User login
High-Dose Barium Enemas Prevent Recurrent Diverticular Bleeding
Clinical question
Does barium impaction therapy using high-dose barium enemas prevent recurrent diverticular bleeding?
Bottom line
This small study demonstrates that barium impaction therapy using high-dose barium enemas is safe and effective at reducing the rate of recurrent diverticular bleeding. Note that this study was conducted in Japan, where the rate of rebleeding for patients with diverticulosis is much higher than in Western populations. (LOE = 1b-)
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis
A high-dose barium enema is thought to prevent recurrent diverticular bleeding through a physical tamponade of bleeding vessels, as well as by a direct hemostatic effect of the barium itself. Retained barium in colonic diverticula over time has previously been shown to be safe.
In this trial, patients hospitalized with diverticular bleeding who had spontaneous cessation of bleeding were randomized, using concealed allocation, to receive either barium impaction therapy (n = 27) or conservative treatment (n = 27). In the barium impaction therapy group, barium sulfate was administered by gastroenterologists via an enema bag at a concentration of 200 g barium per 100 mL tap water for a total volume of 400 mL. X-ray imaging confirmed filling of multiple colonic diverticula with barium and the patient was asked to rotate positions to ensure filling of all diverticula.
Baseline characteristics were similar in the 2 groups: the majority of patients were male, the average age was 70 years, and half had a prior history of diverticular bleeding. The severity of initial bleeding was also similar, as measured by number of units of blood transfused prior to randomization and the number of days until spontaneous cessation of bleeding.
For the primary outcome of recurrence of bleeding at the 1-year follow-up, the barium group fared better than the conservative treatment group (15% vs 43%; P = .04). You would have to treat 4 patients with barium impaction therapy to prevent 1 episode of recurrent bleeding. After adjusting for factors associated with recurrent bleeding, including hypertension, nonsteroidal anti-inflammatory drug use, and chronic renal failure, the risk of bleeding was decreased in the barium group (hazard ratio = 0.34, 95% CI 0.12-0.97). Barium impaction therapy did not result in any complications. Furthermore, over the course of the follow-up period, the barium group had a decreased number of re-hospitalizations, transfusions, and repeat colonoscopies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does barium impaction therapy using high-dose barium enemas prevent recurrent diverticular bleeding?
Bottom line
This small study demonstrates that barium impaction therapy using high-dose barium enemas is safe and effective at reducing the rate of recurrent diverticular bleeding. Note that this study was conducted in Japan, where the rate of rebleeding for patients with diverticulosis is much higher than in Western populations. (LOE = 1b-)
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis
A high-dose barium enema is thought to prevent recurrent diverticular bleeding through a physical tamponade of bleeding vessels, as well as by a direct hemostatic effect of the barium itself. Retained barium in colonic diverticula over time has previously been shown to be safe.
In this trial, patients hospitalized with diverticular bleeding who had spontaneous cessation of bleeding were randomized, using concealed allocation, to receive either barium impaction therapy (n = 27) or conservative treatment (n = 27). In the barium impaction therapy group, barium sulfate was administered by gastroenterologists via an enema bag at a concentration of 200 g barium per 100 mL tap water for a total volume of 400 mL. X-ray imaging confirmed filling of multiple colonic diverticula with barium and the patient was asked to rotate positions to ensure filling of all diverticula.
Baseline characteristics were similar in the 2 groups: the majority of patients were male, the average age was 70 years, and half had a prior history of diverticular bleeding. The severity of initial bleeding was also similar, as measured by number of units of blood transfused prior to randomization and the number of days until spontaneous cessation of bleeding.
For the primary outcome of recurrence of bleeding at the 1-year follow-up, the barium group fared better than the conservative treatment group (15% vs 43%; P = .04). You would have to treat 4 patients with barium impaction therapy to prevent 1 episode of recurrent bleeding. After adjusting for factors associated with recurrent bleeding, including hypertension, nonsteroidal anti-inflammatory drug use, and chronic renal failure, the risk of bleeding was decreased in the barium group (hazard ratio = 0.34, 95% CI 0.12-0.97). Barium impaction therapy did not result in any complications. Furthermore, over the course of the follow-up period, the barium group had a decreased number of re-hospitalizations, transfusions, and repeat colonoscopies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does barium impaction therapy using high-dose barium enemas prevent recurrent diverticular bleeding?
Bottom line
This small study demonstrates that barium impaction therapy using high-dose barium enemas is safe and effective at reducing the rate of recurrent diverticular bleeding. Note that this study was conducted in Japan, where the rate of rebleeding for patients with diverticulosis is much higher than in Western populations. (LOE = 1b-)
Study design: Randomized controlled trial (nonblinded)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis
A high-dose barium enema is thought to prevent recurrent diverticular bleeding through a physical tamponade of bleeding vessels, as well as by a direct hemostatic effect of the barium itself. Retained barium in colonic diverticula over time has previously been shown to be safe.
In this trial, patients hospitalized with diverticular bleeding who had spontaneous cessation of bleeding were randomized, using concealed allocation, to receive either barium impaction therapy (n = 27) or conservative treatment (n = 27). In the barium impaction therapy group, barium sulfate was administered by gastroenterologists via an enema bag at a concentration of 200 g barium per 100 mL tap water for a total volume of 400 mL. X-ray imaging confirmed filling of multiple colonic diverticula with barium and the patient was asked to rotate positions to ensure filling of all diverticula.
Baseline characteristics were similar in the 2 groups: the majority of patients were male, the average age was 70 years, and half had a prior history of diverticular bleeding. The severity of initial bleeding was also similar, as measured by number of units of blood transfused prior to randomization and the number of days until spontaneous cessation of bleeding.
For the primary outcome of recurrence of bleeding at the 1-year follow-up, the barium group fared better than the conservative treatment group (15% vs 43%; P = .04). You would have to treat 4 patients with barium impaction therapy to prevent 1 episode of recurrent bleeding. After adjusting for factors associated with recurrent bleeding, including hypertension, nonsteroidal anti-inflammatory drug use, and chronic renal failure, the risk of bleeding was decreased in the barium group (hazard ratio = 0.34, 95% CI 0.12-0.97). Barium impaction therapy did not result in any complications. Furthermore, over the course of the follow-up period, the barium group had a decreased number of re-hospitalizations, transfusions, and repeat colonoscopies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Steroids May Benefit Patients With Severe CAP and High CRP Levels
Clinical question: Do steroids improve outcomes in patients with severe community-acquired pneumonia and a high inflammatory response?
