User login
Catching up on the latest USPSTF recommendations
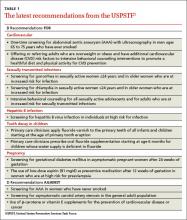
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
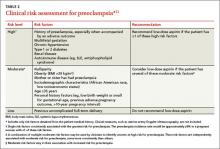
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
Epithelial Ovarian Cancer: Management of Advanced Disease
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Epithelial ovarian cancer is the fifth leading cause of cancer death among women in the United States. Most women with ovarian cancer present at an advanced stage (International Federation of Gynecology and Obstetrics stage III), for which the standard treatment remains cytoreductive surgery followed by platinum- and taxane-based combination chemotherapy. Although this treatment frequently is curative for patients with early-stage disease, more than 60% of women with advanced disease will develop recurrent disease with progressively shorter disease-free intervals. However, there are many clinical trials in progress that are aimed at refining current therapy and evaluating different approaches to postoperative therapy, with the goal of improving prognosis and quality of life.
To read the full article in PDF:
Mind the Gap: Case Study in Toxicology
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
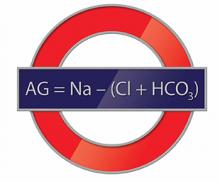
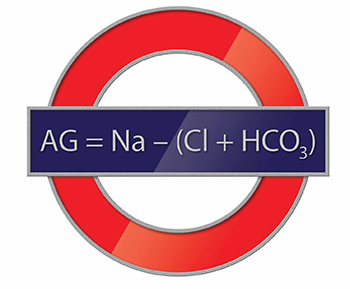
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Malpractice Counsel
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Sepsis Following Vaginal Hysterectomy
| A 45-year-old woman presented to the ED complaining of lower abdominal pain, which she described as gradual, aching, and intermittent. The patient stated that she had undergone a vaginal hysterectomy a few days prior and that the pain started less than 24 hours after discharge from the hospital. She denied fever or chills, nausea, or vomiting, and said that she had a bowel movement earlier that day. She also denied any urinary symptoms. Her medical history was significant only for hypothyroidism, for which she was taking levothyroxine. The patient denied cigarette smoking or alcohol consumption. She said she had been taking acetaminophen-hydrocodone for postoperative pain, but that it did not provide any relief. |
The patient’s vital signs were: temperature, 98.6˚F; blood pressure, 112/65 mm Hg; heart rate, 98 beats/minute; and respiratory rate, 20 breaths/minute. The head, eyes, ears, nose, and throat examination was normal, as were the heart and lung examinations. The patient’s abdomen was soft, with mild diffuse lower abdominal tenderness. There was no guarding, rebound, or mass present. A gross nonspeculum examination of the vaginal area did not reveal any discharge or erythema; a rectal examination was not performed.
The EP ordered a complete blood count (CBC), lipase evaluation, and urinalysis. All test results were normal. The emergency physician (EP) then contacted the obstetrician-gynecologist (OB/GYN) who had performed the hysterectomy. The OB/GYN recommended the EP change the analgesic agent to acetaminophen-oxycodone and to encourage the patient to keep her follow-up postoperative appointment in 1 week. The EP followed these instructions and discharged the patient home with a prescription for the new analgesic.
Three days later, however, the patient presented back to the same ED complaining of increased and now generalized abdominal pain, nausea, and vomiting. She was noted to be febrile, tachycardic, and hypotensive. On physical examination, her abdomen was diffusely tender with guarding and rebound. She was given a 2-L bolus of intravenous (IV) normal saline and started on broad spectrum IV antibiotics. After another consultation with the patient’s OB/GYN surgeon, the patient was taken immediately to the operating room. On exploration, she was found to have a segment of perforated bowel and peritonitis. A portion of the bowel was resected, but her postoperative course was complicated by sepsis. After a 1-month stay in the hospital, she was discharged home.
The patient sued the EP—but not her OB/GYN—for failure to obtain a CT scan of the abdomen/pelvis on her initial ED visit, or at least to admit her to the hospital for observation. The EP argued that even if a computed tomography (CT) scan had been performed on the initial visit, it probably would have been normal, since the bowel had not yet perforated. After trial, a defense verdict was returned.
