User login
Docs need all tools, better education to combat opioid addiction
WASHINGTON– Doctors need a variety of options to treat opioid addiction and more training on safe opioid prescribing, according to experts called to testify before the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations.
One of the primary options – medication-assisted treatment (MAT) utilizing buprenorphine – was criticized by Subcommittee Chairman Tim Murphy (R-Pa.).
Increased availability of MAT is among the recommendations made in late March by the Health & Human Services department.
“We are not going to end this opioid epidemic by increasing the use of opioids,” Rep. Murphy said at the April 23 hearing. “We need an exit strategy that enables Americans to become opioid free altogether. We can do better than addiction maintenance.”
Physicians testifying to the subcommittee unanimously suggested that MAT needs to be a vital part of the treatment toolbox, noting that for some patients, it is an essential component of recovery.
Witnesses called for recognition that opioid addiction is a long-term issue; patients can need life-long treatment to help prevent relapse.
“Opiate dependence is not like the common cold. It does not go away” Dr. Robert DuPont, president of the Institute for Behavior and Health, testified. “It is a lifetime problem. A person who has opiate dependence is going to deal with that problem one way or another for his or her lifetime. People are not fixed in treatment.”
Treatment options, including MAT, almost always are short term, and that needs to change, Dr. DuPont said, adding that it is critically important to get health insurers to recognize longer-term treatment.
Dr. Anna Lembke of the departments of psychiatry and behavioral science at Stanford (Calif.) University, testified regarding the difficulty in getting Medicaid and private insurers to cover addiction treatment.
“When you try to get coverage for addiction treatment, they give you the huge runaround,” she said, noting that ironically, that it’s fairly easy to prescribe opioids and have those covered by most insurers.
“What that means is that if you want to get addiction treatment for patients who are struggling with the disease addiction, you can’t insurance to pay for it, which means that patients don’t access the treatment,” Dr. Lembke said, adding that it leaves patients to find and pay for treatments themselves.
While many insurers cover MAT, their utilization management techniques can make it challenging to get and keep patients in the programs, she said. “Once you start someone on buprenorphine, you don’t want to just suddenly not have it available to them, but that happens frequently because all of a sudden you have been denied coverage.”
Witnesses also called for better guidance on prescribing opioids. Dr. Patrice A. Harris, secretary of the American Medical Association board of trustees, highlighted some of her organizations efforts to improve education and awareness of the dangers of overprescribing opioid pain medications for physicians as well as patients. She noted, and other panelists agreed, that addiction needs to be looked at and treated as any other chronic condition. That shift in perspective could help alleviate the stigma attached to seeking treatment.
Dr. Lembke called for changes to Medicare to lead private insurance to better incentivize the proper use of addiction treatment, including both medication and non–medication based treatments, including setting quality measures to ensure proper use of opioids, removing any connection in customer satisfaction surveys to whether or not they receive prescription opioids, and to incentivize greater use of prescription drug monitoring programs.
She also called for changes in federal regulations to make it easier for doctors to communicate with one another on such issues as addiction and what medications they might be on to help better coordinate treatment and to prevent patients from getting opioid prescriptions from one doctor while getting treatment from another.
WASHINGTON– Doctors need a variety of options to treat opioid addiction and more training on safe opioid prescribing, according to experts called to testify before the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations.
One of the primary options – medication-assisted treatment (MAT) utilizing buprenorphine – was criticized by Subcommittee Chairman Tim Murphy (R-Pa.).
Increased availability of MAT is among the recommendations made in late March by the Health & Human Services department.
“We are not going to end this opioid epidemic by increasing the use of opioids,” Rep. Murphy said at the April 23 hearing. “We need an exit strategy that enables Americans to become opioid free altogether. We can do better than addiction maintenance.”
Physicians testifying to the subcommittee unanimously suggested that MAT needs to be a vital part of the treatment toolbox, noting that for some patients, it is an essential component of recovery.
Witnesses called for recognition that opioid addiction is a long-term issue; patients can need life-long treatment to help prevent relapse.
“Opiate dependence is not like the common cold. It does not go away” Dr. Robert DuPont, president of the Institute for Behavior and Health, testified. “It is a lifetime problem. A person who has opiate dependence is going to deal with that problem one way or another for his or her lifetime. People are not fixed in treatment.”
Treatment options, including MAT, almost always are short term, and that needs to change, Dr. DuPont said, adding that it is critically important to get health insurers to recognize longer-term treatment.
Dr. Anna Lembke of the departments of psychiatry and behavioral science at Stanford (Calif.) University, testified regarding the difficulty in getting Medicaid and private insurers to cover addiction treatment.
“When you try to get coverage for addiction treatment, they give you the huge runaround,” she said, noting that ironically, that it’s fairly easy to prescribe opioids and have those covered by most insurers.
“What that means is that if you want to get addiction treatment for patients who are struggling with the disease addiction, you can’t insurance to pay for it, which means that patients don’t access the treatment,” Dr. Lembke said, adding that it leaves patients to find and pay for treatments themselves.
While many insurers cover MAT, their utilization management techniques can make it challenging to get and keep patients in the programs, she said. “Once you start someone on buprenorphine, you don’t want to just suddenly not have it available to them, but that happens frequently because all of a sudden you have been denied coverage.”
Witnesses also called for better guidance on prescribing opioids. Dr. Patrice A. Harris, secretary of the American Medical Association board of trustees, highlighted some of her organizations efforts to improve education and awareness of the dangers of overprescribing opioid pain medications for physicians as well as patients. She noted, and other panelists agreed, that addiction needs to be looked at and treated as any other chronic condition. That shift in perspective could help alleviate the stigma attached to seeking treatment.
Dr. Lembke called for changes to Medicare to lead private insurance to better incentivize the proper use of addiction treatment, including both medication and non–medication based treatments, including setting quality measures to ensure proper use of opioids, removing any connection in customer satisfaction surveys to whether or not they receive prescription opioids, and to incentivize greater use of prescription drug monitoring programs.
She also called for changes in federal regulations to make it easier for doctors to communicate with one another on such issues as addiction and what medications they might be on to help better coordinate treatment and to prevent patients from getting opioid prescriptions from one doctor while getting treatment from another.
WASHINGTON– Doctors need a variety of options to treat opioid addiction and more training on safe opioid prescribing, according to experts called to testify before the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations.
One of the primary options – medication-assisted treatment (MAT) utilizing buprenorphine – was criticized by Subcommittee Chairman Tim Murphy (R-Pa.).
Increased availability of MAT is among the recommendations made in late March by the Health & Human Services department.
“We are not going to end this opioid epidemic by increasing the use of opioids,” Rep. Murphy said at the April 23 hearing. “We need an exit strategy that enables Americans to become opioid free altogether. We can do better than addiction maintenance.”
Physicians testifying to the subcommittee unanimously suggested that MAT needs to be a vital part of the treatment toolbox, noting that for some patients, it is an essential component of recovery.
Witnesses called for recognition that opioid addiction is a long-term issue; patients can need life-long treatment to help prevent relapse.
“Opiate dependence is not like the common cold. It does not go away” Dr. Robert DuPont, president of the Institute for Behavior and Health, testified. “It is a lifetime problem. A person who has opiate dependence is going to deal with that problem one way or another for his or her lifetime. People are not fixed in treatment.”
Treatment options, including MAT, almost always are short term, and that needs to change, Dr. DuPont said, adding that it is critically important to get health insurers to recognize longer-term treatment.
Dr. Anna Lembke of the departments of psychiatry and behavioral science at Stanford (Calif.) University, testified regarding the difficulty in getting Medicaid and private insurers to cover addiction treatment.
“When you try to get coverage for addiction treatment, they give you the huge runaround,” she said, noting that ironically, that it’s fairly easy to prescribe opioids and have those covered by most insurers.
“What that means is that if you want to get addiction treatment for patients who are struggling with the disease addiction, you can’t insurance to pay for it, which means that patients don’t access the treatment,” Dr. Lembke said, adding that it leaves patients to find and pay for treatments themselves.
While many insurers cover MAT, their utilization management techniques can make it challenging to get and keep patients in the programs, she said. “Once you start someone on buprenorphine, you don’t want to just suddenly not have it available to them, but that happens frequently because all of a sudden you have been denied coverage.”
Witnesses also called for better guidance on prescribing opioids. Dr. Patrice A. Harris, secretary of the American Medical Association board of trustees, highlighted some of her organizations efforts to improve education and awareness of the dangers of overprescribing opioid pain medications for physicians as well as patients. She noted, and other panelists agreed, that addiction needs to be looked at and treated as any other chronic condition. That shift in perspective could help alleviate the stigma attached to seeking treatment.