Bottom line
In patients with severe community-acquired pneumonia (CAP) who have elevated levels of C-reactive protein (CRP), a short course of methylprednisolone decreases treatment failure, mainly by reducing radiographic progression of pulmonary infiltrates within 3 days to 5 days of treatment initiation. The patient population studied here represents a fraction of the patients with severe CAP, so this finding cannot be generalized. Moreover, this study was small and the findings require replication before they can be applied to this population.
Design: Randomized controlled trial (double-blinded); LOE: 1b
Setting: Inpatient (any location)
Synopsis
A previous meta-analysis of randomized controlled trials showed that the addition of steroids for treatment of community-acquired pneumonia decreases hospital length of stay but does not affect other clinical outcomes such as mortality or need for mechanical ventilation (J Hosp Med 2013;8:68-75).
In this study, investigators enrolled patients hospitalized with severe CAP (either risk class V by the Pneumonia Severity Index or as defined by American Thoracic Society) and a CRP level of greater than 15 mg/dL. Immunosuppressed patients or those with diabetes or recent major gastrointestinal bleeding were excluded.
Patients were randomized, using concealed allocation, to receive either methylprednisolone (0.5 mg per kg) every 12 hours (n = 61) or matching placebo (n = 59) for 5 days. The primary outcome was treatment failure. Early treatment failure was defined as clinical deterioration within 72 hours of treatment, whereas late failure was defined as radiographic progression of pulmonary infiltrates by more than 50%, respiratory failure, shock, or death between 3 days and 5 days.
Baseline characteristics were similar in the 2 groups, except for lower procalcitonin levels and less septic shock in the steroid group. Antibiotic treatment on admission was similar in both groups (most commonly ceftriaxone combined with either levofloxacin or azithromycin). The majority of patients were initially admitted to the intensive care unit. In the intention-to-treat analysis, the steroid group had less treatment failure than the placebo group (13% vs 31%; P = .02). This was driven by a higher rate of late treatment failure in the placebo group, primarily due to a greater incidence of radiographic progression of infiltrates. Results were similar after adjusting for potential confounders and imbalances in baseline characteristics (hazard ratio = 0.33, 95% CI 0.12 - 0.90; P = .03). There were no significant differences in length of stay, in-hospital mortality, or adverse events between the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Do steroids improve outcomes in patients with severe community-acquired pneumonia and a high inflammatory response?
Bottom line
In patients with severe community-acquired pneumonia (CAP) who have elevated levels of C-reactive protein (CRP), a short course of methylprednisolone decreases treatment failure, mainly by reducing radiographic progression of pulmonary infiltrates within 3 days to 5 days of treatment initiation. The patient population studied here represents a fraction of the patients with severe CAP, so this finding cannot be generalized. Moreover, this study was small and the findings require replication before they can be applied to this population.
Design: Randomized controlled trial (double-blinded); LOE: 1b
Setting: Inpatient (any location)
Synopsis
A previous meta-analysis of randomized controlled trials showed that the addition of steroids for treatment of community-acquired pneumonia decreases hospital length of stay but does not affect other clinical outcomes such as mortality or need for mechanical ventilation (J Hosp Med 2013;8:68-75).
In this study, investigators enrolled patients hospitalized with severe CAP (either risk class V by the Pneumonia Severity Index or as defined by American Thoracic Society) and a CRP level of greater than 15 mg/dL. Immunosuppressed patients or those with diabetes or recent major gastrointestinal bleeding were excluded.
Patients were randomized, using concealed allocation, to receive either methylprednisolone (0.5 mg per kg) every 12 hours (n = 61) or matching placebo (n = 59) for 5 days. The primary outcome was treatment failure. Early treatment failure was defined as clinical deterioration within 72 hours of treatment, whereas late failure was defined as radiographic progression of pulmonary infiltrates by more than 50%, respiratory failure, shock, or death between 3 days and 5 days.
Baseline characteristics were similar in the 2 groups, except for lower procalcitonin levels and less septic shock in the steroid group. Antibiotic treatment on admission was similar in both groups (most commonly ceftriaxone combined with either levofloxacin or azithromycin). The majority of patients were initially admitted to the intensive care unit. In the intention-to-treat analysis, the steroid group had less treatment failure than the placebo group (13% vs 31%; P = .02). This was driven by a higher rate of late treatment failure in the placebo group, primarily due to a greater incidence of radiographic progression of infiltrates. Results were similar after adjusting for potential confounders and imbalances in baseline characteristics (hazard ratio = 0.33, 95% CI 0.12 - 0.90; P = .03). There were no significant differences in length of stay, in-hospital mortality, or adverse events between the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Do steroids improve outcomes in patients with severe community-acquired pneumonia and a high inflammatory response?
Bottom line
In patients with severe community-acquired pneumonia (CAP) who have elevated levels of C-reactive protein (CRP), a short course of methylprednisolone decreases treatment failure, mainly by reducing radiographic progression of pulmonary infiltrates within 3 days to 5 days of treatment initiation. The patient population studied here represents a fraction of the patients with severe CAP, so this finding cannot be generalized. Moreover, this study was small and the findings require replication before they can be applied to this population.
Design: Randomized controlled trial (double-blinded); LOE: 1b
Setting: Inpatient (any location)
Synopsis
A previous meta-analysis of randomized controlled trials showed that the addition of steroids for treatment of community-acquired pneumonia decreases hospital length of stay but does not affect other clinical outcomes such as mortality or need for mechanical ventilation (J Hosp Med 2013;8:68-75).
In this study, investigators enrolled patients hospitalized with severe CAP (either risk class V by the Pneumonia Severity Index or as defined by American Thoracic Society) and a CRP level of greater than 15 mg/dL. Immunosuppressed patients or those with diabetes or recent major gastrointestinal bleeding were excluded.
Patients were randomized, using concealed allocation, to receive either methylprednisolone (0.5 mg per kg) every 12 hours (n = 61) or matching placebo (n = 59) for 5 days. The primary outcome was treatment failure. Early treatment failure was defined as clinical deterioration within 72 hours of treatment, whereas late failure was defined as radiographic progression of pulmonary infiltrates by more than 50%, respiratory failure, shock, or death between 3 days and 5 days.
