User login
Isolating Suture Slippage During Cadaveric Testing of Knotless Anchors
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
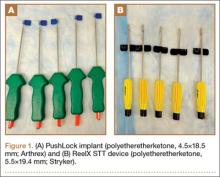
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
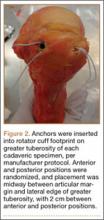
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
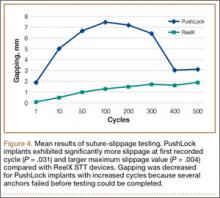
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
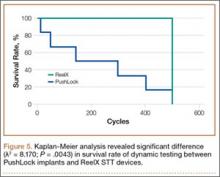
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Second pathology review boosts diagnostic accuracy in lymphoma
In patients with newly diagnosed lymphoma and suspected lymphoma, a second pathological review found inaccuracies in the original diagnosis among 17% of more than 42,000 cases, based on data presented at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In more than 25% of all discrepancies, tumors were reclassified at the second pathology review as the result of findings from additional immunostaining and molecular studies – polymerase chain reaction and fluorescence in situ hybridization.
In 15% of cases, diagnostic changes were expected to result in a change in patient management.
“Our study highlights the importance of specialized centralized review of lymphoma diagnosis, not only in the setting of clinical trials but also in routine clinical practice, for optimal patient management,” reported Dr. Camille Laurent of the Institut Universitaire du Cancer Oncopole, Toulouse, France.
In 2010, the French National Cancer Agency (INCa) established the Lymphopath Network, comprising 33 reference centers, to provide a review by expert hematopathologists of every newly diagnosed lymphoma or suspected lymphoma prior to treatment. These new diagnoses were entered in a central national database. Between 2010 and 2015, 42,146 samples were reviewed: 35,753 were newly diagnosed as lymphomas, while the remaining 6,393 cases included 4,610 reactive lymphoid conditions and 1,783 nonlymphoid malignancies, including especially myeloma and leukemic disorders.
Discordant diagnoses among extra-cutaneous lymphomas were carefully examined by a hematologist and recorded as major or minor depending on the expected therapeutic impact. Dr. Laurent said.
The discordance rate between the referral diagnosis and the final diagnosis was 17.2%. Small B-cell lymphomas and peripheral T-cell lymphoma subtyping were the most common discrepancies; 6.4% of discordances were due to an unspecified lymphoma diagnosis, Dr. Laurent stated.
Less than 2% of discrepancies were due to misclassifications of benign versus malignant lymphoid conditions and of Hodgkin lymphoma versus non-Hodgkin lymphoma. There were minor discrepancies (2.2%) in follicular lymphoma misgrading and diffuse large B-cell lymphoma subtypes.
Given the complexity of lymphoma classification, it is not surprising that expert hematopathologists can refine diagnoses. As we progress in understanding the specific pathogenesis of lymphoma subtypes and utility of targeted therapy, it becomes even more critical to make correct diagnoses. This study reiterates the importance of expert review for many, if not all, lymphoma samples, particularly any T-cell lymphoma and non—follicular small B-cell lymphoma.
Given the complexity of lymphoma classification, it is not surprising that expert hematopathologists can refine diagnoses. As we progress in understanding the specific pathogenesis of lymphoma subtypes and utility of targeted therapy, it becomes even more critical to make correct diagnoses. This study reiterates the importance of expert review for many, if not all, lymphoma samples, particularly any T-cell lymphoma and non—follicular small B-cell lymphoma.
Given the complexity of lymphoma classification, it is not surprising that expert hematopathologists can refine diagnoses. As we progress in understanding the specific pathogenesis of lymphoma subtypes and utility of targeted therapy, it becomes even more critical to make correct diagnoses. This study reiterates the importance of expert review for many, if not all, lymphoma samples, particularly any T-cell lymphoma and non—follicular small B-cell lymphoma.
In patients with newly diagnosed lymphoma and suspected lymphoma, a second pathological review found inaccuracies in the original diagnosis among 17% of more than 42,000 cases, based on data presented at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In more than 25% of all discrepancies, tumors were reclassified at the second pathology review as the result of findings from additional immunostaining and molecular studies – polymerase chain reaction and fluorescence in situ hybridization.
In 15% of cases, diagnostic changes were expected to result in a change in patient management.
“Our study highlights the importance of specialized centralized review of lymphoma diagnosis, not only in the setting of clinical trials but also in routine clinical practice, for optimal patient management,” reported Dr. Camille Laurent of the Institut Universitaire du Cancer Oncopole, Toulouse, France.
In 2010, the French National Cancer Agency (INCa) established the Lymphopath Network, comprising 33 reference centers, to provide a review by expert hematopathologists of every newly diagnosed lymphoma or suspected lymphoma prior to treatment. These new diagnoses were entered in a central national database. Between 2010 and 2015, 42,146 samples were reviewed: 35,753 were newly diagnosed as lymphomas, while the remaining 6,393 cases included 4,610 reactive lymphoid conditions and 1,783 nonlymphoid malignancies, including especially myeloma and leukemic disorders.
Discordant diagnoses among extra-cutaneous lymphomas were carefully examined by a hematologist and recorded as major or minor depending on the expected therapeutic impact. Dr. Laurent said.
The discordance rate between the referral diagnosis and the final diagnosis was 17.2%. Small B-cell lymphomas and peripheral T-cell lymphoma subtyping were the most common discrepancies; 6.4% of discordances were due to an unspecified lymphoma diagnosis, Dr. Laurent stated.
Less than 2% of discrepancies were due to misclassifications of benign versus malignant lymphoid conditions and of Hodgkin lymphoma versus non-Hodgkin lymphoma. There were minor discrepancies (2.2%) in follicular lymphoma misgrading and diffuse large B-cell lymphoma subtypes.
In patients with newly diagnosed lymphoma and suspected lymphoma, a second pathological review found inaccuracies in the original diagnosis among 17% of more than 42,000 cases, based on data presented at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In more than 25% of all discrepancies, tumors were reclassified at the second pathology review as the result of findings from additional immunostaining and molecular studies – polymerase chain reaction and fluorescence in situ hybridization.
In 15% of cases, diagnostic changes were expected to result in a change in patient management.
“Our study highlights the importance of specialized centralized review of lymphoma diagnosis, not only in the setting of clinical trials but also in routine clinical practice, for optimal patient management,” reported Dr. Camille Laurent of the Institut Universitaire du Cancer Oncopole, Toulouse, France.
In 2010, the French National Cancer Agency (INCa) established the Lymphopath Network, comprising 33 reference centers, to provide a review by expert hematopathologists of every newly diagnosed lymphoma or suspected lymphoma prior to treatment. These new diagnoses were entered in a central national database. Between 2010 and 2015, 42,146 samples were reviewed: 35,753 were newly diagnosed as lymphomas, while the remaining 6,393 cases included 4,610 reactive lymphoid conditions and 1,783 nonlymphoid malignancies, including especially myeloma and leukemic disorders.
Discordant diagnoses among extra-cutaneous lymphomas were carefully examined by a hematologist and recorded as major or minor depending on the expected therapeutic impact. Dr. Laurent said.
The discordance rate between the referral diagnosis and the final diagnosis was 17.2%. Small B-cell lymphomas and peripheral T-cell lymphoma subtyping were the most common discrepancies; 6.4% of discordances were due to an unspecified lymphoma diagnosis, Dr. Laurent stated.
Less than 2% of discrepancies were due to misclassifications of benign versus malignant lymphoid conditions and of Hodgkin lymphoma versus non-Hodgkin lymphoma. There were minor discrepancies (2.2%) in follicular lymphoma misgrading and diffuse large B-cell lymphoma subtypes.
FROM 13-ICML
Key clinical point: A second pathological review of newly-diagnosed lymphoma or suspected lymphoma found discrepancies in 17% of cases.
Major finding: Small B-cell lymphomas and peripheral T-cell lymphoma subtyping were the most common discrepancies; 6.4% of discordances were due to an unspecified lymphoma diagnosis.
Data source: 42,146 samples from the French National Cancer Agency’s Lymphopath Network, comprising 33 reference centers.
Disclosures: Dr. Laurent had no relevant financial disclosures.
LISTEN NOW: Hospitalist, Edwin Lopez, PA-C, on Post-Acute Care in the U.S. Health System
Edwin Lopez, PA-C, of St. Elizabeth Hospital in Enumclaw, Wash., offers his views on post-acute care in the U.S. health system, and how his work as a hospitalist has expanded to the nursing home across the street.
Edwin Lopez, PA-C, of St. Elizabeth Hospital in Enumclaw, Wash., offers his views on post-acute care in the U.S. health system, and how his work as a hospitalist has expanded to the nursing home across the street.
Edwin Lopez, PA-C, of St. Elizabeth Hospital in Enumclaw, Wash., offers his views on post-acute care in the U.S. health system, and how his work as a hospitalist has expanded to the nursing home across the street.
Methotrexate could treat MPNs cheaply, team says
Photo courtesy of the
National Cancer Institute
Preclinical research suggests the antineoplastic agent methotrexate (MTX) could be used to treat patients with myeloproliferative neoplasms (MPNs).
Experiments in Drosophila and human cell lines showed that MTX reduces JAK/STAT pathway activity and MPN-associated pathway signaling.
Researchers therefore speculated that low doses of MTX might treat MPNs as effectively as the JAK1/2 inhibitor ruxolitinib, but for a lower cost.
“Given that a year’s course of low-dose MTX costs around £30, the potential to repurpose MTX could provide thousands of patients with a much-needed treatment option and also generate substantial savings for healthcare systems,” said study author Martin Zeidler, DPhil, of The University of Sheffield in the UK.
He and his colleagues noted that, in comparison, ruxolitinib costs more than £40,000 per year per patient.
The researchers made this comparison and described their work with MTX in PLOS ONE.
The team used cells from the fruit fly Drosophila to screen for small molecules that suppress the JAK/STAT signaling pathway, which is central to the development of MPNs in humans. The screen suggested that MTX and a related molecule, aminopterin, suppress STAT activation.
So the researchers conducted experiments in human cell lines and found that MTX suppresses human JAK/STAT signaling without affecting other phosphorylation-dependent pathways.
The team also found that MTX significantly reduces STAT5 phosphorylation in cells expressing JAK2 V617F. However, MTX-treated cells can still respond to physiological levels of erythropoietin.
The researchers are now looking to undertake clinical trials to examine the possibility of repurposing low-dose MTX for the treatment of MPNs.
“We have the potential to revolutionize the treatment of this group of chronic diseases—a breakthrough that may ultimately represent a new treatment option able to bring relief to both patients and health funders,” Dr Zeidler said. ![]()

Photo courtesy of the
National Cancer Institute
Preclinical research suggests the antineoplastic agent methotrexate (MTX) could be used to treat patients with myeloproliferative neoplasms (MPNs).
Experiments in Drosophila and human cell lines showed that MTX reduces JAK/STAT pathway activity and MPN-associated pathway signaling.
Researchers therefore speculated that low doses of MTX might treat MPNs as effectively as the JAK1/2 inhibitor ruxolitinib, but for a lower cost.
“Given that a year’s course of low-dose MTX costs around £30, the potential to repurpose MTX could provide thousands of patients with a much-needed treatment option and also generate substantial savings for healthcare systems,” said study author Martin Zeidler, DPhil, of The University of Sheffield in the UK.
He and his colleagues noted that, in comparison, ruxolitinib costs more than £40,000 per year per patient.
The researchers made this comparison and described their work with MTX in PLOS ONE.
The team used cells from the fruit fly Drosophila to screen for small molecules that suppress the JAK/STAT signaling pathway, which is central to the development of MPNs in humans. The screen suggested that MTX and a related molecule, aminopterin, suppress STAT activation.
So the researchers conducted experiments in human cell lines and found that MTX suppresses human JAK/STAT signaling without affecting other phosphorylation-dependent pathways.
The team also found that MTX significantly reduces STAT5 phosphorylation in cells expressing JAK2 V617F. However, MTX-treated cells can still respond to physiological levels of erythropoietin.
The researchers are now looking to undertake clinical trials to examine the possibility of repurposing low-dose MTX for the treatment of MPNs.
“We have the potential to revolutionize the treatment of this group of chronic diseases—a breakthrough that may ultimately represent a new treatment option able to bring relief to both patients and health funders,” Dr Zeidler said. ![]()

Photo courtesy of the
National Cancer Institute
Preclinical research suggests the antineoplastic agent methotrexate (MTX) could be used to treat patients with myeloproliferative neoplasms (MPNs).
Experiments in Drosophila and human cell lines showed that MTX reduces JAK/STAT pathway activity and MPN-associated pathway signaling.
Researchers therefore speculated that low doses of MTX might treat MPNs as effectively as the JAK1/2 inhibitor ruxolitinib, but for a lower cost.
“Given that a year’s course of low-dose MTX costs around £30, the potential to repurpose MTX could provide thousands of patients with a much-needed treatment option and also generate substantial savings for healthcare systems,” said study author Martin Zeidler, DPhil, of The University of Sheffield in the UK.
He and his colleagues noted that, in comparison, ruxolitinib costs more than £40,000 per year per patient.
The researchers made this comparison and described their work with MTX in PLOS ONE.
The team used cells from the fruit fly Drosophila to screen for small molecules that suppress the JAK/STAT signaling pathway, which is central to the development of MPNs in humans. The screen suggested that MTX and a related molecule, aminopterin, suppress STAT activation.
So the researchers conducted experiments in human cell lines and found that MTX suppresses human JAK/STAT signaling without affecting other phosphorylation-dependent pathways.
The team also found that MTX significantly reduces STAT5 phosphorylation in cells expressing JAK2 V617F. However, MTX-treated cells can still respond to physiological levels of erythropoietin.
The researchers are now looking to undertake clinical trials to examine the possibility of repurposing low-dose MTX for the treatment of MPNs.
“We have the potential to revolutionize the treatment of this group of chronic diseases—a breakthrough that may ultimately represent a new treatment option able to bring relief to both patients and health funders,” Dr Zeidler said. ![]()
Healthcare professionals work while sick despite risk to patients

Photo by Logan Tuttle
Results of a small survey showed that many healthcare professionals reported to work while sick, despite recognizing that this could put their patients at risk.
About 95% of survey respondents acknowledged that working while sick puts patients at risk, but 83% of respondents said they had worked while sick at least once in the past year.
About 9% of respondents reported working while sick at least 5 times.
Julia E. Szymczak, PhD, of the Children’s Hospital of Philadelphia in Pennsylvania, and her colleagues reported these results in JAMA Pediatrics alongside a related editorial.
The researchers administered an anonymous survey to attending physicians and advanced practice clinicians (APCs) at the Children’s Hospital of Philadelphia. APCs included certified registered nurse practitioners, physician assistants, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives.
Overall, 280 attending physicians (61%) and 256 APCs (54.5%) responded. Most respondents (504, 95.3%) said working while sick put patients at risk.
However, 446 respondents (83.1%) reported working while sick at least once in the past year, and 50 respondents (9.3%) reported working while sick at least 5 times.
Respondents reported working with symptoms that included diarrhea, fever, and the onset of significant respiratory symptoms.
The reasons physicians and APCs worked while sick included not wanting to let colleagues down (98.7%), staffing concerns (94.9%), not wanting to let patients down (92.5%), fear of being ostracized by colleagues (64%), and concerns about the continuity of care (63.8%).
An analysis of written comments about why respondents work while sick highlighted 3 areas: challenges in identifying and arranging someone to cover their work and a lack of resources to accommodate sick leave, a strong cultural norm in the hospital to report for work unless one is extremely ill, and ambiguity about what symptoms constitute being too sick to work.
Dr Szymczak and her colleagues said this study suggests complex social and logistical factors cause healthcare workers to report to work sick. But these results could inform efforts to help workers make the right choice to keep their patients and colleagues safe while caring for themselves. ![]()

Photo by Logan Tuttle
Results of a small survey showed that many healthcare professionals reported to work while sick, despite recognizing that this could put their patients at risk.
About 95% of survey respondents acknowledged that working while sick puts patients at risk, but 83% of respondents said they had worked while sick at least once in the past year.
About 9% of respondents reported working while sick at least 5 times.
Julia E. Szymczak, PhD, of the Children’s Hospital of Philadelphia in Pennsylvania, and her colleagues reported these results in JAMA Pediatrics alongside a related editorial.
The researchers administered an anonymous survey to attending physicians and advanced practice clinicians (APCs) at the Children’s Hospital of Philadelphia. APCs included certified registered nurse practitioners, physician assistants, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives.
Overall, 280 attending physicians (61%) and 256 APCs (54.5%) responded. Most respondents (504, 95.3%) said working while sick put patients at risk.
However, 446 respondents (83.1%) reported working while sick at least once in the past year, and 50 respondents (9.3%) reported working while sick at least 5 times.
Respondents reported working with symptoms that included diarrhea, fever, and the onset of significant respiratory symptoms.
The reasons physicians and APCs worked while sick included not wanting to let colleagues down (98.7%), staffing concerns (94.9%), not wanting to let patients down (92.5%), fear of being ostracized by colleagues (64%), and concerns about the continuity of care (63.8%).
An analysis of written comments about why respondents work while sick highlighted 3 areas: challenges in identifying and arranging someone to cover their work and a lack of resources to accommodate sick leave, a strong cultural norm in the hospital to report for work unless one is extremely ill, and ambiguity about what symptoms constitute being too sick to work.
Dr Szymczak and her colleagues said this study suggests complex social and logistical factors cause healthcare workers to report to work sick. But these results could inform efforts to help workers make the right choice to keep their patients and colleagues safe while caring for themselves. ![]()

Photo by Logan Tuttle
Results of a small survey showed that many healthcare professionals reported to work while sick, despite recognizing that this could put their patients at risk.
About 95% of survey respondents acknowledged that working while sick puts patients at risk, but 83% of respondents said they had worked while sick at least once in the past year.
About 9% of respondents reported working while sick at least 5 times.
Julia E. Szymczak, PhD, of the Children’s Hospital of Philadelphia in Pennsylvania, and her colleagues reported these results in JAMA Pediatrics alongside a related editorial.
The researchers administered an anonymous survey to attending physicians and advanced practice clinicians (APCs) at the Children’s Hospital of Philadelphia. APCs included certified registered nurse practitioners, physician assistants, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives.
Overall, 280 attending physicians (61%) and 256 APCs (54.5%) responded. Most respondents (504, 95.3%) said working while sick put patients at risk.
However, 446 respondents (83.1%) reported working while sick at least once in the past year, and 50 respondents (9.3%) reported working while sick at least 5 times.
Respondents reported working with symptoms that included diarrhea, fever, and the onset of significant respiratory symptoms.
The reasons physicians and APCs worked while sick included not wanting to let colleagues down (98.7%), staffing concerns (94.9%), not wanting to let patients down (92.5%), fear of being ostracized by colleagues (64%), and concerns about the continuity of care (63.8%).
An analysis of written comments about why respondents work while sick highlighted 3 areas: challenges in identifying and arranging someone to cover their work and a lack of resources to accommodate sick leave, a strong cultural norm in the hospital to report for work unless one is extremely ill, and ambiguity about what symptoms constitute being too sick to work.
Dr Szymczak and her colleagues said this study suggests complex social and logistical factors cause healthcare workers to report to work sick. But these results could inform efforts to help workers make the right choice to keep their patients and colleagues safe while caring for themselves. ![]()
NICE supports use of apixaban for VTE

Photo courtesy of the CDC
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
According to NICE, data from the AMPLIFY and AMPLIFY-EXT studies suggest apixaban is clinically effective for treating and preventing VTE.
And cost analyses indicate that apixaban is a cost-effective use of National Health Service (NHS) resources.
NICE said apixaban should be available on the NHS within 3 months of the guidance release date. The guidance was made available in June.
Dosing
To treat deep vein thrombosis (DVT) or pulmonary embolism (PE), 10 mg of apixaban should be taken twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months.
To prevent recurrent VTE, patients who have completed 6 months of treatment for DVT or PE should take apixaban at 2.5 mg twice a day.
“[A]pixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
To assess the clinical effectiveness of apixaban, a committee advising NICE evaluated data from the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee also pointed out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of NHS resources. ![]()

Photo courtesy of the CDC
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
According to NICE, data from the AMPLIFY and AMPLIFY-EXT studies suggest apixaban is clinically effective for treating and preventing VTE.
And cost analyses indicate that apixaban is a cost-effective use of National Health Service (NHS) resources.
NICE said apixaban should be available on the NHS within 3 months of the guidance release date. The guidance was made available in June.
Dosing
To treat deep vein thrombosis (DVT) or pulmonary embolism (PE), 10 mg of apixaban should be taken twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months.
To prevent recurrent VTE, patients who have completed 6 months of treatment for DVT or PE should take apixaban at 2.5 mg twice a day.
“[A]pixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
To assess the clinical effectiveness of apixaban, a committee advising NICE evaluated data from the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee also pointed out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of NHS resources. ![]()