Dr. Lembke called for changes to Medicare to lead private insurance to better incentivize the proper use of addiction treatment, including both medication and non–medication based treatments, including setting quality measures to ensure proper use of opioids, removing any connection in customer satisfaction surveys to whether or not they receive prescription opioids, and to incentivize greater use of prescription drug monitoring programs.
She also called for changes in federal regulations to make it easier for doctors to communicate with one another on such issues as addiction and what medications they might be on to help better coordinate treatment and to prevent patients from getting opioid prescriptions from one doctor while getting treatment from another.
AT A HOUSE ENERGY & COMMERCE SUBCOMMITTEE HEARING
Ischemic preconditioning fails to shift CABG outcomes
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
AT ACC 2015
Key clinical point: Remote ischemic conditioning prior to CABG did not improve outcomes at 1 year.
Major finding: Cardiovascular death, MI, stroke, and coronary revascularization rates at 1 year were similar with and without RIC (27% vs. 28%).
Data source: ERICCA, a double-blind, randomized, controlled trial in 1,612 patients undergoing CABG.
Disclosures: The study was funded by the National Institute for Health Research, Medical Research Council, and British Heart Foundation. Dr. Hausenloy and Dr. Fogel reported having no disclosures. Dr. Bates reported consulting fees/honoraria from Merck and Astra Zeneca.
Simplified PESI identified low-risk pulmonary embolism
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care, investigators reported in Academic Emergency Medicine.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr. Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates.
“The results of this analysis provide further support that risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department (Acad. Emerg. Med. 2015;22:299-307).”
The PESI has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The simplified PESI has 6 of the PESI’s 11 variables, but remains accurate in assessing PE severity, the researchers said. They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the phase III Einstein PE study, in which rivaroxaban was found noninferior to an enoxaparin–vitamin K antagonist combination in terms of the risk of recurrent venous thromboembolism and clinically important bleeding events (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received.
However, the incidence of major bleeds up to 30 days was lower in the rivaroxaban group than in the standard treatment group, especially if patients’ simplified PESI scores were greater than 0. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding at all time points and in both treatment groups.
Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr. Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported financial and advisory relationships with several other pharmaceutical companies.
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care, investigators reported in Academic Emergency Medicine.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr. Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates.
“The results of this analysis provide further support that risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department (Acad. Emerg. Med. 2015;22:299-307).”
The PESI has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The simplified PESI has 6 of the PESI’s 11 variables, but remains accurate in assessing PE severity, the researchers said. They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the phase III Einstein PE study, in which rivaroxaban was found noninferior to an enoxaparin–vitamin K antagonist combination in terms of the risk of recurrent venous thromboembolism and clinically important bleeding events (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received.
However, the incidence of major bleeds up to 30 days was lower in the rivaroxaban group than in the standard treatment group, especially if patients’ simplified PESI scores were greater than 0. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding at all time points and in both treatment groups.
Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr. Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported financial and advisory relationships with several other pharmaceutical companies.
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care, investigators reported in Academic Emergency Medicine.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr. Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates.
“The results of this analysis provide further support that risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department (Acad. Emerg. Med. 2015;22:299-307).”
The PESI has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The simplified PESI has 6 of the PESI’s 11 variables, but remains accurate in assessing PE severity, the researchers said. They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the phase III Einstein PE study, in which rivaroxaban was found noninferior to an enoxaparin–vitamin K antagonist combination in terms of the risk of recurrent venous thromboembolism and clinically important bleeding events (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received.
However, the incidence of major bleeds up to 30 days was lower in the rivaroxaban group than in the standard treatment group, especially if patients’ simplified PESI scores were greater than 0. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding at all time points and in both treatment groups.
Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr. Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported financial and advisory relationships with several other pharmaceutical companies.
Key clinical point: The simplified version of the PESI identified low-risk pulmonary embolism patients.
Major finding: Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days, regardless of which treatment they received.
Data source: Post hoc analysis of simplified PESI scores and outcomes among 4,831 patients with acute pulmonary embolism who received either rivaroxaban or an enoxaparin–vitamin K antagonist combination.
Disclosures: Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr. Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported relationships with several other pharmaceutical companies.
U.S. poison center calls nearly quadrupled because of fake pot
Poison control call centers in the United States have received 1,900 exposure calls from people seeking help for adverse reactions to synthetic cannabinoids since Jan. 1, nearly four times the rate of calls received in 2014, the American Association of Poison Control Centers (AAPCC) announced April 23.
“These synthetic drugs present a potentially fatal risk that is not well recognized by people consuming these products,” Jay L. Schauben, Pharm.D., AAPCC president, said in a statement. “The recent death of five people suspected of using this category of drugs underscores the urgency of controlling these drugs and educating the public of their dangers.”
Synthetic “marijuana” products, also known as THC homologs, can induce similar highs to THC but are chemically different from cannabis. These drugs, marketed under such names as “Spice,” “K2,” and “AK-47,” are typically sprayed on plant material, and can be easily purchased in convenience stores and gas stations.
Chemical formulas in a particular brand of fake pot can vary dramatically and are often changed or altered to avoid using specifically banned formulations, Eric Wish, Ph.D., of the National Drug Early Warning System, said in a statement. “People are playing Russian Roulette with their lives because only the chemist creating the synthetic cannabinoid really knows what is in it.”
Emergency physicians should consider synthetic cannabinoids in patients presenting with be alert to the following symptoms in patients presenting with severe agitation and anxiety, nausea and vomiting, muscle spasms, seizures, tremors, psychotic episodes, and suicidal and other harmful thoughts and/or actions.
However, since formulations are ever-changing, recognizing synthetic cannabinoid intoxication can be difficult, according to AAPCC.
Poison control call centers in the United States have received 1,900 exposure calls from people seeking help for adverse reactions to synthetic cannabinoids since Jan. 1, nearly four times the rate of calls received in 2014, the American Association of Poison Control Centers (AAPCC) announced April 23.
“These synthetic drugs present a potentially fatal risk that is not well recognized by people consuming these products,” Jay L. Schauben, Pharm.D., AAPCC president, said in a statement. “The recent death of five people suspected of using this category of drugs underscores the urgency of controlling these drugs and educating the public of their dangers.”
Synthetic “marijuana” products, also known as THC homologs, can induce similar highs to THC but are chemically different from cannabis. These drugs, marketed under such names as “Spice,” “K2,” and “AK-47,” are typically sprayed on plant material, and can be easily purchased in convenience stores and gas stations.
Chemical formulas in a particular brand of fake pot can vary dramatically and are often changed or altered to avoid using specifically banned formulations, Eric Wish, Ph.D., of the National Drug Early Warning System, said in a statement. “People are playing Russian Roulette with their lives because only the chemist creating the synthetic cannabinoid really knows what is in it.”
Emergency physicians should consider synthetic cannabinoids in patients presenting with be alert to the following symptoms in patients presenting with severe agitation and anxiety, nausea and vomiting, muscle spasms, seizures, tremors, psychotic episodes, and suicidal and other harmful thoughts and/or actions.
However, since formulations are ever-changing, recognizing synthetic cannabinoid intoxication can be difficult, according to AAPCC.
Poison control call centers in the United States have received 1,900 exposure calls from people seeking help for adverse reactions to synthetic cannabinoids since Jan. 1, nearly four times the rate of calls received in 2014, the American Association of Poison Control Centers (AAPCC) announced April 23.
“These synthetic drugs present a potentially fatal risk that is not well recognized by people consuming these products,” Jay L. Schauben, Pharm.D., AAPCC president, said in a statement. “The recent death of five people suspected of using this category of drugs underscores the urgency of controlling these drugs and educating the public of their dangers.”
Synthetic “marijuana” products, also known as THC homologs, can induce similar highs to THC but are chemically different from cannabis. These drugs, marketed under such names as “Spice,” “K2,” and “AK-47,” are typically sprayed on plant material, and can be easily purchased in convenience stores and gas stations.
Chemical formulas in a particular brand of fake pot can vary dramatically and are often changed or altered to avoid using specifically banned formulations, Eric Wish, Ph.D., of the National Drug Early Warning System, said in a statement. “People are playing Russian Roulette with their lives because only the chemist creating the synthetic cannabinoid really knows what is in it.”