Baseline characteristics were similar in the 2 groups, except for lower procalcitonin levels and less septic shock in the steroid group. Antibiotic treatment on admission was similar in both groups (most commonly ceftriaxone combined with either levofloxacin or azithromycin). The majority of patients were initially admitted to the intensive care unit. In the intention-to-treat analysis, the steroid group had less treatment failure than the placebo group (13% vs 31%; P = .02). This was driven by a higher rate of late treatment failure in the placebo group, primarily due to a greater incidence of radiographic progression of infiltrates. Results were similar after adjusting for potential confounders and imbalances in baseline characteristics (hazard ratio = 0.33, 95% CI 0.12 - 0.90; P = .03). There were no significant differences in length of stay, in-hospital mortality, or adverse events between the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Inhibitor controls WM long-term

Photo by Sam Odgen
Updated results of a phase 2 trial suggest the Bruton’s tyrosine kinase inhibitor ibrutinib can control Waldenstrom’s macroglobulinemia (WM) long-term.
In previously treated WM patients, ibrutinib produced an overall response rate of 91%.
The 2-year overall survival rate was 95%, and 69% of patients had not progressed at 2 years.
Researchers reported these results in NEJM. The research was supported by Pharmacyclics, Janssen Pharmaceuticals, and several foundations.
An earlier analysis of data from this trial supported the US Food and Drug Administration’s approval of ibrutinib as the first treatment for WM.
“These findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia and show how genome sequencing can lead to the discovery of cancer mutations that can be specifically targeted by new therapies,” said study author Steven Treon, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Treon and his colleagues enrolled 63 WM patients on this trial. The patients had received a median of 2 prior therapies (range, 1-9), 56 patients (89%) had the MYD88L265P mutation, and 21 (34%) had the CXCR4WHIM mutation.
Patients received ibrutinib at 420 mg once daily until disease progression or unacceptable toxicity. After a median treatment duration of 19.1 months (range, 0.5-29.7 months), the overall response rate was 91%. The median time to response was 4 weeks.
Investigator-determined responses were impacted by the MYD88 and CXCR4 mutations. Patients carrying MYD88L265P and CXCR4WT achieved the highest responses, with a 100% overall response rate and 91% major response rate.
For all patients, the estimated progression-free and overall survival rates at 24 months were 69% and 95%, respectively.
“The results are remarkable when you consider that patients had received an average of 2 prior therapies, and 40% showed no response to the previous treatments,” Dr Treon said. “The findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia.”
Dr Treon and his colleagues also said ibrutinib was well-tolerated. At the time of analysis, 68% of patients remained on therapy.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Grade 3 or higher neutropenia and thrombocytopenia occurred in 9 (14%) and 8 (13%) patients, respectively.
Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()

Photo by Sam Odgen
Updated results of a phase 2 trial suggest the Bruton’s tyrosine kinase inhibitor ibrutinib can control Waldenstrom’s macroglobulinemia (WM) long-term.
In previously treated WM patients, ibrutinib produced an overall response rate of 91%.
The 2-year overall survival rate was 95%, and 69% of patients had not progressed at 2 years.
Researchers reported these results in NEJM. The research was supported by Pharmacyclics, Janssen Pharmaceuticals, and several foundations.
An earlier analysis of data from this trial supported the US Food and Drug Administration’s approval of ibrutinib as the first treatment for WM.
“These findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia and show how genome sequencing can lead to the discovery of cancer mutations that can be specifically targeted by new therapies,” said study author Steven Treon, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Treon and his colleagues enrolled 63 WM patients on this trial. The patients had received a median of 2 prior therapies (range, 1-9), 56 patients (89%) had the MYD88L265P mutation, and 21 (34%) had the CXCR4WHIM mutation.
Patients received ibrutinib at 420 mg once daily until disease progression or unacceptable toxicity. After a median treatment duration of 19.1 months (range, 0.5-29.7 months), the overall response rate was 91%. The median time to response was 4 weeks.
Investigator-determined responses were impacted by the MYD88 and CXCR4 mutations. Patients carrying MYD88L265P and CXCR4WT achieved the highest responses, with a 100% overall response rate and 91% major response rate.
For all patients, the estimated progression-free and overall survival rates at 24 months were 69% and 95%, respectively.
“The results are remarkable when you consider that patients had received an average of 2 prior therapies, and 40% showed no response to the previous treatments,” Dr Treon said. “The findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia.”
Dr Treon and his colleagues also said ibrutinib was well-tolerated. At the time of analysis, 68% of patients remained on therapy.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Grade 3 or higher neutropenia and thrombocytopenia occurred in 9 (14%) and 8 (13%) patients, respectively.
Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()

Photo by Sam Odgen
Updated results of a phase 2 trial suggest the Bruton’s tyrosine kinase inhibitor ibrutinib can control Waldenstrom’s macroglobulinemia (WM) long-term.
In previously treated WM patients, ibrutinib produced an overall response rate of 91%.
The 2-year overall survival rate was 95%, and 69% of patients had not progressed at 2 years.
Researchers reported these results in NEJM. The research was supported by Pharmacyclics, Janssen Pharmaceuticals, and several foundations.
An earlier analysis of data from this trial supported the US Food and Drug Administration’s approval of ibrutinib as the first treatment for WM.
“These findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia and show how genome sequencing can lead to the discovery of cancer mutations that can be specifically targeted by new therapies,” said study author Steven Treon, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Treon and his colleagues enrolled 63 WM patients on this trial. The patients had received a median of 2 prior therapies (range, 1-9), 56 patients (89%) had the MYD88L265P mutation, and 21 (34%) had the CXCR4WHIM mutation.
Patients received ibrutinib at 420 mg once daily until disease progression or unacceptable toxicity. After a median treatment duration of 19.1 months (range, 0.5-29.7 months), the overall response rate was 91%. The median time to response was 4 weeks.
Investigator-determined responses were impacted by the MYD88 and CXCR4 mutations. Patients carrying MYD88L265P and CXCR4WT achieved the highest responses, with a 100% overall response rate and 91% major response rate.
For all patients, the estimated progression-free and overall survival rates at 24 months were 69% and 95%, respectively.
“The results are remarkable when you consider that patients had received an average of 2 prior therapies, and 40% showed no response to the previous treatments,” Dr Treon said. “The findings herald a new era for the treatment of Waldenstrom’s macroglobulinemia.”