Photo courtesy of the CDC
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
According to NICE, data from the AMPLIFY and AMPLIFY-EXT studies suggest apixaban is clinically effective for treating and preventing VTE.
And cost analyses indicate that apixaban is a cost-effective use of National Health Service (NHS) resources.
NICE said apixaban should be available on the NHS within 3 months of the guidance release date. The guidance was made available in June.
Dosing
To treat deep vein thrombosis (DVT) or pulmonary embolism (PE), 10 mg of apixaban should be taken twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months.
To prevent recurrent VTE, patients who have completed 6 months of treatment for DVT or PE should take apixaban at 2.5 mg twice a day.
“[A]pixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
To assess the clinical effectiveness of apixaban, a committee advising NICE evaluated data from the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee also pointed out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of NHS resources. ![]()
EAP makes drug available to MM patients

Photo by Linda Bartlett
A new expanded access program (EAP) will make daratumumab, an investigational anti-CD38 monoclonal antibody, available to patients with double-refractory multiple myeloma (MM).
The multicenter EAP is open to MM patients in the US who are 18 years of age or older.
Patients must be refractory to both a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) or have received 3 or more prior lines of therapy, including a PI and an IMiD.
In the US, EAPs are designed to make investigational medicines available for patients with serious or life-threatening illnesses who are ineligible for ongoing interventional trials and have exhausted currently available treatment options.
This EAP will make daratumumab available at up to 40 medical centers in the US while the drug’s Biologics License Application is under review by the US Food and Drug Administration (FDA).
Additional information about the EAP can be found on clinicaltrials.gov (NCT02477891). Janssen Research & Development, LLC, the company developing daratumumab, is collaborating with the Multiple Myeloma Research Foundation on this EAP.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds with high affinity to the transmembrane ectoenzyme CD38 on the surface of MM cells.
Five phase 3 clinical studies with daratumumab in relapsed and frontline settings are currently ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases on which CD38 is expressed, such as smoldering myeloma and non-Hodgkin lymphoma.
On May 1, 2013, daratumumab received breakthrough designation from the FDA for the treatment of patients with MM who have received at least 3 prior lines of therapy, including a PI and an IMiD, or who are double refractory to a PI and an IMiD.
On June 5, 2015, Janssen announced it had initiated the rolling submission of its Biologics License Application for daratumumab to the FDA for the treatment of this set of MM patients. A rolling submission allows the company to submit portions of the regulatory application to the FDA as they are completed.
The regulatory submission for daratumumab will be primarily supported by data from the phase 2 MMY2002 (SIRIUS) study, the results of which were presented at the 2015 ASCO Annual Meeting.
The application will also be supported by data from 4 other studies, including the phase 1/2 GEN501 monotherapy study. ![]()

Photo by Linda Bartlett
A new expanded access program (EAP) will make daratumumab, an investigational anti-CD38 monoclonal antibody, available to patients with double-refractory multiple myeloma (MM).
The multicenter EAP is open to MM patients in the US who are 18 years of age or older.
Patients must be refractory to both a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) or have received 3 or more prior lines of therapy, including a PI and an IMiD.
In the US, EAPs are designed to make investigational medicines available for patients with serious or life-threatening illnesses who are ineligible for ongoing interventional trials and have exhausted currently available treatment options.
This EAP will make daratumumab available at up to 40 medical centers in the US while the drug’s Biologics License Application is under review by the US Food and Drug Administration (FDA).
Additional information about the EAP can be found on clinicaltrials.gov (NCT02477891). Janssen Research & Development, LLC, the company developing daratumumab, is collaborating with the Multiple Myeloma Research Foundation on this EAP.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds with high affinity to the transmembrane ectoenzyme CD38 on the surface of MM cells.
Five phase 3 clinical studies with daratumumab in relapsed and frontline settings are currently ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases on which CD38 is expressed, such as smoldering myeloma and non-Hodgkin lymphoma.
On May 1, 2013, daratumumab received breakthrough designation from the FDA for the treatment of patients with MM who have received at least 3 prior lines of therapy, including a PI and an IMiD, or who are double refractory to a PI and an IMiD.
On June 5, 2015, Janssen announced it had initiated the rolling submission of its Biologics License Application for daratumumab to the FDA for the treatment of this set of MM patients. A rolling submission allows the company to submit portions of the regulatory application to the FDA as they are completed.
The regulatory submission for daratumumab will be primarily supported by data from the phase 2 MMY2002 (SIRIUS) study, the results of which were presented at the 2015 ASCO Annual Meeting.
The application will also be supported by data from 4 other studies, including the phase 1/2 GEN501 monotherapy study. ![]()

Photo by Linda Bartlett
A new expanded access program (EAP) will make daratumumab, an investigational anti-CD38 monoclonal antibody, available to patients with double-refractory multiple myeloma (MM).
The multicenter EAP is open to MM patients in the US who are 18 years of age or older.
Patients must be refractory to both a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) or have received 3 or more prior lines of therapy, including a PI and an IMiD.
In the US, EAPs are designed to make investigational medicines available for patients with serious or life-threatening illnesses who are ineligible for ongoing interventional trials and have exhausted currently available treatment options.
This EAP will make daratumumab available at up to 40 medical centers in the US while the drug’s Biologics License Application is under review by the US Food and Drug Administration (FDA).
Additional information about the EAP can be found on clinicaltrials.gov (NCT02477891). Janssen Research & Development, LLC, the company developing daratumumab, is collaborating with the Multiple Myeloma Research Foundation on this EAP.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds with high affinity to the transmembrane ectoenzyme CD38 on the surface of MM cells.
Five phase 3 clinical studies with daratumumab in relapsed and frontline settings are currently ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases on which CD38 is expressed, such as smoldering myeloma and non-Hodgkin lymphoma.
On May 1, 2013, daratumumab received breakthrough designation from the FDA for the treatment of patients with MM who have received at least 3 prior lines of therapy, including a PI and an IMiD, or who are double refractory to a PI and an IMiD.
On June 5, 2015, Janssen announced it had initiated the rolling submission of its Biologics License Application for daratumumab to the FDA for the treatment of this set of MM patients. A rolling submission allows the company to submit portions of the regulatory application to the FDA as they are completed.
The regulatory submission for daratumumab will be primarily supported by data from the phase 2 MMY2002 (SIRIUS) study, the results of which were presented at the 2015 ASCO Annual Meeting.
The application will also be supported by data from 4 other studies, including the phase 1/2 GEN501 monotherapy study. ![]()
Telemetry Use for LOS and Cost Reduction
Inpatient hospital services are a major component of total US civilian noninstitutionalized healthcare expenses, accounting for 29.3% of spending in 2009[1] when the average cost per stay was $9700.[2] Telemetry monitoring, a widely used resource for the identification of life‐threatening arrhythmias, contributes to these costs. In 1998, Sivaram et al. estimated the cost per patient at $683; in 2010, Ivonye et al. published the cost difference between a telemetry bed and a nonmonitored bed in their inner‐city public teaching facility reached $800.[3, 4]
In 1991, the American College of Cardiology published guidelines for telemetry use, which were later revised by the American Heart Association in 2004.[5, 6] Notably, the guidelines are based on expert opinion and on research data in electrocardiography.[7] The guidelines divide patients into 3 classes based on clinical condition: recommending telemetry monitoring for almost all class I patients, stating possible benefit in class II patients, and discouraging cardiac monitoring for the low‐risk class III patients.[5, 6] The Choosing Wisely campaign, an initiative of the American Board of Internal Medicine and the Society of Hospital Medicine, highlights telemetry monitoring as 1 of the top 5 interventions that physicians and patients should question when determining tests and procedures.[8] Choosing Wisely suggests using a protocol to govern continuation of telemetry outside of the intensive care unit (ICU), as inappropriate monitoring increases care costs and may result in patient harm.[8] The Joint Commission 2014 National Patient Safety Goals notes that numerous alarm signals and the resulting noise and displayed information tends to desensitize staff and cause them to miss or ignore alarm signals or even disable them.[9]
Few studies have examined implementation methods for improved telemetry bed utilization. One study evaluated the impact of a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team, noting improved cardiac monitoring bed utilization and decreased academic hospital closure, which previously resulted in inability to accept new patients or procedure cancellation.[10] Another study provided an orientation handout discussed by the chief resident and telemetry indication reviews during multidisciplinary rounds 3 times a week.[11]
Our study is one the first to demonstrate a model for a hospitalist‐led approach to guide appropriate telemetry use. We investigated the impact of a multipronged approach to guide telemetry usage: (1) a hospitalist‐led, daily review of bed utilization during attending rounds, (2) a hospitalist attending‐driven, trainee‐focused education module on telemetry utilization, (3) quarterly feedback on telemetry bed utilization rates, and (4) financial incentives. We analyzed pre‐ and post‐evaluation results from the education module to measure impact on knowledge, skills, and attitudes. Additionally, we evaluated the effect of the intervention on length of stay (LOS) and bed utilization costs, while monitoring case mix index (CMI) and overall mortality.
METHODS
Setting
This study took place at Stanford Hospital and Clinics, a teaching academic center in Stanford, California. Stanford Hospital is a 444‐bed, urban medical center with 114 telemetry intermediate ICU beds, and 66 ICU beds. The 264 medicalsurgical beds lack telemetry monitoring, which can only be completed in the intermediate and full ICU. All patients on telemetry units receive both cardiac monitoring and increased nursing ratios. Transfer orders are placed in the electronic medical record to shift patients between care levels. Bed control attempts to transfer patients as soon as an open bed in the appropriate care level exists.
The study included all 5 housestaff inpatient general internal medicine wards teams (which excludes cardiology, pulmonary hypertension, hematology, oncology, and post‐transplant patients). Hospitalists and nonhospitalists attend on the wards for 1‐ to 2‐week blocks. Teaching teams are staffed by 1 to 2 medical students, 2 interns, 1 resident, and 1 attending. The university institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Participants
Ten full‐ and part‐time hospitalist physicians participated in the standardized telemetry teaching. Fifty‐six of the approximately 80 medical students and housestaff on hospitalists' teams completed the educational evaluation. Both hospitalist and nonhospitalist teams participated in daily multidisciplinary rounds, focusing on barriers to discharge including telemetry use. Twelve nonhospitalists served on the wards during the intervention period. Hospitalists covered 72% of the internal medicine wards during the intervention period.
Study Design
We investigated the impact of a multipronged approach to guide telemetry usage from January 2013 to August 2013 (intervention period).
Hospitalist‐Led Daily Review of Bed Utilization
Hospitalists were encouraged to discuss the need of telemetry on daily attending rounds and review indications for telemetry while on service. Prior to starting a ward block, attendings were emailed the teaching module with a reminder to discuss the need for telemetry on attending rounds. Reminders to discuss telemetry utilization were also provided during every‐other‐week hospitalist meetings. Compliance of daily discussion was not tracked.
Hospitalist‐Driven, Trainee‐Focused, Education Module on Telemetry Utilization
The educational module was taught during teaching sessions only by the hospitalists. Trainees on nonhospitalist teams did not receive dedicated teaching about telemetry usage. The module was given to learners only once. The module was a 10‐slide, Microsoft PowerPoint (Microsoft Corp., Redmond, WA) presentation that reviewed the history of telemetry, the American College of Cardiology and the American Heart Association guidelines, the cost difference between telemetry and nonmonitored beds, and the perceived barriers to discontinuation. The presentation was accompanied by a pre‐ and post‐evaluation to elicit knowledge, skills, and attitudes of telemetry use (see Supporting Information, Appendix A, in the online version of this article). The pre‐ and post‐evaluations were created through consensus with a multidisciplinary, expert panel after reviewing the evidence‐based literature.
Quarterly Feedback on Telemetry Bed Utilization Rates
Hospital beduse and CMI data were obtained from the Stanford finance department for the intervention period and for the baseline period, which was the year prior to the study, January 1, 2012 to December 31, 2012. Hospital beduse data included the number of days patients were on telemetry units versus medicalsurgical units (nontelemetry units), differentiated by hospitalists and nonhospitalists. Cost savings were calculated by the Stanford finance department that used Stanford‐specific, internal cost accounting data to determine the impact of the intervention. These data were reviewed at hospitalist meetings on a quarterly basis. We also obtained the University Healthsystem Consortium mortality index (observed to expected) for the general internal medicine service during the baseline and intervention periods.
To measure sustainment of telemetry reduction in the postintervention period, we measured telemetry LOS from September 2014 to March 2015 (extension period).
Financial Incentives
Hospitalists were provided a $2000 bonus at the end of fiscal year 2013 if the group showed a decrease in telemetry bed use in comparison to the baseline period.
Statistical Analysis of Clinical Outcome Measures
Continuous outcomes were tested using 2‐tailed t tests. Comparison of continuous outcome included differences in telemetry and nontelemetry LOS and CMI. Pairwise comparisons were made for various time periods. A P value of 0.05 was considered statistically significant. Statistical analyses were performed using Stata 12.0 software (StataCorp, College Station, TX).
RESULTS
Clinical and Value Outcomes
Baseline (January 2012December 2012) Versus Intervention Period (January 2013August 2013)
LOS for telemetry beds was significantly reduced over the intervention period (2.75 days vs 2.13 days, P=0.005) for hospitalists. Notably, there was no significant difference in mean LOS between baseline and intervention periods for nontelemetry beds (2.84 days vs 2.72 days, P=0.32) for hospitalists. In comparison, for nonhospitalists, there was no difference in mean LOS for telemetry beds between baseline and intervention periods (2.75 days vs 2.46 days, P=0.33) and nontelemetry beds (2.64 days vs 2.89 days, P=0.26) (Table 1).
| Baseline Period | Intervention Period | P Value | Extension Period | P Value | |
|---|---|---|---|---|---|
| |||||
| Length of stay | |||||
| Hospitalists | |||||
| Telemetry beds | 2.75 | 2.13 | 0.005 | 1.93 | 0.09 |
| Nontelemetry beds | 2.84 | 2.72 | 0.324 | 2.44 | 0.21 |
| Nonhospitalists | |||||
| Telemetry beds | 2.75 | 2.46 | 0.331 | 2.22 | 0.43 |
| Nontelemetry beds | 2.64 | 2.89 | 0.261 | 2.26 | 0.05 |
| Case mix index | |||||
| Hospitalists | 1.44 | 1.45 | 0.68 | 1.40 | 0.21 |
| Nonhospitalists | 1.46 | 1.40 | 0.53 | 1.53 | 0.18 |
Costs of hospital stay were also reduced in the multipronged, hospitalist‐driven intervention group. Expenditures for telemetry beds were reduced by 22.5% over the intervention period for hospitalists (Table 2).
| Baseline to Intervention Period | Intervention to Extension Period | |
|---|---|---|
| ||
| Hospitalists | ||
| Telemetry beds | 22.55% | 9.55% |
| Nontelemetry beds | 4.23% | 10.14% |
| Nonhospitalists | ||
| Telemetry beds | 10.55% | 9.89% |
| Nontelemetry beds | 9.47% | 21.84% |
The mean CMI of the patient cohort managed by the hospitalists in the baseline and intervention periods was not significantly different (1.44 vs 1.45, P=0.68). The mean CMI of the patients managed by the nonhospitalists in the baseline and intervention periods was also not significantly different (1.46 vs 1.40, P=0.53) (Table 1). Mortality index during the baseline and intervention periods was not significantly different (0.770.22 vs 0.660.23, P=0.54), as during the intervention and extension periods (0.660.23 vs 0.650.15, P=0.95).
Intervention Period (January 2013August 2013) Versus Extension Period (September 2014‐March 2015)
The decreased telemetry LOS for hospitalists was sustained from the intervention period to the extension period, from 2.13 to 1.93 (P=0.09). There was no significant change in the nontelemetry LOS in the intervention period compared to the extension period (2.72 vs 2.44, P=0.21). There was no change in the telemetry LOS for nonhospitalists from the intervention period to the extension period (2.46 vs 2.22, P=0.43).
The mean CMI in the hospitalist group was not significantly different in the intervention period compared to the extension period (1.45 to 1.40, P=0.21). The mean CMI in the nonhospitalist group did not change from the intervention period to the extension period (1.40 vs 1.53, P=0.18) (Table 1).
Education Outcomes
Out of the 56 participants completing the education module and survey, 28.6% were medical students, 53.6% were interns, 12.5% were second‐year residents, and 5.4% were third‐year residents. Several findings were seen at baseline via pretest. In evaluating patterns of current telemetry use, 32.2% of participants reported evaluating the necessity of telemetry for patients on admission only, 26.3% during transitions of care, 5.1% after discharge plans were cemented, 33.1% on a daily basis, and 3.4% rarely. When asked which member of the care team was most likely to encourage use of appropriate telemetry, 20.8% identified another resident, 13.9% nursing, 37.5% attending physician, 20.8% self, 4.2% the team as a whole, and 2.8% as not any.
Figure 1 shows premodule results regarding the trainees perceived percentage of patient encounters during which a participant's team discussed their patient's need for telemetry.
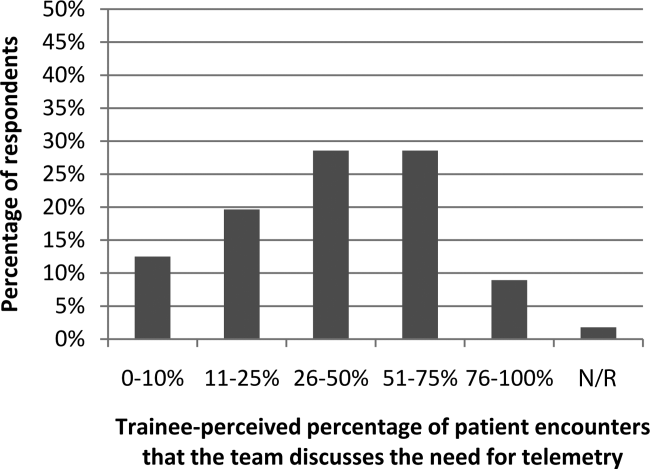
In assessing perception of current telemetry utilization, 1.8% of participants thought 0% to 10% of patients were currently on telemetry, 19.6% thought 11% to 20%, 42.9% thought 21% to 31%, 30.4% thought 31% to 40%, and 3.6% thought 41% to 50%.
Two areas were assessed at both baseline and after the intervention: knowledge of indications of telemetry use and cost related to telemetry use. We saw increased awareness of cost‐saving actions. To assess current knowledge of the indications of proper telemetry use according to American Heart Association guidelines, participants were presented with a list of 5 patients with different clinical indications for telemetry use and asked which patient required telemetry the most. Of the participants, 54.5% identified the correct answer in the pretest and 61.8% identified the correct answer in the post‐test. To assess knowledge of the costs of telemetry relative to other patient care, participants were presented with a patient case and asked to identify the most and least cost‐saving actions to safely care for the patient. When asked to identify the most cost‐saving action, 20.3% identified the correct answer in the pretest and 61.0% identified the correct answer in the post‐test. Of those who answered incorrectly in the pretest, 51.1% answered correctly in the post‐test (P=0.002). When asked to identify the least cost‐saving action, 23.7% identified the correct answer in the pretest and 50.9% identified the correct answer in the posttest. Of those who answered incorrectly in the pretest, 60.0% answered correctly in the post‐test (P=0.003).
In the post‐test, when asked about the importance of appropriate telemetry usage in providing cost‐conscious care and assuring appropriate hospital resource management, 76.8% of participants found the need very important, 21.4% somewhat important, and 1.8% as not applicable. The most commonly perceived barriers impeding discontinuation of telemetry, as reported by participants via post‐test, were nursing desires and time. Figure 2 shows all perceived barriers.
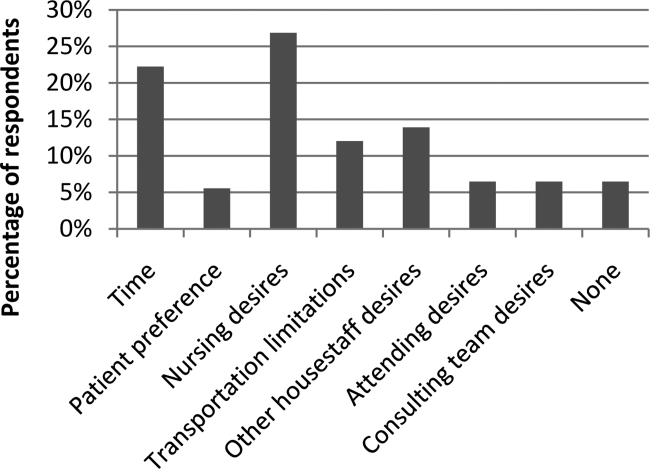
DISCUSSION
Our study is one of the first to our knowledge to demonstrate reductions in telemetry LOS by a hospitalist intervention for telemetry utilization. Others[10, 11] have studied the impact of an orientation handout by chief residents or a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team. Dressler et al. later sustained a 70% reduction in telemetry use without adversely affecting patient safety, as assessed through numbers of rapid response activations, codes, and deaths, through integrating the AHA guidelines into their electronic ordering system.[12] However, our study has the advantage of the primary team, who knows the patient and clinical scenario best, driving the change during attending rounds. In an era where cost consciousness intersects the practice of medicine, any intervention in patient care that demonstrates cost savings without an adverse impact on patient care and resource utilization must be emphasized. This is particularly important in academic institutions, where residents and medical students are learning to integrate the principles of patient safety and quality improvement into their clinical practice.[13] We actually showed sustained telemetry LOS reductions into the extension period after our intervention. We believe this may be due to telemetry triage being integrated into our attending and resident rounding practices. Future work should include integration of telemetry triage into clinical decision support in the electronic medical record and multidisciplinary rounds to disseminate telemetry triage hospital‐wide in both the academic and community settings.
Our study also revealed that nearly half of participants were not aware of the criteria for appropriate utilization of telemetry before our intervention; in the preintervention period, there were many anecdotal and objective findings of inappropriate utilization of telemetry as well as prolonged continuation beyond the clinical needs in both the hospitalist and nonhospitalist group. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
We were able to show increased knowledge of cost‐saving actions among trainees with our educational module. We believe it is imperative to educate our providers (physicians, nurses, case managers, and students within these disciplines) on the appropriate indications for telemetry use, not only to help with cost savings and resource availability (ie, allowing telemetry beds to be available for patients who need them most), but also to instill consistent expectations among our patients. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
Additionally, we feel it is important to consider the impacts of inappropriate use of telemetry from a patient's perspective: it is physically restrictive/emnconvenient, alarms are disruptive, it can be a barrier for other treatments such as physical therapy, it may increase the time it takes for imaging studies, a nurse may be required to accompany patients on telemetry, and poses additional costs to their medical bill.
We believe our success is due to several strategies. First, at the start of the fiscal year when quality improvement metrics are established, this particular metric (improving the appropriate utilization and timely discontinuation of telemetry) was deemed important by all hospitalists, engendering group buy‐in prior to the intervention. Our hospitalists received a detailed and interactive tutorial session in person at the beginning of the study. This tutorial provided the hospitalists with a comprehensive understanding of the appropriate (and inappropriate) indications for telemetry monitoring, hence facilitating guideline‐directed utilization. Email reminders and the tutorial tool were provided each time a hospitalist attended on the wards, and hospitalists received a small financial incentive to comply with appropriate telemetry utilization.
Our study has several strengths. First, the time frame of our study was long enough (8 months) to allow consistent trends to emerge and to optimize exposure of housestaff and medical students to this quality‐improvement initiative. Second, our cost savings came from 2 factors, direct reduction of inappropriate telemetry use and reduction in length of stay, highlighting the dual impact of appropriate telemetry utilization on cost. The overall reductions in telemetry utilization for the intervention group were a result of both reductions in initial placement on telemetry for patients who did not meet criteria for such monitoring as well as timely discontinuation of telemetry during the patient's hospitalization. Third, our study demonstrates that physicians can be effective in driving appropriate telemetry usage by participating in the clinical decision making regarding necessity and educating providers, trainees/students, and patients on appropriate indications. Finally, we show sustainment of our intervention in the extension period, suggesting telemetry triage integration into rounding practice.
Our study has limitations as well. First, our sample size is relatively small at a single academic center. Second, due to complexities in our faculty scheduling, we were unable to completely randomize patients to a hospitalist versus nonhospitalist team. However, we believe that despite the inability to randomize, our study does show the benefit of a hospitalist attending to reduce telemetry LOS given there was no change in nonhospitalist telemetry LOS despite all of the other hospital‐wide interventions (multidisciplinary rounds, similar housestaff). Third, our study was limited in that the CMI was used as a proxy for patient complexity, and the mortality index was used as the overall marker of safety. Further studies should monitor frequency and outcomes of arrhythmic events of patients transferred from telemetry monitoring to medicalsurgical beds. Finally, as the intervention was multipronged, we are unable to determine which component led to the reductions in telemetry utilization. Each component, however, remains easily transferrable to outside institutions. We demonstrated both a reduction in initiation of telemetry as well as timely discontinuation; however, due to the complexity in capturing this accurately, we were unable to numerically quantify these individual outcomes.
Additionally, there were approximately 10 nonhospitalist attendings who also staffed the wards during the intervention time period of our study; these attendings did not undergo the telemetry tutorial/orientation. This difference, along with the Hawthorne effect for the hospitalist attendings, also likely contributed to the difference in outcomes between the 2 attending cohorts in the intervention period.
CONCLUSIONS
Our results demonstrate that a multipronged hospitalist‐driven intervention to improve appropriate use of telemetry reduces telemetry LOS and cost. Hence, we believe that targeted, education‐driven interventions with monitoring of progress can have demonstrable impacts on changing practice. Physicians will need to make trade‐offs in clinical practice to balance efficient resource utilization with the patient's evolving condition in the inpatient setting, the complexities of clinical workflow, and the patient's expectations.[14] Appropriate telemetry utilization is a prime example of what needs to be done well in the future for high‐value care.
Acknowledgements
The authors acknowledge the hospitalists who participated in the intervention: Jeffrey Chi, Willliam Daines, Sumbul Desai, Poonam Hosamani, John Kugler, Charles Liao, Errol Ozdalga, and Sang Hoon Woo. The authors also acknowledge Joan Hendershott in the Finance Department and Joseph Hopkins in the Quality Department.
Disclosures: All coauthors have seen and agree with the contents of the article; submission (aside from abstracts) was not under review by any other publication. The authors report no disclosures of financial support from, or equity positions in, manufacturers of drugs or products mentioned in the article.
- , National health care expenses in the U.S. civilian noninstitutionalized population, 2009. Statistical brief 355. 2012. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Costs for hospital stays in the United States, 2010. Statistical brief 146. 2013. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol. 1998;21(7):503–505.
- , , , et al. Evaluation of telemetry utilization, policy, and outcomes in an inner‐city academic medical center. J Natl Med Assoc. 2010;102(7):598–604.
- , , Recommended guidelines for in‐hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431–1433.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , , , Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368–372.
- Society of Hospital Medicine. Adult Hospital Medicine. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/society‐of‐hospital‐medicine‐adult. Published February 21, 2013. Accessed October 5, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect. 2013;33(7):1–4.
- , , , , Optimizing telemetry utilization in an academic medical center. J Clin Outcomes Manage. 2008;15(9):435–440.
- , Improving utilization of telemetry in a university hospital. J Clin Outcomes Manage. 2005;12(10):519–522.
- , , , , Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174:1852–1854.
- , , "Innovation" institutes in academic health centers: enhancing value through leadership, education, engagement, and scholarship. Acad Med. 2014;89(9):1204–1206.
- , , , , , Controlling health costs: physician responses to patient expectations for medical care. J Gen Intern Med. 2014;29(9):1234–1241.
Inpatient hospital services are a major component of total US civilian noninstitutionalized healthcare expenses, accounting for 29.3% of spending in 2009[1] when the average cost per stay was $9700.[2] Telemetry monitoring, a widely used resource for the identification of life‐threatening arrhythmias, contributes to these costs. In 1998, Sivaram et al. estimated the cost per patient at $683; in 2010, Ivonye et al. published the cost difference between a telemetry bed and a nonmonitored bed in their inner‐city public teaching facility reached $800.[3, 4]
In 1991, the American College of Cardiology published guidelines for telemetry use, which were later revised by the American Heart Association in 2004.[5, 6] Notably, the guidelines are based on expert opinion and on research data in electrocardiography.[7] The guidelines divide patients into 3 classes based on clinical condition: recommending telemetry monitoring for almost all class I patients, stating possible benefit in class II patients, and discouraging cardiac monitoring for the low‐risk class III patients.[5, 6] The Choosing Wisely campaign, an initiative of the American Board of Internal Medicine and the Society of Hospital Medicine, highlights telemetry monitoring as 1 of the top 5 interventions that physicians and patients should question when determining tests and procedures.[8] Choosing Wisely suggests using a protocol to govern continuation of telemetry outside of the intensive care unit (ICU), as inappropriate monitoring increases care costs and may result in patient harm.[8] The Joint Commission 2014 National Patient Safety Goals notes that numerous alarm signals and the resulting noise and displayed information tends to desensitize staff and cause them to miss or ignore alarm signals or even disable them.[9]
Few studies have examined implementation methods for improved telemetry bed utilization. One study evaluated the impact of a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team, noting improved cardiac monitoring bed utilization and decreased academic hospital closure, which previously resulted in inability to accept new patients or procedure cancellation.[10] Another study provided an orientation handout discussed by the chief resident and telemetry indication reviews during multidisciplinary rounds 3 times a week.[11]
Our study is one the first to demonstrate a model for a hospitalist‐led approach to guide appropriate telemetry use. We investigated the impact of a multipronged approach to guide telemetry usage: (1) a hospitalist‐led, daily review of bed utilization during attending rounds, (2) a hospitalist attending‐driven, trainee‐focused education module on telemetry utilization, (3) quarterly feedback on telemetry bed utilization rates, and (4) financial incentives. We analyzed pre‐ and post‐evaluation results from the education module to measure impact on knowledge, skills, and attitudes. Additionally, we evaluated the effect of the intervention on length of stay (LOS) and bed utilization costs, while monitoring case mix index (CMI) and overall mortality.
METHODS
Setting
This study took place at Stanford Hospital and Clinics, a teaching academic center in Stanford, California. Stanford Hospital is a 444‐bed, urban medical center with 114 telemetry intermediate ICU beds, and 66 ICU beds. The 264 medicalsurgical beds lack telemetry monitoring, which can only be completed in the intermediate and full ICU. All patients on telemetry units receive both cardiac monitoring and increased nursing ratios. Transfer orders are placed in the electronic medical record to shift patients between care levels. Bed control attempts to transfer patients as soon as an open bed in the appropriate care level exists.
The study included all 5 housestaff inpatient general internal medicine wards teams (which excludes cardiology, pulmonary hypertension, hematology, oncology, and post‐transplant patients). Hospitalists and nonhospitalists attend on the wards for 1‐ to 2‐week blocks. Teaching teams are staffed by 1 to 2 medical students, 2 interns, 1 resident, and 1 attending. The university institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Participants
Ten full‐ and part‐time hospitalist physicians participated in the standardized telemetry teaching. Fifty‐six of the approximately 80 medical students and housestaff on hospitalists' teams completed the educational evaluation. Both hospitalist and nonhospitalist teams participated in daily multidisciplinary rounds, focusing on barriers to discharge including telemetry use. Twelve nonhospitalists served on the wards during the intervention period. Hospitalists covered 72% of the internal medicine wards during the intervention period.
Study Design
We investigated the impact of a multipronged approach to guide telemetry usage from January 2013 to August 2013 (intervention period).
Hospitalist‐Led Daily Review of Bed Utilization
Hospitalists were encouraged to discuss the need of telemetry on daily attending rounds and review indications for telemetry while on service. Prior to starting a ward block, attendings were emailed the teaching module with a reminder to discuss the need for telemetry on attending rounds. Reminders to discuss telemetry utilization were also provided during every‐other‐week hospitalist meetings. Compliance of daily discussion was not tracked.
Hospitalist‐Driven, Trainee‐Focused, Education Module on Telemetry Utilization
The educational module was taught during teaching sessions only by the hospitalists. Trainees on nonhospitalist teams did not receive dedicated teaching about telemetry usage. The module was given to learners only once. The module was a 10‐slide, Microsoft PowerPoint (Microsoft Corp., Redmond, WA) presentation that reviewed the history of telemetry, the American College of Cardiology and the American Heart Association guidelines, the cost difference between telemetry and nonmonitored beds, and the perceived barriers to discontinuation. The presentation was accompanied by a pre‐ and post‐evaluation to elicit knowledge, skills, and attitudes of telemetry use (see Supporting Information, Appendix A, in the online version of this article). The pre‐ and post‐evaluations were created through consensus with a multidisciplinary, expert panel after reviewing the evidence‐based literature.
Quarterly Feedback on Telemetry Bed Utilization Rates
Hospital beduse and CMI data were obtained from the Stanford finance department for the intervention period and for the baseline period, which was the year prior to the study, January 1, 2012 to December 31, 2012. Hospital beduse data included the number of days patients were on telemetry units versus medicalsurgical units (nontelemetry units), differentiated by hospitalists and nonhospitalists. Cost savings were calculated by the Stanford finance department that used Stanford‐specific, internal cost accounting data to determine the impact of the intervention. These data were reviewed at hospitalist meetings on a quarterly basis. We also obtained the University Healthsystem Consortium mortality index (observed to expected) for the general internal medicine service during the baseline and intervention periods.
To measure sustainment of telemetry reduction in the postintervention period, we measured telemetry LOS from September 2014 to March 2015 (extension period).
Financial Incentives
Hospitalists were provided a $2000 bonus at the end of fiscal year 2013 if the group showed a decrease in telemetry bed use in comparison to the baseline period.
Statistical Analysis of Clinical Outcome Measures
Continuous outcomes were tested using 2‐tailed t tests. Comparison of continuous outcome included differences in telemetry and nontelemetry LOS and CMI. Pairwise comparisons were made for various time periods. A P value of 0.05 was considered statistically significant. Statistical analyses were performed using Stata 12.0 software (StataCorp, College Station, TX).
RESULTS
Clinical and Value Outcomes
Baseline (January 2012December 2012) Versus Intervention Period (January 2013August 2013)
LOS for telemetry beds was significantly reduced over the intervention period (2.75 days vs 2.13 days, P=0.005) for hospitalists. Notably, there was no significant difference in mean LOS between baseline and intervention periods for nontelemetry beds (2.84 days vs 2.72 days, P=0.32) for hospitalists. In comparison, for nonhospitalists, there was no difference in mean LOS for telemetry beds between baseline and intervention periods (2.75 days vs 2.46 days, P=0.33) and nontelemetry beds (2.64 days vs 2.89 days, P=0.26) (Table 1).
| Baseline Period | Intervention Period | P Value | Extension Period | P Value | |
|---|---|---|---|---|---|
| |||||
| Length of stay | |||||
| Hospitalists | |||||
| Telemetry beds | 2.75 | 2.13 | 0.005 | 1.93 | 0.09 |
| Nontelemetry beds | 2.84 | 2.72 | 0.324 | 2.44 | 0.21 |
| Nonhospitalists | |||||
| Telemetry beds | 2.75 | 2.46 | 0.331 | 2.22 | 0.43 |
| Nontelemetry beds | 2.64 | 2.89 | 0.261 | 2.26 | 0.05 |
| Case mix index | |||||
| Hospitalists | 1.44 | 1.45 | 0.68 | 1.40 | 0.21 |
| Nonhospitalists | 1.46 | 1.40 | 0.53 | 1.53 | 0.18 |
Costs of hospital stay were also reduced in the multipronged, hospitalist‐driven intervention group. Expenditures for telemetry beds were reduced by 22.5% over the intervention period for hospitalists (Table 2).
| Baseline to Intervention Period | Intervention to Extension Period | |
|---|---|---|
| ||
| Hospitalists | ||
| Telemetry beds | 22.55% | 9.55% |
| Nontelemetry beds | 4.23% | 10.14% |
| Nonhospitalists | ||
| Telemetry beds | 10.55% | 9.89% |
| Nontelemetry beds | 9.47% | 21.84% |
The mean CMI of the patient cohort managed by the hospitalists in the baseline and intervention periods was not significantly different (1.44 vs 1.45, P=0.68). The mean CMI of the patients managed by the nonhospitalists in the baseline and intervention periods was also not significantly different (1.46 vs 1.40, P=0.53) (Table 1). Mortality index during the baseline and intervention periods was not significantly different (0.770.22 vs 0.660.23, P=0.54), as during the intervention and extension periods (0.660.23 vs 0.650.15, P=0.95).
Intervention Period (January 2013August 2013) Versus Extension Period (September 2014‐March 2015)
The decreased telemetry LOS for hospitalists was sustained from the intervention period to the extension period, from 2.13 to 1.93 (P=0.09). There was no significant change in the nontelemetry LOS in the intervention period compared to the extension period (2.72 vs 2.44, P=0.21). There was no change in the telemetry LOS for nonhospitalists from the intervention period to the extension period (2.46 vs 2.22, P=0.43).
The mean CMI in the hospitalist group was not significantly different in the intervention period compared to the extension period (1.45 to 1.40, P=0.21). The mean CMI in the nonhospitalist group did not change from the intervention period to the extension period (1.40 vs 1.53, P=0.18) (Table 1).
Education Outcomes
Out of the 56 participants completing the education module and survey, 28.6% were medical students, 53.6% were interns, 12.5% were second‐year residents, and 5.4% were third‐year residents. Several findings were seen at baseline via pretest. In evaluating patterns of current telemetry use, 32.2% of participants reported evaluating the necessity of telemetry for patients on admission only, 26.3% during transitions of care, 5.1% after discharge plans were cemented, 33.1% on a daily basis, and 3.4% rarely. When asked which member of the care team was most likely to encourage use of appropriate telemetry, 20.8% identified another resident, 13.9% nursing, 37.5% attending physician, 20.8% self, 4.2% the team as a whole, and 2.8% as not any.
Figure 1 shows premodule results regarding the trainees perceived percentage of patient encounters during which a participant's team discussed their patient's need for telemetry.