Discussion
This case illustrates two important points. First, not every patient with abdominal pain requires a CT scan of the abdomen/pelvis. So many malpractice cases against EPs involve the failure to perform advanced imaging. Unfortunately, that is usually only through the benefit of hindsight. For a patient with mild abdominal pain, only minimal tenderness on examination, and a negative laboratory workup, it can be perfectly appropriate to treat him or her symptomatically with close follow-up and specific instructions to return to the ED if his or her condition worsens (as was the case with this patient).
The second important point is to not over-rely on a consultant(s), especially if she or he has not independently examined the patient. When calling a consultant, it is best to have a specific question (ie, “Can you see the patient in the morning?”) or action (ie, “I would like to admit the patient to your service”). In general, the EP should not rely on the consultant to give “permission” to discharge the patient. As the physician seeing the patient, the EP is the most well-equipped to work up the patient and determine the needed disposition. Rare is the consultant that can arrive at a better disposition than the EP who performed the history and physical examination on the patient.
Regarding the patient’s GYN surgery, vaginal hysterectomy (VH) is preferred over abdominal hysterectomy (AH) for benign disease as it is associated with reduced infective morbidity and earlier return to normal activities.1 With respect to postoperative events, clinicians typically employ the Clavien-Dindo grading system for the classification of surgical complications.2 The system consists of five grades, ranging from Grade I (any deviation from normal postoperative course, without the need for pharmacological intervention) to Grade V (death).
Following hysterectomy, postoperative urinary or pelvic infections are not uncommon, with an incidence of 15% to 20%.1 In the Clavien-Dindo system, these complications would typically be considered Grade II (pharmacological treatment other than what is considered an acceptable therapeutic regimen), requiring antibiotics and no surgical intervention. Grade III complications, however, usually involve postoperative issues that require surgical, endoscopic, or radiological intervention, which in VH would include ureteral, bladder, or bowel injury.1 In a study by Gendy et al,1 the incidence of such complications posthysterectomy, ranged from 1.7% to 5.7%. So while not extremely common, serious complications can occur postoperatively.
The last point is a minor one, but a truth every EP needs to remember: While it may be difficult for a patient to sue her or his own physician, especially one with whom she or he has a longstanding patient-physician relationship, it is much easier for her or him to place blame upon and sue another physician—for example, the EP.
Missed Testicular Torsion?
| A 14-year-old boy presented to the ED with a several day history of abdominal pain with radiation to the right testicle. The patient denied any nausea, vomiting, or changes in bowel habits. He also denied any genitourinary symptoms, including dysuria or urinary frequency. The boy was otherwise in good health, on no medications, and up to date on his immunizations. |
The patient was a well appearing teenager in no acute distress. All vital signs were normal, as were the heart and lung examinations. The abdominal examination revealed mild, generalized tenderness without guarding or rebound. The genitalia examination was normal.
The EP ordered a CBC, urinalysis, and a testicular ultrasound, the results of which were all normal. The patient was discharged home with instructions to follow up with his pediatrician in 2 days and to return to the ED if his symptoms worsened.
The patient was seen by his pediatrician approximately 1 month later for his scheduled annual physical examination. The pediatrician, who was aware of the boy’s prior ED visit, found the patient in good health, and performed no additional testing.
Approximately 9 months after the initial ED visit, the patient was accidently kicked in the groin while jumping on a trampoline. He experienced immediate onset of severe, excruciating right testicular pain and presented to the ED approximately 24 hours later with continued pain and swelling. A testicular ultrasound was immediately ordered and demonstrated an enlarged right testicle due to torsion.
The patient underwent surgery to remove the right testicle. His family sued the EP and hospital from the initial visit (9 months earlier) for missed intermittent testicular torsion. They argued that the patient should have been referred to a urologist for further evaluation. In addition, the plaintiff claimed he could no longer participate in sports and suffered disfigurement as a result of the surgery. The EP asserted that the patient’s pain during that initial visit was primarily abdominal in nature and that an ultrasound of the testicles was normal, and did not reveal any evidence of testicular torsion. The EP further argued that the testicular torsion was due to the trauma incurred on the trampoline. According to published accounts, a defense verdict was returned.