Dr Treon and his colleagues also said ibrutinib was well-tolerated. At the time of analysis, 68% of patients remained on therapy.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Grade 3 or higher neutropenia and thrombocytopenia occurred in 9 (14%) and 8 (13%) patients, respectively.
Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()
Uninsured cancer patients pay more

Photo by Rhoda Baer
Uninsured patients are asked to pay much more for cancer treatments than Medicare and private insurers pay, according to research published in Health Affairs.
Researchers reviewed newly available Medicare data on what physicians charged for intravenous chemotherapy drugs in 2012.
And the results showed that uninsured cancer patients were asked to pay anywhere from 2 to 43 times what Medicare would pay and 2 to 5 times as
much as private insurance would pay.
For example, uninsured patients who did not negotiate the billed amounts could expect to pay $6711 for an infusion of the colorectal cancer drug oxaliplatin. But Medicare and private health plans only pay $3090 and $3616, respectively.
“Patients with Medicare and private insurance don’t pay the sticker price of healthcare,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“They pay a discounted rate. However, uninsured patients don’t have the bargaining power, or they may not try to negotiate for a better price.”
In addition to estimating costs for infused chemotherapy drugs, Dr Dusetzina and her colleagues looked at what cancer patients were asked to pay for a doctor visit.
Uninsured patients were billed between $129 and $391, depending on the complexity of the visit. Medicare paid between $65 and $188, and private insurance paid $78 to $246 for the same visits.
“This is unreasonable,” Dr Dusetzina said. “There needs to be more transparency and less variability in healthcare pricing.”
Under the Affordable Care Act, everyone in the US must be insured or face a tax penalty. However, many Americans remain without insurance. So differences in what the uninsured are charged really matter, Dr Dusetzina said.
“In states like North Carolina that didn’t expand Medicaid, there is a large group of people who can get insurance on the federal exchange but cannot get subsidies because the law assumed they would be covered by Medicaid,” she noted.
This population earns at or near the federal poverty level. Without subsidies to help pay for private insurance, many are likely to remain uninsured.
These drug pricing discrepancies could become even more important depending on the outcome of the pending Supreme Court case King vs Burwell, which challenges the legitimacy of federal healthcare subsidies and could leave as many as 8 million Americans without subsidies and uninsured in 2016. ![]()

Photo by Rhoda Baer
Uninsured patients are asked to pay much more for cancer treatments than Medicare and private insurers pay, according to research published in Health Affairs.
Researchers reviewed newly available Medicare data on what physicians charged for intravenous chemotherapy drugs in 2012.
And the results showed that uninsured cancer patients were asked to pay anywhere from 2 to 43 times what Medicare would pay and 2 to 5 times as
much as private insurance would pay.
For example, uninsured patients who did not negotiate the billed amounts could expect to pay $6711 for an infusion of the colorectal cancer drug oxaliplatin. But Medicare and private health plans only pay $3090 and $3616, respectively.
“Patients with Medicare and private insurance don’t pay the sticker price of healthcare,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“They pay a discounted rate. However, uninsured patients don’t have the bargaining power, or they may not try to negotiate for a better price.”
In addition to estimating costs for infused chemotherapy drugs, Dr Dusetzina and her colleagues looked at what cancer patients were asked to pay for a doctor visit.
Uninsured patients were billed between $129 and $391, depending on the complexity of the visit. Medicare paid between $65 and $188, and private insurance paid $78 to $246 for the same visits.
“This is unreasonable,” Dr Dusetzina said. “There needs to be more transparency and less variability in healthcare pricing.”
Under the Affordable Care Act, everyone in the US must be insured or face a tax penalty. However, many Americans remain without insurance. So differences in what the uninsured are charged really matter, Dr Dusetzina said.
“In states like North Carolina that didn’t expand Medicaid, there is a large group of people who can get insurance on the federal exchange but cannot get subsidies because the law assumed they would be covered by Medicaid,” she noted.
This population earns at or near the federal poverty level. Without subsidies to help pay for private insurance, many are likely to remain uninsured.
These drug pricing discrepancies could become even more important depending on the outcome of the pending Supreme Court case King vs Burwell, which challenges the legitimacy of federal healthcare subsidies and could leave as many as 8 million Americans without subsidies and uninsured in 2016. ![]()

Photo by Rhoda Baer
Uninsured patients are asked to pay much more for cancer treatments than Medicare and private insurers pay, according to research published in Health Affairs.
Researchers reviewed newly available Medicare data on what physicians charged for intravenous chemotherapy drugs in 2012.
And the results showed that uninsured cancer patients were asked to pay anywhere from 2 to 43 times what Medicare would pay and 2 to 5 times as
much as private insurance would pay.
For example, uninsured patients who did not negotiate the billed amounts could expect to pay $6711 for an infusion of the colorectal cancer drug oxaliplatin. But Medicare and private health plans only pay $3090 and $3616, respectively.
“Patients with Medicare and private insurance don’t pay the sticker price of healthcare,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“They pay a discounted rate. However, uninsured patients don’t have the bargaining power, or they may not try to negotiate for a better price.”
In addition to estimating costs for infused chemotherapy drugs, Dr Dusetzina and her colleagues looked at what cancer patients were asked to pay for a doctor visit.
Uninsured patients were billed between $129 and $391, depending on the complexity of the visit. Medicare paid between $65 and $188, and private insurance paid $78 to $246 for the same visits.
“This is unreasonable,” Dr Dusetzina said. “There needs to be more transparency and less variability in healthcare pricing.”
Under the Affordable Care Act, everyone in the US must be insured or face a tax penalty. However, many Americans remain without insurance. So differences in what the uninsured are charged really matter, Dr Dusetzina said.
“In states like North Carolina that didn’t expand Medicaid, there is a large group of people who can get insurance on the federal exchange but cannot get subsidies because the law assumed they would be covered by Medicaid,” she noted.
This population earns at or near the federal poverty level. Without subsidies to help pay for private insurance, many are likely to remain uninsured.
These drug pricing discrepancies could become even more important depending on the outcome of the pending Supreme Court case King vs Burwell, which challenges the legitimacy of federal healthcare subsidies and could leave as many as 8 million Americans without subsidies and uninsured in 2016. ![]()
FDA grants drug orphan designation for SCD
Image by Graham Beards
The US Food and Drug Administration (FDA) has granted orphan drug designation for the bovine PEGylated carboxyhemoglobin product Sanguinate to treat sickle cell disease (SCD).
