User login
Medicare Rankings Favor Small, For-Profit Hospitals
In April, the Centers for Medicare and Medicaid Services (CMS) publicly revealed for the first time which hospitals achieved five stars and which had room for improvement based on patient experience per the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.
Although these measures are not new, this year CMS assembled the star ratings from HCAHPS survey results and made them available on its consumer-facing website, in an effort to increase transparency.
The decision has not been met without controversy, particularly given the fact that just 251 hospitals out of more than 3,500 received five stars, and only two major teaching hospitals achieved the highest rating. Some professional groups, like the American Hospital Association (AHA), which issued a statement the day CMS released its ratings, believe the rankings risk “oversimplifying the complexity of quality care or misinterpreting what is important to a particular patient, especially since patients seek care for many different reasons.”
Others argue that there is a disconnect between what hospital leaders perceive as important drivers of patient experience and what patients really want. For instance, a 2013 Harvard Business Review article cites a 2012 survey in which C-suite leaders suggested new facilities, private rooms, on-demand food, bedside electronics, and more amenities were necessary to improve patient experience in the hospital.1
“I am surprised at how much controversy there is on this,” says Ashish Jha, MD, MPH, hospitalist at the VA Boston Healthcare System and professor of health policy at the Harvard T.H. Chan School of Public Health. “Modestly good evidence suggests that hospitals that do well on patient experience scores are also the hospitals that have better patient outcomes on more hard measures, like mortality and evidence-based guidelines.”
Dr. Jha cites a February 2015 study, published in the Journal of Hospital Medicine, in which patients were moved from one clinical building to a newer one with more patient-centered features.2 The care team remained the same. The study concluded that patients were able to differentiate between satisfactory clinical care and their surroundings, and that clinical care had a greater impact on patient experience than any other factor.
“Was your pain controlled adequately? Were people responsive to your needs? Were you treated with dignity and respect?” Dr. Jha says. “I think it’s disrespectful to say patients can’t tell the difference between high thread-count bed sheets and being treated with disrespect.”
The HCAHPS survey, Dr. Jha notes, reflects important aspects of healthcare that only patients can report. It encompasses 11 measures that gauge, for example, how well patients felt nurses and physicians communicated with them. It also asks patients to provide an overall hospital rank on a 10-point scale (counting only those that receive a nine or 10), according to Kaiser Health News, and to rate the cleanliness and quietness of the rooms.
Hospitals must send surveys to a random sample of adult patients monthly, including those not on Medicare, within six weeks of discharge, and Inpatient Prospective Payment System hospitals should collect at least 300 surveys every four years, CMS says.
“I think one of the most important things for a hospital to understand is [that] the methodology behind creating the star ratings and the way CMS structures the ratings does make it challenging to achieve the very highest score,” says Akin Demehin, AHA senior associate director of policy.
While CMS applies adjustments to account for sampling methods and patient characteristics of hospitals, an analysis by Dr. Jha’s team showed significant disparities between the rankings of large, academic medical centers and those of small, for-profit hospitals, as well as a substantial difference between hospitals that provide for the greatest number of poor patients and those that serve the fewest.3
However, he writes on his blog, "An Ounce of Evidence," that survey methodology is not the problem and that he believes star ratings are a good idea. Although some hospitals might find themselves at score cut-offs—a one-point difference can translate to a full star change—it’s a “small price to pay to make data more accessible to patients,” he writes.
“There is pretty good evidence hospitals are paying attention, and one that gets a one or two-star rating may be motivated to be better,” Dr. Jha says.
“Every hospital is interested in this because it’s part of value-based purchasing,” says Trina Dorrah, MD, MPH, a hospitalist and director of quality at Baylor Scott & White Health in Round Rock, Texas.
Dr. Dorrah has authored two books focused on patient experience, and she suggests simple ways hospitals can work toward improving their HCAHPS scores, and potentially their star ratings, from having nurses round with physicians to installing communication-facilitating whiteboards in every room.
Her hospital also awards bonuses to the hospitalist group for achieving set goals. Some hospitalist programs around the country are also adding questions to their surveys to link individual providers to patient rankings, she said, though many also do it in aggregate, because linking patients to individual physicians can get “very messy.”
CMS advises caution in interpreting star rankings, acknowledging that they are not the only valuable measures of care quality. Despite the concern over the contextual value of the new rankings, Demehin says AHA supports use of the HCAHPS survey and the value of patient experience measures and believes they should be consulted in conjunction with other quality improvement efforts.
“When I’m really sick and I go to the hospital, I want to be treated with dignity and respect and I want my pain treated quickly, but I also want to survive and not develop an infection,” Dr. Jha says. “That’s obviously not in the star ratings.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Merlino J, Raman A. Understanding the drivers of the patient experience. Harvard Business Review. Sept. 17, 2013. Accessed May 14, 2015.
- Siddiqui Z, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: Experience with a new clinical building. J Hosp Med. 2015;10(3):165-171.
- Jha A. Finding the stars of hospital care in the U.S. An Ounce of Evidence blog. April 20, 2015. Accessed June 4, 2015.
In April, the Centers for Medicare and Medicaid Services (CMS) publicly revealed for the first time which hospitals achieved five stars and which had room for improvement based on patient experience per the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.
Although these measures are not new, this year CMS assembled the star ratings from HCAHPS survey results and made them available on its consumer-facing website, in an effort to increase transparency.
The decision has not been met without controversy, particularly given the fact that just 251 hospitals out of more than 3,500 received five stars, and only two major teaching hospitals achieved the highest rating. Some professional groups, like the American Hospital Association (AHA), which issued a statement the day CMS released its ratings, believe the rankings risk “oversimplifying the complexity of quality care or misinterpreting what is important to a particular patient, especially since patients seek care for many different reasons.”
Others argue that there is a disconnect between what hospital leaders perceive as important drivers of patient experience and what patients really want. For instance, a 2013 Harvard Business Review article cites a 2012 survey in which C-suite leaders suggested new facilities, private rooms, on-demand food, bedside electronics, and more amenities were necessary to improve patient experience in the hospital.1
“I am surprised at how much controversy there is on this,” says Ashish Jha, MD, MPH, hospitalist at the VA Boston Healthcare System and professor of health policy at the Harvard T.H. Chan School of Public Health. “Modestly good evidence suggests that hospitals that do well on patient experience scores are also the hospitals that have better patient outcomes on more hard measures, like mortality and evidence-based guidelines.”
Dr. Jha cites a February 2015 study, published in the Journal of Hospital Medicine, in which patients were moved from one clinical building to a newer one with more patient-centered features.2 The care team remained the same. The study concluded that patients were able to differentiate between satisfactory clinical care and their surroundings, and that clinical care had a greater impact on patient experience than any other factor.
“Was your pain controlled adequately? Were people responsive to your needs? Were you treated with dignity and respect?” Dr. Jha says. “I think it’s disrespectful to say patients can’t tell the difference between high thread-count bed sheets and being treated with disrespect.”
The HCAHPS survey, Dr. Jha notes, reflects important aspects of healthcare that only patients can report. It encompasses 11 measures that gauge, for example, how well patients felt nurses and physicians communicated with them. It also asks patients to provide an overall hospital rank on a 10-point scale (counting only those that receive a nine or 10), according to Kaiser Health News, and to rate the cleanliness and quietness of the rooms.
Hospitals must send surveys to a random sample of adult patients monthly, including those not on Medicare, within six weeks of discharge, and Inpatient Prospective Payment System hospitals should collect at least 300 surveys every four years, CMS says.
“I think one of the most important things for a hospital to understand is [that] the methodology behind creating the star ratings and the way CMS structures the ratings does make it challenging to achieve the very highest score,” says Akin Demehin, AHA senior associate director of policy.
While CMS applies adjustments to account for sampling methods and patient characteristics of hospitals, an analysis by Dr. Jha’s team showed significant disparities between the rankings of large, academic medical centers and those of small, for-profit hospitals, as well as a substantial difference between hospitals that provide for the greatest number of poor patients and those that serve the fewest.3
However, he writes on his blog, "An Ounce of Evidence," that survey methodology is not the problem and that he believes star ratings are a good idea. Although some hospitals might find themselves at score cut-offs—a one-point difference can translate to a full star change—it’s a “small price to pay to make data more accessible to patients,” he writes.
“There is pretty good evidence hospitals are paying attention, and one that gets a one or two-star rating may be motivated to be better,” Dr. Jha says.
“Every hospital is interested in this because it’s part of value-based purchasing,” says Trina Dorrah, MD, MPH, a hospitalist and director of quality at Baylor Scott & White Health in Round Rock, Texas.
Dr. Dorrah has authored two books focused on patient experience, and she suggests simple ways hospitals can work toward improving their HCAHPS scores, and potentially their star ratings, from having nurses round with physicians to installing communication-facilitating whiteboards in every room.
Her hospital also awards bonuses to the hospitalist group for achieving set goals. Some hospitalist programs around the country are also adding questions to their surveys to link individual providers to patient rankings, she said, though many also do it in aggregate, because linking patients to individual physicians can get “very messy.”
CMS advises caution in interpreting star rankings, acknowledging that they are not the only valuable measures of care quality. Despite the concern over the contextual value of the new rankings, Demehin says AHA supports use of the HCAHPS survey and the value of patient experience measures and believes they should be consulted in conjunction with other quality improvement efforts.
“When I’m really sick and I go to the hospital, I want to be treated with dignity and respect and I want my pain treated quickly, but I also want to survive and not develop an infection,” Dr. Jha says. “That’s obviously not in the star ratings.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Merlino J, Raman A. Understanding the drivers of the patient experience. Harvard Business Review. Sept. 17, 2013. Accessed May 14, 2015.
- Siddiqui Z, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: Experience with a new clinical building. J Hosp Med. 2015;10(3):165-171.
- Jha A. Finding the stars of hospital care in the U.S. An Ounce of Evidence blog. April 20, 2015. Accessed June 4, 2015.
In April, the Centers for Medicare and Medicaid Services (CMS) publicly revealed for the first time which hospitals achieved five stars and which had room for improvement based on patient experience per the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.
Although these measures are not new, this year CMS assembled the star ratings from HCAHPS survey results and made them available on its consumer-facing website, in an effort to increase transparency.
The decision has not been met without controversy, particularly given the fact that just 251 hospitals out of more than 3,500 received five stars, and only two major teaching hospitals achieved the highest rating. Some professional groups, like the American Hospital Association (AHA), which issued a statement the day CMS released its ratings, believe the rankings risk “oversimplifying the complexity of quality care or misinterpreting what is important to a particular patient, especially since patients seek care for many different reasons.”
Others argue that there is a disconnect between what hospital leaders perceive as important drivers of patient experience and what patients really want. For instance, a 2013 Harvard Business Review article cites a 2012 survey in which C-suite leaders suggested new facilities, private rooms, on-demand food, bedside electronics, and more amenities were necessary to improve patient experience in the hospital.1
“I am surprised at how much controversy there is on this,” says Ashish Jha, MD, MPH, hospitalist at the VA Boston Healthcare System and professor of health policy at the Harvard T.H. Chan School of Public Health. “Modestly good evidence suggests that hospitals that do well on patient experience scores are also the hospitals that have better patient outcomes on more hard measures, like mortality and evidence-based guidelines.”
Dr. Jha cites a February 2015 study, published in the Journal of Hospital Medicine, in which patients were moved from one clinical building to a newer one with more patient-centered features.2 The care team remained the same. The study concluded that patients were able to differentiate between satisfactory clinical care and their surroundings, and that clinical care had a greater impact on patient experience than any other factor.
“Was your pain controlled adequately? Were people responsive to your needs? Were you treated with dignity and respect?” Dr. Jha says. “I think it’s disrespectful to say patients can’t tell the difference between high thread-count bed sheets and being treated with disrespect.”
The HCAHPS survey, Dr. Jha notes, reflects important aspects of healthcare that only patients can report. It encompasses 11 measures that gauge, for example, how well patients felt nurses and physicians communicated with them. It also asks patients to provide an overall hospital rank on a 10-point scale (counting only those that receive a nine or 10), according to Kaiser Health News, and to rate the cleanliness and quietness of the rooms.
Hospitals must send surveys to a random sample of adult patients monthly, including those not on Medicare, within six weeks of discharge, and Inpatient Prospective Payment System hospitals should collect at least 300 surveys every four years, CMS says.
“I think one of the most important things for a hospital to understand is [that] the methodology behind creating the star ratings and the way CMS structures the ratings does make it challenging to achieve the very highest score,” says Akin Demehin, AHA senior associate director of policy.
While CMS applies adjustments to account for sampling methods and patient characteristics of hospitals, an analysis by Dr. Jha’s team showed significant disparities between the rankings of large, academic medical centers and those of small, for-profit hospitals, as well as a substantial difference between hospitals that provide for the greatest number of poor patients and those that serve the fewest.3
However, he writes on his blog, "An Ounce of Evidence," that survey methodology is not the problem and that he believes star ratings are a good idea. Although some hospitals might find themselves at score cut-offs—a one-point difference can translate to a full star change—it’s a “small price to pay to make data more accessible to patients,” he writes.
“There is pretty good evidence hospitals are paying attention, and one that gets a one or two-star rating may be motivated to be better,” Dr. Jha says.
“Every hospital is interested in this because it’s part of value-based purchasing,” says Trina Dorrah, MD, MPH, a hospitalist and director of quality at Baylor Scott & White Health in Round Rock, Texas.
Dr. Dorrah has authored two books focused on patient experience, and she suggests simple ways hospitals can work toward improving their HCAHPS scores, and potentially their star ratings, from having nurses round with physicians to installing communication-facilitating whiteboards in every room.
Her hospital also awards bonuses to the hospitalist group for achieving set goals. Some hospitalist programs around the country are also adding questions to their surveys to link individual providers to patient rankings, she said, though many also do it in aggregate, because linking patients to individual physicians can get “very messy.”
CMS advises caution in interpreting star rankings, acknowledging that they are not the only valuable measures of care quality. Despite the concern over the contextual value of the new rankings, Demehin says AHA supports use of the HCAHPS survey and the value of patient experience measures and believes they should be consulted in conjunction with other quality improvement efforts.
“When I’m really sick and I go to the hospital, I want to be treated with dignity and respect and I want my pain treated quickly, but I also want to survive and not develop an infection,” Dr. Jha says. “That’s obviously not in the star ratings.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Merlino J, Raman A. Understanding the drivers of the patient experience. Harvard Business Review. Sept. 17, 2013. Accessed May 14, 2015.
- Siddiqui Z, Zuccarelli R, Durkin N, Wu AW, Brotman DJ. Changes in patient satisfaction related to hospital renovation: Experience with a new clinical building. J Hosp Med. 2015;10(3):165-171.
- Jha A. Finding the stars of hospital care in the U.S. An Ounce of Evidence blog. April 20, 2015. Accessed June 4, 2015.
The Hospital Leader Explores Ways Hospitalists Can Tackle Healthcare Costs
EDITOR’S NOTE: A version of this article originally appeared on Medpage Today on May 5, 2015.
By now, we have all heard the stories about unconscionable medical bills causing financial harm for patients. We have read about more Americans than ever before on high-deductible health insurance plans. Some of us have even helped our parents navigate the deceptively simple-looking bronze, silver, and gold tiers of the insurance exchanges, weighing the gamble of increasingly unaffordable monthly premiums against catastrophically high deductibles and out-of-pocket costs.
We have accepted the fact that healthcare costs are out of control and causing real constraints on every level, from individuals to communities to businesses to states to our nation.
OK, but now what are we supposed to do about it?
“Remarkably, given the importance of this issue, until now, we lacked a roadmap to attack it,” wrote Bob Wachter, MD, in the foreword to our new book, Understanding Value-Based Healthcare. “Now we have one.”
For starters, we can supply a pipeline for change by embedding the principles of value-based care in the apprenticeship of health professional education. Recently, the leaders of the soon-to-open Dell Medical School at University of Texas-Austin articulated their plan to build their entire curriculum around teaching students to root out waste and to care for the health of the community. This is the clearest example that medical educators are taking seriously the calls to action coming from the Accreditation Council for Graduate Medical Education (ACGME), the Association of American Medical Colleges (AAMC), the American College of Physicians (ACP), and other leaders to address healthcare value in training very seriously. However, the front lines are not waiting for new medical schools to open up or for massive curricular overhauls. The Second Annual Teaching Value and Choosing Wisely Challenge that we organized resulted in 80 submissions spanning the country (and Canada). The authors, including five students, 30 residents or fellows, and at least 41 faculty members, described their bright ideas and innovations for integrating healthcare value into education.
Education is fundamental but will not be enough. We must also practice what we preach. Practicing clinicians can deflate medical bills for their patients by advocating for appropriate care, considering patient affordability in customizing treatments, and leading local initiatives to improve value of care.
Clinicians can advocate for appropriate care by avoiding low-value services at the point of care. Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. Physicians will need to understand the true risks and benefits of recommended therapies and learn ways to communicate this balance with patients.
Patient affordability is increasingly important, with more patients now facing astronomically high out-of-pocket bills, even for simple medical treatments or procedures. A December 2014 CBS/New York Times poll found that 80% of Americans now think their doctor should discuss the cost of recommended medical treatment with them ahead of time. Clinicians can screen their patients for financial harm and can help them navigate the trade-offs of lower cost options. Physicians should seek to provide high-value prescribing, which entails recommending the simplest medication regimen that minimizes physical and financial risk to the patient while achieving the best outcome. In other words, decreasing either cost, complexity, or risk of medications can improve value—and clinicians should aim to improve all three simultaneously.
In addition to reducing waste and considering patient affordability, clinicians are ideal leaders of local value initiatives, whether they accomplish this by running value improvement projects or by launching formal high-value care programs. Our framework to guide value improvement project design is “COST”: culture, oversight accountability, system support, and training. This approach leverages principles from implementation science to ensure that value improvement projects successfully provide multi-pronged tactics for overcoming the many barriers to high-value care delivery. At some locations across the country, individual efforts have matured into entire groups dedicated to designing and implementing value-improvement initiatives, including the UCSF High Value Care Committee, the Johns Hopkins High-Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering (PRO), and “High-Value Carolina” in North Carolina.
Health professionals are faced with a responsibility to help deflate medical bills. To achieve this goal, clinicians can advocate for appropriate care, consider patient affordability, and lead local value improvement initiatives. For those ready to tackle this challenge, we elaborate on and explain some of these necessary tools in our book, Understanding Value-Based Healthcare.
EDITOR’S NOTE: A version of this article originally appeared on Medpage Today on May 5, 2015.
By now, we have all heard the stories about unconscionable medical bills causing financial harm for patients. We have read about more Americans than ever before on high-deductible health insurance plans. Some of us have even helped our parents navigate the deceptively simple-looking bronze, silver, and gold tiers of the insurance exchanges, weighing the gamble of increasingly unaffordable monthly premiums against catastrophically high deductibles and out-of-pocket costs.
We have accepted the fact that healthcare costs are out of control and causing real constraints on every level, from individuals to communities to businesses to states to our nation.
OK, but now what are we supposed to do about it?
“Remarkably, given the importance of this issue, until now, we lacked a roadmap to attack it,” wrote Bob Wachter, MD, in the foreword to our new book, Understanding Value-Based Healthcare. “Now we have one.”
For starters, we can supply a pipeline for change by embedding the principles of value-based care in the apprenticeship of health professional education. Recently, the leaders of the soon-to-open Dell Medical School at University of Texas-Austin articulated their plan to build their entire curriculum around teaching students to root out waste and to care for the health of the community. This is the clearest example that medical educators are taking seriously the calls to action coming from the Accreditation Council for Graduate Medical Education (ACGME), the Association of American Medical Colleges (AAMC), the American College of Physicians (ACP), and other leaders to address healthcare value in training very seriously. However, the front lines are not waiting for new medical schools to open up or for massive curricular overhauls. The Second Annual Teaching Value and Choosing Wisely Challenge that we organized resulted in 80 submissions spanning the country (and Canada). The authors, including five students, 30 residents or fellows, and at least 41 faculty members, described their bright ideas and innovations for integrating healthcare value into education.
Education is fundamental but will not be enough. We must also practice what we preach. Practicing clinicians can deflate medical bills for their patients by advocating for appropriate care, considering patient affordability in customizing treatments, and leading local initiatives to improve value of care.
Clinicians can advocate for appropriate care by avoiding low-value services at the point of care. Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. Physicians will need to understand the true risks and benefits of recommended therapies and learn ways to communicate this balance with patients.
Patient affordability is increasingly important, with more patients now facing astronomically high out-of-pocket bills, even for simple medical treatments or procedures. A December 2014 CBS/New York Times poll found that 80% of Americans now think their doctor should discuss the cost of recommended medical treatment with them ahead of time. Clinicians can screen their patients for financial harm and can help them navigate the trade-offs of lower cost options. Physicians should seek to provide high-value prescribing, which entails recommending the simplest medication regimen that minimizes physical and financial risk to the patient while achieving the best outcome. In other words, decreasing either cost, complexity, or risk of medications can improve value—and clinicians should aim to improve all three simultaneously.
In addition to reducing waste and considering patient affordability, clinicians are ideal leaders of local value initiatives, whether they accomplish this by running value improvement projects or by launching formal high-value care programs. Our framework to guide value improvement project design is “COST”: culture, oversight accountability, system support, and training. This approach leverages principles from implementation science to ensure that value improvement projects successfully provide multi-pronged tactics for overcoming the many barriers to high-value care delivery. At some locations across the country, individual efforts have matured into entire groups dedicated to designing and implementing value-improvement initiatives, including the UCSF High Value Care Committee, the Johns Hopkins High-Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering (PRO), and “High-Value Carolina” in North Carolina.
Health professionals are faced with a responsibility to help deflate medical bills. To achieve this goal, clinicians can advocate for appropriate care, consider patient affordability, and lead local value improvement initiatives. For those ready to tackle this challenge, we elaborate on and explain some of these necessary tools in our book, Understanding Value-Based Healthcare.
EDITOR’S NOTE: A version of this article originally appeared on Medpage Today on May 5, 2015.
By now, we have all heard the stories about unconscionable medical bills causing financial harm for patients. We have read about more Americans than ever before on high-deductible health insurance plans. Some of us have even helped our parents navigate the deceptively simple-looking bronze, silver, and gold tiers of the insurance exchanges, weighing the gamble of increasingly unaffordable monthly premiums against catastrophically high deductibles and out-of-pocket costs.
We have accepted the fact that healthcare costs are out of control and causing real constraints on every level, from individuals to communities to businesses to states to our nation.
OK, but now what are we supposed to do about it?
“Remarkably, given the importance of this issue, until now, we lacked a roadmap to attack it,” wrote Bob Wachter, MD, in the foreword to our new book, Understanding Value-Based Healthcare. “Now we have one.”
For starters, we can supply a pipeline for change by embedding the principles of value-based care in the apprenticeship of health professional education. Recently, the leaders of the soon-to-open Dell Medical School at University of Texas-Austin articulated their plan to build their entire curriculum around teaching students to root out waste and to care for the health of the community. This is the clearest example that medical educators are taking seriously the calls to action coming from the Accreditation Council for Graduate Medical Education (ACGME), the Association of American Medical Colleges (AAMC), the American College of Physicians (ACP), and other leaders to address healthcare value in training very seriously. However, the front lines are not waiting for new medical schools to open up or for massive curricular overhauls. The Second Annual Teaching Value and Choosing Wisely Challenge that we organized resulted in 80 submissions spanning the country (and Canada). The authors, including five students, 30 residents or fellows, and at least 41 faculty members, described their bright ideas and innovations for integrating healthcare value into education.
Education is fundamental but will not be enough. We must also practice what we preach. Practicing clinicians can deflate medical bills for their patients by advocating for appropriate care, considering patient affordability in customizing treatments, and leading local initiatives to improve value of care.
Clinicians can advocate for appropriate care by avoiding low-value services at the point of care. Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. Physicians will need to understand the true risks and benefits of recommended therapies and learn ways to communicate this balance with patients.
Patient affordability is increasingly important, with more patients now facing astronomically high out-of-pocket bills, even for simple medical treatments or procedures. A December 2014 CBS/New York Times poll found that 80% of Americans now think their doctor should discuss the cost of recommended medical treatment with them ahead of time. Clinicians can screen their patients for financial harm and can help them navigate the trade-offs of lower cost options. Physicians should seek to provide high-value prescribing, which entails recommending the simplest medication regimen that minimizes physical and financial risk to the patient while achieving the best outcome. In other words, decreasing either cost, complexity, or risk of medications can improve value—and clinicians should aim to improve all three simultaneously.
In addition to reducing waste and considering patient affordability, clinicians are ideal leaders of local value initiatives, whether they accomplish this by running value improvement projects or by launching formal high-value care programs. Our framework to guide value improvement project design is “COST”: culture, oversight accountability, system support, and training. This approach leverages principles from implementation science to ensure that value improvement projects successfully provide multi-pronged tactics for overcoming the many barriers to high-value care delivery. At some locations across the country, individual efforts have matured into entire groups dedicated to designing and implementing value-improvement initiatives, including the UCSF High Value Care Committee, the Johns Hopkins High-Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering (PRO), and “High-Value Carolina” in North Carolina.
Health professionals are faced with a responsibility to help deflate medical bills. To achieve this goal, clinicians can advocate for appropriate care, consider patient affordability, and lead local value improvement initiatives. For those ready to tackle this challenge, we elaborate on and explain some of these necessary tools in our book, Understanding Value-Based Healthcare.
SGR Repeal: What It Means for Hospitalists
On April 16, President Obama signed into law a bipartisan, bicameral piece of legislation that not only fully repealed the sustainable growth rate (SGR) but also permanently eliminated the recurring threat of physician payment cuts in Medicare.
Along with the SGR repeal, the Medicare Access and CHIP Reauthorization Act, or MACRA, institutes the Merit-based Incentive Payment System (MIPS). Starting in 2019, the MIPS will consolidate all of Medicare’s current quality reporting programs: the Physician Quality Reporting System (PQRS), the Value-Based Payment Modifier (VBPM) Program, and the meaningful use (MU) requirements, and will restructure their associated penalties.
Under current law, however, physicians are still required to participate in PQRS, VBPM, and MU, or face their associated penalties until the MIPS is fully implemented in 2019. MACRA also incentivizes the adoption of alternative payment models (APMs). APMs are broadly defined within the law as models that involve both upside and downside financial risk (e.g. ACOs or bundled payments) or patient-centered medical homes, provided they improve quality without increasing costs or lower costs without decreasing quality. Those participating in and deriving substantial revenue from an approved APM will not only be exempt from reporting within the MIPS, but will also receive an automatic 5% bonus in their Medicare billing.
Pay-for-performance programs lack relevant quality metrics and are structured in ways that do not account for the realities of providing inpatient care, which increasingly result in headaches for hospitalists. MACRA has the potential to alleviate this burden and reshape the way in which hospitalists are measured.
SHM worked closely with key Congressional committees, as they were developing the SGR repeal legislation, to include flexible language that could better align quality measures for hospitalists. As a result, buried deep in the text of MACRA lies a two-sentence section that makes this goal possible. The law authorizes the “use [of] measures used for a payment system other than for physicians, such as measures for inpatient hospitals, for the purposes of the performance categories [quality and resource use].” Permitting the use of measures from other payment systems allows hospitalists to have the opportunity to align their quality and resource use performance with that of their institutions. As this alignment is not allowed under current law, it brings new potential to level the playing field and increase the relevance of hospitalist quality reporting in the future.
SHM has been pressing CMS to pursue this concept for the last three years, and MACRA finally gives CMS clear authority to move ahead.
The law is not overly specific, so it is not exactly clear how this provision will be implemented. SHM will remain vigilant, working with CMS to ensure that the MIPS-related regulations set the stage for more fair assessment of hospitalists when the MIPS goes into effect in 2019.
Although hospitalists face an uphill battle in terms of current PQRS reporting, the flexibility contained in MACRA provides an important first step toward a better pathway to reporting quality measures that are fair and relevant for hospitalists.
Ellen Boyer is SHM’s government relations project coordinator.
On April 16, President Obama signed into law a bipartisan, bicameral piece of legislation that not only fully repealed the sustainable growth rate (SGR) but also permanently eliminated the recurring threat of physician payment cuts in Medicare.
Along with the SGR repeal, the Medicare Access and CHIP Reauthorization Act, or MACRA, institutes the Merit-based Incentive Payment System (MIPS). Starting in 2019, the MIPS will consolidate all of Medicare’s current quality reporting programs: the Physician Quality Reporting System (PQRS), the Value-Based Payment Modifier (VBPM) Program, and the meaningful use (MU) requirements, and will restructure their associated penalties.
Under current law, however, physicians are still required to participate in PQRS, VBPM, and MU, or face their associated penalties until the MIPS is fully implemented in 2019. MACRA also incentivizes the adoption of alternative payment models (APMs). APMs are broadly defined within the law as models that involve both upside and downside financial risk (e.g. ACOs or bundled payments) or patient-centered medical homes, provided they improve quality without increasing costs or lower costs without decreasing quality. Those participating in and deriving substantial revenue from an approved APM will not only be exempt from reporting within the MIPS, but will also receive an automatic 5% bonus in their Medicare billing.
Pay-for-performance programs lack relevant quality metrics and are structured in ways that do not account for the realities of providing inpatient care, which increasingly result in headaches for hospitalists. MACRA has the potential to alleviate this burden and reshape the way in which hospitalists are measured.
SHM worked closely with key Congressional committees, as they were developing the SGR repeal legislation, to include flexible language that could better align quality measures for hospitalists. As a result, buried deep in the text of MACRA lies a two-sentence section that makes this goal possible. The law authorizes the “use [of] measures used for a payment system other than for physicians, such as measures for inpatient hospitals, for the purposes of the performance categories [quality and resource use].” Permitting the use of measures from other payment systems allows hospitalists to have the opportunity to align their quality and resource use performance with that of their institutions. As this alignment is not allowed under current law, it brings new potential to level the playing field and increase the relevance of hospitalist quality reporting in the future.
SHM has been pressing CMS to pursue this concept for the last three years, and MACRA finally gives CMS clear authority to move ahead.
The law is not overly specific, so it is not exactly clear how this provision will be implemented. SHM will remain vigilant, working with CMS to ensure that the MIPS-related regulations set the stage for more fair assessment of hospitalists when the MIPS goes into effect in 2019.
Although hospitalists face an uphill battle in terms of current PQRS reporting, the flexibility contained in MACRA provides an important first step toward a better pathway to reporting quality measures that are fair and relevant for hospitalists.
Ellen Boyer is SHM’s government relations project coordinator.
On April 16, President Obama signed into law a bipartisan, bicameral piece of legislation that not only fully repealed the sustainable growth rate (SGR) but also permanently eliminated the recurring threat of physician payment cuts in Medicare.
Along with the SGR repeal, the Medicare Access and CHIP Reauthorization Act, or MACRA, institutes the Merit-based Incentive Payment System (MIPS). Starting in 2019, the MIPS will consolidate all of Medicare’s current quality reporting programs: the Physician Quality Reporting System (PQRS), the Value-Based Payment Modifier (VBPM) Program, and the meaningful use (MU) requirements, and will restructure their associated penalties.
Under current law, however, physicians are still required to participate in PQRS, VBPM, and MU, or face their associated penalties until the MIPS is fully implemented in 2019. MACRA also incentivizes the adoption of alternative payment models (APMs). APMs are broadly defined within the law as models that involve both upside and downside financial risk (e.g. ACOs or bundled payments) or patient-centered medical homes, provided they improve quality without increasing costs or lower costs without decreasing quality. Those participating in and deriving substantial revenue from an approved APM will not only be exempt from reporting within the MIPS, but will also receive an automatic 5% bonus in their Medicare billing.
Pay-for-performance programs lack relevant quality metrics and are structured in ways that do not account for the realities of providing inpatient care, which increasingly result in headaches for hospitalists. MACRA has the potential to alleviate this burden and reshape the way in which hospitalists are measured.
SHM worked closely with key Congressional committees, as they were developing the SGR repeal legislation, to include flexible language that could better align quality measures for hospitalists. As a result, buried deep in the text of MACRA lies a two-sentence section that makes this goal possible. The law authorizes the “use [of] measures used for a payment system other than for physicians, such as measures for inpatient hospitals, for the purposes of the performance categories [quality and resource use].” Permitting the use of measures from other payment systems allows hospitalists to have the opportunity to align their quality and resource use performance with that of their institutions. As this alignment is not allowed under current law, it brings new potential to level the playing field and increase the relevance of hospitalist quality reporting in the future.
SHM has been pressing CMS to pursue this concept for the last three years, and MACRA finally gives CMS clear authority to move ahead.
The law is not overly specific, so it is not exactly clear how this provision will be implemented. SHM will remain vigilant, working with CMS to ensure that the MIPS-related regulations set the stage for more fair assessment of hospitalists when the MIPS goes into effect in 2019.
Although hospitalists face an uphill battle in terms of current PQRS reporting, the flexibility contained in MACRA provides an important first step toward a better pathway to reporting quality measures that are fair and relevant for hospitalists.
Ellen Boyer is SHM’s government relations project coordinator.
Hospitalists Play Key Role Developing Electronic Health Records
If you are a hospitalist, you know this to be true: Electronic health records (EHRs) run more smoothly because of our input. According to the 2014 State of Hospital Medicine (SOHM) report, hospitalists played a significant role in implementing their hospitals’ EHR systems; 34% of respondent hospital medicine groups (HMGs) serving adults only develop order sets, protocols, and decision support, while 28.3% of HMGs serve as super users. As super users, hospitalists are role models who help other physicians work successfully with the computerized systems and set the tone for acceptance within their medical staff and the larger hospital staff.
When EHRs were implemented a decade ago at Allina Health, a 14-hospital system headquartered in Minneapolis, hospitalists acted as super users and were able to interface closely with IT staff, quickly fixing computer troubles that physicians encountered. We partnered with IT to help them prioritize their work by letting them know which computer issues involved patient safety, which were high frequency, irksome issues, and which EHR quirks could be queued farther down the line for repair.
Although successful EHR implementation is a mammoth accomplishment, it is just the tip of the iceberg. Once a system is implemented, an unending amount of work is needed to make sure EHRs deliver, through data analysis and standardization of care, all the efficiency, safety, and cost containment we hoped for at their inception.
When hospitalists at Allina were asked to be the first group of physicians to convert to professional fee billing using the EHR, we found that we needed to create a check-and-balance reporting system to ensure that our charges were indeed being captured. All of our health system physicians now use the hospitalist-developed reporting system.
Hospitalists in our system also forged the way to getting meaningful data out of the EHR system. When we first implemented our EHR, we found it difficult to get hospitalist-specific data. We worked closely with data analytics staff to develop a hospitalist “flag” that would differentiate hospitalists from other physicians providing inpatient care.
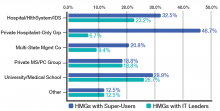
Because hospitalists are involved with patients through all phases of their care, from admission to discharge, they encounter firsthand the benefits and glitches of EHRs. By the very nature of our work, we are positioned to help improve EHRs. Hospitalist input ranges from informal feedback to the IT department to participation in focused work groups dedicated to specific aspects of improving patient care. Not surprisingly, 18.4% of adults-only HMGs who participated in the last SOHM survey have hospitalists serving in an IT leadership role for their organizations.
When looked at by ownership/employment models (see Table 1), this number is higher for the “University, Medical School, or Faculty Practice Plan” (26.7%) and for the “Hospital, Health System, or Integrated Delivery System” (23.2%) and lower for the “Private Local/Regional Hospitalist-Only Medical Groups” (6.7%).
Interestingly, the “Private Local/Regional Hospitalist-Only” employment model has the highest percentage of super users (46.7%).
Although most hospitalists across the nation spend a majority of their time interfacing with EHRs, 4.5% of HMGs in the most recent SOHM responded that their hospitals or health systems have not yet begun EHR implementation. I’m guessing the hospitalists in those organizations have a lot of work ahead of them in the next year or two!
Dr. Stephan is a hospitalist at Abbott Northwestern Hospital in Minneapolis and a member of the SHM Practice Analysis Committee.
If you are a hospitalist, you know this to be true: Electronic health records (EHRs) run more smoothly because of our input. According to the 2014 State of Hospital Medicine (SOHM) report, hospitalists played a significant role in implementing their hospitals’ EHR systems; 34% of respondent hospital medicine groups (HMGs) serving adults only develop order sets, protocols, and decision support, while 28.3% of HMGs serve as super users. As super users, hospitalists are role models who help other physicians work successfully with the computerized systems and set the tone for acceptance within their medical staff and the larger hospital staff.
When EHRs were implemented a decade ago at Allina Health, a 14-hospital system headquartered in Minneapolis, hospitalists acted as super users and were able to interface closely with IT staff, quickly fixing computer troubles that physicians encountered. We partnered with IT to help them prioritize their work by letting them know which computer issues involved patient safety, which were high frequency, irksome issues, and which EHR quirks could be queued farther down the line for repair.
Although successful EHR implementation is a mammoth accomplishment, it is just the tip of the iceberg. Once a system is implemented, an unending amount of work is needed to make sure EHRs deliver, through data analysis and standardization of care, all the efficiency, safety, and cost containment we hoped for at their inception.
When hospitalists at Allina were asked to be the first group of physicians to convert to professional fee billing using the EHR, we found that we needed to create a check-and-balance reporting system to ensure that our charges were indeed being captured. All of our health system physicians now use the hospitalist-developed reporting system.
Hospitalists in our system also forged the way to getting meaningful data out of the EHR system. When we first implemented our EHR, we found it difficult to get hospitalist-specific data. We worked closely with data analytics staff to develop a hospitalist “flag” that would differentiate hospitalists from other physicians providing inpatient care.
Because hospitalists are involved with patients through all phases of their care, from admission to discharge, they encounter firsthand the benefits and glitches of EHRs. By the very nature of our work, we are positioned to help improve EHRs. Hospitalist input ranges from informal feedback to the IT department to participation in focused work groups dedicated to specific aspects of improving patient care. Not surprisingly, 18.4% of adults-only HMGs who participated in the last SOHM survey have hospitalists serving in an IT leadership role for their organizations.
When looked at by ownership/employment models (see Table 1), this number is higher for the “University, Medical School, or Faculty Practice Plan” (26.7%) and for the “Hospital, Health System, or Integrated Delivery System” (23.2%) and lower for the “Private Local/Regional Hospitalist-Only Medical Groups” (6.7%).
Interestingly, the “Private Local/Regional Hospitalist-Only” employment model has the highest percentage of super users (46.7%).
Although most hospitalists across the nation spend a majority of their time interfacing with EHRs, 4.5% of HMGs in the most recent SOHM responded that their hospitals or health systems have not yet begun EHR implementation. I’m guessing the hospitalists in those organizations have a lot of work ahead of them in the next year or two!
Dr. Stephan is a hospitalist at Abbott Northwestern Hospital in Minneapolis and a member of the SHM Practice Analysis Committee.
If you are a hospitalist, you know this to be true: Electronic health records (EHRs) run more smoothly because of our input. According to the 2014 State of Hospital Medicine (SOHM) report, hospitalists played a significant role in implementing their hospitals’ EHR systems; 34% of respondent hospital medicine groups (HMGs) serving adults only develop order sets, protocols, and decision support, while 28.3% of HMGs serve as super users. As super users, hospitalists are role models who help other physicians work successfully with the computerized systems and set the tone for acceptance within their medical staff and the larger hospital staff.
When EHRs were implemented a decade ago at Allina Health, a 14-hospital system headquartered in Minneapolis, hospitalists acted as super users and were able to interface closely with IT staff, quickly fixing computer troubles that physicians encountered. We partnered with IT to help them prioritize their work by letting them know which computer issues involved patient safety, which were high frequency, irksome issues, and which EHR quirks could be queued farther down the line for repair.
Although successful EHR implementation is a mammoth accomplishment, it is just the tip of the iceberg. Once a system is implemented, an unending amount of work is needed to make sure EHRs deliver, through data analysis and standardization of care, all the efficiency, safety, and cost containment we hoped for at their inception.
When hospitalists at Allina were asked to be the first group of physicians to convert to professional fee billing using the EHR, we found that we needed to create a check-and-balance reporting system to ensure that our charges were indeed being captured. All of our health system physicians now use the hospitalist-developed reporting system.
Hospitalists in our system also forged the way to getting meaningful data out of the EHR system. When we first implemented our EHR, we found it difficult to get hospitalist-specific data. We worked closely with data analytics staff to develop a hospitalist “flag” that would differentiate hospitalists from other physicians providing inpatient care.
Because hospitalists are involved with patients through all phases of their care, from admission to discharge, they encounter firsthand the benefits and glitches of EHRs. By the very nature of our work, we are positioned to help improve EHRs. Hospitalist input ranges from informal feedback to the IT department to participation in focused work groups dedicated to specific aspects of improving patient care. Not surprisingly, 18.4% of adults-only HMGs who participated in the last SOHM survey have hospitalists serving in an IT leadership role for their organizations.
When looked at by ownership/employment models (see Table 1), this number is higher for the “University, Medical School, or Faculty Practice Plan” (26.7%) and for the “Hospital, Health System, or Integrated Delivery System” (23.2%) and lower for the “Private Local/Regional Hospitalist-Only Medical Groups” (6.7%).
Interestingly, the “Private Local/Regional Hospitalist-Only” employment model has the highest percentage of super users (46.7%).
Although most hospitalists across the nation spend a majority of their time interfacing with EHRs, 4.5% of HMGs in the most recent SOHM responded that their hospitals or health systems have not yet begun EHR implementation. I’m guessing the hospitalists in those organizations have a lot of work ahead of them in the next year or two!
Dr. Stephan is a hospitalist at Abbott Northwestern Hospital in Minneapolis and a member of the SHM Practice Analysis Committee.
Job Search Tips for First-Time Hospitalists
The best strategy for landing that first job is to start your search early, says Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo.
“Many hospital organizations planning to add to their staff are willing to consider candidates six or 12 months out,” DeVita explains. Required licensing and credentialing “don’t happen fast,” she adds, and you will not be the only applicant. To preserve your range of choices, explore options early, preferably in the fall before your residency concludes.
Tom Baudendistel, MD, FACP, program director of internal medicine residency at Kaiser Permanente in Oakland, Calif., encourages his senior residents to “at least interview at new settings, and test the waters a bit.
“Too often, I see residents trying to find the absolute perfect position for the next 30 years of their career,” he says, “when in reality they are likely to change jobs in a few years for a variety of reasons, from personal to geographic to the job itself. Even if they decide to remain with Kaiser—as many do—they’ll have some perspective on what other systems are up to, which they can file away for future reference.”