Discussion
Testicular torsion occurs in a bimodal age distribution—during the first year of life (perinatal) and between ages 13 and 16 years (as was the case with this patient).1 In approximately 4% to 8% of patients, there is a history of an athletic event, strenuous physical activity, or trauma just prior to the onset of scrotal pain.2
Patients typically present with sudden onset of testicular pain that is frequently associated with nausea and vomiting. However, this condition can present with only lower abdominal pain—in part be due to the fact that adolescents and children may be reluctant to complain of testicular or scrotal pain out of fear or embarrassment.1 In all cases, a genital examination should be performed on every adolescent male with a chief complaint of lower abdominal pain.3
On physical examination, the patient will usually have a swollen tender testicle. In comparison to the opposite side, the affected testicle is frequently raised and rests on a horizontal axis. The cremasteric reflex (ie, scratching the proximal inner thigh causes the ipsilateral testicle to rise) is frequently absent.4
Because of the time sensitive nature of the disease process, in classic presentations, a urologist should be immediately consulted. Ischemic changes to the testicle can begin within hours, and complete testicular atrophy occurs after 24 hours in most cases.4 Detorsion within 6 hours of onset of symptoms has a salvage rate of 90% to 100%, which drops to 25% to 50% after 12 hours and to less than 10% after 24 hours.4
For less obvious cases, color duplex testicular ultrasonography can be very helpful. Demonstration of decreased or absent blood flow is diagnostic and requires operative intervention. If untwisting the testis restores blood flow, then the condition is resolved; if this procedure fails, the testis is removed. Regardless of the outcome, the contralateral testis is fixed to prevent future torsion.
Intermittent testicular torsion is a difficult diagnosis to make. A history of recurrent unilateral scrotal pain is highly suspicious and warrants referral to a urologist. This patient had only one previous episode, which was primarily abdominal pain—not scrotal or testicular pain.
In this case, it appears the jury came to the correct decision. Given the patient had only one previous episode of abdominal pain, and an inciting event (trauma to the testicle) on the second presentation, this does not appear to be a case of missed intermittent testicular torsion. Rather, this was a correctly diagnosed testicular torsion with a delayed presentation, resulting in an unsalvageable testicle.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Reference - Sepsis Following Vaginal Hysterectomy
- Gendy R, Walsh CA, Walsh SR, Karantanis E. Vaginal hysterectomy versus total laparoscopic hysterectomy for benign disease: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204(5):388.e1-8.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009:250(2):187-196.
Reference - Missed Testicular Torsion?
- Pogorelić Z, Mrklić I, Jurić I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J Pediatr Urol. 2013;9(6 Pt B):1161-1165.
- Nicks BA, Manthey DE. Male genital problems. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cine DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill Medical; 2011:649.
- Lopez RN, Beasley SW. Testicular torsion: potential pitfalls in its diagnosis and management. J Paediatr Child Health. 2012;48(2):E30-E32.
- Somani BK, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213.
Memory problems: How best to assess and address
Targeting receptors to better treat AML
Photo courtesy of UT
Southwestern Medical Center
Preclinical research suggests that certain receptors containing the immunoreceptor tyrosine-based inhibition motif (ITIM) are important for the development of acute myeloid leukemia (AML).
“Although counterintuitive, this result is consistent with the generally immune-suppressive and, thus, tumor-promoting roles of inhibitory receptors in the immune system,” said Chengcheng Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“These findings suggest that blocking ITIM-receptor signaling in combination with conventional therapies may represent a novel strategy for AML treatment.”
Dr Zhang and his colleagues reported their findings in Nature Cell Biology.
The team focused mainly on an ITIM-containing receptor called LAIR1. They found that deleting LAIR1 abolished leukemia in several different mouse models, without affecting normal hematopoiesis.
The investigators also identified a pathway that sustains the survival and self-renewal of AML cells, the mechanism by which LAIR1 supports AML development.
They said LAIR1 induces activation of SHP-1, which acts as a phosphatase-independent signaling adaptor to recruit CAMK1 for activation of downstream CREB in AML cells. And the LAIR1–SHP-1–CAMK1–CREB pathway sustains AML stem cells.
So the investigators believe that inhibiting the signaling initiated by LAIR1 and other ITIM-containing receptors could help us treat AML more effectively.
“Our study suggests that current treatment options, including chemotherapy, may not efficiently target cancer stem cells because these inhibitory receptors enable the leukemia stem cells to survive conventional therapies, eventually resulting in tumor relapse,” Dr Zhang said.
“The blockade of ITIM-receptor signaling may prove to be a novel, effective strategy for elimination of leukemia stem cells and lead to complete remission in patients.” ![]()

Photo courtesy of UT
Southwestern Medical Center
Preclinical research suggests that certain receptors containing the immunoreceptor tyrosine-based inhibition motif (ITIM) are important for the development of acute myeloid leukemia (AML).