Emergency physicians should consider synthetic cannabinoids in patients presenting with be alert to the following symptoms in patients presenting with severe agitation and anxiety, nausea and vomiting, muscle spasms, seizures, tremors, psychotic episodes, and suicidal and other harmful thoughts and/or actions.
However, since formulations are ever-changing, recognizing synthetic cannabinoid intoxication can be difficult, according to AAPCC.
Are we taught enough about behavior?
If you ask a primary care pediatrician who has been practicing for more than 2 decades, she will tell you that her practice has tilted steeply toward complaints with a more developmental and behavioral flavor. In the lead article of the April 2015 Pediatrics (“Are We on the Right Track? Examining the Role of Developmental Behavioral Pediatrics”) Dr. Ruth E.K. Stein, a recent recipient of the C. Anderson Aldrich Award given by the American Academy of Pediatrics section on children with developmental and behavioral pediatrics, questions whether we, both general pediatricians and specialists in developmental and behavioral pediatrics, are on the right path in addressing this shifting mix in our patient populations.
Dr. Stein observes that while she and other pioneers in the creation of her specialty began as general pediatricians, today physicians typically enter developmental and behavioral fellowship programs without the benefit of practicing the runny nose–earache–diaper rash kind of pediatrics that many of us enjoy. She worries that from this early branching point in training, developmental and behavioral specialists have become “sequestered and siloed – increasingly seen only as people who take care of children who have special needs.” The problem is that, as Dr. Stein wisely observes, developmental and behavioral issues “are the core constructs of pediatrics and its backbone and that they must be incorporated into every primary care and specialty encounter and included in every educational experience.”
Dr. Stein continues her essay by proposing a handful of strategies for bridging the gap between developmental and behavioral specialists and general pediatricians, and strengthening the training of house officers, which currently requires only a pitifully inadequate month devoted to developmental and behavioral issues. While it is hard to argue with Dr. Stein’s suggestions, they only nibble around the edges of the real problem.
If one really believes as she and I do, that behavior and development must be considered in every patient encounter and educational experience, then the solution lies in changing how we teach medicine from the very beginning instead of waiting until postgraduate education. Everyone mouths the importance of the mind-body connection, but it is often just so much hot air. The relationship between behavior and development, and what Dr. Stein refers to as “biomedical” conditions, exists in every patient. It is often said it is the fact that our patients are growing and developing that keeps pediatrics apart from the rest of medicine. But the same process of change over time occurs in adults as well; we call it aging instead of development. Understanding where our patients are positioned on this trajectory from birth to death is critical in helping us understand what is troubling them, and how best to help them manage their concerns.
For pediatricians, our preverbal patients’ behavior is often the only way we have of knowing there is a problem. Behavior can be their unspoken chief complaint. The failure by a physician to interpret her patient’s behavior as either a result or the cause of the problem can lead to an unfortunate outcome.
This means, as we teach aspiring doctors the art of medicine, we must make it clear that the patient’s behavior and stage of development must be considered equally with the more traditional biomedical etiologies, not as an afterthought. For example, any discussion of nonacute recurrent abdominal pain in children that fails to acknowledge from the outset that most of these patients will not have an abnormality detectable by lab work and imaging studies is doing the young physician and his patients a disservice. I am suggesting that we adopt a more patient-centered rather than a disease-centered approach to training all physicians.
While every patient must be viewed in the proper behavioral and developmental context, there are those in whom a behavior problem dominates. Given the patient mix that the new millennium pediatrician is going to face, 1 month in postgraduate training is clearly insufficient. One cannot begin to learn even the rudiments of managing common problems such as attention-deficit/hyperactivity disorder, disordered sleep, temper tantrums, and school refusal in 30 days. Finding room in a training program to give behavioral and developmental problems more than a quick nod is going to require some rethinking of how we train pediatricians. It may be that training programs will need to selectively trim back some programs that may be of only limited long-term use to most general office-based pediatricians and offer them as electives. For example, how many of us still practice the kind of neonatology we were exposed to in the special care nursery? These are not easy decisions, but as Dr. Reid has suggested, we need to reconsider whether we are on the right track.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
If you ask a primary care pediatrician who has been practicing for more than 2 decades, she will tell you that her practice has tilted steeply toward complaints with a more developmental and behavioral flavor. In the lead article of the April 2015 Pediatrics (“Are We on the Right Track? Examining the Role of Developmental Behavioral Pediatrics”) Dr. Ruth E.K. Stein, a recent recipient of the C. Anderson Aldrich Award given by the American Academy of Pediatrics section on children with developmental and behavioral pediatrics, questions whether we, both general pediatricians and specialists in developmental and behavioral pediatrics, are on the right path in addressing this shifting mix in our patient populations.
Dr. Stein observes that while she and other pioneers in the creation of her specialty began as general pediatricians, today physicians typically enter developmental and behavioral fellowship programs without the benefit of practicing the runny nose–earache–diaper rash kind of pediatrics that many of us enjoy. She worries that from this early branching point in training, developmental and behavioral specialists have become “sequestered and siloed – increasingly seen only as people who take care of children who have special needs.” The problem is that, as Dr. Stein wisely observes, developmental and behavioral issues “are the core constructs of pediatrics and its backbone and that they must be incorporated into every primary care and specialty encounter and included in every educational experience.”
Dr. Stein continues her essay by proposing a handful of strategies for bridging the gap between developmental and behavioral specialists and general pediatricians, and strengthening the training of house officers, which currently requires only a pitifully inadequate month devoted to developmental and behavioral issues. While it is hard to argue with Dr. Stein’s suggestions, they only nibble around the edges of the real problem.
If one really believes as she and I do, that behavior and development must be considered in every patient encounter and educational experience, then the solution lies in changing how we teach medicine from the very beginning instead of waiting until postgraduate education. Everyone mouths the importance of the mind-body connection, but it is often just so much hot air. The relationship between behavior and development, and what Dr. Stein refers to as “biomedical” conditions, exists in every patient. It is often said it is the fact that our patients are growing and developing that keeps pediatrics apart from the rest of medicine. But the same process of change over time occurs in adults as well; we call it aging instead of development. Understanding where our patients are positioned on this trajectory from birth to death is critical in helping us understand what is troubling them, and how best to help them manage their concerns.
For pediatricians, our preverbal patients’ behavior is often the only way we have of knowing there is a problem. Behavior can be their unspoken chief complaint. The failure by a physician to interpret her patient’s behavior as either a result or the cause of the problem can lead to an unfortunate outcome.
This means, as we teach aspiring doctors the art of medicine, we must make it clear that the patient’s behavior and stage of development must be considered equally with the more traditional biomedical etiologies, not as an afterthought. For example, any discussion of nonacute recurrent abdominal pain in children that fails to acknowledge from the outset that most of these patients will not have an abnormality detectable by lab work and imaging studies is doing the young physician and his patients a disservice. I am suggesting that we adopt a more patient-centered rather than a disease-centered approach to training all physicians.
While every patient must be viewed in the proper behavioral and developmental context, there are those in whom a behavior problem dominates. Given the patient mix that the new millennium pediatrician is going to face, 1 month in postgraduate training is clearly insufficient. One cannot begin to learn even the rudiments of managing common problems such as attention-deficit/hyperactivity disorder, disordered sleep, temper tantrums, and school refusal in 30 days. Finding room in a training program to give behavioral and developmental problems more than a quick nod is going to require some rethinking of how we train pediatricians. It may be that training programs will need to selectively trim back some programs that may be of only limited long-term use to most general office-based pediatricians and offer them as electives. For example, how many of us still practice the kind of neonatology we were exposed to in the special care nursery? These are not easy decisions, but as Dr. Reid has suggested, we need to reconsider whether we are on the right track.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
If you ask a primary care pediatrician who has been practicing for more than 2 decades, she will tell you that her practice has tilted steeply toward complaints with a more developmental and behavioral flavor. In the lead article of the April 2015 Pediatrics (“Are We on the Right Track? Examining the Role of Developmental Behavioral Pediatrics”) Dr. Ruth E.K. Stein, a recent recipient of the C. Anderson Aldrich Award given by the American Academy of Pediatrics section on children with developmental and behavioral pediatrics, questions whether we, both general pediatricians and specialists in developmental and behavioral pediatrics, are on the right path in addressing this shifting mix in our patient populations.
Dr. Stein observes that while she and other pioneers in the creation of her specialty began as general pediatricians, today physicians typically enter developmental and behavioral fellowship programs without the benefit of practicing the runny nose–earache–diaper rash kind of pediatrics that many of us enjoy. She worries that from this early branching point in training, developmental and behavioral specialists have become “sequestered and siloed – increasingly seen only as people who take care of children who have special needs.” The problem is that, as Dr. Stein wisely observes, developmental and behavioral issues “are the core constructs of pediatrics and its backbone and that they must be incorporated into every primary care and specialty encounter and included in every educational experience.”