At Stake? More than Money
Salary and benefits are important, but they aren’t the only factors to evaluate during a job search. Dr. Baudendistel offers a few “don’ts” to help guide the job search journey:
- Don’t forget to consider how you will stay up to date: “Residents sometimes take for granted the amount of didactic learning going on every day in the academic environment of residency, only to become disenchanted to take a job at a hospital where there may only be one grand rounds a week [if that], and the group meetings center primarily on business items, such as contracts, coding, and RVUs.”
- Don’t be lured by the money: Debt-ridden residents may be drawn to the quick fix of a nice salary, but this can cloud the fact that the salary might not increase much over the next five to 10 years, that the benefits/retirement/home loan packages are thin, or that there is very little growth potential within the group. To assess the potential for professional growth—a better predictor of job satisfaction—ask the attendings who have been with the group for five to 10 years: “How has your job evolved since you first started?” Be wary if the answer is, “I’ve been doing the same full-time clinical job since I started.”
- Don’t forget to look critically at group happiness. What is the turnover of the group? How is leadership viewed by the rank-and-file attendings? What is the relationship between the HM group and the hospital administration and nurses? A good question to ask is, “Does the group go to lunch?”
- Don’t forget to consider who your mentors will be. Who will help you grow and thrive in your job? Is there a formal mentoring program? If not, how does the group leader mentor the attendings?
The Nuts and Bolts
Once you’ve been offered a contract, it’s not just a simple matter of whether you will be salaried with benefits or a contract employee and have to purchase your own benefits. Legal counsel might be appropriate, DeVita says, to ensure you understand the ramifications of malpractice insurance.
Importantly, find out who pays for “tail insurance” for when you leave a job. This is vital, because physicians remain liable for malpractice acts performed when they were a part of the previous medical group.
You’re In, Now What?
Dr. Baudendistel and DeVita agree that honing your clinical skills will be “job one” once you start to work.
“If you’re averaging 12 patients and your peers are averaging 17, you will be in a position of jeopardy,” DeVita cautions.
For that reason, Baudendistel advises young hospitalists not to overcommit to nonclinical duties.
“There is a temptation to say ‘yes’ to every opportunity that arises in your first job. There will be plenty of time over the years to get involved in committee work, QI [quality improvement], and the like. Sometimes saying ‘no’ is the right approach in your early years,” he says.
Once you’re maintaining the same productivity level as your peers, DeVita points out, then it may be appropriate to participate in committee work—and there may be bonus components for citizenship work.
Gretchen Henkel is a freelance writer in California.
The best strategy for landing that first job is to start your search early, says Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo.
“Many hospital organizations planning to add to their staff are willing to consider candidates six or 12 months out,” DeVita explains. Required licensing and credentialing “don’t happen fast,” she adds, and you will not be the only applicant. To preserve your range of choices, explore options early, preferably in the fall before your residency concludes.
Tom Baudendistel, MD, FACP, program director of internal medicine residency at Kaiser Permanente in Oakland, Calif., encourages his senior residents to “at least interview at new settings, and test the waters a bit.
“Too often, I see residents trying to find the absolute perfect position for the next 30 years of their career,” he says, “when in reality they are likely to change jobs in a few years for a variety of reasons, from personal to geographic to the job itself. Even if they decide to remain with Kaiser—as many do—they’ll have some perspective on what other systems are up to, which they can file away for future reference.”