“Although counterintuitive, this result is consistent with the generally immune-suppressive and, thus, tumor-promoting roles of inhibitory receptors in the immune system,” said Chengcheng Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“These findings suggest that blocking ITIM-receptor signaling in combination with conventional therapies may represent a novel strategy for AML treatment.”
Dr Zhang and his colleagues reported their findings in Nature Cell Biology.
The team focused mainly on an ITIM-containing receptor called LAIR1. They found that deleting LAIR1 abolished leukemia in several different mouse models, without affecting normal hematopoiesis.
The investigators also identified a pathway that sustains the survival and self-renewal of AML cells, the mechanism by which LAIR1 supports AML development.
They said LAIR1 induces activation of SHP-1, which acts as a phosphatase-independent signaling adaptor to recruit CAMK1 for activation of downstream CREB in AML cells. And the LAIR1–SHP-1–CAMK1–CREB pathway sustains AML stem cells.
So the investigators believe that inhibiting the signaling initiated by LAIR1 and other ITIM-containing receptors could help us treat AML more effectively.
“Our study suggests that current treatment options, including chemotherapy, may not efficiently target cancer stem cells because these inhibitory receptors enable the leukemia stem cells to survive conventional therapies, eventually resulting in tumor relapse,” Dr Zhang said.
“The blockade of ITIM-receptor signaling may prove to be a novel, effective strategy for elimination of leukemia stem cells and lead to complete remission in patients.” ![]()

Photo courtesy of UT
Southwestern Medical Center
Preclinical research suggests that certain receptors containing the immunoreceptor tyrosine-based inhibition motif (ITIM) are important for the development of acute myeloid leukemia (AML).
“Although counterintuitive, this result is consistent with the generally immune-suppressive and, thus, tumor-promoting roles of inhibitory receptors in the immune system,” said Chengcheng Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“These findings suggest that blocking ITIM-receptor signaling in combination with conventional therapies may represent a novel strategy for AML treatment.”
Dr Zhang and his colleagues reported their findings in Nature Cell Biology.
The team focused mainly on an ITIM-containing receptor called LAIR1. They found that deleting LAIR1 abolished leukemia in several different mouse models, without affecting normal hematopoiesis.
The investigators also identified a pathway that sustains the survival and self-renewal of AML cells, the mechanism by which LAIR1 supports AML development.
They said LAIR1 induces activation of SHP-1, which acts as a phosphatase-independent signaling adaptor to recruit CAMK1 for activation of downstream CREB in AML cells. And the LAIR1–SHP-1–CAMK1–CREB pathway sustains AML stem cells.
So the investigators believe that inhibiting the signaling initiated by LAIR1 and other ITIM-containing receptors could help us treat AML more effectively.
“Our study suggests that current treatment options, including chemotherapy, may not efficiently target cancer stem cells because these inhibitory receptors enable the leukemia stem cells to survive conventional therapies, eventually resulting in tumor relapse,” Dr Zhang said.
“The blockade of ITIM-receptor signaling may prove to be a novel, effective strategy for elimination of leukemia stem cells and lead to complete remission in patients.” ![]()
Vigorous physical activity may lower risk of NHL

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()
Symptoms confer higher-than-expected risk of HL, NHL
Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()

Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()

Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()
Artificial blood vessels give way to the real thing

biocompatible polymer
Photo courtesy of Vienna
University of Technology
Scientists have created implantable artificial blood vessels using a newly developed polymer that is biodegradable.
In experiments with rats, these thin-walled vascular grafts were replaced by endogenous material, ultimately leaving natural, fully functional blood vessels in their place.
Furthermore, 6 months after the artificial vessels were implanted, none of the animals had experienced thromboses, inflammation, or aneurysms.
Helga Bergmeister, MD, DVM, PhD, of the Medical University of Vienna in Austria, and her colleagues conducted this research and described the results in Acta Biomaterialia.
To create artificial blood vessels that are compatible with body tissue, the researchers developed a new polymer—thermoplastic polyurethane.
“By selecting very specific molecular building blocks, we have succeeded in synthesizing a polymer with the desired properties,” said Robert Liska, PhD, of the Vienna University of Technology.