In assessing perception of current telemetry utilization, 1.8% of participants thought 0% to 10% of patients were currently on telemetry, 19.6% thought 11% to 20%, 42.9% thought 21% to 31%, 30.4% thought 31% to 40%, and 3.6% thought 41% to 50%.
Two areas were assessed at both baseline and after the intervention: knowledge of indications of telemetry use and cost related to telemetry use. We saw increased awareness of cost‐saving actions. To assess current knowledge of the indications of proper telemetry use according to American Heart Association guidelines, participants were presented with a list of 5 patients with different clinical indications for telemetry use and asked which patient required telemetry the most. Of the participants, 54.5% identified the correct answer in the pretest and 61.8% identified the correct answer in the post‐test. To assess knowledge of the costs of telemetry relative to other patient care, participants were presented with a patient case and asked to identify the most and least cost‐saving actions to safely care for the patient. When asked to identify the most cost‐saving action, 20.3% identified the correct answer in the pretest and 61.0% identified the correct answer in the post‐test. Of those who answered incorrectly in the pretest, 51.1% answered correctly in the post‐test (P=0.002). When asked to identify the least cost‐saving action, 23.7% identified the correct answer in the pretest and 50.9% identified the correct answer in the posttest. Of those who answered incorrectly in the pretest, 60.0% answered correctly in the post‐test (P=0.003).
In the post‐test, when asked about the importance of appropriate telemetry usage in providing cost‐conscious care and assuring appropriate hospital resource management, 76.8% of participants found the need very important, 21.4% somewhat important, and 1.8% as not applicable. The most commonly perceived barriers impeding discontinuation of telemetry, as reported by participants via post‐test, were nursing desires and time. Figure 2 shows all perceived barriers.