Through its anti-vaso-constrictive properties, Sanguinate facilitates the transfer of oxygen to oxygen-deprived cells and tissues.
By correcting oxygen levels and downregulating inflammation, the drug could potentially treat many of the comorbidities associated with SCD.
Trials of Sanguinate
The company developing Sanguinate, Prolong Pharmaceuticals, has several clinical studies underway to determine the safety and efficacy of the drug in SCD and other diseases caused by the effects of oxygen deprivation.
In a phase 1 trial, Sanguinate proved safe and well-tolerated in healthy volunteers. Three cohorts of 8 subjects received single, ascending doses of Sanguinate at 80 mg/kg, 120 mg/kg, or 160 mg/kg. Two volunteers in each cohort were control subjects who received saline.
There were no serious adverse events reported with Sanguinate. Subjects experienced decreases in serum haptoglobin, but this did not appear to be dose-related. Sanguinate’s half-life was dose-dependent and ranged from 7.9 hours to 13.8 hours.
A phase 1 study of Sanguinate in SCD patients has been completed, and researchers are now conducting a phase 2 study testing the drug for the reduction or prevention of delayed cerebral ischemia following subarachnoid hemorrhage.
Phase 2 trials are also planned for vaso-occlusive crisis and leg ulcers secondary to SCD, as well as for preventing delayed graft function following kidney transplant. Sanguinate is also being evaluated for the treatment of beta-thalassemia.
About orphan designation
The FDA grants orphan designation to diseases affecting fewer than 200,000 people in the US.
Orphan designation provides the company developing a drug with certain benefits and incentives, including a 7-year period of marketing exclusivity upon regulatory approval, potential tax credits for certain activities, eligibility for orphan drug grants, and the waiver of certain administrative fees. ![]()

Image by Graham Beards
The US Food and Drug Administration (FDA) has granted orphan drug designation for the bovine PEGylated carboxyhemoglobin product Sanguinate to treat sickle cell disease (SCD).
Through its anti-vaso-constrictive properties, Sanguinate facilitates the transfer of oxygen to oxygen-deprived cells and tissues.
By correcting oxygen levels and downregulating inflammation, the drug could potentially treat many of the comorbidities associated with SCD.
Trials of Sanguinate
The company developing Sanguinate, Prolong Pharmaceuticals, has several clinical studies underway to determine the safety and efficacy of the drug in SCD and other diseases caused by the effects of oxygen deprivation.
In a phase 1 trial, Sanguinate proved safe and well-tolerated in healthy volunteers. Three cohorts of 8 subjects received single, ascending doses of Sanguinate at 80 mg/kg, 120 mg/kg, or 160 mg/kg. Two volunteers in each cohort were control subjects who received saline.
There were no serious adverse events reported with Sanguinate. Subjects experienced decreases in serum haptoglobin, but this did not appear to be dose-related. Sanguinate’s half-life was dose-dependent and ranged from 7.9 hours to 13.8 hours.
A phase 1 study of Sanguinate in SCD patients has been completed, and researchers are now conducting a phase 2 study testing the drug for the reduction or prevention of delayed cerebral ischemia following subarachnoid hemorrhage.
Phase 2 trials are also planned for vaso-occlusive crisis and leg ulcers secondary to SCD, as well as for preventing delayed graft function following kidney transplant. Sanguinate is also being evaluated for the treatment of beta-thalassemia.
About orphan designation
The FDA grants orphan designation to diseases affecting fewer than 200,000 people in the US.
Orphan designation provides the company developing a drug with certain benefits and incentives, including a 7-year period of marketing exclusivity upon regulatory approval, potential tax credits for certain activities, eligibility for orphan drug grants, and the waiver of certain administrative fees. ![]()

Image by Graham Beards
The US Food and Drug Administration (FDA) has granted orphan drug designation for the bovine PEGylated carboxyhemoglobin product Sanguinate to treat sickle cell disease (SCD).
Through its anti-vaso-constrictive properties, Sanguinate facilitates the transfer of oxygen to oxygen-deprived cells and tissues.
By correcting oxygen levels and downregulating inflammation, the drug could potentially treat many of the comorbidities associated with SCD.
Trials of Sanguinate
The company developing Sanguinate, Prolong Pharmaceuticals, has several clinical studies underway to determine the safety and efficacy of the drug in SCD and other diseases caused by the effects of oxygen deprivation.
In a phase 1 trial, Sanguinate proved safe and well-tolerated in healthy volunteers. Three cohorts of 8 subjects received single, ascending doses of Sanguinate at 80 mg/kg, 120 mg/kg, or 160 mg/kg. Two volunteers in each cohort were control subjects who received saline.
There were no serious adverse events reported with Sanguinate. Subjects experienced decreases in serum haptoglobin, but this did not appear to be dose-related. Sanguinate’s half-life was dose-dependent and ranged from 7.9 hours to 13.8 hours.
A phase 1 study of Sanguinate in SCD patients has been completed, and researchers are now conducting a phase 2 study testing the drug for the reduction or prevention of delayed cerebral ischemia following subarachnoid hemorrhage.
Phase 2 trials are also planned for vaso-occlusive crisis and leg ulcers secondary to SCD, as well as for preventing delayed graft function following kidney transplant. Sanguinate is also being evaluated for the treatment of beta-thalassemia.
About orphan designation
The FDA grants orphan designation to diseases affecting fewer than 200,000 people in the US.
Orphan designation provides the company developing a drug with certain benefits and incentives, including a 7-year period of marketing exclusivity upon regulatory approval, potential tax credits for certain activities, eligibility for orphan drug grants, and the waiver of certain administrative fees. ![]()
Thrombectomy fails to improve PCI outcomes, ups stroke risk
SAN DIEGO – Routine manual thrombectomy before percutaneous coronary intervention did not improve 180-day outcomes and was linked with an increased risk of stroke in patients with acute ST-segment elevation MI in the TOTAL trial.
Routine thrombectomy had no effect on the primary outcome of cardiovascular death, MI, cardiogenic shock, or New York Heart Association class IV heart failure, occurring in 6.9% of thrombectomy patients and 7% of PCI-only patients.