To produce the grafts, the researchers spun polymer solutions in an electrical field to form very fine threads and wound these threads onto a spool.
“The wall of these artificial blood vessels is very similar to that of natural ones,” said Heinrich Schima, PhD, of the Medical University of Vienna.
The polymer fabric is slightly porous. So, initially, it allows a small amount of blood to seep through, which enriches the wall with growth factors. And this encourages the migration of endogenous cells.
The researchers implanted these artificial blood vessels in rats and found them to be safe and functional long-term.
“The rats’ blood vessels were examined 6 months after insertion of the vascular prostheses,” Dr Bergmeister said.
“We did not find any aneurysms, thrombosis, or inflammation. Endogenous cells had colonized the vascular prostheses and turned the artificial constructs into natural body tissue.”
In fact, natural body tissue regrew much faster than expected.
The researchers said their thin-walled grafts “offer a new and desirable form of biodegradable vascular implant.” ![]()

biocompatible polymer
Photo courtesy of Vienna
University of Technology
Scientists have created implantable artificial blood vessels using a newly developed polymer that is biodegradable.
In experiments with rats, these thin-walled vascular grafts were replaced by endogenous material, ultimately leaving natural, fully functional blood vessels in their place.
Furthermore, 6 months after the artificial vessels were implanted, none of the animals had experienced thromboses, inflammation, or aneurysms.
Helga Bergmeister, MD, DVM, PhD, of the Medical University of Vienna in Austria, and her colleagues conducted this research and described the results in Acta Biomaterialia.
To create artificial blood vessels that are compatible with body tissue, the researchers developed a new polymer—thermoplastic polyurethane.
“By selecting very specific molecular building blocks, we have succeeded in synthesizing a polymer with the desired properties,” said Robert Liska, PhD, of the Vienna University of Technology.
To produce the grafts, the researchers spun polymer solutions in an electrical field to form very fine threads and wound these threads onto a spool.
“The wall of these artificial blood vessels is very similar to that of natural ones,” said Heinrich Schima, PhD, of the Medical University of Vienna.
The polymer fabric is slightly porous. So, initially, it allows a small amount of blood to seep through, which enriches the wall with growth factors. And this encourages the migration of endogenous cells.
The researchers implanted these artificial blood vessels in rats and found them to be safe and functional long-term.
“The rats’ blood vessels were examined 6 months after insertion of the vascular prostheses,” Dr Bergmeister said.
“We did not find any aneurysms, thrombosis, or inflammation. Endogenous cells had colonized the vascular prostheses and turned the artificial constructs into natural body tissue.”
In fact, natural body tissue regrew much faster than expected.
The researchers said their thin-walled grafts “offer a new and desirable form of biodegradable vascular implant.” ![]()

biocompatible polymer
Photo courtesy of Vienna
University of Technology
Scientists have created implantable artificial blood vessels using a newly developed polymer that is biodegradable.
In experiments with rats, these thin-walled vascular grafts were replaced by endogenous material, ultimately leaving natural, fully functional blood vessels in their place.
Furthermore, 6 months after the artificial vessels were implanted, none of the animals had experienced thromboses, inflammation, or aneurysms.
Helga Bergmeister, MD, DVM, PhD, of the Medical University of Vienna in Austria, and her colleagues conducted this research and described the results in Acta Biomaterialia.
To create artificial blood vessels that are compatible with body tissue, the researchers developed a new polymer—thermoplastic polyurethane.
“By selecting very specific molecular building blocks, we have succeeded in synthesizing a polymer with the desired properties,” said Robert Liska, PhD, of the Vienna University of Technology.
To produce the grafts, the researchers spun polymer solutions in an electrical field to form very fine threads and wound these threads onto a spool.
“The wall of these artificial blood vessels is very similar to that of natural ones,” said Heinrich Schima, PhD, of the Medical University of Vienna.
The polymer fabric is slightly porous. So, initially, it allows a small amount of blood to seep through, which enriches the wall with growth factors. And this encourages the migration of endogenous cells.
The researchers implanted these artificial blood vessels in rats and found them to be safe and functional long-term.
“The rats’ blood vessels were examined 6 months after insertion of the vascular prostheses,” Dr Bergmeister said.
“We did not find any aneurysms, thrombosis, or inflammation. Endogenous cells had colonized the vascular prostheses and turned the artificial constructs into natural body tissue.”
In fact, natural body tissue regrew much faster than expected.