At Stake? More than Money
Salary and benefits are important, but they aren’t the only factors to evaluate during a job search. Dr. Baudendistel offers a few “don’ts” to help guide the job search journey:
- Don’t forget to consider how you will stay up to date: “Residents sometimes take for granted the amount of didactic learning going on every day in the academic environment of residency, only to become disenchanted to take a job at a hospital where there may only be one grand rounds a week [if that], and the group meetings center primarily on business items, such as contracts, coding, and RVUs.”
- Don’t be lured by the money: Debt-ridden residents may be drawn to the quick fix of a nice salary, but this can cloud the fact that the salary might not increase much over the next five to 10 years, that the benefits/retirement/home loan packages are thin, or that there is very little growth potential within the group. To assess the potential for professional growth—a better predictor of job satisfaction—ask the attendings who have been with the group for five to 10 years: “How has your job evolved since you first started?” Be wary if the answer is, “I’ve been doing the same full-time clinical job since I started.”
- Don’t forget to look critically at group happiness. What is the turnover of the group? How is leadership viewed by the rank-and-file attendings? What is the relationship between the HM group and the hospital administration and nurses? A good question to ask is, “Does the group go to lunch?”
- Don’t forget to consider who your mentors will be. Who will help you grow and thrive in your job? Is there a formal mentoring program? If not, how does the group leader mentor the attendings?
The Nuts and Bolts
Once you’ve been offered a contract, it’s not just a simple matter of whether you will be salaried with benefits or a contract employee and have to purchase your own benefits. Legal counsel might be appropriate, DeVita says, to ensure you understand the ramifications of malpractice insurance.
Importantly, find out who pays for “tail insurance” for when you leave a job. This is vital, because physicians remain liable for malpractice acts performed when they were a part of the previous medical group.
You’re In, Now What?
Dr. Baudendistel and DeVita agree that honing your clinical skills will be “job one” once you start to work.
“If you’re averaging 12 patients and your peers are averaging 17, you will be in a position of jeopardy,” DeVita cautions.
For that reason, Baudendistel advises young hospitalists not to overcommit to nonclinical duties.
“There is a temptation to say ‘yes’ to every opportunity that arises in your first job. There will be plenty of time over the years to get involved in committee work, QI [quality improvement], and the like. Sometimes saying ‘no’ is the right approach in your early years,” he says.
Once you’re maintaining the same productivity level as your peers, DeVita points out, then it may be appropriate to participate in committee work—and there may be bonus components for citizenship work.
Gretchen Henkel is a freelance writer in California.
The best strategy for landing that first job is to start your search early, says Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo.
“Many hospital organizations planning to add to their staff are willing to consider candidates six or 12 months out,” DeVita explains. Required licensing and credentialing “don’t happen fast,” she adds, and you will not be the only applicant. To preserve your range of choices, explore options early, preferably in the fall before your residency concludes.
Tom Baudendistel, MD, FACP, program director of internal medicine residency at Kaiser Permanente in Oakland, Calif., encourages his senior residents to “at least interview at new settings, and test the waters a bit.
“Too often, I see residents trying to find the absolute perfect position for the next 30 years of their career,” he says, “when in reality they are likely to change jobs in a few years for a variety of reasons, from personal to geographic to the job itself. Even if they decide to remain with Kaiser—as many do—they’ll have some perspective on what other systems are up to, which they can file away for future reference.”