However, the study’s primary safety endpoint of stroke at 30 days doubled in patients undergoing routine thrombectomy before PCI to 33 events (0.7%), compared with those who had PCI with only bailout thrombectomy (16 events [0.3%]; P = .015).
The same pattern was observed with stroke or transient ischemic attack within 30 days (42 vs. 19 events; hazard ratio, 2.21; P = .003) and continued for stroke within 180 days (52 vs. 25 events; HR, 2.08; P = .002).
“The stroke findings are unexpected and we believe require confirmation in other datasets. A detailed case-by-case review is underway to help us understand the etiology and the relationship with the procedure,” lead author Dr. Sanjit S. Jolly said at the annual meeting of the American College of Cardiology.
Enthusiasm for manual thrombus aspiration was sparked by a survival benefit observed in the single-center, prospective TAPAS trial in ST-segment elevation MI patients (STEMI), and the procedure was widely adopted.
The more recent, multicenter TASTE trial, however, reported that routine thrombectomy before PCI failed to significantly reduce 30-day mortality in 7,244 STEMI patients, though there were trends toward reductions in stent thrombosis and hospitalization for recurrent MI.
TOTAL (Manual Aspiration Thrombectomy Plus PCI vs. PCI Alone in STEMI) randomly assigned 10,063 patients within 12 hours of STEMI symptoms to primary PCI either with upfront manual thrombectomy or only bailout thrombectomy if the PCI strategy failed.
The lack of significant differences between groups in the primary outcome was also true in all the components of the primary outcome. Furthermore, there was no effect on the primary outcome based on thrombotic burden, a question that remained unanswered after TASTE, Dr. Jolly reported. The TOTAL results were published online simultaneously with his report (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1415098]).
“TOTAL and TASTE emphasize the need to conduct large randomized trials of common interventions, even when small trials appear positive,” Dr. Jolly said.
Discussant Dr. Steven Nissen, chair of cardiovascular medicine at Cleveland Clinic, described the routine use of thrombectomy as “a sad story about device regulation in the United States” in that the evidence level needed to get a medical device on the market is so far below that required for drug approval that patients undergo procedures without good randomized trial evidence to show they even work.
“We dodged a bullet recently with renal denervation when everyone thought it would work, and when you finally tested it, it didn’t,” Dr. Nissen said. “Let this be a lesson to us: We need to have more rigorous studies of medical devices before they get to market and get used in very large numbers of people.”
Currently, aspiration thrombectomy carries a IIa recommendation for use with PCI in the most recent ACC/American Heart Association guidelines for the management of patients with STEMI (J. Am. Coll. Cardiol. 2009;54:2205-41).
When asked whether the guidelines should change based on the TOTAL and TASTE results, Dr. Jolly said there should be a clear recommendation that routine thrombus aspiration should not be the appropriate approach, while the issue of bailout aspiration may be left to clinician judgment.
The finding of late strokes is difficult to understand and should be interpreted with caution because of the small number of strokes occurring between 30 and 180 days, he said. Detailed analysis of all strokes will be presented at a later meeting, but Rankin Scale scores show several strokes were “very debilitating.” There is a consistency in the data, as a meta-analysis of smaller trials also identified an increased stroke risk with adjunctive thrombectomy.
Discussant Dr. Gregg W. Stone, director of cardiovascular research and education at Columbia University Medical Center in New York, said a mechanism for periprocedural stroke with aspiration can be envisioned, but that understanding the risk of ongoing, late stroke is more difficult.
As for why thrombectomy didn’t work, “aspiration is incredibly inefficient, thromboemboli still occur before, during, and after aspiration, the timing of aspiration is often too late to benefit most patients,” and other mechanisms of myonecrosis may predominate, such as reperfusion injury, he observed.
Dr. Stone said the TOTAL results should change practice and that the guideline recommendation should be downgraded to IIb.
“There are some patients who have a very large thrombus burden who have trouble dealing with all that thrombus in the cath lab who might benefit, and it is impossible to design randomized trials for small sections and groups of patients,” he said. “I wouldn’t make it class III by any means, but I think it’ll take a long time for that reduction in use to actually transmit through clinical practice, because I must say interventional cardiologists love the idea of simply removing thrombus with a relatively easy-to-use device.”
Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said in an interview that TOTAL will make operators much more selective and cautious in their performance of thrombectomy until further insights into the stroke issue are available. Thrombectomy should be reserved for bailout instances and not as a front-line therapy, he said.
On the other hand, the stroke rate in the early phase was not significantly different between groups in an as-treated analysis, and an opportunity exists to investigate potential differences between stroke and nonstroke patients to determine whether other comorbidities rather than thrombectomy per se may account for the stroke signal, Dr. Kandzari observed.
TOTAL was funded by the Canadian Institutes of Health Research, Canadian Network and Centre for Trials Internationally, and Medtronic. Dr. Jolly disclosed receiving consulting fees and honoraria from AstraZeneca, speaking fees for St. Jude, and research grants from Medtronic. Dr. Nissen has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. Dr. Stone reported consulting honoraria from Guided Delivery Systems, Miracor, and Reva, and ownership interest or partnership in Arstasis, Caliber, VNT, Micardia, Biostar family funds, and Medfocus family funds. Dr. Kandzari reported research and grant support from Medtronic, Biotronic, Abbott Vascular, and Boston Scientific.
SAN DIEGO – Routine manual thrombectomy before percutaneous coronary intervention did not improve 180-day outcomes and was linked with an increased risk of stroke in patients with acute ST-segment elevation MI in the TOTAL trial.
Routine thrombectomy had no effect on the primary outcome of cardiovascular death, MI, cardiogenic shock, or New York Heart Association class IV heart failure, occurring in 6.9% of thrombectomy patients and 7% of PCI-only patients.
However, the study’s primary safety endpoint of stroke at 30 days doubled in patients undergoing routine thrombectomy before PCI to 33 events (0.7%), compared with those who had PCI with only bailout thrombectomy (16 events [0.3%]; P = .015).
The same pattern was observed with stroke or transient ischemic attack within 30 days (42 vs. 19 events; hazard ratio, 2.21; P = .003) and continued for stroke within 180 days (52 vs. 25 events; HR, 2.08; P = .002).