DISCUSSION
Our study is one of the first to our knowledge to demonstrate reductions in telemetry LOS by a hospitalist intervention for telemetry utilization. Others[10, 11] have studied the impact of an orientation handout by chief residents or a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team. Dressler et al. later sustained a 70% reduction in telemetry use without adversely affecting patient safety, as assessed through numbers of rapid response activations, codes, and deaths, through integrating the AHA guidelines into their electronic ordering system.[12] However, our study has the advantage of the primary team, who knows the patient and clinical scenario best, driving the change during attending rounds. In an era where cost consciousness intersects the practice of medicine, any intervention in patient care that demonstrates cost savings without an adverse impact on patient care and resource utilization must be emphasized. This is particularly important in academic institutions, where residents and medical students are learning to integrate the principles of patient safety and quality improvement into their clinical practice.[13] We actually showed sustained telemetry LOS reductions into the extension period after our intervention. We believe this may be due to telemetry triage being integrated into our attending and resident rounding practices. Future work should include integration of telemetry triage into clinical decision support in the electronic medical record and multidisciplinary rounds to disseminate telemetry triage hospital‐wide in both the academic and community settings.
Our study also revealed that nearly half of participants were not aware of the criteria for appropriate utilization of telemetry before our intervention; in the preintervention period, there were many anecdotal and objective findings of inappropriate utilization of telemetry as well as prolonged continuation beyond the clinical needs in both the hospitalist and nonhospitalist group. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
We were able to show increased knowledge of cost‐saving actions among trainees with our educational module. We believe it is imperative to educate our providers (physicians, nurses, case managers, and students within these disciplines) on the appropriate indications for telemetry use, not only to help with cost savings and resource availability (ie, allowing telemetry beds to be available for patients who need them most), but also to instill consistent expectations among our patients. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
Additionally, we feel it is important to consider the impacts of inappropriate use of telemetry from a patient's perspective: it is physically restrictive/emnconvenient, alarms are disruptive, it can be a barrier for other treatments such as physical therapy, it may increase the time it takes for imaging studies, a nurse may be required to accompany patients on telemetry, and poses additional costs to their medical bill.
We believe our success is due to several strategies. First, at the start of the fiscal year when quality improvement metrics are established, this particular metric (improving the appropriate utilization and timely discontinuation of telemetry) was deemed important by all hospitalists, engendering group buy‐in prior to the intervention. Our hospitalists received a detailed and interactive tutorial session in person at the beginning of the study. This tutorial provided the hospitalists with a comprehensive understanding of the appropriate (and inappropriate) indications for telemetry monitoring, hence facilitating guideline‐directed utilization. Email reminders and the tutorial tool were provided each time a hospitalist attended on the wards, and hospitalists received a small financial incentive to comply with appropriate telemetry utilization.
Our study has several strengths. First, the time frame of our study was long enough (8 months) to allow consistent trends to emerge and to optimize exposure of housestaff and medical students to this quality‐improvement initiative. Second, our cost savings came from 2 factors, direct reduction of inappropriate telemetry use and reduction in length of stay, highlighting the dual impact of appropriate telemetry utilization on cost. The overall reductions in telemetry utilization for the intervention group were a result of both reductions in initial placement on telemetry for patients who did not meet criteria for such monitoring as well as timely discontinuation of telemetry during the patient's hospitalization. Third, our study demonstrates that physicians can be effective in driving appropriate telemetry usage by participating in the clinical decision making regarding necessity and educating providers, trainees/students, and patients on appropriate indications. Finally, we show sustainment of our intervention in the extension period, suggesting telemetry triage integration into rounding practice.
Our study has limitations as well. First, our sample size is relatively small at a single academic center. Second, due to complexities in our faculty scheduling, we were unable to completely randomize patients to a hospitalist versus nonhospitalist team. However, we believe that despite the inability to randomize, our study does show the benefit of a hospitalist attending to reduce telemetry LOS given there was no change in nonhospitalist telemetry LOS despite all of the other hospital‐wide interventions (multidisciplinary rounds, similar housestaff). Third, our study was limited in that the CMI was used as a proxy for patient complexity, and the mortality index was used as the overall marker of safety. Further studies should monitor frequency and outcomes of arrhythmic events of patients transferred from telemetry monitoring to medicalsurgical beds. Finally, as the intervention was multipronged, we are unable to determine which component led to the reductions in telemetry utilization. Each component, however, remains easily transferrable to outside institutions. We demonstrated both a reduction in initiation of telemetry as well as timely discontinuation; however, due to the complexity in capturing this accurately, we were unable to numerically quantify these individual outcomes.
Additionally, there were approximately 10 nonhospitalist attendings who also staffed the wards during the intervention time period of our study; these attendings did not undergo the telemetry tutorial/orientation. This difference, along with the Hawthorne effect for the hospitalist attendings, also likely contributed to the difference in outcomes between the 2 attending cohorts in the intervention period.
CONCLUSIONS
Our results demonstrate that a multipronged hospitalist‐driven intervention to improve appropriate use of telemetry reduces telemetry LOS and cost. Hence, we believe that targeted, education‐driven interventions with monitoring of progress can have demonstrable impacts on changing practice. Physicians will need to make trade‐offs in clinical practice to balance efficient resource utilization with the patient's evolving condition in the inpatient setting, the complexities of clinical workflow, and the patient's expectations.[14] Appropriate telemetry utilization is a prime example of what needs to be done well in the future for high‐value care.
Acknowledgements
The authors acknowledge the hospitalists who participated in the intervention: Jeffrey Chi, Willliam Daines, Sumbul Desai, Poonam Hosamani, John Kugler, Charles Liao, Errol Ozdalga, and Sang Hoon Woo. The authors also acknowledge Joan Hendershott in the Finance Department and Joseph Hopkins in the Quality Department.
Disclosures: All coauthors have seen and agree with the contents of the article; submission (aside from abstracts) was not under review by any other publication. The authors report no disclosures of financial support from, or equity positions in, manufacturers of drugs or products mentioned in the article.
Inpatient hospital services are a major component of total US civilian noninstitutionalized healthcare expenses, accounting for 29.3% of spending in 2009[1] when the average cost per stay was $9700.[2] Telemetry monitoring, a widely used resource for the identification of life‐threatening arrhythmias, contributes to these costs. In 1998, Sivaram et al. estimated the cost per patient at $683; in 2010, Ivonye et al. published the cost difference between a telemetry bed and a nonmonitored bed in their inner‐city public teaching facility reached $800.[3, 4]
In 1991, the American College of Cardiology published guidelines for telemetry use, which were later revised by the American Heart Association in 2004.[5, 6] Notably, the guidelines are based on expert opinion and on research data in electrocardiography.[7] The guidelines divide patients into 3 classes based on clinical condition: recommending telemetry monitoring for almost all class I patients, stating possible benefit in class II patients, and discouraging cardiac monitoring for the low‐risk class III patients.[5, 6] The Choosing Wisely campaign, an initiative of the American Board of Internal Medicine and the Society of Hospital Medicine, highlights telemetry monitoring as 1 of the top 5 interventions that physicians and patients should question when determining tests and procedures.[8] Choosing Wisely suggests using a protocol to govern continuation of telemetry outside of the intensive care unit (ICU), as inappropriate monitoring increases care costs and may result in patient harm.[8] The Joint Commission 2014 National Patient Safety Goals notes that numerous alarm signals and the resulting noise and displayed information tends to desensitize staff and cause them to miss or ignore alarm signals or even disable them.[9]
Few studies have examined implementation methods for improved telemetry bed utilization. One study evaluated the impact of a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team, noting improved cardiac monitoring bed utilization and decreased academic hospital closure, which previously resulted in inability to accept new patients or procedure cancellation.[10] Another study provided an orientation handout discussed by the chief resident and telemetry indication reviews during multidisciplinary rounds 3 times a week.[11]
Our study is one the first to demonstrate a model for a hospitalist‐led approach to guide appropriate telemetry use. We investigated the impact of a multipronged approach to guide telemetry usage: (1) a hospitalist‐led, daily review of bed utilization during attending rounds, (2) a hospitalist attending‐driven, trainee‐focused education module on telemetry utilization, (3) quarterly feedback on telemetry bed utilization rates, and (4) financial incentives. We analyzed pre‐ and post‐evaluation results from the education module to measure impact on knowledge, skills, and attitudes. Additionally, we evaluated the effect of the intervention on length of stay (LOS) and bed utilization costs, while monitoring case mix index (CMI) and overall mortality.
METHODS
Setting
This study took place at Stanford Hospital and Clinics, a teaching academic center in Stanford, California. Stanford Hospital is a 444‐bed, urban medical center with 114 telemetry intermediate ICU beds, and 66 ICU beds. The 264 medicalsurgical beds lack telemetry monitoring, which can only be completed in the intermediate and full ICU. All patients on telemetry units receive both cardiac monitoring and increased nursing ratios. Transfer orders are placed in the electronic medical record to shift patients between care levels. Bed control attempts to transfer patients as soon as an open bed in the appropriate care level exists.
The study included all 5 housestaff inpatient general internal medicine wards teams (which excludes cardiology, pulmonary hypertension, hematology, oncology, and post‐transplant patients). Hospitalists and nonhospitalists attend on the wards for 1‐ to 2‐week blocks. Teaching teams are staffed by 1 to 2 medical students, 2 interns, 1 resident, and 1 attending. The university institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Participants
Ten full‐ and part‐time hospitalist physicians participated in the standardized telemetry teaching. Fifty‐six of the approximately 80 medical students and housestaff on hospitalists' teams completed the educational evaluation. Both hospitalist and nonhospitalist teams participated in daily multidisciplinary rounds, focusing on barriers to discharge including telemetry use. Twelve nonhospitalists served on the wards during the intervention period. Hospitalists covered 72% of the internal medicine wards during the intervention period.
Study Design
We investigated the impact of a multipronged approach to guide telemetry usage from January 2013 to August 2013 (intervention period).
Hospitalist‐Led Daily Review of Bed Utilization
Hospitalists were encouraged to discuss the need of telemetry on daily attending rounds and review indications for telemetry while on service. Prior to starting a ward block, attendings were emailed the teaching module with a reminder to discuss the need for telemetry on attending rounds. Reminders to discuss telemetry utilization were also provided during every‐other‐week hospitalist meetings. Compliance of daily discussion was not tracked.
Hospitalist‐Driven, Trainee‐Focused, Education Module on Telemetry Utilization
The educational module was taught during teaching sessions only by the hospitalists. Trainees on nonhospitalist teams did not receive dedicated teaching about telemetry usage. The module was given to learners only once. The module was a 10‐slide, Microsoft PowerPoint (Microsoft Corp., Redmond, WA) presentation that reviewed the history of telemetry, the American College of Cardiology and the American Heart Association guidelines, the cost difference between telemetry and nonmonitored beds, and the perceived barriers to discontinuation. The presentation was accompanied by a pre‐ and post‐evaluation to elicit knowledge, skills, and attitudes of telemetry use (see Supporting Information, Appendix A, in the online version of this article). The pre‐ and post‐evaluations were created through consensus with a multidisciplinary, expert panel after reviewing the evidence‐based literature.
Quarterly Feedback on Telemetry Bed Utilization Rates
Hospital beduse and CMI data were obtained from the Stanford finance department for the intervention period and for the baseline period, which was the year prior to the study, January 1, 2012 to December 31, 2012. Hospital beduse data included the number of days patients were on telemetry units versus medicalsurgical units (nontelemetry units), differentiated by hospitalists and nonhospitalists. Cost savings were calculated by the Stanford finance department that used Stanford‐specific, internal cost accounting data to determine the impact of the intervention. These data were reviewed at hospitalist meetings on a quarterly basis. We also obtained the University Healthsystem Consortium mortality index (observed to expected) for the general internal medicine service during the baseline and intervention periods.
To measure sustainment of telemetry reduction in the postintervention period, we measured telemetry LOS from September 2014 to March 2015 (extension period).
Financial Incentives
Hospitalists were provided a $2000 bonus at the end of fiscal year 2013 if the group showed a decrease in telemetry bed use in comparison to the baseline period.
Statistical Analysis of Clinical Outcome Measures
Continuous outcomes were tested using 2‐tailed t tests. Comparison of continuous outcome included differences in telemetry and nontelemetry LOS and CMI. Pairwise comparisons were made for various time periods. A P value of 0.05 was considered statistically significant. Statistical analyses were performed using Stata 12.0 software (StataCorp, College Station, TX).
RESULTS
Clinical and Value Outcomes
Baseline (January 2012December 2012) Versus Intervention Period (January 2013August 2013)
LOS for telemetry beds was significantly reduced over the intervention period (2.75 days vs 2.13 days, P=0.005) for hospitalists. Notably, there was no significant difference in mean LOS between baseline and intervention periods for nontelemetry beds (2.84 days vs 2.72 days, P=0.32) for hospitalists. In comparison, for nonhospitalists, there was no difference in mean LOS for telemetry beds between baseline and intervention periods (2.75 days vs 2.46 days, P=0.33) and nontelemetry beds (2.64 days vs 2.89 days, P=0.26) (Table 1).
| Baseline Period | Intervention Period | P Value | Extension Period | P Value | |
|---|---|---|---|---|---|
| |||||
| Length of stay | |||||
| Hospitalists | |||||
| Telemetry beds | 2.75 | 2.13 | 0.005 | 1.93 | 0.09 |
| Nontelemetry beds | 2.84 | 2.72 | 0.324 | 2.44 | 0.21 |
| Nonhospitalists | |||||
| Telemetry beds | 2.75 | 2.46 | 0.331 | 2.22 | 0.43 |
| Nontelemetry beds | 2.64 | 2.89 | 0.261 | 2.26 | 0.05 |
| Case mix index | |||||
| Hospitalists | 1.44 | 1.45 | 0.68 | 1.40 | 0.21 |
| Nonhospitalists | 1.46 | 1.40 | 0.53 | 1.53 | 0.18 |
Costs of hospital stay were also reduced in the multipronged, hospitalist‐driven intervention group. Expenditures for telemetry beds were reduced by 22.5% over the intervention period for hospitalists (Table 2).
| Baseline to Intervention Period | Intervention to Extension Period | |
|---|---|---|
| ||
| Hospitalists | ||
| Telemetry beds | 22.55% | 9.55% |
| Nontelemetry beds | 4.23% | 10.14% |
| Nonhospitalists | ||
| Telemetry beds | 10.55% | 9.89% |
| Nontelemetry beds | 9.47% | 21.84% |
The mean CMI of the patient cohort managed by the hospitalists in the baseline and intervention periods was not significantly different (1.44 vs 1.45, P=0.68). The mean CMI of the patients managed by the nonhospitalists in the baseline and intervention periods was also not significantly different (1.46 vs 1.40, P=0.53) (Table 1). Mortality index during the baseline and intervention periods was not significantly different (0.770.22 vs 0.660.23, P=0.54), as during the intervention and extension periods (0.660.23 vs 0.650.15, P=0.95).
Intervention Period (January 2013August 2013) Versus Extension Period (September 2014‐March 2015)
The decreased telemetry LOS for hospitalists was sustained from the intervention period to the extension period, from 2.13 to 1.93 (P=0.09). There was no significant change in the nontelemetry LOS in the intervention period compared to the extension period (2.72 vs 2.44, P=0.21). There was no change in the telemetry LOS for nonhospitalists from the intervention period to the extension period (2.46 vs 2.22, P=0.43).
The mean CMI in the hospitalist group was not significantly different in the intervention period compared to the extension period (1.45 to 1.40, P=0.21). The mean CMI in the nonhospitalist group did not change from the intervention period to the extension period (1.40 vs 1.53, P=0.18) (Table 1).
Education Outcomes
Out of the 56 participants completing the education module and survey, 28.6% were medical students, 53.6% were interns, 12.5% were second‐year residents, and 5.4% were third‐year residents. Several findings were seen at baseline via pretest. In evaluating patterns of current telemetry use, 32.2% of participants reported evaluating the necessity of telemetry for patients on admission only, 26.3% during transitions of care, 5.1% after discharge plans were cemented, 33.1% on a daily basis, and 3.4% rarely. When asked which member of the care team was most likely to encourage use of appropriate telemetry, 20.8% identified another resident, 13.9% nursing, 37.5% attending physician, 20.8% self, 4.2% the team as a whole, and 2.8% as not any.
Figure 1 shows premodule results regarding the trainees perceived percentage of patient encounters during which a participant's team discussed their patient's need for telemetry.

In assessing perception of current telemetry utilization, 1.8% of participants thought 0% to 10% of patients were currently on telemetry, 19.6% thought 11% to 20%, 42.9% thought 21% to 31%, 30.4% thought 31% to 40%, and 3.6% thought 41% to 50%.
Two areas were assessed at both baseline and after the intervention: knowledge of indications of telemetry use and cost related to telemetry use. We saw increased awareness of cost‐saving actions. To assess current knowledge of the indications of proper telemetry use according to American Heart Association guidelines, participants were presented with a list of 5 patients with different clinical indications for telemetry use and asked which patient required telemetry the most. Of the participants, 54.5% identified the correct answer in the pretest and 61.8% identified the correct answer in the post‐test. To assess knowledge of the costs of telemetry relative to other patient care, participants were presented with a patient case and asked to identify the most and least cost‐saving actions to safely care for the patient. When asked to identify the most cost‐saving action, 20.3% identified the correct answer in the pretest and 61.0% identified the correct answer in the post‐test. Of those who answered incorrectly in the pretest, 51.1% answered correctly in the post‐test (P=0.002). When asked to identify the least cost‐saving action, 23.7% identified the correct answer in the pretest and 50.9% identified the correct answer in the posttest. Of those who answered incorrectly in the pretest, 60.0% answered correctly in the post‐test (P=0.003).
In the post‐test, when asked about the importance of appropriate telemetry usage in providing cost‐conscious care and assuring appropriate hospital resource management, 76.8% of participants found the need very important, 21.4% somewhat important, and 1.8% as not applicable. The most commonly perceived barriers impeding discontinuation of telemetry, as reported by participants via post‐test, were nursing desires and time. Figure 2 shows all perceived barriers.