At Stake? More than Money
Salary and benefits are important, but they aren’t the only factors to evaluate during a job search. Dr. Baudendistel offers a few “don’ts” to help guide the job search journey:
- Don’t forget to consider how you will stay up to date: “Residents sometimes take for granted the amount of didactic learning going on every day in the academic environment of residency, only to become disenchanted to take a job at a hospital where there may only be one grand rounds a week [if that], and the group meetings center primarily on business items, such as contracts, coding, and RVUs.”
- Don’t be lured by the money: Debt-ridden residents may be drawn to the quick fix of a nice salary, but this can cloud the fact that the salary might not increase much over the next five to 10 years, that the benefits/retirement/home loan packages are thin, or that there is very little growth potential within the group. To assess the potential for professional growth—a better predictor of job satisfaction—ask the attendings who have been with the group for five to 10 years: “How has your job evolved since you first started?” Be wary if the answer is, “I’ve been doing the same full-time clinical job since I started.”
- Don’t forget to look critically at group happiness. What is the turnover of the group? How is leadership viewed by the rank-and-file attendings? What is the relationship between the HM group and the hospital administration and nurses? A good question to ask is, “Does the group go to lunch?”
- Don’t forget to consider who your mentors will be. Who will help you grow and thrive in your job? Is there a formal mentoring program? If not, how does the group leader mentor the attendings?
The Nuts and Bolts
Once you’ve been offered a contract, it’s not just a simple matter of whether you will be salaried with benefits or a contract employee and have to purchase your own benefits. Legal counsel might be appropriate, DeVita says, to ensure you understand the ramifications of malpractice insurance.
Importantly, find out who pays for “tail insurance” for when you leave a job. This is vital, because physicians remain liable for malpractice acts performed when they were a part of the previous medical group.
You’re In, Now What?
Dr. Baudendistel and DeVita agree that honing your clinical skills will be “job one” once you start to work.
“If you’re averaging 12 patients and your peers are averaging 17, you will be in a position of jeopardy,” DeVita cautions.
For that reason, Baudendistel advises young hospitalists not to overcommit to nonclinical duties.
“There is a temptation to say ‘yes’ to every opportunity that arises in your first job. There will be plenty of time over the years to get involved in committee work, QI [quality improvement], and the like. Sometimes saying ‘no’ is the right approach in your early years,” he says.
Once you’re maintaining the same productivity level as your peers, DeVita points out, then it may be appropriate to participate in committee work—and there may be bonus components for citizenship work.
Gretchen Henkel is a freelance writer in California.
Hospital Medicine’s Challenges, Rewards Lure Healthcare Administrator
As a child, Courtney, director of operations for a multi-site hospitalist program at Baptist Health System in Birmingham, Ala., knew a boy who was diagnosed with leukemia.
“I often visited him in the hospital,” she says. “Those visits made me want to be in medicine. As I grew up, I knew I had more of a business mindset verses clinical, but my passion for healthcare remained.”
She’s not kidding. In 2000, she earned a bachelor of science degree in business administration from Mississippi University for Women in Columbus. Five years later, she earned an MBA in healthcare administration from the University of Phoenix. Her career started in marketing in North Carolina, but after five years in her current role, she has been involved in developing new hospitalist programs at three hospital sites.
Courtney is an active SHM member and is in her second year as a member of SHM’s Practice Administrators Committee. Now she is bringing that nonphysician perspective to Team Hospitalist. She is one of seven new members of The Hospitalist’s volunteer editorial advisory board.
Question: Was there a specific person/mentor who steered you to hospital medicine?
Answer: I was over operations of outpatient clinics and one hospitalist location when the opportunity to help start our system-wide hospitalist program became available. My boss approached me with the opportunity. She had once helped start a hospital medicine program and thought I would enjoy the challenge it would bring.