“The stroke findings are unexpected and we believe require confirmation in other datasets. A detailed case-by-case review is underway to help us understand the etiology and the relationship with the procedure,” lead author Dr. Sanjit S. Jolly said at the annual meeting of the American College of Cardiology.
Enthusiasm for manual thrombus aspiration was sparked by a survival benefit observed in the single-center, prospective TAPAS trial in ST-segment elevation MI patients (STEMI), and the procedure was widely adopted.
The more recent, multicenter TASTE trial, however, reported that routine thrombectomy before PCI failed to significantly reduce 30-day mortality in 7,244 STEMI patients, though there were trends toward reductions in stent thrombosis and hospitalization for recurrent MI.
TOTAL (Manual Aspiration Thrombectomy Plus PCI vs. PCI Alone in STEMI) randomly assigned 10,063 patients within 12 hours of STEMI symptoms to primary PCI either with upfront manual thrombectomy or only bailout thrombectomy if the PCI strategy failed.
The lack of significant differences between groups in the primary outcome was also true in all the components of the primary outcome. Furthermore, there was no effect on the primary outcome based on thrombotic burden, a question that remained unanswered after TASTE, Dr. Jolly reported. The TOTAL results were published online simultaneously with his report (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1415098]).
“TOTAL and TASTE emphasize the need to conduct large randomized trials of common interventions, even when small trials appear positive,” Dr. Jolly said.
Discussant Dr. Steven Nissen, chair of cardiovascular medicine at Cleveland Clinic, described the routine use of thrombectomy as “a sad story about device regulation in the United States” in that the evidence level needed to get a medical device on the market is so far below that required for drug approval that patients undergo procedures without good randomized trial evidence to show they even work.
“We dodged a bullet recently with renal denervation when everyone thought it would work, and when you finally tested it, it didn’t,” Dr. Nissen said. “Let this be a lesson to us: We need to have more rigorous studies of medical devices before they get to market and get used in very large numbers of people.”
Currently, aspiration thrombectomy carries a IIa recommendation for use with PCI in the most recent ACC/American Heart Association guidelines for the management of patients with STEMI (J. Am. Coll. Cardiol. 2009;54:2205-41).
When asked whether the guidelines should change based on the TOTAL and TASTE results, Dr. Jolly said there should be a clear recommendation that routine thrombus aspiration should not be the appropriate approach, while the issue of bailout aspiration may be left to clinician judgment.
The finding of late strokes is difficult to understand and should be interpreted with caution because of the small number of strokes occurring between 30 and 180 days, he said. Detailed analysis of all strokes will be presented at a later meeting, but Rankin Scale scores show several strokes were “very debilitating.” There is a consistency in the data, as a meta-analysis of smaller trials also identified an increased stroke risk with adjunctive thrombectomy.
Discussant Dr. Gregg W. Stone, director of cardiovascular research and education at Columbia University Medical Center in New York, said a mechanism for periprocedural stroke with aspiration can be envisioned, but that understanding the risk of ongoing, late stroke is more difficult.
As for why thrombectomy didn’t work, “aspiration is incredibly inefficient, thromboemboli still occur before, during, and after aspiration, the timing of aspiration is often too late to benefit most patients,” and other mechanisms of myonecrosis may predominate, such as reperfusion injury, he observed.
Dr. Stone said the TOTAL results should change practice and that the guideline recommendation should be downgraded to IIb.
“There are some patients who have a very large thrombus burden who have trouble dealing with all that thrombus in the cath lab who might benefit, and it is impossible to design randomized trials for small sections and groups of patients,” he said. “I wouldn’t make it class III by any means, but I think it’ll take a long time for that reduction in use to actually transmit through clinical practice, because I must say interventional cardiologists love the idea of simply removing thrombus with a relatively easy-to-use device.”
Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said in an interview that TOTAL will make operators much more selective and cautious in their performance of thrombectomy until further insights into the stroke issue are available. Thrombectomy should be reserved for bailout instances and not as a front-line therapy, he said.
On the other hand, the stroke rate in the early phase was not significantly different between groups in an as-treated analysis, and an opportunity exists to investigate potential differences between stroke and nonstroke patients to determine whether other comorbidities rather than thrombectomy per se may account for the stroke signal, Dr. Kandzari observed.
TOTAL was funded by the Canadian Institutes of Health Research, Canadian Network and Centre for Trials Internationally, and Medtronic. Dr. Jolly disclosed receiving consulting fees and honoraria from AstraZeneca, speaking fees for St. Jude, and research grants from Medtronic. Dr. Nissen has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. Dr. Stone reported consulting honoraria from Guided Delivery Systems, Miracor, and Reva, and ownership interest or partnership in Arstasis, Caliber, VNT, Micardia, Biostar family funds, and Medfocus family funds. Dr. Kandzari reported research and grant support from Medtronic, Biotronic, Abbott Vascular, and Boston Scientific.
SAN DIEGO – Routine manual thrombectomy before percutaneous coronary intervention did not improve 180-day outcomes and was linked with an increased risk of stroke in patients with acute ST-segment elevation MI in the TOTAL trial.
Routine thrombectomy had no effect on the primary outcome of cardiovascular death, MI, cardiogenic shock, or New York Heart Association class IV heart failure, occurring in 6.9% of thrombectomy patients and 7% of PCI-only patients.
However, the study’s primary safety endpoint of stroke at 30 days doubled in patients undergoing routine thrombectomy before PCI to 33 events (0.7%), compared with those who had PCI with only bailout thrombectomy (16 events [0.3%]; P = .015).
The same pattern was observed with stroke or transient ischemic attack within 30 days (42 vs. 19 events; hazard ratio, 2.21; P = .003) and continued for stroke within 180 days (52 vs. 25 events; HR, 2.08; P = .002).
“The stroke findings are unexpected and we believe require confirmation in other datasets. A detailed case-by-case review is underway to help us understand the etiology and the relationship with the procedure,” lead author Dr. Sanjit S. Jolly said at the annual meeting of the American College of Cardiology.
Enthusiasm for manual thrombus aspiration was sparked by a survival benefit observed in the single-center, prospective TAPAS trial in ST-segment elevation MI patients (STEMI), and the procedure was widely adopted.
The more recent, multicenter TASTE trial, however, reported that routine thrombectomy before PCI failed to significantly reduce 30-day mortality in 7,244 STEMI patients, though there were trends toward reductions in stent thrombosis and hospitalization for recurrent MI.