DISCUSSION
Our study is one of the first to our knowledge to demonstrate reductions in telemetry LOS by a hospitalist intervention for telemetry utilization. Others[10, 11] have studied the impact of an orientation handout by chief residents or a multispecialty telemetry policy with enforcement by an outside cardiologist and nurse team. Dressler et al. later sustained a 70% reduction in telemetry use without adversely affecting patient safety, as assessed through numbers of rapid response activations, codes, and deaths, through integrating the AHA guidelines into their electronic ordering system.[12] However, our study has the advantage of the primary team, who knows the patient and clinical scenario best, driving the change during attending rounds. In an era where cost consciousness intersects the practice of medicine, any intervention in patient care that demonstrates cost savings without an adverse impact on patient care and resource utilization must be emphasized. This is particularly important in academic institutions, where residents and medical students are learning to integrate the principles of patient safety and quality improvement into their clinical practice.[13] We actually showed sustained telemetry LOS reductions into the extension period after our intervention. We believe this may be due to telemetry triage being integrated into our attending and resident rounding practices. Future work should include integration of telemetry triage into clinical decision support in the electronic medical record and multidisciplinary rounds to disseminate telemetry triage hospital‐wide in both the academic and community settings.
Our study also revealed that nearly half of participants were not aware of the criteria for appropriate utilization of telemetry before our intervention; in the preintervention period, there were many anecdotal and objective findings of inappropriate utilization of telemetry as well as prolonged continuation beyond the clinical needs in both the hospitalist and nonhospitalist group. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
We were able to show increased knowledge of cost‐saving actions among trainees with our educational module. We believe it is imperative to educate our providers (physicians, nurses, case managers, and students within these disciplines) on the appropriate indications for telemetry use, not only to help with cost savings and resource availability (ie, allowing telemetry beds to be available for patients who need them most), but also to instill consistent expectations among our patients. For the hospitalist group (ie, the group receiving guideline‐based education on appropriate indications for telemetry utilization), there was an assessment of both appropriate usage and timely discontinuation of telemetry in the postintervention period, which we attribute in large part to adherence to the education provided to this group.
Additionally, we feel it is important to consider the impacts of inappropriate use of telemetry from a patient's perspective: it is physically restrictive/emnconvenient, alarms are disruptive, it can be a barrier for other treatments such as physical therapy, it may increase the time it takes for imaging studies, a nurse may be required to accompany patients on telemetry, and poses additional costs to their medical bill.
We believe our success is due to several strategies. First, at the start of the fiscal year when quality improvement metrics are established, this particular metric (improving the appropriate utilization and timely discontinuation of telemetry) was deemed important by all hospitalists, engendering group buy‐in prior to the intervention. Our hospitalists received a detailed and interactive tutorial session in person at the beginning of the study. This tutorial provided the hospitalists with a comprehensive understanding of the appropriate (and inappropriate) indications for telemetry monitoring, hence facilitating guideline‐directed utilization. Email reminders and the tutorial tool were provided each time a hospitalist attended on the wards, and hospitalists received a small financial incentive to comply with appropriate telemetry utilization.
Our study has several strengths. First, the time frame of our study was long enough (8 months) to allow consistent trends to emerge and to optimize exposure of housestaff and medical students to this quality‐improvement initiative. Second, our cost savings came from 2 factors, direct reduction of inappropriate telemetry use and reduction in length of stay, highlighting the dual impact of appropriate telemetry utilization on cost. The overall reductions in telemetry utilization for the intervention group were a result of both reductions in initial placement on telemetry for patients who did not meet criteria for such monitoring as well as timely discontinuation of telemetry during the patient's hospitalization. Third, our study demonstrates that physicians can be effective in driving appropriate telemetry usage by participating in the clinical decision making regarding necessity and educating providers, trainees/students, and patients on appropriate indications. Finally, we show sustainment of our intervention in the extension period, suggesting telemetry triage integration into rounding practice.
Our study has limitations as well. First, our sample size is relatively small at a single academic center. Second, due to complexities in our faculty scheduling, we were unable to completely randomize patients to a hospitalist versus nonhospitalist team. However, we believe that despite the inability to randomize, our study does show the benefit of a hospitalist attending to reduce telemetry LOS given there was no change in nonhospitalist telemetry LOS despite all of the other hospital‐wide interventions (multidisciplinary rounds, similar housestaff). Third, our study was limited in that the CMI was used as a proxy for patient complexity, and the mortality index was used as the overall marker of safety. Further studies should monitor frequency and outcomes of arrhythmic events of patients transferred from telemetry monitoring to medicalsurgical beds. Finally, as the intervention was multipronged, we are unable to determine which component led to the reductions in telemetry utilization. Each component, however, remains easily transferrable to outside institutions. We demonstrated both a reduction in initiation of telemetry as well as timely discontinuation; however, due to the complexity in capturing this accurately, we were unable to numerically quantify these individual outcomes.
Additionally, there were approximately 10 nonhospitalist attendings who also staffed the wards during the intervention time period of our study; these attendings did not undergo the telemetry tutorial/orientation. This difference, along with the Hawthorne effect for the hospitalist attendings, also likely contributed to the difference in outcomes between the 2 attending cohorts in the intervention period.
CONCLUSIONS
Our results demonstrate that a multipronged hospitalist‐driven intervention to improve appropriate use of telemetry reduces telemetry LOS and cost. Hence, we believe that targeted, education‐driven interventions with monitoring of progress can have demonstrable impacts on changing practice. Physicians will need to make trade‐offs in clinical practice to balance efficient resource utilization with the patient's evolving condition in the inpatient setting, the complexities of clinical workflow, and the patient's expectations.[14] Appropriate telemetry utilization is a prime example of what needs to be done well in the future for high‐value care.
Acknowledgements
The authors acknowledge the hospitalists who participated in the intervention: Jeffrey Chi, Willliam Daines, Sumbul Desai, Poonam Hosamani, John Kugler, Charles Liao, Errol Ozdalga, and Sang Hoon Woo. The authors also acknowledge Joan Hendershott in the Finance Department and Joseph Hopkins in the Quality Department.
Disclosures: All coauthors have seen and agree with the contents of the article; submission (aside from abstracts) was not under review by any other publication. The authors report no disclosures of financial support from, or equity positions in, manufacturers of drugs or products mentioned in the article.
- , National health care expenses in the U.S. civilian noninstitutionalized population, 2009. Statistical brief 355. 2012. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Costs for hospital stays in the United States, 2010. Statistical brief 146. 2013. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol. 1998;21(7):503–505.
- , , , et al. Evaluation of telemetry utilization, policy, and outcomes in an inner‐city academic medical center. J Natl Med Assoc. 2010;102(7):598–604.
- , , Recommended guidelines for in‐hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431–1433.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , , , Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368–372.
- Society of Hospital Medicine. Adult Hospital Medicine. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/society‐of‐hospital‐medicine‐adult. Published February 21, 2013. Accessed October 5, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect. 2013;33(7):1–4.
- , , , , Optimizing telemetry utilization in an academic medical center. J Clin Outcomes Manage. 2008;15(9):435–440.
- , Improving utilization of telemetry in a university hospital. J Clin Outcomes Manage. 2005;12(10):519–522.
- , , , , Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174:1852–1854.
- , , "Innovation" institutes in academic health centers: enhancing value through leadership, education, engagement, and scholarship. Acad Med. 2014;89(9):1204–1206.
- , , , , , Controlling health costs: physician responses to patient expectations for medical care. J Gen Intern Med. 2014;29(9):1234–1241.
- , National health care expenses in the U.S. civilian noninstitutionalized population, 2009. Statistical brief 355. 2012. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Costs for hospital stays in the United States, 2010. Statistical brief 146. 2013. Agency for Healthcare Research and Quality, Rockville, MD.
- , , Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol. 1998;21(7):503–505.
- , , , et al. Evaluation of telemetry utilization, policy, and outcomes in an inner‐city academic medical center. J Natl Med Assoc. 2010;102(7):598–604.
- , , Recommended guidelines for in‐hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431–1433.
- , , , et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical‐Care Nurses. Circulation. 2004;110(17):2721–2746.
- , , , , , Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368–372.
- Society of Hospital Medicine. Adult Hospital Medicine. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/society‐of‐hospital‐medicine‐adult. Published February 21, 2013. Accessed October 5, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect. 2013;33(7):1–4.
- , , , , Optimizing telemetry utilization in an academic medical center. J Clin Outcomes Manage. 2008;15(9):435–440.
- , Improving utilization of telemetry in a university hospital. J Clin Outcomes Manage. 2005;12(10):519–522.
- , , , , Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174:1852–1854.
- , , "Innovation" institutes in academic health centers: enhancing value through leadership, education, engagement, and scholarship. Acad Med. 2014;89(9):1204–1206.
- , , , , , Controlling health costs: physician responses to patient expectations for medical care. J Gen Intern Med. 2014;29(9):1234–1241.
Planned Readmission Algorithm
The Centers for Medicare & Medicaid Services (CMS) publicly reports all‐cause risk‐standardized readmission rates after acute‐care hospitalization for acute myocardial infarction, pneumonia, heart failure, total hip and knee arthroplasty, chronic obstructive pulmonary disease, stroke, and for patients hospital‐wide.[1, 2, 3, 4, 5] Ideally, these measures should capture unplanned readmissions that arise from acute clinical events requiring urgent rehospitalization. Planned readmissions, which are scheduled admissions usually involving nonurgent procedures, may not be a signal of quality of care. Including planned readmissions in readmission quality measures could create a disincentive to provide appropriate care to patients who are scheduled for elective or necessary procedures unrelated to the quality of the prior admission. Accordingly, under contract to the CMS, we were asked to develop an algorithm to identify planned readmissions. A version of this algorithm is now incorporated into all publicly reported readmission measures.
Given the widespread use of the planned readmission algorithm in public reporting and its implications for hospital quality measurement and evaluation, the objective of this study was to describe the development process, and to validate and refine the algorithm by reviewing charts of readmitted patients.
METHODS
Algorithm Development
To create a planned readmission algorithm, we first defined planned. We determined that readmissions for obstetrical delivery, maintenance chemotherapy, major organ transplant, and rehabilitation should always be considered planned in the sense that they are desired and/or inevitable, even if not specifically planned on a certain date. Apart from these specific types of readmissions, we defined planned readmissions as nonacute readmissions for scheduled procedures, because the vast majority of planned admissions are related to procedures. We also defined readmissions for acute illness or for complications of care as unplanned for the purposes of a quality measure. Even if such readmissions included a potentially planned procedure, because complications of care represent an important dimension of quality that should not be excluded from outcome measurement, these admissions should not be removed from the measure outcome. This definition of planned readmissions does not imply that all unplanned readmissions are unexpected or avoidable. However, it has proven very difficult to reliably define avoidable readmissions, even by expert review of charts, and we did not attempt to do so here.[6, 7]
In the second stage, we operationalized this definition into an algorithm. We used the Agency for Healthcare Research and Quality's Clinical Classification Software (CCS) codes to group thousands of individual procedure and diagnosis International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes into clinically coherent, mutually exclusive procedure CCS categories and mutually exclusive diagnosis CCS categories, respectively. Clinicians on the investigative team reviewed the procedure categories to identify those that are commonly planned and that would require inpatient admission. We also reviewed the diagnosis categories to identify acute diagnoses unlikely to accompany elective procedures. We then created a flow diagram through which every readmission could be run to determine whether it was planned or unplanned based on our categorizations of procedures and diagnoses (Figure 1, and Supporting Information, Appendix A, in the online version of this article). This version of the algorithm (v1.0) was submitted to the National Quality Forum (NQF) as part of the hospital‐wide readmission measure. The measure (NQR #1789) received endorsement in April 2012.
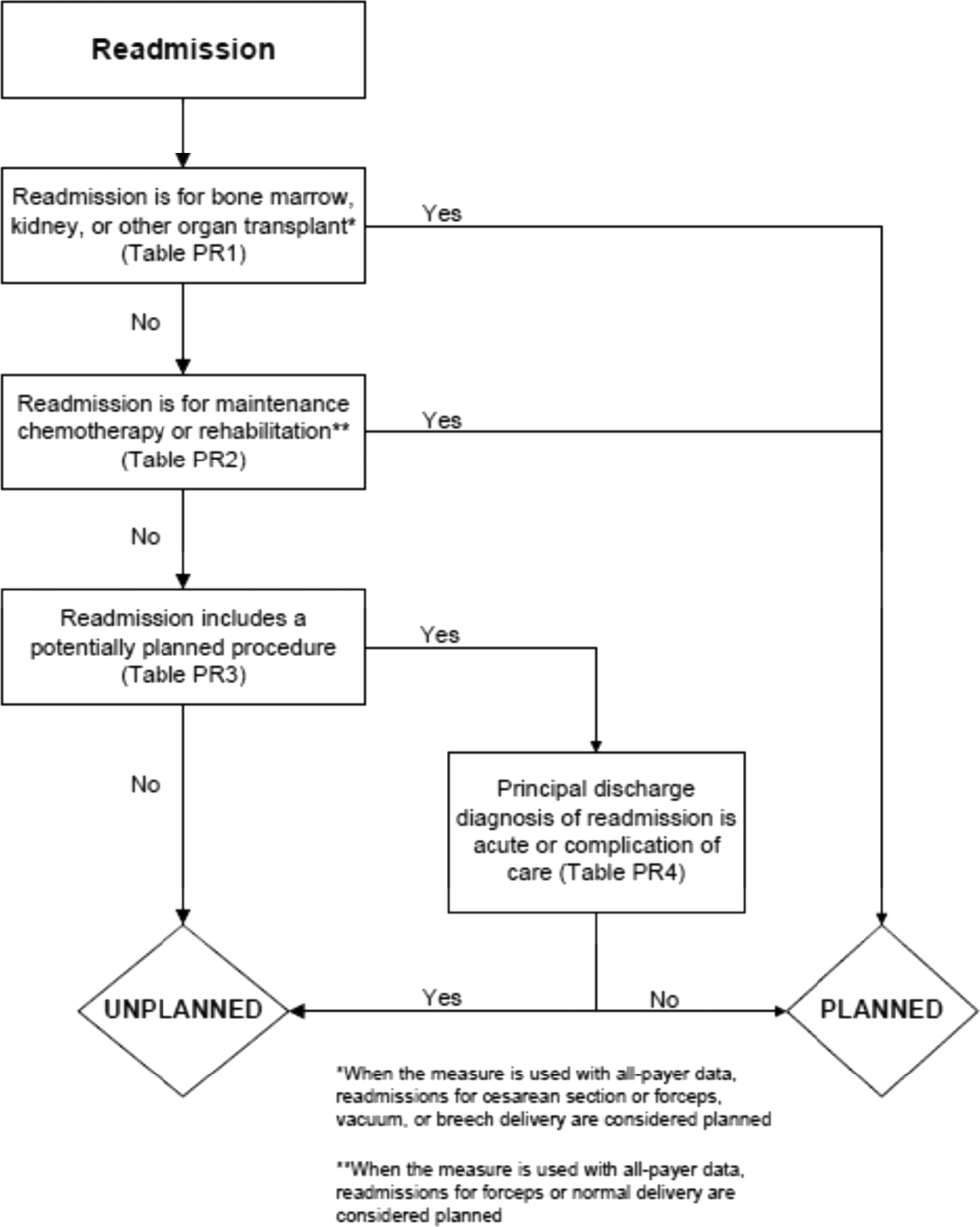
In the third stage of development, we posted the algorithm for 2 public comment periods and recruited 27 outside experts to review and refine the algorithm following a standardized, structured process (see Supporting Information, Appendix B, in the online version of this article). Because the measures publicly report and hold hospitals accountable for unplanned readmission rates, we felt it most important that the algorithm include as few planned readmissions in the reported, unplanned outcome as possible (ie, have high negative predictive value). Therefore, in equivocal situations in which experts felt procedure categories were equally often planned or unplanned, we added those procedures to the potentially planned list. We also solicited feedback from hospitals on algorithm performance during a confidential test run of the hospital‐wide readmission measure in the fall of 2012. Based on all of this feedback, we made a number of changes to the algorithm, which was then identified as v2.1. Version 2.1 of the algorithm was submitted to the NQF as part of the endorsement process for the acute myocardial infarction and heart failure readmission measures and was endorsed by the NQF in January 2013. The algorithm (v2.1) is now applied, adapted if necessary, to all publicly reported readmission measures.[8]
Algorithm Validation: Study Cohort
We recruited 2 hospital systems to participate in a chart validation study of the accuracy of the planned readmission algorithm (v2.1). Within these 2 health systems, we selected 7 hospitals with varying bed size, teaching status, and safety‐net status. Each included 1 large academic teaching hospital that serves as a regional referral center. For each hospital's index admissions, we applied the inclusion and exclusion criteria from the hospital‐wide readmission measure. Index admissions were included for patients age 65 years or older; enrolled in Medicare fee‐for‐service (FFS); discharged from a nonfederal, short‐stay, acute‐care hospital or critical access hospital; without an in‐hospital death; not transferred to another acute‐care facility; and enrolled in Part A Medicare for 1 year prior to discharge. We excluded index admissions for patients without at least 30 days postdischarge enrollment in FFS Medicare, discharged against medical advice, admitted for medical treatment of cancer or primary psychiatric disease, admitted to a Prospective Payment System‐exempt cancer hospital, or who died during the index hospitalization. In addition, for this study, we included only index admissions that were followed by a readmission to a hospital within the participating health system between July 1, 2011 and June 30, 2012. Institutional review board approval was obtained from each of the participating health systems, which granted waivers of signed informed consent and Health Insurance Portability and Accountability Act waivers.
Algorithm Validation: Sample Size Calculation
We determined a priori that the minimum acceptable positive predictive value, or proportion of all readmissions the algorithm labels planned that are truly planned, would be 60%, and the minimum acceptable negative predictive value, or proportion of all readmissions the algorithm labels as unplanned that are truly unplanned, would be 80%. We calculated the sample size required to be confident of these values 10% and determined we would need a total of 291 planned charts and 162 unplanned charts. We inflated these numbers by 20% to account for missing or unobtainable charts for a total of 550 charts. To achieve this sample size, we included all eligible readmissions from all participating hospitals that were categorized as planned. At the 5 smaller hospitals, we randomly selected an equal number of unplanned readmissions occurring at any hospital in its healthcare system. At the 2 largest hospitals, we randomly selected 50 unplanned readmissions occurring at any hospital in its healthcare system.
Algorithm Validation: Data Abstraction
We developed an abstraction tool, tested and refined it using sample charts, and built the final the tool into a secure, password‐protected Microsoft Access 2007 (Microsoft Corp., Redmond, WA) database (see Supporting Information, Appendix C, in the online version of this article). Experienced chart abstractors with RN or MD degrees from each hospital site participated in a 1‐hour training session to become familiar with reviewing medical charts, defining planned/unplanned readmissions, and the data abstraction process. For each readmission, we asked abstractors to review as needed: emergency department triage and physician notes, admission history and physical, operative report, discharge summary, and/or discharge summary from a prior admission. The abstractors verified the accuracy of the administrative billing data, including procedures and principal diagnosis. In addition, they abstracted the source of admission and dates of all major procedures. Then the abstractors provided their opinion and supporting rationale as to whether a readmission was planned or unplanned. They were not asked to determine whether the readmission was preventable. To determine the inter‐rater reliability of data abstraction, an independent abstractor at each health system recoded a random sample of 10% of the charts.
Statistical Analysis
To ensure that we had obtained a representative sample of charts, we identified the 10 most commonly planned procedures among cases identified as planned by the algorithm in the validation cohort and then compared this with planned cases nationally. To confirm the reliability of the abstraction process, we used the kappa statistic to determine the inter‐rater reliability of the determination of planned or unplanned status. Additionally, the full study team, including 5 practicing clinicians, reviewed the details of every chart abstraction in which the algorithm was found to have misclassified the readmission as planned or unplanned. In 11 cases we determined that the abstractor had misunderstood the definition of planned readmission (ie, not all direct admissions are necessarily planned) and we reclassified the chart review assignment accordingly.
We calculated sensitivity, specificity, positive predictive value, and negative predictive value of the algorithm for the validation cohort as a whole, weighted to account for the prevalence of planned readmissions as defined by the algorithm in the national data (7.8%). Weighting is necessary because we did not obtain a pure random sample, but rather selected a stratified sample that oversampled algorithm‐identified planned readmissions.[9] We also calculated these rates separately for large hospitals (>600 beds) and for small hospitals (600 beds).
Finally, we examined performance of the algorithm for individual procedures and diagnoses to determine whether any procedures or diagnoses should be added or removed from the algorithm. First, we reviewed the diagnoses, procedures, and brief narratives provided by the abstractors for all cases in which the algorithm misclassified the readmission as either planned or unplanned. Second, we calculated the positive predictive value for each procedure that had been flagged as planned by the algorithm, and reviewed all readmissions (correctly and incorrectly classified) in which procedures with low positive predictive value took place. We also calculated the frequency with which the procedure was the only qualifying procedure resulting in an accurate or inaccurate classification. Third, to identify changes that should be made to the lists of acute and nonacute diagnoses, we reviewed the principal diagnosis for all readmissions misclassified by the algorithm as either planned or unplanned, and examined the specific ICD‐9‐CM codes within each CCS group that were most commonly associated with misclassifications.
After determining the changes that should be made to the algorithm based on these analyses, we recalculated the sensitivity, specificity, positive predictive value, and negative predictive value of the proposed revised algorithm (v3.0). All analyses used SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Cohort