Q: What do you like most about working in hospital medicine?
A: My favorite part of hospital medicine is working with my medical directors, physicians, and hospital leadership to improve quality and outcomes for our patients. It’s great to see initiatives start from the group up and then watch the improved outcomes take place.
Q: What do you dislike most?
A: Navigating the staffing challenges is the least enjoyable part of my job.
Q: What’s the best advice you ever received?
A: My father always was kind to everyone and [was] respected because of his character. I was taught a person’s character is more important than any professional achievement.
Q: What’s the biggest change you’ve seen in HM in your career?
A: It seems I’ve seen an uptick of specialists wanting to model their practice after hospitalists. I’ve seen neurologists and nephrologists who only want to do inpatient care. I believe this stems from the [interest in] work-/home life balance that is more important to the newest generation of physicians.
Q: What is your biggest professional challenge?
A: Helping start our system hospitalist program has been both my biggest professional challenge and biggest professional reward. It was tough. With one established program already in place, it was decided to bring our two largest hospital medicine programs in-house. The programs were literally starting over from scratch within one month of each other. We started with six (full-time) FT physicians, two office managers, and me between the two locations. There was lots of locum usage, heavy recruiting, physicians working crazy hours to help out, and sleepless nights.
Q: And since then?
A: We have maintained good staffing/quality physicians at our initial location, fully staffed with 28 full-time physicians, four nurse practitioners, and several other support staff at our two startup locations, and have started a program at the fourth hospital. The hospital medicine group and the hospitals have worked together on clinical documentation improvement [and] geographic interdisciplinary rounding and have gone through an EPIC EHR install. It’s been a very challenging but rewarding road to be on.
Q: What SHM event (i.e., Leadership Academy, annual meeting) made the most lasting impression on you?
A: The “Leadership Essentials” course [part of SHM’s three-course Leadership Academy] was very important to me. I look forward to continuing the Leadership Academy courses.
Q: Where do you see yourself in 10 years?
A: I love the organization I currently work for. I hope to continue on my current career path and grow as a leader within the organization.
Q: When you aren’t working, what is important to you?
Answer: My family life is very important. I’m married, and we have one son who is nine. My off time revolves around traveling and sports my son participates in.
Q: Apple or Android?
A: As much as I hate to admit it, I’m an Apple fan. It took me a long time to make the switch, but I’m like the masses and addicted.
Q: What show is sitting in your Netflix queue that you can’t wait to binge watch?
A: I’m a “Walking Dead” fan and am working my way through all of the seasons now. I’ve made it up to last season.
As a child, Courtney, director of operations for a multi-site hospitalist program at Baptist Health System in Birmingham, Ala., knew a boy who was diagnosed with leukemia.
“I often visited him in the hospital,” she says. “Those visits made me want to be in medicine. As I grew up, I knew I had more of a business mindset verses clinical, but my passion for healthcare remained.”
She’s not kidding. In 2000, she earned a bachelor of science degree in business administration from Mississippi University for Women in Columbus. Five years later, she earned an MBA in healthcare administration from the University of Phoenix. Her career started in marketing in North Carolina, but after five years in her current role, she has been involved in developing new hospitalist programs at three hospital sites.
Courtney is an active SHM member and is in her second year as a member of SHM’s Practice Administrators Committee. Now she is bringing that nonphysician perspective to Team Hospitalist. She is one of seven new members of The Hospitalist’s volunteer editorial advisory board.
Question: Was there a specific person/mentor who steered you to hospital medicine?
Answer: I was over operations of outpatient clinics and one hospitalist location when the opportunity to help start our system-wide hospitalist program became available. My boss approached me with the opportunity. She had once helped start a hospital medicine program and thought I would enjoy the challenge it would bring.