The researchers said their thin-walled grafts “offer a new and desirable form of biodegradable vascular implant.” ![]()
Acid exposure time found most useful in pH-impedance testing
pH-impedance testing best predicted response to reflux treatment when patients were off proton pump inhibitors, and abnormal acid exposure time was the single most useful testing parameter, a prospective study has found.
“Impedance-based reflux parameters complement but do not replace acid-based parameters in predicting symptom outcome from both medical and surgical antireflux therapy,” wrote Dr. Amit Patel and his associates at the Washington School of Medicine, St. Louis. The study appears in the May issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2014.08.02).
“Because abnormal acid reflux time and symptom-reflux correlation parameters are detected more often when testing is performed off therapy, pH-impedance testing off antisecretory therapy maximizes prediction of symptomatic outcome from GERD [gastroesophageal reflux disease] therapy,” the researchers wrote.
Clinicians continue to debate the role of pH-impedance testing in management of GERD, whether testing should be performed on or off antisecretory therapy, and which testing parameters are most useful for predicting treatment response, the investigators noted. Therefore, they followed 187 adults with persistent GERD symptoms who underwent pH-impedance testing at their center during a 5-year period. Average age of patients was 54 years, almost 71% were female, and none had histopathologic evidence of esophageal motor disorders. Almost half had been off proton pump inhibitors for 7 days when tested, and 68% were managed medically as opposed to surgically.
Global symptom assessment (GSS) and dominant symptom intensity (DSI) scores both improved significantly during an average of 40 months of follow-up, the researchers said. After the researchers controlled for demographics, symptoms at presentation, use of proton pump inhibitors (PPI), and parameters that predicted treatment response in the univariate analyses, only abnormal acid exposure time (AET) predicted linear improvement in DSI scores (P =.027), while abnormal AET (P =.002) and symptom association probability (P =.026) predicted significant improvements in linear and dichotomous GSS, they reported. Abnormal AET and being off PPIs during testing also more than doubled the odds of at least a 50% improvement in GSS.
In contrast, dichotomous reflux exposure time did not predict response in any of the analyses, said the investigators. “Established thresholds for the total number of reflux events did not predict linear or dichotomous global symptom severity improvement,” they added. “Our data therefore suggest that performing pH-impedance testing off PPI therapy increases the yield of abnormal AET and symptom-reflux association with reflux events, facilitating predicting value for symptom improvement with both medical and surgical antireflux therapy.”
The researchers could not corroborate patients’ compliance with antisecretory therapy, assess the reasons physicians chose medical or surgical management, or evaluate the impact of the placebo effect or other factors unrelated to reflux, they said.
The study was partly funded by the National Institute for Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the Washington University Department of Medicine Mentors in Medicine and Clinical Science Training and Research programs. The authors declared no relevant conflicts of interest.
pH-impedance testing best predicted response to reflux treatment when patients were off proton pump inhibitors, and abnormal acid exposure time was the single most useful testing parameter, a prospective study has found.
“Impedance-based reflux parameters complement but do not replace acid-based parameters in predicting symptom outcome from both medical and surgical antireflux therapy,” wrote Dr. Amit Patel and his associates at the Washington School of Medicine, St. Louis. The study appears in the May issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2014.08.02).
“Because abnormal acid reflux time and symptom-reflux correlation parameters are detected more often when testing is performed off therapy, pH-impedance testing off antisecretory therapy maximizes prediction of symptomatic outcome from GERD [gastroesophageal reflux disease] therapy,” the researchers wrote.
Clinicians continue to debate the role of pH-impedance testing in management of GERD, whether testing should be performed on or off antisecretory therapy, and which testing parameters are most useful for predicting treatment response, the investigators noted. Therefore, they followed 187 adults with persistent GERD symptoms who underwent pH-impedance testing at their center during a 5-year period. Average age of patients was 54 years, almost 71% were female, and none had histopathologic evidence of esophageal motor disorders. Almost half had been off proton pump inhibitors for 7 days when tested, and 68% were managed medically as opposed to surgically.
Global symptom assessment (GSS) and dominant symptom intensity (DSI) scores both improved significantly during an average of 40 months of follow-up, the researchers said. After the researchers controlled for demographics, symptoms at presentation, use of proton pump inhibitors (PPI), and parameters that predicted treatment response in the univariate analyses, only abnormal acid exposure time (AET) predicted linear improvement in DSI scores (P =.027), while abnormal AET (P =.002) and symptom association probability (P =.026) predicted significant improvements in linear and dichotomous GSS, they reported. Abnormal AET and being off PPIs during testing also more than doubled the odds of at least a 50% improvement in GSS.