Characteristics of participating hospitals are shown in Table 1. Hospitals represented in this sample ranged in size, teaching status, and safety net status, although all were nonprofit. We selected 663 readmissions for review, 363 planned and 300 unplanned. Overall we were able to select 80% of hospitals planned cases for review; the remainder occurred at hospitals outside the participating hospital system. Abstractors were able to locate and review 634 (96%) of the eligible charts (range, 86%100% per hospital). The kappa statistic for inter‐rater reliability was 0.83.
| Description | Hospitals, N | Readmissions Selected for Review, N* | Readmissions Reviewed, N (% of Eligible) | Unplanned Readmissions Reviewed, N | Planned Readmissions Reviewed, N | % of Hospital's Planned Readmissions Reviewed* | |
|---|---|---|---|---|---|---|---|
| |||||||
| All hospitals | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| No. of beds | >600 | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| >300600 | 2 | 190 | 173 (91.1) | 85 | 88 | 87.1 | |
| <300 | 3 | 127 | 122 (96.0) | 82 | 40 | 44.9 | |
| Ownership | Government | 0 | |||||
| For profit | 0 | ||||||
| Not for profit | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| Teaching status | Teaching | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonteaching | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Safety net status | Safety net | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonsafety net | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Region | New England | 3 | 409 | 392 (95.8) | 155 | 237 | 85.9 |
| South Central | 4 | 254 | 242 (95.3) | 128 | 114 | 64.0 | |
The study sample included 57/67 (85%) of the procedure or condition categories on the potentially planned list. The most common procedure CCS categories among planned readmissions (v2.1) in the validation cohort were very similar to those in the national dataset (see Supporting Information, Appendix D, in the online version of this article). Of the top 20 most commonly planned procedure CCS categories in the validation set, all but 2, therapeutic radiology for cancer treatment (CCS 211) and peripheral vascular bypass (CCS 55), were among the top 20 most commonly planned procedure CCS categories in the national data.
Test Characteristics of Algorithm
The weighted test characteristics of the current algorithm (v2.1) are shown in Table 2. Overall, the algorithm correctly identified 266 readmissions as unplanned and 181 readmissions as planned, and misidentified 170 readmissions as planned and 15 as unplanned. Once weighted to account for the stratified sampling design, the overall prevalence of true planned readmissions was 8.9% of readmissions. The weighted sensitivity was 45.1% overall and was higher in large teaching centers than in smaller community hospitals. The weighted specificity was 95.9%. The positive predictive value was 51.6%, and the negative predictive value was 94.7%.
| Cohort | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| Algorithm v2.1 | ||||
| Full cohort | 45.1% | 95.9% | 51.6% | 94.7% |
| Large hospitals | 50.9% | 96.1% | 53.8% | 95.6% |
| Small hospitals | 40.2% | 95.5% | 47.7% | 94.0% |
| Revised algorithm v3.0 | ||||
| Full cohort | 49.8% | 96.5% | 58.7% | 94.5% |
| Large hospitals | 57.1% | 96.8% | 63.0% | 95.9% |
| Small hospitals | 42.6% | 95.9% | 52.6% | 93.9% |
Accuracy of Individual Diagnoses and Procedures
The positive predictive value of the algorithm for individual procedure categories varied widely, from 0% to 100% among procedures with at least 10 cases (Table 3). The procedure for which the algorithm was least accurate was CCS 211, therapeutic radiology for cancer treatment (0% positive predictive value). By contrast, maintenance chemotherapy (90%) and other therapeutic procedures, hemic and lymphatic system (100%) were most accurate. Common procedures with less than 50% positive predictive value (ie, that the algorithm commonly misclassified as planned) were diagnostic cardiac catheterization (25%); debridement of wound, infection, or burn (25%); amputation of lower extremity (29%); insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (33%); and other hernia repair (43%). Of these, diagnostic cardiac catheterization and cardiac devices are the first and second most common procedures nationally, respectively.
| Readmission Procedure CCS Code | Total Categorized as Planned by Algorithm, N | Verified as Planned by Chart Review, N | Positive Predictive Value |
|---|---|---|---|
| |||
| 47 Diagnostic cardiac catheterization; coronary arteriography | 44 | 11 | 25% |
| 224 Cancer chemotherapy | 40 | 22 | 55% |
| 157 Amputation of lower extremity | 31 | 9 | 29% |
| 49 Other operating room heart procedures | 27 | 16 | 59% |
| 48 Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator | 24 | 8 | 33% |
| 43 Heart valve procedures | 20 | 16 | 80% |
| Maintenance chemotherapy (diagnosis CCS 45) | 20 | 18 | 90% |
| 78 Colorectal resection | 18 | 9 | 50% |
| 169 Debridement of wound, infection or burn | 16 | 4 | 25% |
| 84 Cholecystectomy and common duct exploration | 16 | 5 | 31% |
| 99 Other OR gastrointestinal therapeutic procedures | 16 | 8 | 50% |
| 158 Spinal fusion | 15 | 11 | 73% |
| 142 Partial excision bone | 14 | 10 | 71% |
| 86 Other hernia repair | 14 | 6 | 42% |
| 44 Coronary artery bypass graft | 13 | 10 | 77% |
| 67 Other therapeutic procedures, hemic and lymphatic system | 13 | 13 | 100% |
| 211 Therapeutic radiology for cancer treatment | 12 | 0 | 0% |
| 45 Percutaneous transluminal coronary angioplasty | 11 | 7 | 64% |
| Total | 497 | 272 | 54.7% |
The readmissions with least abstractor agreement were those involving CCS 157 (amputation of lower extremity) and CCS 169 (debridement of wound, infection or burn). Readmissions for these procedures were nearly always performed as a consequence of acute worsening of chronic conditions such as osteomyelitis or ulceration. Abstractors were divided over whether these readmissions were appropriate to call planned.
Changes to the Algorithm
We determined that the accuracy of the algorithm would be improved by removing 2 procedure categories from the planned procedure list (therapeutic radiation [CCS 211] and cancer chemotherapy [CCS 224]), adding 1 diagnosis category to the acute diagnosis list (hypertension with complications [CCS 99]), and splitting 2 diagnosis condition categories into acute and nonacute ICD‐9‐CM codes (pancreatic disorders [CCS 149] and biliary tract disease [CCS 152]). Detailed rationales for each modification to the planned readmission algorithm are described in Table 4. We felt further examination of diagnostic cardiac catheterization and cardiac devices was warranted given their high frequency, despite low positive predictive value. We also elected not to alter the categorization of amputation or debridement because it was not easy to determine whether these admissions were planned or unplanned even with chart review. We plan further analyses of these procedure categories.
| Action | Diagnosis or Procedure Category | Algorithm | Chart | N | Rationale for Change |
|---|---|---|---|---|---|
| |||||
| Remove from planned procedure list | Therapeutic radiation (CCS 211) | Accurate | The algorithm was inaccurate in every case. All therapeutic radiology during readmissions was performed because of acute illness (pain crisis, neurologic crisis) or because scheduled treatment occurred during an unplanned readmission. In national data, this ranks as the 25th most common planned procedure identified by the algorithm v2.1. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Cancer chemotherapy (CCS 224) | Accurate | Of the 22 correctly identified as planned, 18 (82%) would already have been categorized as planned because of a principal diagnosis of maintenance chemotherapy. Therefore, removing CCS 224 from the planned procedure list would only miss a small fraction of planned readmissions but would avoid a large number of misclassifications. In national data, this ranks as the 8th most common planned procedure identified by the algorithm v2.1. | |||
| Planned | Planned | 22 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 18 | |||
| Add to planned procedure list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. A handful of these cases were missed because the planned procedure was not on the current planned procedure list; however, those procedures (eg, abdominal paracentesis, colonoscopy, endoscopy) were nearly always unplanned overall and should therefore not be added as procedures that potentially qualify as an admission as planned. | |||
| Remove from acute diagnosis list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. The relevant disqualifying acute diagnoses were much more often associated with unplanned readmissions in our dataset. | |||
| Add to acute diagnosis list | Hypertension with complications (CCS 99) | Accurate | This CCS was associated with only 1 planned readmission (for elective nephrectomy, a very rare procedure). Every other time this CCS appeared in the dataset, it was associated with an unplanned readmission (12/13, 92%); 10 of those, however, were misclassified by the algorithm as planned because they were not excluded by diagnosis (91% error rate). Consequently, adding this CCS to the acute diagnosis list is likely to miss only a very small fraction of planned readmissions, while making the overall algorithm much more accurate. | ||
| Planned | Planned | 1 | |||
| Unplanned | Unplanned | 2 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 10 | |||
| Split diagnosis condition category into component ICD‐9 codes | Pancreatic disorders (CCS 152) | Accurate | ICD‐9 code 577.0 (acute pancreatitis) is the only acute code in this CCS. Acute pancreatitis was present in 2 cases that were misclassified as planned. Clinically, there is no situation in which a planned procedure would reasonably be performed in the setting of acute pancreatitis. Moving ICD‐9 code 577.0 to the acute list and leaving the rest of the ICD‐9 codes in CCS 152 on the nonacute list will enable the algorithm to continue to identify planned procedures for chronic pancreatitis. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 2 | |||
| Biliary tract disease (CCS 149) | Accurate | This CCS is a mix of acute and chronic diagnoses. Of 14 charts classified as planned with CCS 149 in the principal diagnosis field, 12 were misclassified (of which 10 were associated with cholecystectomy). Separating out the acute and nonacute diagnoses will increase the accuracy of the algorithm while still ensuring that planned cholecystectomies and other procedures can be identified. Of the ICD‐9 codes in CCS 149, the following will be added to the acute diagnosis list: 574.0, 574.3, 574.6, 574.8, 575.0, 575.12, 576.1. | |||
| Planned | Planned | 2 | |||
| Unplanned | Unplanned | 3 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Consider for change after additional study | Diagnostic cardiac catheterization (CCS 47) | Accurate | The algorithm misclassified as planned 25/38 (66%) unplanned readmissions in which diagnostic catheterizations were the only qualifying planned procedure. It also correctly identified 3/3 (100%) planned readmissions in which diagnostic cardiac catheterizations were the only qualifying planned procedure. This is the highest volume procedure in national data. | ||
| Planned | Planned | 3* | |||
| Unplanned | Unplanned | 13* | |||
| Inaccurate | |||||
| Unplanned | Planned | 0* | |||
| Planned | Unplanned | 25* | |||
| Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (CCS 48) | Accurate | The algorithm misclassified as planned 4/5 (80%) unplanned readmissions in which cardiac devices were the only qualifying procedure. However, it also correctly identified 7/8 (87.5%) planned readmissions in which cardiac devices were the only qualifying planned procedure. CCS 48 is the second most common planned procedure category nationally. | |||
| Planned | Planned | 7 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 1 | |||
| Planned | Unplanned | 4 | |||
The revised algorithm (v3.0) had a weighted sensitivity of 49.8%, weighted specificity of 96.5%, positive predictive value of 58.7%, and negative predictive value of 94.5% (Table 2). In aggregate, these changes would increase the reported unplanned readmission rate from 16.0% to 16.1% in the hospital‐wide readmission measure, using 2011 to 2012 data, and would decrease the fraction of all readmissions considered planned from 7.8% to 7.2%.
DISCUSSION
We developed an algorithm based on administrative data that in its currently implemented form is very accurate at identifying unplanned readmissions, ensuring that readmissions included in publicly reported readmission measures are likely to be truly unplanned. However, nearly half of readmissions the algorithm classifies as planned are actually unplanned. That is, the algorithm is overcautious in excluding unplanned readmissions that could have counted as outcomes, particularly among admissions that include diagnostic cardiac catheterization or placement of cardiac devices (pacemakers, defibrillators). However, these errors only occur within the 7.8% of readmissions that are classified as planned and therefore do not affect overall readmission rates dramatically. A perfect algorithm would reclassify approximately half of these planned readmissions as unplanned, increasing the overall readmission rate by 0.6 percentage points.
On the other hand, the algorithm also only identifies approximately half of true planned readmissions as planned. Because the true prevalence of planned readmissions is low (approximately 9% of readmissions based on weighted chart review prevalence, or an absolute rate of 1.4%), this low sensitivity has a small effect on algorithm performance. Removing all true planned readmissions from the measure outcome would decrease the overall readmission rate by 0.8 percentage points, similar to the expected 0.6 percentage point increase that would result from better identifying unplanned readmissions; thus, a perfect algorithm would likely decrease the reported unplanned readmission rate by a net 0.2%. Overall, the existing algorithm appears to come close to the true prevalence of planned readmissions, despite inaccuracy on an individual‐case basis. The algorithm performed best at large hospitals, which are at greatest risk of being statistical outliers and of accruing penalties under the Hospital Readmissions Reduction Program.[10]
We identified several changes that marginally improved the performance of the algorithm by reducing the number of unplanned readmissions that are incorrectly removed from the measure, while avoiding the inappropriate inclusion of planned readmissions in the outcome. This revised algorithm, v3.0, was applied to public reporting of readmission rates at the end of 2014. Overall, implementing these changes increases the reported readmission rate very slightly. We also identified other procedures associated with high inaccuracy rates, removal of which would have larger impact on reporting rates, and which therefore merit further evaluation.
There are other potential methods of identifying planned readmissions. For instance, as of October 1, 2013, new administrative billing codes were created to allow hospitals to indicate that a patient was discharged with a planned acute‐care hospital inpatient readmission, without limitation as to when it will take place.[11] This code must be used at the time of the index admission to indicate that a future planned admission is expected, and was specified only to be used for neonates and patients with acute myocardial infarction. This approach, however, would omit planned readmissions that are not known to the initial discharging team, potentially missing planned readmissions. Conversely, some patients discharged with a plan for readmission may be unexpectedly readmitted for an unplanned reason. Given that the new codes were not available at the time we conducted the validation study, we were not able to determine how often the billing codes accurately identified planned readmissions. This would be an important area to consider for future study.
An alternative approach would be to create indicator codes to be applied at the time of readmission that would indicate whether that admission was planned or unplanned. Such a code would have the advantage of allowing each planned readmission to be flagged by the admitting clinicians at the time of admission rather than by an algorithm that inherently cannot be perfect. However, identifying planned readmissions at the time of readmission would also create opportunity for gaming and inconsistent application of definitions between hospitals; additional checks would need to be put in place to guard against these possibilities.
Our study has some limitations. We relied on the opinion of chart abstractors to determine whether a readmission was planned or unplanned; in a few cases, such as smoldering wounds that ultimately require surgical intervention, that determination is debatable. Abstractions were done at local institutions to minimize risks to patient privacy, and therefore we could not centrally verify determinations of planned status except by reviewing source of admission, dates of procedures, and narrative comments reported by the abstractors. Finally, we did not have sufficient volume of planned procedures to determine accuracy of the algorithm for less common procedure categories or individual procedures within categories.
In summary, we developed an algorithm to identify planned readmissions from administrative data that had high specificity and moderate sensitivity, and refined it based on chart validation. This algorithm is in use in public reporting of readmission measures to maximize the probability that the reported readmission rates represent truly unplanned readmissions.[12]
Disclosures: Financial supportThis work was performed under contract HHSM‐500‐2008‐0025I/HHSM‐500‐T0001, Modification No. 000008, titled Measure Instrument Development and Support, funded by the Centers for Medicare and Medicaid Services (CMS), an agency of the US Department of Health and Human Services. Drs. Horwitz and Ross are supported by the National Institute on Aging (K08 AG038336 and K08 AG032886, respectively) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. Dr. Krumholz is supported by grant U01 HL105270‐05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; or in the writing of the article. The CMS reviewed and approved the use of its data for this work and approved submission of the manuscript. All authors have completed the Unified Competing Interest form at
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. An administrative claims measure suitable for profiling hospital performance based on 30‐day all‐cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243–252.
- , , , et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30‐day all‐cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37.
- , , , et al. Hospital‐level 30‐day all‐cause risk‐standardized readmission rate following elective primary total hip arthroplasty (THA) and/or total knee arthroplasty (TKA). Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page161(supp10 l):S66–S75.
- , , A meta‐analysis of hospital 30‐day avoidable readmission rates. J Eval Clin Pract. 2011;18(6):1211–1218.
- , , , , Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Centers for Medicare 3(4):477–492.
- , Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- Centers for Medicare and Medicaid Services. Inpatient Prospective Payment System/Long‐Term Care Hospital (IPPS/LTCH) final rule. Fed Regist. 2013;78:50533–50534.
- , , Massachusetts health reforms: uninsurance remains low, self‐reported health status improves as state prepares to tackle costs. Health Aff (Millwood). 2012;31(2):444–451.
The Centers for Medicare & Medicaid Services (CMS) publicly reports all‐cause risk‐standardized readmission rates after acute‐care hospitalization for acute myocardial infarction, pneumonia, heart failure, total hip and knee arthroplasty, chronic obstructive pulmonary disease, stroke, and for patients hospital‐wide.[1, 2, 3, 4, 5] Ideally, these measures should capture unplanned readmissions that arise from acute clinical events requiring urgent rehospitalization. Planned readmissions, which are scheduled admissions usually involving nonurgent procedures, may not be a signal of quality of care. Including planned readmissions in readmission quality measures could create a disincentive to provide appropriate care to patients who are scheduled for elective or necessary procedures unrelated to the quality of the prior admission. Accordingly, under contract to the CMS, we were asked to develop an algorithm to identify planned readmissions. A version of this algorithm is now incorporated into all publicly reported readmission measures.
Given the widespread use of the planned readmission algorithm in public reporting and its implications for hospital quality measurement and evaluation, the objective of this study was to describe the development process, and to validate and refine the algorithm by reviewing charts of readmitted patients.
METHODS
Algorithm Development
To create a planned readmission algorithm, we first defined planned. We determined that readmissions for obstetrical delivery, maintenance chemotherapy, major organ transplant, and rehabilitation should always be considered planned in the sense that they are desired and/or inevitable, even if not specifically planned on a certain date. Apart from these specific types of readmissions, we defined planned readmissions as nonacute readmissions for scheduled procedures, because the vast majority of planned admissions are related to procedures. We also defined readmissions for acute illness or for complications of care as unplanned for the purposes of a quality measure. Even if such readmissions included a potentially planned procedure, because complications of care represent an important dimension of quality that should not be excluded from outcome measurement, these admissions should not be removed from the measure outcome. This definition of planned readmissions does not imply that all unplanned readmissions are unexpected or avoidable. However, it has proven very difficult to reliably define avoidable readmissions, even by expert review of charts, and we did not attempt to do so here.[6, 7]
In the second stage, we operationalized this definition into an algorithm. We used the Agency for Healthcare Research and Quality's Clinical Classification Software (CCS) codes to group thousands of individual procedure and diagnosis International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes into clinically coherent, mutually exclusive procedure CCS categories and mutually exclusive diagnosis CCS categories, respectively. Clinicians on the investigative team reviewed the procedure categories to identify those that are commonly planned and that would require inpatient admission. We also reviewed the diagnosis categories to identify acute diagnoses unlikely to accompany elective procedures. We then created a flow diagram through which every readmission could be run to determine whether it was planned or unplanned based on our categorizations of procedures and diagnoses (Figure 1, and Supporting Information, Appendix A, in the online version of this article). This version of the algorithm (v1.0) was submitted to the National Quality Forum (NQF) as part of the hospital‐wide readmission measure. The measure (NQR #1789) received endorsement in April 2012.