Q: What do you like most about working in hospital medicine?
A: My favorite part of hospital medicine is working with my medical directors, physicians, and hospital leadership to improve quality and outcomes for our patients. It’s great to see initiatives start from the group up and then watch the improved outcomes take place.
Q: What do you dislike most?
A: Navigating the staffing challenges is the least enjoyable part of my job.
Q: What’s the best advice you ever received?
A: My father always was kind to everyone and [was] respected because of his character. I was taught a person’s character is more important than any professional achievement.
Q: What’s the biggest change you’ve seen in HM in your career?
A: It seems I’ve seen an uptick of specialists wanting to model their practice after hospitalists. I’ve seen neurologists and nephrologists who only want to do inpatient care. I believe this stems from the [interest in] work-/home life balance that is more important to the newest generation of physicians.
Q: What is your biggest professional challenge?
A: Helping start our system hospitalist program has been both my biggest professional challenge and biggest professional reward. It was tough. With one established program already in place, it was decided to bring our two largest hospital medicine programs in-house. The programs were literally starting over from scratch within one month of each other. We started with six (full-time) FT physicians, two office managers, and me between the two locations. There was lots of locum usage, heavy recruiting, physicians working crazy hours to help out, and sleepless nights.
Q: And since then?
A: We have maintained good staffing/quality physicians at our initial location, fully staffed with 28 full-time physicians, four nurse practitioners, and several other support staff at our two startup locations, and have started a program at the fourth hospital. The hospital medicine group and the hospitals have worked together on clinical documentation improvement [and] geographic interdisciplinary rounding and have gone through an EPIC EHR install. It’s been a very challenging but rewarding road to be on.
Q: What SHM event (i.e., Leadership Academy, annual meeting) made the most lasting impression on you?
A: The “Leadership Essentials” course [part of SHM’s three-course Leadership Academy] was very important to me. I look forward to continuing the Leadership Academy courses.
Q: Where do you see yourself in 10 years?
A: I love the organization I currently work for. I hope to continue on my current career path and grow as a leader within the organization.
Q: When you aren’t working, what is important to you?
Answer: My family life is very important. I’m married, and we have one son who is nine. My off time revolves around traveling and sports my son participates in.
Q: Apple or Android?
A: As much as I hate to admit it, I’m an Apple fan. It took me a long time to make the switch, but I’m like the masses and addicted.
Q: What show is sitting in your Netflix queue that you can’t wait to binge watch?
A: I’m a “Walking Dead” fan and am working my way through all of the seasons now. I’ve made it up to last season.
As a child, Courtney, director of operations for a multi-site hospitalist program at Baptist Health System in Birmingham, Ala., knew a boy who was diagnosed with leukemia.
“I often visited him in the hospital,” she says. “Those visits made me want to be in medicine. As I grew up, I knew I had more of a business mindset verses clinical, but my passion for healthcare remained.”
She’s not kidding. In 2000, she earned a bachelor of science degree in business administration from Mississippi University for Women in Columbus. Five years later, she earned an MBA in healthcare administration from the University of Phoenix. Her career started in marketing in North Carolina, but after five years in her current role, she has been involved in developing new hospitalist programs at three hospital sites.
Courtney is an active SHM member and is in her second year as a member of SHM’s Practice Administrators Committee. Now she is bringing that nonphysician perspective to Team Hospitalist. She is one of seven new members of The Hospitalist’s volunteer editorial advisory board.
Question: Was there a specific person/mentor who steered you to hospital medicine?
Answer: I was over operations of outpatient clinics and one hospitalist location when the opportunity to help start our system-wide hospitalist program became available. My boss approached me with the opportunity. She had once helped start a hospital medicine program and thought I would enjoy the challenge it would bring.

Q: What do you like most about working in hospital medicine?
A: My favorite part of hospital medicine is working with my medical directors, physicians, and hospital leadership to improve quality and outcomes for our patients. It’s great to see initiatives start from the group up and then watch the improved outcomes take place.
Q: What do you dislike most?
A: Navigating the staffing challenges is the least enjoyable part of my job.
Q: What’s the best advice you ever received?
A: My father always was kind to everyone and [was] respected because of his character. I was taught a person’s character is more important than any professional achievement.
Q: What’s the biggest change you’ve seen in HM in your career?
A: It seems I’ve seen an uptick of specialists wanting to model their practice after hospitalists. I’ve seen neurologists and nephrologists who only want to do inpatient care. I believe this stems from the [interest in] work-/home life balance that is more important to the newest generation of physicians.
Q: What is your biggest professional challenge?
A: Helping start our system hospitalist program has been both my biggest professional challenge and biggest professional reward. It was tough. With one established program already in place, it was decided to bring our two largest hospital medicine programs in-house. The programs were literally starting over from scratch within one month of each other. We started with six (full-time) FT physicians, two office managers, and me between the two locations. There was lots of locum usage, heavy recruiting, physicians working crazy hours to help out, and sleepless nights.
Q: And since then?
A: We have maintained good staffing/quality physicians at our initial location, fully staffed with 28 full-time physicians, four nurse practitioners, and several other support staff at our two startup locations, and have started a program at the fourth hospital. The hospital medicine group and the hospitals have worked together on clinical documentation improvement [and] geographic interdisciplinary rounding and have gone through an EPIC EHR install. It’s been a very challenging but rewarding road to be on.
Q: What SHM event (i.e., Leadership Academy, annual meeting) made the most lasting impression on you?
A: The “Leadership Essentials” course [part of SHM’s three-course Leadership Academy] was very important to me. I look forward to continuing the Leadership Academy courses.
Q: Where do you see yourself in 10 years?
A: I love the organization I currently work for. I hope to continue on my current career path and grow as a leader within the organization.
Q: When you aren’t working, what is important to you?
Answer: My family life is very important. I’m married, and we have one son who is nine. My off time revolves around traveling and sports my son participates in.
Q: Apple or Android?
A: As much as I hate to admit it, I’m an Apple fan. It took me a long time to make the switch, but I’m like the masses and addicted.
Q: What show is sitting in your Netflix queue that you can’t wait to binge watch?
A: I’m a “Walking Dead” fan and am working my way through all of the seasons now. I’ve made it up to last season.
Physician Assistant Hooked on Hospital Medicine's Patient Care Approach
James Levy, PA-C, SFHM, isn’t a doctor. But he’s been a hospitalist for more than 15 years.
A veteran physician assistant (PA), Levy is vice president of human resources (VPHR) for iNDIGO Health Partners of Traverse City, Mich., a firm he has co-owned since it began in 2008. From 2001-2013, he was a PA at Hospitalists of Northern Michigan, also in Traverse City.
A longtime SHM member who serves on the SHM NP/PA Committee and speaks regularly at SHM meetings, Levy finds the joy of HM is simple: It follows a patient from admission to discharge.
“Clinically, I enjoy seeing the patient through the process of initial assessment, enacting a plan, and following the patient through to discharge,” he says. “Making a clinical judgment, acting on it, and being rewarded by an improving patient is deeply satisfying, as is working as part of a team with a common goal.”
His newest team is Team Hospitalist, The Hospitalist newsmagazine’s volunteer editorial advisory group. He is one of seven new members seated this year.
Question: Why did you choose a career in medicine?
Answer: I was interested in biology and science, but after a meandering academic career with the ultimate purpose of avoiding the draft, I was badly injured in a car accident. Having recovered, draft deferment in hand, I wound up as an operating room technician and immediately knew medicine was what I had to do. When I realized I preferred to deal with patients who were actually awake, my direction was established.
Q: Was there a specific person/mentor who steered you to hospital medicine?