Dr. Stein continues her essay by proposing a handful of strategies for bridging the gap between developmental and behavioral specialists and general pediatricians, and strengthening the training of house officers, which currently requires only a pitifully inadequate month devoted to developmental and behavioral issues. While it is hard to argue with Dr. Stein’s suggestions, they only nibble around the edges of the real problem.
If one really believes as she and I do, that behavior and development must be considered in every patient encounter and educational experience, then the solution lies in changing how we teach medicine from the very beginning instead of waiting until postgraduate education. Everyone mouths the importance of the mind-body connection, but it is often just so much hot air. The relationship between behavior and development, and what Dr. Stein refers to as “biomedical” conditions, exists in every patient. It is often said it is the fact that our patients are growing and developing that keeps pediatrics apart from the rest of medicine. But the same process of change over time occurs in adults as well; we call it aging instead of development. Understanding where our patients are positioned on this trajectory from birth to death is critical in helping us understand what is troubling them, and how best to help them manage their concerns.
For pediatricians, our preverbal patients’ behavior is often the only way we have of knowing there is a problem. Behavior can be their unspoken chief complaint. The failure by a physician to interpret her patient’s behavior as either a result or the cause of the problem can lead to an unfortunate outcome.
This means, as we teach aspiring doctors the art of medicine, we must make it clear that the patient’s behavior and stage of development must be considered equally with the more traditional biomedical etiologies, not as an afterthought. For example, any discussion of nonacute recurrent abdominal pain in children that fails to acknowledge from the outset that most of these patients will not have an abnormality detectable by lab work and imaging studies is doing the young physician and his patients a disservice. I am suggesting that we adopt a more patient-centered rather than a disease-centered approach to training all physicians.
While every patient must be viewed in the proper behavioral and developmental context, there are those in whom a behavior problem dominates. Given the patient mix that the new millennium pediatrician is going to face, 1 month in postgraduate training is clearly insufficient. One cannot begin to learn even the rudiments of managing common problems such as attention-deficit/hyperactivity disorder, disordered sleep, temper tantrums, and school refusal in 30 days. Finding room in a training program to give behavioral and developmental problems more than a quick nod is going to require some rethinking of how we train pediatricians. It may be that training programs will need to selectively trim back some programs that may be of only limited long-term use to most general office-based pediatricians and offer them as electives. For example, how many of us still practice the kind of neonatology we were exposed to in the special care nursery? These are not easy decisions, but as Dr. Reid has suggested, we need to reconsider whether we are on the right track.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
Angiojet system found safe, effective in lower-extremity deep venous thrombosis
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
FROM THE JOURNAL OF VASCULAR INTERVENTIONAL RADIOLOGY
Key clinical point: Endovascular treatment with the Angiojet rheolytic thrombectomy systemwas found safe and effective for lower-extremity deep venous thrombosis.
Major finding: 83% of patients were free of rethrombosis a year later, and only one suffered a serious adverse event (acute renal failure).
Data source: A multicenter prospective registry study of 329 patients who underwent Angiojet thrombectomy with or without catheter-directed thrombolysis.
Disclosures: Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
One Palm, Two Soles, Three Guesses on Diagnosis
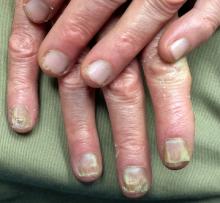
A 27-year-old man, who works as an electrician, self-refers for evaluation of changes on his left palm. He first noticed them three years ago, when a scaly patch appeared on his volar wrist. At the time, his mother sent him an unidentified cream from Mexico, which he used on the rash. Although it resolved, within a few months, his entire palm was similarly affected.
The problem has persisted despite a number of treatment attempts—with, among other things, OTC creams and lotions, topical steroids, antifungals, and two courses of oral antibiotics. He even tried a vegetarian diet. Nothing has worked.
The hand is mildly symptomatic and faintly irritated. He reports similar symptoms on the soles of both feet, which he says date to several years before his hand problem. The fingernails on the affected hand have also changed.
The skin and nails of his right hand have remained completely normal throughout this experience. His health is otherwise excellent.
EXAMINATION
The skin on his left palm and on both soles is identical: uniformly scaly and pink, and in sharp contrast to the normal, smooth skin of his right palm. All five fingernails of his left hand are yellowed, thickened, and dystrophic. Surprisingly, all toenails are normal in appearance. His right hand is unaffected.
What is the diagnosis?
DISCUSSION
This curious phenomenon, known as “two-feet, one-hand disease,” represents a variant of dermatophytosis that is usually remarkably mild in terms of symptoms but often lasts years (if not decades) once the patient develops it. The causative organism is the same dermatophyte that causes jock itch and athlete’s foot: Trichophyton rubrum, the most commonly isolated fungal organism causing disease in humans.
There has never been a satisfactory explanation for why this fairly common condition spares one hand completely. It has been posited that the disease more often affects the dominant hand, but studies haven’t supported that theory. Neither has one hand (left or right) been shown to predominate.
What we do know is that the organisms feed on nonliving skin, which is why they cause minimal symptoms. The palms, soles, and nails represent more tissue for them to feed on and a way to escape the reach of the immune system.
Susceptibility is a key factor in why some people, but not others, develop two-foot-one-hand disease. It is thought that the inability to fend off this organism is related to an inherited, qualitative deficiency of cell-mediated immunity—coupled in some cases with environmental factors such as heat, humidity, and choice of shoes.
We also know that as common and well known as this diagnosis is in dermatology circles, it is far more obscure to nondermatology providers. As a result, the uninitiated provider tends to treat it as he or she would eczema or psoriasis (both of which belong in the differential): with steroids, which are the very thing certain to worsen the condition.
In some cases, before seeking professional care, patients attempt to treat their problem with steroids prescribed for another condition—or even for another patient. The condition will appear to improve but then worsen over time, as the steroids suppress what little immune response exists. The overall effect is thus to worsen the situation considerably, making the condition more difficult to treat.
In the absence of a definite diagnosis, even when antifungals are tried, they are often relatively weak products (eg, tolnaftate or undecylenic acid) that afford little if any relief. The patient (and sometimes provider) then interprets this as a sign that the problem is not of fungal origin and proceeds to other, truly ineffective treatments.
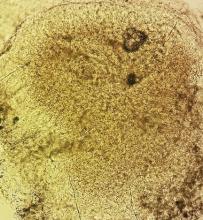
The two key factors that solve the mystery of two-foot-one-hand disease are: knowledge of its existence and the performance of a KOH examination of scrapings from the palm or sole. In this case, KOH revealed numerous fungal hyphae, confirming the diagnosis. Then—and only then—could effective treatment be provided.
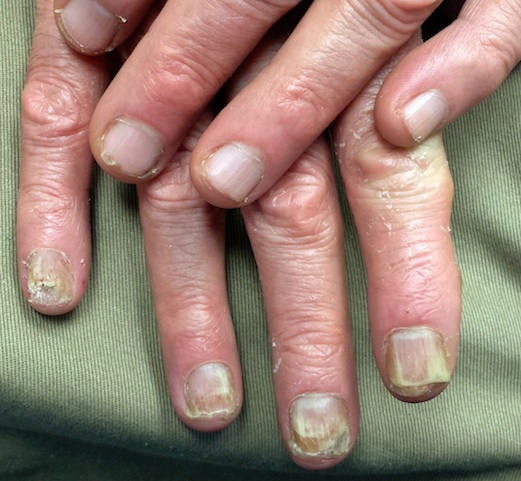
This patient was treated with miconazole cream (bid application) and a month-long course oral terbinafine (250 mg/d). The good news is that this will help control the disease. The bad news? The problem will continue to lurk in the background, because we can do little or nothing to make the patient less susceptible. At least he now knows what he has, and he has the weapons to ward it off in the future.
TAKE-HOME LEARNING POINTS
• “Two feet, one hand” disease is a form of dermatophytosis that affects both feet and one hand while completely sparing the other hand. To date, no one has explained this pattern of distribution.
• As with many dermatologic conditions, the diagnosis of two-feet-one-hand disease is simple if you know it exists and completely obscure if you don’t.
• Individual susceptibility appears to explain why some people develop it and many more (including family/household members of affected individuals) don’t.
• Besides knowledge of its existence, the key to diagnosis is the confirmatory KOH prep.