In the third stage of development, we posted the algorithm for 2 public comment periods and recruited 27 outside experts to review and refine the algorithm following a standardized, structured process (see Supporting Information, Appendix B, in the online version of this article). Because the measures publicly report and hold hospitals accountable for unplanned readmission rates, we felt it most important that the algorithm include as few planned readmissions in the reported, unplanned outcome as possible (ie, have high negative predictive value). Therefore, in equivocal situations in which experts felt procedure categories were equally often planned or unplanned, we added those procedures to the potentially planned list. We also solicited feedback from hospitals on algorithm performance during a confidential test run of the hospital‐wide readmission measure in the fall of 2012. Based on all of this feedback, we made a number of changes to the algorithm, which was then identified as v2.1. Version 2.1 of the algorithm was submitted to the NQF as part of the endorsement process for the acute myocardial infarction and heart failure readmission measures and was endorsed by the NQF in January 2013. The algorithm (v2.1) is now applied, adapted if necessary, to all publicly reported readmission measures.[8]
Algorithm Validation: Study Cohort
We recruited 2 hospital systems to participate in a chart validation study of the accuracy of the planned readmission algorithm (v2.1). Within these 2 health systems, we selected 7 hospitals with varying bed size, teaching status, and safety‐net status. Each included 1 large academic teaching hospital that serves as a regional referral center. For each hospital's index admissions, we applied the inclusion and exclusion criteria from the hospital‐wide readmission measure. Index admissions were included for patients age 65 years or older; enrolled in Medicare fee‐for‐service (FFS); discharged from a nonfederal, short‐stay, acute‐care hospital or critical access hospital; without an in‐hospital death; not transferred to another acute‐care facility; and enrolled in Part A Medicare for 1 year prior to discharge. We excluded index admissions for patients without at least 30 days postdischarge enrollment in FFS Medicare, discharged against medical advice, admitted for medical treatment of cancer or primary psychiatric disease, admitted to a Prospective Payment System‐exempt cancer hospital, or who died during the index hospitalization. In addition, for this study, we included only index admissions that were followed by a readmission to a hospital within the participating health system between July 1, 2011 and June 30, 2012. Institutional review board approval was obtained from each of the participating health systems, which granted waivers of signed informed consent and Health Insurance Portability and Accountability Act waivers.
Algorithm Validation: Sample Size Calculation
We determined a priori that the minimum acceptable positive predictive value, or proportion of all readmissions the algorithm labels planned that are truly planned, would be 60%, and the minimum acceptable negative predictive value, or proportion of all readmissions the algorithm labels as unplanned that are truly unplanned, would be 80%. We calculated the sample size required to be confident of these values 10% and determined we would need a total of 291 planned charts and 162 unplanned charts. We inflated these numbers by 20% to account for missing or unobtainable charts for a total of 550 charts. To achieve this sample size, we included all eligible readmissions from all participating hospitals that were categorized as planned. At the 5 smaller hospitals, we randomly selected an equal number of unplanned readmissions occurring at any hospital in its healthcare system. At the 2 largest hospitals, we randomly selected 50 unplanned readmissions occurring at any hospital in its healthcare system.
Algorithm Validation: Data Abstraction
We developed an abstraction tool, tested and refined it using sample charts, and built the final the tool into a secure, password‐protected Microsoft Access 2007 (Microsoft Corp., Redmond, WA) database (see Supporting Information, Appendix C, in the online version of this article). Experienced chart abstractors with RN or MD degrees from each hospital site participated in a 1‐hour training session to become familiar with reviewing medical charts, defining planned/unplanned readmissions, and the data abstraction process. For each readmission, we asked abstractors to review as needed: emergency department triage and physician notes, admission history and physical, operative report, discharge summary, and/or discharge summary from a prior admission. The abstractors verified the accuracy of the administrative billing data, including procedures and principal diagnosis. In addition, they abstracted the source of admission and dates of all major procedures. Then the abstractors provided their opinion and supporting rationale as to whether a readmission was planned or unplanned. They were not asked to determine whether the readmission was preventable. To determine the inter‐rater reliability of data abstraction, an independent abstractor at each health system recoded a random sample of 10% of the charts.
Statistical Analysis
To ensure that we had obtained a representative sample of charts, we identified the 10 most commonly planned procedures among cases identified as planned by the algorithm in the validation cohort and then compared this with planned cases nationally. To confirm the reliability of the abstraction process, we used the kappa statistic to determine the inter‐rater reliability of the determination of planned or unplanned status. Additionally, the full study team, including 5 practicing clinicians, reviewed the details of every chart abstraction in which the algorithm was found to have misclassified the readmission as planned or unplanned. In 11 cases we determined that the abstractor had misunderstood the definition of planned readmission (ie, not all direct admissions are necessarily planned) and we reclassified the chart review assignment accordingly.
We calculated sensitivity, specificity, positive predictive value, and negative predictive value of the algorithm for the validation cohort as a whole, weighted to account for the prevalence of planned readmissions as defined by the algorithm in the national data (7.8%). Weighting is necessary because we did not obtain a pure random sample, but rather selected a stratified sample that oversampled algorithm‐identified planned readmissions.[9] We also calculated these rates separately for large hospitals (>600 beds) and for small hospitals (600 beds).
Finally, we examined performance of the algorithm for individual procedures and diagnoses to determine whether any procedures or diagnoses should be added or removed from the algorithm. First, we reviewed the diagnoses, procedures, and brief narratives provided by the abstractors for all cases in which the algorithm misclassified the readmission as either planned or unplanned. Second, we calculated the positive predictive value for each procedure that had been flagged as planned by the algorithm, and reviewed all readmissions (correctly and incorrectly classified) in which procedures with low positive predictive value took place. We also calculated the frequency with which the procedure was the only qualifying procedure resulting in an accurate or inaccurate classification. Third, to identify changes that should be made to the lists of acute and nonacute diagnoses, we reviewed the principal diagnosis for all readmissions misclassified by the algorithm as either planned or unplanned, and examined the specific ICD‐9‐CM codes within each CCS group that were most commonly associated with misclassifications.
After determining the changes that should be made to the algorithm based on these analyses, we recalculated the sensitivity, specificity, positive predictive value, and negative predictive value of the proposed revised algorithm (v3.0). All analyses used SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Cohort
Characteristics of participating hospitals are shown in Table 1. Hospitals represented in this sample ranged in size, teaching status, and safety net status, although all were nonprofit. We selected 663 readmissions for review, 363 planned and 300 unplanned. Overall we were able to select 80% of hospitals planned cases for review; the remainder occurred at hospitals outside the participating hospital system. Abstractors were able to locate and review 634 (96%) of the eligible charts (range, 86%100% per hospital). The kappa statistic for inter‐rater reliability was 0.83.
| Description | Hospitals, N | Readmissions Selected for Review, N* | Readmissions Reviewed, N (% of Eligible) | Unplanned Readmissions Reviewed, N | Planned Readmissions Reviewed, N | % of Hospital's Planned Readmissions Reviewed* | |
|---|---|---|---|---|---|---|---|
| |||||||
| All hospitals | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| No. of beds | >600 | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| >300600 | 2 | 190 | 173 (91.1) | 85 | 88 | 87.1 | |
| <300 | 3 | 127 | 122 (96.0) | 82 | 40 | 44.9 | |
| Ownership | Government | 0 | |||||
| For profit | 0 | ||||||
| Not for profit | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| Teaching status | Teaching | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonteaching | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Safety net status | Safety net | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonsafety net | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Region | New England | 3 | 409 | 392 (95.8) | 155 | 237 | 85.9 |
| South Central | 4 | 254 | 242 (95.3) | 128 | 114 | 64.0 | |
The study sample included 57/67 (85%) of the procedure or condition categories on the potentially planned list. The most common procedure CCS categories among planned readmissions (v2.1) in the validation cohort were very similar to those in the national dataset (see Supporting Information, Appendix D, in the online version of this article). Of the top 20 most commonly planned procedure CCS categories in the validation set, all but 2, therapeutic radiology for cancer treatment (CCS 211) and peripheral vascular bypass (CCS 55), were among the top 20 most commonly planned procedure CCS categories in the national data.
Test Characteristics of Algorithm
The weighted test characteristics of the current algorithm (v2.1) are shown in Table 2. Overall, the algorithm correctly identified 266 readmissions as unplanned and 181 readmissions as planned, and misidentified 170 readmissions as planned and 15 as unplanned. Once weighted to account for the stratified sampling design, the overall prevalence of true planned readmissions was 8.9% of readmissions. The weighted sensitivity was 45.1% overall and was higher in large teaching centers than in smaller community hospitals. The weighted specificity was 95.9%. The positive predictive value was 51.6%, and the negative predictive value was 94.7%.
| Cohort | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| Algorithm v2.1 | ||||
| Full cohort | 45.1% | 95.9% | 51.6% | 94.7% |
| Large hospitals | 50.9% | 96.1% | 53.8% | 95.6% |
| Small hospitals | 40.2% | 95.5% | 47.7% | 94.0% |
| Revised algorithm v3.0 | ||||
| Full cohort | 49.8% | 96.5% | 58.7% | 94.5% |
| Large hospitals | 57.1% | 96.8% | 63.0% | 95.9% |
| Small hospitals | 42.6% | 95.9% | 52.6% | 93.9% |
Accuracy of Individual Diagnoses and Procedures
The positive predictive value of the algorithm for individual procedure categories varied widely, from 0% to 100% among procedures with at least 10 cases (Table 3). The procedure for which the algorithm was least accurate was CCS 211, therapeutic radiology for cancer treatment (0% positive predictive value). By contrast, maintenance chemotherapy (90%) and other therapeutic procedures, hemic and lymphatic system (100%) were most accurate. Common procedures with less than 50% positive predictive value (ie, that the algorithm commonly misclassified as planned) were diagnostic cardiac catheterization (25%); debridement of wound, infection, or burn (25%); amputation of lower extremity (29%); insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (33%); and other hernia repair (43%). Of these, diagnostic cardiac catheterization and cardiac devices are the first and second most common procedures nationally, respectively.
| Readmission Procedure CCS Code | Total Categorized as Planned by Algorithm, N | Verified as Planned by Chart Review, N | Positive Predictive Value |
|---|---|---|---|
| |||
| 47 Diagnostic cardiac catheterization; coronary arteriography | 44 | 11 | 25% |
| 224 Cancer chemotherapy | 40 | 22 | 55% |
| 157 Amputation of lower extremity | 31 | 9 | 29% |
| 49 Other operating room heart procedures | 27 | 16 | 59% |
| 48 Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator | 24 | 8 | 33% |
| 43 Heart valve procedures | 20 | 16 | 80% |
| Maintenance chemotherapy (diagnosis CCS 45) | 20 | 18 | 90% |
| 78 Colorectal resection | 18 | 9 | 50% |
| 169 Debridement of wound, infection or burn | 16 | 4 | 25% |
| 84 Cholecystectomy and common duct exploration | 16 | 5 | 31% |
| 99 Other OR gastrointestinal therapeutic procedures | 16 | 8 | 50% |
| 158 Spinal fusion | 15 | 11 | 73% |
| 142 Partial excision bone | 14 | 10 | 71% |
| 86 Other hernia repair | 14 | 6 | 42% |
| 44 Coronary artery bypass graft | 13 | 10 | 77% |
| 67 Other therapeutic procedures, hemic and lymphatic system | 13 | 13 | 100% |
| 211 Therapeutic radiology for cancer treatment | 12 | 0 | 0% |
| 45 Percutaneous transluminal coronary angioplasty | 11 | 7 | 64% |
| Total | 497 | 272 | 54.7% |
The readmissions with least abstractor agreement were those involving CCS 157 (amputation of lower extremity) and CCS 169 (debridement of wound, infection or burn). Readmissions for these procedures were nearly always performed as a consequence of acute worsening of chronic conditions such as osteomyelitis or ulceration. Abstractors were divided over whether these readmissions were appropriate to call planned.
Changes to the Algorithm
We determined that the accuracy of the algorithm would be improved by removing 2 procedure categories from the planned procedure list (therapeutic radiation [CCS 211] and cancer chemotherapy [CCS 224]), adding 1 diagnosis category to the acute diagnosis list (hypertension with complications [CCS 99]), and splitting 2 diagnosis condition categories into acute and nonacute ICD‐9‐CM codes (pancreatic disorders [CCS 149] and biliary tract disease [CCS 152]). Detailed rationales for each modification to the planned readmission algorithm are described in Table 4. We felt further examination of diagnostic cardiac catheterization and cardiac devices was warranted given their high frequency, despite low positive predictive value. We also elected not to alter the categorization of amputation or debridement because it was not easy to determine whether these admissions were planned or unplanned even with chart review. We plan further analyses of these procedure categories.
| Action | Diagnosis or Procedure Category | Algorithm | Chart | N | Rationale for Change |
|---|---|---|---|---|---|
| |||||
| Remove from planned procedure list | Therapeutic radiation (CCS 211) | Accurate | The algorithm was inaccurate in every case. All therapeutic radiology during readmissions was performed because of acute illness (pain crisis, neurologic crisis) or because scheduled treatment occurred during an unplanned readmission. In national data, this ranks as the 25th most common planned procedure identified by the algorithm v2.1. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Cancer chemotherapy (CCS 224) | Accurate | Of the 22 correctly identified as planned, 18 (82%) would already have been categorized as planned because of a principal diagnosis of maintenance chemotherapy. Therefore, removing CCS 224 from the planned procedure list would only miss a small fraction of planned readmissions but would avoid a large number of misclassifications. In national data, this ranks as the 8th most common planned procedure identified by the algorithm v2.1. | |||
| Planned | Planned | 22 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 18 | |||
| Add to planned procedure list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. A handful of these cases were missed because the planned procedure was not on the current planned procedure list; however, those procedures (eg, abdominal paracentesis, colonoscopy, endoscopy) were nearly always unplanned overall and should therefore not be added as procedures that potentially qualify as an admission as planned. | |||
| Remove from acute diagnosis list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. The relevant disqualifying acute diagnoses were much more often associated with unplanned readmissions in our dataset. | |||
| Add to acute diagnosis list | Hypertension with complications (CCS 99) | Accurate | This CCS was associated with only 1 planned readmission (for elective nephrectomy, a very rare procedure). Every other time this CCS appeared in the dataset, it was associated with an unplanned readmission (12/13, 92%); 10 of those, however, were misclassified by the algorithm as planned because they were not excluded by diagnosis (91% error rate). Consequently, adding this CCS to the acute diagnosis list is likely to miss only a very small fraction of planned readmissions, while making the overall algorithm much more accurate. | ||
| Planned | Planned | 1 | |||
| Unplanned | Unplanned | 2 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 10 | |||
| Split diagnosis condition category into component ICD‐9 codes | Pancreatic disorders (CCS 152) | Accurate | ICD‐9 code 577.0 (acute pancreatitis) is the only acute code in this CCS. Acute pancreatitis was present in 2 cases that were misclassified as planned. Clinically, there is no situation in which a planned procedure would reasonably be performed in the setting of acute pancreatitis. Moving ICD‐9 code 577.0 to the acute list and leaving the rest of the ICD‐9 codes in CCS 152 on the nonacute list will enable the algorithm to continue to identify planned procedures for chronic pancreatitis. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 2 | |||
| Biliary tract disease (CCS 149) | Accurate | This CCS is a mix of acute and chronic diagnoses. Of 14 charts classified as planned with CCS 149 in the principal diagnosis field, 12 were misclassified (of which 10 were associated with cholecystectomy). Separating out the acute and nonacute diagnoses will increase the accuracy of the algorithm while still ensuring that planned cholecystectomies and other procedures can be identified. Of the ICD‐9 codes in CCS 149, the following will be added to the acute diagnosis list: 574.0, 574.3, 574.6, 574.8, 575.0, 575.12, 576.1. | |||
| Planned | Planned | 2 | |||
| Unplanned | Unplanned | 3 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Consider for change after additional study | Diagnostic cardiac catheterization (CCS 47) | Accurate | The algorithm misclassified as planned 25/38 (66%) unplanned readmissions in which diagnostic catheterizations were the only qualifying planned procedure. It also correctly identified 3/3 (100%) planned readmissions in which diagnostic cardiac catheterizations were the only qualifying planned procedure. This is the highest volume procedure in national data. | ||
| Planned | Planned | 3* | |||
| Unplanned | Unplanned | 13* | |||
| Inaccurate | |||||
| Unplanned | Planned | 0* | |||
| Planned | Unplanned | 25* | |||
| Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (CCS 48) | Accurate | The algorithm misclassified as planned 4/5 (80%) unplanned readmissions in which cardiac devices were the only qualifying procedure. However, it also correctly identified 7/8 (87.5%) planned readmissions in which cardiac devices were the only qualifying planned procedure. CCS 48 is the second most common planned procedure category nationally. | |||
| Planned | Planned | 7 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 1 | |||
| Planned | Unplanned | 4 | |||
The revised algorithm (v3.0) had a weighted sensitivity of 49.8%, weighted specificity of 96.5%, positive predictive value of 58.7%, and negative predictive value of 94.5% (Table 2). In aggregate, these changes would increase the reported unplanned readmission rate from 16.0% to 16.1% in the hospital‐wide readmission measure, using 2011 to 2012 data, and would decrease the fraction of all readmissions considered planned from 7.8% to 7.2%.
DISCUSSION
We developed an algorithm based on administrative data that in its currently implemented form is very accurate at identifying unplanned readmissions, ensuring that readmissions included in publicly reported readmission measures are likely to be truly unplanned. However, nearly half of readmissions the algorithm classifies as planned are actually unplanned. That is, the algorithm is overcautious in excluding unplanned readmissions that could have counted as outcomes, particularly among admissions that include diagnostic cardiac catheterization or placement of cardiac devices (pacemakers, defibrillators). However, these errors only occur within the 7.8% of readmissions that are classified as planned and therefore do not affect overall readmission rates dramatically. A perfect algorithm would reclassify approximately half of these planned readmissions as unplanned, increasing the overall readmission rate by 0.6 percentage points.
On the other hand, the algorithm also only identifies approximately half of true planned readmissions as planned. Because the true prevalence of planned readmissions is low (approximately 9% of readmissions based on weighted chart review prevalence, or an absolute rate of 1.4%), this low sensitivity has a small effect on algorithm performance. Removing all true planned readmissions from the measure outcome would decrease the overall readmission rate by 0.8 percentage points, similar to the expected 0.6 percentage point increase that would result from better identifying unplanned readmissions; thus, a perfect algorithm would likely decrease the reported unplanned readmission rate by a net 0.2%. Overall, the existing algorithm appears to come close to the true prevalence of planned readmissions, despite inaccuracy on an individual‐case basis. The algorithm performed best at large hospitals, which are at greatest risk of being statistical outliers and of accruing penalties under the Hospital Readmissions Reduction Program.[10]
We identified several changes that marginally improved the performance of the algorithm by reducing the number of unplanned readmissions that are incorrectly removed from the measure, while avoiding the inappropriate inclusion of planned readmissions in the outcome. This revised algorithm, v3.0, was applied to public reporting of readmission rates at the end of 2014. Overall, implementing these changes increases the reported readmission rate very slightly. We also identified other procedures associated with high inaccuracy rates, removal of which would have larger impact on reporting rates, and which therefore merit further evaluation.
There are other potential methods of identifying planned readmissions. For instance, as of October 1, 2013, new administrative billing codes were created to allow hospitals to indicate that a patient was discharged with a planned acute‐care hospital inpatient readmission, without limitation as to when it will take place.[11] This code must be used at the time of the index admission to indicate that a future planned admission is expected, and was specified only to be used for neonates and patients with acute myocardial infarction. This approach, however, would omit planned readmissions that are not known to the initial discharging team, potentially missing planned readmissions. Conversely, some patients discharged with a plan for readmission may be unexpectedly readmitted for an unplanned reason. Given that the new codes were not available at the time we conducted the validation study, we were not able to determine how often the billing codes accurately identified planned readmissions. This would be an important area to consider for future study.
An alternative approach would be to create indicator codes to be applied at the time of readmission that would indicate whether that admission was planned or unplanned. Such a code would have the advantage of allowing each planned readmission to be flagged by the admitting clinicians at the time of admission rather than by an algorithm that inherently cannot be perfect. However, identifying planned readmissions at the time of readmission would also create opportunity for gaming and inconsistent application of definitions between hospitals; additional checks would need to be put in place to guard against these possibilities.
Our study has some limitations. We relied on the opinion of chart abstractors to determine whether a readmission was planned or unplanned; in a few cases, such as smoldering wounds that ultimately require surgical intervention, that determination is debatable. Abstractions were done at local institutions to minimize risks to patient privacy, and therefore we could not centrally verify determinations of planned status except by reviewing source of admission, dates of procedures, and narrative comments reported by the abstractors. Finally, we did not have sufficient volume of planned procedures to determine accuracy of the algorithm for less common procedure categories or individual procedures within categories.
In summary, we developed an algorithm to identify planned readmissions from administrative data that had high specificity and moderate sensitivity, and refined it based on chart validation. This algorithm is in use in public reporting of readmission measures to maximize the probability that the reported readmission rates represent truly unplanned readmissions.[12]
Disclosures: Financial supportThis work was performed under contract HHSM‐500‐2008‐0025I/HHSM‐500‐T0001, Modification No. 000008, titled Measure Instrument Development and Support, funded by the Centers for Medicare and Medicaid Services (CMS), an agency of the US Department of Health and Human Services. Drs. Horwitz and Ross are supported by the National Institute on Aging (K08 AG038336 and K08 AG032886, respectively) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. Dr. Krumholz is supported by grant U01 HL105270‐05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; or in the writing of the article. The CMS reviewed and approved the use of its data for this work and approved submission of the manuscript. All authors have completed the Unified Competing Interest form at
The Centers for Medicare & Medicaid Services (CMS) publicly reports all‐cause risk‐standardized readmission rates after acute‐care hospitalization for acute myocardial infarction, pneumonia, heart failure, total hip and knee arthroplasty, chronic obstructive pulmonary disease, stroke, and for patients hospital‐wide.[1, 2, 3, 4, 5] Ideally, these measures should capture unplanned readmissions that arise from acute clinical events requiring urgent rehospitalization. Planned readmissions, which are scheduled admissions usually involving nonurgent procedures, may not be a signal of quality of care. Including planned readmissions in readmission quality measures could create a disincentive to provide appropriate care to patients who are scheduled for elective or necessary procedures unrelated to the quality of the prior admission. Accordingly, under contract to the CMS, we were asked to develop an algorithm to identify planned readmissions. A version of this algorithm is now incorporated into all publicly reported readmission measures.
Given the widespread use of the planned readmission algorithm in public reporting and its implications for hospital quality measurement and evaluation, the objective of this study was to describe the development process, and to validate and refine the algorithm by reviewing charts of readmitted patients.
METHODS
Algorithm Development
To create a planned readmission algorithm, we first defined planned. We determined that readmissions for obstetrical delivery, maintenance chemotherapy, major organ transplant, and rehabilitation should always be considered planned in the sense that they are desired and/or inevitable, even if not specifically planned on a certain date. Apart from these specific types of readmissions, we defined planned readmissions as nonacute readmissions for scheduled procedures, because the vast majority of planned admissions are related to procedures. We also defined readmissions for acute illness or for complications of care as unplanned for the purposes of a quality measure. Even if such readmissions included a potentially planned procedure, because complications of care represent an important dimension of quality that should not be excluded from outcome measurement, these admissions should not be removed from the measure outcome. This definition of planned readmissions does not imply that all unplanned readmissions are unexpected or avoidable. However, it has proven very difficult to reliably define avoidable readmissions, even by expert review of charts, and we did not attempt to do so here.[6, 7]
In the second stage, we operationalized this definition into an algorithm. We used the Agency for Healthcare Research and Quality's Clinical Classification Software (CCS) codes to group thousands of individual procedure and diagnosis International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes into clinically coherent, mutually exclusive procedure CCS categories and mutually exclusive diagnosis CCS categories, respectively. Clinicians on the investigative team reviewed the procedure categories to identify those that are commonly planned and that would require inpatient admission. We also reviewed the diagnosis categories to identify acute diagnoses unlikely to accompany elective procedures. We then created a flow diagram through which every readmission could be run to determine whether it was planned or unplanned based on our categorizations of procedures and diagnoses (Figure 1, and Supporting Information, Appendix A, in the online version of this article). This version of the algorithm (v1.0) was submitted to the National Quality Forum (NQF) as part of the hospital‐wide readmission measure. The measure (NQR #1789) received endorsement in April 2012.