persistence, and common decency. —James Levy, PA-C, SFHM
A: The hospitalists I initially encountered when I dipped my toe into inpatient medicine were passionate and engaged. Their understanding of the medicine we were doing was profound, and I was hooked. Several of them are still my partners.
Q: Tell me a little more about your training. What did you like most, dislike during the process? Was there a single moment you knew “I can do this?”
A: I’m a PA, which means I do a great deal of what a physician does with a fraction of the training. Much of what I know I’ve learned from patients, but I’m not sure that separates me from many good, experienced physicians. As the VPHR of my hospitalist company, I’ve hired many physicians, PAs, and NPs. I have come to feel that great clinicians are less about training and more about intelligence, commitment, work ethic, honesty, persistence, and common decency. These are qualities that would predict success in any field.
Q: What do you dislike most about HM?
A: All hospitalists share many of the same frustrations and irritations. The thing I hate most is when I feel I’ve done my best and the patient isn’t improving. Both parties have to do their part in order for the encounter to be successful.
Q: What’s the best advice you ever received?
A: An early physician mentor told me, “Take care of the patient, and the money will take care of itself.” My father taught me to avoid debt and live below my means. My kids taught me that often all you have to do is show up and care.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: My early mentor was our family doctor. He took a kindly interest in me and convinced me that I could be someone worthy of his respect and my own.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The advent of EHR has done more to change the mechanics of inpatient practice than anything else.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care do you find most challenging?
A: It’s a real challenge to try to develop a working, human-to-human relationship with every sick stranger you encounter.
Q: What aspect of patient care is most rewarding?
A: When you acquire the skill of gaining the trust of a complete stranger on an initial encounter, it becomes a rewarding thing to do.
Q: Outside of patient care, tell me about your career interests.
A: I am a partner in, and officer of, the hospitalist company I helped start. Our overarching goal is to strengthen and stabilize the small, rural hospitals where we run programs. Without those hospitals, many patients will not be able to overcome the challenges of distance and winter travel to get the care they need. I am absolutely passionate about seeing this mission succeed in as many places as possible.
Q: What is your biggest professional challenge?
A: I spend lots of time talking to and working with the young physicians, PAs, and NPs I’m trying to recruit. This becomes more and more difficult as more hospitalist opportunities open and the pool of good clinicians does not expand. I’m often in the position of trying to interest a physician trained in an urban center in the advantages of working in an outlying location. Some get it; some don’t.
Q: What is your biggest professional reward?
A: Attracting a great clinician to a program where I’m sure s/he will be successful is a great thing for the individual and for that hospitalist team.
Q: What did it mean for you to be selected a Senior Fellow in Hospital Medicine?
A: This was especially meaningful to me as a PA because it was SHM really meaning it when they aspired to be a “big tent” society. I know of no other medical group that is this inclusive.
James Levy, PA-C, SFHM, isn’t a doctor. But he’s been a hospitalist for more than 15 years.
A veteran physician assistant (PA), Levy is vice president of human resources (VPHR) for iNDIGO Health Partners of Traverse City, Mich., a firm he has co-owned since it began in 2008. From 2001-2013, he was a PA at Hospitalists of Northern Michigan, also in Traverse City.
A longtime SHM member who serves on the SHM NP/PA Committee and speaks regularly at SHM meetings, Levy finds the joy of HM is simple: It follows a patient from admission to discharge.
“Clinically, I enjoy seeing the patient through the process of initial assessment, enacting a plan, and following the patient through to discharge,” he says. “Making a clinical judgment, acting on it, and being rewarded by an improving patient is deeply satisfying, as is working as part of a team with a common goal.”
His newest team is Team Hospitalist, The Hospitalist newsmagazine’s volunteer editorial advisory group. He is one of seven new members seated this year.
Question: Why did you choose a career in medicine?
Answer: I was interested in biology and science, but after a meandering academic career with the ultimate purpose of avoiding the draft, I was badly injured in a car accident. Having recovered, draft deferment in hand, I wound up as an operating room technician and immediately knew medicine was what I had to do. When I realized I preferred to deal with patients who were actually awake, my direction was established.
Q: Was there a specific person/mentor who steered you to hospital medicine?

persistence, and common decency. —James Levy, PA-C, SFHM
A: The hospitalists I initially encountered when I dipped my toe into inpatient medicine were passionate and engaged. Their understanding of the medicine we were doing was profound, and I was hooked. Several of them are still my partners.
Q: Tell me a little more about your training. What did you like most, dislike during the process? Was there a single moment you knew “I can do this?”
A: I’m a PA, which means I do a great deal of what a physician does with a fraction of the training. Much of what I know I’ve learned from patients, but I’m not sure that separates me from many good, experienced physicians. As the VPHR of my hospitalist company, I’ve hired many physicians, PAs, and NPs. I have come to feel that great clinicians are less about training and more about intelligence, commitment, work ethic, honesty, persistence, and common decency. These are qualities that would predict success in any field.
Q: What do you dislike most about HM?
A: All hospitalists share many of the same frustrations and irritations. The thing I hate most is when I feel I’ve done my best and the patient isn’t improving. Both parties have to do their part in order for the encounter to be successful.
Q: What’s the best advice you ever received?
A: An early physician mentor told me, “Take care of the patient, and the money will take care of itself.” My father taught me to avoid debt and live below my means. My kids taught me that often all you have to do is show up and care.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: My early mentor was our family doctor. He took a kindly interest in me and convinced me that I could be someone worthy of his respect and my own.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The advent of EHR has done more to change the mechanics of inpatient practice than anything else.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care do you find most challenging?
A: It’s a real challenge to try to develop a working, human-to-human relationship with every sick stranger you encounter.
Q: What aspect of patient care is most rewarding?
A: When you acquire the skill of gaining the trust of a complete stranger on an initial encounter, it becomes a rewarding thing to do.
Q: Outside of patient care, tell me about your career interests.
A: I am a partner in, and officer of, the hospitalist company I helped start. Our overarching goal is to strengthen and stabilize the small, rural hospitals where we run programs. Without those hospitals, many patients will not be able to overcome the challenges of distance and winter travel to get the care they need. I am absolutely passionate about seeing this mission succeed in as many places as possible.
Q: What is your biggest professional challenge?
A: I spend lots of time talking to and working with the young physicians, PAs, and NPs I’m trying to recruit. This becomes more and more difficult as more hospitalist opportunities open and the pool of good clinicians does not expand. I’m often in the position of trying to interest a physician trained in an urban center in the advantages of working in an outlying location. Some get it; some don’t.
Q: What is your biggest professional reward?
A: Attracting a great clinician to a program where I’m sure s/he will be successful is a great thing for the individual and for that hospitalist team.
Q: What did it mean for you to be selected a Senior Fellow in Hospital Medicine?
A: This was especially meaningful to me as a PA because it was SHM really meaning it when they aspired to be a “big tent” society. I know of no other medical group that is this inclusive.
James Levy, PA-C, SFHM, isn’t a doctor. But he’s been a hospitalist for more than 15 years.
A veteran physician assistant (PA), Levy is vice president of human resources (VPHR) for iNDIGO Health Partners of Traverse City, Mich., a firm he has co-owned since it began in 2008. From 2001-2013, he was a PA at Hospitalists of Northern Michigan, also in Traverse City.
A longtime SHM member who serves on the SHM NP/PA Committee and speaks regularly at SHM meetings, Levy finds the joy of HM is simple: It follows a patient from admission to discharge.
“Clinically, I enjoy seeing the patient through the process of initial assessment, enacting a plan, and following the patient through to discharge,” he says. “Making a clinical judgment, acting on it, and being rewarded by an improving patient is deeply satisfying, as is working as part of a team with a common goal.”
His newest team is Team Hospitalist, The Hospitalist newsmagazine’s volunteer editorial advisory group. He is one of seven new members seated this year.
Question: Why did you choose a career in medicine?
Answer: I was interested in biology and science, but after a meandering academic career with the ultimate purpose of avoiding the draft, I was badly injured in a car accident. Having recovered, draft deferment in hand, I wound up as an operating room technician and immediately knew medicine was what I had to do. When I realized I preferred to deal with patients who were actually awake, my direction was established.
Q: Was there a specific person/mentor who steered you to hospital medicine?

persistence, and common decency. —James Levy, PA-C, SFHM
A: The hospitalists I initially encountered when I dipped my toe into inpatient medicine were passionate and engaged. Their understanding of the medicine we were doing was profound, and I was hooked. Several of them are still my partners.
Q: Tell me a little more about your training. What did you like most, dislike during the process? Was there a single moment you knew “I can do this?”
A: I’m a PA, which means I do a great deal of what a physician does with a fraction of the training. Much of what I know I’ve learned from patients, but I’m not sure that separates me from many good, experienced physicians. As the VPHR of my hospitalist company, I’ve hired many physicians, PAs, and NPs. I have come to feel that great clinicians are less about training and more about intelligence, commitment, work ethic, honesty, persistence, and common decency. These are qualities that would predict success in any field.
Q: What do you dislike most about HM?
A: All hospitalists share many of the same frustrations and irritations. The thing I hate most is when I feel I’ve done my best and the patient isn’t improving. Both parties have to do their part in order for the encounter to be successful.
Q: What’s the best advice you ever received?
A: An early physician mentor told me, “Take care of the patient, and the money will take care of itself.” My father taught me to avoid debt and live below my means. My kids taught me that often all you have to do is show up and care.
Q: Did you have a mentor during training or early career? If so, who was the mentor, and what were the most important lessons you learned from him/her?
A: My early mentor was our family doctor. He took a kindly interest in me and convinced me that I could be someone worthy of his respect and my own.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The advent of EHR has done more to change the mechanics of inpatient practice than anything else.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care do you find most challenging?
A: It’s a real challenge to try to develop a working, human-to-human relationship with every sick stranger you encounter.
Q: What aspect of patient care is most rewarding?
A: When you acquire the skill of gaining the trust of a complete stranger on an initial encounter, it becomes a rewarding thing to do.
Q: Outside of patient care, tell me about your career interests.
A: I am a partner in, and officer of, the hospitalist company I helped start. Our overarching goal is to strengthen and stabilize the small, rural hospitals where we run programs. Without those hospitals, many patients will not be able to overcome the challenges of distance and winter travel to get the care they need. I am absolutely passionate about seeing this mission succeed in as many places as possible.
Q: What is your biggest professional challenge?
A: I spend lots of time talking to and working with the young physicians, PAs, and NPs I’m trying to recruit. This becomes more and more difficult as more hospitalist opportunities open and the pool of good clinicians does not expand. I’m often in the position of trying to interest a physician trained in an urban center in the advantages of working in an outlying location. Some get it; some don’t.
Q: What is your biggest professional reward?
A: Attracting a great clinician to a program where I’m sure s/he will be successful is a great thing for the individual and for that hospitalist team.
Q: What did it mean for you to be selected a Senior Fellow in Hospital Medicine?
A: This was especially meaningful to me as a PA because it was SHM really meaning it when they aspired to be a “big tent” society. I know of no other medical group that is this inclusive.
Movers and Shakers in Hospital Medicine, July 2015