• This is a perfect example of the principle of “correct diagnosis dictates correct treatment.”
A 27-year-old man, who works as an electrician, self-refers for evaluation of changes on his left palm. He first noticed them three years ago, when a scaly patch appeared on his volar wrist. At the time, his mother sent him an unidentified cream from Mexico, which he used on the rash. Although it resolved, within a few months, his entire palm was similarly affected.
The problem has persisted despite a number of treatment attempts—with, among other things, OTC creams and lotions, topical steroids, antifungals, and two courses of oral antibiotics. He even tried a vegetarian diet. Nothing has worked.
The hand is mildly symptomatic and faintly irritated. He reports similar symptoms on the soles of both feet, which he says date to several years before his hand problem. The fingernails on the affected hand have also changed.
The skin and nails of his right hand have remained completely normal throughout this experience. His health is otherwise excellent.
EXAMINATION
The skin on his left palm and on both soles is identical: uniformly scaly and pink, and in sharp contrast to the normal, smooth skin of his right palm. All five fingernails of his left hand are yellowed, thickened, and dystrophic. Surprisingly, all toenails are normal in appearance. His right hand is unaffected.
What is the diagnosis?
DISCUSSION
This curious phenomenon, known as “two-feet, one-hand disease,” represents a variant of dermatophytosis that is usually remarkably mild in terms of symptoms but often lasts years (if not decades) once the patient develops it. The causative organism is the same dermatophyte that causes jock itch and athlete’s foot: Trichophyton rubrum, the most commonly isolated fungal organism causing disease in humans.
There has never been a satisfactory explanation for why this fairly common condition spares one hand completely. It has been posited that the disease more often affects the dominant hand, but studies haven’t supported that theory. Neither has one hand (left or right) been shown to predominate.
What we do know is that the organisms feed on nonliving skin, which is why they cause minimal symptoms. The palms, soles, and nails represent more tissue for them to feed on and a way to escape the reach of the immune system.
Susceptibility is a key factor in why some people, but not others, develop two-foot-one-hand disease. It is thought that the inability to fend off this organism is related to an inherited, qualitative deficiency of cell-mediated immunity—coupled in some cases with environmental factors such as heat, humidity, and choice of shoes.
We also know that as common and well known as this diagnosis is in dermatology circles, it is far more obscure to nondermatology providers. As a result, the uninitiated provider tends to treat it as he or she would eczema or psoriasis (both of which belong in the differential): with steroids, which are the very thing certain to worsen the condition.
In some cases, before seeking professional care, patients attempt to treat their problem with steroids prescribed for another condition—or even for another patient. The condition will appear to improve but then worsen over time, as the steroids suppress what little immune response exists. The overall effect is thus to worsen the situation considerably, making the condition more difficult to treat.
In the absence of a definite diagnosis, even when antifungals are tried, they are often relatively weak products (eg, tolnaftate or undecylenic acid) that afford little if any relief. The patient (and sometimes provider) then interprets this as a sign that the problem is not of fungal origin and proceeds to other, truly ineffective treatments.
The two key factors that solve the mystery of two-foot-one-hand disease are: knowledge of its existence and the performance of a KOH examination of scrapings from the palm or sole. In this case, KOH revealed numerous fungal hyphae, confirming the diagnosis. Then—and only then—could effective treatment be provided.
This patient was treated with miconazole cream (bid application) and a month-long course oral terbinafine (250 mg/d). The good news is that this will help control the disease. The bad news? The problem will continue to lurk in the background, because we can do little or nothing to make the patient less susceptible. At least he now knows what he has, and he has the weapons to ward it off in the future.
TAKE-HOME LEARNING POINTS
• “Two feet, one hand” disease is a form of dermatophytosis that affects both feet and one hand while completely sparing the other hand. To date, no one has explained this pattern of distribution.
• As with many dermatologic conditions, the diagnosis of two-feet-one-hand disease is simple if you know it exists and completely obscure if you don’t.
• Individual susceptibility appears to explain why some people develop it and many more (including family/household members of affected individuals) don’t.
• Besides knowledge of its existence, the key to diagnosis is the confirmatory KOH prep.
• This is a perfect example of the principle of “correct diagnosis dictates correct treatment.”
A 27-year-old man, who works as an electrician, self-refers for evaluation of changes on his left palm. He first noticed them three years ago, when a scaly patch appeared on his volar wrist. At the time, his mother sent him an unidentified cream from Mexico, which he used on the rash. Although it resolved, within a few months, his entire palm was similarly affected.
The problem has persisted despite a number of treatment attempts—with, among other things, OTC creams and lotions, topical steroids, antifungals, and two courses of oral antibiotics. He even tried a vegetarian diet. Nothing has worked.
The hand is mildly symptomatic and faintly irritated. He reports similar symptoms on the soles of both feet, which he says date to several years before his hand problem. The fingernails on the affected hand have also changed.
The skin and nails of his right hand have remained completely normal throughout this experience. His health is otherwise excellent.
EXAMINATION
The skin on his left palm and on both soles is identical: uniformly scaly and pink, and in sharp contrast to the normal, smooth skin of his right palm. All five fingernails of his left hand are yellowed, thickened, and dystrophic. Surprisingly, all toenails are normal in appearance. His right hand is unaffected.
What is the diagnosis?
DISCUSSION
This curious phenomenon, known as “two-feet, one-hand disease,” represents a variant of dermatophytosis that is usually remarkably mild in terms of symptoms but often lasts years (if not decades) once the patient develops it. The causative organism is the same dermatophyte that causes jock itch and athlete’s foot: Trichophyton rubrum, the most commonly isolated fungal organism causing disease in humans.
There has never been a satisfactory explanation for why this fairly common condition spares one hand completely. It has been posited that the disease more often affects the dominant hand, but studies haven’t supported that theory. Neither has one hand (left or right) been shown to predominate.
What we do know is that the organisms feed on nonliving skin, which is why they cause minimal symptoms. The palms, soles, and nails represent more tissue for them to feed on and a way to escape the reach of the immune system.
Susceptibility is a key factor in why some people, but not others, develop two-foot-one-hand disease. It is thought that the inability to fend off this organism is related to an inherited, qualitative deficiency of cell-mediated immunity—coupled in some cases with environmental factors such as heat, humidity, and choice of shoes.
We also know that as common and well known as this diagnosis is in dermatology circles, it is far more obscure to nondermatology providers. As a result, the uninitiated provider tends to treat it as he or she would eczema or psoriasis (both of which belong in the differential): with steroids, which are the very thing certain to worsen the condition.
In some cases, before seeking professional care, patients attempt to treat their problem with steroids prescribed for another condition—or even for another patient. The condition will appear to improve but then worsen over time, as the steroids suppress what little immune response exists. The overall effect is thus to worsen the situation considerably, making the condition more difficult to treat.
In the absence of a definite diagnosis, even when antifungals are tried, they are often relatively weak products (eg, tolnaftate or undecylenic acid) that afford little if any relief. The patient (and sometimes provider) then interprets this as a sign that the problem is not of fungal origin and proceeds to other, truly ineffective treatments.
The two key factors that solve the mystery of two-foot-one-hand disease are: knowledge of its existence and the performance of a KOH examination of scrapings from the palm or sole. In this case, KOH revealed numerous fungal hyphae, confirming the diagnosis. Then—and only then—could effective treatment be provided.
This patient was treated with miconazole cream (bid application) and a month-long course oral terbinafine (250 mg/d). The good news is that this will help control the disease. The bad news? The problem will continue to lurk in the background, because we can do little or nothing to make the patient less susceptible. At least he now knows what he has, and he has the weapons to ward it off in the future.
TAKE-HOME LEARNING POINTS
• “Two feet, one hand” disease is a form of dermatophytosis that affects both feet and one hand while completely sparing the other hand. To date, no one has explained this pattern of distribution.
• As with many dermatologic conditions, the diagnosis of two-feet-one-hand disease is simple if you know it exists and completely obscure if you don’t.
• Individual susceptibility appears to explain why some people develop it and many more (including family/household members of affected individuals) don’t.
• Besides knowledge of its existence, the key to diagnosis is the confirmatory KOH prep.
• This is a perfect example of the principle of “correct diagnosis dictates correct treatment.”
Device can test multiple cancer drugs in tumors

Image courtesy of
Presage Biosciences
A device that tests multiple cancer drugs in living tumor tissue could guide treatment selection in patients with lymphoma and other cancers, according to researchers.