In contrast, dichotomous reflux exposure time did not predict response in any of the analyses, said the investigators. “Established thresholds for the total number of reflux events did not predict linear or dichotomous global symptom severity improvement,” they added. “Our data therefore suggest that performing pH-impedance testing off PPI therapy increases the yield of abnormal AET and symptom-reflux association with reflux events, facilitating predicting value for symptom improvement with both medical and surgical antireflux therapy.”
The researchers could not corroborate patients’ compliance with antisecretory therapy, assess the reasons physicians chose medical or surgical management, or evaluate the impact of the placebo effect or other factors unrelated to reflux, they said.
The study was partly funded by the National Institute for Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the Washington University Department of Medicine Mentors in Medicine and Clinical Science Training and Research programs. The authors declared no relevant conflicts of interest.
pH-impedance testing best predicted response to reflux treatment when patients were off proton pump inhibitors, and abnormal acid exposure time was the single most useful testing parameter, a prospective study has found.
“Impedance-based reflux parameters complement but do not replace acid-based parameters in predicting symptom outcome from both medical and surgical antireflux therapy,” wrote Dr. Amit Patel and his associates at the Washington School of Medicine, St. Louis. The study appears in the May issue of Clinical Gastroenterology and Hepatology (doi:10.1016/j.cgh.2014.08.02).
“Because abnormal acid reflux time and symptom-reflux correlation parameters are detected more often when testing is performed off therapy, pH-impedance testing off antisecretory therapy maximizes prediction of symptomatic outcome from GERD [gastroesophageal reflux disease] therapy,” the researchers wrote.
Clinicians continue to debate the role of pH-impedance testing in management of GERD, whether testing should be performed on or off antisecretory therapy, and which testing parameters are most useful for predicting treatment response, the investigators noted. Therefore, they followed 187 adults with persistent GERD symptoms who underwent pH-impedance testing at their center during a 5-year period. Average age of patients was 54 years, almost 71% were female, and none had histopathologic evidence of esophageal motor disorders. Almost half had been off proton pump inhibitors for 7 days when tested, and 68% were managed medically as opposed to surgically.
Global symptom assessment (GSS) and dominant symptom intensity (DSI) scores both improved significantly during an average of 40 months of follow-up, the researchers said. After the researchers controlled for demographics, symptoms at presentation, use of proton pump inhibitors (PPI), and parameters that predicted treatment response in the univariate analyses, only abnormal acid exposure time (AET) predicted linear improvement in DSI scores (P =.027), while abnormal AET (P =.002) and symptom association probability (P =.026) predicted significant improvements in linear and dichotomous GSS, they reported. Abnormal AET and being off PPIs during testing also more than doubled the odds of at least a 50% improvement in GSS.
In contrast, dichotomous reflux exposure time did not predict response in any of the analyses, said the investigators. “Established thresholds for the total number of reflux events did not predict linear or dichotomous global symptom severity improvement,” they added. “Our data therefore suggest that performing pH-impedance testing off PPI therapy increases the yield of abnormal AET and symptom-reflux association with reflux events, facilitating predicting value for symptom improvement with both medical and surgical antireflux therapy.”
The researchers could not corroborate patients’ compliance with antisecretory therapy, assess the reasons physicians chose medical or surgical management, or evaluate the impact of the placebo effect or other factors unrelated to reflux, they said.
The study was partly funded by the National Institute for Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the Washington University Department of Medicine Mentors in Medicine and Clinical Science Training and Research programs. The authors declared no relevant conflicts of interest.
Key clinical point: pH-impedance testing best predicted response to reflux treatment when patients were off proton pump inhibitors, and abnormal acid exposure time was the single most useful parameter.
Major finding: Acid exposure time consistently predicted response to antireflux therapy in univariate and multivariable analyses, while reflux exposure time did not.
Data source: Prospective, single-center study of 187 patients who underwent pH-impedance testing.
Disclosures: This study was partly funded by the National Institute for Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, and the Washington University Department of Medicine Mentors in Medicine and Clinical Science Training and Research programs. The authors declared no relevant conflicts of interest.