Business Moves










Business Moves










Business Moves

Childhood Bacteremia and Antibiotics Usage in the Emergency Department
Clinical question: Have the causative organisms in pediatric bacteremia changed over time concurrent with introduction of the pneumococcal conjugate vaccine?
Background: Previous research has shown introduction of polyvalent pneumococcal conjugate vaccine led to changes in the organisms causing meningitis and otitis media, and patterns of nasopharyngeal colonization. Pneumococcus, historically, was a common cause of bacteremia. The availability of pneumococcal conjugate vaccine may have changed the organisms causing bacteremia in children.
Study design: Retrospective chart review and time series.
Setting: Children presenting to the ED of Alder Hey Children’s Hospital in Liverpool, England, from 2001 to 2011.
Synopsis: Five hundred seventy-five episodes of bacteremia were found in 525 children. Infants most commonly had E. coli and Group B streptococcal infections; children over age five most commonly had S. aureus. The introduction of the pneumococcal conjugate vaccine decreased pneumococcal bacteremia by 49% over the study period. This decrease was accompanied by an increase in Gram-negative bacteremia. Susceptibility to empiric antibiotics (third-generation cephalosporins) dropped from 96% to 83%. Over the study period, more children presented with central venous lines, which was felt to be due to increasing outpatient use of total parenteral nutrition (TPN).
Bottom line: Vaccination against pneumococcus is changing the microbiology of pediatric bacteremia, with fewer vaccine-preventable Gram-positive infections and more Gram-negative infections. This increases the likelihood of resistance to third-generation cephalosporins as empiric antibiotic.
Citation: Irwin AD, Drew RJ, Marshall P, et al. Etiology of childhood bacteremia and timely antibiotics administration in the emergency department. Pediatrics. 2015;135(4): 635-642.

Clinical question: Have the causative organisms in pediatric bacteremia changed over time concurrent with introduction of the pneumococcal conjugate vaccine?
Background: Previous research has shown introduction of polyvalent pneumococcal conjugate vaccine led to changes in the organisms causing meningitis and otitis media, and patterns of nasopharyngeal colonization. Pneumococcus, historically, was a common cause of bacteremia. The availability of pneumococcal conjugate vaccine may have changed the organisms causing bacteremia in children.
Study design: Retrospective chart review and time series.
Setting: Children presenting to the ED of Alder Hey Children’s Hospital in Liverpool, England, from 2001 to 2011.
Synopsis: Five hundred seventy-five episodes of bacteremia were found in 525 children. Infants most commonly had E. coli and Group B streptococcal infections; children over age five most commonly had S. aureus. The introduction of the pneumococcal conjugate vaccine decreased pneumococcal bacteremia by 49% over the study period. This decrease was accompanied by an increase in Gram-negative bacteremia. Susceptibility to empiric antibiotics (third-generation cephalosporins) dropped from 96% to 83%. Over the study period, more children presented with central venous lines, which was felt to be due to increasing outpatient use of total parenteral nutrition (TPN).
Bottom line: Vaccination against pneumococcus is changing the microbiology of pediatric bacteremia, with fewer vaccine-preventable Gram-positive infections and more Gram-negative infections. This increases the likelihood of resistance to third-generation cephalosporins as empiric antibiotic.
Citation: Irwin AD, Drew RJ, Marshall P, et al. Etiology of childhood bacteremia and timely antibiotics administration in the emergency department. Pediatrics. 2015;135(4): 635-642.

Clinical question: Have the causative organisms in pediatric bacteremia changed over time concurrent with introduction of the pneumococcal conjugate vaccine?
Background: Previous research has shown introduction of polyvalent pneumococcal conjugate vaccine led to changes in the organisms causing meningitis and otitis media, and patterns of nasopharyngeal colonization. Pneumococcus, historically, was a common cause of bacteremia. The availability of pneumococcal conjugate vaccine may have changed the organisms causing bacteremia in children.
Study design: Retrospective chart review and time series.
Setting: Children presenting to the ED of Alder Hey Children’s Hospital in Liverpool, England, from 2001 to 2011.
Synopsis: Five hundred seventy-five episodes of bacteremia were found in 525 children. Infants most commonly had E. coli and Group B streptococcal infections; children over age five most commonly had S. aureus. The introduction of the pneumococcal conjugate vaccine decreased pneumococcal bacteremia by 49% over the study period. This decrease was accompanied by an increase in Gram-negative bacteremia. Susceptibility to empiric antibiotics (third-generation cephalosporins) dropped from 96% to 83%. Over the study period, more children presented with central venous lines, which was felt to be due to increasing outpatient use of total parenteral nutrition (TPN).
Bottom line: Vaccination against pneumococcus is changing the microbiology of pediatric bacteremia, with fewer vaccine-preventable Gram-positive infections and more Gram-negative infections. This increases the likelihood of resistance to third-generation cephalosporins as empiric antibiotic.
Citation: Irwin AD, Drew RJ, Marshall P, et al. Etiology of childhood bacteremia and timely antibiotics administration in the emergency department. Pediatrics. 2015;135(4): 635-642.

What Should You Do If You Get a Needlestick?
EDITOR’S NOTE: This month’s KCQ first appeared in October 2010 and since that time has been one of our website’s most-read articles, generating nearly 35,000-plus page views. Enjoy it again this month!
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center, approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione and colleagues surveyed internal medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the CDC in 2004 suggests that because these numbers represent only self-reported injuries, the annual incidence of such injuries is much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
Because decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should be encouraged and supported in reporting all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Widespread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this improvement, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler and colleagues reported that, following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis (PEP), an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, PEP with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source ranges from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, because no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin; however, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing the benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated and should be started as quickly as possible.3,8,10
The 2013 U.S. Public Health Service recommendations for PEP call for initiating three (or more) antiretroviral drugs for all occupational exposures. Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.10
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HBIG prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP.
Dr. Zehnder is a hospitalist and assistant professor of medicine at the University of Colorado Denver in Aurora.
References
- Mangione CM, Gerberding JL, Cummings SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, Cobden D, Pashos CL. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opin. 2005;21(5):741-747.
- Centers for Disease Control and Prevention. Workbook for designing, implementing, and evaluating a sharps injury prevention program. CDC website. Accessed May 31, 2015.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Centers for Disease Control and Prevention. Exposure to blood: What healthcare personnel need to know. CDC website. Accessed May 31, 2015.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Accessed May 31, 2015.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
- Updated US Public Health Service Guidelines for the management of occupational exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Accessed May 31, 2015.
EDITOR’S NOTE: This month’s KCQ first appeared in October 2010 and since that time has been one of our website’s most-read articles, generating nearly 35,000-plus page views. Enjoy it again this month!
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center, approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione and colleagues surveyed internal medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the CDC in 2004 suggests that because these numbers represent only self-reported injuries, the annual incidence of such injuries is much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
Because decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should be encouraged and supported in reporting all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Widespread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this improvement, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler and colleagues reported that, following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis (PEP), an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, PEP with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source ranges from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, because no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin; however, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing the benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated and should be started as quickly as possible.3,8,10
The 2013 U.S. Public Health Service recommendations for PEP call for initiating three (or more) antiretroviral drugs for all occupational exposures. Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.10
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HBIG prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP.
Dr. Zehnder is a hospitalist and assistant professor of medicine at the University of Colorado Denver in Aurora.
References
- Mangione CM, Gerberding JL, Cummings SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, Cobden D, Pashos CL. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opin. 2005;21(5):741-747.
- Centers for Disease Control and Prevention. Workbook for designing, implementing, and evaluating a sharps injury prevention program. CDC website. Accessed May 31, 2015.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Centers for Disease Control and Prevention. Exposure to blood: What healthcare personnel need to know. CDC website. Accessed May 31, 2015.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Accessed May 31, 2015.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
- Updated US Public Health Service Guidelines for the management of occupational exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Accessed May 31, 2015.
EDITOR’S NOTE: This month’s KCQ first appeared in October 2010 and since that time has been one of our website’s most-read articles, generating nearly 35,000-plus page views. Enjoy it again this month!
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center, approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione and colleagues surveyed internal medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the CDC in 2004 suggests that because these numbers represent only self-reported injuries, the annual incidence of such injuries is much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
Because decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should be encouraged and supported in reporting all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Widespread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this improvement, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler and colleagues reported that, following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis (PEP), an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, PEP with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source ranges from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, because no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin; however, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing the benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated and should be started as quickly as possible.3,8,10
The 2013 U.S. Public Health Service recommendations for PEP call for initiating three (or more) antiretroviral drugs for all occupational exposures. Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.10
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HBIG prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP.
Dr. Zehnder is a hospitalist and assistant professor of medicine at the University of Colorado Denver in Aurora.
References
- Mangione CM, Gerberding JL, Cummings SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, Cobden D, Pashos CL. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opin. 2005;21(5):741-747.
- Centers for Disease Control and Prevention. Workbook for designing, implementing, and evaluating a sharps injury prevention program. CDC website. Accessed May 31, 2015.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Centers for Disease Control and Prevention. Exposure to blood: What healthcare personnel need to know. CDC website. Accessed May 31, 2015.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Accessed May 31, 2015.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
- Updated US Public Health Service Guidelines for the management of occupational exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Accessed May 31, 2015.