They also believe the device, called CIVO, could help speed up drug development by testing the efficacy of candidate drugs in very small doses while sparing patients side effects.
CIVO is a handheld microinjection platform that can deliver small doses of up to 8 drugs or combinations of drugs into a tumor.
The device proved effective for testing multiple cancer drugs in xenograft mouse models, dogs, and humans with lymphoma.
Richard Klinghoffer, PhD, of Presage Biosciences in Seattle, Washington, and his colleagues described their research with CIVO in Science Translational Medicine. The research was funded by Presage Biosciences, the National Institutes of Health, and Seattle Children’s Hospital Neuro-Oncology Fund.
About CIVO
CIVO is designed for tumors near the skin surface, such as lymphoma or skin and breast cancer.
The technology enables the placement of multiple columns of drugs directly into the tumor along the needle axis, spanning the full depth of the tumor. This makes it possible to assess drug effects with multiple biomarkers and in multiple regions along the injection axis to capture the heterogeneity of response within the tumor.
Later (typically 24 to 72 hours after injection), the tumor is resected for subsequent analysis, and responses are measured with multiple immunohistochemistry-based assays and high-resolution scanning.
Results in mice
In xenograft lymphoma models, CIVO microinjection of well-characterized anticancer agents (vincristine, doxorubicin, mafosfamide, and prednisolone) induced spatially defined cellular changes around sites of drug exposure, specific to the known mechanisms of each drug. And the observed localized responses predicted responses to the same drugs systemically delivered in animals.
In pair-matched drug-resistant and drug-sensitive lymphoma models, CIVO correctly demonstrated tumor resistance to doxorubicin and vincristine.
The researchers also identified an unexpected enhanced sensitivity to the active form of cyclophosphamide in multidrug-resistant lymphomas compared with chemotherapy-naïve lymphomas.
And a CIVO-enabled in vivo screen of oncology agents revealed that a novel mTOR pathway inhibitor exhibits significantly increased tumor-killing activity in the drug-resistant setting compared with chemotherapy-naïve tumors.
Results in dogs and humans
Dogs with lymphoma showed no toxicity when injected with drugs via CIVO. And the researchers said they observed robust, easily tracked, drug-specific responses in the animals.
For lymphoma patients, the researchers used CIVO to inject microdoses of vincristine into the tumors in patients’ lymph nodes. Cells surrounding the injections died, and there were no serious adverse events, although patients did report mild discomfort.
“This analysis creates a comprehensive portrait of drug response that has never been seen before this early in the drug development process,” Dr Klinghoffer said. “Using this technology, we can assess how drugs, both as single agents and in combinations, impact the biology of tumor cells in the context of the native tumor microenvironment.”
“[T]ranslation of CIVO to the clinical setting has enabled assessment on all aspects of tumor biology, including drug effects on tumor-infiltrating immune cells. This sets the stage for a new type of pre-phase 1 clinical study in which multiple drugs or drug combinations can be tested simultaneously, directly in a patient’s own tumor, without toxicity associated with systemic drug delivery.” ![]()

Image courtesy of
Presage Biosciences
A device that tests multiple cancer drugs in living tumor tissue could guide treatment selection in patients with lymphoma and other cancers, according to researchers.
They also believe the device, called CIVO, could help speed up drug development by testing the efficacy of candidate drugs in very small doses while sparing patients side effects.
CIVO is a handheld microinjection platform that can deliver small doses of up to 8 drugs or combinations of drugs into a tumor.
The device proved effective for testing multiple cancer drugs in xenograft mouse models, dogs, and humans with lymphoma.
Richard Klinghoffer, PhD, of Presage Biosciences in Seattle, Washington, and his colleagues described their research with CIVO in Science Translational Medicine. The research was funded by Presage Biosciences, the National Institutes of Health, and Seattle Children’s Hospital Neuro-Oncology Fund.
About CIVO
CIVO is designed for tumors near the skin surface, such as lymphoma or skin and breast cancer.
The technology enables the placement of multiple columns of drugs directly into the tumor along the needle axis, spanning the full depth of the tumor. This makes it possible to assess drug effects with multiple biomarkers and in multiple regions along the injection axis to capture the heterogeneity of response within the tumor.
Later (typically 24 to 72 hours after injection), the tumor is resected for subsequent analysis, and responses are measured with multiple immunohistochemistry-based assays and high-resolution scanning.
Results in mice
In xenograft lymphoma models, CIVO microinjection of well-characterized anticancer agents (vincristine, doxorubicin, mafosfamide, and prednisolone) induced spatially defined cellular changes around sites of drug exposure, specific to the known mechanisms of each drug. And the observed localized responses predicted responses to the same drugs systemically delivered in animals.
In pair-matched drug-resistant and drug-sensitive lymphoma models, CIVO correctly demonstrated tumor resistance to doxorubicin and vincristine.
The researchers also identified an unexpected enhanced sensitivity to the active form of cyclophosphamide in multidrug-resistant lymphomas compared with chemotherapy-naïve lymphomas.
And a CIVO-enabled in vivo screen of oncology agents revealed that a novel mTOR pathway inhibitor exhibits significantly increased tumor-killing activity in the drug-resistant setting compared with chemotherapy-naïve tumors.
Results in dogs and humans
Dogs with lymphoma showed no toxicity when injected with drugs via CIVO. And the researchers said they observed robust, easily tracked, drug-specific responses in the animals.
For lymphoma patients, the researchers used CIVO to inject microdoses of vincristine into the tumors in patients’ lymph nodes. Cells surrounding the injections died, and there were no serious adverse events, although patients did report mild discomfort.
“This analysis creates a comprehensive portrait of drug response that has never been seen before this early in the drug development process,” Dr Klinghoffer said. “Using this technology, we can assess how drugs, both as single agents and in combinations, impact the biology of tumor cells in the context of the native tumor microenvironment.”
“[T]ranslation of CIVO to the clinical setting has enabled assessment on all aspects of tumor biology, including drug effects on tumor-infiltrating immune cells. This sets the stage for a new type of pre-phase 1 clinical study in which multiple drugs or drug combinations can be tested simultaneously, directly in a patient’s own tumor, without toxicity associated with systemic drug delivery.” ![]()

Image courtesy of
Presage Biosciences
A device that tests multiple cancer drugs in living tumor tissue could guide treatment selection in patients with lymphoma and other cancers, according to researchers.
They also believe the device, called CIVO, could help speed up drug development by testing the efficacy of candidate drugs in very small doses while sparing patients side effects.
CIVO is a handheld microinjection platform that can deliver small doses of up to 8 drugs or combinations of drugs into a tumor.
The device proved effective for testing multiple cancer drugs in xenograft mouse models, dogs, and humans with lymphoma.
Richard Klinghoffer, PhD, of Presage Biosciences in Seattle, Washington, and his colleagues described their research with CIVO in Science Translational Medicine. The research was funded by Presage Biosciences, the National Institutes of Health, and Seattle Children’s Hospital Neuro-Oncology Fund.
About CIVO
CIVO is designed for tumors near the skin surface, such as lymphoma or skin and breast cancer.
The technology enables the placement of multiple columns of drugs directly into the tumor along the needle axis, spanning the full depth of the tumor. This makes it possible to assess drug effects with multiple biomarkers and in multiple regions along the injection axis to capture the heterogeneity of response within the tumor.
Later (typically 24 to 72 hours after injection), the tumor is resected for subsequent analysis, and responses are measured with multiple immunohistochemistry-based assays and high-resolution scanning.
Results in mice
In xenograft lymphoma models, CIVO microinjection of well-characterized anticancer agents (vincristine, doxorubicin, mafosfamide, and prednisolone) induced spatially defined cellular changes around sites of drug exposure, specific to the known mechanisms of each drug. And the observed localized responses predicted responses to the same drugs systemically delivered in animals.
In pair-matched drug-resistant and drug-sensitive lymphoma models, CIVO correctly demonstrated tumor resistance to doxorubicin and vincristine.
The researchers also identified an unexpected enhanced sensitivity to the active form of cyclophosphamide in multidrug-resistant lymphomas compared with chemotherapy-naïve lymphomas.
And a CIVO-enabled in vivo screen of oncology agents revealed that a novel mTOR pathway inhibitor exhibits significantly increased tumor-killing activity in the drug-resistant setting compared with chemotherapy-naïve tumors.
Results in dogs and humans
Dogs with lymphoma showed no toxicity when injected with drugs via CIVO. And the researchers said they observed robust, easily tracked, drug-specific responses in the animals.