TOTAL (Manual Aspiration Thrombectomy Plus PCI vs. PCI Alone in STEMI) randomly assigned 10,063 patients within 12 hours of STEMI symptoms to primary PCI either with upfront manual thrombectomy or only bailout thrombectomy if the PCI strategy failed.
The lack of significant differences between groups in the primary outcome was also true in all the components of the primary outcome. Furthermore, there was no effect on the primary outcome based on thrombotic burden, a question that remained unanswered after TASTE, Dr. Jolly reported. The TOTAL results were published online simultaneously with his report (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1415098]).
“TOTAL and TASTE emphasize the need to conduct large randomized trials of common interventions, even when small trials appear positive,” Dr. Jolly said.
Discussant Dr. Steven Nissen, chair of cardiovascular medicine at Cleveland Clinic, described the routine use of thrombectomy as “a sad story about device regulation in the United States” in that the evidence level needed to get a medical device on the market is so far below that required for drug approval that patients undergo procedures without good randomized trial evidence to show they even work.
“We dodged a bullet recently with renal denervation when everyone thought it would work, and when you finally tested it, it didn’t,” Dr. Nissen said. “Let this be a lesson to us: We need to have more rigorous studies of medical devices before they get to market and get used in very large numbers of people.”
Currently, aspiration thrombectomy carries a IIa recommendation for use with PCI in the most recent ACC/American Heart Association guidelines for the management of patients with STEMI (J. Am. Coll. Cardiol. 2009;54:2205-41).
When asked whether the guidelines should change based on the TOTAL and TASTE results, Dr. Jolly said there should be a clear recommendation that routine thrombus aspiration should not be the appropriate approach, while the issue of bailout aspiration may be left to clinician judgment.
The finding of late strokes is difficult to understand and should be interpreted with caution because of the small number of strokes occurring between 30 and 180 days, he said. Detailed analysis of all strokes will be presented at a later meeting, but Rankin Scale scores show several strokes were “very debilitating.” There is a consistency in the data, as a meta-analysis of smaller trials also identified an increased stroke risk with adjunctive thrombectomy.
Discussant Dr. Gregg W. Stone, director of cardiovascular research and education at Columbia University Medical Center in New York, said a mechanism for periprocedural stroke with aspiration can be envisioned, but that understanding the risk of ongoing, late stroke is more difficult.
As for why thrombectomy didn’t work, “aspiration is incredibly inefficient, thromboemboli still occur before, during, and after aspiration, the timing of aspiration is often too late to benefit most patients,” and other mechanisms of myonecrosis may predominate, such as reperfusion injury, he observed.
Dr. Stone said the TOTAL results should change practice and that the guideline recommendation should be downgraded to IIb.
“There are some patients who have a very large thrombus burden who have trouble dealing with all that thrombus in the cath lab who might benefit, and it is impossible to design randomized trials for small sections and groups of patients,” he said. “I wouldn’t make it class III by any means, but I think it’ll take a long time for that reduction in use to actually transmit through clinical practice, because I must say interventional cardiologists love the idea of simply removing thrombus with a relatively easy-to-use device.”
Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said in an interview that TOTAL will make operators much more selective and cautious in their performance of thrombectomy until further insights into the stroke issue are available. Thrombectomy should be reserved for bailout instances and not as a front-line therapy, he said.
On the other hand, the stroke rate in the early phase was not significantly different between groups in an as-treated analysis, and an opportunity exists to investigate potential differences between stroke and nonstroke patients to determine whether other comorbidities rather than thrombectomy per se may account for the stroke signal, Dr. Kandzari observed.
TOTAL was funded by the Canadian Institutes of Health Research, Canadian Network and Centre for Trials Internationally, and Medtronic. Dr. Jolly disclosed receiving consulting fees and honoraria from AstraZeneca, speaking fees for St. Jude, and research grants from Medtronic. Dr. Nissen has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. Dr. Stone reported consulting honoraria from Guided Delivery Systems, Miracor, and Reva, and ownership interest or partnership in Arstasis, Caliber, VNT, Micardia, Biostar family funds, and Medfocus family funds. Dr. Kandzari reported research and grant support from Medtronic, Biotronic, Abbott Vascular, and Boston Scientific.
AT ACC 2015
Key clinical point: Routine manual thrombectomy did not improve 180-day outcomes and increased the risk of stroke, compared with PCI alone.
Major finding: Thrombectomy plus PCI did not improve the primary outcome vs. PCI alone (6.9% vs. 7%) and doubled the 30-day stroke rate (0.7% vs. 0.3%).
Data source: Prospective study in 10,063 patients with STEMI.
Disclosures: TOTAL was funded by the Canadian Institutes of Health Research, Canadian Network and Centre for Trials Internationally, and Medtronic. Dr. Jolly disclosed receiving consulting fees and honoraria from AstraZeneca, speaking fees for St. Jude, and research grants from Medtronic.
Environmental factors affect bloodstream infections

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()
Cholinergic Urticaria With Anaphylaxis: Hazardous Duty of a Deployed US Marine
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
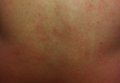
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.
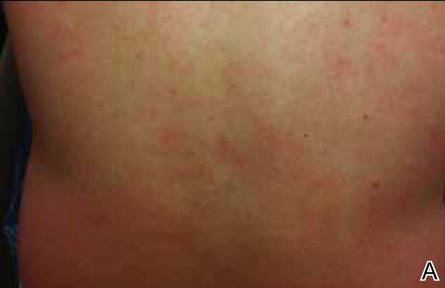
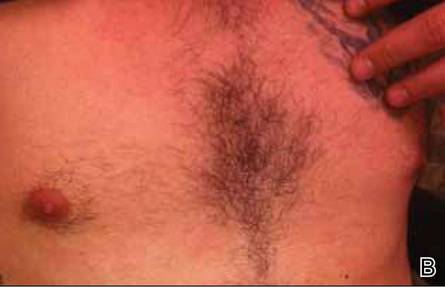
Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.


Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.


Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Practice Points
- Cholinergic urticaria can be a life-threatening condition and should be diagnosed in a controlled clinical setting where airway can be maintained.
- Cholinergic urticaria can be a profession-limiting condition, affecting people whose work involves exposure to heat or physical activity such as members of the military.
Product News: 04 2015
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Failure to warn or report
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].