In the third stage of development, we posted the algorithm for 2 public comment periods and recruited 27 outside experts to review and refine the algorithm following a standardized, structured process (see Supporting Information, Appendix B, in the online version of this article). Because the measures publicly report and hold hospitals accountable for unplanned readmission rates, we felt it most important that the algorithm include as few planned readmissions in the reported, unplanned outcome as possible (ie, have high negative predictive value). Therefore, in equivocal situations in which experts felt procedure categories were equally often planned or unplanned, we added those procedures to the potentially planned list. We also solicited feedback from hospitals on algorithm performance during a confidential test run of the hospital‐wide readmission measure in the fall of 2012. Based on all of this feedback, we made a number of changes to the algorithm, which was then identified as v2.1. Version 2.1 of the algorithm was submitted to the NQF as part of the endorsement process for the acute myocardial infarction and heart failure readmission measures and was endorsed by the NQF in January 2013. The algorithm (v2.1) is now applied, adapted if necessary, to all publicly reported readmission measures.[8]
Algorithm Validation: Study Cohort
We recruited 2 hospital systems to participate in a chart validation study of the accuracy of the planned readmission algorithm (v2.1). Within these 2 health systems, we selected 7 hospitals with varying bed size, teaching status, and safety‐net status. Each included 1 large academic teaching hospital that serves as a regional referral center. For each hospital's index admissions, we applied the inclusion and exclusion criteria from the hospital‐wide readmission measure. Index admissions were included for patients age 65 years or older; enrolled in Medicare fee‐for‐service (FFS); discharged from a nonfederal, short‐stay, acute‐care hospital or critical access hospital; without an in‐hospital death; not transferred to another acute‐care facility; and enrolled in Part A Medicare for 1 year prior to discharge. We excluded index admissions for patients without at least 30 days postdischarge enrollment in FFS Medicare, discharged against medical advice, admitted for medical treatment of cancer or primary psychiatric disease, admitted to a Prospective Payment System‐exempt cancer hospital, or who died during the index hospitalization. In addition, for this study, we included only index admissions that were followed by a readmission to a hospital within the participating health system between July 1, 2011 and June 30, 2012. Institutional review board approval was obtained from each of the participating health systems, which granted waivers of signed informed consent and Health Insurance Portability and Accountability Act waivers.
Algorithm Validation: Sample Size Calculation
We determined a priori that the minimum acceptable positive predictive value, or proportion of all readmissions the algorithm labels planned that are truly planned, would be 60%, and the minimum acceptable negative predictive value, or proportion of all readmissions the algorithm labels as unplanned that are truly unplanned, would be 80%. We calculated the sample size required to be confident of these values 10% and determined we would need a total of 291 planned charts and 162 unplanned charts. We inflated these numbers by 20% to account for missing or unobtainable charts for a total of 550 charts. To achieve this sample size, we included all eligible readmissions from all participating hospitals that were categorized as planned. At the 5 smaller hospitals, we randomly selected an equal number of unplanned readmissions occurring at any hospital in its healthcare system. At the 2 largest hospitals, we randomly selected 50 unplanned readmissions occurring at any hospital in its healthcare system.
Algorithm Validation: Data Abstraction
We developed an abstraction tool, tested and refined it using sample charts, and built the final the tool into a secure, password‐protected Microsoft Access 2007 (Microsoft Corp., Redmond, WA) database (see Supporting Information, Appendix C, in the online version of this article). Experienced chart abstractors with RN or MD degrees from each hospital site participated in a 1‐hour training session to become familiar with reviewing medical charts, defining planned/unplanned readmissions, and the data abstraction process. For each readmission, we asked abstractors to review as needed: emergency department triage and physician notes, admission history and physical, operative report, discharge summary, and/or discharge summary from a prior admission. The abstractors verified the accuracy of the administrative billing data, including procedures and principal diagnosis. In addition, they abstracted the source of admission and dates of all major procedures. Then the abstractors provided their opinion and supporting rationale as to whether a readmission was planned or unplanned. They were not asked to determine whether the readmission was preventable. To determine the inter‐rater reliability of data abstraction, an independent abstractor at each health system recoded a random sample of 10% of the charts.
Statistical Analysis
To ensure that we had obtained a representative sample of charts, we identified the 10 most commonly planned procedures among cases identified as planned by the algorithm in the validation cohort and then compared this with planned cases nationally. To confirm the reliability of the abstraction process, we used the kappa statistic to determine the inter‐rater reliability of the determination of planned or unplanned status. Additionally, the full study team, including 5 practicing clinicians, reviewed the details of every chart abstraction in which the algorithm was found to have misclassified the readmission as planned or unplanned. In 11 cases we determined that the abstractor had misunderstood the definition of planned readmission (ie, not all direct admissions are necessarily planned) and we reclassified the chart review assignment accordingly.
We calculated sensitivity, specificity, positive predictive value, and negative predictive value of the algorithm for the validation cohort as a whole, weighted to account for the prevalence of planned readmissions as defined by the algorithm in the national data (7.8%). Weighting is necessary because we did not obtain a pure random sample, but rather selected a stratified sample that oversampled algorithm‐identified planned readmissions.[9] We also calculated these rates separately for large hospitals (>600 beds) and for small hospitals (600 beds).
Finally, we examined performance of the algorithm for individual procedures and diagnoses to determine whether any procedures or diagnoses should be added or removed from the algorithm. First, we reviewed the diagnoses, procedures, and brief narratives provided by the abstractors for all cases in which the algorithm misclassified the readmission as either planned or unplanned. Second, we calculated the positive predictive value for each procedure that had been flagged as planned by the algorithm, and reviewed all readmissions (correctly and incorrectly classified) in which procedures with low positive predictive value took place. We also calculated the frequency with which the procedure was the only qualifying procedure resulting in an accurate or inaccurate classification. Third, to identify changes that should be made to the lists of acute and nonacute diagnoses, we reviewed the principal diagnosis for all readmissions misclassified by the algorithm as either planned or unplanned, and examined the specific ICD‐9‐CM codes within each CCS group that were most commonly associated with misclassifications.
After determining the changes that should be made to the algorithm based on these analyses, we recalculated the sensitivity, specificity, positive predictive value, and negative predictive value of the proposed revised algorithm (v3.0). All analyses used SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Cohort
Characteristics of participating hospitals are shown in Table 1. Hospitals represented in this sample ranged in size, teaching status, and safety net status, although all were nonprofit. We selected 663 readmissions for review, 363 planned and 300 unplanned. Overall we were able to select 80% of hospitals planned cases for review; the remainder occurred at hospitals outside the participating hospital system. Abstractors were able to locate and review 634 (96%) of the eligible charts (range, 86%100% per hospital). The kappa statistic for inter‐rater reliability was 0.83.
| Description | Hospitals, N | Readmissions Selected for Review, N* | Readmissions Reviewed, N (% of Eligible) | Unplanned Readmissions Reviewed, N | Planned Readmissions Reviewed, N | % of Hospital's Planned Readmissions Reviewed* | |
|---|---|---|---|---|---|---|---|
| |||||||
| All hospitals | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| No. of beds | >600 | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| >300600 | 2 | 190 | 173 (91.1) | 85 | 88 | 87.1 | |
| <300 | 3 | 127 | 122 (96.0) | 82 | 40 | 44.9 | |
| Ownership | Government | 0 | |||||
| For profit | 0 | ||||||
| Not for profit | 7 | 663 | 634 (95.6) | 283 | 351 | 77.3 | |
| Teaching status | Teaching | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonteaching | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Safety net status | Safety net | 2 | 346 | 339 (98.0) | 116 | 223 | 84.5 |
| Nonsafety net | 5 | 317 | 295 (93.1) | 167 | 128 | 67.4 | |
| Region | New England | 3 | 409 | 392 (95.8) | 155 | 237 | 85.9 |
| South Central | 4 | 254 | 242 (95.3) | 128 | 114 | 64.0 | |
The study sample included 57/67 (85%) of the procedure or condition categories on the potentially planned list. The most common procedure CCS categories among planned readmissions (v2.1) in the validation cohort were very similar to those in the national dataset (see Supporting Information, Appendix D, in the online version of this article). Of the top 20 most commonly planned procedure CCS categories in the validation set, all but 2, therapeutic radiology for cancer treatment (CCS 211) and peripheral vascular bypass (CCS 55), were among the top 20 most commonly planned procedure CCS categories in the national data.
Test Characteristics of Algorithm
The weighted test characteristics of the current algorithm (v2.1) are shown in Table 2. Overall, the algorithm correctly identified 266 readmissions as unplanned and 181 readmissions as planned, and misidentified 170 readmissions as planned and 15 as unplanned. Once weighted to account for the stratified sampling design, the overall prevalence of true planned readmissions was 8.9% of readmissions. The weighted sensitivity was 45.1% overall and was higher in large teaching centers than in smaller community hospitals. The weighted specificity was 95.9%. The positive predictive value was 51.6%, and the negative predictive value was 94.7%.
| Cohort | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| Algorithm v2.1 | ||||
| Full cohort | 45.1% | 95.9% | 51.6% | 94.7% |
| Large hospitals | 50.9% | 96.1% | 53.8% | 95.6% |
| Small hospitals | 40.2% | 95.5% | 47.7% | 94.0% |
| Revised algorithm v3.0 | ||||
| Full cohort | 49.8% | 96.5% | 58.7% | 94.5% |
| Large hospitals | 57.1% | 96.8% | 63.0% | 95.9% |
| Small hospitals | 42.6% | 95.9% | 52.6% | 93.9% |
Accuracy of Individual Diagnoses and Procedures
The positive predictive value of the algorithm for individual procedure categories varied widely, from 0% to 100% among procedures with at least 10 cases (Table 3). The procedure for which the algorithm was least accurate was CCS 211, therapeutic radiology for cancer treatment (0% positive predictive value). By contrast, maintenance chemotherapy (90%) and other therapeutic procedures, hemic and lymphatic system (100%) were most accurate. Common procedures with less than 50% positive predictive value (ie, that the algorithm commonly misclassified as planned) were diagnostic cardiac catheterization (25%); debridement of wound, infection, or burn (25%); amputation of lower extremity (29%); insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (33%); and other hernia repair (43%). Of these, diagnostic cardiac catheterization and cardiac devices are the first and second most common procedures nationally, respectively.
| Readmission Procedure CCS Code | Total Categorized as Planned by Algorithm, N | Verified as Planned by Chart Review, N | Positive Predictive Value |
|---|---|---|---|
| |||
| 47 Diagnostic cardiac catheterization; coronary arteriography | 44 | 11 | 25% |
| 224 Cancer chemotherapy | 40 | 22 | 55% |
| 157 Amputation of lower extremity | 31 | 9 | 29% |
| 49 Other operating room heart procedures | 27 | 16 | 59% |
| 48 Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator | 24 | 8 | 33% |
| 43 Heart valve procedures | 20 | 16 | 80% |
| Maintenance chemotherapy (diagnosis CCS 45) | 20 | 18 | 90% |
| 78 Colorectal resection | 18 | 9 | 50% |
| 169 Debridement of wound, infection or burn | 16 | 4 | 25% |
| 84 Cholecystectomy and common duct exploration | 16 | 5 | 31% |
| 99 Other OR gastrointestinal therapeutic procedures | 16 | 8 | 50% |
| 158 Spinal fusion | 15 | 11 | 73% |
| 142 Partial excision bone | 14 | 10 | 71% |
| 86 Other hernia repair | 14 | 6 | 42% |
| 44 Coronary artery bypass graft | 13 | 10 | 77% |
| 67 Other therapeutic procedures, hemic and lymphatic system | 13 | 13 | 100% |
| 211 Therapeutic radiology for cancer treatment | 12 | 0 | 0% |
| 45 Percutaneous transluminal coronary angioplasty | 11 | 7 | 64% |
| Total | 497 | 272 | 54.7% |
The readmissions with least abstractor agreement were those involving CCS 157 (amputation of lower extremity) and CCS 169 (debridement of wound, infection or burn). Readmissions for these procedures were nearly always performed as a consequence of acute worsening of chronic conditions such as osteomyelitis or ulceration. Abstractors were divided over whether these readmissions were appropriate to call planned.
Changes to the Algorithm
We determined that the accuracy of the algorithm would be improved by removing 2 procedure categories from the planned procedure list (therapeutic radiation [CCS 211] and cancer chemotherapy [CCS 224]), adding 1 diagnosis category to the acute diagnosis list (hypertension with complications [CCS 99]), and splitting 2 diagnosis condition categories into acute and nonacute ICD‐9‐CM codes (pancreatic disorders [CCS 149] and biliary tract disease [CCS 152]). Detailed rationales for each modification to the planned readmission algorithm are described in Table 4. We felt further examination of diagnostic cardiac catheterization and cardiac devices was warranted given their high frequency, despite low positive predictive value. We also elected not to alter the categorization of amputation or debridement because it was not easy to determine whether these admissions were planned or unplanned even with chart review. We plan further analyses of these procedure categories.
| Action | Diagnosis or Procedure Category | Algorithm | Chart | N | Rationale for Change |
|---|---|---|---|---|---|
| |||||
| Remove from planned procedure list | Therapeutic radiation (CCS 211) | Accurate | The algorithm was inaccurate in every case. All therapeutic radiology during readmissions was performed because of acute illness (pain crisis, neurologic crisis) or because scheduled treatment occurred during an unplanned readmission. In national data, this ranks as the 25th most common planned procedure identified by the algorithm v2.1. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Cancer chemotherapy (CCS 224) | Accurate | Of the 22 correctly identified as planned, 18 (82%) would already have been categorized as planned because of a principal diagnosis of maintenance chemotherapy. Therefore, removing CCS 224 from the planned procedure list would only miss a small fraction of planned readmissions but would avoid a large number of misclassifications. In national data, this ranks as the 8th most common planned procedure identified by the algorithm v2.1. | |||
| Planned | Planned | 22 | |||
| Unplanned | Unplanned | 0 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 18 | |||
| Add to planned procedure list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. A handful of these cases were missed because the planned procedure was not on the current planned procedure list; however, those procedures (eg, abdominal paracentesis, colonoscopy, endoscopy) were nearly always unplanned overall and should therefore not be added as procedures that potentially qualify as an admission as planned. | |||
| Remove from acute diagnosis list | None | The abstractors felt a planned readmission was missed by the algorithm in 15 cases. The relevant disqualifying acute diagnoses were much more often associated with unplanned readmissions in our dataset. | |||
| Add to acute diagnosis list | Hypertension with complications (CCS 99) | Accurate | This CCS was associated with only 1 planned readmission (for elective nephrectomy, a very rare procedure). Every other time this CCS appeared in the dataset, it was associated with an unplanned readmission (12/13, 92%); 10 of those, however, were misclassified by the algorithm as planned because they were not excluded by diagnosis (91% error rate). Consequently, adding this CCS to the acute diagnosis list is likely to miss only a very small fraction of planned readmissions, while making the overall algorithm much more accurate. | ||
| Planned | Planned | 1 | |||
| Unplanned | Unplanned | 2 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 10 | |||
| Split diagnosis condition category into component ICD‐9 codes | Pancreatic disorders (CCS 152) | Accurate | ICD‐9 code 577.0 (acute pancreatitis) is the only acute code in this CCS. Acute pancreatitis was present in 2 cases that were misclassified as planned. Clinically, there is no situation in which a planned procedure would reasonably be performed in the setting of acute pancreatitis. Moving ICD‐9 code 577.0 to the acute list and leaving the rest of the ICD‐9 codes in CCS 152 on the nonacute list will enable the algorithm to continue to identify planned procedures for chronic pancreatitis. | ||
| Planned | Planned | 0 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 2 | |||
| Biliary tract disease (CCS 149) | Accurate | This CCS is a mix of acute and chronic diagnoses. Of 14 charts classified as planned with CCS 149 in the principal diagnosis field, 12 were misclassified (of which 10 were associated with cholecystectomy). Separating out the acute and nonacute diagnoses will increase the accuracy of the algorithm while still ensuring that planned cholecystectomies and other procedures can be identified. Of the ICD‐9 codes in CCS 149, the following will be added to the acute diagnosis list: 574.0, 574.3, 574.6, 574.8, 575.0, 575.12, 576.1. | |||
| Planned | Planned | 2 | |||
| Unplanned | Unplanned | 3 | |||
| Inaccurate | |||||
| Unplanned | Planned | 0 | |||
| Planned | Unplanned | 12 | |||
| Consider for change after additional study | Diagnostic cardiac catheterization (CCS 47) | Accurate | The algorithm misclassified as planned 25/38 (66%) unplanned readmissions in which diagnostic catheterizations were the only qualifying planned procedure. It also correctly identified 3/3 (100%) planned readmissions in which diagnostic cardiac catheterizations were the only qualifying planned procedure. This is the highest volume procedure in national data. | ||
| Planned | Planned | 3* | |||
| Unplanned | Unplanned | 13* | |||
| Inaccurate | |||||
| Unplanned | Planned | 0* | |||
| Planned | Unplanned | 25* | |||
| Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator (CCS 48) | Accurate | The algorithm misclassified as planned 4/5 (80%) unplanned readmissions in which cardiac devices were the only qualifying procedure. However, it also correctly identified 7/8 (87.5%) planned readmissions in which cardiac devices were the only qualifying planned procedure. CCS 48 is the second most common planned procedure category nationally. | |||
| Planned | Planned | 7 | |||
| Unplanned | Unplanned | 1 | |||
| Inaccurate | |||||
| Unplanned | Planned | 1 | |||
| Planned | Unplanned | 4 | |||
The revised algorithm (v3.0) had a weighted sensitivity of 49.8%, weighted specificity of 96.5%, positive predictive value of 58.7%, and negative predictive value of 94.5% (Table 2). In aggregate, these changes would increase the reported unplanned readmission rate from 16.0% to 16.1% in the hospital‐wide readmission measure, using 2011 to 2012 data, and would decrease the fraction of all readmissions considered planned from 7.8% to 7.2%.
DISCUSSION
We developed an algorithm based on administrative data that in its currently implemented form is very accurate at identifying unplanned readmissions, ensuring that readmissions included in publicly reported readmission measures are likely to be truly unplanned. However, nearly half of readmissions the algorithm classifies as planned are actually unplanned. That is, the algorithm is overcautious in excluding unplanned readmissions that could have counted as outcomes, particularly among admissions that include diagnostic cardiac catheterization or placement of cardiac devices (pacemakers, defibrillators). However, these errors only occur within the 7.8% of readmissions that are classified as planned and therefore do not affect overall readmission rates dramatically. A perfect algorithm would reclassify approximately half of these planned readmissions as unplanned, increasing the overall readmission rate by 0.6 percentage points.
On the other hand, the algorithm also only identifies approximately half of true planned readmissions as planned. Because the true prevalence of planned readmissions is low (approximately 9% of readmissions based on weighted chart review prevalence, or an absolute rate of 1.4%), this low sensitivity has a small effect on algorithm performance. Removing all true planned readmissions from the measure outcome would decrease the overall readmission rate by 0.8 percentage points, similar to the expected 0.6 percentage point increase that would result from better identifying unplanned readmissions; thus, a perfect algorithm would likely decrease the reported unplanned readmission rate by a net 0.2%. Overall, the existing algorithm appears to come close to the true prevalence of planned readmissions, despite inaccuracy on an individual‐case basis. The algorithm performed best at large hospitals, which are at greatest risk of being statistical outliers and of accruing penalties under the Hospital Readmissions Reduction Program.[10]
We identified several changes that marginally improved the performance of the algorithm by reducing the number of unplanned readmissions that are incorrectly removed from the measure, while avoiding the inappropriate inclusion of planned readmissions in the outcome. This revised algorithm, v3.0, was applied to public reporting of readmission rates at the end of 2014. Overall, implementing these changes increases the reported readmission rate very slightly. We also identified other procedures associated with high inaccuracy rates, removal of which would have larger impact on reporting rates, and which therefore merit further evaluation.
There are other potential methods of identifying planned readmissions. For instance, as of October 1, 2013, new administrative billing codes were created to allow hospitals to indicate that a patient was discharged with a planned acute‐care hospital inpatient readmission, without limitation as to when it will take place.[11] This code must be used at the time of the index admission to indicate that a future planned admission is expected, and was specified only to be used for neonates and patients with acute myocardial infarction. This approach, however, would omit planned readmissions that are not known to the initial discharging team, potentially missing planned readmissions. Conversely, some patients discharged with a plan for readmission may be unexpectedly readmitted for an unplanned reason. Given that the new codes were not available at the time we conducted the validation study, we were not able to determine how often the billing codes accurately identified planned readmissions. This would be an important area to consider for future study.
An alternative approach would be to create indicator codes to be applied at the time of readmission that would indicate whether that admission was planned or unplanned. Such a code would have the advantage of allowing each planned readmission to be flagged by the admitting clinicians at the time of admission rather than by an algorithm that inherently cannot be perfect. However, identifying planned readmissions at the time of readmission would also create opportunity for gaming and inconsistent application of definitions between hospitals; additional checks would need to be put in place to guard against these possibilities.
Our study has some limitations. We relied on the opinion of chart abstractors to determine whether a readmission was planned or unplanned; in a few cases, such as smoldering wounds that ultimately require surgical intervention, that determination is debatable. Abstractions were done at local institutions to minimize risks to patient privacy, and therefore we could not centrally verify determinations of planned status except by reviewing source of admission, dates of procedures, and narrative comments reported by the abstractors. Finally, we did not have sufficient volume of planned procedures to determine accuracy of the algorithm for less common procedure categories or individual procedures within categories.
In summary, we developed an algorithm to identify planned readmissions from administrative data that had high specificity and moderate sensitivity, and refined it based on chart validation. This algorithm is in use in public reporting of readmission measures to maximize the probability that the reported readmission rates represent truly unplanned readmissions.[12]
Disclosures: Financial supportThis work was performed under contract HHSM‐500‐2008‐0025I/HHSM‐500‐T0001, Modification No. 000008, titled Measure Instrument Development and Support, funded by the Centers for Medicare and Medicaid Services (CMS), an agency of the US Department of Health and Human Services. Drs. Horwitz and Ross are supported by the National Institute on Aging (K08 AG038336 and K08 AG032886, respectively) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. Dr. Krumholz is supported by grant U01 HL105270‐05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; or in the writing of the article. The CMS reviewed and approved the use of its data for this work and approved submission of the manuscript. All authors have completed the Unified Competing Interest form at
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. An administrative claims measure suitable for profiling hospital performance based on 30‐day all‐cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243–252.
- , , , et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30‐day all‐cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37.
- , , , et al. Hospital‐level 30‐day all‐cause risk‐standardized readmission rate following elective primary total hip arthroplasty (THA) and/or total knee arthroplasty (TKA). Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page161(supp10 l):S66–S75.
- , , A meta‐analysis of hospital 30‐day avoidable readmission rates. J Eval Clin Pract. 2011;18(6):1211–1218.
- , , , , Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Centers for Medicare 3(4):477–492.
- , Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- Centers for Medicare and Medicaid Services. Inpatient Prospective Payment System/Long‐Term Care Hospital (IPPS/LTCH) final rule. Fed Regist. 2013;78:50533–50534.
- , , Massachusetts health reforms: uninsurance remains low, self‐reported health status improves as state prepares to tackle costs. Health Aff (Millwood). 2012;31(2):444–451.
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. An administrative claims measure suitable for profiling hospital performance based on 30‐day all‐cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243–252.
- , , , et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30‐day all‐cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37.
- , , , et al. Hospital‐level 30‐day all‐cause risk‐standardized readmission rate following elective primary total hip arthroplasty (THA) and/or total knee arthroplasty (TKA). Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page161(supp10 l):S66–S75.
- , , A meta‐analysis of hospital 30‐day avoidable readmission rates. J Eval Clin Pract. 2011;18(6):1211–1218.
- , , , , Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Centers for Medicare 3(4):477–492.
- , Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- Centers for Medicare and Medicaid Services. Inpatient Prospective Payment System/Long‐Term Care Hospital (IPPS/LTCH) final rule. Fed Regist. 2013;78:50533–50534.
- , , Massachusetts health reforms: uninsurance remains low, self‐reported health status improves as state prepares to tackle costs. Health Aff (Millwood). 2012;31(2):444–451.
© 2015 Society of Hospital Medicine
Manage Your Dermatology Practice: Offering a Mix of Treatments for Acne and Rosacea
Combination therapy may be essential to treat acne and rosacea and minimize any negative aftereffects. Dr. Gary Goldenberg discusses the options for combination therapy including topical and oral prescription therapies as well as procedures and over-the-counter therapy. Patients also should be counseled on minimizing triggers.
Combination therapy may be essential to treat acne and rosacea and minimize any negative aftereffects. Dr. Gary Goldenberg discusses the options for combination therapy including topical and oral prescription therapies as well as procedures and over-the-counter therapy. Patients also should be counseled on minimizing triggers.
Combination therapy may be essential to treat acne and rosacea and minimize any negative aftereffects. Dr. Gary Goldenberg discusses the options for combination therapy including topical and oral prescription therapies as well as procedures and over-the-counter therapy. Patients also should be counseled on minimizing triggers.