For lymphoma patients, the researchers used CIVO to inject microdoses of vincristine into the tumors in patients’ lymph nodes. Cells surrounding the injections died, and there were no serious adverse events, although patients did report mild discomfort.
“This analysis creates a comprehensive portrait of drug response that has never been seen before this early in the drug development process,” Dr Klinghoffer said. “Using this technology, we can assess how drugs, both as single agents and in combinations, impact the biology of tumor cells in the context of the native tumor microenvironment.”
“[T]ranslation of CIVO to the clinical setting has enabled assessment on all aspects of tumor biology, including drug effects on tumor-infiltrating immune cells. This sets the stage for a new type of pre-phase 1 clinical study in which multiple drugs or drug combinations can be tested simultaneously, directly in a patient’s own tumor, without toxicity associated with systemic drug delivery.” ![]()
CCSs more likely to claim social security support

Photo courtesy of
Huntsman Cancer Institute
A new study indicates that childhood cancer survivors (CCSs) are more likely than individuals without a cancer history to enroll on federal programs that provide disability benefits.
CCSs diagnosed between 1970 and 1986 were about 2 to 5 times as likely as control subjects to utilize such a program.
“The long-term impact of cancer can affect other issues besides health outcomes,” said study author Anne Kirchhoff, PhD, of the Huntsman Cancer Institute at the University of Utah.
“We need to do a better job of helping people function throughout their lives, not just when they’re finishing their cancer therapy.”
Dr Kirchhoff and her colleagues conducted this research and detailed the results in the Journal of the National Cancer Institute.
The researchers looked at health insurance surveys completed in 2011 and 2012 by a random sample of 698 CCSs who were diagnosed between the ages of 0 and 20 years. Today, they range in age from 20s to early 60s.
The patients are part of a National Cancer Institute initiative, called the Childhood Cancer Survivor Study, which has followed more than 14,000 children and adolescents since 1994 who were diagnosed with cancer and survived at least 5 years after diagnosis. A comparison group of 210 siblings without cancer also responded to the survey and were used as controls.
Dr Kirchhoff and her colleagues looked at current or former enrollment on 2 federal disability programs:
- Supplemental security income (SSI), which is for people with limited income who have no prior work history
- Social security disability insurance (SSDI), which pays disability benefits to adults ages 18 years and older who have worked and paid social security taxes.
In all, 13.5% of CCSs reported being enrolled on SSI in the past or present, and 10% of survivors reported being enrolled on SSDI at some point. This was substantially higher than for the comparison group, in which 2.6% of patients reported SSI enrollment and 5.4% reported SSDI enrollment.
In addition, CCSs reported current enrollment in SSI more frequently than the US population, at rates of 7.3% and 2.5%, respectively.
Dr Kirchoff and her colleagues also identified survivor socio-demographic and treatment characteristics that were associated with a higher rate of enrollment in federal support programs.
“Survivors that were younger at diagnosis, age 4 or under, were about 7 times more likely to be on SSI than we see with survivors that were diagnosed in their adolescence,” she said.
SSI enrollment was more likely for female CCSs and for survivors with a history of cranial radiation treatment as well.
Dr Kirchhoff noted that, over the years, research on CCSs has caused hospitals to rethink how to better care for cancer survivors.
“There’s really a growing strategy to support survivors in the long-term,” she said. “For example, here at Huntsman Cancer Institute, we have a pediatric cancer late-effects clinic, which helps manage issues that might come up with childhood cancer survivors in the long term, including health-management support, health-behavior support, and access to providers to help them with other issues.” ![]()

Photo courtesy of
Huntsman Cancer Institute
A new study indicates that childhood cancer survivors (CCSs) are more likely than individuals without a cancer history to enroll on federal programs that provide disability benefits.
CCSs diagnosed between 1970 and 1986 were about 2 to 5 times as likely as control subjects to utilize such a program.
“The long-term impact of cancer can affect other issues besides health outcomes,” said study author Anne Kirchhoff, PhD, of the Huntsman Cancer Institute at the University of Utah.
“We need to do a better job of helping people function throughout their lives, not just when they’re finishing their cancer therapy.”
Dr Kirchhoff and her colleagues conducted this research and detailed the results in the Journal of the National Cancer Institute.
The researchers looked at health insurance surveys completed in 2011 and 2012 by a random sample of 698 CCSs who were diagnosed between the ages of 0 and 20 years. Today, they range in age from 20s to early 60s.
The patients are part of a National Cancer Institute initiative, called the Childhood Cancer Survivor Study, which has followed more than 14,000 children and adolescents since 1994 who were diagnosed with cancer and survived at least 5 years after diagnosis. A comparison group of 210 siblings without cancer also responded to the survey and were used as controls.
Dr Kirchhoff and her colleagues looked at current or former enrollment on 2 federal disability programs:
- Supplemental security income (SSI), which is for people with limited income who have no prior work history
- Social security disability insurance (SSDI), which pays disability benefits to adults ages 18 years and older who have worked and paid social security taxes.
In all, 13.5% of CCSs reported being enrolled on SSI in the past or present, and 10% of survivors reported being enrolled on SSDI at some point. This was substantially higher than for the comparison group, in which 2.6% of patients reported SSI enrollment and 5.4% reported SSDI enrollment.
In addition, CCSs reported current enrollment in SSI more frequently than the US population, at rates of 7.3% and 2.5%, respectively.
Dr Kirchoff and her colleagues also identified survivor socio-demographic and treatment characteristics that were associated with a higher rate of enrollment in federal support programs.
“Survivors that were younger at diagnosis, age 4 or under, were about 7 times more likely to be on SSI than we see with survivors that were diagnosed in their adolescence,” she said.
SSI enrollment was more likely for female CCSs and for survivors with a history of cranial radiation treatment as well.
Dr Kirchhoff noted that, over the years, research on CCSs has caused hospitals to rethink how to better care for cancer survivors.
“There’s really a growing strategy to support survivors in the long-term,” she said. “For example, here at Huntsman Cancer Institute, we have a pediatric cancer late-effects clinic, which helps manage issues that might come up with childhood cancer survivors in the long term, including health-management support, health-behavior support, and access to providers to help them with other issues.” ![]()

Photo courtesy of
Huntsman Cancer Institute
A new study indicates that childhood cancer survivors (CCSs) are more likely than individuals without a cancer history to enroll on federal programs that provide disability benefits.
CCSs diagnosed between 1970 and 1986 were about 2 to 5 times as likely as control subjects to utilize such a program.
“The long-term impact of cancer can affect other issues besides health outcomes,” said study author Anne Kirchhoff, PhD, of the Huntsman Cancer Institute at the University of Utah.
“We need to do a better job of helping people function throughout their lives, not just when they’re finishing their cancer therapy.”
Dr Kirchhoff and her colleagues conducted this research and detailed the results in the Journal of the National Cancer Institute.
The researchers looked at health insurance surveys completed in 2011 and 2012 by a random sample of 698 CCSs who were diagnosed between the ages of 0 and 20 years. Today, they range in age from 20s to early 60s.
The patients are part of a National Cancer Institute initiative, called the Childhood Cancer Survivor Study, which has followed more than 14,000 children and adolescents since 1994 who were diagnosed with cancer and survived at least 5 years after diagnosis. A comparison group of 210 siblings without cancer also responded to the survey and were used as controls.
Dr Kirchhoff and her colleagues looked at current or former enrollment on 2 federal disability programs:
- Supplemental security income (SSI), which is for people with limited income who have no prior work history
- Social security disability insurance (SSDI), which pays disability benefits to adults ages 18 years and older who have worked and paid social security taxes.
In all, 13.5% of CCSs reported being enrolled on SSI in the past or present, and 10% of survivors reported being enrolled on SSDI at some point. This was substantially higher than for the comparison group, in which 2.6% of patients reported SSI enrollment and 5.4% reported SSDI enrollment.
In addition, CCSs reported current enrollment in SSI more frequently than the US population, at rates of 7.3% and 2.5%, respectively.
Dr Kirchoff and her colleagues also identified survivor socio-demographic and treatment characteristics that were associated with a higher rate of enrollment in federal support programs.
“Survivors that were younger at diagnosis, age 4 or under, were about 7 times more likely to be on SSI than we see with survivors that were diagnosed in their adolescence,” she said.
SSI enrollment was more likely for female CCSs and for survivors with a history of cranial radiation treatment as well.
Dr Kirchhoff noted that, over the years, research on CCSs has caused hospitals to rethink how to better care for cancer survivors.
