child is 5 years old and that many pediatric cancers are difficult to treat, and you will understand why we thought it essential to try to tackle this problem in a practical way.”
The resulting report, “The SIOPE Strategic Plan: A European Cancer Plan for Children and Adolescents,” was recently presented at the 2015 European Cancer Congress.
Problem-solving
The report was drawn up after widespread consultation, including discussions with parents, patients, and survivors. It sets out existing problems and proposes solutions to tackle them.
Among these problems are poor access to new drugs for pediatric patients; lack of funding; disparities across Europe in access to treatment and, hence, survival; and the fact that pediatric oncology has been relatively isolated from the adult oncology community.
With the goal of fixing these problems, the report sets out a number of goals and lists the key factors that will be necessary in order to achieve them.
These include a commitment of all funding bodies to finance projects and structures of relevance to pediatric oncology; a strong partnership with patients, parents, and survivors, including better communication and dissemination of information; better collaboration with adult oncology; and transparent partnerships with industry.
Understanding biology
“One of the most important objectives focuses on increasing our knowledge of the biology of pediatric tumors,” said SIOPE President-Elect Martin Schrappe, MD, of the University of Kiel, Germany.
“Cancers in adults result from a multistep process, usually after exposure to external carcinogens such as tobacco, alcohol, and diet, and often progress over many years. Pediatric malignancies develop early in life and over a much shorter time period. This suggests that fewer and stronger events are required for them to progress. Compared with adult cancers, most of them show fewer genetic defects and have a lower genetic complexity.”
“Major progress has been made in understanding pediatric tumor biology, and this has led to the discovery of some unique cancer hallmarks. Now, we need to use modern, innovative technologies to further decipher the mechanisms of pediatric tumor development, progression, and relapse, and speed up its translation to the clinic.”
To do this effectively and fairly, according to the report, interactions need to be strengthened at several levels—between networks of basic research teams, between basic scientists and clinical researchers, and by increasing the involvement of patients and parents in the search for personalized treatments. SIOPE plans to monitor progress through research into outcomes.
Improving quality of life
Another important issue for SIOPE is improving the quality of life for survivors.
“We believe that, in 2020, there will be nearly half a million European pediatric cancer survivors, and many of them will have side effects that are severe enough to affect their daily lives,” Dr Schrappe said. “While the fact that so many survive is a cause for rejoicing, we have a duty to provide them with optimal long-term care so that the rest of their lives may be as normal as possible.”
“One way of doing this would be the creation of a ‘survivorship passport’ for each child and adolescent cured of a cancer. This would contain a history of their disease and treatment, together with relevant follow-up measures aimed at improving their quality of life and a database for storing the clinical data [that would] facilitate monitoring and research.” ![]()

Photo by Logan Tuttle
VIENNA—Despite progress made in recent years, there are “major problems” in pediatric oncology care in Europe, according to a report from the European Society for Paediatric Oncology (SIOPE).
Cancer is still the first cause of death by disease in children age 1 and older in Europe, and more than 300,000 European citizens are pediatric cancer survivors.
These individuals have a higher risk of death at 5 years after diagnosis than that of the general population.
“This is a serious problem for patients, their families, and for health services, with major inequalities existing across Europe,” said SIOPE President Gilles Vassal, MD, PhD, of the Institut Gustave Roussy in Villejuif, France.
“Add to this the fact that 35% of such cancers normally occur before the child is 5 years old and that many pediatric cancers are difficult to treat, and you will understand why we thought it essential to try to tackle this problem in a practical way.”
The resulting report, “The SIOPE Strategic Plan: A European Cancer Plan for Children and Adolescents,” was recently presented at the 2015 European Cancer Congress.
Problem-solving
The report was drawn up after widespread consultation, including discussions with parents, patients, and survivors. It sets out existing problems and proposes solutions to tackle them.
Among these problems are poor access to new drugs for pediatric patients; lack of funding; disparities across Europe in access to treatment and, hence, survival; and the fact that pediatric oncology has been relatively isolated from the adult oncology community.
With the goal of fixing these problems, the report sets out a number of goals and lists the key factors that will be necessary in order to achieve them.
These include a commitment of all funding bodies to finance projects and structures of relevance to pediatric oncology; a strong partnership with patients, parents, and survivors, including better communication and dissemination of information; better collaboration with adult oncology; and transparent partnerships with industry.
Understanding biology
“One of the most important objectives focuses on increasing our knowledge of the biology of pediatric tumors,” said SIOPE President-Elect Martin Schrappe, MD, of the University of Kiel, Germany.
“Cancers in adults result from a multistep process, usually after exposure to external carcinogens such as tobacco, alcohol, and diet, and often progress over many years. Pediatric malignancies develop early in life and over a much shorter time period. This suggests that fewer and stronger events are required for them to progress. Compared with adult cancers, most of them show fewer genetic defects and have a lower genetic complexity.”
“Major progress has been made in understanding pediatric tumor biology, and this has led to the discovery of some unique cancer hallmarks. Now, we need to use modern, innovative technologies to further decipher the mechanisms of pediatric tumor development, progression, and relapse, and speed up its translation to the clinic.”
To do this effectively and fairly, according to the report, interactions need to be strengthened at several levels—between networks of basic research teams, between basic scientists and clinical researchers, and by increasing the involvement of patients and parents in the search for personalized treatments. SIOPE plans to monitor progress through research into outcomes.
Improving quality of life
Another important issue for SIOPE is improving the quality of life for survivors.
“We believe that, in 2020, there will be nearly half a million European pediatric cancer survivors, and many of them will have side effects that are severe enough to affect their daily lives,” Dr Schrappe said. “While the fact that so many survive is a cause for rejoicing, we have a duty to provide them with optimal long-term care so that the rest of their lives may be as normal as possible.”
“One way of doing this would be the creation of a ‘survivorship passport’ for each child and adolescent cured of a cancer. This would contain a history of their disease and treatment, together with relevant follow-up measures aimed at improving their quality of life and a database for storing the clinical data [that would] facilitate monitoring and research.” ![]()
CHMP endorses expanded indication for azacitidine