“There’s really a growing strategy to support survivors in the long-term,” she said. “For example, here at Huntsman Cancer Institute, we have a pediatric cancer late-effects clinic, which helps manage issues that might come up with childhood cancer survivors in the long term, including health-management support, health-behavior support, and access to providers to help them with other issues.” ![]()
Team images immune response

Photo by Aaron Logan
Researchers say they have devised an approach that allows for real-time imaging of the immune system’s response to tumors, without the need for blood draws or biopsies.
The method harnesses PET to identify areas of immune cell activity associated with inflammation or tumor development.
The researchers believe the approach offers a potential breakthrough in diagnostics and the ability to monitor the efficacy of cancer therapies.
The team described their method in PNAS.
Study author Hidde Ploegh, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, said that every experimental immunologist wants to monitor an ongoing immune response, but options are limited.
“One can look at blood, but blood is a vehicle of transport for immune cells and is not where immune responses occur,” he said. “Surgical biopsies are invasive and non-random, so, for example, a fine-needle aspirate of a tumor could miss a significant feature of that condition.”
In search of a better monitoring approach, Dr Ploegh and his colleagues leveraged two research tools that have become staples in his lab in recent years.
The first exploits single-domain antibodies known as VHHs, derived from the heavy chain-only antibodies made by the immune systems of animals in the camelid family. Dr Ploegh’s lab immunizes alpacas—his camelid of choice—to generate VHHs specific to immune cells of interest.
The second tool, sortagging, labels the VHHs in a site-specific fashion so that researchers can track the VHHs and their targets in a living animal.
Knowing that the tissue around tumors often contains immune cells such as neutrophils and macrophages, Dr Ploegh and his colleagues hypothesized that appropriately labeled VHHs might allow them to pinpoint tumor locations by finding the tumor-associated immune cells.
Dr Ploegh noted that VHHs’ extremely small size—approximately one-tenth that of conventional antibodies—are likely responsible for their superior tissue penetration and, thus, makes them particularly well suited for such use.
So the researchers generated VHHs that recognize mouse immune cells, then labeled these VHHs with radioisotopes and injected them into tumor-bearing mice. Subsequent PET imaging detected the location of immune cells around the tumor quickly and accurately.
“We were able to image tumors as small as 1 mm in size and within just a few days of their starting to grow,” said Mohammad Rashidian, PhD, a researcher in Dr Ploegh’s lab.
“We’re very excited about this because it’s a powerful approach to pick up inflammation in and around the tumor.”
Drs Rashidian and Ploegh believe that, with further refinement, the method could be used to monitor response to—and perhaps modify—cancer immunotherapy.
“To succeed with immunotherapy, we need more information about the tumor microenvironment,” Dr Rashidian said. “With this method, you could perhaps start immunotherapy and then, a few weeks later, image with VHHs to figure out progress and success of treatment.”
“PET imaging should allow a much more comprehensive look at the entire tumor in its environment,” Dr Ploegh added. “Then we can ask, ‘Did the tumor grow? Did immune cells invade? What has happened to the tumor?’ And to be able to see this without going in invasively is a significant achievement.” ![]()

Photo by Aaron Logan
Researchers say they have devised an approach that allows for real-time imaging of the immune system’s response to tumors, without the need for blood draws or biopsies.
The method harnesses PET to identify areas of immune cell activity associated with inflammation or tumor development.
The researchers believe the approach offers a potential breakthrough in diagnostics and the ability to monitor the efficacy of cancer therapies.
The team described their method in PNAS.
Study author Hidde Ploegh, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, said that every experimental immunologist wants to monitor an ongoing immune response, but options are limited.
“One can look at blood, but blood is a vehicle of transport for immune cells and is not where immune responses occur,” he said. “Surgical biopsies are invasive and non-random, so, for example, a fine-needle aspirate of a tumor could miss a significant feature of that condition.”
In search of a better monitoring approach, Dr Ploegh and his colleagues leveraged two research tools that have become staples in his lab in recent years.
The first exploits single-domain antibodies known as VHHs, derived from the heavy chain-only antibodies made by the immune systems of animals in the camelid family. Dr Ploegh’s lab immunizes alpacas—his camelid of choice—to generate VHHs specific to immune cells of interest.
The second tool, sortagging, labels the VHHs in a site-specific fashion so that researchers can track the VHHs and their targets in a living animal.
Knowing that the tissue around tumors often contains immune cells such as neutrophils and macrophages, Dr Ploegh and his colleagues hypothesized that appropriately labeled VHHs might allow them to pinpoint tumor locations by finding the tumor-associated immune cells.
Dr Ploegh noted that VHHs’ extremely small size—approximately one-tenth that of conventional antibodies—are likely responsible for their superior tissue penetration and, thus, makes them particularly well suited for such use.
So the researchers generated VHHs that recognize mouse immune cells, then labeled these VHHs with radioisotopes and injected them into tumor-bearing mice. Subsequent PET imaging detected the location of immune cells around the tumor quickly and accurately.
“We were able to image tumors as small as 1 mm in size and within just a few days of their starting to grow,” said Mohammad Rashidian, PhD, a researcher in Dr Ploegh’s lab.
“We’re very excited about this because it’s a powerful approach to pick up inflammation in and around the tumor.”
Drs Rashidian and Ploegh believe that, with further refinement, the method could be used to monitor response to—and perhaps modify—cancer immunotherapy.
“To succeed with immunotherapy, we need more information about the tumor microenvironment,” Dr Rashidian said. “With this method, you could perhaps start immunotherapy and then, a few weeks later, image with VHHs to figure out progress and success of treatment.”
“PET imaging should allow a much more comprehensive look at the entire tumor in its environment,” Dr Ploegh added. “Then we can ask, ‘Did the tumor grow? Did immune cells invade? What has happened to the tumor?’ And to be able to see this without going in invasively is a significant achievement.” ![]()

Photo by Aaron Logan
Researchers say they have devised an approach that allows for real-time imaging of the immune system’s response to tumors, without the need for blood draws or biopsies.
The method harnesses PET to identify areas of immune cell activity associated with inflammation or tumor development.
The researchers believe the approach offers a potential breakthrough in diagnostics and the ability to monitor the efficacy of cancer therapies.
The team described their method in PNAS.
Study author Hidde Ploegh, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, said that every experimental immunologist wants to monitor an ongoing immune response, but options are limited.
“One can look at blood, but blood is a vehicle of transport for immune cells and is not where immune responses occur,” he said. “Surgical biopsies are invasive and non-random, so, for example, a fine-needle aspirate of a tumor could miss a significant feature of that condition.”
In search of a better monitoring approach, Dr Ploegh and his colleagues leveraged two research tools that have become staples in his lab in recent years.
The first exploits single-domain antibodies known as VHHs, derived from the heavy chain-only antibodies made by the immune systems of animals in the camelid family. Dr Ploegh’s lab immunizes alpacas—his camelid of choice—to generate VHHs specific to immune cells of interest.
The second tool, sortagging, labels the VHHs in a site-specific fashion so that researchers can track the VHHs and their targets in a living animal.
Knowing that the tissue around tumors often contains immune cells such as neutrophils and macrophages, Dr Ploegh and his colleagues hypothesized that appropriately labeled VHHs might allow them to pinpoint tumor locations by finding the tumor-associated immune cells.
Dr Ploegh noted that VHHs’ extremely small size—approximately one-tenth that of conventional antibodies—are likely responsible for their superior tissue penetration and, thus, makes them particularly well suited for such use.
So the researchers generated VHHs that recognize mouse immune cells, then labeled these VHHs with radioisotopes and injected them into tumor-bearing mice. Subsequent PET imaging detected the location of immune cells around the tumor quickly and accurately.
“We were able to image tumors as small as 1 mm in size and within just a few days of their starting to grow,” said Mohammad Rashidian, PhD, a researcher in Dr Ploegh’s lab.
“We’re very excited about this because it’s a powerful approach to pick up inflammation in and around the tumor.”
Drs Rashidian and Ploegh believe that, with further refinement, the method could be used to monitor response to—and perhaps modify—cancer immunotherapy.
“To succeed with immunotherapy, we need more information about the tumor microenvironment,” Dr Rashidian said. “With this method, you could perhaps start immunotherapy and then, a few weeks later, image with VHHs to figure out progress and success of treatment.”
“PET imaging should allow a much more comprehensive look at the entire tumor in its environment,” Dr Ploegh added. “Then we can ask, ‘Did the tumor grow? Did immune cells invade? What has happened to the tumor?’ And to be able to see this without going in invasively is a significant achievement.” ![]()