Image by Lance Liotta
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for azacitidine for injection (Vidaza).
The CHMP is recommending that azacitidine be approved to treat adults age 65 and older with acute myeloid leukemia (AML) who are not eligible for hematopoietic stem cell transplant (HSCT) and have more than 30% blasts according to the WHO classification.
The CHMP’s recommendation will be reviewed by the European Commission (EC). The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision in 2 months.
The CHMP said this new indication for azacitidine would bring significant clinical benefit in comparison with existing therapies. If the EC follows the CHMP’s recommendation, azacitidine will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
Azacitidine is already approved in the European Economic Area for the treatment of HSCT-ineligible adults diagnosed with intermediate-2- and high-risk myelodysplastic syndromes; chronic myelomonocytic leukemia with 10%-29% marrow blasts without myeloproliferative disorder; or AML with 20%-30% blasts and multi-lineage dysplasia.
AML-001 trial
The CHMP’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle) or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33%, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

Image by Lance Liotta
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for azacitidine for injection (Vidaza).
The CHMP is recommending that azacitidine be approved to treat adults age 65 and older with acute myeloid leukemia (AML) who are not eligible for hematopoietic stem cell transplant (HSCT) and have more than 30% blasts according to the WHO classification.
The CHMP’s recommendation will be reviewed by the European Commission (EC). The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision in 2 months.
The CHMP said this new indication for azacitidine would bring significant clinical benefit in comparison with existing therapies. If the EC follows the CHMP’s recommendation, azacitidine will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
Azacitidine is already approved in the European Economic Area for the treatment of HSCT-ineligible adults diagnosed with intermediate-2- and high-risk myelodysplastic syndromes; chronic myelomonocytic leukemia with 10%-29% marrow blasts without myeloproliferative disorder; or AML with 20%-30% blasts and multi-lineage dysplasia.
AML-001 trial
The CHMP’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle) or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33%, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()

Image by Lance Liotta
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for azacitidine for injection (Vidaza).
The CHMP is recommending that azacitidine be approved to treat adults age 65 and older with acute myeloid leukemia (AML) who are not eligible for hematopoietic stem cell transplant (HSCT) and have more than 30% blasts according to the WHO classification.
The CHMP’s recommendation will be reviewed by the European Commission (EC). The EC usually follows the CHMP’s recommendations and is expected to deliver its final decision in 2 months.
The CHMP said this new indication for azacitidine would bring significant clinical benefit in comparison with existing therapies. If the EC follows the CHMP’s recommendation, azacitidine will receive extended market protection in all its indications for an additional year throughout the European Economic Area.
Azacitidine is already approved in the European Economic Area for the treatment of HSCT-ineligible adults diagnosed with intermediate-2- and high-risk myelodysplastic syndromes; chronic myelomonocytic leukemia with 10%-29% marrow blasts without myeloproliferative disorder; or AML with 20%-30% blasts and multi-lineage dysplasia.
AML-001 trial
The CHMP’s recommendation to expand the indication of azacitidine in AML was based on data from the AML-001 trial. This randomized study included patients age 65 and older with newly diagnosed or secondary AML with greater than 30% blasts.
Patients were pre-selected to receive 1 of 3 regimens per investigator’s choice. This included intensive chemotherapy (standard 7+3 regimen), low-dose cytarabine (20 mg subcutaneously twice a day for 10 days of each 28-day cycle) or best supportive care only.
Patients were then randomized to receive either azacitidine (75 mg/m2/day subcutaneously for 7 days of each 28-day cycle, n=241) or their predetermined conventional care regimen (CCR, n=247).
Median overall survival, the study’s primary endpoint, was 10.4 months for patients receiving azacitidine and 6.5 months for patients receiving CCR (hazard ratio=0.85, P=0.1009).
One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively.
Grade 3/4 anemia occurred in 16% of patients who received azacitidine, 5% who received best supportive care, 23% who received low-dose cytarabine, and 14% who received intensive chemotherapy.
Grade 3/4 neutropenia occurred in 26%, 5%, 25%, and 33%, respectively. Grade 3/4 febrile neutropenia occurred in 28%, 28%, 30%, and 31%, respectively. And grade 3/4 thrombocytopenia occurred in 24%, 5%, 28%, and 21%, respectively. ![]()
My go-to Web resources for quick ICD-10 coding questions
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.